Abstract
Simple Summary
Aromatase inhibitors (AIs) remain key elements of endocrine therapy for patients with hormone receptor positive HER2 negative metastatic breast cancer (HR+ HER2− MBC). Recent advances include the use of CDK 4/6 inhibitors or PIK3CA inhibitor in the clinic; however, few immunotherapy trials have been conducted in HR+ HER2− metastatic disease. The current phase II trial was designed to test the safety and efficacy of AIs in combination with immune checkpoint inhibitor pembrolizumab in patients with HR+ HER2− MBC (NCT 02648477). This combination was well tolerated, but minimal clinical activity was observed likely due to lack of PD-L1 pre-selection and heavily pretreated patient population.
Abstract
This study investigated the safety and antitumor activity of aromatase inhibitors (AI) with immune checkpoint inhibitor (ICI) pembrolizumab in patients with hormone receptor positive (HR+) human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) in a phase II study with a safety lead-in (NCT 02648477). Patients received pembrolizumab plus AI up to 2 years or until confirmed progression or unacceptable toxicity. Key eligibility criteria were HR+ HER2− MBC; RECIST v1.1 measurable disease; adequate organ function; and ECOG 0-1. Primary endpoints were safety and overall response rate. A 3-at-risk design was used for the safety lead-in with a targeted accrual of 20 patients. Grade 2 adverse events (AEs) included 35% fatigue, 20% rash, and 10% hot flashes. Grade 3 immune-related AEs (irAEs) related to pembrolizumab included 5% elevated AST/ALT, 5% rash, and 5% lymphopenia. Two (10%) patients had partial responses, three (15%) had stable disease, and 15 (75%) had progression of disease. Median progression-free survival was 1.8 months (95% CI 1.6, 2.6), median overall survival was 17.2 months (95% CI 9.4, NA), and median follow-up time was 40.1 months (range 31.3–46.8 months). The combination was well tolerated, but clinical activity was comparable to AI alone.
Keywords: pembrolizumab, aromatase inhibitor, metastatic, hormone receptor positive, breast cancer
1. Introduction
Hormone receptor positive (HR+) human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) accounts for approximately 60% of all breast cancers [1]. Despite multiple currently available treatment modalities such as selective ER modulators (SERMs), aromatase inhibitors (AIs), and selective ER degraders (SERDs), both alone or in combination with cyclin-dependent kinase 4/6 inhibitors (CDK4/6i), mammalian target of rapamycin (mTOR) inhibitors, or PIK3CA inhibitor, HR+ MBC patients eventually develop endocrine resistance and are considered incurable [1,2,3,4,5,6,7]. Thus, there is an unmet need to discover novel treatment strategies that may overcome innate or acquired endocrine resistance.
The immune checkpoint inhibitor (ICI) pembrolizumab in combination with chemotherapy has shown significant progression-free survival (PFS) benefit for first-line therapy of metastatic triple-negative breast cancer (mTNBC) observed in the KEYNOTE 355 trial. In the KEYNOTE 522 trial, addition of pembrolizumab to chemotherapy significantly increased pathological complete response (pCR) and prolonged event-free survival (EFS) in patients with high-risk early-stage TNBC [8,9,10]. However, the role of ICIs is not well established in HR+ metastatic breast cancer (MBC).
HR+ HER2− BC is well known for its lack of tumor infiltrating lymphocytes (TILs) and PD-L1 expression and is considered “immune-cold.” Approximately 10–15% of ER+ HER2− BC expresses PD-L1 detected by 22C3 antibody testing [11]. Preclinical evidence has shown that high ER levels decrease PD-1/PD-L1 expression and CD8+T cell infiltration by suppressing Th17 cell infiltration and IL-17 signal transduction in breast cancer [12]. Modulation of host immune and tumor microenvironment (TME) may be the key to effective immune responses in HR+ HER2 MBC. Due to potentially synergistic effects and non-overlapping toxicities, there is good rationale to assess the combination of pembrolizumab and AI in patients with HR+ HER2− MBC. The current trial was designed to evaluate the safety and efficacy of AI and pembrolizumab.
2. Materials and Methods
Clinical trial: This phase II trial for patients with metastatic hormone receptor positive (HR+) BC was conducted at the City of Hope National Cancer Center between March 2016 and October 2019 with the approval of the institutional review board (IRB), the World Medical Association’s Ethical Principles, the International Conference on Harmonization of Technical Requirements, Good Clinical Practice guidelines, and U.S. federal regulations. Informed voluntary consent was signed by all patients prior to study entry (NCT02648477). HR+ patients received pembrolizumab 200 mg IV every 3 weeks, and anastrozole (1 mg), letrozole (2.5 mg), or exemestane (25 mg) daily until progression.
Eligibility criteria: Patients were eligible if they were 18 years or older and had metastatic HR+ BC defined by ASCO/CAP guidelines, measurable disease based on RECIST 1.1, a performance status of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) performance scale, life expectancy of 3 months or more, and adequate organ function. Prior exposure to aromatase inhibitor, fulvestrant, CDK4/6i, everolimus, or chemotherapy were not excluded, unless patient was deemed to be resistant to all three (anastrozole, letrozole, exemestane) approved AIs (defined as progression within 12 months or while on an AI).
Study objectives: Objectives included evaluation of overall response rate (ORR) of the combination of pembrolizumab and oral aromatase inhibitor in patients with stage IV HR+ HER2− BC, and assessment of clinical benefit rate (CBR) (no progression for >24 weeks), progression-free survival (PFS), and overall survival (OS) based on RECIST 1.1 and irRECIST. In addition, safety and tolerability were assessed based on toxicities by CTCAE 4.0.
Best response: CT scans were performed at baseline and every 9 weeks after the first dose, and patients with complete response (CR) or partial response (PR) were confirmed by a second examination 4 weeks or more later. Patients were deemed to have stable disease (SD) if one or more post-treatment assessments of SD were observed 8 weeks after treatment started.
Statistical analysis: During the safety lead-in, at most three patients were allowed to be at risk for first cycle toxicities at any one time [13]. When the first six patients completed cycle 1 with at most one dose limiting toxicity (DLT), the safety-lead in for the doublet was considered successful, and accrual proceeded based on the Phase II considerations. If two DLTs were observed on dose level 1 in the first six patients, the study would hold accrual for the selection of a dose level −1 or termination of study. For patients who recurred on or after, or progressed on prior endocrine therapy, objective response in this setting was rare for AI (~10%) [14,15]. With 20 patients, if three or more patients experienced a response, this was considered worthy of further evaluation. This rule had a power of over 80% to detect a true response rate of 20% (encouraging, alternative hypothesis) and a type I error of less than 8% for falsely declaring a 5% response rate (discouraging, null hypothesis) as promising. This rule established the minimum response rate for declaring the combination promising; however, it did not preclude that the combination could be promising based on secondary endpoints such as progression-free survival. Analysis by prior treatment may also influence the overall recommendation. Kaplan–Meier analysis was used for clinical outcomes, and median follow-up was calculated using reverse Kaplan–Meier. The 95% CI for ORR and CBR were calculated using the Clopper–Pearson method.
TIL analysis: Stromal TILs were measured by hematoxylin and eosin (H&E) staining of formalin-fixed paraffin-embedded (FFPE) tumor biopsies according to the International TIL Work Group guideline [16].
PD-L1 analysis: QualTek Molecular Laboratory (Goleta, CA, USA) performed PD-L1 on FFPE tumor sections from baseline testing using 22C3 antibody (Merck & Co., Kenilworth, NJ, USA) for immunohistochemistry (IHC). PD-L1 positivity was defined as membrane staining in at least 1% of cells [17,18,19].
3. Results
3.1. Patient Characteristics
Twenty eligible patients were accrued from March 2016 to October 2019 and received 200 mg pembrolizumab IV (every 3 weeks) plus AI. The median age was 62 years (34–79 years); 15 (75%) were Caucasian, 3 (15%) Asian, 1 (5%) Pacific Islander, and 1 (5%) unknown (Table 1). Major sites of metastasis were bone 90%, liver 60%, and lung 20%. Median lines of therapy were 2 (0, 7). Fourteen (70%) patients had prior CDK4/6 inhibitor (CDK4/6i), 15 (75%) patients received prior fulvestrant, 14 (70%) received prior AI (18 patients had fulvestrant and/or AI), and seven (35%) received prior everolimus.
Table 1.
Patient characteristics and treatment variables (N = 20).
| Characteristic | N (%) |
|---|---|
| Age (median, range) | 62 (34–79) |
| Gender | |
| Female | 20 (100%) |
| Race | |
| White | 15 (75%) |
| Asian | 3 (15%) |
| Pacific Islander | 1 (5%) |
| Unknown | 1 (5%) |
| Performance status | |
| 0 | 8 (40%) |
| 1 | 12 (60%) |
| Initial tumor stage | |
| Stage I | 2 (10%) |
| Stage II | 5 (25%) |
| Stage III | 10 (50%) |
| Stage IV | 3 (15%) |
| Initial histology grade | |
| Grade II | 15 (75%) |
| Grade III | 1 (5%) |
| Not done | 1 (5%) |
| Unknown | 3 (15%) |
| Prior surgery | 17 (85%) |
| Prior radiation | 12 (60%) |
| Lines of chemotherapy for MBC | |
| 0 | 1 (5%) |
| 1 | 3 (15%) |
| 2 | 7 (35%) |
| 3+ | 9 (45%) |
| Sites of metastasis | |
| Bone | 18 (90%) |
| Liver | 12 (60%) |
| Lung | 4 (20%) |
| Brain | 1 (5%) |
| Other * | 11 (55%) |
| Prior CDK 4/6i | 14 (70%) |
| Prior Fulvestrant | 15 (75%) |
| Prior AI | 14 (70%) |
| Prior Everolimus | 7 (35%) |
* Skin, chest wall, pleural effusion, pleural nodule, adrenal gland, bladder, colon, lymph nodes.
3.2. Efficacy
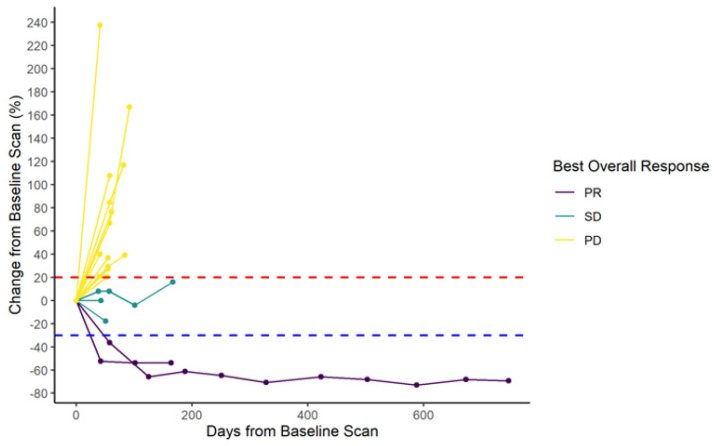
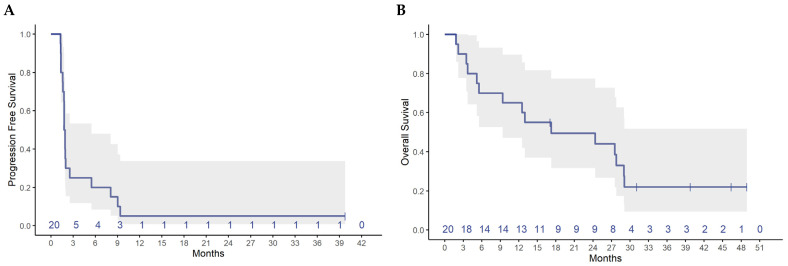
Treatment with AI plus pembrolizumab resulted in a clinical benefit rate (CBR) at 6 months of 20% (95% CI 5.7, 43.7) and an overall response rate (ORR) of 10% (95% CI 0.3, 44.5) (Table 2). Two (10%) patients had PR, three (15%) patients had SD, and 15 (75%) patients had PD as best response. The spider plot shows relative changes in tumor size from baseline over time (Figure 1). One patient had sustained PR until end of treatment (35 cycles) of pembrolizumab plus exemestane and maintained response on single agent exemestane until data cut-off of July 2021. Median follow-up time was 40.1 months (range 31.3–46.8 months). Median PFS was 1.8 months (95% CI 1.6, 2.6), and median OS was 17.2 months (95% CI 9.4, NA) (Figure 2).
Table 2.
Evaluable Response per RECIST 1.1 (N = 20).
| Best Response | N (%) |
|---|---|
| PR | 2 (10%) |
| SD | 3 (15%) |
| PD | 15 (75%) |
| CBR (6 months) | 4 (20%) (95% CI 5.7, 43.7) |
| ORR (CR + PR) | 2 (10%) (95% CI 0.3, 44.5) |
PR, partial response; SD, stable disease; PD, progression of disease; CBR, clinical benefit rate; ORR, overall response rate.
Figure 1.
Response to AI plus pembrolizumab (N = 20). Spider plot showing relative change in tumor size from baseline. Each line represents one patient in study. Two patients had partial response (PR), three had stable disease (SD), and 15 had progression of disease (PD). One patient had sustained PR until end of treatment (35 cycles) of pembrolizumab plus exemestane and maintained response on single agent exemestane until data cut-off of July 2021. Red line indicates 20% of increased tumor size per RECIST 1.1. Blue line indicates 30% of reduced tumor size per RECIST 1.1.
Figure 2.
Progression-free survival and overall survival (N = 20). (A) Median PFS was 1.8 months (95% CI 1.6, 2.6), and (B) median OS was 17.2 months (95% CI 9.4, NA). Numbers above x-axis refer to number of patients.
The first partial responder was a 61-year-old woman with de novo stage IV ER+ PR+ HER2− metastasis to bone and lung, PD-L1 negative by 22C3. This patient received exemestane and pembrolizumab in this study as front-line treatment for metastatic disease. Initial diagnosis was February 2017. The patient started C1D1 on 7 March 2017 and achieved PR by RECIST 1.1 after 12 weeks (four cycles) of treatment. PR was sustained until end of treatment (35 cycles) of pembrolizumab plus exemestane. The patient maintained response until data cut-off of July 2021 while on single agent exemestane.
The second partial responder was a 67-year-old woman with stage IV ER+ PR+ HER2− primary breast cancer with metastasis to lymph node, bone, lung, and liver with two prior lines of treatment (fulvestrant and letrozole/palbociclib) for metastatic disease. The patient initially was diagnosed in December 2006 with left-sided stage IIA IDC, ER+ 90% PR- HER2neu 2+ IHC, FISH negative primary (T1cN1M0), PD-L1 negative by 22C3. She underwent left-sided mastectomy and sentinel lymph node biopsy followed by adjuvant chemotherapy with dose-dense Adriamycin, cyclophosphamide, and paclitaxel. The patient received endocrine therapy with anastrozole from June 2007 to July 2014. Metastatic disease was diagnosed in August 2014 with biopsy-proven ER+ 95% PR 1% HER2neu- disease with bone, neck, and LN metastasis. She received first-line therapy with fulvestrant from August 2014 to April 2015 with best response as SD. She progressed in May 2015 with a new lytic bone lesion. She started on second-line therapy with letrozole and palbociclib from May 2015 to March 2017 with best response of SD. She progressed with multiple liver metastases (the largest measured 4.2 cm by 1.4 cm) on 4 March 2017. The patient enrolled in this study on 27 March 2017 and received exemestane plus pembrolizumab through late November 2017 with best response as PR and PFS of 33 weeks. Upon progression, patient continued to receive four additional lines of therapy, including capecitabine, exemestane and everolimus, eribulin, and as of July 2021 (data cut-off), nab-paclitaxel.
3.3. Safety
Grade 3 adverse events (AE) per CTCAE 4.0 were rash (5%), elevated liver enzymes (5%) and lymphopenia (5%) (Table 3). Grade 2 AEs were fatigue (35%), rash (15%), hot flashes (10%), insomnia (5%), headache (5%), pruritis (5%), arthralgia (5%), dry mouth (5%), localized edema (5%), cough (5%), hypertension (5%), and hypothyroidism (5%). Immune toxicity was consistent with known pembrolizumab toxicity, as listed in package insert.
Table 3.
Adverse events with attributions in “Definite”, “Possible”, “Probable” (grade 2+ only, and for all courses) per CTCAE 4.0 (N = 20).
| Adverse Event | Grade 2 | Grade 3 |
|---|---|---|
| All adverse events (worst grade per patient) | 12 | 2 |
| Rash | 4 (20%) | 1 (5%) * |
| Elevated AST/ALT | 1 (5%) | 1 (5%) |
| Lymphopenia | 1 (5%) * | |
| Fatigue | 7 (35%) | |
| Hot flashes | 2 (10%) | |
| Insomnia | 1 (5%) | |
| Headache | 1 (5%) | |
| Pruritus | 1 (5%) | |
| Arthralgia | 1 (5%) | |
| Dry mouth | 1 (5%) | |
| Localized edema | 1 (5%) | |
| Cough | 1 (5%) | |
| Hypertension | 1 (5%) | |
| Hypothyroidism | 1 (5%) |
* Same patient.
3.4. Immune Biomarkers
A total of 14 (70%) patients had PD-L1 results by IHC using the 22C3 antibody, with six lacking available tissue for testing. Of those 14, three (21%) patients were PD-L1 positive (PD-L1 ≥ 1% by 22C3 IHC testing), and 11 (79%) patients were PD-L1 negative (PD-L1 < 1% by 22C3 IHC testing). Of the two patients with PR, both were negative for PD-L1. One patient with SD had 50% PD-L1 staining (H score = 70), one patient with PD had 2% PD-L1 staining (H score = 4), and one patient with PD had 1% PD-L1 staining (H score = 1).
Stromal TILs (sTILs) were analyzed for nine patients with available H&E slides (Table 4). Of these patients, one PR patient had 5% sTILs (supraclavicular mass), two SD patients had 5–10% sTILs (breast and chest wall), one PD patient had 70% sTILs (axillary LN), and five PD patients had 2–10% sTILs (two skin, two liver, one chest wall).
Table 4.
Tumor Immune Biomarker sTILs and PD-L1 with 22C3 antibody (N = 20).
| Patient ID | Best Response | Tissue | % sTILs | PD-L1 |
|---|---|---|---|---|
| COH-01 | PD | NA | NA | NA |
| COH-02 | PD | NA | NA | NA |
| COH-03 | SD | NA | NA | NA |
| COH-04 | PD | Skin | 5% | Neg |
| COH-05 | PD | Liver | 10 | 1% |
| COH-06 | SD | Breast | 5% | Neg |
| COH-07 | PD | Frontal tumor | NA | Neg |
| COH-08 | PR | Breast | NA | Neg |
| COH-09 | PD | Liver | NA | NA |
| COH-10 | PR | Supraclavicular mass | 5% | Neg |
| COH-11 | PD | Liver | 5% | Neg |
| COH-12 | PD | Bone | NA | Neg |
| COH-13 | SD | Chest wall | 10% | 50% |
| COH-14 | PD | Axillary LN | 70% | NA |
| COH-15 | PD | Ovary | NA | 2% |
| COH-16 | PD | Skin | 2% | Neg |
| COH-17 | PD | LN | NA | NA |
| COH-18 | PD | Liver | NA | Neg |
| COH-19 | PD | Chest wall | 10% | Neg |
| COH-20 | PD | Liver | NA | Neg |
sTILs, stromal tumor infiltrating lymphocytes; PR, partial response; SD, stable disease; PD, progression of disease; NA, not applicable; LN, lymph node; Pos, positive; Neg, negative.
4. Discussion
In the current study, the combination of AI and pembrolizumab was well tolerated with a modest CBR of 20% (95% CI 5.7, 43.7) and ORR of 10% (95% CI 0.3, 44.5). The immune-related AEs were consistent with known profiles of pembrolizumab. Our result is comparable with the single agent pembrolizumab historical data in HR+ BC. Rugo et al. studied a cohort of 25 patients with HR+ MBC and PD-L1 ≥ 1% by 22C3 IHC testing [20]. The incidence of treatment-related adverse events was 64%, with grade 1 or 2 nausea (20%) and fatigue (12%). Rugo et al. reported a single agent pembrolizumab response rate of 12% in a cohort of 25 patients with PD-L1+ HR+ MBC [21]. The combination of CTLA-4 inhibitor tremelimumab and exemestane showed 42% SD only in a cohort of 26 patients with heavily pretreated HR+ MBC [22]. The low immunogenicity of HR+ HER2− MBC compared with TNBC may explain this modest clinical activity [23,24]. HR+ HER2− breast cancer is well known for its lack of TIL and PD-L1 expression, hence considered “immune-cold tumors” [11]. Approximately 10−15% of HR+ HER2− BC expresses PD-L1 detected by 22C3 antibody testing. Preclinical evidence has shown that high ER levels decrease PD-1/PD-L1 expression and CD8+ T cell infiltration by suppressing Th17 cell infiltration and IL-17 signal transduction in breast cancer [25]. Antiestrogen therapy with SERDs, particularly when administered in combination with anti-PD-L1 antibodies, acts to inhibit BC progression in part by blocking the expansion and mobilization of myeloid-derived stem cells (MDSC), suppress regulatory T cells (Tregs), and increase Th1 response [25]. These preclinical data indicate that SERDs in combination with ICI may be worth further testing in HR+ MBC.
The modest clinical activity observed in the current and earlier immune checkpoint inhibitor trials indicates that novel combination therapy eliciting robust immune response to turn “immune-cold” tumors into “immune-hot” tumors is necessary to enhance the efficacy of checkpoint inhibitors in HR+ MBC. Modulation of the host immune and tumor microenvironment may be the key to effective immune responses in HR+ HER2− MBC. In theory, a number of targeted therapies could act as an “immune-switch”, such as chemotherapy, radiation therapy, epigenetic targeting drugs, oncolytic viruses, anti-angiogenic therapies, immune cytokines, tumor vaccines, and cellular therapies [26].
CDK4/6 inhibitors have demonstrated PFS and OS benefit in HR+ HER2− MBC. Beyond cell cycle suppression, immune-modulatory effects of CDK 4/6 inhibitors were well documented in both preclinical and clinical trial settings. Goel et al. demonstrated that CDK4/6 inhibition stimulates production of type III interferons, enhances tumor antigen presentation, and markedly suppresses the proliferation of regulator T cells [27]. CDK4/6i was also shown to increase tumor infiltration and activation of effector T cells and augment the response to PD-1 blockade in a novel ex vivo organotypic tumor spheroid culture system and in multiple in vivo murine syngeneic models by Deng et al. [28]. In a recent phase I trial, we tested the combination of AI, palbociclib, and pembrolizumab in patients with ER+ HER2− MBC [29], with a CR of 31%. In our peripheral blood mononuclear cells (PBMCs) flow cytometry analysis, CDK4/6i showed immune-priming effect with significantly increased fractions of type 1 conventional dendritic cells (cDC1s) within circulating dendritic cells and decreased classical monocytes (cMO) within circulating monocytes only in patients treated with palbociclib [30]. To fully exploit the immune-priming effect of CDK4/6i, future confirmatory trials are needed.
The current study is limited by its small sample size and the trial design, which was a non-randomized, single-arm trial in endocrine-resistant patients who received prior lines of endocrine therapy and were unselected for PD-L1 expression. Future studies with larger patient cohorts and randomized designs are necessary to explore novel combinations with ICI in incurable HR+ HER2− MBC patients.
5. Conclusions
The combination of pembrolizumab and AI is well tolerated in PD-L1 unselected patients with HR+ HER2− MBC. The modest clinical activity observed in this study indicates that novel combination therapies are required to boost immune-mediated responses and clinical benefit ratio and duration in HR+ MBC patients.
Author Contributions
Conceptualization, G.S.; methodology, G.S.; software, P.H.F., C.R., Y.C.; validation, G.S., J.W., Y.Y.; formal analysis, P.H.F., C.R., Y.C.; investigation, Y.Y.; resources, M.M., A.T., N.M., S.C., C.Y., D.S., D.L., S.R., G.S., J.M., J.W., Y.Y.; data curation, X.G., S.E.Y., J.S.L.; writing—original draft preparation, X.G.; writing—review and editing, J.S.L., S.E.Y., Y.Y.; visualization, Y.Y.; supervision, Y.Y.; project administration, Y.Y.; funding acquisition, G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the City of Hope National Medical Center (NCT02648477, 24 December 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data can be shared upon reasonable request.
Conflicts of Interest
Y.Y. has contracted research with Merck, Eisai, Novartis, Puma, Genentech, Celgene, and Pfizer; consults for Puma, Pfizer, and Immunomedics; and is on the Eisai, Novartis, Genentech, AstraZeneca, Daiichi Sankyo, and Immunomedics Speakers Bureau. The other authors have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was supported by Merck. The Biostatistics Core at City of Hope was supported by the National Cancer Institute of the National Institutes of Health (P30CA033572).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rozeboom B., Dey N., De P. ER+ metastatic breast cancer: Past, present, and a prescription for an apoptosis-targeted future. Am. J. Cancer Res. 2019;9:2821–2831. [PMC free article] [PubMed] [Google Scholar]
- 2.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S., et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 3.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.-S., Sonke G.S., Paluch-Shimon S., Campone M., Blackwell K.L., André F., Winer E.P., et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 5.Dickler M.N., Tolaney S.M., Rugo H.S., Cortés J., Diéras V., Patt D., Wildiers H., Hudis C.A., O’Shaughnessy J., Zamora Z., et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(−) Metastatic Breast Cancer. Clin. Cancer Res. 2017;23:5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselga J., Campone M., Piccart M., Burris H.A., 3rd, Rugo H.S., Sahmoud T., Noguchi S., Gnant M., Pritchard K.I., Lebrun F., et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.André F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., Iwata H., Conte P., Kaufman B., Yamashita T., et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 8.PD-1 Inhibitor Promising in Treatment of Triple-Negative Breast Cancer. [(accessed on 29 August 2022)];Hum. Vaccin Immunother. 2015 11:1298. Available online: https://pubmed.ncbi.nlm.nih.gov/26290901/ [Google Scholar]
- 9.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.-A., Shaw Wright G., et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 10.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.A., Yusof M.M., Gallardo C., Lipatov O., Barrios C.H., Holgado E., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 11.Guo H., Ding Q., Gong Y., Gilcrease M.Z., Zhao M., Zhao J., Sui D., Wu Y., Chen H., Liu H., et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. BCR. 2020;22:69. doi: 10.1186/s13058-020-01303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuai C., Yang X., Pan H., Han W. Estrogen Receptor Downregulates Expression of PD-1/PD-L1 and Infiltration of CD8(+) T Cells by Inhibiting IL-17 Signaling Transduction in Breast Cancer. Front. Oncol. 2020;10:582863. doi: 10.3389/fonc.2020.582863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel P.H., Chung V., Tuscano J., Siddiqi T., Sampath S., Longmate J., Groshen S., Newman E.M. Model of a Queuing Approach for Patient Accrual in Phase 1 Oncology Studies. JAMA Netw. Open. 2020;3:e204787. doi: 10.1001/jamanetworkopen.2020.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thürlimann B., Robertson J., Nabholtz J., Buzdar A., Bonneterre J. Efficacy of tamoxifen following anastrozole (‘Arimidex’) compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur. J. Cancer. 2003;39:2310–2317. doi: 10.1016/S0959-8049(03)00602-6. [DOI] [PubMed] [Google Scholar]
- 15.Schiavon G., Smith I.E. Endocrine Therapy for Advanced/Metastatic Breast Cancer. Hematol. Clin. North Am. 2013;27:715–736. doi: 10.1016/j.hoc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., Van den Eynden G., Baehner F.L., Penault-Llorca F., et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro K., Chung H.C., Shankaran V., Geva R., Catenacci D., Gupta S., Eder J.P., Golan T., Le D.T., Burtness B., et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 18.Dolled-Filhart M., Locke D., Murphy T., Lynch F., Yearley J.H., Frisman D., Pierce R., Weiner R., Wu D., Emancipator K. Development of a Prototype Immunohistochemistry Assay to Measure Programmed Death Ligand-1 Expression in Tumor Tissue. Arch. Pathol. Lab. Med. 2016;140:1259–1266. doi: 10.5858/arpa.2015-0544-OA. [DOI] [PubMed] [Google Scholar]
- 19.Clouthier D.L., Lien S.C., Yang S.Y.C., Nguyen L.T., Manem V.S.K., Gray D., Ryczko M., Razak A.R.A., Lewin J., Lheureux S., et al. An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE) J. Immunother. Cancer. 2019;7:72. doi: 10.1186/s40425-019-0541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo H.S., Lerebours F., Ciruelos E., Drullinsky P., Ruiz-Borrego M., Neven P., Park Y.H., Prat A., Bachelot T., Juric D., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 21.Rugo H.S., Delord J.-P., Im S.-A., Ott P.A., Piha-Paul S.A., Bedard P.L., Sachdev J., Le Tourneau C., van Brummelen E.M., Varga A., et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. Clin. Cancer Res. 2018;24:2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 22.Vonderheide R.H., LoRusso P.M., Khalil M., Gartner E.M., Khaira D., Soulieres D., Dorazio P., Trosko J.A., Rüter J., Mariani G.L., et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 23.Sabatier R., Finetti P., Mamessier E., Adelaide J., Chaffanet M., Ali H.R., Viens P., Caldas C., Birnbaum D., Bertucci F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2014;6:5449–5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barroso-Sousa R., Jain E., Cohen O., Kim D., Buendia-Buendia J., Winer E., Lin N., Tolaney S., Wagle N. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann. Oncol. 2020;31:387–394. doi: 10.1016/j.annonc.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Márquez-Garbán D.C., Deng G., Comin-Anduix B., Garcia A.J., Xing Y., Chen H.-W., Cheung-Lau G., Hamilton N., Jung M.E., Pietras R.J. Antiestrogens in combination with immune checkpoint inhibitors in breast cancer immunotherapy. J. Steroid Biochem. Mol. Biol. 2019;193:105415. doi: 10.1016/j.jsbmb.2019.105415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., Caux C., Depil S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel S., DeCristo M.J., Watt A.C., BrinJones H., Sceneay J., Li B.B., Khan N., Ubellacker J.M., Xie S., Metzger-Filho O., et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng J., Wang E.S., Jenkins R.W., Li S., Dries R., Yates K., Chhabra S., Huang W., Liu H., Aref A.R., et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Y., Lee J.S., Yost S.E., Frankel P.H., Ruel C., Egelston C.A., Guo W., Padam S., Tang A., Martinez N., et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer. 2021;154:11–20. doi: 10.1016/j.ejca.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egelston C., Guo W., Yost S., Lee J.S., Rose D., Avalos C., Ye J., Frankel P., Schmolze D., Waisman J., et al. Pre-existing effector T-cell levels and augmented myeloid cell composition denote response to CDK4/6 inhibitor palbociclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. J. Immunother. Cancer. 2021;9:e002084. doi: 10.1136/jitc-2020-002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared upon reasonable request.