Abstract
Calcific aortic valve disease (CAVD) is a major cause of cardiovascular mortality and morbidity, with increased prevalence and incidence. The underlying mechanisms behind CAVD are complex, and are mainly illustrated by inflammation, mechanical stress (which induces prolonged aortic valve endothelial dysfunction), increased oxidative stress (OS) (which trigger fibrosis), and calcification of valve leaflets. To date, besides aortic valve replacement, there are no specific pharmacological treatments for CAVD. In this review, we describe the mechanisms behind aortic valvular disease, the involvement of OS as a fundamental element in disease progression with predilection in AS, and its two most frequent etiologies (calcific aortic valve disease and bicuspid aortic valve); moreover, we highlight the potential of OS as a future therapeutic target.
Keywords: oxidative stress, calcific aortic valve disease, bicuspid aortic valve, valvular interstitial cells, antioxidants, therapeutic targets
1. Introduction
Valvular heart disease (VHD) is currently a major contributor to the loss of physical functioning, quality of life, and longevity [1]. Between 1990 and 2017, the global burden of VHDs showed a wide range of variations; over this time, period rheumatic heart disease, considered the leading cause of VHD in developing countries, substantially decreased. In this matter, The Euro Heart Survey (EHS) noted a predominance of functional and degenerative valvular diseases in high-income countries, where degenerative aortic valve disease and degenerative mitral valve disease represented 70% and 46% of VHD cases [1,2,3,4]. Additionally, the elderly population (≥60 years) is expected to grow from 962.3 million in 2017 to 2080.5 million in 2050, which will lead to a substantial increase in the incidence of non-rheumatic VHD (NRVHD), and its most frequent subtype—non-rheumatic calcific aortic valve disease (NRCAVD) [1,2].
Aortic stenosis (AS) is the most common type of valvular heart disease [5]; its prevalence varies between 2% and 7% in patients over 65 years old and it is estimated that by the year 2030, 4.5 million patients will be diagnosed with this VHD [6]. The most common etiology of aortic stenosis is calcific disease; however, the most common congenital valve defect causing AS is the bicuspid aortic valve (BAV) [7]. Calcific aortic valve disease (CAVD) is no longer considered a simple passive calcium depositing process with consequent fibrosis and valve leaflets thickening, it is also an active cellular process that causes endothelial dysfunction throughout oxidative stress (OS) and inflammatory activation [8,9]. BAV is characterized by the abnormal cusp development where two of the three cusps fuse into a single cusp resulting in abnormal leaflet architecture; it has a prevalence of 0.5–2% in the general population and is three times more prevalent in males than in females [10,11]. One of the most frequent complications of this congenital malformation is BAV-associated aortopathy (BAVAA), which predisposes patients to the risk of developing thoracic aortic aneurysm, aortic dissection in the ascending aorta, and is linked to a faster aortic valve degeneration [10,12]. In both aortic diseases, valvular interstitial cells initiate and maintain mineralization, calcification, and fibrosis; all of these processes are supported by mechanical and biochemical pathways that include lipid deposition, inflammation, and OS [13,14].
In this review, we describe the mechanisms behind aortic valvular disease and the involvement of oxidative stress as a key element in disease progression, with predilection in AS and its two most frequent etiologies (calcific aortic valve disease and BAV); moreover, we highlight the potential of OS as a future therapeutic target.
2. Immunopathogenesis behind Aortic Disease
Aortic valve disease is a growing health condition that has a considerable impact on quality of life. The lack of effective strategies to prevent aortic valve degeneration and progression into calcific aortic valve stenosis (CAVS), besides the surgical option, reinforces the necessity of comprehending the immunopathogenic mechanism in order to develop targeted therapies [15,16].
Genetic susceptibility, endothelial shear stress, chronic inflammation, oxidative stress, lipid deposition, and valve calcification are all contributors to the progression of fibrocalcific remodeling of the aortic valve. The presence of overlapping disease development risk variables and a link between the severity of CAVD and coronary calcification suggest a shared disease pathway, at least in the early stages between CAVD and atherosclerosis [8,17]. As seen in atherosclerosis, endothelial dysfunction secondary to mechanical stress is the initiating event in CAVD. Damaging the structure of the endothelium enables lipids, primarily low-density lipoproteins (LDL) and lipoprotein (a) (Lp(a)) to penetrate the aortic leaflets, and can also trigger the migration of inflammatory cells within [17,18]. Plaque-like lesions consisting of LDL, Lp(a), and inflammatory cells form in the subendothelial region contributing to the production of reactive oxygen species (ROS) that promotes the oxidation of LDL and vascular dysfunction [17,19,20]. The persistent inflammatory process enables valvular interstitial cells (VICs) to attain an osteogenic phenotype resulting in the production of microcalcifications and leaflet mineralization [7,11]. The end stages of CAVD and atherosclerosis differ in the main cell types involved. Whilst in atherosclerosis, smooth muscle cells are known to be the major effectors in chronic inflammation, in CAVD, the pathophysiological process is enhanced by the addition of fibroblasts [17,21,22].
Given the various treatments that have been proven to delay the progression of atherosclerosis, none are effective at preventing aortic stenosis [7]. By stimulating inflammation through a deficiency of antioxidant protection (superoxide dismutase, heme oxygenase-1, glutathione peroxidases) or excessive formation of ROS, OS draws attention as one of the key mechanisms in the early stages of the disease [23,24]. Standard cardiovascular medications evaluated in clinical trials failed to slow disease progression or reduce negative outcomes. More research into alternative therapeutic options that target oxidative stress in VHD is needed [25].
2.1. Mechanical Stress and Endothelial Dysfunction
Endothelial dysfunction is recognized as a primary phase in the genesis of vascular disease and one of the most promising domains for the development of new therapeutic targets [26]. Mechanical stress, lipid infiltration, OS, inflammation, and the fibro-calcific response are all examples of interrelated events that trigger endothelial dysfunction, translated as the loss of its barrier properties [18,27]. The repeated opening and closing of aortic valvular cusps during the cardiac cycle is a continuous source of mechanical stress, which contributes directly to compromising the structural stability of the endothelial layer [5,18,28].
The aortic side of the valve is usually the area of choice for the above-mentioned pathological modifications, especially the noncoronary leaflet [28]. During systole, the blood is forcibly ejected past the ventricle leaving the aortic surface of the valve exposed during the diastole to a higher velocity gradient blood flow and oscillatory shear stress [29,30]. The bicuspid aortic valve also depicts the key role of hemodynamic stress through the high prevalence, accelerated initiation, and progression of CAVD [27,28], mainly at the site of the fused BAV leaflet [11]. In order to properly maintain endothelial homeostasis on both valvular and vascular endothelial cells (VECs), laminar blood flow is a mandatory condition. Fulfilling this requirement, the endothelial relaxing factors (mainly the nitric oxide signaling pathway) are stimulated while the expression of adhesion molecules (vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), E-selectin), and vasoconstrictors (endothelin-1, angiotensin II, thromboxane A2) are suppressed [27,31]. The most common local laminar flow disturbances are inflicted by atherosclerosis, arterial stiffening, and remodeling [32], arterial hypertension, or valvular disorders [27,31]. Valvular endothelial cell changes relate to shear stress by aligning perpendicular to the flow direction opposed to vascular endothelial cells, which align parallel to the flow [29,30].
Ontological and transcriptional profiles suggest that valve endothelial cells (VECs) have the particularity to display side-specific heterogeneity being innate suppressors of calcification with antioxidative and anti-inflammatory phenotypes [30,33,34,35]. VECs have the ability to not only detect alterations in sheer stress but also modulate these signals by regulating valvular interstitial cell (VIC) functions, preventing them from differentiating into osteoblast-like cells [11]. The process is very similar to the endothelial to mesenchymal transition (EndMT) seen throughout valve development and formation [21,36].
Apart from the classic wear and tear pathogeny, biomechanical factors are now recognized as driving forces in the development of CAVD [37,38]. For example, genes, such as the KLF2/PLPP3 axis, inhibitor of DNA binding-1 (ID1), heme oxygenase 1 (HMOX1), and nitric oxide synthase 3 (NOS3) may play hemodynamic-related roles in the development of CAVD by regulating other procalcification genes, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [39], by promoting endothelial repair [40] and by protecting against OS and atherosclerosis [37].
The association between hemodynamics and endothelial oxidative stress in the genesis of CAVD is not yet completely defined and lacks sufficient research [37].
2.2. Lipid Deposition and Oxidative Stress in SA and BAV
Nowadays, western dietary patterns (consisting of a high intake of saturated fatty acids, processed foods, and low intakes of unsaturated fatty acids, dietary fiber, and micronutrients) combined with sedentary lifestyles and increased life expectancies have, as a consequence, contributed to the increase in noncommunicable diseases (NCDs), such as cardiovascular diseases, diabetes, and cancers [41,42]. Vascular OS and inflammation are clearly distinguished mechanisms underlying endothelial dysfunction, which lowers the bioavailability of the key vasodilator molecule nitric oxide (NO) and promotes inflammatory cell recruitment, lipid peroxidation, and aortic valve remodeling [32,43].
Uncoupled nitric oxide synthases (NOS), nicotinamide adenine dinucleotide phosphate oxidases (NOXs), and mitochondrial ROS (mitoROS) are the main sources of ROS in the cardiovascular system [44]; they maintain endothelium homeostasis and smooth muscle cell contraction [45]. Since its first definition in 1985, OS has evolved into a global concept featuring an imbalance between ROS formation and antioxidant response [46]. Interactions between the key ROS generating systems (NOXs, xanthine oxidase, NOS, myeloperoxidase, lipoxygenases) and the major antioxidant systems (catalase, superoxide dismutase (SOD), glutathione peroxidase, glutathione S transferases (GST), α-tocopherol, ascorbic acid, reduced glutathione, and protein thiols) has generated a balanced redox state [47,48,49]. In recent years, the nuclear factor erythroid 2-related factor 2 (Nrf2) has gained attention as an important mediator in the homeostasis of ROS via the Keap1/Nrf2 axis [50,51,52,53].
Although oxidative stress and atherosclerosis are prominent traits in CAVD, statins used as successful monotherapies in atherosclerosis have not been proven effective in CAVD treatment amidst the significant evidence that LDL contributes to disease progression [6,18,20,54]. The depositions of oxidized LDL and LP(a) molecules in the subendothelial layer sustain chronic inflammation at the expense of proinflammatory cytokines and fibrocalcific remodeling by promoting osteogenic differentiation of VICs (via toll-like receptors 2 and 4) and the upregulation of bone morphogenetic protein 2 (BMP-2) (via extracellular signal-regulated kinase (ERK)1/2 and NF-κB) [7,14,21,27,55].
From the four NOS subtypes, endothelial nitric oxide synthase (eNOS) plays a key role in NO modulation being diminished under elevated oxidized LDL (oxLDL) exposure. eNOS uncoupling [31,37,56] and tetrahydrobiopterin (BH4) depletion (NOS co-factor) are two major traits in CAVD that lead to ROS cell injury [57,58]. In addition to eNOS uncoupling, several more pathways, such as mechanical stress [34,37], transforming growth factor beta 1 (TGF-β1) stimulation [36], and S-glutathionylation protein are involved in NO impaired production [59]. BAV aortopathy studies on OS reiterate the role of NO in endothelial homeostasis [60,61,62].
At the ROS imbalance, besides eNOS uncoupling in BAV aortopathy, the ineffective antioxidant protection is demonstrated by the inadequate SOD activity in the ascending aorta [11,63,64,65], whose expression is also dependent on the valve morphotypes [10,12,66,67]. Alongside NOS, NOXs are also part of the ROS milieu seen in CAVD. NOX2 and NOX4 are the main members of the NOX assembly (NOX1 to 5 and DUOX1 and 2) in the vascular system that are required for LDL oxidation, as shown in experimental mice studies [68]. However, the exact role of every NOX in CAVD is not very well defined and further studies are needed. The significance of NOX2 in CAVD has been illustrated in hypercholesterolemia-induced mice who received western-type diets [69], in a rabbit model fed cholesterol-enriched chow and vitamin D [70], and in human aortic tissue [44,71]. From the standpoint of mitoROS, oxidative stress generated through this pathway is observed in the VIC initiation of calcific degeneration [72].
In conjunction with the direct mechanism that NOX exerts, it is also intricated in the crosstalk between OS and inflammation through the cytokine release, controlling the nod-like receptor protein 3 (NLRP3) inflammasome [25,73,74,75,76]. Additionally, ROS assists various inflammation pathways via the redox modulation of inflammation mediators (damage-associated molecular patterns (DAMP)s, high mobility group box 1 (HMGB1), and S100 proteins), transcription factors (Nrf2, NF-κB, HIF- 1α, and AP-1), and by the formation of redox-dependent protein complexes (Nrf2-Keap1) [25,49]. Semicarbazide-sensitive amine oxidase (SSAO)/vascular adhesion protein-1 (VAP-1) has been identified as an additional injurious component of ROS in CAVD and has been linked to disease activity in several research studies [16,44,77]. ROS components cannot be viewed as separate effectors because they are part of an intricate crosstalk process supporting self-propagation and amplification of oxidative stress [25,49].
2.3. Inflammation Mechanism
Apart from being present from the onset of CAVD, inflammation orchestrates all subsequent events that lead to aortic valve remodeling [78]. In summary, as part of the initial innate immune response, activated VICs from prior exposure to mechanical stress and cytotoxic oxLDL prompt the recruitment and infiltration of macrophages, T cells, and mast cells via toll-like receptors 2, 4 (TLRs-2,4) and the NF-kB pathway [6,14,15,17,21,37].
At the same time, extracellular matrix remodeling and osteogenic gene expression take place under the effects of profibrotic cytokines (IL-2, IL-1β, TNF-α, IL-6) and proteolytic enzymes (collagenase-1/MMP-1, collagenase-3/MMP-13, gelatinase-A/MMP-2, and gelatinase-B/MMP-9) [20,34,79]. For the induction of vascular inflammation, the activation of the NF-kB pathway is pivotal and can be achieved either by the canonical (mediated by tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), angiotensin II-induced ROS), or by the non-canonical pathway (mediated by vasoactive peptides, ox-LDL, activated CD40 receptor, B cell-activating factor (BAFF), lymphotoxin b (LTb) monocyte-released cytokines, and advanced glycation end-products) [40,80,81].
Amongst the interleukins, the IL-6 through NF-kB/ interleukin-6/bone morphogenic protein (BMP) signaling pathway [14,82] expression of the receptor activator of the NF-kB ligand superfamily member 11 (RANKL) [7] and IL-1 through enhancing the expression of matrix metalloproteinases (MMPs) [8,17,73] sustain inflammation involvement in aortic valve remodeling and calcification. Amidst the cytokines, TNF-α is of particular importance as a direct inductor of the NF-κB canonical pathway, further mediating biomineralization of the valve [27] and activation of ERK1/2, c-Jun N-terminal kinase (JNK), and p38/MAPK [55]. Being part of a complex, intricate, and self-amplifying mechanism, the roles of IL-1, IL-6, and TNF-α cannot be fully divided, together with IL-18 and IL-32 promoting the secretion of IL-8, MCP-1/CCL2, and adhesion molecules [5,17,27,83].
As a peculiarity in BAV, enhanced densities of macrophages [11] that produce IL-12 and Th17 activation have been observed, as well as changes in the proteomics of canonical pathways characterized by significant upregulation of α-1-antitrypsin (AAT), α-1-antichymotrypsin (ACT), and α-1-acid glycoprotein 2 (AGP) [64]. In addition to macrophages and inflammatory cells, a cluster of CD4+ and CD8+/CD28− T cells are present in early valve lesions adjacent to calcific loci [82,84].
As far as the adaptive immune response in aortic stenosis is concerned, this remains a topic with considerable potential for further research, especially with regard to intracellular pathogens [17] or epitopes [82].
2.4. Extracellular Matrix Remodeling and Biomineralization
A starting point in extracellular matrix fibrosis is the differentiation of VICs into active myofibroblasts via the Wnt3a/β-catenin pathway and TGF-β1-SMAD2/3 activation that upregulates the SMC markers α-SMA and SM22αn [34,79,85]. α-SMA levels are also increased by the renin-angiotensin system (RAS), which alongside BMP-2, ALP, MSX2, RANK, and RANKL subsequently favor the VIC osteogenic phenotype [31,86]. The positive pro-fibrotic feedback loop created by TGF-β1 is further sustained by integrins and adhesion kinases being mediated by phosphoinositide 3-kinase (PI3K)/Akt, the Ras homolog gene family, member A and its associated kinase (RhoA/ROCK), ERK1/2, JNK, p38/MAPK [55], and NF-κB [24,37,72,87].
Over the course of CAVD progression under the influence of periostin, TNF-α, IL-1β, TGF-β1, RANKL, and excessive strain matrix metalloproteinases (MMPs) (especially MMP-1/9/12) and their tissue inhibitors (TIMPs) are secreted by ECs, myofibroblasts, macrophages, and T cells modulating collagen turnover in fibrotic aortic leaflets [6,19,37,88,89,90]. In addition to MMPs and TIMPs, cathepsins S/K/V [10,11,66,84] and elastin-degrading proteases enhance the remodeling and degradation of the vascular extracellular matrix (VECM) [89,91]. Driven by hypoxia in thickened leaflets, neoangiogenesis occurs in areas of calcification surrounded by intensive inflammation and is accompanied by circulating osteogenic progenitor cells [7,8,11].
Degradation and fibrosis of the extracellular matrix serve as the foundations for the two subsequent calcification processes that follow. Dystrophic calcification is enabled by the precipitation of calcium and phosphates in potent calcification nuclei, such as degraded collagen and elastin fibers in the absence of the active participation of osteoblasts [77]. In the definitive stages of CAVD evolution, dystrophic calcification is completed by biomineralization. The process is governed by VICs differentiated into osteoblasts under the transcription factor RUNX2, acquiring the expressions of ALP, BMP-2, BMP-4, osteopontin (SPP1), osteocalcin (BGLAP), Sry-related HMG box-9 (SOX9), and bone sialoprotein [6,34,53]. Under hypercholesterolemia and oxidative stress, endothelial valvular cells secrete Wnt3 that binds to receptors LRP5 and Frizzled on the VIC surface [34,58,82], further inducing the RUNX2 expression via the Wnt pathway [7]. The Notch signaling pathway, consisting of four receptors (Notch1/2/3/4), ligands, Jagged-1/2, and delta-like proteins (DLL1/3/4), is a physiological neutralizer of the Wnt pathway, upholding indirect regulatory effects on RUNX2 [20], being in close conjunction with NF-κB and TLR4 activation in calcific areas of aortic leaflets [60,82].
The osteoprotegerin (OPG)/RANKL/RANK axis represents an alternative route that controls osteoclastogenesis through its receptor RANK and VIC osteoblastic transformation under RANKL overly-expressed by T cells and macrophages in stenotic valves [21,84]. Increased RANKL in atherosclerotic aortic valves interferes with OPG, the antagonist of RANKL that precludes osteoblast maturation [5,17]. In osteogenic transformation, bone morphogenetic proteins (BMPs) in conjunction with the SMAD pathway, RUNX2, Osterix, MSX2 [57], and DIX5/6 uphold significant roles for osteoblasts and chondrocytes [6,20]. As far as VIC osteoblastic transformation is concerned, BMP-2 and BMP-4 play key roles in aortic stenosis alongside ERK1/2, SMAD, ALP, RUNX2, and osteopontin [21,22,28,34,80,84].
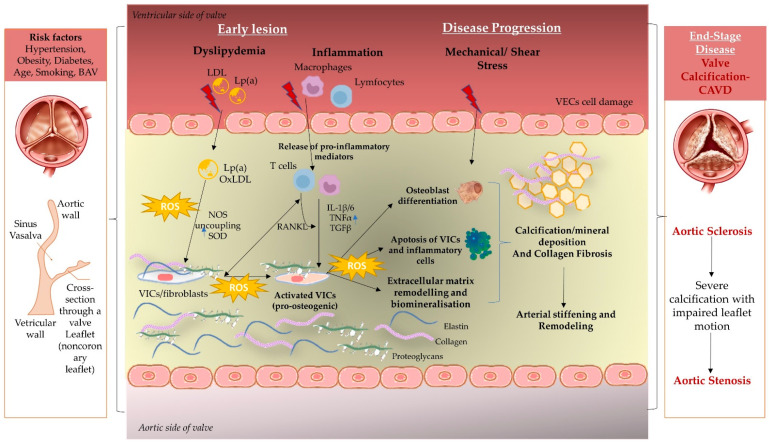
In summary, the interplay of oxidative stress, endothelial dysfunction, and inflammation alter valvular homeostasis expressed as a damaged and hyper-vascularized extracellular matrix containing VICs differentiated into active myofibroblasts (Figure 1). The anarchic organizational pattern of collagen fibers promotes calcium and phosphate deposition, and VIC osteogenic differentiation, conclusively leading to valve ossification, and ending the complex active chain reaction of biomineralization [27].
Figure 1.
Contribution of oxidative stress in calcific aortic valve disease. Calcific aortic valve disease (CAVD); the bicuspid aortic valve (BAV); reactive oxygen species (ROS); low-density lipoproteins (LDL); lipoprotein (a) (Lp(a)); vascular endothelial cells (VECs); valvular interstitial cells (VICs); nitric oxide synthases (NOS); superoxide dismutase (SOD); receptor activator of NF- kB ligand superfamily member 11 (RANKL); transforming growth factor beta (TGFβ); tumor necrosis factor-alpha (TNF-α).
3. Therapeutic Targets of Oxidative Stress
CAVD is extremely common and a rapidly advancing disease with no effective drug therapies at present. Surgical aortic valve replacement (SAVR) is the last resort in severe cases;the window of opportunity being limited for elderly frail patients as they represent the majority of concerned patients and, thus, it is not the most desirable option [4,7,92]. In the last decades, transcatheter aortic valve replacement (TAVR) gained popularity as a more tolerable procedure with promising outcomes [48,93,94,95].
Several therapies that were attempted, including statins, antihypertensives, and medications that target the phospho-calcic pathways, were unable to arrest the naturally occurring progression of CAVD [14,20,96]. As the research into the pathology of calcific aortic stenosis progressed and revealed the multifactorial processes and cellular mechanisms, the demand for targeted therapeutics effectively preventing or delaying the progression of CAVD has emerged [17,20,97,98,99].
Regarding oxidative stress as a unifying mechanism involved in the initiation and propagation phases of aortic stenosis, counteracting it may represent a major future in targeted therapy. Thus, Table 1 provides an overview of potential strategies for reducing the interplay of oxidative stress in CAVD.
3.1. Targeting Lipid Oxidation
Since atherosclerosis and CAVD share similar risk factors and pathways in the initial pathological stages, targeting LDL to further prevent oxLDL formation was the first subject of interest in regard to medical therapies for aortic stenosis.
Four large-scale randomized controlled clinical trials (RCT), registering a total of 2388 patients, showed no significant improvements in CAVD progression after LDL cholesterol-lowering treatment [18,54,100], with only statins (rosuvastatin, atorvastatin) in ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) (NCT00800800) [101], RAAVE (Rosuvastatin Affecting Aortic Valve Endothelium) (NCT00114491) [102], and SALTIRE II (Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression) (NCT02132026) trials [103], or statins in association with fibrates (simvastatin/ezetimibe) in the SEAS (the Simvastatin and Ezetimibe in Aortic Stenosis) (NCT00092677) trial [104]. The conflicting outcomes were proven to be the result of increased levels of Lp(a) [101,105,106,107]. Hence, studies started to focus on the correlation between Lp(a) and CAVD [14,108], confirming that Lp(a) levels are associated with an increased risk of surgical replacement supplemented by complications, hemodynamic alterations [109,110,111,112], and cardiac death [113,114], reinforcing the premise that lowering Lp(a) may be a future effective treatment.
Proprotein-converting enzyme subtilisin/kexin type 9 (PCSK9) inhibitors can simultaneously decrease the level of LDL-C via LDL-R internalization and Lp(a) synthesis [115,116]. The FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) (NCT01764633) [115,117] and the GLAGOV (Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound) (NCT01813422) studies [118] reinforced the important roles of PCSK9 inhibitors in CAVD treatment.
Niacin (nicotinic acid) also raises awareness by reducing Lp(a), LDL-C, and triglycerides plasma levels and by increasing HDL-C [119]. There are currently only two trials evaluating niacin (the Early Aortic Valve Lipoprotein (a) Lowering (EAVaLL; NCT02109614)) and PCSK9 inhibitor (South Korean clinical trial (NCT03051360)), supporting the necessity of additional studies on this matter [6,14,34,108].
Presently, there is a paucity of data from RCTs to endorse the notion that lowering Lp(a) levels alone can improve the CAVD outcome without taking into account other lipid risk factors. New therapies consisting of antisense oligonucleotide against apolipoprotein(a), which consistently lowers plasma Lp(a) levels, such as IONIS-APO(a)-Rx, IONIS-APO(a)-LRx [120], and hepatocyte-directed antisense oligonucleotide AKCEA-APO(a)-LRx (TQJ230) have recently emerged [106]. Additionally, E06, a natural antibody that prevents macrophages from absorbing oxLDL, demonstrated a 49% reduction in the AV gradient and a lower overall calcium load in E06 mice [121].
Alternative therapies that inhibit LDL oxidation and that are not yet proven to be directly correlated to CAVD may be represented by probucol [24], sitagliptin, and alogliptin [34,122]. Moreover, aegeline, a compound anti-lectin-like oxidized-LDL receptor-1 (LOX-1) [77], as well as capsaicin [123], kefir peptides [73], and p38 MAPK inhibitors dilmapimod (SB681323, GlaxoSmithKline, London, UK) and losmapimod (GW856553, GlaxoSmithKline, London, UK) [55], are currently under investigation.
3.2. Antioxidants from Natural Compounds to Targeted Experimental Therapies
A general trait observed in calcified aortic valves is the general reduction of the major antioxidant systems and excessive ROS accumulation; hence, mechanisms approaching these imbalances were sought as novel therapeutic strategies [44,124,125]. Most natural antioxidants are obtained from herbal sources, as will be described in the following paragraphs.
3.2.1. Phenolic Acids, Flavonoids, Anthocyanins, Lignans, and Stilbenes
The most known polyphenolic representative is likely resveratrol (3,5,4′-trihydroxytrans-stilbene), which is found in berries and grapes [81]. The major antioxidant effects are mediated by SIRT1, Nrf2, and the ER (estrogen receptor) reducing arterial calcification by downregulating RUNX2, osteocalcin, and ALP [83,126,127,128]. In addition to having an anti-inflammatory impact, precursors of resveratrol (palmitoylethanolamide, polydatin) significantly reduced the expression of VCAM, ICAM-1, TNF- α, IL-1, alleviating vascular injury [19,49,129,130,131,132].
Curcumin, historically used as a spice, and the main component of Curcuma longa (turmeric), reduces oxidative stress by inhibiting osteogenic VIC differentiation across NF-κB, protein kinase B (PKB/AKT), and extracellular signal-regulated kinase (ERK) routes, and modulates the formation of ox-LDL [133,134,135,136,137] while reducing the expression of pro-atherogenic cytokines [49,138].
Cardamonin, primarily available in Ginkgo biloba and Amomum subulatum, exhibited strong anticalcific effects by suppressing the activation of p-AKT, p-ERK1/2, as well as the expressions of NLRP3 inflammasome and IL-1 [139,140,141].
Improved endothelial dysfunction and anti-proliferative impacts on smooth muscle cells were exemplified by ellagic acid, naturally present in nuts and red berries [142,143], and gallic acid that further decreased the expressions of the calcification markers RUNX2, BMP2, osteocalcin, and MSX2 [87,144]. Anticalcific and anti-inflammatory properties by interfering with the PI3K-AKT, ERK1/2, and NF-κB/NLRP3 inflammasome pathways are also displayed by caffeic acid phenethyl ester (CAPE), found in honeybee propolis and conifer barks [145,146].
Flavonoids are potent calcification inhibitors interfering with PI3K-Akt, mTOR, NF-kB, and ROS/TLR4 via nobiletin found in citrus fruits [147,148,149], and quercetin, which additionally modulates the iNOS/p38 MAPK pathway [49,150,151,152,153].
Further anti-inflammatory, anti-calcific, and ROS-downregulating properties were observed in anthocyanins [26,154], phytoestrogen puerarin [155,156,157,158], diosgenin [159,160,161], and 10-dehydrogingerdione (10-DHGD) [162].
3.2.2. Vitamins
Inhibitory effects on lipid oxidation, cardiovascular inflammation, vascular calcification, and the improvement of vascular endothelial dysfunction are depicted by vitamins C and E (α-tocopherol), which have been extensively researched in cardiovascular preventive medicine [19,163,164]. Several observational studies have revealed an unfavorable connection between the nutrient intake of antioxidants and cardiovascular morbidity and mortality, despite the supporting idea that antioxidant vitamins help reduce oxidative stress and vascular illnesses [165].
The cardiovascular morbidity and mortality rates in the placebo and antioxidant groups were found to be identical in meta-analyses of RCTs on the benefits of vitamins B, C, E, and S, or b-carotene [166,167]. The unsatisfactory results can be explained by a number of factors, such as the lack of standard dosages and the follow-up periods of insufficient lengths, but further research is needed on the so-called antioxidant paradox in therapeutics [45].
3.2.3. Other Antioxidant Compounds
Fucoxanthin (found in brown seaweed and micro and macro algae) [87,168], Andrographolide extracted from a Chinese medicinal herb (Andrographis paniculata) [169,170,171], and Apocynin found in Apocynum cannabinum and Picrorhiza kurroa [172,173] have potent antioxidant properties and attenuated calcification by interfering with the AKT, NF-κB, and ERK1/2 signaling pathways. Additionally, apocynin and celastrol are isolated from the Tripterygium wilfordii Hook F (TWHF) plant, also known as Thunder God Vine lowered NOX expression [70,174,175].
Compounds found in Hengshun aromatic vinegar (HSAV), also from the Asian cuisine, display a myriad of antioxidants, such as polyphenols, flavonoids, and melanosine, enhancing SIRT1, Nrf2, and NQO1 expressions [176], alongside capsaicin, proving antioxidant efficacy, which may be considered for future studies [123].
Recently, the pineal hormone melatonin showed antioxidant effects in atherosclerotic lesions excising NLRP3 inflammasome activation [49,177]. In patients with underlying pathologies that additionally amplify ROS production, such as rheumatic valvular heart disease, or those undergoing cardiopulmonary bypass or diabetes, L-carnitine and glycine represent promising potential in targeting NF-κB, Nrf2 [178], and the RAGE-NOX-NF-κB signaling pathway [75]. Studies have also been conducted on ascending aortic aneurysms and patients with Marfan syndrome. Thus far, Hibiscus sabdariffa Linne (HSL), which is rich in polyphenols, anthocyanin, flavonoids, and L-ascorbic acid [19], and the inhibitor of the kappa B kinase epsilon (IKKε) inhibitor, amlexanox [179], are potential antioxidants meriting further research.
3.2.4. Targeting mitoROS and SOD
One of the most important systems involved in maintaining redox balance is SOD, which is downregulated in aortic stenosis [24]. In an attempt to promote SOD upregulation, polyethylene glycol-SOD (peg-SOD) and other SOD-mimetics consisting of cerium oxide nanoparticle (CNPs) rods and sphere-shapes [23] were utilized [180,181]. With the application of several aortic VICs, controversial outcomes were observed in terms of the calcification process [70], raising the question as to whether this therapeutic method depends on the dosage or a specific CAVD model. A redox-active MnSOD mimetic, manganese compound MnTnBuOE-2-PyP5 (MnBuOE) reduced aortic valve remodeling by targeting collagen buildup [182].
Research on mitoROS is somewhat underdeveloped with only one compound being mentioned. Mitoquinone (MitoQ), a derivative of ubiquinone, downregulated OS through the Nrf2/Keap1 pathway [52], with further antifibrotic properties obtained by TGF-β1-NOX4-ROS axis suppression [85].
3.3. NO Bioavailability and NOX Inhibition
Increasing NO bioavailability was proposed as an alternative to reduce oxidative stress relying on the concomitant Notch1 regulation to also target osteogenic VICs differentiation [183]. From the aforementioned potential therapies, statins illustrate pleiotropic NO increased levels but their failure in aortic stenosis therapy mandates the use of alternative approaches [34].
These included treatment with NO donors application (DETA-NONOate) [184], sodium nitroprusside (SNP) [185], NO precursor L-arginine [186], and co-treatment with BH4, which mitigates eNOS uncoupling, showing modest results in attenuating osteogenic differentiation and endothelial dysfunction [15,187,188]. Consecrated therapies, such as angiotensin-converting enzyme (ACE) inhibitors [96], nebivolol [45], inhibitors of dipeptidyl peptidase 4 (DPP4), such as sitagliptin and anagliptin [89,122,189,190], and empagliflozin, a sodium–glucose cotransporter-2 (SGLT2) inhibitor [25], also interfere and prevent eNOS uncoupling and suppress superoxide production. Currently, there are no RCTs testing the aforementioned theories, lending credence that NO bioavailability modulators might be a cutting-edge therapy in CAVD in the future.
In the hope of finding a more targeted approach, NOX-specific inhibitors were explored. Apocynin and celastrol from the antioxidant category demonstrated their potential as NOX2-selective inhibitors in addition to other non-specific antioxidant activities described in previous paragraphs. Apocynin significantly prevents the phosphorylation of the NOX2 cytosolic component [172], and celastrol demonstrates a general favorable effect in terms of calcium deposition and AV fibrotic remodeling by blocking the NOX2-driven GSK3β/β-catenin pathway [70,191].
Antidiabetic therapies, such as sitagliptin, showed pleiotropic effects in NOX by additionally inhibiting the cross-talk between RAGE/NF-κB with implications in calcific remodeling [21,89,122]. By reducing TNF-α, Il-6, iNOS, and NOX2 expression, the GLP-1 analog liraglutide also reduced vascular dysfunction, aortic inflammation, and oxidative stress in mice with polymicrobial sepsis [192]. Given that numerous other anti-inflammatory properties of pioglitazone were documented, such as improving TNF and IL-6 levels and cusp mobility, it is unclear as to the specific role it plays in NOX2 inhibition [69,193]. Triazolopyrimidines, such as VAS2870 and VAS3947 [194,195,196], and pyrazolopyridine derivatives, such as GKT136901/137831 [197], were developed under the idea of creating substances that specifically block NOX enzymes, although more research is needed to determine their safety and precise effects in CAVD [45,56,63]. As seen, efforts are being made in finding new therapeutic approaches via OS in CAVD, with potential for future implementation as additional therapy to current approaches (Table 1, Table 2 and Table 3).
Table 2.
Human experimental research of therapeutic strategies via OS in CAVD.
| Refs. | Compound | Administration and Doses/Researched Cells | Salient Findings |
|---|---|---|---|
| Experimental Human Studies | |||
| [120] | IONIS-APO(a)Rx vs. placebo | 100 mg, 200 mg, and then 300 mg once a week for 4 weeks each /1 dose of 10–120 mg sq /multiple doses of 10 mg, 20 mg, or 40 mg sq |
-Reduced Lp(a) levels in a dose-dependent manner. |
| [137] | Curcumin | hVICs | -Inhibition of NF-κB, AKT, ERK. |
| [141] | Cardamonin | In Vitro: hVICs Ex Vivo: human aortic valve leaflet | -Inhibition of VIC osteogenic differentiation through the NF-κB/NLRP3 inflammasome pathway. |
| [145] | Caffeic Acid | hVICs | -Inhibition of the ERK/AKT/NF-κB/NLRP3 inflammasome pathway. |
| [152] | Quercetin | HUVECs | -Attenuated atherosclerotic inflammation and adhesion molecule expression by the TLR-NF-κB pathway. |
| [26] | Anthocyanins | D-HAEC | -Inhibition of the NF-κB pathway. |
| [169] | Andrographolide | hVICs (from patients undergoing Bentall surgery due to acute type I aortic dissection) | -Inhibition of the NF-κB/Akt/ERK pathway. |
| [176] | HSAV | HUVECs | -Inhibited apoptosis, decreased serum Hcy, ET-1), ox-LDL levels, MDA level; -Increased NO, eNOS, SOD, GSH, and GSH-Px levels; -Downregulated the expression of PKCζ and regulated the SIRT1-mediated pathway. |
| [178] | L-carnitine | Patients with RVHD with CPB-induced MIRI (myocardial ischemia-reperfusion injury) | -Increased levels of SOD, CAT; -Suppressed activation of NF-κB and Nrf2. |
| [24] | Adenoviral SOD delivery | hVIC | -Reduced VIC osteoblastic differentiation by reducing RUNX2, MSX2, and OPN. |
| [23,181] | CNPs | hVIC | -Scavenged ROS, acted as SOD-mimetics, and reduced VIC osteoblastic differentiation. |
Subcutaneously (sq); human valve interstitial cells (hVICs); human umbilical vein endothelial cells (HUVECs); diabetic human aortic endothelial cells (D-HAEC); Hengshun aromatic vinegar (HSAV); malondialdehyde (MDA); glutathione peroxidase (GSH-Px); protein kinase C zeta (PKCζ); homocysteine (Hcy); endothelin 1 (ET-1).
Table 3.
Animal experimental studies of antioxidant compounds and other therapeutic options that target OS with potential in CAVD treatment.
| Ref. | Compound | Species and/or Cells Researched | Meaningful Findings |
|---|---|---|---|
| [121] | E06 natural antibody | E06-scFv transgenic mice | -Counteracted the proinflammatory and proatherogenic OxPL effects. |
| [126] | Resveratrol | Ovariectomized rats | -Reduced RUNX2, ALP expression, and aortic calcification. |
| [127] | Resveratrol | Rat vascular smooth muscle cells (RASMCs) | -Prevents vascular calcification and mitochondria dysfunction through SIRT1 and Nrf2. |
| [128] | Resveratrol | Mouse model of uremia | -Fewer aortic atherosclerotic lesions at the site of the ascending aorta. |
| [131] | Resveratrol | Porcine aortic valve interstitial cells (pVICs) | -Inhibition of osteogenic pVIC differentiation through the AKT/SMAD1/5/8 signaling pathway. |
| [129] | PLD the natural precursor of resveratrol | Mice with complete ligatures of the left carotid arteries for 14 days | -Reduced adhesion molecule expression (ICAM-1, VCAM-1), proinflammatory cytokine production (TNF-α, IL-1β), iNOS, NF-κB expression, and BAX, Fas-Ligand activation. |
| [136] | Curcumin | Apolipoprotein E-knockout mice | -Reduced TLR4 expression, macrophage infiltration in atherosclerotic plaque, aortic IL-1β, TNF-α, VCAM-1, ICAM-1 expression, NF-κB activity, and plasma IL-1β, TNF-α, soluble VCAM-1, and ICAM-1 levels; -Reduced the extent of atherosclerotic lesions and inhibited atherosclerosis development. |
| [138] | Curcumin | Different types of mice, all treated with HF and mice fed with a normal chow diet | -Reduced serum lipid levels, TNF-α, IL-1β, and the aortic atherosclerotic lesion area. |
| [141] | Cardamonin | In Vivo: mice model fed with a HF diet | -Inhibition of VIC osteogenic differentiation through the NF-κB/NLRP3 inflammasome pathway. |
| [143] | Ellagic acid | Rat model | -Improved nitric oxide bioavailability and reduced ROS formation. |
| [144] | Gallic acid | Vascular smooth muscle cell | -Inhibition of vascular calcification through the BMP2-SMAD1/5/8 signaling pathway. |
| [149] | Nobiletin | Male Wistar rats | -Increased intracellular cGMP (activation of cGC, opening BK channels and KATP channels). |
| [151] | Quercetin | Adenine-induced chronic renal failure rats | -Modulation of vascular calcification through the iNOS/p38 MAPK pathway. |
| [154] | Anthocyanins | Tac-induced myocardial dysfunction in mice | -Ameliorated Tac-induced myocardial dysfunction, oxidative stress, and apoptosis via the DDAH1/ADMA/no pathway. |
| [155] | Puerarin | In Vitro; rat vascular smooth muscle cells In Vivo; uremic rats |
-Modulated NLRP3/CASPASE1/IL-1β, NF-κB, and ER/PI3K-AK signaling pathways; -Prevents calcium deposition and inhibits the expression of RUNX2 and BMP2. |
| [158] | Puerarin | VSMCs | -Inhibited oxLDL-induced VSMC viability via inhibition of the p38 MAPK and JNK signaling pathways; -Decreased the levels of IL-6 and TNF-α and increased SOD activity. |
| [161] | Diosgenin | Adenine-induced chronic renal failure rats | -Inhibited the c/Akt/ERK, p38 pathway. |
| [162] | 10-DHGD | HCD-fed rabbits | -Alleviated calcium deposition via the downregulation of the BMP2/Wnt3a pathway, OPG/RANK modulation, and raised HDL-C levels. |
| [164] | Vitamin E | Uremic obese rats | -Prevents osteoblastic differentiation in VSMC and inhibits dephosphorylation of Akt. |
| [87] | Fucoxanthin | In Vitro; rat heart VIC In Vivo; dog model | -Inhibition of the Akt/ERK pathway. |
| [172] | Apocynin | VSMCs | -Enhanced expression of α-SMA, reduced expression of BMP2, RUNX2, OPN, suppressed the ERK1/2 pathway and phosphorylation of p47phox (cytosolic NOX2 component). |
| [70] | Celastrol | In Vitro; porcine AVIC In Vivo; rabbit CAVD model | -Inhibition of NADPH Oxidase 2 and the GSK3β/β-catenin pathway |
| [174] | Celastrol | Macrophages in mice | -Attenuated oxLDL-induced excessive expression of LOX-1; -Decreased IkB phosphorylation and degradation, reduced production of iNOS, NO, TNF-α, and IL-6; -Reduced atherosclerotic plaque size. |
| [75] | Glycine | Streptozotocin-induced diabetic rats and HUVECs | -Downregulating the AGE/RAGE signaling pathway by decreasing levels of AGEs, RAGE, NOX4, and NF-κB p65, and by restoring GLO1 function. |
| [182] | MnBuOE | hVIC and murine model of aortic valve sclerosis | -Inhibited aortic valve remodeling and α-SMA upregulation via TGF-β1; -Upregulated MnSOD via activation of Nrf2. |
| [52,85] | Mitoquinone | Male Sprague–Dawley rats and adult C57BL/6J mice | -Reduced vascular calcification through the Nrf2/Keap1 pathway and fibrosis by inhibiting the TGF-β1-NOX4-ROS axis. |
| [184] | DETA NONOate |
PAVEC and aortic VIC PAVIC | -Inhibited VIC osteogenic differentiation and calcification. |
| [186] | L-arginine | Bovine aortic VICs | -Inhibited VIC osteogenic differentiation and remodeling by downregulating ADAMTSL4 and fibrillin-1. |
| [89] | Anagliptin | Eight-week-old male BALB/c mice | -Activated the PI3K/Akt signaling pathway; -Downregulated the expression of MCP-1, ICAM-1, VCAM-1; -Reduced proteolysis via MMP-2/-9 and CatS/K. |
| [122,190] | Sitagliptin | Weaned male low-density lipoprotein receptor knockout mice | -Blocked NADPH activation; -Inhibited calcification by downregulating RAGE expression and NF-κB activation. |
| [189] | Sitagliptin | Rabbit model of CAVD fed with HCD and vitamin D2 | -Reduced osteogenic transformation of VICs by reinstating IGF-1 activity. |
| [198] | Evogliptin | hVIC, endothelial nitric oxide synthase-deficient, male New Zealand white rabbits | -Reduced TNF-α, IL-1β, and IL-6 levels; -Reduced RUNX2 expression. |
| [69] | Pioglitazone | Mice fed a western-type diet | -Attenuated cusp mobility and inhibited valve calcification by reducing TNFα, IL-6, and BMP2. |
| [193] | Pioglitazone | Male New Zealand rabbits | -Reduced RAGE activation and inhibited NF-κB p65 intranuclear translocation. |
High-fat diet (HF); transverse aortic constriction (Tac); vascular smooth muscle cells (VSMCs); 10-dehydrogingerdione (10-DHGD); high cholesterol diet (HCD); MnTnBuOE-2-PyP5+ (MnBuOE); NO donors application (DETA-NONOate); Porcine aortic VEC (PAVEC); aortic VIC (PAVIC); Polydatin (PLD).
Table 1.
Human trials of therapeutic strategies via OS in CAVD.
| Study and Refs. | Compound | Administration and Doses/Researched Cells | Salient Findings |
|---|---|---|---|
| Clinical Trials | |||
| ASTRONOMER [101] | Rosuvastatin vs. placebo | 40 mg/day | -Lp(a) and OxPL-apoB levels are associated with faster AS progression; -OxPL-apoB levels were higher after one year in the rosuvastatin arm. |
| RAAVE [102] | Rosuvastatin vs. placebo | 20 mg/day | -Precocious statin treatment is more effective in the progression of aortic valve stenosis. |
| SALTIRE [103] | Atorvastatin vs. placebo | 80 mg/day | -Intensive lipid-lowering therapy delays the progression of calcific aortic stenosis. |
| SEAS [104] | Simvastatin + Ezetimibe vs. placebo | 40 mg + 10 mg/day | -No reduction in valvular or ischemic events in patients with aortic stenosis. |
| FOURIER [115,117] | Evolocumab | 140 mg every 2 weeks or 420 mg every month | -After 1-year of reduced LDL cholesterol levels and cardiovascular events; -Higher Lp(a) levels were associated with a higher risk of AS events. |
| GLAGOV [118] | Evolocumab | 420 mg every month | -Added statin treatment in angiographic coronary artery disease decreased atheroma volume. |
Subcutaneously (sq); human valve interstitial cells (hVICs); human umbilical vein endothelial cells (HUVECs); diabetic human aortic endothelial cells (D-HAEC); Hengshun aromatic vinegar (HSAV); malondialdehyde (MDA); glutathione peroxidase (GSH-Px); protein kinase C zeta (PKCζ); homocysteine (Hcy); endothelin 1 (ET-1).
4. Conclusions
Aortic stenosis is a significant worldwide medical issue and a disabling disease that diminishes a patient’s life quality. Even though the pathogenesis of aortic stenosis cannot be explained by a single mechanism, current data reveal the deleterious effects of increased OS activity in the complex heterogeneity of active cellular and molecular mechanisms that support the progression of CAVD and endothelial dysfunction through various pathways. Nonetheless, research continues to address new molecules that, in addition to playing roles in cardiovascular pathophysiology, may also represent novel therapeutic targets. Due in part to the fact that the multifactorial, self-replicating cellular mechanisms causing CAVD have already been established by the time a patient presents with the disease, proposed strategies to inhibit oxidative stress, either systemically or locally in CAVD, have so far delivered unclear results.
Furthermore, the spectrum of early paraclinical diagnosis might be expanded; the risk stratification and long-term prognosis could be improved through the development of new protein assays and technologies that speed up and increase the detectability of aortic stenosis. Currently, there are few in vivo/in vitro and cohort observational studies that could determine whether there is a relationship between the degree of valvular oxidative stress, the rate of disease progression, and its relationship to clinical severity. Therefore, more research studies with adequate follow-up periods are essential for translating the OS concept into clinical practice therapies.
Author Contributions
Conceptualization, D.M.T., M.A.M., E.M.G. and M.F.; methodology, N.D.; software, C.F.C.; validation, D.M.T., M.F., I.L.S. and E.M.G.; formal analysis, C.F.C.; investigation, M.A.M.; resources, I.T.; data curation, E.V.; writing—original draft preparation, D.M.T., E.V., E.M.G. and I.L.S.; writing—review and editing, M.A.M., C.F.C. and N.D.; visualization, M.A.M.; supervision, I.L.S.; project administration, D.M.T. and E.M.G.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen J., Li W., Xiang M. Burden of Valvular Heart Disease, 1990–2017: Results from the Global Burden of Disease Study 2017. J. Glob. Health. 2020;10:020404. doi: 10.7189/jogh.10.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iung B. A Prospective Survey of Patients with Valvular Heart Disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003;24:1231–1243. doi: 10.1016/S0195-668X(03)00201-X. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A., Beyersdorf F., Bauersachs J., Capodanno D., Conradi L., Bonis M.D., Paulis R.D., Delgado V., Freemantle N., Jeppsson A., et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 5.Conte M., Petraglia L., Campana P., Gerundo G., Caruso A., Grimaldi M.G., Russo V., Attena E., Leosco D., Parisi V. The Role of Inflammation and Metabolic Risk Factors in the Pathogenesis of Calcific Aortic Valve Stenosis. Aging Clin. Exp. Res. 2021;33:1765–1770. doi: 10.1007/s40520-020-01681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natorska J., Kopytek M., Undas A. Aortic Valvular Stenosis: Novel Therapeutic Strategies. Eur. J. Clin. Investig. 2021;51:e13527. doi: 10.1111/eci.13527. [DOI] [PubMed] [Google Scholar]
- 7.Akahori H., Tsujino T., Masuyama T., Ishihara M. Mechanisms of Aortic Stenosis. J. Cardiol. 2018;71:215–220. doi: 10.1016/j.jjcc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Tretjakovs P., Lurins J., Svirskis S., Gersone G., Lurina D., Rozenberga U., Blumfelds L., Bahs G., Lejnieks A., Mackevics V. Thioredoxin-1 and Correlations of the Plasma Cytokines Regarding Aortic Valve Stenosis Severity. Biomedicines. 2021;9:1041. doi: 10.3390/biomedicines9081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phua K., Chew N.W., Kong W.K., Tan R.-S., Ye L., Poh K.-K. The Mechanistic Pathways of Oxidative Stress in Aortic Stenosis and Clinical Implications. Theranostics. 2022;12:5189–5203. doi: 10.7150/thno.71813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozzini C., Girelli D., Cominacini L., Soresi M. An Exploratory Look at Bicuspid Aortic Valve (Bav) Aortopathy: Focus on Molecular and Cellular Mechanisms. Curr. Probl. Cardiol. 2021;46:100425. doi: 10.1016/j.cpcardiol.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Manno G., Bentivegna R., Morreale P., Nobile D., Santangelo A., Novo S., Novo G. Chronic Inflammation: A Key Role in Degeneration of Bicuspid Aortic Valve. J. Mol. Cell. Cardiol. 2019;130:59–64. doi: 10.1016/j.yjmcc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Phillippi J.A., Hill J.C., Billaud M., Green B.R., Kotlarczyk M.P., Gleason T.G. Bicuspid Aortic Valve Morphotype Correlates With Regional Antioxidant Gene Expression Profiles in the Proximal Ascending Aorta. Ann. Thorac. Surg. 2017;104:79–87. doi: 10.1016/j.athoracsur.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu P., Bossé Y., Huggins G.S., Della Corte A., Pibarot P., Michelena H.I., Limongelli G., Boulanger M.-C., Evangelista A., Bédard E., et al. The Pathology and Pathobiology of Bicuspid Aortic Valve: State of the Art and Novel Research Perspectives: Pathology and Pathobiology of Bicuspid Aortic Valve. J. Pathol. Clin. Res. 2015;1:195–206. doi: 10.1002/cjp2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng K.H., Tzolos E., Dweck M.R. Pathophysiology of Aortic Stenosis and Future Perspectives for Medical Therapy. Cardiol. Clin. 2020;38:1–12. doi: 10.1016/j.ccl.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Farrar E.J., Huntley G.D., Butcher J. Endothelial-Derived Oxidative Stress Drives Myofibroblastic Activation and Calcification of the Aortic Valve. PLoS ONE. 2015;10:e0123257. doi: 10.1371/journal.pone.0123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercier N., Pawelzik S.-C., Pirault J., Carracedo M., Persson O., Wollensack B., Franco-Cereceda A., Bäck M. Semicarbazide-Sensitive Amine Oxidase Increases in Calcific Aortic Valve Stenosis and Contributes to Valvular Interstitial Cell Calcification. Oxid. Med. Cell. Longev. 2020;2020:5197376. doi: 10.1155/2020/5197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alushi B., Curini L., Christopher M.R., Grubitzch H., Landmesser U., Amedei A., Lauten A. Calcific Aortic Valve Disease-Natural History and Future Therapeutic Strategies. Front. Pharmacol. 2020;11:685. doi: 10.3389/fphar.2020.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmanis J., Hofmane D., Svirskis S., Mackevics V., Tretjakovs P., Lejnieks A., Signorelli S.S. HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress. Medicina. 2019;55:416. doi: 10.3390/medicina55080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rysz J., Gluba-Brzózka A., Rokicki R., Franczyk B. Oxidative Stress-Related Susceptibility to Aneurysm in Marfan’s Syndrome. Biomedicines. 2021;9:1171. doi: 10.3390/biomedicines9091171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho K.I., Sakuma I., Sohn I.S., Jo S.-H., Koh K.K. Inflammatory and Metabolic Mechanisms Underlying the Calcific Aortic Valve Disease. Atherosclerosis. 2018;277:60–65. doi: 10.1016/j.atherosclerosis.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Goody P.R., Hosen M.R., Christmann D., Niepmann S.T., Zietzer A., Adam M., Bönner F., Zimmer S., Nickenig G., Jansen F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020;40:885–900. doi: 10.1161/ATVBAHA.119.313067. [DOI] [PubMed] [Google Scholar]
- 22.Sun J.T., Chen Y.Y., Mao J.Y., Wang Y.P., Chen Y.F., Hu X., Yang K., Liu Y. Oxidized HDL, as a Novel Biomarker for Calcific Aortic Valve Disease, Promotes the Calcification of Aortic Valve Interstitial Cells. J. Cardiovasc. Transl. Res. 2019;12:560–568. doi: 10.1007/s12265-019-09903-3. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y., St. Hilaire C., Hortells L., Phillippi J.A., Sant V., Sant S. Shape-Specific Nanoceria Mitigate Oxidative Stress-Induced Calcification in Primary Human Valvular Interstitial Cell Culture. Cell. Mol. Bioeng. 2017;10:483–500. doi: 10.1007/s12195-017-0495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branchetti E., Sainger R., Poggio P., Grau J.B., Patterson-Fortin J., Bavaria J.E., Chorny M., Lai E., Gorman R.C., Levy R.J., et al. Antioxidant Enzymes Reduce DNA Damage and Early Activation of Valvular Interstitial Cells in Aortic Valve Sclerosis. Arterioscler. Thromb. Vasc. Biol. 2013;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboonabi A., Singh I., Rose’ Meyer R. Cytoprotective Effects of Berry Anthocyanins against Induced Oxidative Stress and Inflammation in Primary Human Diabetic Aortic Endothelial Cells. Chem. Biol. Interact. 2020;317:108940. doi: 10.1016/j.cbi.2020.108940. [DOI] [PubMed] [Google Scholar]
- 27.Kostyunin A.E., Yuzhalin A.E., Ovcharenko E.A., Kutikhin A.G. Development of Calcific Aortic Valve Disease: Do We Know Enough for New Clinical Trials? J. Mol. Cell. Cardiol. 2019;132:189–209. doi: 10.1016/j.yjmcc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Honda S., Miyamoto T., Watanabe T., Narumi T., Kadowaki S., Honda Y., Otaki Y., Hasegawa H., Netsu S., Funayama A., et al. A Novel Mouse Model of Aortic Valve Stenosis Induced by Direct Wire Injury. Arterioscler. Thromb. Vasc. Biol. 2014;34:270–278. doi: 10.1161/ATVBAHA.113.302610. [DOI] [PubMed] [Google Scholar]
- 29.Heistad D.D. Endothelial Function in the Time of the Giants. J. Cardiovasc. Pharmacol. 2008;52:385–392. doi: 10.1097/FJC.0b013e31818a403b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher J.T., Tressel S., Johnson T., Turner D., Sorescu G., Jo H., Nerem R.M. Transcriptional Profiles of Valvular and Vascular Endothelial Cells Reveal Phenotypic Differences: Influence of Shear Stress. Arterioscler. Thromb. Vasc. Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Martínez E., Souza-Neto F., Jiménez-González S., Cachofeiro V. Oxidative Stress and Vascular Damage in the Context of Obesity: The Hidden Guest. Antioxidants. 2021;10:406. doi: 10.3390/antiox10030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioscia-Ryan R.A., Clayton Z.S., Zigler M.C., Richey J.J., Cuevas L.M., Rossman M.J., Battson M.L., Ziemba B.P., Hutton D.A., VanDongen N.S., et al. Lifelong Voluntary Aerobic Exercise Prevents Age- and Western Diet- Induced Vascular Dysfunction, Mitochondrial Oxidative Stress and Inflammation in Mice. J. Physiol. 2021;599:911–925. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons C.A., Grant G.R., Manduchi E., Davies P.F. Spatial Heterogeneity of Endothelial Phenotypes Correlates with Side-Specific Vulnerability to Calcification in Normal Porcine Aortic Valves. Circ. Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulin A., Hego A., Lancellotti P., Oury C. Advances in Pathophysiology of Calcific Aortic Valve Disease Propose Novel Molecular Therapeutic Targets. Front. Cardiovasc. Med. 2018;5:21. doi: 10.3389/fcvm.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theodoris C.V., Li M., White M.P., Liu L., He D., Pollard K.S., Bruneau B.G., Srivastava D. Human Disease Modeling Reveals Integrated Transcriptional and Epigenetic Mechanisms of NOTCH1 Haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjortnaes J., Shapero K., Goettsch C., Hutcheson J.D., Keegan J., Kluin J., Mayer J.E., Bischoff J., Aikawa E. Valvular Interstitial Cells Suppress Calcification of Valvular Endothelial Cells. Atherosclerosis. 2015;242:251–260. doi: 10.1016/j.atherosclerosis.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M., Luo M., Sun H., Ni B., Shao Y. Integrated Bioinformatics Analysis Predicts the Key Genes Involved in Aortic Valve Calcification: From Hemodynamic Changes to Extracellular Remodeling. Tohoku J. Exp. Med. 2017;243:263–273. doi: 10.1620/tjem.243.263. [DOI] [PubMed] [Google Scholar]
- 38.Bäck M., Gasser T.C., Michel J.-B., Caligiuri G. Biomechanical Factors in the Biology of Aortic Wall and Aortic Valve Diseases. Cardiovasc. Res. 2013;99:232–241. doi: 10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C., Huang R.-T., Kuo C.-H., Kumar S., Kim C.W., Lin Y.-C., Chen Y.-J., Birukova A., Birukov K.G., Dulin N.O., et al. Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ. Res. 2015;117 doi: 10.1161/CIRCRESAHA.117.306457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W., Wang H., Kuang C., Zhu J., Yu Y., Qin Z., Liu J., Huang L. An Essential Role for the Id1/PI3K/Akt/NFkB/Survivin Signalling Pathway in Promoting the Proliferation of Endothelial Progenitor Cells In Vitro. Mol. Cell. Biochem. 2012;363:135–145. doi: 10.1007/s11010-011-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Gil J.F., Tárraga-López P.J. Research on Diet and Human Health. Int. J. Environ. Res. Public. Health. 2022;19:6526. doi: 10.3390/ijerph19116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshin A., Sur P.J., Fay K.A., Cornaby L., Ferrara G., Salama J.S., Mullany E.C., Abate K.H., Abbafati C., Abebe Z., et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donato A.J., Machin D.R., Lesniewski L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg H.Z.E., Zhao G., Shah A.M., Zhang M. Role of Oxidative Stress in Calcific Aortic Valve Disease and Its Therapeutic Implications. Cardiovasc. Res. 2021;118:1433–1451. doi: 10.1093/cvr/cvab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q., Wang Q., Zhu J., Xiao Q., Zhang L. Reactive Oxygen Species: Key Regulators in Vascular Health and Diseases: ROS in Vascular Diseases. Br. J. Pharmacol. 2018;175:1279–1292. doi: 10.1111/bph.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sies H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants. 2020;9:852. doi: 10.3390/antiox9090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaito A., Aramouni K., Assaf R., Parenti A., Orekhov A., Yazbi A.E., Pintus G., Eid A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci.-Landmark. 2022;27:0105. doi: 10.31083/j.fbl2703105. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoudi M., Gormaz J.G., Erazo M., Howard M., Baeza C., Feelisch M., Curzen N., Olechowski B., Fernandez B., Minnion M., et al. Early Oxidative Stress Response in Patients with Severe Aortic Stenosis Undergoing Transcatheter and Surgical Aortic Valve Replacement: A Transatlantic Study. Oxid. Med. Cell. Longev. 2019;2019:1–8. doi: 10.1155/2019/6217837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poznyak A.V., Grechko A.V., Orekhova V.A., Chegodaev Y.S., Wu W.-K., Orekhov A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology. 2020;9:60. doi: 10.3390/biology9030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Q., He X., Virginia W. Molecular Basis of Electrophilic and Oxidative Defense: Promises and Perils of Nrf2. Pharmacol. Rev. 2012;64:1055–1081. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui L., Zhou Q., Zheng X., Sun B., Zhao S. Mitoquinone Attenuates Vascular Calcification by Suppressing Oxidative Stress and Reducing Apoptosis of Vascular Smooth Muscle Cells via the Keap1/Nrf2 Pathway. Free Radic. Biol. Med. 2020;161:23–31. doi: 10.1016/j.freeradbiomed.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Balogh E., Chowdhury A., Ababneh H., Csiki D.M., Tóth A., Jeney V. Heme-Mediated Activation of the Nrf2/HO-1 Axis Attenuates Calcification of Valve Interstitial Cells. Biomedicines. 2021;9:427. doi: 10.3390/biomedicines9040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heistad D.D., Wakisaka Y., Miller J., Chu Y., Pena-Silva R. Novel Aspects of Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009;73:201–207. doi: 10.1253/circj.CJ-08-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reustle A., Torzewski M. Role of P38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int. J. Mol. Sci. 2018;19:3761. doi: 10.3390/ijms19123761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daiber A., Xia N., Steven S., Oelze M., Hanf A., Kröller-Schön S., Münzel T., Li H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (ENOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019;20:187. doi: 10.3390/ijms20010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller J.D., Chu Y., Brooks R.M., Richenbacher W.E., Peña-Silva R., Heistad D.D. Dysregulation of Antioxidant Mechanisms Contributes to Increased Oxidative Stress in Calcific Aortic Valvular Stenosis in Humans. J. Am. Coll. Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajamannan N.M. Bicuspid Aortic Valve Disease: The Role of Oxidative Stress in Lrp5 Bone Formation. Cardiovasc. Pathol. 2011;20:168–176. doi: 10.1016/j.carpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valerio V., Myasoedova V.A., Moschetta D., Porro B., Perrucci G.L., Cavalca V., Cavallotti L., Songia P., Poggio P. Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis. J. Clin. Med. 2019;8:552. doi: 10.3390/jcm8040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junco-Vicente A., del Río-García Á., Martín M., Rodríguez I. Update in Biomolecular and Genetic Bases of Bicuspid Aortopathy. Int. J. Mol. Sci. 2021;22:5694. doi: 10.3390/ijms22115694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo Presti F., Guzzardi D.G., Bancone C., Fedak P.W.M., Della Corte A. The Science of BAV Aortopathy. Prog. Cardiovasc. Dis. 2020;63:465–474. doi: 10.1016/j.pcad.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Mennander A.A. Biochemistry: On Bicuspid Aortic Valve’s Aortic Service. J. Thorac. Cardiovasc. Surg. 2017;154:1763. doi: 10.1016/j.jtcvs.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Portelli S.S., Hambly B.D., Jeremy R.W., Robertson E.N. Oxidative Stress in Genetically Triggered Thoracic Aortic Aneurysm: Role in Pathogenesis and Therapeutic Opportunities. Redox Rep. 2021;26:45–52. doi: 10.1080/13510002.2021.1899473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skeffington K., Bond A., Bigotti M.G., Abdulghani S., Iacobazzi D., Kang S.-L., Heesom K., Wilson M., Stoica S., Martin R., et al. Changes in Inflammation and Oxidative Stress Signalling Pathways in Coarcted Aorta Triggered by Bicuspid Aortic Valve and Growth in Young Children. Exp. Ther. Med. 2020;20:1. doi: 10.3892/etm.2020.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Billaud M., Phillippi J.A., Kotlarczyk M.P., Hill J.C., Ellis B.W., St Croix C.M., Cantu-Medéllin N., Kelley E.E., Gleason T.G. Elevated Oxidative Stress in the Aortic Media of Patients with Bicuspid Aortic Valve. J. Thorac. Cardiovasc. Surg. 2017;154:1756–1762. doi: 10.1016/j.jtcvs.2017.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillippi J.A., Klyachko E.A., Kenny J.P., Eskay M.A., Gorman R.C., Gleason T.G. Basal and Oxidative Stress–Induced Expression of Metallothionein Is Decreased in Ascending Aortic Aneurysms of Bicuspid Aortic Valve Patients. Circulation. 2009;119:2498–2506. doi: 10.1161/CIRCULATIONAHA.108.770776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillippi J.A., Eskay M.A., Kubala A.A., Pitt B.R., Gleason T.G. Altered Oxidative Stress Responses and Increased Type I Collagen Expression in Bicuspid Aortic Valve Patients. Ann. Thorac. Surg. 2010;90:1893–1898. doi: 10.1016/j.athoracsur.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Förstermann U., Xia N., Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 69.Chu Y., Lund D.D., Weiss R.M., Brooks R.M., Doshi H., Hajj G.P., Sigmund C.D., Heistad D.D. Pioglitazone Attenuates Valvular Calcification Induced by Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2013;33:523–532. doi: 10.1161/ATVBAHA.112.300794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H., Wang L., Pan Y., Wang X., Ding Y., Zhou C., Shah A.M., Zhao G., Zhang M. Celastrol Alleviates Aortic Valve Calcification Via Inhibition of NADPH Oxidase 2 in Valvular Interstitial Cells. JACC Basic Transl. Sci. 2019;5:35–49. doi: 10.1016/j.jacbts.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liberman M., Bassi E., Martinatti M.K., Lario F.C., Wosniak J., Pomerantzeff P.M.A., Laurindo F.R.M. Oxidant Generation Predominates Around Calcifying Foci and Enhances Progression of Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 72.Canugovi C., Stevenson M.D., Vendrov A.E., Hayami T., Robidoux J., Xiao H., Zhang Y.-Y., Eitzman D.T., Runge M.S., Madamanchi N.R. Increased Mitochondrial NADPH Oxidase 4 (NOX4) Expression in Aging Is a Causative Factor in Aortic Stiffening. Redox Biol. 2019;26:101288. doi: 10.1016/j.redox.2019.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tung M.-C., Lan Y.-W., Li H.-H., Chen H.-L., Chen S.-Y., Chen Y.-H., Lin C.-C., Tu M.-Y., Chen C.-M. Kefir Peptides Alleviate High-Fat Diet-Induced Atherosclerosis by Attenuating Macrophage Accumulation and Oxidative Stress in ApoE Knockout Mice. Sci. Rep. 2020;10:8802. doi: 10.1038/s41598-020-65782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.-H., Tian B., Sadoshima J. Increased Oxidative Stress in the Nucleus Caused by Nox4 Mediates Oxidation of HDAC4 and Cardiac Hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z., Zhang J., Chen L., Li J., Zhang H., Guo X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxid. Med. Cell. Longev. 2019;2019:4628962. doi: 10.1155/2019/4628962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou B., Qiu Y., Wu N., Chen A.-D., Zhou H., Chen Q., Kang Y.-M., Li Y.-H., Zhu G.-Q. FNDC5 Attenuates Oxidative Stress and NLRP3 Inflammasome Activation in Vascular Smooth Muscle Cells via Activating the AMPK-SIRT1 Signal Pathway. Oxid. Med. Cell. Longev. 2020;2020:6384803. doi: 10.1155/2020/6384803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doroszko A., Radziwon-Balicka A., Skomro R. Novel Approaches for Diagnosing and Management of Cardiovascular Disorders Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2020;2020:7096727. doi: 10.1155/2020/7096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plunde O., Bäck M. Fatty Acids and Aortic Valve Stenosis. Kardiol. Pol. 2021;79:614–621. doi: 10.33963/KP.a2021.0003. [DOI] [PubMed] [Google Scholar]
- 79.Di Vito A., Donato A., Presta I., Mancuso T., Brunetti F.S., Mastroroberto P., Amorosi A., Malara N., Donato G. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int. J. Mol. Sci. 2021;22:913. doi: 10.3390/ijms22020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopytek M., Mazur P., Ząbczyk M., Undas A., Natorska J. Diabetes Concomitant to Aortic Stenosis Is Associated with Increased Expression of NF-ΚB and More Pronounced Valve Calcification. Diabetologia. 2021;64:2562–2574. doi: 10.1007/s00125-021-05545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Izzo C., Vitillo P., Di Pietro P., Visco V., Strianese A., Virtuoso N., Ciccarelli M., Galasso G., Carrizzo A., Vecchione C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life. 2021;11:60. doi: 10.3390/life11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathieu P., Bouchareb R., Boulanger M.-C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J. Immunol. Res. 2015;2015:851945. doi: 10.1155/2015/851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matilla L., Ibarrola J., Arrieta V., Garcia-Peña A., Martinez-Martinez E., Sádaba R., Alvarez V., Navarro A., Fernández-Celis A., Gainza A., et al. Soluble ST2 Promotes Oxidative Stress and Inflammation in Cardiac Fibroblasts: An In Vitro and In Vivo Study in Aortic Stenosis. Clin. Sci. 2019;133:1537–1548. doi: 10.1042/CS20190475. [DOI] [PubMed] [Google Scholar]
- 84.Kapelouzou A., Tsourelis L., Kaklamanis L., Degiannis D., Kogerakis N., Cokkinos D.V. Serum and Tissue Biomarkers in Aortic Stenosis. Glob. Cardiol. Sci. Pract. 2015;2015:49. doi: 10.5339/gcsp.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goh K.Y., He L., Song J., Jinno M., Rogers A.J., Sethu P., Halade G.V., Rajasekaran N.S., Liu X., Prabhu S.D., et al. Mitoquinone Ameliorates Pressure Overload-Induced Cardiac Fibrosis and Left Ventricular Dysfunction in Mice. Redox Biol. 2019;21:101100. doi: 10.1016/j.redox.2019.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel V.B., Zhong J.-C., Fan D., Basu R., Morton J.S., Parajuli N., McMurtry M.S., Davidge S.T., Kassiri Z., Oudit G.Y. Angiotensin-Converting Enzyme 2 Is a Critical Determinant of Angiotensin II–Induced Loss of Vascular Smooth Muscle Cells and Adverse Vascular Remodeling. Hypertension. 2014;64:157–164. doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- 87.Chiang Y.-F., Tsai C.-H., Chen H.-Y., Wang K.-L., Chang H.-Y., Huang Y.-J., Hong Y.-H., Ali M., Shieh T.-M., Huang T.-C., et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs. 2021;19:307. doi: 10.3390/md19060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henderson B.C., Tyagi N., Ovechkin A., Kartha G.K., Moshal K.S., Tyagi S.C. Oxidative Remodeling in Pressure Overload Induced Chronic Heart Failure. Eur. J. Heart Fail. 2007;9:450–457. doi: 10.1016/j.ejheart.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Xin M., Jin X., Cui X., Jin C., Piao L., Wan Y., Xu S., Zhang S., Yue X., Wang H., et al. Dipeptidyl Peptidase-4 Inhibition Prevents Vascular Aging in Mice under Chronic Stress: Modulation of Oxidative Stress and Inflammation. Chem. Biol. Interact. 2019;314:108842. doi: 10.1016/j.cbi.2019.108842. [DOI] [PubMed] [Google Scholar]
- 90.Jones J.A. Oxidative Stress in Bicuspid Aortic Valve-Related Aortopathy: Hand-Me-Downs and Yoga Pants. J. Thorac. Cardiovasc. Surg. 2017;154:1764–1765. doi: 10.1016/j.jtcvs.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nachar W., Merlet N., Maafi F., Shi Y., Mihalache-Avram T., Mecteau M., Ferron M., Rhéaume E., Tardif J.-C. Cardiac Inflammation and Diastolic Dysfunction in Hypercholesterolemic Rabbits. PLoS ONE. 2019;14:e0220707. doi: 10.1371/journal.pone.0220707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Navaratnarajah A., Bhan A., Alcock E., Dew T., Monaghan M., Shah A.M., Wendler O., MacCarthy P., Dworakowski R. Systemic Inflammation and Oxidative Stress Contribute to Acute Kidney Injury after Transcatheter Aortic Valve Implantation. Cardiol. J. 2020 doi: 10.5603/CJ.a2020.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heldmaier K., Stoppe C., Goetzenich A., Foldenauer A.-C., Zayat R., Breuer T., Schälte G. Oxidation-Reduction Potential in Patients Undergoing Transcatheter or Surgical Aortic Valve Replacement. BioMed Res. Int. 2018;2018:8469383. doi: 10.1155/2018/8469383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komosa A., Perek B., Rzymski P., Lesiak M., Siller-Matula J.M., Grygier M., Puślecki M., Misterski M., Olasińska-Wiśniewska A., Ropacka-Lesiak M., et al. Transcatheter Aortic Valve Replacement Is Associated with Less Oxidative Stress and Faster Recovery of Antioxidant Capacity than Surgical Aortic Valve Replacement. J. Clin. Med. 2019;8:1364. doi: 10.3390/jcm8091364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchandot B., Kibler M., Charles A.L., Trinh A., Petit Eisenmann H., Zeyons F., Von Hunolstein J.J., Reydel A., Matsushita K., Kindo M., et al. Does Transcatheter Aortic Valve Replacement Modulate the Kinetic of Superoxide Anion Generation? Antioxid. Redox Signal. 2019;31:420–426. doi: 10.1089/ars.2018.7689. [DOI] [PubMed] [Google Scholar]
- 96.Marquis-Gravel G., Redfors B., Leon M.B., Généreux P. Medical Treatment of Aortic Stenosis. Circulation. 2016;134:1766–1784. doi: 10.1161/CIRCULATIONAHA.116.023997. [DOI] [PubMed] [Google Scholar]
- 97.Towler D.A. Molecular and Cellular Aspects of Calcific Aortic Valve Disease. Circ. Res. 2013;113:198–208. doi: 10.1161/CIRCRESAHA.113.300155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pedriali G., Morciano G., Patergnani S., Cimaglia P., Morelli C., Mikus E., Ferrari R., Gasbarro V., Giorgi C., Wieckowski M.R., et al. Aortic Valve Stenosis and Mitochondrial Dysfunctions: Clinical and Molecular Perspectives. Int. J. Mol. Sci. 2020;21:4899. doi: 10.3390/ijms21144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong S., Li L., Shen X., Li Q., Xu W., Wang X., Tao Y., Yin H. An Update on Lipid Oxidation and Inflammation in Cardiovascular Diseases. Free Radic. Biol. Med. 2019;144:266–278. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 100.Miller J.D., Weiss R.M., Serrano K.M., Brooks R.M., Berry C.J., Zimmerman K., Young S.G., Heistad D.D. Lowering Plasma Cholesterol Levels Halts Progression of Aortic Valve Disease in Mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Capoulade R., Chan K.L., Yeang C., Mathieu P., Bossé Y., Dumesnil J.G., Tam J.W., Teo K.K., Mahmut A., Yang X., et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 102.Moura L.M., Ramos S.F., Zamorano J.L., Barros I.M., Azevedo L.F., Rocha-Gonçalves F., Rajamannan N.M. Rosuvastatin Affecting Aortic Valve Endothelium to Slow the Progression of Aortic Stenosis. J. Am. Coll. Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cowell S.J., Newby D.E., Prescott R.J., Bloomfield P., Reid J., Northridge D.B., Boon N.A. A Randomized Trial of Intensive Lipid-Lowering Therapy in Calcific Aortic Stenosis. N. Engl. J. Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 104.Rossebø A.B., Pedersen T.R., Boman K., Brudi P., Chambers J.B., Egstrup K., Gerdts E., Gohlke-Bärwolf C., Holme I., Kesäniemi Y.A., et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 105.Capoulade R., Yeang C., Chan K.L., Pibarot P., Tsimikas S. Association of Mild to Moderate Aortic Valve Stenosis Progression With Higher Lipoprotein(a) and Oxidized Phospholipid Levels: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2018;3:1212. doi: 10.1001/jamacardio.2018.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I., Tardif J.-C., Baum S.J., Steinhagen-Thiessen E., Shapiro M.D., Stroes E.S., Moriarty P.M., Nordestgaard B.G., et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 107.Yeang C., Hung M.-Y., Byun Y.-S., Clopton P., Yang X., Witztum J.L., Tsimikas S. Effect of Therapeutic Interventions on Oxidized Phospholipids on Apolipoprotein B100 and Lipoprotein(a) J. Clin. Lipidol. 2016;10:594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Hu J., Lei H., Liu L., Xu D. Lipoprotein(a), a Lethal Player in Calcific Aortic Valve Disease. Front. Cell Dev. Biol. 2022;10:812368. doi: 10.3389/fcell.2022.812368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guddeti R.R., Patil S., Ahmed A., Sharma A., Aboeata A., Lavie C.J., Alla V.M. Lipoprotein(a) and Calcific Aortic Valve Stenosis: A Systematic Review. Prog. Cardiovasc. Dis. 2020;63:496–502. doi: 10.1016/j.pcad.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Ljungberg J., Holmgren A., Bergdahl I.A., Hultdin J., Norberg M., Näslund U., Johansson B., Söderberg S. Lipoprotein(a) and the Apolipoprotein B/A1 Ratio Independently Associate with Surgery for Aortic Stenosis Only in Patients With Concomitant Coronary Artery Disease. J. Am. Heart Assoc. 2017;6:e007160. doi: 10.1161/JAHA.117.007160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma G.S., Wilkinson M.J., Reeves R.R., Yeang C., DeMaria A.N., Cotter B., Patel M., Mahmud E., Tsimikas S. Lipoprotein(a) in Patients Undergoing Transcatheter Aortic Valve Replacement. Angiology. 2019;70:332–336. doi: 10.1177/0003319719826461. [DOI] [PubMed] [Google Scholar]
- 112.Pérez de Isla L., Watts G.F., Alonso R., Díaz-Díaz J.L., Muñiz-Grijalvo O., Zambón D., Fuentes F., de Andrés R., Padró T., López-Miranda J., et al. Lipoprotein(a), LDL-Cholesterol, and Hypertension: Predictors of the Need for Aortic Valve Replacement in Familial Hypercholesterolaemia. Eur. Heart J. 2021;42:2201–2211. doi: 10.1093/eurheartj/ehaa1066. [DOI] [PubMed] [Google Scholar]
- 113.Capoulade R., Torzewski M., Mayr M., Chan K.-L., Mathieu P., Bossé Y., Dumesnil J.G., Tam J., Teo K.K., Burnap S.A., et al. ApoCIII-Lp(a) Complexes in Conjunction with Lp(a)-OxPL Predict Rapid Progression of Aortic Stenosis. Heart. 2020;106:738–745. doi: 10.1136/heartjnl-2019-315840. [DOI] [PubMed] [Google Scholar]
- 114.Vongpromek R., Bos S., ten Kate G.-J.R., Yahya R., Verhoeven A.J.M., de Feyter P.J., Kronenberg F., Roeters van Lennep J.E., Sijbrands E.J.G., Mulder M.T. Lipoprotein(a) Levels Are Associated with Aortic Valve Calcification in Asymptomatic Patients with Familial Hypercholesterolaemia. J. Intern. Med. 2015;278:166–173. doi: 10.1111/joim.12335. [DOI] [PubMed] [Google Scholar]
- 115.Sabatine M.S., Giugliano R.P., Wiviott S.D., Raal F.J., Blom D.J., Robinson J., Ballantyne C.M., Somaratne R., Legg J., Wasserman S.M., et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 116.O’Donoghue M.L., Fazio S., Giugliano R.P., Stroes E.S.G., Kanevsky E., Gouni-Berthold I., Im K., Lira Pineda A., Wasserman S.M., Češka R., et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk: Insights from the FOURIER Trial. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 117.Bergmark B.A., O’Donoghue M.L., Murphy S.A., Kuder J.F., Ezhov M.V., Ceška R., Gouni-Berthold I., Jensen H.K., Tokgozoglu S.L., Mach F., et al. An Exploratory Analysis of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition and Aortic Stenosis in the FOURIER Trial. JAMA Cardiol. 2020;5:709. doi: 10.1001/jamacardio.2020.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J.P., Koenig W., Somaratne R., Kassahun H., Yang J., et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016;316:2373. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 119.van Capelleveen J.C., van der Valk F.M., Stroes E.S.G. Current Therapies for Lowering Lipoprotein (a) J. Lipid Res. 2016;57:1612–1618. doi: 10.1194/jlr.R053066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Viney N.J., van Capelleveen J.C., Geary R.S., Xia S., Tami J.A., Yu R.Z., Marcovina S.M., Hughes S.G., Graham M.J., Crooke R.M., et al. Antisense Oligonucleotides Targeting Apolipoprotein(a) in People with Raised Lipoprotein(a): Two Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 121.Que X., Hung M.-Y., Yeang C., Gonen A., Prohaska T.A., Sun X., Diehl C., Määttä A., Gaddis D.E., Bowden K., et al. Oxidized Phospholipids Are Proinflammatory and Proatherogenic in Hypercholesterolaemic Mice. Nature. 2018;558:301–306. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin C.P., Huang P.-H., Chen C.-Y., Chen J.-S., Chen J.-W., Lin S.-J. Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Attenuates Arterial Calcification By Downregulating Oxidative Stress-Induced Receptor for Advanced Glycation End Products in Low-Density Lipoprotein Receptor Knockout Mice. Res. Square. 2021 doi: 10.21203/rs.3.rs-498043/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang S., Liu L., Meng L., Hu X. Capsaicin Is Beneficial to Hyperlipidemia, Oxidative Stress, Endothelial Dysfunction, and Atherosclerosis in Guinea Pigs Fed on a High-Fat Diet. Chem. Biol. Interact. 2019;297:1–7. doi: 10.1016/j.cbi.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 124.Adhikari R., Shiwakoti S., Ko J.-Y., Dhakal B., Park S.-H., Choi I.J., Kim H.J., Oak M.-H. Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants. 2022;11:1169. doi: 10.3390/antiox11061169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chao C.-T., Yeh H.-Y., Tsai Y.-T., Chuang P.-H., Yuan T.-H., Huang J.-W., Chen H.-W. Natural and Non-Natural Antioxidative Compounds: Potential Candidates for Treatment of Vascular Calcification. Cell Death Discov. 2019;5:145. doi: 10.1038/s41420-019-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hammad S.K., Eissa R.G., Shaheen M.A., Younis N.N. Resveratrol Ameliorates Aortic Calcification in Ovariectomized Rats via SIRT1 Signaling. Curr. Issues Mol. Biol. 2021;43:1057–1071. doi: 10.3390/cimb43020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang P., Li Y., Du Y., Li G., Wang L., Zhou F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016;48:3378–3386. doi: 10.1016/j.transproceed.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 128.Tomayko E.J., Cachia A.J., Chung H.R., Wilund K.R. Resveratrol Supplementation Reduces Aortic Atherosclerosis and Calcification and Attenuates Loss of Aerobic Capacity in a Mouse Model of Uremia. J. Med. Food. 2014;17:278–283. doi: 10.1089/jmf.2012.0219. [DOI] [PubMed] [Google Scholar]
- 129.Gugliandolo E., Fusco R., Biundo F., D’Amico R., Benedetto F., Di Paola R., Cuzzocrea S. Palmitoylethanolamide and Polydatin Combination Reduces Inflammation and Oxidative Stress in Vascular Injury. Pharmacol. Res. 2017;123:83–92. doi: 10.1016/j.phrs.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 130.Xia N., Daiber A., Förstermann U., Li H. Antioxidant Effects of Resveratrol in the Cardiovascular System: Antioxidant Effects of Resveratrol. Br. J. Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin Y., Wang Y., Weng Y., Li X., Huang Q., Liu Y., Xiang Y., Li X., Jiang P., He W., et al. Resveratrol Exhibits Inhibition Effects on Osteogenic Differentiation of Aortic Valve Interstitial Cells by Interfering with the AKT Pathway. J. Funct. Foods. 2022;91:105002. doi: 10.1016/j.jff.2022.105002. [DOI] [Google Scholar]
- 132.van Andel M., Groenink M., Zwinderman A., Mulder B., de Waard V. The Potential Beneficial Effects of Resveratrol on Cardiovascular Complications in Marfan Syndrome Patients–Insights from Rodent-Based Animal Studies. Int. J. Mol. Sci. 2019;20:1122. doi: 10.3390/ijms20051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Assis R., Arcaro C., Gutierres V., Oliveira J., Costa P., Baviera A., Brunetti I. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017;18:332. doi: 10.3390/ijms18040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hewlings S., Kalman D. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin S., Huang L., Gong J., Shen S., Huang J., Ren H., Hu H. Efficacy and Safety of Turmeric and Curcumin in Lowering Blood Lipid Levels in Patients with Cardiovascular Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2017;16:68. doi: 10.1186/s12937-017-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang S., Zou J., Li P., Zheng X., Feng D. Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J. Agric. Food Chem. 2018;66:449–456. doi: 10.1021/acs.jafc.7b04260. [DOI] [PubMed] [Google Scholar]
- 137.Zhou T., Wang Y., Liu M., Huang Y., Shi J., Dong N., Xu K. Curcumin Inhibits Calcification of Human Aortic Valve Interstitial Cells by Interfering NF-κB, AKT, and ERK Pathways. Phyther. Res. 2020;34:2074–2081. doi: 10.1002/ptr.6674. [DOI] [PubMed] [Google Scholar]
- 138.Lin K., Chen H., Chen X., Qian J., Huang S., Huang W. Efficacy of Curcumin on Aortic Atherosclerosis: A Systematic Review and Meta-Analysis in Mouse Studies and Insights into Possible Mechanisms. Oxid. Med. Cell. Longev. 2020;2020:1520747. doi: 10.1155/2020/1520747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daimary U.D., Parama D., Rana V., Banik K., Kumar A., Harsha C., Kunnumakkara A.B. Emerging Roles of Cardamonin, a Multitargeted Nutraceutical in the Prevention and Treatment of Chronic Diseases. Curr. Res. Pharmacol. Drug Discov. 2021;2:100008. doi: 10.1016/j.crphar.2020.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gonçalves L.M., Valente I.M., Rodrigues J.A. An Overview on Cardamonin. J. Med. Food. 2014;17:633–640. doi: 10.1089/jmf.2013.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C., Xia Y., Qu L., Liu Y., Liu X., Xu K. Cardamonin Inhibits Osteogenic Differentiation of Human Valve Interstitial Cells and Ameliorates Aortic Valve Calcification via Interfering NF-ΚB/NLRP3 Inflammasome Pathway. Food Funct. 2021 doi: 10.1039/D1FO00813G. [DOI] [PubMed] [Google Scholar]
- 142.Sharifi-Rad J., Quispe C., Castillo C.M.S., Caroca R., Lazo-Vélez M.A., Antonyak H., Polishchuk A., Lysiuk R., Oliinyk P., De Masi L., et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022;2022:3848084. doi: 10.1155/2022/3848084. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Jordão J., Porto H., Lopes F., Batista A., Rocha M. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017;83:830–836. doi: 10.1055/s-0043-103281. [DOI] [PubMed] [Google Scholar]
- 144.Kee H.J., Cho S.-N., Kim G.R., Choi S.Y., Ryu Y., Kim I.K., Hong Y.J., Park H.W., Ahn Y., Cho J.G., et al. Gallic Acid Inhibits Vascular Calcification through the Blockade of BMP2–Smad1/5/8 Signaling Pathway. Vascul. Pharmacol. 2014;63:71–78. doi: 10.1016/j.vph.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Liu M., Li F., Huang Y., Zhou T., Chen S., Li G., Shi J., Dong N., Xu K. Caffeic Acid Phenethyl Ester Ameliorates Calcification by Inhibiting Activation of the AKT/NF-ΚB/NLRP3 Inflammasome Pathway in Human Aortic Valve Interstitial Cells. Front. Pharmacol. 2020;11:826. doi: 10.3389/fphar.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nie J., Chang Y., Li Y., Zhou Y., Qin J., Sun Z., Li H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-ΚB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J. Agric. Food Chem. 2017;65:9041–9053. doi: 10.1021/acs.jafc.7b02880. [DOI] [PubMed] [Google Scholar]
- 147.Huang H., Li L., Shi W., Liu H., Yang J., Yuan X., Wu L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Alternat. Med. 2016;2016:2918796. doi: 10.1155/2016/2918796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu K., Huang Y., Zhou T., Wang C., Chi Q., Shi J., Zhu P., Dong N. Nobiletin Exhibits Potent Inhibition on Tumor Necrosis Factor Alpha-induced Calcification of Human Aortic Valve Interstitial Cells via Targeting ABCG2 and AKR1B1. Phytother. Res. 2019;33:1717–1725. doi: 10.1002/ptr.6360. [DOI] [PubMed] [Google Scholar]
- 149.Kaneda H., Otomo R., Sasaki N., Omi T., Sato T., Kaneda T. Endothelium-Independent Vasodilator Effects of Nobiletin in Rat Aorta. J. Pharmacol. Sci. 2019;140:48–53. doi: 10.1016/j.jphs.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 150.Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schäfer B., Hirsch-Ernst K.I., Lampen A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018;62:1700447. doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 151.Chang X., Cui L., Wang X., Zhang L., Zhu D., Zhou X., Hao L. Quercetin Attenuates Vascular Calcification through Suppressed Oxidative Stress in Adenine-Induced Chronic Renal Failure Rats. BioMed Res. Int. 2017;2017:5716204. doi: 10.1155/2017/5716204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhaskar S., Sudhakaran P.R., Helen A. Quercetin Attenuates Atherosclerotic Inflammation and Adhesion Molecule Expression by Modulating TLR-NF-ΚB Signaling Pathway. Cell. Immunol. 2016;310:131–140. doi: 10.1016/j.cellimm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 153.Cao H., Jia Q., Shen D., Yan L., Chen C., Xing S. Quercetin Has a Protective Effect on Atherosclerosis via Enhancement of Autophagy in ApoE-/- Mice. Exp. Ther. Med. 2019;18:2451–2458. doi: 10.3892/etm.2019.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu W., Wang W., Ma Q., Liu T., Zhang J., Zhang J. Blueberry Anthocyanin-enriched Extract Ameliorates Transverse Aortic Constriction-induced Myocardial Dysfunction via the DDAH1/ADMA/NO Signaling Pathway in Mice. Mol. Med. Rep. 2019;21:454–462. doi: 10.3892/mmr.2019.10800. [DOI] [PubMed] [Google Scholar]
- 155.Liu H., Zhang X., Zhong X., Li Z., Cai S., Yang P., Ou C., Chen M. Puerarin Inhibits Vascular Calcification of Uremic Rats. Eur. J. Pharmacol. 2019;855:235–243. doi: 10.1016/j.ejphar.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 156.Jiang Z., Cui X., Qu P., Shang C., Xiang M., Wang J. Roles and Mechanisms of Puerarin on Cardiovascular Disease: A Review. Biomed. Pharmacother. 2022;147:112655. doi: 10.1016/j.biopha.2022.112655. [DOI] [PubMed] [Google Scholar]
- 157.Cai S.-A., Hou N., Zhao G.-J., Liu X.-W., He Y.-Y., Liu H.-L., Hua Y.-Q., Li L.-R., Huang Y., Ou C.-W., et al. Nrf2 Is a Key Regulator on Puerarin Preventing Cardiac Fibrosis and Upregulating Metabolic Enzymes UGT1A1 in Rats. Front. Pharmacol. 2018;9:540. doi: 10.3389/fphar.2018.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu Y., Li H., Li R., Wu Z., Yang W., Qu W. Puerarin Protects Vascular Smooth Muscle Cells from Oxidized Low-density Lipoprotein-induced Reductions in Viability via Inhibition of the P38 MAPK and JNK Signaling Pathways. Exp. Ther. Med. 2020;20:1. doi: 10.3892/etm.2020.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu S., Tang S., Su F. Dioscin Inhibits Ischemic Stroke-induced Inflammation through Inhibition of the TLR4/MyD88/NF-κB Signaling Pathway in a Rat Model. Mol. Med. Rep. 2017;17:660–666. doi: 10.3892/mmr.2017.7900. [DOI] [PubMed] [Google Scholar]
- 160.Pari L., Monisha P., Mohamed Jalaludeen A. Beneficial Role of Diosgenin on Oxidative Stress in Aorta of Streptozotocin Induced Diabetic Rats. Eur. J. Pharmacol. 2012;691:143–150. doi: 10.1016/j.ejphar.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 161.Manivannan J., Balamurugan E., Thangarasu S., Raja B. Diosgenin Improves Vascular Function by Increasing Aortic ENOS Expression, Normalize Dyslipidemia and ACE Activity in Chronic Renal Failure Rats. Mol. Cell. Biochem. 2013;384:s11010–s11013. doi: 10.1007/s11010-013-1788-2. [DOI] [PubMed] [Google Scholar]
- 162.Elseweidy M.M., Mohamed H.E., Elrashidy R.A., Atteia H.H., Elnagar G.M., Ali A.E.-M. Potential Therapeutic Roles of 10-Dehydrogingerdione and/or Pentoxifylline against Calcium Deposition in Aortic Tissues of High Dietary Cholesterol-Fed Rabbits. Mol. Cell. Biochem. 2019;453:131–142. doi: 10.1007/s11010-018-3438-1. [DOI] [PubMed] [Google Scholar]
- 163.Böhm V. Vitamin, E. Antioxidants. 2018;7:44. doi: 10.3390/antiox7030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peralta-Ramírez A., Montes de Oca A., Raya A.I., Pineda C., López I., Guerrero F., Diez E., Muñoz-Castañeda J.R., Martinez J., Almaden Y., et al. Vitamin E Protection of Obesity-Enhanced Vascular Calcification in Uremic Rats. Am. J. Physiol.-Ren. Physiol. 2014;306:F422–F429. doi: 10.1152/ajprenal.00355.2013. [DOI] [PubMed] [Google Scholar]
- 165.Knekt P., Reunanen A., Jävinen R., Seppänen R., Heliövaara M., Aromaa A. Antioxidant Vitamin Intake and Coronary Mortality in a Longitudinal Population Study. Am. J. Epidemiol. 1994;139:1180–1189. doi: 10.1093/oxfordjournals.aje.a116964. [DOI] [PubMed] [Google Scholar]
- 166.Bleys J., Miller E.R., Pastor-Barriuso R., Appel L.J., Guallar E. Vitamin-Mineral Supplementation and the Progression of Atherosclerosis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2006;84:880–887. doi: 10.1093/ajcn/84.4.880. [DOI] [PubMed] [Google Scholar]
- 167.Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. Use of Antioxidant Vitamins for the Prevention of Cardiovascular Disease: Meta-Analysis of Randomised Trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 168.Gammone M.A. Prevention of Cardiovascular Diseases with Carotenoids. Front. Biosci. 2017;9:165–171. doi: 10.2741/s480. [DOI] [PubMed] [Google Scholar]
- 169.Huang Y., Zhou X., Liu M., Zhou T., Shi J., Dong N., Xu K. The Natural Compound Andrographolide Inhibits Human Aortic Valve Interstitial Cell Calcification via the NF-Kappa B/Akt/ERK Pathway. Biomed. Pharmacother. 2020;125:109985. doi: 10.1016/j.biopha.2020.109985. [DOI] [PubMed] [Google Scholar]
- 170.Kandanur S.G.S., Tamang N., Golakoti N.R., Nanduri S. Andrographolide: A Natural Product Template for the Generation of Structurally and Biologically Diverse Diterpenes. Eur. J. Med. Chem. 2019;176:513–533. doi: 10.1016/j.ejmech.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 171.Zhu T., Wang D., Zhang W., Liao X., Guan X., Bo H., Sun J., Huang N., He J., Zhang Y., et al. Andrographolide Protects against LPS-Induced Acute Lung Injury by Inactivation of NF-ΚB. PLoS ONE. 2013;8:e56407. doi: 10.1371/journal.pone.0056407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feng W., Zhang K., Liu Y., Chen J., Cai Q., Zhang Y., Wang M., Wang J., Huang H. Apocynin Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cells Osteogenic Switching via Suppressing Extracellular Signal-Regulated Kinase 1/2. Oncotarget. 2016;7:83588–83600. doi: 10.18632/oncotarget.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Watanabe S., Fujii H., Kono K., Watanabe K., Goto S., Nishi S. Influence of Oxidative Stress on Vascular Calcification in the Setting of Coexisting Chronic Kidney Disease and Diabetes Mellitus. Sci. Rep. 2020;10:20708. doi: 10.1038/s41598-020-76838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gu L., Bai W., Li S., Zhang Y., Han Y., Gu Y., Meng G., Xie L., Wang J., Xiao Y., et al. Celastrol Prevents Atherosclerosis via Inhibiting LOX-1 and Oxidative Stress. PLoS ONE. 2013;8:e65477. doi: 10.1371/journal.pone.0065477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shi J., Li J., Xu Z., Chen L., Luo R., Zhang C., Gao F., Zhang J., Fu C. Celastrol: A Review of Useful Strategies Overcoming Its Limitation in Anticancer Application. Front. Pharmacol. 2020;11:558741. doi: 10.3389/fphar.2020.558741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X., Gao M., Zhu S., Yin L., Zhang B., Qi Y., Zhao Y., Yu Y., Xu L. Hengshun Aromatic Vinegar Ameliorates Vascular Endothelial Injury via Regulating PKCζ-Mediated Oxidative Stress and Apoptosis. Front. Nutr. 2021;8:635232. doi: 10.3389/fnut.2021.635232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ma S., Chen J., Feng J., Zhang R., Fan M., Han D., Li X., Li C., Ren J., Wang Y., et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018;2018:9286458. doi: 10.1155/2018/9286458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li M., Xu S., Geng Y., Sun L., Wang R., Yan Y., Wang H., Li Y., Yi Q., Zhang Y., et al. The Protective Effects of L-carnitine on Myocardial Ischaemia–Reperfusion Injury in Patients with Rheumatic Valvular Heart Disease Undergoing CPB Surgery Are Associated with the Suppression of NF -κB Pathway and the Activation of Nrf2 Pathway. Clin. Exp. Pharmacol. Physiol. 2019;46:1001–1012. doi: 10.1111/1440-1681.13155. [DOI] [PubMed] [Google Scholar]
- 179.Chai H., Tao Z., Qi Y., Qi H., Chen W., Xu Y., Zhang L., Chen H., Chen X. IKK Epsilon Deficiency Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice by Inhibiting Inflammation, Oxidative Stress, and Apoptosis. Oxid. Med. Cell. Longev. 2020;2020:3602824. doi: 10.1155/2020/3602824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nelson B., Johnson M., Walker M., Riley K., Sims C. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants. 2016;5:15. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xue Y., Balmuri S.R., Patel A., Sant V., Sant S. Synthesis, Physico-Chemical Characterization, and Antioxidant Effect of PEGylated Cerium Oxide Nanoparticles. Drug Deliv. Transl. Res. 2018;8:357–367. doi: 10.1007/s13346-017-0396-1. [DOI] [PubMed] [Google Scholar]
- 182.Anselmo W., Branchetti E., Grau J.B., Li G., Ayoub S., Lai E.K., Rioux N., Tovmasyan A., Fortier J.H., Sacks M.S., et al. Porphyrin-Based SOD Mimic MnTnBuOE-2-PyP 5+ Inhibits Mechanisms of Aortic Valve Remodeling in Human and Murine Models of Aortic Valve Sclerosis. J. Am. Heart Assoc. 2018;7:e007861. doi: 10.1161/JAHA.117.007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Majumdar U., Manivannan S., Basu M., Ueyama Y., Blaser M.C., Cameron E., McDermott M.R., Lincoln J., Cole S.E., Wood S., et al. Nitric Oxide Prevents Aortic Valve Calcification by S-Nitrosylation of USP9X to Activate NOTCH Signaling. Sci. Adv. 2021;7:eabe3706. doi: 10.1126/sciadv.abe3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Richards J., El-Hamamsy I., Chen S., Sarang Z., Sarathchandra P., Yacoub M.H., Chester A.H., Butcher J.T. Side-Specific Endothelial-Dependent Regulation of Aortic Valve Calcification. Am. J. Pathol. 2013;182:1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tyagi N., Vacek T. Tyagi Hydrogen Sulfide and Sodium Nitroprusside Compete to Activate/Deactivate MMPs in Bone Tissue Homogenates. Vasc. Health Risk Manag. 2013;9:117. doi: 10.2147/VHRM.S39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rattazzi M., Donato M., Bertacco E., Millioni R., Franchin C., Mortarino C., Faggin E., Nardin C., Scarpa R., Cinetto F., et al. L-Arginine Prevents Inflammatory and pro-Calcific Differentiation of Interstitial Aortic Valve Cells. Atherosclerosis. 2020;298:27–35. doi: 10.1016/j.atherosclerosis.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 187.Lindman B.R., Chakinala M.M. Modulating the Nitric Oxide—Cyclic GMP Pathway in the Pressure-Overloaded Left Ventricle and Group II Pulmonary Hypertension: Group II PH and NO-CGMP Signalling Pathway. Int. J. Clin. Pract. 2010;64:15–22. doi: 10.1111/j.1742-1241.2010.02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu Z., Dong N., Hui H., Wang Y., Liu F., Xu L., Liu M., Rao Z., Yuan Z., Shang Y., et al. Endothelial Cell-Derived Tetrahydrobiopterin Prevents Aortic Valve Calcification. Eur. Heart J. 2022;43:1652–1664. doi: 10.1093/eurheartj/ehac037. [DOI] [PubMed] [Google Scholar]
- 189.Choi B., Lee S., Kim S.-M., Lee E.-J., Lee S.R., Kim D.-H., Jang J.Y., Kang S.-W., Lee K.-U., Chang E.-J., et al. Dipeptidyl Peptidase-4 Induces Aortic Valve Calcification by Inhibiting Insulin-Like Growth Factor-1 Signaling in Valvular Interstitial Cells. Circulation. 2017;135:1935–1950. doi: 10.1161/CIRCULATIONAHA.116.024270. [DOI] [PubMed] [Google Scholar]
- 190.Lin C.-P., Huang P.-H., Chen C.-Y., Wu M.-Y., Chen J.-S., Chen J.-W., Lin S.-J. Sitagliptin Attenuates Arterial Calcification by Downregulating Oxidative Stress-Induced Receptor for Advanced Glycation End Products in LDLR Knockout Mice. Sci. Rep. 2021;11:17851. doi: 10.1038/s41598-021-97361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yeang C., Tsimikas S. Ancient Remedy for a Modern Disease. JACC Basic Transl. Sci. 2020;5:50–52. doi: 10.1016/j.jacbts.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Helmstädter J., Keppeler K., Aust F., Küster L., Frenis K., Filippou K., Vujacic-Mirski K., Tsohataridis S., Kalinovic S., Kröller-Schön S., et al. GLP-1 Analog Liraglutide Improves Vascular Function in Polymicrobial Sepsis by Reduction of Oxidative Stress and Inflammation. Antioxidants. 2021;10:1175. doi: 10.3390/antiox10081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li F., Cai Z., Chen F., Shi X., Zhang Q., Chen S., Shi J., Wang D.W., Dong N. Pioglitazone Attenuates Progression of Aortic Valve Calcification via Down-Regulating Receptor for Advanced Glycation End Products. Basic Res. Cardiol. 2012;107:306. doi: 10.1007/s00395-012-0306-0. [DOI] [PubMed] [Google Scholar]
- 194.Drummond G.R., Selemidis S., Griendling K.K., Sobey C.G. Combating Oxidative Stress in Vascular Disease: NADPH Oxidases as Therapeutic Targets. Nat. Rev. Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu W.J., Li J.Y., Chen R.J., Huang L.T., Lee T.Y., Lin K.H. VAS2870 and VAS3947 Attenuate Platelet Activation and Thrombus Formation via a NOX-Independent Pathway Downstream of PKC. Sci. Rep. 2019;9:18852. doi: 10.1038/s41598-019-55189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reis J., Massari M., Marchese S., Ceccon M., Aalbers F.S., Corana F., Valente S., Mai A., Magnani F., Mattevi A. A Closer Look into NADPH Oxidase Inhibitors: Validation and Insight into Their Mechanism of Action. Redox Biol. 2020;32:101466. doi: 10.1016/j.redox.2020.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Teixeira G., Szyndralewiez C., Molango S., Carnesecchi S., Heitz F., Wiesel P., Wood J.M. Therapeutic Potential of NADPH Oxidase 1/4 Inhibitors: Pharmacology of NOX Inhibition. Br. J. Pharmacol. 2017;174:1647–1669. doi: 10.1111/bph.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choi B., Kim E.-Y., Kim J.-E., Oh S., Park S.-O., Kim S.-M., Choi H., Song J.-K., Chang E.-J. Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification. Cells. 2021;10:57. doi: 10.3390/cells10010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.