Abstract
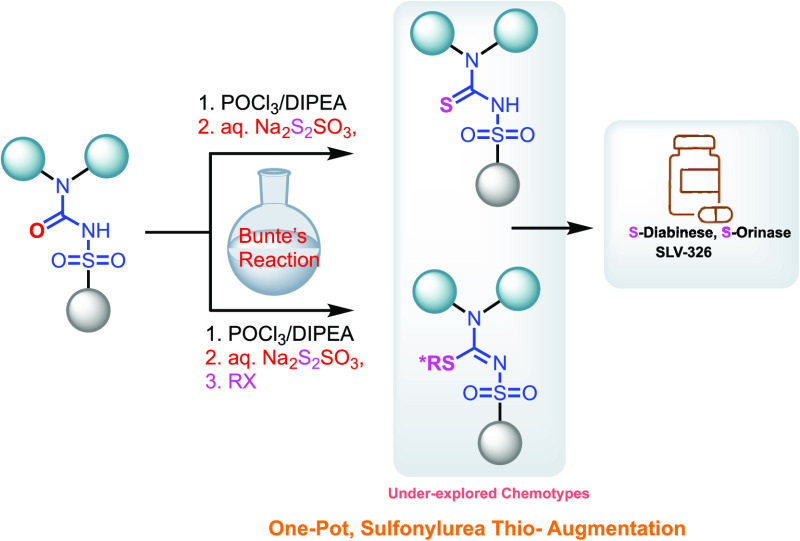
We report the development of a one-pot Bunte’s reaction-enabled expeditious platform under aqueous conditions for the scalable conversion of sulfonylureas to synthetically versatile thio-sulfonylureas. The reaction was further propagated in the same pot to yield diverse chiral and achiral isothiosulfonyl analogs. The protocol enabled the synthesis of various drug-like molecules and was applied to an enantiomeric synthesis of a cannabinoid receptor antagonist SLV326.
Introduction
Chalcogenide elements of the periodic table, namely, sulfur in its various oxidation states are proving to be indispensable in modern medicinal and environmental applications.1,2 Interrogating biological problems and treating complex diseases require new approaches to the generation of novel chemical probes and drug-like molecules often in scalable quantities. Synthetic innovation can steer the exploration of new chemical space around under-utilized scaffolds to expand their potential further in biochemical applications. In this regard, a class of ureas—sulfonylureas (SUs)—have long been used as antidiabetic agents and impact a range of biological pathways.3−6 They are reported to have anti-aging properties7 and are proposed as an auxiliary therapy for Alzheimer’s disease.8 They have a role in ameliorating inflammatory pathways by acting as inflammasome inhibitors.9,10 They can also act as potassium ATP channel modulators11 and ACAT inhibitors.12 (Figure 1A).9 They are also used widely as herbicides,13 antihelminthics,14 and acaricides.15−17 Antimicrobial and fungicidal activities are also attributed to sulfonylurea compounds.18 Sulfonylthioureas (STUs) are also purported to have insecticidal activities19 and anticonvulsant properties.20
Figure 1.

Background of sulfonylurea motifs and chalcogenide sulfonylurea synthesis.
Synthetic routes to obtain sulfonylureas have been reviewed recently.21 As for STUs, many applications explore a limited number of these analogs, possibly due to the lack of robust routes to generate them.22,23 Thiourea analogs have important utility in the synthesis of therapeutically important guanidine compounds in the presence of HgCl2.24 Generation of STUs would also enable the synthesis of isothiourea derivatives, which can have novel properties and applications.25−27 An additional innovation would be to generate novel, nontraditional chemotypes with S-bearing stereocenters, which are one of the underexplored pharmacophores in the biological realm (Figure 1A).28
In this report, we highlight a simple, one-pot sequential approach to generate terminal sulfonyl thiocarbamide analogs or its propagation to obtain sulfonylisothiocarbamide analogs in a complex scaffold. The utility of the protocol was further demonstrated in a supercritical chromatography (SFC)-free assembly of an enantiomeric active pharmaceutical ingredient (API).
Current methods to convert urea to thiourea-containing derivatives involve the use of volatile and flammable carbon disulfide and toxic and corrosive thiophosgene and malodourous reagents like Lawesson’s reagents, P2S5, NaHS, and H2S at high refluxing temperatures.29−31 A commonly used process for the synthesis of thioureas involves unstable isothiocyanates (Figure 1D).32 These routes however remain mercurial for the generation of chalcogenide analogs of SU. A recent approach showed that aryl sulfonylisoureas and isothioureas can be generated from the alcohol/thiol source using dibromoarylsulfonamides and isocyanides.33 Thus, a practical and environment-friendly process for sulfonyl thiocarbamide construction in complex molecular scaffolds is highly desirable but still remains a challenge in organic synthesis.
Organic thiosulfates (RSSO3M, M = Na, K) are commonly known as Bunte salts named after Hans Bunte, who first reported them in 1874.34,35 They are known for their ability to be used as “sulfur surrogates.”36 A direct synthesis of thiols from halo-heterocycles using sodium thiosulfate has been reported by Foye and co-workers.37 Recently, 2-chloro-4,5-dihydroimidazole hemisulfate was subjected to a reaction with sodium thiosulfate in aqueous solution at room temperature.38 An exothermic nucleophilic substitution reaction forming the internal Bunte salt, followed by a vigorous evolution of SO3, gave imidazolidine-2-thione in good yields (Figure 1E).38 Many methodologies have appeared expanding the use of Bunte salts.36 Despite the well-established application of SUs in various biological and environmental settings, there is a paucity of routes for STU and sulfonylisothiourea (SITU) generation. We questioned whether we could integrate Bunte salt to an SU motif to selectively deliver STU, which could also be propagated to generate SITU analogs for diverse applications. On this basis, we hypothesized that a sulfonylurea imidoylhalide may serve as an intermediate precursor to sulfonylurea Bunte salt under right conditions, which may then collapse to yield isolable STUs. This pathway, if successful, would generate sulfonyl-bearing chalcogenide adducts with myriad provisions for amino inclusions/alterations. A protocol that allows for the generation of chalcogenide analogs based on SUs and concurrent augmentation to a chiral and prochiral handle will be a formidable platform for diversity-oriented synthesis (Figure 1F).
Results and Discussion
Optimization of Reaction Conditions
FDA approved drugs like chlorpropamide and tolbutamide, which are sulfonylureas that are commercially available and could support feasibility studies. 3,4-Diarylpyrazoline sulfonylureas and thioureas are purported to have insecticidal activity.19 They also serve as precursors to potent cannabinoid-1 (CB1) receptor blockers, which are currently being explored for ameliorating a range of health conditions.39 Hence, we were tempted to initially explore the generation of 3,4-diarylpyrazoline sulfonylthioureas directly from the sulfonylurea intermediate precursors.40−43 Additionally, a robust way of generating sulfonylthioureas would help practicing chemists to assay the properties of SU and STU in parallel. In an optimized approach, we now show the successful execution of the reaction depicted in Table 1. We present a protocol, where an inexpensive, odor-free and safe inorganic thiosulfate can displace an SU imidoylchloride to deliver clean, isolable sulfonyl-containing thioureas. Using 1a as the model system, imidoylchloride 2a could be obtained cleanly from substrate 1a by treatment with POCl3/N,N-diisopropylethylamine (DIPEA) at 90 °C. Upon subsequent treatment with Na2S2O3 ($0.05/g), the putative formation of a Bunte intermediate (not detected) led to the generation of carbothioamide analogs in excellent yield (Table 1C).
Table 1. Screening of Reaction Conditions Using 1a (100 mg Scale/1 mmol Scale)f.
|
C optimization conditionsa | ||||
|---|---|---|---|---|
| entry | reagent/equiv | solventb | temp (°C) | time to complete conversionc/% yield from 1ad,e |
| 1. | Na2S2O3 (5 equiv) | MeOH–H2O | RT | 12/c85% |
| 2. | Na2S2O3 (5 equiv) | MeOH–H2O | 55 | 2 h/c80% |
| 3. | Na2S2O3 (2 equiv) | MeOH–H2O | 90 | 20 min/d91% |
| 4. | Na2S2O3 (5 equiv) | MeOH | 55 | 3 h/c70% |
| 5. | Na2S2O3 (2 equiv) | MeOH | 85 | 1 h/c84% |
| 6. | Na2S2O3 (2 equiv) | EtOH–H2O | 90 | 20 min/cNA |
| 7. | Na2S2O3, (2 equiv) | dioxane–H2O | 85 | 30 min/d72% |
| 8. | Na2S2O3, (2 equiv) | toluene–H2O | 90 | -/traces |
| 9. | Na2S2O3, (2 equiv) | DMF–H2O | 85 | 30 min/d68% |
| 10. | Na2S2O3, (2 equiv) | acetonitrile–H2O | 85 | 1 h/c68% |
Reaction conditions: 1a (0.2 mmol scale/see the Supporting Information, SI, for additional details).
Water was used at a maximum of 10% solvent combination.
Conversion based on liquid chromatography–mass spectrometry (LCMS) comparison with intermediate imidoylchloride 2a.
Yield based on work-up/MeOH-IPA (1:1) trituration.
Yield based on work-up/flash chromatography (40% hex/EtOAc).
[A] Streamlined process for sulfonyl thiourea synthesis. [B] Reaction monitoring of crude thiourea. [C] Optimization of reaction conditions.
This one-pot, two-step protocol with or without the isolation of the intermediate imidoylchloride bypasses the need for sensitive isothiocyanate precursors. The imidoylchloride intermediate could also be generated from PCl5 in refluxing chlorobenzene although decomposition side products were seen alongside (SI). The nucleophilic displacement of imidoylchloride 2a by the thiosulfate ion worked best in methanol/H2O at 90 °C, and the thiourea analog could be generated within 20 min. The nucleophilic displacement also worked well in methanol/H2O at 55 °C with excess (5 equiv) Na2S2O3 over a period of 2 h. The reaction worked sluggishly at room temperature and proceeded to give 3a over extended reaction times (∼12 h). Dimethylformamide (DMF)/H2O, ethanol/H2O, and acetonitrile/water were acceptable solvents for the reaction. The reaction also proceeded in aqueous dioxane although somewhat slower, and only traces of the product could be obtained in aqueous toluene. Water was deemed essential for solubilization of the inorganic reactant, albeit the reaction did proceed in alcoholic solvents likely due to adventitious water. In general, we observed that with substrate 1a, the reaction had a large range of temperature flexibility, where the STU product 3a could be obtained without significant loss of yield.
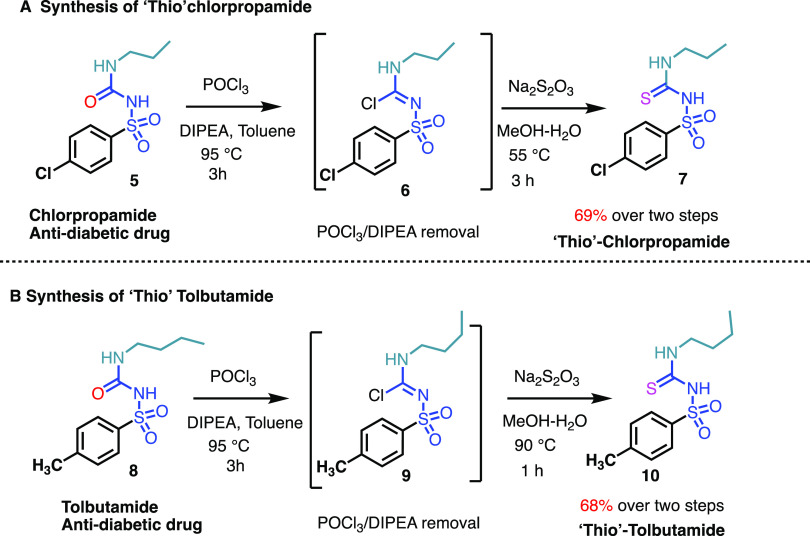
With the optimized protocol in hand, we tested whether the antidiabetic drugs chlorpropamide and tolbutamide would undergo the oxo-edits. Indeed, on a gram-scale, both compounds underwent the imidolylchloride conversion and the subsequent thiosulfate displacement, leading to clean corresponding thiourea products in yields greater than 65% (Scheme 1).
Scheme 1. Thio-Antidiabetic FDA-Approved Drugs Synthesized through the Present Protocol [A] and Thio-Chlorpropamide (Diabinese) (1 g Scale) and [B] Thio-Tolbutamide Synthesis (Orinase) (1 g Scale).
Substrate Scope
Having ready access to a range of 3,4-diarylpyrazoline SU products, we then proceeded to test the scope of conversion of 3,4-dihydropyrazoline sulfonylurea precursors to substituents that could be tolerated on the arylsulfonyl part of the molecule. To our delight, many of the commonly used groups for structure–activity relationship (SAR) studies in biological applications (1a–t) were very well tolerated under the optimized reaction conditions. As seen in Scheme 2, a wide variety of substituted sulfonylurea precursors40 proceeded to provide the STU in good to excellent yields. 3,4-Dihydropyrazoline compounds of type 1/3 bear a stereocenter at the C4 pyrazoline ring.
Scheme 2. Scope and Skeletal Diversity for the Bunte Reaction of Sulfonylureas (See the Yield Color Code for Conditions#).

Therefore, in the case of example 1q (4-chlorophenyl substituent), we used the racemic R as well as S-enantiomer of the SU precursor to generate the corresponding thiourea products. Gratifyingly, we saw that the two-step, one-pot protocol proceeded to give the thio-products with no erosion of chirality as documented by chiral high-performance liquid chromatography (HPLC) (SI). Replacement of the aryl groups at the sulfonyl end with dialkyl or cyclic amino pendants in the 3,4-diarylpyrazoline SU precursors (1u–w) also provided the sulfonylthioureas (3u–w) in yields above 50%. Conditions 2 in Table 1C were deemed to be optimal for substrates 1u–w. We also observed that yields of the products were largely unaffected by the electronics on the sulfonyl end of the molecule.
We then proceeded to test the scope of the substituents at the nonsulfonyl end of the molecule. Various amino precursors could be utilized at this end of the molecule. Replacement of the pyrazoline core with pyridazinyl attachment also led to the conversion of urea to thiourea sulfonyl moieties (Scheme 2). Subtle differences in temperature to affect the thiosulfate displacement and use of aqueous dioxane in lieu of methanol were required to obtain the thiourea products (12a–d). Additionally, substituents like a dense adamantyl group and simple rings were also well tolerated as seen in examples 13a–d, 15.
Modification Approaches
To maximize the utility of this new protocol, we posited that the Bunte salts can be alkylated “in situ” to generate sulfonylisothiourea analogs. Treatment of 3a reaction mixture containing the preformed “masked STU” underwent facile alkylation in the same pot upon reaction with methyl iodide, resulting in a clean conversion to the S-methylated analog 17 (Scheme 3). This analog has potent cannabinoid-1 receptor antagonist activity.40 It should be noted that the reaction required a slight excess of the alkylating precursor for complete transformation. A clear hint that the reaction occurs on the putative Bunte salt 4a with concomitant alkylation along with SO3 extrusion was seen with faster reaction times of the in situ alkylation as opposed to the alkylation in methanol after thiourea isolation (Scheme 3A,B).
Scheme 3. Modification to Diverse Thiosulfonyl Functionality [A] Building Block Diversity for Three-Step, One-Pot Chalcogenide Sulfonylurea Functional Group Propagation and [B] Mechanistic Insights in Three-Step, One-Pot Thiosulfonyl Augmentation.

That this reaction could proceed smoothly under wet alcoholic conditions gave us hope that other alkylation agents/STU may also be amenable to this transformation, yielding novel isothiosulfonyl molecular scaffolds. Importantly, we were able to utilize unprotected alkylating precursors like butynyl bromide (18/23) bromo-acetic acid and bromoethanol to yield chemotypes 22 and 25, respectively, on different STU scaffolds, which could be primed for downstream coupling manipulations. It became clear to us that we could also use chiral alkylating agents to drive this synthesis toward stereoselective products. Expanding on those lines, the strategy was successfully executed by the reaction of racemic 1a with methyl (S)-3-bromo-2-methylpropanoate to uneventfully yield 19 as a diastereomeric mixture. Similarly, racemic 1q could be turned into a diastereomeric mixture 20 using a chiral alkylating agent ((S)-1-bromo-2-methylbutane). As seen in Scheme 3B, for the alkylation procedure, in situ trapping of Bunte salt without thiourea isolation (step C/D) is the method of choice. The alkylation of thiourea of type 3a proceeds to give products in good yields even under aqueous methanol or dioxane. When the thiourea was isolated and alkylation attempted in methanol/95 °C (step E/F), the reaction proceeded sluggishly (MeI) or small traces of products were seen in the case of alkylating agents like (S)-3-bromo-2-methylpropanoate.
With the wealth of combinations available as building blocks for amino, sulfonyl, and alkylating agents, one could envision constructing a diverse screening library based on this synthetic platform.
Also, this protocol would open avenues for the generation of novel thio-mimics and warheads.
Finally, we sought to exploit this methodology in the preparation of substituted 4,5-dihydro-1H-pyrazoles that are useful as potent cannabinoid receptor antagonists. Recently, we and others have employed such scaffolds to generate novel, selective agents that have the potential in treating fibrosis, obesity, and related metabolic disorders.39−41,44−46 Thus, there remains a need for an efficient, high-yielding, and scalable synthetic approach to provide substantial amounts of enantiomerically pure active pharmaceutical ingredients (APIs) in the chiral pyrazoline series for biological evaluation. As a proof of principle, our current protocol provided validation of its orthogonal utility by enabling an expeditious assembly of a chiral pyrazoline sulfonyl carboximdamide CB1 receptor antagonist, SLV326.47 We decided to apply our newly developed protocol by utilizing an isothiourea-based chiral auxiliary/decoy intermediate to generate diastereomeric isothiourea precursors, which could be displaced by an amino pendant to give a chiral supercritical chromatography (SFC)-free approach to generate enantiomeric APIs.
Compounds of type 1a have a singular quaternary carbon stereocenter at the C4 position of the pyrazoline ring. Previous methods have used chiral HPLC/SFC separation of the final racemic mixture to yield the biologically active S-enantiomer.40,41 Since our methodology was amenable to multigram-scale synthesis of STU precursor 3a (71% yield on a standalone 2.0 g scale), we chose to synthesize the precursor of type 28 (via 19) through an in situ chiral alkylating agent bearing a known stereocenter. This ensured access to a separable diastereomeric mixture, which could afford a single diastereomer of type 28. Displacement of the thioalkyl group under controlled basic conditions can then deliver the requisite enantiomeric API (Scheme 4). The diastereomeric mixture 19 thus enabled the generation of single diastereomer 28 through preferential recrystallization. Chiral HPLC analysis showed that the crystals were indeed enriched in one of the diastereomeric forms (S,S). The stereochemistry of the diastereomer was assigned by separation on R,R-Whelk-O chiral column and X-ray crystallography (SI). Knowing the stereochemistry at the S-alkylation center, the S,S-diastereomer was treated individually with methyl amine in a DCM:MeOH/Et3N mixture to obtain SLV326. Isolation of SLV326 and comparison of literature data showed that the transformation proceeded with 97.9% ee and in 84% yield for the final step.
Scheme 4. Gram-Scale Synthesis and Application of the Protocol in Chiral Synthesis of Cannabinoid-1 Receptor Antagonists.
Conclusions
In summary, we have successfully achieved a facile protocol for oxo-edits based on a modified Bunte salt approach in sulfonylurea molecules to the sulfur atom. The reaction relies on a sequential one-pot generation of an imidoylchloride, followed by a putative Bunte salt formation, which expels sulfur trioxide to create a new C–S bond. Additionally, common sulfonyl thiourea intermediates could be alkylated in the same pot to various sulfur pendant side chains. We have demonstrated a high sulfonylurea substrate tolerance of this method and its application to diverse chiral and achiral de novo sulfur-containing drug-like molecules. A further validation of this protocol was achieved by a successful application of this strategy in a high-yield synthesis of potent dihydropyrazoline class of cannabinoid antagonists, enabled by a key diastereomeric intermediate bearing a chiral isothiourea handle. An added advantage of this protocol is the use of cheap feedstock (inorganic thiosulfate) under aqueous conditions with clean, isolable products under controlled temperatures and solvents employed. The robust and expedient reaction conditions along with adaptable precursors in this transformation will help expand the underexplored chemical space of isothio-based (chiral) analogs and provide downstream access to expanding S-oxidation states or utilizing them as decoy substrates for synthetic transformation. We reckon that this protocol will serve as a versatile tool in many milieus and will help influence the assembly of diverse target libraries based on thio-sulfonylurea entities.
Acknowledgments
This work was supported by intramural funds from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to M.R.I. The authors thank Dr. George Kunos for reading the manuscript and helpful suggestions. For help with HRMS data, John Lloyd is acknowledged. Dr. Klaus Gawrisch and Dr. Walter Teague are acknowledged for their help with NMR experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04816.
The authors declare the following competing financial interest(s): The United States of America as represented by the Secretary, Department of Health and Human Services has filed provisional patent applications on compounds and the subject matter related to this study.
Notes
The United States of America as represented by the Secretary, Department of Health and Human Services, has filed provisional patent applications on compounds and the subject matter related to this study.
Supplementary Material
References
- Beno B. R.; Yeung K.-S.; Bartberger M. D.; Pennington L. D.; Meanwell N. A. A Survey of the Role of Noncovalent Sulfur Interactions in Drug Design. J. Med. Chem. 2015, 58, 4383–4438. 10.1021/jm501853m. [DOI] [PubMed] [Google Scholar]
- Mustafa M.; Winum J.-Y. The Importance of Sulfur-Containing Motifs in Drug Design and Discovery. Expert Opin. Drug Discovery 2022, 17, 1–12. 10.1080/17460441.2022.2044783. [DOI] [PubMed] [Google Scholar]
- Proks P.; Reimann F.; Green N.; Gribble F.; Ashcroft F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 2002, 51, S368–S376. 10.2337/diabetes.51.2007.S368. [DOI] [PubMed] [Google Scholar]
- Patlak M. New Weapons to Combat an Ancient Disease: Treating Diabetes. FASEB J. 2002, 16, 1853 10.1096/fj.02-0974bkt. [DOI] [PubMed] [Google Scholar]
- Seino S. Cell Signalling in Insulin Secretion: The Molecular Targets of ATP, CAMP and Sulfonylurea. Diabetologia 2012, 55, 2096–2108. 10.1007/s00125-012-2562-9. [DOI] [PubMed] [Google Scholar]
- Sola D.; Rossi L.; Schianca G. P. C.; Maffioli P.; Bigliocca M.; Mella R.; Corlianò F.; Fra G. P.; Bartoli E.; Derosa G. Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 11, 840–848. 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z.; Liu W.; Huang Y.; Sun T.; Bao K.; Feng J.; Moskalev A.; Hu Z.; Li J. Anti-Aging Effects of Chlorpropamide Depend on Mitochondrial Complex-II and the Production of Mitochondrial Reactive Oxygen Species. Acta Pharm. Sin. B 2022, 12, 665–677. 10.1016/j.apsb.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah P.; Das A.; Paul D.; Chakrabarty S.; Aguan K.; Mitra S. Sulfonylurea Class of Antidiabetic Drugs Inhibit Acetylcholinesterase Activity: Unexplored Auxiliary Pharmacological Benefit toward Alzheimer’s Disease. ACS Pharmacol. Transl. Sci. 2021, 4, 193–205. 10.1021/acsptsci.0c00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll R. C.; Robertson A. A. B.; Chae J. J.; Higgins S. C.; Muñoz-Planillo R.; Inserra M. C.; Vetter I.; Dungan L. S.; Monks B. G.; Stutz A.; et al. A Small-Molecule Inhibitor of the NLRP3 Inflammasome for the Treatment of Inflammatory Diseases. Nat. Med. 2015, 21, 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux D. G.; McNiff P.; Laliberte R.; Hawryluk N.; Peurano H.; Stam E.; Eggler J.; Griffiths R.; Dombroski M. A.; Gabel C. A. Identification and Characterization of a Novel Class of Interleukin-1 Post-Translational Processing Inhibitors. J. Pharmacol. Exp. Ther. 2001, 299, 187–197. [PubMed] [Google Scholar]
- Amoroso S.; Schmid-Antomarchi H.; Fosset M.; Lazdunski M. Glucose, Sulfonylureas, and Neurotransmitter Release: Role of ATP-Sensitive K+ Channels. Science 1990, 247, 852–854. 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Ohgami N.; Kuniyasu A.; Furukawa K.; Miyazaki A.; Hakamata H.; Horiuchi S.; Nakayama H. Glibenclamide Acts as an Inhibitor of Acyl-CoA:Cholesterol Acyltransferase Enzyme. Biochem. Biophys. Res. Commun. 2000, 277, 417–422. 10.1006/bbrc.2000.3681. [DOI] [PubMed] [Google Scholar]
- Appleby A. P.; Müller F.; Carpy S.. Weed Control. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Loos J. A.; Churio M. S.; Cumino A. C. Anthelminthic Activity of Glibenclamide on Secondary Cystic Echinococcosis in Mice. PLoS Neglected Trop. Dis. 2017, 11, e0006111 10.1371/journal.pntd.0006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. V. Chemistry of Sulfonylurea Herbicides. Pestic. Sci. 1990, 29, 247–261. 10.1002/ps.2780290303. [DOI] [Google Scholar]
- Brown H. M. Mode of Action, Crop Selectivity, and Soil Relations of the Sulfonylurea Herbicides. Pestic. Sci. 1990, 29, 263–281. 10.1002/ps.2780290304. [DOI] [Google Scholar]
- Sarmah A. K.; Sabadie J. Hydrolysis of Sulfonylurea Herbicides in Soils and Aqueous Solutions: A Review. J. Agric. Food Chem. 2002, 50, 6253–6265. 10.1021/jf025575p. [DOI] [PubMed] [Google Scholar]
- Faidallah H. M.; Khan K. A.; Asiri A. M. Synthesis and Characterization of a Novel Series of Benzenesulfonylurea and Thiourea Derivatives of 2H-Pyran and 2H-Pyridine-2-Ones as Antibacterial, Antimycobacterial and Antifungal Agents. Eur. J. Chem. 2011, 2, 243–250. 10.5155/eurjchem.2.2.243-250.257. [DOI] [Google Scholar]
- Grosscurt A. C.; Van Hes R.; Wellinga K. 1-Phenylcarbamoyl-2-Pyrazolines, a New Class of Insecticides. 3. Synthesis and Insecticidal Properties of 3,4-Diphenyl-1-Phenylcarbamoyl-2-Pyrazolines. J. Agric. Food Chem. 1979, 27, 406–409. 10.1021/jf60222a061. [DOI] [Google Scholar]
- Masereel B.; Lambert D. M.; Dogné J. M.; Poupaert J. H.; Delarge J. Anticonvulsant Activity of Pyrid-3-Yl-Sulfonyl Ureas and Thioureas. Epilepsia 1997, 38, 334–337. 10.1111/j.1528-1157.1997.tb01125.x. [DOI] [PubMed] [Google Scholar]
- De Ventura T.; Zanirato V. Recent Advances in the Synthesis of Sulfonylureas. Eur. J. Org. Chem. 2021, 2021, 1201–1214. 10.1002/ejoc.202001437. [DOI] [Google Scholar]
- Ding C.; Wang S.; Sheng Y.; Dai Q.; Zhao Y.; Liang G.; Song Z. One-Step Construction of Unsymmetrical Thioureas and Oxazolidinethiones from Amines and Carbon Disulfide via a Cascade Reaction Sequence. RSC Adv. 2019, 9, 26768–26772. 10.1039/C9RA04540F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti R.; Moroni G.; Carotti A.; Gioiello A.; Camaioni E. Recent Advances in Urea- and Thiourea-Containing Compounds: Focus on Innovative Approaches in Medicinal Chemistry and Organic Synthesis. RSC Med. Chem. 2021, 12, 1046–1064. 10.1039/D1MD00058F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. H. M.; Verhoog S.; Sanders H. J.; van Loevezijn A.; Kruse C. G. A Novel Atom-Efficient, One-Pot Synthesis of Sulfonylguanidines and Sulfamoylguanidines. Tetrahedron Lett. 2011, 52, 3198–3200. 10.1016/j.tetlet.2011.04.031. [DOI] [Google Scholar]
- Perlovich G. L.; Proshin A. N.; Volkova T. V.; Kurkov S. V.; Grigoriev V. V.; Petrova L. N.; Bachurin S. O. Novel Isothiourea Derivatives as Potent Neuroprotectors and Cognition Enhancers: Synthesis, Biological and Physicochemical Properties. J. Med. Chem. 2009, 52, 1845–1852. 10.1021/jm8012882. [DOI] [PubMed] [Google Scholar]
- Southan G. J.; Szabó C.; Thiemermann C. Isothioureas: Potent Inhibitors of Nitric Oxide Synthases with Variable Isoform Selectivity. Br. J. Pharmacol. 1995, 114, 510–516. 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wahaibi L. H.; Hassan H. M.; Abo-Kamar A. M.; Ghabbour H. A.; El-Emam A. A. Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities. Molecules 2017, 22, 5089 10.3390/molecules22050710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Ang E. C. X.; Yang Z.; Kee C. W.; Tan C.-H. Synthesis of Chiral Sulfinate Esters by Asymmetric Condensation. Nature 2022, 604, 298–303. 10.1038/s41586-022-04524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk T.; Ertas E.; Mert O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- Ozturk T.; Ertas E.; Mert O. A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses. Chem. Rev. 2010, 110, 3419–3478. 10.1021/cr900243d. [DOI] [PubMed] [Google Scholar]
- Sharma S. Thiophosgene in Organic Synthesis. Synthesis 1978, 1978, 803–820. 10.1055/s-1978-24896. [DOI] [Google Scholar]
- Hermanson G. T.The Reactions of Bioconjugation. In Bioconjugate Techniques; Elsevier, 2013; pp 229–258. [Google Scholar]
- Mishra D.; Phukan P. A Unified Approach for the Synthesis of Isourea and Isothiourea from Isonitrile and N,N-Dibromoarylsulfonamides. J. Org. Chem. 2021, 86, 17581–17593. 10.1021/acs.joc.1c01578. [DOI] [PubMed] [Google Scholar]
- Distler H. The Chemistry of Bunte Salts. Angew. Chem., Int. Ed. 1967, 6, 544–553. 10.1002/anie.196705441. [DOI] [Google Scholar]
- Bunte H. Zur Constitution Der Unterschwefligen Säure. Ber. Dtsch. Chem. Ges. 1874, 7, 646–648. 10.1002/cber.187400701201. [DOI] [Google Scholar]
- Biswas K.; Basu B. Bunte Salts and Congeners as Efficient Sulfur Surrogates: Recent Advances. Curr. Organocatal. 2019, 5, 182–195. 10.2174/2213337206666181122101209. [DOI] [Google Scholar]
- Foye W. O.; Abood N.; Kauffman J. M.; Kim Y.-H.; Patel B. R. A Direct Synthesis of Heterocyclic Thiols. Phosphorus Sulfur Relat. Elem. 1980, 8, 205–207. 10.1080/03086648008078190. [DOI] [Google Scholar]
- Sączewski F.; Gdaniec M.; Data K. A New Imidazoline-Containing Bunte Salt: Synthesis, Molecular and Electronic Structure. Heterocycl. Commun. 2017, 23, 359–363. 10.1515/hc-2017-0177. [DOI] [Google Scholar]
- Cinar R.; Iyer M. R.; Kunos G. The Therapeutic Potential of Second and Third Generation CB1R Antagonists. Pharmacol. Ther. 2020, 208, 107477 10.1016/j.pharmthera.2020.107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. H. M.; Coolen H. K. A. C.; van Stuivenberg H. H.; Dijksman J. A. R.; Herremans A. H. J.; Ronken E.; Keizer H. G.; Tipker K.; McCreary A. C.; Veerman W.; et al. Synthesis, Biological Properties, and Molecular Modeling Investigations of Novel 3,4-Diarylpyrazolines as Potent and Selective CB(1) Cannabinoid Receptor Antagonists. J. Med. Chem. 2004, 47, 627–643. 10.1021/jm031019q. [DOI] [PubMed] [Google Scholar]
- Iyer M. R.; Cinar R.; Katz A.; Gao M.; Erdelyi K.; Jourdan T.; Coffey N. J.; Pacher P.; Kunos G. Design, Synthesis, and Biological Evaluation of Novel, Non-Brain-Penetrant, Hybrid Cannabinoid CB1R Inverse Agonist/Inducible Nitric Oxide Synthase (INOS) Inhibitors for the Treatment of Liver Fibrosis. J. Med. Chem. 2017, 60, 1126–1141. 10.1021/acs.jmedchem.6b01504. [DOI] [PubMed] [Google Scholar]
- Cinar R.; Iyer M. R.; Liu Z.; Cao Z.; Jourdan T.; Erdelyi K.; Godlewski G.; Szanda G.; Liu J.; Park J. K.; et al. Hybrid Inhibitor of Peripheral Cannabinoid-1 Receptors and Inducible Nitric Oxide Synthase Mitigates Liver Fibrosis. JCI Insight 2016, 1, e87336 10.1172/jci.insight.87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex J. M.; Kumar R. 4,5-Dihydro-1H-Pyrazole: An Indispensable Scaffold. J. Enzyme Inhib. Med. Chem. 2014, 29, 427–442. 10.3109/14756366.2013.795956. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Iyer M. R.; Godlewski G.; Jourdan T.; Liu J.; Coffey N. J.; Zawatsky C. N.; Puhl H. L.; Wess J.; Meister J.; et al. Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist. ACS Pharmacol. Transl. Sci. 2021, 4, 1175–1187. 10.1021/acsptsci.1c00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorvat R. J. Peripherally Restricted CB1 Receptor Blockers. Bioorg. Med. Chem. Lett. 2013, 23, 4751–4760. 10.1016/j.bmcl.2013.06.066. [DOI] [PubMed] [Google Scholar]
- Iyer M. R.; Cinar R.; Wood C. M.; Zawatsky C. N.; Coffey N. J.; Kim K. A.; Liu Z.; Katz A.; Abdalla J.; Hassan S. A.; Lee Y. S. Synthesis, Biological Evaluation, and Molecular Modeling Studies of 3,4-Diarylpyrazoline Series of Compounds as Potent, Nonbrain Penetrant Antagonists of Cannabinoid-1 (CB1R) Receptor with Reduced Lipophilicity. J. Med. Chem. 2022, 65, 2374–2387. 10.1021/acs.jmedchem.1c01836. [DOI] [PubMed] [Google Scholar]
- Lange J. H. M.; van Stuivenberg H. H.; Veerman W.; Wals H. C.; Stork B.; Coolen H. K. A. C.; McCreary A. C.; Adolfs T. J. P.; Kruse C. G. Novel 3,4-Diarylpyrazolines as Potent Cannabinoid CB1 Receptor Antagonists with Lower Lipophilicity. Bioorg. Med. Chem. Lett. 2005, 15, 4794–4798. 10.1016/j.bmcl.2005.07.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.