Abstract
Introduction
Mozambique suffers from regular floods along its principal river basins and periodic cyclones that resulted in several cholera epidemics during the last decades. Cholera outbreaks in the recent 5 years affected particularly the northern provinces of the country including Nampula and Niassa provinces. A pre-emptive oral cholera vaccine (OCV) mass vaccination campaign was conducted in Cuamba District, Niassa Province, and the feasibility, costs, and vaccination coverage assessed.
Methods
WHO prequalified OCV (Euvichol-Plus), a killed whole-cell bivalent vaccine containing Vibrio cholerae O1 (classical and El Tor) and O139, was administered in two doses with a 15-day interval during 7–31 August 2018, targeting around 180 000 people aged above 1 year in Cuamba District. Microplanning, community sensitisation, and training of local public health professionals and field enumerators were conducted. Feasibility and costs of vaccination were assessed using CholTool. Vaccination coverage and barriers were assessed through community surveys.
Results
The administrative coverage of the first and second rounds of the campaign were 98.9% (194 581) and 98.8% (194 325), respectively, based on the available population data that estimated total 196 652 inhabitants in the target area. The vaccination coverage survey exhibited 75.9% (±2.2%) and 68.5% (±3.3%) coverage for the first and second rounds, respectively. Overall, 60.4% (±3.4%) of the target population received full two doses of OCV. Barriers to vaccination included incompatibility between working hours and campaign time. No severe adverse events were notified. The total financial cost per dose delivered was US$0.60 without vaccine cost and US$1.98 including vaccine costs.
Conclusion
The pre-emptive OCV mass vaccination campaign in remote setting in Mozambique was feasible with reasonable full-dose vaccination coverage to confer sufficient herd immunity for at least the next 3 to 5 years. The delivery cost estimate indicates that the OCV campaign is affordable as it is comparable with Gavi’s operational support for vaccination campaigns.
Keywords: public health, health economics, infectious diseases, preventive medicine, infection control
Strengths and limitations of this study.
This study has successfully demonstrated the feasibility of an oral cholera vaccine (OCV) mass vaccination campaign in a remote setting in Mozambique.
The cost of a mass vaccination campaign for the two-dose OCV administrations has been analysed for the first time in Mozambique, which can serve as a reference cost estimate when planning for any OCV vaccination programmes in a similar setting in Mozambique or other countries.
Vaccination coverage estimates may be affected if there are people movements in and out of the study area. A substudy on this and a focused community engagement strategy to reduce the identified barriers to vaccination should be considered in future vaccination programmes.
Newly introduced vaccination monitoring/coverage survey engaging the same survey team enabled quick availability of the vaccination coverage during or immediately after the campaign, but at the same time the team could be overburdened.
Introduction
Cholera is a vaccine-preventable disease that remains as a major public health concern in many parts of low-and-middle-income countries. A comprehensive policy measure is warranted to control and prevent cholera including investments in improving infrastructure and knowledge, attitude and behaviour associated with water, sanitation and hygiene (WaSH), strengthening health system and adequate use of oral cholera vaccine (OCV).1 In Mozambique, cholera has been endemic since the early 1970s when the first cholera outbreak was reported in the country. Several epidemics followed since then including the outbreaks in 1997–1999 and 2012–2016.2 3 Cholera outbreaks are more frequent in the country’s northern provinces including Nampula, Cabo Delgado, Tete and Niassa.4 Following the reinforcement of cholera outbreak response strategies, the Ministry of Health (MOH) of Mozambique has carried out several OCV mass vaccination campaigns, as recommended by WHO as an integral part of a comprehensive strategy for cholera prevention and control in endemic setting along with primary interventions of WaSH measures.5 Recent cholera outbreaks in these cholera endemic and hotspot areas in December 2015 resulted in the use of global OCV emergency stockpile to vaccinate approximately 212 745 people living in six neighbourhoods of Nampula city in 20164; and in April 2017, another 709 077 doses were used from the stockpile to vaccinate approximately 354 550 people in Tete City and Moatize and Mutarara districts, in response to the cholera outbreak with over 3592 cholera cases.
In addition to these reactive vaccination campaigns supported by the WHO International Coordinating Group on vaccine provision for cholera, a growing need for a preventive public health intervention using a targeted vaccination approach in cholera priority areas in-country was identified. The past records of numerous episodes of cholera epidemics in Mozambique have spotted at-risk districts in the most cholera endemic provinces such as Nampula (particularly Nampula City), Niassa (Lichinga city and Cuamba and Lago Districts) and Cabo Delgado (Pemba City and Ancuabe District), and to a lesser degree, other provinces and districts with limited sanitary conditions.5 Niassa province, one of the cholera endemic regions with annual cholera outbreaks affecting largely the Lichinga City and Lago and Cuamba Districts, was identified for a planned pre-emptive vaccine introduction to prevent subsequent cholera outbreaks. Cuamba District, with an estimated population of 264 572,6 reports over 200 suspected cholera and 2000 diarrheal cases almost every year, with an exception of 2014 and 2016.7 Here, we describe the feasibility, costs and coverage estimates associated with a pre-emptive OCV mass vaccination campaign conducted in Cuamba District using two-dose OCVs (Euvichol-Plus) administered to approximately 180 000 people with a 15-day interval between the doses, as well as challenges of delivering healthcare in resource-limited rural setting in Mozambique.
Methods
Study site and population
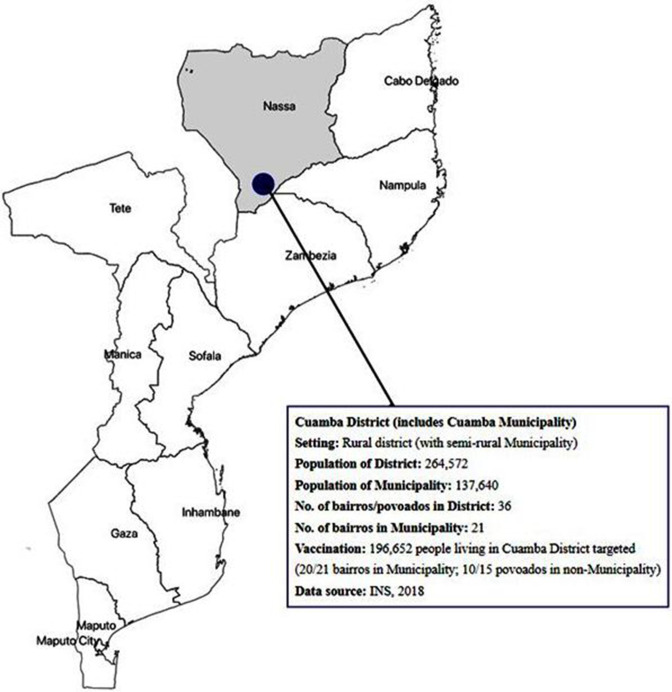
The Cuamba District is located in Niassa Province with a population size of around 264 572.6 The site was selected for a pre-emptive OCV mass vaccination campaign as the district includes the Cuamba Municipality area where cholera is found to be endemic with periodic outbreaks. The area was also highlighted by WHO as one of the priority sites to consider for a potential OCV intervention during a needs assessment performed in September 2015.1 The District of Cuamba is composed of a total 36 bairros and povoados with population size of approximately 264 572,6 which includes 21 bairros in the Cuamba Municipality area with around 137 640 residents.8 In total, approximately 180 000 individuals living in Cuamba District were targeted initially, and ultimately around 196 652 people living in Cuamba District were targeted, which included 20 bairros in the Municipality area and 10 povoados in the outskirts of the Municipality area (figure 1). Selection of bairros and povoados in the outskirts of Cuamba Municipality within the District was made not only based on the high number of doses destined for the target population in the municipality area but also the records of cholera cases during the outbreaks. Everyone above 1 year of age was eligible for the two-dose OCV administration.
Figure 1.
Pre-emptive OCV mass vaccination site. Location of the pre-emptive OCV vaccination campaign site in Cuamba District, Mozambique, included bairros and povoados in the municipality and district. OCV, oral cholera vaccine.
Vaccine delivery, storage and handling
Approximately 360 000 doses of WHO pre-qualified Euvichol-Plus, a killed whole-cell bivalent OCV containing Vibrio cholerae O1 (classical and El Tor) and O139, were procured from the manufacturer (EuBiologics) and shipped to the entry port in Pemba, Mozambique, in cold chain. On arrival in Mozambique, the vaccines were delivered to Lichinga by airfreight and transported to a central vaccine storage room in Cuamba project site, and kept in refrigerators with temperature maintained within range between 2 and 8℃ until and throughout the campaign. The vaccine vial monitor and electronic shipping indicators (Q-Tag) were used to monitor the temperature of the vaccines during delivery, storage and handling. During the vaccination campaign, cool boxes with dry ice maintained within 2–8℃ were used to carry the vaccines to the vaccination posts.
Cost of vaccine delivery
An openly available, standardised and validated Excel-based tool known as the CholTool was used for estimating vaccine delivery costs.9 This tool comprehensively estimates programmatic costs such as microplanning, communication and training materials development, sensitisation/social mobilisation and personnel training, as well as costs related to vaccine delivery such as vaccine procurement, handling, storage and transport, vaccination administration, adverse events following immunisation (AEFI) management, monitoring supervision and field support. The CholTool has the ability to estimate both financial and economic costs. Financial costs refer to the monetary costs to the payer (eg, allowances, supplies, transport and resources used in microplanning, training and sensitisation/social mobilisation) while economic costs include financial costs along with non-monetary costs of donated goods and resources already available (eg, health personnel time). Key informant interviews were conducted at various administrative levels before, during and after the vaccination campaign in order to identify the resources necessary for each vaccination-related activity and costs of respective resources for each of the two rounds of vaccination. The resource and cost data were entered in CholTool which auto-calculates OCV delivery costs. The costs were reported in 2018 in US dollars (US$) based on government and payer perspective.
Vaccination strategy and microplanning
A fixed-post vaccination strategy with additional mobile teams was adapted for the microplanning of the vaccination campaign. The vaccination teams for 15 fixed posts and 33 mobile teams were identified and trained prior to the campaign. The fixed posts included existing healthcare facilities such as primary health centres and secondary and referral hospital, schools and market areas where many people have easy access to. The mobile teams were deployed to households remotely located with limited access to these fixed posts. This adopted mixed vaccination strategy aimed to improve quality, accessibility and coverage. Each post was staffed with around five field workers including two health workers and three community engagement workers. Five days prior to the vaccination campaign, microplans for each cluster were prepared with postal addresses, target populations, vaccination dates, teams and other site-specific resources. The health workers obtained verbal informed consents from the individuals visiting the vaccination posts for the OCV administration. Pregnant women by self-report or infants below 1 year old were excluded from the vaccination. Vaccination cards and vaccination registry book were developed and deployed, specific to this vaccination that included variables such as name, age, address and vaccination date. The collected data in the vaccine registry book were entered in an Excel-based database. The number of doses planned and administered was also recorded daily for each round of the vaccination campaign.
Vaccination, adverse event monitoring and coverage estimate
The vaccination campaign occurred in two rounds with a 15-day interval. The first round took place during 7–11 August, followed by the second round during 27–31 August 2018. Provision was made for mop-up activities after the second round for those who missed the second dose. To detect any possible AEFI during and after the campaign, health workers were trained to monitor and notify any adverse events encountered in inpatient and outpatient admissions at Cuamba health facilities from the first day of each round throughout the 15 days after the last day of each round.
The vaccination coverage estimates were assessed twofold: administrative coverage and coverage surveys. The administrative coverage was recorded by the local government health office in charge of the vaccination campaign by tracking the number of vaccine doses administered compared with doses that had been planned in the vaccination target areas, at the end of vaccination activities every day during the two rounds of the OCV vaccination campaign. For the vaccination coverage surveys, around 520–650 households, subject to the vaccination schedule including the mop-up vaccination, were estimated to ensure more than 550 samples for each age group (1–4 years, 5–14 years, 15 years and above) assuming 80% coverage with a design effect of 2 to achieve around 5% of prevision. Sampled households were organised per cluster; total 20–25 clusters with 26 households per cluster. The households were selected using a two-stage cluster random sampling methodology. Clusters (primary sampling unit) were selected from the list of villages in the Health Zones, according to the probability proportional to population size, and households (secondary sampling unit) were chosen randomly. For the household random sampling, the enumerators identified the centre point and boundary of the survey target area and applied random selection of households. The surveyors were recruited based on their knowledge on the local area and level of education to conduct the survey, and trained on household sampling methodology, structured survey questionnaire and process of conducting a survey interview, including verbal informed consent and data capturing on the paper-based survey questionnaires.
Five survey teams were deployed to the predetermined clusters for daily vaccination monitoring, where randomly identified 26 households per cluster (5 clusters with total 130 households per day) were visited for 4–5 days (total 520–650 households) from the second or third day of the campaign until 1 day after the last vaccination day. This was applied for each round of the two-dose OCV vaccination campaigns. The information gathered through the survey on the vaccine uptake in the previous day, barriers against the vaccination and the information source on the campaign were analysed and fed daily to the vaccination campaign coordinators and supervisors in order to facilitate overall vaccine uptakes. During the second round of campaign, the survey team collected data for the first-round coverage using the same questionnaire for monitoring, which enabled the first-round vaccine coverage available before the completion of the second round. After the second round, the enumerators continued the household survey for additional 3 days (total 4 days, including the last survey day for monitoring of the second round, which was 1 day after the mop-up campaign) to estimate the coverage for the second round and two full doses of vaccination.
Patient and public involvement
The vaccination campaign was conducted as part of the government’s public health intervention, approved by the MOH in Mozambique. The participants in this study were people living in the cholera endemic and hotspot area, targeted for OCV vaccination campaign as an integral part of the government’s cholera prevention efforts. The vaccination target population living in Cuamba District were sensitised and engaged, prior to and during the vaccination campaign, by the district and provincial health officials, study team that included the MOH and National Institute of Health government officials, and local public health professionals at healthcare facilities. The participants were provided with information on the planned OCV mass vaccination such as the purpose of pre-emptive vaccination and detailed information on where and when the vaccination campaigns were to take place. The vaccination campaign was also announced through various press and social media in Mozambique for public awareness and involvement. The study was conducted in a transparent manner with open communication and information sharing in the community, and participants to the OCV vaccination and vaccination coverage surveys were informed for oral consent. For children, consents were obtained from parents/guardian and all adult participants provided their own consent. The study did not present any risk of harm to subjects. No biological samples were collected. Minimum data were collected from participants, whereby privacy and confidentiality of the data were ensured during the survey implementation and data entry and management. Stakeholder meetings were conducted prior to, during and after the vaccination campaign to further disseminate the campaign plan and results to the community members.
Results
OCV vaccination coverage
The administrative coverage of the first and the second rounds of the campaign were 98.9% (194 581) and 98.8% (194 325), respectively, based on the available census data of vaccination target population in Cuamba Municipality and outskirts, estimated at around 196 6526 inhabitants (table 1). A total of 194 581 people over 1 year old received the first dose, out of whom 99 275 were females and 122 592 were children aged less than 15 years. For the second round, total 194 325 people were vaccinated, including 99 275 females and 120 169 children less than 15 years old. Notably, the vaccination coverage survey conducted in the target community during each round and post-vaccination exhibited approximate coverage estimates of 75.9% (95% CI, 78.10% to 73.70%) for the first round and 68.5% (71.80% to 65.20%) for the second round. The coverage rate for the full two doses was estimated at 60.4% (63.80% to 57.00%), whereby the coverage of children aged 1–4 years was around 64.4% (57.10% to 71.10%) (table 1). The coverage rates in each round were higher in male (76.3% and 77.8%) than female (75.4% and 67.7%), but coverage rate of full doses was higher in female (64.4%) than male (57.3%). No adverse events were reported during and after the vaccination activities, monitored up to 14 days post-vaccination campaign.
Table 1.
OCV vaccination coverage estimates, Cuamba District, 2018
| (A) Administrative vaccination coverage rates | ||||||||
| Number of people vaccinated (N) | ||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Total | ||
| First dose | ||||||||
| Individuals vaccinated per age group (years) | 1–4 | 6493 | 9283 | 12 394 | 12 506 | 7691 | – | 48 367 |
| 5–15 | 7050 | 16 705 | 21 590 | 17 536 | 11 344 | – | 74 225 | |
| ≥15 | 10 136 | 12 400 | 18 835 | 18 798 | 11 820 | – | 71 989 | |
| Total no of daily vaccinated | 23 679 | 38 388 | 52 819 | 48 840 | 30 855 | – | 194 581 | |
| Cumulative no of vaccinated | 23 679 | 62 067 | 114 886 | 163 726 | 194 581 | – | ||
| Cumulative administrative coverage | 12.04% | 31.56% | 58.42% | 83.26% | 98.95% | – | 98.95% | |
| Second dose | ||||||||
| Individuals vaccinated per age group (years) | 1–4 | 5479 | 6484 | 11 117 | 9596 | 7760 | 7586 | 48 022 |
| 5–15 | 9355 | 8796 | 15 679 | 13 208 | 14 444 | 10 665 | 72 147 | |
| ≥15 | 9416 | 9275 | 14 271 | 14 265 | 14 848 | 12 081 | 74 156 | |
| Total no of daily vaccinated | 24 250 | 24 555 | 41 067 | 37 069 | 37 052 | 30 332 | 194 325 | |
| Cumulative no of vaccinated | 24 250 | 48 805 | 89 872 | 126 941 | 163 993 | 194 325 | ||
| Cumulative administrative coverage | 12.33% | 24.82% | 45.70% | 64.55% | 83.39% | 98.82% | 98.82% | |
| (B) Vaccination coverage rates through coverage surveys | |||
| First round | Second round | Full two doses | |
| Age (years) | |||
| 1–4 | 81.1±4.5% | 72.2±6.9% | 64.4±7.3% |
| 5–14 | 86.4±3.1% | 71.3±5.8% | 65.2±6.1% |
| ≥15 | 67.6±3.3% | 65.2±4.8% | 55.7±5.0% |
| Sex | |||
| Male | 76.3±2.9% | 77.8±3.9% | 57.3±4.6% |
| Female | 75.4±3.2% | 67.7±5.0% | 64.4±5.1% |
| Total | 75.9±2.2% | 68.5±3.3% | 60.4±3.4% |
OCV, oral cholera vaccine.
Source of information and acceptability
The source of information on the OCV vaccination campaign, identified by the populations living in the vaccination target areas, showed use of megaphone as the most effective tool in disseminating information on the vaccination plan and mobilising the community to get immunised for both rounds: 24% and 34% at the first and second rounds, respectively (table 2). Around 15% of the surveyed people in the target community indicated that they have learnt about the vaccination campaign through radio broadcast for the first round, but its communication impact decreased in the second round (4%). This was different for the community leaders, whose contribution increased from 5% in the first round to 19% in the following round, reflecting their active engagement and communication efforts in close coordination with the vaccination teams on the ground.
Table 2.
Source of information on OCV campaign, Cuamba District, 2018
| Source of information | First round* N=646 n (%=n/N) |
Second round† N=578 n (%=n/N) |
| Megaphone | 152 (24) | 195 (34) |
| Family | 60 (9) | 53 (9) |
| Radio | 96 (15) | 23 (4) |
| Religious leader | 82 (13) | 25 (4) |
| Health workers | 74 (11) | 120 (21) |
| Activists | 55 (9) | 9 (2) |
| Community leader | 33 (5) | 108 (19) |
| TV | 14 (2) | 11 (2) |
| Others‡ | 78 (12) | 33 (6) |
*1st round: 646 households/or people were interviewed.
†2nd round: 578 households/or people were interviewed.
‡Others included: list other source of info if such data were collected.
OCV, oral cholera vaccine.
Reasons for not being vaccinated
The unavailability (absence) of the target population for vaccination and incompatibility between working hours and campaign schedule were commonly cited as barriers for vaccination in both the first (35%) and the second round (51%) (table 3). Absence of vaccinators at the vaccination sites were also mentioned, 12% and 18% for the first and second rounds, respectively, despite the pre-vaccination planning and programmatic organisation. Notably, around 10% of the target population have indicated that they have not been informed about the vaccination campaign even in the second round, although this was a reduction compared with 18% in the first round. In order to address the most common barriers identified in the first round, the second round of the vaccination campaign was further extended for additional few days including the weekends, enabling more people to get vaccinated.
Table 3.
Reasons for non-vaccination during the OCV campaign, Cuamba District, 2018
| Reasons for non-vaccination | First dose | Second dose | ||
| N=361 | %(=n/N) | N=222 | %(=n/N) | |
| Unavailable | 63 | 17 | 96 | 43 |
| Incompatibility between working hours and campaign time | 53 | 15 | 18 | 8 |
| Vaccination post without vaccinator | 40 | 11 | 41 | 18 |
| Did not have information | 66 | 18 | 23 | 10 |
| Ill during the vaccination period | 30 | 8 | 10 | 5 |
| Does not believe in vaccine efficacy | 24 | 7 | 2 | 1 |
| Afraid of adverse events | 8 | 2 | 0 | 0 |
| Head of the family did not authorise | 4 | 1 | 2 | 1 |
| Religious leader forbid | 2 | 1 | 0 | 0 |
| Considered not safe for pregnant women | 1 | 0 | 2 | 1 |
| Other | 70 | 19 | 28 | 13 |
OCV, oral cholera vaccine.
OCV delivery costs
The total financial cost of campaign was US$768 904 of which vaccine acquisition including vaccine shipment constituted 69% (US$533 659) (table 4). The vaccine delivery costs including microplanning, training, communication and social mobilisation, and vaccination implementation (rounds 1 and 2) constituted rest 31% (US$235 245). The total financial cost per dose delivered was US$0.60 without the vaccine cost and US$1.98 including the vaccine costs in 2018 price. The economic cost per dose delivered excluding vaccine costs was five times higher at US$3.02. The total financial cost of delivery per fully immunised person excluding vaccine costs was US$1.21.
Table 4.
Costs of OCV vaccine delivery and immunisation in Cuamba District
| Vaccine delivery costs | Financial cost (Mzn) | Economic cost (Mzn) | Financial cost (US$) | Economic cost (US$) |
| Vaccine acquisition | 32 179 644 | 42 081 073 | 533 659 | 697 862 |
| Microplanning | 640 415 | 7 596 625 | 10 620 | 125 981 |
| Training | 265 186 | 299 419 | 4398 | 4965 |
| Communication and social mobilisation | 1 912 520 | 4 301 342 | 31 717 | 71 332 |
| Vaccination implementation (rounds 1 and 2) | 11 367 160 | 58 510 806 | 188 510 | 970 328 |
| Total | 46 364 925 | 112 789 265 | 786 904 | 1 870 469 |
| Immunisation costs | Financial cost (Mzn) | Economic cost (Mzn) | Financial cost (US$) | Economic cost (US$) |
| Cost per vaccine administered (including vaccine) | 119 | 290 | 1.98 | 4.81 |
| Cost per vaccine administered (without vaccine cost) | 36 | 182 | 0.60 | 3.02 |
| Cost per partially immunised person | 238 | 580 | 3.95 | 9.61 |
| Cost per fully immunised person (with vaccine) | 239 | 580 | 3.96 | 9.63 |
| Cost per fully immunised person (without vaccine) | 73 | 364 | 1.21 | 6.03 |
OCV, oral cholera vaccine.
Discussion
The OCV campaign in Cuamba District was organised without major logistical and programmatic challenges, and no adverse events were reported throughout the vaccination activities and up to 14 days after the campaign. Despite the similarity in the number of people vaccinated in the first and second rounds, the vaccination coverage survey of the second round showed lower coverage estimates than the first round. This may be due to possible cross-border movement of people from untargeted districts to get vaccination during the second round. The vaccination coverage for the full two doses was over 60% that may confer sufficient herd immunity for the following several years based on the existing literature on a cholera transmission model using the Matlab data from Bangladesh,10 11 which predicted that 50% coverage with OCV in cholera endemic areas may result in 89% reduction in cholera cases in unvaccinated.12
In our study, children aged 5–14 years exhibited the highest coverage. This may be due to the vaccination posts in both schools (fixed vaccination post) and near homes (mobile vaccination posts), which facilitated the school-aged children to access the immunisation health service more easily. The female group also presented higher full vaccination coverage rate compared with the male group, who showed higher drop-out after the first dose, likely associated with their routine boundaries of livelihood near their houses or their child/children’s schools as they take care of children while the male group typically work outside. This assumption is supported by the fact that the absence during the campaign was identified as a significant barrier against vaccination during both rounds of the campaign. A similar pattern was consistently prevalent in the previous OCV campaigns in Beira13 and Nampula,4 whereby absence was the main barrier for vaccination. The second round of the campaign coincided with the period of school holidays when most households move to farming and food production, resulting in higher absence rate in the second round (43.0%) than in the first round (17.0%). Furthermore, it is encouraging to observe more than 60% vaccination coverage rate among children aged 1–4 years, the most at-risk population age group concerning cholera outbreaks. Considering that caregivers for these younger children are mostly women, higher vaccination coverage for these toddlers and younger children and women is as anticipated in accordance with other studies published in similar settings.14
For real-time monitoring of the OCV vaccination campaign, the researchers have employed a representative sampling (two-stage cluster sampling) instead of conventional convenient sampling, where the new approach assessed only 1/5 of the predetermined households and demanding 5 days reaching full households for optimal precision. This new approach has several advantages including (1) availability of representative daily coverage, and barriers, which were fed to the coordination team on a real-time basis despite limited precision; (2) the first round vaccine coverage that became available before the end of the second round; and finally (3) the vaccine coverage that was available immediately after each round without a separate post vaccination coverage survey using ‘measurement error approach’15 (the details have not been discussed here, but in a separate article currently under development). Again, the second and full-dose vaccine coverage were estimated within a week after the campaign by extension of the survey days by three more days. However, the survey extension and additional questions for the final coverages (the first, second and full) made some survey team members exhausted, which might have affected survey quality.
In order to enhance the vaccination coverage, it is paramount to better understand the effective means of communications for community sensitisation and engagements, as well as barriers towards participating in a vaccination programme such as this campaign. Here, we showed that the use of megaphone proved to be the most effective advocacy tool for disseminating information on the vaccination to our target community, which may have allowed the field workers to reach out to families without access to other sources of information. This may also indicate the need to better understand the inter-personnel communication and community mobilisation approach for future vaccination campaigns. For those with missed opportunities to receive the OCV doses during the two rounds, a mop-up vaccination can be considered, although it is often more laborious and costly, requiring a complex management.13 Furthermore, informing the public on the availability of a mop-up prior to or during the campaign may negatively affect their participation in the regular vaccination schedule set-up. Hence, a mop-up was not considered after the first round in our approach but pursued after the second round in order to enhance the full two-dose vaccination and verify vaccination data records submitted during the regular programme. Approximately 15.4% (32 775/212 824) of the delivered second doses were through this mop-up campaign indicative of an effective strategy.
The financial costs of OCV delivery per fully immunised person in this campaign were lower than delivery costs reported in other African countries using the same CholTool (US$1.8 in Shashemene district of Ethiopia; US$2.5 in Nsanje district of Malawi; US$3.5 in Machinga, Phalombe and Zomba districts of Malawi per the US$ price value of 2016), but closer to that reported in Puri district of India (US$1.14 per the US$ price value of 2016).9 One reason could be that Mozambique has experience of conducting several OCV campaigns in recent years, and hence there were already resources and expertise available for microplanning, communication, sensitisation, training and so on, which might have reduced the costs associated with introduction of vaccines in comparison with a vaccination programme in naive setting. The financial cost of US$0.60 per dose delivered (excluding vaccine procurement) is comparable with the operational support ranging between US$0.30 and US$0.80 per person targeted for vaccination campaigns, recommended by the Gavi, the Vaccine Alliance.16 17 This indicates the affordability of OCV campaign in the current setting. To economise the healthcare provider time and efforts and incentivise beneficiaries for greater uptake of vaccines, delivery of multiple products at vaccination posts or on household visits may potentially synergise the delivery cost associated with vaccination campaigns.
Overall, our study proved the feasibility of conducting a pre-emptive OCV mass vaccination campaign in a rural and semi-rural setting in Cuamba District and Cuamba Municipality areas, respectively, with sufficient coverage rate and relatively lower delivery cost. The success of vaccination was a result of effective coordination and microplanning among stakeholders despite some field challenges. The vaccination strategy using both fixed and mobile posts, as well as the daily feedback to the coordination team on the preliminary coverage survey result and data related to barriers and source of information on the vaccination campaign, proved valuable to prospectively refine the campaign and mobilisation strategy every day on a real-time basis.
However, there are several limitations. First, the operational challenges concerning poor road conditions resulted in the accessibility to the target area difficult. Second, the programmatic support required sufficient and trained human resources and budget for a sustained field monitoring activity and close on-site supervision prior to and during the vaccination campaign and coverage survey activities. Third, the differences in the coverage rates of administrative data and survey result are due to the lack of accurate up-to-date census data of local population. In addition, in order to avoid any conflict with the measles and rubella national immunisation campaign that was taking place across the country at the time of this vaccination campaign, we had to delay our OCV vaccination campaign for about 2 months to obtain support from immunisation-related stakeholders, particularly the expanded programme of immunisation for cold chain space and logistics. Any mass vaccination campaigns should also consider seasonality and other major community activities and/or any political issues.
Supplementary Material
Acknowledgments
The authors acknowledge the local government officials and healthcare professionals in Cuamba District, Niassa Province for engaging the community on the OCV vaccination program throughout the planning and implementation period. Thanks are also extended to the people who consented and took part in the coverage survey. We thank our research partners and staff at the MOCA sentinel site networks in Mozambique, and Ms Somyoung Cho for the project management and Ms Jihyun Han and Ms Nozipho Manjate for the project administrative support.
Footnotes
Twitter: @DrSeEunPark
Contributors: SEP conceptualised the overall study design of the Mozambique Cholera Prevention and Surveillance (MOCA) project. CSB supervised the MOCA project in Mozambique. NSB supervised the overall vaccination campaign and monitoring and evaluation. All authors participated in the vaccination campaign. JJEC, NL, LDB, JPL, NSB, SEP, SA, AO, MM and the project field team in Cuamba and Niassa contributed to data acquisition on the community vaccination coverage surveys, and interpretation of results under the supervision of NSB. RBJM, JAM, SA, AO, MM and others in the vaccination teams of Cuamba District and Niassa Province contributed to acquisition, review and report of the administrative coverage data. IC contributed to data acquisition and analysis on vaccination costs; VM and CVR reviewed the cost analysis. JJEC drafted and edited the paper under the scientific guidance from NSB and SEP. All authors read and approved the final draft. SEP (corresponding author) is responsible for the overall content as the guarantor.
Funding: This study was supported by the Korea International Cooperation Agency (KOICA), government of the Republic of Korea. The International Vaccine Institute acknowledges its donors, including the Government of Republic of Korea and the Swedish International Development Cooperation Agency (SIDA).
Disclaimer: The findings and conclusions are our own and do not necessarily reflect positions of the KOICA.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and the Institutional Ethical Committee of the National Institute of Health (Ref: 116/CNBS/19) and ethical review board of the International Vaccine Institute, Seoul, Korea (IRB number 2017-006) approved the study protocol for the OCV mass vaccination campaign monitoring and coverage survey. Participants gave verbal informed consent to participate in the study before taking part.
References
- 1.Fung IC-H, Fitter DL, Borse RH, et al. Modeling the effect of water, sanitation, and hygiene and oral cholera vaccine implementation in Haiti. Am J Trop Med Hyg 2013;89:633–40. 10.4269/ajtmh.13-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langa JP, Sema C, De Deus N, et al. Epidemic waves of cholera in the last two decades in Mozambique. J Infect Dev Ctries 2015;9:635–41 http://www.jidc.org/index.php/journal/article/view/6943 10.3855/jidc.6943 [DOI] [PubMed] [Google Scholar]
- 3.WHO . Cholera country profile: Mozambique. Global Task Force on Cholera Control, 2013. [Google Scholar]
- 4.Semá Baltazar C, Rafael F, Langa JPM, et al. Oral cholera vaccine coverage during a preventive door-to-door mass vaccination campaign in Nampula, Mozambique. PLoS One 2018;13:e0198592. 10.1371/journal.pone.0198592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Cholera vaccines: WHO position paper – August 2017. Wkly Epidemiol Rec 2017;92:477–98. [PubMed] [Google Scholar]
- 6.Estatística INde. Divulgação os resultados preliminares IV RGPH Habitação, agregados familiares e população, Província de Niassa. In: IV Recenseamento Geral da População e Habitação, 2017: 3. [Google Scholar]
- 7.Gujral L, Sema C, Rebaudet S, et al. Cholera epidemiology in Mozambique using national surveillance data. J Infect Dis 2013;208 Suppl 1:S107–14. 10.1093/infdis/jit212 [DOI] [PubMed] [Google Scholar]
- 8.Estatística INde. Recenseamento Geral da População e Habitação. 63. Indicadores Sócio Demográficos, Província de Niassa, 2007. [Google Scholar]
- 9.Morgan W, Levin A, Hutubessy RC, et al. Costing oral cholera vaccine delivery using a generic oral cholera vaccine delivery planning and costing tool (CholTool). Hum Vaccin Immunother 2020;16:3111–8. 10.1080/21645515.2020.1747930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet 1986;2:124–7. 10.1016/S0140-6736(86)91944-6 [DOI] [PubMed] [Google Scholar]
- 11.Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990;335:270–3. 10.1016/0140-6736(90)90080-O [DOI] [PubMed] [Google Scholar]
- 12.Longini IM, Nizam A, Ali M, et al. Controlling endemic cholera with oral vaccines. PLoS Med 2007;4:e336. 10.1371/journal.pmed.0040336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas MES, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352:757–67. 10.1056/NEJMoa043323 [DOI] [PubMed] [Google Scholar]
- 14.Legros D, Paquet C, Perea W. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp in Sudan, Uganda. Bulletin of the World Health Organization. 77, 1999: 10. [PMC free article] [PubMed] [Google Scholar]
- 15.Berg EJ, Fuller WA. Small area prediction of proportions with applications to the Canadian labour force survey. J Surv Stat Methodol 2014;2:227–56. 10.1093/jssam/smu011 [DOI] [Google Scholar]
- 16. GavitheVaccineAlliance. Vaccine introduction grants and operational support for campaigns. Report to the GAVI alliance Board, 2012. Available: https://www.gavi.org/sites/default/files/board/minutes/2012/12-june/13%20-%20Vaccine%20introduction%20grants%20and%20operation%20support%20for%20campaigns%20document.pdf [Accessed 9 Feb 2021].
- 17. GavitheVaccineAlliance. GAVI alliance board meeting. 12–13 June 2012, Washington, DC, USA, final minutes. Available: https://www.gavi.org/sites/default/files/board/minutes/2012/Board-2012-Mtg-02%20-%20Minutes.pdf [Accessed 9 Feb 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.