Abstract
Background
The oral protease inhibitor nirmatrelvir has shown substantial efficacy in high-risk, unvaccinated patients infected with the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Data regarding the effectiveness of nirmatrelvir in preventing severe coronavirus disease 2019 (Covid-19) outcomes from the B.1.1.529 (omicron) variant are limited.
Methods
We obtained data for all members of Clalit Health Services who were 40 years of age or older at the start of the study period and were assessed as being eligible to receive nirmatrelvir therapy during the omicron surge. A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of nirmatrelvir treatment with hospitalization and death due to Covid-19, with adjustment for sociodemographic factors, coexisting conditions, and previous SARS-CoV-2 immunity status.
Results
A total of 109,254 patients met the eligibility criteria, of whom 3902 (4%) received nirmatrelvir during the study period. Among patients 65 years of age or older, the rate of hospitalization due to Covid-19 was 14.7 cases per 100,000 person-days among treated patients as compared with 58.9 cases per 100,000 person-days among untreated patients (adjusted hazard ratio, 0.27; 95% confidence interval [CI], 0.15 to 0.49). The adjusted hazard ratio for death due to Covid-19 was 0.21 (95% CI, 0.05 to 0.82). Among patients 40 to 64 years of age, the rate of hospitalization due to Covid-19 was 15.2 cases per 100,000 person-days among treated patients and 15.8 cases per 100,000 person-days among untreated patients (adjusted hazard ratio, 0.74; 95% CI, 0.35 to 1.58). The adjusted hazard ratio for death due to Covid-19 was 1.32 (95% CI, 0.16 to 10.75).
Conclusions
Among patients 65 years of age or older, the rates of hospitalization and death due to Covid-19 were significantly lower among those who received nirmatrelvir than among those who did not. No evidence of benefit was found in younger adults.
The emergence of the fast-spreading B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2021 led to a worldwide resurgence of coronavirus disease 2019 (Covid-19). By early January 2022, omicron had spread widely, even among communities with high levels of preexisting immunity.1
On December 22, 2021, the Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the oral antiviral nirmatrelvir for the treatment of mild-to-moderate Covid-19 in patients who had a high risk of progression to severe disease.2 Patients receive nirmatrelvir for 5 consecutive days, beginning within 5 days after the onset of symptoms.3 Data supporting the FDA EUA were from the EPIC-HR trial (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) — a randomized, controlled trial in which nirmatrelvir was evaluated in nonhospitalized, high-risk, symptomatic adults without previous immunity against SARS-CoV-2, either through vaccination or through confirmed SARS-CoV-2 infection,4 when the B.1.617.2 (delta) variant was the predominant circulating variant.5 However, data regarding the effectiveness of nirmatrelvir against the omicron variant and in patients with previous immunity against SARS-CoV-2 are limited. Therefore, our objective was to assess the effectiveness of nirmatrelvir in preventing severe Covid-19 outcomes during the omicron surge in a population with widespread SARS-CoV-2 immunity.
Methods
Study Design
This observational, retrospective cohort study was based on data obtained from electronic medical records for members of Clalit Health Services (CHS), a large health care organization that covers approximately 52% of the entire Israeli population and almost two thirds of older adults. The study period started on January 9, 2022, which was the first day that nirmatrelvir was administered to CHS members, and ended on March 31, 2022. During the study period, the omicron variant was the dominant strain in Israel (see Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Study Population
The study population comprised all CHS members who were 40 years of age or older, had confirmed SARS-CoV-2 infection, received a diagnosis of Covid-19 as outpatients, were assessed as being at high risk for progression to severe disease, and were deemed eligible to receive nirmatrelvir therapy. High-risk patients were identified on the basis of a risk model that was developed by CHS to evaluate the risk of severe Covid-19 in patients infected with SARS-CoV-2; details are provided in the Supplementary Appendix. Patients were included in the study cohort if they had a risk score of at least 2 points; details are provided in Table S1. Patients were eligible for inclusion if they received the Covid-19 diagnosis on or before February 24, 2022. Eligibility for receipt of the antiviral therapy took into account drug interactions and other contraindications, as described in the nirmatrelvir prescribing information.3 For each patient, follow-up ended at the earliest of the following time points: 35 days after the diagnosis of Covid-19, the end of the study period, or the time of data censoring if the patient died during the study period from reasons unrelated to Covid-19.
Most of the patients who were tested for Covid-19 during the study period underwent such testing because of the occurrence of symptoms. Polymerase-chain-reaction (PCR) and state-regulated antigen tests were freely available at the request of the patient. However, no screening for SARS-CoV-2 was performed, even when a patient had been exposed to a person with confirmed Covid-19. CHS policy stipulated that the antiviral therapy be initiated in eligible patients as soon as possible after a positive SARS-CoV-2 test, in accordance with FDA prescribing information.3 Each CHS district was responsible for the delivery of nirmatrelvir therapy to the patients’ homes and for verification of adherence to the treatment regimen. High-risk patients who had a contraindication to nirmatrelvir were offered treatment with molnupiravir, which was available in Israel beginning on January 16, 2022. Patients who were residing in long-term care facilities and patients who had been hospitalized before or on the same day as a positive SARS-CoV-2 test were excluded from the study, as were patients who had received treatment with molnupiravir or with the anti–SARS-CoV-2 monoclonal antibodies tixagevimab and cilgavimab.
The study was approved by the Community Helsinki and Data Utilization committees of CHS. The study was exempt from the requirement to obtain informed consent owing to the retrospective design.
Data Sources and Organization
We evaluated integrated patient-level data that were maintained by CHS from two primary sources: the primary care operational database and the Covid-19 database. The operational database includes sociodemographic data and comprehensive clinical information, such as coexisting chronic illnesses, community-care visits, medications, and results of laboratory tests. The Covid-19 database includes results of PCR and state-regulated rapid antigen tests, vaccinations, and hospitalizations and deaths related to Covid-19. These same databases were used in the primary studies that evaluated the effectiveness of the BNT162b2 vaccine (Pfizer–BioNTech) in a real-world setting in Israel.6,7 A description of the data repositories that were used in this study is provided in the Supplementary Appendix. For each patient in the study, the following sociodemographic data were extracted: age, sex, population sector (general Jewish, ultra-Orthodox Jewish, or Arab), and score for socioeconomic status (ranging from 1 [lowest] to 10 [highest]; details are provided in the Supplementary Appendix). The following clinical data were extracted: Covid-19 vaccination dates, PCR and state-regulated rapid antigen test dates and results, Covid-19 antiviral therapies, hospitalizations, and deaths. Data regarding the following clinical risk factors for severe Covid-19 were also collected: immunosuppression, diabetes mellitus, asthma, hypertension, neurologic disease, current cancer disease, chronic hepatic disease, chronic obstructive pulmonary disease, chronic kidney failure, chronic heart failure, obesity, history of stroke or smoking, and recent hospitalizations (in the preceding 3 years) for any cause. In addition, the estimated glomerular filtration rate was extracted when available.
Study Outcomes
The primary outcome of the study was hospitalization due to Covid-19. The secondary outcome was death due to Covid-19.
Subgroup analyses of the primary and secondary outcomes were performed to determine the effect of SARS-CoV-2 immunity status. Patients were divided into one of two categories according to their immunity status: those who had already acquired previous immunity (vaccine-induced, infection-induced, or a hybrid of both) and those with no previous immunity (unvaccinated or vaccinated with only one mRNA vaccine dose and with no previous documented SARS-CoV-2 infection). This classification was based on the Israeli Ministry of Health guidelines, which refer to persons who receive only one mRNA vaccine dose and persons who are unvaccinated as having similar immunity.
Statistical Analysis
All eligible CHS members were included in the analysis, in accordance with the study design. Descriptive statistics were used to characterize the study patients. Because the independent variable (nirmatrelvir treatment) varied over time, univariate and multivariate survival analyses were performed with time-dependent covariates.
For patients who did not receive treatment with nirmatrelvir, time zero corresponded to the time at which each patient received a diagnosis of Covid-19. For patients who received treatment with nirmatrelvir, time zero corresponded to the time at which a patient began the treatment. In order to avoid immortal time bias,8 we performed a time-dependent analysis in which a time-varying covariate was used to indicate the initiation of treatment for each treated patient. In this analysis, patients who received nirmatrelvir were transferred from the “untreated” risk set to the “treated” risk set when treatment was initiated, thereby modifying their treatment status from untreated to treated. Consequently, the follow-up of nirmatrelvir-treated patients started at the end of the immortal period.
A sensitivity analysis assessed the effect size of nirmatrelvir treatment beginning on day 3 of follow-up by excluding patients who had been hospitalized within 2 days after the start of follow-up. This approach allowed for comparability with the EPIC-HR trial, in which patients were excluded if the need for hospitalization within 2 days after randomization was anticipated.4
The association between nirmatrelvir therapy and Covid-19 outcomes was estimated with the use of a multivariate Cox proportional-hazards regression model with time-dependent covariates; adjustment was made for sociodemographic factors and coexisting illnesses. Given that many clinical and sociodemographic factors are potential confounders, two-step testing criteria were applied for the selection of covariates. First, a univariate Kaplan–Meier analysis with a log-rank test was applied to evaluate the associations between each independent candidate variable and the time-dependent primary outcome. Then, a comparison of the survival curves and Schoenfeld’s global test was used to test the proportional-hazards assumption for those variables. Variates that met these two testing criteria served as the inputs for the multivariate regression analysis. An additional multivariate Cox proportional-hazards regression model was used to estimate the association between each of the covariates and uptake of nirmatrelvir therapy.
R statistical software, version 3.5.0 (R Foundation), was used for the univariate and multivariate survival analyses with time-dependent covariates. SPSS software, version 26 (IBM), was used for all other statistical analyses.
Results
Patient Population
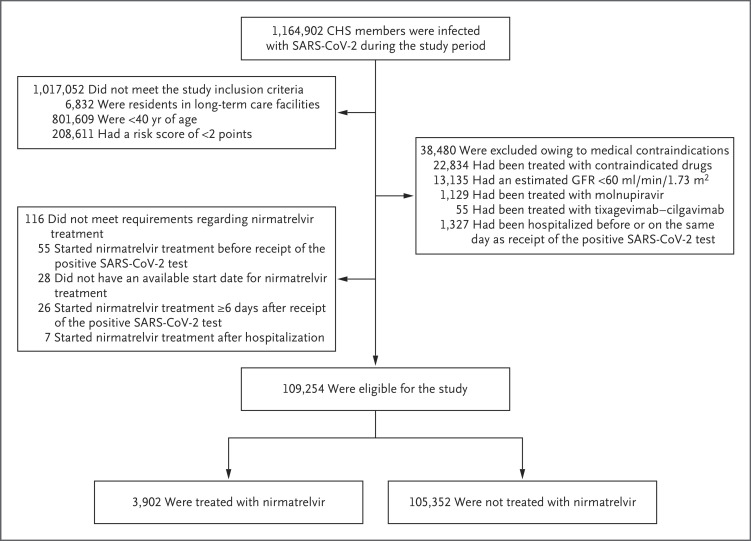
A total of 1,164,902 CHS members were infected with SARS-CoV-2 during the study period (Table S2), 109,254 of whom met the study eligibility criteria. The assessment for eligibility is detailed in Figure 1. The mean age of the study patients was 60 years; 39% of the patients were 65 years of age or older, and 60% were women. The most common coexisting conditions were obesity, hypertension, and diabetes. Overall, 78% of the patients had previous SARS-CoV-2 immunity induced by vaccination, previous SARS-CoV-2 infection, or a hybrid of both (Table 1).
Figure 1. Assessment for Eligibility.
CHS denotes Clalit Health Services, GFR glomerular filtration rate, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Table 1. Characteristics of the Patients at Baseline.*.
| Characteristic | All Patients (N=109,254) |
Patients Treated with Nirmatrelvir (N=3,902) |
Untreated Patients (N=105,352) |
|---|---|---|---|
| Age | |||
| Mean ±SD — yr | 59.9±12.8 | 67.4 ±11.2 | 59.6±12.8 |
| Age group — no. (%) | |||
| 40–64 yr | 66,433 (61) | 1,418 (36) | 65,015 (62) |
| ≥65 yr | 42,821 (39) | 2,484 (64) | 40,337 (38) |
| Female sex — no. (%) | 65,714 (60) | 2,349 (60) | 63,365 (60) |
| Population sector — no. (%) | |||
| General Jewish | 81,886 (75) | 3,381 (87) | 78,505 (75) |
| Ultra-Orthodox Jewish | 6,354 (6) | 176 (5) | 6,178 (6) |
| Arab | 21,014 (19) | 345 (9) | 20,669 (20) |
| Score for socioeconomic status† | |||
| Mean ±SD | 5.5±1.9 | 6.1±1.9 | 5.5±1.9 |
| Median (IQR) | 5.0 (2.0–8.0) | 6.0 (3.0–9.0) | 5.0 (2.0–8.0) |
| Clinical risk factors — no. (%) | |||
| Obesity | 37,766 (35) | 1,626 (42) | 36,140 (34) |
| Hypertension | 37,138 (34) | 1,920 (49) | 35,218 (33) |
| Diabetes | 28,668 (26) | 1,565 (40) | 27,103 (26) |
| History of smoking | 26,647 (24) | 1,010 (26) | 25,637 (24) |
| Immunosuppression | 10,777 (10) | 881 (23) | 9,896 (9) |
| Neurologic disease | 9,257 (8) | 503 (13) | 8,754 (8) |
| Current cancer | 7,588 (7) | 738 (19) | 6,850 (7) |
| Asthma | 6,443 (6) | 368 (9) | 6,075 (6) |
| History of stroke | 5,693 (5) | 363 (9) | 5,330 (5) |
| Chronic hepatic disease | 4,854 (4) | 217 (6) | 4,638 (4) |
| Chronic obstructive pulmonary disease | 3,495 (3) | 290 (7) | 3,205 (3) |
| Chronic heart failure | 3,174 (3) | 211 (5) | 2,963 (3) |
| Chronic kidney failure | 1,471 (1) | 85 (2) | 1,386 (1) |
| Recent hospitalization | 31,815 (29) | 1,748 (45) | 30,067 (29) |
| SARS-CoV-2 immunity status — no. (%) | |||
| No previous immunity | 23,873 (22) | 382 (10) | 23,491 (22) |
| Previous immunity induced by vaccination, infection, or both | 85,381 (78) | 3,520 (90) | 81,861 (78) |
Percentages may not total 100 because of rounding. IQR denotes interquartile range, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Scores for socioeconomic status range from 1 (lowest) to 10 (highest).
Nirmatrelvir Uptake
Among the 109,254 patients in the study cohort, 3902 (4%) received at least one dose of nirmatrelvir during the study period, including 2484 of 42,821 patients (6%) who were 65 years of age or older and 1418 of 66,433 patients (2%) who were 40 to 64 years of age. The association between patient characteristics and nirmatrelvir uptake is summarized in Table S3. Nirmatrelvir uptake was notably higher among patients 65 years of age or older and among patients with current cancer or immunosuppression. Uptake was significantly lower among patients with no previous SARS-CoV-2 immunity and within the Arab minority population. The median time from a positive test to initiation of therapy was 2 days (range, 1 to 5). According to CHS district reports, 3774 patients (97%) completed the 5-day treatment course.
Primary Outcome
Testing of the interaction of the nirmatrelvir treatment status with the other variables revealed a significant interaction with age group (40 to 64 years vs. ≥65 years). Therefore, we report efficacy results separately for these two age groups (Table 2). The decision to divide the cohort at 65 years of age was made on the basis of the results of the subgroup analysis in the EPIC-HR trial.4
Table 2. Association between Confounding Variables and Hospitalization Due to Covid-19, According to Age Group.*.
| Variable | Hazard Ratio for Hospitalization Due to Covid-19 (95% CI) | |
|---|---|---|
| 40–64 yr (N=66,433) |
≥65 yr (N=42,821) |
|
| Demographic and other variables at baseline | ||
| Nirmatrelvir therapy | 0.74 (0.35–1.58) | 0.27 (0.15–0.49) |
| Male sex | 1.41 (1.13–1.75) | 1.65 (1.43–1.91) |
| Age | 1.06 (1.04–1.08) | 1.09 (1.08–1.09) |
| Score for socioeconomic status | 1.01 (0.95–1.07) | 0.89 (0.86–0.93) |
| No previous immunity | 5.79 (4.58–7.32) | 5.82 (4.99–6.78) |
| Clinical risk factors | ||
| Recent hospitalizations | 3.36 (2.66–4.24) | 2.09 (1.80–2.43) |
| Obesity | 1.29 (1.03–1.63) | 1.07 (0.91–1.25) |
| Diabetes | 1.34 (1.04–1.74) | 1.36 (1.18–1.58) |
| Chronic hepatic disease | 1.78 (1.24–2.53) | 1.11 (0.82–1.50) |
| Neurologic disease | 1.82 (1.30–2.53) | 1.58 (1.34–1.87) |
| Chronic heart failure | 2.41 (1.61–3.61) | 1.44 (1.17–1.78) |
| Chronic obstructive pulmonary disease | 2.26 (1.52–3.35) | 1.74 (1.40–2.15) |
| History of stroke | 1.81 (1.24–2.63) | 1.39 (1.16–1.67) |
| Chronic kidney failure | 1.82 (0.96–3.44) | 1.78 (1.33–2.38) |
The association between nirmatrelvir therapy and hospitalization due to coronavirus disease 2019 (Covid-19) was estimated with the use of a multivariate Cox proportional-hazards regression model after adjustment for sociodemographic factors, coexisting illnesses, and SARS-CoV-2 immunity status. Variables that met the testing criteria and were significantly associated with the outcome served as the inputs for the multivariate regression analysis. CI denotes confidence interval.
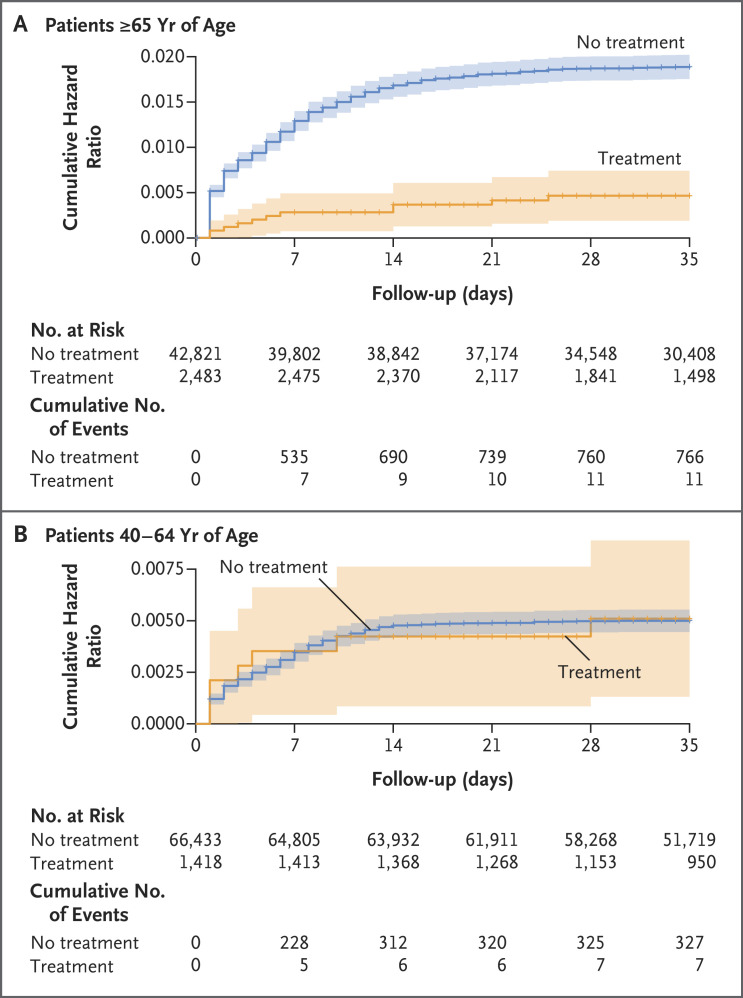
Among the 42,821 patients who were 65 years of age or older, hospitalization due to Covid-19 occurred in 11 treated patients (14.7 cases per 100,000 person-days) and in 766 untreated patients (58.9 cases per 100,000 person-days). The adjusted hazard ratio for hospitalization due to Covid-19 was 0.27 (95% confidence interval [CI], 0.15 to 0.49). Results of the sensitivity analysis, which assessed the effect size of treatment beginning on day 3 of follow-up, were similar to those of the main analysis (Table S4). Among the 66,433 patients who were 40 to 64 years of age, hospitalization due to Covid-19 occurred in 7 treated patients (15.2 cases per 100,000 person-days) and in 327 untreated patients (15.8 cases per 100,000 person-days). The adjusted hazard ratio for hospitalization was 0.74 (95% CI, 0.35 to 1.58). The cumulative hazard-ratio curves are shown in Figure 2. Across both age groups, a lack of previous SARS-CoV-2 immunity and a previous hospitalization were the variables that were most strongly associated with high rates of hospitalization due to Covid-19 (Table 2).
Figure 2. Cumulative Hazard Ratio for Hospitalization Due to Covid-19, According to Age Group and Treatment Status.
For patients who did not receive treatment with nirmatrelvir, time zero corresponds to the time at which each patient received the diagnosis of coronavirus disease 2019 (Covid-19). For patients who received treatment with nirmatrelvir, time zero corresponds to the time at which a patient began the treatment. The shaded areas indicate the 95% confidence intervals.
Subgroup Analysis, According to Immunity Status
Among patients 65 years of age or older who had received nirmatrelvir, the adjusted hazard ratio for hospitalization due to Covid-19 was 0.32 (95% CI, 0.17 to 0.63) among patients who had previous immunity and 0.15 (95% CI, 0.04 to 0.60) among those without previous immunity. Among patients 40 to 64 years of age who had received nirmatrelvir, the adjusted hazard ratio for hospitalization was 1.13 (95% CI, 0.50 to 2.58) among patients who had previous immunity and 0.23 (95% CI, 0.03 to 1.67) among those without previous immunity (Table 3 and Tables S5 and S6).
Table 3. Hazard Ratios for Hospitalization Due to Covid-19, According to Immunity Status and Age Group.
| Variable | All Patients | Patients without Previous Immunity |
Patients with Previous Immunity |
|||
|---|---|---|---|---|---|---|
| 40–64 yr (N=66,433) |
≥65 yr (N=42,821) |
40–64 yr (N=20,555) |
≥65 yr (N=3318) |
40–64 yr (N=45,878) |
≥65 yr (N=39,503) |
|
| Hazard ratio for hospitalization (95% CI) | 0.74 (0.35 to 1.58) | 0.27 (0.15 to 0.49) | 0.23 (0.03 to 1.67) | 0.15 (0.04 to 0.60) | 1.13 (0.50 to 2.58) | 0.32 (0.17 to 0.63) |
Secondary Outcome
Among patients 65 years of age or older, death due to Covid-19 occurred in 2 of 2484 treated patients and in 158 of 40,337 untreated patients (adjusted hazard ratio, 0.21; 95% CI, 0.05 to 0.82). Among patients 40 to 64 years of age, death due to Covid-19 occurred in 1 of 1418 treated patients and in 16 of 65,015 untreated patients (adjusted hazard ratio, 1.32; 95% CI, 0.16 to 10.75) (Table S7).
Discussion
At the beginning of the surge of the omicron variant in January 2022, the Israeli authorities decided to pursue two lines of defense to protect the vulnerable and high-risk populations from severe Covid-19: a second booster dose9 and antiviral therapy. Treatment with nirmatrelvir is currently recommended by the National Institutes of Health (NIH) as the first choice for antiviral therapy for nonhospitalized adults who are at high risk for disease progression, regardless of vaccination status.10 Nevertheless, the NIH stated that data on the clinical efficacy of nirmatrelvir against the omicron variant are lacking,11 despite in vitro studies that showed potent inhibition of this variant by nirmatrelvir.12-15
Our study suggests that during the omicron surge, the rates of hospitalization and death due to Covid-19 were significantly lower among adults 65 years of age or older who had received treatment with nirmatrelvir than among younger adults who had received such treatment, regardless of whether a patient had previous SARS-CoV-2 immunity. An observed difference in the efficacy of nirmatrelvir treatment between patients who were 65 years of age or older and those who were younger than 65 years of age was also shown in the published subgroup analysis of the EPIC-HR trial.4 As expected, the observed risk of hospitalization due to Covid-19 in our study (which was conducted during the omicron-predominant period) was substantially lower than that reported in the EPIC-HR trial during the delta wave.16 However, within this older age group, the incidence of events was higher in the subgroup of patients without previous SARS-CoV-2 immunity (277 of 3318 patients [8%]) than in the subgroup with previous immunity (505 of 39,503 patents [1%]).
Our study has several limitations. As in any retrospective cohort study, various confounders may have caused bias in the observed effectiveness. We attempted to overcome biases in the risk of hospitalization by adjusting for the variables that are known to affect severe Covid-19 outcomes. Nevertheless, we may not have measured, or corrected adequately for, some sources of residual confounding and selection bias, such as differences in early diagnosis and differential access to nirmatrelvir therapy.17 Our study showed that only a minority of patients who were identified as high risk and eligible for nirmatrelvir therapy received the antiviral therapy. We do not know why the other eligible patients did not receive treatment, and there may be some selection mechanism that is not explained by the observed confounders; therefore, this observation remains our primary concern regarding residual bias.
We assume that the patients who were likely to be hospitalized because of severe symptoms were systematically treated at a higher rate. Since these symptoms were not reported consistently, it is likely that the treatment effect was underestimated in this study. Moreover, there is a concern that many of the hospitalizations occurred during the first 2 days of follow-up (Figure 2A), which may have resulted in bias if patients who were unwell enough to be hospitalized very soon after the positive PCR test were more or less likely to receive nirmatrelvir treatment. The sensitivity analysis, which assessed the effect size of nirmatrelvir treatment beginning on day 3 of follow-up, showed a hazard ratio of 0.28 (95% CI, 0.15 to 0.55); this result was similar to that of the main analysis, which suggests that this bias does not explain a large proportion of the effectiveness.
The EPIC-HR trial did not show a substantial effect of the treatment during the first 2 days of follow-up. However, patients who were likely to be hospitalized within 2 days after randomization were excluded.4 As a result, the clinical trial did not provide evidence regarding whether the drug had a more immediate effect. It should be noted that the subjective clinical impression of the treating physicians at CHS has been that the effect occurs quickly after nirmatrelvir administration.
A further limitation of our study is the heterogeneity of degrees of immunity in the subgroup of patients with previous immunity, including differences in the time since the patient’s last vaccine dose. This group included patients with immunity induced by infection, vaccination, or both, and any waning of such immunity over time was not taken into account. Nevertheless, the results seen in the subgroup of patients 65 years of age or older who had no previous immunity are similar to those seen in the EPIC-HR trial.
It should be noted that the evaluation of adverse events and safety data reports was beyond the scope of this study. Future studies will be needed to assess the short- and long-term safety of nirmatrelvir treatment in real-world settings.
During the omicron variant surge, among adults 65 years of age or older, the rates of severe Covid-19 outcomes were significantly lower among those who received nirmatrelvir than among those who did not receive nirmatrelvir. However, no evidence of benefit was found in younger adults.
Supplementary Appendix
Disclosure Forms
This article was published on August 24, 2022, at NEJM.org.
Footnotes
The study did not receive any financial or in-kind support.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Rubin EJ, Baden LR, Morrissey S. Audio interview: understanding the omicron variant of SARS-CoV-2. N Engl J Med 2022;386(8):e27-e27. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. December 22, 2021. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19).
- 3.Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. December 2021. (https://www.fda.gov/media/155050/download).
- 4.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin EJ, Baden LR. The potential of intentional drug development. N Engl J Med 2022;386:1463-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021;385:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087-b5087. [DOI] [PubMed] [Google Scholar]
- 9.Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med 2022;28:1486-1490. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. August 8, 2022. (https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management).
- 11.National Institutes of Health. Ritonavir-boosted nirmatrelvir (Paxlovid). May 13, 2022. (https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/#:~:text=The%20COVID%2D19%20Treatment%20Guidelines,risk%20of%20disease%20progression%3B4).
- 12.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N Engl J Med 2022;386:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med 2022;386:1475-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Wang Y, Lavrijsen M, et al. SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res 2022;32:322-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. Antiviral Res 2022;198:105252-105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaway E, Ledford H. How bad is omicron? What scientists know so far. Nature 2021;600:197-199. [DOI] [PubMed] [Google Scholar]
- 17.Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. June 17, 2022. (https://www.medrxiv.org/content/10.1101/2022.06.14.22276393v2). preprint. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.