Abstract
Simple Summary
Wild carnivores can harbor a dangerous tapeworm Echinococcus sp. that causes an important food-borne disease called echinococcosis. This study uses molecular methods to assess the prevalence of the tapeworm Echinococcus multilocularis, E. canadensis, and E. granulosus sensu stricto in the stool of red foxes, wolves, golden jackals, martens, lynxes, badgers, and otter. Red foxes (29.1%) and golden jackals (18%) were positive for E. multilocularis (EM), while all other animals were negative for all Echinococcus species tested in this study. Statistical analysis showed that the prevalence of EM is associated only with the region where the sample originated and not by species, age, or sex of the animal. Central and south regions of Slovenia have a higher EM prevalence and risk of infection. Due to habitat expansion and an increasing population, golden jackal may soon become an important source for human infection with EM.
Abstract
Wild carnivores are definitive hosts and potential reservoirs for the tapeworm Echinococcus sp. which can cause cystic and alveolar echinococcosis. Both are considered neglected and important food-borne pandemics. This study is the first to molecularly test Slovenian wild carnivores for Echinococcus species that can cause disease in humans. Fecal samples from 210 red foxes (Vulpes vulpes), 42 wolves (Canis lupus), 39 golden jackals (Canis aureus), 18 martens (Marten sp.), 2 Eurasian lynx (Lynx lynx), 2 European badger (Meles meles), and 1 Eurasian otter (Lutra lutra) were examined for Echinococcus granulosus sensu lato (EGsl: E. granulosus sensu stricto, E. canadensis) and E. multilocularis (EM) using real-time PCR. Red foxes (29.1%) and golden jackals (18%) were positive for EM. All animals examined were negative for EGsl. Univariate analysis showed no significant differences in EM prevalence with respect to animal species (red fox vs., golden jackal) (p = 0.22), age (p = 0.12), and sex (p = 0.18). Prevalence of EM was associated with the region (p < 0.001), with regions in central and southern Slovenia having higher EM prevalence and risk of infection. Due to the increase in population and expansion of habitat, the golden jackal may soon become as important definitive host for EM as the red fox.
Keywords: Echinococcus multilocularis, Echinococcus granulosus sensu stricto, Echinococcus canadensis, real time PCR, red fox, golden jackal, wildlife
1. Introduction
Described already by the ancient Greek physicians [1,2], Echinococcus sp. is a life-threatening zoonotic parasite that is still relevant today. The small tapeworm has two obligate mammalian hosts, and its life cycle depends on predator–prey association [2]. The definitive host is always a carnivore, in which the adult tapeworm develops in the small intestine. When eggs are shed by the definitive host and ingested by the herbivorous or omnivorous intermediate host, the metacestode develops, usually in the viscera (liver, lungs) of the mammal [3]. Due to low host specificity, humans are accidental hosts for the larvae of Echinococcus granulosus sensu latu complex (EGsl) and Echinococcus multilocularis (EM), where it can cause cystic or alveolar echinococcosis, respectively [2,3]. Several genotypes have been described in the EGsl complex, namely E. granulosus sensu stricto (EGss) genotype G1 and G3, E. equinus (genotype G4), E. ortleppi (genotype G5), E. canadensis cluster (EC) (genotype G6-G8, G10), and E. felidis [2,4,5]. Cystic echinococcosis is predominantly caused by EGss, but infections with EC (G6 and G7) are also common [4,6]. Both cystic echinococcosis (CE) and alveolar echinococcosis (AE) are among the top four foodborne parasitosis in Europe [7,8]. Echinococcus granulosus s.l. has a more domestic life cycle (dog/domestic ungulate), but wild canids such as wolf (Canis lupus), golden jackal (Canis aureus), and red fox (Vulpes vulpes) also act as final hosts, that may pose a threat to humans or serve as a wildlife reservoir [9,10]. The sylvatic cycle (fox/rodent) is, however, more common for EM, where the main reservoir for the parasite is the red fox population. Other wild (wolf, golden jackal) and domestic (dog, cat) canids can be infected with EM, bringing the parasite closer to humans [9,10,11,12,13,14,15]. Unlike EGsl, which has a more global distribution, EM is mainly found in the northern hemisphere [8]. Several reports from European countries show a high prevalence of up to 62% in North-Eastern Europe [16] in the red fox population and almost 50% in Sweden and central Europe (France, Switzerland) [17]. Because EM prevalence can vary greatly from region to region within the same country, data must be carefully assessed to avoid its biased interpretation [13,16]. In Slovenia, a study of EM from the 2002–2005 red fox population revealed a relatively low prevalence of 2.6% [18]. Echinococcus granulosus s.l. has only been detected in intermediate hosts (pig, cattle) [19], and although its presence in the definitive hosts has not yet been studied, genotyping of human CE cases showed that EGss (G1-G3) and EC (G7) are present in the country [4].
The aim of this study is to evaluate the prevalence of EGsl and to reassess the prevalence of EM among wild carnivores in Slovenia using molecular methods to assess the regional distribution and risk of animal and human infection.
2. Materials and Methods
2.1. Samples
Fecal samples from 210 red foxes (Vulpes vulpes), 42 wolves (Canis lupus), and 39 golden jackals (Canis aureus) were collected at the Institute of Pathology, Wild Animals, Fish and Bees (Veterinary faculty, University of Ljubljana, Slovenia) along with 18 samples from martens (Marten sp.), two samples each from Eurasian lynx (Lynx lynx) and European badger (Meles meles), and one sample from Eurasian otter (Lutra lutra). The carcasses were collected as part of the regular annual hunting bag throughout the Slovenian territory. Samples from red foxes were collected between 2019 and 2022, while samples from wolves, golden jackals, and other animals were collected over a ten-year period. All samples were stored at –80 °C for at least one month prior to processing and DNA isolation to avoid contamination with viable Echinococcus eggs.
All samples were collected postmortem, so the ethics committee/welfare authority approval was not required.
2.2. Methods
2.2.1. Age Determination
The age of older animals (one year and older) was determined by counting the increment layers of the secondary dental cementum of a cut lower canine root and, in young animals, by the size of pulp cavity according to Roulichova and Andera [20]. The age of wolves was determined by counting the cementum layers on cross-sections of the first premolar in the Matson laboratory (Missoula, MT, USA).
2.2.2. Molecular Methods
Fecal DNA isolation was performed using a protocol from the SmartHelix™ First DNAid kit (IFB, Ljubljana, Slovenia) as previously described [21]. Three different published real time polymerase chain reactions (separately fPCR) were used for the detection of EM (G5), EGss (G1-G3), and EC (G6/G7). Echinococcus multilocularis was detected using a protocol by Knapp et al. [22] with a detection limit of 5 × 10−5 ng/µL, corresponding to one EM egg. For EGss, a protocol by Maksimov et al. [23] was used, while EC was screened using a protocol published by Grech-Angelini et al. [24]. All qPCRs were performed in a 96-well plate format using ABI 7500 Fast (Applied Biosystems®, Waltham, MA, USA) with the same thermal cycling conditions consisting of a preheating step at 50 °C for 2 min, followed by 95 °C for 10 min and 45 cycles of denaturation at 95 °C for 15 s with annealing and extension at 60 °C for 1 min. When optimizing the protocols, the optimal total DNA/PCR mix volume was set at 2/20 μL. Fast Start Universal Probe Master (Rox; Roche®, Basel, Swizerland) was used for the EM and EG protocols, whereas Maxima Probe qPCR Master Mix (Thermo Scientific®, Waltham, MA, USA) was used for the EC protocol. The EC protocol showed marked inhibition when Fast Start Universal Probe Master (Rox; Roche®) or TaqMan Universal Master Mix (Applied Biosystems®) were used to detect EC DNA in clinical samples, whereas no inhibition was detected when Maxima Probe qPCR Master Mix (Thermo Scientific®) was used. All samples were tested twice, and DNA from negative samples was diluted (1:10) and retested to rule out possible inhibition. Inhibition of the PCR is common in complex clinical samples, such as feces, where there is excess DNA that can interfere/inhibit with the amplification of the targeted DNA [25]. No additional positive samples were found when DNA was retested and diluted. The limit of detection for EM was 4.4 × 10−5 ng/µL, confirming the detection limit established by Knapp et al [22]. The detection limit was 2.44 × 10−2 ng/µL for EGss and 3.2 × 10−5 ng/µL for EC. Positive reference samples for EM, Egss and EC were provided by the European Union Reference Laboratory for Parasites (Istituto Superiore di Sanità, Rome, Italy) (Table 1).
Table 1.
Description of the different primers and probes used in three separate qPCR to detect E. multilocularis (EM), E. granulosus s.s. (Egss) and E. canadensis (EC).
| Assay Name (Gene Targeted) | Primer/ Probe | Oligonucleotide Sequences (5′-3′) | Product Size | Reference |
|---|---|---|---|---|
| EM (rrnL) |
Forward | CTGTGATCTTGGTGTAGTAGTTGAGATTT | (bp) 84 |
[22] |
| Reverse | GGCTTACGCCGGTCTTAACTC | |||
| Probe | FAM -TGGTCTGTTCGACCTTTTTAGCCTCCAT – TAMRA | |||
| Egss (cox1) |
Forward | AGGGGCTGGTGTTGGTTGGA | 80 | [23] |
| Reverse | TGAAACACCAGCCAAATGCAGAGA | |||
| Probe | FAM – TCCGCCGTTGTCCTCGTCGT – BHQ1 | |||
| EC (nad5) |
Forward | TCTTTCTGATAGACGAGGTTAGG |
109 |
[24] |
| Reverse | TCCATAAAGCCAAAAATTGTAC | |||
| Probe | Cy5 – CGGTGGTTTGTAGTGTGAGTTTGGTG – BHQ2 |
2.2.3. Statistical Analysis
Data were summarized as frequencies (%). Univariate associations between EM prevalence (positive vs. negative) and sex (male, female), age (3 categories: 0 years, 1 year, ≥2 years), and region (11 regions) (Table 2) were tested using chi-square test with Yates continuity correction. The multivariate analysis was performed using a binary logistic regression. Random intercept by region was included in the model to account for potential effect of the region (due to the large number of regions, the region could not be considered as a fixed effect). Results are presented as conditional odds ratios (Ors) with corresponding 95% confidence intervals (Cis). Effects were considered significant when the p-value was lower than 0.05. R language for statistical computing (R version 3.6.1) was used for the analyses [26]. R package lme4 was used to fit the model using 10 points per axis for evaluating the Gauss–Hermite approximation to the log-likelihood.
Table 2.
Prevalence of E. multilocularis in red fox and golden jackal populations by species, sex, age, and region.
| . | Tested Animals | EM Positive (%) | p * | |||
|---|---|---|---|---|---|---|
| Species | 0.22 | |||||
| Red fox | 210 | 61 (29.1) | ||||
| Golden jackal | 39 | 7 (18) | ||||
| Red fox | Golden jackal | Red fox | Golden jackal | Red fox + Golden jackal | ||
| Sex | 0.18 | |||||
| male | 133 | 28 | 43 (32.3) | 6 (21.4) | 49/161 (30.4) | |
| female | 77 | 11 | 18 (23.4) | 1 (9.1) | 19/88 (21.6) | |
| Age (years) | 0.12 | |||||
| juvenile (0) | 57 | 6 | 19 (33.3) | 0 | 19/63 (30.2) | |
| young adult (1) | 108 | 30 | 24 (22.2) | 7 (23.3) | 31/138 (22.5) | |
| adult (≥2) | 45 | 3 | 18 (40) | 0 | 18/48 (37.5) | |
| Region | <0.001 | |||||
| R1 obalno kraska | 2 | 8 | 0 | 1 (12.5) | 1/10 (10) | |
| R2 goriska | 8 | 2 | 0 | 0 | 0/10 (0) | |
| R3 primorsko notranjska | 12 | 3 | 6 (50) | 0 | 6/15 (40) | |
| R4 osrednjeslovenska | 51 | 13 | 24 (47.1) | 3 (23.1) | 27/64 (42.2) | |
| R5 gorenjska | 9 | 2 | 1 (11.1) | 1 (50) | 2/11 (18.2) | |
| R6 jugovzhodna slovenija | 66 | / | 25 (37.9) | / | 25/66 (37.9) | |
| R7 posavska | 11 | 9 | 1 (9.1) | 2 (22.2) | 3/20 (15) | |
| R8 zasavska | / | / | / | / | / | |
| R9 savinjska | 30 | 2 | 1 (3.3) | / | 1/32 (3.1) | |
| R10 podravska | 9 | / | 1 (11.1) | / | 1/9 (11.1) | |
| R11 pomurska | 10 | / | 0 | / | 0/10 (0) | |
| R12 koroska | 2 | / | 2 (100) | / | 2/2 (100) | |
Data are frequencies (%), * p-value from a chi-squared test with continuity correction.
3. Results
Echinococcus multilocularis DNA was found in the feces of 61/210 (29.1%; CI 0.23–0.36) red foxes and 7/39 (18%; CI 0.07–0.34) golden jackals (Table 2). Wolves (n = 42), martens (n = 18), Eurasian lynx (n = 2), European badger (n = 2), and Eurasian otter (n = 1) were negative for the presence of EM. All samples were negative for EGss genotype G1-G3 and EC genotype G6 and G7.
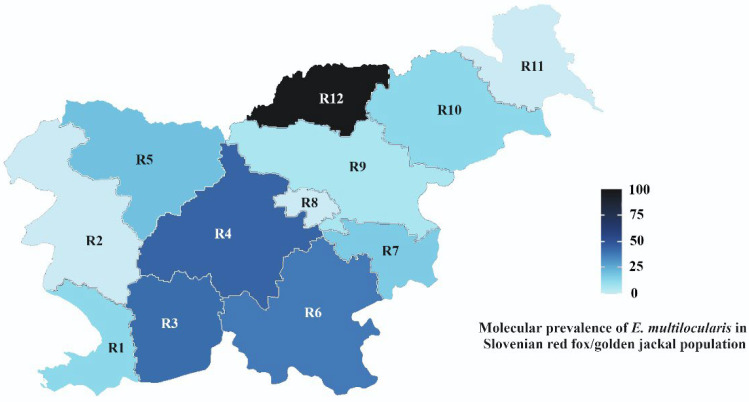
Univariate analysis revealed no statistically significant association between the prevalence of EM and animal species (red fox and golden jackal) (p = 0.22), sex (p = 0.18), and age groups (p = 0.12) (Table 2). However, sampling location was highly significant (p < 0.001) (Table 2). Regions R3 (primorsko notranjska), R4 (osrednjeslovenska), and R6 (jugovzhodna Slovenia) had a prevalence of 37.9% to 42.2% in the red fox/golden jackal population (Table 2, Figure 1). Region R12 (koroska) had an EM prevalence of 100%, but only two samples were collected and tested from this region.
Figure 1.
Molecular prevalence of E. multilocularis in the Slovenian red fox/golden jackal population (%). Regions (R): R1—obalno kraska, R2—goriska, R3—primorsko notranjska, R4—osrednjeslovenska, R5—gorenjska, R6— jugovzhodna Slovenija, R7—posavska, R8—zasavska, R9—savinjska, R10—podravska, R11—pomurska, R12—koroska.
In the multivariate analysis, when adjusted for region, no statistically significant association for an EM positive outcome was observed; species: OR 0.57 (95% CI: 0.2–1.57), sex: OR 1.77 (95% CI: 0.91–3.44), and age: 1 year vs. 0 years, OR 0.91 (95% CI: 0.42–1.92); ≥2 years vs. 1 year, OR 1.75 (95% CI: 0.79–3.87) (Table 3).
Table 3.
Risk factors for prevalence of E. multilocularis in red fox and golden jackal. Results are conditional odds ratio (OR), 95% confidence intervals (CI), and p-values (model area under the curve 0.77).
| Red Fox/ Golden Jackal. | OR | 95% CI | p-Values |
|---|---|---|---|
| Species (red fox vs. golden jackal) | 0.57 | 0.2–1.57 | 0.28 |
| Sex (male vs. female) | 1.77 | 0.91–3.44 | 0.1 |
| Age (y) | 0.37 | ||
| 1 vs. 0 | 0.91 | 0.42–1.92 | 0.8 |
| ≥2 vs. 1 | 1.75 | 0.79–3.87 | 0.17 |
4. Discussion
Wild carnivores are definitive hosts and potential reservoirs for the tapeworm Echinococcus sp. which can cause severe disease in animals and humans [27,28]. Cystic and alveolar echinococcosis, caused by EGsl and EM, respectively, are recognized as neglected and important food-borne pandemics [29,30]. This study is the first to address the need for molecular testing of Slovenian wild carnivores for the presence of European Echinococcus species capable of causing disease in humans.
The EGss and EC are part of the EGsl complex and have been recognized as the cause of disease in several patients in Slovenia [4]. Sporadic cases of cystic echinococcosis were reported in intermediate hosts, such as pigs and cattle [19], but no surveys were conducted in wild or domestic animals in Slovenia. Echinococcus granulosus s.s. and EC (G6/G7) have adapted to a domestic life cycle over the years. Their life cycle in the wildlife (wild sheep, wild goat, cervids, wild boar/wolves, jackals, foxes) is considered more ancestral [28,31,32]. However, due to the increase in wild prey-predator populations susceptible to the parasite, sylvatic transmission is predicted to regain at least some of its former role in Europe [28,32]. Echinococcus granulosus (genotype 1) was found in a jackal and a wolf from Bulgaria [28,33] and the occurrence of EGsl in Iberian wolves was 1.5% in Portugal [34] and 5.6–15% in the Italian Northern Apennine population of wolves [35,36]. Red foxes in Great Britain and Corsica (France) served as a definite host for EGsl when access to sheep or pigs’ carcasses was available [28]. However, our study showed zero prevalence of EGss and EC in wolves, golden jackals, red foxes, martens, lynxes, badgers, and otter, suggesting that the EGsl parasite is still mainly maintained in the domestic life cycle in Slovenia. Further studies are needed to assess the prevalence of the parasite in domestic definite and intermediate hosts. In Slovenia, sustainable livestock production in mountainous/hilly perennial grasslands with shallow soil of poor quality (Dinaric Karst area) is mainly limited to sheep and goat farming, which is also the main Slovenian habitat for wolves [37]. Small ruminants are usually pastured near forest edges, where wolves reside, further increasing the risk of attacks on livestock. The wolf density in Slovenia of 1 wolf/100 km2 is considered low, but as the population increases, the number of attacks on sheep also increases [37]. Domestic livestock represents 10% of the wolves’ diet [38], which could serve as an entry point for a possible reinstation of EGsl from the domestic to a semi-domestic and sylvatic life cycle. This could reinforce the presence of EGsl on Slovenian territory as has done in Italy [35].
While this study is the first to assess the occurrence of EGsl in animals from Slovenia, EM was previously reported in the 2002–2005 in the red fox population with a prevalence of 2.6% [18]. In this study, a prevalence of 29.1% was found in Slovenian red foxes sampled between 2019 and 2022, which is similar to the reported pooled prevalence in other countries with high occurrence of EM in Central and North-Eastern Europe [16]. Based on the results of this study, Slovenia has moved from a low prevalence group [16] to a high prevalence group in less than two decades. The reasons for the high prevalence of EM in red foxes; could be due to a successful rabies vaccination campaign, the subsequent increase in their population [39], and the introduction of a new predator, the golden jackal. They have been sporadically sighted in Slovenia since 1952, but permanent territorial jackal families were first reported in Central Slovenia (Region 4 in this study) in 2008 [40]. Since then, they have spread throughout the country and into Central and Eastern Europe [41]. Golden jackals are susceptible to Echinococcus species and may serve as the second definitive hosts, contributing to an increase in the overall prevalence of EM [10,16,42,43]. A report from Serbia suggests a prevalence of EM in golden jackals of 14.3% [44], which is only slightly lower than the 18% prevalence found in the Slovenian jackal population from this study. In our study, the prevalence of EM did not differ between animal species (red fox vs. golden jackal), which is consistent with previous published studies from Hungary and Serbia [28,44,45]. This suggests that red fox and golden jackal have similar feeding behavior, with the main prey being small mammals (rodents), while wolves in Slovenia feed mainly on cervids, wild boar, and small domestic ruminants [28,37,38,44]. The pooled prevalence in the European wolf population is 1.4% [16], so it is not surprising, that no wolf in this study tested positive for EM. All other animals tested (lynx, otter, martens, badgers) from this study were negative for EM, which was expected based on other published studies [16,28]. Overall, only one lynx from Turkey [46], stone and pine martens from the European part of Russia [28] were found to be positive for EM. However, more samples should be tested for EM to properly assess the prevalence of EM in these species. The dramatic increase in EM prevalence observed in red foxes and jackals in Slovenia, combined with the zoonotic potential of the parasite, warrants a more vigilant approach even in species that are not considered primary hosts for the tapeworm.
In the red fox and golden jackal populations from our study, no association was found between EM prevalence and age, and sex. This is consistent with previously published studies [47,48,49]. However, the prevalence of EM was associated with region where the sampled animals were collected. Thus, EM prevalence in Slovenia ranged from 0 (R2 – goriska, R11 – pomurska) to 42.19% (R4 – osrednjeslovenska) and 100% in region R12 (koroska). However, only two foxes were sampled in region R12, which cannot represent a true red fox population EM prevalence in this region. As shown previously, studies that focus on limited areas within a country and are not accompanied by other studies from different regions, may not show the true EM prevalence at the national level [16,28]. Accordingly, 11 of the 12 regions in Slovenia were included in this study. The only region that did not have a sample was region R8 (Zasavska), which is also the smallest region in Slovenia [50]. Region R4 (osrednjeslovenska) had a high EM prevalence of 42.19% and is also the region with the highest population density of 228 person/km2 in Slovenia [51]. Several studies have found a higher prevalence of EM positive foxes in an urban versus a rural environment and referred to it as EM urbanization [39,52]. On the national level this study shows a more complete assessment of the EM prevalence in definitive sylvatic hosts and a distinct prevalence in central and South regions of Slovenia.
5. Conclusions
In conclusion, this is the first study in which molecular methods were used to assess EGsl and EM in Slovenian wildlife. Although no animal was positive for EGsl, the prevalence of EM in the red fox population increased dramatically since 2002–2005 [18]. This study is also the first to report EM positive golden jackals in Slovenia. Their EM prevalence and habitat expansion suggest that they may soon be considered as important definitive host as the red fox. Both species are well acclimated to urban environments and come close to human residencies [52]. It is inevitable that humans and their pets (dogs, cats) will come in contact with EM in a contaminated environment or through an infected intermediate host. Based on the results of this study, regions in central and southern Slovenia should be considered at higher risk for human and pet exposure. Further studies are needed to determine the risk factors associated with the high prevalence of EM and the prevalence of Echinococcus sp. in intermediate hosts, and to evaluate the domestic cycles of EGsl and EM.
Acknowledgments
The authors thank all hunters for their participation in the study by collecting the samples. We thank Darja Kušar, Alenka Magdalena Usenik, and Maja Kavalič for their help with processing of the samples and qPCR protocol optimization. We would like to thank Aleksandra Vergles Rataj for her guidance. For providing us with positive controls and their overall help, we would like to thank Adriano Casulli and Azzurra Santoro.
Author Contributions
Conceptualization: G.V., D.Ž.V. and P.B.; methodology: P.B. and R.B.; software: P.B.; validation: P.B. and R.B.; formal analysis: P.B. and R.B.; investigation: G.V., D.Ž.V. and P.B.; resources: G.V. and D.Ž.V.; data curation: P.B. and D.Ž.V.; writing—original draft preparation: P.B.; writing—review and editing: P.B., D.Ž.V., G.V. and R.B.; visualization: G.V. and D.Ž.V.; supervision: G.V.; project administration: P.B. and D.Ž.V.; funding acquisition: G.V. and P.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Slovenian Research Agency (research core funding No. P4-0092).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beaver P.C., Jung R.C., Cupp E.W. Clinical Parasitology. 9th ed. Lea & Febiger; Philadelphia, PA, USA: 1984. [Google Scholar]
- 2.Nakao M., Lavikainen A., Yanagida T., Ito A. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae) Int. J. Parasitol. 2013;43:1017–1029. doi: 10.1016/j.ijpara.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Thompson R.C.A. Biology and systematics of Echinococcus. Adv. Parasitol. 2017;95:65–109. doi: 10.1016/bs.apar.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Šoba B., Gašperšič Š., Keše D., Kotar T. Molecular characterization of Echinococcus granulosus sensu lato from humans in Slovenia. Pathogens. 2020;9:562. doi: 10.3390/pathogens9070562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuitton D.A., McManus D.P., Rogan M.T., Romig T., Gottstein B., Naidich A., Tuxun T., Hao W., Menezes da Silva A., World Association of Echinococcosis International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41. doi: 10.1051/parasite/2020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvares Rojas C.A., Romig T., Lightowlers M.W. Echinococcus granulosus sensu lato genotypes infecting humans—Review of current knowledge. Int. J. Parasitol. 2014;44:9–18. doi: 10.1016/j.ijpara.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Bouwknegt M., Devleesschauwer B., Graham H., Robertson L.J., van der Giessen J.W. The Euro-Fbp Workshop Participants. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance. 2018;23:17-00161. doi: 10.2807/1560-7917.ES.2018.23.9.17-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolsey I.D., Miller A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res. Vet. Sci. 2021;135:517–522. doi: 10.1016/j.rvsc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Craig P., Mastin A., van Kesteren F., Boufana B. Echinococcus granulosus: Epidemiology and state-of-the-art of diagnostics in animals. Vet. Parasitol. 2015;213:132–148. doi: 10.1016/j.vetpar.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Balog T., Nagy G., Halász T., Csányi E., Zomborsszky Z., Csivincsik Á. The occurrence of Echinococcus spp. in golden jackal (Canis aureus) in southwestern Hungary: Should we need to rethink its expansion? Parasitol. Int. 2021;80:102214. doi: 10.1016/j.parint.2020.102214. [DOI] [PubMed] [Google Scholar]
- 11.Vuitton D.A., Zhou H., Bresson-Hadni S., Wang Q., Piarroux M., Raoul F., Giraudoux P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. 2003;127:S87–S107. doi: 10.1017/S0031182003004153. [DOI] [PubMed] [Google Scholar]
- 12.Kapel C.M., Torgerson P.R., Thompson R.C., Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Otero-Abad B., Torgerson P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl. Trop. Dis. 2013;7:e2249. doi: 10.1371/journal.pntd.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sindičić M., Bujanić M., Štimac I., Martinković F., Tuškan N., Špehar M., Konjević D. First identification of Echinococcus multilocularis in golden jackals in Croatia. Acta Parasitol. 2018;63:654–656. doi: 10.1515/ap-2018-0076. [DOI] [PubMed] [Google Scholar]
- 15.Miljević M., Lalošević D., Simin V., Blagojević J., Čabrilo B., Bjelić Čabrilo O. Intestinal helminth infections in the golden jackal (Canis aureus L.) from Vojvodina: Hotspot area of multilocular echinococcosis in Serbia. Acta Vet. Hung. 2021;69:274–281. doi: 10.1556/004.2021.00030. [DOI] [PubMed] [Google Scholar]
- 16.Oksanen A., Siles-Lucas M., Karamon J., Possenti A., Conraths F.J., Romig T., Wysocki P., Mannocci A., Mipatrini D., La Torre G., et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasites Vectors. 2016;9:519. doi: 10.1186/s13071-016-1746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European Union One Health 2018 Zoonoses Report. EFSA J. 2019;17:e05926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergles Rataj A., Bidovec A., Zele D., Vengust G. Echinococcus multilocularis in the red fox (Vulpes vulpes) in Slovenia. Eur. J. Wildl. Res. 2010;56:819–822. doi: 10.1007/s10344-010-0417-6. [DOI] [Google Scholar]
- 19.Administration of the Republic of Slovenia for Food Safety, Veterinary and Plant Protection (UVHVVR) Letno Poročilo o Zoonozah in Povzročiteljih Zoonoz. UVHVVR; Ljubljana, Slovenia: 2020. [(accessed on 7 July 2022)]. Annual Report on Zoonoses in Slovenia, 2018. Available online: https://www.gov.si/assets/organi-v-sestavi/UVHVVR/Varna-hrana/Porocila-bioloska-varnost/Nacionalno-porocilo-monitoringa-zoonoz-2020.pdf. [Google Scholar]
- 20.Roulichova J., Andera M. Simple method of age determination in red foxes, Vulpes vulpes. Folia Zool. 2007;56:440–444. [Google Scholar]
- 21.Bandelj P., Logar K., Usenik A.M., Vengust M., Ocepek M. An improved qPCR protocol for rapid detection and quantification of Clostridium difficile in cattle feces. FEMS Microbiol. Lett. 2013;341:115–121. doi: 10.1111/1574-6968.12102. [DOI] [PubMed] [Google Scholar]
- 22.Knapp J., Millon L., Mouzon L., Umhang G., Raoul F., Ali Z.S., Combes B., Comte S., Gbaguidi-Haore H., Grenouillet F., et al. Real time PCR to detect the environmental faecal contamination by Echinococcus multilocularis from red fox stools. Vet. Parasitol. 2014;201:40–47. doi: 10.1016/j.vetpar.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Maksimov P., Bergmann H., Wassermann M., Romig T., Gottstein B., Casulli A., Conraths F.J. Species Detection within the Echinococcus granulosus sensu lato complex by novel probe-based real-time PCRs. Pathogens. 2020;9:791. doi: 10.3390/pathogens9100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grech-Angelini S., Richomme C., Peytavin de Garam C., Boucher J.M., Maestrini O., Grenouillet F., Casabianca F., Boué F., Umhang G. Identification and molecular characterization of Echinococcus canadensis G6/7 in dogs from Corsica, France. Parasitol. Res. 2019;118:1313–1319. doi: 10.1007/s00436-019-06261-6. [DOI] [PubMed] [Google Scholar]
- 25.Wilson I.G. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team . R: A Language and Environment for Statistical Computing: c2013. R Foundation for Statistical Computing; Vienna, Austria: 2013. [(accessed on 18 July 2022)]. Available online: http://www.R-project.org/ [Google Scholar]
- 27.Deplazes P., Rinaldi L., Alvarez Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G., et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Romig T., Deplazes P., Jenkins D., Giraudoux P., Massolo A., Craig P.S., Wassermann M., Takahashi K., de la Rue M. Ecology and life cycle patterns of Echinococcus species. In: Thompson R.C.A., Deplazes P., Lymbery A.J., editors. Echinococcus and Echinococcosis, Part A. Volume 95. Elsevier; Amsterdam, The Netherlands: 2017. pp. 213–314. [DOI] [PubMed] [Google Scholar]
- 29.FAO—Food and Agriculture Organization of the UN. WHO . Multicriteria-Based Ranking for Risk Management of Food-borne Parasites. Volume 23. WHO; Geneva, Switzerland: 2014. [(accessed on 19 February 2020)]. (Microbiological Risk Assessment Series). Available online: https://apps.who.int/iris/bitstream/handle/10665/112672/9789241564700_eng.pdf?sequence=1. [Google Scholar]
- 30.Casulli A. Recognizing the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob. Health. 2020;8:e470–e471. doi: 10.1016/S2214-109X(20)30066-8. [DOI] [PubMed] [Google Scholar]
- 31.Rojo-Vazquez F.A., Pardo-Lledias J., Francos-Von Hunefeld M., Cordero-Sanchez M., Alamo-Sanz R., Hernandez-Gonzalez A., Brunetti E., Siles-Lucas M. Cystic echinococcosis in Spain: Current situation and relevance for other endemic areas in Europe. PLoS Negl. Trop. Dis. 2011;5:e893. doi: 10.1371/journal.pntd.0000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onac D., Gyorke A., Oltean M., Gavrea R., Cozma V. First detection of Echinococcus granulosus G1 and G7 in wild boars (Sus scrofa) and red deer (Cervus elaphus) in Romania using PCR and PCR-RFLP techniques. Vet. Parasitol. 2013;193:289–291. doi: 10.1016/j.vetpar.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Breyer I., Georgieva D., Kurdova R., Gottstein B. Echinococcus granulosus strain typing in Bulgaria: The G1 genotype is predominant in intermediate and definitive wild hosts. Parasitol. Res. 2004;93:127–130. doi: 10.1007/s00436-004-1116-6. [DOI] [PubMed] [Google Scholar]
- 34.Guerra D., Armua-Fernandez M.T., Silva M., Bravo I., Santos N., Deplazes P., Carvalho L.M. Taeniid species of the Iberian wolf (Canis lupus signatus) in Portugal with special focus on Echinococcus spp. Int. J. Parasitol. Parasites Wildl. 2012;2:50–53. doi: 10.1016/j.ijppaw.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guberti V., Bolognini M., Lanfranchi P., Battelli G. Echinococcus granulosus in the wold in Italy. Parassitologia. 2004;46:425–427. [PubMed] [Google Scholar]
- 36.Gori F., Armua-Fernandez M.T., Milanesi P., Serafini M., Magi M., Deplazes P., Macchioni F. The occurrence of taeniids of wolves in Liguria (northern Italy) Int. J. Parasitol. Parasites Wildl. 2015;4:252–255. doi: 10.1016/j.ijppaw.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Liere D., Dwyer C., Jordan D., Premik-Banič A., Valenčič A., Kompan D., Siard N. Farm characteristics in Slovene wolf habitat related to attacks on sheep. Appl. Anim. Behav. Sci. 2013;144:46–56. doi: 10.1016/j.applanim.2012.12.005. [DOI] [Google Scholar]
- 38.Krofel M., Kos I. Analiza vsebine iztrebkov volka (Canis lupus) v Sloveniji (Scat analysis of grey wolves (Canis lupus) in Slovenia) Zb. Gozdarstva Lesar. 2010;91:3–12. [Google Scholar]
- 39.Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: The urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Krofel M. Confirmed presence of territorial groups of golden jackals (Canis aureus) in Slovenia. Nat. Slov. 2009;11:65–68. [Google Scholar]
- 41.Arnold J., Humer A., Heltai M., Murariu D., Spassov N., Hackländer K. Current status and distribution of golden jackals Canis aureus in Europe. Mammal Rev. 2012;42:1–11. doi: 10.1111/j.1365-2907.2011.00185.x. [DOI] [Google Scholar]
- 42.Széll Z., Marucci G., Pozio E., Sréter T. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Vet. Parasitol. 2013;197:393–396. doi: 10.1016/j.vetpar.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Frey C.F., Basso W.U., Zürcher-Giovannini S., Marti I., Borel S., Guthruf S., Gliga D., Lundström-Stadelmann B., Origgi F.C., Ryser-Degiorgis M.P. The golden jackal (Canis aureus): A new host for Echinococcus multilocularis and Trichinella britovi in Switzerland. Schweiz. Arch. Tierheilkd. 2022;164:71–78. doi: 10.17236/sat00338. [DOI] [PubMed] [Google Scholar]
- 44.Lalošević D., Lalošević V., Simin V., Miljevic M., Cabrilo B., Bjelic Cabrilo O. Spreading of multilocular echinococcosis in southern Europe: The first record in foxes and jackals in Serbia, Vojvodina Province. Eur. J. Wildl. Res. 2016;62:793–796. [Google Scholar]
- 45.Lanszki J., Heltai M., Szabó L. Feeding habits and trophic niche overlap between sympatric golden jackal (Canis aureus) and red fox (Vulpes vulpes) in the Pannonian ecoregion (Hungary) Can. J. Zool. 2006;84:1647–1656. doi: 10.1139/z06-147. [DOI] [Google Scholar]
- 46.Avcioglu H., Guven E., Balkaya I., Kirman R. Echinococcus multilocularis in a Eurasian lynx (Lynx lynx) in Turkey. Parasitology. 2018;145:1147–1150. doi: 10.1017/S0031182018000082. [DOI] [PubMed] [Google Scholar]
- 47.Kritsky D.C., Leiby P.D. Studies on sylvatic echinococcosis. V. Factors influencing prevalence of Echinococcus multilocularis Leuckart 1863, in red foxes from North Dakota, 1965–1972. J. Parasitol. 1978;64:625–634. [PubMed] [Google Scholar]
- 48.Hofer S., Gloor S., Müller U., Mathis A., Hegglin D., Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- 49.di Cerbo A.R., Manfredi M.T., Bregoli M., Ferro Milone N., Cova M. Wild carnivores as source of zoonotic helminths in north-eastern Italy. Helminthologia. 2008;45:13–19. doi: 10.2478/s11687-008-0002-7. [DOI] [Google Scholar]
- 50.RS Statistical Office, 2022a. [(accessed on 18 July 2022)]. Available online: https://www.stat.si/obcine/en/Region/Index/5.
- 51.RS Statistical Office, 2022b. [(accessed on 18 July 2022)]. Available online: https://www.stat.si/obcine/en/Region/Index/8.
- 52.Avcioglu H., Guven E., Balkaya I., Kirman R., Akyuz M., Bia M.M., Gulbeyen H., Yaya S. Echinococcus multilocularis in Red Foxes in Turkey: Increasing risk in urban. Acta Trop. 2021;216:105826. doi: 10.1016/j.actatropica.2021.105826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.