Abstract
Purpose:
Stereotactic body radiation therapy (SBRT) has become standard of care for inoperable early stage non-small cell lung cancer and is often used for recurrent lung cancer and pulmonary metastases. Radiation-induced lung toxicity (RILT), including radiation pneumonitis and pulmonary fibrosis, is a major concern for which it is important to understand dosimetric and clinical predictors.
Methods:
This study was undertaken through the American Association of Physicists in Medicine’s Working Group on Biological Effects of Stereotactic Body Radiotherapy. Data from studies of lung SBRT published through the summer of 2016 that provided detailed information about RILT were analyzed.
Results:
Ninety-seven studies were ultimately considered. Definitions of the risk organ and complication endpoints as well as dose-volume information presented varied among studies. The risk of RILT, including radiation pneumonitis and pulmonary fibrosis, was reported to be associated with the size and location of the tumor. Patients with interstitial lung disease appear to be especially susceptible to severe RILT. A variety of dosimetric parameters were reported to be associated with RILT. There was no apparent threshold ‘tolerance dose-volume’ level. However, most studies note safe treatment with a rate of symptomatic RILT of < 10–15% after lung SBRT with a mean lung dose (MLD) of the combined lungs ≤ 8 Gy in 3–5 fractions, and the percent of total lung volume receiving more than 20 Gy (V20) <10–15%.
Conclusions:
To allow more rigorous analysis of this complication, future studies should standardize reporting by including standardized endpoint and volume definitions and providing dose-volume information for all patients, with and without RILT.
Keywords: Hypofractionated Treatment Effects in the Clinic (HyTEC), Stereotactic Body Radiation Therapy (SBRT), Normal Tissue Complication Probability (NTCP), Radiation-Induced Lung Toxicity (RILT), Radiation Pneumonitis (RP), Pulmonary Fibrosis (PF)
Summary
Data were pooled from published reports to analyze dosimetric and clinical predictors of radiation-induced lung toxicity (RILT) after thoracic stereotactic body radiotherapy. Most reviewed studies report safe treatment for mean combined lung dose ≤ 8 Gy (3–5 fractions) and percent total combined lung V20≤10–15%. Interstitial lung disease is a particular risk factor. More detailed dosimetric and endpoint reporting is needed to facilitate future development of accurate quantitative models of RILT.
1. CLINICAL SIGNIFICANCE
Stereotactic body radiation therapy (SBRT) or stereotactic ablative body radiotherapy (SABR) has become the standard of care for inoperable early stage non-small cell lung cancer (NSCLC) and an emerging treatment option for recurrent lung cancer and pulmonary metastasis. A pooled-analysis and results from two prematurely closed randomized trials suggested that for early stage operable NSCLC, SBRT appears to provide equivalent survival outcomes to surgical resection (1,2). For medically inoperable NSCLC, the Phase II Radiation Therapy Oncology Group (RTOG) 0236 cooperative group study reported a 98% 3-year tumor control rate (3). For recurrent lung cancer, with disease limited in size and extent, SBRT may provide high local control rates of 95% and 87% at 1- and 2-year, respectively (4). For cancers metastasized to the lung, excellent 3-year local control (70%–100%) has also been reported (5–7).
Lung SBRT can cause serious or life-threatening treatment toxicity from damage to thoracic organs at risk (OARs) (8–10). For example with 60–66 Gy in 3 fractions, Timmerman et al. from Indiana University reported 6 out of 70 patients (8.6%) with centrally located NSCLC died from treatment related toxicity after SBRT (8). Radiation pneumonitis and fibrosis can be clinically symptomatic in over 30% patients (3,11–16) and serious in ≥10% of patients (3,11,13–21). While it is challenging to generate predictive models, an objective of the American Association of Physicists in Medicine (AAPM) Working Group on Biological Effects of Hypofractionated Radiotherapy/SBRT (WGSBRT) is to review and summarize the published data on Hypofractionated Treatment Effects in the Clinic (HyTEC) and provide guidance for safe practice in the clinic.
The subject of this review is symptomatic radiation-induced lung toxicity (RILT) resulting from damage to the lung parenchyma. This endpoint is chosen since it is one of the most common toxicities after thoracic SBRT (18,22–27), is clinically relevant, can be serious, and is the most-commonly studied toxicity in lung SBRT (in terms of the number of publications to date). As clinicians have gained confidence, SBRT is increasingly being used for treatment of central lesions, where toxicity of other OARs including esophagus, great vessel, heart and proximal bronchial tree becomes more clinically pertinent and will be the subject of future HyTEC reports; some of these are currently discussed in references (28–34).
A recently published pooled review of 88 studies analyzed the simple risk factors associated with RILT (35) and demonstrated that older age and larger tumor diameter were significant adverse risk factors associated with RILT, while histology and tumor location (central vs peripheral) were not significant. Patients with grade 2 and above (G2+) RILT had a significantly higher mean lung dose (MLD) and percent of lung volume receiving more than 20 Gy (V20) than those with grade 0–1 RILT. We herein update that review and perform a more detailed appraisal of predictive dose/volume factors and associated possibilities for modelling. Supplemental material Table 1 (S-Table-1) (11,12,17,18,22,25–27,36–51) summarizes the details of key papers we reviewed that provided quantitative dose-volume information and data on risk of complications.
2. ENDPOINTS
Several endpoints can be considered when quantifying RILT, including clinical symptoms, radiologic abnormalities (e.g. CT density changes), and changes in functional assessments (e.g. pulmonary function tests, hemoglobin oxygen saturation). We will focus on moderate and severe RILT that presents as radiation pneumonitis (RP) or pulmonary fibrosis (PF), as they are the most clinically relevant toxicity endpoints. RP is the most frequently reported in the literature. We also elected to include fibrosis as 1) it is commonly seen in the clinic, 2) it can develop concurrently with the earlier inflammatory stage, 3) it is often difficult to distinguish fibrosis from RP, and 4) it is likely that many of the published reports of dyspnea attributed to RP may have been due to symptomatic PF.
A variety of grading systems are used to quantify the severity of RILT. The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) was used in the vast majority of studies that we reviewed, as it is the standard for all NIH sponsored cooperative group trials in the United States. However, CTCAE criteria represent adverse events from any or all treatments, without specific attribution of toxicity to radiotherapy. As shown in Table 1, pneumonitis or fibrosis in the CTCAE criteria utilize broad groupings that describe the extent of symptoms, but not the underlying cause (i.e. radiation or chemotherapy). The RTOG and the South West Oncology Group (SWOG) (Supplemental material, S-Table-2a) (52,53) scales were used in early studies. Quantitative Analysis of Normal Tissue Effect in the Clinic (QUANTEC) recommended using the Late Effects of Normal Tissue–Subjective, Objective, Management, and Analytic (LENT-SOMA) scoring system (Supplemental material, S-Table-2b), but that has not been implemented widely in the clinic or in clinical trials. There are differences in grading criteria such as the use of steroids to differentiate grade 2 and 3 (grade 3 for RTOG, grade 2 for SWOG, grade 2 or 3 for CTCAE) (54). To capture data from the literature meaningfully and consistently, we will focus on G2+, symptomatic toxicity, requiring medical intervention, which is similar across all the systems, as the primary endpoint of this review. For future studies, we recommend consideration of a system for more detailed description for RILT diagnosis as presented in Table 5.
Table 2.
Variations in RILT grading and lung definitions among 24 recent publications
|
|
|
|
|||
|---|---|---|---|---|---|
| RILT grading criteria | Lung definition for analysis | T arget exclusion | |||
|
|
|
|
|||
| CTCAE only | 17 | Whole (bilateral) lungs | 16 | GTV | 7 |
| CTCAE & SWOG | 2 | Bilateral lungs and ipsilateral lung | 2 | IGTV | 1 |
| CTCAE & RTOG | 1 | Ipsilateral lung | 2 | CTV | 2 |
| RTOG | 2 | NCS | 3 | ITV | 1 |
| SWOG | 1 | NS | 1 | PTV | 3 |
|
|
|||||
| NS | 1 | None or NS | 10 | ||
|
|
|
||||
RILT=Radiation-Induced Lung Toxicity
NS=Not Specified
NCS=Not Clearly Specified (but can infer bilateral from text)
GTV=Gross Tumor Volume
IGTV=Internal Gross Tumor Volume
ITV=Internal Target Volume
CTV=Clinical Target Volume
PTV=Planning Target Volume
3. DEFINITION OF LUNG VOLUMES
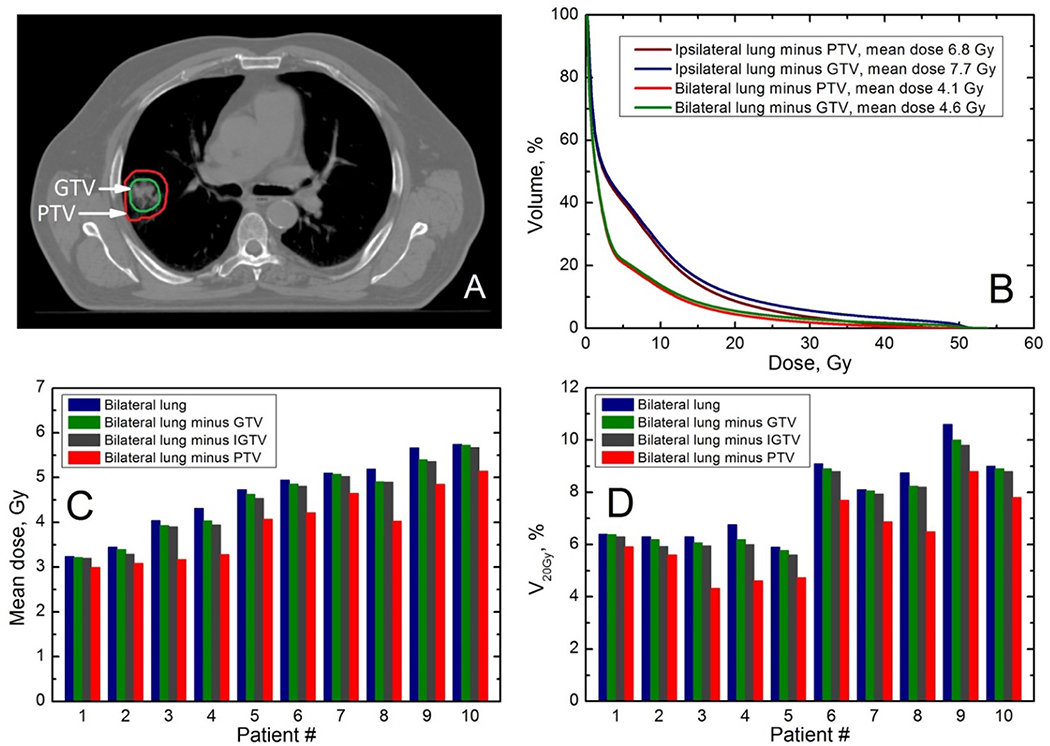
The definition of lung volumes is important for dosimetric analyses, but was found to be inconsistent in the literature. Variations that are not specific for SBRT, may result from 1) considering the whole lung versus ipsilateral lung, 2) different breathing phases, 3) subtraction of tumor or target volumes, and 4) inclusion of air in major airways and blood vessels around the hilar region (55). Additionally, auto-segmentation algorithms can introduce errors. There is no consensus on how much of the bronchus should be defined as “lung”, and there is some uncertainty as to the lung boundaries since the apparent edges may vary with the window/level setting (55). Studies reporting rates of RILT linked to dose/volume parameters varied widely in how the lung was defined (see Table 2) (11,12,17,18,25–27,37,38,40–43,46,47,49,56). As for breathing, three studies used “slow” CT scans, one used a breath-hold scan, one used average intensity projection, and the others mentioned free breathing or did not specify. Since dose/volume metrics will differ with different methods of defining the lung volume (Figure 1), inter-study comparisons are often difficult, and a more-standardized approach to defining and reporting such metrics would facilitate data comparisons/pooling.
Table 3.
Risk of severe radiation pneumonitis in all patients and those with interstitial lung disease (ILD)
| Study (year of publication) (ref#) | Dose Fractionation Schemes | Patients Without ILD | Patients with ILD | ||
|---|---|---|---|---|---|
| Rate of G3-5 RP % (fraction) | Rate of G4-5 RP % (fraction) | Rate of G3-5 RP % (fraction) | Rate of G4-5 RP % (fraction) | ||
| Takeda (2010)(68) | 48 Gy in 4 fx^ | 4% (5/125) | 0% (0/125) | 67% (2/3) | 0% (0/3) |
| Yamashita (2010) (58) | 48 Gy in 4 fx | 2% (2/104)# | 2% (2/104) | 69% (9/13) | 57% (7*/13) |
| Ueki (2015) (69) | 48 Gy in 4 fx or 60 Gy in 8 fx others | 2% (2/137)** | 2% (2/137) | 10% (2/20)*** | 5% (1/20) |
| Bahig (2016) (36) | Median BED# 142 Gy | 2% (10/476) | 0% (0/476) | 32% (9/28) | 18% (5/28) |
* Includes 5 patients with G5 radiation pneumonitis. # Authors did not report G3 for this group.
** 29 patients (18%) with G2 RP
*** 11 cases (55%) with G2 RP
Fractionation regimen not reported. BED=biologically effective dose using α/β of 10 Gy.
used in most of the 133 treated tumors in 128 patients
Figure 1. Variance in normal lung definition and examples of its effect on lung dose volume histograms (DVH).
DVH vary with the kind of target subtracted from the lung. Panel A shows an axial slice with PTV contour in red and GTV in green; panel B shows DVH for ipsilateral lung with either PTV (purple line) or GTV (blue line) subtracted and bilateral lung with either PTV (red line) or GTV (green line) subtracted Panels C and D show mean doses and V20 for 10 patients for bilateral lung with either GTV or PTV subtracted. GTV= Gross Tumor Volume. IGTV=internal GTV, i.e. composite GTV of all phases of 4DCT. IGTV=ITV when CTV margin is zero. PTV = Planning Target Volume.
The QUANTEC report, that focused on conventionally fractionated radiation therapy (54), advised that the lung be considered as a single, paired-organ (total lung tissue) with the internal gross tumor volume (IGTV) excluded and that an average CT scan be used for computation for patients treated under free breathing. This method may predict RILT more accurately than those with no target exclusion or excluding the clinical target volume (CTV) or planning target volume (PTV), after 3D conformal radiotherapy (57). Excluding PTV may also increase inter-institutional variations as PTV margins may vary with institution. Modern RTOG trials such as RTOG 1106 require lungs to exclude GTV according to a recently published atlas (55). Until more data are available, we recommend using the lung volume definition according to RTOG recommendation.
4. REVIEW OF TOXICITY OUTCOMES DATA AND SIMPLE DOSIMETRIC FACTORS
Many publications reported incidence or prevalence of lung toxicity after SBRT and some reported dosimetric correlates. A prior pooled analysis of 88 published studies (7752 patients) (35) reported weighted average rates of G2+ and G3+ RILT of 9% (95% CI: 7–11) and 2% (95% CI: 1–3), respectively, though some studies reported G2+ exceeding 30% (3,11–16). There was no significant difference between studies with primary vs. metastatic diseases with regard to the rates of G2+ or G3+ RILT. In addition to including the larger series from a prior review (28), the present review included an additional 9 studies (all references are listed in S-Table-1) (11,12,17,18,22,25–27,36–51). Our literature search concluded in the summer of 2016.
In contrast to our traditional assumption, total prescription dose was not significantly correlated with RILT in the meta-analysis (35). This may be explained by the fact that the total physical doses are quite similar across studies despite the various fractionations (such as 48–50 Gy in 4–5 fractions, 45–54 Gy in 3 fractions, 50–55 Gy in 5 fractions). Further, inter-study and inter-patient variations in the prescription isodose line reduce the association between prescription dose and lung doses. Additionally, dose per fraction will need to be considered when evaluating the biological effect of a prescribed dose (further discussed in section 6).
The mean lung dose (MLD) is a simple dose-volume metric readily available from planning systems and is often used as a constraint for conventional fractionation. The prior analysis of pooled data from 21 studies did not show a significant correlation between median MLD values and the overall rate of G2 RILT at study level (35). However, 14 of the 21 studies (10 of the studies in S-Table-1) reported a significant within-study correlation of different grades of RILT with MLD (35). For example, Bahig et al. (36) reported a significant univariate correlation between G3+ RILT and MLD of bilateral lungs in a study of 504 patients with 4% incidence of G3+ RILT: patients without G3+ RILT had mean MLD of 4 Gy (range 3–5) while those with G3+ RILT had MLD of 7 Gy (range 5–9). Hayashi et al. (45) reported borderline significant correlation (p = 0.052) with G2+ RILT and a MLD (bilateral lungs minus PTV) cutpoint of 4.8 Gy in a study of 81 elderly patients where the overall G2+ RILT rate was 11%. In 6 studies reporting individual data for either bilateral or ipsilateral lung, Zhao et al reported that MLD was significantly higher in patients with G2+ RILT than those with grade 0–1 RILT (p = 0.027) (35). Thus, the significant association in the individual studies is lost when study outcomes are pooled, probably as a result of inconsistencies in calculation of MLD (e.g. varying definitions of lung volumes, and/or the use of physical vs fraction-size adjusted doses), inconsistencies in toxicity grading, and the relatively narrow range of MLDs across studies. In fact, the median physical MLD of bilateral lungs were below 8 Gy in all studies, and G2+ RILT of all studies were less than 25%, regardless of the number of SBRT fractions (Figure 2).
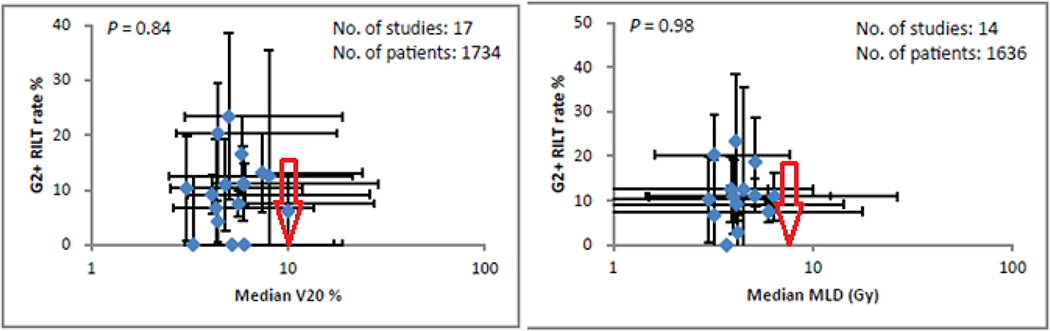
Figure 2: Simple dosimetric factors and grade 2+ radiation-induced lung toxicities (G2+ RILT).
Data shown were from published studies (35). Error bars show the ranges. MLD=mean lung dose of whole lung. V20=% volume of lungs receiving greater than 20 Gy. Based on our review, we recommend keeping the bilateral lung V20 and MLD below the values indicated by the red arrows.
Vdose (percent of lung volume receiving > “dose” Gy) is another simple dose-volume metric, frequently used as a clinical planning constraint for conventionally fractionated treatment, that has also been studied for association with RILT after SBRT. V20 is a common choice in treatment planning. Grills et al. (43) did not find any significant association between V20 and RP in 483 patients from 5 centers using the same planning system, with a median follow-up of 1.6 years and a crude G2+ RILT rate of 7%. Allibhai et al. (25) also found no association between RILT and bilateral lung V20 or MLD. Although the meta-analysis (35) did not show a significant relationship between G2+ RILT and V20, 16 of these publications individually reported correlations between symptomatic RILT and V20 and/or other Vdose metrics as did 9 of the publications listed in S-Table-1. As with MLD, variation between studies may explain why some individual studies found significant correlations but the pooled analysis did not.
Figure 2 shows that most of the studies had low RILT rates and the majority had low V20 (study medians were all ≤10%). Nine of the larger studies found a significant or borderline significant correlation between V20 and the rate of RILT (35,56), but the absolute rates of RILT and corresponding levels of V20 were variable (see Figure 4). A number of studies also investigated Vdose for doses other than 20 Gy, such as V5 and V13 and in some cases found better correlations (11,12,17,18,22,25–27,36–50,58) (S-Table-1). Going forward, studies with more detailed information and possibly with wider ranges of lung DVHs may help us determine the Vdose-RILT relationship to better balance the risks of RILT against those of local failure.
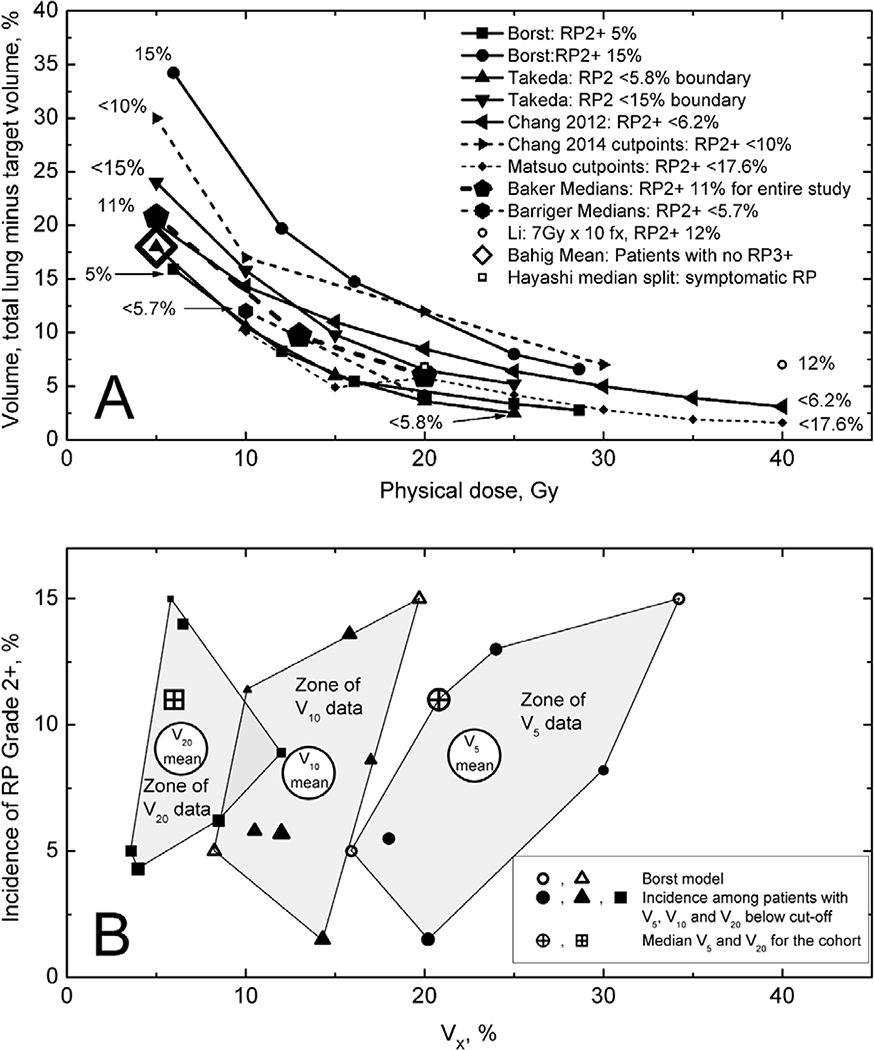
Figure 4: Dosimetric factors and risk of RILT.
RILT=radiation-induced lung toxicity. Figure 4A shows total lung significant or near-significant Vdose cutpoints related to symptomatic RILT from studies with >50 patients. “Dose” plotted on the X axis is the ‘physical dose’ that one obtains from a well-commissioned planning system. The legend and labels adjacent to each line show the rate of G2+ RP below the cutpoints or curves as given in the publications. Supplemental Figure 1 replots the data of Figure 4A, with equivalent dose (EQD2) at α/β of 3 Gy on the x axis, which displays the effect of fractionation correction. Different total lung definitions were used in different studies: gross tumor volume was subtracted by Barriger (38), Borst (39), Chang (40,41), Li (22) and Bahig (36); planning target volume was subtracted by Matsuo (46); subtraction was not mentioned for Baker (37), Li (22) and Takeda (27). The points for Chang 2014 are based on 82 patients who received 4 fractions (total study was 100 patients). Numbers corresponding to the rates of G2+ RP are shown adjacent to the lines.
Figure 4B shows the range of G2+ RILT incidence for published ranges of V5, V10, and V20, with the weighted mean shown as well.
5. CLINICAL RISK FACTORS
Several non-dosimetric factors are associated with RILT risks. Zhao et al. found that studies with older patients had a significantly higher rate of G2+ RILT (p = 0.049) (35) whereas gender, race, and histology (including primary lung tumors vs metastases) were not significantly correlated with the risk of G2+ RILT. Patients with larger tumor sizes (measured by the greatest diameter) had significantly higher risk of G2+ (p = 0.049) and G3+ RILT (p = 0.001). Patients with stage IB NSCLC had a 17% risk of G2+ RILT compared with 8% in stage IA NSCLC (p < 0.0001) (23). An early study reported that the location of the tumor is an important factor, with centrally located disease having higher risk of G5 lung toxicity after 3-fraction regimens (8). As a result, many clinicians either treat central disease with 5–12 hypofractionated image-guided/stereotactic treatments or use conventional fractionation for such tumors. RTOG 0813, which tested the safety of a 5-fraction regimen for central disease, reported a 7% rate of G3+RILT at the highest (12 Gy × 5) dose level (59,60); a final toxicity report from this trial is pending. An 8-fraction regimen of 7.5 Gy was reported to have acceptable toxicity (61). A 10-fraction regimen of 7 Gy was considered to have acceptable risk of RILT, but may not be suitable for ultra-central disease due to other serious toxicity such as bleeding (22). A 10-fraction regimen of 4–5 Gy was safe though local tumor control rates may be compromised (62). A 15-fraction regimen of 4 Gy reported 10% rate of pneumonitis with 91% 2-year control rate including patients with central diseases (63). One should also note that the toxicity profile of central disease varies from that of peripheral disease, toxicities other than RILT may be more dose limiting. For example, a regimen of 5 Gy × 12 applied to a series of 47 patients with ‘ultra-central’ lesions (where the PTV overlaps with the trachea or main bronchus) reported a 38% rate of grade 3 toxicity, a 21% rate of ‘likely/possible’ grade 5 events, and a 15% rate of fatal bleeding (64). While location may not be a risk factor for RILT, central location is clearly a risk factor for bleeding which will be covered in a separate review.
Baseline lung function is often considered to be a risk factor for RILT after conventional fractionated RT (albeit based on relatively limited data), but this has not been seen for RILT after SBRT (65–67), likely due to the limited lung volume receiving high dose with SBRT. Conversely, interstitial lung disease (ILD), a group of lung diseases that share a pathogenic pathway leading to irreversible fibrosis, has been associated with an elevated risk of RILT after SBRT (Table 3). Indeed, some investigators consider ILD as a contraindication to SBRT (51,58).
Table 4.
Examples of dose response modeling: dose-volume metric and probit model parameters recomputed
| Source | Diagnosis; #patients/#lesions | Prescription Dose | OAR definition | Toxicity scheme, grade | Vdose | MLD/MLD2 | Probit model parameters (95% CI) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Ipsilateral lung | ||||||||
|
| ||||||||
| Ricardi 2009 (48) | NSCLC+lung metastases; 60/63 | 3×15Gy; 1×26Gy | Ipsilateral lung minus CTV | RTOG, grade 2+ | MLD2 | D50=19.7 Gy (17.5, 24.0); | γ50=1.90 (1.13, 3.07) | |
|
| ||||||||
| Guckenberger 2010 (44) | NSCLC+lung metastases; 59/68 | 3×12.5Gy at 65%; 1×26Gy at 80%; 8×6Gy at 65%; 5×6Gy at 65%; 3×10Gy at 65% | Ipsilateral lung minus CTV | SWOG, grade 2+ | MLD2 | D50=32.2 Gy (18.7, ∞) | γ50=0.59 (0.30, 0.92) | |
|
| ||||||||
| Ong 2010 (18) | All NSCLC 18/18 | 5×11Gy; 8×7.5Gy | Ipsilateral lung minus PTV | CTCAE v4.0, grade 2+ | V5 | V5,50=54.2 (47.0, 68.3) | γ50=2.46 (0.87, 5.11) | |
|
| ||||||||
| Combined lung | ||||||||
|
| ||||||||
| MLD | D50=7.9 (6.7, 9.2) | γ50=4.85 (1.21, m) | ||||||
|
|
||||||||
| V10 | V10,50=25.8 (19.9, 46.3) | γ50=1.25 (0.41, 2.51) | ||||||
|
|
||||||||
| V15 | V15,50=19.6 (at limit) | γ50=0.87 (0.00, 1.93) | ||||||
|
|
||||||||
| Ong 2010 (18) | All NSCLC 18/18 | 5×11Gy; 8×7.5Gy | Combined lung minus PTV | CTCAE v4.0, grade 2+ | V20 | V20,50=16.6 (at limit) | γ50=0.74 (−0.03, 1.71) | |
|
|
||||||||
| V5 | Threshold behavior, 0% toxicity for V5<36.3%; 100% toxicity for V20>42.8% | Threshold behavior, 0% toxicity for V5<36.3%; 100% toxicity for V20>42.8% | ||||||
|
| ||||||||
| Borst 2010 (39) | NSCLC/lung metastases; 128/161+ | 4×8.75Gy; 4×10Gy; 8×6Gy; 8×7.5Gy; 4×12Gy | Combined lung minus GTV | CTC v2.0, grade 2+ | MLD2 | D50=23.1 (17.4, 46.7) | γ50=0.82 (0.58 1.08) | |
|
| ||||||||
| MLD | D50=14.9 (11.2, 29.0) | γ50=0.82 (0.58, 1.08) | ||||||
OAR=organ at risk, CI=confidence interval, NSCLC=Non-small cell lung cancer, GTV=gross tumor volume, CTV=clinical target volume, PTV=planning target volume, RTOG=radiation therapy oncology group, SWOG=southwest oncology group, CTCAE=common terminology criteria for adverse events, MLD=mean lung dose, MLD2=fraction-number-corrected MLD, D50=dose to cause 50% toxicity, Vdose,50=Volume to cause 50% toxicity at dose, γ50=slope parameter, i.e. the percentage point change in response probability per 1% change in dose at the 50% response level.
The association between ILD and RILT, particularly RP, following SBRT is also supported by radiation dose-response data. Yamashita et al. (51) reported dosimetry data for 25 patients, including 3 with ILD who were treated with SBRT for lung tumors. Seven patients (35%) had grade 2–5 RP (3 grade 5) (51). A probit model of the probability of symptomatic RP as a function of MLD that was generated from the data in Ref 51 had D50=6.9 Gy, which is substantially lower (suggesting increased sensitivity) than the values reported by Ong and Borst for the broader patient population (18,26). In the follow-up study of 117 patients including those from the earlier study, Yamashita et al. (58) reported that many patients who developed severe complications exhibited interstitial pneumonitis prior to SBRT as well as high levels of serum Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D). Of 117 patients, 9 (7.7%) developed G4+ RP, of which 7 (6%) were G5. Most of the patients with severe RP were in the previously reported group. Following those early experiences, the authors began to pre-screen patients to exclude high risk patients from SBRT and noted a significant decrease in the incidence of G4+ RP. Grade 4 or higher RP was noted in six out of 32 patients (18.8%) before 2005 and in only three out of 85 patients (3.5%) after 2006 (p = 0.018). As treatment technique was unchanged and no dose-volume metric was found to be significant predictors of toxicity, this decrease in incidence of RP was attributed to use of biomarkers and to exclude patients with ILD. Unfortunately, this study only provided dosimetric data for patients with G4–5 radiation pneumonitis.
In a series of 128 patients treated mostly with 5 fractions, Takeda et al (68) had 3 patients with ideopathic pulmonary fibrosis (IPF). In this study there were 7 instances of G3+ RP, of which 2 were patients with IPF. Ueki et al (69) reported outcomes for 157 consecutive patients treated with SBRT alone for Stage I NSCLC and whose pre-treatment images could be retrospectively assessed for ILD. ILD was found in 20 patients. The treatment approach was largely similar in the ILD vs. non-ILD patients: 90% of the ILD patients and 84% of those without ILD were treated in 4 fractions, with 70% with ILD and 78% without receiving 48 Gy in 4 fractions, prescribed to isocenter. Patients with ILD developed significantly more RILT than those without: G2+ RP was 55% among patients with ILD vs. 13.3% among those without; G3+ RP was 10% for those with ILD vs. 1.5% for those without. In 72 patients for whom pre-treatment KL-6 level was available, it was significantly-higher in those with ILD than those without.
Finally, in a recent study of 504 patients (36) treated with SBRT between 2009–2014 with prescribed biologically effective dose with α/β=10 Gy (BED10) 112–164 Gy, the rate of G3+ RP was 3.8% for the entire cohort but 32.1% in the 28-patient subset with ILD. Five patients with ILD and no patients without ILD had G5 RP. Future studies of the relevance of ILD and of radiological and tissue biomarkers for adverse response to SBRT may be warranted to improve the safety of lung SBRT. Presently, it seems prudent to be extremely cautious in using SBRT in patients with ILD, and consider alternative treatment options. In patients with ILD who are desirous of curative therapy for pulmonary lesions, and where there are limited alternative treatment options, SBRT can be considered with strategies to minimize toxicity; e.g. strict adherence to conservative dose/volume limits, delivering biologically equivalent tumoricidal doses over ≥5 fractions, treatment on alternate days (vs. consecutive days), consideration of functional imaging to reduce damaging to the functional lung (e.g. ventilation/perfusion, V/Q scans), and applying IGRT methods for reduced PTV margins (e.g. tracking or gating instead of free breathing treatment) to reduce dose to normal lungs. Physicians should weigh benefit over risk of SBRT over other options in each patient with ILD. When SBRT is selected as the treatment option, more stringent dosimetric limits should be considered.
6. MATHEMATICAL/BIOLOGICAL DOSE RESPONSE MODELS FOR RELATIIONSHIP
The charge of the WGSBRT is to “generate reports, including but not limited to, critically surveying the published data regarding….(2) Normal tissue response: review of the effect of hypofractionation normal tissue tolerances…(3).” Mathematical dose response models are one way to summarize the review findings. The strength of such models depends critically on the available data. A statistically strong model would allow users to confidently interpolate and slightly extrapolate beyond the individual data points and even a weaker model may suggest trends worthy of further investigation. But in general, clinical use of models must be approached with great caution, particularly if they are based on sparse data with major sources of uncertainty.
Ideally, models of SBRT dose effects in lung should be based on treatment planning dose calculation algorithms that account accurately for tissue inhomogeneity. The majority of papers discussed in this section used some inhomogeneity correction and reported its nature, though 4/23 did not specify this factor. A variety of algorithms were employed. This information is included in S-Table-1.
Mathematical/biological models for SBRT dose effects should account for the different fractionation regimens. For example, V20 is often reported as the selected metric regardless of fractionation, but the biological effect is dependent on the number of fractions. For example, in a standard conventional 60 Gy in 30 fraction regimen (with a single treatment plan used throughout), the lung receiving 20 Gy has an equi-effective dose at 2 Gy per fraction of only 14.7 Gy (based on the LQ model with α/β = 3 Gy), because the dose per fraction of the pertinent lung is 0.67 Gy (i.e. less than 2 Gy). EQD23 is 20 Gy at V20 when only 10 fractions are used. In hypofractionated schedules, for 3, 5 and 7 fractions, the EQD23 for V20 is higher than 20 Gy: 38.7 Gy, 28.0 Gy and 23.4 Gy, respectively. Similarly, identical physical DVHs delivered with different fractionations have very different calculated biological mean doses. Future investigation of the applicability of radiobiological models other than LQ might prove interesting, though few RILT studies have done this to date (Ref 26 as an example).
The report of AAPM’s TG 101 on stereotactic radiotherapy recognized the problems of accurate fractionation modeling and suggested different absolute limits of MLD and V20 for different fractionations (70), but emphasized that “Because of the sparseness of long-term follow-up for SBRT, it should be recognized that the data in both TG101 Table III and the published reports represent, at best, a first approximation of normal tissue tolerance.” Four of the reviewed individual reports presented detailed dose-response data (18,39,44,48) associating the probability of RILT and physical dose or fraction-number corrected MLD (called MLD2 when the DVH dose axis is expressed as EQD23) in ipsilateral (44,48) or whole lung (18,39). These studies used different methods to compute and present their findings. In order to facilitate comparison, data were extracted from the original publications, either digitized from published graphs or transcribed from published tables, and were individually fit to a probit model using the maximum likelihood method. The profile likelihood method was used to calculate confidence intervals. Individual patient data were used when available (18,48). The model parameter sets and 95% confidence intervals for these parameters were derived and are presented for each dataset. The individual patient Vdose data from one study (18) was similarly processed. Table 4 shows brief descriptions of the original reports and a summary of model parameters from our reanalysis. D50 is the dose for 50% rate of toxicity; and γ50 is the slope parameter i.e. the percentage point change in response probability per 1% change in dose at the 50% response level.
Table 5.
Diagnosis and grading for radiation-induced pneumonitis and fibrosis*
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
|
Radiation Pneumonitis (Radiographic evidence of acute radiation pneumonitis required for the diagnosis) |
Radiographic evidence only, or minimal or mild symptoms of cough or dyspnea not requiring medication | Cough or dyspnea, requires prescribed medications or increase in steroid use from baseline, but does not interfere with ADL | Severe cough or dyspnea, interferes with ADL, or requiring oxygen (intermittent or continuous), or increase in baseline oxygen use | Respiratory insufficiency requiring assisted ventilation | Respiratory insufficiency directly contributing to death |
| Radiation pulmonary fibrosis (radiographic evidence of radiation fibrosis needed for the diagnosis) | Radiographic evidence of radiation fibrosis with no or mild dyspnea | Radiation fibrosis causing dyspnea but does not interfere with ADL | Radiation fibrosis causing dyspnea that interferes with ADL, or requiring oxygen or increase in baseline home oxygen use | Radiation fibrosis that causes respiratory insufficiency, requires assisted ventilation | Radiation fibrosis directly contributing to death |
• ADL=Activities of Daily Life
Mean dose of ipsilateral lung:
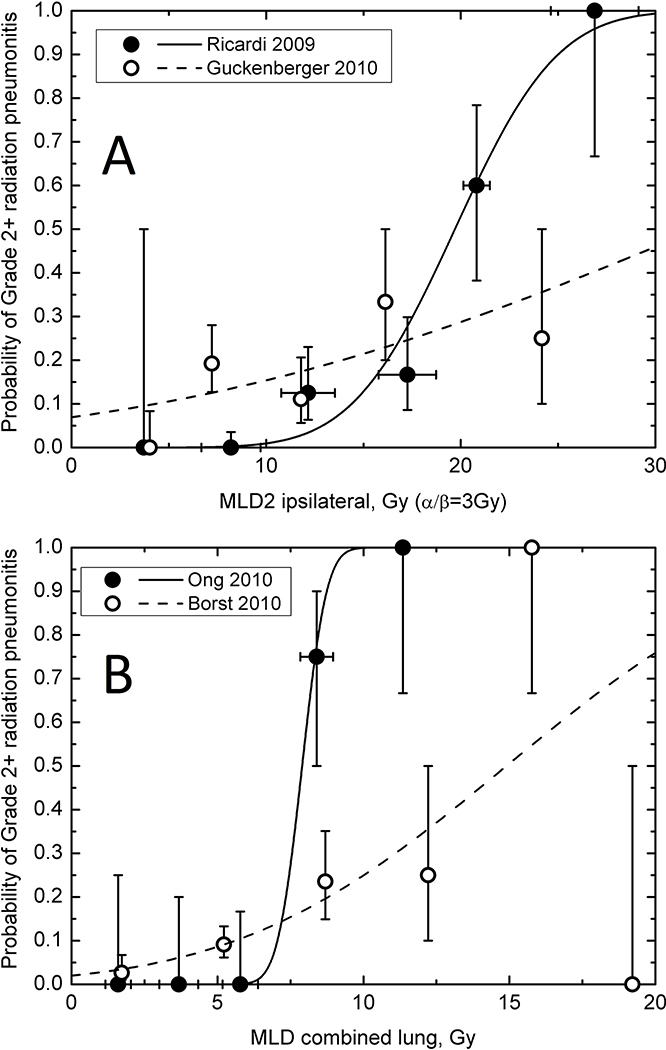
The dose-RILT relationship for ipsilateral lung was studied by Ricardi et al. (48) (60 patients) and Guckenberger et al. (44)(59 patients) (Figure 3A). Both defined the OAR as ipsilateral lung minus CTV and “corrected” the DVH with the LQ model with α/β =3 Gy. Interestingly these two studies showed remarkably different dose-response relationships. The latter exhibits a shallow dose response with RP probability ranging from 10 to 20% for MLD2 ranging from 5 to 15 Gy, while the incidence of G2+ RP is almost zero for MLD2 below 10 Gy and rises to 20% at approximately 17 Gy in Ricardi’s study. Part of the explanation for this discrepancy may be the use of different diagnosis criteria and grading scales. Ricardi et al.(48) diagnosed RP only if the symptoms worsen from baseline while Guckenberger et al. (44) classified any symptoms as RP (SWOG system) and gave steroids to all patients with clinical and radiological symptoms, which possibly reclassified all patients from grade 1 into grade 2. Despite this disagreement, if MLD2 in the ipsilateral lung is kept below 15 Gy the probability of G2+ RP remains below 20% in both data sets (Figure 3A).
Figure 3. Radiation lung dose and radiation pneumonitis relationship.
Incidence of G2+ RP as a function of the fraction-size adjusted mean lung dose (MLD2) in ipsilateral lung (A) and physical mean lung dose in combined lung (B). Vertical error bars indicate 68% confidence intervals. Horizontal bars, when shown, are ±1 standard deviation calculated for grouped data. Lines show probit model curves obtained by independently fitting the incidence of pneumonitis as a function of ipsilateral MLD2 (44,48) or combined MLD (18,39); see Table 4 for other model studies.
Mean dose of bilateral lungs:
Ong et al. (18) and Borst et al. (39) presented data points for G2+ RILT as a function of physical mean dose to the lung OAR defined as both lungs minus target. In our probit model fits, the MLD for 50% complication (D50) was 7.9 Gy (95%CI 6.7–9.2) (18) and 14.9 Gy (11.2–29.0) (39). These were remarkably lower than the value of 31.4 Gy (29.0–34.7) reported by the QUANTEC review of RILT after conventional fractionated RT (54). The fit of physical MLD of both lungs to the 18-patient dataset of Ong et al. (18) resulted in a steep dose-response, γ50=4.85 (95%CI 1.21−∞) (Figure 3B). The larger dataset of 128 patients from Borst et al. (39) yielded a shallower dose response, γ50 = 0.82 (0.58–1.08) and incidence of radiation pneumonitis was less than 10% for MLD below 6 Gy. Barriger et al. (38) reported that in 143 patients, the rate of G2+ RP was 4.3% in patients with median physical MLD ≤4 Gy, compared with 17.6% for patients with MLD >4 Gy (p = 0.02) (lung was bilateral lungs minus GTV), suggesting the complexity of the MLD response relationship with RILT.
Vdose of lungs:
We identified several studies where the OAR is combined lungs, with or without a subtracted target, that reported either a Vdose response curve or a significantly correlated cut-point. Borst et al. (39) presented a probit model with “Dose” being EQD23 for bilateral lungs minus GTV. Using the median number of fractions, 5, the physical dose could be back-calculated and the curve of iso-complication vs physical dose could be plotted. Takeda et al.(27) provided curves of iso-complication vs Vdose for 5% and 15% complications (no target subtracted from lung).
Several other papers (36,38,40,41,46) provided a variety of dose response relationships. The larger studies (with >50 patients) are plotted together in Figure 4 and study characteristics are summarized in S-Table-1. As with MLD, there is considerable variability between the studies. Indeed, Figure 4 implies a range of relatively safe Vdoses. Using V20 as an example, it appears that keeping it below 12% would keep the G2+ RP rate below 15%. Nevertheless, there does appear to be a trend of inter-study consistency in the data in Figure 4. The studies with a lower Vdose threshold (smaller x-coordinate), are largely towards the bottom (lower complication), and those with a higher one are largely towards the top.
7. SPECIAL SITUATIONS
A). Single fraction and shorter schedules
Although single fraction treatment or more compressed schedules are attractive and convenient options for patients, the relative safety of these approaches compared to 3–5 daily or every other day treatment is poorly studied. The RTOG 0915, a phase 2 randomized trial (71), compared the outcome of a single fraction of 34 Gy (Arm 1) to 48 Gy in 4 fractions in 4 days (Arm 2) for patients with peripheral T1 or T2 lesions. The lung constraints were V20< 10% for both arms, D1500 cc < 7 Gy for Arm 1 (single fraction) and 11.6 Gy for arm 2 and D1000 cc < 7.4 Gy for Arm 1 and < 12.4 Gy for arm 2. The median tumor size was 2 cm. With a median follow-up of 30 months, the rates of toxicity were similar in the two groups; grade 3 pulmonary toxicity occurred in 4/39 (10%) of patients in arm 1 vs. 6/45 (13%) in arm 2; with one additional grade 5 event in each arm. The estimated difference in these two toxicity rates (3%) has a 95% CI from −14% to +19%, illustrating that a larger study is required to say with reasonable precision that a single fraction might be safe for lung in patients with small peripheral tumors.
The impact of treatment duration for fractionated SBRT has been studied. A single-institution randomized study from the United Kingdom compared the results of 48 Gy delivered in 4 fractions over four consecutive days vs. 11 days at the same dose per fraction with 27 patients in each arm (72). G2+ acute toxicity was suggested to be more common in the 4-day group (56%) than 11-day arm (33%), approaching statistical significance (p = 0.085). Of note, G3 RP was the highest grade of pneumonitis and there was just one in each group; dyspnea was the most prevalent respiratory toxicity. However, a recent retrospective study (107 patients, 117 tumors) comparing 5 consecutive daily SBRT fractions versus a schedule of every other day treatment of the same prescription dose (50 Gy in 88% of the patients), reported similar toxicity for the two groups. The rate of G2+ pulmonary toxicity was 13.9% in the consecutively treated group vs. 10.8% in the nonconsecutively treated group (p = 0.78) (73).
B). SBRT for Re-irradiation
A few studies with limited patient numbers are concerned with SBRT for re-irradiation of NSCLC (10,74–78). Dose reduction for cases with direct overlap of previous radiation fields appears to result in acceptable re-irradiation toxicity profile. Liu et al. (74) analyzed 72 patients treated with SBRT of 48 Gy in 4 fractions after previous thoracic RT and reported that 20.8% of the patients developed G3+ lung toxicity. Eastern Cooperative Oncology Group (ECOG) performance status scores of 2–3, forced expiration volume in 1-second (FEV1) ≤ 65%, V20 ≥ 30% of the composite plan, and an initial planning target volume in the bilateral mediastinum were significantly associated with increased risk. Reyngold et al. (76) studied 39 patients with prior conventional lung RT and observed G2 RILT in 7 cases and G3 in 2 cases. They reported that SBRT prescription doses with a BED10 ≥100 Gy vs. <100 Gy and overlap of prior conventionally fractionated RT with SBRT fields were not predictive of pulmonary toxicity, while local control was better with the higher BED10. They concluded that for their patients, lower rates of RILT are largely due to lower SBRT prescription doses. In another study of 29 patients receiving a second course of SBRT with overlapping target volumes, 8 of 11 patients with central tumors experienced G2+ toxicity (mostly pulmonary), of which 3 were G5. There were no G4 or 5 toxicities in patients receiving a second course of SBRT to peripheral tumors (10). They concluded that overlapping SBRT should only be considered for small recurrent tumors at peripheral locations. Milano et al (79) recently reviewed 28 published studies describing toxicity in lung cancer patients who received SBRT re-irradiation in the lung. RILT was the most commonly reported toxicity. Although they could not extract robust composite dose constraints, they concluded that with careful weighing of risks vs benefits for individual patients, SBRT reirradiation was a feasible option.
In summary, previous thoracic RT is not a contraindication for SBRT for recurrent or secondary lung tumors. However, care should be taken in this setting. Factors that should be considered include performance status, pulmonary function such as FEV1, retreatment location, comprehensive assessment of lung dosimetry of the SBRT including V20 and MLD of the composite plan, and overlap status with the previous radiation fields. For less favorable cases, lung functional mapping using V/Q single-photon emission CT may be considered to avoid further damage of the functioning lung. Other organs at risk than lung, such as spinal cord, heart, esophagus (some of which are discussed in other WGSBRT reviews) are also potentially affected by the cumulative dose exposure from 2 courses of radiation and should also be considered. Nevertheless, re-irradiation may be the only curative option for many patients with recurrent cancer.
C). Combination with systemic therapy
There are no reports documenting the safety of concurrent systemic agents with lung SBRT, and such strategies should thus be considered in the clinical trial setting. Nevertheless, in practice, patients with metastatic cancers are often on systemic therapies that might not be practical to discontinue and/or where the agents have a long half-life. In this setting, it might be reasonable to deliver SBRT during the middle cycle of systemic therapy (not the same day) but with appropriate informed consent. For the setting of systemic therapy followed by SBRT, the agent’s half-life should be considered when determining the interval between modalities. In all settings, clinicians should be cautious about the use of concurrent systemic therapy and cognizant of the associated potential for unexpected interactions and excessive normal tissue toxicity.
8. RECOMMENDED DOSE/VOLUME OBJECTIVES
Although SBRT is increasingly becoming the standard of care for medically inoperable primary and metastatic lung cancer and RILT is the most feared complication of such treatments, peer-reviewed evidence upon which to base dosimetric models and guidelines is limited. The publications summarized in Sections 4 and 6 of this report suggest that thoracic SBRT of BED10>100 Gy in 3–5 fractions can be delivered safely with limited risk of RILT for patients with small peripheral tumors, if the bilateral MLD is limited to < 8 Gy, and the V20 is < 10–15%. Dose is expected to be among the most important factors, but when dose constraints are sufficiently conservative to prevent complications, the effects are not often observed in small datasets.
In general, data with larger sample sizes are needed to generate and validate safe dose-volume lung limits for lung SBRT. In all cases, care should be taken in defining dose/volume limits since overly-conservative constraints might preclude adequate dose to the tumor while inadequate limits risk serious RILT. Additionally, further studies are needed to develop guidelines for more hypofractionated protocols (8–15 fractions with total doses ≥ 50 Gy) and/or larger or more central tumors.
9. FUTURE STUDIES
Future studies should implement prospective data collection using standardized criteria for grading of the lung toxicity endpoints, consistent delineation and definition of the lungs, accurate dose computation, and comprehensive description and accounting for clinical risk factors. Such effort is urgently needed to generate predictive dose-volume-effect models that could guide SBRT practice. To the extent that journals make feasible, full dose-volume-outcome data should be made electronically available. With these datasets, modelers may be able to generate the most clinically useful safe limits of dosimetric parameters such as mean lung dose, Vdose or the recently emphasized critical volume (CV) (80) as has been used in RTOG lung SBRT trials (S-Table-3) (71).
9.1. BIOMARKERS: THE ROLE OF RILT PREDICTION FOR SBRT
The risk of RILT after SBRT is also associated with the intrinsic radiosensitivity of each individual patient determined by biologic factors. A number of biomarkers such as single nuclear polymorphism (SNPs) in genes of DNA repair, inflammation, ATM and transforming growth factor-beta (TGF-β1) pathways, microRNA, proteomic and proinflammatory cytokines, have been investigated for RILT, mostly in patients treated with conventionally fractionated radiotherapy, recently summarized by Kong et al. (81). Despite the extensive literature, only a few of them have been validated by relatively small studies. For patients treated with SBRT, only KL-6 and SP-D have been reported to be predictive. In Yamashita’s study (58), a correlation was found between the incidence of RP and higher serum KL-6 and SP-D levels. Iwata et al. (82), noted that high pre-treatment levels of KL-6 might be a significant factor predictive for G2+ RP. The predictive ability of these biomarkers needs to be further evaluated. Future studies are needed to consider the combined utility of biomarkers, clinical and dosimetric factors, as potential predictors for RILT. Future studies should investigate whether biomarkers with potentials for RILT prediction after conventional RT can also be applied in patients treated with SBRT.
10. FUTURE REPORTING STANDARDS
Quantitative synthesis of published data is hampered by inconsistent reporting of many patient and disease characteristics, inconsistent lung volume definition, target delineation and insufficient detail regarding lung dose volume histograms, and incomplete definition of clinical outcomes. For future reports, we recommend the use of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement so that essential items be recorded consistently (83).
Lung definition: the lung should be segmented consistently, according to the RTOG atlas (55). Attention should be paid to exclude air in the proximal bronchial tree, GTV and collapsed lung, and include the small vessels in the parenchyma. Consistent with the QUANTEC recommendation regarding RILT after conventionally fractionated RT (54), the lung should be considered as a single, paired-organ (total lung tissue) with the GTV (for breathing controlled treatment) or IGTV (for free breathing treatment after 4D simulation) excluded. IGTV is a composite volume of GTVs from all breathing phases, not the GTV from average CT scan. For free breathing treatments, we recommend that the average scan of 4DCT be used for lung definition and treatment planning dose computation. For breathingcontrolled treatments, the CT corresponding to the particular respiratory phase for treatment delivery (e.g. for breath-hold treatments, the scan during breath hold) should be used for treatment planning.
Complete physical DVHs of the combined, ipsilateral and contralateral lungs (with GTV subtracted) should be computed together with common summary indices and, if possible, their correlation with toxicity. Dosimetric computation should account for tissue inhomogeneities with algorithms that would be acceptable according to modern national guidelines. Care should be taken to consider the impact of contrast given during the planning scan since it might influence the dose computation.
The toxicity endpoints of radiation pneumonitis and radiation fibrosis should be diagnosed and graded as carefully and consistently as possible, and a variety of grading systems are available (e.g. see section 2). An alternative grading system outlined in Table 5, which is largely based on CTCAE and a prior study (84) as well as RTOG1106, might more specifically address issues pertinent to RILT. Future trials and practice might consider reporting per both the CTCAE and this modified scale. Regardless of the system used, the diagnosis of RILT should consider the clinical history, exam, and radiologic/laboratory findings, including the exclusion of alternative conditions (e.g. tumor recurrence, infection, chronic obstructive pulmonary disease, heart failure, anemia) whose symptoms may mimic RILT. Nevertheless, we recognize that the diagnosis of RILT can be challenging, and many patients with lung cancer often also have some of these other diagnosis, and that patient’s symptoms may be multifactorial. Thus, the input of a multidisciplinary team is recommended in diagnosing, grading and managing RILT.
Investigators should provide comprehensive data regarding patient and treatment factors of each individual that might be relevant to potential risk factors (e.g. previous treatment, specifics of systemic treatment and RILT). These factors include age, gender, smoking status, comorbidities, tumor stage, size, volume, GTV/IGTV, CTV, ITV, PTV, prescriptions, fractionation, detailed patient-specific DVHs, regimen of systemic therapy, lung definition, dose computation/heterogeneity correction algorithm, and diagnosis criteria of toxicity endpoints.
To facilitate the presentation of this large quantity of data, investigators should also take advantage of electronic supplements available in many journals to provide anonymized data in a format conducive to pooled analyses and journals should encourage this practice, as in example publications on a similar topic (85,86). Alternatively, investigators are encouraged to submit data-only papers, which were recently implemented as a special category by Medical Physics (87). This will provide data to help develop and validate models that relate dose-volume and other factors with RILT.
11. CONCLUSIONS
In summary, the risk of RILT has been reported to be associated with the size and location of the tumor as well as a variety of dosimetric parameters. There is no apparent threshold ‘tolerance dose-volume’ level. However, most studies note safe treatment with a rate of symptomatic RILT of < 10–15% after lung SBRT with a mean lung dose (MLD) of the combined lungs < 8 Gy, and the percent of total lung volume receiving >20 Gy (V20) <10–15%. Future studies should standardize reporting by including endpoint and volume definitions and providing prescription dose including fractionation and dose-volume information for all patients, with and without RILT.
Supplementary Material
Table 1.
CTCAE$ 3.0/4.0 grading system for pneumonitis and pulmonary fibrosis
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
|
| |||||
|
| |||||
| Pneumonitis | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; medical intervention indicated; limiting instrumental ADL* | Severe symptoms; limiting self care ADL*; oxygen indicated | Life-threatening respiratory compromise; urgent intervention indicated (e.g.,tracheotomy or intubation) | Death |
|
| |||||
| Pulmonary Fibrosis | Mild hypoxemia; radiologic pulmonary fibrosis <25% of lung volume | Moderate hypoxemia; evidence of pulmonary hypertension; radiographic pulmonary fibrosis 25 – 50% | Severe hypoxemia; evidence of rightsided heart failure; radiographic pulmonary fibrosis >50 – 75% | Life-threatening consequences (e.g., hemodynamic/pulmonary complications); intubation with ventilatory support indicated; radiographic pulmonary fibrosis >75% with severe honeycombing | Death |
CTCAE=Common Terminology Criteria for Adverse Events
ADL= Activities of Daily Living.
Funding Statement:
The HyTEC papers are from the Working Group on Biological Effects of Hypofractionated Radiotherapy/SBRT (WGSBRT) within the American Association of Physicists in Medicine (AAPM).
Footnotes
| Name | COI Information |
|---|---|
| Feng-Ming Kong, Vitali Moiseenko, Jing Zhao, Ling Li, Shiva Das, X. Allen Li, Moyed Miften, Zhong Xing Liao, Mary Martel, Soren M. Bentzen | No COI |
| Michael T. Milano | Personal fees: UpToDate |
| Andreas Rimner | Institutional Core NIH Grant P30 CA008748; Outside Submitted Work: Grants and personal fees: Varian Medical Systems, Astra Zeneca; Grants: Boehringer Ingelheim, Pfizer; Personal fees – Merck; non-financial support: Elekta |
| Andrew Jackson | NIH Grant: RO1CA129182, Institutional Core NIH Grant P30 CA008748 |
| Jimm Grimm | Outside Submitted Work: Grants: Accuray, NovoCure Patent: DVH Evaluator |
| Lawrence B. Marks | Outside this work: Departmental support from CDC, NIH, Elekta, Accuray, AHRQ, Lance Armstrong, Department of Defense, Vision RT, Morphormics, Sun Nuclear, Communify |
| Ellen Yorke | Institutional core NIH grant: P30 CA008748 |
References
- 1.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage i non-small cell lung cancer: A meta-analysis. International journal of radiation oncology, biology, physics 2014;90:603–611. [DOI] [PubMed] [Google Scholar]
- 2.Chang JY, Bezjak A, Mornex F, et al. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: What we have learned. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:577–585. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda A, Sanuki N, Eriguchi T, et al. Salvage stereotactic ablative irradiation for isolated postsurgical local recurrence of lung cancer. The Annals of thoracic surgery 2013;96:1776–1782. [DOI] [PubMed] [Google Scholar]
- 5.Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta oncologica 2009;48:578–583. [DOI] [PubMed] [Google Scholar]
- 6.Baschnagel AM, Mangona VS, Robertson JM, et al. Lung metastases treated with image-guided stereotactic body radiation therapy. Clinical oncology 2013;25:236–241. [DOI] [PubMed] [Google Scholar]
- 7.Shultz DB, Filippi AR, Thariat J, et al. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2014;9:1426–1433. [DOI] [PubMed] [Google Scholar]
- 8.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase ii study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24:4833–4839. [DOI] [PubMed] [Google Scholar]
- 9.Corradetti MN, Haas AR Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. The New England journal of medicine 2012;366:2327–2329. [DOI] [PubMed] [Google Scholar]
- 10.Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;101:260–266. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Oh RJ, Shiomi H, et al. Stereotactic body radiotherapy for pulmonary metastases. Prognostic factors and adverse respiratory events. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2013;189:285–292. [DOI] [PubMed] [Google Scholar]
- 12.Kimura T, Matsuura K, Murakami Y, et al. Ct appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (sbrt) for lung cancers: Are patients with pulmonary emphysema also candidates for sbrt for lung cancers? International journal of radiation oncology, biology, physics 2006;66:483–491. [DOI] [PubMed] [Google Scholar]
- 13.Cuaron JJ, Yorke ED, Foster A, et al. Stereotactic body radiation therapy for primary lung cancers >3 centimeters. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013;8:1396–1401. [DOI] [PubMed] [Google Scholar]
- 14.Xiong W, Xu Q, Xu Y, et al. Stereotactic body radiation therapy for post-pulmonary lobectomy isolated lung metastasis of thoracic tumor: Survival and side effects. BMC cancer 2014;14:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: Results of a phase ii trial. International journal of radiation oncology, biology, physics 2011;80:1343–1349. [DOI] [PubMed] [Google Scholar]
- 16.Nuyttens JJ, van der Voort van Zyp NC, Praag J, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2012;102:383–387. [DOI] [PubMed] [Google Scholar]
- 17.Kundu S, Mathew A, Munshi A, et al. Stereotactic body radiotherapy in early stage non-small cell lung cancer: First experience from an indian centre. Indian journal of cancer 2013;50:227–232. [DOI] [PubMed] [Google Scholar]
- 18.Ong CL, Palma D, Verbakel WF, et al. Treatment of large stage i-ii lung tumors using stereotactic body radiotherapy (sbrt): Planning considerations and early toxicity. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2010;97:431–436. [DOI] [PubMed] [Google Scholar]
- 19.Shintani T, Masago K, Takayama K, et al. Stereotactic body radiotherapy for synchronous primary lung cancer: Clinical outcome of 18 cases. Clinical lung cancer 2015;16:e91–96. [DOI] [PubMed] [Google Scholar]
- 20.Thompson R, Giuliani M, Yap ML, et al. Stereotactic body radiotherapy in patients with previous pneumonectomy: Safety and efficacy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2014;9:843–847. [DOI] [PubMed] [Google Scholar]
- 21.Mattonen SA, Palma DA, Haasbeek CJ, et al. Distinguishing radiation fibrosis from tumour recurrence after stereotactic ablative radiotherapy (sabr) for lung cancer: A quantitative analysis of ct density changes. Acta oncologica 2013;52:910–918. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Swanick CW, Allen PK, et al. Stereotactic ablative radiotherapy (sabr) using 70 gy in 10 fractions for non-small cell lung cancer: Exploration of clinical indications. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2014;112:256–261. [DOI] [PubMed] [Google Scholar]
- 23.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011;6:2036–2043. [DOI] [PubMed] [Google Scholar]
- 24.Nagata Y, Hiraoka M, Mizowaki T, et al. Survey of stereotactic body radiation therapy in japan by the japan 3-d conformal external beam radiotherapy group. International journal of radiation oncology, biology, physics 2009;75:343–347. [DOI] [PubMed] [Google Scholar]
- 25.Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. International journal of radiation oncology, biology, physics 2013;87:1064–1070. [DOI] [PubMed] [Google Scholar]
- 26.Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2009;91:307–313. [DOI] [PubMed] [Google Scholar]
- 27.Takeda A, Ohashi T, Kunieda E, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. The British journal of radiology 2012;85:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang KH, Okoye CC, Patel RB, et al. Complications from stereotactic body radiotherapy for lung cancer. Cancers 2015;7:981–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuyttens JJ, Moiseenko V, McLaughlin M, et al. Esophageal dose tolerance in patients treated with stereotactic body radiation therapy. Seminars in radiation oncology 2016;26:120–128. [DOI] [PubMed] [Google Scholar]
- 30.Duijm M, Tekatli H, Oomen-de Hoop E, et al. Esophagus toxicity after stereotactic and hypofractionated radiotherapy for central lung tumors: Normal tissue complication probability modeling. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2018. [DOI] [PubMed] [Google Scholar]
- 31.Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Practical radiation oncology 2016;6:e27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duijm M, Schillemans W, Aerts JG, et al. Dose and volume of the irradiated main bronchi and related side effects in the treatment of central lung tumors with stereotactic radiotherapy. Seminars in radiation oncology 2016;26:140–148. [DOI] [PubMed] [Google Scholar]
- 33.Schanne DH, Nestle U, Allgauer M, et al. Stereotactic body radiotherapy for centrally located stage i nsclc: A multicenter analysis. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2015;191:125–132. [DOI] [PubMed] [Google Scholar]
- 34.Wu AJ, Williams E, Modh A, et al. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2014;112:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Yorke ED, Li L, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: A pooled analysis of 88 studies. International journal of radiation oncology, biology, physics 2016;95:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahig H, Filion E, Vu T, et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Practical radiation oncology 2016;6:367–374. [DOI] [PubMed] [Google Scholar]
- 37.Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. International journal of radiation oncology, biology, physics 2013;85:190–195. [DOI] [PubMed] [Google Scholar]
- 38.Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. International journal of radiation oncology, biology, physics 2012;82:457–462. [DOI] [PubMed] [Google Scholar]
- 39.Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis after hypofractionated radiotherapy: Evaluation of the lq(l) model and different dose parameters. International journal of radiation oncology, biology, physics 2010;77:1596–1603. [DOI] [PubMed] [Google Scholar]
- 40.Chang JY, Liu H, Balter P, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage i non-small cell lung cancer. Radiation oncology 2012;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone”. International journal of radiation oncology, biology, physics 2014;88:1120–1128. [DOI] [PubMed] [Google Scholar]
- 42.Duncker-Rohr V, Nestle U, Momm F, et al. Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2013;189:33–40. [DOI] [PubMed] [Google Scholar]
- 43.Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:1382–1393. [DOI] [PubMed] [Google Scholar]
- 44.Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2010;97:65–70. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi A, Shibamoto Y, Hattori Y, et al. Dose-volume histogram comparison between static 5-field imrt with 18-mv x-rays and helical tomotherapy with 6-mv x-rays. Journal of radiation research 2015;56:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuo Y, Shibuya K, Nakamura M, et al. Dose--volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. International journal of radiation oncology, biology, physics 2012;83:e545–549. [DOI] [PubMed] [Google Scholar]
- 47.Ohashi T, Takeda A, Shigematsu N, et al. Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. International journal of radiation oncology, biology, physics 2005;62:1003–1008. [DOI] [PubMed] [Google Scholar]
- 48.Ricardi U, Filippi AR, Guarneri A, et al. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta oncologica 2009;48:571–577. [DOI] [PubMed] [Google Scholar]
- 49.Stauder MC, Macdonald OK, Olivier KR, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;99:166–171. [DOI] [PubMed] [Google Scholar]
- 50.Westover KD, Seco J, Adams JA, et al. Proton sbrt for medically inoperable stage i nsclc. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiation oncology 2007;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox JD, Stetz J Pajak TF. Toxicity criteria of the radiation therapy oncology group (rtog) and the european organization for research and treatment of cancer (eortc). International journal of radiation oncology, biology, physics 1995;31:1341–1346. [DOI] [PubMed] [Google Scholar]
- 53.Lent soma scales for all anatomic sites. International journal of radiation oncology, biology, physics 1995;31. [DOI] [PubMed] [Google Scholar]
- 54.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. International journal of radiation oncology, biology, physics 2010;76:S70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: Atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. International journal of radiation oncology, biology, physics 2011;81:1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda A, Kunieda E, Sanuki N, et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. International journal of radiation oncology, biology, physics 2009;74:363–369. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Xu Y, Schipper M, et al. Effect of normal lung definition on lung dosimetry and lung toxicity prediction in radiation therapy treatment planning. International journal of radiation oncology, biology, physics 2013;86:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamashita H, Kobayashi-Shibata S, Terahara A, et al. Prescreening based on the presence of ct-scan abnormalities and biomarkers (kl-6 and sp-d) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiation oncology 2010;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bezjak A, Paulus R, Gaspar LE, et al. Primary study endpoint analysis for nrg oncology/rtog 0813 trial of stereotactic body radiation therapy (sbrt) for centrally located non-small cell lung cancer (nsclc). International journal of radiation oncology, biology, physics 2016;94:5–6. [Google Scholar]
- 60.Bezjak A, Paulus R, Gaspar LE, et al. Efficacy and toxicity analysis of nrg oncology/rtog 0813 trial of stereotactic body radiation therapy (sbrt) for centrally located non-small cell lung cancer (nsclc). International journal of radiation oncology, biology, physics 2016;96:S8. [Google Scholar]
- 61.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage i non-small-cell lung cancer. International journal of radiation oncology, biology, physics 2008;70:685–692. [DOI] [PubMed] [Google Scholar]
- 62.Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2009;91:301–306. [DOI] [PubMed] [Google Scholar]
- 63.Cheung P, Faria S, Ahmed S, et al. Phase ii study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage t1–3 n0 m0 non-small cell lung cancer: Ncic ctg br.25. Journal of the National Cancer Institute 2014;106. [DOI] [PubMed] [Google Scholar]
- 64.Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016;11:1081–1089. [DOI] [PubMed] [Google Scholar]
- 65.Navarro-Martin A, Aso S, Cacicedo J, et al. Phase ii trial of sbrt for stage i nsclc: Survival, local control, and lung function at 36 months. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016;11:1101–1111. [DOI] [PubMed] [Google Scholar]
- 66.Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: An analysis of rtog 0236. International journal of radiation oncology, biology, physics 2014;88:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012;7:542–551. [DOI] [PubMed] [Google Scholar]
- 68.Takeda A, Ohashi T, Kunieda E, et al. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (sbrt) in patients with lung tumors. International journal of radiation oncology, biology, physics 2010;77:685–690. [DOI] [PubMed] [Google Scholar]
- 69.Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:116–125. [DOI] [PubMed] [Google Scholar]
- 70.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of aapm task group 101. Medical physics 2010;37:4078–4101. [DOI] [PubMed] [Google Scholar]
- 71.Videtic GM, Hu C, Singh AK, et al. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage i peripheral non-small cell lung cancer: Nrg oncology rtog 0915 (ncctg n0927). International journal of radiation oncology, biology, physics 2015;93:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain S, Poon I, Soliman H, et al. Lung stereotactic body radiation therapy (sbrt) delivered over 4 or 11 days: A comparison of acute toxicity and quality of life. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2013;108:320–325. [DOI] [PubMed] [Google Scholar]
- 73.Alite F, Stang K, Balasubramanian N, et al. Local control dependence on consecutive vs. Nonconsecutive fractionation in lung stereotactic body radiation therapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2016;121:9–14. [DOI] [PubMed] [Google Scholar]
- 74.Liu H, Zhang X, Vinogradskiy YY, et al. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. International journal of radiation oncology, biology, physics 2012;84:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trovo M, Minatel E, Durofil E, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. International journal of radiation oncology, biology, physics 2014;88:1114–1119. [DOI] [PubMed] [Google Scholar]
- 76.Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (sbrt). Radiation oncology 2013;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Bari B, Filippi AR, Mazzola R, et al. Available evidence on re-irradiation with stereotactic ablative radiotherapy following high-dose previous thoracic radiotherapy for lung malignancies. Cancer treatment reviews 2015;41:511–518. [DOI] [PubMed] [Google Scholar]
- 78.Amini A, Yeh N, Gaspar LE, et al. Stereotactic body radiation therapy (sbrt) for lung cancer patients previously treated with conventional radiotherapy: A review. Radiation oncology 2014;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milano MT, Mihai A Kong FM. Review of thoracic reirradiation with stereotactic body radiation therapy: A focus on toxicity risks. Prac Radiat Oncol 2018;In Press. [DOI] [PubMed] [Google Scholar]
- 80.Ritter TA, Matuszak M, Chetty IJ, et al. Application of critical volume-dose constraints for stereotactic body radiation therapy in NRG radiation therapy trials. International journal of radiation oncology, biology, physics 2017;98:34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong FM Wang S Nondosimetric risk factors for radiation-induced lung toxicity. Seminars in radiation oncology 2015;25:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwata H, Shibamoto Y, Baba F, et al. Correlation between the serum kl-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage i lung cancer or small lung metastasis. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2011;101:267–270. [DOI] [PubMed] [Google Scholar]
- 83.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Journal of clinical epidemiology 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 84.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (nsclc): Predictors for radiation pneumonitis and fibrosis. International journal of radiation oncology, biology, physics 2006;65:1075–1086. [DOI] [PubMed] [Google Scholar]
- 85.Mutter R, Liu F, Yorke E, et al. Dose-volume dependency of chestwall pain in patients treated with SBRT for NSCLC. International journal of radiation oncology, biology, physics 2012;82:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu F, Yorke ED, Belderbos JSA, et al. Using gEUD Atlases to Combine and Analyze Prospective Dosimetric and Radiation Pneumonitis Data from Two Non-Small Cell Lung Cancer Dose Escalation Protocols. International journal of radiation oncology, biology, physics 2013;85:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williamson JF, Das SK, Goodsitt MS, Deasy JO. Introducing the Medical Physics Dataset Article. Med. Phys. 2017; 44: 349–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.