Abstract
Reactive oxygen species (ROS) is considered a double-edged sword. The slightly elevated level of ROS helps in wound healing by inhibiting microbial infection. In contrast, excessive ROS levels in the wound site show deleterious effects on wound healing by extending the inflammation phase. Understanding the ROS-mediated molecular and biomolecular mechanisms and their effect on cellular homeostasis and inflammation thus substantially improves the possibility of exogenously augmenting and manipulating wound healing with the emerging antioxidant therapeutics. This review comprehensively delves into the relationship between ROS and critical phases of wound healing and the processes underpinning antioxidant therapies. The manuscript also discusses cutting-edge antioxidant therapeutics that act via ROS scavenging to enhance chronic wound healing.
1. Introduction
The skin is the body’s outermost layer, protecting it from toxic elements and serving various essential functions. When skin is damaged, a multistep process begins that offers at least some restoration of the afflicted skin. Wound healing is a well-known process in which the wound recovers through three overlapping phases: inflammation, proliferation, and remodeling.1 The wounded vascular system forms a platelet plug shortly after an injury or damage to temporarily stop blood loss from the wound. In addition, the inflammatory phase attracts a variety of immune cells, which generate pro-inflammatory cytokines and enzymes to speed up the healing process.
The slightly elevated ROS produced during wound healing plays an essential role in avoiding bacteria and other microbial infections.2 ROS also plays a crucial role in intracellular signaling. The production of elevated level of different ROS at the wound site from the different cells are known as a respiratory burst. The production of a higher level of hydroxyl radicals (OH•), superoxide (O–2), hydrogen peroxide (H2O2), iron, and copper ions causes significant damage to the cells.3 Oxidative stress caused due to a rise in ROS levels induces tissue damage and cell death.4 Antioxidant therapies can considerably reduce the elevated level of ROS produced during the wound healing phases, making them a viable treatment option for nonhealing wounds. This review delves into the relationship between ROS and essential phases of wound healing and the processes underpinning antioxidant therapies. We also spoke about some of the most cutting-edge, innovative antioxidant therapies for quick wound healing.
2. Link between the Free Radicals in Cellular Homeostasis during Wound Healing
Adenosine triphosphate (ATP) synthesis during mitochondrial oxidative phosphorylation requires oxygen as a substrate for energy production in the cells. Moreover, rapid wound healing also needs a great deal of energy. As a result, ROS and its derivatives may play an essential part in wound healing. The ROS family includes oxygen derivatives such as OH•, peroxide, superoxide anion, hydrogen peroxide (H2O2), etc. In mitochondria during ATP production, endogenous ROS species are generated due to enzymatic activity. During nonhealing wounds, these ROS are produced in a considerabe amount. Further, these elevated ROS scavenges electrons from neighboring molecules or cells through oxidation and causes cellular damage. According to research, a small amount of ROS keeps cells in equilibrium and elevated levels of ROS show deleterious effects on wound healing.
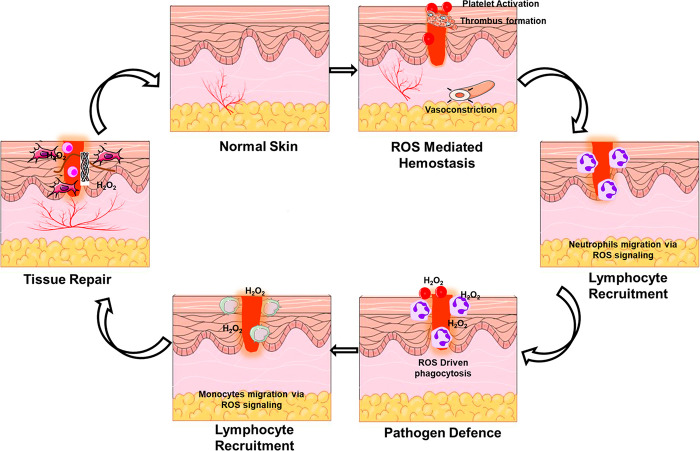
Generally, cell function and homeostasis are maintained when the ROS level remains constant at the baseline level. Increased production of ROS activates pro-apoptotic proteins, resulting in cell death and necrosis.5 ROS also acts in charge of vasoconstriction and vasorelaxation. Furthermore, nitric oxide (NO) is a ROS that naturally combines with various molecules (e.g., oxygen, transition metals, s-nitrosothiols, other ROS, and ONOO–) to function as an antioxidant. Many cells, including fibroblasts, keratinocytes, platelets, endothelial cells, and macrophages, employ these radicals during wound healing.6 The role of a moderate level of ROS in various phases of wound healing through cellular interactions are depicted in Figure 1.
Figure 1.
ROS and their role in the various process of wound healing.
Oxygen stimulates re-epithelialization, wound healing, keratinocyte differentiation, fibroblast proliferation, and migration, angiogenesis, collagen production, and wound contraction during tissue regeneration. Optimal oxygenation of cells and tissues is essential for tissue regeneration and wound healing. Choi et al. prepared oxygen-releasing polymeric microspheres and embedded them in alginate-based hydrogels. Double emulsion was used to incorporate H2O2 into PLGA to make oxygen-releasing microspheres (ORMs). The alginate-based hydrogel was implanted with H2O2–PLGA microspheres to generate an oxygen-releasing sponge (ORHS). In vivo research tested the ORHS for wound healing safety and effectiveness. Experiments demonstrated that oxygen produced from ORM and ORHS promotes cell growth and wound healing. The ORM may give oxygen to cells and tissues that need it, while the ORHS can heal wounds by enhancing angiogenesis with oxygen. Oxygen-releasing polymeric microspheres and hydrogel scaffolds offers immense promise for tissue engineering applications requiring oxygen.7
The earliest step in battling invading pathogens and boosting cellular signal transduction pathways in response to skin injury starts with the increased ROS generation.8 Nuclear factor E2 p45-related factor 2 (Nrf2) supports wound healing in damaged and inflammatory tissue.9 This shows that moderate level of epithelial ROS works as a sensor for Nrf2 expression. ROS build-up prevents wound infections.10 NO- and H2S-driven signals may also modulate the Nrf2 transcription factor.11
Nrf2/Keap1 protects against ROS, electrophilic, and proteotoxic stress.12 Nrf2 reduces cellular stress and restores redox equilibrium by regulating the expression of over 1000 genes, including HMOX1, TRX, GSR, Gclc, Gclm, SOD1, and catalase.13 Nrf2 signaling also governs essential cellular processes such as apoptosis, autophagy, angiogenesis, proliferation, and cell migration.14 Nrf2 targets antioxidant enzymes, detoxifying enzymes, proteases, chaperones, inflammatory factors, and growth factors.15 NO, siRNA, bee venom, and other active substances are Nrf2 activators that enhance wound healing.8
Low levels of caNrf2 in keratinocytes protect mice against UVB-induced cell death.16 More robust expression of caNrf2, reflecting endogenous Nrf2 activation by chemical activators, induced skin abnormalities, including moderate inflammation, hyperkeratosis, sebaceous gland enlargement, and epidermal barrier function deficiencies.17 However, these animals had quicker wound closure due to increased re-epithelialization. While caNrf2 expression promoted wound healing, keratinocyte migration and proliferation in the wound epidermis were unaffected. CaNrf2 promoted hair follicle and sebaceous gland growth in the wound periphery.10 Instead, caNRF2 increased hair follicle bulge, junctional zone, and upper isthmus stem cells. All of these processes were previously shown to contribute to wound re-epithelialization.18 The extra pool of cells formed near the wound edge, possibly by stem cell growth, appears to serve as a reserve of cells to move into and repopulate the wound. Nrf2-mediated epigen expression may stimulate stem cell growth. This epidermal growth factor family member, encoded by Nrf2,17 promotes hair follicle stem cell proliferation in vivo.19 Faster wound closure in caNrf2-transgenic animals needed significant Nrf2 activation since mice expressing lower amounts of the transgene failed to demonstrate differences despite a minor increase in Nrf2 target gene expression.10 These observations suggest that Nrf2 activation may promote wound re-epithelialization and that the degree of activation may be significant when assessing its efficacy.
Effect of Nrf2’s on fibroblasts have also been studied. Skin fibroblasts produce cytokines and growth factors, deposit ECM, and constrict wounds.20 Activating Nrf2 in fibroblasts causes senescence in both in vitro and in vivo conditions. Nrf2-mediated deposition of an altered matrisome with high quantities of senescence-promoting factor plasminogen activator inhibitor-1 drove this (PAI-1, serpine1). Accelerated senescence was found in both caNrf2-expressing cells and wild-type fibroblasts treated with the Nrf2 activator tert-butylhydroquinone.21 Senescent cells offer key biochemical signals to surrounding cells through a growth-promoting secretome called the senescence-associated secretory phenotype (SASP). Senescent cells aid in wound healing.22 caNrf2 fibroblasts secrete factors that promote keratinocyte growth.21 Mice expressing caNrf2 in fibroblasts had quicker wound closure due to increased keratinocyte proliferation in the wound epidermis.
3. ROS and Its Direct Connection with Inflammation in Wound Healing
According to the previously reported studies, ROS aid in various stages of tissue repair. ROS is a secondary messenger for various immunocytes and nonlymphoid cells involved in wound healing. They have a remarkable ability to modulate angiogenesis and blood perfusion in the wound healing domain.8 Furthermore, they have evolved into a critical coordinator in deploying lymphoid cells to the site of interest (i.e., wound regions) and effective tissue recovery. A moderate level of ROS generation is essential for fighting microbes and cell survival signaling. Furthermore, both increased and decreased ROS levels can cause oxidative damage, which can delay the healing process of chronic wounds.23,24
Inflammation plays a critical role in the initiation of wound healing.25 An elevated and prolonged ROS production at the wound site leads to chronic inflammation. Deregulation of the inflammatory phase and chronic response cause tissue damage.26 Fascinatingly, re-establishing the ROS equilibrium can significantly improve the damaged skin condition. Excess ROS in injured skin tissue activates transcription factors such as activator protein 1 (AP-1), mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), and nuclear factor erythroid-derived 2-like 2 (Nrf2).3,27,28 Among these transcription factors, Nrf2 controls the transcription of the antioxidant gene by binding to the antioxidant response element (ARE) to increase the transcription of its target genes.29,30 Nrf2 is primarily involved in defending against high levels of endogenous ROS build-up, which aids in wound healing. According to reports, Nrf2 overexpression can significantly protect cells against cellular damage induced by oxidative stress. Furthermore, studies have shown that Nrf2 overexpression is critical for controlling the re-epithelialization process.30,31
In contrast, NF-κB and AP-1 activation increased the number of matrices metalloproteins (MMPs) in dermal fibroblasts, leading to extracellular matrix protein breakdown (ECM), which leads to the delay in the wound healing process. However, the published studies indicate Nrf2’s beneficial function in healing chronic wounds.31,32 In diabetic wounds, persistent pro-inflammatory macrophages and dysregulation of the wound healing inflammatory phase link to persistent nod-like receptor (NLR)-3-containing inflammasome activity.33 NLR family pyrin domain-containing 3 (NLRP3) demonstrates innate immune responses after sensing damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) via NLR receptors. When activated by DAMPs and PAMPs, NLRP3 interacts with the apoptosis-associated spike-like protein (ASC). Following that, ASC interacts with procaspase-1 to generate NLRP3 inflammasomes. Thus, the created complex promotes proteolytic cleavage, which aids in expressing interleukins (IL-1 and IL-18), which are responsible for various inflammatory and immunological activities (Figure 2). Inflammasomes have multiple features, including an autoimmune impact, antibacterial activity, and autoinflammatory reactions.
Figure 2.
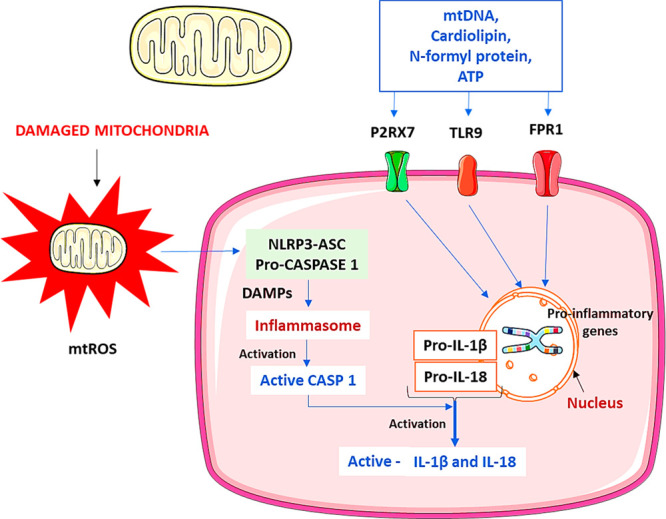
Mitochondria dysfunction mediated activation of the inflammasome. Damaged mitochondria secrete molecular patterns identified by the cytosolic and membrane receptors such as toll-like receptors (TLR) 9. NLRP3 inflammasomes get activated by interacting with various responses, including DAMPs. Further, the NLRP3 forms caspase activation and recruitment domain (CARD) with ASC and CASP1 called inflammasomes. Caspase-1 stimulates the inflammasome complex for activation, and the activated inflammasomes help convert the pro-IL-1β and pro-IL-18 into matured forms. Mitochondrial DNA (mtDNA) formyl proteins, ATP, and mitochondria ROS also help to start the NLRP3 inflammasomes directly or indirectly by receptor-mediated (FPR1, P2RX7). TLR9 specifically interacts with the mitochondrial DNA motifs and activates the signaling cascades resulting in a pro-inflammatory cytokine response. TLR-9, toll-like receptor-9; CARD, caspase activation, and recruitment domain; CASP1, caspase-1; DAMPs, danger-associated molecular patterns; IL, interleukin; mtDNA, mitochondrial DNA; mtROS, mitochondrial reactive oxygen species; FPR1, formyl peptide receptor 1; P2RX7, P2X purinoceptor.
4. Biomolecular Links of Oxidative Stress with Wound Healing
Due to ROS’s limited half-life, it is challenging to determine their concentration in vivo. Recently, efforts were made to determine the ROS concentration in the murine wound models at the wound site using electrochemical techniques. The study findings indicated a low concentration of H2O2 in the wound (range from 100 to 250 μM). The early wound healing phase (day 2 following damage) had a greater concentration of H2O2 than the later wound healing phase. In addition, the dihydroethidium staining experiment reveals the presence of superoxide as well as H2O2 near the wound margins.34 Another study found that superoxide levels peaked 2 days postwounding in the inflammatory phase.35 Superoxide ion generation was reduced 3-fold in the mice with the missing Rac2 gene compared to the control animals. In these mice, a shallow level of superoxide production at the wound site leads to compromised wound closure. Thus, these findings suggest that a modest level of ROS is essential for the normal wound healing process.
Most studies used indirect measurement of lipids, deoxyribonucleic acid (DNA), and oxidative protein products to determine ROS concentrations at the wound site.36 Immunohistochemistry was also used to assess the formation of 4-hydroxy-2-nonenal (4-HNE) due to lipid peroxidation. Co-immunostaining reveals 4-HNE as the primary lipid peroxidation product in neutrophils. Further, a respiratory burst of these inflammatory cells produces superoxide, which increases superoxide generation and exaggerates lipid peroxidation.35
Malondialdehyde (MDA) is a byproduct of lipid peroxidation caused by various oxidative stressors.37 Elevated levels of the free radicals promote over production of MDA.38 These elevated levels of MDA promote pro-inflammatory molecules. This might lead to increased lymphocyte activation, which worsens diabetic problems.39 Musalmah et al. tested the antioxidant activity of α-tocopherol on the plasma MDA levels and its role in normal and diabetic wound closure rate in the rats. The results showed that α-tocopherol decreased plasma malondialdehyde, enhanced glutathione peroxidase activity, and sped wound healing in rats.40
Gupta et al. found that compared to control animals, significant MDA levels are present in the damaged wounds. Surprisingly, no significant variation in MDA levels was seen in this investigation in both chronic and acute wound fluids.41 Another fatty acid peroxidation product is 8-isoprostanes, found in greater concentrations in the fluid of chronic wounds than in acute human wounds.42 These findings show that oxidative stress plays a significant role in chronic ulcers, causing the inflammatory phase to continue.43 Also, the considerable rise in the allantoin to the uric acid proportion in chronic wounds strengthens the hypothesis. This ratio is the indication of oxidative stress.44
Another possible measure for understanding oxidative stress is to measure the oxidized protein level at the wound site in preclinical animal models. The Oxyblot (protein standard) revealed a considerable increase in oxidized protein levels in chronic wounds compared to healthy skin. Importantly, oxidized proteins were found in more significant quantities in male mice than in female mice.45 The rise in oxidative protein levels may contribute to more inflammation and worse wound health in the male mice.46 However, reactive protein concentrations were higher in acute wound fluids than in chronic wound fluids. More research is needed to flesh out the findings. Another oxidative protein marker that the immunohistochemistry assay can detect is nitrotyrosine. Reactive molecules bind to the protein’s tyrosine residues, resulting in the formation of 3-nitrotyrosine.
Wound healing is governed by various hormones, growth factors, and cytokines.47 Recent research has found that ROS and NO are also essential regulators of wound healing.27 In general, ROS protects against invading pathogens. In addition, a modest level of ROS also participates in intracellular signaling pathways.48 For example, injecting H2O2 in small amounts into a wound enhances wound angiogenesis.34 On the other hand, surplus levels of ROS will be detrimental to wound healing due to their high reactivity.13
5. Mechanistic Look at Antioxidants and Their Role in Controlling Oxidative Stress
According to research and clinical data, antioxidants, and anti-inflammatory approaches have shown potential effects in the treatment of wounds. Currently, among all available antioxidant techniques, mitochondrial-targeted antioxidants have piqued the interest of researchers. Most notably, peptides used to target mitochondria have shown enormous promise. Elamipretide, for example, has a solid ability to alleviate mitochondrial dysfunction and abnormal inflammatory effects via stimulation of nucleotide-attached oligomerization domain (NOD)-like receptors (e.g., NLRP3), inflammasome, inhibition of the NF-κB signaling pathway, and so on.23 The presence of exceptionally high levels of ROS in tissues (for example, skin) promotes the activation of specific types of transcription factors such as Nrf2, AP-1, NF-κB, and MAPK pathways.
Overactivation of the NF-κB pathway, on the other hand, impedes wound healing in type 2 diabetes patients. Some pioneering studies indicated that miR-146a and SIRT1 treatment targets and limits the previously active NF-κB pathway. However, Nrf2 is the primary controller of the antioxidant gene that regulates the transcription of cytoprotective genes by binding to the critical ARE and initiating the transcription via its target genes.29,30 Furthermore, studies show that antioxidants have a role in the stimulating Nrf2/Kelch-like ECH-associated protein-1 (KEAP1) pathway, particularly in the healing of diabetic wounds.
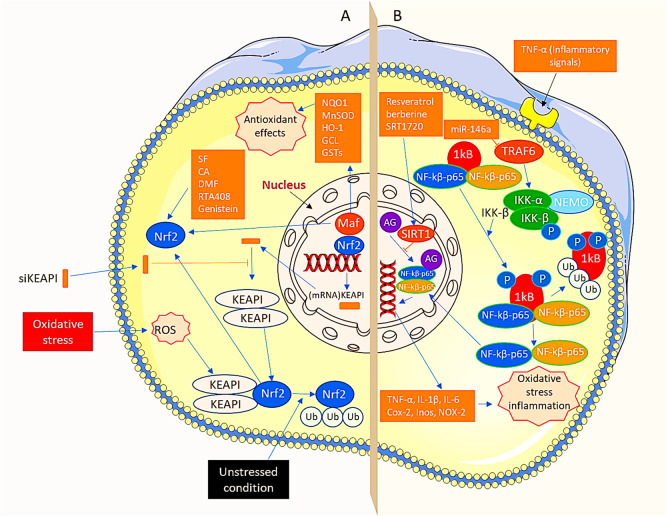
These findings imply that addressing Nrf2 and KEAP1 can be effective with gene therapy and molecular wound repair in diabetes patients (Figure 3A,B).
Figure 3.
Two major pathways in antioxidant therapy “Nrf2 pathway and the NFκB pathway”. (A) Under unstressed events, KEAP1 interrelates with Nrf2 and actin cytoskeleton to retain Nrf2 in dormant form and thus encourage both ubiquitination and deprivation of Nrf2. In addition, oxidative stress not only separates Nrf2 from KEAP1 but also translocates it to the nucleus. In the nucleus, Nrf2 heterodimerizes with Maf. It produces Nrf2-Maf heterodimer, which further attaches to ARE to form metabolic genes. For instance, NQO1, heme oxygenase-1 (HO-1), GSTs, GCL, and manganese superoxide dismutase (MnSOD) produce antioxidant effects. Additionally, oxidative stress is controlled via the activation of the Nrf2 pathway. In addition, the levels of KEAP1 are lowered by the siKEAP1 after loading them into RISC and by eliminating complementary mRNA of the KEAP1. Furthermore, Nrf2 activators, for instance, SF, CA, DMF, RTA408, and genistein, also induce the Nrf2 pathway and improve oxidative stress. (B) In resting conditions, NFκB dimers develop a complex with IkB protein in the cytoplasm. TNF-α, an inflammatory signal, encourages phosphorylation of IkB protein due to the involvement of IKK, which leads to ubiquitination and ultimately degradation of IkB. In addition, after moving active NFκB into the nucleus, it stimulates target genes such as TNF-α, NADPH oxidase (NOX)-2, cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), IL-6, and IL-1b causing both oxidative stresses as well as inflammation. Moreover, oxidative stress is controlled by hindering the NFκB pathway. Furthermore, MiR-146a shows aptitude toward targeting, inhibiting TRAF6, impeding the stimulation of IKK, and the NFκB pathway. Finally, SIRT1 activators, such as SRT1720, resveratrol, and berberine, overcome attaching of NFκB to inflammation-initiating gene promoters and their transcriptional events via stimulating SIRT1. RISC, RNA-induced silencing complex; TRAF6, tumor necrosis factor receptor-associated factor 6; ARE, antioxidant response element.
Most patients (diabetic and nondiabetic) with oxidative stress-dependent long-term wounds are treated with antibiotics, moisture dressing, pressure off-loading, and surgical excision of the lesion.49 Current research focuses on specific strategies for improving the recovery speed of chronic wounds, such as using collagen-derived tissue-engineered grafts, various growth factors for topical use, and the engagement of different cells obtained through bone marrow (e.g., endothelial/epithelial cells), etc. Precise regulation of ROS expression using antioxidants and antioxidative enzymes can also significantly reduce oxidative stress-induced cellular harm.50
6. Emerging Antioxidant Novel Therapeutics for Combating the Oxidative Stress in Wound Healing
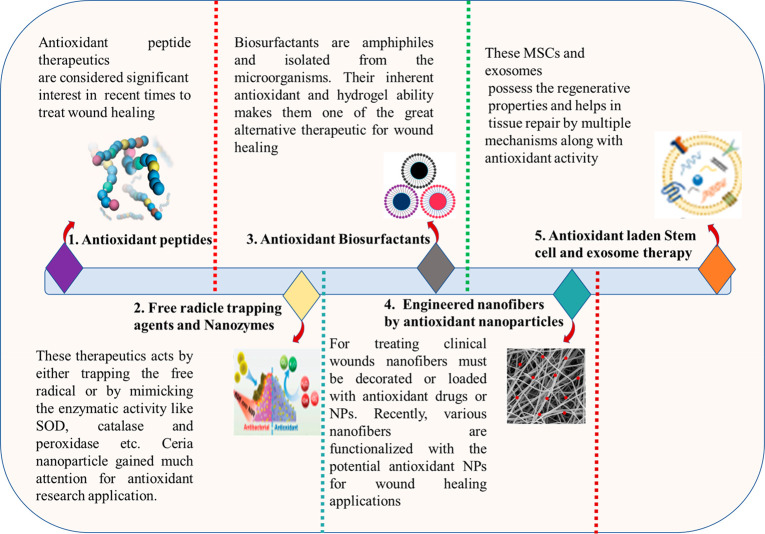
The elevated levels of ROS in the wound are managed by antioxidants such as phytochemicals, endogenous compounds, peptides, and polymers are commonly employed. Several antioxidants effectively control the oxidative stress in the wound and aid wound healing. A detailed overview of the antioxidant phytochemicals in wound healing applications has been explicitly discussed elsewhere.51−56 This work examined the innovative antioxidant techniques used for quick wound healing in recent years. Figure 4 depicts several unique antioxidant treatment methods for fast wound healing.
Figure 4.
Emerging novel antioxidant therapeutic approaches for rapid wound healing.
6.1. Leading Antioxidative Enzymes for Wound Healing
Antioxidant enzymes help with oxidative stress and diabetic wounds. SOD, catalase, glutathione peroxidase, and heme oxygenase are antioxidant enzymes.57 Antioxidant enzymes have been used in diabetic wound healing in recent years.
6.1.1. Superoxide Dismutase
Superoxide dismutase is an antioxidant enzyme involved in oxidative stress. As an endogenous factor capable of scavenging free radicals, SOD catalyzes the breakdown of superoxide radicals into hydrogen peroxide which is transformed into water and oxygen.58 However, SOD is produced at insufficient levels in diabetic wound healing, resulting in increased oxidative stress and poor wound healing. In recent years, research have documented usage of SOD in diabetic wound healing, with SOD-loaded hydrogels are successfully encouraging the repair of chronic diabetic wounds.59
In a murine model with full-thickness burns, intravenous Cu/Zn-SOD at 10 mg/kg (poly(butyl ester) bound SOD) affected plasma thiobarbituric acid reactive (TBAR) metabolite after prewound dosing.60 At 3 h after burn, SOD-treated mice showed lower plasma TBAR metabolite levels than saline-treated animals. SOD-treated animals survived burns 7 days longer than control rats.
6.1.2. Glutathione Peroxidase (GPX)
GPX proteins decrease H2O2 and organic peroxides glutathione-dependently. Humans have eight GPX genes (GPX1–8). GPX1–4 are selenoproteins with a selenocysteine (SeCys) residue in the catalytic core.61 Because GPX needs glutathione as an electron donor, a wound lesion should reduce glutathione levels and GPX activity.62
GPX1 mRNA is elevated in a wound lesion,63 although GPX1 protein levels are low in damaged skin.64 Normal rat wounds have lower protein and GPX levels.64 Immunocompromised rats had similar outcomes.65 Alkylation or nitric oxide-dependent oxidation of SeCys lowers GPX activity, but high H2O2 and a SOD1 deficit convert SeCys to dehydroalanine, causing irreversible inactivation and degradation.66 GPX1 protein levels are elevated between days 3 and 7 following a cutaneous injury, according to another research.67 Thus, our existing evidence on GPX1 protein levels after injury is inconsistent, and it is not apparent what explains the disparity.
Some GPX family members need selenium for SeCys biosynthesis and translational inclusion.68 Selenium reduces GPX1 protein abundance. A quarter of trauma patients with skin wound healing abnormalities had low serum selenium,69 confirming GPX’s role in etiology. Selenium may convert macrophages from M1, which creates ROS, to M2, which stimulates cell growth.70 GPX1-deficient animals may convert macrophages to M2. Selenium may work independently of GPX production and activity. Selenium may be used to treat wound healing issues, albeit the process requires further study.
6.1.3. Heme Oxygenases (HO)
HO degrades heme to biliverdin, iron, and CO and fights oxidative damage. Biliverdin reductase converts biliverdin to bilirubin. Appropriate quantities of bilirubin operate as a powerful antioxidant in the blood.
HO-1 is inducible by oxidative stress and hypoxia.71 HO-1 mRNA and protein are elevated in wounds, suggesting that HO-1 protects against ROS by producing bilirubin.72 HO-1 knockout mice demonstrate delayed wound healing due to poor re-epithelialization and angiogenesis.73
HO-1 regulates diabetic wound healing, according to studies. Hemin, a potent inducer of HO-1, may expedite wound healing in diabetic rat wounds by lowering inflammatory cytokines including TNF- and IL-6, boosting antioxidants, and encouraging angiogenesis.74 HO-1 inhibitor in-protoporphyrin IX (SnPPIX) worsened oxidative stress and delayed wound closure in nondiabetic mice. Recent investigations corroborate HO-1’s usefulness in diabetic wound healing.75
6.2. Antioxidant Peptides with Potential for Wound Healing
A slew of research back up the idea that antioxidant and antimicrobial peptides enhance wound healing in the skin through various mechanisms, including neutralizing the oxidative species, regulating cytokine production, cell migration, proliferation, and, in some circumstances, angiogenesis.76 For example, by activating the epidermal growth factor in human skin, these peptides cause the expression of human-defensin (hBD)-2 and boost cytokine production in keratinocytes and cell migration.77 The recent finding of the antioxidant peptides in wound healing suggests that cathelicidins are essential in enhancing the host defensive system. Cathelicidins are more abundant invertebrates than amphibians. Some cathelicidins have recently exhibited possible antioxidant action in wound healing activities. Gj-CATH3, derived from Gekko Japonicus, is a significant example of a cathelicidin peptide. In vitro investigations demonstrated that they had a high antioxidant capacity. However, their application does not emerge because of the high manufacturing cost and related toxicity.
Cai et al. created a plethora of analogues for the Gj-CATH3 short peptide to improve cell sensitivity. 2,2′-Azinobis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay results showed that the two analogues of the Gj-CATH3 such as Gj-CATH3-(38–42), and Gj-CATH3-(33–42)-peptide had high antioxidant activity. Cytotoxic and hemolytic experiments, on the other hand, revealed much-decreased cell toxicity and hemolysis when compared to Gj-CATH3. Further in vitro cell line investigations on HaCaT cells showed that cell proliferation had improved significantly. On the other hand, preclinical trials revealed robust wound healing activity and increased superoxide dismutase (SOD) activity. Treatment with Gj-CATH3 derivatives such as Gj-CATH3-(38–42)-peptide and Gj-CATH3-(33–42)-peptides resulted in much lower MDA concentrations in the excisional wound model tissues. According to the authors, this was the first study to look at the wound healing function of cathelicidins. This study adds to the interest in the research of cathelicidins as an alternative wound healing therapy.78
Similarly, Cao et al. discovered cathelicidin-OA1 from Odorrana andersonii, an antioxidant cathelicidin. This peptide’s functional investigation found that it lacked antibacterial, hemolytic, and poisonous properties. However, cathelicidin-OA1 demonstrated considerable antioxidant activity in the ABTS+ and DPPH tests. Furthermore, in vitro tests on HaCaT and fibroblasts revealed enhanced cell proliferation in a dose-dependent and time-dependent manner. Cathelicidin-OA1 was used to treat full-thickness wounds in mice and displayed improved wound healing.
Furthermore, at lower concentrations, the study found dose-dependent wound healing activity. Histopathological findings revealed that the peptide also enhanced tissue re-epithelialization and granulation during the early stages of wound healing. Interestingly, granulation tissue production dramatically decreases in the later stages of wound healing. Compared to control and epidermal growth factor (EGF) treated rats, cathelicidin-OA1 treated rats displayed vigorous wound healing activity. Furthermore, wound healing aids by a transient increase in tumor necrotic factor (TNF-α) production following a protein therapy. This may aid in releasing growth factors and the recruitment of cells at the wound site. Furthermore, increased expression of transforming growth factor (TGF-β) leads to faster wound healing. Overall, the suggested peptide aids in the recruitment of macrophages, consequently increasing TNF-α and TGF-β production and, as a result, the potential wound healing activity.79
Wound infections cause inflammation and immunological responses, which delay wound healing and cause increased tissue damage.80 Furthermore, a disproportionate quantity of ROS in infected wounds causes an imbalance in the antioxidant defense system, impacting inflammatory responses, inhibiting angiogenesis, and slowing wound regeneration.81 However, treating wounds with ROS scavenging material increased the therapeutic effect with rapid wound healing. Dopamine-containing compounds with intrinsic antibacterial and ROS scavenging action treat therapeutically relevant infected wounds. Dopamine conjugation with catechols benefits ROS scavenging38 by enhancing dopamine’s dose-dependent antioxidant capacity.
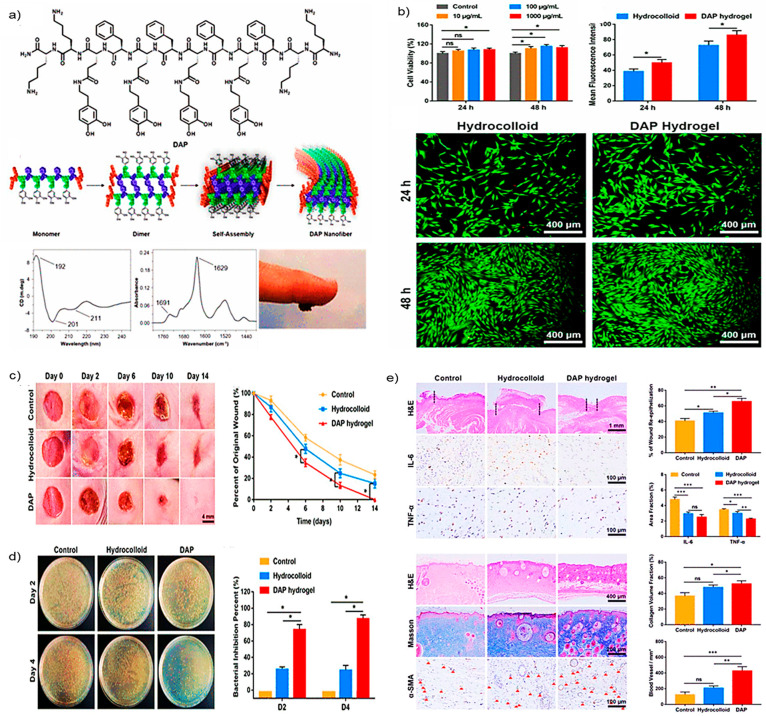
Hussain et al. created a dopamine-substituted multidomain peptide (DAP) with various favorable wound healing properties. DAP contains unique qualities such as antibacterial activity, ROS scavenging activity, and firm skin adherence. DAP has the potential to produce sheets comprising hydrogels with dopamine at the surface (Figure 5a). Adding multivalent ions or exposing hydrogel to UV radiation improves the mechanical characteristics of the hydrogel. Examination of DAP hydrogels for antimicrobial capabilities discovers that DAP exhibits broad-spectrum antibacterial activities. This broad-spectrum antibacterial action attributes to the DAP’s positively charged lysine residues and sheet formation. The OH radical clearance test analyzes the DAP’s ROS scavenging activity and discovers the scavenging impact of the DAP is dose-dependent.
Figure 5.
Antioxidant and wound healing activity of DAP: (a) Chemical structure and self-assembled structural alignment of DAP; (b) percentage cell viability, mean fluorescence intensity, and live/dead assay for biocompatibility estimation of DAP; (c) photographs of wound images and their respective wound closure rates; (d) bacterial burden isolated from the infected wound in different groups at days 2 and 4 and quantitative analysis of the bacterial inhibition by various treatment at days 2 and 4; (e) immunohistological evaluation of different treatment groups H&E, IL-6, and TNF-α staining on day 6 and H&E, Masson’s trichrome, and α-smooth muscle actin (α-SMA) staining on day 14, wound re-epithelialization, number of a blood vessel per area, and fraction of collagen volume in wounded tissue. Adapted with the permissions from ref (82). Copyright 2021 American Chemical Society.
Furthermore, at 2 mg/mL, DAP had the most incredible scavenging action. Compared to the hydrocolloid treatment, 3T3 fibroblast cells treated with the DAP enhanced significant cell proliferation (Figure 5b). Effective wound healing was seen when this DAP was administered to the wounds in a full-thickness model of mice (Figure 5c). A brief inflammatory phase was also detected due to the DAP’s natural antibacterial and antioxidant action (Figure 5d). On day 6 of therapy, immunohistochemistry examination revealed significantly fewer pro-inflammatory markers such as IL-6 and TNF-α. In the DAP-treated rats, substantial blood vessel development and collagen deposition were observed (Figure 5e). This research suggests that peptides with engineered antimicrobial and antioxidant properties can scavenge ROS, reduce the bacterial burden at the wound site, reduce wound closure time, promote granular tissue formation, improve collagen synthesis, and rapid vascularization for rapid wound healing.82
Several studies have shown that fish and their byproducts have wound healing properties. The wound healing activity of the fish and its products is primarily related to the antioxidant capacity of the peptides found in them.83 Potential antioxidant peptides can aid wound healing by neutralizing ROS, acting as a chelator, or decreasing lipid peroxidation.84 However, the in vivo antioxidant capacity of the many peptides is yet unknown. The KEAP1-Nrf2 signaling pathway was identified as one of the most plausible explanations in several investigations for wound healing activity of the antioxidant peptides.85 This pathway plays a critical regulatory function in displaying the cytoprotective impact on oxidative stress. Nrf2 plays an essential role in the cellular antioxidant process; nevertheless, KEAP1 promotes Nrf2 degradation.86 As a result, antioxidant peptides that can interact with KEAP1 and block the formation of the Keasp1-Nrf2 complex can potentially boost antioxidant activity in vivo.87
Zhang et al. isolated a novel antioxidant peptide after digesting the snakehead with simulated gastrointestinal fluid. To study the antioxidant potential of newly isolated peptides, antioxidant tests such as DPPH and hydroxyl radical assays were performed. In the DPPH experiment, a peptide having the sequences SDGSNIHFPN and PGMLGGSPPGLLGGSPP had the best antioxidant potential among the four new peptides tested. In silico molecular docking of peptides with KEAP1 indicated an acceptable ligand binding efficacy. Furthermore, the cell viability findings revealed cytoprotective activity against H2O2-treated cells. This study discovered that peptides could not only operate as possible new antioxidants by stimulating the KEAP1-Nrf2 signaling but also be exploited as potential treatments for fast wound healing.88
6.3. Nanomaterials That Trap Free Radicals for Wound Healing
Many diseases, including cancer, inflammatory disorders, and neurological problems, are caused by an imbalance in free radical production in the human body.89 Endogenous enzymes such as glutathione (GSH) peroxidase, superoxide dismutase (SOD), catalase, and others have successfully reduced free radical levels. Scavengers of free radicals may interact with free radicals to interrupt the peroxidation chain events. However, significantly high quantities of free radicals (e.g., O2-) can inhibit their action. As the illness worsens, it cannot replenish an appropriate amount of these enzymes. As a result, highly biocompatible synthetic antioxidant materials are necessary to fight this problem. Polydopamine (PDA) contains antioxidation characteristics such as melanin. PDA’s antioxidant mechanism is currently being studied due to its intricacy. The free-radical scavenging mechanism of PDA may be connected to the redox chemistry of the polycatechol structure, lifetime of inner radical, and rapid energy transfer. The catechol may quench free radicals by giving hydrogen atoms on the phenolic hydroxyl group and reduce specific molecules by electron transfer, generating a stable quinone structure via the interaction of generated phenoxyl radicals and second quenching free radicals.90 PDA’s ROS-scavenging capacity is frequently used in oxidative stress-induced disease treatment. Polydopamine (PDA) nanoparticles (NPs) are well-known for scavenging free radicals and are employed to neutralize a range of ROS types.91
Jing et al. used a bottom-up strategy to create innovative PDA 2D nanosheets (NSs) (PDA NSs). This non-enzymatic NS exhibits outstanding free radical scavenging action against a broad spectrum of free radicals, including O2–, ABTS+, and DPPH. Furthermore, PDA NSs demonstrated considerable antioxidant activity against O2–free radicals. Aside from that, PDA NSs have anti-inflammatory properties. PDA NSs were administered to 14 mm full-thickness damaged rat models to test if they may speed up wound healing. Before being investigated for wound healing capabilities in animals, the NSs were tested for biocompatibility on mouse fibroblasts (L-929). Wounds treated with 60 g/mL exhibited complete healing with no apparent scar on day 14. Compared to the control group, histological evaluation of the treated group demonstrated a substantial reduction in inflammatory cells and an enhanced degree of collagen deposition at the wound site. As a result, nanomaterials such as PDA NSs have the potential to speed up wound healing and reduce scar formation.92
2,2,6,6-Tetramethylpiperidinenoxyl (TEMPO) may grab unpaired electrons from other radicals by a single electron on nitroxide, and the redox process switches between nitroxide, oxoammonium cation, and hydroxylamine. The nitroxide/oxoammonium redox pair enhances catalysis through reversible one-electron redox reaction and hydroxylamine functions as an antioxidant hydrogen-atom donor. TEMPO conducts Fenton reactions and radical–radical recombination as a membrane-permeable stable nitroxide radical.93
TEMPO may cure different disorders, according to studies.94 Polynitroxyl albumin and TEMPO lowered ROS levels in a hepatic liver ischemia and reperfusion injury model, reducing liver damage and inflammatory response symptoms, especially ICAM-1 and neutrophil accumulation.95 TEMPO ameliorated dehydroepiandrosterone-induced polycystic ovarian syndrome (PCOS) by reducing oxidative stress in the stomach, reversing gut dysbiosis, and altering the interaction between host metabolites and gut microbiota. TEMPO therapy for PCOS is promising.96 Calabrese et al. studied TEMPO’s anti-inflammatory effect in osteoarthritis (OA). TEMPO reduces inflammation, oxidative stress, nitrite production, and pro-inflammatory mediators. TEMPO was predicted to be a novel therapy for oxidative stress-induced inflammation.97
Yin et al. prepared three forms of water-soluble fullerenes and assessed the ROS scavenging efficacy. The three fullerene materials C60(C(COOH)2)2, C60(OH)22, and Gd@C82(OH)22 protect cells from H2O2-induced oxidative damage, maintain mitochondrial membrane potential, and inhibit intracellular ROS generation. The ROS scavenging efficacies for the fullerene materials are as follows Gd@C82(OH)22 ≥ C60(OH)22 > C60(C(COOH)2)2. Consistent with their cytoprotective abilities, these derivatives can scavenge the stable DPPH, ROS, O2•-, HO•, and inhibit lipid peroxidation in vitro. The differences in free radical-scavenging abilities support the hypothesis that chemical properties, such as surface chemistry-induced differences in electron affinity, and physical properties, such as degree of aggregation, influence the biological and biomedical activities of functionalized fullerenes. This is the first report of fullerene compounds scavenging all physiologically relevant ROS. The involvement of oxidative stress and damage in many illnesses implies that fullerene derivatives may be useful in vivo cytoprotective and therapeutic agents.98 Thus, free radical trapping characteristics of TEMPO and fullerene NPs are comparable and can be explored to treat chronic wounds by regulating the ROS level at the wound site in the future.99
6.4. Nanozymes (Antioxidant Enzymes Mimicking Nanomaterials) for Wound Healing
The diabetic wound’s hyperglycemic environment dramatically increases the likelihood of bacterial infections, making wound healing a problematic task. Aside from bacterial infections, ROS is another risk factor that slows wound healing. Furthermore, accumulating ROS might cause an inflammatory reaction at the wound site, slowing recovery. Cerium NPs (CeO2 NPs) have high photolytic and antioxidant activity, making them excellent alternative therapies for wound healing and other disorders.100 Previous research on CeO2 has found that it promotes cell proliferation and migration and neutralizes the ROS level in chronic ulcers, hence expediting wound healing.101 Furthermore, CeO2 NPs imitate SOD and catalase (CAT) imitating activities.102 Combining antioxidant CeO2 with the antibacterial substance, on the other hand, will significantly reduce bacterial growth and decrease the ROS load, which might potentially expedite wound healing.
Ma et al. created a biocompatible nanocomposite by combining a PEG-MoS2 and CeO2 (MoS2–CeO2) via simple electrostatic interactions with exceptional photothermal antibacterial activity against both Gram-negative and Gram-positive bacteria, as well as antioxidant activity for wound infection reduction and wound repair (Figure 6a). The reversible transformational character of the Ce3+ and Ce4+ (biological antioxidant mimics) is offered by the MoS2–CeO2 nanocomposite with exceptional antioxidant activity (Figure 6b). The in vivo streptozotocin-diabetic rat wound model verified wound healing at day 14 after NIR laser and nanocomposite therapy. Histopathological examination of the nanocomposite and the NIR laser-treated groups revealed fewer inflammatory cells and fibroblast migration. In addition, parallel alignment of dense collagen fibers was seen in the nanocomposite and the NIR 808 nm laser-treated group.
Figure 6.
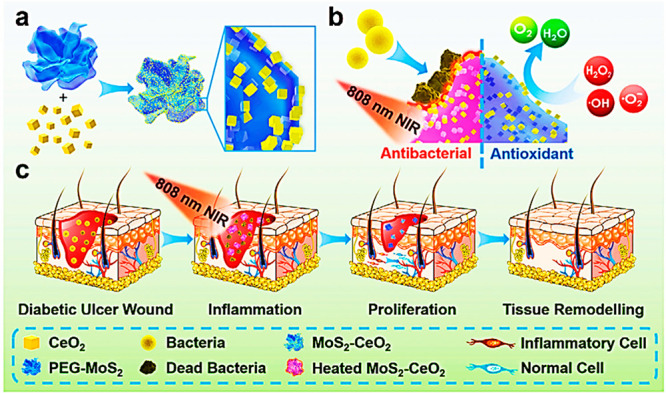
Illustration of MoS2–CeO2 nanocomposite for wound healing: (a) synthetic scheme of MoS2–CeO2 nanocomposite; (b) MoS2–CeO2 nanocomposite antioxidant and antibacterial mechanism; (c) MoS2–CeO2 nanocomposite actions on various phases of wound healing for promoting wound healing. Adapted with permission from ref (103). Copyright 2021 John Wiley and Sons.
Surprisingly, collagen production at the wound site was uneven in the remaining groups. Collagen synthesis was high and stayed the same in all treatment groups compared to the control group animals, regardless of the treatment methods with or without the NIR laser. Overall, the MoS2–CeO2 nanocomposite, combined with the NIR 808 nm laser therapy, demonstrated remarkable antibacterial, antioxidant, anti-inflammatory, and regenerative characteristics for the quick healing of chronic wounds (Figure 6c). Finally, the findings revealed that MoS2–CeO2 nanocomposite might be deemed therapeutically viable alternative treatments for treating chronic nonhealing wounds with laser aid.103 Similarly, platinum, copper, and Prussian blue imitate endogenous antioxidant enzymes (e.g., peroxidase, catalase, etc.) and SOD-like action. These carriers can be used to investigate wound healing activities.104
6.5. Antioxidant Biosurfactants Used in Wound Healing
Biosurfactants are molecules that resemble surfactants.105 These biosurfactants are highly biocompatible, environment-friendly, biodegradable, and stable across various pH and temperature conditions. Biosurfactants are employed in cosmetic and medicinal applications due to their inherent antioxidant, antibacterial, and antifungal activity and their perfect physicochemical qualities.106 Biosurfactants are classified into several groups: flavolipids, lipopeptides, phospholipids, fatty acids, and glycolipids.107 Because of their antioxidant and antibacterial capabilities, they were appealing carriers for testing their wound healing potential.
Lipopeptide biosurfactants, for example, have potential physicochemical features such as antiwrinkle, free radical scavenging, moisturizing, and antibacterial qualities. They are found in dermatological products intended for cosmetic purposes. Zouari et al. isolated an SPB1 lipopeptide biosurfactant (LBS) from Bacillus subtilis and assessed its antioxidant properties using the DPPH assay. The wound healing ability of the SPB1 lipopeptide was also studied in excisional rat wound models. Using the DPPH test, the SPB1 free radical scavenging activity was 70.4% at 1 mg/mL with an IC50 = 0.55 mg/mL. SPB1 LBS was then added into the gel and administered topically to the wound site in rats every 2 days for 13 days. Finally, data showed a considerable reduction in wound area on day 13 compared to control, CICAFLORATM, and glycerol-treated animals.
Histopathological examinations indicated that the epithelial layer of the skin had been completely repaired. Furthermore, on day 13, complete wound healing was seen in the animals treated with the 15 mg/mL SPB1 LBS-based gels compared to the 5 mg/mL SPB1 LBS-based gels. Finally, the scientists stated that SPB1 LBS might be a possible wound healing therapy. However, before contemplating this peptide as a potential therapy for wound healing, its comprehensive biocompatibility, safety, and toxicity tests are needed.108
Similarly, Ohadi et al. isolated LBS from Acinetobacter Junii B6 and studied its antioxidant and wound healing properties. The antioxidant capacity of the extracted lipopeptide biosurfactant was determined using FRAP and DPPH tests. Not only that, but they also used kits to test MDA, H2O2, and GSH. The DPPH test revealed significant scavenging action, with an IC50 of 0.7 mg/mL. The LBS’s ROS scavenging action was supported by the findings of the MDA, H2O2, and GSH tests. The 5 mg/mL LBS-loaded gels were used to treat the injured animals. On day 13, the wound length was significantly shorter in the LBS-treated group than in the control group. On day 13, histopathological examination revealed a small lesion area, better re-epithelialization, and decreased neutrophil inflammation in the LBS-treated mice compared to the control animals. The significant wound healing action of the LBS isolated from Acinetobacter Junii B6 is related to its intrinsic antioxidant potential.109
In continuation of this study, Mehrabani et al. studied LBS’s angiogenic and proliferative capabilities on human umbilical vein endothelial cells (HUVECs). Treatment with 300 g/mL LBS increased cell motility and vascular endothelial growth factor (VEGF) levels in HUVECs.110 Other biosurfactants produced from Bacillus amyloliquefaciens, Pseudomonas aeruginosa, Bacillus strains, and Lactobacillus casei have high antioxidant and antibacterial activity.111 However, the potential of these biosurfactants’ wound healing action has not yet been studied. Shortly, preclinical wound models can be used to investigate the efficacy of these kinds of biosurfactants in wound healing.
6.6. Nanoparticle Engineered Nanofibers with Antioxidant and Wound Healing Properties
Nanofibers are a nanomaterial created as an intriguing material for healing wounds and restoring damaged skin. Nanofibers are polymers that can be natural or manufactured and have variable releasing characteristics and morphological alignment.112 They are ideal drug delivery systems and employed for oxygen transportation because of their extraordinary features, including porous nature, strong biocompatibility, mechanical qualities, and high surface to volume ratio.113
Nanofibers must be adorned or loaded with antioxidant medicines or NPs to treat clinical wounds. Several nanofibers have recently been functionalized with potent antioxidants for wound healing applications. For example, the curcumin-encapsulated zein-silk fibroin-chitosan nanofiber has optimal physicochemical properties for wound healing114 with good mechanical stability, biodegradability, and biocompatibility. On the other hand, chitosan is a natural antioxidant, anti-inflammatory, and antibacterial agent with remarkable wound-healing capabilities. In an in vitro fibroblast cell culture investigation, these nanofibers improved adhesion and cell proliferation while causing little toxicity. This combination produced an outstanding mechanical performance and antioxidant, biodegradability, biocompatibility, and wound healing capabilities.
Similarly, in another work, silver nanoparticles were loaded into hyaluronic acid and polygalacturonic acid nanofibers to obtain antioxidant and anti-inflammatory capabilities to accelerate wound healing.115 Silver nanoparticles aid in the scavenging of ROS and reduce their concentration in the wound during wound healing. They can also function as antibacterial agents by damaging their cell membrane and interacting with their DNA simultaneously. Polygalacturonic acid, on the other hand, aids in the stabilization of silver nanoparticles. Furthermore, because of its hydrophilic nature, hyaluronic acid aids in the reduction of wound exudate formation. Thus, their collaboration results in effective nanofibers mat for wound healing applications. The preclinical investigations for wound healing assessment of the nanofiber mat on the rat wound model revealed quicker wound healing when compared to the commercially available ointment formulations.
6.7. Cell-Based Therapeutics for Wound Healing
Exosomes, mesenchymal stem cells (MSCs), and growth factors provide a glimmer of hope in wound healing treatments. MSCs, in general, promote the migration and proliferation of critical cells to the wound bed and regulate ECM-related proteins by generating fibronectin and collagen.116 In particular, epidermal growth factors (EGF), in particular, aids in EGF receptor activation and promote the migration and proliferation of keratinocytes, endothelial cells, and fibroblasts at the wound site.117 However, EGF degrades at the wound site due to protease activity and persistent wound infections.118
Recently, bone marrow MSCs have sparked interest in treating diabetic wound healing. They do, however, have several limitations, such as limited viability and growth near the wound bed. Mohanty and Pradhan created an EGF-curcumin bandage bioconjugate and put it into MSCs (MSCs-EGF-Cur B) to address these constraints. Furthermore, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) studies establish the EGF conjugation effectiveness. In contrast, FTIR was used to examine the chemical integrity of the curcumin in the matrix. In addition, the TNFα level was determined using an ELISA test, and the mouse embryonic fibroblasts were subjected to an inflammatory assay. TNF-α levels in EGF-Cur B treated cells were the same as in healthy control cells. The lipopolysaccharide (LPS) treated group, on the other hand, saw a considerable drop in TNF-α levels. EGF-Cur B treated MSCs showed a significant increase in the MSC transcription factors compared to the MSCs grown in standard conditions. The cell proliferation potential of EGF-Cur B was significantly increased compared to curcumin B.
Furthermore, for 12 days, a diabetic full-thickness rat model was utilized to assess the effectiveness of the MSCs-EGF-Cur B, EGF-Cur B, MSCs, and MSCs-Cur. Significant improvements in wound closure, collagen production, granular tissue development, and angiogenesis were reported in MSCs-EGF-Cur B treated rats. As a result, hybrid systems containing growth factors, MSCs, and antioxidant chemicals may offer the most excellent alternative treatments for treating difficult-to-heal wounds by working on several processes.119
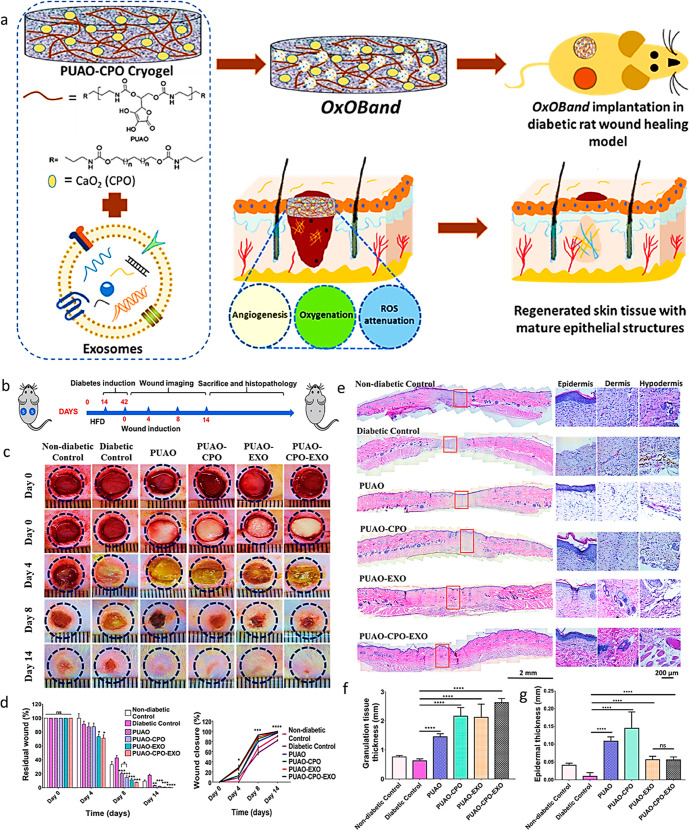
6.7.1. Exosome Laden Oxygen Releasing Antioxidant and Antibacterial Cryogel (OxOBand)
Infections, oxidative stress, decreased angiogenesis, and a low quantity of oxygen at the wound are essential clinical features of nonhealing chronic wounds.120 As a result, increasing angiogenesis, neutralizing oxidative stress, supplying oxygen, and avoiding infections might be a unique approach to managing chronic wounds and improving clinical outcomes.
Shiekh et al. created an exosome-loaded oxygen-releasing antioxidant wound dressing (OxOBand) to aid chronic wound closure and skin regeneration (Figure 7a). The OxOBand comprises polyurethane cryogels with high porosity and regulated oxygen release capabilities and an exosome as a supplement. The usage of OxOBand increases the pace of wound healing closure boosts collagen production and enhances angiogenesis. Exosomes employed in OxOBand aid in the migration of human fibroblasts and keratinocytes. Exosomes also allow fibroblasts and keratinocytes to have longer lives under hyperglycemic conditions.
Figure 7.
(a) Schematic illustration of OxOBand and their properties; (b) study design for diabetic wound production and subsequent healing; (c) representative images of the wound healing on different days; (d) residual wound and % wound closure; (e) H&E staining of the wounds (left) and higher magnification images (right) on day 14 after treatment; (f) granulation tissue quantification results on day 14 after treatment; (g) quantitative epidermal thickness at the center of the wound. Adapted with permission from ref (121). Copyright 2020 Elsevier.
It dramatically decreased oxidative stress in diabetes wounds after 14 days of wound progression compared to diabetic control wounds (Figure 7b–d). The OxOBand dressings aided in developing epithelial cells and hair follicles, much like healthy skin. The OxOBand therapy of clinically complex diabetic wounds reduced ulceration and infection, increasing collagen deposition, wound healing, and re-epithelialization (Figure 7e–g). Novel treatments, such as the OxOBand therapy method, can help to open up new avenues of therapeutic development for treating chronic wounds.121
7. Conclusions
Many studies demonstrate the importance of ROS in wound healing. On the one hand, a low amount of ROS aids in defense of wounds against microbial infections and promotes vascularization by activating multiple cellular signaling pathways. On the other hand, excessive production of ROS impedes wound healing by creating oxidative stress and, as a result, causing inflammation. If ROS detoxification is not performed at the appropriate time, the wound frequently becomes nonhealing and chronic, making treatment challenging. As a result, we require effective ROS detoxifying agents and antibacterial and anti-inflammatory capabilities that comprise medicines. We reviewed new therapeutics with remarkable antioxidant, antimicrobial, and anti-inflammatory characteristics to aid wound healing. As a result, in this review, we have included many potential innovative treatment methods that essentially stimulate wound healing by detoxifying ROS. This information expects researchers to attempt to create and describe clinically relevant numerous innovative systems with advanced features, such as antioxidant, anti-inflammatory, and antibacterial capabilities, to improve wound healing.
Acknowledgments
We acknowledge the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, India, for supporting our research on different ailments at NIPER Ahmedabad. R.K.T. acknowledges the Science and Engineering Research Board (Statutory Body Established through an Act of Parliament: SERB Act 2008), Department of Science and Technology, Government of India for a grant (Grant No. ECR/2016/001964), Core Research Grant funding (File No. CRG/2021/005402) and ICMR, New Delhi for funding Proposal No. ID 2021-14161 for work on arthritis in Dr. Tekade’s Laboaratory in Dr. Tekade’s Laboratory. R.K.T. also acknowledges the Indian Council of Medical Research (ICMR), New Delhi, for the research associate (RA) grant (File No. 5/3/8/23/ITR-/2020-ITR) to K.R. for work on targeted cancer and arthritis therapy in Dr. Tekade’s Laboaratory. R.K.T. also acknowledges the ICMR, New Delhi, for the senior research fellowship grant of S.P. (File No. 5/3/8/53/ITR-F/2020).
The authors declare no competing financial interest.
References
- Martin P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276 (5309), 75–81. 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]; Gurtner G. C.; Werner S.; Barrandon Y.; Longaker M. T. Wound repair and regeneration. Nature 2008, 453 (7193), 314–321. 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Clark R. A.Wound repair. In The molecular and cellular biology of wound repair; Springer, 1988; pp 3–50. [Google Scholar]
- Schafer M.; Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58 (2), 165–171. 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Homayouni-Tabrizi M.; Asoodeh A.; Abbaszadegan M. R.; Shahrokhabadi K.; Nakhaie Moghaddam M. An identified antioxidant peptide obtained from ostrich (Struthio camelus) egg white protein hydrolysate shows wound healing properties. Pharm. Biol. 2015, 53 (8), 1155–1162. 10.3109/13880209.2014.962061. [DOI] [PubMed] [Google Scholar]
- Trachootham D.; Lu W.; Ogasawara M. A.; Rivera-Del Valle N.; Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10 (8), 1343–1374. 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yin X.-M.; Dong Z.. Essentials of apoptosis-A guide for basic and clinical research; Humana Press, 2009. [Google Scholar]
- Soneja A.; Drews M.; Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57 (Suppl), 108–119. [PubMed] [Google Scholar]
- Choi J.; Hong G.; Kwon T.; Lim J. O. Fabrication of oxygen releasing scaffold by embedding H2O2-PLGA microspheres into alginate-based hydrogel sponge and its application for wound healing. Appl. Sci. 2018, 8 (9), 1492. 10.3390/app8091492. [DOI] [Google Scholar]
- Hiebert P.; Werner S. Regulation of wound healing by the NRF2 transcription factor—More than cytoprotection. Int. J. Mol. Sci. 2019, 20 (16), 3856. 10.3390/ijms20163856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrozova N.; Ulrichova J.; Galandakova A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc Czech. Repub. 2017, 161 (1), 1–13. 10.5507/bp.2016.063. [DOI] [PubMed] [Google Scholar]
- Muzumdar S.; Hiebert H.; Haertel E.; Ben-Yehuda Greenwald M.; Bloch W.; Werner S.; Schafer M. Nrf2-Mediated expansion of pilosebaceous cells accelerates cutaneous wound healing. Amer. J. Pathol. 2019, 189 (3), 568–579. 10.1016/j.ajpath.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Goren I.; Köhler Y.; Aglan A.; Pfeilschifter J.; Beck K.-F.; Frank S. Increase of cystathionine-γ-lyase (CSE) during late wound repair: Hydrogen sulfide triggers cytokeratin 10 expression in keratinocytes. Nitric Oxide 2019, 87, 31–42. 10.1016/j.niox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Uruno A.; Yagishita Y.; Yamamoto M. The Keap1–Nrf2 system and diabetes mellitus. Arch. Biochem. Biophys. 2015, 566, 76–84. 10.1016/j.abb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Tonelli C.; Chio I. I. C.; Tuveson D. A. Transcriptional regulation by Nrf2. Antiox. Redox Signal. 2018, 29 (17), 1727–1745. 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo de la Vega M.; Chapman E.; Zhang D. D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34 (1), 21–43. 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi C. K.; Jena G. Nrf2, a novel molecular target to reduce type 1 diabetes associated secondary complications: The basic considerations. Eur. J. Pharmacol. 2019, 843, 12–26. 10.1016/j.ejphar.2018.10.026. [DOI] [PubMed] [Google Scholar]
- Schafer M.; Dutsch S.; Auf dem Keller U.; Navid F.; Schwarz A.; Johnson D.; Johnson J.; Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010, 24 (10), 1045–1058. 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M.; Willrodt A.; Kurinna S.; Link A.; Farwanah H.; Geusau A.; et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014, 6 (4), 442–457. 10.1002/emmm.201303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M.; Liu Y.; Yang Z.; Nguyen J.; Liang F.; Morris R. J.; Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11 (12), 1351–1354. 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M.; Frances D.; Kloepper J. E.; Paus R.; Schäfer M.; Niemann C.; Schneider M. R. Overexpression of epigen during embryonic development induces reversible, epidermal growth factor receptor-dependent sebaceous gland hyperplasia. Mol. Cell. Biol. 2014, 34 (16), 3086–3095. 10.1128/MCB.00302-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S.; Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83 (3), 835–870. 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Hiebert P.; Wietecha M. S.; Cangkrama M.; Haertel E.; Mavrogonatou E.; Stumpe M.; Steenbock H.; Grossi S.; Beer H.-D.; Angel P.; et al. Nrf2-mediated fibroblast reprogramming drives cellular senescence by targeting the matrisome. Dev. Cell 2018, 46 (2), 145–161.e10. 10.1016/j.devcel.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Demaria M.; Ohtani N.; Youssef S. A.; Rodier F.; Toussaint W.; Mitchell J. R.; Laberge R.-M.; Vijg J.; Van Steeg H.; Dollé M. E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31 (6), 722–733. 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano Sanchez M.; Lancel S.; Boulanger E.; Neviere R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants (Basel) 2018, 7 (8), 98. 10.3390/antiox7080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves R. V.; Costa A. M. A.; Grzeskowiak L. Oxidative Stress and Tissue Repair: Mechanism, Biomarkers, and Therapeutics. Oxid. Med. Cell Longevity 2021, 2021, 6204096. 10.1155/2021/6204096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya A.; Tokura Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76 (3), 169–172. 10.1016/j.jdermsci.2014.11.001. [DOI] [PubMed] [Google Scholar]; Portou M. J.; Baker D.; Abraham D.; Tsui J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vascul. Pharmacol. 2015, 71, 31–36. 10.1016/j.vph.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Zhang Y.; Dusting G. J. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63 (1), 218–242. 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- Sen C. K.; Roy S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780 (11), 1348–1361. 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnill C.; Patton T.; Brennan J.; Barrett J.; Dryden M.; Cooke J.; Leaper D.; Georgopoulos N. T. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14 (1), 89–96. 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]; (accessed 2021-07-09)
- Bitar M. S.; Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301 (6), E1119–1129. 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]; (accessed 2021-07-07)
- David J. A.; Rifkin W. J.; Rabbani P. S.; Ceradini D. J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. A.; Cohen O. D.; Low Y. C.; Sartor R. A.; Ellison T.; Anil U.; Anzai L.; Chang J. B.; Saadeh P. B.; Rabbani P. S.; et al. Restoration of Nrf2 Signaling Normalizes the Regenerative Niche. Diabetes 2016, 65 (3), 633–646. 10.2337/db15-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrozova N.; Ulrichova J.; Galandakova A. Models for the study of skin wound healing. The role of Nrf2 and NF-kappaB. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161 (1), 1–13. 10.5507/bp.2016.063. [DOI] [PubMed] [Google Scholar]
- Artlett C. M. Inflammasomes in wound healing and fibrosis. J. Pathol. 2013, 229 (2), 157–167. 10.1002/path.4116. [DOI] [PubMed] [Google Scholar]
- Roy S.; Khanna S.; Nallu K.; Hunt T. K.; Sen C. K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13 (1), 211–220. 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha N.; Roy S.; He G.; Biswas S.; Velayutham M.; Khanna S.; Kuppusamy P.; Zweier J. L.; Sen C. K. Assessment of wound-site redox environment and the significance of Rac2 in cutaneous healing. Free Radic. Biol. Med. 2008, 44 (4), 682–691. 10.1016/j.freeradbiomed.2007.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso M. L.; Clarkson P. M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189 (1–2), 41–54. 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Muguregowda H. T.; Kumar P.; Govindarama P. U. E. Wound healing potential of intermittent negative pressure under limited access dressing in burn patients: Biochemical and histopathological study. World J. Plast. Surg. 2018, 7 (1), 58–66. [PMC free article] [PubMed] [Google Scholar]
- Gaweł S.; Wardas M.; Niedworok E.; Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57 (9–10), 453–455. [PubMed] [Google Scholar]
- Raghavan S.; Subramaniyam G.; Shanmugam N. Proinflammatory effects of malondialdehyde in lymphocytes. J. Leukocyte Biol. 2012, 92 (5), 1055–1067. 10.1189/jlb.1211617. [DOI] [PubMed] [Google Scholar]
- Musalmah M.; Fairuz A. H.; Gapor M. T.; Wan Ngah W. Z. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pacific J. Clin. Nutr. 2002, 11, S448–S451. 10.1046/j.1440-6047.11.s.7.6.x. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Singh R. L.; Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol. Cell. Biochem. 2002, 241 (1–2), 1–7. 10.1023/A:1020804916733. [DOI] [PubMed] [Google Scholar]; Moseley R.; Hilton J. R.; Waddington R. J.; Harding K. G.; Stephens P.; Thomas D. W. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen. 2004, 12 (4), 419–429. 10.1111/j.1067-1927.2004.12406.x. [DOI] [PubMed] [Google Scholar]
- Yeoh-Ellerton S.; Stacey M. C. Iron and 8-isoprostane levels in acute and chronic wounds. J. Invest. Dermatol. 2003, 121 (4), 918–925. 10.1046/j.1523-1747.2003.12471.x. [DOI] [PubMed] [Google Scholar]
- Wlaschek M.; Scharffetter-Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005, 13 (5), 452–461. 10.1111/j.1067-1927.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- James T. J.; Hughes M. A.; Cherry G. W.; Taylor R. P. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen. 2003, 11 (3), 172–176. 10.1046/j.1524-475X.2003.11304.x. [DOI] [PubMed] [Google Scholar]
- Kümin A.; Schäfer M.; Epp N.; Bugnon P.; Born-Berclaz C.; Oxenius A.; Klippel A.; Bloch W.; Werner S. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J. Cell Biol. 2007, 179 (4), 747–760. 10.1083/jcb.200706090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft G. S.; Mills S. J. Androgen receptor-mediated inhibition of cutaneous wound healing. J. Clin. Invest. 2002, 110 (5), 615–624. 10.1172/JCI0215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M.; Werner S. Transcriptional control of wound repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 69–92. 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- D’Autréaux B.; Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8 (10), 813–824. 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Guo S.; Dipietro L. A. Factors affecting wound healing. J. Dent. Res. 2010, 89 (3), 219–229. 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (accessed 2021-07-07); Janis J. E.; Harrison B. Wound Healing: Part I. Basic Science. Plast. Reconstr. Surg. 2016, 138 (3S), 9S–17S. 10.1097/PRS.0000000000002773. [DOI] [PubMed] [Google Scholar]
- Zielins E. R.; Brett E. A.; Luan A.; Hu M. S.; Walmsley G. G.; Paik K.; Senarath-Yapa K.; Atashroo D. A.; Wearda T.; Lorenz H. P.; et al. Emerging drugs for the treatment of wound healing. Expert Opin. Emerg. Drugs 2015, 20 (2), 235–246. 10.1517/14728214.2015.1018176. [DOI] [PubMed] [Google Scholar]
- Rex J.; Muthukumar N.; Selvakumar P. J. M. B. O. C. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing-A review. MOJ Biorg. Org. Chem. 2018, 2 (2), 61–70. [Google Scholar]
- Johnson J. B.; Broszczak D. A.; Mani J. S.; Anesi J.; Naiker M. J. J. o. P. Pharmacology. A cut above the rest: Oxidative stress in chronic wounds and the potential role of polyphenols as therapeutics. J. Pharm. Pharmacol. (Chichester, United Kingdom) 2022, 74 (4), 485–502. 10.1093/jpp/rgab038. [DOI] [PubMed] [Google Scholar]
- Guimarães I.; Baptista-Silva S.; Pintado M.; Oliveira A. L. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11 (3), 1230. 10.3390/app11031230. [DOI] [Google Scholar]
- Sharma A.; Puri V.; Bakshi I. S.; Kumar P.. Herbal bioactive-incorporated scaffolds for wound healing applications. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier, 2022; pp 311–330. 10.1016/B978-0-12-824385-5.00018-2. [DOI] [Google Scholar]
- Viaña-Mendieta P.; Sánchez M. L.; Benavides J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19 (1), 100–113. 10.1111/iwj.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazarlu O.; Iranshahi M.; Kashani H. R. K.; Reshadat S.; Habtemariam S.; Iranshahy M.; Hasanpour M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. 10.1016/j.phrs.2021.105841. [DOI] [PubMed] [Google Scholar]
- Panayi A. C.; Endo Y.; Karvar M.; Sensharma P.; Haug V.; Fu S.; Mi B.; An Y.; Orgill D. P. Low mortality oxidative stress murine chronic wound model. BMJ Open Diabetes Res. Care 2020, 8 (1), e001221. 10.1136/bmjdrc-2020-001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Chen L.; Xiong Y.; Panayi A. C.; Abududilibaier A.; Hu Y.; Yu C.; Zhou W.; Sun Y.; Liu M.; et al. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. 10.3389/fbioe.2021.707479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Ma Y.; Pan X.; Chen S.; Zhuang H.; Wang S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. 10.1016/j.carbpol.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice S.; Sivamani R. K.; Isseroff R. R. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24 (3), 113–126. 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R.; Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta, Gen. Subj. 2013, 1830 (5), 3289–3303. 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Rasik A. M.; Shukla A. Antioxidant status in delayed healing type of wounds. Int. J. Exp. Pathol. 2000, 81 (4), 257–263. 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiling H.; Munz B.; Werner S.; Brauchle M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999, 247 (2), 484–494. 10.1006/excr.1998.4366. [DOI] [PubMed] [Google Scholar]
- Iuchi Y.; Roy D.; Okada F.; Kibe N.; Tsunoda S.; Suzuki S.; Takahashi M.; Yokoyama H.; Yoshitake J.; Kondo S.; et al. Spontaneous skin damage and delayed wound healing in SOD1-deficient mice. Mol. Cell. Biochem. 2010, 341 (1), 181–194. 10.1007/s11010-010-0449-y. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Singh R. L.; Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol. Cell. Biochem. 2002, 241 (1), 1–7. 10.1023/A:1020804916733. [DOI] [PubMed] [Google Scholar]
- Wang S. K.; Weaver J. D.; Zhang S.; Lei X. G. Knockout of SOD1 promotes conversion of selenocysteine to dehydroalanine in murine hepatic GPX1 protein. Free Radical Biol. Med. 2011, 51 (1), 197–204. 10.1016/j.freeradbiomed.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H. A.; Yim S. H.; Shin D. H.; Kang D.; Yu D.-Y.; Rhee S. G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 2010, 140 (4), 517–528. 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Kümin A.; Huber C.; Rülicke T.; Wolf E.; Werner S. Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis. Am. J. Pathol. 2006, 169 (4), 1194–1205. 10.2353/ajpath.2006.060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass S. C.; Goost H.; Burger C.; Tolba R. H.; Stoffel-Wagner B.; Stehle P.; Ellinger S. Extracellular micronutrient levels and pro-/antioxidant status in trauma patients with wound healing disorders: results of a cross-sectional study. Nutr. J. 2013, 12 (1), 157. 10.1186/1475-2891-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. M.; Lei X.; Prabhu K. S. Selenium levels affect the IL-4–induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141 (9), 1754–1761. 10.3945/jn.111.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. J.; Jiang B.-H.; Chin B. Y.; Iyer N. V.; Alam J.; Semenza G. L.; Choi A. K. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997, 272 (9), 5375–5381. 10.1074/jbc.272.9.5375. [DOI] [PubMed] [Google Scholar]
- Hanselmann C.; Mauch C.; Werner S. Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis?. Biochem. J. 2001, 353 (3), 459–466. 10.1042/bj3530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshane J.; Chen S.; Caballero S.; Grochot-Przeczek A.; Was H.; Li Calzi S.; Lach R.; Hock T. D.; Chen B.; Hill-Kapturczak N.; et al. Stromal cell–derived factor 1 promotes angiogenesis via a heme oxygenase 1–dependent mechanism. J. Exp. Med. 2007, 204 (3), 605–618. 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.-Y.; Wang G.-G.; Li W.; Jiang Y.-X.; Lu X.-H.; Zhou P.-P. Heme oxygenase-1 promotes delayed wound healing in diabetic rats. J. Diabetes Res. 2016, 2016, 9726503. 10.1155/2016/9726503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D.; Jena G. R.; Ram M.; Lingaraju M. C.; Singh V.; Prasad R.; Kumawat S.; Kant V.; Gupta P.; Tandan S. K. Hemin attenuated oxidative stress and inflammation to improve wound healing in diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392 (11), 1435–1445. 10.1007/s00210-019-01682-7. [DOI] [PubMed] [Google Scholar]
- Steinstraesser L.; Koehler T.; Jacobsen F.; Daigeler A.; Goertz O.; Langer S.; Kesting M.; Steinau H.; Eriksson E.; Hirsch T. Host defense peptides in wound healing. Mol. Med. 2008, 14 (7), 528–537. 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyonsaba F.; Ushio H.; Nakano N.; Ng W.; Sayama K.; Hashimoto K.; Nagaoka I.; Okumura K.; Ogawa H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 2007, 127 (3), 594–604. 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]; Roupé K. M.; Nybo M.; Sjöbring U.; Alberius P.; Schmidtchen A.; Sørensen O. E. Injury is a major inducer of epidermal innate immune responses during wound healing. J. Invest. Dermatol. 2010, 130 (4), 1167–1177. 10.1038/jid.2009.284. [DOI] [PubMed] [Google Scholar]
- Cai S.; Lu C.; Liu Z.; Wang W.; Lu S.; Sun Z.; Wang G. Derivatives of gecko cathelicidin-related antioxidant peptide facilitate skin wound healing. Eur. J. Pharmacol. 2021, 890, 173649. 10.1016/j.ejphar.2020.173649. [DOI] [PubMed] [Google Scholar]
- Cao X.; Wang Y.; Wu C.; Li X.; Fu Z.; Yang M.; Bian W.; Wang S.; Song Y.; Tang J.; et al. Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing. Sci. Rep. 2018, 8 (1), 943. 10.1038/s41598-018-33558-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M.; Kosaric N.; Bonham C. A.; Gurtner G. C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99 (1), 665–706. 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano Sanchez M.; Lancel S.; Boulanger E.; Neviere R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants (Basel) 2018, 7 (8), 98. 10.3390/antiox7080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M.; Suo H.; Xie Y.; Wang K.; Wang H.; Hou Z.; Gao Y.; Zhang L.; Tao J.; Jiang H.; et al. Dopamine-Substituted Multidomain Peptide Hydrogel with Inherent Antimicrobial Activity and Antioxidant Capability for Infected Wound Healing. ACS Appl. Mater. Interfaces 2021, 13 (25), 29380–29391. 10.1021/acsami.1c07656. [DOI] [PubMed] [Google Scholar]
- Wang C.-H.; Doan C. T.; Nguyen V. B.; Nguyen A. D.; Wang S.-L. Reclamation of Fishery Processing Waste: A Mini-Review. Molecules 2019, 24 (12), 2234. 10.3390/molecules24122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sila A.; Bougatef A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. 10.1016/j.jff.2015.11.007. [DOI] [Google Scholar]
- Han J.; Tang S.; Li Y.; Bao W.; Wan H.; Lu C.; Zhou J.; Li Y.; Cheong L.; Su X. In silico analysis and in vivo tests of the tuna dark muscle hydrolysate anti-oxidation effect. RSC Adv. 2018, 8 (25), 14109–14119. 10.1039/C8RA00889B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. D.; Lo S. C.; Sun Z.; Habib G. M.; Lieberman M. W.; Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J. Biol. Chem. 2005, 280 (34), 30091–30099. 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- Li L.; Liu J.; Nie S.; Ding L.; Wang L.; Liu J.; Liu W.; Zhang T. Direct inhibition of Keap1–Nrf2 interaction by egg-derived peptides DKK and DDW revealed by molecular docking and fluorescence polarization. RSC Adv. 2017, 7 (56), 34963–34971. 10.1039/C7RA04352J. [DOI] [Google Scholar]
- Zhang J.; Li M.; Zhang G.; Tian Y.; Kong F.; Xiong S.; Zhao S.; Jia D.; Manyande A.; Du H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. 10.1016/j.foodchem.2020.127921. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ai K.; Ji X.; Askhatova D.; Du R.; Lu L.; Shi J. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017, 139 (2), 856–862. 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Yang L.; Yang P.; Jiang S.; Liu X.; Li Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8 (18), 4940–4950. 10.1039/D0BM01070G. [DOI] [PubMed] [Google Scholar]
- Zhong G.; Yang X.; Jiang X.; Kumar A.; Long H.; Xie J.; Zheng L.; Zhao J. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale 2019, 11 (24), 11605–11616. 10.1039/C9NR03060C. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hunyadi A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39 (6), 2505–2533. 10.1002/med.21592. [DOI] [PubMed] [Google Scholar]
- Jing Y.; Deng Z.; Yang X.; Li L.; Gao Y.; Li W. Ultrathin two-dimensional polydopamine nanosheets for multiple free radical scavenging and wound healing. Chem. Commun. (Cambridge, U. K.) 2020, 56 (74), 10875–10878. 10.1039/D0CC02888F. [DOI] [PubMed] [Google Scholar]
- Soule B. P.; Hyodo F.; Matsumoto K.-i.; Simone N. L.; Cook J. A.; Krishna M. C.; Mitchell J. B. The chemistry and biology of nitroxide compounds. Free Radical Biol. Med. 2007, 42 (11), 1632–1650. 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J.; Yoshimura S.; Yamakawa H.; Sawada M.; Nakagawa M.; Hara S.; Kaku Y.; Iwama T.; Naganawa T.; Banno Y.; et al. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci. Res. 2003, 45 (1), 1–8. 10.1016/S0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]; Marciniak A.; Walczyna B.; Rajtar G.; Marciniak S.; Wojtak A.; Lasiecka K. Tempol, a membrane-permeable radical scavenger, exhibits anti-inflammatory and cardioprotective effects in the cerulein-induced pancreatitis rat model. Oxid. Med. Cell Longevity 2016, 2016, 4139851. 10.1155/2016/4139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder J. M.; McCalden T. A.; Hsia C. J.; Billings R. E. Polynitroxyl albumin plus tempol attenuates liver injury and inflammation after hepatic ischemia and reperfusion. Life Sci. 2000, 67 (26), 3231–3239. 10.1016/S0024-3205(00)00907-3. [DOI] [PubMed] [Google Scholar]
- Li T.; Zhang T.; Gao H.; Liu R.; Gu M.; Yang Y.; Cui T.; Lu Z.; Yin C. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021, 41, 101886. 10.1016/j.redox.2021.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese G.; Ardizzone A.; Campolo M.; Conoci S.; Esposito E.; Paterniti I. Beneficial effect of tempol, a membrane-permeable radical scavenger, on inflammation and osteoarthritis in in vitro models. Biomolecules 2021, 11 (3), 352. 10.3390/biom11030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.-J.; Lao F.; Fu P. P.; Wamer W. G.; Zhao Y.; Wang P. C.; Qiu Y.; Sun B.; Xing G.; Dong J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30 (4), 611–621. 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule B. P.; Hyodo F.; Matsumoto K.; Simone N. L.; Cook J. A.; Krishna M. C.; Mitchell J. B. The chemistry and biology of nitroxide compounds. Free Radical Biol. Med. 2007, 42 (11), 1632–1650. 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yin J. J.; Lao F.; Fu P. P.; Wamer W. G.; Zhao Y.; Wang P. C.; Qiu Y.; Sun B.; Xing G.; Dong J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30 (4), 611–621. 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Chen R.; Yu Q.; Huang W.; Lai P.; Tang J.; Nie L. Near-Infrared Plasmon-Boosted Heat/Oxygen Enrichment for Reversing Rheumatoid Arthritis with Metal/Semiconductor Composites. ACS Appl. Mater. Interfaces 2020, 12 (41), 45796–45806. 10.1021/acsami.0c13261. [DOI] [PubMed] [Google Scholar]; Liu T.; Xiao B.; Xiang F.; Tan J.; Chen Z.; Zhang X.; Wu C.; Mao Z.; Luo G.; Chen X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11 (1), 2788. 10.1038/s41467-020-16544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Li L.-D.; Lyu G.-M.; Shen B.-Y.; Han Y.-F.; Shi J.-L.; Teng J.-L.; Feng L.; Si S.-Y.; Wu J.-H.; et al. Chitosan-coated cerium oxide nanocubes accelerate cutaneous wound healing by curtailing persistent inflammation. Inorg. Chem. Front. 2018, 5 (2), 386–393. 10.1039/C7QI00707H. [DOI] [Google Scholar]
- Celardo I.; Pedersen J. Z.; Traversa E.; Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3 (4), 1411–1420. 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]; Singh S.; Dosani T.; Karakoti A. S.; Kumar A.; Seal S.; Self W. T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32 (28), 6745–6753. 10.1016/j.biomaterials.2011.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T.; Zhai X.; Huang Y.; Zhang M.; Zhao X.; Du Y.; Yan C. A Smart Nanoplatform with Photothermal Antibacterial Capability and Antioxidant Activity for Chronic Wound Healing. Adv. Healthcare Mater. 2021, 10 (13), 2100033. 10.1002/adhm.202100033. [DOI] [PubMed] [Google Scholar]
- Cai X.; Gao W.; Zhang L.; Ma M.; Liu T.; Du W.; Zheng Y.; Chen H.; Shi J. Enabling Prussian Blue with Tunable Localized Surface Plasmon Resonances: Simultaneously Enhanced Dual-Mode Imaging and Tumor Photothermal Therapy. ACS Nano 2016, 10 (12), 11115–11126. 10.1021/acsnano.6b05990. [DOI] [PubMed] [Google Scholar]; Cheng H.; Liu Y.; Hu Y.; Ding Y.; Lin S.; Cao W.; Wang Q.; Wu J.; Muhammad F.; Zhao X.; et al. Monitoring of heparin activity in live rats using metal–organic framework nanosheets as peroxidase mimics. Anal. Chem. 2017, 89 (21), 11552–11559. 10.1021/acs.analchem.7b02895. [DOI] [PubMed] [Google Scholar]; Zhang L.; Zhang Y.; Wang Z.; Cao F.; Sang Y.; Dong K.; Pu F.; Ren J.; Qu X. Constructing metal–organic framework nanodots as bio-inspired artificial superoxide dismutase for alleviating endotoxemia. Mater. Horizons 2019, 6 (8), 1682–1687. 10.1039/C9MH00339H. [DOI] [Google Scholar]; Nash K. M.; Ahmed S. Nanomedicine in the ROS-mediated pathophysiology: Applications and clinical advances. Nanomedicine 2015, 11 (8), 2033–2040. 10.1016/j.nano.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Mawgoud A. M.; Lepine F.; Deziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86 (5), 1323–1336. 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L.; Banat I. M.; Teixeira J.; Oliveira R. Biosurfactants: potential applications in medicine. J. Antimicrob. Chemother. 2006, 57 (4), 609–618. 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- Saharan B.; Sahu R.; Sharma D. A review on biosurfactants: fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2011, 330 (1), 124963. 10.1016/j.biortech.2021.124963. [DOI] [Google Scholar]
- Zouari R.; Moalla-Rekik D.; Sahnoun Z.; Rebai T.; Ellouze-Chaabouni S.; Ghribi-Aydi D. Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed. Pharmacother. 2016, 84, 878–891. 10.1016/j.biopha.2016.09.084. [DOI] [PubMed] [Google Scholar]
- Ohadi M.; Forootanfar H.; Rahimi H. R.; Jafari E.; Shakibaie M.; Eslaminejad T.; Dehghannoudeh G. Antioxidant Potential and Wound Healing Activity of Biosurfactant Produced by Acinetobacter junii B6. Curr. Pharm. Biotechnol. 2018, 18 (11), 900–908. 10.2174/1389201018666171122121350. [DOI] [PubMed] [Google Scholar]
- Mehrabani M.; Esmaeili-Tarzi M.; Forootanfar H.; Nematollahi M. H.; Banat I. M.; Ohadi M.; Dehghannoudeh G. Lipopeptide Biosurfactant from Acinetobacter junii B6: A Promising Natural Surfactant for Promoting Angiogenesis. Int. J. Peptide Res. Ther. 2021, 27 (2), 1197–1203. 10.1007/s10989-021-10160-9. [DOI] [Google Scholar]
- Merghni A.; Dallel I.; Noumi E.; Kadmi Y.; Hentati H.; Tobji S.; Ben Amor A.; Mastouri M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. 10.1016/j.micpath.2017.01.017. [DOI] [PubMed] [Google Scholar]; Giri S. S.; Ryu E. C.; Sukumaran V.; Park S. C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. 10.1016/j.micpath.2019.04.035. [DOI] [PubMed] [Google Scholar]; Abdollahi S.; Tofighi Z.; Babaee T.; Shamsi M.; Rahimzadeh G.; Rezvanifar H.; Saeidi E.; Mohajeri Amiri M.; Saffari Ashtiani Y.; Samadi N. Evaluation of Anti-oxidant and Anti-biofilm Activities of Biogenic Surfactants Derived from Bacillus amyloliquefaciens and Pseudomonas aeruginosa. Iran J. Pharm. Res. 2020, 19 (2), 115–126. 10.22037/IJPR.2020.1101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabra S.; Ragab D. M.; Agwa M. M.; Rohani S. Recent advances in electrospun nanofibers for some biomedical applications. Eur. J. Pharm. Sci. 2020, 144, 105224. 10.1016/j.ejps.2020.105224. [DOI] [PubMed] [Google Scholar]
- Fereydouni N.; Darroudi M.; Movaffagh J.; Shahroodi A.; Butler A. E.; Ganjali S.; Sahebkar A. Curcumin nanofibers for the purpose of wound healing. J. Cell Physiol. 2019, 234 (5), 5537–5554. 10.1002/jcp.27362. [DOI] [PubMed] [Google Scholar]
- Akrami-Hasan-Kohal M.; Tayebi L.; Ghorbani M. Curcumin-loaded naturally-based nanofibers as active wound dressing mats: morphology, drug release, cell proliferation, and cell adhesion studies. New J. Chem. 2020, 44 (25), 10343–10351. 10.1039/D0NJ01594F. [DOI] [Google Scholar]
- El-Aassar M. R.; Ibrahim O. M.; Fouda M. M. G.; El-Beheri N. G.; Agwa M. M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. 10.1016/j.carbpol.2020.116175. [DOI] [PubMed] [Google Scholar]
- Chen J. S.; Wong V. W.; Gurtner G. C. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front. Immunol. 2012, 3, 192. 10.3389/fimmu.2012.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar R. J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care (New Rochelle) 2013, 2 (1), 24–29. 10.1089/wound.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R.; Liang H.; Clarke E.; Jackson C.; Xue M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17 (12), 2085. 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty C.; Pradhan J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. C 2020, 111, 110751. 10.1016/j.msec.2020.110751. [DOI] [PubMed] [Google Scholar]
- Nunan R.; Harding K. G.; Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis. Models Mechanisms 2014, 7 (11), 1205–1213. 10.1242/dmm.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiekh P. A.; Singh A.; Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]