Abstract

The frequency of overweight and obesity is rising globally. These disorders are prevalent health problems. It has a substantial correlation with a number of health issues, including cardiovascular, metabolic, and diabetes mellitus disorders. Lycopene (Lyc) is an acyclic structural isomer of β-carotene and has powerful antioxidant properties with various promising therapeutic effects. In this study, rats fed a high-fat diet were examined to determine how lycopene affected metabolic syndrome and kidney damage. After being acclimated, rats were divided into 5 groups (n = 8/group) as follows: the first group served as the control and was fed on a normal pelleted diet (4.25% fat) until the end of the experiment. The second group (high-fat diet; HFD) was fed on a high-fat diet (45.5 kcal% fat) composed of 24% fat, 24% protein, and 41% carbohydrate. The third and fourth groups were fed on HFD and administered lycopene at 25 and 50 mg/kg bodyweight orally every day. The fifth group (standard drug group) received HFD and simvastatin (SVS; 10 mg/kg bodyweight orally daily) for 3 months. Tissue samples from the kidney were taken for determination of the biochemical parameters, lipid peroxidation (LPO), protein carbonyl (PC), reduced glutathione (GSH), total thiol group, antioxidant enzymes, namely, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR), in addition to renal mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2), renal levels of inflammatory markers [tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)], and apoptotic markers (BCL2 Associated X (Bax), B-cell lymphoma 2 (Bcl-2), and Bax/Bcl-2 ratio). When compared to the control group, the HFD group’s food consumption, body weight, serum levels of glucose, uric acid, creatinine, LPO, PC, TNF-α, IL-1β, Bax, and the Bax/Bcl-2 ratio all increased significantly. In the kidney sample of HFD-fed rats, there was a downregulation of Nrf2 mRNA expression along with a significant reduction in the enzymatic activity of SOD, CAT, GR, and GPx. Lyc treatment was able to successfully reverse HFD-mediated changes as compared to the HFD group. Consuming lyc helps to prevent fat and renal damage in a positive way.
1. Introduction
Obesity prevalence has risen dramatically during the last 50 years, reaching epidemic proportions. It is a significant public health issue since it drastically increases the risk of type 2 diabetes, myocardial infarction, stroke, and numerous malignancies, decreasing both life expectancy and quality of life.1 Obesity prevalence grew everywhere, and it nearly tripled between 1975 and 2020.2 Moreover, more than 2 billion adults (or 39% of the worldwide adult population) will be overweight by 2020. Over 600 million of them were obese.3
Over the last three decades, dietary fat consumption has risen dramatically.4 Diets high in saturated fat have been shown to be harmful,5 according to a large body of evidence. A high-fat diet (HFD) induces cellular and molecular damage, as well as an oxidative stress response.6 Furthermore, previous research has demonstrated that HFD causes oxidative stress by increasing the overproduction of reactive oxygen species (ROS) in adipocytes and several organs.7
Simvastatin (SVS) is a statin that lowers cholesterol by blocking the enzyme 3-hydroxy-3-methyl glutaryl coenzyme-A reductase, which is involved in cholesterol biosynthesis.8,9 In addition, SVS has antioxidant properties in rats fed an HFD diet.10 However, as the other statins, SVS may cause muscle pain, digestive problems, diabetes mellitus, kidney injury, and mental fuzziness in some people who take them and may rarely cause hepatic dysfunction.11 As a result, scientists began to focus their efforts on natural bioactive substances with antiobesity and antioxidant properties with no side effects. Antioxidants are substances that reduce ROS levels by modifying glucose, lipid, and amino acid homeostasis pathways and decreasing inflammation.12 Total antioxidant capacity, superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities can all be used to establish the animal’s oxidative status. A crucial transcription factor that regulates the antioxidant response is nuclear factor erythroid 2-related factor 2 (Nrf2)13 via controlling the expression of several phase II detoxifying and antioxidant enzymes, including GPx, SOD, catalase, heme oxygenase-1, glutamate-cysteine ligase, and glutathione S-transferase.14
In today’s world, traditional obesity treatment primarily consists of synthetic molecules and surgical treatments, both of which have several negative side effects and high recurrence rates, restricting their use.15 Therefore, creating easily accessible, safe, and cost-effective entities is essential. The first defense in the fight against diseases and associated complications is thought to be plant-based remedies.15 As a result, a range of plant metabolites have been exploited as an alternate technique for the treatment of obesity in several antiobesity products.9,16 Lycopene (Lyc), a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables like red carrots, watermelons, grapefruits, and papaya,17 is one of these natural chemicals. Lyc is well known for its antioxidant and anti-inflammatory activities as well as its capacity to influence key metabolic processes in the body.18 In this regard, Al-Brakati et al.19 recently were found that biosynthesis of selenium nanoparticles using Lyc has a renoprotective effect on acute kidney injury via antioxidant, anti-inflammatory, antiapoptotic, and antinecroptotic properties. Furthermore, we previously showed the antilipidemic effect of Lyc in HFD-induced dyslipidaemia in rats.20 Given the widespread consumption of fat-rich foods in modern countries, this study aimed to evaluate the possible effects of Lyc on metabolic syndrome and renal injury in rats fed a high-fat diet.
2. Materials and Methods
2.1. Animal
Male Wistar rats weighing 200–220 g were kept in a controlled environment with a temperature of 25 ± 1 °C, humidity level of 50 ± 10%, and 12 h cycle of light and darkness. Rats were habituated with free access to food and water for 2 weeks prior to beginning the experiment.
2.2. Experimental Design
After acclimating, the rats were allocated to five experimental groups (each with eight rats) as follows: the first group acted as the control group. Until the completion of the experiment, it was fed a normal pelleted diet (3.7 kcal/g; 4.2 percent fat). A high-fat diet (4.9 kcal/g; 29.3 percent fat) was given to the second group (HFD), which consisted of 29.3% fat, 25.5% protein, and 29.9% carbohydrate (TD.07011; Envigo, USA). The third and fourth groups were fed an HFD and given Lyc (Sigma Chemicals, St. Louis, MO, USA) orally every day at doses of 25 and 50 mg/kg bodyweight, respectively. HFD and simvastatin (SVS; 10 mg/kg bodyweight orally daily) were given to the fifth group (standard group). The lycopene doses chosen were based on previous research.21,22 Dimethyl sulfoxide (DMSO) was used to dissolve the lycopene before it was diluted with normal saline solution (0.9 percent NaCl) until it contained less than 0.25 percent DMSO, or 10 μL per rat. As a result, untreated lycopene groups (control, HFD, and SVS-treated rats) were given 10 μL of DMSO per rat. To determine food intake, pelleted diets were weighed and distributed daily. Consumption was defined as the difference in pellet weight per group within a 24 h period. While for determining the bodyweight gain, initial bodyweight of all rats was determined before they were fed with either normal diet or HFD for 3 months. At the end of the experiment, the weight gain (%) was calculated as follows:
W is week.
2.3. Sampling and Tissue Preparation
Rats were euthanized by an excess dose of pentobarbital (300 mg/kg, i.p.) after a 3 month intervention. A syringe puncture was utilized to collect blood from the heart, which was kept at 37 °C for 30 min and then centrifuged at 3000g for 10 min to separate the serum, which was then stored at 80 °C for biochemical analysis. The kidney tissue was taken as soon as possible. The extracted kidney tissue was homogenized in a 10-fold volume of ice-cold 5 mM potassium phosphate buffer (pH 7.4). The supernatant was separated by centrifugation for 10 min at 3000g (4 °C). The supernatants were kept at a temperature of 80 °C and used for biochemical analysis. Some kidney tissues were taken and preserved at −80 °C until gene expression assays were performed, or they were fixed in 10% buffered formalin for histopathological examination.
2.4. Measurement of Kidney Function Biomarkers in Serum
Utilizing colorimetric kits, the serum concentrations of uric acid, creatinine, and urea were assessed using the same procedures used by RANDOX Reagents (USA). However, neutrophil gelatinase-associated lipocalin (NGAL), one of the most promising novel biomarkers of renal epithelial damage, was evaluated using an enzyme-linked immunosorbent assay (ELISA) according to Abcam’s recommendations (Cambridge, UK).
2.5. Renal Oxidative Stress Markers
To assess oxidant/antioxidant imbalance in kidney tissue, malondialdehyde (MDA), which is the byproduct of lipid peroxidation (LPO), and nitric oxide (NO) levels were assessed using the Ohkawa et al.23 and Griess reagent24 protocols, respectively. Protein carbonyl content (PC) was determined using a colorimetric kit obtained from Abcam (Cambridge, UK) and followed the manufacturer’s directions. In addition, the contents of reduced glutathione (GSH) and total thiol group (T-SH) were determined using Ellman’s method25 with some modifications to measure T-SH.
2.6. Antioxidant Enzyme Activity in the Kidneys
Superoxide dismutase (SOD) and catalase (CAT) activity in the kidney were evaluated using the techniques reported by Nishikimi et al.26 and Aebi,27 respectively. Additionally, glutathione reductase (GR) was calculated using De Vega et al. procedures’29 and the renal activities of glutathione peroxidase (GPx) were evaluated using Paglia and Valentine’s methods.28
2.7. Inflammatory and Apoptotic Markers in Kidney Tissue Determination
Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), B-cell lymphoma 2 (Bcl-2), and BCL2 associated X, apoptosis regulator (Bax)levels in kidney tissue were assessed using ELISA kits from Abcam in Cambridge, UK, in accordance with the manufacturer’s recommendations. Using ELISA kits purchased from ThermoFisher Scientific, the level of NF-κB (nuclear factor kappa light chain enhancer of activated B cells) p65 was also measured (Waltham, MA, USA).
2.8. Quantitative RT- PCR Analysis
Using the Qiazol reagent and the manufacturer’s recommendations, total RNA was extracted from freshly isolated kidney tissues (Qiagen, Germantown, MD, USA). The RevertAid H Minus Reverse Transcriptase kit (Fermentas, Thermo Fisher Scientific Inc., Canada) was used to synthesize cDNA in accordance with the manufacturer’s instructions after a Nanodrop was used to measure RNA quantities. The SYBR green PCR kit was used to determine the Nrf2 mRNA levels (Qiagen, Germany). The quantitative PCR was carried out in duplicate on the ViiATM 7 PCR equipment (Applied Biosystems, USA). The relative levels of Nrf2 mRNA were calculated using the 2–ΔΔCt method and normalized to the mRNA level of the GAPDH housekeeping gene. The Nrf2 primer sequences were forward 5′-CAG CAT GAT GGA CTT GGA ATT G-3′ and reverse 5′-GCA AGC GAC TCA TGG TCA TC-3′, and the primer sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward 5′-AGT GCC AGC CTC GTC TCA TA-3′ and reverse 5′-TCC CGT TGA TGA CCA GCT TC-3′.
2.9. Histological Examination of Kidney
Histological studies were used to assess kidney injuries. Kidney tissues were fixed in a buffered 10% formaldehyde solution before being embedded in paraffin. To assess general histological features, the implanted tissue samples were sectioned (5 μm) and stained with hematoxylin and eosin.
2.10. Statistic Evaluation
Using SPSS software, all data were statistically examined. Tukey’s post-hoc test was conducted after the one-way ANOVA to determine whether there were any significant differences between the groups. The obtained data were presented as mean standard deviation. P values lower than 0.05 were regarded as significant.
3. Results
In the present investigation, we found that rats supplemented with the HFD had significantly higher food intake and weight gains (p < 0.05) than rats supplied with a conventional diet (Figure S1). In contrast to HFD rats, rats co-treated with 50 mg/kg Lyc or SVS dramatically reduced the increase in food intake and weight gain. In comparison to the HFD-treated group, the Lyc (at both doses) or SVS co-treated rats showed substantial decreases (p < 0.05) in the aforementioned metrics.20
Figure 1 demonstrates that rats on the HFD had substantial increases (p < 0.05) in serum levels of urea, creatinine, and neutrophil gelatinase-associated lipocalin (NGAL) but no significant changes in serum uric acid, compared to rats on a regular diet. The levels of urea, creatinine, and NGAL in HFD-fed rats received SVS or Lyc at both doses, on the other hand, were drastically reduced (p < 0.05). HFD fed rats given a high dose of Lyc had a better effect, and all examined parameters were recovered to their control values.
Figure 1.
Serum levels of urea, uric acid, creatinine, and neutrophil gelatinase-associated lipocalin following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 7). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
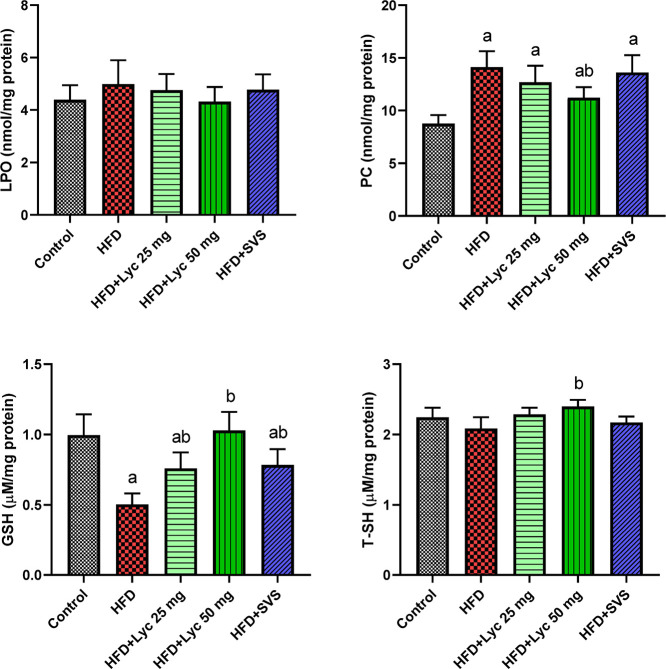
When compared to rats fed a normal diet, Figure 2 indicates a substantial increment (p < 0.05) in PC accompanied by a significant decrease in GSH in rats subjected to HFD primarily and HFD-treated groups. When HFD supplemented rats were compared to those fed a regular diet, no substantial differences in LPO and T-SH levels were found. Lyc treatment at a high dose to HFD supplemented rats, on the other hand, resulted in significant antioxidant effects, as evidenced by rises (p < 0.05) in T-SH and GSH levels compared to the HFD-supplemented rats. In addition, when compared to the HFD-supplemented group, there were marked decreases (p < 0.05) in renal PC levels.
Figure 2.
Renal levels of lipid peroxidation, protein carbonyl, glutathione, and total thiol groups following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 7). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
In the kidney sample of HFD fed rats, there were considerable declines (p < 0.05) in the enzymatic activities of SOD, CAT, and GR, as well as the activity of GPx, compared to the normal control group, as shown in Figure 3. SVS or Lyc supplementation at low and high dosages to HFD supplemented rats, on the other hand, resulted in significant antioxidant effects, as evidenced by increases (p < 0.05) in SOD, GPx, and GR levels when compared to the control group. In addition, when compared to the control group, there was a marked elevation (p < 0.05) in renal CAT activity.
Figure 3.
Renal activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 7). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
Rats fed on HFD had significantly lower Nrf2 expression (p < 0.05) when compared to the normal control group, as seen in Figure 4. In comparison to HFD-treated rats, HFD + Lyc 25 mg-treated rats showed an increase in Nrf2 expression. Furthermore, when compared to control and HFD-supplemented rats, Lyc treatment at a high dose increased Nrf2 expression.
Figure 4.

Renal mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2) following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 3). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
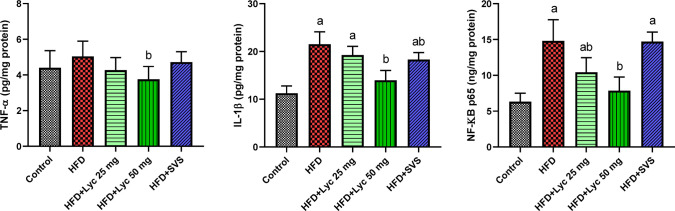
Figure 5 shows that there were significant increases (p < 0.05) in IL-1β accompanied with a non-significant elevation in TNF-α when compared to the normal control group. However, Lyc administration at the low dose was successfully restrained the inflammatory markers in the renal tissue when compared with the HFD-supplemented rats. Furthermore, there was a decrease in those investigated cytokines in HFD + Lyc 50 mg-treated rats compared to the normal control group and HFD treated rats. To understand the anti-inflammatory effect of Lyc, NK-κB p65 levels were measured in renal tissues of all investigated groups. The obtained data revealed that the NK-κB p65 level was increased significantly in HFD-supplemented rats compared with the control fed rats. Notably, Lyc treatment at both doses restrained the NK-κB p65 level compared with the HFD-supplemented rats.
Figure 5.
Renal levels of inflammatory markers (tumor necrosis factor-α, interleukin-1β, and nuclear factor kappa-light-chain-enhancer of activated B cells) following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 7). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
When compared to the normal control group, HFD-supplemented rats had a significant rise (p < 0.05) in Bax and Bax/Bcl-2 ratio as well as a non-significant drop in Bcl-2 levels in renal tissue (Figure 6). When compared to HFD-supplemented rats, SVS or Lyc treatment induced substantial antiapoptotic activity, as evidenced by lower levels (p < 0.05) of renal Bax and Bax/Bcl-2 ratio in renal tissue as well as a significant rise in Bcl-2 level.
Figure 6.
Renal levels of apoptotic markers (Bax, Bcl-2, and Bax/Bcl-2 ratio) following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Data are expressed as the mean ± SD (n = 7). Letters a and b indicate statistically significant differences between control and HFD groups, respectively, at p < 0.05.
Normal glomeruli and tubules were seen in the control rats’ renal histopathology (Figure 7a). HFD supplementation, on the other hand, revealed necrotic and deteriorated renal tubules as well as inflammatory cell infiltration and clogged glomeruli in rats (Figure 7b). Surprisingly, co-treatment with Lyc improved renal injury, as seen by less crowded glomeruli and reduced renal cortex damage without inflammatory cell infiltration (Figure 7c,d). Furthermore, the SVS group’s histological features of renal tubular structures improved (Figure 7e).
Figure 7.
Photomicrographs of histological changes in the kidney tissue following treatment with lycopene (Lyc) or simvastatin (SVS) in high-fat diet (HFD) fed rats. Scale bar = 100 μm. (a) Control, (b) HFD, (c) HFD-Lyc 25 mg, (d) HFD-Lyc 50 mg, (e) HFD-SVS. Black stars: apoptotic and necrotic area; white stars: deteriorated renal tubules; blue stars: congested glomeruli and black arrow: inflammatory cell infiltration.
4. Discussion
Several studies have demonstrated that employing natural antioxidant compounds is effective in controlling HFD-related disorders,6,30 and more emphasis is being paid to the various techniques and prospective remedies aimed at obesity. Antioxidants have been used in the past to protect against chemical-induced kidney damage.31 Lycopene is a powerful free radical scavenger that has been shown in various investigations to protect against chemically induced kidney injury.32 The potential of Lyc on rats fed on HFD with kidney injury was investigated in this study.
In several stages of kidney illness, including the progression of obesity and metabolic syndrome, and the onset of obesity-related glomerulopathy, a high-fat diet plays a critical role. These are linked to increased oxidative stress and cell death in the kidney, resulting in inflammation and renal function impairment.33 In the current investigation, we demonstrated that feeding on an HFD causes considerable elevations in serum urea and creatinine, which is an indicator of impaired kidney function in HFD-fed rats, which is consistent with previous results.3 The HFD increases protein catabolism, resulting in elevated urea generation in these animals. Furthermore, under HFD-fed settings, reduced renal clearance due to compromised renal function may have worsened the situation.33 However, combining Lyc with the HFD provided protection against HFD-induced kidney dysfunction by bringing serum urea, creatinine, and other renal indicators back to normal in Lyc-treated groups. These protective benefits of Lyc could be attributed to its ameliorative activity on metabolic changes reported in HFD-fed circumstances as well as its antioxidant characteristics. In rats, a similar renoprotective effect of Lyc was shown against increasing serum creatinine and uric acid levels.22
According to our findings, eating a diet high in fat caused a considerable decline in antioxidant enzyme activity (SOD, CAT, GPx, and GR) as well as a reduction in the amount of GSH content. Renal damage reported under HFD-fed conditions could be explained by HFD-induced changes in renal lipid metabolism caused by an imbalance between lipogenesis and lipolysis as well as systemic metabolic abnormalities, leading to renal lipid buildup and LPO. Damaged renal tissue generates ROS and triggers oxidative stress. Previous research has found that oxidative stress caused by the HFD increases lipid peroxidation and decreases antioxidant enzyme activity.33 Furthermore, reduced biosynthesis or enhanced degradation/utilization of GSH by increased oxidative stress and impaired regeneration could be the cause of the considerable drop in GSH levels in HFD-fed rats.34 Nevertheless, Lyc supplementation provoked significant antioxidant effects that may probably be due to stimulation of Nrf2. By coordinating the cellular antioxidant capacity through the synthesis of antioxidant enzymes to detoxify excess ROS as well as other electrophilic compounds through conjugative processes, Nrf2 exerts its cytoprotective impact.35 Previous reports stated that carotenoids including Lyc have the ability to interact with ROS via electron transfer reaction, removal of hydrogen, or addition of a radical species.36 A study stated that Lyc exhibits antiobesity effects on different tissues and/or organs, including the kidney. Its antioxidant and anti-inflammatory activities, ability to control AGE/RAGE, JNK/MAPK, PI3K/Akt, and SIRT1/FoxO1/PPAR signaling pathways, and ability to modulate acetylcholinesterase activity may be responsible for the underlying process.37 The study also stated that Lyc supplementation resulted in a preservation of the antioxidant characteristics with respect to SOD, CAT, GPx, and GSH with a concentration range of 1 and 2 mmol/L Lyc revealed to be the most effective, which is similar to our current findings.38 Additionally, According to a study, Lyc, when given before renal I/R injury, protected against kidney damage using biochemical and histological indicators.39
Among the diverse processes involved in oxidative stress, protein carbonylation and lipid peroxidation are both important modifications associated with the pathophysiology of numerous diseases, including renal injury.40 PCs often employed indicators of protein oxidation. Additionally, compared to lipid peroxidation products or other products of protein oxidation, PCs are more stable and remain in circulation for a longer time after exposure, and they are thought to be susceptible to antioxidant treatment.41 In the current investigation, there were no discernible differences between the HFD group and the control group in the levels of LPO and PC. An earlier study stated that Lyc supplementation decreased lipid peroxidation and reduced the lipid profile, providing a successful method to lower the risk of developing oxidative stress-related illnesses.42 In accordance with another study, Lyc supplementation inhibited protein carbonylation in both high-oil and high-carbohydrate diets, and it also reduced lipid accumulation and reduced inflammation.43
Obesity caused by a poor diet is a well-known risk factor for kidney disease.44 The key findings are that a high-fat diet causes inflammation in rat kidneys, as demonstrated by a marked rise in IL-1β, TNF-α, and NF-κB. Chronic inflammation and lipid disorders are two significant synergistic variables that cause renal pathology. HFD causes kidney damage, which is accompanied by monocyte infiltration and the discharge of inflammatory signaling cytokines like IL-1β and TNF-α, according to a number of recent studies.44 Chronic inflammatory infiltration may damage the structure and function of renal tubules at an advanced stage, resulting in renal tubular fibrosis.45 Inflammatory cytokines protect the renal tubules from damage during the early stages of injury,45 and renal tubular fibrosis can develop as a result of extensive renal tubular injury caused by continuous inflammatory infiltration. Furthermore, the HFD can enhance the production of pro-inflammatory cytokines and adhesion molecules as well as activate the NF-B signaling pathway, which can lead to excessive fatty acid accumulation in the renal tubules and promote inflammation.44 A study found that inhibiting the NF-κB signaling pathway by lowering ROS production can stop the release of pro-inflammatory cytokines.46 Another study discovered that HFD-induced renal tubular inflammatory damage and oxidative stress are caused by the NF-κB and Nrf-2/HO-1 signaling pathways.47 As a result, we utilized Lyc in the current investigation to provide protection against HFD-induced kidney impairment, as demonstrated by a considerable reduction in inflammatory cytokines and mediators. The obtained results were in agreement with the previous study of Ramadan et al.22 who found that Lyc has the ability to restrain inflammation in mice intoxicated with arsenate. Another study was also reported that Lyc significantly restrained the level and gene expression of inflammation-related transcription factors including NF-κB in lipopolysaccharides-induced acute kidney injury in mice.48
It has been suggested that apoptosis significantly contributes to the development of renal scarring. Complex systems influence whether a cell begins the apoptotic program. These events have been shown to be modulated by Bax and Bcl-2, and cells’ outcomes depend on their relative levels. The pro-apoptosis protein Bax and antiapoptosis protein Bcl-2 are well-known members of the Bcl-2 family, acting as opposing apoptosis regulators. The oxidative stress damaged cells accumulated in the kidney, leading to renal tissue apoptosis.22 Furthermore, inflammatory mediators and/or ROS overproduction in mitochondria due to metabolic disorder can liberate cytochrome c into the cytoplasm, which then forms apoptosome to activate executioner caspase-3. Thus, restraining increased oxidative stress and inflammation can be ideal for suppressing apoptosis in the kidney. In this study, we found that there were significant increases (p < 0.05) in Bax, Bcl-2, and Bax/Bcl-2 ratio in the HFD-fed rats when compared to the normal control group. However, there was a significant difference between obtained data of HDF and HFD + Lyc groups in apoptosis markers. The reduction in kidney apoptosis observed following Lyc therapy could be attributable to the observed control of oxidative stress and inflammation. The antiapoptotic impact of Lyc on arsenate-induced apoptosis, as demonstrated by Ramadan et al.,22 reveals the inhibitory activity of Lyc on apoptosis. Histopathological studies demonstrated considerable improvement in kidney architecture after treatment of HFD-fed rats with Lyc, corroborating biochemical and molecular findings.
5. Conclusions
Lycopene administration protected HFD fed rats’ kidneys from harm by decreasing oxidative stress and increasing the antioxidant defense system, Nrf2. Furthermore, lycopene activated the Bcl-2 survival signaling system, which protects kidney tissue from harm. Furthermore, lycopene inhibited NF-κB activation, which lowered their downstream effectors, inflammatory cytokines (IL-1β and TNF-α), and pro-apoptotic mediators, resulting in a strong antiapoptotic effect (Bax). These data show that lycopene is a promising hypolipidemic candidate and can be recommended as an important part of a dietary approach for obesity prevention and management.
Acknowledgments
This research was funded by Princess Nourah bint Abdulrahman University Research Supporting Project number (PNURS2022R69), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02796.
Food intake and body weight gain following treatment with lycopene (PDF)
Author Contributions
§ TA and AAR are contributed equally to this work.
This research was funded by Princess Nourah Bint Abdulrahman University Research Supporting Project number (PNURS2022R69), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Notes
All experimental procedures were by the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Princess Nourah bint Abdulrahman University (approval no HAP-01-R-059; IRB Log Number: 21–0290).
Notes
All relevant data are within the paper.
Supplementary Material
References
- a Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]; b Pinzón-García A. D.; Orellano L. A. A.; de Lazari M. G. T.; Campos P. P.; Cortes M. E.; Sinisterra R. D. Evidence of hypoglycemic, lipid-lowering and hepatoprotective effects of the bixin and bixin: β-CD inclusion compound in high-fat-fed obese mice. Biomed. Pharmacother. 2018, 106, 363–372. 10.1016/j.biopha.2018.06.144. [DOI] [PubMed] [Google Scholar]; c Zhang S.; Yan L.; Kim S. M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of β-catenin and LKB1/AMPK signaling pathway. PLoS One 2020, 15, e0239547 10.1371/journal.pone.0239547. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ortega-Loubon C.; Fernández-Molina M.; Singh G.; Correa R. Obesity and its cardiovascular effects. Diabetes Metab. Res. Rev. 2019, 35, e3135 10.1002/dmrr.3135. [DOI] [PubMed] [Google Scholar]
- WHO . WHO Obesity and overweight; 2021, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 9 June 2021).
- Othman M. S.; Khaled A. M.; Aleid G. M.; Fareid M. A.; Hameed R. A.; Abdelfattah M. S.; Aldin D. E.; Moneim A. E. A. Evaluation of antiobesity and hepatorenal protective activities of Salvia officinalis extracts pre-treatment in high-fat diet-induced obese rats. Environ. Sci. Pollut. Res. Int. 2022, 1–14. 10.1007/s11356-022-21092-2. [DOI] [PubMed] [Google Scholar]
- Vadiveloo M.; Scott M.; Quatromoni P.; Jacques P.; Parekh N. Trends in dietary fat and high-fat food intakes from 1991 to 2008 in the Framingham Heart Study participants. J. Geophys. Res. Oceans 2014, 111, 724–734. 10.1017/s0007114513002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio J. J.; Lucan S. C.; O’Keefe J. H. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. 10.1016/j.pcad.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman M. S.; Khaled A. M.; Al-Bagawi A. H.; Fareid M. A.; Ghany R. A.; Habotta O. A.; Abdel Moneim A. E. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287 10.1016/j.biopha.2021.112287. [DOI] [PubMed] [Google Scholar]
- Kakimoto P. A.; Kowaltowski A. J. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016, 8, 216–225. 10.1016/j.redox.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-R.; Kim J.-W.; Park J. B.; Hong Y.-K.; Ku S. K.; Choi J.-S. Anti-obesity effects of yellow catfish protein hydrolysate on mice fed a 45% kcal high-fat diet. Int. J. Mol. Med. 2017, 40, 784–800. 10.3892/ijmm.2017.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubaa-Ghorbel F.; Chaâbane M.; Turki M.; Makni-Ayadi F.; El Feki A. The protective effects of Salvia officinalis essential oil compared to simvastatin against hyperlipidemia, liver, and kidney injuries in mice submitted to a high-fat diet. J. Food Biochem. 2020, 44, e13160. [DOI] [PubMed] [Google Scholar]
- Abbas A. M.; Sakr H. F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J. Physiol. Biochem. 2013, 69, 737–750. 10.1007/s13105-013-0250-y. [DOI] [PubMed] [Google Scholar]
- Ramkumar S.; Raghunath A.; Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol. Sin. 2016, 32, 631–639. 10.6515/acs20160611a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M. M.; Althagafi H. A.; Alharthi F.; Albrakati A.; Alsharif K. F.; Theyab A.; Kassab R. B.; Mufti A. H.; Algahtani M.; Oyouni A. A. A.; et al. Apigenin attenuates molecular, biochemical, and histopathological changes associated with renal impairments induced by gentamicin exposure in rats. Environ. Sci. Pollut. Res. 2022, 1. 10.1007/s11356-022-20235-9. [DOI] [PubMed] [Google Scholar]
- Sahin K.; Orhan C.; Tuzcu M.; Sahin N.; Hayirli A.; Bilgili S.; Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016, 95, 1088–1095. 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- a Latchman D. S. Transcription factors: an overview. Int. J. Biochem. Cell Biol. 1997, 29, 1305–1312. 10.1016/s1357-2725(97)00085-x. [DOI] [PubMed] [Google Scholar]; b Surh Y. J.; Kundu J. K.; Na H. K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Karri S.; Sharma S.; Hatware K.; Patil K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. 10.1016/j.biopha.2018.11.076. [DOI] [PubMed] [Google Scholar]
- Bouhlali E. D. T.; Hmidani A.; Bourkhis B.; Khouya T.; Harnafi H.; Filali-Zegzouti Y.; Alem C. Effect of Phoenix dactylifera seeds (dates) extract in triton WR-1339 and high fat diet induced hyperlipidaemia in rats: A comparison with simvastatin. J. Ethnopharmacol. 2020, 259, 112961 10.1016/j.jep.2020.112961. [DOI] [PubMed] [Google Scholar]
- Róvero Costa M.; Leite Garcia J.; Cristina Vágula de Almeida Silva C.; Junio Togneri Ferron A.; Valentini Francisqueti-Ferron F.; Kurokawa Hasimoto F.; Schmitt Gregolin C.; Henrique Salomé de Campos D.; Roberto de Andrade C.; Dos Anjos Ferreira A. L. Lycopene modulates pathophysiological processes of non-alcoholic fatty liver disease in obese rats. Antioxidants 2019, 8, 276. 10.3390/antiox8080276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Han G.-M.; Soliman G. A.; Meza J. L.; Islam K. M.; Watanabe-Galloway S. The influence of BMI on the association between serum lycopene and the metabolic syndrome. J. Geophys. Res. Oceans 2016, 115, 1292–1300. 10.1017/S0007114516000179. [DOI] [PubMed] [Google Scholar]; b Luvizotto R. D. A. M.; Nascimento A. F.; Imaizumi E.; Pierine D. T.; Conde S. J.; Correa C. R.; Yeum K.-J.; Ferreira A. L. A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. J. Geophys. Res. Oceans 2013, 110, 1803–1809. 10.1017/S0007114513001256. [DOI] [PubMed] [Google Scholar]
- Al-Brakati A.; Alsharif K. F.; Alzahrani K. J.; Kabrah S.; Al-Amer O.; Oyouni A. A.; Habotta O. A.; Lokman M. S.; Bauomy A. A.; Kassab R. B.; et al. Using Green Biosynthesized Lycopene-Coated Selenium Nanoparticles to Rescue Renal Damage in Glycerol-Induced Acute Kidney Injury in Rats. Int. J. Nanomed. 2021, 16, 4335–4349. 10.2147/IJN.S306186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrahim T.; Alonazi M. A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831 10.1016/j.biopha.2021.111831. [DOI] [PubMed] [Google Scholar]
- Breinholt V.; Lauridsen S. T.; Daneshvar B.; Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. 10.1016/S0304-3835(00)00401-8. [DOI] [PubMed] [Google Scholar]
- Ramadan S. S.; Almeer R.; Albasher G.; Abdel Moneim A. E. Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Rev. 2021, 1–11. 10.1080/15569543.2021.1891938. [DOI] [Google Scholar]
- Ohkawa H.; Ohishi N.; Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Green L. C.; Wagner D. A.; Glogowski J.; Skipper P. L.; Wishnok J. S.; Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]; Research Support, U.S. Gov’t, P.H.S
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Nishikimi M.; Rao N. A.; Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Paglia D. E.; Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [PubMed] [Google Scholar]
- De Vega L.; Fernandez R. P.; Mateo M. C.; Bustamante J. B.; Herrero A. M.; Munguira E. B. Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren. Fail 2002, 24, 421–432. 10.1081/JDI-120006769. [DOI] [PubMed] [Google Scholar]
- Al-Megrin W. A.; El-Khadragy M. F.; Hussein M. H.; Mahgoub S.; Abdel-Mohsen D. M.; Taha H.; Bakkar A. A. A.; Abdel Moneim A. E.; Amin H. K. Green Coffea arabica Extract Ameliorates Testicular Injury in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. J Diabetes Res 2020, 2020, 6762709. 10.1155/2020/6762709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ramesh B.; Viswanathan P.; Pugalendi K. V. Protective effect of Umbelliferone on membranous fatty acid composition in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2007, 566, 231–239. 10.1016/j.ejphar.2007.03.045. [DOI] [PubMed] [Google Scholar]; b Kalender S.; Apaydin F. G.; Bas H.; Kalender Y. Protective effects of sodium selenite on lead nitrate-induced hepatotoxicity in diabetic and non-diabetic rats. Environ. Toxicol. Pharmacol. 2015, 40, 568–574. 10.1016/j.etap.2015.08.011. [DOI] [PubMed] [Google Scholar]
- a Palabiyik S. S.; Erkekoglu P.; Zeybek N. D.; Kizilgun M.; Baydar D. E.; Sahin G.; Giray B. K. Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp. Toxicol. Pathol. 2013, 65, 853–861. 10.1016/j.etp.2012.12.004. [DOI] [PubMed] [Google Scholar]; b Aydin S.; Palabiyik S. S.; Erkekoglu P.; Sahin G.; Başaran N.; Giray B. K. The carotenoid lycopene protects rats against DNA damage induced by Ochratoxin A. Toxicon 2013, 73, 96–103. 10.1016/j.toxicon.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Gujjala S.; Putakala M.; Nukala S.; Bangeppagari M.; Ramaswamy R.; Desireddy S. Renoprotective effect of Caralluma fimbriata against high-fat diet-induced oxidative stress in Wistar rats. J. Food Drug Anal. 2016, 24, 586–593. 10.1016/j.jfda.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman M. S.; Khaled A. M.; Al-Bagawi A. H.; Fareid M. A.; Hameed R. A.; Zahra F. A. A.; Moneim A. E. A. Echinops spinosus effect against diabetes and its hepatorenal complications: total extract and flavonoids fraction. Environ. Sci. Pollut. Res. Int. 2022, 10.1007/s11356-022-18824-9. [DOI] [PubMed] [Google Scholar]
- a Kassab R. B.; Lokman M. S.; Daabo H. M.; Gaber D. A.; Habotta O. A.; Hafez M. M.; Zhery A. S.; Moneim A. E. A.; Fouda M. S. Ferulic acid influences Nrf2 activation to restore testicular tissue from cadmium-induced oxidative challenge, inflammation, and apoptosis in rats. J. Food Biochem. 2020, e13505 10.1111/jfbc.13505. [DOI] [PubMed] [Google Scholar]; b El-Khadragy M. F.; Al-Megrin W. A.; Alomar S.; Alkhuriji A. F.; Metwally D. M.; Mahgoub S.; Amin H. K.; Habotta O. A.; Moneim A. E. A.; Albeltagy R. S. Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem.-Biol. Interact. 2021, 109333 10.1016/j.cbi.2020.109333. [DOI] [PubMed] [Google Scholar]
- Brito A. K. d. S.; Lima G. D. M.; Farias L. M. D.; Rodrigues L. A. R. L.; Carvalho V. B. L. D.; Pereira C. F. D. C.; Frota K. D. M. G.; Conde-Júnior A. M.; Silva A. M. O.; Rizzo M. D. S. Lycopene-Rich Extract from Red Guava (Psidium guajava L.) Decreases Plasma Triglycerides and Improves Oxidative Stress Biomarkers on Experimentally-Induced Dyslipidemia in Hamsters. Nutrients 2019, 11, 393. 10.3390/nu11020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R.; Chen B.; Bai Y.; Miao T.; Rui L.; Zhang H.; Xia B.; Li Y.; Gao S.; Wang X. D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966 10.1016/j.phrs.2020.104966. [DOI] [PubMed] [Google Scholar]
- Tvrdá E.; Kováčik A.; Tušimová E.; Paál D.; Mackovich A.; Alimov J.; Lukáč N. Antioxidant efficiency of lycopene on oxidative stress - induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 50. 10.1186/s40104-016-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C.; Karabulut R.; Turkyilmaz Z.; Sonmez K.; Kulduk G.; Gülbahar Ö.; Köse F.; Basaklar A. C. Lycopene has reduced renal damage histopathologically and biochemically in experimental renal ischemia-reperfusion injury. Ren. Fail. 2015, 37, 1390–1395. 10.3109/0886022x.2015.1064742. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García A.; García-Vicente R.; Morales M. L.; Ortiz-Ruiz A.; Martínez-López J.; Linares M. Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies. Antioxidants 2020, 9, 12. 10.3390/antiox9121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I.; Giustarini D.; Colombo R.; Rossi R.; Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Wang Z.; Ma Y.; Qu Y.; Lu X.; Luo H. Effects of Dietary Lycopene Supplementation on Plasma Lipid Profile, Lipid Peroxidation and Antioxidant Defense System in Feedlot Bamei Lamb. Asian-Aust. J. Anim. Sci. 2015, 28, 958–965. 10.5713/ajas.14.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreto F.; Ferron A. J. T.; Francisqueti F. V.; Garcia J. L.; Kitawara K. A. H.; Correa-Camacho C. R.; Anjos Ferreira A. L. d. 222 - Lycopene Shows Anti-protein Carbonylation and Anti-inflammatory Properties in Liver of Rats Treated with a Hypercaloric Refined-Carbohydrate Rich Diet. Free Radical Biol. Med. 2017, 112, 153–154. 10.1016/j.freeradbiomed.2017.10.235. [DOI] [Google Scholar]
- Chen S.; Chen J.; Li S.; Guo F.; Li A.; Wu H.; Pan Q.; Liao S.; Liu H. F. High-Fat Diet-Induced Renal Proximal Tubular Inflammatory Injury: Emerging Risk Factor of Chronic Kidney Disease. Front. Physiol. 2021, 12, 786599 10.3389/fphys.2021.786599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. M. Inflammatory Mediators and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 381–406. 10.1007/978-981-13-8871-2_18. [DOI] [PubMed] [Google Scholar]
- Rehman M. U.; Rashid S. M.; Rasool S.; Shakeel S.; Ahmad B.; Ahmad S. B.; Madkhali H.; Ganaie M. A.; Majid S.; Bhat S. A. Zingerone (4-(4-hydroxy-3-methylphenyl)butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch. Physiol. Biochem. 2019, 125, 201–209. 10.1080/13813455.2018.1448422. [DOI] [PubMed] [Google Scholar]
- Chenxu G.; Xianling D.; Qin K.; Linfeng H.; Yan S.; Mingxin X.; Jun T.; Minxuan X. Fisetin protects against high fat diet-induced nephropathy by inhibiting inflammation and oxidative stress via the blockage of iRhom2/NF-kappaB signaling. Int. Immunopharmacol. 2021, 92, 107353 10.1016/j.intimp.2020.107353. [DOI] [PubMed] [Google Scholar]
- Salari S.; Ghorbanpour A.; Marefati N.; Baluchnejadmojarad T.; Roghani M. Therapeutic effect of lycopene in lipopolysaccharide nephrotoxicity through alleviation of mitochondrial dysfunction, inflammation, and oxidative stress. Mol. Biol. Rep. 2022, 1. 10.1007/s11033-022-07661-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.