Abstract
Members of the protein family of immunoglobulin A1 protease-like autotransporters comprise multidomain precursors consisting of a C-terminal autotransporter domain that promotes the translocation of N-terminally attached passenger domains across the cell envelopes of gram-negative bacteria. Several autotransporter domains have recently been shown to efficiently promote the export of heterologous passenger domains, opening up an effective tool for surface display of heterologous proteins. Here we report on the autotransporter domain of the Escherichia coli adhesin involved in diffuse adherence (AIDA-I), which was genetically fused to the C terminus of the periplasmic enzyme β-lactamase, leading to efficient expression of the fusion protein in E. coli. The β-lactamase moiety of the fusion protein was presented on the bacterial surface in a stable manner, and the surface-located β-lactamase was shown to be enzymatically active. Enzymatic activity was completely removed by protease treatment, indicating that surface display of β-lactamase was almost quantitative. The periplasmic domain of the outer membrane protein OmpA was not affected by externally added proteases, demonstrating that the outer membranes of E. coli cells expressing the β-lactamase AIDA-I fusion protein remained physiologically intact.
The secretion of proteins into the extracellular environment and surface display by gram-negative bacteria are of rising interest (13, 14). However, the translocation of high-molecular-weight molecules in gram-negative bacteria is hampered by the cell envelope, consisting of two membranes that are separated by the periplasmic space. The outer membrane acts as a physiological barrier, allowing the uptake or secretion of low-molecular-weight compounds by diffusion through the porins, while larger molecules require specialized transport mechanisms to cross the cell wall.
To facilitate the export of large proteins, gram-negative bacteria have evolved complex secretion pathways characterized by varying numbers of accessory proteins that are required for the translocation of specific target proteins across both membranes of the cell envelope. In both the type I secretion of Escherichia coli hemolysin (33) and the type II secretion of pullulanase from Klebsiella oxytoca (31), the translocated proteins are secreted into the medium, whereas proteins secreted by the type III secretion systems of Salmonella (5), Shigella (27), and Yersinia (6) species have been shown to be injected into eukaryotic cells (16). However, in all three secretion pathways the complex interplay of the exported proteins with the accessory components is required.
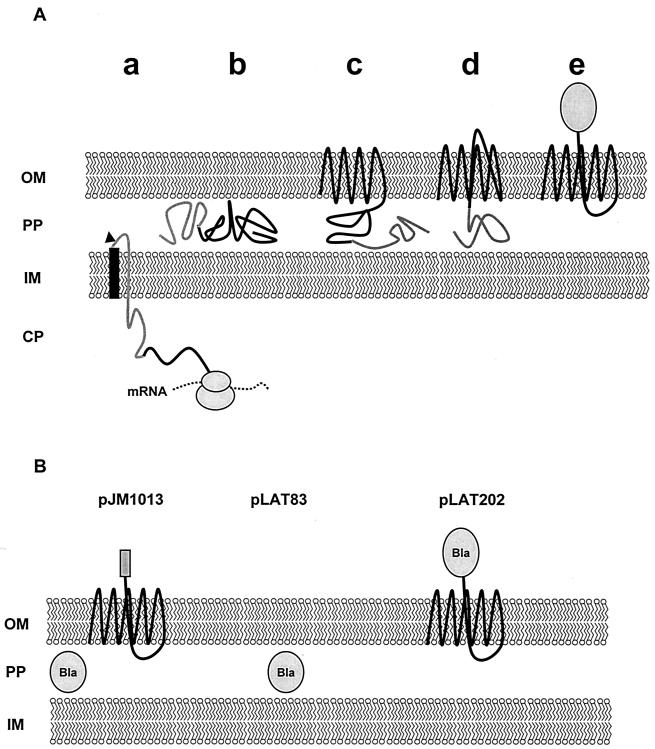
In contrast, the immunoglobulin A1 (IgA1)-protease-like autotransporter secretion pathway (9, 18), a system that was first discovered and extensively investigated for the IgA1 protease of Neisseria gonorrhoeae (20, 21, 30), is characterized by a single self-translocating protein precursor. This precursor consists of a classic signal peptide for Sec-dependent secretion into the periplasm and a C-terminal autotransporter domain that mediates the translocation of one or more N-terminal passenger domains through the outer membrane (30, 34, 35, 38). The autotransporter domain consists of a β-barrel, made up by 14 antiparallel membrane-spanning β-sheets, that is assumed to insert as a porin-like structure into the outer membrane, directing the export of the passenger domain (18, 22). Figure 1A is a schematic illustration of the mode of action of autotransporters.
FIG. 1.
(A) Schematic representation of surface display by autotransporters in gram-negative bacteria. The protein precursor is secreted in a classical Sec-dependent manner into the periplasm, where the signal peptide is cleaved off (a and b). The C-terminal domain is assumed to insert into the outer membrane, forming a β-barrel (c) that mediates the translocation of the passenger domain, probably through the hydrophilic pore in the center of the β-barrel, to the cell surface (d). The export results in a stable presentation of the passenger domain on the cell surface (e). The signal peptide is shown as a solid bar, the passenger domain is shaded, and the action of the signal peptidase is symbolized by an arrowhead. (B) Expected membrane phenotypes of JK321 strains conferred by pLAT83, pLAT202, or pJM1013. CP, cytoplasm; IM, inner membrane; OM, outer membrane; PP, periplasm.
The ability of autotransporter domains to direct heterologous passenger proteins to the surface has been investigated in our laboratory for the autotransporter domains of the IgA1 protease of N. gonorrhoeae (20, 21) and AIDA-I (24), the E. coli adhesin involved in diffuse adherence (2). In these studies, both autotransporter domains were shown to efficiently mediate the export of heterologous passenger proteins, such as the B subunit of cholera toxin (CTB), as well as defined epitopes, to the surfaces of E. coli and Salmonella enterica serovar Typhimurium cells. The amount of the heterologous passenger protein presented on the surface was up to 5% of the total bacterial protein in the AIDA-I system (24), demonstrating the potential of autotransporters for biotechnological applications. The autotransporter domains of the Serratia marcescens serine protease (35) and the Shigella flexneri VirG protein (38) have also been successfully employed for surface display.
Possible applications for autotransporters include (i) the development of recombinant, live oral vaccines using attenuated bacterial vaccine strains, (ii) construction of bacterial whole-cell absorbents, (iii) export of protein domains for the study of receptor-ligand interactions, (iv) surface display of random peptide libraries, and (v) the export of biologically active proteins for biomedical and biotechnological use. In this communication we report on an example of the latter application, the export of enzymatic activity to the surfaces of E. coli cells by the autotransporter domain of AIDA-I using the periplasmic enzyme β-lactamase (Bla). We have constructed a genetic fusion of the bla gene and the gene encoding the autotransporter domain of AIDA-I, resulting in the export of Bla to the surfaces of E. coli cells and the stable display of active Bla on physiologically intact cells.
MATERIALS AND METHODS
Bacterial strains.
All E. coli strains employed in this study are listed in Table 1. For all purposes, the bacteria were grown at 28 or 37°C on Luria-Bertani (LB) agar plates supplemented with ampicillin (100 mg/liter) or chloramphenicol (30 mg/liter) when required.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype or phenotype | Reference or source |
|---|---|---|
| DH5α | F− (φ80dlacZΔM15) Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 λ− thi-1 gyrA96 relA1 | GIBCO BRL |
| JCB570 | araD139 Δ(araABC-leu)7679 galU galK Δ(lac)X74 rpsL thi phoR zih-12::Tn10 | 1 |
| JCB571 | araD139 Δ(araABC-leu)7679 galU galK Δ(lac)X74 rpsL thi phoR zih-12::Tn10 dsbA::kan | 1 |
| JK321 | UT5600 zih::Tn10 dsbA::kan | 19 |
| UT2300 | azi-6 fhuA23 lacY1 leu-6 mtl-1 proC14 purE42 rpsL109 thi-1 trpE38 tsx-67 Δ(entD-fepC) | 7 |
| UT5600 | azi-6 fhuA23 lacY1 leu-6 mtl-1 proC14 purE42 rpsL109 thi-1 trpE38 tsx-67 Δ(ompT-fepC) | 7 |
| XL1-Blue | F′::Tn10 proA+B+ lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rK− mK+) supE44 relA1 lac | Stratagene |
| pJM1013 | Periplasmic Bla, FP50 in the outer membrane, derivative of pJM7 (24) | This study |
| pLAT83 | Periplasmic Bla, derivative of pACYC184 | This study |
| pLAT202 | Surface-displayed Bla (FP77), derivative of pACYC184 | This study |
Recombinant DNA and protein techniques.
For the construction of the Bla-AIDA fusion, the bla gene was amplified by PCR from plasmid pJM7 (24) using oligonucleotide primers WS34 (5′-CCTTTCACCACCAGACGG-3′) and A3 (5′-GATCAGATCTAGACCAATGCTTAATCAGTGA-3′). The PCR fragment was hydrolyzed with ClaI and BglII, fused with the autotransporter portion of the gene encoding AIDA-I, and inserted into the Tetr gene of plasmid vector pACYC184 (32). The resulting plasmid (pLAT202) contains a genetic fusion of the bla gene with the AIDA-I autotransporter domain; the expression of the corresponding fusion protein is driven by the promoter of the bla gene. The control construct (pLAT83) expressing wild-type Bla in the periplasm was obtained by the same strategy, replacing primer A3 with A2 (5′-GATCAGATCTAGATTACCAATGCTTAATCAGTG-3′), incorporating the stop codon of the bla gene. Plasmid pJM1013 is a medium-copy-number vector expressing the reporter epitope PEYFK fused to the AIDA-I autotransporter domain under the strong PTK promoter, similar to pJM22 (24). The expression of the AIDA-I fusion proteins was analyzed by the separation of the outer membrane fraction of E. coli by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or by Western blotting using a rabbit antiserum raised against Bla. The trypsin accessibility of OmpA was examined by SDS-PAGE of E. coli membrane fractions and subsequent Western blot analysis. After SDS-PAGE the samples were transferred onto a Immobilon-P membrane (Millipore) and probed with the OmpA-specific antiserum AK57 (20) diluted 1:10,000 or the Bla-specific antiserum diluted 1:2,000 in Tris-buffered saline (TBS, consisting of 150 mM NaCl–50 mM Tris-HCl [pH 7.4]) supplemented with 5% skim milk powder (5% M-TBS). Unbound antibodies were removed by washes with TBS-T (0.05% Tween 20 in TBS), and the bound antibodies were detected by enhanced chemiluminescence (Amersham) using a goat anti-rabbit IgG-peroxidase conjugate (Sigma).
In vivo techniques.
For whole-cell trypsin treatment, the bacteria were collected from the agar plates and resuspended in phosphate-buffered saline (PBS). Subsequently, the bacterial suspension was adjusted to an optical density at 575 nm (OD575) of 10.0 and surface-exposed protein domains were cleaved by incubation of the suspension at 37°C for 10 min with trypsin at a final concentration of 50 mg/liter. To remove the trypsin after the reaction, the cells were washed twice in PBS by gentle centrifugation and subjected to further manipulations.
To determine whole-cell Bla activity, the cells were collected from agar plates and resuspended in PBS. The suspension was adjusted to an OD575 of 10.0. Subsequently, 0.02 ml of this suspension was incubated at room temperature either with 50 μl of a penicillin G solution (10 mg/ml) or with 10 μl of a cephaloridine solution (5 mg/ml). After 10 min, PBS was added to a final volume of 1.0 ml. After a brief centrifugation at 13,000 × g to remove the cells, the penicillin G or cephaloridine content of the supernatant was analyzed by spectrophotometry at 240 or 260 nm, respectively. As a control, the same assay was performed without incubation for 10 min to obtain normalized ΔOD values for each experiment.
Purified TEM-Bla from E. coli was obtained lyophilized from Sigma (catalog no. P3553) and was reconstituted in PBS and adjusted to a concentration of 0.002 mg of protein/μl, corresponding to a calculated enzymatic activity of 0.8 U/μl with penicillin G as the substrate and 0.13 U/μl with cephaloridine as the substrate.
Preparation of outer membranes of E. coli.
Bacteria grown overnight were harvested from agar plates and resuspended in PBS. The suspension was passaged once through a French pressure cell at 20,000 lb/in2 to lyse the cells. Large bacterial fragments and intact cells were sedimented from the opaque solution by centrifugation at 5,000 × g for 5 min. To solubilize the inner membrane, l-laurylsarcosinate was added to a final concentration of 1% to the cleared solution. Subsequently, the outer membrane was separated from the cytoplasm and inner membrane by centrifugation at 20,000 × g for 30 min.
RESULTS
Genetic fusion of the bla gene to the autotransporter domain of AIDA-I.
The bla gene was amplified by PCR from plasmid pJM7 and genetically fused to the autotransporter domain of AIDA-I, and the gene fusion was inserted into the Tetr gene of plasmid vector pACYC184. The resulting plasmid (pLAT202) contains the Bla–AIDA-1 gene fusion, and the expression of the Bla–AIDA-I fusion protein is controlled by the native promoter of the bla gene. The fusion protein was termed FP77, according to the predicted molecular mass of 77.4 kDa resulting after processing by the signal peptidase in the periplasm. For the subsequent assays, the E. coli strains listed in Table 1 transformed with pLAT202 were used. The E. coli strains JK321(pLAT83), expressing wild-type Bla from the pACYC184 backbone, and JK321(pJM1013), expressing wild-type Bla in addition to FP50, a reporter epitope fused to the AIDA-I autotransporter domain under the control of a strong constitutive promoter, were employed as controls. The expected membrane phenotypes of JK321 harboring pLAT83, pLAT202, or pJM1013 are diagrammed in Fig. 1B.
Targeting of FP77 to the surfaces of E. coli cells.
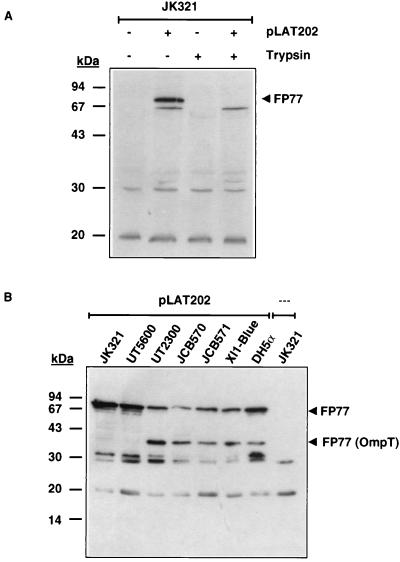
FP77 was expressed in a stable manner in JK321(pLAT202), migrating in SDS-PAGE at approximately 77 kDa, as predicted (Fig. 2A). Since protease treatment of physiologically intact cells is a suitable tool for testing the surface exposure of a heterologous passenger domain at the bacterial cell surface (21, 24), physiologically intact JK321(pLAT202) cells were subjected to trypsin treatment prior to the Western blot analysis. Trypsin treatment of JK321(pLAT202) cells resulted in the complete disappearance of FP77, indicating that the Bla moiety of FP77 is exposed on the cell surface.
FIG. 2.
(A) Expression of FP77 in JK321(pLAT202) as assessed by Western blot analysis. Whole-cell lysates of JK321 and JK321(pLAT202) cells corresponding to the amount of bacteria in 1 ml of a suspension with an OD575 of 0.1 were subjected to SDS-PAGE (12.5% gel) and subsequent Western blot analysis. The membrane was probed with a Bla-specific antiserum that binds to FP77. Tryptic digestion of physiologically intact cells was performed as described in the text. (B) Expression of FP77 in different E. coli strains. Whole-cell lysates of E. coli strains harboring pLAT202 were prepared and analyzed as described above. FP77 (OmpT), OmpT degradation product of FP77.
The stability of the heterologous passenger domain on the bacterial cell surface and the efficiency of translocation across the outer membrane are crucial issues in the process of autodisplay. The outer membrane protease OmpT is known to degrade heterologous passenger proteins displayed by autotransporters on the cell surface (21, 24), and the presence of the periplasmic oxidoreductase DsbA, required for the efficient formation of disulfide bonds in the periplasm (1), has been shown to hamper the translocation of passenger domains containing stable tertiary structures that contain disulfide bonds (19). Interestingly, full-length FP77 was expressed in all E. coli strains examined (Fig. 2B). However, the presentation was most efficient in the ompT strains JK321(pLAT202) and UT5600(pLAT202). In the isogenic strains JCB570(pLAT202) and JCB571(pLAT202), the export of FP77 was more efficient in the dsbA mutant JCB571, whereas no difference was seen between the levels of FP77 expression in freshly transformed JK321(pLAT202) and UT5600(pLAT202). However, a striking instability of FP77 was observed in UT5600(pLAT202) after passaging on solid medium. In contrast, JK321(pLAT202) showed stable FP77 expression even after multiple passages (data not shown). FP77 was completely removed by tryptic digestion of physiologically intact cells in JK321, UT5600, and DH5α and was almost completely removed in UT2300, JCB570, JCB571 and XL1-Blue, confirming the surface localization of the Bla moiety (data not shown). The differences observed might reflect distinct phenotypes of the outer membrane.
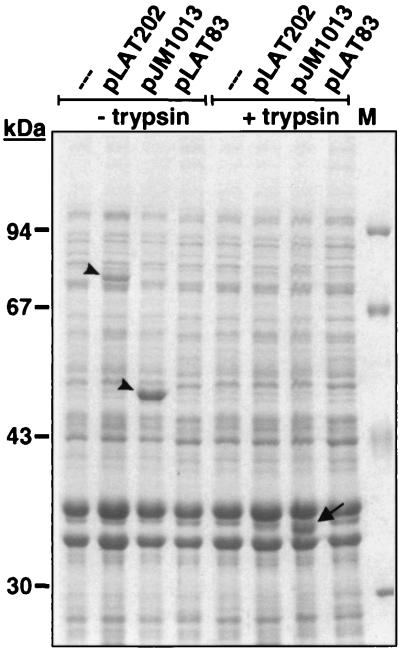
The subcellular localization of FP77 was examined by the preparation of outer membranes of JK321 cells carrying pLAT83, pLAT202, or pJM1013 using the sarcosyl method (8). SDS-PAGE revealed that FP50 and FP77 are integrated into the outer membrane, migrating at approximately 50 and 77 kDa, respectively (Fig. 3). Additionally, the Bla moiety of FP77 is cleaved from the surface of JK321(pLAT202) cells by the action of trypsin, as is the reporter epitope from FP50 in JK321(pJM1013) cells. The protease-resistant core of the AIDA-I autotransporter domain, migrating at 37 kDa, remains embedded in the outer membrane after trypsin treatment, confirming previous findings obtained with other heterologous passenger domains (24).
FIG. 3.
Surface targeting and protease accessibility of FP77 and FP50. Membrane preparations of E. coli JK321 expressing surface-located Bla (pLAT202) or wild-type Bla (pLAT83 and pJM1013) or of JK321 without plasmid (–––) were separated by SDS-PAGE (9% gel) and stained with Coomassie brilliant blue. To determine surface location, the bacterial samples either were subjected to trypsin treatment (+ trypsin) prior to membrane preparation or remained untreated (− trypsin). FP77 and FP50 are marked by arrowheads; the protease-resistant core of AIDA-I migrating at 37 kDa is indicated by an arrow.
Integrity of the outer membrane of FP77-expressing cells.
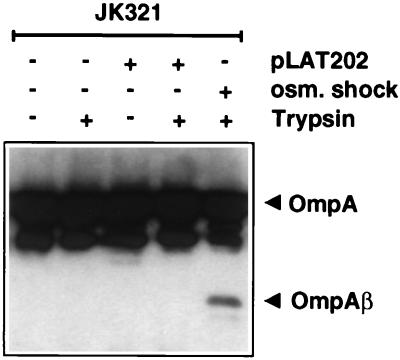
The integrity of the outer membrane was assessed by a control experiment using the outer membrane protein OmpA as a marker (14, 20). In cells displaying a high degree of membrane disorder, the periplasmic C-terminal domain of OmpA becomes sensitive to trypsin when whole cells are treated. We subjected JK321 cells expressing FP77 to trypsin digestion in order to monitor membrane integrity. While tryptic digestion of whole cells led to the removal of the Bla moiety from JK321(pLAT202) cells (Fig. 2), the molecular weight of OmpA was not altered (Fig. 4). This experiment was also performed with JK321(pJM1013) cells, leading to the same results (data not shown). These results clearly indicate that the periplasmic domain of OmpA was not affected by the action of trypsin, demonstrating that the outer membranes of JK321 cells expressing FP50 or FP77 are physiologically intact.
FIG. 4.
Protease accessibility of OmpA. E. coli strains JK321 and JK321(pLAT202) were subjected to trypsin treatment prior to membrane fractionation. Outer membranes were separated by SDS-PAGE (15% gel) and transferred to an Immobilon-P membrane. The filters were probed with the OmpA-specific antiserum AK57, which binds to full-size OmpA migrating at 36 kDa. As a control, the 28-kDa protease-resistant membrane-embedded core of OmpA (OmpAβ) was generated by trypsin treatment of osmotically shocked JK321 cells.
Functional surface expression of Bla.
JK321(pLAT202) cells were able to grow normally on solid LB medium containing ampicillin (100 mg/liter) when plated at a density of ∼100 CFU in a volume of 100 μl, providing the first evidence for a functional Bla–AIDA-I fusion protein. The growth of the cells was significantly retarded on LB medium containing 200 mg of ampicillin/liter, while no growth could be observed on media containing higher concentrations of the antibiotic.
To distinguish between periplasmic and surface-located activity of this enzyme, we set up an in vivo assay for the cleavage of penicillin G using physiologically intact JK321(pLAT202) cells, since penicillin G penetrates the outer membrane poorly (29). The following strains were employed as controls: (i) JK321, expressing no Bla, (ii) JK321(pLAT83), expressing periplasmic Bla, and (iii) JK321(pJM1013), expressing periplasmic Bla and high levels of FP50. Control ii allows periplasmic and surface-exposed Bla activity to be distinguished, while control iii permits the detection of potential membrane disorders caused by the artificial AIDA-I fusion proteins. Such disorders might lead to enhanced diffusion of penicillin G into the periplasm and subsequent degradation by the action of prematurely folded but not exported Bla.
Table 2 shows that JK321(pLAT202), displaying Bla on the cell surface, has high whole-cell Bla activity, leading to the rapid cleavage of penicillin G. The whole-cell penicillinase activity of JK321(pLAT202) cells is 148 mU, contrasting with that of the control strains JK321(pLAT83) and JK321(pJM1013), which express periplasmic Bla and have low levels of whole-cell Bla activity (4 and 11 mU, respectively). However, the Bla activity of JK321(pJM1013) does not differ significantly from that of JK321(pLAT83) despite the higher copy number of the former plasmid and the expression of large amounts of FP50 in the outer membrane. In another experiment, JK321(pLAT202) cells were treated with trypsin before analysis of Bla activity of whole cells. Bla activity was decreased by 95% from that of JK321 expressing FP77 (Table 2), whereas the low level of penicillinase activity of the control strains remained unaltered. This could also be demonstrated using cephaloridine as the substrate. Interestingly, in this assay, the whole-cell Bla activity of the dsbA+ wild-type strain UT5600(pLAT202) was about twofold higher than that of the dsbA strain JK321(pLAT202), which expressed the same amount of FP77 on the cell surface (Fig. 2B). The lower whole-cell activity of UT2300(pLAT202) in comparison to UT5600(pLAT202) correlates with the degradation of full-length FP77 by OmpT (Fig. 2B).
TABLE 2.
Whole-cell penicillinase activity of E. coli cells expressing surface-displayed or periplasmic Bla
| Strain and plasmid (cultivation conditionsa) | Bla activityb on the following substrate:
|
|||||
|---|---|---|---|---|---|---|
| Penicillin G
|
Cephaloridine
|
|||||
| Activityc (mU) | Activity after trypsin treatment (mU) | % Reduction | Activity (mU) | Activity after trypsin treatment (mU) | % Reduction | |
| JK321 | ||||||
| pJM1013 (37°C; Amp) | 11 (±12) | 14 (±14) | NS | 4.5 (±1.8) | 5.1 (±1.4) | NS |
| pLAT83 (37°C; Amp) | 4 (±18) | 3 (±1) | NS | 3.4 (±1.9) | 3.3 (±1.8) | NS |
| pLAT202 (37°C; Amp) | 148 (±10) | 6 (±30) | 95 | 5.48 (±1) | 0.7 (±2.2) | 86.3 |
| pLAT202 (28°C; Amp) | 182 (±20) | ND | ND | ND | ND | ND |
| pLAT202 (37°C; Cam) | 100 (±18) | ND | ND | ND | ND | ND |
| UT2300/pLAT202 (37°C; Amp) | ND | ND | ND | 7.1 (±5.5) | 4.3 (±3.0) | 39.8 |
| UT5600/pLAT202 (37°C; Amp) | ND | ND | ND | 11.0 (±3.7) | 2.3 (±1.9) | 78.7 |
Amp, ampicillin; Cam, chloramphenicol.
One unit of Bla activity is defined as the amount of enzyme that hydrolyzes 1 μmol of penicillin G or cephaloridine per min.
Mean values, representing the results of five independent experiments, are calculated for the amount of bacteria present in 1 ml of a suspension with an OD575 of 1.0 (∼2.5 × 109 CFU). Standard deviations are shown in parentheses. ND, no data; NS, not significant.
These results demonstrate that the Bla moiety of FP77 is surface exposed in the dsbA strain JK321(pLAT202) and in the corresponding wild-type strains UT5600(pLAT202) and UT2300(pLAT202) and that the fusion of heterologous passenger domains to the autotransporter domain of AIDA-I does not affect the integrity of the outer membrane.
Assessing the enzymatic activity of surface-displayed Bla.
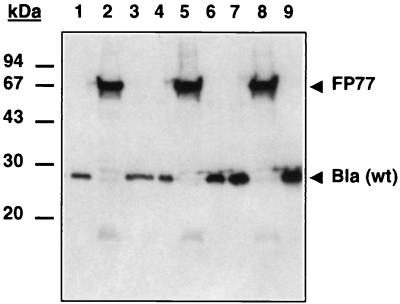
According to the model of autodisplay established by Pohlner et al. (30), surface display of heterologous domains by autotransporters requires the passage of two membranes in a conformation that is compatible for outer membrane translocation. Consequently, it is important to determine what percentage of the molecules in which the passenger domain is displayed on the bacterial surface are functional. To compare the enzymatic activity of the surface-displayed Bla with the purified periplasmic enzyme, the Bla activity of JK321(pLAT202) cells was determined with cephaloridine as the substrate (Table 2). Subsequently, a stock solution of the purified periplasmic enzyme was diluted until the activity of the diluted Bla solution was equal to the activity obtained with the amount of JK321(pLAT202) cells present in 1 ml of a suspension with an OD575 of 0.1 (∼2.5 × 108 CFU). The amount of soluble Bla present in the diluted Bla solution was compared semiquantitatively by Western blotting to the amount of FP77 present in 2.5 × 108 CFU of JK321(pLAT202) cells. Figure 5 shows that the amount of Bla in JK321(pLAT202) cells (lane 2) is significantly higher than the amount in the Bla solution with the same enzymatic activity (lane 1). The Bla sample with a fivefold-higher concentration of the enzyme (lane 7) shows a signal of the same strength as that in 2.5 × 108 CFU of JK321(pLAT202) cells. According to these data, we estimate the enzymatic activity of surface-displayed Bla to be about 20% of that of purified wild-type Bla.
FIG. 5.
Semiquantitative analysis of the enzymatic activity of surface-displayed Bla. Increasing amounts of a solution of commercially available purified TEM-Bla were compared by Western blot analysis with the amount present in 2.5 × 108 CFU of JK321(pLAT202). Lane 1, 0.54 mU of Bla; lane 2, JK321(pLAT202); lane 3, 1.08 mU of Bla; lane 4, 1.62 mU of Bla; lane 5, JK321(pLAT202); lane 6, 2.16 mU of Bla; lane 7, 2.7 mU of Bla; lane 8, JK321(pLAT202); lane 9, 3.24 mU of Bla.
Influence of cultivation conditions on whole-cell penicillinase activity.
To determine the influence of cultivation conditions on the penicillinase activities of cells expressing FP77, bacteria were grown overnight on LB plates containing ampicillin at either 28 or 37°C. Whole-cell penicillinase activity was assessed as described above. Cells grown at 28°C showed a 25% increase in penicillinase activity compared to cells grown at 37°C (Table 2). This effect is not due to down-regulation of FP77 expression at 37°C, since the amount of FP77 produced by JK321(pLAT202) grown at 28°C was identical to that of cells grown at 37°C, as shown by Western blot analysis (data not shown). Interestingly, when cells displaying FP77 on the surface were grown in the absence of ampicillin, the whole-cell penicillinase activity was found to decrease significantly (Table 2). Again, the amounts of FP77 expressed by JK321(pLAT202) grown in the presence and absence of ampicillin were compared by Western blotting and shown to be similar. Thus, the differences observed in whole-cell penicillinase activity are not due to down-regulation of FP77 (data not shown).
DISCUSSION
In this report, we describe the export of an active enzyme to the surfaces of E. coli cells by the autotransporter secretion pathway. The Bla-AIDA-I fusion protein was efficiently targeted to the surfaces of E. coli JK321(pLAT202) cells, and the enzymatically active Bla moiety of FP77 was shown to be surface exposed. This was demonstrated by the accessibility of the Bla moiety to the exogenously added protease trypsin and by monitoring the Bla activity of physiologically intact cells using penicillin G and cephaloridine as the substrates.
Surface display has become a rising focus of interest due to possible applications in biotechnology, biomedicine, and vaccine development. Filamentous bacteriophage have been successfully employed for the display of random peptide libraries (3) and single-chain (scFv) antibodies and combinatorial libraries thereof (15). Peptide and scFv antibody libraries of high diversity have been established, and screening for appropriate ligands has become very efficient by virtue of high-throughput panning procedures (26) or the selectively infective phage technology (37). Despite the high degree of sophistication achieved in phage display technology, bacterial systems for surface display offer distinct advantages, including the strict linkage of genotype and phenotype, constant growth under selective conditions, the high copy number of the passenger proteins on the bacterial surface (14, 24), and the ease of reamplification of selected bacteria expressing peptide libraries on the surface (23).
For the targeting of an scFv molecule to the surfaces of E. coli cells, the peptidoglycan-associated lipoprotein (PAL) of E. coli has been utilized (11). The PAL-scFv fusion was located in the periplasm and bound to the murein layer, and after permeabilization of the outer membrane, the scFv became accessible to externally added antigen. Another system for bacterial surface display is based on the C-terminal fusion of a heterologous passenger protein to a genetically engineered hybrid molecule of the major E. coli lipoprotein (Lpp) and the outer membrane protein OmpA (10). By use of this system, export to the surface of E. coli cells of a number of enzymes, such as Bla and the Cex exoglucanase of Cellulomonas fimi, and of an scFv antibody has been reported (10, 14). Furthermore, the functionality of all three fusions was demonstrated by the degradation of externally added penicillin G by surface-displayed Bla and substrate binding for the Cex exoglucanase and the scFV antibody (10). However, E. coli strains expressing Lpp-OmpA-Bla tripartite fusions have been shown to have major alterations of the outer membrane (14). As discussed by Georgiou et al. (14), periplasmic markers, such as the periplasmic domain of OmpA and the peptidoglycan backbone, were accessible from the extracellular space. Thus, in these strains differentiation between periplasmic and surface-located enzymatic activities was not possible.
A third system comprises the fusion of heterologous passenger moieties to autotransporter domains of various proteins that are members of the autotransporter family present in gram-negative species, resulting in the export of the passenger domain to the surfaces of E. coli, Salmonella serovar Typhimurium, and S. flexneri cells. These passenger proteins included CTB (19, 20, 21, 24), pseudoazurin of Alcaligenes faecalis (36), MalE and PhoA (38), and various defined epitopes (24; C. T. Lattemann, unpublished data). Display of functional protein domains has not been demonstrated for this system until recently (39). A limitation, however, is the incompatibility for the translocation of passenger domains containing extensive tertiary structures such as disulfide bonds (20). This restriction could be overcome by inactivating the dsbA gene product of E. coli, leading to the export of wild-type CTB fused to the autotransporter domain of the IgA1 protease of N. gonorrhoeae in the dsbA strain JK321 (19).
The Bla–AIDA-I fusion protein FP77 was efficiently targeted to the surfaces of E. coli JK321(pLAT202) (dsbA ompT) cells and of cells of the corresponding wild-type strain, UT5600(pLAT202) (dsbA+ ompT) irrespective of the disulfide bond present within the Bla moiety. The Bla moiety was clearly shown to be surface exposed and retained functionality on the bacterial surface. Penicillin G is efficiently hydrolyzed by the surface-exposed Bla domain of FP77 in physiologically intact JK321(pLAT202) cells, while JK321(pLAT83) and JK321(pJM1013) cells expressing periplasmic Bla show low whole-cell Bla activity due to the low capacity of penicillin G to diffuse into the periplasmic space (29). In contrast, the differences observed between surface-located and periplasmic Bla obtained with cephaloridine as the substrate were less prominent due to the enhanced capacity of cephaloridine to penetrate the periplasm. However, trypsin treatment abolished Bla activity in JK321(pLAT202), while the Bla activities of JK321(pJM1013) and JK321(pLAT83) remained unaltered with regard to the cephaloridine substrate. Thus, these experiments demonstrate that the Bla activity observed in JK321(pLAT202) is surface located and is not due to penetration of penicillin G into the periplasm, as is seen in JK321(pJM1013) and JK321(pLAT83). In addition, our data indicate that the membranes of E. coli cells expressing AIDA-I fusion proteins remain intact. The whole-cell Bla activity of JK321(pJM1013), expressing large amounts of FP50 in the outer membrane, remains at the same low level as that of JK321(pLAT83) cells, which do not express an AIDA-I fusion protein. Thus, large amounts of AIDA-I fusion proteins inserted into the outer membrane do not appear to cause membrane disorders that might promote the influx of penicillin G into the periplasm. Additionally, the periplasmic domain of OmpA was not accessible to trypsin in JK321 cells expressing FP77 or FP50, providing further evidence that the outer membrane is intact in these strains.
We estimate the activity of surface-displayed Bla in JK321(pLAT202) cells to be approximately 20% compared with that of purified commercially available TEM-Bla. However, it is difficult to assess the molecular basis of the reduction of enzymatic activity on the cell surface. A decrease in the enzymatic activity of surface-displayed Bla might be caused by conformational changes of the enzyme due to the C-terminal attachment of Bla to the autotransporter domain. Alternatively, only 20% of the surface-exposed Bla molecules might adopt the correct conformation after the translocation process, whereas 80% may remain in an inactive state in JK321(pLAT202). Nevertheless, at this point it is not clear what factors are responsible for the decrease in Bla activity on the cell surface. As discussed above, the activity of the surface-displayed enzyme is about twofold higher in the dsbA+ background of UT5600. It is likely that in this strain enzymatically active Bla moieties with preformed disulfide bonds are translocated across the outer membrane, leading to enhanced whole-cell Bla activity.
Recently Veiga et al. reported the export of an scFv molecule by the autotransporter domain of the IgA1 protease, demonstrating the binding of E. coli cells expressing the scFv-IgA autotransporter fusion to the cognate antigen of the scFv antibody (39). Interestingly, binding of cells expressing the scFv molecule was observed only in the presence of the dsbA gene product, although the relative amount of active scFv molecules on the bacterial surface was not quantified. Based on these data, Veiga et al. postulated that the scFv molecule had to fold in a correct manner in the periplasm, prior to translocation across the outer membrane, in order to be functionally expressed on the bacterial cell surface. In contrast, the Bla moiety of FP77 is displayed functionally on the cell surface in a dsbA background, although FP77 is also expressed functionally in the presence of the dsbA gene product. Additionally, the highest rate of expression and stabilization of the full-length gene product was achieved in JK321 (dsbA ompT). Higher expression of FP77 was observed in the dsbA mutant JCB571 in comparison to its parental strain, JCB570. This is in accordance with the findings of Klauser et al., who reported on limitations of the autosecretion pathway with respect to stable tertiary structures of the passenger proteins that form disulfide bonds (20). In contrast to CTB, where the two Cys residues lie far apart (Cys9 and Cys86), the two Cys residues of the IgA1 protease domain are separated by 11 residues (30); thus, disulfide bond formation in the IgA1 protease is unlikely to result in bulky tertiary structures capable of interfering with outer membrane translocation. In the case of TEM-Bla, 44 residues are located between the Cys residues forming the disulfide bond. Apparently, these 44 residues do not form an extensive secondary structure (25). In addition, the AIDA-I adhesin itself appears to be posttranslationally modified, carrying a modification of approximately 18 kDa (2). This characteristic of the AIDA-I autotransporter might account for the ability of the disulfide-containing Bla moiety of FP77 in UT5600(pLAT202) to translocate in a presumably oxidized manner through the pore.
Maurer et al. (24) have reported the release of a heterologous passenger protein by the outer membrane protease OmpT. Interestingly, the Bla-AIDA fusion is only partially released from the surface in an ompT-positive background, indicating that OmpT cleavage sites located in the linker region of the autotransporter domain (24) and Bla were accessible only to a limited extent to OmpT. It is intriguing to speculate that this phenomenon is related to the folding of the surface-displayed Bla, providing protection of the linker region against proteolytic cleavage.
The cultivation conditions have been shown to influence the whole-cell activity of JK321 cells expressing FP77. Cultivation of the bacteria at 28°C significantly enhanced the overall activity of the surface-displayed Bla. This effect might be due to a delayed kinetics of protein folding on the cell surface, thereby promoting the formation of stable, enzymatically active conformations of Bla displayed on the surfaces of JK321 cells. Additionally, higher overall activity was also observed when cells were grown in the presence of the substrate. It is possible that the presence of the β-lactam structure in the culture medium facilitates the correct folding of the exported enzyme on the bacterial-cell surface. Another explanation for this effect might be a prolonged half-life of the enzyme in the presence of substrate.
It is noteworthy that the expression of FP77 enables JK321(pLAT202) cells to grow on solid medium in the presence of ampicillin (100 mg/ml). It has been reported that Bla secreted by the α-hemolysin secretion apparatus retained functionality but did not protect the cells significantly against the action of ampicillin (4). JK321(pLAT202) cells might be resistant to ampicillin because functional β-lactamase displayed on the surface is able to degrade the β-lactam before it reaches the periplasm by diffusion through the porins. Another hypothesis might be that residual Bla activity in the periplasm, resulting from proteolytic degradation of FP77, might protect the penicillin binding proteins from inactivation by the antibiotic.
Our data suggest that autodisplay using the autotransporter domain of AIDA-I is a promising tool for various approaches in biotechnology and biomedicine, demonstrating that, in addition to the export of peptides, proteins retaining their enzymatic activity can be displayed successfully on the bacterial cell surface. Therefore, surface display might be an efficient way to present complex proteinaceous antigens on the surfaces of cells of bacterial live-vaccine strains, since the display of conformational epitopes might be more advantageous than the insertion of antigenic polypeptides into loops of outer membrane proteins (17) or into flagellin (28). Biological activity of proteins exported by autotransporters (36, 38) has not been demonstrated so far. The lack of biological activity of surface-displayed passenger proteins is likely due to the structural complexity of the passenger domains examined in addition to the limitation with regard to disulfide bonds. Thus, the rational selection of passenger domains may provide further information about the mechanisms that define the translocation capability of the passenger.
ACKNOWLEDGMENTS
We thank C. P. Gibbs for critical comments on the manuscript and A. Tehrani for providing oligonucleotides A2 and A3.
REFERENCES
- 1.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 2.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 3.Cesareni G. Peptide display on filamentous phage capsids. A new powerful tool to study protein-ligand interaction. FEBS Lett. 1992;307:66–70. doi: 10.1016/0014-5793(92)80903-t. [DOI] [PubMed] [Google Scholar]
- 4.Chervaux C, Sauvonnet N, Le Clainche A, Kenny B, Hung A L, Broome-Smith J K, Holland I B. Secretion of active β-lactamase to the medium mediated by the Escherichia coli haemolysin transport pathway. Mol Gen Genet. 1995;249:237–245. doi: 10.1007/BF00290371. [DOI] [PubMed] [Google Scholar]
- 5.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 7.Elish M E, Pierce J R, Earhart C F. Biochemical analysis of spontaneous fepA mutants of Escherichia coli. J Gen Microbiol. 1988;134:1355–1364. doi: 10.1099/00221287-134-5-1355. [DOI] [PubMed] [Google Scholar]
- 8.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco J A, Georgiou G. The expression of recombinant proteins on the external surface of Escherichia coli. Biotechnological applications. Ann N Y Acad Sci. 1994;745:372–382. doi: 10.1111/j.1749-6632.1994.tb44389.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs P, Breitling F, Dübel S, Seehaus T, Little M. Targeting recombinant antibodies to the surface of Escherichia coli: fusion to a peptidoglycan associated lipoprotein. Bio/Technology. 1991;9:1369–1372. doi: 10.1038/nbt1291-1369. [DOI] [PubMed] [Google Scholar]
- 12.Georgiou G, Poetschke H L, Stathopoulos C, Francisco J A. Practical applications of engineering Gram-negative bacterial cell surfaces. Trends Biotechnol. 1993;1:6–10. doi: 10.1016/0167-7799(93)90068-K. [DOI] [PubMed] [Google Scholar]
- 13.Georgiou G, Stathopoulos C, Daugherty P S, Nayak A R, Iverson B L, Curtiss R., III Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat Biotechnol. 1997;15:29–34. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 14.Georgiou G, Stephens D L, Stathopoulos C, Poetschke H L, Mendenhall J, Earhart C F. Display of β-lactamase on the Escherichia coli surface: outer membrane phenotypes conferred by Lpp′-OmpA′-β-lactamase fusions. Protein Eng. 1996;9:239–247. doi: 10.1093/protein/9.2.239. [DOI] [PubMed] [Google Scholar]
- 15.Hoogenboom H R. Designing and optimizing library selection strategies for generating high-affinity antibodies. Trends Biotechnol. 1997;15:62–70. doi: 10.1016/S0167-7799(97)84205-9. [DOI] [PubMed] [Google Scholar]
- 16.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen R, Tommassen J. PhoE protein as a carrier for foreign epitopes. Int Rev Immunol. 1994;11:113–121. doi: 10.3109/08830189409061719. [DOI] [PubMed] [Google Scholar]
- 18.Jose J, Jähnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:378–380. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 19.Jose J, Krämer J, Klauser T, Pohlner J, Meyer T F. Absence of periplasmic DsbA oxidoreductase facilitates export of cysteine-containing passenger proteins to the Escherichia coli cell surface via the Igaβ autotransporter pathway. Gene. 1996;178:107–110. doi: 10.1016/0378-1119(96)00343-5. [DOI] [PubMed] [Google Scholar]
- 20.Klauser T, Pohlner J, Meyer T F. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease β-domain: conformation-dependent outer membrane translocation. EMBO J. 1990;9:1991–1999. doi: 10.1002/j.1460-2075.1990.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klauser T, Pohlner J, Meyer T F. Selective extracellular release of cholera toxin B subunit by Escherichia coli: dissection of Neisseria Igaβ-mediated outer membrane transport. EMBO J. 1992;11:2327–2335. doi: 10.1002/j.1460-2075.1992.tb05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loveless B J, Saier M H., Jr A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol Mem Biol. 1997;14:113–123. doi: 10.3109/09687689709048171. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Murray K S, Van Cleave V, Lavallie E R, Stahl M L, McCoy J M. Expression of thioredoxin random peptide libraries on the Escherichia coli cell surface as functional fusions to flagellin: a system designed for exploring protein-protein interactions. Bio/Technology. 1995;13:366–372. doi: 10.1038/nbt0495-366. [DOI] [PubMed] [Google Scholar]
- 24.Maurer J, Jose J, Meyer T F. Autodisplay: one-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J Bacteriol. 1997;179:794–804. doi: 10.1128/jb.179.3.794-804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maveyraud L, Pratt R F, Samama J P. Crystal structure of an acylation transition-state analog of the TEM-1 beta-lactamase. Mechanistic implications for class A beta-lactamases. Biochemistry. 1998;37:2622–2628. doi: 10.1021/bi972501b. [DOI] [PubMed] [Google Scholar]
- 26.McGregor D. Selection of proteins and peptides from libraries displayed on filamentous bacteriophage. Mol Biotechnol. 1996;6:155–162. doi: 10.1007/BF02740770. [DOI] [PubMed] [Google Scholar]
- 27.Menard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton S M C, Jacob C O, Stocker B A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido H, Normark S. Sensitivity of Escherichia coli to various β-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic β-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987;1:29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 30.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 31.Pugsley A P, Francetic O, Hardie K, Possot O M, Sauvonnet N, Seydel A. Pullulanase: model protein substrate for the general secretory pathway of gram-negative bacteria. Fol Microbiol. 1997;42:184–192. doi: 10.1007/BF02818976. [DOI] [PubMed] [Google Scholar]
- 32.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlör S, Schmidt A, Maier E, Benz R, Goebel W, Gentschev I. In vivo and in vitro studies on interactions between the components of the haemolysin (HlyA) secretion machinery of Escherichia coli. Mol Gen Genet. 1997;256:306–319. doi: 10.1007/s004380050574. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 35.Shikata S, Shimada K, Ohnishi Y, Horinouchi S, Beppu T. Characterization of secretory intermediates of Serratia marcescens serine protease produced during its extracellular secretion from Escherichia coli cells. J Biochem. 1993;114:723–731. doi: 10.1093/oxfordjournals.jbchem.a124244. [DOI] [PubMed] [Google Scholar]
- 36.Shimada K, Ohnishi Y, Horinouchi S, Beppu T. Extracellular transport of pseudoazurin of Alcaligenes faecalis in Escherichia coli using the COOH-terminal domain of Serratia marcescens serine protease. J Biochem. 1994;116:327–334. doi: 10.1093/oxfordjournals.jbchem.a124527. [DOI] [PubMed] [Google Scholar]
- 37.Spada S, Krebber C, Plückthun A. Selectively infective phages (SIP) Biol Chem. 1997;378:445–456. doi: 10.1515/bchm.1997.378.6.445. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Lett M C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 39.Veiga E, de Lorenzo V, Fernandez L A. Probing secretion and translocation of a β-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol Microbiol. 1999;33:1232–1243. doi: 10.1046/j.1365-2958.1999.01571.x. [DOI] [PubMed] [Google Scholar]