Abstract
Simple Summary
The gut microbiome is crucial for lactation sow health and production. This study aimed to investigate the effect of dietary silymarin supplementation on the inflammatory responses and fecal microbiota in lactation sow and to evaluate the relationship between the inflammatory factor and fecal microbiota in lactation sow. It is suggested in our results that silymarin supplementation decreased inflammatory responses of lactation sow and changed fecal microbiota composition at different taxonomic levels. The altered fecal microbiota was associated with variations in inflammatory factors, indicating that silymarin modulates microbiota in the gut and may improve the health of lactation sow.
Abstract
Inflammatory responses reduce milk production in lactating sows. Silymarin may modulate inflammatory reactions. Here, we aimed to verify whether dietary silymarin supplementation could alleviate inflammatory responses in lactating sows through microbiota change in the gut. We also investigated how silymarin impacts inflammatory response in lactating sows. One hundred and ten sows were randomly assigned to a control diet (basal diet) or treatment diet (basal diet and 40 g/d silymarin) from the 108th day of gestation to weaning. Blood, milk, and feces from sows were collected for analysis. It was shown in the results that dietary silymarin supplementation decreased the level of pro-inflammatory cytokine IL-1β (p < 0.05) on the 18th day of lactation in the blood of the sows. Dietary silymarin supplementation tended to decrease (p = 0.06) somatic cell count in the colostrum of sows. Dietary silymarin supplementation reduced the gut bacterial community and the richness of the gut microbial community (p < 0.01) using 16S rRNA gene sequencing. The fecal microbes varied at different taxonomic levels in the lactating sows with silymarin supplementation. The most representative changes included an increase in the relative abundance of Fibrobacteres and Actinobacteria (p < 0.05) and tended to reduce the relative abundance of Spirochaetaes and Tenericutes (p = 0.09, 0.06) at the phylum level. It is suggested that dietary silymarin supplementation in late gestation until lactation has anti-inflammatory effects in lactation sow, which could be associated with the modulation of gut microbiota.
Keywords: inflammation, microbiota, silymarin, sow
1. Introduction
Silymarin is a 25-carbon flavonolignan mixture extracted from the plant Silybum marianum (L.) Gaertn. (Asteraceae) (milk thistle) [1]. The main components of silymarin are four isomers: silybin, isosilybin, silydianin, and silychristin [2]. In our previous study, dietary silymarin supplementation during the sow transition and lactation reduced oxidative stress, decreased TNF-α concentration in serum on the seventh day of lactation, and increased the colostrum yield of sows [3]. Sows fed micelle silymarin from the 109th prenatal day to the 21st postnatal day increased their feed intake and milk yields during lactation, along with increased sow antioxidant capacity [4]. It has been found in previous studies that the functional mechanism of silymarin includes anti-inflammatory, anti-oxidation, scavenging free radicals, regulating intracellular glutathione peroxidase content, stabilizing cell membrane, regulating cell permeability, and improving liver regeneration ability [5,6,7]. However, there remains little knowledge of the maternal diversity of gut microbiota following silymarin supplementation during late gestation to lactation. The flavonoids extracted from S. marianum were active against Staphylococcus aureus, Staphylococcus albus, Candida albicans, and Saccharomyces cerevisiae [8]. This suggests that dietary silymarin supplementation leads to variation in the microbiota in the gut of sows.
Placental mitochondria and mitochondrial electron chain enzyme activity increase in sows, especially in those before farrowing, with the rise of gestational age and fetal metabolic intensity. This process increases reactive oxygen species (ROS) and maternal oxidative stress [9]. Cell-generated ROS can activate the tumor necrosis factor-α (NF-κB) signaling pathway [10], leading to an inflammation response that adversely affects sow reproductive performance and health [11,12]. Furthermore, gut microbiota plays a critical role in the metabolism of nutrients, immune function, and the health of animals. At the same time, the microbiota is also regulated by the nutrient intake and immune and metabolic status of the host. The transition period from gestation to lactation is a crucial window for microbiota remodeling in sows [13]. The top dominant phyla were Firmicutes (35.67–73.96%), Bacteroidetes (11.95–51.43%), Proteobacteria (3.40–6.54%), Tenericutes (2.50–5.69%), and Spirochaetes (0.98–9.87%) on the 14th and the 21st day of lactation of sows [13,14]. However, whether silymarin supplementation in the diet of sows from late gestation to lactation has a correlation between sow gut microbiota and inflammation and then affects the health of the sow remains to be further investigated. Therefore, this study aimed to evaluate the hypothesis that silymarin supplementation might modulate microbiota in the gut of sows and change the inflammatory response, thus affecting the health of lactation sows.
2. Materials and Methods
The experimental procedures were approved by the Guide for the Care and Use of Laboratory Animals prepared by the Animal Care and Use Committee of Sichuan Agricultural University. Silymarin was provided by Tianben Bio-Engineering Co., Ltd. (Xi’an, China), which contained 10.32% silybin, 15.64% silydianin plus silychristin, and 6.91% isosilybin as the main ingredients.
2.1. Animals and Experimental Design
A total of 110 sows (Landrace × Yorkshire, parity 1–8, 19.50 ± 0.36 mm backfat thickness) were used. Half of the sows in each parity were fed a control diet (CTL) (basic diet, n = 55) or silymarin diet (TRT) (basic diet + 40 g/d silymarin, n = 55) from the 108th day of gestation until weaning. The basal diet for this study was the same as in our previous study [3]. The basal lactation diet contained 14.22 MJ of digestible energy per kilogram (DE/kg), 17.00% crude protein, 1.06% Lys, 0.80% calcium, and 0.35% standardized total tract digestible phosphorus.
Sows were housed in individual farrowing crates on the 108th day of gestation. Sows were fed an average diet of 3.5 kg/d and two times/d during the late gestation stage, fed a 1.34 kg of diet on the day of farrowing, and then gradually increased by 0.5 kg/d and two times/d up to the maximum amount of feeding. During the experiment, free access to water was maintained.
2.2. Sample Collection
Colostrum samples were collected within one hour after the end of farrowing from multiparous sows (n = 10/treatment group, parity 2–4, randomly selected) and stored at -20 °C until analysis. On the 18th day of lactation, 20 mL milk samples (n = 10/treatment group, same sow that collected colostrum sample) were collected after injection of 1.0 mL of oxytocin (20 IU/mL, Hangzhou Animal Drug Factory, Hangzhou, China) via the ear vein. One tube of milk sample was stored at −20 °C until analysis. Another milk tube was centrifuged at 8000× g for 15 min at 4 °C to obtain milk serum and then stored in a −20 °C freezer for later use.
Blood samples were collected (n = 10/treatment group, sow, which collected milk samples) on the 18th day of lactation through ear venipuncture. After centrifuging at 3000× g for 15 min at 4 °C, a serum sample was obtained, kept in liquid nitrogen, and stored at –20 °C until analysis.
For each treatment, 10 sows were selected, and their colostrum was taken. In the early morning on the 18th day of lactation, three tubes of fresh feces (≈5 g/tube) were directly collected by massaging the rectum of each sow. Fresh feces were stored in a sterile tube and kept in liquid nitrogen before being transferred to −80 °C.
2.3. Somatic Cell and Cytokine Analyses
The somatic cell count (SCC) in milk was determined using a FOSS MATIC 5000 (Foss Electric A/S, Hillerød, Denmark). Serum and milk serum interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) concentrations were analyzed by corresponding ELISA assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China; porcine specific antibodies) according to the manufacturer’s instructions.
2.4. Bacterial Community Analysis
The composition of the microbial community in the feces (n = 10/treatment group) was analyzed by high-throughput pyrosequencing, as previously described [15]. The sequencing and bioinformatics analyses were performed by Novogene Bioinformatics Technology Co. (Beijing, China).
The data were analyzed following our previous protocol [13]. Briefly, high-quality tags were filtered and clustered into operational taxonomic unit (OTU) utilizing Uparse v7.0.1001 (http://drive5.com/uparse/, accessed on 20 December 2019) at 97% sequence similarity. The Ribosomal Database Project (RDP) classifier Version 2.2 (http://github.com/rdpstaff/ (accessed on 20 December 2019)) was applied to assign a taxonomy for 16S rRNA gene sequences. The representative sequence of OTUs was annotated. A Venn diagram was generated to compare the OTUs of the treatment groups. Alpha diversity values for each sample were assessed using Qiime 1.7.0. Principal coordinates analysis (PCoA) plots were produced using unweighted UniFrac metrics.
2.5. Statistical Analysis
Before the analyses, descriptive statistics were performed to check the normality and homogeneity of variances. The data were analyzed using t-tests in SAS 9.4 (SAS Institute, Cary, NC, USA). The results are presented as mean ± SE, except for microbial data, which are represented by mean ± SD. p < 0.05 was considered statistically significant, and 0.05 ≤ p < 0.10 was considered a tendency.
Correlations between gut microbiota and sow serum cytokines were analyzed with Spearman’s correlation in R 3.0.2 with the Rstudio 0.97.310 package. Differences of p < 0.05 were considered statistically significant, and 0.05 ≤ p < 0.10 was considered a tendency.
3. Results
3.1. Effect of Dietary Silymarin Supplementation on the Somatic Cell Count of Lactating Sows
Dietary supplementation with silymarin in late gestation and lactation tended to decrease somatic cell count in the colostrum of sows (p = 0.06, Table 1). Still, no effect on milk somatic cell count on the 18th day of lactation was detected in the sows.
Table 1.
Effects of dietary silymarin supplementation during late gestation and lactation on the somatic cell count in sow milk.
| CTL | TRT | p-Value | |
|---|---|---|---|
| Colostrum (log10/mL) | 5.83 ± 0.10 | 5.57 ± 0.08 | 0.06 |
| Milk (log10/mL) | 5.69 ± 0.11 | 5.51 ± 0.07 | 0.17 |
Data are expressed as means ± standard error, n = 10 for each group (parity 2 to 4). CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation.
3.2. Effect of Dietary Silymarin Supplementation on the Cytokines of Lactating Sows
Dietary silymarin supplementation in late gestation and lactating sows decreased IL-1β concentration in the blood on the 18th day of lactation (p < 0.01, Table 2). No difference in blood serum was observed in IL-6, IL-10, and TNF-α between the treatment and control groups. No difference was observed in the cytokines in the milk serum of lactating sows.
Table 2.
Effects of dietary silymarin supplementation during late gestation and lactation on the cytokines in blood and milk serum of lactating sows.
| CTL | TRT | p-Value | |
|---|---|---|---|
| Blood | |||
| IL-1β, ng/mL | 0.10 ± 0.02 a | 0.04 ± 0.01 b | <0.01 |
| IL-6, ng/mL | 1.13 ± 0.26 | 0.78 ± 0.18 | 0.48 |
| IL-10, ng/mL | 0.69 ± 0.13 | 0.72 ± 0.13 | 0.89 |
| TNF-α, ng/mL | 0.30 ± 0.06 | 0.23 ± 0.04 | 0.59 |
| Milk serum | |||
| IL-1β, pg/L | 73.66 ± 6.41 | 85.23 ± 8.69 | 0.30 |
| IL-6, pg/mL | 84.71 ± 11.22 | 75.10 ± 12.63 | 0.58 |
| IL-10, pg/mL | 17.43 ± 3.09 | 12.71 ± 1.95 | 0.23 |
| TNF-α, pg/mL | 25.57 ± 2.08 | 28.04 ± 2.36 | 0.44 |
Data are expressed as means ± standard error, n = 10 for each group (parity 2 to 4). CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation. a,b Means not sharing identical superscripts in the same row are significantly different (p < 0.05).
3.3. Effect of Silymarin on Microbiota in the Feces of Lactating Sows
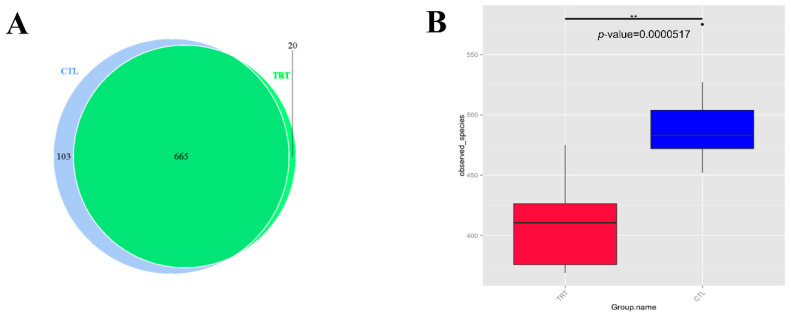
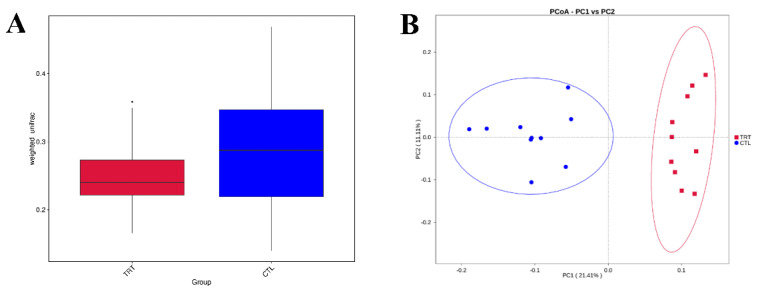
A total of 20 fecal samples were subjected to 16S rRNA gene sequencing. A set of 665 OTUs existed in the two groups and was defined as core OTUs (Figure 1). Dietary supplementation with silymarin in late gestation and lactation reduced the microbial diversity in the feces of lactating sows (Figure 1A,B). Silymarin also reduced the richness (ACE, Chao1 index, p < 0.01, Figure 2A,B) and diversity (p < 0.1, Figure 2C) of the sow gut microbial community. For beta diversity analysis, the distribution of microbiota in sow feces with a diet supplemented with silymarin in late pregnancy and lactation was significantly different from that in the control group (Figure 3A). Cluster analysis was performed on the samples, and a PCoA cluster diagram was constructed. The silymarin treatment group and the control group had apparent clusters according to the principal coordinate analysis (Figure 3B).
Figure 1.
Dietary silymarin supplementation modulated microbiota diversity in lactation sow. (A) A Venn diagram was generated to describe the common and unique OTUs between the treatment groups. (B) The effect of silymarin on the observed species. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation. ** are significantly different (p < 0.01).
Figure 2.
Dietary silymarin supplementation modulated the microbiota alpha diversity index of lactation sow, including richness (ACE, Chao 1 index) and diversity (Shannon, Simpson index). (A) ACE index: p < 0.01. (B) Chao 1 index: p < 0.01. (C) Shannon index: p = 0.097. (D) Simpson index: p = 0.39. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation. ** are significantly different (p < 0.01).
Figure 3.
Dietary silymarin supplementation modulated microbiota beta diversity in lactation sow. (A) Beta diversity analysis based on Weighted Unifrac distance. (B) Principal coordinates analysis of each sample based on unweighted UniFrac metric. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation.
3.4. Changes in Fecal Microbiota Composition by Silymarin Supplementation in Lactating Sows
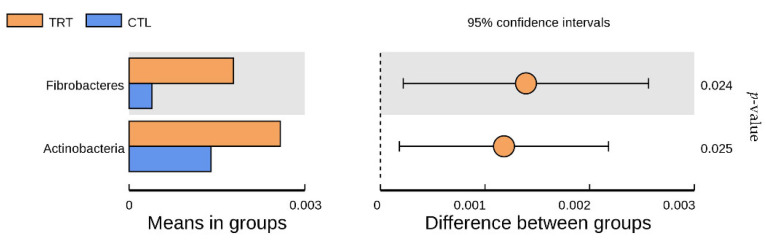
When silymarin was supplemented to the diet of sows during late gestation and lactation, there were eight dominant phyla with an average relative abundance > 0.1% in the treatment and control groups: Firmicutes, Bacteroidetes, Spirochaetaes, Tenericutes, Proteobacteria, Melainabacteria, Fibrobacteres, and Actinobacteria (Figure 4). Among them, Firmicutes and Bacteroidetes were the most abundant, accounting for more than 95% of the phyla in the samples. Silymarin significantly increased the relative abundance of Fibrobacteres and Actinobacteria (p < 0.05, Figure 5), and tended to reduce the relative abundance of Spirochaetaes and Tenericutes (p = 0.09, 0.06, Table 3) at the phylum level.
Figure 4.
16S rRNA gene analysis revealed phylum-level differences in lactation sow feces between treatments. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation.
Figure 5.
Analysis of distinct species between treatment groups at the phylum level. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation.
Table 3.
The effect of dietary silymarin supplementation on the relative abundances of eight phyla (%, the relative abundance > 0.1%) in sow feces and Firmicutes/Bacteroidetes ratio.
| Item | CTL | TRT | p-Value |
|---|---|---|---|
| Firmicutes | 78.25 ± 6.99 | 80.23 ± 5.54 | 0.49 |
| Bacteroidetes | 17.19 ± 6.07 | 15.94 ± 5.24 | 0.63 |
| Spirochaetes | 1.16 ± 0.96 | 0.51 ± 0.62 | 0.09 |
| Tenericutes | 1.53 ± 0.86 | 0.77 ± 0.79 | 0.06 |
| Proteobacteria | 1.28 ± 0.32 | 1.79 ± 0.82 | 0.10 |
| Melainabacteria | 0.12 ± 0.21 | 0.01 ± 0.01 | 0.12 |
| Fibrobacteres | 0.04 ± 0.06 b | 0.18 ± 0.16 a | 0.02 |
| Actinobacteria | 0.14 ± 0.04 b | 0.26 ± 0.14 a | 0.02 |
| Firmicutes/Bacteroidetes | 5.77 ± 2.19 | 5.26 ± 2.43 | 0.93 |
Data are expressed as mean ± SD. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation. a,b Means not sharing identical superscripts in the same row are significantly different (p < 0.05).
At the genus level, 11 genera relative abundances changed on the 18th day of lactation based on the dietary silymarin supplementation during late gestation and lactation (Figure 6, Table 4). Silymarin supplementation reduced the relative abundance of unidentified_Ruminococcaceae, unidentified_Bacteroidales, Terrisporobacter, and unidentified_Spirochaetaceae (p < 0.05), and increased the relative abundance of Oscillibacter, Candidatus_Soleaferrea, Succinivibrio, Lachnoclostridium, Fibrobacter, and Marvinbryantia at the genus level (p < 0.05).
Figure 6.
Analysis of distinct species between treatment groups at the genus level. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation.
Table 4.
The effect of dietary silymarin supplementation on the relative abundances at the genus level (%, the relative abundance > 0.15%) in lactation sow feces.
| Item | CTL | TRT | p-Value |
|---|---|---|---|
| unidentified_Clostridiales | 7.11 ± 4.46 | 8.65 ± 5.28 | 0.83 |
| Faecalibacterium | 2.29 ± 1.63 | 0.72 ± 0.93 | 0.91 |
| Lactobacillus | 4.49 ± 2.96 | 2.09 ± 2.09 | 0.93 |
| unidentified_Ruminococcaceae | 5.19 ± 1.70 a | 3.32 ± 1.69 b | 0.02 |
| Parabacteroides | 1.10 ± 0.54 | 2.29 ± 1.83 | 0.18 |
| Subdoligranulum | 0.85 ± 1.61 | 0.09 ± 0.12 | 0.31 |
| unidentified_Christensenellaceae | 1.92 ± 1.69 a | 0.17 ± 0.13 b | 0.01 |
| unidentified_Bacteroidales | 1.16 ± 1.57 | 0.03 ± 0.03 | <0.05 |
| unidentified_Prevotellaceae | 1.48 ± 1.35 | 0.57 ± 0.84 | 0.20 |
| Oscillibacter | 0.64 ± 0.27 b | 1.55 ± 0.58 a | <0.01 |
| Cellulosilyticum | 0.20 ± 0.26 | 0.38 ± 0.11 | 0.27 |
| Candidatus_Soleaferrea | 0.39 ± 0.23 | 0.84 ± 0.60 | <0.05 |
| Terrisporobacter | 0.78 ± 0.51 a | 0.34 ± 0.22 b | 0.03 |
| Butyricicoccus | 0.65 ± 0.35 | 0.56 ± 0.51 | 0.90 |
| Desulfovibrio | 0.88 ± 0.27 | 0.81 ± 0.47 | 0.93 |
| unidentified_Spirochaetaceae | 0.49 ± 0.48 a | 0.08 ± 0.08 b | 0.03 |
| Catenisphaera | 0.18 ± 0.12 | 0.43 ± 0.39 | 0.19 |
| Succinivibrio | 0.17 ± 0.10 | 0.52 ± 0.47 | 0.04 |
| Phascolarctobacterium | 0.75 ± 0.34 | 0.44 ± 0.28 | 0.06 |
| Romboutsia | 0.37 ± 0.34 | 0.36 ± 0.21 | 0.99 |
| Lachnoclostridium | 0.31 ± 0.19 b | 0.59 ± 0.32 a | 0.03 |
| Alloprevotella | 0.39 ± 0.36 | 0.18 ± 0.12 | 0.09 |
| Blautia | 0.13 ± 0.12 | 0.32 ± 0.28 | 0.17 |
| Fibrobacter | 0.04 ± 0.07 b | 0.18 ± 0.16 a | 0.02 |
| Marvinbryantia | 0.06 ± 0.05 b | 0.17 ± 0.10 a | <0.01 |
Data are expressed as mean ± SD. n = 10. CTL, sow fed the basal diet; TRT, sow fed basal diet with silymarin 40 g/d from late gestation to lactation. a,b Means not sharing identical superscripts in the same row are significantly different (p < 0.05).
3.5. Relationship between Gut Microbiota and Blood Cytokines in Lactating Sows
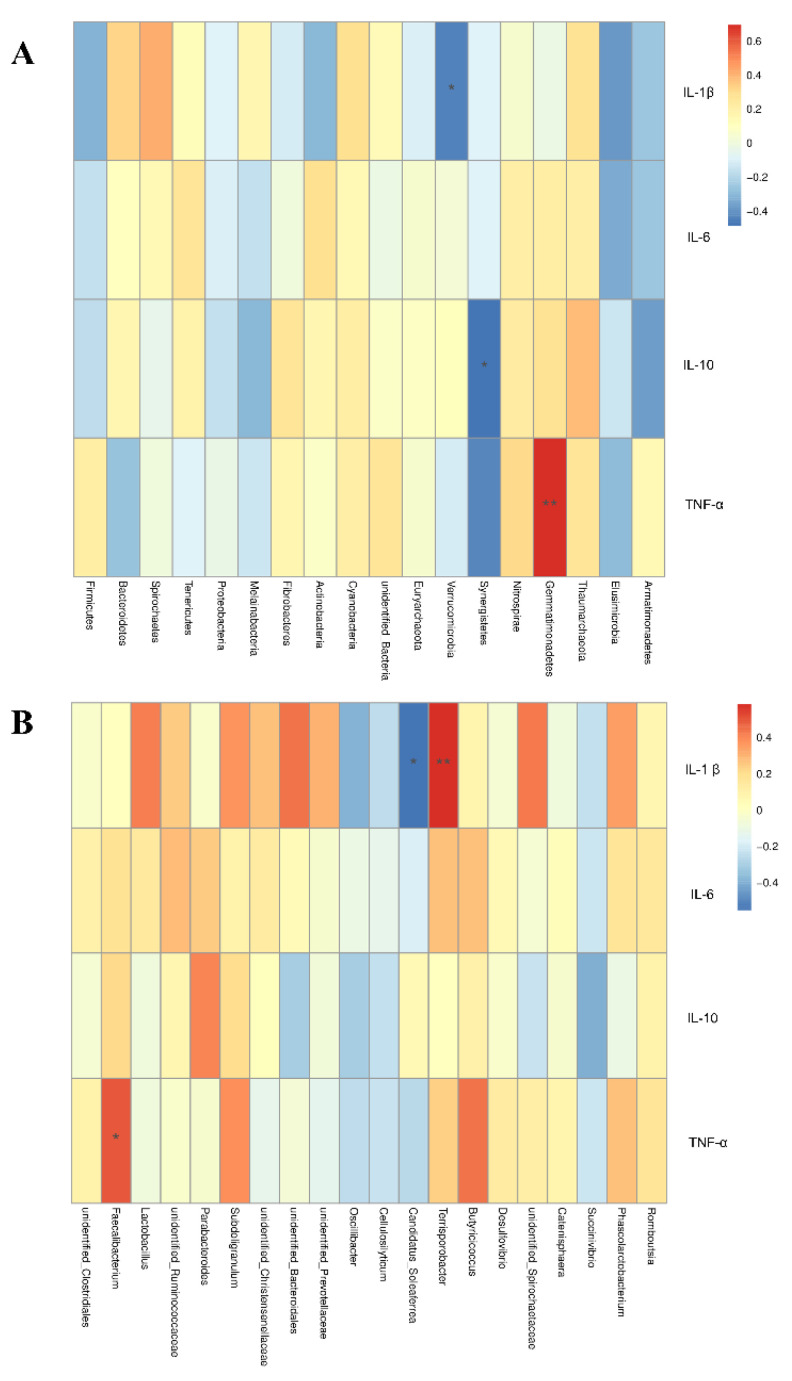
Spearman correlation analysis was used to study the relationship between serum cytokines and microbial species richness. At the phylum level, Gemmatimonadetes was positively correlated with plasma TNF-α (r = 0.69, p < 0.01; Figure 7A). Spirochaetes tended to be positively correlated with serum IL-1β (r = 0.41, p = 0.07). Verrucomicrobia was negatively correlated with serum IL-1β (r = −0.45, p < 0.05). Synergistetes was negatively correlated with serum IL-10 (r = −0.49, p < 0.05). At the genus level, Terrisporobacter was positively correlated with serum IL-1β (r = 0.58, p < 0.01; Figure 7B). However, Candidatus_Soleaferrea was negatively correlated with serum IL-1β (r = −0.55, p < 0.05). Faecalibacterium was positively correlated with serum TNF-α (r = 0.50, p < 0.05).
Figure 7.
Heat map of the Spearman r correlations between gut microbiota and plasma cytokines in lactation sow at the phylum level (A) and the genus level (B). n = 10. * is significantly different (p < 0.05). ** are significantly different (p < 0.01) (following the Spearman correlation analysis).
4. Discussion
In late gestation and lactation, sows face stresses, such as changing stalls, changes in diet, and parturition. These factors change the intestinal microflora, reduce the disease resistance of sow, and are prone to bacterial infection and cause mastitis, which will seriously affect milk production [16]. In this study, although no significant effect on inflammatory factors was found in milk, serum IL-lβ was significantly reduced on the 18th day of lactation after dietary silymarin supplementation, and there were no significant changes in TNF-α, IL-6, and IL-10. However, our previous study found a decrease in TNF-α concentration in serum on the seventh day of lactation. This agrees with Giorgi et al. [17], who found that silibinin can reduce the levels of NF-κB and cytokines TNF-α and IL-1β in preeclamptic women. Studies have shown that silymarin can inhibit the expression of TNF-α [3,18], IL-2, IL-4, and IFN-γ mRNA expression and, at the same time, increase the expression of IL-10 [19], thereby inhibiting the occurrence of inflammation. The dynamics of pro- and anti-inflammatory cytokines changes in sow serum during the peripartum period (day −28 to +28) evaluation found that the levels of IL-8 and IL-10 were stable in healthy sows [20]. No significant changes were observed in IL-10. This result may reflect the fact that the sows in the present experiment were in a healthy state. Interestingly, we also found a downward trend in the log10 somatic cell count in colostrum in this study, one of the best indicators of mastitis status [21]. It has been found in other studies that animals with lower somatic cell counts have higher milk production [21,22,23]. Therefore, this could reflect that silymarin can reduce the sow inflammatory response, improve mammary gland health, and thus improve lactation performance. This was consistent with our previously reported findings that silymarin supplementation in the sow before farrowing and lactation increased sow colostrum production and significantly increased average piglet weaning weight and daily gain [3].
This study was the first to focus on the effect of dietary silymarin supplementation from late gestation to lactation on gut microbial composition in lactation sow. Standardized silymarin extracts contain approximately 65–80% flavonolignans, which were obtained from S. marianum seeds with small amounts of flavonoids [24]. Flavonolignans have an inhibitory effect on intestinal flora metabolism at a concentration of 200 mg/L [25]. It was observed in this study that the supplementation of silymarin in the sow diet in late gestation and lactation reduced the number of OUTs and community richness indices (observed_species, ACE, Chao1) of intestinal flora and changed the structure of intestinal flora. An experiment in mice revealed that silibinin modulates the abundance of several critical bacterial groups involved in the development of Alzheimer’s disease and tends to reduce the diversity of bacterial flora [26]. The gut microbial diversity of sow decreased significantly after receiving 1.0 kg/t lysozyme for 21 days before farrowing (the study started 24 days before the expected farrowing date) [27]. Reduced microbial diversity may indicate a better intestinal condition and physiological preparation for parturition [14,28]. However, a formula diet alters the colon microbiota and appears to shift the tryptophan metabolism from serotonin to tryptamine, leading to greater histamine levels and the risk of allergies in infants [29]. It was found in this study that dietary silymarin supplementation reduced lactation sow gut microbiota diversity. Further studies are needed to elucidate the impact of this reduction in microbial diversity on sows or offspring.
Abundant Firmicutes and Bacteroidetes were found in the feces of the sows evaluated in this study. The abundance of the two groups accounted for more than 95%. The ratio of Firmicutes and Bacteroidetes is related to the energy metabolism of the body [30]. The difference in the ratio was insignificant in this study, indicating that silymarin supplementation did not change the ratio of Firmicutes and Bacteroidetes and further affected intestinal energy metabolism in sows. However, silymarin treatment tended to reduce the relative abundance of Spirochaetaes and Tenericutes at the phylum level and reduced genera abundance, including unidentified_Ruminococcaceae, Terrisporobacter, unidentified_Christensenellaceae, unidentified_Bacteroidales, and unidentified_Spirochaetaceae, which reduced the relative abundance of some potential pathogenic bacteria [31,32,33]. Similar to the results of this study, our previous study found that lysozyme supplementation in sow diets reduced the concentration of inflammatory factors and, at the same time, decreased the abundance of Spirochaetaes and Tenericutes at the phylum level in sow gut microbiota [13]. In this study, Spearman correlation analysis found that Spirochaetaes had a positive correlation trend with IL-1β. This finding was confirmed with dietary supplementation with silymarin-reduced serum IL-1β concentrations. It has been found in previous studies that silymarin has inhibitory effects on both gram-positive and gram-negative bacteria. Additionally, silymarin has a more substantial inhibitory effect on gram-positive bacteria, where a minimum inhibitory concentration of 12.5–1000 μg/mL also inhibited the formation of biofilms [34,35]. No significant differences were observed between Gram-negative and Gram-positive bacteria in this study. Following silymarin supplementation, it was found that the relative abundance of phyla Fibrobacteres, Actinobacteria, and genera Oscillibacter, Candidatus_Soleaferrea, Succinivibrio, Lachnoclostridium, Fibrobacter, and Marvinbryantia increased significantly. These bacteria may be associated with the metabolism of anti-inflammatory products and cellulolysis [36,37,38]. These results suggest that silymarin supplementation can reduce inflammatory responses while reducing harmful microbes and increasing the abundance of beneficial microbes.
5. Conclusions
In conclusion, in this study, we suggested that dietary silymarin supplementation from late gestation to lactation altered gut microbiota composition, reducing inflammatory responses. This was indicated by the decreased blood concentration of IL-1β and a tendency to decrease colostrum somatic cell count in lactating sows. There has been little knowledge about the lactation sow microbial alterations induced by silymarin supplementation, meaning that more attention should be given to future research. Further studies are needed to elucidate the mechanisms underlying the interaction between gut microbiota and inflammatory responses in sows following dietary silymarin supplementation.
Author Contributions
X.J. (Xiaojun Jiang) and D.W. conceived and designed the experiments. X.J. (Xiaojun Jiang), X.J. (Xinlin Jia) and S.X. carried out the animal experiments and performed the laboratory work. X.J. (Xuemei Jiang), L.C., Y.L., J.L., Z.F. and S.X. performed the statistical analysis. Y.Z. and B.F. did the formal analysis. J.W. and Z.R. provided the resources. S.X. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Present study was conducted according to the guidelines of the Care and Use of Laboratory Animals approved by the Animal Experimental Committee of Sichuan Agricultural University (Ethic Approval Number: SCAUAC201408-3).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found online repositories. The names of the repository/repositories and accession number can be found at: https://www.ncbi.nlm.nih.gov/, BioProject ID PRJNA842556. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding Statement
The present study was funded by Major Scientific and Technological Special Project of Sichuan Province (No. 2021ZDZX0009), National Natural Science Foundation of China (32102554), Natural Science Foundation of Sichuan Province (2022NSFSC1628), Sichuan Province “145” Breeding Tackle Project (2021YFYZ0008).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Surai P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek J.B., Pastorino J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/S0741-8329(02)00215-X. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X., Lin S., Lin Y., Fang Z., Xu S., Feng B., Zhuo Y., Li J., Che L.Q., Jiang X.M., et al. Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. Anim. Nutr. 2020;104:1896–1903. doi: 10.1111/jpn.13425. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q., Ahn J.M., Kim I.H. Micelle silymarin supplementation to sows’ diet from day 109 of gestation to entire lactation period enhances reproductive performance and affects serum hormones and metabolites. J. Anim. Sci. 2021;99:skab354. doi: 10.1093/jas/skab354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C.H., Huang S.M., Yen G.C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox. Signal. 2011;14:353–366. doi: 10.1089/ars.2010.3134. [DOI] [PubMed] [Google Scholar]
- 6.Karimi G., Vahabzadeh M., Lari P., Rashedinia M., Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- 7.Hajiaghamohammadi A.A., Ziaee A., Oveisi S., Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: A randomized controlled pilot study. Hepat Mon. 2012;12:e6099. doi: 10.5812/hepatmon.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahlah Z.F., Meziani M., Maza A. Silymarin natural antimicrobiol agent extracted from Silybum marianum. J. Acad. 2012;2:164–169. [Google Scholar]
- 9.Wu F., Tian F.J., Lin Y. Oxidative stress in placenta: Health and diseases. Biomed Res. Int. 2015;2015:293271. doi: 10.1155/2015/293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenório M.B., Ferreira R.C., Moura F.A., Bueno N.B., de Oliveira A.C.M., Goulart M.O.F. Cross-Talk between oxidative stress and inflammation in preeclampsia. Oxid. Med. Cell. Longev. 2019;2019:8238727. doi: 10.1155/2019/8238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo W., Xu W., Zhang J., Yao J., Xu J. The maternal diet with fish oil might decrease the oxidative stress and inflammatory response in sows, but increase the susceptibility to inflammatory stimulation in their offspring. Animals. 2020;10:1455. doi: 10.3390/ani10091455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.W., Weaver A.C., Shen Y.B., Zhao Y. Improving efficiency of sow productivity: Nutrition and health. J. Anim. Sci. Biotechnol. 2013;4:26. doi: 10.1186/2049-1891-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu S., Shi J., Dong Y., Li Z., Wu X., Lin Y., Che L., Li J., Feng B., Fang Z., et al. Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci. Rep. 2020;10:3210. doi: 10.1038/s41598-020-60131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng C., Wei H., Yu H., Xu C., Jiang S., Peng J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front Microbiol. 2018;9:1989. doi: 10.3389/fmicb.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Dong Y., Chen S., Jia X., Jiang X., Che L., Lin Y., Li J., Feng B., Fang Z., et al. Organic selenium increased gilts antioxidant capacity, immune function, and changed intestinal microbiota. Front. Microbiol. 2021;12:723190. doi: 10.3389/fmicb.2021.723190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seegers H., Fourichon C., Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003;34:475–491. doi: 10.1051/vetres:2003027. [DOI] [PubMed] [Google Scholar]
- 17.Giorgi V.S., Peracoli M.T., Peracoli J.C., Witkin S.S., Bannwart-Castro C.F. Silibinin modulates the NF-κb pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women. J. Reprod Immunol. 2012;95:67–72. doi: 10.1016/j.jri.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 18.He Q., Osuchowski M.F., Johnson V.J., Sharma R.P. Physiological responses to a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: I induction of transforming growth factor beta1 and c-myc in liver with marginal effects on other genes. Planta Med. 2002;68:676–679. doi: 10.1055/s-2002-33801. [DOI] [PubMed] [Google Scholar]
- 19.Schümann J., Prockl J., Kiemer A.K., Vollmar A.M., Bang R., Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J. Hepatol. 2003;39:333–340. doi: 10.1016/S0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 20.Pomorska-Mól M., Wierzchosławski K., Włodarek J., Gogulski M., Pejsak Z. Dynamics of pro- and anti-inflammatory cytokine changes in serum and assessment of their diagnostic utility during lactation impairment in pigs. Res. Vet. Sci. 2020;128:9–15. doi: 10.1016/j.rvsc.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Costa A., Neglia G., Campanile G., De Marchi M. Milk somatic cell count and its relationship with milk yield and quality traits in Italian water buffaloes. J. Dairy Sci. 2020;103:5485–5494. doi: 10.3168/jds.2019-18009. [DOI] [PubMed] [Google Scholar]
- 22.Song D., Li X., Cheng Y., Wu G., Xiao X., Wang F., Wang Y., Lu Z. Effects of supplementing sow diets with Saccharomyces cerevisiae refermented sorghum dried distiller’s grains with solubles from late gestation to weaning on the performance of sows and progeny. J. Anim. Sci. 2017;95:2025–2031. doi: 10.2527/jas.2017.1438. [DOI] [PubMed] [Google Scholar]
- 23.Hashemzadeh-Cigari F., Khorvash M., Ghorbani G.R., Kadivar M., Riasi A., Zebeli Q. Effects of supplementation with a phytobiotics-rich herbal mixture on performance, udder health, and metabolic status of Holstein cows with various levels of milk somatic cell counts. J. Dairy Sci. 2014;97:7487–7497. doi: 10.3168/jds.2014-7989. [DOI] [PubMed] [Google Scholar]
- 24.Comelli M.C., Mengs U., Schneider C., Prosdocimi M. Toward the definition of the mechanism of action of silymarin: Activities related to cellular protection from toxic damage induced by chemotherapy. Integr. Cancer Ther. 2007;6:120–129. doi: 10.1177/1534735407302349. [DOI] [PubMed] [Google Scholar]
- 25.Valentová K., Havlík J., Kosina P., Papoušková B., Jaimes J.D., Káňová K., Petrásková L., Ulrichová J., Křen V. Biotransformation of silymarin flavonolignans by human fecal microbiota. Metabolites. 2020;10:29. doi: 10.3390/metabo10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen L., Liu L., Li X.Y., Ji H.F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019;103:7141–7149. doi: 10.1007/s00253-019-09950-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J., Xiong X., Yin J., Zou L., Wang K., Shao Y., Yin Y. Dietary lysozyme alters sow’s gut microbiota, serum immunity and milk metabolite profile. Front. Microbiol. 2019;10:177. doi: 10.3389/fmicb.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veljović K., Dinić M., Lukić J., Mihajlović S., Tolinački M., Živković M., Begović J., Mrvaljević I., Golić N., Terzić-Vidojević A. Promotion of early gut colonization by probiotic intervention on microbiota diversity in pregnant sows. Front. Microbiol. 2017;8:2028. doi: 10.3389/fmicb.2017.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraf M.K., Piccolo B.D., Bowlin A.K., Mercer K.E., LeRoith T., Chintapalli S.V., Shankar K., Badger T.M., Yeruva L. Formula diet driven microbiota shifts tryptophan metabolism from serotonin to tryptamine in neonatal porcine colon. Microbiome. 2017;5:77. doi: 10.1186/s40168-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley R., Turnbaugh P., Klein S., Gordon J.I. Human gut microbes associated with obesity. Nature. 2006;444:1022. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen R., Ingerslev H.C., Sturek M., Alloosh M., Cirera S., Christoffersen B.Ø., Moesgaard S.G., Larsen N., Boye M. Characterisation of gut microbiota in Ossabaw and Göttingen minipigs as models of obesity and metabolic syndrome. PLoS ONE. 2013;8:e56612. doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund L.C., Sydenham T.V., Høgh S.V., Skov M., Kemp M., Justesen U.S. Draft genome sequence of “Terrisporobacter othiniensis” isolated from a blood culture from a human patient. Genome Announc. 2015;3:e00042-15. doi: 10.1128/genomeA.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporale D.A., Johnson C.M., Millard B.J. Presence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in southern Kettle Moraine State Forest, Wisconsin, and characterization of strain W97F51. J. Med. Entomol. 2005;42:457–472. doi: 10.1093/jmedent/42.3.457. [DOI] [PubMed] [Google Scholar]
- 34.Bessam F.Z., Mehdadi Z. Evaluation of the antibacterial and antifongigal activity of different extract of flavonoïques Silybum marianum L. Adv. Environ. Biol. 2014;8:1–9. [Google Scholar]
- 35.Evren E., Yurtcu E. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia Microbiol. 2015;60:351–356. doi: 10.1007/s12223-015-0399-6. [DOI] [PubMed] [Google Scholar]
- 36.Yeoman C.J., Fields C.J., Lepercq P., Ruiz P., Forano E., White B.A., Mosoni P. In vivo competitions between Fibrobacter succinogenes, Ruminococcus flavefaciens, and Ruminoccus albus in a gnotobiotic sheep model revealed by multi-omic analyses. mBio. 2021;12:e03533-20. doi: 10.1128/mBio.03533-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Z., Xie X., Xue M., Valencak T.G., Liu J., Sun H. The effects of non-fiber carbohydrate content and forage type on rumen microbiome of dairy cows. Animals. 2021;11:3519. doi: 10.3390/ani11123519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia B., Wu W., Fang W., Wen X., Xie J., Zhang H. Heat stress-induced mucosal barrier dysfunction is potentially associated with gut microbiota dysbiosis in pigs. Anim. Nutr. 2022;8:289–299. doi: 10.1016/j.aninu.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found online repositories. The names of the repository/repositories and accession number can be found at: https://www.ncbi.nlm.nih.gov/, BioProject ID PRJNA842556. Further inquiries can be directed to the corresponding author.