Abstract
Introduction
Sleep-wake and circadian disturbance is a key feature of mood disorders with a potential causal role and particular relevance to young people. Brexpiprazole is a second-generation antipsychotic medication with demonstrated efficacy as an adjunct to antidepressant treatment for major depressive disorder (MDD) in adults, with preliminary evidence suggesting greater effectiveness in subgroups of depressed patients with sleep disturbances. This clinical trial aims to evaluate the relationships between changes in sleep-wake and circadian parameters and changes in depressive symptoms following adjunctive brexpiprazole treatment in young adults with MDD and sleep-wake disturbance.
Methods and analysis
This study is designed as a 16 week (8 weeks active treatment, 8 weeks follow-up) mechanistic, open-label, single-arm, phase IV clinical trial and aims to recruit 50 young people aged 18–30 with MDD and sleep-wake cycle disturbance through an early intervention youth mental health clinic in Sydney, Australia. At baseline, participants will undergo multidimensional outcome assessment and subsequently receive 8 weeks of open-label treatment with brexpiprazole as adjunctive to their stable psychotropic medication. Following 4 weeks of treatment, clinical and self-report measures will be repeated. Ambulatory sleep-wake monitoring will be conducted continuously for the duration of treatment. After 8 weeks of treatment, all multidimensional outcome assessments will be repeated. Follow-up visits will be conducted 4 and 8 weeks after trial completion (including sleep-wake, clinical and self-report assessments). Circadian rhythm biomarkers including salivary melatonin, cortisol and core body temperature will be collected during an in-lab assessment. Additionally, metabolic, inflammatory and genetic risk markers will be collected at baseline and after 8 weeks of treatment.
Ethics and dissemination
This trial protocol has been approved by the Human Research Ethics Committee of the Sydney Local Health District (X19-0417 and 2019/ETH12986, Protocol Version 1–3, dated 25 February 2021). The results of this study, in deidentified form, will be disseminated through publication in peer-reviewed journals, scholarly book chapters, presentation at conferences and publication in conference proceedings.
Trial registration number
ACTRN12619001456145.
Keywords: mental health, psychiatry, depression & mood disorders, neurobiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Use of a comprehensive battery that includes ecologically valid and laboratory-based circadian assessments (alongside genetic, metabolic and inflammatory markers) may provide greater insights into the antidepressant mechanisms of adjunctive brexpiprazole.
Participants will receive a psychoeducational session about sleep and circadian rhythms, including information on how to improve their sleep-wake cycle based on their individual actigraphy data.
This trial focuses on ascertainment of the current 24-hour sleep-wake cycle and will not examine possible factors that may contribute to sleep/circadian disruption in the long term (eg, stressors) or the physiologic properties of sleep.
Some extraneous factors that influence the sleep-wake cycle and/or circadian rhythms (eg, ambient temperature, natural light exposure) are not measured in this study.
Introduction
In young adults, major depressive disorder (MDD) is highly prevalent, recurrent and comorbid with other mental and physical conditions, generating a substantial burden of disease and disability.1 2 While multiple psychological and pharmacological treatments are commonly provided, a large proportion of patients fail to respond to first-line psychotherapy or antidepressant treatments,3–10 and augmentation with a second-generation antipsychotic is often recommended in these treatment-resistant cases.11 12 Sleep-wake cycle disturbances are common features of depressive disorders, including insomnia,13–15 hypersomnia,16 17 abnormal sleep duration13 14 and abnormal timing of 24-hour patterns of rest/activity.18 19 Moreover, abnormalities in biological circadian rhythms (eg, melatonin) have been reported,20 21 suggesting that in some cases sleep disturbances are accompanied or underpinned by disturbances of the underlying circadian system.22 23
The human circadian system is controlled by a master oscillator in the brain’s hypothalamus (suprachiasmatic nucleus) which projects to circuits that govern biobehavioural processes often altered in depression (eg, mood, vigilance and 24-hour sleep-wake cycle). The circadian system is primarily entrained by bright light, and its functioning can be disrupted by factors including aberrant light exposure and irregular sleep-wake behaviours.24 25 Adolescents and young adults are particularly vulnerable to circadian perturbations due to significant developmental changes in circadian rhythms across this age period,26 and sleep-wake phase delays are common in young people with depressive disorders.27 Recently, we reported delayed and disrupted circadian rhythms in a subgroup of young people with depressive disorders (who also presented with greater symptom severity).28 During adolescence there is a phase shift in the circadian rhythm of the sleep-wake cycle, such that adolescents typically develop a biobehavioural preference for going to sleep later and waking later (a manifestation of changes in the biology of the circadian system).29 30 Furthermore, there is some evidence that correction of circadian abnormalities is associated with antidepressant effects of treatments targeting the circadian system such as agomelatine31 and light therapy.32
Brexpiprazole is a second-generation antipsychotic with demonstrated efficacy by multiple randomised controlled trials as an adjunct to antidepressant treatment in MDD in adults33–36; however, the mechanism of antidepressant action is unknown.37 The pharmacodynamic properties of brexpiprazole, together with evidence from preclinical studies, suggest that there may be specific effects on anxiety, cognition and sleep.37 38 Further, there is preliminary evidence to suggest that brexpiprazole may have greater effectiveness in subgroups of depressed patients with sleep disturbances, anxiety or irritability.22 39 As an adjunctive treatment in patients with MDD with inadequate response to antidepressant treatment, brexpiprazole has been reported to lead to clinical improvement of sleep disturbances (eg, insomnia) and depressive symptoms, as well as improvement of daytime alertness and functioning.40 This pattern of changes is consistent with effects on circadian rhythms, with potential influences on the entire 24-hour pattern of rest/activity, rather than simply on sleep period (in isolation). To improve the personalisation of treatment selection for mood disorders, a greater understanding of the mechanisms of antidepressant action of specific compounds is needed.
The goal of this clinical trial is to investigate whether the effect of brexpiprazole on depressive symptoms is associated with changes in 24-hour sleep-wake or circadian parameters in young people with MDD. While disturbances in electrophysiological measures of sleep (eg, REM sleep) have been considered to represent biomarkers for depression,41–44 this trial focuses instead on the investigation and measurement of biobehavioural changes associated with the circadian system (eg, rest/activity, melatonin, cortisol and core body temperature rhythms), rather than changes in electrophysiological sleep architecture (beyond the scope of this study).
Methods and analysis
Study objectives
The primary objective of this study is to determine if changes in depressive symptoms following adjunctive brexpiprazole treatment are correlated with changes in sleep-wake or circadian parameters in youth with depressive disorders.
The secondary objective is to determine if changes in social and occupational functioning following adjunctive brexpiprazole are correlated with changes in sleep-wake or circadian parameters in youth with depressive disorders.
The tertiary objectives of this study are to determine if changes in depressive symptoms or changes in sleep-wake or circadian parameters following adjunctive brexpiprazole treatment are associated with a range of multidimensional outcome measures in youth with depressive disorders45 (eg, other mental illness symptoms, self-harm and suicidal thoughts and behaviours, physical health, alcohol/substance use and genetic markers).
Trial design
This investigator-initiated, mechanistic study involving 50 young people with depressive disorders and sleep-wake cycle disturbances is designed as a 16-week (8 weeks active treatment, 8 weeks follow-up) open-label, single-arm, phase IV clinical trial.
Participants
Participants aged 18–30 years with a diagnosis of MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria on a current antidepressant treatment of either selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) with a disrupted sleep-wake cycle will be recruited through the youth mental health clinics associated with the Brain and Mind Centre (BMC), University of Sydney. All participants will provide written informed consent. The research team will make explicit to any potential participants both verbally and in writing (in the Participant Information Statement) that participation is voluntary and will not affect the patient’s care.
The inclusion criteria for this trial are as follows: (1) aged 18–30, (2) diagnosis of MDD as per DSM-5 (Structured Clinical Interview for DSM; SCID46) criteria, (3) current major depressive episode of moderate severity as defined by a Quick Inventory of Depressive Symptomatology47 rating ≥11 at two assessments 2 weeks apart, (4) failure to respond to at least one adequate (minimum 4 weeks) trial of pharmacological treatment, (5) current antidepressant treatment with an SSRI or SNRI (including citalopram, fluoxetine, paroxetine, sertraline, escitalopram, venlafaxine, desvenlafaxine or duloxetine) for at least 6 weeks, at a stable dose for 2 weeks prior to study commencement and (6) a perturbed sleep-wake cycle as evidenced by: delayed sleep onset; delayed sleep offset; disrupted sleep; high day-to-day variability of sleep-wake cycle; non-restorative sleep or daytime fatigue.
Exclusion criteria are as follows: (1) any adjunctive antipsychotic treatment for the current episode in the past month, (2) use of medications which affect sleep, melatonin, circadian rhythms or alertness (eg, agomelatine, modafinil), (3) primary psychotic disorder, (4) acute suicidal behaviour (score of 6 on Comprehensive Assessment of At-Risk Mental States (CAARMS) item 7.3,48 (5) evidence of a medical condition (primary, respiratory, neurological) that could contribute to sleep-wake dysfunction, (6) significant alcohol or substance misuse or dependence (assessed via DSM-5 SCID46 and WHO Alcohol, Smoking and Substance Involvement Screening Test),49 50 (7) shift work or (8) recent transmeridian travel (ie, participants will be required to wait 3 days for each jet lag hour before entering the study), (9) previous hypersensitivity to brexpiprazole, (10) taking CYP2D6 or CYP3A4 inhibitors (or other contraindicated medications listed in the Rexulti product information) and (11) pregnancy or lactation.
Study course and procedure
Patients presenting for mental healthcare who may be eligible for the study will be screened by phone before being invited to participate and attend an enrolment visit. The enrolment visit will formally assess eligibility criteria and confirm the presence of MDD as per DSM-5 (SCID).46 Participants will be provided with an actigraphy device (non-invasive wrist-worn device used to objectively measure rest/activity patterns) and will be given instructions to wear the device for the following 2 week period.
Visit 1 (Baseline): Within 2 weeks of completing the diagnostic and screening assessments, data from the actigraphy device will be downloaded and reviewed. A further assessment of depressive symptom severity (QIDS Clinician-Rated; QIDS-CR)47 will be conducted to ensure participants meet all inclusion criteria. Bloods will be collected for assessment of metabolic and inflammatory measures and genomic analysis. Clinical and self-report assessments will be conducted, as well as circadian assessments in which participants will remain in the sleep lab overnight. The following morning, participants will attend a 1-hour psychoeducation session about sleep and circadian rhythms covering the following topics: (1) sleep and circadian education with tailored discussion based on their personal actigraphy data; (2) individualised plan for progressive sleep rescheduling; and (3) lifestyle factors and behaviours impacting on sleep (eg, exercise, light, sleep environment, sleep regulation, foods, stress, anxiety and mood).
Once all baseline clinical and self-report assessments have been conducted, and the medical assessment completed by the study doctor to confirm inclusion and exclusion criteria, participants will be issued with the study medication to receive 8 weeks of open-label pharmacotherapy with brexpiprazole (REXULTI-Lundbeck) as adjunctive to their stable psychotropic medication (treatment as usual). Brexpiprazole will be provided to participants at visit 1 (baseline) and visit 2 (week 4) for the following 4 weeks and will be titrated from 1 mg once daily in week 1, to 2 mg once daily in weeks 2–8.
Patients will receive 2 mg/day, once daily as tablets, for oral use. The brexpiprazole dosage will be steadily increased from 1 mg/day during week 1, to 2 mg/day during weeks 2–8 (up-titration). This is the dosage and titration regimen recommended for adjunctive use in MDD by the Federal Drug Administration (USA). Several previous clinical trials have used this titration regimen from 1 mg to 2 mg,34 40 51 and a dose of 2 mg has been shown to be effective in reducing depressive symptoms.33 As doses higher than 2 mg have been shown to increase incidence of akathisia,37 a maximum dose of 2 mg will be used in the present study to minimise side effects.
Monitoring visits: Participants will be contacted by telephone on a weekly basis for the duration of the 8-week treatment period to monitor adverse events and adherence. Any changes in concomitant medications will also be investigated and recorded. In addition, participants will be provided with a medication diary and asked to complete during the study to monitor adherence. More detailed information about potential side effects will be further assessed by the study doctor at visits 2, 3, 4 and 5 using the UKU Side Effect Rating Scale,52 Abnormal Involuntary Movement Scale53 and the Simpson-Angus Scale54 for evaluation of extrapyramidal symptoms.
Visit 2 (week 4): Following 4 weeks of the treatment phase, participants will return for clinical and self-report assessments to assess changes in clinical and functional measures.
Visit 3 (week 8): Following 8 weeks of the treatment phase, participants will return to complete clinical and self-report assessments and will also complete a second circadian (overnight) in-lab assessment. Bloods will be collected at this visit for follow-up metabolic and inflammatory markers. Participants will continue to be provided the brexpiprazole for up to 12 months following completion of the treatment phase as clinically indicated, at the discretion of their treating clinician. Analyses will account for whether participants have continued or discontinued the medication during the follow-up period.
Visits 4 and 5 (follow-up visits 1 and 2, 12 and 16 weeks, respectively): 12 and 16 weeks after commencing treatment (ie, 4 and 8 weeks after completing the 8-week treatment period, respectively), participants will return to complete clinical and self-report assessments. Participants will be provided with an actigraphy device to wear for 2 weeks prior to these assessments.
Participants will be reimbursed for their time and the cost of transportation to and from the research sites.
Outcomes
Outcome measures are summarised in table 1.
Table 1.
Primary, secondary, and tertiary outcome measures.
| Primary outcome measures (Correlation between change in sleep-wake and circadian parameters and change in depressive symptoms from baseline to week eight) |
Secondary outcome measures (Correlation between change in sleep-wake and circadian parameters and change in functioning from baseline to week eight) |
Tertiary outcome measures (Correlation between change in sleep-wake and circadian parameters and other multidimensional outcome measures) |
Primary depressive symptom measures:
Sleep-wake and circadian variables: Actigraphy parameters (in the 2-week period prior to baseline and prior to week eight):
In-lab circadian measures:
Self-report measures:
|
Functioning measures:
|
Symptom measures:
Self-harm and suicidal thoughts and behaviours:
Physical health:
Alcohol and substance Use:
Comparison of primary endpoints between:
|
*Baseline scores will be used rather than change scores as these are trait measures.
QIDS-CR, Clinical-rated Quick Inventory of Depressive Symptomatolog; QIDS-SR, Quick Inventory of Depressive Symptomatology-self-report.
Primary outcomes
The primary endpoint will be the correlation between change in sleep-wake and circadian parameters and change in depressive symptoms from baseline to week 8. Sleep items in the primary depressive symptom measures (QIDS-CR total score, QIDS-Self Report (QIDS-SR) total score, The Montgomery-Åsberg Depression Rating Scale (MADRS) total score) will be removed in analyses to provide a measure of depressive symptoms not biased by changes in sleep-wake parameters.
Secondary outcomes
The secondary endpoint will be correlation between change in sleep-wake and circadian parameters and change in functioning from baseline to week 8.
Tertiary outcomes
Tertiary endpoints will be correlation between change in sleep-wake and circadian parameters and other multidimensional outcome measures based on assessments of symptoms, self-harm and suicidal thoughts and behaviours, physical health and alcohol/substance use. Further tertiary endpoints will be comparison of primary endpoints between Clinical Stages,55–58 illness trajectories59 and genetic variants with potential relevance to mood disorders and/or circadian rhythms (eg, CLOCK and BMAL1).
Assessments
Assessments used here are based on the multidimensional assessment and outcomes framework45 and include clinical and self-report ratings of mental health symptoms, social and occupational functioning, self-harm, suicidal thoughts and behaviours, physical health, alcohol and substance use, illness type, stage and trajectory, as well as circadian parameters and metabolic, inflammatory and genetic markers. Our recent research45 60–62 indicates the capacity of the multidimensional outcomes framework to further our understanding of the pathophysiological mechanisms and illness progression in this cohort, as well as to inform more personalised and measurement-based models of care.
Diagnostic assessments
The presence of MDD and any comorbidity will be evaluated using SCID46 (30–75 min to complete).
Mental risk assessment
Acute suicidal behaviour will be assessed by the relevant subscale of the CAARMS48 (5 min to complete).
Clinical assessments
The clinical interview is expected to take around 35 min to complete.
Clinician-rated Quick Inventory of Depressive Symptomatology (QIDS-CR)47: assesses the nine criterion symptom domains designated by the DSM to diagnose an MDE.
MADRS63: will also be used to assess depressive symptoms, to allow for direct comparisons with previous studies of brexpiprazole in MDD.
Young Mania Rating Scale64: an 11-item, multiple-choice diagnostic questionnaire used to measure severity of manic episodes.
Brief Psychiatric Rating Scale65: used to measure psychiatric symptoms (eg, depression, anxiety, hallucinations).
Social and Occupational Functioning Assessment Scale66: used to assess functioning on a 0–100 scale (lower scores suggesting greater impairment).
Clinical Global Impressions scale67: used to measure of clinical improvement.
Participants will also be rated on previously established clinical stage55–58 and illness trajectory59 models on the basis of information collected throughout the clinical interview. The clinical staging framework differentiates those in the earliest phases of mental health problems with non-specific clinical presentations (stage 1a; ‘help-seeking’) from those at greater-risk with more specific, subthreshold presentations (stage 1b; ‘attenuated syndromes’) and those who have already reached a threshold for a progressive or recurrent disorder meeting diagnostic criteria (stages 2, 3 or 4). The illness trajectory model is a novel tripartite framework based on three proposed pathophysiological pathways leading to youth-onset mental disorders: (1) neurodevelopmental-psychosis, (2) circadian-bipolar spectrum; and (3) hyperarousal-anxious depression.45 59 68
WHO Alcohol, Smoking and Substance Involvement Screening Test49 50: a reliable, culturally adaptable, valid screener for problematic or risky substance use.
Self-report assessments
The self-report questionnaires are tailored to the individual (using skip logic) so the amount of time taken to complete the questionnaire varies, but we estimate the assessment will take 45 min to complete.
Demographics and Mental Health History: including details of work and education, physical health (height, weight, waist circumference), history of mental health and family history
International Physical Activity Questionnaire-short version69 70: seven-item questionnaire providing internationally comparable data on health-related physical activity.
Alcohol Use Disorders Identification Test-Consumption71: includes three short questions that estimate alcohol consumption in a standard, meaningful, non-judgemental manner. Additional questions assessing age of onset of alcohol consumption will also be used.
Suicidal Ideation Attributes Scale (SIDAS)72: five-item self-report questionnaire assessing the frequency, controllability, closeness to attempt, distress and interference with daily activities on a 10-point Likert scale over the past month.
Columbia-Suicide Severity Rating Scale73: The scale comprises three sections: suicidal ideation, intensity of ideation and suicidal behaviour. A self-rating adaptation will be used in combination with the SIDAS.
Brief Non-Suicidal Self-Injury Assessment Tool74: designed to assess primary (such as form, frequency, and function) and secondary (including but not limited to NSSI habituation; contexts in which NSSI is practiced; and NSSI perceived life interference, treatment, and impacts) NSSI characteristics.
Quick Inventory of Depressive Symptomatology – self-report (QIDS-SR)75: a self-rating version includes 16 questions with equivalent weightings (0–3) for each symptom item that assesses the nine criterion symptom domains designated by the DSM-IV to diagnose an MDE.
Overall Anxiety Severity Impairment Scale76: five-item self-report measure used to assess severity and impairment associated with any anxiety disorder or multiple anxiety disorders.
Altman Self-Rating Mania Scale77: five-item self-rating scale designed to assess the presence and/or severity of manic symptoms.
Primary Care Post-Traumatic Stress Disorder Screen for DSM-578: five-item screen designed for use in primary care settings to identify respondents with probable PTSD.
Prodromal Questionnaire79: self-report screen for use in secondary mental healthcare services to select subjects for psychosis risk.
Eating Disorder Examination80 81: comprises a range of health-related and demographic questions, including present height and weight, and a detailed and comprehensive assessment of symptoms, particularly binge eating. In this study, three eating disorder behaviours are assessed: binge eating, purging and strict dieting or fasting.
Sleep questions: seven questions regarding time falling asleep, waking up during weekdays and weekends, hours of sleep, feelings when waking up. Sleep timing items are based on the Pittsburgh Sleep Quality Index (PSQI) and Munich Chrono Type Questionnaire, while sleep quality items are based on expert consensus in the literature.
Work and Social Adjustment Scale82: five-item scale of functional impairment attributable to an identified problem.
Schuster Social Support Scale83: 15-item measure of social support used to examine an individual’s social relationships with others (relatives, friends, spouse) and the associated impact on their emotional functioning.
Additional sleep questionnaires: participants will complete the BMC sleep-wake self-report questionnaire battery including questions regarding ethnicity, caffeine consumption, menstrual cycle, visual impairments, and non-restorative sleep, as well as the PPSQI,84 Epworth Sleepiness Scale,85 Insomnia Severity Index,86 Horne-Ostberg Morningness-Eveningness Questionnaire87 and the Seasonal Patterns Assessment Questionnaire.88
Sleep wake assessments
24-hour sleep-wake and circadian rest-activity parameters will be measured by actigraphy recordings. Actigraphy is a non-invasive tool to objectively measure activity profiles and used to estimate sleep and circadian patterns based on validated algorithms. Participants will be asked to complete a sleep diary and to wear an actigraph (GENEActiv device; Activinsights, Kimbolton, UK) on the non-dominant wrist for at least 10 days prior to commencing the study, and continuously for the 8-week treatment phase. The device is a comfortable and easy to use, battery operated wrist-worn device (similar in appearance to a Fitbit), designed to record and provide data on movement, light, temperature and sleep patterns. The device will provide objective monitoring of participants’ 24-hour circadian rhythm, including their sleep onset and duration, rise time, night-time sleep interruptions, and activity patterns (see table 1). The device allows for the outputting of real-time raw measurement data for up to a month without charging. Instructions will be provided to participants on how to use the device on their recruitment into the study. Two-week actigraphy recordings will also be completed at the 12-week and 16-week follow-up assessments. GENEActiv devices have been validated against several types of accelerometry-based activity monitors89–92 as well as for sleep-wake scoring.93 94 For decades, actigraphy monitors like the GENEActiv devices have been used extensively in research to measure sleep and activity patterns in diverse clinical settings including sleep disorders, medical illnesses (eg, neurodegenerative diseases) and various major mental disorders.19 95 96
In-lab circadian assessment
Circadian rhythms will be measured in an evening/overnight recording period, including 24-hour rhythms of salivary melatonin, salivary cortisol and core body temperature (table 1). Circadian assessments will be performed in accordance with established dim light melatonin onset protocols.97–100
Metabolic and inflammatory markers
The following markers will be collected at baseline and 8 weeks follow-up visits: (1) triglycerides, (2) cholesterol (including total, high-density lipoprotein and low-density lipoprotein), (3) fasting glucose, (4) fasting insulin, (5) interleukin (IL)-1β, (6) IL-6, (7) tissue necrosis factor-α and (8) C-reactive protein. Height, weight and waist circumference will be recorded. Insulin resistance will be estimated based on paired fasting plasma glucose and insulin levels101 by the updated homeostatic model assessment (HOMA2-IR) using iHOMA2 software V.8.8.102
Genetics
Additional blood (30 mL) will be collected at baseline for assessment of genetic markers as per procedures from the University of Queensland Human Studies Unit.
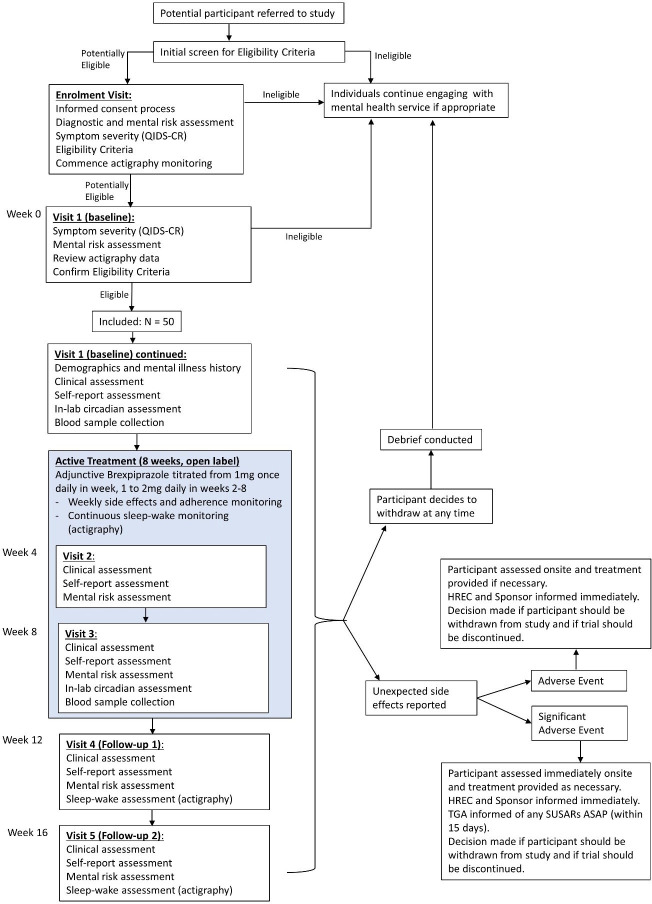
The schedule of trial assessments is summarised in table 2 and the participant timeline is presented in figure 1.
Table 2.
Schedule of assessments
| Enrolment visit | Visit 1 (baseline) | Visit 2 (week 4) |
Visit 3 (week 8) |
Visit 4 (follow-up 1) |
Visit 5 (follow-up 2) |
|
| Study week | 0 | 4 | 8 | 12 | 16 | |
| Informed consent | ✓ | |||||
| Inclusion/exclusion criteria | ✓ | ✓ | ||||
| Diagnostic Assessment (SCID) | ✓ | |||||
| Mental Risk Assessment (CAARMS) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Demographics and mental illness history | ✓ | |||||
| Clinical Assessment (QIDS-CR, MADRS, YMRS, BPRS, SOFAS) | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Self-report assessment | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Sleep-wake assessment (actigraphy monitoring) | ✓ | ✓ | ✓ | ✓ | ✓ | |
| In-lab circadian assessment | ✓ | ✓ | ||||
| Blood sample collection (Metabolic and inflammatory markers) |
✓ | ✓ | ||||
| Safety, side effects and adherence assessment | ✓ | ✓ | ✓ | ✓ | ||
| IP dispensing | ✓ | ✓ | ✓* | ✓* | ✓* | |
| IP return | ✓ | ✓ |
*If clinically indicated, at the discretion of the treating clinician.
BPRS, Brief Psychiatric Rating Scale; CAARMS, Comprehensive Assessment of At-Risk Mental States; IP, investigational product; MADRS, Montgomery-Åsberg Depression Rating Scale; QIDS-CR, Clinician-rated Quick Inventory of Depressive Symptomatology; SCID, Structured Clinical Interview for DSM; SOFAS, Social and Occupational Functioning Assessment Scale; YMRS, Young Mania Rating Scale.
Figure 1.
Study flow chart. This figure illustrates the study design and participant timeline from referral to the last follow-up visit, including withdrawal and safety procedures. QIDS-CR, Clinician-rated Quick Inventory of Depressive Symptomatology; HREC, Human Research Ethics Committee; TGA, Therapeutic Goods Administration; SUSAR, Suspected unexpected serious adverse reaction.
Safety and side effects monitoring
Studies have shown that brexpiprazole is generally well tolerated, with no unexpected or severe side effects.33 36 103 104 Safety and tolerability of the investigational product (IP) will be closely monitored throughout the trial. For the duration of the 8-week treatment period, weekly phone calls will be conducted to monitor safety and elicit information regarding side effects and potential adverse events. Any changes in concomitant medications will also be investigated and recorded. In addition, participants will be provided with a medication diary and asked to complete during the study to monitor tolerability and adherence. According to the IP information, participants will be advised to not drive a car, operate machinery or do other dangerous activities until they know how the IP affects them, as it may induce drowsiness in some subjects.
Further formal assessment of side effects and potential adverse events following the end of the treatment phase will be conducted at the two follow-up visits (visits 4 and 5). At the baseline visit, participants will be informed that any serious negative side effects should be reported to the study doctor immediately and will be provided with the relevant contact details to do so. All adverse events will be assessed for causality and symptom severity according to the study protocol and followed up by the study doctor if required. In the occurrence of a serious adverse event, appropriate diagnostic and therapeutic measures will be taken and the participant will be kept under observation for as long as is medically indicated. The principal investigator will then determine if the seriousness of the event warrants the removal of the participant from the study or abandonment of the study. For serious side effects or medical problems, the patient may be taken immediately to Royal Prince Alfred Hospital for treatment. The principal investigator will ensure that follow-up of the participant is appropriate to the nature of any event, and that it continues until resolution.
Throughout the trial the study doctor will monitor participants for pregnancy. It is not currently included in the protocol, but the trial Standard Operating Procedures manual explains that the study doctor will give relevant contraception advice to participants based on the current IP information.
Sample size calculation
Sample size was determined based on a study of circadian changes in response to agomelatine31 where a coefficient of 0.54 was found for the correlation between change in DLMO and change in depressive symptoms. We conservatively estimated that the coefficient for brexpiprazole would be smaller (~0.35; ie, medium effect size), as effects on the circadian system may be less direct. Assuming α=0.05 and 80% power for a one-tailed correlation analysis, a sample size of n=49 is required to detect this effect.
Data analysis plan
Correlations will be performed between change scores (ie, visit 2 score minus visit 1 score) for sleep and circadian measures, and change scores for depressive symptoms. The intention-to-treat principle will be used for missing data (last observations carried forward). Before analysis, plots with outlier and normality statistics will be generated for all variables to identify outliers and issues with variable distribution. Extreme outliers (±3 z-scores) will be checked against source data and testing notes to verify whether they are the result of an error in data entry or a specific issue during testing (eg, equipment failure), with errors rectified. If not the result of data entry error, outliers will be curtailed. Pearson’s or Spearman’s correlations will be selected to perform analyses based on normative or non-normative data distribution (significance level α=0.05). Correlations will also be examined between other change scores to assess secondary and tertiary endpoints. Further tertiary endpoints will be assessed by partial correlations comparing categorical variables.
Patient and public involvement
Clinical professionals working with young people with mental health problems were invited to comment on the study design and procedures. Findings will be disseminated to scientific, clinical and wider communities.
Ethics and dissemination
This trial has been approved by the Human Research Ethics Committee of the Sydney Local Health District (X19-0417 and 2019/ETH12986, Protocol Version 1–3, dated 25 February 2021). The study will be conducted in compliance with all stipulations of the protocol, the conditions of ethics committee approval, the NHMRC National Statement on Ethical Conduct in Human Research and the Good Clinical Practice guidelines. The results of this study will be disseminated as widely as possible into the scientific and broader community. This will include publication in peer-reviewed journals, scholarly book chapters, presentation at conferences and publication in conference proceedings. Publications arising from this project will be deposited into an open access institutional repository, where possible. Results will also be disseminated into the wider community in a format appropriate for a lay audience, through links including the BMC website and social media, as well as newsletters.
Supplementary Material
Acknowledgments
This work was supported in-part by philanthropic funding, for which donor(s) are families affected by mental illness who wish to remain anonymous.
Footnotes
Contributors: JSC and IBH conceived the study, led the proposal and protocol development. JSC wrote the trial protocol. AG drafted the first version of the manuscript. NZ drafted the final version of the manuscript with input from other authors. JSC, AG, AN, NZ, YJCS, CW, CR, JC, CM, FML, DK and EMS were all involved with modifications to the design of the study and with drafting of this paper. All authors have read and approved the final manuscript.
Funding: JC is supported by a Breakthrough Mental Health Research Foundation Fellowship and an NHMRC Emerging Leadership Fellowship (GNT2008197).
Competing interests: IBH is the Co-Director, Health and Policy at the Brain and Mind Centre (BMC) University of Sydney, Australia. The BMC operates an early-intervention youth services at Camperdown under contract to headspace. Professor Hickie has previously led community-based and pharmaceutical industry-supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) projects focused on the identification and better management of anxiety and depression. He is the Chief Scientific Advisor to, and a 3.2% equity shareholder in, InnoWell Pty Ltd. InnoWell was formed by the University of Sydney (45% equity) and PwC (Australia; 45% equity) to deliver the $30M Australian Government-funded Project Synergy (2017-20) and to lead transformation of mental health services internationally through the use of innovative technologies. EMS is the Medical Director, Young Adult Mental Health Unit, St Vincent’s Hospital Darlinghurst, Discipline Leader of Adult Mental Health, School of Medicine, University of Notre Dame, Research Affiliate, The University of Sydney and Consultant Psychiatrist. She has received honoraria for educational seminars related to the clinical management of depressive disorders supported by Servier and Eli-Lilly pharmaceuticals. She has participated in a national advisory board for the antidepressant compound Pristiq, manufactured by Pfizer. She was the National Coordinator of an antidepressant trial sponsored by Servier.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the who world mental health (WMH) surveys. Epidemiol Psichiatr Soc 2009;18:23–33. 10.1017/S1121189X00001421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore FM, Bloem PJN, Patton GC, et al. Global burden of disease in young people aged 10-24 years: a systematic analysis. Lancet 2011;377:2093–102. 10.1016/S0140-6736(11)60512-6 [DOI] [PubMed] [Google Scholar]
- 3.Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the treatment for adolescents with depression study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry 2009;48:186–95. 10.1097/CHI.0b013e31819176f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA 2004;292:807–20. 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 6.Murphy SE, Capitão LP, Giles SLC, et al. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021;8:824–35. 10.1016/S2215-0366(21)00154-1 [DOI] [PubMed] [Google Scholar]
- 7.Cuijpers P, Karyotaki E, Eckshtain D, et al. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiatry 2020;77:694–702. 10.1001/jamapsychiatry.2020.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolpert M, Dalzell K, Ullman R, et al. Strategies not accompanied by a mental health professional to address anxiety and depression in children and young people: a scoping review of range and a systematic review of effectiveness. Lancet Psychiatry 2019;6:46–60. 10.1016/S2215-0366(18)30465-6 [DOI] [PubMed] [Google Scholar]
- 9.Eckshtain D, Kuppens S, Ugueto A, et al. Meta-Analysis: 13-year follow-up of psychotherapy effects on youth depression. J Am Acad Child Adolesc Psychiatry 2020;59:45–63. 10.1016/j.jaac.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 10.Bear HA, Edbrooke-Childs J, Norton S, et al. Systematic review and meta-analysis: outcomes of routine specialist mental health care for young people with depression and/or anxiety. J Am Acad Child Adolesc Psychiatry 2020;59:810–41. 10.1016/j.jaac.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Ravindran AV, Qin B, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 2015;76:e487–98. 10.4088/JCP.14r09204 [DOI] [PubMed] [Google Scholar]
- 12.Fornaro M, Fusco A, Anastasia A, et al. Brexpiprazole for treatment-resistant major depressive disorder. Expert Opin Pharmacother 2019;20:1925–33. 10.1080/14656566.2019.1654457 [DOI] [PubMed] [Google Scholar]
- 13.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry 1992;49:651–68. 10.1001/archpsyc.1992.01820080059010 [DOI] [PubMed] [Google Scholar]
- 14.van Mill JG, Hoogendijk WJG, Vogelzangs N, et al. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry 2010;71:239–46. 10.4088/JCP.09m05218gry [DOI] [PubMed] [Google Scholar]
- 15.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011;135:10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Buysse DJ, Gentzler AL, et al. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep 2007;30:83–90. 10.1093/sleep/30.1.83 [DOI] [PubMed] [Google Scholar]
- 17.Geoffroy PA, Hoertel N, Etain B, et al. Insomnia and hypersomnia in major depressive episode: prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord 2018;226:132–41. 10.1016/j.jad.2017.09.032 [DOI] [PubMed] [Google Scholar]
- 18.Merikangas KR, Swendsen J, Hickie IB, et al. Real-Time mobile monitoring of the dynamic associations among motor activity, energy, mood, and sleep in adults with bipolar disorder. JAMA Psychiatry 2019;76:190–8. 10.1001/jamapsychiatry.2018.3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Difrancesco S, Lamers F, Riese H, et al. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: a 2-week ambulatory assessment study. Depress Anxiety 2019;36:975–86. 10.1002/da.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol 2008;23:571–85. 10.1002/hup.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther 2013;138:176–84. 10.1016/j.pharmthera.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 22.Krystal AD, Mittoux A, Lindsten A, et al. Chronobiologic parameter changes in patients with major depressive disorder and sleep disturbance treated with adjunctive brexpiprazole: an open-label, flexible-dose, exploratory substudy. J Affect Disord 2021;278:288–95. 10.1016/j.jad.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 23.Carpenter JS, Crouse JJ, Scott EM, et al. Circadian depression: a mood disorder phenotype. Neurosci Biobehav Rev 2021;126:79–101. 10.1016/j.neubiorev.2021.02.045 [DOI] [PubMed] [Google Scholar]
- 24.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 2006;15 Spec No 2:R271–7. 10.1093/hmg/ddl207 [DOI] [PubMed] [Google Scholar]
- 25.Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 2019;20:49–65. 10.1038/s41583-018-0088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter JS, Robillard R, Hickie IB. Variations in the sleep-wake cycle from childhood to adulthood: chronobiological perspectives. ChronoPhysiology and Therapy 2015;5:37–49. [Google Scholar]
- 27.Robillard R, Naismith SL, Rogers NL, et al. Delayed sleep phase in young people with unipolar or bipolar affective disorders. J Affect Disord 2013;145:260–3. 10.1016/j.jad.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Robillard R, Carpenter JS, Rogers NL, et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl Psychiatry 2018;8:213. 10.1038/s41398-018-0255-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med 2007;8:602–12. 10.1016/j.sleep.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol 2004;14:R1038–9. 10.1016/j.cub.2004.11.039 [DOI] [PubMed] [Google Scholar]
- 31.Robillard R, Carpenter JS, Feilds K-L, et al. Parallel changes in mood and melatonin rhythm following an adjunctive multimodal chronobiological intervention with agomelatine in people with depression: a proof of concept open label study. Front Psychiatry 2018;9:624. 10.3389/fpsyt.2018.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedetti F, Dallaspezia S, Fulgosi MC, et al. Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int 2007;24:921–37. 10.1080/07420520701649455 [DOI] [PubMed] [Google Scholar]
- 33.Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose Brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry 2018;79:17m12058. 10.4088/JCP.17m12058 [DOI] [PubMed] [Google Scholar]
- 34.Bauer M, Hefting N, Lindsten A, et al. A randomised, placebo-controlled 24-week study evaluating adjunctive brexpiprazole in patients with major depressive disorder. Acta Neuropsychiatr 2019;31:1–9. 10.1017/neu.2018.23 [DOI] [PubMed] [Google Scholar]
- 35.Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 Mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry 2015;76:1232–40. 10.4088/JCP.14m09689 [DOI] [PubMed] [Google Scholar]
- 36.Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 Mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 2015;76:1224–31. 10.4088/JCP.14m09688 [DOI] [PubMed] [Google Scholar]
- 37.Yoon S, Jeon SW, Ko Y-H, et al. Adjunctive Brexpiprazole as a novel effective strategy for treating major depressive disorder: a systematic review and meta-analysis. J Clin Psychopharmacol 2017;37:46–53. 10.1097/JCP.0000000000000622 [DOI] [PubMed] [Google Scholar]
- 38.Citrome L, Stensbøl TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother 2015;15:1219–29. 10.1586/14737175.2015.1086269 [DOI] [PubMed] [Google Scholar]
- 39.Beyer JL, Weisler RH. Adjunctive brexpiprazole for the treatment of major depressive disorder. Expert Opin Pharmacother 2016;17:2331–9. 10.1080/14656566.2016.1254188 [DOI] [PubMed] [Google Scholar]
- 40.Krystal AD, Mittoux A, Meisels P. Effects of adjunctive Brexpiprazole on sleep disturbances in patients with major depressive disorder: an open-label, Flexible-Dose, exploratory study. Prim Care Companion CNS Disord 2016;18:15m01914. 10.4088/PCC.15m01914 [DOI] [PubMed] [Google Scholar]
- 41.Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry 2011;70:912–9. 10.1016/j.biopsych.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 42.Palagini L, Baglioni C, Ciapparelli A, et al. Rem sleep dysregulation in depression: state of the art. Sleep Med Rev 2013;17:377–90. 10.1016/j.smrv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y-Q, Li R, Zhang M-Q, et al. The neurobiological mechanisms and treatments of REM sleep disturbances in depression. Curr Neuropharmacol 2015;13:543–53. 10.2174/1570159x13666150310002540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Ren R, Yang L, et al. Polysomnographically measured sleep changes in idiopathic REM sleep behavior disorder: a systematic review and meta-analysis. Sleep Med Rev 2020;54:101362. 10.1016/j.smrv.2020.101362 [DOI] [PubMed] [Google Scholar]
- 45.Hickie IB, Scott EM, Cross SP, et al. Right care, first time: a highly personalised and measurement-based care model to manage youth mental health. Med J Aust 2019;211 Suppl 9:S3–46. 10.5694/mja2.50383 [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association, . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Pub, 2013. [Google Scholar]
- 47.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–83. 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 48.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry 2005;39:964–71. 10.1080/j.1440-1614.2005.01714.x [DOI] [PubMed] [Google Scholar]
- 49.WHO ASSIST Working Group . The alcohol, smoking and substance involvement screening test (assist): development, reliability and feasibility. Addiction 2002;97:1183–94. 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- 50.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (assist). Addiction 2008;103:1039–47. 10.1111/j.1360-0443.2007.02114.x [DOI] [PubMed] [Google Scholar]
- 51.Fava M, Ménard F, Davidsen CK, et al. Adjunctive Brexpiprazole in patients with major depressive disorder and irritability: an exploratory study. J Clin Psychiatry 2016;77:1695–701. 10.4088/JCP.15m10470 [DOI] [PubMed] [Google Scholar]
- 52.Lingjærde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand 1987;76:1–100. 10.1111/j.1600-0447.1987.tb10566.x [DOI] [PubMed] [Google Scholar]
- 53.Guy WA. Abnormal Involuntary Movement Scale (AIMS). In: ECDEU assessment manual for psychopharmacology. Washington, DC: U.S. Department of Health Education and Welfare, 1976: 534–7. [Google Scholar]
- 54.Simpson GM, B. M, B. GH, et al. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970;45:11–19. 10.1111/j.1600-0447.1970.tb02066.x [DOI] [PubMed] [Google Scholar]
- 55.Hickie IB, Scott EM, Hermens DF, et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry 2013;7:31–43. 10.1111/j.1751-7893.2012.00366.x [DOI] [PubMed] [Google Scholar]
- 56.McGorry PD, Hickie IB, Yung AR, et al. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry 2006;40:616–22. 10.1080/j.1440-1614.2006.01860.x [DOI] [PubMed] [Google Scholar]
- 57.Scott J, Leboyer M, Hickie I, et al. Clinical staging in psychiatry: a cross-cutting model of diagnosis with heuristic and practical value. Br J Psychiatry 2013;202:243–5. 10.1192/bjp.bp.112.110858 [DOI] [PubMed] [Google Scholar]
- 58.Hickie IB, Scott J, McGorry PD. Clinical staging for mental disorders: a new development in diagnostic practice in mental health. Med J Aust 2013;198:461–2. 10.5694/mja13.10431 [DOI] [PubMed] [Google Scholar]
- 59.Hickie IB, Hermens DF, Naismith SL, et al. Evaluating differential developmental trajectories to adolescent-onset mood and psychotic disorders. BMC Psychiatry 2013;13:303. 10.1186/1471-244X-13-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crouse JJ, Chitty KM, Iorfino F, et al. Transdiagnostic neurocognitive subgroups and functional course in young people with emerging mental disorders: a cohort study. BJPsych Open 2020;6:e31. 10.1192/bjo.2020.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carpenter JS, Iorfino F, Cross S, et al. Cohort profile: the Brain and Mind Centre Optymise cohort: tracking multidimensional outcomes in young people presenting for mental healthcare. BMJ Open 2020;10:e030985. 10.1136/bmjopen-2019-030985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott EM, et al. Early intervention, prevention, and prediction in mood disorders: Tracking multidimensional outcomes in young people presenting for mental health care. In: Personalized psychiatry. Elsevier, 2020: 39–62. [Google Scholar]
- 63.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 64.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429-35. 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 65.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799–812. 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- 66.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992;149:1148–56. 10.1176/ajp.149.9.1148 [DOI] [PubMed] [Google Scholar]
- 67.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 2007;4:28. [PMC free article] [PubMed] [Google Scholar]
- 68.Crouse JJ, Carpenter JS, Song YJC, et al. Circadian rhythm sleep-wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry 2021;8:813–23. 10.1016/S2215-0366(21)00034-1 [DOI] [PubMed] [Google Scholar]
- 69.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 70.Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000;71:114–20. 10.1080/02701367.2000.11082794 [DOI] [PubMed] [Google Scholar]
- 71.Bush K, Kivlahan DR, McDonell MB, et al. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). alcohol use disorders identification test. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 72.van Spijker BAJ, Batterham PJ, Calear AL, et al. The suicidal ideation attributes scale (SIDAS): community-based validation study of a new scale for the measurement of suicidal ideation. Suicide Life Threat Behav 2014;44:408–19. 10.1111/sltb.12084 [DOI] [PubMed] [Google Scholar]
- 73.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whitlock J, Exner-Cortens D, Purington A. Assessment of nonsuicidal self-injury: development and initial validation of the Non-Suicidal Self-Injury-Assessment tool (NSSI-AT). Psychol Assess 2014;26:935–46. 10.1037/a0036611 [DOI] [PubMed] [Google Scholar]
- 75.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–83. 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 76.Norman SB, Cissell SH, Means-Christensen AJ, et al. Development and validation of an overall anxiety severity and impairment scale (OASIS). Depress Anxiety 2006;23:245–9. 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- 77.Campbell-Sills L, Norman SB, Craske MG, et al. Validation of a brief measure of anxiety-related severity and impairment: the overall anxiety severity and impairment scale (OASIS). J Affect Disord 2009;112:92–101. 10.1016/j.jad.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prins A, Bovin MJ, Smolenski DJ, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med 2016;31:1206–11. 10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ising HK, Veling W, Loewy RL, et al. The validity of the 16-item version of the prodromal questionnaire (PQ-16) to screen for ultra high risk of developing psychosis in the general help-seeking population. Schizophr Bull 2012;38:1288–96. 10.1093/schbul/sbs068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hay PJ, Mond J, Buttner P, et al. Eating disorder behaviors are increasing: findings from two sequential community surveys in South Australia. PLoS One 2008;3:e1541. 10.1371/journal.pone.0001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchison D, Hay P, Slewa-Younan S, et al. The changing demographic profile of eating disorder behaviors in the community. BMC Public Health 2014;14:943. 10.1186/1471-2458-14-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mundt JC, Marks IM, Shear MK, et al. The work and social adjustment scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461–4. 10.1192/bjp.180.5.461 [DOI] [PubMed] [Google Scholar]
- 83.Schuster TL, Kessler RC, Aseltine RH. Supportive interactions, negative interactions, and depressed mood. Am J Community Psychol 1990;18:423–38. 10.1007/BF00938116 [DOI] [PubMed] [Google Scholar]
- 84.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 85.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 86.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 87.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4:97–110. [PubMed] [Google Scholar]
- 88.Rosenthal NE, et al. Seasonal affective disorder and its relevance for the understanding and treatment of bulimia.. In: Hudson JI, Pope HG, eds. The Psychobiology of Bulimia. Washington, DC: American Psychiatric Press, 1987: 205–28. [Google Scholar]
- 89.Schaefer CA, Nigg CR, Hill JO, et al. Establishing and evaluating wrist cutpoints for the GENEActiv accelerometer in youth. Med Sci Sports Exerc 2014;46:826–33. 10.1249/MSS.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hildebrand M, VAN Hees VT, Hansen BH, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816–24. 10.1249/MSS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 91.Esliger DW, Rowlands AV, Hurst TL, et al. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085–93. 10.1249/MSS.0b013e31820513be [DOI] [PubMed] [Google Scholar]
- 92.van Hees VT, Gorzelniak L, Dean León EC, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One 2013;8:e61691. 10.1371/journal.pone.0061691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.te Lindert BHW, Van Someren EJW. Sleep estimates using microelectromechanical systems (MEMS). Sleep 2013;36:781–9. 10.5665/sleep.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a Wrist-Worn Accelerometer. PLoS One 2015;10:e0142533. 10.1371/journal.pone.0142533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stone JE, McGlashan EM, Facer-Childs ER, et al. Accuracy of the GENEActiv device for measuring light exposure in sleep and circadian research. Clocks Sleep 2020;2:143–52. 10.3390/clockssleep2020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavey TG, Gomersall SR, Clark BK, et al. The validity of the GENEActiv wrist-worn accelerometer for measuring adult sedentary time in free living. J Sci Med Sport 2016;19:395–9. 10.1016/j.jsams.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 97.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for Orcadian phase position. Chronobiol Int 1989;6:93–102. 10.3109/07420528909059144 [DOI] [PubMed] [Google Scholar]
- 98.de Almeida EA, Di Mascio P, Harumi T, et al. Measurement of melatonin in body fluids: Standards, protocols and procedures. Childs Nerv Syst 2011;27:879–91. 10.1007/s00381-010-1278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 1997;12:457–66. 10.1177/074873049701200507 [DOI] [PubMed] [Google Scholar]
- 100.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (Dlmo): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1–11. 10.1016/j.pnpbp.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 101.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 102.Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of β-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care 2013;36:2324–30. 10.2337/dc12-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lepola U, Hefting N, Zhang D, et al. Adjunctive brexpiprazole for elderly patients with major depressive disorder: an open-label, long-term safety and tolerability study. Int J Geriatr Psychiatry 2018;33:1403–10. 10.1002/gps.4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hakala M, Gislum M, Skuban A, et al. O7.5. long-term safety and tolerability of BREXPIPRAZOLE in patients with schizophrenia. Schizophr Bull 2018;44:S94–5. 10.1093/schbul/sby015.234 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.