Abstract
Acute pancreatitis is a common indication for hospital admission, increasing in incidence, including in children, pregnancy and the elderly. Moderately severe acute pancreatitis with fluid and/or necrotic collections causes substantial morbidity, and severe disease with persistent organ failure causes significant mortality. The diagnosis requires two of upper abdominal pain, amylase/lipase ≥ 3 ×upper limit of normal, and/or cross-sectional imaging findings. Gallstones and ethanol predominate while hypertriglyceridaemia and drugs are notable among many causes. Serum triglycerides, full blood count, renal and liver function tests, glucose, calcium, transabdominal ultrasound, and chest imaging are indicated, with abdominal cross-sectional imaging if there is diagnostic uncertainty. Subsequent imaging is undertaken to detect complications, for example, if C-reactive protein exceeds 150 mg/L, or rarer aetiologies. Pancreatic intracellular calcium overload, mitochondrial impairment, and inflammatory responses are critical in pathogenesis, targeted in current treatment trials, which are crucially important as there is no internationally licenced drug to treat acute pancreatitis and prevent complications. Initial priorities are intravenous fluid resuscitation, analgesia, and enteral nutrition, and when necessary, critical care and organ support, parenteral nutrition, antibiotics, pancreatic exocrine and endocrine replacement therapy; all may have adverse effects. Patients with local complications should be referred to specialist tertiary centres to guide further management, which may include drainage and/or necrosectomy. The impact of acute pancreatitis can be devastating, so prevention or reduction of the risk of recurrence and progression to chronic pancreatitis with an increased risk of pancreas cancer requires proactive management that should be long term for some patients.
Key Points
| Acute pancreatitis is a major disease that cannot be considered self-limiting, as it has serious early and long-term impact. Interventional clinical trials of new and repurposed drugs are crucial to address the absence of a definitive, internationally licensed treatment. Many drugs, however, have a role in treating complications of acute pancreatitis or, in specific sub-groups, preventing recurrence. A definitive drug, however, would reduce complications and the adverse effects of their treatments. |
| Initially intravenous fluids, opiates, and oral or enteral if not parenteral nutrition are indicated; occasionally antivirals, or antivenom for scorpion stings in endemic areas. |
| Insulin is used in hypertriglcyeridaemia-associated acute pancreatitis, which has a range of drugs including antisense therapies in specific groups to prevent recurrence. |
| Inotropes maintain cardiac output and assist renal perfusion in patients with severe acute pancreatitis, but may cause gut and/or peripheral ischaemia. |
| Antibiotics are the mainstay to treat, but not recommended to prevent, all infection; these may be complicated by dysbiosis and/or fungal infection. |
| Pancreatic enzyme replacement therapy is necessary in many patients, notably after pancreatic necrosis; pancreatic endocrine insufficiency is likely to require insulin. |
| Interventions for source control are usually delayed for several weeks because early infection is poorly localised amid intra-abdominal inflammation and necrosis. |
Introduction
Acute pancreatitis is a common inflammatory disease of the exocrine pancreas that causes severe abdominal pain and multiple organ dysfunction that may lead to pancreatic necrosis and persistent organ failure, with a mortality of 1–5% [1]. Overall, it has a global incidence of 30–40 cases per 100,000 population per year [1] and over twice that in some regions [2], contributing an average cost of circa €10,000 per patient [3]. The incidence in children is not far behind at 10–15 cases per 100,000 children [4]. The global incidence is rising, although studies suggest rates are currently more stable in Asia [2, 5]. Acute pancreatitis leads to significant short- and long-term morbidity, which in a significant minority causes prolonged debility, recurrent disease, and pancreatic exocrine and/or endocrine insufficiency. There can be a significant but often ignored impact on quality of life due to chronic pain as well as the socio-economic consequences of prolonged hospitalisation.
A search for ‘acute pancreatitis’ in July 2022 yields over 75,000 references in PubMed, including articles relating to diagnosis, classification and overall management, with a small but critically important fraction reporting randomised trials of targeted treatments. Despite this, there is no specific internationally licensed drug therapy that can change the course of acute pancreatitis. Among reasons for this failure are insufficient understanding of pathophysiology and inappropriate drug choice. Trial designs remain problematic, as underlying assumptions may be incorrect, for example, therapeutic windows and complication rates [6, 7]. Most established therapies address the complications of the disease, among which are generic treatments, for example, for organ failure and infection. Here we review state-of-the-art therapy in practice for acute pancreatitis, highlighting the importance of diagnosis to determine aetiology and identify complications, both of which alter management acutely and during follow up.
Diagnosis
Diagnostic Criteria
Presentations of pancreatitis include epigastric or diffuse abdominal pain (80–95%), nausea and vomiting (40–80%), abdominal distension, fever, breathlessness, irritability, and impaired consciousness, with pyrexia, low oxygen saturation, tachypnoea, tachycardia, hypotension, abdominal guarding, ileus and/or oliguria [8]. The medical history should include careful enquiry directed at aetiology including gallstones, obesity, alcohol excess, smoking, hyperlipidaemia, and drugs that can induce the disease, recognising that more than one precipitant may cause the disease. Identification of alcohol excess is assisted by use of the 10-item alcohol-use disorders identification test (AUDIT), for which both clinician-administered and self-report versions are available [9] as well as abbreviated versions [10] or alternatives [11], including single-item screening questions [12]. A diagnosis of acute pancreatitis requires two out of three criteria: (1) abdominal pain consistent with pancreatitis, (2) a serum amylase or lipase three or more times the upper limit of normal, and (3) findings consistent with pancreatitis on cross-sectional abdominal imaging [in adults: computed tomography (CT) or magnetic resonance imaging (MRI); in children CT, MRI or in some cases transabdominal ultrasound (TUS)] [13, 14]. Care is required, as the first two criteria alone may fail to identify a quarter of patients with acute pancreatitis, while diagnosing one in ten patients with acute pancreatitis incorrectly [15].
Aetiology
Cholelithiasis
Gallstone disease is the leading cause of acute pancreatitis world-wide [16] and accounts for around 20–70% of all cases in the West [17]. Epidemiological studies show an increasing prevalence of cholelithiasis with age [18], accounting partly for the increasing incidence of acute pancreatitis associated with demographic changes. A stone obstructing the sphincter of Oddi was the first ever reported confirmed aetiology of acute necrotising pancreatitis [19]; obstruction of the pancreatic duct and/or ductal infusion of bile acid is an effective way to produce experimental acute pancreatitis [20]. Around two-thirds of patients with acute biliary pancreatitis are female [17], as gallstones are more common in women. Gallstones can cause acute pancreatitis in children, but less commonly than drugs [21]. Patients admitted with a diagnosis of acute pancreatitis should have a TUS as part of their initial work-up to identify gallbladder stones [11], usually taken to indicate gallstone aetiology, and to identify biliary ductal dilatation, which may support a diagnosis of cholangitis.
Ethanol
Heavy alcohol consumption is a well-known cause of acute pancreatitis, the incidence of which differs widely by geography [22]. It is often reported that alcohol is the second most common cause of acute pancreatitis in North America and Europe, accounting for up to a third of cases [17, 23, 24]; in Eastern Europe it is the leading cause [5]. The incidence of acute alcoholic pancreatitis has been reported to be as low as 2% in Latin America [16] and < 10% in China [25] or as high as 46% in Japan [26] and 70% in Finland [27].
Binge drinking, that is five or more alcoholic drinks per session [in the UK 80 mL (~ 65 g) ethanol/session in men and 60 (~ 47.5 g) mL/session in women; in the USA 70 g (~ 90 mL) and 56 g (~ 70 mL) respectively], confers an increased risk of acute pancreatitis, notably on a background of heavy alcohol consumption (in the USA heavy drinking is defined as 210 g (15 drinks of 14 g each; ~ 270 mL) ethanol/week in men and 112 g (eight drinks of 14 g each; ~ 140 mL) in women). The impact of binge drinking is evident in the spike in incidence during national holidays and festivals [28–30]. Patients with alcohol-induced pancreatitis have similar drinking patterns to those with alcohol abuse disorders, and may have high levels of alcohol intake over several years prior to pancreatitis [31]. Only a minority of heavy drinkers develop identifiable episodes of acute pancreatitis, indicative of co-factors that include genetic risk [32], hypertriglyceridaemia [33] and smoking [34]. The use of the AUDIT tool is described above (Sect. 2.1) to support identification of an alcoholic aetiology.
Smoking is a risk factor for acute pancreatitis, recurrent acute pancreatitis and chronic pancreatitis [35], most notably in heavy drinkers [34]. In contrast, low alcohol consumption (< two drinks/day in men and < one drink/day in women) by non-smokers may protect against a first but not subsequent attack of acute pancreatitis [34, 36], possibly by stimulating pancreatic ductal secretion [37].
Hypertriglyceridaemia
Hypertriglyceridaemia was found to be the cause of acute pancreatitis in ~ 9% in a recent global systematic review [38], making it the third most common cause. One high-volume centre in China reported acute pancreatitis secondary to hypertriglyceridaemia in 33% of cases, the second most common cause in this cohort [39]. The Endocrine Society differentiates primary (genetic) from secondary (e.g. metabolic syndrome, diabetes mellitus, alcohol or pregnancy) hypertriglyceridaemia as mild (150–500 mg/dL; 1.7–5.6 mmol/L), moderate (500–1000 mg/dL; 5.6–11.3 mmol/L) or severe (> 1000 mg/dL; > 11.3 mmol/L) dependent on serum triglyceride levels [40]; much is polygenic in nature [41]. There is an approximate 4% increase in the incidence of acute pancreatitis for every 100 mg/dL rise in serum triglyceride levels above 1000 mg/dL [42, 43], a level frequently used to define hypertriglyceridaemia as the cause of acute pancreatitis [39]. Nevertheless, as there is an increased but lower risk of acute pancreatitis at lower levels, also proportional to the increase in triglycerides, 500 mg/dL appears preferable for this definition [39, 44]. It is most important that levels are taken on admission to identify hypertriglyceridaemia [14], either as sole cause or a co-factor conferring a worse prognosis, since hypertriglyceridaemia-associated acute pancreatitis is severe more frequently than from other causes [39].
Drugs
Although a well-recognised category accounting for up to 5% of acute pancreatitis [45], possibly with higher frequencies as co-factors [46], there are relatively few studies of drug-associated acute pancreatitis. A large multi-national collaboration is underway to develop biomarkers that may help to address this deficit (www.transbioline.com). An important focus of this collaboration is to develop novel biomarkers for accurate identification and prognostication of pancreatic injury induced by drugs in early-phase trials, to determine whether to continue, modify or abandon drug development. Most published reviews to date focus on acute pancreatitis associated with drugs already licensed for other indications, the strongest evidence for which is recurrence of acute pancreatitis if the implicated drug is reintroduced, after withdrawal and recovery from a prior attack. Trivedi and Pitchumoni [47] described three categories that Badalov et al. refined into four categories [48]. Class I drugs are defined as those featured in at least one case report describing recurrence of acute pancreatitis following drug rechallenge, where class Ia drugs have had other causes excluded but class Ib have not. Exclusion of other causes is, however, not uniform as tests vary. Class II drugs have a consistent latency in 75% or more of reported cases, latency being short (> 24 h), intermediate (> 1 and < 30 days) or long (> 30 days), although this criterion may be inapplicable to immune-mediated drug-induced pancreatic injury. Class III drugs have two or more case reports without rechallenge or consistent latency. Class IV drugs are like class III but with only one published case report. Despite the first category depending on exclusion of other causes, there may be a continuum in which drugs contribute to acute pancreatitis with other causes, as can hypertriglyceridaemia [39]. Pharmaceuticals in class I include antiretrovirals (e.g. didanosine), chemotherapeutics (e.g. asparaginase, cytarabine), antibiotics (e.g. tetracyclines, cotrimoxazole), steroids (e.g. dexamethasone, prednisolone, estradiol), 5-aminosalicylates (e.g. sulphasalazine, mesalazine), antiepileptics (e.g. valproic acid, carbamazepine), antihypertensives (e.g. enalapril, lisinopril, losartan, furosemide), opiates (e.g. codeine), and thiopurines (e.g. azathioprine, mercaptopurine) [47]. Data on mechanisms of drug-induced pancreatic injury are sparse, although there is evidence to suggest intrinsic mechanisms in drug or metabolite dose-dependent toxicity from some drugs [49] and idiosyncratic mechanisms in the interplay of drugs or metabolites with host susceptibility from others [50], as in drug-induced liver injury [51]. Interestingly, although most drugs are reported to cause acute pancreatitis within 1 week of initiation, many agents will take weeks or months to trigger acute pancreatitis, or in the case of some oestrogens, years [48, 52]. A careful drug history is essential when assessing patients presenting with acute pancreatitis.
Endoscopic Retrograde Cholangiopancreatography (ERCP)
ERCP is associated with a significant risk of acute pancreatitis, assessed in one systematic review of > 100 randomised clinical trials at ~ 9% and up to 14% in high-risk patients [53], although there are effective strategies to reduce this risk [54]. Patients at greatest risk are young women with small or normal bile ducts and sphincter of Oddi dysfunction [55]. The presence of a patent accessory pancreatic duct may help to decompress the main pancreatic duct, thereby preventing acute pancreatitis [56]. Prolonged procedures or repeated attempts at bile duct cannulation or unintended pancreatic duct cannulation also increase the risk of post-ERCP pancreatitis.
Other Causes of Acute Pancreatitis
There are many rarer causes of acute pancreatitis [57] for clinicians to be aware of so patients can be best managed to address potential complications and/or prevent recurrence. These include trauma, hypercalcaemia, viral infections [e.g. mumps, cytomegalovirus, coxsackie B virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)], tumours, anatomical variants (pancreas divisum, pancreatobiliary ductal malunion), cardiac bypass surgery, scorpion bites (notably from Tityus trinitatis) and organophosphate poisoning. SARS-CoV-2 likely enters cells via the angiotensin converting enzyme II receptor, present on pancreatic acinar and especially islet cells, causing or contributing significantly to the severity of acute pancreatitis that may be complicated by diabetes mellitus and metabolic dysfunction [58–60].
Acute on Chronic Pancreatitis
Acute, recurrent acute and chronic pancreatitis represent a continuum of progressive injury, inflammation, fibrosis, scarring and loss of function from multiple aetiologies. Although sentinel (first) attacks of and recurrent acute pancreatitis do not occur in all patients who develop chronic pancreatitis, acute attacks may be superimposed on established chronic pancreatitis. A recent study from the Hungarian Pancreatitis Study Group found ~ 50% of patients who had suffered three or more episodes of acute pancreatitis had coincident chronic pancreatitis, confirming that recurrent acute pancreatitis helps identify early chronic pancreatitis [61]. Alcohol is the most common aetiology to account for these scenarios, and even moderate alcohol intake has been shown to accelerate the progression of such pancreatic injury. Smoking and hypertriglyceridaemia are other causes of this progression.
Genetic Factors
Hereditary pancreatitis is a rare familial form of chronic pancreatitis that usually presents in childhood or adolescence with recurrent attacks of acute pancreatitis. Autosomal dominant mutations of the PRSS1 cationic trypsinogen gene, notably R122H and N29I, account for many cases [62]. In some families with two or more affected first-degree or three or more second-degree relatives in two or more generations, no mutation can be identified. There are many mutations of pancreatic digestive enzymes (e.g. chymotrypsin C, CTRC; carboxypeptidase A1, CPA1, pancreatic lipase, PNLIP; carboxyl ester lipase, CEL) enzyme inhibitors (serine protease inhibitor Kazal type 1, SPINK1) and ion channels (cystic fibrosis transmembrane conductance regulator, CFTR; claudin 2, CLDN2) that induce cell stress, increase intracellular pancreatic enzyme activation, impair acinar or ductal secretion and/or amplify symptoms, conferring an increased risk of chronic pancreatitis [62]. Affected individuals may present with a sentinel attack and subsequent recurrent attacks of acute pancreatitis before chronic pancreatitis becomes evident on CT, MRI and/or endoscopic ultrasound (EUS), or in children by TUS. Such disease is usually described as associated with the mutation or idiopathic (not hereditary) when no direct precipitating cause (e.g. alcohol, hypertriglyceridaemia) can be identified. Further gene variants may contribute to acute pancreatitis via other causes, as in hypertriglyceridaemia from type I, 4 or 5 hyperlipidaemia from mutations of lipoprotein lipase, LPL, or other genes [63].
Autoimmune Pancreatitis
Autoimmune pancreatitis is a rare, distinct form of chronic pancreatitis that may present acutely. It was initially described as chronic pancreatitis with hypergammaglobulinaemia [64], prior to introduction of the term ‘autoimmune pancreatitis’ [65]. It was then variously described as chronic pancreatitis with autoimmune features, non-alcoholic duct-destructive chronic pancreatitis, lymphoplasmocytic sclerosing pancreatitis with cholangitis, chronic sclerosing pancreatitis, pseudotumorous pancreatitis and duct-narrowing chronic pancreatitis. The current definition of two types is based on histopathology: type 1 for lymphoplasmacytic sclerosing pancreatitis and type 2 for idiopathic duct centric chronic pancreatitis or autoimmune pancreatitis with granulocytic epithelial lesions. These lesions have also been reported in children with autoimmune sclerosing cholangitis but no pancreatic disease involvement [66, 67].
Type 1 autoimmune pancreatitis is a feature of IgG4-related systemic disease [68]; others include sclerosing cholangitis, sclerosing sialadenitis, retroperitoneal fibrosis, interstitial nephritis, chronic thyroiditis, interstitial pneumonia and lymphadenopathy. IgG4-related systemic disease is typified by tumour-like mass formation in affected organs that may feature high serum IgG4 concentrations or increased numbers of IgG4 plasma cells in tissues. Type 2 autoimmune pancreatitis is more often associated with inflammatory bowel disease, which confers a less favourable prognosis [69].
Disease Course
Pathophysiology
Understanding critical mechanisms in acute pancreatitis is essential to the development of specific, effective therapies to minimise pancreatic and systemic injury [7] (see Sect. 6, Clinical Trials). On a molecular level, the triggers of acute pancreatitis induce injury of pancreatic acinar and ductal cells by disrupting normal intracellular calcium signalling that maintains stimulus-secretion coupling [70] (Fig. 1). Acute biliary pancreatitis is caused by gallstone occlusion of the ampulla of Vater, resulting in elevated pressure and/or entry of bile into the pancreatic duct [19]. High pressures induce abnormal calcium entry into pancreatic acinar cells (normally maintaining nanomolar cytosolic calcium concentrations) via Piezo 1 and transient receptor potential vanilloid cation 4 (TRPV4) channels in the plasmalemma [71], while bile acids ligate G protein-coupled bile acid receptor 1 [72], inducing sustained intracellular release of calcium via endoplasmic reticulum inositol trisphosphate and ryanodine receptor (IP3R and RyR) calcium channels [73–75]. Intracellular calcium release from the endoplasmic reticulum calcium store lowers the calcium concentration within the endoplasmic reticulum, which prompts the endoplasmic reticulum to form puncta with the plasmalemma, comprised of stromal interaction molecule 1 (STIM1), ORAI and TRP canonical cation (TRPC) channels, to allow extracellular calcium to replenish the endoplasmic reticulum [70]. Although calcium entry is normally dampened by store-operated calcium entry-associated regulatory factor (SARAF), expression of SARAF becomes downregulated [76], as is micro-RNA 26a, which normally dampens expression of TRPC channels [77], removing controls on calcium entry. Oxidative stress results, opening non-selective TRP melastatin 2 (TRPM2) channels [78]. If intracellular calcium release from the endoplasmic reticulum continues unfettered, further calcium entry to replenish endoplasmic reticulum stores also continues, resulting in cytosolic calcium overload that swamps mitochondria [79]. There, calcium overload induces the mitochondrial permeability transition pore, causing a loss of the mitochondrial membrane potential that drives production of adenosine triphosphate (ATP), the universal energy currency within cells [73]. These effects may be partially offset by insulin, which preserves glycolytic ATP supply [80], although glycolysis is less efficient than oxidative phosphorylation. Normally the sarco-endoplasmic reticulum and plasma membrane calcium ATPase (SERCA and PMCA) pumps clear cytosolic calcium to restore resting cytosolic levels, but with less ATP these pumps fail, exacerbating calcium toxicity [7]. Similar second messenger release mechanisms are induced in alcohol-associated acute pancreatitis by fatty acid ethyl esters (FAEEs) [81], and by fatty acids released from FAEEs or lipolysis of triglycerides in hypertriglyceridaemia [82], to inhibit mitochondria and aerobic ATP production. In general, there is a direct correlation between the severity of toxin exposure and that of acute pancreatitis, as in the case of serum triglyceride levels and the severity of acute pancreatitis [39, 43, 83].
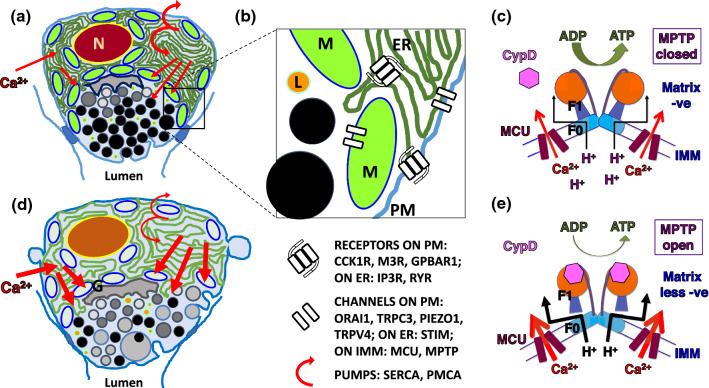
Fig. 1.
Pathophysiology of acute pancreatitis illustrating effects of calcium overload within acinar cells and the consequent failure of adenosine triphosphate (ATP) generation from adenosine diphosphate (ADP); Ca2+ calcium, CypD cyclophilin D, ER endoplasmic reticulum, G Golgi, IMM inner mitochondrial membrane, L lysosome, MCU mitochondrial calcium uniporter, MPTP mitochondrial permeability transition pore, M mitochondrion, N nucleus, PM plasma membrane, Z zymogen granule. a Cartoon of normal pancreatic acinar cell showing typical apical granular pole facing lumen, with peri-granular, peri-nuclear and sub-plasmalemmal mitochondria; red arrows indicate calcium flux into the cell, which triggers apical stimulus-secretion and stimulus-metabolism coupling; the semi-circular arrows represent sarco-endoplasmic reticulum calcium ATP-ase (SERCA) and plasmalemmal ATP-ase (PMCA), pumps that restore low resting calcium levels within the cell. b Higher power cartoon of small part of an acinar cell representing PM receptors for the two secretagogues cholecystokin (cholecystokin 1 receptor, CCK1R) and acetylcholine (muscarinic type 3 receptor, M3R), and for bile acid (G-protein bile acid receptor 1, GPBAR1). ER receptors are those for second messengers, released after CCK1R and M3R ligation, which are the inositol trisphosphate receptor (IP3R) and ryanodine receptor (RYR); after their ligation, IP3R and RYR release calcium from the ER. Also represented are the PM calcium channels Orai1, transient receptor potential cation channel 3 (TRPC3), Piezo1, and transient receptor potential vanilloid subtype 4 (TRPV4); the former two coordinate normal calcium entry through stromal interaction molecules 1 and 2 (STIM), calcium channels on the ER. Calcium uptake into the mitochondria is mediated by the MCU on the IMM for stimulus-metabolism coupling, and while the sodium calcium exchanger removes this calcium, the MPTP in low conductance mode may assist mitochondrial calcium removal. c Normal mitochondrial generation of ATP is undertaken by the F0F1ATP synthase using the H+ electrochemical gradient produced by the electron transport chain in response to calcium entry through the MCU into the electronegative mitochondrial matrix; cyclophilin D in the matrix is not bound to the F0F1ATP synthase and the MPTP is closed. d Calcium overload within acinar cells is caused by excessive intracellular release of calcium from the ER induced by pancreatitis toxins, for example bile acids, fatty acid ethyl esters or hyperstimution, provoking excessive calcium entry in response to low ER concentrations, or is caused by excessive calcium entry induced by pressure activation of Piezo1 and TRPV4. Thick red arrows indicate excessive calcium flux disrupting organellar and regulatory functions, resulting in impaired, basolateral secretion; SERCA and PMCA function are diminished, exacerbating calcium overload. e Impaired mitochondrial generation of ATP is a result of mitochondrial calcium overload, which induces binding of the MPTP regulator cyclophilin D and disruption of the F0F1ATP synthase forming the MPTP, which in high conductance mode allows particles up to 1,500 daltons into the matrix, with collapse of the H+ electrochemical gradient that normally drives ATP production. Derived from [7, 70–75, 79, 81, 96]
Diminished ATP production results in defective autophagy, co-localisation of zymogen granules and endolysosomes, zymogen activation, cytoskeletal disruption, diminished zymogen secretion, inflammasome activation, cytokine release and cellular necrosis [7, 84]. Intracellular calcium overload also activates the calcineurin-nuclear factor of activated T cells (NFAT) pathway [85], nuclear factor kappa B (NF-κB) and cytokine production [86, 87], and the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome [86, 88, 89], driving pancreatic and systemic inflammation. Other cells within the pancreas contribute to this, notably ductal cells [90], stellate cells [70, 91], macrophages [92], and neutrophils [93]. Damage-associated molecular patterns, for example ATP, nucleic acids and cytokines released from necrotic and stressed cells further drive innate immune activation [94].
Multiple cytokines mediate a powerful pro-inflammatory immune response, notably tumour necrosis factor-α and interleukins 1α, 1β, 6 and 18, exacerbating the initial pancreatic injury [95, 96], and extending inflammatory cascades via the lymphatic and systemic circulations into the liver, lungs, heart, kidneys and gastrointestinal tract. This causes the systemic inflammatory response syndrome, an early clinical feature that persists in moderately severe and severe acute pancreatitis. Most extremely, a cytokine storm syndrome results through positive feedback loops between cytokine release and programmed immune cell death (pyroptosis, apoptosis and necrosis), exacerbating multi-organ dysfunction [7, 94]. Compensatory anti-inflammatory responses occur, exemplified by increases in regulatory T cells within lymphoid tissue [89], although this may be as much a feature of dysregulation as restoration of balance; with superimposed infection, a persistent inflammation, immunosuppression and catabolism syndrome may ensue [97]. Inflammation of, and damage to, the gastrointestinal tract results in bacterial translocation [98], endotoxaemia and portal bacteraemia, infecting pancreatic necrosis and exacerbating systemic inflammation, all of which may result in multi-organ failure and death [99]. The species of bacteria present in the gastrointestinal tract of patients with acute pancreatitis is predictive of disease severity, with the facultative anaerobic Enterococcidae most frequently associated with severe disease [100]. Obesity adds further deleterious effects through adipocyte lipolysis in the pancreas and adipose tissue, leading to an increase in local and systemic concentrations of triglycerides that are hydrolysed by pancreatic [101, 102] and adipocyte triglyceride lipase [103]. These and other critical pathways reviewed elsewhere [7] provide multiple targets for drug development.
Severity
Predicted Severity
Prediction of severity is made as early as possible after admission to determine which patients are likely or not to develop local and/or systemic complications, and who may benefit from early, more intensive management. It is distinct from actual severity, determined once sufficient time has elapsed to ensure accurate grading, which may take a number of days or occasionally weeks. Many scoring systems using clinical and laboratory measures with or without imaging features have been developed for severity prediction, several designed to differentiate mild from severe acute pancreatitis, as in the Original Atlanta Classification [104, 105]. More recent studies have grouped the moderately severe category (RAC) [13] with the mild or severe category for binary prediction of severity. Of those clinical scores that can be assessed on admission, accuracy is at best about 70% in differentiating mild from more severe acute pancreatitis; accuracy can increase to 80% on the second day of admission [106]. Delayed prognostication, although not optimal, remains helpful because this may be when patients reach specialist service provision in some geographical areas [16]. Machine-learning approaches have been applied to increase the accuracy of prediction [107], although extensive evaluation of these and other approaches, for example omics technologies, is required [108, 109]. The simpler the method, the more applicable it becomes; point-of-care technology is required for omics applications [110, 111]. The Hungarian Pancreatic Study Group used machine learning to develop EASY-APP to predict severe disease (RAC), which during validation on > 3000 patients had an accuracy of 89%, now available on the web and built to improve with use [112]. Of readily available single markers, C-reactive protein ≥ 150 mg/L is useful on the second day of admission [106], indicative of more severe systemic inflammation. Alongside other indicators, this may be considered sufficient evidence to undertake CECT to detect local complications, at a suitable interval after disease onset (see Sect. 4.3). The pancreatitis activity scoring system (PASS) can have a similar function to monitor patient progress [113, 114], which may be more accurate without inclusion of pain medication [115]. Respiratory, cardiac and renal function assessment should be undertaken frequently to detect organ dysfunction and institute prompt organ support when required.
Actual Severity
The most widely accepted classification of severity is the Revised Atlanta Classification (RAC), derived from expert opinion [13] that identifies: (1) mild acute pancreatitis with no local complication or organ failure; (2) moderately severe acute pancreatitis with transient organ failure (< 48 h) and/or local complications and/or exacerbation of comorbidity; and (3) severe acute pancreatitis with persistent organ failure (≥ 48 h), most often respiratory, with or without local complication(s) (Fig. 2). Around 65–70% of patients with acute pancreatitis follow an uncomplicated course [13, 99], with resolution of symptoms within several days. After discharge patients typically require 3–6 weeks off work, plus further time dependent on cause and requisite management. Some 20–25% of patients develop moderately severe acute pancreatitis that may feature local pancreatic injury with acute fluid collections and/or necrosis that may become infected. Such patients experience more prolonged pain, nutritional deficit, and hospital stays over 2 weeks. After discharge, these patients will typically require 6–12 weeks or more off work. Around 10% of patients develop severe acute pancreatitis, likely to be accompanied by more prolonged, severe pain, nutritional deficit and hospital stays over 4 weeks. A significant component is systemic cytokine activation associated with acute respiratory and/or cardiac and/or renal failure, coagulopathy and sepsis. Critical and/or high-dependency care is required, often with intervention for infected pancreatic necrosis. Death is likely in up to half of this group [116], resulting in an overall likelihood of death in all cases of 1–5%, depending on demographics and service provision [13, 14]. After discharge, surviving patients will typically have to take 12 or more weeks off or may never return to work.
Fig. 2.
Contrasts of severity in uncomplicated acute pancreatitis (‘mild’ in the Revised Atlanta Classification [13]) versus severe acute pancreatitis that features persistent failure of respiratory, cardiac or renal function and, typically, pancreatic necrosis; SIRS = systemic inflammatory response syndrome; MODS = multi-organ dysfunction syndrome. Upper panel shows a cartoon of acute pancreatitis with inflammatory swelling and circulating inflammation represented by neutrophils, but no local complications and no significant impact on respiratory, cardiac or renal function. Lower panel shows cartoon of acute pancreatitis with marked glandular change and necrosis in the pancreatic body, also found in some patients with moderately severe acute pancreatitis (‘moderate’ in the Revised Atlanta Classfication); in severe acute pancreatitis extensive circulatory inflammation with profuse neutrophilia extends the disease impact systemically, with exacerbation through vicious circles of damage [7], inducing dysfunction and failure of lungs, heart and/or kidneys that persists > 48 h (‘severe’ in the Revised Atlanta Classification)
The Determinants-based Classification (DBC) [99] is an alternative scheme derived from published evidence [117] with four categories: mild—no necrosis or organ failure; moderate—sterile necrosis or transient (< 48 h) organ failure; severe—infected necrosis or persistent (≥ 48 h) organ failure; critical—infected necrosis and persistent organ failure. Newer evidence indicates that infected pancreatic necrosis now has a lesser impact on mortality [118]. A modified scheme was developed [119] and validated [120] by the Epidemiología de la Pancreatitis Aguda en Medicina Intensiva group, separating transient from persistent organ failure, each further separated by the presence or absence of infected necrosis (sterile necrosis was not included). These categories, almost a hybrid between RAC and DBC, correlated more closely with mortality and morbidity than RAC or DBC in intensive care settings. Another validation study divided the severe DBC category into two, keeping the other DBC categories intact (sterile necrosis included) [121]; while this provides greater insight into the course of acute pancreatitis, optimal categorisation remains elusive. It is important to note, however, that whatever classification is used, actual severity can only be assessed once sufficient time has elapsed for this.
Acute Pancreatitis in Children
Much of the natural history and management of paediatric acute pancreatitis aligns with that in adults, and while there is a lower annual incidence at 10–15 per 100,000, this is also rising. A lower incidence was recorded by the British Paediatric Surveillance Unit [21], likely the result of incomplete case ascertainment. The diagnostic criteria are the same, for example as defined by the INSPPIRE paediatric working group [122, 123], excepting that transabdominal ultrasound is the preferred imaging technique; in infants and toddlers, irritability, vomiting and/or abdominal distension may also suggest acute pancreatitis [124]. Further investigations parallel those in adults, with CECT to be conducted 5–7 days after disease onset to identify pancreatic and/or peri-pancreatic necrosis. An MRI pancreas with MRCP may delineate pancreatic duct abnormalities while minimising risks of radiation exposure. Some attempts have been made to formulate severity criteria in children including age, white cell count, serum albumin, calcium, urea, lactate dehydrogenase and fluid collections, but with no consensus [125]. One of the major limitations resulting from the lower incidence of acute pancreatitis in children is the concomitantly lower incidence of severe acute pancreatitis, so collaboration amongst specialised centres looking after paediatric pancreatitis cases is recommended.
The distribution of aetiologies is different from adults, with lifestyle being less prominent. Many medications are associated with pancreatitis in children, the most reported being asparaginase, azathioprine, 6-mercaptopurine, sodium valproate/valproic acid, tetracyclines, aminosalicylic acid, steroids, sulfasalazine and non-steroidal anti-inflammatory drugs (NSAIDs) [126]. Other causes of pancreatitis are cholelithiasis, anatomical anomalies (pancreas divisum, long common channel, duodenal diverticulum, biliary obstruction), alcohol, metabolic conditions (hypertriglyceridaemia, hypercalcaemia, methylmalonic acidaemia), trauma (accidental, child abuse or ERCP induced), infection, hereditary causes (PRSS1, SPINK1, CFTR, CPA1, see Sect. 2.3.1), autoimmune, and idiopathic [127].
Acute Pancreatitis in Pregnancy
The incidence in pregnancy is between 1 in 500 to 1 in 5000 pregnancies [128–131], higher than in the general population. Hormonal changes alter bile flow and composition, generating a pro-lithogenic state, resulting in a higher proportion of cases attributable to gallstones than in the general population, with alcohol and hypertriglyceridaemia as other principal causes [132]. Hypertriglyceridaemia accounts for up to a third of all cases in pregnancy in some Chinese series [133, 134], likely in part because oestrogen alters lipid metabolism; preventative measures are desirable in those identified with high triglyceride levels [135]. Hypertriglyceridaemia-associated acute pancreatitis has been linked to oestrogen therapy for fertility treatment [136] and the oral contraceptive pill [137]; one cohort study of 31,494 women found a relative risk of 1.57 for acute pancreatitis in those receiving hormone replacement therapy [138]. As in non-pregnant patients, hypertriglyceridaemia-associated acute pancreatitis tends to be more severe than from other aetiologies, and in the context of pregnancy leads to greater maternal and fetal mortality [133]. Although the role and indications for caesarean section and termination of pregnancy are undefined, preliminary evidence suggests early intervention is preferable [133].
Acute Pancreatitis in the Elderly
The incidence of gallstones increases with age [139], with biliary pancreatitis the most common aetiology in elderly patients [140]; overall, acute pancreatitis increases in incidence with age [1]. Cholecystectomy is the management of choice to prevent recurrence, except in the frail for whom endoscopic sphincterotomy is an alternative [141]. Frailty and comorbidity increase the likelihood of less favourable outcomes from acute pancreatitis [142]. The rate of idiopathic acute pancreatitis was reported to be as high as 30–40% in elderly patients in the late twentieth century [143, 144]. This high rate of idiopathic pancreatitis remains in more recent series, despite the availability of modern imaging and advanced endoscopy [145]. Polypharmacy may account for some of these cases, as drug-induced pancreatitis often goes unrecognised. Importantly, there is a significant risk of malignancy in this age group. Pancreatitis secondary to obstruction of the pancreatic duct from both benign and malignant tumours is more likely to be mild and recurrent [146, 147], as ductal obstruction is usually partial. The treatment of choice is surgical resection or palliative stenting. Autoimmune pancreatitis is also more common in the elderly, with a male preponderance [148, 149], so measuring serum IgG4 should be considered. Significantly, up to 50% of patients with autoimmune pancreatitis are diagnosed with a distant malignancy (often gastric, lung or prostate carcinoma) at the time or within 1 year of their admission with pancreatitis, leading to the suggestion that autoimmune pancreatitis can represent a paraneoplastic syndrome [150]. Malignancies including myeloma [151], parathyroid cancer [152, 153], leukaemia [154] or small-cell lung cancer [155] can cause acute pancreatitis via hypercalcaemia.
Inpatient Management
Investigations on Admission
As for any sick patient, vital signs and oxygen saturation must be assessed; arterial blood gases can be measured, but oxygen saturation is simpler and quicker in the emergency room; SARS-CoV-2 testing is done. Prior to diagnosis, oxygen, intravenous fluid resuscitation and pain relief are usually indicated; importantly, analgesia does not impair the accurate diagnosis of abdominal pain [156]. Initial investigations for suspected acute pancreatitis include serum amylase and/or lipase, triglycerides and lipid panel, full blood count, renal and liver function tests, glucose, HbA1c, calcium and TUS (Fig. 3) [157]. Chest x-ray or ultrasound should be done to identify pleural effusion, an indicator of more severe disease; this is also important in the assessment of patients with an acute abdomen. Both amylase and lipase lack specificity as either can be elevated by other acute conditions including peptic ulceration, cholecystitis, mesenteric ischaemia and macroamylasemia. If the diagnosis remains uncertain, whether amylase and/or lipase are elevated or not, MRI or CT are indicated to identify features of acute pancreatitis (swelling of the pancreas, inflammatory fat stranding, peri-pancreatic fluid collections) or any other diagnosis. Further tests are dependent on the patient’s presentation, for example, electrocardiography, other blood tests, and/or other imaging. Most patients with gallstone-induced acute pancreatitis pass their stones into the duodenum early during their disease; transient elevation of alanine or aspartate amino transferase is typical. If the patient’s condition includes pyrexia, with or without rigors, jaundice and bile duct dilatation on TUS indicating cholangitis, ERC (without pancreatic duct cannulation), sphincterotomy and stone extraction are appropriate; immediately prior to ERCP, EUS is advised to determine whether such treatment is necessary [158]. Without cholangitis, a conservative strategy is preferable [159].
Fig. 3.
Rationale and plan of investigation for patients with acute pancreatitis to guide treatment. a Determining aetiology is a priority in initial investigations [e.g., serum triglyceride measurement, planning early laparoscopic cholecystectomy, contrast-enhanced CT to differentiate perforation from acute pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP)]. There are many tools to assess if acute pancreatitis is severe, more likely in the very young, very old or those with co-morbidities; identification of complications is central to planning management and assessing when and how to prevent recurrence. b Early investigations should ensure differentiation of acute pancreatitis from other emergencies, confirmed by two of characteristic abdominal pain, amylase or lipase ≥ 3 × upper normal limit, and characteristic 3D imaging that may be done if there is diagnostic uncertainty. The listed assessments are important in establishing aetiology and severity. c Further tests for aetiology, severity and the identification and/or assessment of progression of complications. The extent of testing is dependent on whether an aetiology is evident from b, and disease severity, as more severe disease requires more extensive investigation. Specific investigations may include those to identify or exclude further complications, for example, pulmonary or mesenteric angiography, further contrast-enhanced CT or MRI or other tests after discharge from hospital during early or long-term follow-up
Initial Therapy
Oxygen, intravenous fluid resuscitation, analgesia and nutrition are fundamental [160] (Fig. 4). A nil-by-mouth regimen to rest the gut, routine use of prophylactic antibiotics, and avoidance of early opiate analgesia have been invalidated in randomised trials, and do not feature in international guidelines [14, 161–165]. Antiviral therapy may be appropriate for example for SARS-CoV-2 infection [166], and antivenom for those stung by Tityus trinitatis or other scorpions in endemic areas [167]. Critical care, for respiratory and/or other organ support, may be required from the outset in severe cases.
Fig. 4.
Generalised treatment strategy for acute pancreatitis at the admitting hospital, after referral to specialist, tertiary services for complex pancreatic disease, and subsequently to prevent recurrence as well as address the aftermath of the disease. a Initial treatment at the admitting hospital, scaled to the severity of disease. A low index of suspicion is recommended for 3D abdominal imaging, otherwise complications are likely to be missed and patients readmitted in a more compromised state, with delay in referral for specialist opinion and/or transfer. Specific treatments include insulin and/or plasmapheresis for hypertriglyceridaemia and antivenom for scorpion or snake bites in endemic areas throughout the world. b There are many options required for the specialist management of complex acute pancreatitis, many of which are itemised. Necrosectomy is better delayed for some 4 weeks but may have to be brought forward if there is uncontrollable sepsis or other organ injury. Embolisation is required for pseudoaneurysms; contrarily, anticoagulation is indicated for recent thrombosis at or near the portal venous confluence. Pancreatic ductal stenting together with glyceryl trinitrate (GTN, to relax the smooth muscle of the sphincter of Oddi) and octreotide (to reduce secretion) may assist healing of pancreatic ductal rupture. c Measures to prevent recurrent acute pancreatitis are displayed as these save lives, reduce morbidity, reduce healthcare costs, and halt or slow progression of disease. Local complications of complex acute pancreatitis include recurrent pseudocyst formation, recollection of abscesses, ductal strictures, progression to chronic pancreatitis—all of which can be causes of recurrent pain and/or sepsis, and for which patients should be kept under review. Appropriately thorough investigation of acute pancreatitis may have identified benign or malignant neoplasms, for which surgical resection +/– chemotherapy may be most appropriate
Oxygen
For most patients an oxygen saturation (SpO2) of 94–98% is an appropriate target range to prescribe, to be given by trained staff, with regular recording of inspired oxygen, delivery system, flow rate and saturation, linked to a track-and-trigger early warning system [168]. Lower levels (88–92%) are appropriate to those at risk of hypercapnic respiratory failure, as from chronic obstructive pulmonary disease or morbid obesity. Nasal cannulae or simple face masks deliver lower while Venturi and reservoir masks deliver higher concentrations of oxygen; if initial saturation is < 85%, 15 L/min via a reservoir mask should be started and reduced if the patient stabilises [168]. Oxygen delivery should not be stopped for oximetry on room air; arterial blood gas measurement should be undertaken if there is deterioration, and if occurring with higher delivery, critical care advice should be sought.
Intravenous Fluid Resuscitation
Immediate administration of intravenous fluids is pivotal in acute pancreatitis, as this corrects third-space volume loss and tissue hypoperfusion, counteracting pancreatic and systemic microcirculatory impairment consequent on many inflammatory cascades [169]. Early intravenous fluid resuscitation within 24 h of disease onset results in lower rates of persistent systemic inflammatory response syndrome and organ failure, recommended to be given at 5–10 mL/kg/h [14]. Aims of fluid resuscitation are to decrease and/or maintain heart rate to < 120/min and urine output measured via catheter at > 0.5 mL/kg/h, and if non-invasive continuous arterial pressure measurement is available, maintain mean arterial pressure of 65–85 mm Hg, with a haematocrit at 35–44%. In critically ill patients invasive monitoring may include determination of stroke volume variation and intrathoracic blood volume. In mild and moderately severe cases, organ dysfunction is likely to resolve with intravenous fluid resuscitation; in severe disease, there is a significant risk of overly aggressive intravenous fluid therapy. Fluid overload in the critical care setting is associated with an increased risk of death, as there are negative effects on all major organ systems [170]. Assessment of responsiveness is necessary to continue higher rates when required, but not when there is a risk of overload, and caution is necessary as clinical assessment has its limitations; passive leg raising followed by measurement of cardiac parameters may be an effective method for assessment [171], but this requires confirmation. Recent trials have tested strategies to prevent progression to moderate or severe disease. A trial including 40 patients suggested lactated Ringer’s solution is preferable to saline in the initial resuscitation phase of pancreatitis [172]. The findings of this small trial have been upheld in systematic reviews [173], but larger trials are required and ongoing; at present Ringer’s lactate is the preferred solution for fluid resuscitation in acute pancreatitis. Further research is necessary to improve strategies for each phase of fluid therapy, i.e., resuscitation, optimisation, stabilisation and evacuation (ROSE) [170].
Pain Management
Abdominal pain can be profound in acute pancreatitis, and for most patients, strong opioid analgesia is appropriate, reducing the need for supplementary analgesia over other regimens; analgesia ladders can be used for those with less severe pain, mindful that early and effective relief is a priority [174]. NSAIDs are an opiate-sparing alternative for uncomplicated disease [175], but run the risk of renal injury in more severe disease. Experimental evidence indicates opiates increase sphincter of Oddi phasic contractions that may increase pressure within the bile duct [176], but randomised trials have demonstrated that opiates are as, if not more, effective than alternatives, and as safe in acute pancreatitis [177]. Early oral nutrition that does not exacerbate abdominal pain, as in less severe disease, may reduce pain intensity and duration, analgesic use, and the risk of oral food intolerance [178]; in more severe disease, recurrence or exacerbation of pain with eating may delay resumption of solid food intake. The intensity and duration of pain is broadly proportional to disease severity and total opiate administration [179]. Continuous intravenous opiate infusions may be used for persisting, severe pain; the relative merits of patient compared to nurse control are unclear, although patient control is more effective after surgery [180]; input from a local pain team is desirable. Epidural anaesthesia has not been found in small trials to present significant advantage over alternatives [174], but it is hoped that larger trials will assess whether more is gained than analgesia, for example from increased pancreatic perfusion [181]. The management of pain and other physical and psychological issues are interdependent, exacerbated by anxiety; patients require comprehensive, understandable information about their illness, counselling about their progress, confidence in the staff, prompt attention to their needs, and timely interventions to address complications [165]. Analgesia should not be used as an alternative for adequate discussion with patients about their illness and expectations, feedback, and judicious guidance about opioid requirements to avoid long-term dependency. Psychological support should be offered to build a relationship with patients and help with anxiety, especially for adolescents and young adults. Most patients who have had acute pancreatitis should not leave hospital continuing on strong opiates.
Nutrition
Acute pancreatitis induces a hypermetabolic state, with lipolysis, protein catabolism, insulin resistance and loss of body mass, all of which are far more marked in severe disease, and exacerbated by inadequate nutrition and infection [182]. Early oral refeeding should be encouraged as soon as tolerated, and if not, liquid food supplements or enteral tube feeding given within a day or two of admission, not likely necessary for mild acute pancreatitis [183]. The nasogastric rather than nasojejunal route is easier, but some patients may require the latter when intolerant of the former, as from delayed gastric emptying. Oral or enteral feeding is associated with lower pro-inflammatory responses, and reduces the likelihood of bacterial translocation across the gastrointestinal permeability barrier, compared to parenteral nutrition, with its attendant risks of catheter placement and infection [184]. Enteral tube feeding, however, is limited in patients who are haemodynamically unstable, display gastrointestinal intolerance, or have frequent interruptions required for investigations or interventions; delays then develop in the provision of nutritional support. Attempts to maximise enteral nutrition should be avoided in patients who are not volume replete, because of the risk of inducing gut injury through non-occlusive mesenteric ischaemia [185, 186]. Inadequate nutrition has prompted the use of combined enteral and parenteral nutrition, the latter begun before, during or after enteral intake is considered insufficient. Current trials and meta-analyses do not, however, provide definitive evidence of superiority for this combined approach [187].
The treatment of hypertriglyceridaemia-associated acute pancreatitis aims to reduce serum triglyceride levels, while supporting the patient in the same way as for other causes of acute pancreatitis [188]. Unlike for other aetiologies, however, this may include gut rest with no oral intake, to expedite clearance of circulating triglycerides. The metabolic/endocrine team should be involved, the choices being intravenous insulin alongside fluid resuscitation or plasmapheresis for more severe or obstinate HTG [189], monitoring high triglyceride levels; optimal therapeutic strategies are currently under investigation in randomised trials [82, 190]. Resumption of oral/enteral intake should include fibrates, and if not possible, parenteral nutrition with minimal lipid content [191]. Subsequently, maintenance therapies can be instituted (see section 4.4.6, Prevention of Recurrence).
Subsequent Investigations
Further investigations are required to confirm the need to treat complications—notably infection, necrosis, pancreatic endocrine and exocrine insufficiency, and to prevent recurrence. C-reactive protein is a useful biomarker to gauge the level of systemic inflammatory response syndrome that may be accompanied by organ dysfunction, but elevation may take at least 48 h, so measurement is best delayed until the day after admission. C-reactive protein can also be used to monitor progress, with further elevation paralleling pancreatic and/or peri-pancreatic necrosis, infection and/or organ failure; interleukin-6 [106], procalcitonin and lactate dehydrogenase are alternatives [192]. Elevation of C-reactive protein to ≥ 150 mg/L, persistently elevated white cell count, organ dysfunction and/or other clinical deterioration are indications for an abdominal contrast enhanced CT (CECT). Unless for initial diagnosis, this should not normally be done until a week has elapsed from the onset of acute pancreatitis to plan management, as necrosis may take several days to become detectable; by then, CECT indicates severity [193], likely already apparent. Radiological classification of acute pancreatitis is primarily either acute interstitial oedematous pancreatitis with or without heterogeneous pancreatic enhancement and peripancreatic fatty changes, or acute necrotising pancreatitis. Clinical suspicion of local complications may warrant earlier imaging, although subsequently delay may be required for anticipated intervention. Acute necrotic collections (< 4 weeks from presentation) and walled-off necrosis are differentiated from pancreatic pseudocysts that appear > 4 weeks from presentation, with a clear wall containing no solid material. Evaluation of findings on CECT has been formalised in the Balthazar CT severity score [194] and its modifications [195], which assess the pancreas, fluid collections, pleural effusion, necrosis and vascular complications. CECT is also useful to evaluate progress following interventions such as percutaneous drainage or necrosectomy.
Further tests follow clinical indications, for example to identify or exclude specific complications such as pulmonary angiography for pulmonary embolus, stool culture and sensitivity for Clostridium difficile or other infective diarrhoea, specific tests to identify underlying mechanisms of hypertriglyceridaemia, haemolysis, cholestasis or hypercalcaemia, and endoscopic or percutaneous biopsies to identify tumours.
If no aetiology is established, imaging with MRCP and MRI may identify anatomical abnormalities, early chronic pancreatitis, or tumours, while EUS is an alternative that may also identify microlithiasis. Genetic screening for PRSS1, SPINK1 and CFTR is appropriate for a second or subsequent attack when there is no other obvious cause, but current health service provision does not cover analysis of all known genetic variants associated with pancreatitis. Faecal elastase may be used to assess exocrine insufficiency [196], but this test is not accurate, and can be misleading. In patients with recurrent acute pancreatitis, fat-soluble vitamin (A, D, E, K) levels may provide evidence of malabsorption and the requirement for replacement therapy of both vitamins and pancreatic enzymes. If autoimmune pancreatitis is suspected from imaging demonstrating a halo around the pancreas, generalised pancreatic enlargement and/or irregular pancreatic or intrahepatic bile ducts, then raised levels of γ-globulin, IgG or particularly IgG4 subclass may be supportive of this diagnosis, as may the presence of non-specific autoantibodies (smooth muscle, antinuclear, anti-lactoferrin, anti-carbonic anhydrase). A pancreatic biopsy via endoscopic ultrasound or laparoscopy may be considered, with detection of IgG4-positive plasma cell infiltration strongly indicative of autoimmune pancreatitis; findings may include interlobular fibrosis, acinar atrophy, tissue infiltration, and obliterative phlebitis.
Therapy for Complications
Critical Care
This is a demanding aspect of the treatment of patients with severe acute pancreatitis, whose lives are particularly in danger. Organ failure featuring respiratory, cardiac and/or renal dysfunction that is persistent (lasting 48 h or more) requires substantial respiratory, cardiac and/or renal support [13, 14, 116]. Endotracheal intubation with mechanical ventilation is most likely to be required; hypotension despite resuscitation necessitates inotropes; and for acute kidney injury with persistent oliguria or anuria, renal replacement therapy using haemofiltration and/or haemodialysis is the mainstay. Complications of these invasive treatments include ventilator-induced lung injury, inotrope-induced ischaemic injury and haemodynamic instability from renal replacement therapy; care in the use of sedation, early weaning and conservative fluid regimens may reduce such complications [197]. Correction of haemodynamic instability, avoidance of NSAIDs and nephrotoxic antibiotics, and careful management of fluid and electrolyte balance remain necessary. Sedation, with analgesia and nutritional support as previously described, are given with close monitoring of essential organ function, early identification of intra-abdominal complications by CECT, and appropriate management of infection and necrosis as described below. Often the critical illness of severe acute pancreatitis features persistent organ failure together with intra-abdominal necrosis and infection. There is continued endeavour to define this group of patients from among all those with critical illness, for the development of effective, targeted therapies [198] (see Sect. 6).
Treatment of Infection
Randomised clinical trials have not shown sufficient advantage for prophylactic antibiotics during hospital admission for acute pancreatitis [14], yet recent surveys in 22 countries indicate global overuse [199]. Patients who develop moderately severe and severe acute pancreatitis, however, are at increased risk of infective complications, in association with either persistent organ failure or local complications. As discussed above (Sect. 3.1), bacterial translocation across the injured gut and a defective gastrointestinal permeability barrier is a consequence of the systemic inflammatory response. Despite enteral nutrition, bacterial translocation may still occur, an important although not the only route of infective complications, notably infecting necrosis; early infection is associated with increased mortality [200]. Prompt recognition, for example gas bubbles in necrosis on CECT, source control that may necessitate percutaneous drainage, appropriate antibiotics, physiological stabilisation, and optimal further interventional approaches are fundamental [201]. Nevertheless, during the first 4 weeks of acute pancreatitis inflammation, fluid collections and necrosis are diffuse, such that major interventions are less likely to be effective and can increase patient instability. The mainstay of treatment may need to be antibiotics appropriate to Gram-negative intra-abdominal infection, for example piperacillin-tazobactam or tigecycline or a third-generation cephalosporin with metronidazole [201]; the choice depends in part on the availability of samples for culture and patient response, best determined in consultation with clinical microbiologists. In the most severe disease, fungal infection is characteristic, may be brought on by extensive antibacterial treatments, and requires early and prolonged treatment with antifungals [202].
Management of Necrosis
Timely identification of local complications is important to determine continued need for hospitalisation with a view to localised intervention; all clinical, laboratory and imaging data available should be used to assess this need. If local complications are missed and patients discharged, they may require readmission in a worse condition, which is physically and psychologically deleterious. Should severe pain and/or inability to eat solid food continue for 3 or more days, or laboratory measures, for example C-reactive protein ≥ 150 mg/L or any scoring system, be indicative of more than mild acute pancreatitis, continued effective analgesia and/or nutritional support are likely to be needed, and CECT should be undertaken some 7 days after disease onset. Local complications found on CECT (Fig. 5) require review by a specialist tertiary centre to guide further management, assessing the need to transfer patients for specialist intervention [203]. Management of local complications, such as pancreatic necrosis or fluid collections, or later pseudocysts, should be undertaken in a specialist centre and follow a selective step-up approach, with drainage of necrosis or fluid achieved endoscopically or percutaneously [204–206]. These approaches should be considered before attempting minimally invasive or more hazardous open surgery, exceptionally mandated by necrosis of adjacent organs. As discussed, local complications are almost always diffuse in the early days of acute pancreatitis, so intervention is better delayed until these are walled off and more suitable for drainage, preferably over 4 weeks, with appropriate patient counselling. The presence of infection, as may be evident from the presence of gas on CECT, and critical illness are relative indications to expedite drainage and/or necrosectomy, but randomised trial evidence favours delayed intervention [207]. While endoscopic necrosectomy often requires several repeated procedures, it has the advantage of internal drainage without external irrigation, allowing patients to be discharged at an earlier date for repeat outpatient procedures, reducing health service usage and costs [208–210]. In contrast, minimally invasive or open necrosectomy requires continued external irrigation to flush away build-up of septic material, prolonging hospital stays, and imposing additional burdens on patients and hospital staff.
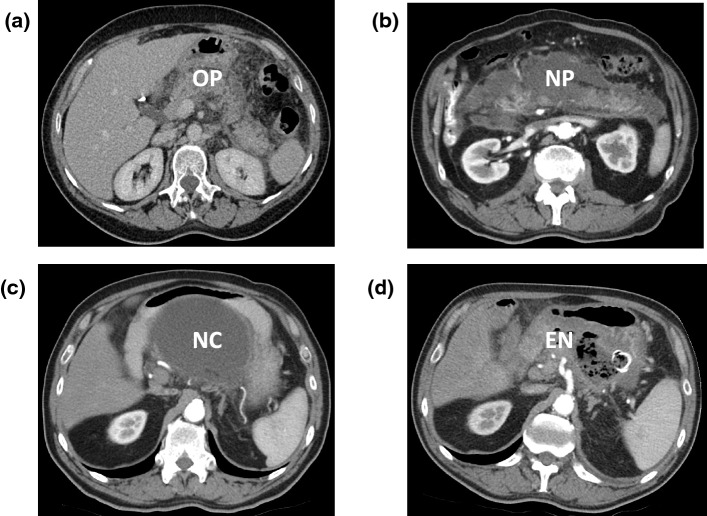
Fig. 5.
Contrast-enhanced computerised tomography scans of patients with acute pancreatitis, with application of specialist interventions for pancreatic necrosis. a Uncomplicated acute oedematous pancreatitis (OP) is seen with perfusion of the pancreatic parenchyma, surrounded by inflammation. b Acute necrotising pancreatitis (NP) with loss of most of the parenchyma, excepting a small portion in the head and separate small portion of the tail of the gland. This scan was taken 2 weeks into the attack and the necrosis with associated inflammation is diffuse and poorly localised. c More than 4 weeks after the onset of acute pancreatitis in the same patient as in b, the necrotic collection (NC) has become localised and walled off close to the posterior wall of the stomach, making it suitable for endoscopic drainage. (d) In the same patient as in b, infection has supervened, identified by the presence of radiolucent black gas bubbles within the collection, which was treated by endoscopic necrosectomy (EN). The endoscopically inserted self-expanding metal stent between the stomach and necrotic cavity is visible as a radiopaque white ring; flushing the cavity endoscopically every 7–10 days was necessary to empty and allow collapse of the cavity, following which the stent was removed
Management of Diabetes Mellitus
Elevated blood glucose is indicative of more severe acute pancreatitis. Development of impaired glucose tolerance can be as high as 60% 5 years following a first attack of acute pancreatitis [211]; unsurprisingingly, the greatest risk is in those who develop necrosis. Those requiring necrosectomy are among those who lose the most pancreatic parenchyma and therefore the most islets. Development of clinical diabetes has been estimated around 15% and 40% following mild or severe acute pancreatitis, respectively [212]. Due to the loss of pancreatic parenchyma, insulin production is reduced, when multiple daily insulin injections may be appropriate. For those with recurrent hypoglycaemia despite optimised regimens, continuous subcutaneous insulin pump therapy with or without continuous glucose monitoring is an alternative, guided by specialists in diabetes care [213].
Pancreatic Enzyme Replacement Therapy
Pancreatic exocrine insufficiency can be identified in > 50% of patients during their inpatient stay with acute pancreatitis, although this frequency falls during follow-up, persisting in a minority, including > 50% of those with pancreatic necrosis [214] (Fig. 6). The normal adult human pancreas secretes between one and two million units of lipase daily, with many proteases, carbohydrate hydrolases, lipid hydrolases and nucleases [215], whereas acute pancreatitis inhibits secretion [214]. Pancreatic enzyme replacement therapy is likely to be beneficial in moderately severe and severe acute pancreatitis with oral/enteral feeding until faecal elastase-1 testing is repeatedly normal (≥ 200 μg/g); this therapy is recommended routinely for such patients, and long term for patients with > 50% necrosis. The presence of exocrine pancreatic insufficiency suggested by steatorrhoea warrants pancreatic enzyme replacement therapy after acute pancreatitis of any severity, and indefinitely should faecal elastase-1 remain < 100 μg/g (this therapy does not have to be stopped for faecal elastase-1 testing) [214]. A minimum standard dose for adult patients of one of the licensed preparations (Creon, Nutrizyme, Pancrease HL and Pancrex V in the UK) is 50,000 (lipase) units with a meal and half that with a snack, increased if steatorrhoea is insufficiently ameliorated. The total daily dose should be tailored to oral/enteral intake and in children should be adjusted based on a combination of body weight and intake, not exceeding a maximum daily dose of 10,000 IU/kg to achieve satisfactory growth and normal fat-soluble vitamin levels.
Fig. 6.
Prevalence of exocrine pancreatic insufficiency (EPI) during inpatient stay and over 5 or more years after mild or severe acute pancreatitis. Data are derived from a systematic review of multiple studies during follow-up after an attack of acute pancreatitis [214]. Separate data are given on mild and severe acute pancreatitis as two categories of Original Atlanta Classification [104], and combining as severe acute pancreatitis data on the moderate and severe categories in studies that used the Revised Atlanta Classification [13]. Studies were either prospective observational or interventional, the latter assessing the effectiveness of pancreatic enzyme replacement therapy. The number of patients with either mild or severe acute pancreatitis for whom there were data are given underneath the histogram
Prevention of Recurrence
The most effective method of preventing recurrent biliary pancreatitis is cholecystectomy. The recent PONCHO trial from the Dutch Pancreatitis Study Group confirmed cholecystectomy during the index admission to be cost effective [216], as found by others [217]. ERCP and sphincterotomy alone can halve the risk of recurrent pancreatitis in those unfit for surgery, but does not reduce the risk to the same extent as cholecystectomy [141], and increases the risk of subsequent cholecystitis from compromise of sphincter of Oddi function. If extensive investigation for aetiology is negative, resulting in an idiopathic diagnosis, cholecystectomy may still be justified; recurrence after cholecystectomy in idiopathic acute pancreatitis is lower than with conservative management [218].
After a first attack of acute pancreatitis, at least 20% of patients have a recurrence, approaching half of whom subsequently develop chronic pancreatitis, notably in males who continue to smoke and/or consume alcohol [219]. Prevention of recurrent acute alcoholic pancreatitis necessitates abstinence (defined as < 24 g alcohol per 2 months). An Icelandic study demonstrated that recurrent attacks were observed in one-third of persistent drinkers, while there were none in abstainers during 5 years of follow-up [220]. If a little is allowed, a lot may be consumed, so advice must be for abstinence; if active counselling is provided, randomised trial [221] and observational [222] evidence indicates abstinence is more likely to be achieved. Specialist alcohol services can assist, including managing withdrawal during admission, counselling and selection of patients for medication to reduce dependence [223]. Similar lifestyle advice applies to smoking, which increases the risks of recurrent and chronic pancreatitis [224, 225] and pancreas cancer [226].
Prevention of recurrent episodes of hypertriglyceridaemia-associated acute pancreatitis aims to reduce serum triglycerides at least below 1000 mg/dL (11.3 mmol/L), preferably below 500 mg/dL (5.65 mmol/L). First-line therapy includes life-style changes (weight management, exercise, low-fat diet, alcohol cessation), omega-3 fatty acids, and lipid-lowering mediation (fibrates and niacin, with/without statins) [40]. In treatment-resistant hypertriglyceridaemia, plasmapheresis may help reduce recurrence [227]. There is an increasing range of licenced therapies for rarer forms of hypertriglyceridaemia: alipogene tiparvovec for lipoprotein lipase deficiency; mipomersen (antisense oligonucleotide against apolipoprotein B messenger ribonucleic acid) or evinacumab (monoclonal against angiopoietin-like protein 3) or lomitapide (microsomal triglyceride transfer protein inhibitor) for homozygous familial hypercholesterolaemia; volanesorsen (antisense oligonucleotide against apolipoprotein C3) or pradigastat (diacylglycerol acyltransferase-1 inhibitor) for familial chylonomicronaemia syndrome) [228, 229].
When a drug is considered the cause of, or to have contributed to, acute pancreatitis, cessation of the drug is appropriate. Later resumption of the drug will depend on the availability of alternatives, the clinical indication, the severity of acute pancreatitis, any data on the frequency and severity of acute pancreatitis known to be induced by the drug, and, assuming intrinsic toxicity, options for reduced dosage. Thus, asparaginase for acute lymphoblastic leukaemia may induce acute necrotising pancreatitis and resumption would be better avoided unless there is a high risk of relapse [230], whereas interferon alpha causes relatively mild acute pancreatitis—and that very infrequently—so a trial of resumption may be appropriate [231]. Biomarkers that might be used to identify drug-induced pancreatic injury are in their infancy, and, at present, assumed to be congruent with biomarkers of pancreatic injury from other causes. Amylase and lipase lack specificity and in humans do not indicate the severity of pancreatic injury, so there is a need for alternatives [232].
Patients with pancreas-sufficient cystic fibrosis are at risk of acute pancreatitis, a risk that can be reduced by CFTR modulator therapy as with ivacaftor and/or tezacaftor [233], although CFTR modulator therapy with ivacaftor or lumacaftor-ivacaftor that restores exocrine function in pancreas-insufficient cystic fibrosis may increase the risk of acute pancreatitis [234]. In patients with disabling recurrent idiopathic pancreatitis with or without associated mutations, for example in CFTR or SPINK-1, consideration should be given to more invasive measures, although surgery is unlikely to be appropriate unless there is clear morphological evidence of chronic pancreatitis. Endoscopic interventions may be tried if significant ductal changes are identified, for example with ductal stenting, but such measures are less successful than surgery when appropriate [235]; total pancreatectomy and islet autotransplantation is another option [236].
Recommended first-line treatment for autoimmune pancreatitis is oral prednisolone (2 mg/kg, max 60 mg daily) tapered slowly by 5–10 mg, aiming to keep the patient on a maintenance dose (5–7.5 mg/day) for some 6 months and then close follow up. During treatment patients should be monitored closely for biochemical, clinical or radiological progress and side effects such as hypertension and diabetes [237]. Escalating immunomodulatory treatment with azathioprine, 6-mercaptopurine or rituximab are alternatives for steroid-resistant or relapsing disease [238].
Addressing the Aftermath of Acute Pancreatitis
The mortality rate of acute pancreatitis has decreased over the last five decades, but the incidence of acute pancreatitis has risen continuously in those countries for which there are data, such that improvements in diagnosis and treatment do not appear to have had a major impact on population mortality or the burden of morbidity [1]. There are several very significant impacts that indicate it is not appropriate to consider acute pancreatitis a self-limiting disease. For those surviving severe acute pancreatitis, the impact can be devastating, including debilitating sequelae of critical illness [239], pancreatic exocrine [214] and endocrine insufficiency [240], loss of employment, and poor quality of life, a context in which prevention of recurrence or progression to chronic pancreatitis requires active, positive, long-term, specialist management [22]. Alcohol addiction, hypertriglyceridaemia and smoking are stubborn to change; yet, randomised evidence shows the benefit of proactive management with counselling [221]. Moderately severe acute pancreatitis with necrosis may also result in substantial morbidity from local complications, after repeated interventions to achieve debridement. Even in patients with mild acute pancreatitis, there is a significant incidence and long-term prevalence of pancreatic exocrine [241] and endocrine insufficiency [242]. In such patients, the first attack of acute pancreatitis may be sentinel to progression [243], including a long-term increased risk of pancreatic cancer at ~ 1%, clustered in those with progressive disease [244]. One-fifth of those who suffer acute pancreatitis develop recurrent attacks, and of this fifth, at least one-third develop chronic pancreatitis [1]. In long-term follow-up, quality of life, for example as measured with Short Form 36, EQ-5D-5L [245] and PAN-PROMISE [246], and underlying biological determinants remain to be explored, which may enable management to be adjusted to improve outcomes, and/or demonstrate efficacy in clinical trials.
Clinical Trials
Many randomised trials of treatments have been undertaken to improve outcomes from acute pancreatitis, a number of which have been discussed above as individual trials [159, 172, 178, 185, 205, 208, 209, 217, 221, 247, 248] or meta-analyses [6, 54, 156, 173–175, 177, 183, 187, 214, 218, 249, 250]. These have helped shape intravenous fluid administration, analgesia, nutrition and critical care for acute pancreatitis, and treatment of infection, necrosis, pancreatic endocrine and exocrine insufficiency. There is, however, no internationally accepted, licenced, specific drug for acute pancreatitis, evident in international guidelines [14, 40, 124, 158, 161–165, 251]. Nevertheless, there are lessons to be learnt (see Table 1), for example from the phase 3 lexipafant trial about which there was so much hope two decades ago, underpowered to detect a significant change in the primary endpoint of new-onset organ failure [252]. The disappointing phase 3 ACCESS trial of eritoran in severe sepsis targeting toll-like receptor 4 included heterogenous infective pathologies [253]. Meta-analyses suggest potential merit in intravenous heparin, glutamine, ω-3 fatty acids and/or traditional Chinese medicine in severe acute pancreatitis [254–259], but the majority of included trials have been compromised by the absence of allocation concealment and double-blinding, awaiting well-designed, large-scale, double-blind, placebo-controlled, multicentre trials with patient inclusion appropriate to primary outcome.
Table 1.
Drug targets and the development of drug treatments with potential application for acute pancreatitis
In acute pancreatitis prevention is easier to achieve than cure, as shown by the success of post-ERCP acute pancreatitis prophylaxis trials [249]. Small-scale randomised trials established non-ionic, iso-osmolar contrast agents as superior to hyperosmolar, ionic contrast agents [260, 261]. Prophylactic pancreatic stent placement has a role in high-risk cases [262] and prophylactic administration of NSAIDs (indomethacin or diclofenac) administered per rectum significantly reduce the risk of post-ERCP pancreatitis, if administered 30–60 min prior to the procedure [247, 263]. A recent network meta-analysis found rectal diclofenac to be the best performing rectal NSAID, although there is insufficient evidence to conclude whether combinations of prophylaxis may be more effective [249].
Significant endeavour has focussed on addressing issues presented by local complications specific to acute pancreatitis, with important incremental advances pioneered by the Dutch Pancreatitis Study Group, notably in the timing and technique of necrosectomy (see Sect. 4.4.3). They and others have demonstrated the benefits of reduced trauma of access with a minimal access step-up approach [204], or better still, an endoscopic approach that is easier for patients and more cost-effective [208–210], as well as the benefits of delaying intervention to reduce the need for the same [207].
A Cochrane review of pharmacological interventions for acute pancreatitis found no effect on short-term mortality or consistent benefits from any treatment [6], borne out by the absence of any internationally licensed medication. The authors recommended wide entry criteria in future trials, measuring health-related quality of life and costs as outcomes, with follow-up for at least 3 months. Currently there are only three randomised, double-blind, placebo-controlled, multicentre trials testing the efficacy of intravenous drugs in acute pancreatitis registered as actively recruiting on the ClinicalTrials.gov website (see Table 2); review of the International Clinical Trial Registry identifies none further with this design. Such design is less prone to bias, more likely to satisfy regulatory requirements, and ensures effective, early drug delivery. The first is evaluating the new agent Auxora, an Orai Ca2+ channel inhibitor [248], in a phase IIb trial in the USA (CARPO, NCT04681066) targeting calcium entry and intracellular overload, a central disease mechanism highlighted in Sect. 3.1. CM4620 (Auxora) is also being trialled in children and young adults for acute pancreatitis attributed to asparaginase (NCT04195347). The second is a phase IIb trial testing the biologic infliximab in the UK (RAPID-I, NCT03684278), targeting tumour necrosis factor-α [264, 265]. RAPID-I has wide entry criteria, includes health-related quality of life and costs as outcomes, with follow-up of 3 months. The third is a Danish trial of the peripherally acting µ-opioid receptor antagonist methylnaltrexone, which counteracts inhibitory effects of opiates on gut function and immune responses, without affecting analgesia [266]. It is to be hoped these trials will be completed soon and that the international impetus for such trials will grow. New or repositioned drugs targeting pivotal disease mechanisms (see Table 1) are vital in this pursuit, as is reduced door-to-needle time, unlike in a recently reported negative trial of thymosin alpha 1 that featured a recruitment window of 7 days [267]. Establishing pipelines of double-blind, placebo-controlled, multicentre trials of drugs will bring the prospect of success closer, an imperative for affected patients.
Table 2.
Current double-blind, placebo-controlled, multicentre randomised trials of drug treatments for acute pancreatitis registered as actively recruiting in the ClinicalTrials.gov and International Clinical Trial Registry websites as of July 2022.
| CARPO | RAPID-I | PAMORA-AP | |
|---|---|---|---|
| Agent | Auxora 3 daily 4-h infusions | Infliximab single infusion | Methylnaltrexone 5-day infusion |
| Mechanism | Orai calcium channel inhibitor | Anti-tumour necrosis factor-⍺ | Peripherally acting µ-opioid RA1 |
| Centres | Multicentre USA | Multicentre UK | Multicentre Denmark |
| Inclusion | Two SIRS2 cirteria plus other features3 | Onset in 2 days before day of admission | Onset within 48 h with SIRS3 |
| Patients | 216 | 290 | 90 |
| Groups | 2 mg or 1 mg or 0.5 mg/kg vs. placebo | 5 mg/kg vs. 10 mg/kg vs. placebo | 0.15 mg/kg vs. placebo |
| 1o outcome4 | Time to solid food tolerance | Cumlative CRP5 on days 2, 4 and 14 | PASS6 48 h after randomisation |
1RA = receptor antagonist
2SIRS = systemic inflammatory response syndrome
3Either abdominal guarding, abdominal rebound, haematocrit ≥ 44% (men), haematocrit ≥ 40% (women), fluid collection on computed tomography or pleural effusion on computed tomography
4Primary outcome
5CRP = C-reactive protein
6PASS = Pancreatitis Activity Scoring System
Conclusion and Future Perspectives
Acute pancreatitis is a common disease affecting all ages, with aetiologies displaying great variation with geography. Initial management remains supportive, with specific pharmacological, endoscopic, radiological and/or surgical interventions limited to the management of complications or, for a small minority of aetiologies, prevention of recurrence. Care should taken to determine accurately the cause of pancreatitis, as misidentification will lead to recurrent disease and worse outcomes. Sustained endeavour is required to pursue clinical trials of new and repurposed drugs with broad generalisability in acute pancreatitis, so that, sooner rather than later, there are internationally licensed drug therapies for acute pancreatitis.
Declarations
Funding
WC is supported by the China Scholarship Council (no. 201806240274) and West China-Liverpool Clinician Scientist Development Award from West China Hospital of Sichuan University. WH is supported by the National Natural Science Foundation of China (no. 81973632). GB is supported by German Research Council (DFG BE 6395-1/1) and the TransBioLine Consortium. RS is supported by an NIHR Senior Investigator Award, an EME Award (15/20/01), an MRC Award (MR/T002220/1) and the TransBioLine Consortium. The TransBioLine project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (Grant agreement no. 821283). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. This communication reflects the authors’ view and neither IMI nor the European Union or EFPIA are responsible for any use that may be made of the information contained therein.
Conflicts of interest/competing interests
TG has consulted for Albireo (ongoing). WH has received research funding from Cypralis, Farsight, and Corvidia, with all funds paid to West China Hospital of Sichuan University. RS has consulted for AbbVie CalciMedica, GlaxoSmithKline (GSK), Novartis and Takeda, and has received research funding from CalciMedica, EA Pharma, GSK, Lilly, Merck/MSD, Pfizer as well as multiple public sources in the last three years. RS is collaborating in the IMI2 TransBioLine Consortium with Janssen, Lilly, Merck/MSD, Novartis, Pfizer, Roche, and Sanofi-Aventis. All funds received have been paid to the University of Liverpool and/or Liverpool University Hospitals NHS Foundation Trust.
Author contributions
This article was written by RS, PS and TG with critical intellectual input from WC, WH, CH and GB. All authors meet the International Committee of Medical Journal Editors criteria for authorship of this paper, take full responsibility for the integrity of the work, and have given their approval for this final version to be published.
Ethics approval
Not applicable.
Consent to participate and consent for publication
All authors have contributed to review and enhancement of the quality of this paper and have reviewed and approved the final version of the manuscript.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
- 1.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–134. doi: 10.1053/j.gastro.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Andersson B, Appelgren B, Sjodin V, Ansari D, Nilsson J, Persson U, et al. Acute pancreatitis–costs for healthcare and loss of production. Scand J Gastroenterol. 2013;48(12):1459–1465. doi: 10.3109/00365521.2013.843201. [DOI] [PubMed] [Google Scholar]
- 4.Della Corte C, Faraci S, Majo F, Lucidi V, Fishman DS, Nobili V. Pancreatic disorders in children: new clues on the horizon. Dig Liver Dis. 2018;50(9):886–893. doi: 10.1016/j.dld.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17(2):155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev. 2017;4:CD011384. doi: 10.1002/14651858.CD011384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreto SG, Habtezion A, Gukovskaya A, Lugea A, Jeon C, Yadav D, et al. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut. 2021;70(1):194–203. doi: 10.1136/gutjnl-2020-322163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiriyama S, Gabata T, Takada T, Hirata K, Yoshida M, Mayumi T, et al. New diagnostic criteria of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(1):24–36. doi: 10.1007/s00534-009-0214-3. [DOI] [PubMed] [Google Scholar]
- 9.World Health O. AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care. In: Babor TF, et al, editor. 2nd edn. Geneva: World Health Organization; 2001.
- 10.Meneses-Gaya C, Zuardi AW, Loureiro SR, Hallak JE, Trzesniak C, de Azevedo Marques JM, et al. Is the full version of the AUDIT really necessary? Study of the validity and internal construct of its abbreviated versions. Alcohol Clin Exp Res. 2010;34(8):1417–1424. doi: 10.1111/j.1530-0277.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson RJ, John B, Abbasi T, Hodgson RC, Waller S, Thom B, et al. Fast screening for alcohol misuse. Addict Behav. 2003;28(8):1453–1463. doi: 10.1016/S0306-4603(02)00246-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Hendershot CS. A review of performance indicators of single-item alcohol screening questions in clinical and population settings. J Subst Abuse Treat. 2020;111:73–85. doi: 10.1016/j.jsat.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 14.Working Group IAPAPAAPG IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 15.Rompianesi G, Hann A, Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database Syst Rev. 2017;4(4):Cd012010. doi: 10.1002/14651858.CD012010.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18(7):1567–75. [DOI] [PMC free article] [PubMed]
- 17.Lankisch PG, Assmus C, Lehnick D, Maisonneuve P, Lowenfels AB. Acute pancreatitis: does gender matter? Dig Dis Sci. 2001;46(11):2470–2474. doi: 10.1023/A:1012332121574. [DOI] [PubMed] [Google Scholar]
- 18.Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers. 2016;28(2):16024. doi: 10.1038/nrdp.2016.24. [DOI] [PubMed] [Google Scholar]
- 19.Opie EL. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp. 1901;12:182–188. [Google Scholar]
- 20.Gorelick FS, Lerch MM. Do animal models of acute pancreatitis reproduce human disease? Cell Mol Gastroenterol Hepatol. 2017;4(2):251–262. doi: 10.1016/j.jcmgh.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majbar AA, Cusick E, Johnson P, Lynn RM, Hunt LP, Shield JP. Incidence and clinical associations of childhood acute pancreatitis. Pediatrics. 2016 Sep;138(3):e20161198. [DOI] [PubMed]
- 22.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7(3):131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 23.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33(4):323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 24.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Yang L, Bian Z, et al. Metabolic and lifestyle risk factors for acute pancreatitis in Chinese adults: a prospective cohort study of 0.5 million people. PLoS Med. 2018;15(8):e1002618. doi: 10.1371/journal.pmed.1002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamada S, Masamune A, Kikuta K, Hirota M, Tsuji I, Shimosegawa T, et al. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2014;43(8):1244–1248. doi: 10.1097/MPA.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989. Gut. 1993;34(9):1255–1260. doi: 10.1136/gut.34.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38(5):539–548. doi: 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raty S, Sand J, Alho H, Nordback I. Alcoholic, but not biliary, pancreatitis varies seasonally in occurrence. Scand J Gastroenterol. 2003;38(7):794–797. doi: 10.1080/00365520310003499. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Tang M, Zhao Y, Zhou S, Xu X, Wang F, et al. Impact of seasons and festivals on the onset of acute pancreatitis in Shanghai, China. Pancreas. 2017;46(4):496–503. doi: 10.1097/MPA.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen JK, Olafsson S, Bergmann OM, Runarsdottir V, Hansdottir I, Sigurdardottir R, et al. Lifetime drinking history in patients with alcoholic liver disease and patients with alcohol use disorder without liver disease. Scand J Gastroenterol. 2017;52(6–7):762–767. doi: 10.1080/00365521.2017.1295466. [DOI] [PubMed] [Google Scholar]
- 32.Whitcomb DC, LaRusch J, Krasinskas AM, Klei L, Smith JP, Brand RE, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44(12):1349–1354. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pownall HJ, Ballantyne CM, Kimball KT, Simpson SL, Yeshurun D, Gotto AM., Jr Effect of moderate alcohol consumption on hypertriglyceridemia: a study in the fasting state. Arch Intern Med. 1999;159(9):981–987. doi: 10.1001/archinte.159.9.981. [DOI] [PubMed] [Google Scholar]
- 34.Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, et al. Prospective study of alcohol drinking, smoking, and pancreatitis: the multiethnic cohort. Pancreas. 2016;45(6):819–825. doi: 10.1097/MPA.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169(11):1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav D, O'Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096–1103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 37.Maleth J, Balazs A, Pallagi P, Balla Z, Kui B, Katona M, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148(2):427 e16–439 e16. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16(4):469–476. doi: 10.1016/j.pan.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Deng L, Jin T, Zhu P, Shi N, Jiang K, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 2019;21(9):1240–1249. doi: 10.1016/j.hpb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969–2989. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2(8):655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164. doi: 10.1001/2013.jamainternmed.477. [DOI] [PubMed] [Google Scholar]
- 43.Kiss L, Fur G, Matrai P, Hegyi P, Ivany E, Cazacu IM, et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep. 2018;8(1):14096. doi: 10.1038/s41598-018-32337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Wang G, Qiu Z, He X, Liu C. Elevated serum triglycerides in the prognostic assessment of acute pancreatitis: a systematic review and meta-analysis of observational studies. J Clin Gastroenterol. 2017;51(7):586–593. doi: 10.1097/MCG.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 45.Vinklerová I, Procházka M, Procházka V, Urbánek K. Incidence, severity, and etiology of drug-induced acute pancreatitis. Dig Dis Sci. 2010;55(10):2977–2981. doi: 10.1007/s10620-010-1277-3. [DOI] [PubMed] [Google Scholar]
- 46.Spanier BW, Tuynman HA, van der Hulst RW, Dijkgraaf MG, Bruno MJ. Acute pancreatitis and concomitant use of pancreatitis-associated drugs. Am J Gastroenterol. 2011;106(12):2183–2188. doi: 10.1038/ajg.2011.303. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39(8):709–716. doi: 10.1097/01.mcg.0000173929.60115.b4. [DOI] [PubMed] [Google Scholar]
- 48.Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5(6):648–661. doi: 10.1016/j.cgh.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Tenenbein MS, Tenenbein M. Acute pancreatitis due to erythromycin overdose. Pediatr Emerg Care. 2005;21(10):675–676. doi: 10.1097/01.pec.0000181419.49106.ec. [DOI] [PubMed] [Google Scholar]
- 50.Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46(10):1131–1134. doi: 10.1038/ng.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5(1):58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 52.Balani AR, Grendell JH. Drug-induced pancreatitis: incidence, management and prevention. Drug Saf. 2008;31(10):823–837. doi: 10.2165/00002018-200831100-00002. [DOI] [PubMed] [Google Scholar]
- 53.Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81(1):143 e9–149 e9. doi: 10.1016/j.gie.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 54.Njei B, McCarty TR, Muniraj T, Sharma P, Jamidar PA, Aslanian HR, et al. Comparative effectiveness of pharmacologic and endoscopic interventions for prevention of post-ERCP pancreatitis: a network meta-analysis. Endosc Int Open. 2020;8(1):E29–e40. doi: 10.1055/a-1005-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Funatsu E, Masuda A, Takenaka M, Nakagawa T, Shiomi H, Yoshinaka H, et al. History of post-endoscopic retrograde cholangiopancreatography pancreatitis and acute pancreatitis as risk factors for post-ERCP pancreatitis. Kobe J Med Sci. 2017;63(1):E1–E8. [PMC free article] [PubMed] [Google Scholar]
- 56.Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004;39(7):605–615. doi: 10.1007/s00535-004-1390-1. [DOI] [PubMed] [Google Scholar]
- 57.Gardner TB. Acute pancreatitis. Ann Intern Med. 2021;174(2):itc17–itc32. doi: 10.7326/AITC202102160. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee R, Smith A, Sutton R. Covid-19-related pancreatic injury. Br J Surg. 2020;107(7):e190. [DOI] [PMC free article] [PubMed]
- 59.Szatmary P, Arora A, Thomas Raraty MG, Joseph Dunne DF, Baron RD, Halloran CM. Emerging phenotype of severe acute respiratory syndrome-coronavirus 2-associated pancreatitis. Gastroenterology. 2020;159(4):1551–1554. doi: 10.1053/j.gastro.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, et al. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut. 2021;70(6):1061–1069. doi: 10.1136/gutjnl-2020-323364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hegyi PJ, Soós A, Tóth E, Ébert A, Venglovecz V, Márta K, et al. Evidence for diagnosis of early chronic pancreatitis after three episodes of acute pancreatitis: a cross-sectional multicentre international study with experimental animal model. Sci Rep. 2021;11(1):1367. doi: 10.1038/s41598-020-80532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, cell biology, and pathophysiology of pancreatitis. Gastroenterology. 2019;156(7):1951–68.e1. doi: 10.1053/j.gastro.2018.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss FU, Laemmerhirt F, Lerch MM. Acute pancreatitis: genetic risk and clinical implications. J Clin Med. 2021;10(2):190. doi: 10.3390/jcm10020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40(7):1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 66.Zen Y, Grammatikopoulos T, Hadzic N. Autoimmune pancreatitis in children: insights into the diagnostic challenge. J Pediatr Gastroenterol Nutr. 2014;59(5):e42–e45. doi: 10.1097/MPG.0b013e3182994559. [DOI] [PubMed] [Google Scholar]
- 67.Zen Y, Grammatikopoulos T, Heneghan MA, Vergani D, Mieli-Vergani G, Portmann BC. Sclerosing cholangitis with granulocytic epithelial lesion: a benign form of sclerosing cholangiopathy. Am J Surg Pathol. 2012;36(10):1555–1561. doi: 10.1097/PAS.0b013e31825faae0. [DOI] [PubMed] [Google Scholar]
- 68.Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16(12):702–714. doi: 10.1038/s41584-020-0500-7. [DOI] [PubMed] [Google Scholar]
- 69.Lorenzo D, Maire F, Stefanescu C, Gornet JM, Seksik P, Serrero M, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(1):59–67. doi: 10.1016/j.cgh.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 70.Petersen OH, Gerasimenko JV, Gerasimenko OV, Gryshchenko O, Peng S. The roles of calcium and ATP in the physiology and pathology of the exocrine pancreas. Physiol Rev. 2021;101(4):1691–1744. doi: 10.1152/physrev.00003.2021. [DOI] [PubMed] [Google Scholar]
- 71.Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, et al. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Investig. 2020;130(5):2527–2541. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138(2):715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee R, Mareninova OA, Odinokova IV, Huang W, Murphy J, Chvanov M, et al. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut. 2016;65(8):1333–1346. doi: 10.1136/gutjnl-2014-308553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W, Cane MC, Mukherjee R, Szatmary P, Zhang X, Elliott V, et al. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut. 2017;66(2):301–313. doi: 10.1136/gutjnl-2015-309363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutton R. Parenchymal pressure injury Ca(2+) entry mechanism in pancreatitis. Cell Calcium. 2020;19(88):102208. doi: 10.1016/j.ceca.2020.102208. [DOI] [PubMed] [Google Scholar]
- 76.Son A, Ahuja M, Schwartz DM, Varga A, Swaim W, Kang N, et al. Ca(2+) influx channel inhibitor SARAF protects mice from acute pancreatitis. Gastroenterology. 2019;157(6):1660–72.e2. doi: 10.1053/j.gastro.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 77.Du W, Liu G, Shi N, Tang D, Ferdek PE, Jakubowska MA, et al. A microRNA checkpoint for Ca(2+) signaling and overload in acute pancreatitis. Mol Ther. 2022;30:1754–1774. doi: 10.1016/j.ymthe.2022.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fanczal J, Pallagi P, Görög M, Diszházi G, Almássy J, Madácsy T, et al. TRPM2-mediated extracellular Ca(2+) entry promotes acinar cell necrosis in biliary acute pancreatitis. J Physiol. 2020;598(6):1253–1270. doi: 10.1113/JP279047. [DOI] [PubMed] [Google Scholar]
- 79.Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology. 2015;149(2):481–92.e7. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruce JIE, Sánchez-Alvarez R, Sans MD, Sugden SA, Qi N, James AD, et al. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat Commun. 2021;12(1):4386. doi: 10.1038/s41467-021-24506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63(8):1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zadori N, Gede N, Antal J, Szentesi A, Alizadeh H, Vincze A, et al. EarLy Elimination of Fatty Acids iN hypertriglyceridemia-induced acuTe pancreatitis (ELEFANT trial): protocol of an open-label, multicenter, adaptive randomized clinical trial. Pancreatology 2020;20(3):369–76 [DOI] [PubMed]
- 83.Lu Z, Zhang G, Guo F, Li M, Ding Y, Zheng H, et al. Elevated triglycerides on admission positively correlate with the severity of hypertriglyceridaemic pancreatitis. Int J Clin Pract. 2020;74(3):e13458. doi: 10.1111/ijcp.13458. [DOI] [PubMed] [Google Scholar]
- 84.Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology. 2018;154(3):689–703. doi: 10.1053/j.gastro.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wen L, Javed TA, Dobbs AK, Brown R, Niu M, Li L, et al. The protective effects of calcineurin on pancreatitis in mice depend on the cellular source. Gastroenterology. 2020;159(3):1036–50.e8. doi: 10.1053/j.gastro.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146(7):1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122(2):448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 88.Gao L, Dong X, Gong W, Huang W, Xue J, Zhu Q, et al. Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br J Pharmacol. 2021;178(17):3533–3552. doi: 10.1111/bph.15499. [DOI] [PubMed] [Google Scholar]
- 89.Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 2020;158(1):253–69.e14. doi: 10.1053/j.gastro.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 90.Maléth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014;55(6):337–345. doi: 10.1016/j.ceca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Jakubowska MA, Ferdek PE, Gerasimenko OV, Gerasimenko JV, Petersen OH. Nitric oxide signals are interlinked with calcium signals in normal pancreatic stellate cells upon oxidative stress and inflammation. Open Biol. 2016;6(8):160149. doi: 10.1098/rsob.160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154(3):704 e10–718 e10. doi: 10.1053/j.gastro.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122(4):974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 94.Karki R, Kanneganti TD. The 'cytokine storm': molecular mechanisms and therapeutic prospects. Trends Immunol. 2021;42(8):681–705. doi: 10.1016/j.it.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, Scharffetter-Kochanek K, et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62(3):430–439. doi: 10.1136/gutjnl-2011-300771. [DOI] [PubMed] [Google Scholar]
- 96.Gukovskaya AS, Gukovsky I, Algul H, Habtezion A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153(5):1212–1226. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hawkins RB, Raymond SL, Stortz JA, Horiguchi H, Brakenridge SC, Gardner A, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Immunol. 2018;9:1511. doi: 10.3389/fimmu.2018.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Huang L, Luo M, Xia X. Bacterial translocation in acute pancreatitis. Crit Rev Microbiol. 2019;45(5–6):539–547. doi: 10.1080/1040841X.2019.1621795. [DOI] [PubMed] [Google Scholar]
- 99.Dellinger EP, Forsmark CE, Layer P, Levy P, Maravi-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–880. doi: 10.1097/SLA.0b013e318256f778. [DOI] [PubMed] [Google Scholar]
- 100.Yu S, Xiong Y, Xu J, Liang X, Fu Y, Liu D, et al. Identification of dysfunctional gut microbiota through rectal swab in patients with different severity of acute pancreatitis. Dig Dis Sci. 2020;65(11):3223–37. [DOI] [PubMed]
- 101.Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3(107):107ra10. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Investig. 2020;130(4):1931–1947. doi: 10.1172/JCI132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X, Yao L, Dai L, Yuan M, He W, Liu T, et al. Alcohol predisposes obese mice to acute pancreatitis via adipose triglyceride lipase-dependent visceral adipocyte lipolysis.Gut. 2022 Apr 22; Online ahead of print. [DOI] [PMC free article] [PubMed]
- 104.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128(5):586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 105.Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476–1482. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 106.van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; a systematic review and meta-analysis. Pancreatology. 2020;20(7):1302–1311. doi: 10.1016/j.pan.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Thapa R, Iqbal Z, Garikipati A, Siefkas A, Hoffman J, Mao Q, et al. Early prediction of severe acute pancreatitis using machine learning. Pancreatology. 2022;22(1):43–50. doi: 10.1016/j.pan.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Y, Ge YT, Shi XL, Wu KY, Chen WW, Ding YB, et al. Machine learning predictive models for acute pancreatitis: a systematic review. Int J Med Inform. 2022;157:104641. doi: 10.1016/j.ijmedinf.2021.104641. [DOI] [PubMed] [Google Scholar]
- 109.Dzobo K, Adotey S, Thomford NE, Dzobo W. Integrating artificial and human intelligence: a partnership for responsible innovation in biomedical engineering and medicine. OMICS. 2020;24(5):247–263. doi: 10.1089/omi.2019.0038. [DOI] [PubMed] [Google Scholar]
- 110.McDermott JH, Burn J, Donnai D, Newman WG. The rise of point-of-care genetics: how the SARS-CoV-2 pandemic will accelerate adoption of genetic testing in the acute setting. Eur J Hum Genet. 2021;29(6):891–893. doi: 10.1038/s41431-021-00816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castelli FA, Rosati G, Moguet C, Fuentes C, Marrugo-Ramírez J, Lefebvre T, et al. Metabolomics for personalized medicine: the input of analytical chemistry from biomarker discovery to point-of-care tests. Anal Bioanal Chem. 2022;414(2):759–789. doi: 10.1007/s00216-021-03586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, et al. EASY-APP: an artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12(6):e842. doi: 10.1002/ctm2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu BU, Batech M, Quezada M, Lew D, Fujikawa K, Kung J, et al. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system. Am J Gastroenterol. 2017;112(7):1144–1152. doi: 10.1038/ajg.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buxbaum J, Quezada M, Chong B, Gupta N, Yu CY, Lane C, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113(5):755–764. doi: 10.1038/s41395-018-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paragomi P, Hinton A, Pothoulakis I, Talukdar R, Kochhar R, Goenka MK, et al. The modified pancreatitis activity scoring system shows distinct trajectories in acute pancreatitis: an international study. Clin Gastroenterol Hepatol. 2022;20(6):1334–42. [DOI] [PMC free article] [PubMed]
- 116.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 118.Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68(6):1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 119.Zubia-Olaskoaga F, Maraví-Poma E, Urreta-Barallobre I, Ramírez-Puerta MR, Mourelo-Fariña M, Marcos-Neira MP. Comparison between revised atlanta classification and determinant-based classification for acute pancreatitis in intensive care medicine. Why Do not use a modified determinant-based classification? Crit Care Med. 2016;44(5):910–917. doi: 10.1097/CCM.0000000000001565. [DOI] [PubMed] [Google Scholar]
- 120.Zubia-Olaskoaga F, Maraví-Poma E, Urreta-Barallobre I, Ramírez-Puerta MR, Mourelo-Fariña M, Marcos-Neira MP. Validation of the modified determinant-based classification for patients with acute pancreatitis in intensive care medicine. Pancreas. 2021;50(6):867–872. doi: 10.1097/MPA.0000000000001855. [DOI] [PubMed] [Google Scholar]
- 121.Wu D, Lu B, Xue HD, Yang H, Qian JM, Lee P, et al. Validation of modified determinant-based classification of severity for acute pancreatitis in a tertiary teaching hospital. Pancreatology. 2019;19(2):217–223. doi: 10.1016/j.pan.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 122.Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012;55(3):261–265. doi: 10.1097/MPG.0b013e31824f1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morinville VD, Lowe ME, Ahuja M, Barth B, Bellin MD, Davis H, et al. Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr. 2014;59(3):360–364. doi: 10.1097/MPG.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Párniczky A, Abu-El-Haija M, Husain S, Lowe M, Oracz G, Sahin-Tóth M, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18(2):146–160. doi: 10.1016/j.pan.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 125.DeBanto JR, Goday PS, Pedroso MR, Iftikhar R, Fazel A, Nayyar S, et al. Acute pancreatitis in children. Am J Gastroenterol. 2002;97(7):1726–1731. doi: 10.1111/j.1572-0241.2002.05833.x. [DOI] [PubMed] [Google Scholar]
- 126.Bai HX, Ma MH, Orabi AI, Park A, Latif SU, Bhandari V, et al. Novel characterization of drug-associated pancreatitis in children. J Pediatr Gastroenterol Nutr. 2011;53(4):423–428. doi: 10.1097/MPG.0b013e318228574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30(4):343–356. doi: 10.1097/00004836-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 128.Ramin KD, Ramin SM, Richey SD, Cunningham FG. Acute pancreatitis in pregnancy. Am J Obstet Gynecol. 1995;173(1):187–191. doi: 10.1016/0002-9378(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 129.Hernandez A, Petrov MS, Brooks DC, Banks PA, Ashley SW, Tavakkolizadeh A. Acute pancreatitis and pregnancy: a 10-year single center experience. J Gastrointest Surg. 2007;11(12):1623–1627. doi: 10.1007/s11605-007-0329-2. [DOI] [PubMed] [Google Scholar]
- 130.Eddy JJ, Gideonsen MD, Song JY, Grobman WA, O'Halloran P. Pancreatitis in pregnancy. Obstet Gynecol. 2008;112(5):1075–1081. doi: 10.1097/AOG.0b013e318185a032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Z, Guo G, Li H. Predicting fetal loss in severe acute pancreatitis during pregnancy: a 5-year single-tertiary-center retrospective analysis. Postgrad Med. 2020;132(5):473–478. doi: 10.1080/00325481.2020.1752010. [DOI] [PubMed] [Google Scholar]
- 132.Ducarme G, Maire F, Chatel P, Luton D, Hammel P. Acute pancreatitis during pregnancy: a review. J Perinatol. 2014;34(2):87–94. doi: 10.1038/jp.2013.161. [DOI] [PubMed] [Google Scholar]
- 133.Luo L, Zen H, Xu H, Zhu Y, Liu P, Xia L, et al. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet. 2018;297(2):333–339. doi: 10.1007/s00404-017-4558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yin BL, Fu XD. A clinical analysis of acute pancreatitis in pregnancy. Hepatobiliary Pancreat Dis Int. 2017;16(3):323–325. doi: 10.1016/S1499-3872(17)60017-1. [DOI] [PubMed] [Google Scholar]
- 135.Gupta M, Liti B, Barrett C, Thompson PD, Fernandez AB. Prevention and management of hypertriglyceridemia-induced acute pancreatitis during pregnancy: a systematic review. Am J Med. 2022;135(6):709–14. [DOI] [PubMed]
- 136.Aljenedil S, Hegele RA, Genest J, Awan Z. Estrogen-associated severe hypertriglyceridemia with pancreatitis. J Clin Lipidol. 2017;11(1):297–300. doi: 10.1016/j.jacl.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Stumpf MAM, Kluthcovsky A, Okamoto JM, Schrut GCA, Cajoeiro PO, Chacra APM, et al. Acute pancreatitis secondary to oral contraceptive-induced hypertriglyceridemia: a case report. Gynecol Endocrinol. 2018;34(11):930–932. doi: 10.1080/09513590.2018.1473365. [DOI] [PubMed] [Google Scholar]
- 138.Oskarsson V, Orsini N, Sadr-Azodi O, Wolk A. Postmenopausal hormone replacement therapy and risk of acute pancreatitis: a prospective cohort study. CMAJ. 2014;186(5):338–344. doi: 10.1503/cmaj.131064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shabanzadeh DM. Incidence of gallstone disease and complications. Curr Opin Gastroenterol. 2018;34(2):81–89. doi: 10.1097/MOG.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 140.Somasekar K, Foulkes R, Morris-Stiff G, Hassn A. Acute pancreatitis in the elderly—can we perform better? Surgeon. 2011;9(6):305–308. doi: 10.1016/j.surge.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Qayed E, Shah R, Haddad YK. Endoscopic retrograde cholangiopancreatography decreases all-cause and pancreatitis readmissions in patients with acute gallstone pancreatitis who do not undergo cholecystectomy: a nationwide 5-year analysis. Pancreas. 2018;47(4):425–435. doi: 10.1097/MPA.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 142.Baeza-Zapata AA, García-Compeán D, Jaquez-Quintana JO. Acute pancreatitis in elderly patients. Gastroenterology. 2021;161(6):1736–1740. doi: 10.1053/j.gastro.2021.06.081. [DOI] [PubMed] [Google Scholar]
- 143.Park J, Fromkes J, Cooperman M. Acute pancreatitis in elderly patients. Pathogenesis and outcome. Am J Surg. 1986;152(6):638–642. doi: 10.1016/0002-9610(86)90440-X. [DOI] [PubMed] [Google Scholar]
- 144.Browder W, Patterson MD, Thompson JL, Walters DN. Acute pancreatitis of unknown etiology in the elderly. Ann Surg. 1993;217(5):469–474. doi: 10.1097/00000658-199305010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koziel D, Gluszek-Osuch M, Suliga E, Zak M, Gluszek S. Elderly persons with acute pancreatitis—specifics of the clinical course of the disease. Clin Interv Aging. 2019;14:33–41. doi: 10.2147/CIA.S188520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Minato Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, et al. Pancreatic cancer causing acute pancreatitis: a comparative study with cancer patients without pancreatitis and pancreatitis patients without cancer. J Hepatobiliary Pancreat Sci. 2013;20(6):628–633. doi: 10.1007/s00534-013-0598-y. [DOI] [PubMed] [Google Scholar]
- 147.Venkatesh PG, Navaneethan U, Vege SS. Intraductal papillary mucinous neoplasm and acute pancreatitis. J Clin Gastroenterol. 2011;45(9):755–758. doi: 10.1097/MCG.0b013e31821b1081. [DOI] [PubMed] [Google Scholar]
- 148.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41(7):613–625. doi: 10.1007/s00535-006-1862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Majumder S, Takahashi N, Chari ST. Autoimmune pancreatitis. Dig Dis Sci. 2017;62(7):1762–1769. doi: 10.1007/s10620-017-4541-y. [DOI] [PubMed] [Google Scholar]
- 150.Okamoto A, Watanabe T, Kamata K, Minaga K, Kudo M. Recent updates on the relationship between cancer and autoimmune pancreatitis. Intern Med. 2019;58(11):1533–1539. doi: 10.2169/internalmedicine.2210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mishra SB, Azim A, Mukherjee A. Multiple myeloma presenting as acute pancreatitis. Am J Emerg Med. 2017;35(9):1385 e1–1385 e2. doi: 10.1016/j.ajem.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 152.Pal A, Sonavane A, Agrawal R, Rathi P. Parathyroid cancer causing acute severe pancreatitis. J Assoc Physicians India. 2017;65(12):95–97. [PubMed] [Google Scholar]
- 153.Gao Y, Yu C, Xiang F, Xie M, Fang L. Acute pancreatitis as an initial manifestation of parathyroid carcinoma: a case report and literature review. Medicine (Baltimore) 2017;96(44):e8420. doi: 10.1097/MD.0000000000008420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abdullah AS, Adel AM, Hussein RM, Abdullah MA, Yousaf A, Mudawi D, et al. Hypercalcemia and acute pancreatitis in a male patient with acute promyelocytic leukemia and pulmonary tuberculosis. Acta Biomed. 2018;89(3-S):23–27. doi: 10.23750/abm.v89i3-S.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hussain A, Adnan A, El-Hasani S. Small cell carcinoma of the lung presented as acute pancreatitis. Case report and review of the literature. JOP. 2012;13(6):702–704. doi: 10.6092/1590-8577/1181. [DOI] [PubMed] [Google Scholar]
- 156.Manterola C, Vial M, Moraga J, Astudillo P. Analgesia in patients with acute abdominal pain. Cochrane Database Syst Rev. 2011;1:Cd005660. doi: 10.1002/14651858.CD005660.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang X, Zhang R, Jin T, Zhu P, Yao L, Li L, et al. Stress hyperglycemia is independently associated with persistent organ failure in acute pancreatitis. Dig Dis Sci. 2021;67(5):1879–89. [DOI] [PMC free article] [PubMed]
- 158.Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51(5):472–491. doi: 10.1055/a-0862-0346. [DOI] [PubMed] [Google Scholar]
- 159.Schepers NJ, Hallensleben NDL, Besselink MG, Anten MGF, Bollen TL, da Costa DW, et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): a multicentre randomised controlled trial. Lancet. 2020;396(10245):167–176. doi: 10.1016/S0140-6736(20)30539-0. [DOI] [PubMed] [Google Scholar]
- 160.Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103–1139. doi: 10.1053/j.gastro.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 161.Isaji S, Takada T, Mayumi T, Yoshida M, Wada K, Yokoe M, et al. Revised Japanese guidelines for the management of acute pancreatitis 2015: revised concepts and updated points. J Hepatobiliary Pancreat Sci. 2015;22(6):433–445. doi: 10.1002/jhbp.260. [DOI] [PubMed] [Google Scholar]
- 162.Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59(2):128–140. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sellers ZM, Abu-El-Haija M, Husain SZ, Morinville V. New management guidelines for both children and adults with acute pancreatitis. Gastroenterology. 2018;155(1):234–235. doi: 10.1053/j.gastro.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 164.Leppaniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.National Institute for Health and Care Excellence. Clinical guidelines. Pancreatitis. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2020; 2020.
- 166.Iacopetta D, Ceramella J, Catalano A, Saturnino C, Pellegrino M, Mariconda A, et al. COVID-19 at a glance: an up-to-date overview on variants, drug design and therapies. Viruses. 2022;14(3):573. doi: 10.3390/v14030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Dev Ther. 2012;6:165–173. doi: 10.2147/DDDT.S24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O'Driscoll BR, Howard LS, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–ii90. doi: 10.1136/thoraxjnl-2016-209729. [DOI] [PubMed] [Google Scholar]
- 169.Machicado JD, Papachristou GI. Intravenous fluid resuscitation in the management of acute pancreatitis. Curr Opin Gastroenterol. 2020;36(5):409–416. doi: 10.1097/MOG.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 170.Malbrain M, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA) Ann Intensive Care. 2020;10(1):64. doi: 10.1186/s13613-020-00679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298–1309. doi: 10.1001/jama.2016.12310. [DOI] [PubMed] [Google Scholar]
- 172.Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710 e1–717 e1. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 173.Iqbal U, Anwar H, Scribani M. Ringer’s lactate versus normal saline in acute pancreatitis: a systematic review and meta-analysis. J Dig Dis. 2018;19(6):335–341. doi: 10.1111/1751-2980.12606. [DOI] [PubMed] [Google Scholar]
- 174.Cai W, Liu F, Wen Y, Han C, Prasad M, Xia Q, et al. Pain management in acute pancreatitis: a systematic review and meta-analysis of randomised controlled trials. Front Med (Lausanne). 2021;8:782151. doi: 10.3389/fmed.2021.782151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thavanesan N, White S, Lee S, Ratnayake B, Oppong KW, Nayar MK, et al. Analgesia in the initial management of acute pancreatitis: a systematic review and meta-analysis of randomised controlled trials. World J Surg. 2022;46(4):878–890. doi: 10.1007/s00268-021-06420-w. [DOI] [PubMed] [Google Scholar]
- 176.Thompson DR. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol. 2001;96(4):1266–1272. doi: 10.1111/j.1572-0241.2001.03536.x. [DOI] [PubMed] [Google Scholar]
- 177.Basurto Ona X, Rigau Comas D, Urrútia G. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev. 2013;26(7):Cd009179. doi: 10.1002/14651858.CD009179.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Petrov MS, McIlroy K, Grayson L, Phillips AR, Windsor JA. Early nasogastric tube feeding versus nil per os in mild to moderate acute pancreatitis: a randomized controlled trial. Clin Nutr. 2013;32(5):697–703. doi: 10.1016/j.clnu.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 179.Moore D, Sud A, Cheng J, Alves D, Huang W, Sutton R. Clinical measures to capture stratified outcomes of mild, moderate and severe acute pancreatitis. Int J Surg. 2018;2018(55):S64. doi: 10.1016/j.ijsu.2018.05.293. [DOI] [Google Scholar]
- 180.Bainbridge D, Martin JE, Cheng DC. Patient-controlled versus nurse-controlled analgesia after cardiac surgery—a meta-analysis. Can J Anaesth. 2006;53(5):492–499. doi: 10.1007/BF03022623. [DOI] [PubMed] [Google Scholar]
- 181.Jabaudon M, Belhadj-Tahar N, Rimmelé T, Joannes-Boyau O, Bulyez S, Lefrant JY, et al. Thoracic epidural analgesia and mortality in acute pancreatitis: a multicenter propensity analysis. Crit Care Med. 2018;46(3):e198–e205. doi: 10.1097/CCM.0000000000002874. [DOI] [PubMed] [Google Scholar]
- 182.Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101(13):1644–1656. doi: 10.1002/bjs.9665. [DOI] [PubMed] [Google Scholar]
- 183.Yao Q, Liu P, Peng S, Xu X, Wu Y. Effects of immediate or early oral feeding on acute pancreatitis: a systematic review and meta-analysis. Pancreatology. 2022;22(2):175–184. doi: 10.1016/j.pan.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 184.Lakananurak N, Gramlich L. Nutrition management in acute pancreatitis: clinical practice consideration. World J Clin Cases. 2020;8(9):1561–1573. doi: 10.12998/wjcc.v8.i9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2) Lancet. 2018;391(10116):133–143. doi: 10.1016/S0140-6736(17)32146-3. [DOI] [PubMed] [Google Scholar]
- 186.Reintam Blaser A, Preiser JC, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24(1):224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alsharif DJ, Alsharif FJ, Aljuraiban GS, Abulmeaty MMA. Effect of supplemental parenteral nutrition versus enteral nutrition alone on clinical outcomes in critically ill adult patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(10):2968. doi: 10.3390/nu12102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Simha V. Management of hypertriglyceridemia. BMJ. 2020;12(371):m3109. doi: 10.1136/bmj.m3109. [DOI] [PubMed] [Google Scholar]
- 189.Fei F, Boshell N, Williams LA., 3rd Predictability and efficacy of therapeutic plasma exchange for hypertriglyceridemia induced acute pancreatitis. Transfus Apher Sci. 2019;24:102699. doi: 10.1016/j.transci.2019.102699. [DOI] [PubMed] [Google Scholar]
- 190.Song X, Shi D, Cui Q, Yu S, Yang J, Song P, et al. Intensive insulin therapy versus plasmapheresis in the management of hypertriglyceridemia-induced acute pancreatitis (Bi-TPAI trial): study protocol for a randomized controlled trial. Trials. 2019;20(1):365. doi: 10.1186/s13063-019-3498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral nutrition and lipids. Nutrients. 2017;9(4):388. doi: 10.3390/nu9040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev. 2017;4(4):Cd012645. doi: 10.1002/14651858.CD012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. Front Physiol. 2019;10:1002. doi: 10.3389/fphys.2019.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 195.Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183(5):1261–1265. doi: 10.2214/ajr.183.5.1831261. [DOI] [PubMed] [Google Scholar]
- 196.Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011;8(7):405–415. doi: 10.1038/nrgastro.2011.91. [DOI] [PubMed] [Google Scholar]
- 197.Weinberger J, Cocoros N, Klompas M. Ventilator-associated events: epidemiology, risk factors, and prevention. Infect Dis Clin N Am. 2021;35(4):871–899. doi: 10.1016/j.idc.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 198.Reddy K, Sinha P, O'Kane CM, Gordon AC, Calfee CS, McAuley DF. Subphenotypes in critical care: translation into clinical practice. Lancet Respir Med. 2020;8(6):631–643. doi: 10.1016/S2213-2600(20)30124-7. [DOI] [PubMed] [Google Scholar]
- 199.Parniczky A, Lantos T, Toth EM, Szakacs Z, Godi S, Hagendorn R, et al. Antibiotic therapy in acute pancreatitis: from global overuse to evidence based recommendations. Pancreatology. 2019;19(4):488–499. doi: 10.1016/j.pan.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 200.Moran RA, Halloran C, Guo Q, Umapathy C, Jalaly NY, Jain S, et al. Early infection is an independent risk factor for increased mortality in patients with culture-confirmed infected pancreatic necrosis. Pancreatology. 2022;22(1):67–73. doi: 10.1016/j.pan.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 201.Fugazzola P, Ceresoli M, Coccolini F, Gabrielli F, Puzziello A, Monzani F, et al. The WSES/SICG/ACOI/SICUT/AcEMC/SIFIPAC guidelines for diagnosis and treatment of acute left colonic diverticulitis in the elderly. World J Emerg Surg. 2022;17(1):5. doi: 10.1186/s13017-022-00408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Singh RR, Mitchell W, David Y, Cheesman A, Dixon RE, Nagula S, et al. Pancreatic fungal infection in patients with necrotizing pancreatitis: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55(3):218–226. doi: 10.1097/MCG.0000000000001467. [DOI] [PubMed] [Google Scholar]
- 203.Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67–75.e1. doi: 10.1053/j.gastro.2019.07.064. [DOI] [PubMed] [Google Scholar]
- 204.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 205.Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, et al. Superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 2019;156(4):1016–1026. doi: 10.1053/j.gastro.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 206.Saunders R, Neoptolemos JP, Hughes F, Ghaneh P, Halloran CM. Single port retroperitoneal pancreatic necrosectomy for the treatment of extra-pancreatic walled off necrotic collections. Ann Surg Open. 2021;2(1):e019. doi: 10.1097/AS9.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Boxhoorn L, van Dijk SM, van Grinsven J, Verdonk RC, Boermeester MA, Bollen TL, et al. Immediate versus postponed intervention for infected necrotizing pancreatitis. N Engl J Med. 2021;385(15):1372–1381. doi: 10.1056/NEJMoa2100826. [DOI] [PubMed] [Google Scholar]
- 208.van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391(10115):51–58. doi: 10.1016/S0140-6736(17)32404-2. [DOI] [PubMed] [Google Scholar]
- 209.Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, et al. An endoscopic transluminal approach, compared with minimally invasive surgery, reduces complications and costs for patients with necrotizing pancreatitis. Gastroenterology. 2019;156(4):1027–40.e3. doi: 10.1053/j.gastro.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 210.Saunders R, Hughes FE, Evans JC, Smart HL, Ghaneh P, Ramesh J, et al. Cost analysis and outcomes of endoscopic, minimal access and open pancreatic necrosectomy. Ann Surg Open. 2021;2(2):e068. doi: 10.1097/AS9.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, et al. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore) 2018;97(22):e10713. doi: 10.1097/MD.0000000000010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: a systematic review and meta-analysis. Front Physiol. 2019;10:637. doi: 10.3389/fphys.2019.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dovc K, Battelino T. Evolution of diabetes technology. Endocrinol Metab Clin N Am. 2020;49(1):1–18. doi: 10.1016/j.ecl.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 214.Huang W, de la Iglesia-Garcia D, Baston-Rey I, Calvino-Suarez C, Larino-Noia J, Iglesias-Garcia J, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci. 2019;64(7):1985–2005. doi: 10.1007/s10620-019-05568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(Suppl 6):vi1–vi28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.da Costa DW, Dijksman LM, Bouwense SA, Schepers NJ, Besselink MG, van Santvoort HC, et al. Cost-effectiveness of same-admission versus interval cholecystectomy after mild gallstone pancreatitis in the PONCHO trial. Br J Surg. 2016;103(12):1695–1703. doi: 10.1002/bjs.10222. [DOI] [PubMed] [Google Scholar]
- 217.Jee SL, Jarmin R, Lim KF, Raman K. Outcomes of early versus delayed cholecystectomy in patients with mild to moderate acute biliary pancreatitis: a randomized prospective study. Asian J Surg. 2018;41(1):47–54. doi: 10.1016/j.asjsur.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 218.Umans DS, Hallensleben ND, Verdonk RC, Bouwense SAW, Fockens P, van Santvoort HC, et al. Recurrence of idiopathic acute pancreatitis after cholecystectomy: systematic review and meta-analysis. Br J Surg. 2020;107(3):191–199. doi: 10.1002/bjs.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149(6):1490–500.e1. doi: 10.1053/j.gastro.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 220.Nikkola J, Raty S, Laukkarinen J, Seppanen H, Lappalainen-Lehto R, Jarvinen S, et al. Abstinence after first acute alcohol-associated pancreatitis protects against recurrent pancreatitis and minimizes the risk of pancreatic dysfunction. Alcohol Alcohol. 2013;48(4):483–486. doi: 10.1093/alcalc/agt019. [DOI] [PubMed] [Google Scholar]
- 221.Nordback I, Pelli H, Lappalainen-Lehto R, Järvinen S, Räty S, Sand J. The recurrence of acute alcohol-associated pancreatitis can be reduced: a randomized controlled trial. Gastroenterology. 2009;136(3):848–855. doi: 10.1053/j.gastro.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 222.Sorrento C, Shah I, Yakah W, Ahmed A, Tintara S, Kandasamy C, et al. Inpatient alcohol cessation counseling is associated with a lower 30-day hospital readmission in acute alcoholic pancreatitis. J Clin Gastroenterol. 2022 Jan 10; Online ahead of print. [DOI] [PubMed]
- 223.Pilling S, Yesufu-Udechuku A, Taylor C, Drummond C. Diagnosis, assessment, and management of harmful drinking and alcohol dependence: summary of NICE guidance. BMJ. 2011;23(342):d700. doi: 10.1136/bmj.d700. [DOI] [PubMed] [Google Scholar]
- 224.Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Tobacco smoking and the risk of pancreatitis: a systematic review and meta-analysis of prospective studies. Pancreatology. 2019;19(8):1009–1022. doi: 10.1016/j.pan.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 225.Spagnolo DM, Greer PJ, Ohlsen CS, Mance S, Ellison M, Breze C, et al. Acute and chronic pancreatitis disease prevalence, classification, and comorbidities: a cohort study of the UK BioBank. Clin Transl Gastroenterol. 2022;13(1):e00455. doi: 10.14309/ctg.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fitts E, Lee PDK, Yates SG. Efficacy of therapeutic plasma exchange in reducing the incidence of recurrent pancreatitis related to familial chylomicronemia. Transfusion. 2019;59(11):3324–3328. doi: 10.1111/trf.15532. [DOI] [PubMed] [Google Scholar]
- 228.de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United Eur Gastroenterol J. 2018;6(5):649–655. doi: 10.1177/2050640618755002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381(6):531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 230.Rank CU, Wolthers BO, Grell K, Albertsen BK, Frandsen TL, Overgaard UM, et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL2008 treatment of patients 1–45 years of age. J Clin Oncol. 2020;38(2):145–154. doi: 10.1200/JCO.19.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bazerbachi F, Haffar S, Hussain MT, Vargas EJ, Watt KD, Murad MH, et al. Systematic review of acute pancreatitis associated with interferon-α or pegylated interferon-α: possible or definitive causation? Pancreatology. 2018;18(7):691–699. doi: 10.1016/j.pan.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 232.Wolfe D, Kanji S, Yazdi F, Skidmore B, Moher D, Hutton B. Methods for the early detection of drug-induced pancreatitis: a systematic review of the literature. BMJ Open. 2019;9(11):e027451. doi: 10.1136/bmjopen-2018-027451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Akshintala VS, Kamal A, Faghih M, Cutting GR, Cebotaru L, West NE, et al. Cystic fibrosis transmembrane conductance regulator modulators reduce the risk of recurrent acute pancreatitis among adult patients with pancreas sufficient cystic fibrosis. Pancreatology. 2019;19(8):1023–1026. doi: 10.1016/j.pan.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 234.Gould MJ, Smith H, Rayment JH, Machida H, Gonska T, Galante GJ. CFTR modulators increase risk of acute pancreatitis in pancreatic insufficient patients with cystic fibrosis. J Cyst Fibros. 2022;21(4):600–2. [DOI] [PubMed]
- 235.Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356(7):676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 236.Chinnakotla S, Bellin MD, Schwarzenberg SJ, Radosevich DM, Cook M, Dunn TB, et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. 2014;260(1):56–64. doi: 10.1097/SLA.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee HM, Deheragoda M, Harrison P, Devlin J, Sellars M, Hadzic N, et al. Autoimmune pancreatitis in children: a single centre experience in diagnosis, management and long term follow up. Pancreatology. 2019;19(1):169–176. doi: 10.1016/j.pan.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 238.Matsubayashi H, Ishiwatari H, Imai K, Kishida Y, Ito S, Hotta K, et al. Steroid therapy and steroid response in autoimmune pancreatitis. Int J Mol Sci. 2019;21(1):257. doi: 10.3390/ijms21010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25(1):108. doi: 10.1186/s13054-021-03535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6(8):668–675. doi: 10.1016/S2468-1253(21)00019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Huang W, de la Iglesia-García D, Baston-Rey I, Calviño-Suarez C, Lariño-Noia J, Iglesias-Garcia J, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci. 2019;64(7):1985–2005. doi: 10.1007/s10620-019-05568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63(5):818–831. doi: 10.1136/gutjnl-2013-305062. [DOI] [PubMed] [Google Scholar]
- 243.Whitcomb DC. Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut. 1999;45(3):317–322. doi: 10.1136/gut.45.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(6):1729–1736. doi: 10.1053/j.gastro.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 245.Pendharkar SA, Salt K, Plank LD, Windsor JA, Petrov MS. Quality of life after acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2014;43(8):1194–1200. doi: 10.1097/MPA.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 246.de-Madaria E, Sánchez-Marin C, Carrillo I, Vege SS, Chooklin S, Bilyak A, et al. Design and validation of a patient-reported outcome measure scale in acute pancreatitis: the PAN-PROMISE study. Gut. 2021;70(1):139–147. doi: 10.1136/gutjnl-2020-320729. [DOI] [PubMed] [Google Scholar]
- 247.Geraci G, Palumbo VD, D'Orazio B, Maffongelli A, Fazzotta S, Lo Monte AI. Rectal Diclofenac administration for prevention of post-Endoscopic Retrograde Cholangio-Pancreatography (ERCP) acute pancreatitis. Randomized prospective study. Clin Ter. 2019;170(5):e332–e336. doi: 10.7417/CT.2019.2156. [DOI] [PubMed] [Google Scholar]
- 248.Bruen C, Miller J, Wilburn J, Mackey C, Bollen TL, Stauderman K, et al. Auxora for the treatment of patients with acute pancreatitis and accompanying systemic inflammatory response syndrome: clinical development of a calcium release-activated calcium channel inhibitor. Pancreas. 2021;50(4):537–543. doi: 10.1097/MPA.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Akshintala VS, Sperna Weiland CJ, Bhullar FA, Kamal A, Kanthasamy K, Kuo A, et al. Non-steroidal anti-inflammatory drugs, intravenous fluids, pancreatic stents, or their combinations for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(9):733–742. doi: 10.1016/S2468-1253(21)00170-9. [DOI] [PubMed] [Google Scholar]
- 250.Tse F, Yuan Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst Rev. 2012;16(5):CD009779. doi: 10.1002/14651858.CD009779.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22(6):405–432. doi: 10.1002/jhbp.259. [DOI] [PubMed] [Google Scholar]
- 252.Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48(1):62–69. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen F, Zou L, Williams B, Chao W. Targeting Toll-like receptors in sepsis: from bench to clinical trials. Antioxid Redox Signal. 2021;35(15):1324–1339. doi: 10.1089/ars.2021.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dong S, Zhao Z, Li X, Chen Z, Jiang W, Zhou W. Efficacy of glutamine in treating severe acute pancreatitis: a systematic review and meta-analysis. Front Nutr. 2022;9:865102. doi: 10.3389/fnut.2022.865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhou J, Xue Y, Liu Y, Li XK, Tong ZH, Li WQ. The effect of immunonutrition in patients with acute pancreatitis: a systematic review and meta-analysis. J Hum Nutr Diet. 2021;34(2):429–439. doi: 10.1111/jhn.12816. [DOI] [PubMed] [Google Scholar]
- 256.Wolbrink DRJ, Grundsell JR, Witteman B, Poll MV, Santvoort HCV, Issa E, et al. Are omega-3 fatty acids safe and effective in acute pancreatitis or sepsis? A systematic review and meta-analysis. Clin Nutr. 2020;39(9):2686–2694. doi: 10.1016/j.clnu.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 257.Qiu Q, Li GJ, Tang L, Guo Y, Wen LZ, Wang B, et al. The efficacy of low molecular weight heparin in severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. J Dig Dis. 2019;20(10):512–522. doi: 10.1111/1751-2980.12815. [DOI] [PubMed] [Google Scholar]
- 258.Chen H, Bai Z, Li H, Wu Y, Yao H, Wang L, et al. Efficacy of Xuebijing injection for acute pancreatitis: a systematic review and meta-analysis of randomized controlled trialS. Evid Based Complement Alternat Med. 2021;2021:6621368. doi: 10.1155/2021/6621368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang G, Shang D, Zhang G, Zhang S, Jiang N, Liu H, et al. Effects of QingYi decoction on inflammatory markers in patients with acute pancreatitis: a meta-analysis. Phytomedicine. 2022;95:153738. doi: 10.1016/j.phymed.2021.153738. [DOI] [PubMed] [Google Scholar]
- 260.Barkin JS, Casal GL, Reiner DK, Goldberg RI, Phillips RS, Kaplan S. A comparative study of contrast agents for endoscopic retrograde pancreatography. Am J Gastroenterol. 1991;86(10):1437–1441. [PubMed] [Google Scholar]
- 261.Banerjee AK, Grainger SL, Thompson RP. Trial of low versus high osmolar contrast media in endoscopic retrograde cholangiopancreatography. Br J Clin Pract. 1990;44(11):445–447. [PubMed] [Google Scholar]
- 262.Takenaka M, Fujita T, Sugiyama D, Masuda A, Shiomi H, Sugimoto M, et al. What is the most adapted indication of prophylactic pancreatic duct stent within the high-risk group of post-endoscopic retrograde cholangiopancreatography pancreatitis? Using the propensity score analysis. J Hepatobiliary Pancreat Sci. 2014;21(4):275–280. doi: 10.1002/jhbp.24. [DOI] [PubMed] [Google Scholar]
- 263.Elmunzer BJ, Higgins PD, Saini SD, Scheiman JM, Parker RA, Chak A, et al. Does rectal indomethacin eliminate the need for prophylactic pancreatic stent placement in patients undergoing high-risk ERCP? Post hoc efficacy and cost-benefit analyses using prospective clinical trial data. Am J Gastroenterol. 2013;108(3):410–415. doi: 10.1038/ajg.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28(2):130–140. doi: 10.1097/shk.0b013e3180487ba1. [DOI] [PubMed] [Google Scholar]
- 265.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 266.Knoph CS, Cook ME, Fjelsted CA, Novovic S, Mortensen MB, Nielsen LBJ, et al. Effects of the peripherally acting μ-opioid receptor antagonist methylnaltrexone on acute pancreatitis severity: study protocol for a multicentre double-blind randomised placebo-controlled interventional trial, the PAMORA-AP trial. Trials. 2021;22(1):940. doi: 10.1186/s13063-021-05885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ke L, Zhou J, Mao W, Chen T, Zhu Y, Pan X, et al. Immune enhancement in patients with predicted severe acute necrotising pancreatitis: a multicentre double-blind randomised controlled trial. Intensive Care Med. 2022;48(7):899–909. doi: 10.1007/s00134-022-06745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nagib MM, Zhang S, Yasmen N, Li L, Hou R, Yu Y, et al. Inhibition of TRPC3 channels by a novel pyrazole compound confers antiseizure effects. Epilepsia. 2022;63(4):1003–1015. doi: 10.1111/epi.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. 2018;175(10):1744–1759. doi: 10.1111/bph.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thapak P, Vaidya B, Joshi HC, Singh JN, Sharma SS. Therapeutic potential of pharmacological agents targeting TRP channels in CNS disorders. Pharmacol Res. 2020;159:105026. doi: 10.1016/j.phrs.2020.105026. [DOI] [PubMed] [Google Scholar]
- 271.Wen L, Javed TA, Yimlamai D, Mukherjee A, Xiao X, Husain SZ. Transient high pressure in pancreatic ducts promotes inflammation and alters tight junctions via calcineurin signaling in mice. Gastroenterology. 2018;155(4):1250–63.e5. doi: 10.1053/j.gastro.2018.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Narazaki M, Kishimoto T. Current status and prospects of IL-6-targeting therapy. Expert Rev Clin Pharmacol. 2022;7:1–18. doi: 10.1080/17512433.2022.2097905. [DOI] [PubMed] [Google Scholar]
- 274.Walker AL, Ancellin N, Beaufils B, Bergeal M, Binnie M, Bouillot A, et al. Development of a series of kynurenine 3-monooxygenase inhibitors leading to a clinical candidate for the treatment of acute pancreatitis. J Med Chem. 2017;60(8):3383–3404. doi: 10.1021/acs.jmedchem.7b00055. [DOI] [PubMed] [Google Scholar]
- 275.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]