Abstract
Simple Summary
Poly(ADP-ribose) polymerase 1 (PARP1) is perhaps the most studied member of the PARP superfamily and participates in numerous cellular processes. PARP1 inhibitors have been approved as drugs to treat various cancers in clinics, based on its role in DNA repair. Yet, there is a growing body of evidence showing multitasking function of PARP1 in regulation of gene expression. In this review article, we discuss the current knowledge of PARP1 and its conducted enzymatic process, i.e., PARylation, with an emphasis on gene expression by the interaction with transcription factors and regulation of chromatin conformation, dependent or independent of DNA damage. The molecular action mode of PARP1 in gene transcription may present as a potential target for therapeutic intervention of inflammation-related diseases and also for cancer therapy.
Abstract
Poly(ADP-ribosyl)ation (PARylation) is a covalent post-translational modification and plays a key role in the immediate response of cells to stress signals. Poly(ADP-ribose) polymerase 1 (PARP1), the founding member of the PARP superfamily, synthesizes long and branched polymers of ADP-ribose (PAR) onto acceptor proteins, thereby modulating their function and their local surrounding. PARP1 is the most prominent of the PARPs and is responsible for the production of about 90% of PAR in the cell. Therefore, PARP1 and PARylation play a pleotropic role in a wide range of cellular processes, such as DNA repair and genomic stability, cell death, chromatin remodeling, inflammatory response and gene transcription. PARP1 has DNA-binding and catalytic activities that are important for DNA repair, yet also modulate chromatin conformation and gene transcription, which can be independent of DNA damage response. PARP1 and PARylation homeostasis have also been implicated in multiple diseases, including inflammation, stroke, diabetes and cancer. Studies of the molecular action and biological function of PARP1 and PARylation provide a basis for the development of pharmaceutic strategies for clinical applications. This review focuses primarily on the role of PARP1 in the regulation of chromatin remodeling and transcriptional activation.
Keywords: PARP1, PARylation, chromatin, transcription, inflammatory response
1. Introduction
Poly(ADP-ribosyl)ation (or PARylation) is an abundant post-translational modification (PTM) that regulates a variety of cellular pathways in both prokaryotes and eukaryotes. The poly(ADP-ribose) polymerases (PARPs) are a major family of enzymes capable of modifying proteins by PARylation [1]. PARPs can be found in almost all subcellular compartments within the cell, including in the nucleus, cytosol, mitochondria, endoplasmic reticulum, etc. They use intracellular nicotinamide adenine dinucleotide (NAD+) to modify acceptor proteins with mono-ADP-ribose (MAR) moieties or long (up to 200 units) branched poly-ADP-ribose (PAR) [1]. PARP family contains 17 members in humans, but not all of them are enzymatically active. PARP1, PARP2 and tankyrases (i.e., PARP5a (tankyrase-1) and PARP5b (tankyrase-2)) are bona-fide PARPs, generating PARs [1,2]. PARP13 is a pseudo-enzyme without catalytic activity, although it possesses a structurally similar domain to the catalytic domain of active PARP members [1,3]. The activity of PARP9 has not been fully clarified [1,2,3]. Others are mono-ADP-ribose transferases that generate MAR [1,2]. PAR levels are tightly controlled in the cell by timely degradation via ADP-ribosyl hydrolases, including PAR glycohydrolase (PARG), ADP-ribosyl hydrolase 3 (ARH3), macrodomain-containing proteins MacroD1 (LPR16) and MacroD2, as well as terminal ADP-ribose hydrolase (TARG1 or C6orf130). While PARG and ARH3 degrade the PAR chains, MacroD1, MacroD2 and TARG1 remove the protein proximal mono-ADP-ribose and enable the complete reversal of the modification [4].
PARP1 (also known as diphtheria toxin-like ADP-ribosyltransferase, ARTD1), is the founding member of the PARP superfamily. It is the most active member of the PARPs, and believed to execute approximately 90% of total PARylation activity [5,6]. PARP1 adds a negatively charged PAR to various acceptor proteins, including DNA repair proteins, histones, chromatin remodelers, transcription factors and signal transduction elements, yet mainly to PARP1 itself (called auto-PARylation). PARP1 has been widely and extensively studied. The most known function of PARP1 is its function in DNA damage repair, including pathways of base excision repair (BER), the repair of single-strand breaks (SSBs), double-strand breaks (DSBs, by homologous recombination (HR), non-homologous end joining (NHEJ) and alternative end-joining (Alt-EJ)), as well as of stalled replication forks [7,8]. In addition, PARP1 has been shown to be activated by chromatin conformation changes, and thereby modulates chromatin remodeling, which can be independent of DNA breaks [9,10]. Another well-known DNA break-independent function of PARP1 is its role in transcriptional regulation. PARP1 has been involved in various aspects of the transcription process through a variety of mechanisms, including regulation of chromatin remodeling, DNA methylation and coregulation of transcription factors. There have been comprehensive reviews regarding the function of PARP1 and PARylation in DNA repair and damage signaling [7,8]; this review article focuses on the function of PARP1 in chromatin remodeling (chromatin conformation change, but DNA break independent), DNA methylation and transcription (as a cofactor for transcription factors and also modulator of chromatin).
2. PARP1 Structure and Activation
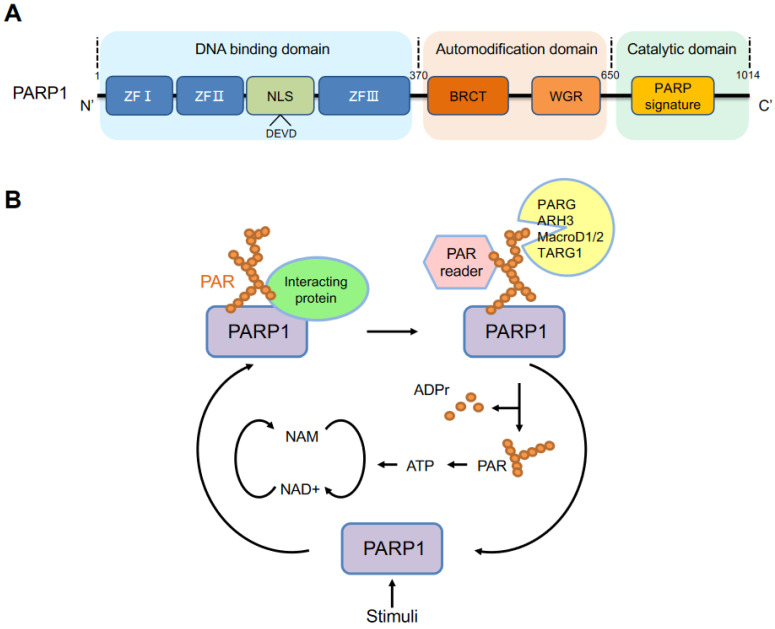
The PARP1 gene, the first PARP family member, was cloned in 1987 [11,12,13] and mapped to chromosome 1 (1q42.12) in humans. Its encoded protein PARP1 contains 1014 amino acids, with a molecular weight of approximately 113 kDa [14]. PARP1 is an evolutionarily conserved, multifunctional enzyme that is found in all eukaryotes, except yeast, enigmatically [15]. Structurally, PARP1 consists of the following three major functional domains (Figure 1A): an N-terminal DNA-binding domain (DBD), an automodification (or auto-PARylation) domain, and a catalytic domain in the C-terminus (CAT) that contains a highly conserved sequence within the active site, defined as the PARP signature.
Figure 1.
PARP1 structure and PARylation. (A) Structural and functional domains of human PARP1. ZFI, ZFII and ZFIII: zinc finger motifs I, II and III, respectively; NLS: nuclear localization signal; DEVD: a caspase cleavage site; BRCA1 C-terminus (BRCT); WGR (Trp–Gly–Arg). A highly conserved PARP signature is located in the C-terminal catalytic domain. (B) The cycle of PARylation as depicted by auto-PARylation of PARP1, a well-documented acceptor. Stimuli, including but not limit to, DNA damage, ERK1/2, hormone, JIL-1, activate PARP1, which catalyzes the formation of long and branched poly (ADP-ribose) (PAR), using NAD+ as a substrate. PARP1 can interact with other partners, either by itself as a scaffold protein or via its enzymatic product PAR. PAR can be recognized by PAR readers and be rapidly degraded by hydrolytic enzymes, PARG, ARH3, MacroD1 and D2, TARG1. NAM: nicotinamide; ADPr: ADP-ribose; PAR: poly (ADP-ribose).
The N-terminal domain contains three zinc finger DNA-binding domains, namely ZFI, ZFII and ZFIII. ZFI and ZFII are critical for the recognition of various DNA structures with high affinity for DNA [16,17,18]. ZFIII is used to couple DNA-binding and catalytic activities of PARP1 and has a structure and function that different from ZFI and ZFII [18,19]. In the N-terminus, there is a nuclear localization sequence (NLS) (KRK-X (11)-KKKSKK) that leads PARP1 to the nucleus. During apoptosis, PARP1 is cleaved by caspases at the conserved site 211DEVD214, generating 24 kDa and 89 kDa fragments. The auto-modification domain has a BRCA1 C-terminal (BRCT) domain and tryptophan-glycine-arginine-rich (WGR) domain. The BRCT domain provides sites of auto-PARylation and regulates protein–protein interaction. A recent study proposed that this BRCT domain can bind to intact DNA without activation of PARP1 and mediate rapid movement of the PARP1 molecule to scan damaged DNA [20]. The WGR domain, ZFI and ZFIII together interact with DNA to link the DNA damage interface to the catalytic domain [18]. Besides the BRCT domain, the auto-modification domain is rich in serine, glutamate and lysine residues, which are the main sites of auto-PARylation. Serine is a major residue for ADP-ribosylation upon DNA damage [21,22]. The C-terminal catalytic domain contains the (ADP-ribosyl) transferase (ART) motif, called the PARP signature, which is a NAD+ binding site and the residues that contribute to the initiation, elongation, and branching of PAR [23].
PARP1 can be activated by multiple stimuli, including but not limited to, DNA damage, ERK1/2 [24], hormones [25], JIL-1 [26] and NAD+ [27]. The activated PARP1 forms long and branched PAR chains mainly to its auto-modification domain but also to other acceptor proteins, such as histones, chromatin regulators and transcription factors. PARP1 uses NAD+ as a substrate for PAR formation, while releasing nicotinamide. PAR chains are then rapidly catabolized by PAR degrading enzymes, including PARG, ARH3, MacroD1, MacroD2 and TARG1 [28,29,30,31]. PARG is the main and robust enzyme that cleaves the ribose–ribose bonds of PAR to release free PAR and ADP-ribose (ADPr) [32]. ARH3 can also catalyze the removal of PAR but not MAR [29]. Furthermore, ARH3 removes the O-acetyl group from the NAD+ metabolite O-acetyl-ADP-ribose [33]. TARG1 cleaves the terminal ADP-ribose moiety, resulting in the release of the last protein proximal ADPr moiety [34]. Finally, the free PAR can be recycled for ATP production and PARP1 can be activated again [35] (Figure 1B).
3. PARP1 and Chromatin Remodeling
Chromatin remodeling, a dynamic modification of chromatin architecture from a condensed state to a DNA-binding protein accessible state, plays a crucial role in regulating various cellular processes [36]. Chromatin consists of genomic DNA and proteins. The major proteins in chromatin are histones, which compose of linker histones (H1) and core histones (H2A, H2B, H3 and H4) and help package DNA into a compact form that fits into the cell nucleus. Chromatin restricts the accessibility of DNA-binding factors to DNA [37]. Cells have evolved the mechanism of chromatin remodeling to change chromatin conformation that allows for better or full access of DNA repair machineries, DNA replication factors, transcription factors and chromatin condensation factors to DNA [38]. PARP1 is an important factor that participates in this process, and hence regulates numerous cellular processes, including DNA repair, DNA replication and gene expression. Chromatin remodeling is partially regulated by histone modification, by various PTMs, which are as follows: acetylation, methylation, phosphorylation, PARylation, sumoylation, ubiquitylation and crotonylation, and chromatin remodeller complexes [39]. PARP1 is able to orchestrate these changes to chromatin architecture, and in turn chromatin-related functions. Below, we discuss the function of PARP1 in the regulation of histones (H1, core histone and histone variants) and chromatin remodeling enzyme complexes.
3.1. Linker Histone H1
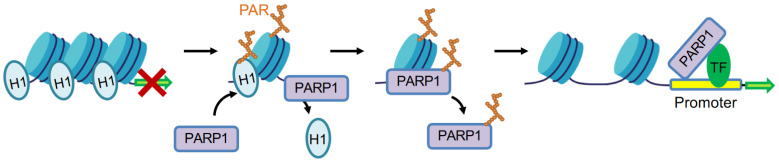
The first association of PARylation with chromatin was reported as early as the 1960s [40,41]. In the early 1980s, Poirier et al. reported that PARP1 could PARylate H1 to promote the decondensation of native polynucleosomes in vitro, mimicking the effects of linker histone H1 depletion [42]. H1 has been identified as a major acceptor of PARylation in native chromatin [43]. In Drosophila, activated PARP1 strips chromatin proteins off DNA to form a puff structure, which makes transcription machinery complexes accessible to promoters [44]. However, H1 and PARP1 bind in a competitive manner to target gene promoters and have distinct roles in determining gene expression outcomes in vivo [45,46]. It is plausible that the highly negatively charged PAR on H1 induces the repulsion of nucleosomes, thereby leading to chromatin relaxation (Figure 2). For example, PARP1 cooperates with GATA3 to compete with linker histone H1 to maintain a transcriptionally competent chromatin environment for CCND1 [47]. H1 is PARylated and released at promoters of genes that are involved in the reprogramming of neuronal gene expression, which is required for learning and memory in the hippocampus of mice [48]. Hormone-dependent phosphorylation of PARP1 by CDK2 activates PARP1 to PARylate H1, which induces H1 release from chromatin. The opened chromatin is essential for the majority of progesterone-responsive genes [49]. Upon differentiation of mouse neuroprogenitor cells into neurons, PARP1 is recruited to the doublecortin (Dcx) promoter and mediates H1 PARylation and eviction of H1 from chromatin, thereby facilitating Dcx gene expression [25]. In primary mouse cortical neurons, KCl-induced depolarization results in H1 PARylation by PARP1, contributing to H1 release from chromatin and the simultaneous activation of immediate early gene (IEG) expression [50]. Activated PARP1 conducts PARylation and displacement of H1 from chromatin to form an euchromatin environment, leading to the activation of aromatase promoter I.3/II [51]. By using spFRET microscopy, PARP1 is found to interact with linker DNA of nucleosomes, promoting reorganization of the nucleosome, which is independent of PARylation activity [52]. Nucleosome reorganization occurs by PARP1 binding to linker DNA, where it displaces linker histone H1 to promote chromatin conformation that is permissive to gene expression [52]. Interestingly, PARP1 helps to maintain chromatin condensation, whereas linker DNA bound to PARP1 prefers to displace H1 to decondense chromatin at transcriptional active regions to facilitate transcription [53] (Figure 2).
Figure 2.
PARP1 regulates transcription by facilitating local chromatin decondensation at active gene sites. Activated PARP1 mediates H1 PARylation and displacement from chromatin to form euchromatin environment. PARP1 also physically interacts with transcription factors (TF), with or without its enzymatic activity, to modulate gene transcription.
3.2. Core Histones and Other Histone Variants
In addition to linker histone H1, PARP1 has been shown to PARylate all four core histones [43]. Early studies that focused on the PARylation site of core histones proposed that PARP1 modifies the important regulatory lysine residue of the core histone tails [54,55]. In LPS-stimulated macrophages, PARP1 enzymatic activity promotes gene transcription by increasing the promoter accessibility via PARylation of all four histones [56]. A recent study showed that PARylation of H2B-Glu35 by PARP1 inhibits AMPK-mediated phosphorylation of H2B-Ser36, which regulates proadipogenic gene expression and fat metabolism in vivo [57]. By PARylating H2B that hinders the occupancy of H2B at the NFATc1 promoter, PARP1 represses the expression of the nuclear factor of activated T cell cytoplasmic 1 (NFATc1) and osteoclast differentiation [58].
Of note, the relationship between PARP1 and histones is complex. In addition to PARP1, which modifies histones by PARylation, histones and their PTMs can modulate the behaviors and activity of PARP1. In Drosophila, histones H2A and H2B inhibit the catalytic activity of PARP1, whereas H3 and H4 can bind to the catalytic domain of PARP1 to induce a H4-mediated induction of PARylation. H4 interacts with the C-terminal domain of PARP1, resulting in long-term activation of PARP1, and hence a sustained accumulation of PAR, which prolongs chromatin relaxation to facilitate the transcription factors’ access to DNA [59]. In Drosophila, the H2Av histone variant (Drosophila homolog of the mammalian H2AZ and H2AX variants) colocalizes together with Drosophila PARP1 (dPARP) on chromatin. Irradiation-induced phosphorylation of H2Av stimulates PARP1 enzymatic activity, which in turn triggers transcriptional activation [60]. Phosphorylation of H2Av by JIL-1 kinase increases the interaction between PARP1 and H4, leading to transcriptional initiation [26]. Moreover, the authors showed that, since H2A inhibits PARP1 activity by its N-terminal tail, the acetylation of H2A in the nucleosome disrupts the inhibitory effect of H2A on PARP1, thus promoting PARP1 activity [26]. Because of the high conservation of these Drosophila genes with mammals and humans, these results can be referenced for the function of PARP1 in chromatin remodeling and transcription in higher organisms. The non-histone domain (NHD) of macroH2A1.2 binds and inhibits PARP1 enzymatic activity, which silences an X chromosome [61]. Finally, PARP1 PARylates the lysine demethylase 5B (KDM5) and inhibits the binding of KDM5 to chromatin. This refracts the demethylation of H3K4me3 and maintains an open chromatin structure to positively regulate gene expression [62].
PARP1 has also a functional association with histone variants such as H2A.Z and macroH2A. The activation of the ERK pathway induces PARP1 activation and promotes binding of PARP1 to the c-fos promoter region, where PARP1 orchestrates the exchange of the histone variant H2A.Z with H2A to allow transcriptional activation in Hela cells and mouse embryonic fibroblasts (MEFs) [63]. In IMR90 primary human fetal lung fibroblast cells, PARP1 cooperates with macroH2A1.1 to regulate the transcription of macroH2A1-target genes, including HDAC9, RGS4, CPA4, ANKRD1, EREG, FBLN1, FMO2 and SHISA3, by enhancing CREB-binding protein (CBP)/p300-mediated H2B acetylation. [64].
4. Chromatin Remodeling Complexes
PARP1 can function as a histone chaperone by recruiting chromatin remodeling enzymes, for example, histone PARylation factor 1 (HPF1 or C4orf27) and others that contain PAR-binding domains, to facilitate chromatin assembly upon DNA damage [65,66]. HPF1 is a key regulator of PARP1-dependent PARylation signaling, acting to promote serine ADP-ribosylation of histones, to limit DNA damage-induced PARP1 hyper-automodification, and to switch the protein modification from PARylation to MARylation [66,67,68,69]. HPF1-mediated serine ADP-ribosylation is a key step in DDR [21]. Of note, the blockage of PARP1 activity that inhibits the repair of DNA damages in tumor cells is a promising approach for cancer treatment [70,71]. Thus, it is worth studying inhibitors that target HPF1-dependent serine ADP-ribosylation. To date, there have been only studies of HPF1 regulating PARP1 in the context of DDR. Whether HPF1 is directly involved in PARP1-dependent transcription activation (see below) requires further investigation. PARylation can also recruit different chromatin remodeling enzymes via their PAR-binding domains [5,8], a range of motifs, including macrodomains, PAR-binding zinc finger (PBZ), PAR-binding motif (PBM), lysine/arginine (KR)-rich domain, high-mobility group (HMG) box-like domain, and PAR-binding regulatory motif (PbR) [5,72]. The macrodomain of chromatin-bound macroH2A1.1 binds to PAR that is locally produced by PARP1, resulting in macroH2A1.1-dependent chromatin compaction [73]. A recent study reported that macroH2A1.1 inhibits PARP1 activity, thereby preventing NAD+ depletion mediated necrosis, and meanwhile stabilizes PAR chains to facilitate DNA repair [74]. It is worth noting that unlike macroH2A1.1, macroH2A1.2 does not bind to PAR [75]. PAR binding by macroH2A1.1 represses cellular proliferation and regulation of gene expression [74,76]. MacroH2A1.1 is specially lost in a majority of cancer types, and thus acts as a tumor suppressor [76]. The interaction of macroH2A1.1 with PAR may explain its different function from its variant macroH2A1.2.
Chromodomain-helicase-DNA-binding protein (CHD) 1-like (CHD1L, also known as ALC1) is an SNF2-like ATPase that is recruited during DNA repair by PAR through its macrodomain motif to trigger chromatin relaxation [77,78]. Aprataxin- and PNK-like factor (APLF or C2orf13) is a well-known histone chaperon, which is recruited to DNA damage sites via interaction of its PBZ domains with PAR. It helps PARP1 to initiate a recruitment cascade of chromatin remodelers by facilitating ALC1 binding to histones and the recruitment of macroH2A1.1 to PAR [79]. CHD6 is another chromatin remodeler that is recruited to DNA damage sites via a conserved KR-rich domain that binds to PAR [80]. The human oncoprotein DEK is a non-histone chromatin architectural protein and, by its interaction with PAR via its PBM domain, it maintains a heterochromatic status in Drosophila [81]. CHD4 is a component of the nucleosome remodeling and deacetylase (NuRD) complex that binds to PAR with its high-mobility group (HMG) box-like domain and promotes the deacetylation of histone, in order to control chromatin reorganization and transcriptional repression [82,83].
As discussed above, PARylation generates long or short, branched or linear PAR, which recruits the chromatin remodeling factors to perform their functions. On the one hand, several chromatin remodeling complexes trigger chromatin relaxation, and thereby regulate the accessibility of DNA in favor of DNA repair. On the other hand, repressive chromatin modifiers are also recruited to sites of DNA damage, which by binding to PAR inhibits transcription. Therefore, the interaction spectrum between PAR and target proteins (PAR readers) may explain different functions of chromatin remodelers.
5. PARP1 as a Modulator of DNA Methylation
Mammalian DNA methylation is an epigenetic mechanism that involves the following two antagonizing processes: the transfer of 5-methylcytosine (5mC) on CpG dinucleotides (CG) by the DNA methyltransferase (DNMT) enzymes (DNMT1, DNMT3a, DNMT3b) [84,85], and demethylation by the action of the TET family of dioxygenases (TET1, TET2 and TET3). DNA methylation controls gene expression by altering chromatin conformation [86]. PARP1-mediated PARylation has been reported to coordinate the dynamics of either methylation or demethylation.
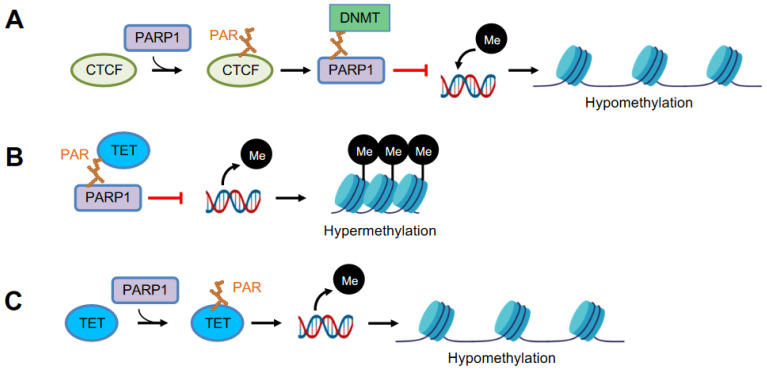
In 1997, Caiafa’s group provided the first evidence that PARylation is associated with DNA methylation [87]. A block of PARylation by PARP inhibitors induces hypermethylation of the CpG island in L929 mouse fibroblasts, while an active PARylation maintains the unmethylated state of the CpG island [88,89]. PAR from auto-PARylated PARP1 binds to DNMT1 and inhibits its enzymatic activity, which prevents DNMT1’s access to DNA, abrogating the methylation of CpG [90]. Chromatin insulator protein CTCF (CCCTC-binding factor) is a key factor in the functional interplay between PARP1 and DNA methylation [91]. CTCF stimulates PARylation activity of PARP1, which in turn maintains the unmethylated status of specific CTCF-bound CpGs, while inhibiting DNMT1’s activity [90,92]. Thus, PARP1 controls DNA methylation patterns by a combined regulatory mode of DNMT1 expression and activity (Figure 3A).
Figure 3.
PARP1 modulates DNA methylation. (A) PARP1 PARylates chromatin insulator CTCF (CCCTC-binding factor), which in turn stimulates auto-PARylation of PARP1. Auto-PARylated PARP1 inhibits the catalytic activity of DNMT through non-covalent binding between PAR-DNMT, resulting in DNA hypomethylation. (B) Non-covalent binding of TET and PAR negatively regulates TET activity, resulting in DNA hypermethylation. (C) Covalent PARylation of TET promotes TET1 activity, causing DNA hypomethylation.
PARP1 is also involved in the maintenance of the unmethylated state of the regulatory sequences of other genes (Figure 3B,C). TET converts 5mC to 5-hydroxymethylcytosine (5hmC) that drives DNA demethylation [93]. Impaired PARP1 activity causes a significant reduction in the expression of TET1 [94]. In HEK293T cells, PARP1 is activated by interacting with TET1 independent of DNA damage; non-covalent PAR binding of TET1 resulted in the negative regulation of TET1 activity, whereas covalent PARylation had a stimulatory effect on TET1 activity [95]. In PARP1 deleted cells, increased mRNA expression of Tet correlates with an increased level of 5hmC and Cxcl12 promoter demethylation increases the Cxcl12 expression [96]. Of note, the histone demethylase KDM5A binds non-covalently with PAR, presented by chromatin remodeler NuRD after PARylation in response to DNA damage, and promotes demethylation of H3K4me3, which stabilizes NuRD at chromatin [97].
The crosstalk between PARP1 activity and DNA demethylation is crucial for gene transcription during cellular differentiation. For example, the interaction of PARP1 with TET2 stimulates conversion of 5mC to 5hmC, which is crucial for early-stage epigenetic modification during somatic cell reprogramming [98]. NAD+ supplementation stimulates PARP1 activity and produced PAR that inhibits DNMT1 activity, resulting in demethylation and transcriptional activation of the CEBPA gene that may be related to myeloid differentiation [27]. Hyperactivation of PARP1 is associated with impaired DNA methylation processes in peripheral blood mononuclear cells from type 2 diabetes mellitus patients [99]. PARylation regulates methylation of histone H3 and DNA methylation during the first cell cycle of mouse development [100]. The mutual exclusivity between the binding of PARP1 to chromatin genome-wide and DNA methylation pattern suggests a functional interplay between PARP1 and DNA methylation [101].
6. PARP1 and PARylation Regulate Gene Transcription
In addition to its involvement in the processes of DNA repair and chromatin homeostasis, PARP1 has been shown to play a prominent role in gene transcription by interacting with and modulating transcriptional machineries at gene promoters. The PARP1 protein directly interacts with numerous transcription factors, such as NF-κB [102], HES1 [103], Elk-1 [24], SOX2 [104], NFAT [105], and AP-1 [106]. PARP1 can regulate transcription positively with co-activators or negatively with repressors. Thus, PARP1 regulates many signaling pathways, thereby controlling a wide array of patho-physiological processes [107].
6.1. PARP1 in Inflammatory Response
For a long time, PARP1 has been shown to be a key mediator of inflammatory response [108,109]. In response to LPS treatment, PARP1−/− mice were resistant to septic shock because of the greatly reduced expression of pro-inflammatory cytokines TNF, IL-6 and iNOS [110]. PARP1 also promotes the pathological inflammatory response in the central nervous and cardiovascular systems [111]. Drosophila that lacks PARP showed insufficient innate immune response and were under an increased risk of bacterial infection [44].
The Hottiger lab first described the mechanism of PARP1 in inflammatory response and demonstrated that PARP1 acts as a transcriptional co-activator to bind both p50 and p65 subunits of NF-κB [112]. PARP1 also interacts with histone acetyltransferases CBP/p300 to promote PARP1’s interactions with p50, which results in NF-κB activation [113,114]. Not only via its protein scaffold, PARP1’s enzymatic activity also influences NF-κB-dependent transcription. However, the conclusion of PARP1 or PARylation activity in NF-κB signaling is complex. An early report using cell extracts showed that PARP1 repressed the DNA-binding activity of NF-κB and PARylation of p50 and p65 inhibited NF-κB-dependent transcription [115]. Many other studies show that PARP1 is positively involved in the NF-κB pathway. For example, in murine macrophage cells, after LPS treatment, PARP1 PARylates p65 and increases the NF-κB-mediated transcription of inflammatory cytokines (such as Il-1β and Il-18) [116]. PARP1 auto-PARylation enhances the DNA-binding activity of p50 [117]. In mouse glia cells, auto-PARylated PARP1 and PARP1 activity increase the LPS-induced DNA binding of NF-κB and the production of cytokines TNFα and iNOS [118]. PARP1 knockout and knockdown, as well as PARP1 inhibitors, can all blunt the interaction between p65-NF-κB and exportin Crm1, thereby attenuating p65 NF-κB nuclear retention, ultimately reducing the expression of NF-κB -targeted genes, such as iNOS and ICAM-1, in LPS-stimulated mouse smooth muscle cells [119]. In the lumbar 5 spinal nerve ligation (SNL) model, SNL increases histone H1 PARylation and PARP inhibitors or PARP1 knockdown repress the binding of NF-κB p65 at the TNFα promoter and downregulates TNFα expression in dorsal root ganglia and spinal dorsal horn [120]. Furthermore, LPS treatment induces PARP1-mediated PARylation of histones at transcription active chromatin regions and the promoters of Il-1β, MIP-2 and CSF2, which facilitates NF-κB recruitment to these promoters [56]. Interestingly, a caspase-resistant PARP1 impairs NF-κB-mediated transcription activity and proinflammatory cytokine production in response to LPS-induced sepsis and intestinal and renal ischemia-reperfusions [121], indicating that PARP1 cleavage at the DNA-binding domain is involved in NF-κB transcription activation.
Apart from auto-PARylation, phosphorylateion of PARP1 by c-Abl under inflammatory agent exposure activated PARylation of p65 and the transcription of NF-κB-target genes [122]. In addition, PARP1 activation achieved by ERK2-mediated phosphorylation, in turn, enhances ERK-induced Elk1-phosphorylation and the transcription of the Elk1-target gene c-fos [24]. Similar to the LPS-mediated sepsis model, PARP1−/− mice were protected from ulcerative colitis induced by trinitrobenzene sulfonic acid (TNBS) treatment, because PARP1 can activate c-Jun of the AP-1 transcription factor [123]. PARP1 can also interact with and PARylate the transcription factor NFAT and increase the binding of NFAT to DNA. PARP1 deficiency or PARP1 inhibitors compromise the expression of cytokines [105,124]. However, PARP1-mediated PARylation of STAT3 inhibits the transcriptional activity of STAT3 and suppresses the expression of PD-L1 [125,126]. The scaffold function of the PARP1 protein and its enzymatic activity seem to complicate the role of PARP1 in transcriptional activation. Nevertheless, these studies demonstrate multiple layers of the gene regulation scheme by PARP1 and PARylation.
6.2. Embryonic Development and Cell Differentiation
Despite the normal development of PARP1−/− mice, PARP1/PARP2 double knockout mice that mostly lack PARylation capacity were embryonic lethal [127], indicating that PARylation homeostasis is crucial for embryo development. The specific tempo-spatial mode of transcription is important for embryonic development. Transcription factors play an important role in maintaining the pluripotency of embryonic stem cells. PARP1 interaction or PARylation modulates the affinity of transcription factors to DNA, and thereby their transcription activity. PARP1 interacts with and PARylates SOX2, thereby reducing SOX2 binding at the enhancer of FGF4 to promote the expression of FGF4 [104]. Another study, however, showed that PARP1 cooperates with SOX2 to facilitate its binding to poorly accessible chromatin and maintains pluripotency in mouse embryonic stem cells, independent of its catalytic activity [128]. The transcription regulation of specific genes dictates cell differentiation. PARP1-mediated PARylation dissociates the SMAD complex from DNA and impairs TGFβ in the differentiation and migration of cells during embryonic development [129]. An activation of calcium-dependent protein kinase (CaMKIIδ) induces phosphorylation and the activation of PARP1, which dissociates the PARylated TLE1 corepressor from HES1 to switch the HES1 function from gene repression to gene activation during neuronal differentiation [103]. In addition, PARP1 directly interacts with ERK2, which then enhances phosphorylation of the transcription activator ELK1 to activate c-fos in growth factor stimulated neural cells and cardiomocytes [24]. PARP1 PARylates C/EBPβ, a key pro-adipogenic transcription factor, and abrogates its DNA-binding and transcriptional activities, thereby affecting the differentiation of adipocyte precursors [130].
6.3. Other Cellular Processes
PARP1 or PARylation also participate in the transcription regulation of many other genes and cellular processes, such as in fat production, cardiovascular and cancer formation. The binding of PARP1 to transcription factor E2F-1 promotes the expression of MYC (c-Myc) for tumorigenesis [131]. PARP1 binds and PARylates FOXO1 to inhibit FOXO1-induced transcription of cell cycle inhibitor p27Kip1 [132]. PARP1 can PARylate estrogen receptor alpha (ERα) and promote its binding to the estrogen reaction element (ERE) in the promoter to activate the transcription of ERα-mediated genes for cardiovascular protection [133]. PARP1 binds to MAF proteins and the antioxidant response elements (ARE) of Nrf2 target genes to activate the transcription of Nrf2 target genes [134]. PARP1, as a co-repressor of transcription together with MATIIα, represses the expression of MAF and Bach1 target genes [135]. PARP1 interacts with the mEH gene (EPHX1) proximal promoter and the linker histone complex H1.2 to stimulate EPHX1 transcription [136]. PARP inhibitor (olaparib, rucaparib) administration decreased the activity and expression of the stress sensor LKB1, although the underlying mechanisms are unknown [137].
In summary, PARP1 can modulate gene expression by direct binding and/or PARylating transcription factors to control gene transcription in response to extracellular signals. This can be achieved by modulating transcription factors’ stability, PTM, or chromatin conformation, all of which affect the DNA binding and/or activity of these transcription machineries.
7. Perspectives
Over the past 60 years, the biochemical and biological functions of PARP1 and PARylation have been extensively studied. The most well-known function is evidently its DNA damage detection, repair and signaling. This function is prominent because PARP1 is largely activated by acute DNA damage and PAR formation. Because of PARP1’s auto-PARylation activity in the process of the DNA damage response and repair, PARP inhibitors have been used to develop a “synthetic lethality” strategy to treat cancers that carry genetically mutated DSB repair pathways, e.g., BRCAs [71,138,139]. Several PARP inhibitors have been recently approved by the Food and Drug Administration (FDA) and European Union (EU) for their efficacy against a variety of cancers [70,71]. However, a growing amount of studies has revealed an important role of PARP1 in other biochemical activities, such as chromatin remodeling, DNA methylation and transcriptional regulation, independent of DNA damage. PARP1 is an abundant and stable nuclear protein. PARP1 changes the chromatin architecture by modifying histones and influencing DNA methylation to facilitate the compaction or loosening of the chromatin structure. PARP1 participates in transcription either as a co-activator or co-repressor, depending on the DNA-binding sequence specificity of the transcription factors, histones of promoter regions, and its interaction with the subunits of the transcription machinery. In addition, the dynamic production and degradation of PAR polymers can influence PAR-binding proteins to specific chromatin loci during transcription. Moreover, PARP1 PARylates target proteins to regulate their activity at these chromatin sites or the promoters. For the same protein that interacts with PARP1, covalently or non-covalently binding of PAR may result in a different outcome of transcription activation. We need a better characterization of the specific targets of PARylation at the promoters in a chromatin context to understand how PARP1 and PARylation change the behaviors of chromatin conformation and transcription factors, under diverse physiological and pathological conditions.
8. Conclusions
PARP1 is well documented to be activated not just by damaged DNA but also by various environmental and developmental stimuli. PARP1 has been broadly related to gene expression, for example by PARylating histones and chromatin remodeling proteins that modulate chromatin architecture and behaviors of transcription machineries at gene promoters. PARP1 controls expression of many genes operating immune responses, cell survival and inflammation. The function of PARP1 in gene regulation thus may contribute to the establishment of the molecular actions of PARP inhibitors for their clinical applications in treatment of inflammatory diseases, as well as, cancer.
Acknowledgments
There is a large quantity of excellent papers published by many laboratories in the field of PARPs and PARylation. We apologize to those whose work could not be discussed due to the focused theme and limited space allowed in the manuscript. We thank Eileen Stöckl for editing the manuscript. We are grateful to the members of the Wang laboratory for their critical and helpful discussions.
Author Contributions
All authors contributed to the literature search and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the DFG grant to Z.-Q.W. (WA2627/11-1) and the Qilu Youth Scholar Startup Fund of Shandong University to T.L.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luscher B., Ahel I., Altmeyer M., Ashworth A., Bai P., Chang P., Cohen M., Corda D., Dantzer F., Daugherty M.D., et al. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2021 doi: 10.1111/febs.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyas S., Matic I., Uchima L., Rood J., Zaja R., Hay R.T., Ahel I., Chang P. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat. Commun. 2014;5:4426. doi: 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlberg T., Klepsch M., Thorsell A.G., Andersson C.D., Linusson A., Schuler H. Structural basis for lack of ADP-ribosyltransferase activity in poly(ADP-ribose) polymerase-13/zinc finger antiviral protein. J. Biol. Chem. 2015;290:7336–7344. doi: 10.1074/jbc.M114.630160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rack J.G.M., Palazzo L., Ahel I. (ADP-ribosyl) hydrolases: Structure, function, and biology. Genes Dev. 2020;34:263–284. doi: 10.1101/gad.334631.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamaletdinova T., Fanaei-Kahrani Z., Wang Z.Q. The Enigmatic Function of PARP1: From PARylation Activity to PAR Readers. Cells. 2019;8:1625. doi: 10.3390/cells8121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shieh W.M., Ame J.C., Wilson M.V., Wang Z.Q., Koh D.W., Jacobson M.K., Jacobson E.L. Poly(ADP-ribose) polymerase null mouse cells synthesize ADP-ribose polymers. J. Biol. Chem. 1998;273:30069–30072. doi: 10.1074/jbc.273.46.30069. [DOI] [PubMed] [Google Scholar]
- 7.Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei H., Yu X. Functions of PARylation in DNA Damage Repair Pathways. Genom. Proteom. Bioinform. 2016;14:131–139. doi: 10.1016/j.gpb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posavec Marjanović M., Crawford K., Ahel I. PARP, transcription and chromatin modeling. Semin. Cell Dev. Biol. 2017;63:102–113. doi: 10.1016/j.semcdb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Ciccarone F., Zampieri M., Caiafa P. PARP1 orchestrates epigenetic events setting up chromatin domains. Semin. Cell Dev. Biol. 2017;63:123–134. doi: 10.1016/j.semcdb.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Alkhatib H.M., Chen D.F., Cherney B., Bhatia K., Notario V., Giri C., Stein G., Slattery E., Roeder R.G., Smulson M.E. Cloning and expression of cDNA for human poly(ADP-ribose) Proc. Natl. Acad. Sci. USA. 1987;84:1224–1228. doi: 10.1073/pnas.84.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida K., Morita T., Sato T., Ogura T., Yamashita R., Noguchi S., Suzuki H., Nyunoya H., Miwa M., Sugimura T. Nucleotide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 1987;148:617–622. doi: 10.1016/0006-291X(87)90921-1. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaki T., Ushiro H., Mitsuuchi Y., Suzuki S., Matsuda M., Matsuda Y., Katunuma N., Kangawa K., Matsuo H., Hirose T. Primary structure of human poly(ADP-ribose) synthetase as deduced from cDNA sequence. J. Biol. Chem. 1987;262:15990–15997. doi: 10.1016/S0021-9258(18)47687-9. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner M., Schneider R., Auer B., Herzog H., Schweiger M., Hirsch-Kauffmann M. Fluorescence in situ mapping of the human nuclear NAD+ ADP-ribosyltransferase gene (ADPRT) and two secondary sites to human chromosomal bands 1q42, 13q34, and 14q24. Cytogenet. Cell Genet. 1992;61:172–174. doi: 10.1159/000133400. [DOI] [PubMed] [Google Scholar]
- 15.Perina D., Mikoc A., Ahel J., Cetkovic H., Zaja R., Ahel I. Distribution of protein poly(ADP-ribosyl)ation systems across all domains of life. DNA Repair. 2014;23:4–16. doi: 10.1016/j.dnarep.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikejima M., Noguchi S., Yamashita R., Ogura T., Sugimura T., Gill D.M., Miwa M. The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J. Biol. Chem. 1990;265:21907–21913. doi: 10.1016/S0021-9258(18)45824-3. [DOI] [PubMed] [Google Scholar]
- 17.Gradwohl G., Ménissier de Murcia J.M., Molinete M., Simonin F., Koken M., Hoeijmakers J.H., de Murcia G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc. Natl. Acad. Sci. USA. 1990;87:2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langelier M.F., Planck J.L., Roy S., Pascal J.M. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel J.O., Van Houten B., Durrant J.D. PARP1: Structural insights and pharmacological targets for inhibition. DNA Repair. 2021;103:103125. doi: 10.1016/j.dnarep.2021.103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudolph J., Muthurajan U.M., Palacio M., Mahadevan J., Roberts G., Erbse A.H., Dyer P.N., Luger K. The BRCT domain of PARP1 binds intact DNA and mediates intrastrand transfer. Mol. Cell. 2021;81:4994–5006.e5. doi: 10.1016/j.molcel.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palazzo L., Leidecker O., Prokhorova E., Dauben H., Matic I., Ahel I. Serine is the major residue for ADP-ribosylation upon DNA damage. Elife. 2018;7:e34334. doi: 10.7554/eLife.34334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prokhorova E., Zobel F., Smith R., Zentout S., Gibbs-Seymour I., Schützenhofer K., Peters A., Groslambert J., Zorzini V., Agnew T., et al. Serine-linked PARP1 auto-modification controls PARP inhibitor response. Nat. Commun. 2021;12:4055. doi: 10.1038/s41467-021-24361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruf A., Mennissier de Murcia J., de Murcia G., Schulz G.E. Structure of the catalytic fragment of poly(AD-ribose) polymerase from chicken. Proc. Natl. Acad. Sci. USA. 1996;93:7481–7485. doi: 10.1073/pnas.93.15.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen-Armon M., Visochek L., Rozensal D., Kalal A., Geistrikh I., Klein R., Bendetz-Nezer S., Yao Z., Seger R. DNA-Independent PARP-1 Activation by Phosphorylated ERK2 Increases Elk1 Activity: A Link to Histone Acetylation. Mol. Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Hau A.C., Grebbin B.M., Agoston Z., Anders-Maurer M., Müller T., Groß A., Kolb J., Langer J.D., Döring C., Schulte D. MEIS homeodomain proteins facilitate PARP1/ARTD1-mediated eviction of histone H1. J. Cell Biol. 2017;216:2715–2729. doi: 10.1083/jcb.201701154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas C.J., Kotova E., Andrake M., Adolf-Bryfogle J., Glaser R., Regnard C., Tulin A.V. Kinase-Mediated Changes in Nucleosome Conformation Trigger Chromatin Decondensation via Poly(ADP-Ribosyl)ation. Mol. Cell. 2014;53:831–842. doi: 10.1016/j.molcel.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ummarino S., Hausman C., Gaggi G., Rinaldi L., Bassal M.A., Zhang Y., Seelam A.J., Kobayashi I.S., Borchiellini M., Ebralidze A.K., et al. NAD Modulates DNA Methylation and Cell Differentiation. Cells. 2021;10:2986. doi: 10.3390/cells10112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochu G., Duchaine C., Thibeault L., Lagueux J., Shah G.M., Poirier G.G. Mode of action of poly (ADP-ribose) glycohydrolase. Biochim. Biophys. Acta. 1994;1219:342–350. doi: 10.1016/0167-4781(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 29.Oka S., Kato J., Moss J. Identification and Characterization of a Mammalian 39-kDa Poly(ADP-ribose) Glycohydrolase. J. Biol. Chem. 2006;281:705–713. doi: 10.1074/jbc.M510290200. [DOI] [PubMed] [Google Scholar]
- 30.Jankevicius G., Hassler M., Golia B., Rybin V., Zacharias M., Timinszky G., Ladurner A.G. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat. Struct. Mol. Biol. 2013;20:508–514. doi: 10.1038/nsmb.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharifi R., Morra R., Appel C.D., Tallis M., Chioza B., Jankevicius G., Simpson M.A., Matic I., Ozkan E., Golia B., et al. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 2013;32:1225–1237. doi: 10.1038/emboj.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slade D., Dunstan M.S., Barkauskaite E., Weston R., Lafite P., Dixon N., Ahel M., Leys D., Ahel I. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature. 2011;477:616–620. doi: 10.1038/nature10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono T., Kasamatsu A., Oka S., Moss J. The 39-kDa poly(ADP-ribose) glycohydrolase ARH3 hydrolyzes O-acetyl-ADP-ribose, a product of the Sir2 family of acetyl-histone deacetylases. Proc. Natl. Acad. Sci. USA. 2006;103:16687–16691. doi: 10.1073/pnas.0607911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson F.C., Chen D., Lytle B.L., Rossi M.N., Ahel I., Denu J.M., Volkman B.F. Orphan macrodomain protein (human C6orf130) is an O-acyl-ADP-ribose deacylase: Solution structure and catalytic properties. J. Biol. Chem. 2011;286:35955–35965. doi: 10.1074/jbc.M111.276238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruta H., Okita N., Takasawa R., Uchiumi F., Hatano T., Tanuma S. The involvement of ATP produced via (ADP-Ribose)n in the maintenance of DNA replication apparatus during DNA repair. Biol. Pharm. Bull. 2007;30:447–450. doi: 10.1248/bpb.30.447. [DOI] [PubMed] [Google Scholar]
- 36.Morrison A.J. Chromatin-remodeling links metabolic signaling to gene expression. Mol. Metab. 2020;38:100973. doi: 10.1016/j.molmet.2020.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bintu L., Ishibashi T., Dangkulwanich M., Wu Y.Y., Lubkowska L., Kashlev M., Bustamante C. Nucleosomal elements that control the topography of the barrier to transcription. Cell. 2012;151:738–749. doi: 10.1016/j.cell.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes A.A., Marcum R.D., He Y. Structure and Function of Chromatin Remodelers. J. Mol. Biol. 2021;433:166929. doi: 10.1016/j.jmb.2021.166929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan M.A.J., Shilatifard A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat. Genet. 2020;52:1271–1281. doi: 10.1038/s41588-020-00736-4. [DOI] [PubMed] [Google Scholar]
- 40.Otake H., Miwa M., Fujimura S., Sugimura T. Binding of ADP-ribose polymer with histone. J. Biochem. 1969;65:145–146. [PubMed] [Google Scholar]
- 41.Ueda K., Reeder R.H., Honjo T., Nishizuka Y., Hayaishi O. Poly adenosine diphosphate ribose synthesis associated with chromatin. Biochem. Biophys. Res. Commun. 1968;31:379–385. doi: 10.1016/0006-291X(68)90486-5. [DOI] [PubMed] [Google Scholar]
- 42.Poirier G.G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc. Natl. Acad. Sci. USA. 1982;79:3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huletsky A., de Murcia G., Muller S., Hengartner M., Ménard L., Lamarre D., Poirier G.G. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. J. Biol. Chem. 1989;264:8878–8886. doi: 10.1016/S0021-9258(18)81875-0. [DOI] [PubMed] [Google Scholar]
- 44.Tulin A., Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–562. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- 45.Kim M.Y., Mauro S., Gevry N., Lis J.T., Kraus W.L. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Krishnakumar R., Gamble M.J., Frizzell K.M., Berrocal J.G., Kininis M., Kraus W.L. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–821. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- 47.Shan L., Li X., Liu L., Ding X., Wang Q., Zheng Y., Duan Y., Xuan C., Wang Y., Yang F., et al. GATA3 cooperates with PARP1 to regulate CCND1 transcription through modulating histone H1 incorporation. Oncogene. 2014;33:3205–3216. doi: 10.1038/onc.2013.270. [DOI] [PubMed] [Google Scholar]
- 48.Fontán-Lozano A., Suárez-Pereira I., Horrillo A., del-Pozo-Martín Y., Hmadcha A., Carrión A.M. Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation. J. Neurosci. 2010;30:13305–13313. doi: 10.1523/JNEUROSCI.3010-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright R.H., Castellano G., Bonet J., Le Dily F., Font-Mateu J., Ballaré C., Nacht A.S., Soronellas D., Oliva B., Beato M. CDK2-dependent activation of PARP-1 is required for hormonal gene regulation in breast cancer cells. Genes Dev. 2012;26:1972–1983. doi: 10.1101/gad.193193.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azad G.K., Ito K., Sailaja B.S., Biran A., Nissim-Rafinia M., Yamada Y., Brown D.T., Takizawa T., Meshorer E. PARP1-dependent eviction of the linker histone H1 mediates immediate early gene expression during neuronal activation. J. Cell Biol. 2018;217:473–481. doi: 10.1083/jcb.201703141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaiser A., Kruger T., Eiselt G., Bechler J., Kniemeyer O., Huber O., Schmidt M. Identification of PARP-1, Histone H1 and SIRT-1 as New Regulators of Breast Cancer-Related Aromatase Promoter I.3/II. Cells. 2020;9:427. doi: 10.3390/cells9020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maluchenko N.V., Nilov D.K., Pushkarev S.V., Kotova E.Y., Gerasimova N.S., Kirpichnikov M.P., Langelier M.F., Pascal J.M., Akhtar M.S., Feofanov A.V., et al. Mechanisms of Nucleosome Reorganization by PARP1. Int. J. Mol. Sci. 2021;22:12127. doi: 10.3390/ijms222212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell N.A.W., Haynes P.J., Brunner K., de Oliveira T.M., Flocco M.M., Hoogenboom B.W., Molloy J.E. Single-molecule measurements reveal that PARP1 condenses DNA by loop stabilization. Sci. Adv. 2021;7:33. doi: 10.1126/sciadv.abf3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altmeyer M., Messner S., Hassa P.O., Fey M., Hottiger M.O. Molecular mechanism of poly(ADP-ribosyl)ation by PARP1 and identification of lysine residues as ADP-ribose acceptor sites. Nucleic Acids Res. 2009;37:3723–3738. doi: 10.1093/nar/gkp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Messner S., Altmeyer M., Zhao H., Pozivil A., Roschitzki B., Gehrig P., Rutishauser D., Huang D., Caflisch A., Hottiger M.O. PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Res. 2010;38:6350–6362. doi: 10.1093/nar/gkq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Zamudio R., Ha H.C. Histone ADP-ribosylation facilitates gene transcription by directly remodeling nucleosomes. Mol. Cell. Biol. 2012;32:2490–2502. doi: 10.1128/MCB.06667-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang D., Camacho C.V., Setlem R., Ryu K.W., Parameswaran B., Gupta R.K., Kraus W.L. Functional Interplay between Histone H2B ADP-Ribosylation and Phosphorylation Controls Adipogenesis. Mol. Cell. 2020;79:934–949.e14. doi: 10.1016/j.molcel.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C., Xiao J., Nowak K., Gunasekera K., Alippe Y., Speckman S., Yang T., Kress D., Abu-Amer Y., Hottiger M.O., et al. PARP1 Hinders Histone H2B Occupancy at the NFATc1 Promoter to Restrain Osteoclast Differentiation. J. Bone Miner. Res. 2020;35:776–788. doi: 10.1002/jbmr.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas C., Ji Y., Wu C., Datz H., Boyle C., MacLeod B., Patel S., Ampofo M., Currie M., Harbin J., et al. Hit and run versus long-term activation of PARP-1 by its different domains fine-tunes nuclear processes. Proc. Natl. Acad. Sci. USA. 2019;116:9941–9946. doi: 10.1073/pnas.1901183116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotova E., Lodhi N., Jarnik M., Pinnola A.D., Ji Y., Tulin A.V. Drosophila histone H2A variant (H2Av) controls poly(ADP-ribose) polymerase 1 (PARP1) activation in chromatin. Proc. Natl. Acad. Sci. USA. 2011;108:6205–6210. doi: 10.1073/pnas.1019644108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nusinow D.A., Hernández-Muñoz I., Fazzio T.G., Shah G.M., Kraus W.L., Panning B. Poly(ADP-ribose) Polymerase 1 Is Inhibited by a Histone H2A Variant, MacroH2A, and Contributes to Silencing of the Inactive X Chromosome. J. Biol. Chem. 2007;282:12851–12859. doi: 10.1074/jbc.M610502200. [DOI] [PubMed] [Google Scholar]
- 62.Krishnakumar R., Kraus W.L. PARP-1 regulates chromatin structure and transcription through a KDM5B-dependent pathway. Mol. Cell. 2010;39:736–749. doi: 10.1016/j.molcel.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donnell A., Yang S.H., Sharrocks A.D. PARP1 orchestrates variant histone exchange in signal-mediated transcriptional activation. EMBO Rep. 2013;14:1084–1091. doi: 10.1038/embor.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H., Ruiz P.D., Novikov L., Casill A.D., Park J.W., Gamble M.J. MacroH2A1.1 and PARP-1 cooperate to regulate transcription by promoting CBP-mediated H2B acetylation. Nat. Struct. Mol. Biol. 2014;21:981–989. doi: 10.1038/nsmb.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutcu H.H., Matta E., Ishchenko A.A. Role of PARP-catalyzed ADP-ribosylation in the Crosstalk between DNA Strand Breaks and Epigenetic Regulation. J. Mol. Biol. 2019;432:1769–1791. doi: 10.1016/j.jmb.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Gibbs-Seymour I., Fontana P., Rack J.G.M., Ahel I. HPF1/C4orf27 Is a PARP-1-Interacting Protein that Regulates PARP-1 ADP-Ribosylation Activity. Mol. Cell. 2016;62:432–442. doi: 10.1016/j.molcel.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suskiewicz M.J., Zobel F., Ogden T.E.H., Fontana P., Ariza A., Yang J.C., Zhu K., Bracken L., Hawthorne W.J., Ahel D., et al. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature. 2020;579:598–602. doi: 10.1038/s41586-020-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun F.H., Zhao P., Zhang N., Kong L.L., Wong C.C.L., Yun C.H. HPF1 remodels the active site of PARP1 to enable the serine ADP-ribosylation of histones. Nat. Commun. 2021;12:1028. doi: 10.1038/s41467-021-21302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendriks I.A., Buch-Larsen S.C., Prokhorova E., Elsborg J.D., Rebak A., Zhu K., Ahel D., Lukas C., Ahel I., Nielsen M.L. The regulatory landscape of the human HPF1- and ARH3-dependent ADP-ribosylome. Nat. Commun. 2021;12:5893. doi: 10.1038/s41467-021-26172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LaFargue C.J., Dal Molin G.Z., Sood A.K., Coleman R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:e15–e28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curtin N.J., Szabo C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020;19:711–736. doi: 10.1038/s41573-020-0076-6. [DOI] [PubMed] [Google Scholar]
- 72.Andronikou C., Rottenberg S. Studying PAR-Dependent Chromatin Remodeling to Tackle PARPi Resistance. Trends Mol. Med. 2021;27:630–642. doi: 10.1016/j.molmed.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Timinszky G., Till S., Hassa P.O., Hothorn M., Kustatscher G., Nijmeijer B., Colombelli J., Altmeyer M., Stelzer E.H., Scheffzek K., et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 2009;16:923–929. doi: 10.1038/nsmb.1664. [DOI] [PubMed] [Google Scholar]
- 74.Ruiz P.D., Hamilton G.A., Park J.W., Gamble M.J. MacroH2A1 Regulation of Poly(ADP-Ribose) Synthesis and Stability Prevents Necrosis and Promotes DNA Repair. Mol. Cell. Biol. 2019;40:e00230-19. doi: 10.1128/MCB.00230-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozlowski M., Corujo D., Hothorn M., Guberovic I., Mandemaker I.K., Blessing C., Sporn J., Gutierrez-Triana A., Smith R., Portmann T., et al. MacroH2A histone variants limit chromatin plasticity through two distinct mechanisms. EMBO Rep. 2018;19:e44445. doi: 10.15252/embr.201744445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corujo D., Buschbeck M. Post-Translational Modifications of H2A Histone Variants and Their Role in Cancer. Cancers. 2018;10:59. doi: 10.3390/cancers10030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gottschalk A.J., Trivedi R.D., Conaway J.W., Conaway R.C. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1·PARP1·nucleosome intermediate. J. Biol. Chem. 2012;287:43527–43532. doi: 10.1074/jbc.M112.401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahel D., Horejsí Z., Wiechens N., Polo S.E., Garcia-Wilson E., Ahel I., Flynn H., Skehel M., West S.C., Jackson S.P., et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325:1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehrotra P.V., Ahel D., Ryan D.P., Weston R., Wiechens N., Kraehenbuehl R., Owen-Hughes T., Ahel I. DNA Repair Factor APLF Is a Histone Chaperone. Mol. Cell. 2011;41:46–55. doi: 10.1016/j.molcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore S., Berger N.D., Luijsterburg M.S., Piett C.G., Stanley F.K.T., Schrader C.U., Fang S., Chan J.A., Schriemer D.C., Nagel Z.D., et al. The CHD6 chromatin remodeler is an oxidative DNA damage response factor. Nat. Commun. 2019;10:241. doi: 10.1038/s41467-018-08111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fahrer J., Popp O., Malanga M., Beneke S., Markovitz D.M., Ferrando-May E., Burkle A., Kappes F. High-affinity interaction of poly(ADP-ribose) and the human DEK oncoprotein depends upon chain length. Biochemistry. 2010;49:7119–7130. doi: 10.1021/bi1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva A.P.G., Ryan D.P., Galanty Y., Low J.K.K., Vandevenne M., Jackson S.P., Mackay J.P. The N-terminal Region of Chromodomain Helicase DNA-binding Protein 4 (CHD4) Is Essential for Activity and Contains a High Mobility Group (HMG) Box-like-domain That Can Bind Poly(ADP-ribose)*. J. Biol. Chem. 2016;291:924–938. doi: 10.1074/jbc.M115.683227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou D.M., Adamson B., Dephoure N.E., Tan X., Nottke A.C., Hurov K.E., Gygi S.P., Colaiácovo M.P., Elledge S.J. A chromatin localization screen reveals poly(ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc. Natl. Acad. Sci. USA. 2010;107:18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hermann A., Goyal R., Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 85.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 86.Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 87.Zardo G., D’Erme M., Reale A., Strom R., Perilli M., Caiafa P. Does Poly(ADP-ribosyl)ation Regulate the DNA Methylation Pattern? Biochemistry. 1997;36:7937–7943. doi: 10.1021/bi970241s. [DOI] [PubMed] [Google Scholar]
- 88.Zardo G., Caiafa P. The unmethylated state of CpG islands in mouse fibroblasts depends on the poly(ADP-ribosyl)ation process. J. Biol. Chem. 1998;273:16517–16520. doi: 10.1074/jbc.273.26.16517. [DOI] [PubMed] [Google Scholar]
- 89.de Capoa A., Febbo F.R., Giovannelli F., Niveleau A., Zardo G., Marenzi S., Caiafa P. Reduced levels of poly(ADP-ribosyl)ation result in chromatin compaction and hypermethylation as shown by cell-by-cell computer-assisted quantitative analysis. FASEB J. 1999;13:89–93. doi: 10.1096/fasebj.13.1.89. [DOI] [PubMed] [Google Scholar]
- 90.Zampieri M., Guastafierro T., Calabrese R., Ciccarone F., Bacalini M.G., Reale A., Perilli M., Passananti C., Caiafa P. ADP-ribose polymers localized on Ctcf-Parp1-Dnmt1 complex prevent methylation of Ctcf target sites. Biochem. J. 2012;441:645–652. doi: 10.1042/BJ20111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu W., Ginjala V., Pant V., Chernukhin I., Whitehead J., Docquier F., Farrar D., Tavoosidana G., Mukhopadhyay R., Kanduri C., et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat. Genet. 2004;36:1105–1110. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- 92.Guastafierro T., Cecchinelli B., Zampieri M., Reale A., Riggio G., Sthandier O., Zupi G., Calabrese L., Caiafa P. CCCTC-binding Factor Activates PARP-1 Affecting DNA Methylation Machinery. J. Biol. Chem. 2008;283:21873–21880. doi: 10.1074/jbc.M801170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciccarone F., Klinger F.G., Catizone A., Calabrese R., Zampieri M., Bacalini M.G., De Felici M., Caiafa P. Poly(ADP-ribosyl)ation acts in the DNA demethylation of mouse primordial germ cells also with DNA damage-independent roles. PLoS ONE. 2012;7:e46927. doi: 10.1371/journal.pone.0046927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciccarone F., Valentini E., Zampieri M., Caiafa P. 5mC-hydroxylase activity is influenced by the PARylation of TET1 enzyme. Oncotarget. 2015;6:24333–24347. doi: 10.18632/oncotarget.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tolić A., Grdović N., Dinić S., Rajić J., Đorđević M., Sinadinović M., Arambašić Jovanović J., Mihailović M., Poznanović G., Uskoković A., et al. Absence of PARP-1 affects Cxcl12 expression by increasing DNA demethylation. J. Cell. Mol. Med. 2019;23:2610–2618. doi: 10.1111/jcmm.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumbhar R., Sanchez A., Perren J., Gong F., Corujo D., Medina F., Devanathan S.K., Xhemalce B., Matouschek A., Buschbeck M., et al. Poly(ADP-ribose) binding and macroH2A mediate recruitment and functions of KDM5A at DNA lesions. J. Cell Biol. 2021;220:e202006149. doi: 10.1083/jcb.202006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doege C.A., Inoue K., Yamashita T., Rhee D.B., Travis S., Fujita R., Guarnieri P., Bhagat G., Vanti W.B., Shih A., et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zampieri M., Bacalini M.G., Barchetta I., Scalea S., Cimini F.A., Bertoccini L., Tagliatesta S., De Matteis G., Zardo G., Cavallo M.G., et al. Increased PARylation impacts the DNA methylation process in type 2 diabetes mellitus. Clin. Epigenetics. 2021;13:114. doi: 10.1186/s13148-021-01099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Osada T., Rydén A.M., Masutani M. Poly(ADP-ribosylation) regulates chromatin organization through histone H3 modification and DNA methylation of the first cell cycle of mouse embryos. Biochem. Biophys. Res. Commun. 2013;434:15–21. doi: 10.1016/j.bbrc.2013.03.074. [DOI] [PubMed] [Google Scholar]
- 101.Nalabothula N., Al-jumaily T., Eteleeb A.M., Flight R.M., Xiaorong S., Moseley H., Rouchka E.C., Fondufe-Mittendorf Y.N. Genome-Wide Profiling of PARP1 Reveals an Interplay with Gene Regulatory Regions and DNA Methylation. PLoS ONE. 2015;10:e0135410. doi: 10.1371/journal.pone.0135410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hassa P., Hottiger M. The functional role of poly(ADP-ribose) polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell Mol. Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ju B.-G., Solum D., Song E.J., Lee K.-J., Rose D.W., Glass C.K., Rosenfeld M.G. Activating the PARP-1 Sensor Component of the Groucho/TLE1 Corepressor Complex Mediates a CaMKinase IIδ-Dependent Neurogenic Gene Activation Pathway. Cell. 2004;119:815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 104.Gao F., Kwon S.W., Zhao Y., Jin Y. PARP1 Poly(ADP-ribosyl)ates Sox2 to Control Sox2 Protein Levels and FGF4 Expression during Embryonic Stem Cell Differentiation. J. Biol. Chem. 2009;284:22263–22273. doi: 10.1074/jbc.M109.033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valdor R., Schreiber V., Saenz L., Martínez T., Muñoz-Suano A., Dominguez-Villar M., Ramírez P., Parrilla P., Aguado E., García-Cózar F. Regulation of NFAT by poly(ADP-ribose) polymerase activity in T cells. Mol. Immunol. 2008;45:1863–1871. doi: 10.1016/j.molimm.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 106.Choudhuri S., Garg N.J. Trypanosoma cruzi Induces the PARP1/AP-1 Pathway for Upregulation of Metalloproteinases and Transforming Growth Factor β in Macrophages: Role in Cardiac Fibroblast Differentiation and Fibrosis in Chagas Disease. mBio. 2020;11:e01853-20. doi: 10.1128/mBio.01853-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao K., Zhang G. The role of PARP1 in neurodegenerative diseases and aging. FEBS J. 2021;289:2013–2024. doi: 10.1111/febs.15716. [DOI] [PubMed] [Google Scholar]
- 108.Rosado M.M., Bennici E., Novelli F., Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013;139:428–437. doi: 10.1111/imm.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Welsby I., Hutin D., Leo O. Complex roles of members of the ADP-ribosyl transferase super family in immune defences: Looking beyond PARP1. Biochem. Pharmacol. 2012;84:11–20. doi: 10.1016/j.bcp.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 110.Oliver F.J., Menissier-de Murcia J., Nacci C., Decker P., Andriantsitohaina R., Muller S., de la Rubia G., Stoclet J.C., de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly(ADP-ribose) polymerase-1 deficient mice. Embo J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pacher P., Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: The therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hassa P.O., Covic M., Hasan S., Imhof R., Hottiger M.O. The Enzymatic and DNA Binding Activity of PARP-1 Are Not Required for NF-κB Coactivator Function. J. Biol. Chem. 2001;276:45588–45597. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- 113.Hassa P.O., Buerki C., Lombardi C., Imhof R., Hottiger M.O. Transcriptional coactivation of nuclear factor-kappaB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J. Biol. Chem. 2003;278:45145–45153. doi: 10.1074/jbc.M307957200. [DOI] [PubMed] [Google Scholar]
- 114.Hassa P.O., Haenni S.S., Buerki C., Meier N.I., Lane W.S., Owen H., Gersbach M., Imhof R., Hottiger M.O. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J. Biol. Chem. 2005;280:40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 115.Kameoka M., Ota K., Tetsuka T., Tanaka Y., Itaya A., Okamoto T., Yoshihara K. Evidence for regulation of NF-kappaB by poly(ADP-ribose) polymerase. Biochem. J. 2000;346:641–649. doi: 10.1042/bj3460641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu L., Ke Y., Jiang X., He F., Pan L., Xu L., Zeng X., Ba X. Lipopolysaccharide activates ERK-PARP-1-RelA pathway and promotes nuclear factor-κB transcription in murine macrophages. Hum. Immunol. 2012;73:439–447. doi: 10.1016/j.humimm.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Chang W.-J., Alvarez-Gonzalez R. The Sequence-specific DNA Binding of NF-κB Is Reversibly Regulated by the Automodification Reaction of Poly(ADP-ribose) Polymerase 1. J. Biol. Chem. 2001;276:47664–47670. doi: 10.1074/jbc.M104666200. [DOI] [PubMed] [Google Scholar]
- 118.Nakajima H., Nagaso H., Kakui N., Ishikawa M., Hiranuma T., Hoshiko S. Critical role of the automodification of poly(ADP-ribose) polymerase-1 in nuclear factor-kappaB-dependent gene expression in primary cultured mouse glial cells. J. Biol. Chem. 2004;279:42774–42786. doi: 10.1074/jbc.M407923200. [DOI] [PubMed] [Google Scholar]
- 119.Zerfaoui M., Errami Y., Naura A.S., Suzuki Y., Kim H., Ju J., Liu T., Hans C.P., Kim J.G., Abd Elmageed Z.Y., et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J. Immunol. 2010;185:1894–1902. doi: 10.4049/jimmunol.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao Y., Bai L., Zhou W., Yang Y., Zhang J., Li L., Jiang M., Mi Y., Li T.T., Zhang X., et al. PARP-1-regulated TNF-α expression in the dorsal root ganglia and spinal dorsal horn contributes to the pathogenesis of neuropathic pain in rats. Brain Behav. Immun. 2020;88:482–496. doi: 10.1016/j.bbi.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Petrilli V., Herceg Z., Hassa P.O., Patel N.S., Di Paola R., Cortes U., Dugo L., Filipe H.M., Thiemermann C., Hottiger M.O., et al. Noncleavable poly(ADP-ribose) polymerase-1 regulates the inflammation response in mice. J. Clin. Investig. 2004;114:1072–1081. doi: 10.1172/JCI200421854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bohio A.A., Sattout A., Wang R., Wang K., Sah R.K., Guo X., Zeng X., Ke Y., Boldogh I., Ba X. c-Abl-Mediated Tyrosine Phosphorylation of PARP1 Is Crucial for Expression of Proinflammatory Genes. J. Immunol. 2019;203:1521–1531. doi: 10.4049/jimmunol.1801616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zingarelli B., Hake P.W., Burroughs T.J., Piraino G., O’Connor M., Denenberg A. Activator protein-1 signalling pathway and apoptosis are modulated by poly(ADP-ribose) polymerase-1 in experimental colitis. Immunology. 2004;113:509–517. doi: 10.1111/j.1365-2567.2004.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olabisi O.A., Soto-Nieves N., Nieves E., Yang T.T.C., Yang X., Yu R.Y.L., Suk H.Y., Macian F., Chow C.-W. Regulation of Transcription Factor NFAT by ADP-Ribosylation. Mol. Cell. Biol. 2008;28:2860–2871. doi: 10.1128/MCB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ding L., Chen X., Xu X., Qian Y., Liang G., Yao F., Yao Z., Wu H., Zhang J., He Q., et al. PARP1 Suppresses the Transcription of PD-L1 by Poly(ADP-Ribosyl)ating STAT3. Cancer Immunol. Res. 2019;7:136–149. doi: 10.1158/2326-6066.CIR-18-0071. [DOI] [PubMed] [Google Scholar]
- 126.Jiao S., Xia W., Yamaguchi H., Wei Y., Chen M.K., Hsu J.M., Hsu J.L., Yu W.H., Du Y., Lee H.H., et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Menissier de Murcia J., Ricoul M., Tartier L., Niedergang C., Huber A., Dantzer F., Schreiber V., Ame J.C., Dierich A., LeMeur M., et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. Embo J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Z., Kraus W.L. Catalytic-independent functions of PARP-1 determine Sox2 pioneer activity at intractable genomic loci. Mol. Cell. 2017;65:589–603.e9. doi: 10.1016/j.molcel.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lönn P., van der Heide L.P., Dahl M., Hellman U., Heldin C.-H., Moustakas A. PARP-1 Attenuates Smad-Mediated Transcription. Mol. Cell. 2010;40:521–532. doi: 10.1016/j.molcel.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 130.Luo X., Ryu K.W., Kim D.S., Nandu T., Medina C.J., Gupte R., Gibson B.A., Soccio R.E., Yu Y., Gupta R.K., et al. PARP-1 Controls the Adipogenic Transcriptional Program by PARylating C/EBPβ and Modulating Its Transcriptional Activity. Mol. Cell. 2017;65:260–271. doi: 10.1016/j.molcel.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simbulan-Rosenthal C.M., Rosenthal D.S., Luo R., Samara R., Espinoza L.A., Hassa P.O., Hottiger M.O., Smulson M.E. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene. 2003;22:8460–8471. doi: 10.1038/sj.onc.1206897. [DOI] [PubMed] [Google Scholar]
- 132.Sakamaki J., Daitoku H., Yoshimochi K., Miwa M., Fukamizu A. Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem. Biophys. Res. Commun. 2009;382:497–502. doi: 10.1016/j.bbrc.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 133.Zhang F., Wang Y., Wang L., Luo X., Huang K., Wang C., Du M., Liu F., Luo T., Huang D., et al. Poly(ADP-ribose) Polymerase 1 Is a Key Regulator of Estrogen Receptor α-dependent Gene Transcription. J. Biol. Chem. 2013;288:11348–11357. doi: 10.1074/jbc.M112.429134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu T., Wang X.-J., Tian W., Jaramillo M.C., Lau A., Zhang D.D. Poly(ADP-ribose) polymerase-1 modulates Nrf2-dependent transcription. Free Radic. Biol. Med. 2014;67:69–80. doi: 10.1016/j.freeradbiomed.2013.10.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Katoh Y., Ikura T., Hoshikawa Y., Tashiro S., Ito T., Ohta M., Kera Y., Noda T., Igarashi K. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol. Cell. 2011;41:554–566. doi: 10.1016/j.molcel.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 136.Peng H., Zhu Q.S., Zhong S., Levy D. Transcription of the Human Microsomal Epoxide Hydrolase Gene (EPHX1) Is Regulated by PARP-1 and Histone H1.2. Association with Sodium-Dependent Bile Acid Transport. PLoS ONE. 2015;10:e0125318. doi: 10.1371/journal.pone.0125318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cardnell R.J., Feng Y., Mukherjee S., Diao L., Tong P., Stewart C.A., Masrorpour F., Fan Y., Nilsson M., Shen Y., et al. Activation of the PI3K/mTOR Pathway following PARP Inhibition in Small Cell Lung Cancer. PLoS ONE. 2016;11:e0152584. doi: 10.1371/journal.pone.0152584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vikas P., Borcherding N., Chennamadhavuni A., Garje R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020;10:570. doi: 10.3389/fonc.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lord C.J., Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]