Abstract
Background
In the present study, we aimed to compare the efficacy and safety of plazomicin with comparators for the treatment of Enterobacterales infections.
Methods
Randomized controlled trials (RCTs) assessing plazomicin for Enterobacterales infections were searched on the PubMed, Embase, and Cochrane Library databases. Meta-analyses were used to evaluate the efficacy and safety in RCTs.
Results
A total of 3 RCTs consisting of 761 patients were included in the present analysis. The study population included complex urinary tract infections (cUTIs), bloodstream infections (BSIs), and hospital-acquired pneumonia (HAP). Plazomicin had a clinical remission rate in the modified intention-to-treat (MITT) population that was similar to that of comparators (odds ratio [OR], 1.02; 95% CI, 0.60–1.73; I2 = 45%) in the pooled analysis of the 3 studies. The overall microbiologic eradication rate in the microbiological MITT (mMITT) population was similar to that of the comparators group (OR, 1.46; 95% CI, 0.72–2.95; I2 = 0%). However, the microbiologic recurrence rate of plazomicin for Enterobacterales was lower than that in the comparators group (OR, 0.38; 95% CI, 0.17–0.86; P = .02; I2 = 0%). No significant differences were found between plazomicin and comparators for the risk of any adverse events (OR, 0.78; 95% CI, 0.55–1.11; I2 = 0%).
Conclusions
Plazomicin is as good as comparators in terms of efficacy and tolerance in the treatment of Enterobacterales infections. Therefore, plazomicin is a suitable choice for antibiotic treatment in adult patients with cUTIs, BSIs, or HAP.
Keywords: plazomicin, adverse events, efficacy, Enterobacterales, meta-analysis
The burden of antibiotic-resistant infections among gram-negative bacteria is increasing, one of the consequences of which is the excessive prescription of last-line antibiotics such as carbapenems as definitive therapy with a high risk of the emergence of multidrug resistance (MDR) [1]. Enterobacterales, particularly Escherichia coli and Klebsiella pneumoniae, are the most common pathogens causing bloodstream infection (BSI), hospital-acquired pneumonia (HAP), complex urinary tract infections (cUTIs), and complicated intra-abdominal infections [2–4]. The emergence of antibiotic-resistant Enterobacterales, such as extended-spectrum β-lactamase (ESBL), AmpC β-lactamases, and carbapenemases, has become the major concern surrounding this clinical entity and further limits the choice of optimal antibiotic treatment. Patients with Enterobacterales are 3 times more likely to receive inappropriate empirical antibiotics than patients without MDR infections [5]. They also have longer hospital stays, higher hospitalization costs, and a higher risk of septic shock and death.
Aminoglycoside antibiotics are a recognized class of antibiotics that are particularly useful in the treatment of serious infections caused by gram-negative bacteria due to their rapid, concentration-dependent bactericidal action and ability to act synergistically with other antibiotics [6]. Although the use of aminoglycosides has declined in recent years due to concerns about toxicity, they have recently regained their utility in the treatment of infections caused by MDR Enterobacteriaceae [7, 8]. Aminoglycosides are administered once daily, a treatment strategy that has been shown to maintain efficacy while reducing toxicity compared with multiple daily dosing [9]. The main mechanism of aminoglycoside resistance in Enterobacteriaceae is via aminoglycoside-modifying enzymes (AMEs), and these enzymes have limited the utility of aminoglycosides in the management of infections due to ESBL-producing Enterobacteriaceae and CRE. According to monitoring data from 2014 to 2015 in the United States, about 80% of carbapenem-resistant Enterobacterales tested positive for ≥1 AME genes [10]. The activity of amikacin, gentamicin, and tobramycin to these Enterobacterales isolated decreased, with only 59.7% of these isolates being sensitive to amikacin, 49.4% to gentamicin, and 0% to tobramycin.
Plazomicin (formerly ACHN-490) is a novel aminoglycoside antibiotic that binds to the bacterial 30S ribosomal subunit, thus inhibiting protein synthesis in a concentration-dependent manner [11]. In June 2018, the US Food and Drug Administration (FDA) approved this agent for treatment of cUTIs, including pyelonephritis [12]. Plazomicin has demonstrated excellent activity against Enterobacterales. In vitro studies have shown that plazomicin has rapid bactericidal effect and strong activity against MDR Enterobacterales, including the mutation of fluoroquinolone target sites and the production of AMEs, ESBL, and carbapenemases [13–15]. However, clinical studies of plazomicin in the treatment of Enterobacterales are limited. To update the evidence on the use of plazomicin in the treatment of acute infections caused by Enterobacterales, we conducted a comprehensive review and meta-analysis to evaluate the efficacy and safety of plazomicin in the treatment of acute infections caused by Enterobacterales.
METHODS
Study Search and Selection
Studies were identified by a systematic review of the literature in the PubMed, Embase, and Cochrane Central Register of Controlled Trials databases from inception to January 2022 using the following search terms: “plazomicin,” “ACHN-490,” and “Enterobacterales.” Articles published in either English or non-English were reviewed. Studies were considered to meet the inclusion criteria if they directly compared the clinical efficacy and safety of plazomicin with other antibiotics in patients with Enterobacterales infection. Studies were excluded if they focused on in vitro activities, animal studies, or pharmacokinetic/pharmacodynamic evaluation. Data extracted from each study in the meta-analysis included year of publication, study design, type of infection, antimicrobial regimen, clinical and microbiological results, and adverse reactions. The quality of enrolled randomized controlled trials (RCTs) and risk of bias were assessed using the Cochrane Risk of Bias Assessment tool [16].
Definitions
The modified intention-to-treat (MITT) population included all intention-to-treat patients who received at least 1 dose of the study drug. The microbiological MITT population was defined as mMITT patients who met the definition of cUTI or BSI disease and identified baseline pathogens in 3 studies [17–19] and all randomized patients who identified at least 1 baseline pathogen in urine or plasma. Complicated UTI is a urinary tract infection in a patient who has underlying conditions, such as anatomical abnormalities, or risk factors, such as indwelling urinary catheters. BSI is a bloodstream infection caused by various pathogenic microorganisms (bacteria or fungi) and toxins invading the bloodstream. The clinically evaluable (CE) population included patients who received the study drug, complied with the protocol, and had a clinical response assessed at the test-of-cure (TOC) visit. The microbiologically evaluable (ME) population included CE patients who had identified baseline pathogens and assessed microbiological responses. The safety population included all patients receiving any medication study treatment.
Outcome Measurement
The primary outcome of this meta-analysis was clinical response assessed at TOC. Clinical remission was defined as complete or near resolution of signs and symptoms, with no further antibiotics needed [20]. Microbiologic eradication was defined as a reduction in the baseline pathogen from ≥105 colony-forming units (CFU) per mL to <104 CFU/mL [21]. Clinical relapse was defined as the return of clinical signs and symptoms requiring antibiotic therapy in patients who were clinically cured at TOC [22]. Microbiological recurrence was defined as culture with 105 CFU/mL of regrowth of a baseline pathogen that was eradicated at TOC [17]. Clinical relapse and microbiological recurrence were long-term follow-up for CE and ME populations. Adverse events (AEs) during treatment were defined as AEs that began during or after the administration of the study drug or increased in severity or relationship with the study drug during the study period.
Data Analysis
Review Manager, version 5.1, was used to perform statistical analysis. Heterogeneity was defined as significant when the P value was <.10 or I2 was >50% [23]. The fixed-effects model was used when the data were homogenous, and the random-effects model was used when they were heterogenous. The pooled odds ratio (OR) and 95% confidence interval were calculated for outcome analysis. The significance of the pooled ratios was determined by Z test, and a P value <.05 was considered statistically significant.
This review was registered and approved in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42022311387).
RESULTS
Study Selection and Characteristics
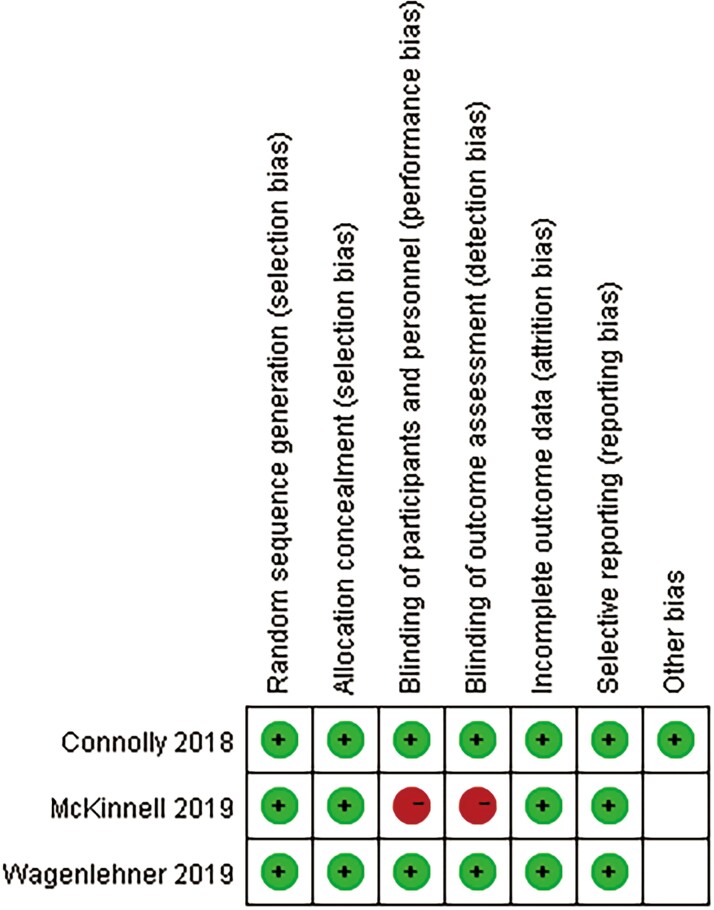
A total of 411 relevant articles were searched through the retrieval strategy. After excluding 99 repetitions, the remaining 312 abstracts were screened. Then, studies that were non-RCTs, pharmacokinetics, in vitro susceptibility testing, or experimental animal studies were also excluded. Finally, 3 studies consisting of 761 patients that met the inclusion criteria were included in this meta-analysis (Figure 1) [17–19]. All studies were randomized, multicenter studies designed to compare the clinical efficacy and safety of plazomicin 15 mg once daily with a control group for patients with cUTIs, BSI, or HAP (Table 1). The patients included in the 3 studies were all older than age 18 years, and their geographic and ethnic characteristics were multicenter, including countries in the Americas, Europe, and India in Asia. Plazomicin 10 mg (unapproved doses) were excluded. Two of the 3 studies focused on cUTIs and compared plazomicin combined with levofloxacin or meropenem [17, 19]. The third study investigated BSI or HAP and compared plazomicin with colistin [18]. Carbapenem-resistant Enterobacterales (CRE) were mainly Klebsiella pneumoniae, accounting for 18%. In the other 2 studies on cUTIs, Enterobacterales accounted for 87.5% and 99%, respectively, mainly Escherichia coli. These Enterobacterales were not susceptible to carbapenem, some quinolones, trimethoprim-sulfamethoxazole, or other aminoglycosides. With the exception of the CRE study using meropenem or tigecycline as an adjunctive antibiotic, the other 2 studies on complicated urinary tract infections had no prior antimicrobial exposures. All the domains in each study were classified as having a low risk of bias (Figure 2).
Figure 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of Included Study
| Author,Year (Region) |
Study Design | Study Duration | Study Population | Enterobacterales (%) |
Dose Regimen | No. of Patients (MITT Population) | Mean Age (SD), y | Male (%) | Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plazomicin | Comparator | Plazomicin | Comparator | Plazomicin | Comparator | Plazomicin | Comparator | ||||||
| Connolly et al., 2018 (USA) |
Multicenter, double-blind, randomized, comparator-controlled | 2010.07–2012.04 | Complicated urinary tract infection and acute pyelonephritis |
Escherichia coli (82.14%) Klebsiella pneumoniae (8.33%) |
Plazomicin 15 mg/kg once daily for 5 d | Levofloxacin 750 mg once daily for 5 d | 51 | 29 | 39.5 (15.2) | 47.9 (15.1) | 17.6 | 13.8 | Clinical remission: 70.6% Plazomicin, 65.5% Levofloxacin Microbiologic eradication: 60.8% Plazomicin, 58.6% Levofloxacin Adverse events: 35.1% Plazomicin, 47.7% Levofloxacin (headache, nausea, vomiting, diarrhea, and dizziness) |
| McKinnell et al., 2019 (USA) |
Multicenter, randomized, open-label trial | 2014.09–2016.09 | Bloodstream infection and hospital-acquired or ventilator-associated bacterial pneumonia |
Klebsiella pneumoniae
(97.29%) Enterobacter aerogenes (2.7%) |
Plazomicin 15 mg/kg once daily in combination with adjunctive meropenem or tigecycline for 7 to 14 d | Colistin 5 mg/kg once daily in combination with adjunctive meropenem or tigecycline for 7 to 14 d | 17 | 20 | 66.7 (12.0) | 63.1 (19.0) | 70.6 | 50 | Clinical remission: 88.2% Plazomicin, 65.0% Colistin Microbiologic eradication: 64.7% Plazomicin, 45% Colistin Adverse events: 88.9% Plazomicin, 100% Colistin (acute kidney injury, septic shock, and anemia) |
| Wagenlehner et al., 2019 (USA) |
Multicenter, multinational, randomized, double-blind | 2016.01–2016.09 | Complicated urinary tract infection and acute pyelonephritis |
Escherichia coli
(66.51%) Klebsiella pneumoniae (18.97%) |
Plazomicin 15 mg/kg once daily for 7 to 10 d | Meropenem 1 g every 8 h for 7 to 10 d | 191 | 197 | 58.8 (18) | 60.0 (17.9) | 44 | 50.3 | Clinical remission: 89.5% Plazomicin, 92.4% Meropenem Microbiologic eradication: 98.4% Plazomicin, 98.0% Meropenem Adverse events: 19.5% Plazomicin, 21.6% Meropenem (diarrhea, hypertension, headache, nausea, vomiting, and hypotension) |
Abbreviation: MITT, modified intention-to-treat.
Figure 2.
Summary graph of the risk of bias.
Clinical Response
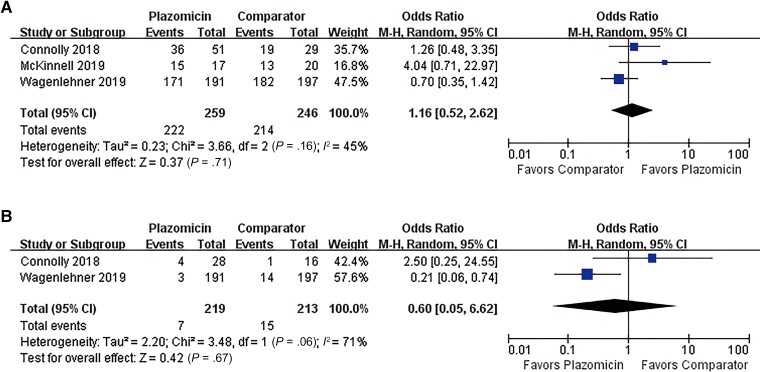
Overall, plazomicin had a clinical remission rate in the MITT population that was similar to that for the comparators (OR, 1.02; 95% CI, 0.60–1.73; I2 = 45%) (Figure 3A) in the pooled analysis of the 3 studies. In the CE population, there was no difference in clinical relapse rates between plazomicin and comparators in the pooled analysis of the 2 studies of cUTIs (OR, 0.60; 95% CI, 0.05–6.62; I2 = 71%) (Figure 3B). The 2 studies showed great heterogeneity in clinical relapse rate.
Figure 3.
Overall clinical response rates of plazomicin and comparators. A, Clinical remission rate in the MITT population. B, Clinical relapse rate in the CE population. Abbreviations: CE, clinically evaluable; MITT, modified intention-to-treat.
Microbiological Response
The overall microbiologic eradication rate in the mMITT population was reported in all 3 studies, and the pooled analysis showed that the Enterobacterales eradication rate of plazomicin was similar to that of the comparator group (OR, 1.46; 95% CI, 0.72–2.95; I2 = 0%) (Figure 4A). In the ME population, the microbiologic recurrence rate of Enterobacterales in those on plazomicin was lower than that in the comparator group in the pooled analysis of the 2 studies of cUTIs (OR, 0.38; 95% CI, 0.17–0.86; P = .02; I2 = 0%) (Figure 4B).
Figure 4.
Overall microbiological response rates of plazomicin and comparators. A, Microbiologic eradication rate in the mMITT population. B, Microbiologic recurrence rate in the ME population. Abbreviations: ME, microbiologically evaluable; mMITT, microbiological modified intention-to-treat.
Adverse Events
No significant differences were found between plazomicin and comparators for the risk of any AEs (OR, 0.78; 95% CI, 0.55–1.11; I2 = 0%) (Figure 5A), serious AEs (OR, 0.49; 95% CI, 0.21–1.14; I2 = 18%) (Figure 5B), or AEs related to study drug (OR, 0.58; 95% CI, 0.26–1.30; I2 = 0%) (Figure 5C). Regarding common nephrotoxicity and ototoxicity of aminoglycosides, no significant difference was observed between plazomicin and comparators in terms of renal function (OR, 1.36; 95% CI, 0.34–5.47; I2 = 56%) (Figure 5D) or vestibular and cochlear function (OR, 1.10; 95% CI, 0.18–6.83; I2 = 0%) (Figure 5E). AEs related to study drugs and ototoxicity were only included in the pooled analysis of 2 studies of cUTIs.
Figure 5.
Overall adverse events rates of plazomicin and comparator. A, Any AEs. B, Serious AEs. C, AEs related to study drug. D, Functional change of renal. E, Functional change of vestibular or cochlear. Abbreviation: AE, adverse event.
DISCUSSION
This meta-analysis based on 3 RCTs showed that the clinical efficacy of plazomicin was not inferior to comparators in the treatment of Enterobacterales infections. The overall pooled clinical remission rate of plazomicin in treating cUTIs, BSI, or HAP was as high as 85.7% in the MITT population, and it was as good as the comparator. The clinical relapse rate of plazomicin was 3.19%, which was lower than that of the levofloxacin and meropenem groups. The overall clinical remission rate heterogeneity of 45% and 71% in clinical relapse in our study resulted from 2 studies of cUTIs (Connolly et al. 2018 [17] and Wagenlehner et al. 2019 [19]). In meta-analyses, heterogeneity is naturally present. For small meta-analyses, it is more important to find the source of heterogeneity. The difference between the 2 studies may be explained by the disparity in the number of included samples, with Wagenlehner’s study being almost 4 times larger than Connolly’s. This pooled comparison due to sample size may be more meaningful for meta-analysis findings.
Plazomicin has received FDA approval for the treatment of adults with cUTIs or pyelonephritis caused by susceptible microorganisms [24]. The primary objectives of phase II and III trials of plazomicin in the treatment of cUTIs were to prove the noninferior treatment of plazomicin compared with levofloxacin and meropenem according to the comprehensive difference in clinical cure rates of the MITT population and the cure visit testing at later follow-up [17, 19]. This clinical effect, especially in the treatment of Enterobacterales infections, could achieve an ideal outcome. In the trial where plazomicin was used to combat CRE with BSI or HAP, plazomicin treatment was associated with an 86% reduction in the rate of death over 28 days and a 63% reduction in rate of death over 60 days compared with colistin treatment, with the separation between treatment arms evident by day 14 and sustained through day 60 [18]. The present findings indicate that plazomicin could be as effective as comparators in the treatment of Enterobacterales infections in adult patients. The overall treatment difference in favor of plazomicin here was probably driven to an appreciable extent by plazomicin’s lower nephrotoxicity than colistin, a known independent risk factor for mortality. Enrolled patients were acutely ill, presenting with multiple comorbidities, prior infections, and complex hospitalizations, reflecting real-world clinical practice. At the time of randomization, 1 adjunctive antibiotic, either tigecycline or meropenem, was selected by the investigator to be added to plazomicin or colistin. Before randomization, patients may have received treatment with empirical therapy according to local standards of care. However, patients who have received >72 hours of empirical therapy for presumed CRE infection were not eligible for the study. Therefore, the comparison of the efficacy and safety of adjuvant antibiotics for plazomicin and polymyxin under equally randomized conditions is modest.
In terms of ME, plazomicin was superior to comparators. In 2 large in vitro studies [10, 25], the minimum inhibitory concentration of plazomicin needed to inhibit 50% and 90% of the tested isolates, respectively (MIC 50/90), was 0.5 µg/mL/2 µg/mL with susceptibility of >95% in both studies. Against Escherichia, Klebsiella, Enterobacter, Serratia, and Citrobacter species, plazomicin exhibited MIC 50/90 = 0.25–0.5 µg/mL/0.5–1 µg/mL. In addition, the difference between plazomicin and other aminoglycosides is that it is more active against Enterobacterales [24]. Aminoglycoside resistance can be mediated by 3 types of mechanisms: enzymatic modification, target site modification, and porin channel/efflux pump expression changes. The most common mechanism in Enterobacterales species is enzymatic modification, mostly via 3 AME classes: n-acetyltransferases (AACs), o-adenyltransferases, and o-phosphotransferases [26]. Plazomicin is protected from nearly all clinically relevant AMEs due to structural differences. However, the only AME currently identified among gram-negative organisms with activity against plazomicin is AAC(2’)-I, which is chromosomally expressed in some Providencia stuartii isolates [27]. Although plazomicin is protected from AMEs, 16S rRNA methyltransferases prevent plazomicin activity, as with all other clinically utilized aminoglycosides [28]. In an evaluation of plazomicin against ESBL-producing Enterobacterales, it was more active than other tested aminoglycosides and comparable to meropenem/vaborbactam and avibactam/ceftazidime [29, 30]. Another study tested plazomicin in 110 unique CRE patient isolates, including 107 Klebsiella pneumonia–producing carbapenemase isolates, and only 1 strain was found to be resistant to plazomicin [31]. Only 3 strains (2.7%) were sensitive to meropenem and imipenem. These isolates showed different sensitivities to amikacin (23.6%), gentamicin (81.8%), kanamycin (8.2%), and tobramycin (3.6%). The MIC 90 value of plazomicin was the lowest of all tested drugs (1.0 mg/L), and for 96 carbapenemase-producing Klebsiella pneumonia isolates, the MIC 90 value was 0.5 mg/L. When plazomicin was tested against 95 polymyxin-resistant Enterobacterales isolates, including both mcr-1-positive isolates and mcr-1-negative isolates, it inhibited 89.5% of these isolates at an MIC of ≤2 mg/L. When evaluating comparator antibiotics including amikacin, gentamicin, tobramycin, doripenem, meropenem, tigecycline, levofloxacin, aztreonam, piperacillin/tazobactam, trimethoprim/sulfamethoxazole, ceftriaxone, and ceftazidime, the highest susceptibility percentage among all these agents was only 21% for amikacin [32].
Any AEs reported in the included trials were comparable between plazomicin and the comparison arms. Plazomicin has demonstrated a not-inferior AE profile when compared with other commonly used antibiotics in the treatment of Enterobacterales infection. The most common AEs in the plazomicin group were headache (8.3%), dizziness (4.2%), nausea (4.2%), vomiting (4.2%), and diarrhea (4.2%), which were similar to the phase I trial data [33]. In addition, serious AEs and drug-related AEs may mainly lead to death, sepsis, or acute renal injury. The plazomicin group was superior to the comparators, but there was no significant difference. Furthermore, most patients in the plazomicin group had full renal recovery by the final follow-up visit (81.0%) [19]. AEs related to renal function changes included elevated serum creatinine levels, decreased creatinine clearance, acute renal injury, renal failure, renal injury, and chronic kidney disease, which occurred in 11 (3.6%) patients in the plazomicin arm vs 4 (1.3%) in the meropenem arm. Increases in serum creatinine of ≥0.5 mg/dL occurred in 21 (7.0%) patients in the plazomicin arm vs 12 (4.0%) in the meropenem arm. Full recovery for the increase in serum creatinine at the end of intravenous therapy occurred in 6 of 11 (54.5%) in the plazomicin arm vs 4 of 9 (44.4%) in the meropenem arm [19]. In another phase II study on cUTIs, the incidence of levofloxacin in renal function events was 0. Among patients with evaluable data, 2/12 (16.7%) in the plazomicin group vs 8/16 (50.0%) in the colistin group had a serum creatinine increase of ≥0.5 mg/dL above baseline at any time during the study, and 1/12 (8.3%) in the plazomicin group vs 6/16 (37.5%) in the colistin group had a serum creatinine increase of ≥0.5 mg/dL above baseline while on study drug therapy [18]. AEs related to potential ototoxicity did not appear to be common, as the report was not entirely based on cochlear and vestibular assessments in the phase 3 trial. Although aminoglycosides have been known to be associated with ototoxicity risks, the possibility of ototoxicity associated with plazomicin treatment could not be determined from the trial. In an earlier study, cochlear and vestibular function was assessed at baseline and up to 6 months after plazomicin treatment [33]. Although the evaluation was conducted in healthy subjects, no evidence of ototoxicity was found, which further supported the low potential ototoxicity of plazomicin.
This study has several limitations. First, only 3 RCTs with limited patients were included in this meta-analysis, so generalization of the findings of this meta-analysis might be limited. Further clinical study is warranted to clarify the effectiveness and safety of plazomicin compared with other antibiotics in the treatment of Enterobacterales infections. Second, this study also did not provide sufficient evidence for treatment of BSI or HAP with plazomicin. In addition to clinical research, more real-world evidence studies are needed to provide evidence. Third, although most of the Enterobacterales included in the study were antibiotic-resistant, there were antibiotic-susceptible Enterobacterales and gram-positive bacteria.
CONCLUSIONS
Plazomicin is as good as comparators in terms of efficacy and tolerance in the treatment of Enterobacterales infections. Therefore, plazomicin is a suitable choice for antibiotic treatment in adult patients with cUTIs, BSI, or HAP. However, plazomicin should not be routinely used as a first-line treatment for these infections because it does not show superiority and there is some uncertainty regarding risks of renal impairment and ototoxicity. Clinicians should reserve these broad-spectrum antibiotics for use when there are special indications.
Acknowledgments
Financial support. This work was supported by the National Natural Science Foundations of China (81770004 and 82073894), Cultivation Project of PLA General Hospital for Distinguished Young Scientists (2020-JQPY-004), and New Medicine Clinical Research Fund (4246Z512).
Author contributions. Kaicheng Yan and Guanxuanzi Zhang reviewed the literature, extracted and analyzed the data, and drafted the manuscript; Beibei Liang and Jin Wang extracted and analyzed the data; as corresponding authors, Man Zhu and Yun Cai contributed to study design, protocol, data extraction, data analysis, and writing.
Availability of data and material. The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Kaicheng Yan, Medical School of Chinese PLA, Beijing, China; Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
Beibei Liang, Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
Guanxuanzi Zhang, Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
Jin Wang, Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
Man Zhu, Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
Yun Cai, Department of Pharmacy, Medical Supplies Center of Chinese PLA General Hospital, Beijing, China.
References
- 1. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 2017; 50:1700582. [DOI] [PubMed] [Google Scholar]
- 3. Lüthje P, Brauner A. Virulence factors of uropathogenic E. coli and their interaction with the host. Adv Microb Physiol 2014; 65:337–72. [DOI] [PubMed] [Google Scholar]
- 4. Brink AJ, Botha RF, Poswa X, et al. Antimicrobial susceptibility of gram-negative pathogens isolated from patients with complicated intra-abdominal infections in South African hospitals (SMART study 2004–2009): impact of the new carbapenem breakpoints. Surg Infect (Larchmt) 2012; 13:43–9. [DOI] [PubMed] [Google Scholar]
- 5. Decousser JW, Poirel L, Nordmann P. Recent advances in biochemical and molecular diagnostics for the rapid detection of antibiotic-resistant Enterobacteriaceae: a focus on ss-lactam resistance. Expert Rev Mol Diagn 2017; 17:327–50. [DOI] [PubMed] [Google Scholar]
- 6. Dozzo P, Moser HE. New aminoglycoside antibiotics. Expert Opin Ther Pat 2010; 20:1321–41. [DOI] [PubMed] [Google Scholar]
- 7. Pogue JM, Potoski BA, Kaye KS. Aminoglycoside use in intensive care units and aminoglycoside nephrotoxicity. Comment letter 1. Antimicrob Agents Chemother 2010; 54:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bottger EC, Crich D. Aminoglycosides: time for the resurrection of a neglected class of antibacterials? ACS Infect Dis 2020; 6:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stankowicz MS, Ibrahim J, Brown DL. Once-daily aminoglycoside dosing: an update on current literature. Am J Health Syst Pharm 2015; 72:1357–64. [DOI] [PubMed] [Google Scholar]
- 10. Castanheira M, Deshpande LM, Woosley LN, Serio AW, Krause KM, Flamm RK. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother 2018; 73:3346–54. [DOI] [PubMed] [Google Scholar]
- 11. Landman D, Babu E, Shah N, et al. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J Antimicrob Chemother 2010; 65:2123–27. [DOI] [PubMed] [Google Scholar]
- 12. Saravolatz LD, Stein GE. Plazomicin: a new aminoglycoside. Clin Infect Dis 2020; 70:704–9. [DOI] [PubMed] [Google Scholar]
- 13. Fleischmann WA, Greenwood-Quaintance KE, Patel R. In vitro activity of plazomicin compared to amikacin, gentamicin, and tobramycin against multidrug-resistant aerobic gram-negative bacilli. Antimicrob Agents Chemother 2020; 64:e01711–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark JA, Kulengowski B, Burgess DS. In vitro activity of plazomicin compared to other clinically relevant aminoglycosides in carbapenem-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis 2020; 98: 115117. [DOI] [PubMed] [Google Scholar]
- 15. Albano M, Fleischmann WA, Greenwood-Quaintance KE, et al. In vitro activity of arbekacin against multidrug-resistant gram-negative bacilli. J Microbiol Immunol Infect 2021; 54:1118–21. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly LE, Riddle V, Cebrik D, et al. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother 2018; 62:e01989–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019; 380:791–3. [DOI] [PubMed] [Google Scholar]
- 19. Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 2019; 380:729–40. [DOI] [PubMed] [Google Scholar]
- 20. Madej A, Pullman J, Popescu M, et al. 2234. Outcomes by age and gender from a global phase 3 study of delafloxacin (DLX) in community-acquired bacterial pneumonia (CABP). Open Forum Infect Dis 2019; 6(Suppl 2):S763. [Google Scholar]
- 21. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan K, Zhu M, Jia Y, et al. Efficacy and safety of quinolones vs. other antimicrobials for the treatment of uncomplicated urinary tract infections in adults: a systematic review and meta-analysis. Int Urogynecol J 2022; 33:1103–23. [DOI] [PubMed] [Google Scholar]
- 23. Ko JH, Kang C-I, Cornejo-Juárez P, et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:546–54. [DOI] [PubMed] [Google Scholar]
- 24. Castanheira M, Davis AP, Mendes RE, et al. In vitro activity of plazomicin against gram-negative and gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrobial Agents Chemotherapy 2018; 62:e00313–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martins AF, Bail L, Ito CAS, et al. Antimicrobial activity of plazomicin against Enterobacteriaceae-producing carbapenemases from 50 Brazilian medical centers. Diagn Microbiol Infect Dis 2018; 90:228–32. [DOI] [PubMed] [Google Scholar]
- 26. Castanheira M, Davis AP, Serio AW, et al. In vitro activity of plazomicin against Enterobacteriaceae isolates carrying genes encoding aminoglycoside-modifying enzymes most common in US Census divisions. Diagn Microbiol Infect Dis 2019; 94:73–7. [DOI] [PubMed] [Google Scholar]
- 27. Cox G, Ejim L, Stogios PJ, et al. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 2018; 4:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aggen JB, Armstrong ES, Goldblum AA, et al. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 2010; 54:4636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thwaites M, Hall D, Shinabarger D, et al. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e00236–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hackel MA, et al. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e01904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Kashikar A, Bush K. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J Antimicrob Chemother 2017; 72:2792–5. [DOI] [PubMed] [Google Scholar]
- 32. Denervaud-Tendon V, Poirel L, Connolly LE, et al. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. J Antimicrob Chemother 2017; 72:2787–91. [DOI] [PubMed] [Google Scholar]
- 33. Cass RT, Brooks CD, Havrilla NA, et al. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother 2011; 55:5874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]