Abstract
Background
Applicability of comprehensive assessment of integrated care services in real world settings is an unmet need. To this end, a Triple Aim evaluation of Hospital at Home (HaH), as use case, was done. As ancillary aim, we explored use of the approach for monitoring the impact of adoption of integrated care at health system level in Catalonia (Spain).
Methods
Prospective cohort study over one year period, 2017–2018, comparing hospital avoidance (HaH-HA) with conventional hospitalization (UC) using propensity score matching. Participants were after the first episode directly admitted to HaH-HA or the corresponding control group. Triple Aim assessment using multiple criteria decision analysis (MCDA) was done. Moreover, applicability of a Triple Aim approach at health system level was explored using registry data.
Results
HaH-HA depicted lower: i) Emergency Room Department (ER) visits (p < .001), ii) Unplanned re-admissions (p = .012); and iii) costs (p < .001) than UC. The weighted aggregation of the standardized values of each of the eight outcomes, weighted by the opinions of the stakeholder groups considered in the MCDA: i) enjoyment of life; ii) resilience; iii) physical functioning; iv) continuity of care; v) psychological wellbeing; (vi) social relationships & participation; (vii) person-centeredness; and (viii) costs, indicated better performance of HaH-HA than UC (p < .05). Actionable factors for Triple Aim assessment of the health system with a population-health approach were identified.
Conclusions
We confirmed health value generation of HaH-HA. The study identified actionable factors to enhance applicability of Triple Aim assessment at health system level for monitoring the impact of adoption of integrated care.
Registration
ClinicalTrials.gov (26/04/2017; NCT03130283).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-022-08496-z.
Keywords: Chronic care, Health Delivery Assessment, Health Services Research, Hospital at Home, Implementation Science, Multiple Criteria Decision Analysis, Triple Aim
Background
Over the last decade, implementation science [1, 2] has experienced significant progresses resulting in well-accepted conceptual frameworks for assessment of healthcare services [3–6]; that should cover the following three areas: i) deployment strategies aiming at identifying barriers/facilitators for service adoption; ii) comprehensive approaches to assess outcomes; and iii) identification of key performance indicators (KPI) feeding customized dashboards for continuous long-term quality monitoring of complex interventions [7–10]. Applicability of such recommendations for evaluation of integrated care services (ICS) [3, 11–13] in real-world settings is still an issue limiting service adoption, as well as comparability and transferability across sites [14, 15].
With respect to the comprehensive assessment of outcomes, the Triple and Quadruple Aim approaches [16, 17] are consolidated conceptual strategies for evaluation of value generation [18] of ICS. The Triple Aim takes into consideration the following three outcome categories: i) health and well-being; ii) patients’ experience and perception of care; and iii) costs; whereas the Quadruple Aim approach incorporates healthcare professionals’ engagement as an additional key determinant to assess healthcare delivery.
The current research should be envisaged as part of the strategy for exploring applicability of a comprehensive framework for evaluation of ICS, covering both vertical and horizontal integration, in the region of Catalonia (Spain) [19]. To this end, we analyzed feasibility of the Triple Aim approach at health system level aiming at monitoring the impact of the process of large-scale adoption of integrated care in Catalonia during the period 2011–2017. Accordingly, a retrospective population-health study was done using registry data [20, 21].
However, the main objective of the current study was to prospectively assess value-based healthcare of Hospital at Home (HaH) over one-year period (2017–2018), as an example of ICS. To this end, information obtained from a Triple Aim assessment of HaH was elaborated using a Multiple Criteria Decision Analysis (MCDA) [22–24], as an academically sound methodology for evaluation of health value generation, aiming to provide the basis for innovative reimbursement incentives and financial sustainability of health-services.
Material and methods
The context
HaH [25–27] refers to home-based delivery of acute hospital services to patients for a condition that otherwise would require acute hospital inpatient care. Such a modality of care encompasses home-based, short-term, services aiming at fully or partially substituting conventional hospitalization; that is, Hospital Avoidance (HaH-HA) and Early Discharge (HaH-ED), respectively.
In 2006, Hospital Clínic de Barcelona, a tertiary reference centre covering a population of 520 k citizens pioneered the deployment of HaH as a mainstream transversal service [28–30]. A recent Cost-Consequence Analysis (CCA) of HaH-HA (Carme H, Carme H, Erik B, Nuria S, Ruben G, Asenjo M, David N, Enric C, Fernandez J, Isaac C, Roca J. Assessment of Hospital Avoidance in a Real-World Setting: a Prospective Cohort Study, Submitted), carried out during the same period, clearly showed higher performance and reduction of both hospital and community-based direct costs, compared to conventional hospitalization.
The Catalan healthcare system (7,7 million citizens), with one-single public payer and high heterogeneity of providers, is organized in three layers being the seven health regions at the top level. Each region includes several geographical areas called health districts covering specialized, intermediate, and primary care needs of the population. It is of note that intermediate care plays a key role facilitating vertical integration [31–34]. The third level, community-based teams, corresponds to clusters of primary care centres within each healthcare district. Catalonia has a total of 369 primary care units covering approximately 20,000 citizens, on average, each of them.
Regional deployment of the Chronic Care model [35] and integration of health and social services has been promoted under the umbrella of the five-yearly regional health plans. Key goals in terms of deployment of the integrated care were established during the 2011–2015 Plan and consolidation of the program was done during the 2016–2020 period [36, 37].
Study groups and design for HaH-HA assessment
The current prospective cohort study performs a real-world data analysis with a Triple Aim approach of the first episode of a subset of non-surgical patients consecutively admitted to HaH-HA, directly from the Emergency Room Department (ER) over one year, from 31st October 2017 to 1st November 2018. Patients undergoing HaH-ED were not considered in the current study to have a more homogeneous intervention group. Inclusion/exclusion criteria, as well as characteristics of the intervention (HaH-HA) have been extensively reported in [28].
A control group (Usual Care, UC), under conventional hospitalization for their first acute episode during the study period, was generated with patients admitted through the ER directly to a general ward, excluding surgical wards and intensive care units, attempting to mimic patients’ clinical profiles between HaH-HA and UC. Comparability between HaH-HA and UC groups was improved with a 1-to-1 Propensity Score Matching (PSM) [38, 39] and Genetic Matching [40] taking simultaneously into account two sets of matching variables to ensure patients’ comparability before admission and during the acute episode.
The first set of matching variables were: i) age; ii) gender; iii) number of admissions during the previous year; iv) patient’s healthcare costs across the health system in the previous year; and v) patient’s population-based risk in terms of Adjusted Morbidity Groups (GMA) [41] scoring. Two additional matching variables, characterizing the acute episode, were included to enhance comparability of the two groups; that is: i) main diagnosis at discharge using the ICD10-CM categories, and ii) case mix index (CMI)[42]. In this regard, GMA is an aggregative index which indicate the burden of an individual’s morbid conditions through a disease-specific weighting deduced from statistical analysis based on mortality and the utilisation of health services; whereas CMI reflects both severity/complexity of main diagnosis, as well as the complications occurring during the admission.
The matching parameters were tuned according to overall performance on covariate balancing, assessed by the Mahalanobis distance [43], Rubin’s B (the absolute standardized difference of the means of the linear index of the propensity score in the HaH-HA and UC groups) and Rubin’s R (the ratio of HaH-HA to UC variances of the propensity score index) metrics [44]. Quality of comparability between HaH-HA and UC after PSM was considered acceptable if Rubin’s B was less than 0.25 and Rubin’s R was between 0.5 and 2.
Triple aim assessment and MCDA of HaH-HA
As indicated above, HaH-HA was assessed with a Triple Aim approach and the outcomes were elaborated using MCDA. Briefly, we aimed to evaluate outcomes that go beyond traditional health variables and include patient reported outcomes and their broader sense of wellbeing, experience with care, and costs from a hospital perspective. For the MCDA, we used the following eight outcomes: i) enjoyment of life [45]; ii) resilience [46]; iii) physical functioning [47]; iv) continuity of care [48]; v) psychological wellbeing [49];(vi) social relationships & participation [50]; (vii) person-centeredness [51]; and, (viii) costs. The questionnaires for all items were administered by a nurse, at 30 days after discharge, in the patients’ home. Moreover, two of these questionnaires, explicitly: i) continuity of care; and ii) person-centeredness were administered again at 90 days after discharge.
Additionally, Discrete Choice Experiments (DCE) [52] were used to obtain weights for the following set of outcomes. They were elicited in an online weight elicitation study among five stakeholder groups: i) patients; ii) informal caregivers; iii) professional care providers; iv) payers; and v) policy makers (we pooled responses for payers and policy makers).
Statistical analysis
For every key outcome, MCDA inputs for the performance scores were calculated using the following linear mixed regression models applied to the matched patient groups, missing data was not considered to build the performance regression models:
Where only T0 (30 days after discharge) available,
where variable time is available,
The resulting performance regression models were used to calculate corresponding predicted values. In each group, HaH-HA and UC, the mean score of the predicted values was standardized relatively to the mean score of the predicted values in both groups. The corresponding formulas are:
For each stakeholder group, the mean predicted outcome values were weighted and subsequently summed to obtain a single overall value score for the HaH-HA group and a single overall value score for the UC group.
Finally, a Monte Carlo simulation addressed parameter uncertainty in both performance outcomes and weights. The required number of bootstrap replications (1,000 iterations) was determined checking stability of the results (95% uncertainty interval around the mean overall value scores).
Population-health assessment using a Triple Aim approach
The population-health impact of the regional deployment of integrated care over the period 2011–2017 was assessed using registry data [20, 21]. The Catalan Health Surveillance System (CHSS) [20] was the main data source for analysis of the evolution of traditional health outcomes and costs on a yearly basis for the period 2011–2017; whereas the potential for a Triple Aim evaluation with a population-health approach; that is, including patient reported outcomes (PROMs) and patient reported experience (PREMs) was explored using the ESCA survey (Enquesta de Salut de Catalunya) [21]. This survey periodically assesses health status, behavioural changes in use of healthcare resources and perception of the healthcare system in Catalonia. The survey is done, on a yearly basis, on a randomly selected sample (n = six thousand citizens) of the Catalan population taking as a basis the seven healthcare regions.
The CHSS includes updated registries of the region of Catalonia from primary care, hospital-related events (hospitalizations, emergency room consultations and specialized outpatient visits), pharmacy, mental health, socio-sanitary services and other items (home-based respiratory therapies, dialysis, outpatient rehabilitation and non-urgent healthcare transportation) since 2011 [20]. It allows analyses on use of healthcare resources, pharmacy consumption, prevalence of key disorders and population-based health risk assessment. Data analysis can be done considering different levels of aggregation: Primary Care Units, Primary Care Areas, Health Districts, Health Regions or the entire Catalonia.
In the current study, the CHSS analysis of health outcomes was constrained to the temporal evolution of three selected KPI of quality of management of chronic patients: i) unplanned hospitalizations in emergency rooms of acute hospitals; ii) potentially avoidable hospitalizations; and iii) hospitalizations due to chronic conditions in acute hospitals. The analysis assumes that lower values for the 3 KPI reflect better health outcomes. In the study, we compared three different geographical areas: i) the health district of Barcelona-Esquerra (AISBE, 520 thousand citizens) wherein HCB is the reference hospital and pioneered in the deployment of integrated care services; ii) the entire city of Barcelona (1,7 million citizens) and iii) the entire Catalonia (7,7 million citizens). Rates of the 3 KPIs were adjusted by the corresponding KPI rate for Catalonia in each year, and a 95% confidence interval was fixed based on the Byar's approximation of the exact Poisson distribution which is extremely accurate even with small numbers [53].
Statistics
Categorical variables were summarized as absolute values and frequencies, whereas continuous variables were represented by the mean and the standard deviation or the median and interquartile range, as appropriate. Unpaired Student T tests, Mann–Whitney and Chi-squared test comparing HaH-HA with UC were used to assess changes in the outcomes. Data analyses were conducted using R [54], version 3.6.1 The threshold for significance was set at 0.05.
Results
Triple Aim assessment and MCDA of HaH-HA
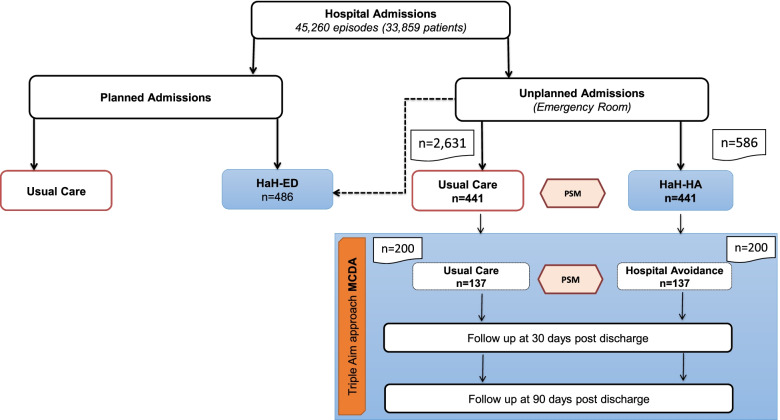
Figure 1 displays the distribution of hospital admissions to conventional hospitalization and to the HaH program: HaH-ED and HaH-HA during the study period. A total of 200 consecutive HaH-HA patients, and their corresponding controls, were studied as candidates for Triple Aim assessment. Application of PSM techniques described above reduced the study groups to 137 HaH-Ha cases, and the corresponding controls.
Fig. 1.
Distribution of hospital admissions during the study period. Five-hundred eighty-six first episodes of HaH admissions, directly from the Emergency Room (HaH-HA), were registered during the study period. A sample of 2.631 conventional hospitalizations was used to generate a usual care (UC) group, as described in the text. The entire intervention group, after propensity score matching (PSM), consisted of 441 HaH-HA patients that were compared with the corresponding matched controls (UC), as reported in (Carme H, Carme H, Erik B, Nuria S, Ruben G, Asenjo M, David N, Enric C, Fernandez J, Isaac C, Roca J. Assessment of Hospital Avoidance in a Real-World Setting: a Prospective Cohort Study, Submitted) During the study period, two-hundred consecutive HaH-HA patients were assessed with a Triple Aim approach to perform Multiple Criteria Decision Analysis (MCDA) that was finally done in 137 HaH-HA patients after PSM with a UC group. Comparisons between the entire HaH-HA population (n = 586), the CCA study (n = 441) and the current study (n = 137) are reported in Tables 1S-3S
Table 1 displays the baseline characteristics of the two groups before admission and the clinical outcomes during the acute episode until 30 days after discharge. The comparability analysis between HaH-HA and UC shows a highly acceptable matching. It is of note that the two groups showed no differences in mortality. However, patients under HaH-HA presented significantly lower rates of emergency room visits (p = < 0.001) and hospital admissions, unplanned (p = 0.012) and planned (p = 0.031), during the 30-day period after discharge than the UC group.
Table 1.
Characteristics of the study groups after propensity score matching before admission and clinical outcomes after discharge
| HaH-HA_MCDA | UC_MCDA | P- value | |
|---|---|---|---|
| (n = 137) | (n = 137) | ||
| BASELINE CHARACTERISTICS | |||
| Socio-demographics | |||
| Age (years), mean (SD)* | 72.42 (13.92) | 72.79 (15.37) | .836 |
| Gender (male), n (%)* | 80 (58.39) | 79 (57.66) | .902 |
| Gender (female), n (%)* | 57 (41.61) | 58 (42.34) | .902 |
| Use of healthcare resources | |||
| Hospital resources in previous 12 months | |||
| Rate of all-cause emergency room visit, mean (SD) | 1.85 (1.19) | 1.76 (1.23) | .231 |
| Rate of all-cause Hospital admissions, mean (SD)* | 1.45 (0.9) | 2.02 (1.54) | .171 |
| Rate of planned admissions, mean (SD) | 1.32 (0.67) | 1.67 (0.91) | .152 |
| Last visit (days) to outpatient clinic before admission, mean (SD) | 78.36 (86.09) | 78.07 (81.86) | .981 |
| Last hospitalisation (days) before admission, mean (SD) | 202.12 (108.21) | 222.25 (110.74) | .413 |
| Length of stay in days (total days per year), mean (total) | 11.45 (458) | 14.75 (590) | .408 |
| Intensive care unit stays, n (%) | 7 (12.10) | 4 (5.70) | .405 |
| Outpatient visits, mean (SD) | 6.14 (7.19) | 5.64 (5.55) | .604 |
| Hospital resources in previous 7 days | |||
| Outpatient visits, mean (SD) | 1.20 (0.63) | 1.25 (0.62) | .405 |
| Healthcare costs across tiers in previous year | |||
| € per year, mean (SD)* | 7,023.81 (9,478.93) | 8,138.43 (8,083.83) | .854 |
| Multimorbidity and severity | |||
| GMA scoring, mean (SD)* | 27.93 (14.72) | 28.8 (18.74) | .872 |
| CLINICAL OUTCOMES AT DISCHARGE | |||
| Total length of stay (days), mean (SD) | 8.20 (4.70) | 7.74 (5.96) | .479 |
| Case Mix Index | 0.72 | 0.75 | .792 |
| Mortality during episode, n (%) | 0 (0) | 0 (0) | 1 |
| Use of resources during Hospital Avoidance | |||
| All-cause Emergency Room visits, n (%) | 2 (1.46) | N/A | NA |
| All-cause In-Hospital re-admissions, n (%) | 4 (2.92) | N/A | NA |
| Outcomes at 30 days after discharge | |||
| All-cause Emergency Room visits, n (%) | 5 (3.65) | 20 (14.6) | < .001 |
| Unplanned Hospital admissions, n (%) | 5 (3.65) | 15 (10.95) | .012 |
| Planned admissions, n (%) | 2 (1.46) | 9 (6.57) | .031 |
| Mortality, n(%) | 1 (0.73) | 1 (0.73) | 1 |
| MATCHING ASSESSMENT METRICS | |||
| Rubin's B | 0.050 | ||
| Rubin's R | 0.598 | ||
Legend. HaH-HA, Hospital al Home-Hospital Avoidance; UC, Usual Care; GMA, Adjusted Morbidity Groups scoring; N/A, not applicable
The raw data of the eight outcomes before the MCDA (Table 2) indicate that HaH-HA group showed slightly better physical functioning (p = 0.019), and higher scores for both patient-reported experience measures: continuity of care (p < 0.001) and person-centeredness (p < 0.001) than the UC group.
Table 2.
Raw data of the eight outcomes before the MCDA
| Criteria/Outcomes | Questionnaire | Score: Mean (SD) | P-value | ||
|---|---|---|---|---|---|
| Range | HaH-HA | UC | |||
| n = 137 | n = 137 | ||||
| Enjoyment of life | ICECAP-O | 1–4 | 2.50 (0.89) | 2.48 (0.87) | .496 |
| Resilience | BRS | 6–30 | 20.52 (5.48) | 19.72 (5.99) | .278 |
| Physical functioning | SF-36 | 0–100 | 55.27 (35.43) | 45.45 (31.87) | .019 |
| Continuity of care | NCQ | 0–5 | 3.82 (1.20) | 3.30 (1.15) | < .001 |
| Psychological well-being | MHI-5 | 0–100 | 69.32 (23.52) | 68.07 (22.11) | .623 |
| Social participation | IPA | 0–24 | 7.13 (3.95) | 7.00 (3.77) | .768 |
| Person-centeredness | P3CEQ | 0–18 | 15.94 (2.95) | 14.62 (3.80) | .001 |
| Health care costs | € | 1,126.76 (226.17) | 2,346.33 (519.14) | < .001 | |
Results expressed as mean (standard deviation). Questionnaires used for the MCDA (Multiple Criteria Decision Analysis) and CCA (Cost Consequence Analysis) are indicated in the Methods section. HaH-HA, (Hospital at Home-Hospital Avoidance); UC, Usual Care
Two outcomes reflecting patients’ experience with care: i) continuity of care; and ii) person-centeredness (Table 2, 30 days after discharge) were also assessed at 90 days after discharge without showing significant changes: mean change (95%CI), 0.022 (-0.25–0.29) and -0.3 (-1.16–0.51), respectively.
The direct HaH-HA costs per patient during the episode was, on average 1,127€; whereas the direct UC costs per patient was slightly more than double, mean 2,346€. It is of note that the average cost savings per patient in HaH-HA (1,220€) were mostly generated (95%) by a reduction in staff (54%), testing (17%), catering (12%) and infrastructure (12%) costs (Table 3S). Likewise, healthcare expenses across the health system during the 30-days transitional care period after discharge; that is, including use of healthcare resources at community level, were 62% lower in HaH-HA than in UC (p < 0.001).
The main results of the MCDA of HaH-HA are depicted in Table 3. The table indicates standardized values of each of the outcomes indicating (PROMs) and patient experience with care (PREMs), as well as health care costs, weighted by the opinions of the stakeholder groups, i.e.: patients, carers, health professionals and policy makers/payers. It is of note that from all stakeholder perspectives, HaH-HA consistently showed higher overall value scores than UC (Table 4S). In addition, the results of the Monte Carlo simulation disclosed that the weighted aggregation of the performance scores is significantly greater in HaH-HA group, showing no overlap of the corresponding 95%CI. This finding is also observable assessing the intervention using the weighting preferences from every stakeholder, showing no overlap of the corresponding 95%CI (Fig. 2).
Table 3.
Overall scores of the multiple criteria decision analysis (MCDA)
| Case Mix MCDA Sub-Group | |||||
|---|---|---|---|---|---|
| (n = 137) | |||||
| Criteria/Outcomes | Relative Weights | Standardized performance | Weighted aggregation | ||
| HaH-HA | UC | HaH-HA | UC | ||
| Enjoyment of life | 0.22 | 0.71 | 0.71 | 0.15 | 0.15 |
| Resilience | 0.13 | 0.72 | 0.69 | 0.09 | 0.09 |
| Physical functioning | 0.12 | 0.77 | 0.64 | 0.09 | 0.07 |
| Continuity of care | 0.15 | 0.75 | 0.66 | 0.11 | 0.10 |
| Psychological well-being | 0.14 | 0.71 | 0.70 | 0.10 | 0.10 |
| Social participation | 0.11 | 0.72 | 0.70 | 0.08 | 0.08 |
| Person-centeredness | 0.09 | 0.74 | 0.67 | 0.07 | 0.06 |
| Health care costs | 0.05 | 0.87 | 0.49 | 0.04 | 0.02 |
| Overall value score | 0.73 | 0.67 | |||
| (95% CI) | (0.71–0.76) | (0.65–0.70) | |||
| Percentage HaH-HA > UC | 98.4 | ||||
Criteria/Outcomes: 8 outcomes categories assessed in the MCDA at 30 days after discharge; Relative weights: pooled weights of all five stakeholder groups; Standardized performance: Overall scoring for each outcome, HaH-HA: Hospital at Home-Hospital Avoidance, UC: Usual Care; Weighted aggregation: Standardized performance times corresponding relative weight. HaH-HA, Hospital at Home-Hospital Avoidance; UC, Usual Care; Overall value score: mean overall scores for HaH-HA and UC; 95% Uncertainty Interval; Percentage HaH-HA > UC, percentage of iterations in the Monte Carlo simulation showing higher overall value scores in HaH-HA than UC.
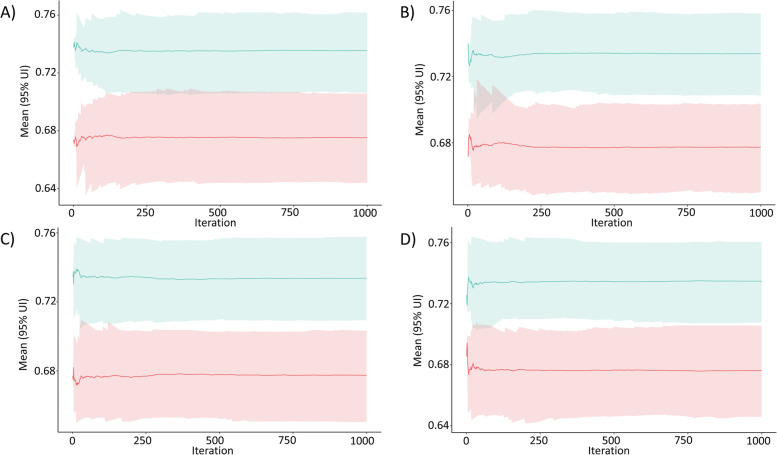
Fig. 2.
Sensitivity analysis of MCDA with DCE weights based on a Bootstrap analysis (1,000 iterations) of the MCDA overall score between hospital avoidance (HaH-HA) (green) and usual care (UC) (red) groups. In each iteration, the values of the eight outcomes considered in the MCDA were weighted according to the DCE results and subsequently summed to obtain a single overall value score. The panels show the mean overall value score across all bootstrap iterations (set to 1000) and their 95% Uncertainty Intervals (UI) for HaH-HA and UC and for each stakeholder group: A) Patients; B) Carers; C) Professionals; D) Payers + Policy Makers. The four panels show no overlap between intervention and control groups along the bootstrap replications
Population-health assessment using a Triple Aim approach
Figure 3 displays the yearly evolution, from 2011–2017, of the three selected KPI reflecting quality of chronic care in the study areas: i) health district of Barcelona-Esquerra; ii) city of Barcelona; and iii) Catalonia. From top to bottom, the panels depict adjusted rates per 10 thousand citizens for: i) unplanned ER visits in acute hospitals, ii) potentially avoidable hospitalizations, and iii) hospitalizations due to chronic conditions in acute hospitals. Over the period 2011–2014, both avoidable hospitalizations and hospitalizations of chronic patients showed a reduction in both the city of Barcelona and the entire Catalonia; whereas AISBE clearly presented a plateau with lower adjusted rates for all KPI than Barcelona and Catalonia. Interestingly, all KPIs in the three geographical areas presented a sustained increase (worsening) after 2013, reaching convergence at the end of the study period. The CHSS allowed such analysis, with different levels of data aggregation, for a large amount of health outcomes and for costs.
Fig. 3.
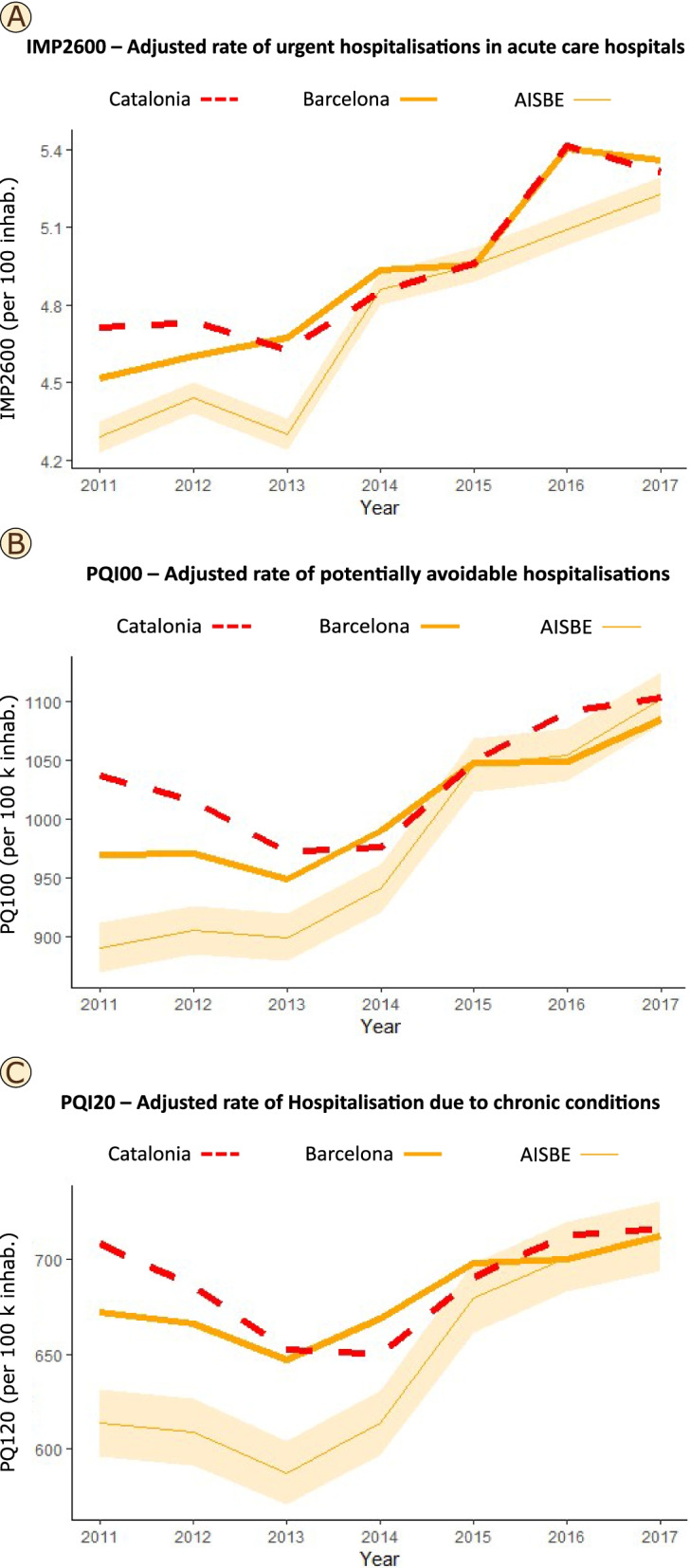
Quality of chronic care in the study areas
The feasibility analysis of the ESCA survey to assess PROMs and PREMs by geographical areas identified some limitations. The ESCA outcomes could partly be mapped to the MCDA variables displayed in Tables 2 and 3. Briefly, four domains: i) psychological well-being; ii) social relationships and participation; iii) resilience; and iv) enjoyment of life were assessed with well-identified measurement tools and showed equivalences with specific MCDA outcomes. Other three domains did not have a standardized measurement tool but showed some equivalences with specific MCDA outcomes, namely: i) physical functioning; ii) activation and engagement; and iii) person centeredness. Only one domain, continuity of care, could not be mapped into the ESCA survey. Moreover, the analysis of the yearly evolution, by geographical areas, of the ESCA outcomes could not be done because of two main technical issues. Firstly, changes introduced in ESCA during 2016–2017 did not allow comparability during the entire study period. A second limitation was that the characteristics of the sampling: size and distribution of data did not allow appropriate comparisons among the geographical areas considered in the current research. Up to 4000 surveys were done in the city of Barcelona with unknown distribution by health districts and approximately 400 surveys corresponded to each of the other six health regions.
Discussion
Hospital avoidance: main findings and context of the analysis
The current report provides a value-based assessment of Hospital at Home-hospital avoidance taking into consideration health and well-being, experience with care and healthcare costs, considering the acute episode and the 30-day period post-discharge. The MCDA clearly showed that HaH-HA was superior to UC, for those candidates meeting the inclusion/exclusion criteria. The research, taking HaH-HA as use case, clearly shows feasibility and applicability of the Triple Aim approach for comprehensive assessment of specific integrated care services, as proposed in [19]. The results are along with those seen in the retrospective CCA carried out with the entire population of patients included in HaH-HA during the study period (Carme H, Carme H, Erik B, Nuria S, Ruben G, Asenjo M, David N, Enric C, Fernandez J, Isaac C, Roca J. Assessment of Hospital Avoidance in a Real-World Setting: a Prospective Cohort Study, Submitted), which strengthens the messages of the current research and reinforces the interest for a comprehensive health delivery assessment (Tables 1S, 2S and 3S). It is of note that cost-savings associated to HaH-HA are mostly attributable to organizational changes and digital support of the service rather than to specificities of the patients’ traits. Accordingly, our results can be reasonably generalized for this type of intervention provided that the general characteristics of the HaH-HA service are maintained [55].
Triple aim assessment of specific integrated care services
The use of MCDA allowed measured outcomes to be balanced by the opinions of relevant stakeholders which shows high potential to facilitate decision making at policy level. We believe that the HaH-HA study provides a methodological approach that can be easily generalizable for assessment of other ICS involving complex interventions.
It is of note that our research aimed to explore applicability of the Triple Aim approach for assessment of ICS in real world settings. One key limiting finding was that the administration of the questionnaires assessing the seven qualitative variables included in the MCDA required approximately 45 min per patient which precludes use in routine testing. Pilot testing carried out before the initiation of the study protocol indicated that administration of questionnaires earlier during admission might not provide robust results because of limitations for data capture due to patients’ clinical conditions. Moreover, the lack of significant changes between 30-days and 90-days after discharge might indicate the need for improving discriminative power of administered questionnaires.
One lesson learnt is that capturing relevant information from patients in real-world scenarios requires refinement of the measurement tools including questionnaires and characteristics of the digital support. We could hypothesize that comprehensive outcomes’ assessment using properly validated visual-analogic scales and/or artificial intelligence-powered brief questionnaires [56] could be alternative approaches to be explored for a desirable adoption of Triple Aim assessment in routine healthcare delivery assessment. The use of user-friendly digital tools conceived to support collaborative work and adaptive case management, as described in [57], should greatly facilitate capturing patients’ information. Such digital tools should also allow a fluent capture of professional inputs allowing Quadruple Aim [16] assessment of healthcare services.
Lessons learnt from population-health study
It is widely accepted that generation of evidence on value-based integrated care at health system level is still an unmet need. The current study indicates that the characteristics of the CHSS fulfill the requirements of health policy makers at regional level and constitute an appropriate tool for assessment of traditional health outcomes, as well as analysis of associated direct costs. In contrast, the ESCA survey shows clear limitations for a Triple Aim assessment that could be easily by overcome by refining the digital support by capturing citizens’ answers with visual-analogic scales and modifying the sampling characteristics aiming at enhancing robustness of the analysis of specific geographical areas.
The results displayed in Fig. 3 reflect highly complex phenomena and may require deeper analysis for a proper understanding of the information. A possible interpretation of the evolution of the selected KPIs indicating quality of chronic care could be as follows. Convergence of selected KPIs between AISBE (early adopter of integrated care services since 2006), the city of Barcelona and the region of Catalonia might be explained by preparation and active deployment of the 2011–2015 Catalan Health Plan heavily promoting integrated care and digitalization of healthcare. However, worse health outcomes in the three areas after 2013 might likely reflect the negative effects of a marked (-10%) reduction of financial healthcare resources during the period 2011–2018, due to the 2008 economic crisis.
Future perspectives
In our setting, the 2011–2015 Health Plan for Catalonia (Spain) stimulated a substantial expansion of HaH with various approaches and overall positive results. It is of note that the successful regional deployment of HaH during the period 2015–2019 is currently being analysed as an original Good Practice (oGP) within the Joint Action on implementation of digitally enabled integrated person-centred care (JADECARE, 2020–2023). We believe that proposals generated by the current research should trigger improvements in the assessment integrated care services facilitating comparability and transferability of Good Practices across sites.
Conclusions
The current study performed a comprehensive assessment of HaH-HA in a real-life setting using the novel methodological approach of MCDA and produced evidence on health value generation of the intervention. The research identifies key actionable factors for Triple Aim assessment of the impact of integrated care interventions at health system level.
Supplementary Information
Acknowledgements
The authors thank the Hospital At Home team who cared for the patients, the Emergency Medicine, Pharmacy, Laboratory, image units at the Hospital Clinic, that were instrumental to the success of the Hospital at Home service, the various departments for their support in carrying out the study, especially internal medicine, the medical informatics teams of the Hospital Clinic for facilitating access to medical records and the coordinator of Information System of the Emergency room for the support for the direct cost analysis during admission. Thanks to the Catalan Health Service for trusting us in the deployment of the service in the territory with different health care providers. Thanks to the management team of Hospital Plato y Sagrat Cor for their collaboration in the deployment of Hospital At Home. Special thanks to the Medical and Nurse Direction at the Hospital Clinic, for betting decisively on cross-disciplinary Hospital at Home unit. We acknowledge the support provided by the projects: Smart PITES (FIS-PI045409, supported by IsCIII, ES) and SELFIE (HP-JA-2019 -951442) a European Union’s Health Program 2014-2020).
Abbreviations
- HaH
Hospital at home
- HaH-HA
Hospital avoidance
- UC
Conventional hospitalization
- MCDA
Multiple criteria decision analysis
- ER
Emergency room department
- KPI
Key performance indicators
- ICS
Integrated care services
- HaH-ED
Early discharge
- CCA
Cost-consequence analysis
- PSM
Propensity score matching
- DCE
Discrete choice experiments
- CHSS
Catalan health surveillance system
- PROM
Patient reported outcome
- PREM
Patient reported experience
- ESCA
Catalan health survey
- AISBE
The health district of Barcelona-Esquerra
- GMA
Adjusted Morbidity Groups scoring
- oGP
Original Good Practice
Authors’ contributions
Conception and design: Herranz C, González, R, Hernández C, Roca J.
Analysis and interpretation of the data: Herranz C, González-Colom C, Baltaxe E, Seijas N, Asenjo M, Hoedemakers M, Nicolas D, Coloma E, Fernandez J, Vela E, Cano I, Rutten-van Mölken M, Roca J and Hernandez C. Drafting of the article: Herranz C, González R, Hernandez C, Roca J. Critical revision of the article for important intellectual content: Herranz C, González-Colom C, Baltaxe E, Seijas N, Asenjo M, Hoedemakers M, Nicolas D, Coloma E, Fernandez J, Vela E, Cano I, Rutten-van Mölken M, Roca J and Hernandez C. Final approval of the article: Herranz C, González-Colom C, Baltaxe E, Seijas N, Asenjo M, Hoedemakers M, Nicolas D, Coloma E, Fernandez J, Vela E, Cano I, Rutten-van Mölken M, Roca J and Hernandez C. Statistical expertise: Gonzalez R, Cano I, Vela E, Asenjo M.
Collection and assembly of data: Hernández C, Seijas N, Herranz C, Baltaxe E, Vela E, Asenjo M.The author(s) read and approved the final manuscript.
Funding
This article was funded by JADECARE project- HP-JA-2019—Grant Agreement nº 951442 a European Union’s Health Program 2014–2020.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to administrative reasons but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics study was approved by the Credited Research Ethics Committee of the Hospital Clinic of Barcelona (Ref. 2017–0451 and 2017–0452). The current data analysis was developed under the umbrella of the EU project SELFIE [58]. All methods were performed in accordance with the relevant guidelines and regulations (i.e., Declaration of Helsinki). All participants in the HaH-MCDA protocol signed an informed consent ahead of any procedure. The population-based study was carried out using deidentified and aggregated data from health registries (CHSS and ESCA), not requiring written informed consent. Registration of the study protocol at ClinicalTrials.gov was done (26/04/2017, NCT03130283), as part of the analysis of the service.thics approval and consent to participate.
Consent for publication
Not applicable.
Competing interest
All authors have disclosed no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carme Herranz and Rubèn González-Colom are equal contributors.
Contributor Information
Carme Herranz, Email: cherranz@clinic.cat.
Carme Hernandez, Email: chernan@clinic.cat.
References
- 1.Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, et al. Standards for Reporting Implementation Studies (StaRI): explanation and elaboration document. Open. 2017;7:13318. doi: 10.1136/bmjopen-2016-013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Republished research: Implementation research: What it is and how to do it. Br J Sports Med. 2014;48(8):731–6. doi: 10.1136/bmj.f6753. [DOI] [PubMed] [Google Scholar]
- 3.Struckmann V, Leijten FRM, van Ginneken E, Kraus M, Reiss M, Spranger A, et al. Relevant models and elements of integrated care for multi-morbidity: Results of a scoping review. Health Policy (New York) 2018;122(1):23–35. doi: 10.1016/j.healthpol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Low L-F, Fletcher J. Models of home care services for persons with dementia: a narrative review. Int Psychogeriatrics. 2015;27(10):1593–600. doi: 10.1017/S1041610215000137. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Integrated care models: an overview [Internet]. Health Services Delivery Programme. 2016. Available from: http://www.euro.who.int/pubrequest%0Ahttp://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf%0Ahttp://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf%0Ahttp://www.euro.who.int/
- 6.Kadu M, Ehrenberg N, Stein V, Tsiachristas A. Methodological quality of economic evaluations in integrated care: Evidence from a systematic review. Int J Integr Care. 2019;19(3):1–34. doi: 10.5334/ijic.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ [Internet]. 2007 Mar 3 [cited 2022 Jul 24];334(7591):455–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17332585/ [DOI] [PMC free article] [PubMed]
- 8.Jebraeily M, Amin VALIZADE HASANLOEI M, Rahimi B, Valizade Hasanloei MA. Design of a Management Dashboard for the Intensive Care Unit: Determining Key Performance Indicators and their Required Capabilities. Appl Med Informatics Orig Res. 2019;41(3):111–21.
- 9.Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc [Internet]. 2019 Mar 1 [cited 2022 Jul 24];59(2):S96-S103.e3. Available from: http://www.japha.org/article/S1544319118305697/fulltext [DOI] [PubMed]
- 10.Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ [Internet]. 2021 Sep 30 [cited 2022 Jul 24];374. Available from: https://pubmed.ncbi.nlm.nih.gov/34593508/ [DOI] [PMC free article] [PubMed]
- 11.Yang H, Dervin G, Madden S, Beaulé PE, Gagné S, Crossan ML, et al. Postoperative home monitoring after joint replacement: Feasibility study. J Med Internet Res. 2018;20(9):e10168. doi: 10.2196/10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looman W, Struckmann V, Köppen J, Baltaxe E, Czypionka T, Huic M, et al. Drivers of successful implementation of integrated care for multi-morbidity: Mechanisms identified in 17 case studies from 8 European countries. Soc Sci Med [Internet]. 2021 May 1 [cited 2022 Apr 4];277:113728. Available from: http://creativecommons.org/licenses/by/4.0/ [DOI] [PubMed]
- 13.Czypionka T, Kraus M, Reiss M, Baltaxe E, Roca J, Ruths S, et al. The patient at the centre: evidence from 17 European integrated care programmes for persons with complex needs. Available from: 10.1186/s12913-020-05917-9 [DOI] [PMC free article] [PubMed]
- 14.European Commission. Jadecare | Joint Action on implementing digitally integrated person-centred care [Internet]. 2022 [cited 2022 Apr 4]. Available from: https://www.jadecare.eu/
- 15.Rutten-van Mölken M. Common Challenges Faced in EU-funded Projects on Integrated Care for Vulnerable Persons. 2017; Available from: 10.5334/ijic.3104 [DOI] [PMC free article] [PubMed]
- 16.Bodenbeimer T, Sinsky C. From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider. Ann Fam Med. 2014;12(6):573–576. doi: 10.1370/afm.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berwick DM, Nolan TW, Whittington J. The Triple Aim: Care, Health, And Cost. 101377/hlthaff273759. 2017 Aug 2;27(3):759–69. [DOI] [PubMed]
- 18.Porter ME. Value-based health care delivery. Ann Surg [Internet]. 2008 Oct [cited 2022 Apr 6];248(4):503–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18936561/ [DOI] [PubMed]
- 19.Baltaxe E, Cano I, Herranz C, Barberan-Garcia A, Alonso A. Evaluation of integrated care services in Catalonia: population-based and service-based real-life deployment protocols. BMC Heal Serv Res. 2019;19(1):370. doi: 10.1186/s12913-019-4174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vela E, Tényi Á, Cano I, Monterde D, Cleries M, Garcia-Altes A, et al. Population-based analysis of patients with COPD in Catalonia: A cohort study with implications for clinical management. BMJ Open. 2018;8(3). [DOI] [PMC free article] [PubMed]
- 21.Medina A, Schiaffino A. Health Survey of Catalunya. Health status, health-related behaviours and use of health services in Catalunya. Main results ESCA 2017. Executive summary [Internet]. 2018. Available from: http://salutweb.gencat.cat/web/.content/_departament/estadistiques-sanitaries/enquestes/Enquesta-de-salut-de-Catalunya/Resultats-de-lenquesta-de-salut-de-Catalunya/documents/summary_esca_2017.pdf
- 22.Rutten-Van Mölken M, Leijten F, Hoedemakers M, Tsiachristas A, Verbeek N, Karimi M, et al. Strengthening the evidence-base of integrated care for people with multi-morbidity in Europe using Multi-Criteria Decision Analysis (MCDA). Available from: 10.1186/s12913-018-3367-4 [DOI] [PMC free article] [PubMed]
- 23.Leijten FRM, Struckmann V, van Ginneken E, Czypionka T, Kraus M, Reiss M, et al. The SELFIE framework for integrated care for multi-morbidity: Development and description. Health Policy (New York) 2018;122(1):12–22. doi: 10.1016/j.healthpol.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Tsiachristas A, Cramm JM, Nieboer A, Rutten-Van Mölken M. Broader economic evaluation of disease management programs using multi-criteria decision analysis. Int J Technol Assess Health Care [Internet]. 2013 Jul [cited 2022 Apr 4];29(3):301–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23759317/ [DOI] [PubMed]
- 25.Leff B. Defining and disseminating the hospital-at-home model Key points. CMAJ. 2009;180(2):156–7. doi: 10.1503/cmaj.081891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepperd S, Iliffe S, Doll HA, Clarke MJ, Kalra L, Wilson AD, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev [Internet]. 2016 Sep 1 [cited 2022 Apr 4];2016(9). Available from: /pmc/articles/PMC6457791/ [DOI] [PMC free article] [PubMed]
- 27.Gonçalves-Bradley DC, Iliffe S, Doll HA, Broad J, Gladman J, Langhorne P, et al. Early discharge hospital at home. Cochrane Database Syst Rev [Internet]. 2017 Jun 26 [cited 2022 Apr 4];2017(6). Available from: /pmc/articles/PMC6481686/ [DOI] [PMC free article] [PubMed]
- 28.Hernández C, Aibar J, Seijas N, Puig I, Alonso A, Garcia-Aymerich J, et al. Implementation of home hospitalization and early discharge as an integrated care service: A ten years pragmatic assessment. Int J Integr Care [Internet]. 2018 Apr 1 [cited 2022 Apr 19];18(2). Available from: http://www.ijic.org/articles/10.5334/ijic.3431/ [DOI] [PMC free article] [PubMed]
- 29.Hernández C, Alonso A, Garcia-Aymerich J, Grimsmo A, Vontetsianos T, García Cuyàs F, et al. Integrated care services: lessons learned from the deployment of the NEXES project. Int J Integr Care. 2015;15:e006. doi: 10.5334/ijic.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salut S C. Model organitzatiu d’hospitalització a domicili de Catalunya. CatSalut Serv Català la Salut [Internet]. Available from: https://catsalut.gencat.cat/ca/detalls/articles/model-organitzatiu-hospitalitzacio-domicili#googtrans(ca
- 31.Mas M, Santaeugènia SJ, Tarazona-Santabalbina FJ, Gámez S, Inzitari M. Effectiveness of a Hospital-at-Home Integrated Care Program as Alternative Resource for Medical Crises Care in Older Adults With Complex Chronic Conditions. J Am Med Dir Assoc [Internet]. 2018 Oct 1 [cited 2022 Jul 24];19(10):860–3. Available from: https://pubmed.ncbi.nlm.nih.gov/30268290/ [DOI] [PubMed]
- 32.Mas M, Inzitari M, Sabaté S, Santaeugènia SJ, Miralles R. Hospital-at-home Integrated Care Programme for the management of disabling health crises in older patients: comparison with bed-based Intermediate Care. Age Ageing [Internet]. 2017 Nov 1 [cited 2022 Jul 22];46(6):925–31. Available from: https://pubmed.ncbi.nlm.nih.gov/28655169/ [DOI] [PubMed]
- 33.Ribbink ME, Gual N, MacNeil-Vroomen JL, Ars Ricart J, Buurman BM, Inzitari M, et al. Two European Examples of Acute Geriatric Units Located Outside of a General Hospital for Older Adults With Exacerbated Chronic Conditions. J Am Med Dir Assoc [Internet]. 2021 Jun 1 [cited 2022 Jul 24];22(6):1228–34. Available from: https://pubmed.ncbi.nlm.nih.gov/33524341/ [DOI] [PubMed]
- 34.Colprim D, Martin R, Parer M, Prieto J, Espinosa L, Inzitari M. Direct admission to intermediate care for older adults with reactivated chronic diseases as an alternative to conventional hospitalization. J Am Med Dir Assoc [Internet]. 2013 [cited 2022 Jul 24];14(4):300–2. Available from: https://pubmed.ncbi.nlm.nih.gov/23294969/ [DOI] [PubMed]
- 35.Epping-Jordan J, Pruitt S, Bengoa R, Wagner E. Improving the quality of health care for chronic conditions. Qual Saf Health Care [Internet]. 2004 Aug 1 [cited 2022 Apr 6];13(4):299–305. Available from: https://pubmed.ncbi.nlm.nih.gov/15289634/ [DOI] [PMC free article] [PubMed]
- 36.Government of Catalonia M of H. Health Plan for Catalonia 2011–2015. 2012; Available from: http://salutweb.gencat.cat/ca/el_departament/pla_de_salut_2011_2015/pla_de_salut_documents/
- 37.Generalitat de Catalunya, Departament de Salut. Pla de Salut de Catalunya 2016–2020. Dir Gen Planif en Salut [Internet]. 2016;162. Available from: https://salutweb.gencat.cat/web/.content/_departament/pla-de-salut/Pla-de-salut-2016-2020/documents/Pla_salut_Catalunya_2016_2020.pdf
- 38.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=hmbr20 [DOI] [PMC free article] [PubMed]
- 39.Tsiachristas A, Ellis G, Buchanan S, Langhorne P, Stott DJ, Shepperd S. Should I stay or should I go? A retrospective propensity score-matched analysis using administrative data of hospital-at-home for older people in Scotland. BMJ Open [Internet]. 2019 May 1 [cited 2022 Apr 4];9(5):23350. Available from: /pmc/articles/PMC6527981/ [DOI] [PMC free article] [PubMed]
- 40.Diamond A, Sekhon JS. Genetic Matching for Estimating Causal Effects: A General Multivariate Matching Method for Achieving Balance in Observational Studies. Rev Econ Stat [Internet]. 2013 Jul 1 [cited 2022 Apr 4];95(3):932–45. Available from: https://direct.mit.edu/rest/article/95/3/932/58101/Genetic-Matching-for-Estimating-Causal-Effects-A
- 41.Monterde D, Vela E, Clèries M, Garcia-Eroles L, Roca J, Pérez-Sust P. Multimorbidity as a predictor of health service utilization in primary care: a registry-based study of the Catalan population. Available from: 10.1186/s12875-020-01104-1 [DOI] [PMC free article] [PubMed]
- 42.Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18(Suppl 2):1–53. [PubMed] [Google Scholar]
- 43.Mahalanobis Distance. In: The Concise Encyclopedia of Statistics [Internet]. New York, NY: Springer New York; 2008. p. 325–6. Available from: 10.1007/978-0-387-32833-1_240
- 44.Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Matched Sampl Causal Eff. 2006;365–82.
- 45.Coast J, Peters TJ, Natarajan L, Sproston K, Flynn T. An assessment of the construct validity of the descriptive system for the ICECAP capability measure for older people. Qual Life Res. 2008;17(7):967–976. doi: 10.1007/s11136-008-9372-z. [DOI] [PubMed] [Google Scholar]
- 46.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: Assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 47.Jenkinson C, Coulter A, Wright L. Short form 36 (SF 36) health survey questionnaire: Normative data for adults of working age. Br Med J [Internet]. 1993;306(6890):1437–40. Available from: http://www.bmj.com/ [DOI] [PMC free article] [PubMed]
- 48.Uijen AA, Schellevis FG, van den Bosch WJHM, Mokkink HGA, van Weel C, Schers HJ. Nijmegen Continuity Questionnaire: Development and testing of a questionnaire that measures continuity of care. J Clin Epidemiol. 2011;64(12):1391–1399. doi: 10.1016/j.jclinepi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Berwick DM, Murphy JM, Goldman PA, Ware JE, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Cardol M, de Haan RJ, van den Bos GAM, de Jong BA, de Groot IJM. The development of a handicap assessment questionnaire: the Impact on Participation and Autonomy (IPA) Clin Rehabil. 1999;13(5):411–419. doi: 10.1191/026921599668601325. [DOI] [PubMed] [Google Scholar]
- 51.Lloyd H, Fosh B, Whalley B, Byng R, Close J. Validation of the person-centred coordinated care experience questionnaire (P3CEQ) Int J Qual Heal Care. 2019;31(7):506–512. doi: 10.1093/intqhc/mzy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutten-Van Mölken M, Karimi M, Leijten F, Hoedemakers M, Looman W, Islam K, et al. Comparing patients’ and other stakeholders’ preferences for outcomes of integrated care for multimorbidity: a discrete choice experiment in eight European countries. BMJ Open. 2020;10:37547. doi: 10.1136/bmjopen-2020-037547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1–406. [PubMed]
- 54.Zeileis A. R: The R Project for Statistical Computing [Internet]. 2022 [cited 2022 Apr 4]. Available from: https://www.r-project.org/
- 55.Hernández C, Aibar J, Seijas N, Puig I, Alonso A, Garcia-Aymerich J, et al. Implementation of home hospitalization and early discharge as an integrated care service: A ten years pragmatic assessment. Int J Integr Care. 2018;18(2):12. doi: 10.5334/ijic.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz AR, Cohen-Zion M, Pham L V., Gal A, Sowho M, Sgambati FP, et al. Brief digital sleep questionnaire powered by machine learning prediction models identifies common sleep disorders. Sleep Med [Internet]. 2020 Jul 1 [cited 2022 Apr 6];71:66–76. Available from: https://pubmed.ncbi.nlm.nih.gov/32502852/ [DOI] [PubMed]
- 57.Barberan-Garcia A, Cano I, Bongers BC, Seyfried S, Ganslandt T, Herrle F, et al. Digital Support to Multimodal Community-Based Prehabilitation: Looking for Optimization of Health Value Generation. Vol. 11, Frontiers in Oncology. 2021. [DOI] [PMC free article] [PubMed]
- 58.Home - Selfie 2020 - [Internet]. [cited 2018 Mar 7]. Available from: https://www.selfie2020.eu/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to administrative reasons but are available from the corresponding author on reasonable request.