Abstract
Background
Increased intracranial pressure (ICP) frequently complicates cryptococcal meningitis. Therapeutic lumbar punctures (LPs) have acute survival benefits in the first week, and we sought to understand the longer-term survival impact of therapeutic LPs.
Methods
We prospectively enrolled human immunodeficiency virus (HIV)–seropositive adults with cryptococcal meningitis from 2013 to 2017 in Uganda. We assessed the association between clinical characteristics, CSF parameters, and 14- and 30-day mortality by baseline ICP. We also assessed 30-day mortality by number of follow-up therapeutic LPs performed within 7 days.
Results
Our analysis included 533 participants. Participants with baseline ICP >350 mm H2O were more likely to have Glasgow Coma Scale (GCS) score <15 (P < .001), seizures (P < .01), and higher quantitative cryptococcal cultures (P < .001), whereas participants with ICP <200 mm H2O were more likely to have baseline sterile CSF cultures (P < .001) and CSF white blood cell count ≥5 cells/µL (P = .02). Thirty-day mortality was higher in participants with baseline ICP >350 mm H2O and ICP <200 mm H2O as compared with baseline ICP 200–350 mm H2O (hazard ratio, 1.55 [95% confidence interval, 1.10–2.19]; P = .02). Among survivors at least 7 days, the 30-day relative mortality was 50% higher among participants who did not receive any additional therapeutic LPs compared to those with ≥1 additional follow-up LP (33% vs 22%; P = .04), irrespective of baseline ICP.
Conclusions
Management of increased ICP remains crucial in improving clinical outcomes in cryptococcal meningitis. Guidelines should consider an approach to therapeutic LPs that is not dictated by baseline ICP.
Keywords: baseline opening pressure, cryptococcal meningitis, mortality, therapeutic lumbar puncture
Baseline opening pressure was associated with mortality in cryptococcal meningitis; 2 therapeutic lumbar punctures had survival benefit irrespective of baseline opening pressure.
Cryptococcal meningitis is the most common cause of human immunodeficiency virus (HIV)–associated adult meningitis in sub-Saharan Africa and is associated with up to 40%–70% 1-year mortality [1–3]. Increased intracranial pressure (ICP), defined as cerebrospinal fluid (CSF) opening pressure >200 mm H2O, is a common presentation in cryptococcal meningitis and contributes to mortality and disability [4–11]. Management of increased ICP involves serial therapeutic lumbar punctures (LPs), lumbar drain placement, ventriculostomy, or ventriculoperitoneal shunting [10, 12–14]. Lumbar drains carry the risk of infection, and ventriculostomies or ventriculoperitoneal shunts are not easily accessible in many resource-limited settings, such as in sub-Saharan Africa, where the burden of cryptococcal disease is highest [1].
World Health Organization (WHO) (2018 and 2022) and 2010 Infectious Diseases Society of America recommendations for the management of increased ICP in cryptococcal meningitis depend on the critical ability to measure baseline CSF opening pressure [10, 15, 16]. CSF drainage is recommended whenever ICP is >250 mm H2O (25 cm H2O) or there are symptoms of increased ICP. Therapeutic LPs and CSF drainage are to be repeated daily if there is persistent elevation of CSF pressure >250 mm H2O until ICP and symptoms have stabilized for 2 consecutive days [10, 15]. While an additional LP at the conclusion of induction antifungal therapy is often recommended to document culture sterility, therapeutic LPs following normalization of ICP by manometry are generally symptom-directed. Existing guidelines do not suggest a role of therapeutic LPs in cryptococcal meningitis when baseline ICP is not elevated or in persons without classical signs and symptoms of increased ICP [10, 15, 16].
While guidelines emphasize therapeutic LPs based on baseline ICP, several studies have demonstrated that additional therapeutic LPs, irrespective of baseline ICP or symptoms, improve overall survival [17, 18]. We previously demonstrated a 69% reduction in approximately 10-day survival for persons receiving 1 therapeutic LP in the first week, irrespective of baseline ICP [17]. In this study, we investigate the role of repeat therapeutic LPs in the first week, for survival outcomes after the first week through 30 days. Additionally, we evaluate risk factors for elevated ICP in HIV-associated cryptococcal meningitis and its relationship with survival.
METHODS
Data from the Adjunctive Sertraline for the Treatment of HIV-Associated Cryptococcal Meningitis (ASTRO-CM) pilot study and randomized controlled trial were used in this analysis.
The ASTRO-CM phase 2 trial, conducted from August 2013 to August 2014, was an open-label dose-finding study to evaluate the safety and tolerability of escalating doses of adjunctive sertraline in addition to standard therapy for cryptococcal meningitis [19]. Eligible participants in the ASTRO-CM phase 3 randomized clinical trial, conducted from March 2015 to May 2017, received standard antifungal therapy with either adjunctive sertraline or placebo [20]. Sertraline was administered at a dose of 400 mg/day for 2 weeks followed by 200 mg/day for 12 weeks. Eligible participants in both studies included HIV-seropositive adults (≥18 years old) diagnosed with first-episode cryptococcal meningitis. Diagnosis of cryptococcal meningitis was made via a positive finger stick and CSF cryptococcal antigen lateral flow assay (IMMY, Norman, Oklahoma) [21, 22]. All participants received standard antifungal therapy of up to 14 days of amphotericin B (0.7–1 mg/kg/day) plus fluconazole (800 mg/day for 4 weeks followed by 400 mg/day for 8 weeks). For the purposes of our analyses, randomized groups were combined as sertraline had no effect on ICP or survival [20].
Enrolled participants received a baseline LP for the diagnosis of cryptococcal meningitis, measurement of CSF opening pressure in the lateral decubitus position using a manometer (hereafter referred to as ICP), and CSF quantitative cryptococcal culture performed [23]. Serial scheduled therapeutic LPs were performed to control elevated ICP and perform quantitative cultures on days 3, 7, 10, and 14 (±1-day window for performing an LP). While the protocol specified therapeutic LPs at scheduled intervals (days 3, 7, 10, and 14) regardless of ICP, additional therapeutic LPs (LPs performed on nonscheduled days) were performed at the study physician’s discretion based on clinical judgement for concern of symptomatic increased ICP. Participants, however, could decline LPs.
Patient Consent Statement
The design of both clinical trials was approved by local ethical committees and conform to Ugandan clinical trial standards. Ethical approval for this study was granted from the institutional review boards at the University of Minnesota, Makerere University, and Mbarara University of Science and Technology. All participants provided their written consent for study participation.
Statistical Analysis
We summarized demographic variables and baseline characteristics by ICP <200 mm H2O (intended to represent low-normal ICP), 200–350 mm H2O (moderately elevated ICP), and >350 mm H2O (severely elevated ICP) [24], which roughly divided the study cohort into tertiles. Association between baseline ICP groups and binary characteristics were determined using Cochran-Mantel-Haenszel test. Association between baseline ICP groups and continuous characteristics were determined using rank-based methods (Kruskal-Wallis). Groups were compared for mortality using a log-rank test and hazard ratios (HRs) were estimated using Cox proportional hazard models (unadjusted and adjusted) using the 200–350 mm H2O group as the reference, as mortality was lowest in this ICP group.
RESULTS
Of 632 total participants enrolled in the ASTRO-CM studies, 533 with first-episode cryptococcal meningitis had a baseline CSF opening pressure recorded and were included in this analysis. Demographic and clinical characteristics are summarized by baseline ICP of <200 mm H2O, 200–350 mm H2O, and >350 mm H2O in Table 1. Participants with severely elevated baseline ICP (>350 mm H2O) were more likely to present with a Glasgow Coma Scale (GCS) score <15 (P < .01), self-reported baseline seizures (P < .01), focal neurologic deficit (P = .03), and vision changes (P < .001) compared with participants who had a baseline ICP of <200 mm H2O and 200–350 mm H2O. Persons with a severely elevated baseline ICP also had higher CSF quantitative cryptococcal culture (median ∼160 000 CFU/mL) as compared to ICP of 200–350 mm H2O (median ∼50 000 CFU/mL) and <200 mm H2O (median ∼32 000 CFU/mL) (P < .001). A lower frequency of those with severely elevated baseline ICP were receiving antiretroviral therapy (ART) (40% compared to 50% or 51% in low-normal or moderately elevated baseline ICP groups), though this was not significantly significant (P = .08). A higher proportion of participants with a low-normal baseline ICP (<200 mm H2O) had sterile cultures (P < .001), CSF white cells ≥5 cells/μL (P = .02), and lower serum hemoglobin (P < .001) as compared to those with elevated baseline ICP. Baseline CD4+ T-cell counts and proportion of participants receiving ART did not differ across the ICP groups.
Table 1.
Baseline Demographics by Baseline Cerebrospinal Fluid Opening Pressure
| Characteristic | No. | OP <200 mm H2O | No. | OP 200–350 mm H2O | No. | OP >350 mm H2O | P Valuea |
| No. with baseline LP | 163 | 197 | 173 | ||||
| Demographics | |||||||
| Age, y, median (IQR) | 163 | 35 (30–42) | 197 | 35 (30–40) | 173 | 34 (29–40) | .06 |
| Female sex | 163 | 72 (44.2) | 197 | 64 (32.5) | 173 | 68 (39.3) | .07 |
| Weight, kg, median (IQR) | 142 | 52 (46–58) | 166 | 53.5 (48–60) | 127 | 53 (50–60) | .12 |
| ART | |||||||
| Currently on ART | 163 | 81 (49.7) | 197 | 101 (51.3) | 172 | 69 (40.1) | .08 |
| Months on ARTb, median (IQR) | 80 | 4.2 (0.9–18.2) | 101 | 6.6 (1.1–37.7) | 68 | 3.3 (0.5–26.2) | .26 |
| Clinical symptoms | |||||||
| GCS score <15 | 163 | 66 (40.5) | 197 | 71 (36.0) | 173 | 93 (53.8) | <.01 |
| Focal neurologic deficit | 163 | 4 (2.5) | 197 | 5 (2.5) | 173 | 15 (8.7) | .03 |
| Seizures | 163 | 16 (9.8) | 197 | 25 (12.7) | 173 | 37 (21.4) | <.01 |
| Fever | 163 | 87 (53.4) | 197 | 97 (49.2) | 173 | 99 (57.2) | .31 |
| Headache | 163 | 158 (96.9) | 197 | 194 (98.5) | 173 | 171 (98.8) | .39 |
| Vision changes | 163 | 50 (30.7) | 197 | 50 (25.4) | 173 | 75 (43.4) | <.001 |
| Photophobia | 163 | 39 (23.9) | 197 | 51 (25.9) | 173 | 59 (34.1) | .08 |
| Confusion | 163 | 60 (36.8) | 197 | 61 (31.0) | 173 | 73 (42.2) | .08 |
| Vomiting | 163 | 89 (54.6) | 197 | 108 (54.8) | 173 | 115 (66.5) | .04 |
| Laboratory | |||||||
| CD4+ count, cells/µL, median (IQR) | 155 | 17 (7–53) | 191 | 16 (6–49) | 163 | 12 (5–35) | .14 |
| Sodium <130 mEq/L | 118 | 64 (54.2) | 134 | 72 (53.7) | 110 | 67 (60.9) | .47 |
| WBC count ≥3.5 × 103 cells/μL | 151 | 73 (48.3) | 178 | 89 (50.0) | 159 | 98 (61.6) | .03 |
| Hemoglobin, g/dL, median (IQR) | 151 | 11.0 (9.0–12.3) | 178 | 11.5 (10.0–13.1) | 159 | 12.5 (11.0–13.7) | <.001 |
| ANC, 103 cells/μL, median (IQR) | 151 | 1.9 (1.1–2.9) | 178 | 1.8 (1.3–2.8) | 159 | 2.6 (1.8–4.0) | <.001 |
| CSF | |||||||
| Cryptococcus, log10 CFU/mLc, median (IQR) | 133 | 4.5 (3.0–5.3) | 183 | 4.7 (3.5–5.3) | 170 | 5.2 (4.4–5.8) | <.001 |
| Sterile cryptococcal culture | 162 | 29 (17.9) | 196 | 13 (6.6) | 172 | 2 (1.2) | <.001 |
| WBC count, cells/μL, median (IQR) | 152 | <5 (<5–50) | 194 | <5 (<5–45) | 171 | <5 (<5–45) | .10 |
| WBC count ≥5 cells/μL | 152 | 68 (44.7) | 194 | 64 (33.0) | 171 | 53 (31.0) | .02 |
| Protein, mg/dL, median (IQR) | 134 | 60 (24–129) | 171 | 46 (22–100) | 155 | 47 (20–91) | .25 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ANC, absolute neutrophil count; ART, antiretroviral therapy; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; H2O, water; IQR, interquartile range; LP, lumbar puncture; OP, opening pressure; WBC, white blood cell.
Kruskal-Wallis test for medians; χ2 test for proportions.
Among those on ART at cryptococcal meningitis diagnosis.
Excludes CSF sterile cultures at baseline in performing quantitative CSF cultures.
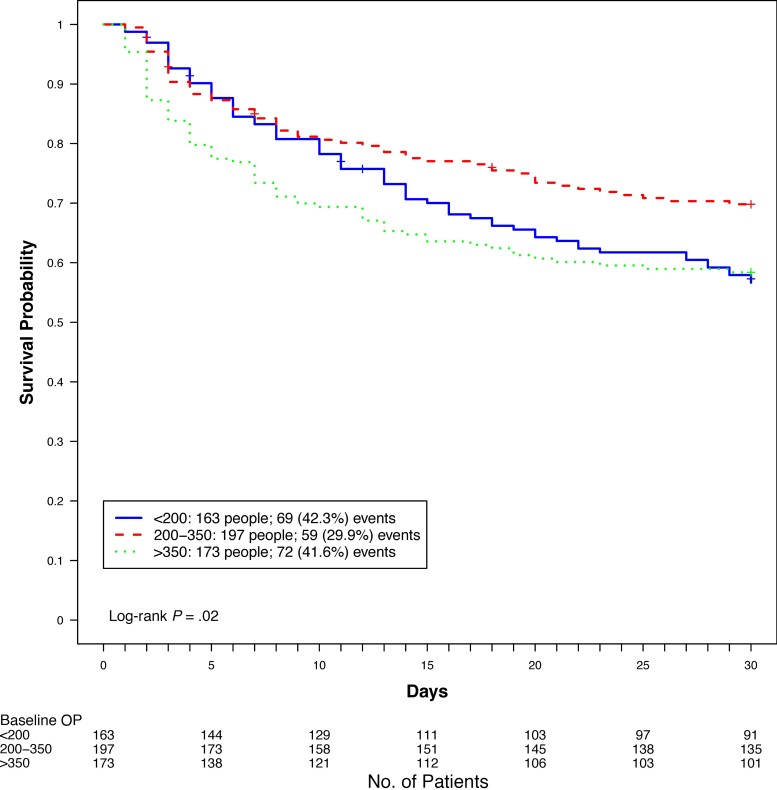
Mortality through 30 days differed between ICP groups (log-rank P = .02) as shown in Figure 1, with the lowest proportion of deaths occurring in the 200–350 mm H2O ICP group (30%). Although the proportion of deaths in the <200 mm H2O and the >350 mm H2O groups were similar, we observed higher early (<14 days) mortality in the >350 mm H2O group. HRs for 14- and 30-day mortality, using baseline ICP of 200–350 mm H2O as the reference group, are shown in Table 2. In unadjusted analysis, baseline ICP >350 mm H2O was associated with an increased risk of 14-day (HR, 1.73 [95% confidence interval {CI}, 1.18–2.55]) and 30-day (HR, 1.55 [95% CI, 1.10–2.19]) mortality. This significance was not retained after adjusting for GCS score, seizures, focal neurologic deficit, CSF white blood cell (WBC) count >5 cells/μL, quantitative cryptococcal culture, and hemoglobin, although some of these are on the causal pathway of elevated ICP. We did not find a statistically significant increased risk of mortality at 14 days in those with a baseline ICP <200 mm H2O in either the unadjusted model (HR, 1.31 [95% CI, .87–1.98]) or the adjusted model (adjusted HR, 1.34 [95% CI, .83–2.14]). In both the adjusted and unadjusted analysis, however, participants with a baseline ICP <200 mm H2O had an increased risk of death at 30 days (HR, 1.48 [95% CI, 1.05–2.10]; adjusted HR, 1.50 [95% CI, 1.01–2.23]).
Figure 1.
Thirty-day mortality by baseline cerebrospinal fluid opening pressure (OP).
Table 2.
Overall Hazard Ratio by Baseline Cerebrospinal Fluid Opening Pressure
| Model and Baseline OP | HR | (95% CI) | P Value |
| 14-day mortality (n = 533): unadjusted model | |||
| Baseline OP | |||
| <200 mm H2O | 1.31 | (.87–1.98) | .20 |
| 200–350 mm H2O | REF | … | |
| >350 mm H2O | 1.73 | (1.18–2.55) | <.01 |
| 14-day mortality (n = 470): adjusted modela | |||
| Baseline OP | |||
| <200 mm H2O | 1.34 | (.83–2.14) | .23 |
| 200–350 mm H2O | REF | … | |
| >350 mm H2O | 1.53 | (.98–2.38) | .06 |
| 30-day mortality (n = 533): unadjusted model | |||
| Baseline OP | |||
| <200 mm H2O | 1.48 | (1.05–2.10) | .03 |
| 200–350 mm H2O | REF | … | |
| >350 mm H2O | 1.55 | (1.10–2.19) | .01 |
| 30-day mortality (n = 470): adjusted modela | |||
| Baseline OP | |||
| <200 mm H2O | 1.50 | (1.01–2.23) | .04 |
| 200–350 mm H2O | REF | … | |
| >350 mm H2O | 1.44 | (.97–2.13) | .07 |
Abbreviations: CI, confidence interval; H2O, water; HR, hazard ratio; OP, opening pressure.
Model adjusted for baseline Glasgow Coma Scale (GCS) score, seizures, focal neurologic deficits, cerebrospinal fluid (CSF) white blood cell count <5 cells/mL, CSF quantitative culture (log10 CFU/mL), hemoglobin. GCS score, seizures, and focal neurologic deficits are likely all on the causal pathway of the effects of elevated intracranial pressure.
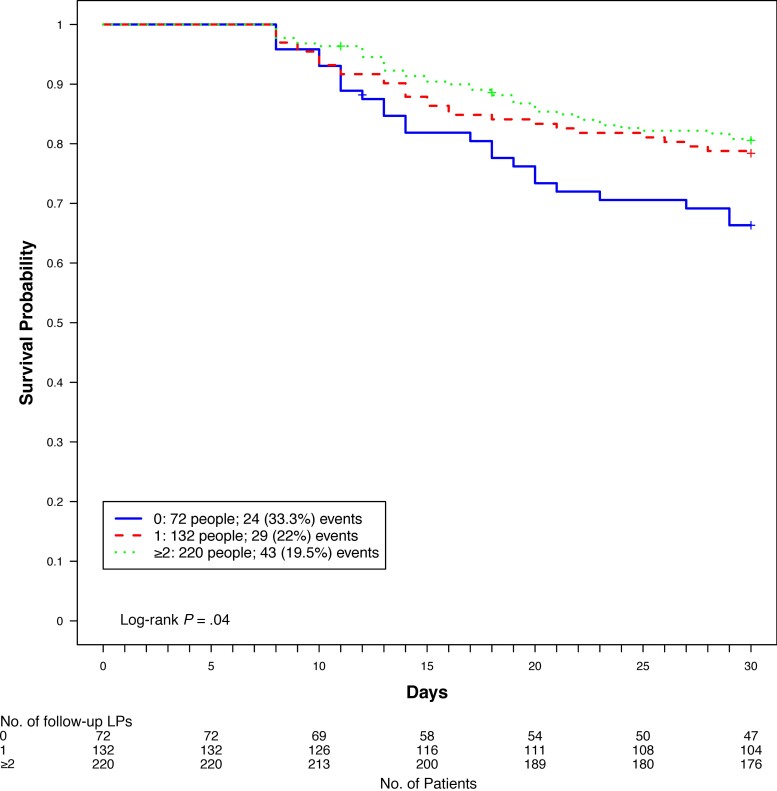
Among participants who survived at least 7 days (n = 424) and were eligible for 2 protocol-specified LPs, 72 (17%) received no further LPs, 132 (31%) received 1 therapeutic LP, and 220 (52%) received 2 or more therapeutic LPs in the first 7 days of follow-up. The clinical characteristics by number of LPs received is shown in Supplementary Table 1. Participants who received 2 or more additional LPs were more likely to have presented with a GCS score <15 (P < .001), self-reported seizures (P = .02), photophobia (P < .001), higher baseline ICP (P < .001), and higher CSF quantitative cryptococcal culture (P < .001). In contrast, participants who had no additional follow-up therapeutic LPs had lower serum hemoglobin levels (P < .001), lower CSF ICP (P < .001), lower CSF quantitative cryptococcal culture (P < .001), and a higher percentage of sterile CSF cultures (P = .03). The association between the number of follow-up LPs received in the first 7 days and 30-day mortality is shown in Figure 2. Among participants who had survived at least 7 days, participants who received no additional LPs had a higher mortality (n = 24/72 [33%]) compared to participants who had received either 1 additional LP (n = 29/132 [22%]) or 2 or more LPs (n = 43/220 [19.5%]) (P = .04) over the first 7 days of induction therapy, regardless of baseline ICP.
Figure 2.
Thirty-day mortality by number of follow-up lumbar punctures (LPs) in the first 7 days for participants who survived at least 7 days.
DISCUSSION
The role of LPs and CSF drainage in the management of increased ICP in HIV-associated cryptococcal meningitis has been well documented to improve CSF opening pressures, relieve symptoms of ICP, and reduce neurological sequalae [7, 17]. No current studies, however, have been able to properly inform on the optimal frequency that LPs should be performed in cryptococcal meningitis. Observational studies looking at the association between mortality and baseline opening pressures, however, have had mixed results. Graybill et al found an association between high baseline ICP and increased mortality [7], whereas Bicanic et al found no association between elevated baseline ICP and 2- or 10-week mortality when performing scheduled LPs [18]. Our study adds to current published literature supporting the association between high baseline ICP and increased mortality as well as support the role of scheduled follow-up therapeutic LPs irrespective of baseline ICP.
Current guidelines place emphasis on performing therapeutic LPs when baseline opening pressures are elevated or if there are symptoms of increased ICP [10]. There is no guidance on performing follow-up therapeutic LPs when baseline opening pressures are <200 mm H2O or symptoms of increased ICP are absent. In our study, 31% of participants had opening pressures <200 mm H2O and would not have received any additional therapeutic LPs during their hospitalization. For participants who did not receive at least 1 therapeutic LP, our data showed a 50% higher relative mortality by 30 days regardless of baseline opening pressure. Therefore, basing recommendations on the use of opening pressures as well as signs and symptoms of increased ICP to dictate the need for therapeutic LPs has its limitations, and further research is needed to investigate the impact of performing therapeutic LPs regardless of the opening pressure and the presence of symptoms.
Participants presenting with severely elevated baseline opening pressures (>350 mm H2O) had a higher 30-day mortality, with the majority of deaths occurring within the first 14 days of hospitalization. Having a baseline opening pressure >350 mm H2O was associated with an increased risk of mortality at both 14 and 30 days. This association was lost when adjusting for altered mental status, seizures, focal neurological deficits, CSF WBCs, quantitative cryptococcal culture, and hemoglobin, which demonstrates that severely elevated baseline pressure is a manifestation of advanced disease. Many of the variables are a direct sequelae of elevated ICP being on the causal pathway toward mortality, such as altered mental status, seizures, and neurologic deficits. While participants with a higher opening pressure are more likely to receive repeated therapeutic LPs throughout their hospitalization, they continued to have higher early mortality as compared to those with lower baseline opening pressures. Our data support consideration of early and aggressive management of severely elevated ICP, including placement of lumbar drains or ventriculoperitoneal shunting in settings where it is possible and if opening pressures remain elevated beyond the first week of cryptococcal meningitis therapy [25–27].
We also observed that baseline opening pressures of <200 mm H2O were associated with increased mortality, but with a higher proportion of deaths occurring later in the disease course. This has also been observed in other large cohorts of individuals with HIV-associated cryptococcal meningitis. Jarvis et al similarly found that low CSF opening pressure (<250 mm H2O) was independently associated with 10-week, but not 2-week, mortality [28]. We found that although participants with a baseline opening pressure <200 mm H2O presented less frequently with classic or even subtle symptoms of ICP, they were more likely to be anemic, have sterile CSF cultures at baseline, have an increased frequency of CSF pleocytosis, and receive fewer LPs throughout their hospitalization. Since current guidelines do not suggest a need for therapeutic LPs in the context of normal baseline opening pressure, it is possible that a lack of therapeutic LPs, which we observed to be beneficial regardless of baseline ICP, may partially explain this increased mortality. One possible explanation for improved outcomes with therapeutic LPs, even at low baseline ICP, is a falsely low baseline measurement. Other possible explanations include a delayed increase in ICP without symptoms over the course of the illness or decreasing CSF fungal burden through manual draining with repeated LPs.
The observation that moderately elevated baseline opening pressure is associated with a lower mortality than those with a low-normal baseline opening pressure (<200 mm H2O) might be considered counterintuitive, though this has been observed in other large studies investigating determinants of mortality in cryptococcal meningitis as well [28]. It is possible that physiologic compensatory mechanisms and proinflammatory cytokines associated with moderately elevated opening pressure are protective. Following this line of reasoning, additional likely explanations for increased mortality at low-normal baseline opening pressure in cryptococcal meningitis could include (1) the presence of an overactive or aberrant proinflammatory immune response [29] or (2) the absence of protective physiologic mechanisms that could be exacerbated by the presence of anemia that we observed in this group.
We also observed that participants with a baseline ICP <200 mm H2O had lower hemoglobin levels at baseline. Anemia has been linked to worse survival in cryptococcal meningitis in several studies [28, 30, 31]. Tugume et al previously reported that in those with hemoglobin <8.5 g/dL at diagnosis, cryptococcal mortality hazard was 2.7-fold elevated at 2 weeks [30]. In cryptococcal meningitis, hemoglobin levels were found to be associated with regional cerebral oxygen saturation of tissue delivery of oxygen; hemoglobin positively correlated with cerebral oxygen saturation [32]. Low regional cerebral oxygen saturation <30% was found to be associated with mortality through 30 days [32]. Anemia may be an important modifiable risk factor for mortality, decreasing oxygen delivery to the brain, whereby even small increases in ICP may have a detrimental effect in persons with decreased oxygen carrying capacity to the brain.
Participants in our study who did not receive any therapeutic LPs were more likely to have normal baseline opening pressures and were less likely to present with signs and symptoms of raised ICP. We observed higher 30-day mortality among participants not receiving a therapeutic LP in the first 7 days of therapy irrespective of baseline opening pressures; those receiving 1, 2 or more additional LPs during the first 7 days had relatively similar 30-day mortality. The survival benefit of therapeutic LPs irrespective of opening pressures is further supported in other studies [17]. Manometers for measuring opening pressure are not commonly available in settings where cryptococcal meningitis is most prevalent, forcing alternatives to opening pressure-directed management of raised ICP. Scheduled LPs during induction antifungal therapy, for example, have been shown to have mortality benefits. In South Africa, Mkoko et al found that in the absence of manometers, performing 4 or more LPs in the first 7 days reduced in-hospital mortality by 60% as compared to having <4 LPs performed (11.6% vs 29% mortality) [33]. In Tanzania, Meda et al found that following a strict schedule of performing LPs on study days 0, 3, 7, and 14, rather than leaving the decision to the discretion of the treating clinicians, reduced in-hospital mortality [34]. Our data demonstrate that scheduled LPs provide a mortality benefit even where manometers are widely available, an assertion that other researchers have previously made as long ago as 1994 [35]. The improved survival demonstrated in studies where therapeutic LPs are scheduled would suggest that all individuals should receive repeat therapeutic LPs during their hospitalization irrespective of baseline opening pressure measurement and provides a basis for management where manometers are not available. We have previously recommended removal of 20 mL of CSF in the absence of a manometer, being the median volume removed in this cohort [17].
Our analysis is one of the largest to date investigating the effects of baseline CSF opening pressures and therapeutic LPs on survival. In the context of existing guidelines, we found that symptom-based monitoring for raised ICP has its limitations, which could result in missed recognition of increased ICP. Most guidelines recommend that a repeat LP be performed at 2 weeks to document mycological sterility. Our data suggest that at a minimum, 1 additional therapeutic LP be performed within the first 7 days of antifungal therapy. As evidence mounts that shorter courses of amphotericin-based induction regimens may be preferred [36, 37], therapeutic LPs will remain necessary. Ultimately, further investigations are needed to understand the ideal timing and frequency of LPs that should be performed. Further research is also needed to understand the driving force behind the late mortality seen in persons who present with low CSF opening pressures.
Our study has several limitations inherent in the observational nature of the study. First, our analysis may be confounded by time-dependent bias. We attempted to minimize the effect of time-dependent bias by restricting our mortality analysis only to those who had survived the first 7 days of hospitalization, thereby excluding participants who died shortly after hospitalization and were unable to receive any additional LPs. Second, the number of LPs each participant received varied. Protocol-specified LPs applied to all participants; however, the decision to decline LPs was often influenced by participant or family hesitancy coupled with the severity of illness. This may have biased our mortality analysis. Third, our study was conducted in the context of changing ART availability and standards of care. Antifungal therapy for cryptococcal meningitis in resource-limited settings at the time included a combination of amphotericin B and high-dose fluconazole. We recognize that with the revised WHO guidelines (2018 and 2022) and increasing access to flucytosine and liposomal amphotericin, globally, there will be improved mortality among cryptococcal cohorts. That said, even with improved antifungal regimens, we believe that LPs will remain a crucial tool in the treatment of cryptococcal meningitis. While those with severely elevated baseline ICP were more likely to be ART naive, this was not statistically significant and a link between baseline ICP and ART status has not been previously observed [38].
In conclusion, the management of ICP in cryptococcal meningitis remains a challenge and contributes to both short- and long-term mortality. We recommend, at a minimum, scheduled LPs at day 3 and 7 after diagnosis in all persons with cryptococcal meningitis. Future studies are needed to further understand contributing risk factors in persons with normal opening pressure and to understand the optimal number and scheduling of LPs in cryptococcal meningitis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Enock Kagimu, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Nicole Engen, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
Kenneth Ssebambulidde, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
John Kasibante, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Tadeo K Kiiza, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Edward Mpoza, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Lillian Tugume, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Edwin Nuwagira, Department of Internal Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Laura Nsangi, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Darlisha A Williams, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda; Division of Infectious Diseases, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota, USA.
Kathy Huppler Hullsiek, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
David R Boulware, Division of Infectious Diseases, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota, USA.
David B Meya, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda; Division of Infectious Diseases, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota, USA; School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Joshua Rhein, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda; Division of Infectious Diseases, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota, USA.
Mahsa Abassi, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda; Division of Infectious Diseases, Department of Medicine, University of Minnesota Medical School, Minneapolis, Minnesota, USA.
Abdu K Musubire, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Notes
Adjunctive Sertraline for the Treatment of HIV-Associated Cryptococcal Meningitis (ASTRO-CM) Team Members. Reuben Kiggundu, Andrew Akampurira, Paul Kirumira, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Tadeo Kiiza Kandole, Tony Luggya, Julian Kaboggoza, Eva Laker, Alice Namudde, Sarah Lofgren, Richard Kwizera, and Ananta S. Bangdiwala.
Financial support. This research was supported by the National Institute of Neurologic Diseases and Stroke (grant/award numbers R01NS086312, K23NS122601, and K43TW010718); the Fogarty International Center (award number K01TW010268); the National Institute of Allergy and Infectious Diseases (award number T32AI055433); the United Kingdom Medical Research Council/Department for International Development/Wellcome Trust Global Clinical Trials (grant number M007413/1); and Grand Challenges Canada (grant number S4-0296-01). D. B. M. is also supported by the DELTAS Africa Initiative (grant number DEL-15-011 to THRiVE-2).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajasingham R, Rolfes MA, Birkenkamp KE, Meya DB, Boulware DR. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS Med 2012; 9:e1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med 1974; 80:176–81. [DOI] [PubMed] [Google Scholar]
- 5. Denning DW, Armstrong RW, Lewis BH, Stevens DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. Am J Med 1991; 91:267–72. [DOI] [PubMed] [Google Scholar]
- 6. van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med 1997; 337:15–21. [DOI] [PubMed] [Google Scholar]
- 7. Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis 2000; 30:47–54. [DOI] [PubMed] [Google Scholar]
- 8. Zhao T, Xu XL, Nie JM, et al. Establishment of a novel scoring model for mortality risk prediction in HIV-infected patients with cryptococcal meningitis. BMC Infect Dis 2021; 21:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One 2013; 8:e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pappas PG. Editorial commentary: an expanded role for therapeutic lumbar punctures in newly diagnosed AIDS-associated cryptococcal meningitis? Clin Infect Dis 2014; 59:1615–7. [DOI] [PubMed] [Google Scholar]
- 12. Cherian J, Atmar RL, Gopinath SP. Shunting in cryptococcal meningitis. J Neurosurg 2016; 125:177–86. [DOI] [PubMed] [Google Scholar]
- 13. Fessler RD, Sobel J, Guyot L, et al. Management of elevated intracranial pressure in patients with cryptococcal meningitis. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17:137–42. [DOI] [PubMed] [Google Scholar]
- 14. Corti M, Priarone M, Negroni R, et al. Ventriculoperitoneal shunts for treating increased intracranial pressure in cryptococcal meningitis with or without ventriculomegaly. Rev Soc Bras Med Trop 2014; 47:524–7. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. 2018. https://apps.who.int/iris/bitstream/handle/10665/260399/9789241550277-eng.pdf. Accessed 25 October 2021. [PubMed]
- 16. World Health Organization . Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. 2022. https://www.who.int/publications/i/item/9789240052178. Accessed 26 July 2022. [PubMed]
- 17. Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bicanic T, Brouwer AE, Meintjes G, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009; 23:701–6. [DOI] [PubMed] [Google Scholar]
- 19. Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhein J, Huppler Hullsiek K, Tugume L, et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 2019; 19:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 2014; 20:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams DA, Kiiza T, Kwizera R, et al. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 2015; 61:464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dyal J, Akampurira A, Rhein J, et al. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology 2006; 67:1690–1. [DOI] [PubMed] [Google Scholar]
- 25. Baddley JW, Thompson GR 3rd, Riley KO, Moore MK, Moser SA, Pappas PG. Factors associated with ventriculoperitoneal shunt placement in patients with cryptococcal meningitis. Open Forum Infect Dis 2019; 6:ofz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Peng X, Weng W, Zhu J, Cao H, Xie S. Efficacy of ventriculoperitoneal shunting in patients with cryptococcal meningitis with intracranial hypertension. Int J Infect Dis 2019; 88:102–9. [DOI] [PubMed] [Google Scholar]
- 27. Park MK, Hospenthal DR, Bennett JE. Treatment of hydrocephalus secondary to cryptococcal meningitis by use of shunting. Clin Infect Dis 1999; 28:629–33. [DOI] [PubMed] [Google Scholar]
- 28. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scriven JE, Rhein J, Hullsiek KH, et al. Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 2015; 212:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tugume L, Morawski BM, Abassi M, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med 2017; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bicanic T, Bottomley C, Loyse A, et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob Agents Chemother 2015; 59:7224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diehl JW, Hullsiek KH, Okirwoth M, et al. Cerebral oximetry for detecting high-mortality risk patients with cryptococcal meningitis. Open Forum Infect Dis 2018; 5:ofy105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mkoko P, Du Preez J, Naidoo S. Intracranial pressure management in patients with human immunodeficiency virus-associated cryptococcal meningitis in a resource-constrained setting. South Afr J HIV Med 2020; 21:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meda J, Kalluvya S, Downs JA, et al. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: an operational research study. J Acquir Immune Defic Syndr 2014; 66:e31–6. [DOI] [PubMed] [Google Scholar]
- 35. Malessa R, Krams M, Hengge U, et al. Elevation of intracranial pressure in acute AIDS-related cryptococcal meningitis. Clinical Investig 1994; 72:1020–6. [DOI] [PubMed] [Google Scholar]
- 36. Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 37. Jarvis JN, Lawrence DS, Meya DB, et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 2022; 386:1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rhein J, Hullsiek KH, Evans EE, et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.