Abstract
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a rapidly growing superfamily of natural products. RiPPs exhibit an extraordinary range of structures, but they all begin as gene-encoded precursor peptides that are linear chains of amino acids produced by ribosomes. Given the gene-encoded nature of RiPP precursor peptides, the toolbox of protein engineering can be directly applied to these precursors. This perspective will discuss examples of site-directed mutagenesis, noncanonical amino acid mutagenesis, and the construction and screening of combinatorial libraries as applied to RiPPs. These studies have led to important insights into the biosynthesis and bioactivity of RiPPs as well reengineering RiPPs for entirely new functions.
Graphical Abstract

Introduction
RiPPs, short for ribosomally synthesized and post-translationally modified peptides [1], are a sprawling collection of natural products from all of the kingdoms of life. RiPPs exhibit diverse bioactivities that have been recently reviewed from a physiological [2] and a molecular [3] perspective. The single feature that ties this motley crew of molecules together is that their biosynthesis always begins from a precursor peptide that is assembled by the ribosome, the cellular machine responsible for protein synthesis. These precursor peptides are subsequently modified by the action of one of more enzymes, in some cases making the final product barely recognizable as a peptide. Current knowledge about the biosynthesis of all known RiPP families has been recently reviewed [4]. Briefly, ribosomally synthesized RiPP precursors are usually comprised of two segments, a leader peptide and a core peptide; some RiPPs also have a follower peptide (FIGURE 1). The core peptide becomes modified by RiPP maturation enzymes while the leader peptide often binds and directs the maturation enzymes. In many RiPPs, the leader peptide must be removed in order for the modified core peptide to be bioactive.
Figure 1:
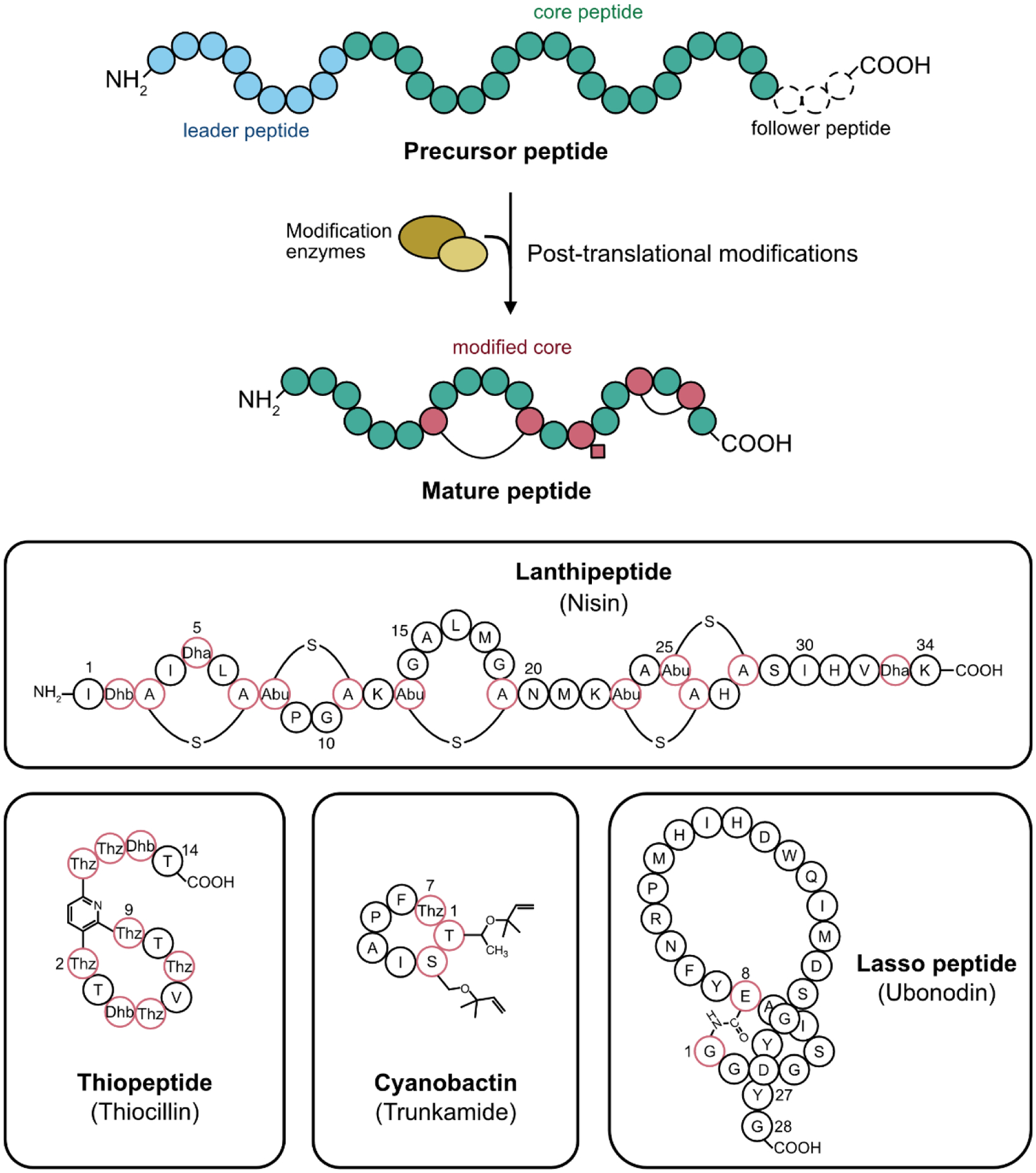
Biosynthesis and examples of RiPPs. Top: schematic of RiPPs biosynthesis. A ribosomally synthesized precursor peptide comprised of leader (blue) and core (green) peptide segments (and sometimes follower segments) is acted on by enzymes (yellow) to install modifications such as macrocyclization and sidechain alterations (red residues). Bottom: examples of RiPPs discussed here. Lanthipeptides include thioether linkages and dehydroamino acids (Dha and Dhb). Thiopeptides are cyclized via a pyridine/piperidine linkage and include thiazoles (Thz) and dehydroamino acids. Cyanobactins are head-to-tail cyclized, and include thiazoles and prenylation. Lasso peptides contain an N-terminal isopeptide bonded macrocycle through which the C-terminus threads.
The RiPPs field draws on knowledge from disparate disciplines: microbiology, synthetic biology, genetics and genomics, enzymology, structural biology, and bioinformatics, to name several. An argument can be made, however, that protein engineering is at the heart of most research on RiPPs. After all, RiPP biosynthetic pathways are fairly unique in that both the precursor peptides and the enzymes that modify them are gene-encoded polypeptides that can be subjected to the creativity and whims of protein engineers. In this perspective we will highlight key examples of protein engineering in the RiPPs field and end by discussing ways in which protein engineering can be brought to bear on future research on RiPPs.
With modern recombinant DNA techniques, protein engineers have a variety of tools to modify proteins. One of the earliest techniques to emerge from the recombinant DNA revolution was site-directed mutagenesis [5]. By modifying a specific codon in the gene encoding for a protein of interest, the protein engineer can probe the role of specific amino acids. An extension of site-directed mutagenesis is saturation mutagenesis, wherein a specific codon is allowed to vary to all (or a subset of all) possible other codons, generating a library of protein variants. Libraries of protein variants can also be generated in a more random fashion by utilizing error-prone PCR. The creation of these libraries is the first step in directed evolution experiments. Another key technique in the protein engineering toolbox is the ability to introduce amino acids beyond the natural set of 22 into proteins. These non-canonical amino acids (ncAAs) can be introduced into proteins in either a residue-specific or site-specific fashion [6]. In residue-specific incorporation, ncAAs are introduced into a protein in place of one of the natural amino acids. The most well-known example of residue-specific incorporation is the incorporation of selenomethionine to assist in solving the phasing problem in x-ray crystallography [7]. Site-specific incorporation of ncAAs involves the addition of one or more ncAAs into proteins on top of the natural set of amino acids. These techniques rely on the addition of additional tRNAs and engineered aminoacyl-tRNA synthetases (aaRSs) to cells or cell-free protein synthesis reactions [8]. All of these protein engineering techniques have been employed on RiPP precursors (FIGURE 2) and will be discussed below.
Figure 2:
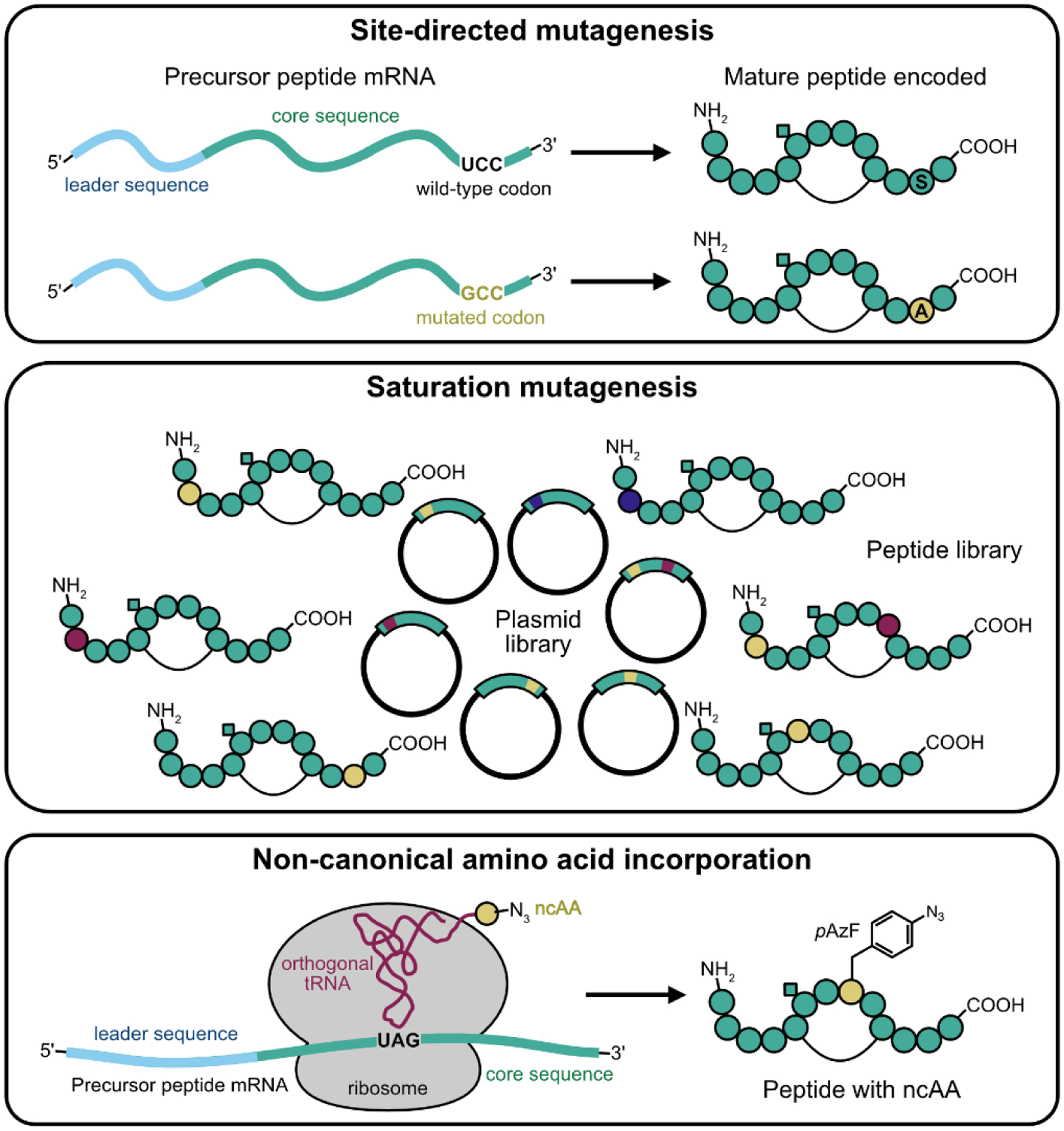
Protein engineering techniques applied to RiPPs. Top: site-directed mutagenesis of a gene encoding a RiPP precursor peptide. Middle: Creation of a gene library of RiPP precursors via saturation mutagenesis. Yellow, blue, and burgundy positions correspond to degenerate (i.e., mixed) codons. Bottom: non-canonical amino acid (ncAA) incorporation into RiPP precursors. Site specific incorporation, in which a 21st amino acid is added to the genetic code via amber suppression is depicted.
Site-directed mutagenesis of RiPPs to probe biosynthesis and activity
The use of site-directed mutagenesis is now commonplace upon the discovery of a new RiPP. For example, conducting alanine scans on a RiPP can provide information about which amino acids are important for its proper maturation. These targeted mutagenesis studies can also provide information about the structure-activity relationship of those RiPPs that have measurable bioactivity. Several seminal examples of using site-directed mutagenesis to study the maturation and function of RiPPs are discussed here.
The earliest studies using site-directed mutagenesis on RiPPs were carried out on lantibiotics, now known as lanthipeptides (FIGURE 1). The class-defining post-translational modifications (PTMs) of lanthipeptides are thioether linkages formed between the sidechain of a dehydrated Ser or Thr sidechain and a Cys sidechain [9]. A review article by Kuipers et al. [10] describes these early studies on the protein engineering of lanthipeptides. In one such study, the nisZ gene, encoding the precursor to lanthipeptide nisin Z [11], and several site-directed mutants of nisZ were introduced into a strain of Lactococcus lactis encoding the nisin maturation enzymes and immunity factors [12]. Conversion of a Ser5 residue in the nisin Z core peptide into Thr resulted in a swap from dehydroalanine (Dha) to dehydrobutyrine (Dhb) in mature nisin Z. This subtle chemical change, the addition of a single methyl group to a single sidechain, led to decreased antimicrobial activity of the nisin Z variant. Another site-directed mutant of nisin Z constructed in this study converted Met17 to Gln and Gly18 to Thr, making the Thr18 position a substrate for lanthipeptide dehydratases. Indeed, maturation of this NisZ variant led to a mixture of products with either unmodified Thr or Dhb at position 18. Site-directed mutagenesis studies on NisZ also probed the function of the leader peptide [13]. While most amino acid variants in the leader peptide were tolerated, some substitutions either abolished production of nisin Z or interfered with proteolysis of the leader peptide, the final step in nisin Z maturation.
Another example of an early study on RiPPs using site-directed mutagenesis was carried out by Pavlova et al. on the lasso peptide microcin J25 (MccJ25) [14]. Lasso peptides like MccJ25 contain an isopeptide bond between the N-terminus of the core peptide and a Glu or Asp sidechain, generating a macrocycle [15,16]. The C-terminal tail of the peptide threads through this macrocycle forming a rotaxane structure (FIGURE 1). A subset of lasso peptides, including MccJ25, exert antimicrobial activity via inhibition of RNA polymerase (RNAP) [17–20]. Pavlova et al. carried out a comprehensive site-directed mutagenesis study, generating single amino acid substitutions at every position in the 21 aa MccJ25 core peptide except at Glu8, the isopeptide bond-forming sidechain, for which a single E8D variant was tested. The authors then subjected this set of 381 variants to three levels of testing for production/stability, RNAP inhibition in vitro, and antimicrobial activity. Despite the unusual, highly-specific lasso structure, 242 of the 381 single amino acid variants of MccJ25 were produced as assessed by mass spectrometry. Of these variants, 155 inhibited RNAP in vitro; 70 of those RNAP-inhibiting variants also had antimicrobial activity. This study was the first indication that lasso peptides could be highly reprogrammable using the tools of protein engineering.
Thiopeptides are a class of highly-modified RiPPs that harbor both backbone heterocycles (azoles) and macrocyclization via a unique pyridine/piperidine moiety [21–24]. Acker et al. carried out a protein engineering study on the thiopeptide thiocillin (FIGURE 1) which contains 6 thiazole rings within its 14 aa [25]. Focusing on the positions within thiocillin that are not heterocyclized, the authors generated a set of 14 thiocillin variants at the gene level, 12 of which were successfully expressed. Since each of these variants exhibited different degrees of posttranslational modifications, a total of 65 different variants of thiocillin were identified using mass spectrometry. Of particular note is that some of the thiocillin variants in this study exhibited improved antimicrobial activity relative to the wild-type RiPP.
Deane et al. carried out a protein engineering study on plantazolicin, a member of the RiPP subfamily of linear azol(in)e peptides [26]. The Bacillus velezensis-derived RiPP plantazolicin, exhibits highly specific activity against Bacillus anthracis [27,28]. A total of 72 single point mutants of the gene encoding the plantazolicin precursor protein bamA were generated and expressed in an E. coli heterologous expression system. Of this set, 29 of the plantazolicin variants could be detected via mass spectrometry. These studies revealed that the Cys, Ser, and Thr residues that are converted into thiazoles and oxazoles are critical for the production of plantazolicin. For example, two Cys residues at the N-terminus of plantazolicin that are matured into thiazoles cannot be substituted with any other amino acid tested without a total loss of production. Positions within plantazolicin that are not posttranslationally modified were more tolerant to substitutions. Of the 29 variants of plantazolicin observed, 10 could be produced at levels sufficient for antimicrobial testing against B. anthracis, but all 10 of the variants exhibited weaker antimicrobial activity than native plantazolicin. These studies revealed that plantazolicin, an antimicrobial peptide with a hyperspecific spectrum of activity, has also highly tuned its core peptide sequence for production and antimicrobial activity.
Diversification of RiPPs with noncanonical amino acids
Noncanonical amino acids (ncAAs) have been introduced into RiPPs via both residue-specific and site-specific incorporation techniques using cells to produce the RiPPs. A further method for introducing non-proteinogenic building blocks into RiPP precursors is via direct solid-phase synthesis of these precursors. Our discussion below will focus on studies of RiPPs produced in cells, but studies employing solid phase synthesis have been reviewed elsewhere [4].
Residue-specific incorporation, also called selective pressure incorporation, has been used to add ncAAs to several lanthipeptides. Oldach et al. carried out residue-specific incorporation studies on the two-component lanthipeptide lichenicidin using an E. coli heterologous host [29]. Strains auxotrophic for Met, Pro, and Trp were used as hosts to incorporate ncAAs structurally similar to these canonical amino acids, including the Met analogs homopropargylglycine (HPG) and azidohomoalanine (AHA). The alkyne and azide moieties of these amino acids can participate in the copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC), the most well-known of the click chemistry reactions [30]. The ncAA-modified lichenicidin components were readily produced as judged by mass spectrometry, and the substitutions had a minimal effect on antimicrobial activity of lichenicidin. Conjugates to HPG- and AHA-modified lichenicidins were also demonstrated. Using a similar residue-specific approach, ncAAs were also introduced into the antimicrobial lasso peptide capistruin [31,32]. Met residues were first introduced to three different positions within the capistruin core peptide sequence corresponding to the ring, loop, and tail of the mature lasso peptide. The ncAAs AHA and HPG were again tested for incorporation, but both ncAAs led to either a decrease or total loss of production of capistruin. These same authors utilized site-specific incorporation techniques to modify capistruin as well. In this case, E. coli cells were equipped with a promiscuous pyrrolysyl-tRNA synthetase (PylRS) and its cognate tRNA, tRNAPyl. A series of Lys analogs harboring reactive alkene, alkyne, azide, and norbornyl moieties were tested for incorporation at the same three positions of capistruin via reengineering of these positions to an amber stop codon, UAG (FIGURE 2). This approach proved more successful than the residue-specific approach as 12 of the 15 variants attempted were produced, at levels ranging from well below the wild-type titer to similar or higher than that of the wild-type peptide.
Site-specific incorporation has also been applied to multiple RiPP families. The first demonstration of this technology was carried out on cyanobactins, a family of RiPPs with head-to-tail cyclization, azoline moiety, and isoprene modifications (FIGURE 1) [33,34]. A series of para-substituted Phe analogs were introduced into the cyanobactins patellin-2 and trunkamide using a promiscuous TyrRS from M. janaschii and a cognate tRNA [35]. All of the ncAA-substituted cyanobactins attempted (FIGURE 3) were produced at varying yields. Lanthipeptides have also been modified with ncAAs using the usual workhorse heterologous host for ncAA incorporation, E. coli [36]. The lanthipeptide cinnamycin has also been modified with ncAAs in the host Streptomyces albus [37]. A different Gram-positive host, Bacillus cereus, was outfitted with PylRS/tRNAPyl in order to generate ncAA-substituted variants of the thiopeptide thiocillin [38].
Figure 3:
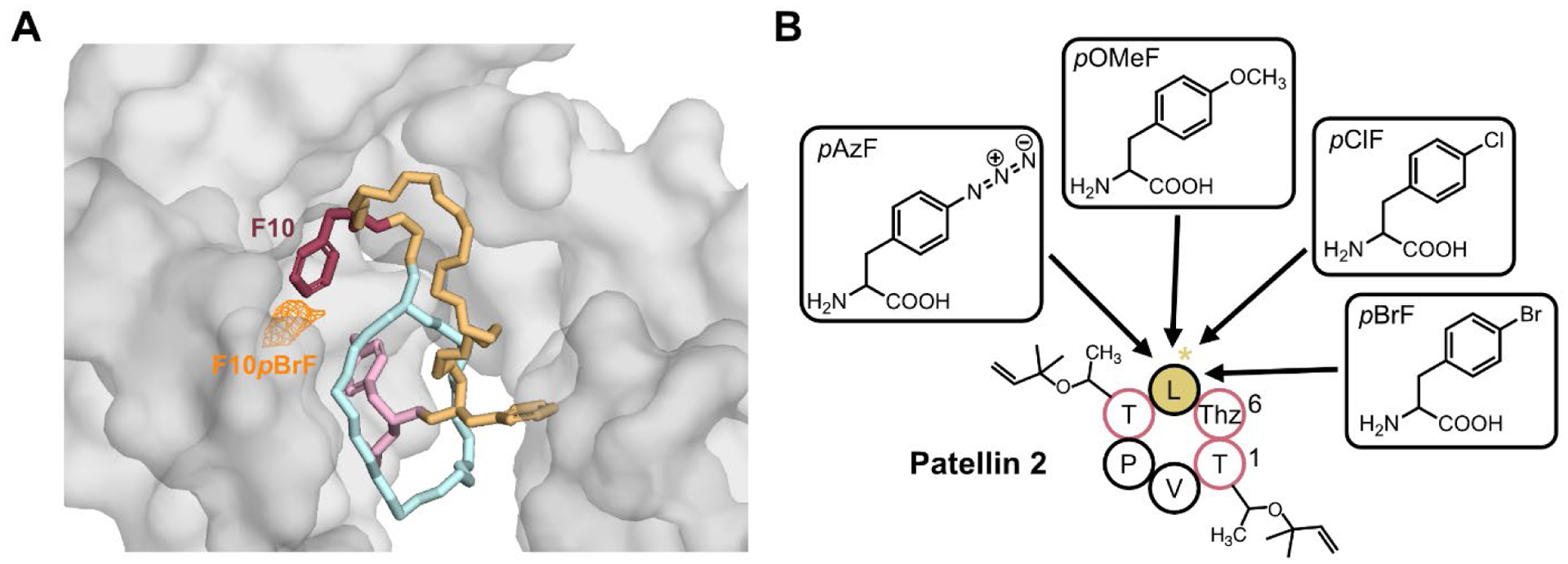
Non-canonical amino acids in RiPP engineering. A: Structure of microcin J25 (MccJ25, sticks) bound to E. coli RNA polymerase (RNAP, space-filling). The precise positioning of MccJ25 within RNAP was enabled by the incorporation of p-bromophenylalanine (pBrF) into MccJ25. The orange mesh shows the anomalous bromine signal from pBrF. Image derived from PDB file 6N60. B: The cyanobactin patellin 2 was engineered with multiple para- substituted phenylalanine analogs.
Our group has also investigated the site-specific incorporation of meta-substituted Phe analogs in the lasso peptide MccJ25 [39]. In these studies, an engineered substrate-tolerant variant of PylRS [40,41] enabled the incorporation of the Phe analogs. Four different ncAAs were tested at each of four different positions within MccJ25. All 16 of these variants were produced, as judged by mass spectrometry, and 15 of the 16 were produced at levels detectable by HPLC analysis. The introduction of the ncAA negatively affected the production yield of the substituted MccJ25 variants, with yields ranging from ~2–30% of the wild-type titer. Likewise, many of the ncAA-substituted MccJ25 variants had reduced antimicrobial activity relative to the wild-type peptide, though some bromophenylalanine and chlorophenylalanine variants retained native antimicrobial activity. The ability to generate brominated derivatives of MccJ25 was key to solving a crystal structure of this RiPP bound to its cytoplasmic target, RNAP [42]. A novel crystal form of RNAP supported the binding of MccJ25, and electron density for MccJ25 was observed in the secondary channel of RNAP. However, the resolution of this structure was insufficient to orient the peptide with RNAP unambiguously. MccJ25 substituted with p-bromophenylalanine in two locations, His5 and Phe10, also bound the RNAP crystals, and the anomalous signal from the bromine atoms in these structures allowed for precise positioning of MccJ25 within the RNAP secondary channel (FIGURE 3). This work shows how ncAA incorporation into RiPPs can be utilized not only as a tool for generating new variants, but also understanding RiPP mechanisms of action.
Construction and screening of RiPP libraries
In an extension of the site-directed mutagenesis studies described above, combinatorial mutant libraries of genes encoding RiPP precursors can be generated using standard recombinant DNA techniques. Below we will discuss examples of this library approach aimed at improving the natural function of RiPPs as well as library screens aimed at repurposing a RiPP for an entirely different function. The key to the success of any library screening effort is the design of the screen, so much of our discussion below focuses on the screens employed.
Our group generated libraries of the lasso peptide MccJ25 in which three of the 21 aa positions were mutagenized using the NNT degenerate codon (N = A,T,G,C) [43]. This degenerate codon covers 15 of the 20 amino acids in 16 codons, reducing library bias and the number of clones that must be examined for full coverage of the library. Two separate libraries were constructed, one that focused on the loop region of MccJ25 and one that focused on its ring. To screen the MccJ25 variants for antimicrobial activity, an indicator strain of E. coli was engineered in which production of MccJ25 and its immunity factor, an exporter, were orthogonally inducible. The library was assessed using a replica plating strategy in which plates in the permissive condition (MccJ25 produced and immunity factor produced) were compared to plates in the restrictive condition (MccJ25 produced, immunity factor repressed). This strategy allowed for the entire libraries to be screened in a semi-high-throughput fashion and revealed that roughly half of the triple mutants in the library retained antimicrobial activity, at least when produced directly in the cytoplasm of the cell. Ultimately, 12 MccJ25 variants were discovered that had increased potency relative to wild-type MccJ25.
Schmitt et al. generated a library of chimeric lanthipeptide precursor genes by mixing and matching fragments of 12 distinct lanthipeptides in a process akin to nonhomologous recombination [44]. This library was screened by encapsulating a single L. lactis producer cell expressing a lanthipeptide variant and ~150 reporter cells (Micrococcus flavus) in 500 μm alginate droplets. After incubation of these droplets, the cell mixture was treated with SYTO 9, a dye that causes live cells to fluoresce green. Flow cytometry was employed to sort droplets with low green fluorescence, and sequencing of the lanthipeptide genes from these droplets allowed for identification of active variants. This procedure, which the authors term nanoFleming, resulted in 126 new hybrid lanthipeptides, some of which had improved activity or altered spectra of activity relative to known lanthipeptides. This large scale library study also revealed design rules for future efforts aimed at engineering antimicrobial lanthipeptides.
While the two studies discussed above have focused on improvement of the natural function of a RiPP (namely antimicrobial activity), in principle library screening can be used to steer a RiPP toward an entirely different function. Yang et al. published a seminal demonstration of this idea utilizing a library of lanthipeptides [45]. The prochlorosins are unique among the lanthipeptides because a single lanthipeptide synthetase, ProcM, can process dozens of distinct natural precursor peptides (ProcAs) into mature lanthipeptides [46]. Thus, ProcM is a naturally promiscuous maturation enzyme especially well-suited for the construction of combinatorial libraries of ProcA precursors. The specific ProcA precursor chosen for mutagenesis, mProcA2.8, is comprised of two 7 aa lanthionine rings (FIGURE 4), leaving five positions within each ring available for mutagenesis. The authors used the degenerate codon NWY, which encodes a set of 8 aa, at all 10 variable positions, resulting in a library with 810 or roughly 109 protein variants of mProcA2.8. The quality of the library was assessed using next generation sequencing, revealing roughly 106 distinct mutants in 2 × 106 reads. A total of 33 members of the library were randomly selected to ensure that the variants would be correctly processed by ProcM. All 33 variants were indeed processed, underscoring the promiscuity of ProcM. This library was screened to identify inhibitors of the protein-protein interaction between the p6 protein from HIV and the UEV (ubiquitin E2 variant) domain of the human protein TSG101, an interaction crucial for HIV budding and cell-to-cell propagation. A reverse two-hybrid growth selection [47] in E. coli was employed such that if an mProcA2.8 variant inhibited the protein-protein interaction, the E. coli cell harboring that variant would survive on selective media. This plate-based growth assay allowed for rapid screening of the large library, and ultimately a single hit, termed XY3–3 was isolated. The XY3–3 peptide inhibited the protein-protein interaction with an IC50 in the single micromolar range, and when fused to the Tat cell penetrating peptide could also inhibit viral budding in a cell-based assay. Overall, this is a remarkable example of repurposing a RiPP scaffold for an entirely unrelated function.
Figure 4:
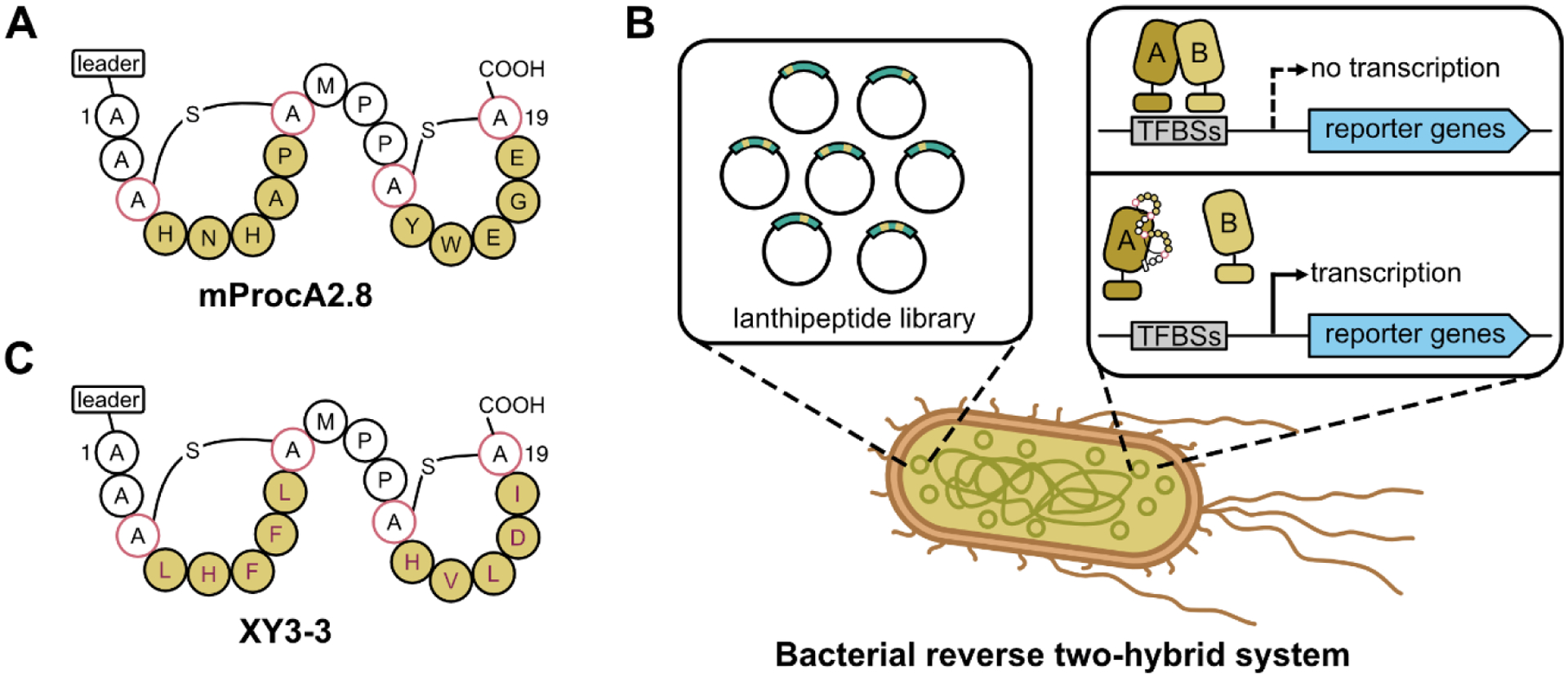
Engineering a lanthipeptide into a protein-protein interaction inhibitor. A: Native sequence of the lanthipeptide mProcA2.8. Positions colored maize were subjected to saturation mutagenesis to create a ~109 member library. B: Bacterial reverse two-hybrid screen to identify library members capable of inhibiting the interaction between two proteins, schematized here as A and B. Proteins A and B are fused to transcription factors that bind to transcription factor binding sites (TFBSs). Successful disruption of the interaction between A and B allows for cell growth. C: Hit from the library screen XY3–3. Note that all 10 positions have been modified relative to the native lanthipeptide.
Engineering of RiPP enzymes and pathways
While this perspective has thus far focused on protein engineering of RiPP precursors (i.e., the substrates in RiPP biosynthesis), the enzymes that mature RiPPs have also been engineered. This section highlights examples of enzyme engineering across multiple RiPP classes as well as efforts to generate RiPP hybrids containing modifications from multiple classes. A compelling site-directed mutagenesis study was carried out by Yang et al. on the peptidyl cyclase OaAEP1 [48]. This enzyme, found in the plant Oldenlandia affinis, catalyzes the cyclization of cyclotides, a RiPP family exhibiting head-to-tail cyclization [49]. OaAEP1 is homologous to asparaginyl endopeptidases (AEPs), but catalyze peptidyl ligation rather than hydrolysis. Structural analysis of OaAEP1 revealed the presence of a potential “gatekeeper” residue, Cys247, near the active site of OaAEP1. Site-directed mutagenesis was carried out on this residue. While the C247G substitution led to OaAEP1 favoring peptide hydrolysis instead of ligation, the most interesting results were obtained with the C247A variant. Kinetic studies on the C247A variant of OaAEP1 revealed that it was much more catalytically competent than the native enzyme with an increase in the specificity factor (kcat/Km) of more than 100-fold on a linear peptide substrate. This allowed the authors to use OaAEP1 C247A as a protein ligation catalyst to assemble polyubiquitin chains on a protein of interest with only nanomolar concentrations of the enzyme.
A classic application of library-based protein engineering is the expansion or alteration of the substrate specificity of an enzyme. Engineering studies of this type have been carried out on NisB, a key enzyme for thioether formation in the lanthipeptide nisin. NisB functions in two steps: first a Ser or Thr residue in the substrate is glutamylated followed by elimination of glutamate to afford dehydroalanine from Ser or dehydrobutyrine from Thr [50]. The residue in the substrate immediately preceding the Ser/Thr to be dehydrated influences the activity of NisB. For example, Zhao et al. showed that an Asp residue immediately preceding Ser prevented dehydration of the Ser residue [51]. To engineer NisB to accept this alternative substrate, the authors created a random NisB library by error-prone PCR with ~105 members. The library was screened in a high-throughput fashion by leveraging the fact that cyclic peptides containing the tetrapeptide HPQF bind streptavidin ~103 more avidly than their linear counterparts. A hexapeptide SHPQFC that can be cyclized by NisB between the underlined residues was fused to the first 12 aa of nisin. While NisB was able to cyclize a peptide in which the SHPQFC sequence was preceded by Lys, NisB had no activity toward a substrate in which Lys was swapped for Asp. The authors displayed the nisin-SHPQFC chimera on the cell surface of L. lactis and screened the library of NisB variants using streptavidin-coated magnetic beads to separate out those cells that had successfully installed a thioether into the SHPQFC peptide. Sequencing of the winners from this screen revealed a variant with two point mutations in 18/20 clones assessed. This NisB variant was able to dehydrate the alternative substrates against which it was screened as well as maintaining activity on conventional nisin substrates.
As described in the introduction, the leader peptide is a key component in the biosynthesis of RiPPs that often guides the RiPP biosynthetic enzymes. But the leader peptide can also be a liability. For many RiPPs it must be cleaved for the final product to have bioactivity. The leader peptide also represents an additional protein synthesis burden, which is especially keen if precursor peptides are being assembled via solid-phase peptide synthesis for biocatalytic production of RiPPs. Oman et al. asked whether the biosynthesis of the lanthipeptide lacticin 481 could proceed with the lacticin 481 precursor peptide LctA split into two separate polypeptides, the leader peptide and core peptide [52]. This amounts to providing the core peptide in trans to the lanthipeptide synthetase LctM. This strategy was successful, but required large quantities of the leader peptide. As an improvement to this strategy, the authors decided to provide the leader peptide in cis to LctM by directly fusing it to this enzyme (FIGURE 5). The fusion of the LctA leader to LctM provides an effectively infinite concentration of the leader peptide to LctM. Careful selection of the linker length between the LctA leader peptide and LctM led to a highly efficient lanthipeptide synthetase that could process the core peptide alone. Since this initial demonstration of leader peptide fusion to RiPP maturation enzymes, similar systems have been developed for the biosynthesis of cyanobactins [53] and microviridins [54].
Figure 5:
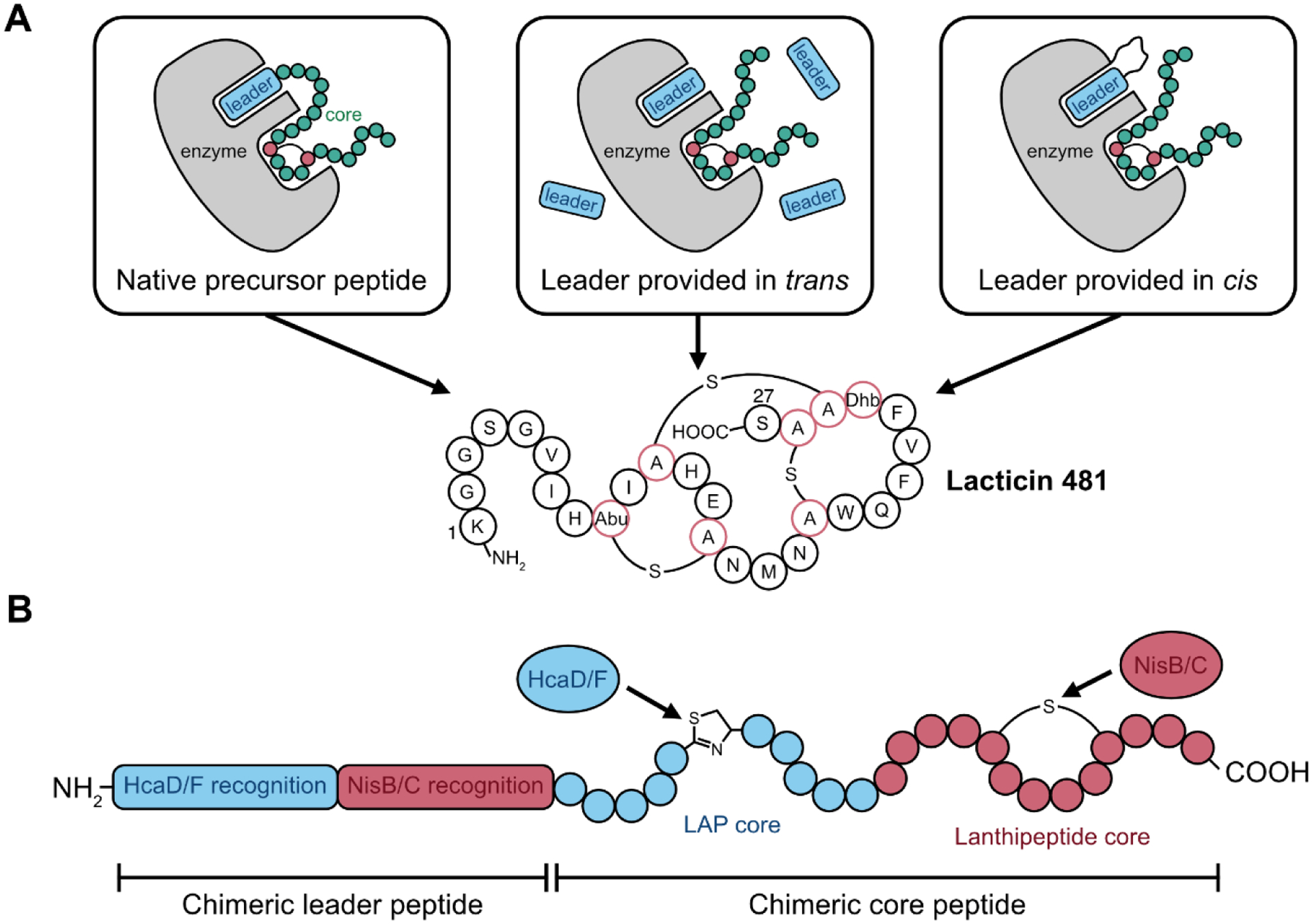
Examples of RiPP enzyme and pathway engineering. A: Proper maturation of RiPPs can occur when the leader peptide is provided in trans or in cis. Left panel: native presentation of the leader peptide, covalently fused to the core peptide. Middle panel: leader peptide provided separately in excess. Right panel: fusion of the leader peptide to the maturation enzyme leads to more efficient maturation than when the leader peptide is provided in trans. B: Chimeric RiPPs can be generated by mixing two different RiPP enzymes and by generating a chimeric leader peptide that is recognized by both enzymes.
A hallmark of RiPPs biosynthesis is its modularity; leader peptides direct specific enzymes to modify the core peptide. As a test of the engineerability of this modularity, Burkhart et al. developed hybrid leader peptides containing recognition elements for two distinct RiPP biosynthetic enzymes [55]. These leader peptides were fused to chimeric core peptides capable of modifications from both enzymes (FIGURE 5). This strategy was successful in generating two different chimeras of lanthipeptides and linear azol(in)e-containing peptides (LAPs) as well as a chimera of a sactipeptide and LAP. Franz and Koehnke recently demonstrated an alternative route to generating RiPP chimeras. Rather than fusing multiple recognition sequences within a single leader peptide, these authors employed the transpeptidase sortase A to swap out the entire leader peptide for a different leader peptide. This strategy, referred to as leader peptide exchange, was demonstrated with leader peptides and enzymes from the cyanobactin and graspetide families, resulting in a chimeric RiPP with both thiazolines and sidechain-sidechain esters [56]. These studies show the power of the modular logic of RiPP biosynthesis and set the stage for engineering even more exotic RiPP chimeras.
Outlook for protein engineering in RiPPs
Protein engineering will continue to play a role in understanding the residues most important for RiPP maturation as well as in determining structure-activity relationships. With the ever-increasing deluge of sequencing data and improvements in synthetic biology and heterologous expression, most RiPP discovery today is via a genomics-first approach. This is in stark contrast to natural product discovery efforts in the pre-genomic era, which relied on bioactivity-guided analysis. As a consequence, many RiPPs have either unknown function or lack detailed mechanism of action studies. Protein engineering can play a role in closing this gap between RiPP discovery and RiPP function. For example, RiPPs with known antimicrobial activity can be prepared with photocrosslinking ncAAs, such as p-benzoylphenylalanine and p-azidophenylalanine. These engineered RiPPs can be incubated with susceptible cells, irradiated, and then analyzed using proteomic or metabolomic techniques to identify cellular targets of the RiPP. Photocrosslinking approaches enabled by ncAAs can also be useful in defining the interactions between RiPP precursors and maturation proteins [57]. One important area of protein engineering that, to our knowledge, has not yet made its way to work on RiPPs is de novo design of proteins. With powerful protein structure prediction algorithms like AlphaFold being made available to the public [58], one could imagine designing RiPP biosynthetic enzymes tailored to specific unnatural precursor peptides. Another more ambitious use of de novo protein design would be to generate chimeric multi-enzyme complexes that can more efficiently process hybrid RiPPs like the lanthipeptide/LAP and sactipeptide/LAP crossovers discussed above. These types of protein engineering will require continued studies on the mechanisms of RiPP enzymes. The future of RiPPs research is bright, and protein engineering is poised to continue playing an outsized role in moving the field forward.
References
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian K-D, Fischbach MA, Garavelli JS, Goransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Muller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl H-G, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Sussmuth RD, Tagg JR, Tang G-L, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA: Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Natural Product Reports 2013, 30:108–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YY, Rebuffat S: The manifold roles of microbial ribosomal peptide-based natural products in physiology and ecology. Journal of Biological Chemistry 2020, 295:34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Do T, Link AJ: Mechanisms of action of ribosomally synthesized and posttranslationally modified peptides (RiPPs). Journal of Industrial Microbiology & Biotechnology 2021, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalban-Lopez M, Scott TA, Ramesh S, Rahman IR, van Heel AJ, Viel JH, Bandarian V, Dittmann E, Genilloud O, Goto Y, Burgos MJG, Hill C, Kim S, Koehnke J, Latham JA, Link AJ, Martinez B, Nair SK, Nicolet Y, Rebuffat S, Sahl HG, Sareen D, Schmidt EW, Schmitt L, Severinov K, Sussmuth RD, Truman AW, Wang H, Weng JK, van Wezel GP, Zhang Q, Zhong J, Piel J, Mitchell DA, Kuipers OP, van der Donk WA: New developments in RiPP discovery, enzymology and engineering. Natural Product Reports 2021, 38:130–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter G, Fersht AR, Wilkinson AJ, Zoller M, Smith M: Redesigning enzyme structure by site-directed mutagenesis: tyrosyl tRNA synthetase and ATP binding. Nature 1982, 299:756–758. [DOI] [PubMed] [Google Scholar]
- 6.Link AJ, Mock ML, Tirrell DA: Non-canonical amino acids in protein engineering. Current Opinion in Biotechnology 2003, 14:603–609. [DOI] [PubMed] [Google Scholar]
- 7.Hendrickson WA, Horton JR, Lemaster DM: Selenomethionyl Proteins Produced for Analysis by Multiwavelength Anomalous Diffraction (MAD) - a Vehicle for Direct Determination of 3-Dimensional Structure. EMBO Journal 1990, 9:1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Schultz PG: Expanding the genetic code. Angewandte Chemie-International Edition 2005, 44:34–66. [DOI] [PubMed] [Google Scholar]
- 9.Jung G: Lantibiotics—Ribosomally Synthesized Biologically Active Polypeptides containing Sulfide Bridges and α,β-Didehydroamino Acids. Angewandte Chemie-International Edition 1991, 30:1051–1068. [Google Scholar]
- 10.Kuipers OP, Bierbaum G, Ottenwalder B, Dodd HM, Horn N, Metzger J, Kupke T, Gnau V, Bongers R, vandenBogaard P, Kosters H, Rollema HS, deVos WM, Siezen RJ, Jung G, Gotz F, Sahl HG, Gasson MJ: Protein engineering of lantibiotics. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology 1996, 69:161–169. [DOI] [PubMed] [Google Scholar]
- 11.Mulders JWM, Boerrigter IJ, Rollema HS, Siezen RJ, Devos WM: Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. European Journal of Biochemistry 1991, 201:581–584. [DOI] [PubMed] [Google Scholar]
- 12.Kuipers OP, Rollema HS, Yap W, Boot HJ, Siezen RJ, Devos WM: Engineering Dehydrated Amino Acid Residues in the Antimicrobial Peptide Nisin. Journal of Biological Chemistry 1992, 267:24340–24346. [PubMed] [Google Scholar]
- 13.van der Meer JR, Rollema HS, Siezen RJ, Beerthuyzen MM, Kuipers OP, Devos WM: Influence of Amino Acid Substitutions in the Nisin Leader Peptide on Biosynthesis and Secretion of Nisin by Lactococcus lactis. Journal of Biological Chemistry 1994, 269:3555–3562. [PubMed] [Google Scholar]
- 14.Pavlova O, Mukhopadhyay J, Sineva E, Ebright RH, Severinov K: Systematic structure-activity analysis of microcin J25. Journal of Biological Chemistry 2008, 283:25589–25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maksimov MO, Pan SJ, Link AJ: Lasso peptides: structure, function, biosynthesis, and engineering. Natural Product Reports 2012, 29:996–1006. [DOI] [PubMed] [Google Scholar]
- 16.Hegemann JD, Zimmermann M, Xie X, Marahiel MA: Lasso Peptides: An Intriguing Class of Bacterial Natural Products. Accounts of Chemical Research 2015, 48:1909–1919. [DOI] [PubMed] [Google Scholar]
- 17.Adelman K, Yuzenkova J, La Porta A, Zenkin N, Lee J, Lis JT, Borukhov S, Wang MD, Severinov K: Molecular mechanism of transcription inhibition by peptide antibiotic microcin J25. Molecular Cell 2004, 14:753–762. [DOI] [PubMed] [Google Scholar]
- 18.Cheung-Lee WL, Parry ME, Zong CH, Cartagena AJ, Darst SA, Connell ND, Russo R, Link AJ: Discovery of Ubonodin, an Antimicrobial Lasso Peptide Active against Members of the Burkholderia cepacia Complex. Chembiochem 2020, 21:1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metelev M, Arseniev A, Bushin LB, Kuznedelov K, Artamonova TO, Kondratenko R, Khodorkovskii M, Seyedsayamdost MR, Severinov K: Acinetodin and Klebsidin, RNA Polymerase Targeting Lasso Peptides Produced by Human Isolates of Acinetobacter gyllenbergii and Klebsiella pneumoniae. ACS Chemical Biology 2017, 12:814–824. [DOI] [PubMed] [Google Scholar]
- 20.Cheung-Lee WL, Parry ME, Cartagena AJ, Darst SA, Link AJ: Discovery and structure of the antimicrobial lasso peptide citrocin. Journal of Biological Chemistry 2019, 294:6822–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly WL, Pan L, Li CX: Thiostrepton Biosynthesis: Prototype for a New Family of Bacteriocins. Journal of the American Chemical Society 2009, 131:4327–4334. [DOI] [PubMed] [Google Scholar]
- 22.Brown LCW, Acker MG, Clardy J, Walsh CT, Fischbach MA: Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin. Proceedings of the National Academy of Sciences of the United States of America 2009, 106:2549–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao RJ, Duan L, Lei C, Pan HX, Ding Y, Zhang Q, Chen DJ, Shen B, Yu Y, Liu W: Thiopeptide Biosynthesis Featuring Ribosomally Synthesized Precursor Peptides and Conserved Posttranslational Modifications. Chemistry & Biology 2009, 16:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris RP, Leeds JA, Naegeli HU, Oberer L, Memmert K, Weber E, LaMarche MJ, Parker CN, Burrer N, Esterow S, Hein AE, Schmitt EK, Krastel P: Ribosomally Synthesized Thiopeptide Antibiotics Targeting Elongation Factor Tu. Journal of the American Chemical Society 2009, 131:5946–5955. [DOI] [PubMed] [Google Scholar]
- 25.Acker MG, Bowers AA, Walsh CT: Generation of Thiocillin Variants by Prepeptide Gene Replacement and in Vivo Processing by Bacillus cereus. Journal of the American Chemical Society 2009, 131:17563–17565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deane CD, Melby JO, Molohon KJ, Susarrey AR, Mitchell DA: Engineering Unnatural Variants of Plantazolicin through Codon Reprogramming. ACS Chemical Biology 2013, 8:1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz R, Molohon KJ, Nachtigall J, Vater J, Markley AL, Sussmuth RD, Mitchell DA, Borriss R: Plantazolicin, a Novel Microcin B17/Streptolysin S-Like Natural Product from Bacillus amyloliquefaciens FZB42. Journal of Bacteriology 2011, 193:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molohon KJ, Blair PM, Park S, Doroghazi JR, Maxson T, Hershfield JR, Flatt KM, Schroeder NE, Ha T, Mitchell DA: Plantazolicin Is an Ultranarrow-Spectrum Antibiotic That Targets the Bacillus anthracis Membrane. ACS Infectious Diseases 2016, 2:207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldach F, Al Toma R, Kuthning A, Caetano T, Mendo S, Budisa N, Sussmuth RD: Congeneric Lantibiotics from Ribosomal In Vivo Peptide Synthesis with Noncanonical Amino Acids. Angewandte Chemie-International Edition 2012, 51:415–418. [DOI] [PubMed] [Google Scholar]
- 30.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB: A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angewandte Chemie-International Edition 2002, 41:2596–2599. [DOI] [PubMed] [Google Scholar]
- 31.Al Toma RS, Kuthning A, Exner MP, Denisiuk A, Ziegler J, Budisa N, Sussmuth RD: Site-Directed and Global Incorporation of Orthogonal and Isostructural Noncanonical Amino Acids into the Ribosomal Lasso Peptide Capistruin. Chembiochem 2015, 16:503–509. [DOI] [PubMed] [Google Scholar]
- 32.Knappe TA, Linne U, Zirah S, Rebuffat S, Xie XL, Marahiel MA: Isolation and structural characterization of capistruin, a lasso peptide predicted from the genome sequence of Burkholderia thailandensis E264. Journal of the American Chemical Society 2008, 130:11446–11454. [DOI] [PubMed] [Google Scholar]
- 33.Donia MS, Ravel J, Schmidt EW: A global assembly line for cyanobactins. Nature Chemical Biology 2008, 4:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tianero MDB, Donia MS, Young TS, Schultz PG, Schmidt EW: Ribosomal Route to Small-Molecule Diversity. Journal of the American Chemical Society 2012, 134:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young TS, Ahmad I, Yin JA, Schultz PG: An Enhanced System for Unnatural Amino Acid Mutagenesis in E. coli. Journal of Molecular Biology 2010, 395:361–374. [DOI] [PubMed] [Google Scholar]
- 36.Shi YX, Yang XA, Garg N, van der Donk WA: Production of Lantipeptides in Escherichia coli. Journal of the American Chemical Society 2011, 133:2338–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopatniuk M, Myronovskyi M, Luzhetskyy A: Streptomyces albus: A New Cell Factory for Non-Canonical Amino Acids Incorporation into Ribosomally Synthesized Natural Products. Acs Chemical Biology 2017, 12:2362–2370. [DOI] [PubMed] [Google Scholar]
- 38.Luo XZ, Zambaldo C, Liu T, Zhang YH, Xuan WM, Wang C, Reed SA, Yang PY, Wang RE, Javahishvili T, Schultz PG, Young TS: Recombinant thiopeptides containing noncanonical amino acids. Proceedings of the National Academy of Sciences of the United States of America 2016, 113:3615–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piscotta FJ, Tharp JM, Liu WR, Link AJ: Expanding the chemical diversity of lasso peptide MccJ25 with genetically encoded noncanonical amino acids. Chemical Communications 2015, 51:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YS, Fang XQ, Chen HY, Wu B, Wang ZYU, Hilty C, Liu WSR: Genetic Incorporation of Twelve meta-Substituted Phenylalanine Derivatives Using a Single Pyrrolysyl-tRNA Synthetase Mutant. ACS Chemical Biology 2013, 8:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YS, Fang XQ, Wallace AL, Wu B, Liu WSR: A Rationally Designed Pyrrolysyl-tRNA Synthetase Mutant with a Broad Substrate Spectrum. Journal of the American Chemical Society 2012, 134:2950–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braffman N, Piscotta FJ, Hauver J, Campbell EA, Link AJ, Darst SA: Structural mechanism of transcription inhibition by lasso peptides microcin J25 and capistruin. Proceedings of the National Academy of Sciences of the United States of America 2019, 116:1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan SJ, Link AJ: Sequence Diversity in the Lasso Peptide Framework: Discovery of Functional Microcin J25 Variants with Multiple Amino Acid Substitutions. Journal of the American Chemical Society 2011, 133:5016–5023. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt S, Montalban-Lopez M, Peterhoff D, Deng JJ, Wagner R, Held M, Kuipers OP, Panke S: Analysis of modular bioengineered antimicrobial lanthipeptides at nanoliter scale. Nature Chemical Biology 2019, 15:437–443. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Lennard KR, He C, Walker MC, Ball AT, Doigneaux C, Tavassoli A, van der Donk WA: A lanthipeptide library used to identify a protein-protein interaction inhibitor. Nature Chemical Biology 2018, 14:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Sher D, Kelly L, Shi YX, Huang K, Knerr PJ, Joewono I, Rusch D, Chisholm SW, van der Donk WA: Catalytic promiscuity in the biosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America 2010, 107:10430–10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavassoli A, Lu Q, Gam J, Pan H, Benkovic SJ, Cohen SN: Inhibition of HIV Budding by a Genetically Selected Cyclic Peptide Targeting the Gag-TSG101 Interaction. Acs Chemical Biology 2008, 3:757–764. [DOI] [PubMed] [Google Scholar]
- 48.Yang RL, Wong YH, Nguyen GKT, Tam JP, Lescar J, Wu B: Engineering a Catalytically Efficient Recombinant Protein Ligase. Journal of the American Chemical Society 2017, 139:5351–5358. [DOI] [PubMed] [Google Scholar]
- 49.Craik DJ, Malik U: Cyclotide biosynthesis. Current Opinion in Chemical Biology 2013, 17:546–554. [DOI] [PubMed] [Google Scholar]
- 50.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK: Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 2015, 517:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao XH, Cebrian R, Fu YX, Rink R, Bosma T, Moll GN, Kuipers OP: High-Throughput Screening for Substrate Specificity-Adapted Mutants of the Nisin Dehydratase NisB. Acs Synthetic Biology 2020, 9:1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oman TJ, Knerr PJ, Bindman NA, Velasquez JE, van der Donk WA: An Engineered Lantibiotic Synthetase That Does Not Require a Leader Peptide on Its Substrate. Journal of the American Chemical Society 2012, 134:6952–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koehnke J, Mann G, Bent AF, Ludewig H, Shirran S, Botting C, Lebl T, Houssen WE, Jaspars M, Naismith JH: Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nature Chemical Biology 2015, 11:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reyna-Gonzalez E, Schmid B, Petras D, Sussmuth RD, Dittmann E: Leader Peptide-Free In Vitro Reconstitution of Microviridin Biosynthesis Enables Design of Synthetic Protease-Targeted Libraries. Angewandte Chemie-International Edition 2016, 55:9398–9401. [DOI] [PubMed] [Google Scholar]
- 55.Burkhart BJ, Kakkar N, Hudson GA, van der Donk WA, Mitchell DA: Chimeric Leader Peptides for the Generation of Non-Natural Hybrid RiPP Products. ACS Central Science 2017, 3:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franz L, Koehnke J: Leader peptide exchange to produce hybrid, new-to-nature ribosomal natural products. Chemical Communications 2021, 57:6372–6375. [DOI] [PubMed] [Google Scholar]
- 57.Cheung WL, Chen MY, Maksimov MO, Link AJ: Lasso Peptide Biosynthetic Protein LarB1 Binds Both Leader and Core Peptide Regions of the Precursor Protein LarA. ACS Central Science 2016, 2:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D: Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]


