Abstract
Background
Alcohol and tobacco use disorders (AUD, TUD) are frequent, both worldwide and in the German population, and cognitive impairments are known to facilitate instances of relapse. Cognitive training has been proposed for enhancing cognitive functioning and possibly improving treatment outcome in mental disorders. However, these effects and underlying neurobiological mechanisms are not yet fully understood regarding AUD and TUD. Examining the effect of chess-based cognitive remediation training (CB-CRT) on neurobiological, neuropsychological and psychosocial aspects as well as treatment outcomes will provide insights into mechanisms underlying relapse and abstinence and might help to improve health behaviour in affected individuals if used as therapy add-on.
Methods and analysis
N=96 individuals with either AUD (N=48) or TUD (N=48) between 18 and 65 years of age will participate in a randomised, controlled clinical functional MRI (fMRI) trial. Two control groups will receive treatment as usual, that is, AUD treatment in a clinic, TUD outpatient treatment. Two therapy add-on groups will receive a 6-week CB-CRT as a therapy add-on. FMRI tasks, neurocognitive tests will be administered before and afterwards. All individuals will be followed up on monthly for 3 months. Endpoints include alterations in neural activation and neuropsychological task performance, psychosocial functioning, and relapse or substance intake. Regarding fMRI analyses, a general linear model will be applied, and t-tests, full factorial models and regression analyses will be conducted on the second level. Behavioural and psychometric data will be analysed using t-tests, regression analyses, repeated measures and one-way analyses of variance.
Ethics and dissemination
This study has been approved by the ethics committee of the medical faculty Mannheim of the University of Heidelberg (2017-647N-MA). The findings of this study will be presented at conferences and published in peer-reviewed journals.
Trial registration
The study was registered in the Clinical Trials Register (trial identifier: NCT04057534 at clinicaltrials.gov).
Keywords: Substance misuse, Adult psychiatry, Magnetic resonance imaging
Strengths and limitations of this study.
The evaluation of the efficacy of chess-based cognitive remediation training as a supportive therapy add-on for substance use disorders might lead to cost-efficient positive treatment outcomes.
The use of objective measures to examine underlying neurobiopsychological mechanisms expands the current research on risk factors for relapse.
The inclusion of two substances (alcohol and tobacco) increases the generalisability of the findings.
The 6-week-long therapy add-on might lead to drop-outs due to the large amount of time participants have to commit to the programme.
Introduction
Substance use, including alcohol and tobacco use, is widespread both worldwide and in the German population. Worldwide, the prevalence for heavy episodic drinking of alcohol was estimated at 18.4% for adults, while daily smoking was estimated at 15.2%.1 In 2018 in Germany, the prevalence of hazardous consumption of alcohol was estimated at 19.1%, and the 12-month prevalence for alcohol use disorder (AUD) at 5.9%. The prevalence of daily consumption of tobacco was estimated at 15.1%, and the 12-month prevalence for tobacco use disorder (TUD) at 8.6%.2 In Germany, follow-up costs of alcohol use are estimated at €21 billion3 and for tobacco use at €24 billion.4 Furthermore, negative effects on health and on mortality rates are associated with TUD.5
For individuals with AUD having undergone treatment, relapse rates between 22% and 86% have been observed during short-term follow-ups (16 weeks) up to a long-term follow-up of 16 years.6–8 Following treatment, the relapse rate for TUD after 1 year is estimated to be between 2% and 17%.9 A relapse can be brought on by heightened stress sensitivity, depressive mood, increased anxiety or confrontation with a substance-related stimulus.10–12
Even though some studies postulate intact, goal-directed behaviour in individuals with substance use disorders (SUD),13–15 others observed neurobiological impairments in brain areas involved in inhibitory control in individuals with SUD.16–19 In a model proposed by Bechara, SUD is viewed as an imbalance between two distinct, but closely interacting neural systems,20 which are essential for decision-making: The impulsive system is involved in the prediction and valuation of immediate rewards and includes such regions as the amygdala and the striatum. The reflective system signals long-term consequences of actions and involves the ventromedial prefrontal cortex, the dorsolateral prefrontal cortex, the anterior cingulate, the insula and the hippocampus. In SUD, it is assumed that the impulsive system becomes overactive, preventing the reflective system from exerting executive cognitive control over substance use. It might be those immediate rewards, such as pleasant effects derived from alcohol or nicotine consumption, are overvalued, and give preference over future rewards, such as health benefits associated with abstinence. Individuals with SUD also demonstrate a preference for smaller, immediate monetary rewards over larger, delayed ones.21 Furthermore, the imbalance between impulsive and reflective systems reveals itself in dysfunctional inhibitory control, leading to increased risk taking.20 Beyond these impairments, individuals with SUD also demonstrate reduced cognitive functioning in the domains of problem-solving, mental flexibility, forming judgments and working memory.22 A study using functional MRI (fMRI)23 found less activation in the right frontal cortex during a response inhibition task was associated with more cigarettes smoked in participants wanting to quit smoking. Other studies using fMRI have revealed a shift of neural activation from the ventral (nucleus accumbens) to the dorsal striatum (putamen and nucleus caudate), which was suggested to reflect a decrease in cortical control when viewing substance related cues.24 Being related to executive functions, metacognitive abilities and beliefs play a major role in addiction.25 In general, metacognition refers to the ability to know about cognition in general but, more importantly, to be aware of and know about one’s own cognition.26 Prefrontal regions, as well as the precuneus or dorsal anterior cingulate cortex, seem to play an important role.27 Not only generic and dysfunctional metacognitive beliefs but also metacognitive beliefs about addiction-related thoughts or craving can predict the severity of addictive behaviour, craving and relapse.25
‘Cognitive remediation’ (cognitive remediation therapy or training (CRT)) is a psychotherapeutic approach to improve cognitive deficits.28 Cognitive training exercises span functional domains from executive functioning (inhibition, decision-making, cognitive flexibility and working memory) to attention. Through repeated training, CRT can systematically stimulate and strengthen cognitive processes. A primary therapeutic objective is to improve the efficacy of other psychotherapeutic interventions, which require a minimal level of cognitive skill.29 For example, it has been demonstrated that executive functioning skills can influence the efficacy of cognitive behavioural therapy.30 CRT, specifically, has already been demonstrated to be successful as an add-on therapy in treating schizophrenia and eating disorders.31 However, it has been suggested to explicitly teach metacognitive abilities in order to improve the outcome of CRT,32 since this might be a significant mechanism contributing to the effects of CRT in patients with schizophrenia.33 Indeed, recent observations indicate a beneficial effect of CRT on metacognitive abilities, for example, in schizophrenia.34 As an add-on therapy to treat substance use disorders, CRT seems promising35 and cognitive training mostly results in improvements within the respective domains.36 However, there is a lack of studies examining the efficacy of CRT as a modulator of cognition to improve treatment outcomes37 and findings on the positive outcome following cognitive trainings in AUD are still mixed38 or not present.39 A review on AUD40 discussed that CRT improves split attention, recognition of warning signals, working memory, as well as episodic memory. Most relevantly, an improvement in working memory and inhibitory control was able to exert a positive influence on substance use patterns.40 Additionally, including metacognitive trainings when treating individuals with SUD might be advantageous.25 41
Finally, promising studies have demonstrated a potential beneficial effect of classical chess training on the treatment of attention deficit hyperactivity disorder (ADHD) and schizophrenia as an add-on therapy. In the case of ADHD, classical chess training was able to effectively reduce disease severity.42 A further study in patients with ADHD showed an improvement in the ability to concentrate.43 Negative symptoms common to patients suffering from schizophrenia include a wide variety of cognitive deficits, including impaired attention-solving, memory-solving, learning-solving and problem-solving skills.44 Chess training was able to rescue some of these deficits experienced by schizophrenic patients, improving voluntary processing, inhibitory capacity and planning proficiencies.45 Examining the effects of chess training on mathematical problem-solving and metacognitive abilities in school children, no significant effects were observed compared with an active control group playing checkers and a passive control group.46
Besides the known effects of CRT on metacognition, the beneficial effect of chess-based CRT (CB-CRT) still remains unclear. However, present findings suggest that CB-CRT might be able to improve cognitive functioning in domains which can be improved by classical CRT, while simultaneously potentially improving specific domains modulated by chess-based interventions.
Consequently, our study aims to assess the effects of CB-CRT on underlying neurobiological mechanisms of CB-CRT in AUD and TUD. We will use a novel and structured training programme that, besides training cognitive functioning, includes metacognitive methods and social reinforcement. As a result of the comprehensiveness of the proposed study and the novel CB-CRT, we will further assess the influence of CB-CRT on different aspects of cognition and psychosocial functioning as well as treatment outcome in individuals with AUD and TUD.
Method and analyses
To investigate the effects of CB-CRT as a therapy add-on in alcohol and TUDs, N=96 individuals will be examined in a randomised, controlled clinical fMRI trial. N=48 AUD participants undergoing a qualified therapy or rehabilitation treatment for AUD and N=48 TUD participants who participate in a qualified smoking cessation group therapy will be included in the study. The Consolidated Standards Reporting Trials statement was used for developing the study framework. Individuals with a diagnosis of AUD will be recruited from the out-patient and in-patient clinics of the department of addictive behaviour and addiction medicine at the Central Institute of Mental Health and from the residential addiction treatment centre MEDIAN Klinik Wilhelmsheim, Germany. Individuals with TUD will be recruited using public announcements including, flyers and social media posts.
Half of each group (AUD, TUD) will be randomly assigned to either the control group or experimental group. Regarding the control groups, N=24 AUD participants receive an in-patient qualified detoxification treatment programme, an in-patient or out-patient rehabilitation programme, or semi-inpatient therapy in a day clinic. N=24 TUD participants receive qualified smoking cessation group therapy following study inclusion. The out-patient smoking cessation therapy lasts for 6 weeks with one group therapy session a 1.5 hours per week. Individuals randomly allocated to the experimental group (24 individuals with AUD and 24 individuals with TUD) will receive CB-CRT for 1.5 hours two times per week for 6 weeks in addition to the standard treatment.
Patient and public involvement
Individuals currently or formerly affected by either AUD or TUD were involved in the development of the study design including outcome measurements. Two research colleagues with insight from both perspectives were consulted and supported the development and implementation of the study. The chess-based cognitive remediation training was used in practice as described in the following including patients with diverse mental disorders. It therefore grew in correspondence with the patients’ feedback. In addition, a pilot study with patient from an addiction rehabilitation centre resulted in good to very good patient ratings regarding helpfulness and acceptance. We will disseminate study results to interested patients. Also, all study participants will always be able to discuss open questions throughout the process of the training with qualified research staff and they will receive feedback regarding the goals of the training and study and the background of the methods used for training and study examination.
No patients are involved in the recruitment procedure and conduct of the study and the burden of study participation was not assessed beforehand by patients.
Examination procedure
Eligible participants between 18 and 65 years will be informed about the purpose and all aspects of the study. They will be provided with written study information according to the ethics regulations. Participants will be able to ask questions regarding the study. Afterwards, written informed consent will be obtained. All participants can withdraw their consent at any time. Then, study exclusion and inclusion criteria will be examined. To do so, a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-5—Clinician Version47 will be performed to assess a possible history of lifetime and current mental disorders. Individuals with a diagnosis of severe mental or personality disorders will be excluded, for example, lifetime bipolar disorder or schizophrenia or current severe depression, post-traumatic stress disorder. Current mild or moderate mental or personality disorders, such as mild anxiety, adaptation, personality disorders or depression, will be tolerated. Individuals with AUD are included in the study after controlled abstinence for at least 72 hours, including completion of medically supervised detoxification (treatment of withdrawal symptoms with short-acting benzodiazepines or chlormethiazole must have been completed for at least 3 days). Individuals with TUD will be included following the intention to quit smoking. A detailed list of all inclusion and exclusion criteria regarding AUD and TUD are shown in table 1. Following study inclusion, participants will be randomly assigned to either the control or experimental group.
Table 1.
Inclusion and exclusion criteria of the overall study sample
| Inclusion criteria | Exclusion criteria | ||
|
|
||
| AUD | TUD | AUD | TUD |
|
|
|
|
|
|
||
Specific criteria for AUD and TUD are highlighted.
AUD, alcohol use disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; MRI, Magnetic resonance imaging; TUD, tobacco use disorder.
At the baseline, examination appointment (T1) all participants will provide sociodemographic information and perform several neuropsychological tasks. An fMRI assessment will then take place. Participants will also fill out several questionnaires directly after the baseline assessment. After the 6-week-long intervention period—either standard treatment alone or with CB-CRT as therapy add-on—a second examination appointment (T2) takes place. All participants will perform the same neuropsychological tasks again and the same fMRI assessment as conducted in T1 will take place. Participants will also fill out the same questionnaires as for T1. During a follow-up period of 12 weeks following the intervention, three telephone interviews (FU1, FU2, T3) will be conducted once a month. Instances of relapse and amount of tobacco or alcohol consumption will be documented. Beyond this, the same questionnaires as for T1 and T2 will be completed.
Please see figure 1 for a detailed description of the study procedure and tables 2 and 3 for list of assessments used, including fMRI and neuropsychological paradigms and questionnaires.
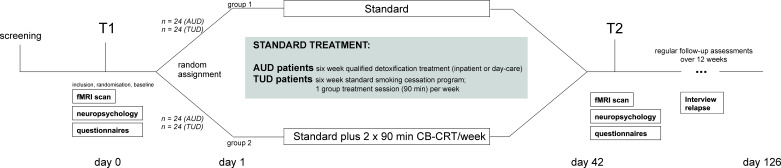
Figure 1.
Study design. Following a screening, all participants will undergo a baseline (T1) appointment with diagnostic interviews, questionnaires and functional MRI measurements. Participants with tobacco or alcohol use disorder will be randomly assigned to the control group or intervention group. All participants will receive their respective treatment as usual. The intervention groups will additionally receive chess-based cognitive remediation training (CB-CRT). After the 6-week-long treatment as usual with/without CB-CRT (T2), the same measurements as for T1 will take place. During the follow-up period of 12 weeks, all participants will be contacted via telephone once a month. AUD, alcohol use disorder; fMRI, functional MRI; TUD, tobacco use disorder.
Table 2.
Self-rating questionnaires
| Questionnaire | Short term | Measurement aim | Reference |
| Goal Attainment Scale | GAS | Abstinence-related goals | 71 |
| Rosenberg Scale | Self-esteem | 72 | |
| General Self-Efficacy Scale | GSE | Self-efficacy | 73 |
| Perceived Social Support Questionnaire | F-SozU | Perceived social support | 74 |
| Habitual Subjective Well-Being Questionnaire | SWLS | Psychological well-being | 75 |
| Satisfaction with Life Scale | SWLS | Life satisfaction | 76 |
| Positive and Negative Affect Schedule | PANAS | Affect | 77 |
| State-Trait Anxiety Inventory | STAI X2 STAI X1 |
Anxiety Personality trait Temporary state |
78 |
| Beck Depression Inventory II | BDI II | Depression | 79 80 |
| Percived Stress Scale | PSS | Perceived stress | 81 |
| Barratt Impulsiveness Scale | BIS-15 | Impulsivity | 64 65 |
| Adult ADHD Self-Report Scale-V.1.1 Symptoms Checklist | ASRS-V.1.1 | ADHD symptoms | 82 |
| ADHD Self-Rating Scale | ADHD-SB | ADHD symptoms | 83 |
| Creature of Habit Scale | COHS | Automatic behaviour | 84 |
| Self-Report Habit Index | SRHI | Substance-related habits | 85 |
| Fagerström Test for Nicotine Dependence | FTND | Intensity of physical nicotine dependence | 86 |
| Alcohol Use Disorder Identification Test | AUDIT | Screening for alcohol use disorder | 87 |
| Clinical Institute Withdrawal Assessment for Alcohol | CIWA-Ar | Alcohol withdrawal symptoms | 70 |
| Form90 | Alcohol or nicotine consumption | 88 | |
| Visual Analog Craving Scales | VACS | Alcohol or nicotine craving | 89 |
| Obsessive Compulsive Drinking Scale | OCDS-G | Thoughts about alcohol and drinking behaviour | 90 |
| Alcohol Craving Questionnaire | ACQ-SF-R | Acute alcohol craving | 91 |
| Craving Automated Scale for Alcohol | CAS-A | Alcohol craving and automated drinking behaviour | 92 |
| Alcohol Urge Questionnaire | AUQ | Alcohol urges | 93 |
| Alcohol Dependence Scale | ADS | Severity of alcohol dependence | 94 |
| Questionnaire on Smoking Urges | QSU | Smoking urges | 95 |
| Craving Automated Scale for Cigarette Smoking | CAS-CS | Nicotine craving and automated smoking behaviour | 60 |
| Obsessive Compulsive Smoking Scale | OCSS | Thoughts about tobacco and smoking behaviour | 96 |
| Smoking Consequences Questionnaire for Adults | SCQ-A | Smoking outcome expectancies | 97 |
| Wisconsin Smoking Withdrawal Scale | WSWS | Nicotine withdrawal symptoms | 98 |
ADHD, Attention Deficit Hyperactivity Disorder.
Table 3.
Schedule of measurement during study participation
| Measurement time point | S | T1 | T2 | FU1 | FU2 | T3 | ||||||
| Baseline | T | A | T | A | T | A | T | A | T | A | T | A |
| Demographic information | x | x | x | x | ||||||||
| Current medication* | x | x | x | x | x | x | x | x | ||||
| Current somatic or mental conditions* | x | x | x | x | x | x | x | x | ||||
| Structured Clinical Interview (SCID-5-CV) | x | x | ||||||||||
| Smoking history | x | x | ||||||||||
| Current smoking behaviour* | x | x | x | x | x | |||||||
| Smoking Assessment Interview | x | x | x | |||||||||
| (Current) drinking behaviour* | x | x | x | x | x | x | x | |||||
| CIWA-Ar | x | x | ||||||||||
| Current drug use* | x | x | x | x | ||||||||
| Urine pregnancy and drugs screening | x | x | x | x | ||||||||
| Breath alcohol test | x | x | x | x | ||||||||
| Breath carbon monoxide test | x | x | ||||||||||
| Goal attainment scaling | x | x | x | x | ||||||||
| Neuropsychology | T | A | T | A | T | A | T | A | T | A | T | A |
| MWT-B | x | |||||||||||
| LNS-Task | x | x | ||||||||||
| D2-R | x | x | x | x | ||||||||
| IGT | x | x | x | x | ||||||||
| DCCS | x | x | x | x | ||||||||
| MRI | T | A | T | A | T | A | T | A | T | A | T | A |
| Field-Map | x | x | x | x | ||||||||
| Resting-State | x | x | x | x | ||||||||
| NICUETINE | x | x | x | x | ||||||||
| N-Back | x | x | x | x | ||||||||
| SST | x | x | x | x | ||||||||
| ALCUE | x | x | x | x | ||||||||
| MPRAGE | x | x | x | x | ||||||||
| General questionnaires | T | A | T | A | T | A | T | A | T | A | T | A |
| PANAS | x | x | x | x | x | x | x | x | x | x | ||
| HSWBS | x | x | x | x | ||||||||
| GSE | x | x | ||||||||||
| Rosenberg | x | x | ||||||||||
| SWLS | x | x | x | x | x | x | ||||||
| FSozU | x | x | ||||||||||
| Questionnaires—depression and anxiety | T | A | T | A | T | A | T | A | T | A | T | A |
| BDI II | x | x | x | x | x | x | ||||||
| PSS | x | x | x | x | x | x | ||||||
| STAI (X1) | x | x | x | x | x | x | ||||||
| STAI (X2) | x | x | ||||||||||
| Questionnaires—impulsivity and ADHD | T | A | T | A | T | A | T | A | T | A | T | A |
| ASRS-V.1.1 | x | x | x | x | ||||||||
| ADHS-SB | x | x | ||||||||||
| BIS-15 | x | x | x | x | x | x | ||||||
| COHS | x | x | ||||||||||
| Questionnaires—alcohol | T | A | T | A | T | A | T | A | T | A | T | A |
| ACQ-SF-R | x | x | x | |||||||||
| ADS | x | |||||||||||
| AUDIT | x | x | ||||||||||
| AUQ | x | x | x | x | ||||||||
| CAS-A | x | x | x | |||||||||
| OCDS-G | x | x | x | x | x | |||||||
| SRHI (alcohol) | x | x | x | |||||||||
| VACS for MRI (alcohol) | x | x | x | x | ||||||||
| Questionnaires—tobacco | T | A | T | A | T | A | T | A | T | A | T | A |
| OCSS | x | x | x | x | x | |||||||
| CAS-CS | x | x | x | x | ||||||||
| QSU | x | x | x | x | x | |||||||
| SCQ-A | x | x | x | |||||||||
| WSWS | x | x | x | |||||||||
| SRHI (tobacco) | x | x | x | |||||||||
| FTND | x | x | x | x | ||||||||
| VACS for MRI (tobacco) | x | x | ||||||||||
*Self-report.
A, alcohol use disorder; ACQ-SF-R, Alcohol Craving Questionnaire—short form revised; ADHS-SB, ADHD Self-rating Scale; ADS, Alcohol Dependence Scale; ALCUE, fMRI alcohol cue reactivity task; ASRS-V.1.1, Adult ADHD Self-Report Scale Symptom Checklist, Part A; AUDIT, Alcohol Use Disorder Identification Test; AUQ, Alcohol Urge Questionnaire; BDI II, Beck-Depression Inventory; BIS-15, Barrett Impulsiveness Scale; CAS-A, Craving Automated Scale for Alcohol; CAS-CS, Craving Automated Scale for Cigarette Smoking; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol; COHS, Creature of Habit Scale; DCCS, Dimensional Change Card Sort; D2-R, d2 R Test of Attention; FSozU, Perceived Social Support Questionnaire; FTND, Fagerström Test for Nicotine Dependence; FU, monthly follow-ups via telephone; GSE, General Self-Efficacy Scale; HSWBS, Habitual Subjective Well-Being Questionnaire; IGT, Iowa Gambling Task; LNS, letter–number sequencing (Wechsler Memory Scale-3); MPRAGE, Magnetization Prepared - RApid Gradient Echo sequence; MWT-B, Multiple-choice vocabulary test (German version); N-Back, N back fMRI task; NICUETINE, fMRI tobacco cue reactivity task; OCDS-G, Obsessive Compulsive Drinking Scale - German; OCSS, Obsessive Compulsive Smoking Scale; PANAS, Positive and Negative Affect Schedule; PSS, Perceived Stress Scale; QSU, Questionnaire on Smoking Urges; Rosenberg, Rosenberg self-esteem scale; S, screening measurement; SCID-5-CV, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-5—Clinician Version; SCQ-A, Smoking Consequences Questionnaire for Adults; SRHI, Self-Report Habit Index (tobacco); SRHI, Self-Report Habit Index (German translation, adapted for alcohol); SST, Stop-Signal-Reaction-Time Task for fMRI; SWLS, Satisfaction with Life Scale; T1, baseline and MRI assessment; T2, MRI assessment; T3, final follow-up via telephone; T, tobacco use disorder; VACS, Visual Analog Craving Scales before and after fMRI for tobacco; VACS, Visual Analog Craving Scales for alcohol before and after fMRI for alcohol; WSWS, Wisconsin Smoking Withdrawal Scale; STAI (X1, X2), State/Trait Anxiety Inventory.
Standard treatment
All study participants (TUD and AUD) will follow their respective treatment as usual (TAU). With regard to TUD, a qualified smoking cessation group therapy with one therapy session per week (90 min) will be held by a trained and certified psychologist. This intervention is strongly recommended in the latest version of the S3 guidelines for TUD.48 A superior effect on smoking cessation was observed following group therapy compared with, for example, self-help or less intense interventions.49 During the qualified smoking cessation group therapy, interventions following a cognitive-behavioural psychotherapy approach will be applied.50 Study participants with AUD will follow the respective in-house or day clinic therapeutic programme, as recommended by the respective S3 guidelines for AUD.51 This standard treatment includes medical and psychological interventions.
Chess-based cognitive remediation training
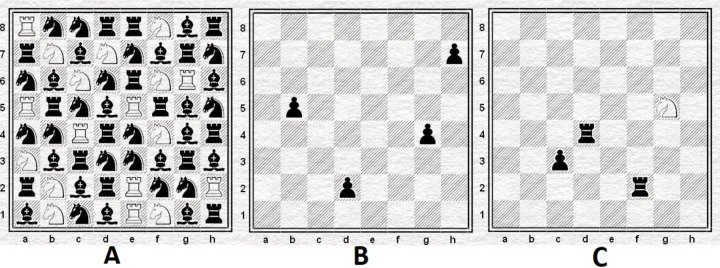
The planned CB-CRT ‘Entrenamiento Cognitivo a través del Ajedrez’ (cognitive training through chess, https://ajedrezmagic.es/el-entrenamiento-cognitivo-a-traves-del-ajedrez/) consists of a battery of tasks and was developed by one of the coauthors (JAM). The training battery, which is administered in a group setting using mainly a chess demonstration board, is designed to strengthen cognitive functioning in specific domains such as selective attention (figure 2A), short-term memory (figure 2B), focal attention, pattern recognition, visuospatial abilities, planification skills (figure 2C) and inhibition. Participants do not need to know the game of chess. They will receive general information about the rules and strategies used for the corresponding training day. Overall, metacognitive abilities are trained as well, for example, by giving psychoeducational information regarding different concepts of cognitive functioning, questioning, and identifying the underlying cognitive process, and enhancing the awareness of before mentioned aspects. Participants perform most of the specific tasks in front of the group and, for a social reinforcement effect, everyone will applaud the respective participant. Some of the tasks are conducted via paper–pencil. The training battery has been used for more than 10 years by JAM and his colleagues as an add-on therapy for elderly individuals, children with autism and/or ADHD, individuals with Down syndrome, mental and other disorders, and in adults with SUD. The scientific evaluation of the programme is one of the goals of the current study.
Figure 2.
Examples of the chess-based cognitive remediation training. (A) Selective attention. Participants are asked to count the number of white knights on white squares (right answer: 5, squares: b1, (b7, c6, d7, f1). During the training, participants receive six boards within a maximum of 3 min. (B) Short-term memory. Participants are focused on the board and see the position for a few seconds up to 1 min. Afterwards, the instructor asks the participants to reconstruct the position. Participants are asked to go to the front of the group and rebuild the position. (C) Executive functions, planification skills. Participants must find out the shortest route the knight can go to capture the pawn. The knight must not stop on any square controlled by the rooks. The participant is asked to announce the number of moves before showing them on the board (correct answer: 4 moves—g5-e6-c7-b5-c3 or g5-e6-d4-b5-c3).
In an unpublished pilot study in the rehabilitation clinic at Comunidad Terapéutica La Garrovilla, N=26 patients with SUD (N=22 male; substances: alcohol, opiates, cocaine, benzodiazepines, cannabis) were examined. CB-CRT was applied in a group setting two times per week for a duration of 90 min each. Cognitive functioning, especially in executive functions, was assessed at admission to the clinic at Comunidad Terapéutica La Garrovilla, Badajoz (Extremadura, Spain) and again after 14 weeks. The neuropsychological testing battery included measures of general processing speed (trail-making test A), cognitive flexibility (trail-making test B),52 planning abilities (Tower of London)53 and intelligence (Wechsler Adult Intelligence Scale (WAIS)). Significant increases in performance were found after 14 weeks of treatment in general processing speed (trail-making test A; p=0.001), cognitive flexibility (trail-making test B; p=0.013), the Tower of London test (p=0.001) as well as in the WAIS measures for verbal comprehension (‘similarities’, p=0.019) and for working memory (‘letter–number sequencing’, p=0.030, ‘digit span forward’, p=0.044, ‘digit span backward’, p=0.018, ‘digit span total’, p=0.007). Performance in the WAIS measures ‘coding’ (processing speed) and ‘matrix reasoning’ (perceptual reasoning) did not differ significantly. In another sample of N=15, patients receiving the chess-based add-on treatment for 3.5 months, subjective satisfaction was evaluated. On scales ranging from 1 (very unsatisfied/very poor) to 4 (very satisfied/very good), 73% of the patients rated the overall programme as very good (ie, score of 4). Overall, 67% of the patients found the programme very helpful in treating their SUD (score of 4), 27% found it helpful (score of 3). Further, when asked how the programme influenced other domains being negatively affected by SUD before admission, 53% found the programme very supportive (score of 4), 27% found it supportive (score of 3). Besides this, 87% reported that the programme helped them to increase their memory capabilities, 93% stated a subjective increase in attention performance and 93% reported an enhancement in decision-making.
Self-rating questionnaires
Self-rating questionnaires will be administered to address factors related to, for example, impulsiveness and inhibitory control, mood, psychosocial functioning, as well as substance consumption, or craving. Please see table 2 for a detailed list.
Neuropsychological assessments
Tasks investigating components of working memory (Wechsler Memory Scale-3),54 decision-making (Iowa Gambling Task),55 as well as mental flexibility (Dimensional Change Card Sort)56 and attentional capacity (d2-R Test of Attention)57 will be administered.
fMRI assessments
During the fMRI scanner examination, study participants will perform a stop-signal task,58 alcohol-based and tobacco-based cue reactivity tasks,59 60 an N-back task61 and a resting-state MRI. Scanning will be performed with a 3T whole-body tomograph (MAGNETOM Prisma; Siemens, Erlangen, Germany). T2* weighted multiband echoplanar images using a multiband acceleration factor 6 will be acquired in a transversal orientation 20° clockwise to AC‐PC (anterior commissure - posterior commissure) line covering the whole brain (Repetition Time (TR)=869 ms, Echo Time (TE)=38 ms, 60 slices, slice thickness=2.4 mm, voxel size 2.4×2.4×2.4 mm, no interslice gap, field of view (FoV)=210 mm, matrix size 88×88, acquisition orientation T>C, interleaved slice order, acceleration factor slice=6, flip angle=58°, bandwidth=1832 Hz/Px, prescan normalise, weak raw data filter, LeakBlock kernel, fat sat). This short TE and the 20° flip to AC‐PC orientation is chosen to minimise susceptibility artefacts. Scanner sequences are provided by the Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, Minnesota, USA (https://www.cmrr.umn.edu/multiband/).62 In addition, a T1-weighted 3D MPRAGE (Magnetization Prepared—RApid Gradient Echo) dataset consisting of 208 sagittal slices (slice thickness 1 mm, 1×1×1 mm voxel size, FOV 256×256 mm2, TR=2000 ms, TE=2.01 ms, Inversion Time (TI)=800 ms, flip angle=8°) will be acquired.
Endpoints are changes in neural alcohol and tobacco cue reactivity59 60 (eg, reduction in substance-related activation of striatal brain regions), neural correlates of inhibition (stop-signal task)63 (eg, increased dorsolateral prefrontal neural activation) and working memory (N-back task)61 (eg, increased inferior frontal neural activation), as well as functional connectivity within the salience network (SN; insula, anterior cingulate cortex) and executive control network (ECN; dorsolateral frontal and lateral posterior parietal cortices) using resting-state fMRI data. Also, working memory capacity (letter–number sequencing task of the Wechsler Memory Scale-3),54 impulsivity (Barratt Impulsiveness Scale-15),64 65 mental flexibility (Dimensional Change Card Sort),56 decision-making (Iowa Gambling Task)55 66 and attentional capacity (d2-R Test of Attention),67 summarised as cognitive functioning, are endpoints of interest. Additionally, the duration until the first severe relapse (daily smoking of at least one cigarette at day, consumption of more than 48 g (females) or 60 g (males) of alcohol) during the follow-up periods and amount of substance consumption in case of a relapse as well as improvements in psychosocial functioning will be examined.
Sample size calculation
Using the software package G*Power,68 the sample size calculation was conducted for the main primary outcomes, that is, neurobiological correlates underlying adaptations following the CB-CRT, where we expected a minimum effect size of f=0.2 for all constructs (analyses of variance (ANOVA) with repeated measures, within-subject and between-subject factors and interactions). In this case, ideal sample coverage would be 24 individuals per group (at 80% power, alpha level 5%).
Data analysis plan
To analyse psychometric and neuropsychological data, SPSS (Statistics for Windows, V.27.0) will be used. The various dependent variables will be evaluated using multivariate analyses of variance with repeated measures. To counteract possible group differences at baseline, a percentage in change (divide by T1 values) or variable values at T1 can be incorporated in subsequent statistical analyses as a covariate. In addition, linear regression models will be calculated to examine the influence of confounding variables (eg, severity of tobacco or alcohol dependence) on the observed change in dependent variables as described previously (eg, craving, task performance, psychosocial well-being). Cox-regression analyses, including, for example, brain activation in the dorsolateral prefrontal or inferior frontal regions during inhibition and executive functioning, or the ventral striatum during cue reactivity tasks as predictors, will be conducted to examine the association with relapse. To analyse the fMRI data, SPM V.12 (Wellcome Department of Cognitive Neurology, London, UK) running under Matlab will be used. The preprocessing pipeline will include motion correction, normalisation to the Montreal Neurological Institute template, and a spatial smoothing with Gaussian kernel of 8 mm full width at half maximum will be conducted. The preprocessed data will then be used for first-level and second-level analyses. On the first level (within-subject), neural activation associated with task conditions (contrasts) will be modelled via a convolution with a canonical haemodynamic response function following a general linear model. A high-pass filter to remove low-frequency components of fMRI time series will be used. Depending on the fMRI tasks, specific contrasts regarding task conditions will be modelled as described in the above cited literature. On the second level (between-subject) and regarding the effects of group and time, paired t-tests (eg, pre vs post intervention within one group) and full factorial models will be used. Additionally, regression models including clinical variables, such as severity of TUD or AUD, will be calculated. To control for multiple statistical testing, we will use established correction procedures, for example, whole-brain family-wise error correction for fMRI analyses or Bonferroni correction for other statistical analyses.
Hypotheses
Primary hypotheses
CB-CRT improves aberrant neural alcohol cue reactivity (measured by alcohol and tobacco cue reactivity fMRI tasks) in AUD/TUD in comparison to standard treatment alone
CB-CRT improves neuronal aberrations present when executing cognitive tasks (measured by N-back and stop-signal fMRI task) in individuals with AUD/TUD in comparison to standard treatment alone.
CB-CRT decreases functional connectivity within the SN in individuals with AUD/TUD in comparison to standard treatment alone.
CB-CRT decreases functional connectivity within the ECN in individuals with AUD/TUD in comparison to standard treatment alone.
CB-CRT improves cognitive functioning (measured by neuropsychological tasks) in AUD/TUD individuals in comparison to standard treatment alone.
CB-CRT improves psychosocial functioning (measured by, eg, Habitual Subjective Well-Being Questionnaire, Satisfaction with Life Scale) in AUD/TUD individuals in comparison to standard treatment alone. CB-CRT influences the treatment process, for example, time to first severe relapse, for AUD/TUD individuals positively in comparison to standard treatment alone.
Secondary hypotheses
CB-CRT might be more efficacious in individuals with impaired cognitive functioning, low self-esteem, self-efficacy and social support.
Chess as a 3-week add-on therapy influences the treatment process, for example, time to first severe relapse for AUD/TUD individuals moderated and mediated by cognitive, affective and psychosocial factors.
Discussion
The here presented study aims to examine the effect of CB-CRT as treatment add-on on neurobiological processes but also neuropsychological and psychosocial functioning known to contribute to the development and maintenance of AUD and TUD. The effect of CB-CRT might also result in longer times of abstinence or reduced substance consumption. If CB-CRT as therapy add-on, as examined in this comprehensive study, shows to be more effective than standard treatment alone, this intervention might help to improve health behaviour in affected individuals.
Limitations with respect to the interpretability of the data might derive from the study design. We aim to examine the superior effect of CB-CRT compared with TAU in therapy outcomes that might rely on neurobiological alterations following this training. As postulated by Sala and Gobet,69 a third, active control group might be needed to ultimately evaluate the chess-specific mechanisms and outcomes. Therefore and in case of successfully demonstrating a superior effect of our CB-CRT, a subsequent study might be needed to address this question. Further, even in light of our future results confirming a superior effect of CB-CRT as therapy add-on on neurobiological and neuropsychological processes, these improvements might to translate to longer abstinence or a reduction in the amount of substance consumption. Previously, this has been demonstrated in AUD: even though an improvement in working memory functioning has been observed following an active working memory training in patients with AUD, heavy drinking and neuropsychological functioning in other domains remained unchanged.39
Since the described study includes a cognitive remediation training that exceeds merely training individual domains, we hope to counteract limitations of previous studies. Including social (training in the group) and metacognitive aspects, the CB-CRT might generalise from altering neurobiological processing to behavioural changes, that is, substance consumption.
Ethics and dissemination
The study was approved by the local ethics committee of the medical faculty Mannheim at the University of Heidelberg, Germany (reference number 2017-647N-MA). Before study inclusion and after a detailed explanation of all procedures, all participants will provide written informed consent. The study was registered in the Clinical Trials Register (trial identifier: NCT04057534) on 8 December 2019. The study results will be disseminated by peer-review publications and conference presentations. Open-access publication is planned for all peer-reviewed publications. All participants are offered to receive a print of the final, published version of peer-reviewed publications. For protection of personal rights, and due to the sensitivity of the clinical and neuroimaging data, data will not be made publicly available. On direct request by other researchers and in mutual agreements (eg, regarding data protection), anonymised data can be made available. On request, analysis procedures and codes will be shared with other researchers.
Risks associated with participation
Participants will be asked several questions regarding their substance consumption, mood, quality of life. They will additionally perform neuropsychological and fMRI tasks. Both excerpts a strain on the participants in terms of time and effort. Further, it may cause emotional discomfort in some participants. To counteract these possible negative consequences of study participations, the research team, also consisting of psychologists and psychotherapists in training, will regularly check if participants and evaluate their (dis)comfort. Contact to qualified clinicians will be made possible in case of severe emotional discomfort. Due to the length of the study appointments, we will offer participants the option to flexibly answer most of the questionnaires at home.
Supplementary Material
Acknowledgments
We would like to thank Carmen Gaitan Coronado for their training in Entrenamiento Cognitivo a través del Ajedrez and their help in creating the therapy manual and Alycia Lee for English editing. We gratefully acknowledge the support of our colleagues, formerly affected by alcohol use disorder or tobacco use disorder, and our study participants regarding the planning, implementation and conduct of the study.
Footnotes
Contributors: SV-K designed the study. TW, GL and JH helped with designing the study. JAM developed the chess-based remediation training Entrenamiento Cognitivo a través del Ajedrez. GL, JH, RS, AJR, DK, AW, SV-K and SG adapted the training. SG, GL and SV-K wrote the manuscript. All authors read and approved the manuscript.
Funding: This study is supported by a grant from the Deutsche Forschungsgemeinschaft (Grant ID 421888313).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analyses section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Peacock A, Leung J, Larney S, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018;113:1905–26. 10.1111/add.14234 [DOI] [PubMed] [Google Scholar]
- 2.Atzendorf J, Rauschert C, Seitz NN, et al. The use of alcohol, tobacco, illegal drugs and medicines. Dtsch Arztebl Int 2019;116:577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konnopka A, König H-H. Direct and indirect costs attributable to alcohol consumption in Germany. Pharmacoeconomics 2007;25:605–18. 10.2165/00019053-200725070-00006 [DOI] [PubMed] [Google Scholar]
- 4.Neubauer S, Welte R, Beiche A, et al. Mortality, morbidity and costs attributable to smoking in Germany: update and a 10-year comparison. Tob Control 2006;15:464–71. 10.1136/tc.2006.016030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newcomb PA, Carbone PP. The health consequences of smoking. cancer. Med Clin North Am 1992;76:305–31. 10.1016/s0025-7125(16)30355-8 [DOI] [PubMed] [Google Scholar]
- 6.Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 2006;101:212–22. 10.1111/j.1360-0443.2006.01310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol 2001;62:211–20. 10.15288/jsa.2001.62.211 [DOI] [PubMed] [Google Scholar]
- 8.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the combine study: a randomized controlled trial. JAMA 2006;295:2003–17. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 9.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav 2008;33:1516–20. 10.1016/j.addbeh.2008.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von der Goltz C, Kiefer F. Learning and memory in the aetiopathogenesis of addiction: future implications for therapy? Eur Arch Psychiatry Clin Neurosci 2009;259 Suppl 2:S183–7. 10.1007/s00406-009-0057-6 [DOI] [PubMed] [Google Scholar]
- 11.West R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol Health 2017;32:1018–36. 10.1080/08870446.2017.1325890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 2011;13:398–405. 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nebe S, Kroemer NB, Schad DJ, et al. No association of goal-directed and habitual control with alcohol consumption in young adults. Addict Biol 2018;23:379–93. 10.1111/adb.12490 [DOI] [PubMed] [Google Scholar]
- 14.Hogarth L, Lam-Cassettari C, Pacitti H, et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci 2019;50:2513–25. 10.1111/ejn.13961 [DOI] [PubMed] [Google Scholar]
- 15.Hogarth L. Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology 2020;45:720–35. 10.1038/s41386-020-0600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev 2007;17:337–45. 10.1007/s11065-007-9034-x [DOI] [PubMed] [Google Scholar]
- 17.Sjoerds Z, de Wit S, van den Brink W, et al. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry 2013;3:e337–e. 10.1038/tp.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ersche KD, Gillan CM, Jones PS, et al. Carrots and sticks fail to change behavior in cocaine addiction. Science 2016;352:1468–71. 10.1126/science.aaf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebold M, Deserno L, Nebe S, et al. Model-Based and model-free decisions in alcohol dependence. Neuropsychobiology 2014;70:122–31. 10.1159/000362840 [DOI] [PubMed] [Google Scholar]
- 20.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 2005;8:1458–63. 10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- 21.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 2004;99:461–71. 10.1111/j.1360-0443.2003.00669.x [DOI] [PubMed] [Google Scholar]
- 22.Yücel M, Lubman DI, Solowij N, et al. Understanding drug addiction: a neuropsychological perspective. Aust N Z J Psychiatry 2007;41:957–68. 10.1080/00048670701689444 [DOI] [PubMed] [Google Scholar]
- 23.Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychol Sci 2011;22:498–506. 10.1177/0956797611400918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollstädt-Klein S, Hermann D, Rabinstein J, et al. Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res 2010;34:771–6. 10.1111/j.1530-0277.2010.01149.x [DOI] [PubMed] [Google Scholar]
- 25.Hamonniere T, Varescon I. Metacognitive beliefs in addictive behaviours: a systematic review. Addict Behav 2018;85:51–63. 10.1016/j.addbeh.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 26.Pintrich PR. The role of metacognitive knowledge in learning, teaching, and assessing. Theory Into Practice 2002;41:219–25. [Google Scholar]
- 27.Fleur DS, Bredeweg B, van den Bos W. Metacognition: ideas and insights from neuro- and educational sciences. NPJ Sci Learn 2021;6:13. 10.1038/s41539-021-00089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella M, Reeder C, Wykes T. Group cognitive remediation for schizophrenia: exploring the role of therapist support and metacognition. Psychol Psychother 2016;89:1-14. 10.1111/papt.12062 [DOI] [PubMed] [Google Scholar]
- 29.Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy--what works and could we do better? Schizophr Bull 2011;37 Suppl 2:S80–90. 10.1093/schbul/sbr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiluk BD, Nich C, Babuscio T, et al. Quality versus quantity: acquisition of coping skills following computerized cognitive-behavioral therapy for substance use disorders. Addiction 2010;105:2120–7. 10.1111/j.1360-0443.2010.03076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danner UN, Dingemans AE, Steinglass J. Cognitive remediation therapy for eating disorders. Curr Opin Psychiatry 2015;28:468–72. 10.1097/YCO.0000000000000192 [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Reeder C, Wykes T. Lessons learnt? the importance of metacognition and its implications for cognitive remediation in schizophrenia. Front Psychol 2015;6:1259. 10.3389/fpsyg.2015.01259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella M, Edwards C, Swan S, et al. Exploring the effects of cognitive remediation on metacognition in people with schizophrenia. Journal of Experimental Psychopathology 2019;10:2043808719826846. [Google Scholar]
- 34.Montemagni C, Del Favero E, Riccardi C, et al. Effects of cognitive remediation on cognition, Metacognition, and social cognition in patients with schizophrenia. Front Psychiatry 2021;12:649737. 10.3389/fpsyt.2021.649737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates ME, Buckman JF, Nguyen TT. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev 2013;23:27–47. 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caetano T, Pinho MS, Ramadas E, et al. Cognitive training effectiveness on memory, executive functioning, and processing speed in individuals with substance use disorders: a systematic review. Front Psychol 2021;12:730165. 10.3389/fpsyg.2021.730165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sofuoglu M, DeVito EE, Waters AJ, et al. Cognitive function as a Transdiagnostic treatment target in stimulant use disorders. J Dual Diagn 2016;12:90–106. 10.1080/15504263.2016.1146383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nixon SJ, Lewis B. Cognitive training as a component of treatment of alcohol use disorder: a review. Neuropsychology 2019;33:822–41. 10.1037/neu0000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khemiri L, Brynte C, Stunkel A, et al. Working memory training in alcohol use disorder: a randomized controlled trial. Alcohol Clin Exp Res 2019;43:135–46. 10.1111/acer.13910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol-dependent subjects. Front Psychiatry 2014;5:78. 10.3389/fpsyt.2014.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spada MM, Caselli G, Wells A. A triphasic metacognitive formulation of problem drinking. Clin Psychol Psychother 2013;20:494–500. 10.1002/cpp.1791 [DOI] [PubMed] [Google Scholar]
- 42.Blasco-Fontecilla H, Gonzalez-Perez M, Garcia-Lopez R, et al. Efficacy of chess training for the treatment of ADHD: a prospective, open label study. Rev Psiquiatr Salud Ment 2016;9:13–21. 10.1016/j.rpsm.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Nour ElDaou BM, El-Shamieh SI. The effect of playing chess on the concentration of ADHD students in the 2nd cycle. Procedia - Social and Behavioral Sciences 2015;192:638–43. [Google Scholar]
- 44.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12:426–45. 10.1037//0894-4105.12.3.426 [DOI] [PubMed] [Google Scholar]
- 45.Demily C, Cavézian C, Desmurget M, et al. The game of chess enhances cognitive abilities in schizophrenia. Schizophr Res 2009;107:112–3. 10.1016/j.schres.2008.09.024 [DOI] [PubMed] [Google Scholar]
- 46.Sala G, Gobet F. Does chess instruction improve mathematical problem-solving ability? two experimental studies with an active control group. Learn Behav 2017;45:414–21. 10.3758/s13420-017-0280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.First MB. Structured Clinical Interview for theDSM(SCID). In: Cautin RL, Lilienfeld SO, eds. The encyclopedia of clinical psychology, 2015: 1–6. [Google Scholar]
- 48.Batra A, Kiefer F, Andreas S. S3-Leitlinie "Rauchen und Tabakabhängigkeit: Screening, Diagnostik und Behandlung". Sucht 2021. [Google Scholar]
- 49.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2017;3:CD001007. 10.1002/14651858.CD001007.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batra ABuchkremer G, ed. Tabakentwöhnung. 70565 Stuttgart: W. Kohlhammer Verlag, 2012. [Google Scholar]
- 51.Kiefer F, Batra A, Bischof G. S3-Leitlinie "Screening, Diagnose und Behandlung alkoholbezogener Störungen". Sucht 2021. [Google Scholar]
- 52.Tombaugh TN. Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–14. 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 53.Keith Berg W, Byrd D. The Tower of London spatial problem-solving task: enhancing clinical and research implementation. J Clin Exp Neuropsychol 2002;24:586–604. 10.1076/jcen.24.5.586.1006 [DOI] [PubMed] [Google Scholar]
- 54.Kent P. The evolution of the Wechsler memory scale: a selective review. Appl Neuropsychol Adult 2013;20:277–91. 10.1080/09084282.2012.689267 [DOI] [PubMed] [Google Scholar]
- 55.Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994;50:7–15. 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- 56.Zelazo PD, Anderson JE, Richler J, et al. Nih Toolbox cognition battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc 2014;20:620–9. 10.1017/S1355617714000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brickenkamp R. Aufmerksamkeits-Belastungs-Test. 9., berarbeitete und neu normierte Auflage [d2 Test of attention, 9th revised edn.]. Hogrefe V, editor. G”ttingen, 2002. [Google Scholar]
- 58.Whelan R, Conrod PJ, Poline JB. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci 2012;15:920–5. [DOI] [PubMed] [Google Scholar]
- 59.Vollstädt-Klein S, Loeber S, Kirsch M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry 2011;69:1060–6. 10.1016/j.biopsych.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 60.Vollstädt-Klein S, Kobiella A, Bühler M, et al. Severity of dependence modulates smokers' neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol 2011;16:166–75. 10.1111/j.1369-1600.2010.00207.x [DOI] [PubMed] [Google Scholar]
- 61.Charlet K, Beck A, Jorde A, et al. Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addict Biol 2014;19:402–14. 10.1111/adb.12103 [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Moeller S, Auerbach EJ, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage 2013;83:991–1001. 10.1016/j.neuroimage.2013.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whelan R, Conrod PJ, Poline J-B, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci 2012;15:920–5. 10.1038/nn.3092 [DOI] [PubMed] [Google Scholar]
- 64.Meule A, Vögele C, Kübler A. Psychometrische evaluation Der deutschen Barratt Impulsiveness scale – Kurzversion (BIS-15). Diagnostica 2011;57:126–33. [Google Scholar]
- 65.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51:768–74. [DOI] [PubMed] [Google Scholar]
- 66.Brevers D, Bechara A, Cleeremans A, et al. Iowa Gambling Task (IGT): twenty years after - gambling disorder and IGT. Front Psychol 2013;4:665. 10.3389/fpsyg.2013.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bates ME, Lemay EP. The D2 test of attention: construct validity and extensions in scoring techniques. J Int Neuropsychol Soc 2004;10:392–400. 10.1017/S135561770410307X [DOI] [PubMed] [Google Scholar]
- 68.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 69.Sala G, Gobet F. Do the benefits of chess instruction transfer to academic and cognitive skills? A meta-analysis. Educational Research Review 2016;18:46–57. [Google Scholar]
- 70.Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical Institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84:1353–7. 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- 71.Kiresuk TJ, Lund SH, Larsen NE. Measurement of goal attainment in clinical and health care programs. Drug Intell Clin Pharm 1982;16:145–53. 10.1177/106002808201600210 [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg MJ. Society and the adolescent self-image. Princeton University Press: Princeton, 1965. [Google Scholar]
- 73.Schwarzer RJ M. (Hrsg.). Skalen Zur Erfassung von Lehrer- und Schülermerkmalen. Dokumentation Der psychometrischen Verfahren Im Rahmen Der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen. Berlin: Freie Universität, 1999. [Google Scholar]
- 74.Kliem S, Mößle T, Rehbein F, et al. A brief form of the perceived social support questionnaire (F-SozU) was developed, validated, and standardized. J Clin Epidemiol 2015;68:551–62. 10.1016/j.jclinepi.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 75.Pouwer F, Snoek FJ, van der Ploeg HM, et al. The well-being questionnaire: evidence for a three-factor structure with 12 items (W-BQ12). Psychol Med 2000;30:455–62. 10.1017/s0033291700001719 [DOI] [PubMed] [Google Scholar]
- 76.Diener E, Emmons RA, Larsen RJ, et al. The satisfaction with life scale. J Pers Assess 1985;49:71–5. 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 77.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063–70. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 78.Laux L, Glanzmann P, Schaffner P, et al. STAI. State-Trait-Angstinventar Göttingen: Beltz Test GmbH, 1981. [Google Scholar]
- 79.Hautzinger M, Bailer M, Worrall H, et al. Das Beck-Depressions-Inventar (BDI). Überarbeitet und ergänzte Neuauflage. Bern: Hans Huber, 1995. [Google Scholar]
- 80.Kühner C, Bürger C, Keller F, et al. [Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples]. Nervenarzt 2007;78:651–6. 10.1007/s00115-006-2098-7 [DOI] [PubMed] [Google Scholar]
- 81.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 82.Reyes MM, Schneekloth TD, Hitschfeld MJ, et al. The clinical utility of ASRS-v1.1 for identifying ADHD in alcoholics using prism as the reference standard. J Atten Disord 2019;23:1119–25. 10.1177/1087054716646450 [DOI] [PubMed] [Google Scholar]
- 83.Rösler M, Retz W, Retz-Junginger P, et al. [Tools for the diagnosis of attention-deficit/hyperactivity disorder in adults. Self-rating behaviour questionnaire and diagnostic checklist]. Nervenarzt 2004;75:888–95. 10.1007/s00115-003-1622-2 [DOI] [PubMed] [Google Scholar]
- 84.Ersche KD, Lim T-V, Ward LHE, et al. Creature of habit: a self-report measure of habitual routines and automatic tendencies in everyday life. Pers Individ Dif 2017;116:73–85. 10.1016/j.paid.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verplanken B, Orbell S. Reflections on past behavior: a Self‐Report index of habit strength. Journal of Applied Social Psychology 2003;33:1313–30. [Google Scholar]
- 86.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict 1991;86:1119–27. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 87.Babor TF, Higgins-Biddle JC, Saunders JB, et al. Geneva: World Health organization, 2001. Available: http://www.who.int/iris/handle/10665/67205
- 88.Scheurich A, Müller MJ, Anghelescu I, et al. Reliability and validity of the form 90 interview. Eur Addict Res 2005;11:50–6. 10.1159/000081417 [DOI] [PubMed] [Google Scholar]
- 89.Sayette MA, Shiffman S, Tiffany ST, et al. The measurement of drug craving. Addiction 2000;95 Suppl 2:S189–210. 10.1080/09652140050111762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakovics H, Diehl A, Croissant B, et al. Modifications of the obsessive compulsive drinking scale (OCDS-G) for use in longitudinal studies. Addict Behav 2008;33:1276–81. 10.1016/j.addbeh.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 91.Singleton T H. Development and validation of a new questionnaire to assess craving for alcohol. Problems of Drug Dependence, 1994 1995;II:1. [Google Scholar]
- 92.Vollstädt-Klein S, Leménager T, Jorde A, et al. Development and validation of the craving automated scale for alcohol. Alcohol Clin Exp Res 2015;39:333–42. 10.1111/acer.12636 [DOI] [PubMed] [Google Scholar]
- 93.Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 1995;19:600–6. 10.1111/j.1530-0277.1995.tb01554.x [DOI] [PubMed] [Google Scholar]
- 94.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 1982;91:199–209. 10.1037//0021-843x.91.3.199 [DOI] [PubMed] [Google Scholar]
- 95.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict 1991;86:1467–76. 10.1111/j.1360-0443.1991.tb01732.x [DOI] [PubMed] [Google Scholar]
- 96.Hitsman B, Shen B-J, Cohen RA, et al. Measuring smoking-related preoccupation and compulsive drive: evaluation of the obsessive compulsive smoking scale. Psychopharmacology 2010;211:377–87. 10.1007/s00213-010-1910-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rash CJ, Copeland AL. The brief smoking consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res 2008;10:1633–43. 10.1080/14622200802409990 [DOI] [PubMed] [Google Scholar]
- 98.Castro Y, Kendzor DE, Businelle MS, et al. Structural and predictive equivalency of the Wisconsin smoking withdrawal scale across three racial/ethnic groups. Nicotine Tob Res 2011;13:548–55. 10.1093/ntr/ntr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.