Abstract
The role of surgery for metastases to the vertebra from yolk sac tumours has not been established. The main treatment for disseminated disease is chemotherapy. We present a man in his 30s with a left orchiectomy for a testicular mixed germ cell tumour with a prominent yolk sac component who, 12 months later, developed an asymptomatic metastasis to the L2 vertebra unresponsive to chemotherapy and radiotherapy. The patient underwent resection of the L2 vertebral body, leaving a small residual tumour anterior to the vertebra attached to the great vessels. Pathology confirmed the diagnosis of a metastatic testicular yolk sac tumour in the vertebra. The postoperative MRI 6 months later demonstrated significant expansion of the tumour at the soft tissues anterior to the expandable titanium cage encasing the great vessels and extending to the paraspinal areas. Additional salvage surgery was not recommended because of the advanced stage of the tumour.
Keywords: Neuroimaging, Neurooncology, Urological cancer, Pathology, Neurosurgery
Background
Yolk sac tumour, also known as endodermal sinus tumour, is the most common malignant germ cell tumour of the testis in infants and children.1 2 This tumour presents more frequently in children under the age of 3 years.3 Another peak occurs in adults around their early 30s.4 Yolk sac tumours may contain other histological cells and are grouped as mixed germ cell tumours. After the 2016 WHO classification update, Stang et al4 reclassified their cases of testicular tumours and noted that yolk sac tumours accounted for only 0.6% among pure testicular germ cell tumours and 12.3% among mixed testicular germ cell tumours.
Spinal metastases from testicular yolk sac tumours are rare, occurring more frequently at the epidural or paraspinal region.1 2 5 6 The adjuvant surgical management of vertebral body metastases from yolk sac tumours has not been established, as chemotherapy is the main treatment for disseminated disease.1 3 5 However, in the presence of neurological deficits, surgery and radiotherapy can be added to control the disease and improve neurological function.7 This report describes a patient who developed an asymptomatic yolk sac metastasis to the L2 vertebra, unresponsive to chemotherapy and conventional radiotherapy. The patient was treated with surgical resection of the vertebral body to control the disease and preserve spinal column stability.
Case presentation
A man in his 30s with a history of testicular stage IIIC mixed germ cell tumour with a prominent yolk sac component treated 12 months before with a left orchiectomy and adjuvant chemotherapy (bleomycin, etoposide and cisplatin) developed an asymptomatic lesion at the L2 vertebral body discovered on a CT positron emission tomography (figure 1). The study showed erosion and loss of the anterior third of the vertebral body without bone retropulsion or gross instability. Laboratory testing revealed a serum alpha-fetoprotein (AFP) level of 2399 ng/dL. A needle biopsy was performed to the L2 vertebral lesion, which was compatible with a metastatic yolk sac tumour. He received 30 Gray of radiotherapy in 10 fractions using volumetric modulated arc therapy to the L2 vertebra. Salvage chemotherapy (paclitaxel, ifosfamide and cisplatin) was given. Despite the chemotherapy and radiotherapy treatment, the tumour increased in size and the oncology tumour board recommended the removal of the L2 vertebral body. The serum AFP increased to 12 639 ng/dL. The patient was referred to the neurosurgery department for evaluation and management. Neurological examination showed motor strength 5/5 in all extremities without sensory deficits. Deep tendon reflexes were 2+ bilateral. He was independent in the activities of daily living.
Figure 1.
Preoperative CT positron emission tomography shows a lesion (yellow arrows) at the L2 vertebral body with erosion and loss of the anterior third of the vertebral body. (A) CT sagittal bone reconstruction of the thoracolumbar region. (B) Image fusion.
Investigations
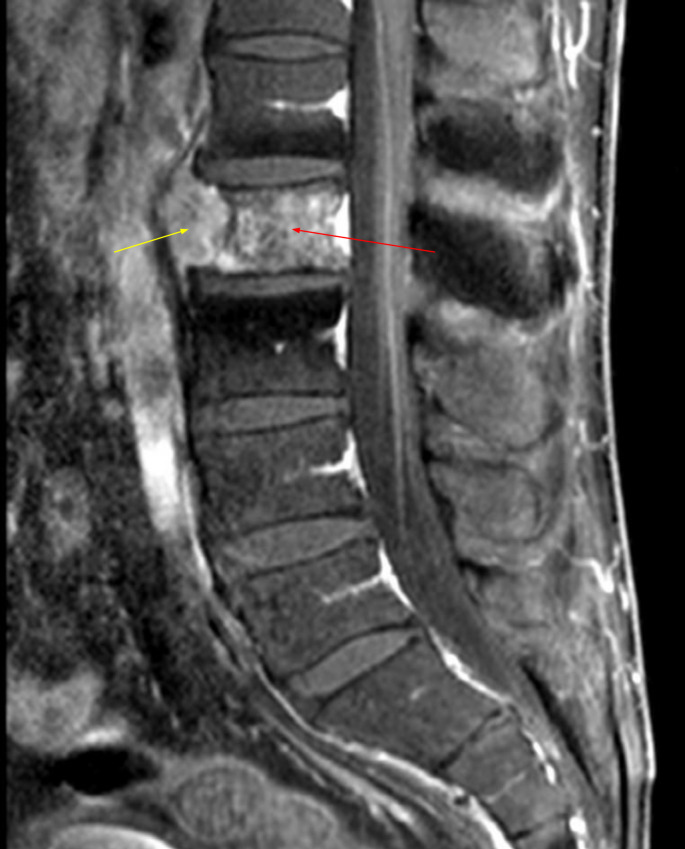
A lumbar MRI showed the L2 vertebral metastasis with a small soft tissue tumourous component at the anterior aspect of the vertebra but without an epidural component (figure 2). A chest and abdomen CT scan with contrast showed no hilar, mediastinal or axillary adenopathy. The patient was admitted for preoperative embolisation of the tumour and resection of the L2 vertebral body. A thoracolumbar spine digital subtraction angiography was performed, and the tumour feeders from both L2 segmentary arteries were embolised with polyvinyl alcohol particles and fibre coils.
Figure 2.
Preoperative lumbar MRI demonstrates the L2 vertebra body metastasis (red arrow) with a small soft tissue tumour component at the anterior aspect of the vertebra (yellow arrow) but without an epidural component.
Treatment
A left retroperitoneal retropleural lateral L2 corpectomy was performed 1 day after the embolisation. An expandable titanium cage was placed between the L1 and L3 vertebral bodies followed by posterior instrumentation using percutaneous pedicle screws at L1 and L3 (figure 3). A small soft tissue tumour component attached to the great vessels anterior to the L2 vertebral body was not resected to avoid vascular injury. The postoperative lumbar CT scan showed the L2 vertebral body corpectomy with an adequate position of the titanium cage vertebral body replacement and posterior instrumentation (figure 4). There were no postoperative complications. Post-op strength examination was 5/5 bilateral at iliopsoas, quadriceps and hamstrings.
Figure 3.

Postoperative X-ray shows the expandable titanium cage (black arrow) between the L1 and L3 vertebral bodies and the posterior fixation (black arrowhead) from L1–L3 with bilateral transpedicular screws at L1 and L3.
Figure 4.

Postoperative lumbar spine CT scan showing the corpectomy of the L2 vertebral body with the placement of an expandable titanium cage from L1 to L3 (A: sagittal reconstruction, B: coronal reconstruction).
The histopathological diagnosis was a metastatic yolk sac tumour to the L2 vertebra (figure 5). Immunohistochemistry showed a diffuse strong positive immunoreaction for AFP and strong positive immunoreaction for pankeratin. There was a weakly positive immunoreaction for placental-like alkaline phosphatase (PLAP) in occasional cells. Human chorionic gonadotropin, octamer-binding transcription factor 4 (OCT4), CD30, c-Kit and S100 protein markers were negative. Programmed death-ligand 1 immunohistochemistry was not performed.
Figure 5.
(A) Nests of tumour cells amidst fibrinoid deposits, with tumour necrosis at the bottom of the image H&E 200×. (B) Tumour at higher magnification H&e 400×. (C) Pleomorphic tumour cells with vesicular chromatin and single prominent nucleolus H&e 600×. (D) Tumour field with tumourous necrosis at the left upper field, H&e 600×. (E) Alpha-fetoprotein immunostain with strong positive and diffuse positive immunoreaction 200×. (F) Pankeratin with strong positive immunoreaction 200×.
Outcome and follow-up
The oncology service evaluated the patient for an autologous stem cell transplant, but it was not recommended because of the advanced stage of the disease. After the patient consented to the treatment, he was started off-label on immunotherapy with pembrolizumab. However, 6 months after the surgery, a follow-up MRI demonstrated that the tumour had expanded significantly to the paraspinal areas and soft tissues anterior to the expandable titanium cage, completely encasing the great vessels (figure 6). The lumbar CT scan showed tumour infiltration at the posterior L1–L2 bony elements and the inferior portion of the L1 vertebral body (figure 7). After three doses, pembrolizumab was discontinued as it did not demonstrate any clinical benefit to the patient. Additional salvage surgery was not recommended as the tumour had grown extensively to adjacent vital tissues. Three months later, he died from multiorgan failure.
Figure 6.
MRI T2-weighted images of the thoracolumbar region (A: sagittal, B: coronal) performed 6 months after the surgery demonstrate significant tumour expansion anterior to the expandable titanium cage encasing the great vessels with bilateral extension to the paraspinal areas more prominent on the left side (yellow arrows).
Figure 7.

Lumbar CT scan sagittal reconstruction image performed 6 months after the surgery shows tumour expansion anterior to the expandable titanium cage and involvement of the L1–L2 posterior elements and the inferior portion of the L1 vertebral body (white arrows).
Discussion
Bone metastases from germ cell tumours are rare, occurring in up to 9% of the cases.8–12 Bone metastases occur more frequently in patients with yolk sac tumour histology with widespread systemic disease.9 12 The most frequent sites of metastases from testicular yolk sac tumours are the lungs, retroperitoneal lymph nodes, liver and bone.5 13 Testicular yolk sac tumours rarely develop metastases to the vertebrae.2 5 A spine MRI with contrast is highly recommended during the workup for metastasis as the CT scan is not as sensitive as the MRI to detect bone involvement in metastatic germ cell tumours.11 14
Yolk sac tumours typically secrete AFP. Elevation of AFP can be detected in over 80% of patients with testicular yolk sac tumours and can be used as a reliable marker in the diagnosis and follow-up of the disease.6 15 A small number of patients can have a normal AFP level at the time of the diagnosis of the primary tumour, but the level rapidly rises when the patient develops metastases.5 Several other diagnostic tumour markers have been used to identify or exclude testicular yolk sac tumours. In the study by Zhang et al,3 PLAP was detected in 81.3% of the yolk sac tumours, while OCT4 was not detected in any case.
For malignant germ cell tumours, complete resection of the primary tumour has been established as the most potent prognostic parameter.16 After the introduction of cisplatin, the outcome of patients with malignant germ cell tumours improved to a 90% 5-year survival.15 The most current chemotherapy protocol with proven efficacy for malignant germ cell tumours includes a combination of cisplatin, etoposide and bleomycin.15 High-dose platinum-based chemotherapy is recommended for patients presenting germ cell tumours with bone and spinal involvement, whereas salvage radiotherapy and chemotherapy are suggested for platinum-resistant germ cell tumours.9 10 Surgical decompression, tumour removal and adjuvant radiotherapy/chemotherapy have been recommended to treat spinal metastatic yolk sac tumours producing cord compression.6 17 18 Metastases to bones, brain and liver are associated with a poor prognosis.10 Patients relapsing with bone metastases have a dismal outcome, despite cisplatin or carboplatin chemotherapy as salvage treatment.8 19 Recently, immunotherapy with checkpoint inhibitors like pembrolizumab has been used as a treatment for platinum-refractory germ cell tumours.20–23 Pembrolizumab did not produce any clinical benefit to our patient, in line with most published literature that shows no improvement in the progression-free survival or the overall survival for refractory testicular germ cell tumours.20–23 The case presented is particularly rare as the patient had no instability or neurological compromise but had significant vertebral body involvement. The decompression and surgical stabilisation prevented the development of instability and neurological deficit that would likely have occurred if not performed.
Learning points.
Vertebral metastases from yolk sac tumours are rare and often present rapid growth.
Metastatic yolk sac tumours are frequently resistant to chemotherapy and radiotherapy.
The prognosis for patients with vertebral metastases from yolk sac tumours is poor.
Surgical stabilisation of the spine in patients with spinal metastasis from malignant germ cell tumours helps to prevent instability and possible neurological deterioration.
Footnotes
Contributors: Conception and design: GM and ODJ. Drafting the manuscript: GM, ODJ, MC-R and JCO. Approval of manuscript: GM, ODJ, MC-R and JCO.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Liu HC, Liang DC, Chen SH, et al. The stage I yolk sac tumor of testis in children younger than 2 years, chemotherapy or not? Pediatr Hematol Oncol 1998;15:223–8. 10.3109/08880019809028788 [DOI] [PubMed] [Google Scholar]
- 2.Unal O, Beyazal M, Avcu S, et al. Metastasis of testicular yolk sac tumor to cauda equina. Fetal Pediatr Pathol 2011;30:150–5. 10.3109/15513815.2010.547553 [DOI] [PubMed] [Google Scholar]
- 3.Zhang T, Ji L, Liu B, et al. Testicular germ cell tumors: a clinicopathological and immunohistochemical analysis of 145 cases. Int J Clin Exp Pathol 2018;11:4622–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Stang A, Rusner C, Trabert B, et al. Incidence of testicular tumor subtypes according to the updated WHO classification, North Rhine-Westphalia, Germany, 2008-2013. Andrology 2019;7:402–7. 10.1111/andr.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J-K, Kim S-H, Kim J-H, et al. Metastatic spinal cord compression of testicular yolk sac tumor. Childs Nerv Syst 2002;18:171–4. 10.1007/s00381-002-0554-7 [DOI] [PubMed] [Google Scholar]
- 6.Colak A, Benli K, Berker M, et al. Epidural metastasis of testicular yolk sac tumor: an unusual cause of spinal cord compression. Case report. Pediatr Neurosurg 1991-1992;17:139–41. 10.1159/000120584 [DOI] [PubMed] [Google Scholar]
- 7.Almouhissen T, Badr H, AlMatrafi B, et al. Testicular cancer in Down syndrome with spinal cord metastases. Urol Ann 2016;8:503–5. 10.4103/0974-7796.192109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oing C, Lorch A, Bokemeyer C, et al. First salvage treatment of germ cell tumor patients with bone metastases: retrospective analysis of a large international database. J Cancer Res Clin Oncol 2015;141:923–31. 10.1007/s00432-014-1876-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oechsle K, Bokemeyer C, Kollmannsberger C, et al. Bone metastases in germ cell tumor patients. J Cancer Res Clin Oncol 2012;138:947–52. 10.1007/s00432-012-1169-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi L, Martignano F, Gallà V, et al. Impact of non-pulmonary visceral metastases in the prognosis and practice of metastatic testicular germ cell tumors. Oncol Rev 2016;10:292. 10.4081/oncol.2016.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biebighauser KC, Gao J, Rao P, et al. Non-Seminomatous germ cell tumor with bone metastasis only at diagnosis: a rare clinical presentation. Asian J Urol 2017;4:124–7. 10.1016/j.ajur.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitchins RN, Philip PA, Wignall B, et al. Bone disease in testicular and extragonadal germ cell tumours. Br J Cancer 1988;58:793–6. 10.1038/bjc.1988.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold PM, Morgan CJ, Morantz RA, et al. Metastatic testicular cancer presenting as spinal cord compression: report of two cases. Surg Neurol 2000;54:27–33. 10.1016/S0090-3019(00)00251-2 [DOI] [PubMed] [Google Scholar]
- 14.Froehner M, Aikele P, Beuthien-Baumann B, et al. Magnetic resonance imaging of bone metastases in patients with nonseminomatous germ cell tumors. Urol Oncol 2007;25:201–6. 10.1016/j.urolonc.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 15.Agarwala S, Mitra A, Bansal D, et al. Management of pediatric malignant germ cell tumors: ICMR consensus document. Indian J Pediatr 2017;84:465–72. 10.1007/s12098-017-2308-2 [DOI] [PubMed] [Google Scholar]
- 16.Göbel U, Schneider DT, Calaminus G, et al. Germ-Cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann Oncol 2000;11:263–71. 10.1023/a:1008360523160 [DOI] [PubMed] [Google Scholar]
- 17.Gale J, Mead GM, Simmonds PD. Management of spinal cord and cauda equina compression secondary to epidural metastatic disease in adults with malignant germ cell tumours. Clin Oncol 2002;14:481–90. 10.1053/clon.2002.0167 [DOI] [PubMed] [Google Scholar]
- 18.Jonska-Gmyrek J, Peczkowski P, Michalski W, et al. Radiotherapy in testicular germ cell tumours - a literature review. Contemp Oncol 2017;21:203–8. 10.5114/wo.2017.69592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamal-Hanjani M, Karpathakis A, Kwan A, et al. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int 2013;112:176–81. 10.1111/bju.12218 [DOI] [PubMed] [Google Scholar]
- 20.Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier cancer research network study GU14-206. Ann Oncol 2018;29:209–14. 10.1093/annonc/mdx680 [DOI] [PubMed] [Google Scholar]
- 21.Semaan A, Haddad FG, Eid R, et al. Immunotherapy: last bullet in platinum refractory germ cell testicular cancer. Future Oncol 2019;15:533–41. 10.2217/fon-2018-0571 [DOI] [PubMed] [Google Scholar]
- 22.Kalavska K, Schmidtova S, Chovanec M, et al. Immunotherapy in testicular germ cell tumors. Front Oncol 2020;10:573977. 10.3389/fonc.2020.573977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsimberidou A-M, Vo HH, Subbiah V, et al. Pembrolizumab in patients with advanced metastatic germ cell tumors. Oncologist 2021;26:558–1098. 10.1002/onco.13682 [DOI] [PMC free article] [PubMed] [Google Scholar]