Abstract
The symbiotic soil bacterium Sinorhizobium meliloti uses the compatible solutes glycine betaine and proline betaine for both protection against osmotic stress and, at low osmolarities, as an energy source. A PCR strategy based on conserved domains in components of the glycine betaine uptake systems from Escherichia coli (ProU) and Bacillus subtilis (OpuA and OpuC) allowed us to identify a highly homologous ATP-binding cassette (ABC) binding protein-dependent transporter in S. meliloti. This system was encoded by three genes (hutXWV) of an operon which also contained a fourth gene (hutH2) encoding a putative histidase, which is an enzyme involved in the first step of histidine catabolism. Site-directed mutagenesis of the gene encoding the periplasmic binding protein (hutX) and of the gene encoding the cytoplasmic ATPase (hutV) was done to study the substrate specificity of this transporter and its contribution in betaine uptake. These mutants showed a 50% reduction in high-affinity uptake of histidine, proline, and proline betaine and about a 30% reduction in low-affinity glycine betaine transport. When histidine was used as a nitrogen source, a 30% inhibition of growth was observed in hut mutants (hutX and hutH2). Expression analysis of the hut operon determined using a hutX-lacZ fusion revealed induction by histidine, but not by salt stress, suggesting this uptake system has a catabolic role rather than being involved in osmoprotection. To our knowledge, Hut is the first characterized histidine ABC transporter also involved in proline and betaine uptake.
In its natural habitat Sinorhizobium meliloti, the alfalfa symbiotic species, regularly encounters osmotic modifications and frequent changes in the availability of water which influence the physiology of the bacterial cells. Study of osmoregulation in S. meliloti has important applications to plant-microbe interactions, since variations of the osmotic environment within the rhizosphere may affect root colonization, nodule development, and atmospheric nitrogen fixation efficiency (12, 17). S. meliloti has the capacity to overcome growth inhibition caused by osmotic stress by uptake of osmoprotectants such as proline betaine (20) and glycine betaine or its precursors choline or choline-O-sulfate (3, 45, 47). Unlike choline or choline-O-sulfate, which are enzymatically converted into glycine betaine immediately after uptake, glycine betaine can be accumulated to high intracellular concentrations without producing adverse effects on essential cellular functions (34). This potent osmoprotectant is widely found in nature and has been adopted by microorganisms, plants, and animals among the most-effective compatible solutes. In contrast to Escherichia coli (46), Bacillus subtilis (4), and other bacteria, S. meliloti can use glycine betaine and proline betaine not only as osmoprotectants but as carbon, nitrogen, and energy sources as well (3, 53, 20).
Although the presence of uptake systems for glycine betaine has been reported for a variety of gram-negative and gram-positive bacteria (7, 19) and also in members of the Archaea (49), such transport systems have been studied at the molecular level in only a few microorganisms. One of the most extensively studied uptake systems is the osmoregulatory locus known as proU, which is an operon that encodes a high-affinity ATP-binding cassette (ABC) transport system consisting of three proteins (ProV, ProW, and ProX), that is found both in E. coli and Salmonella enterica serovar Typhimurium (5, 21, 37, 55). ProV is a peripheral membrane protein found on the cytoplasmic side which shares considerable sequence identity with ATP-binding proteins from other ABC systems. ProW is the integral membrane component of the transport system, and ProX represents the periplasmic glycine betaine-binding protein (GBBP) (21, 55). Within the gram-positive bacteria, molecular aspects of glycine betaine uptake have been recently studied in details in B. subtilis. OpuA and OpuC are members of the superfamily of prokaryotic and eukaryotic ABC uptake systems (32, 36). The OpuA system is the predominant transporter for glycine betaine and consists of three components: an ATPase (OpuAA), an integral membrane protein (OpuAB), and a hydrophilic polypeptide (OpuAC) which functions as the GBBP (32). The OpuC glycine betaine uptake system is related to OpuA but contains an additional integral inner membrane component (36). Both OpuA and OpuC exhibit structural and functional similarities to the ProU system from E. coli. Except for OpuA which is highly specific for glycine betaine, the transport capacity of ProU and OpuC could be extended to other substrates. Upon osmotic stress, ProU is also involved in proline and proline betaine uptake, which both play a role in osmoadaptation in E. coli. OpuC is less specific since besides glycine betaine, choline, choline-O-sulfate, carnitine, crotonobetaine, γ-butyrobetaine, and ectoine can enter the cell via this ABC transporter (26, 28, 30, 40). In addition to these multicomponent binding protein-dependent systems, glycine betaine transporters composed of only one integral membrane protein have also been reported in many bacteria, such as ProP in the enteric bacteria (6, 8) and OpuD in B. subtilis (29).
In S. meliloti, glycine betaine transport activity is strongly stimulated when the cells are grown in media of elevated osmolarity (3), and the existence of a glycine betaine-binding protein in the periplasm of such cells has been demonstrated (35, 57). However, the genes responsible for glycine betaine transport have not been identified. The present study was initiated to gain an understanding of the betaine accumulation mechanism in this bacterium. We used a PCR strategy to isolate an E. coli proU locus analogue in S. meliloti. Our study allowed us to characterize a histidine transport system, called Hut, which is to our knowledge the first histidine transporter also involved in proline and betaine uptake. The role of Hut in S. meliloti and evolutionary aspects are also discussed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. The genomic bank, made up of an EcoRI partial digest of S. meliloti 1021 DNA cloned into pLAFRI (18), was kindly provided by Garry Ditta (University of California, San Diego). E. coli strains were grown at 37°C in Luria-Bertani medium (51). Strains of S. meliloti and A. tumefaciens were grown at 30°C in LBmc (Luria-Bertani medium containing 2.5 mM MgSO4 and 2.5 mM CaCl2). For transport assays or periplasmic protein extraction, cells were grown in M9 minimal medium (39) supplemented with 0.2% mannitol as the carbon source or in MCAA medium (53). When histidine was used as the sole nitrogen source, the M9 medium was depleted of NH4Cl and histidine was added to final concentrations ranging from 10 μM to 5 mM. Cultures used for β-galactosidase assays were realized in an M9-mannitol medium without NaCl in order to obtain a low osmotic strength. When used, amino acids and osmolytes were added at a final concentration of 1 mM. When required, antibiotics were added at concentrations described previously (15, 47). The osmotic strength of media was increased by the addition of NaCl 0.3 M.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| S. meliloti | ||
| RCR2011 | SU47, wild-type | 50 |
| 1021 | RCR2011 Str, Strr derivative of RCR2011 | 38 |
| Rm5000 | rif-5 | 16 |
| RmHY210 | Rm5000 hutV::Ω BglII deletion and insertion | This work |
| RmHY220 | Rm5000 hutX::Ω StuI insertion | This work |
| RmHY230 | Rm5000 hutH2::Ω SmaI insertion | This work |
| RmHZ240 | Rm5000 hutX::lacZ XhoI insertion | This work |
| A. tumefaciens | ||
| GMI9023 | C58 cured of pAtC58 and pTiC58 | 50 |
| At125 | GMI9023 pRmeSU47b | 15 |
| At128 | GMI9023 pRmeSU47a | 15 |
| E. coli | ||
| DH5α | F−supE44 ΔlacU169 (φ80dlacZΔM15) hsdR17(rK− mK+) recA1 endA1 gyrA96 thi-1 relA1 | Bethesda Research Laboratories |
| MT607 | pro-82 thi-1 hsdR17 supE44 recA56 | 15 |
| MT616 | MT607(pRK600) | 15 |
| Plasmids | ||
| pLAFR1 | IncP cosmid cloning vector, Tcr | 18 |
| pRK415 | pRK290 derivative with pUC9 polylinker, Tcr | 31 |
| pRK600 | ColE1 replicon with RK2 transfer region, Cmr | 15 |
| pBluescript SK(−) | Derivative of pUC19 with f1(−)oriR, Apr | Stratagene |
| pGEM −3Zf(+) | Cloning vector | Promega |
| pGM2 | IncP Smr Gmr | 25 |
| pSUP202 | ColE1 Mob+ Tcr Apr Cmr | 52 |
| pKOK5 | Apr Kmr pSUP202 derivative; source of lacZ-Kmr cartridge | 33 |
| pHP45-Ω | Apr pBR322 derivative with interposon Ω Smr/Spr | 48 |
| pLS1 | pLAFR1, S. meliloti 26.5-kb cosmid clone with hut locus | This work |
| pGS4 | 4.0-kb EcoRI fragment from pLS1 cloned into pGEM vector | This work |
| pBS2 | 2.0-kb EcoRI fragment from pBS6 cloned into pBSSK− vector | This work |
| pSUPS2 | EcoRI-EcoRI fragment of pBS2 cloned into pSUP202 | This work |
DNA manipulations and sequencing.
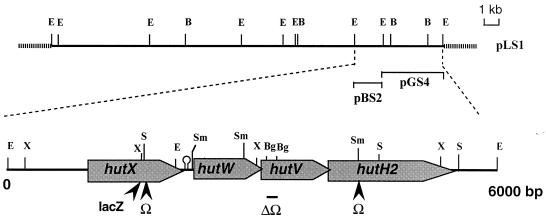
Restriction analysis, ligation, transformation, plasmid DNA extraction, and Southern hybridization were performed by standard methods (51). DNA probes were labeled by using the Prime-a-Gene random priming system (Promega, Charbonnières, France) and [α-32P]dCTP (Amersham Corp., Little Chalfont, United Kingdom). Total DNA from S. meliloti was isolated as described previously (38). The genomic library of S. meliloti 1021 (18) was screened according to standard procedures (51). The nucleotide sequences of pGS4 and pBS2 EcoRI fragments (Fig. 1) were obtained using the fluorescent ABI dye-labeled deoxy-terminator method by Genome Express (Grenoble, France). DNA and protein sequences were analyzed by using Wisconsin Genetics Computer Group (GCG) programs (10) and BLAST protocols (1).
FIG. 1.
Organization of the hut locus. Restriction map of the 26.5-kb EcoRI fragment of S. meliloti Rm5000 cloned into pLAFR1 is shown above the physical and genetic organization of the hut locus. The genes deduced from the nucleotide sequence analysis are represented by boxed arrows. The positions of the Ω insertions and lacZ fusion are indicated below. The hairpin indicates the position of a stem-loop structure able to form a secondary structure. Abbreviations for restriction enzymes: Bg, BglII; E, EcoRI; S, StuI; Sm, SmaI; X, XhoI.
PCR amplification of the S. meliloti hut operon.
PCR mixtures contained 100 pmol of each degenerated primer; 300 ng of Rm5000 genomic DNA or 100 ng of amplified DNA; a 200 μM concentration (each) of dATP, dTTP, dGTP, and dCTP; 1× Taq polymerase buffer (Appligene, Illkirch, France); and 1 U of Taq DNA polymerase (Appligene) in a final volume of 50 μl. Samples were overlaid with mineral oil. Reaction mixtures were cycled automatically using a PHC-3 Thermal Cycler (Techne-Cambridge Ltd., Cambridge, United Kingdom) through temperature and time cycles as follows: denaturation, 96°C for 1 min; annealing, 48°C for 1 min; extension, 72°C for 1 min. The denaturation time of the first cycle was prolonged to 4 min to ensure a single-stranded template for the PCR, and the final extension time was increased to 10 min to ensure completion of strand synthesis. Twenty microliters of reaction mixture was analyzed by electrophoresis on 1.5% agarose gels. PCR-amplified DNA was excised and recovered using the QiaexII kit (Qiagen, Courtabœuf, France) as substrate for reamplification step. The sequence of the two degenerate primers used were 5′ GAR ATI TTY GTI ATI ATG GG 3′ (bup1) and 5′ GCI SIR AAI GCY TCR TCC AT 3′ (bup2).
Mutagenesis of S. meliloti Rm5000.
The hut gene mutagenesis was performed by insertion of a Ω interposon (Sp/Sm) which carries transcription terminators (14). The hutX mutant was constructed by insertion of the SmaI-digested Ω cassette from pHP45-Ω into the StuI site of pBS2 plasmid. The hutV mutant was constructed by deletion of the pGS4 BglII fragment followed by BamHI-digested Ω insertion. To generate a hutH2 mutant, the SmaI Ω cassette was introduced into the pGS4 plasmid partially digested with SmaI (Fig. 1). The EcoRIΩ fragments of these constructions were subcloned into EcoRI-restricted pRK415. Triparental spot matings were used to introduce recombinant plasmids from E. coli to S. meliloti as previously described (11), using E. coli MT616 as a helper strain (15). The Ω insertions were finally recombined into the S. meliloti Rm5000 genome by the plasmid incompatibility technique according to established procedures (44).
Construction of a hutX::lacZ fusion and β-galactosidase assays.
The pSUPS2 was obtained by subcloning the 2-kb EcoRI fragment of pBS2 (Table 1) at the EcoRI site of the suicide vector pSUP202. To construct a transcriptional lacZ fusion in the hutX gene, a SalI-SalI lacZ-Kmr cartridge purified from plasmid pKOK5 was inserted in an XhoI partial digestion of pSUPS2. The resulting plasmid with the lacZ-Kmr cassette inserted at the XhoI site of hutX (Fig. 1) was transferred by conjugation into S. meliloti Rm5000. A recombinant clone, designated RmHZ240, was isolated as Rifr, Nmr, and Tcs. Genetic exchange of the wild-type hutX gene by the hutX-lacZ fusion was confirmed by hybridization analysis. β-Galactosidase activity was determined by the method of Miller (39) using overnight induced cultures at an optical density at 600 nm (OD600) of 0.2 to 0.3.
Transport assays.
Radioactive [methyl-14C]glycine betaine was prepared from [methyl-14C]choline (2.04 GBq/mmol; Amersham Corp.) as previously described (46). [U-14C]histidine (10.6 GBq/mmol) was purchased from Amersham, and [U-14C]proline (9.62 GBq/mmol) and [U-14C]proline betaine (4.6 GBq/mmol) were obtained from the Commissariat à l'Energie Atomique (Gif-sur-Yvette, France). Cells were harvested at an OD420 of 0.8 to 1.0, washed twice in the medium used for the culture, and diluted at a final OD of 0.2 to 0.3. All assays were carried out at 30°C with 1 ml of cell suspension and radioactive substrates (100,000 dpm), at 2 μM for histidine and proline, 10 μM for proline betaine and, 1 μM or 200 μM for glycine betaine for 1 to 5 min. Uptake was determined by rapid filtration through GF/F glass microfiber filters (Whatman), and rinsed with 3 ml of the corresponding medium. The radioactivity remaining on the filters was determined with a liquid scintillation spectrometer (model LS6000SC; Beckman Instruments, Villepinte, France). For competition experiments, cold histidine, proline, arginine, proline betaine, glycine betaine, trigonelline, ectoine, choline, and imidazole were added at a final concentration of 20 or 200 μM into a 2 μM [14C]histidine solution (100,000 dpm). Competition uptakes were run on a 5-min incubation period before filtration.
Periplasmic protein extraction and binding assays.
Rm5000 and RmHY220 strains were grown to an OD420 of 1.5 in MCAA medium or M9 minimal medium supplemented or not with 1 mM histidine. Cells were collected by centrifugation (10,000 × g, 10 min, 20°C) and resuspended in 10 mM Tris-HCl, pH 7.5. Periplasmic proteins were released by cold osmotic shock according to the method of Neu and Heppel (41) and concentrated as described previously (35). Binding activities were detected by using 500 μg of periplasmic proteins in 10 mM Tris-HCl buffer (pH 7.5), incubated for 30 min (cells grown in MCAA medium) or 14 h (cells grown in M9 medium), with 10 μM labeled substrates at 28°C in a sample volume of 400 μl. The amount of substrate bound to periplasmic proteins was determined as described by Walshaw and Poole (61).
Nodulation and nitrogen fixation assays.
The symbiotic proficiency of S. meliloti strains was assayed on alfalfa (Medicago sativa L., cv. Europe) seedlings. Plants were grown in sterile tubes (three plantlets per tube) containing 20 ml of N-free nutrient medium (47) with 0.8% agarose prepared as a slope. The plants were inoculated twice, 5 and 10 days after germination, with the appropriate S. meliloti strains. The number of nodules was determined 4 and 6 weeks after the second inoculation. Phenotypes of bacteria recovered from nodules were checked on the appropriate media. Nitrogen fixation activity was determined by C2H2 reduction, using a gas chromatograph (60).
Nucleotide sequence accession number.
The nucleotide sequence of hut genes has been deposited in the GenBank database under accession no. AF 111939.
RESULTS
Cloning and sequence analysis of a ProU-like ABC transporter in S. meliloti.
Earlier studies (35) have shown the presence of a GBBP in S. meliloti, suggesting that an ABC transport system analogue to the E. coli and S. enterica serovar Typhimurium ProU, and B. subtilis OpuA and OpuC systems might exist in S. meliloti. Among the three proteins involved in these high-affinity transport systems, the ATPase is the best conserved protein between E. coli (ProV), S. enterica serovar Typhimurium (ProV), and B. subtilis (OpuAA and OpuCA). Alignment of the amino acid sequences of these proteins exhibited a highly conserved region ranging from amino acid (aa) 50 to 280 (Fig. 2A). Homology boxes correspond to motifs conserved in the ATP binding-domain of all ATPases from ABC transport systems, including the Walker A and B sites, the linker peptide, and the switch motif and also amino acid boxes specifically conserved in these three proteins involved in glycine betaine transport. In order to isolate a ProV homologue in S. meliloti, two stretches of amino acids, EIFVIMG (positions 62 to 68) and MDEAFSA (positions 206 to 212), were selected to design degenerated primers, called bup1 and bup2 (see Materials and Methods). These primers were used in a PCR amplification of total DNA isolated from S. meliloti Rm5000. A 420-bp amplified fragment of the expected size was obtained, purified, and used as a probe to screen a genomic DNA library of S. meliloti 1021. One positive clone was detected and further analyzed. It contained a recombinant cosmid named pLS1, carrying a 26.5-kb EcoRI insert. By restriction analysis and Southern hybridization studies, the region homologous to the PCR-amplified fragment was restricted to a 4-kb EcoRI fragment (pGS4) (Fig. 1). Sequencing of this fragment, and of the 2-kb EcoRI adjacent fragment (pBS2) led to the determination of a 5,869-bp nucleotide sequence.
FIG. 2.
Comparison of the amino acid sequences of the S. meliloti Hut-component transport system with those of glycine betaine uptake systems of B. subtilis OpuA and OpuC (accession numbers U17292 and AF009352, respectively), E. coli ProU (accession number M24856), and E. coli histidine transport system His (accession numbers U47027 and Y00455). (A) Alignment of ATPases ProV from E. coli and OpuAA and OpuCA from B. subtilis and comparison with the S. meliloti HutV and E. coli HisP proteins. The numbers above the alignment correspond to the amino acid residue position of OpuAA. The Walker A and B ATP binding sites and the ABC transporter family signatures are boxed. Amino acids chosen for the synthesis of PCR primers (bup1 and bup2) are underlined. (B) Partial alignment of S. meliloti HutW with the membrane integral proteins from B. subtilis (BsOpuAB, BsOpuCB, and BsOpuCD), and from E. coli (EcProW, EcHisM, and EcHisQ). The EAA loop is indicated. The alignments were made with the Clustal program from the GCG.
By comparison with the codon usage established for S. meliloti, four genes in the same orientation were deduced: hutX, hutW, hutV, and hutH2 (Fig. 1). Methionine start codons of the four open reading frames are all preceded by a ribosome-binding site. While hutX and hutW are spaced by an intergenic region of 140 bp, hutW and hutV overlap on 8 bp as hutV and hutH2. Upstream of hutX, the EcoRI cloning site might interrupt an open reading frame of unknown function, spaced by 31 bp from hutX. No typical promoter or rho-independent terminator sequences were found in the nucleotide sequence. An inverted repeat which may form a strong hairpin structure (12 bases for the stem, 4 bases for the loop) was detected in the hutX-hutW intergenic region (Fig. 1).
The amino acid sequence of the four gene products shows significant homology with proteins in the databases. The hutX gene encodes a 346-aa hydrophilic protein with a predicted Mr of 37,355 and a calculated isoelectric point of 4.47. This protein shares 26% identical residues with the GBBP ProX of E. coli (21), only 17% identity with the B. subtilis homologue OpuAC, and no significant homology with OpuCC (32, 36). The amino-terminal sequence of HutX of S. meliloti exhibits the characteristic signature of a signal peptide: positively charged amino acids followed by a hydrophobic stretch of amino acids with a sequence L-A-A very similar to the pattern recognized by the E. coli signal peptidase I. The hutW gene codes for a hydrophobic polypeptide (Mr = 30,450) with 46% identity to the inner membrane proteins ProW from E. coli (21) and OpuAB from B. subtilis (32). Much less homology (27% identity) was obtained with the B. subtilis OpuCB and OpuCD hydrophobic proteins involved in the second glycine betaine uptake system, OpuC (Fig. 2B). The hutV gene encodes a hydrophilic protein of 275 aa (Mr = 30,363) which shows strong homology with prokaryotic ATPases involved in ABC transport systems. The best homology is reached with E. coli ProV and B. subtilis OpuAA ATPases (55% identical residues). The identity is only 44% with the ATPase protein OpuCA from the B. subtilis OpuC system. Motifs typical of the ATP cassette of ATPases are also present in S. meliloti HutV (Fig. 2A). Downstream of hutV, hutH2's gene product consists of a 478-aa hydrophilic protein with a predicted molecular mass of 49.7 kDa. This protein shows significant homology (38% identical residues) with human (56) and bacterial histidases (43, 62), including a putative HutH already described in S. meliloti (accession number no. AF032903). Such enzymes are involved in the first step of histidine degradation.
The hut operon expression is induced by histidine.
Upon saline stress, expression of the E. coli proU operon is stimulated by a 100-fold induction factor at the transcriptional level (5). To study the effect of an osmotic shock on S. meliloti hut expression and also to understand the role of a gene encoding a putative histidase downstream hut operon, a chromosomal hutX-lacZ fusion was constructed, resulting in strain RmHZ240. The β-galactosidase activity of RmHZ240 was measured in cells grown in minimal medium (Table 2). Compared to the control (low-osmolarity M9 medium), salt addition did not induce hutX transcription, and instead a threefold repression was observed. The presence of substrates as glycine betaine, proline, and proline betaine, which are molecules transported by E. coli ProU, did not induce hutX gene expression.
TABLE 2.
β-Galactosidase activity of the S. meliloti Rm5000 strain carrying a hutX-lacZ fusion integrated into the chromosome (strain Rm HZ240) under various physiological conditionsa
| Culture condition | β-Galactosidase activity (Miller units) |
|---|---|
| Control | 39 |
| NaCl (0.3 M) | 12 |
| Glycine betaine | 22 |
| Proline | 27 |
| Proline betaine | 29 |
| Histidine | 204 |
Cells were grown in a low-osmolarity M9 minimal medium (Control). Betaines and amino acids were added at a final concentration of 1 mM. Values are means from duplicates of three independent cultures.
The overlap between hutV and hutH2 genes (Fig. 1) suggests that hutH2, which possibly encodes an enzyme involved in histidine catabolism, is cotranscribed with the hut transporter operon. This could signify that Hut is involved in histidine transport, although the best scores of homology of hut encoded proteins were reached with the components of glycine betaine uptake systems (55% identity with the B. subtilis OpuAA and E. coli ProV ATPases) and not with histidine transporters (only 38% identity with the HisP protein of the E. coli histidine ABC transporter [Fig. 2A]). Indeed, when added into the minimal medium, histidine led to a five- to sixfold induction of β-galactosidase-specific activity. These results indicate that hut expression is not regulated by osmotic strength but by histidine and strongly suggest that Hut may be involved in histidine uptake.
Histidine and glycine betaine transport activities in Rm5000 and in hut mutants.
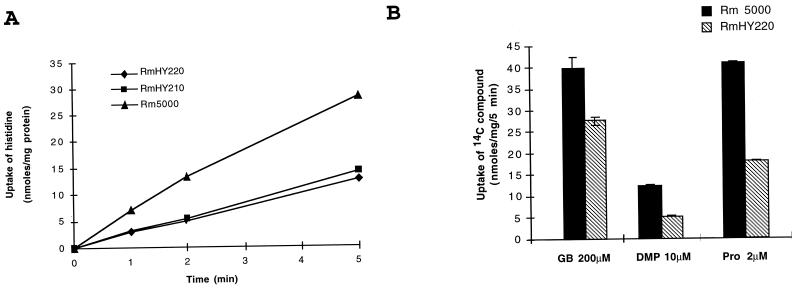
Recombinant Rm5000 strains carrying the Ω interposon in the hutX gene (RmHY220) or in the hutV gene (RmHY210) were constructed as described in Materials and Methods and the legend to Fig. 1. To determine whether these mutants were affected in histidine and glycine betaine uptake, [14C]histidine (2 μM) and [14C]glycine betaine (1 μM and 200 μM) transport assays were realized. When the Rm5000 wild-type strain was grown in M9 medium with histidine (1 mM), a twofold stimulation of the apparent Vmax for histidine uptake was observed (data not shown), suggesting the presence of a histidine-induced transporter(s) in this strain. Under induced conditions, histidine uptake was reduced by 50% in hutX and hutV mutants compared to the parental strain (Fig. 3A). When the Rm5000 strain was grown at high osmolarity, histidine transport activity was not stimulated (data not shown). The high-affinity (1 μM) glycine betaine uptake activities were identical in the wild-type strain Rm5000 and the hutX mutant (data not shown). However, glycine betaine uptake was reduced by 30% in RmHY220 when a high concentration of substrate (200 μM) was used (Fig. 3B). The addition of histidine to the growth medium did not induce glycine betaine transport in the wild-type strain. Taken together, the expression data and the uptake measurements indicate that Hut is a high-affinity histidine ABC transporter regulated by histidine and not by osmotic stress. Furthermore, the Hut transporter is not involved in high-affinity osmoregulated uptake of glycine betaine in S. meliloti.
FIG. 3.
Histidine and glycine betaine uptake activities in S. meliloti Rm5000 and hut mutants. (A) Histidine uptake. Rm5000, RmHY220, and RmHY210 were grown to mid-log phase in M9 medium with histidine (1 mM) and assayed for uptake of [14C]histidine at a final concentration of 2 μM. Values are means from duplicates of two independent cultures with standard errors of less than 3%. (B) Uptake of [14C]glycine betaine (GB 200 μM), [14C]proline betaine (DMP 10 μM), and [14C]proline (Pro 2 μM) in the wild-type strain Rm5000 and the RmHY220 mutant hutX::Ω. Cultures were realized in M9 minimal medium with 1 mM histidine and harvested in the mid-log phase for transport assays.
Periplasmic histidine- and glycine betaine-binding activities.
In order to demonstrate that hutX encodes a histidine-binding protein, periplasmic fractions from Rm5000 and RmHY220 (hutX mutant) strains grown in MCAA medium were prepared and incubated with labeled histidine, and the histidine-binding activity was measured as described in Materials and Methods. With extracts from Rm5000, 1630 pmol of histidine was bound per mg of periplasmic protein compared to 357 pmol per mg of protein detected in the case of Rhizobium leguminosarum (61). With the periplasmic fraction from the RmHY220 strain, only 380 pmol per mg of protein was bound. The strong reduction of the histidine-binding capacity (4.3-fold) in the mutant strain indicates that hutX clearly encodes the histidine-binding protein of the Hut transporter involved in histidine uptake. In the mutant strain, the presence of remaining histidine-binding activity together with 50% histidine uptake activity suggests that other binding protein-dependent ABC transporter(s) for this amino acid exists in S. meliloti.
Since the presence of histidine in M9 medium stimulated histidine uptake in Rm5000, we tested the histidine binding activity in periplasmic fractions from cells grown in the absence or in the presence of histidine (1 mM). Under the experimental conditions used here, no binding activity could be detected with periplasmic extracts from cells grown without histidine whereas the addition of histidine in the growth medium led to a significant binding activity (2,310 pmol per mg protein).
Furthermore, periplasmic fractions from Rm5000 and RmHY220 grown in MCAA medium were tested for their ability to bind glycine betaine when present at a concentration of 10 μM. Extracts from both strains showed the same activity, indicating that HutX was not involved in binding of glycine betaine at high affinities. This result is consistent with the glycine betaine uptake data which did not show, at high affinity (1 μM), any difference between both strains.
Hut transporter is involved in the uptake of several substrates.
Based on amino acid homology, Hut is closer to a glycine betaine uptake system than to a histidine transporter. However, expression studies and uptake experiments clearly show that hut encodes a multicomponent system which is involved in the high-affinity uptake of histidine and not in high- affinity glycine betaine transport. To better understand this paradox, we analyzed the Hut specificity and more precisely its capacity to transport other substrates usually transported by the E. coli ProU system, i.e., proline and proline betaine, or to transport various molecules such as arginine, choline, carnitine, and ectoine. This specificity was first analyzed by competition experiments using the wild-type strain Rm5000. The uptake of [14C]histidine (Table 3) was mainly inhibited by the addition of cold proline and proline betaine with 30 and 45% inhibition, respectively, with 20 μM competitor. Increasing the concentration of these competitors (to 200 μM) enhanced the inhibition only in the case of proline. With cold glycine betaine, ectoine, and carnitine, a 100-fold excess of competitor (200 μM) was necessary to obtain a significant inhibition. The addition of arginine, choline, or imidazole had no effect on histidine uptake activity. These results suggest that, under the experimental conditions used, proline and proline betaine are competitors of histidine uptake activity in Rm5000 even at a low concentration (20 μM), whereas in the case of glycine betaine, ectoine, and carnitine a much higher concentration is needed.
TABLE 3.
Effect of various compounds on histidine uptake
| Competitor | Mean % inhibition of uptake with the following concn of cold competitora:
|
|
|---|---|---|
| 20 μM | 200 μM | |
| Proline | 30 | 43 |
| Proline betaine | 45 | 43 |
| Glycine betaine | 8 | 27 |
| Ectoine | 15 | 56 |
| Carnitine | 15 | 14 |
| Choline | 0 | 0 |
| Arginine | 0 | 0 |
| Imidazole | 0 | 0 |
The results are expressed as percent inhibition of histidine uptake and are means of three independent experiments. Uptake was realized with 2 μM of [14C]histidine. The control value was 21.5 nmol of histidine transported/5 min/mg of protein.
In order to investigate if histidine uptake inhibition was specifically correlated to the inhibition of Hut, transport activities of [14C]proline betaine and [14C]proline were determined in wild-type (Rm5000) and hutX mutant (RmHY220) strains. Indeed, in RmHY220 the proline betaine and proline uptake activities were reduced by 55 to 60% compared to the wild-type strain (Fig. 3B), at a concentration of 10 and 2 μM, respectively. These data confirm that the Hut transporter is also involved in proline betaine and proline uptake at high affinities. The differences observed in the amount of substrates transported during 5 min between the wild-type strain and the mutant RmHY220, showed that Hut allowed the uptake of 14 nmol of histidine per mg of protein instead of 23 and 7 nmol of proline and proline betaine, respectively, per mg of protein. However, the kinetics parameters (Kms and Vmax) of Hut for the different substrates could not be determined, since residual uptake activities were still observed in the mutant strain. This suggests that other high-affinity transporter(s) participates in the entry of these molecules.
hut operon plays a role in histidine catabolism.
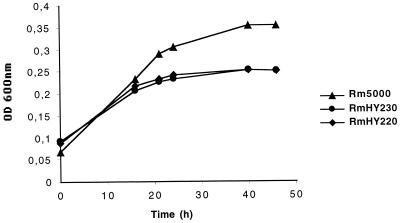
The regulation of hut expression by histidine and not by salt, together with the presence of a putative histidase gene (hutH2) downstream of hutXWV, suggests that the Hut transporter is not involved in osmoprotection but rather in histidine utilization as a carbon and/or nitrogen source. To establish the role of Hut with regard to histidine catabolism, the growth capacity of hut mutants in liquid M9 minimal medium was compared to that of the wild-type strain. Since histidine used as the sole carbon and nitrogen source did not allow an efficient growth of the Rm5000 strain (data not shown), this amino acid was only used as a nitrogen source with mannitol as a carbon source (Fig. 4). Mutants affected either in the uptake of histidine (RmHY220) or in the first step of histidine degradation (RmHY230) were equally affected in their growth capacity: compared to the wild-type strain, a 30% reduction of bacterial yield was observed when histidine was used at a final concentration of 40 μM. Similar results were obtained with 10 or 20 μM histidine. The remaining growth of hut mutants implies (i) the existence of other histidine transporter(s) as expected from uptake data and (ii) the persistence of a histidine degradation pathway in these strains. Indeed, another hutH gene located downstream of a gene encoding an imidazolone-5-propionate hydrolase (hutI) has been reported in S. meliloti (accession number AF032903). When the histidine concentration was increased from 80 μM to 5 mM, the level of inhibition decreased from 30 to 12% (data not shown). These results suggest that the Hut transporter might have a higher affinity for histidine than the other transporter(s) still active in the hut mutants.
FIG. 4.
Growth of S. meliloti Rm5000 wild-type and hut mutant strains. Cells were grown in M9 minimal medium containing mannitol as the carbon source and histidine instead of NH4Cl as the nitrogen source, at a final concentration of 40 μM. Values are means from duplicates, and the standard deviation was less than 5%.
Genomic localization of the hut locus and symbiotic proficiency.
The S. meliloti 2011 genome, besides the chromosome (3.4 Mb), contains two megaplasmids, pSyma (pRmSU47a) (1.4 Mb) and pSymb (pRmSU47b) (1.7 Mb). The location of the hut locus was determined by using Agrobacterium tumefaciens strains containing either pSyma or pSymb from S. meliloti (15). Total DNA from hybrid strains was tested by Southern analysis using two radiolabeled probes, a 2-kb EcoRI fragment from pBS2 and a 4-kb EcoRI fragment from pGS4, corresponding to the entire hut locus. With S. meliloti Rm5000, two strong hybridization signals corresponding to the 2-kb and 4-kb probes were observed (data not shown). Under the stringency conditions used for this analysis, no hybridization band was detected between these probes and DNA from E. coli. With the A. tumefaciens derivatives, both probes strongly hybridized to a 6-kb fragment present in all three strains, probably corresponding to the hut locus of A. tumefaciens. No additional hybridization signal corresponding to the hut locus from S. meliloti could be detected, indicating that this operon is not located on the megaplasmids.
To test the capacity of the Ω insertion mutants to nodulate the alfalfa host plants, seedlings were inoculated with the RmHY210 and RmHY220 strains and the wild-type Rm5000 strain. All strains were similarly efficient in inducing nodulation, and acetylene reduction activities observed with nodules obtained with the mutant strains were not significantly different from that measured with nodules produced by Rm5000 during the first 6 weeks of nitrogen fixation (data not shown). Thus, the hut mutant strains have a Nod+ and Fix+ phenotype.
DISCUSSION
In this study, we have characterized, in S. meliloti, a proU-like operon involved in the high-affinity uptake of histidine and not of glycine betaine. This system (Hut) belongs to the family of ABC transporters (22), which involves multiple components: a periplasmic histidine-binding protein, HutX; a hydrophobic protein, HutW, spanning the internal membrane; and a cytoplasmic protein linked to the membrane, HutV, which plays a role in the active transport due to its ATPase activity. Within the hut operon, while hutW and hutV overlap, hutX and hutV are spaced by a 140-bp region containing an inverted repeat able to form a hairpin structure, which might play a role in hut regulation either as an mRNA stability structure or as a pause site for transcription and translation. Such a structure is analogous to that described in the S. enterica serovar Typhimurium histidine uptake operon, where a repetitive extragenic palindrome is present in the 102-bp intergenic space between hisJ, the gene encoding the binding protein, and the following gene, hisQ. It has been shown that this repetitive extragenic palindrome sequence element may protect mRNA molecules against exonucleolytic 3′→5′ degradation in order to favor expression of the 5′ binding protein gene compared to the genes encoding inner membrane-associated compounds, resulting in a gradient of expression (54). The genetic organization of the hut locus follows the “binding protein first” rule mainly encountered in binding protein transport operons, since the gene arrangement follows the order hutX-hutW-hutV. This may help to increase hutX expression relative to that of other genes downstream, since the amount of periplasmic binding proteins largely exceeds that of integral and ATPase proteins. Such organization is also found in the S. enterica serovar Typhimurium hisJQMP operon (24). In this bacterium, two different genes encode the integral heterodimeric proteins, HisQ and HisM, while only one gene, hutW, encodes the integral homodimeric complex in S. meliloti.
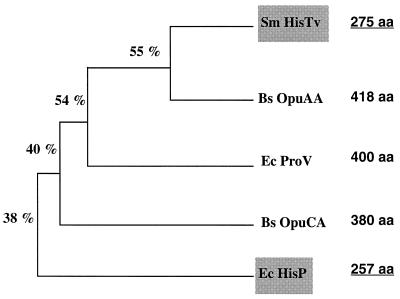
Surprisingly, amino acid homology shows that HutXWV are closer to the corresponding ProU glycine betaine transport proteins than to the E. coli histidine transport proteins. While low structure conservation is usually observed for the ligand binding proteins involved in ABC transporters (59), significant homology was found between HutX and the GBBPs ProX and OpuAC, and no homology with the HisJ protein was found. In addition, the integral membrane protein HutW shows higher homology with ProW-like proteins (ProW, OpuAB, OpuCB, and OpuCD) than with HisQ and HisM proteins (Fig. 2B). The size of HutW (285 aa) is similar to that of OpuAB (282 aa) and slightly larger than those of OpuCB (217 aa), OpuCD (229 aa), HisQ (228 aa), and HisM (238 aa) proteins. These two last proteins exhibit only five transmembrane segments, whereas the largest ProW protein (354 aa) contains two additional N-terminal spanning domains. It has been suggested that this large N-terminal domain (100 aa) is present in the periplasmic space and may be involved in measuring the cell turgor and transducing the mechanical stimulus into alterations of the glycine betaine transport activity (13). More generally, it is conjectured that N-terminal extensions beyond the five C-terminal transmembrane helices present in all inner membrane proteins of ABC systems play an accessory role, such as structural stabilization of the inner membrane complex or regulation of transport activity (2). A similarity tree (Fig. 5) shows that HutV is also closer to ATPases of glycine betaine uptake systems than to HisP protein on the 200-aa N-terminal overlap containing the ABC domain (Walker A and B motifs, ABC signature, switch motif). However, based on the size of the proteins, HutV (275 aa) is closer to HisP (257 aa) than to glycine betaine ATPases (∼400 aa) due to the absence of a large C-terminal domain. Such a short ATPase has also been found in the E. coli glutamine transport system GlnQ (42).
FIG. 5.
Similarity relationships between S. meliloti HutV, the members of the ProV-like family, and HisP protein. The size of each protein is shown on the right. The percentage indicated at each branch point of the dendrogram corresponds to the percentage of identical amino acids between HutV and the different proteins considered as determined with the GAP program of the GCG. Bs OpuAA, B. subtilis ATPase of glycine betaine uptake system OpuA; Ec ProV, E. coli ATPase of glycine betaine uptake system ProU; Bs ProV, B. subtilis ATPase of glycine betaine uptake system OpuC; Ec HisP, E. coli ATPase of histidine uptake system.
While the S. meliloti Hut system is closely related to glycine betaine ABC transporters, uptake experiments have shown the Hut system to be involved in the high-affinity uptake of histidine (Fig. 3A), which is not stimulated by high osmolarity, as expected for a ProU-like system, but rather by histidine. Indeed, hutX-lacZ fusion has shown that hut expression is transcriptionally induced by histidine and not by increasing osmolarity (Table 2). As histidine per se is not used as an osmoprotectant by S. meliloti, Hut does not play a role in osmoprotection but rather in nitrogen assimilation, since the growth capacity of hut mutants on histidine as the only source of nitrogen is significantly reduced. This conclusion is corroborated by the presence of the hutH2 gene, encoding a putative histidase involved in the first step of histidine degradation to glutamate. The overlap between hutH2 and hutV suggests that these genes are cotranscribed on the same operon, and histidine uptake might be coupled with its utilization as a sole source of nitrogen. Such a situation is clearly different from that described in E. coli, where the histidine transport operon (hisJQMP) is not closely linked to the histidine catabolism operon (hut) on the genetic map.
Another notable feature of the S. meliloti Hut transport system is its substrate specificity. Transport activities measured in hutX mutant and wild-type strains have shown that besides histidine, Hut was also involved in proline and proline betaine uptake at high affinities and in glycine betaine at low affinities (Fig. 3B), with all compounds taken up by the E. coli ProU system. Competition experiments also pointed out that histidine transport activity in the wild-type strain was affected by the presence of carnitine or ectoine, the latter of which is a nonaccumulated osmoprotectant in S. meliloti (58). Further experiments are necessary to clearly demonstrate that Hut is directly involved in the entry of these two molecules as it is the case for B. subtilis OpuC. In B. subtilis, while the OpuA system is highly specific for glycine betaine, the related OpuC system transports a wide range of substrates including, beside glycine betaine, ectoine, carnitine, γ-butyrobetaine, crotonobetaine, and choline. Our results suggest that choline is not transported by Hut, since no histidine transport competition could be observed. This is also true for arginine, which may not be transported by the Hut system in S. meliloti, whereas in E. coli, arginine uptake is dependent on the histidine-LAO system which uses a binding protein specific for lysine, arginine, and ornithine (23). Based on the molecular structure of these molecules, it is tempting to speculate that Hut allows the preferential entry of compounds containing a nitrogen heterocycle with a carboxyl residue (histidine, proline, proline betaine, or ectoine), since imidazole, which does not possess a carboxyl, is not a competitor for histidine uptake. Less affinity was obtained for linear molecules that also contain a quaternary ammonium and a carboxyl group, such as glycine betaine and carnitine. Again, uptake was not observed in the absence of a carboxyl group (choline).
To our knowledge, the Hut system characterized here is the first described histidine transport system which is also involved in proline and betaine uptake. However, histidine and proline are not osmoprotectants per se in S. meliloti. This could explain why the Hut system does not play a role in osmoprotection and why its contribution to increased betaine transport under salt stress is low (data not shown). We postulate that in the absence of osmotic stress, proline, proline betaine, and, to a lesser extent, glycine betaine, which enter the cell via the Hut system, are also used as carbon and/or nitrogen sources. Indeed, at low osmolarities, S. meliloti catabolizes proline betaine and glycine betaine very efficiently (20, 53). Such catabolism does not occur in other bacteria like E. coli and B. subtilis, for which a hyperosmotic shock followed by normal osmotic growth conditions results in betaine efflux. In addition, S. meliloti, as a free-living bacterium and also during the establishment of the symbiotic interaction with alfalfa, uses proline as an important energy source (27).
Finally, based on the phylogenetic tree of E. coli ATP-binding proteins established by Dassa et al. (9), it is worth considering that the group of ATPases involved in osmoprotectant transport, such as ProV, and the group of ATPases implicated in polar amino acid uptake, such as HisP, are closely related. This suggests that the two groups have evolved after duplication of a common ancestor, probably able to transport both categories of compounds, which are structurally close.
ACKNOWLEDGMENTS
E.B. and L.D. contributed equally to this work.
This work was funded by the Centre National de la Recherche Scientifique and by the European Communities BIOTECH Programme, as part of the Project of Technological Priority 1993–1997 (BIO2CT930400 [D.L.R.]). E.B. received a doctoral fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche.
We are grateful to the colleagues cited in Table 1 who generously provided strains and the genomic bank of S. meliloti used in this study. We thank E. A. Galinski for the gift of cold ectoine.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment research tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ames G F-L, Lecar H. ATP-dependent bacterial transporters and cystic fibrosis: analogy between channels and transporters. FASEB J. 1992;6:2660–2666. doi: 10.1096/fasebj.6.9.1377140. [DOI] [PubMed] [Google Scholar]
- 3.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 4.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairney J, Booth I R, Higgins C F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985;164:1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairney J, Booth I R, Higgins C F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985;164:1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1210–1233. [Google Scholar]
- 8.Culham D E, Lasby B, Marangoni A G, Milner J L, Steer B A, van Nues R W, Wood J M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transport, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- 9.Dassa E, Hofnung M, Paulsen I A, Saier M H., Jr The Escherichia coli ABC transporters: an update. Mol Microbiol. 1999;32:887–889. doi: 10.1046/j.1365-2958.1999.01392.x. [DOI] [PubMed] [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ditta G, Stanfield S, Corbin D, Helinski D R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dylan T, Helinski D R, Ditta G. Hypoosmotic adaptation in Rhizobium meliloti requires β-(1→2)-glucan. J Bacteriol. 1990;172:1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faatz E, Middendorf A, Bremer E. Cloned structural genes for the osmotically regulated binding-protein-dependent glycine betaine transport system (ProU) of Escherichia coli K-12. Mol Microbiol. 1988;2:265–279. doi: 10.1111/j.1365-2958.1988.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 14.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertion mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 15.Finan T M, Hartwieg E K, LeMieux K, Bergman K, Walker G C, Signer E R. General transduction in Rhizobium meliloti. J Bacteriol. 1984;159:120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finan T M, Kunkel B, DeVos G F, Signer E R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fougère F, Le Rudulier D, Streeter J G. Effects of salts stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.) Plant Physiol. 1991;96:1228–1236. doi: 10.1104/pp.96.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman A M, Long S R, Brown S E, Buikema W J, Ausubel F M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 19.Glaasker E, Heuberger E H M L, Konings W N, Poolman B. Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactobacillus plantarum. J Bacteriol. 1998;180:5540–5546. doi: 10.1128/jb.180.21.5540-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloux K, Le Rudulier D. Transport and catabolism of proline betaine in salt-stressed Rhizobium meliloti. Arch Microbiol. 1989;151:143–148. [Google Scholar]
- 21.Gowrishankar J. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J Bacteriol. 1989;171:1923–1931. doi: 10.1128/jb.171.4.1923-1931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 23.Higgins C F, Ames G F-L. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci USA. 1981;78:6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins C F, Haag P D, Nikaido K, Ardeshir F, Garcia G, Ames G F-L. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982;298:723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby G A, Jacob A E, Hedges R W. Recombination between plasmids of incompatibility groups P-1 and P-2. J Bacteriol. 1976;127:1278–1285. doi: 10.1128/jb.127.3.1278-1285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jebbar M, von Blohn C, Bremer E. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol Lett. 1997;154:325–330. [Google Scholar]
- 27.Jimenez-Zurdo J I, Garcia-Rodriguez F M, Toro N. The Rhizobium meliloti putA gene: its role in the establishment of the symbiotic interaction with alfalfa. Mol Microbiol. 1997;23:85–93. doi: 10.1046/j.1365-2958.1997.1861555.x. [DOI] [PubMed] [Google Scholar]
- 28.Kappes R M, Bremer E. Response of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine and γ-butyrobetaine via the ABC-transport system OpuC. Microbiology. 1998;144:83–90. doi: 10.1099/00221287-144-1-83. [DOI] [PubMed] [Google Scholar]
- 29.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kappes R M, Kempf B, Kneip S, Boch J, Gade J, Meier-Wagner J, Bremer E. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol Microbiol. 1999;32:203–216. doi: 10.1046/j.1365-2958.1999.01354.x. [DOI] [PubMed] [Google Scholar]
- 31.Keen N T, Tamaki S, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 32.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Biol Chem. 1995;270:16701–16713. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 33.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 34.Le Rudulier D, Strøm A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 35.Le Rudulier D, Gloux K, Riou N. Identification of an osmotically induced periplasmic glycine betaine-binding protein from Rhizobium meliloti. Biochim Biophys Acta. 1991;1061:197–205. doi: 10.1016/0005-2736(91)90285-g. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Hansen J N. Characterization of a chimeric proU operon in a subtilin-producing mutant of Bacillus subtilis 168. J Bacteriol. 1995;177:6874–6880. doi: 10.1128/jb.177.23.6874-6880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 38.Meade H M, Long S R, Ruvkun G B, Brown S E, Ausubel F M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 40.Nau-Wagner G, Boch J, Le Good J, Bremer E. High-affinity transport of choline-O-sulfate and its use as a compatible solute in Bacillus subtilis. Appl Environ Microbiol. 1999;65:560–568. doi: 10.1128/aem.65.2.560-568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neu H C, Heppel L A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 42.Nohno T, Saito T, Hong J S. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ) Mol Gen Genet. 1986;205:260–269. doi: 10.1007/BF00430437. [DOI] [PubMed] [Google Scholar]
- 43.Oda M, Sugishita A, Furukawa K. Cloning and nucleotide sequences of histidase and regulatory genes in the Bacillus subtilis hut operon and positive regulation of the operon. J Bacteriol. 1988;170:3199–3205. doi: 10.1128/jb.170.7.3199-3205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Østeräs M, Stanley J, Finan T M. Identification of Rhizobium-specific intergenic mosaic elements within an essential two-component regulatory system of Rhizobium species. J Bacteriol. 1995;177:5485–5494. doi: 10.1128/jb.177.19.5485-5494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Østeräs M, Boncompagni E, Vincent N, Poggi M C, Le Rudulier D. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc Natl Acad Sci USA. 1998;95:11394–11399. doi: 10.1073/pnas.95.19.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perroud B, Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985;161:393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pocard J-A, Vincent N, Boncompagni E, Smith L T, Poggi M C, Le Rudulier D. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology. 1997;143:1369–1379. doi: 10.1099/00221287-143-4-1369. [DOI] [PubMed] [Google Scholar]
- 48.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 49.Proctor L M, Lai R, Gunsalus R P. The methanogenic archaeon Methanosarcina thermophila TM-1 possesses a high-affinity glycine betaine transporter involved in osmotic adaptation. Appl Environ Microbiol. 1997;63:2252–2257. doi: 10.1128/aem.63.6.2252-2257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg C, Huguet T. The pAtC58 plasmid is not essential for tumor induction. Mol Gen Genet. 1984;196:533–536. [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 53.Smith L T, Pocard J-A, Bernard T, Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988;170:3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stern M J, Prossnitz E, Ames G F-L. Role of the intercistronic region in post-transcriptional control of gene expression in the histidine transport operon of Salmonella typhimurium: involvement of REP sequences. Mol Microbiol. 1988;2:141–152. doi: 10.1111/j.1365-2958.1988.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 55.Stirling D A, Hulton C S, Waddell L, Park S F, Stewart G S, Booth I R, Higgins C F. Molecular characterization of the proU loci of Salmonella typhimurium and Escherichia coli encoding osmoregulated glycine betaine transport systems. Mol Microbiol. 1989;3:1025–1038. doi: 10.1111/j.1365-2958.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 56.Suchi M, Harada N, Wada Y, Takagi Y. Molecular cloning of a cDNA encoding human histidase. Biochim Biophys Acta. 1993;1216:293–295. doi: 10.1016/0167-4781(93)90157-9. [DOI] [PubMed] [Google Scholar]
- 57.Talibart R, Le Hénaff M, Bernard T, Wroblewski H. Identification of bacterial periplasmic glycine betaine-binding protein after electrophoresis and affinity labeling. J Biochem Biophys Methods. 1990;21:155–164. doi: 10.1016/0165-022x(90)90062-h. [DOI] [PubMed] [Google Scholar]
- 58.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T. Osmoadaptation in Rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trinchant J C, Birot A M, Rigaud J. Oxygen supply and energy-yielding substrates for nitrogen fixation (acetylene reduction) by bacteroid preparations. J Gen Microbiol. 1981;125:159–165. [Google Scholar]
- 61.Walshaw D L, Poole P S. The general L-amino acid permease of Rhizobium leguminosarum is an ABC uptake system that also influences efflux of solutes. Mol Microbiol. 1996;21:1239–1252. doi: 10.1046/j.1365-2958.1996.00078.x. [DOI] [PubMed] [Google Scholar]
- 62.Wu P C, Kroening T A, White P J, Kendrick K E. Purification of histidase from Streptomyces griseus and nucleotide sequence of the hutH structural gene. J Bacteriol. 1992;174:1647–1655. doi: 10.1128/jb.174.5.1647-1655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]