Abstract
COVID-19 has had a detrimental effect on the provision of cancer surgery, but its impact beyond the first 6 months of the pandemic remains unclear. We used data on 799 220 cancer surgeries performed in Ontario, Canada, during 2018-2021 and segmented regression to address this knowledge gap. With the arrival of the first COVID-19 wave (March 2020), mean cancer surgical volume decreased by 57%. Surgical volume then rose by 2.5% weekly and reached prepandemic levels in 8 months. The surgical backlog after the first wave was 47 639 cases. At the beginning of the second COVID-19 wave (January 2021), mean cancer surgical volume dropped by 22%. Afterward, surgical volume did not actively recover (2-sided P = .25), resulting in a cumulative backlog of 66 376 cases as of August 2021. These data urge the strengthening of the surgical system to quickly clear the backlog in anticipation of a tsunami of newly diagnosed cancer patients in need of surgery.
Like many time-sensitive and potentially lifesaving procedures, cancer surgeries were dramatically reduced by hospitals worldwide during the first wave of COVID-19 (1,2). Treatment delays are associated with worse quality of life and even lower survival in some cases (2,3). We examined the weekly volume of cancer surgical procedures from January 2018 to August 2021, along with COVID-19–related hospitalizations, to demonstrate how the surgical system has responded to 2 distinct waves of COVID-19 and to assess if a full recovery of surgical volume has occurred (1).
This retrospective population-based cohort study was based in Ontario, Canada, where permanent residents are insured under a universal health-care system (4). Administrative datasets were linked using unique encoded identifiers and analyzed at ICES, a nonprofit research institute legalized by section 45 of Ontario’s Personal Health Information Protection Act to collect and analyze health care and demographic data, without consent, for health system research. The use of the data in this study is approved by ICES’ Privacy and Legal Office and, therefore, does not require review by a research ethics board. Records of cancer surgical procedures and hospitalizations were aggregated by week using hospital-matched procedure codes from the Canadian Institute for Health Information (1).
Three COVID-19 periods were defined relative to Ontario’s 2 declared provincial states of emergency (5): prepandemic (January 7, 2018, to March 14, 2020), the first wave of COVID-19 (March 15, 2020, to January 9, 2021), and the combined second and third wave of COVID-19 (January 10, 2021, to August 28, 2021). Comparisons of patient characteristics were carried out between periods, where a standardized mean difference exceeding 0.1 indicated a statistically significant difference (6). Segmented negative binomial regression models were used to quantify the weekly surgical volume trend (slope) within each period and the change in mean volume (intercept) at the beginning of each COVID-19 wave. General and standard parameterizations were used to provide a fulsome depiction of the surgical trend. Surgical backlog was computed at the end of both waves as the difference between the observed and predicted (had there been no pandemic) volume over all weeks of that wave (7). A cumulative backlog was also calculated as the total missed cases from March 15, 2020, to August 28, 2021. Regression analyses were 2-sided using a P value less than .05 to indicate statistical significance. Analyses were performed on SAS Enterprise Guide 7.15 (SAS Institute, Cary, NC, USA).
We observed 799 220 cancer surgical procedures over the study period (mean [standard deviation] age = 56.6 [17.0] years; n = 492 590 [61.6%] women and n = 306 630 [38.4%] men). In the prepandemic period, Ontario hospitals performed an average of 237 545 cancer surgeries annually or 19 524 procedures monthly. These numbers dropped to 179 789 and 14 777, respectively, during the first COVID-19 wave and recovered slightly back to 207 955 and 17 093 during the second wave. A higher proportion of cancer surgeries was performed urgently (ie, patient was admitted to hospital via the emergency department or arrived at hospital by ambulance) during the first wave than in prepandemic (28 220 [19.0%] vs 74 277 [14.3%]; standardized mean difference = 0.13); otherwise, patient characteristics were identical throughout the 3 periods (Table 1).
Table 1.
Distribution of sociodemographic, clinical, and hospital characteristics for cancer surgical procedures performed during the 3 COVID-19 periods in Ontario, Canada
| Variable | Patients by COVID-19 period, No. (%)a |
Standardized mean difference |
||||
|---|---|---|---|---|---|---|
| Prepandemic | First wave | Second wave | First wave vs prepandemic | Second wave vs prepandemic | Second vs first wave | |
| (n = 519 346) | (n = 148 264) | (n = 131 610) | ||||
| Age, mean (SD), y | 56.39 (16.92) | 56.90 (17.23) | 56.89 (17.23) | 0.03 | 0.03 | 0 |
| Sex | ||||||
| Men | 196 838 (37.9) | 58 444 (39.4) | 51 348 (39.0) | 0.03 | 0.02 | 0.01 |
| Women | 322 508 (62.1) | 89 820 (60.6) | 80 262 (61.0) | 0.03 | 0.02 | 0.01 |
| Rural scoreb | ||||||
| 0-9, less rural | 340 434 (65.6) | 96 649 (65.2) | 85 436 (64.9) | 0.01 | 0.01 | 0.01 |
| 10-30 | 93 990 (18.1) | 26 912 (18.2) | 24 507 (18.6) | 0 | 0.01 | 0.01 |
| 31-50 | 55 720 (10.7) | 16 274 (11.0) | 14 196 (10.8) | 0.01 | 0 | 0.01 |
| 51-70 | 15 700 (3.0) | 4422 (3.0) | 3880 (2.9) | 0 | 0 | 0 |
| ≥71, more rural | 7183 (1.4) | 2165 (1.5) | 1864 (1.4) | 0.01 | 0 | 0 |
| Immigrantsc | 77 172 (14.9) | 21 131 (14.3) | 19 031 (14.5) | 0.02 | 0.01 | 0.01 |
| Material deprivationd | ||||||
| 1, least deprived | 114 056 (22.0) | 33 277 (22.4) | 29 407 (22.3) | 0.01 | 0.01 | 0 |
| 2 | 108 543 (20.9) | 30 863 (20.8) | 27 660 (21.0) | 0 | 0 | 0 |
| 3 | 100 053 (19.3) | 28 394 (19.2) | 25 174 (19.1) | 0 | 0 | 0 |
| 4 | 96 108 (18.5) | 27 287 (18.4) | 24 154 (18.4) | 0 | 0 | 0 |
| 5, most deprived | 95 860 (18.5) | 27 058 (18.2) | 23 906 (18.2) | 0.01 | 0.01 | 0 |
| Region | ||||||
| Toronto | 37 593 (7.2) | 10 778 (7.3) | 9608 (7.3) | 0 | 0 | 0 |
| Central | 153 648 (29.6) | 43 325 (29.2) | 37 143 (28.2) | 0.01 | 0.03 | 0.02 |
| East | 133 431 (25.7) | 37 706 (25.4) | 33 088 (25.1) | 0.01 | 0.01 | 0.01 |
| North | 39 042 (7.5) | 11 342 (7.6) | 10 244 (7.8) | 0 | 0.01 | 0.01 |
| West | 155 632 (30.0) | 45 113 (30.4) | 41 527 (31.6) | 0.01 | 0.03 | 0.02 |
| Elixhauser groupinge | ||||||
| 0 | 73 842 (14.2) | 21 321 (14.4) | 18 576 (14.1) | 0 | 0 | 0.01 |
| 1 | 44 736 (8.6) | 13 882 (9.4) | 11 624 (8.8) | 0.03 | 0.01 | 0.02 |
| 2 | 28 076 (5.4) | 8547 (5.8) | 7448 (5.7) | 0.02 | 0.01 | 0 |
| ≥3 | 37 579 (7.2) | 11 746 (7.9) | 9836 (7.5) | 0.03 | 0.01 | 0.02 |
| No hospitalization | 335 113 (64.5) | 92 768 (62.6) | 84 126 (63.9) | 0.04 | 0.01 | 0.03 |
| Cancer typef | ||||||
| Breast | 38 557 (7.4) | 11 470 (7.7) | 10 607 (8.1) | 0.01 | 0.02 | 0.01 |
| Colorectal | 83 853 (16.1) | 26 241 (17.7) | 22 989 (17.5) | 0.04 | 0.04 | 0.01 |
| Endocrine | 14 053 (2.7) | 4209 (2.8) | 3507 (2.7) | 0.01 | 0 | 0.01 |
| Esophagus | 2093 (0.4) | 595 (0.4) | 516 (0.4) | 0 | 0 | 0 |
| Genitourinary | 37 403 (7.2) | 11 978 (8.1) | 10 228 (7.8) | 0.03 | 0.02 | 0.01 |
| Gynecological | 111 377 (21.4) | 28 111 (19.0) | 25 256 (19.2) | 0.06 | 0.06 | 0.01 |
| Head and neck | 27 504 (5.3) | 6915 (4.7) | 6658 (5.1) | 0.03 | 0.01 | 0.02 |
| Hepatobiliary | 63 284 (12.2) | 19 853 (13.4) | 17 027 (12.9) | 0.04 | 0.02 | 0.01 |
| Lung | 10 299 (2.0) | 3168 (2.1) | 2590 (2.0) | 0.01 | 0 | 0.01 |
| Lymphoma | 941 (0.2) | 298 (0.2) | 246 (0.2) | 0 | 0 | 0 |
| Prostate | 22 684 (4.4) | 6417 (4.3) | 5214 (4.0) | 0 | 0.02 | 0.02 |
| Sarcoma (bone) | 20 029 (3.9) | 6290 (4.2) | 5195 (3.9) | 0.02 | 0 | 0.01 |
| Sarcoma (soft tissue) | 3795 (0.7) | 983 (0.7) | 834 (0.6) | 0.01 | 0.01 | 0 |
| Stomach | 14 363 (2.8) | 3841 (2.6) | 4016 (3.1) | 0.01 | 0.02 | 0.03 |
| Other | 69 111 (13.3) | 17 895 (12.1) | 16 727 (12.7) | 0.04 | 0.02 | 0.02 |
| Nonteaching hospital | 382 747 (73.7) | 107 557 (72.5) | 96 464 (73.3) | 0.03 | 0.01 | 0.02 |
| Urgentg | 74 277 (14.3) | 28 220 (19.0) | 22 885 (17.4) | 0.13 | 0.08 | 0.04 |
We defined 3 COVID-19 periods based on the 2 times Ontario declared state of emergency amid rising infection cases. As such, the prepandemic period was from January 7, 2018, to March 14, 2020; the first wave of the pandemic was from March 15, 2020, to January 9, 2021; and the second wave of the pandemic was from January 10, 2021, to August 28, 2021. Missing data were found for rural score (1.3%) and material deprivation (1.0%) that did not differ by COVID-19 period (standardized mean differences < 0.02).
We used the Rurality Index for Ontario version 2008 (rio2008) to define rurality. This measure is computed using the latest census data and accounts for the size and density of population in a neighborhood, travel time to the nearest basic referral center, and travel time to the nearest advanced referral center.
Individuals who immigrated to Ontario between January 1985 and May 2017 were identified from the Immigration, Refugee and Citizenship Canada Permanent Resident Database (with data from that period).
Material deprivation was computed using the latest census data and obtained from the Ontario Marginalization Index database. This composite measure of socioeconomic status encompasses the proportion of a population that is without a high school diploma, lone-parent families, receiving government transfer payments, unemployed, low income, and living in dwellings in need of major repair.
The Elixhauser comorbidity index was computed using hospital administrative data during a 5-year look-back window from the date of cancer surgery.
Cancer type refers to the cancer surgical site determined from hospital procedural code. “Other” includes melanoma, ophthalmologic, paraneoplastic neurological syndromes, central nervous system, and skin cancers.
A surgical procedure is considered urgent if a patient was admitted to hospital via the emergency department or if the patient arrived at hospital by ambulance.
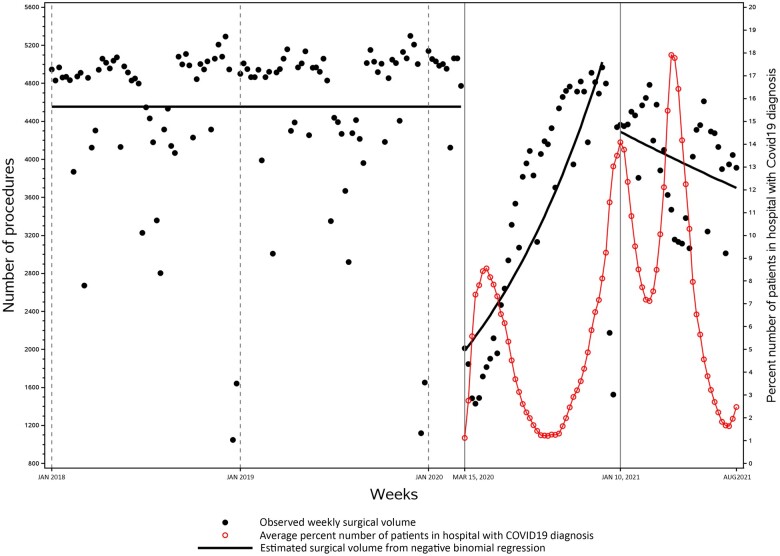
In the first week of the first COVID-19 wave, mean cancer surgical volume declined by 57% compared with the week prior (Supplementary Tables 1 and 2, available online). Cancer surgical volume then increased consistently by 2.5% (rate ratio [RR] = 1.025, 95% confidence interval = 1.02 to 1.03; P < .001) each week and reached prepandemic levels 8 months later. The surgical backlog after the first wave was 47 639 cases in total, or 1108 cases in each week. At the beginning of the second COVID-19 wave, mean cancer surgical volume dropped by 22%. Afterward, surgical volume did not show any weekly growth (RR = 0.995, 95% confidence interval = 0.99 to 1.00; P = .25), resulting in a cumulative backlog of 66 376 cases 6.5 months later or an additional backlog of 18 737 cases after the end of the first wave. This means during the second wave, hospitals performed 568 fewer cancer surgeries per week. The cumulative backlog was equivalent to 28% of the prepandemic annual surgical volume or 3.4-fold of the prepandemic monthly volume. Overall, cancer surgical volume was inversely associated with COVID-19–related hospitalizations (Figure 1).
Figure 1.
Weekly cancer surgical volume and the proportion of hospitalizations related to COVID-19 in Ontario, Canada, during January 7, 2018, to August 28, 2021. COVID-19–related hospitalizations included patients who were admitted to hospital because of a laboratory-confirmed COVID-19 diagnosis as the most responsible diagnosis and those who got infected with COVID-19 during the hospital stay. During the week of November 8-14, 2020 (ie, week 35 since the start of the first wave of COVID-19), cancer surgical volume has returned to prepandemic levels. As of August 28, 2021, there was a surgical backlog of 66 376 missed cases.
At the start of 2 COVID-19 waves, cancer surgeries were ramped down across patient populations to preserve capacity for rising COVID-19–related hospitalizations. Cancer surgical volume was found to decrease by a smaller extent during the first week of the second COVID-19 wave than that of the first wave, but unlike the first wave, surgical volume did not actively recover. This led to a considerable additional backlog even with declining COVID-19–related hospitalizations. On a positive note, we observed the weekly surgical backlog reduce by half during the second wave. Because surgical volume did not rise on a weekly basis over this period, this improved weekly backlog likely reflects a further reduction in the demand for cancer surgery as activities of cancer screening, diagnosis, staging, and symptom assessment continued to be negatively impacted by the pandemic (7-9). As such, our findings suggest that to tackle an anticipated tsunami of new cancer (and noncancer) patients requiring surgery, there is an urgent need to direct hospital resources to strengthen the surgical system. Data from this analysis can be used to inform microsimulation models to quantify how much extra capacity is required to minimize the long-term repercussions especially to prevent excessive cancer mortality (10).
This analysis has limitations. Although a rise in COVID-19–related hospitalizations and a drop in cancer surgical volume around April 2021 was noted, we did not examine a distinct third COVID-19 wave. This is because we only captured the first half time period of this wave (11) and, therefore, had only 19 weekly data points to estimate a new segment in the regression model. Future research with more recent data needs to incorporate more pandemic-related milestones including the strategic reopening of the province and the discovery of the Omicron subvariants in the analysis. We also did not examine cancer staging because these data take at least 3 years in our cancer registry to accrue. With staging data, we would be able to quantify how much the observed shift in surgery utilization can be attributed to the potential stage migration during the pandemic (12,13).
Funding
This work was supported by a Sunnybrook Research Institute and Sunnybrook Foundation COVID-19 Response Grant and a Canadian Institutes of Health Research Operating Grant (#179892).
Notes
Role of the funder: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: JH reported receiving personal fees from Ipsen, Advanced Accelerators Applications, Bristol-Myers, and Medtronic. AE reported receiving grants from Merck and personal fees from Bristol-Myers Squibb. No other disclosures were reported.
Author contributions: RF: conceptualization, methodology, writing—original draft, writing—reviewing and editing. PK: project administration, writing—original draft, writing—reviewing and editing. QL: methodology, data curation, formal analysis, writing—reviewing and editing. JH: conceptualization, methodology, writing—reviewing and editing. DG: conceptualization, methodology, writing—reviewing and editing. RS: conceptualization, methodology, writing—reviewing and editing, supervision. AE: conceptualization, methodology, writing—reviewing and editing, supervision, funding acquisition.
Acknowledgements: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. Parts of this material are based on data and/or information compiled and provided by Cancer Care Ontario; the Canadian Institute for Health Information; and Immigration, Refugees and Citizenship Canada current to May 31, 2017. Parts of this report are based on Ontario Registrar General information on deaths, the original source of which is ServiceOntario. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
We thank the investigators of the Pandemic—Ontario Collaborative in Cancer Research (POCCR) group for contributing to this work in the form of study design, interpretation of the data, review and approval of the manuscript, and decision to submit for publication: Natalie Coburn, MD, MPH (Department of Surgery, University of Toronto); Jonathan Irish, MD, MSc (Department of Otolaryngology–Head and Neck Surgery, University of Toronto); Timothy P. Hanna, MD, PhD (Department of Oncology, Queen’s University); Anna Dare, MBChB, PhD (Department of Surgery, University of Toronto); Kelvin KW Chan, MD, PhD (Department of Medical Oncology, University of Toronto); Simron Singh, MD, MPH (Department of Medical Oncology, University of Toronto); Ambica Parmar, MD, MSc (Department of Medical Oncology, University of Toronto); Craig C. Earle, MD, MSc (Department of Medical Oncology, University of Toronto); Lauren Lapointe-Shaw, MD, PhD (Department of Medicine, University of Toronto); Monika K. Krzyzanowska, MD, MPH (Department of Medical Oncology, University of Toronto); Alexander V. Louie, MD, PhD (Department of Radiation Oncology, University of Toronto); Nicole J. Look Hong, MD, MSc (Department of Surgery, University of Toronto); Ian Witterick, MD, MSc (Department of Otolaryngology–Head and Neck Surgery, University of Toronto); Alyson Mahar, PhD (School of Nursing, Queen’s University); Tony Finelli, MD, MSc (Division of Urology, Department of Surgery, University of Toronto); David Urbach, MD, MSc (Department of Surgery, University of Toronto); Danny Enepekides, MD, MPH (Department of Otolaryngology–Head and Neck Surgery, University of Toronto).
Disclaimers: Part of this cohort and data has been previously reported in: Eskander A, Li Q, Hallet J, Coburn N, Hanna TP, Irish J, Sutradhar R. Access to cancer surgery in a universal health care system during the COVID-19 pandemic. JAMA Network Open. 2021; 4(3):e211104. 10.1001/jamanetworkopen.2021.1104.
Supplementary Material
Contributor Information
Rui Fu, ICES, Toronto, ON, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada; Department of Otolaryngology–Head and Neck Surgery, Michael Garron Hospital and Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Pabiththa Kamalraj, Department of Otolaryngology–Head and Neck Surgery, Michael Garron Hospital and Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Qing Li, ICES, Toronto, ON, Canada.
Julie Hallet, ICES, Toronto, ON, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada; Odette Cancer Centre–Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Department of Surgery, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
David Gomez, ICES, Toronto, ON, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada; Department of Surgery, Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Division of General Surgery, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada.
Rinku Sutradhar, ICES, Toronto, ON, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada.
Antoine Eskander, ICES, Toronto, ON, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada; Department of Otolaryngology–Head and Neck Surgery, Michael Garron Hospital and Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Data Availability
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
- 1. Eskander A, Li Q, Hallet J, et al. Access to cancer surgery in a universal health care system during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e211104.doi: 10.1001/jamanetworkopen.2021.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22(11):1507-1517. doi: 10.1016/S1470-2045(21)00493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087.doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ontario Health Insurance Plan (OHIP). The schedule of benefits: physician services under the Health Insurance Act (January 24, 2022. [Effective November 1, 2021]). https://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Published January 24, 2022. Accessed March 15, 2022.
- 5. Office of the Premier. Ontario declares second provincial emergency to address COVID-19 crisis and save lives. Ontario Newsroom. January 12, 2021. https://news.ontario.ca/en/release/59922/ontario-declares-second-provincial-emergency-to-address-covid-19-crisis-and-save-lives. Accessed February 10, 2022.
- 6. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eskander A, Li Q, Yu J, et al. Incident cancer detection during the COVID-19 pandemic. J Natl Comprehens Cancer Netw. 2022;20(3):276-284. doi: 10.6004/jnccn.2021.7114. [DOI] [PubMed] [Google Scholar]
- 8. Fu R, Sutradhar R, Li Q, et al. ; for the Pandemic–Ontario Collaborative in Cancer Research (POCCR). Imaging and physician visits at cancer diagnosis: COVID-19 pandemic impact on cancer care. J Clin Oncol. 2022;40(suppl 16):1522-1522. doi:10.1200/J Clin Oncol.2022.40.16_suppl.1522.35077203 [Google Scholar]
- 9. Walker MJ, Meggetto O, Gao J, et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: a provincial, population-based study. Prev Med. 2021;151:106586.doi: 10.1016/j.ypmed.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malagón T, Yong JHE, Tope P, Miller WJ, Franco EL; for the McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi: 10.1002/ijc.33884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jüni P, Costa B, Maltsev A, et al. Ontario dashboard: science briefs of the Ontario COVID-19 Science Advisory Table. 10.47326/ocsat.dashboard.2021.1.0. Published 2021. Accessed July 10, 2022. [DOI]
- 12. Guven DC, Sahin TK, Yildirim HC, et al. Newly diagnosed cancer and the COVID-19 pandemic: tumour stage migration and higher early mortality [published online ahead of print October 28, 2021]. BMJ Support Palliat Care. 2021; doi:10.1136/bmjspcare-2021-003301. [DOI] [PubMed] [Google Scholar]
- 13. Liyanage A, Gokul K, Babu B, Ainsworth P.. Stage migration of colorectal cancer during COVID-19 pandemic. Br J Surg. 2020;107(11):E477. doi: 10.1002/bjs.11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.