Abstract
Background:
Pigmentary skin disorders impair the quality of life, leading to the development of therapeutic modalities. However, these treatments should focus more on effectiveness and safety.
Aims and Objectives:
To evaluate the effect of a temperature-adjustable cryotherapy device on the expression of pigmentation-related biomarkers.
Methods and Results:
A temperature- and time-adjustable cryotherapy device was employed to improve 200 mJ UVB-induced pigmentation on mice at −5°C (for 5, 10 or 20 s), 0°C (for 5, 10 or 20 s), 5°C (for 5, 10 or 20 s), or 10°C (for 5, 10 or 20 s). Expression of pigmentation-related biomarkers, such as tyrosinase, c-kit, melanocortin 1 receptor and microphthalmia-associated transcription factor before and after treatment with the cryotherapy device was investigated with real-time polymerase chain reaction and immunohistochemistry.
Results:
Expression of pigmentation-related biomarkers was decreased after the treatment of the temperature-adjustable cryotherapy device. Gene expression of the pigmentation-related biomarkers was decreased under the above conditions with some exception. Protein expression of the pigmentation-related biomarkers showed decreased tendency under the conditions with some exceptions.
Conclusion:
The temperature-adjustable cryotherapy device used in this study reduced the expression of pigmentation-related biomarkers on mice and may be used to treat patients with skin pigmentation.
KEY WORDS: Cryotherapy device, pigmentation, UVB
Introduction
Pigmentary skin disorders, such as melasma, solar lentigines, freckles, café au lait spot and post-inflammatory hyperpigmentation can damage the quality of life.[1] Skin pigmentation results from melanogenesis by melanocytes,[2] which are predominantly found in the basal layer of the epidermis and hair follicles[3,4] and can be detected by the expression of melanocyte-specific markers, such as tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), tyrosinase-related protein 2 (TYRP2), premelanosome protein 17 (Pmel17/gp1000), melan-A or melanoma antigen recognized by T cells 1 (MART-1) and microphthalmia-associated transcription factor (MITF).[5] The melanocyte-specific markers are related to the production of the melanin pigment. Melanin pigment produced by melanocytes in the skin is transferred to the surrounding keratinocytes.[6,7,8,9,10] The initiation and extent of pigmentation can be influenced by a number of intrinsic and extrinsic factors.[11] Therapeutic modalities of pigmentary skin disorders, such as light therapy, laser therapy, topical agents, oral medications and chemical peels have been developed. However, more effective and safer therapeutic modalities are still needed.
This study aimed to evaluate the effect of a temperature-adjustable cryotherapy device on the expression of pigmentation-related biomarkers.
Materials and Methods
Preparation of a device for cryotherapy
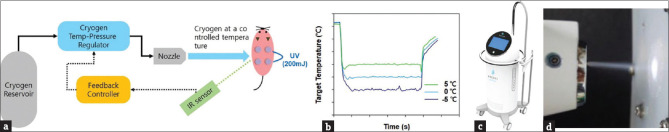
A novel cryotherapy system, which can precisely cool a target area from −20°C to 10°C, was developed and provided by Recens Medical Inc. This cryotherapy system has a unique capability of regulating the thermodynamic state (temperature, pressure) of cryogenic substances (e.g., carbon dioxide) by applying heat to cryogenic substances. A real-time temperature reading by an IR sensor was used to measure the error between the set cooling temperature and the target temperature, and a feedback controller was used to calculate the heat required to achieve a desired thermodynamic state of cryogen substance, leading to rapid and precision cooling at the target area [Figure 1a-d].
Figure 1.
Novel cryotherapy system that can produce precise cooling temperature at a target area, enabled by feedback control of cryogen thermodynamic state based on real-time temperature reading at the target area. (a) Illustration of the cryotherapy system. (b) Temperature control at the target area by the cryotherapy system. (c) Image of prototype device. (d) Spouting cryogens and light-emitting diode guiding injection point
Animal study
UVB 200 mJ was irradiated three times a week for two weeks on the back of 7-week-old female HRM2 mice (SLC Inc., Japan). The mice were once treated with the cryotherapy device at -5°C for 5, 10, or 20 s, 0°C for 5, 10, or 20 s, 5°C for 5, 10, or 20 s, or 10°C for 5, 10, or 20 s after the irradiation. The mice were once treated with the cryotherapy device. Real-time polymerase chain reaction (PCR) and immunohistochemistry were performed. This study was approved by the Institutional Animal Care and Use Committee of KNU (No. 2018-0167).
Real-time-PCR
Real-time PCR was conducted in triplicate with Power SYBR Green premix (Applied Biosystems) using 50 ng cDNA and 10 pM tyrosinase, MITF, melanocortin 1 receptor (MC1R) and c-kit. Cycling conditions for amplification were as follows: 95°C for 10 min, 40 cycles at 95°C for 15 s and 60°C for 60 s. The products of PCR were evaluated with a real-time PCR analysis software, Step One Plus (Applied Biosystems). Tyrosinase was detected with forward primer 5′-TCAGCCCAGCATCCTTCTTC-3′ and reverse primer 5′-TGGCTTTGTCATGGTTTCCAG-3’; MITF, with forward primer 5’-ATGATCCAGACATGC GGTGG-3′and reverse primer 5′-TGCTCCAGCTTCTTCTGTCG-3′; MC1R, with 5′-CC CAATGCCAGCAACATCTC-3′and 5′-CCACAATGCCCAGGAGACAG-3′; and c-kit with 5′- GACAGTTGCCGTGAAGATGC-3′ and 5′- GATTGCCCAGGTAGCTCAGG-3′.
Immunohistochemistry
Tissue samples were obtained from the mice, put in cryomolds with embedding medium and frozen at −80°C. Samples were sliced (7 μm thick) and then fixed with 4% paraformaldehyde and 0.1% Triton X-100 for 10 min. After 1 h of preparation with 5% normal donkey serum (Jackson ImmunoResearch), they were incubated at 4°C overnight with antibodies for tyrosinase (1:200 dilution; Abcam), MITF (1:200 dilution; Santacruz), MC1R (1:200 dilution; Abcam) and c-kit (1:200 dilution; Abcam) and with secondary antibody (1:100; Molecular Probes) for 1 h. The slides were counterstained with DAPI for 10 min. Immunohistochemistry was conducted in duplicate. The expression level of protein was quantified using the Image-J program.
Statistical methods
Statistical analysis was done using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) for Windows. ANOVA was performed. The level of significance was established at 0.05.
Results
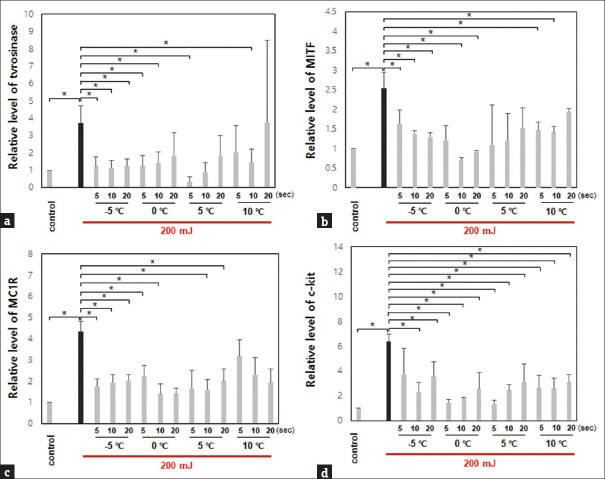
Gene and protein expressions were evaluated in UVB-irradiated mice before and after treatment with the temperature-adjustable cryotherapy device. The gene expressions of tyrosinase, MITF, MC1R, and c-kit were decreased after treatment at −5°C (for 5, 10 or 20 s), 0°C (for 5, 10 or 20 s), 5°C (for 5, 10 or 20 s), or 10°C (for 5, 10 or 20 s) after irradiation of UVB 200 mJ, except in tyrosinase at 10°C for 20 s [Figure 2].
Figure 2.
Gene expression of pigmentation-related biomarkers in mice with UVB irradiation was decreased after treatment with the cryotherapy device (*P < 0.05). (a) Tyrosinase. (b) Microphthalmia-associated transcription factor. (c) Melanocortin 1 receptor. (d) c-kit
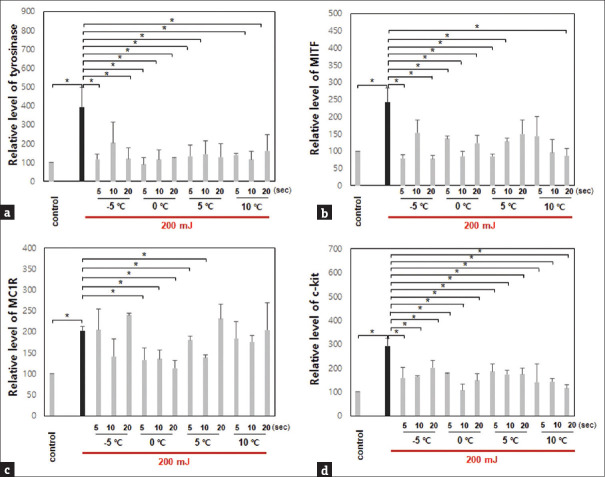
Through immunohistochemistry, the protein expression of the biomarkers was decreased after treatment under the same conditions, except for MC1R at −5°C for 5 or 20 s, 5°C for 20 s, and 10°C for 20 s after irradiation with UVB 200 mJ [Figure 3].
Figure 3.
Protein expression of pigmentation-related biomarkers in mice with UVB irradiation was decreased after treatment with the cryotherapy device (*P < 0.05). (a) Tyrosinase. (b) Microphthalmia-associated transcription factor. (c) Melanocortin 1 receptor. (d) c-kit
Discussion
Melanocyte-specific markers, such as TYR, TYRP1, TYRP2, Pmel17/gp1000, melan-A or MART-1 and MITF, are related to the production of the melanin pigment.[5] Melanin pigment is synthesized and stored in melanosomes before transferring to the surrounding keratinocytes.[12] TYR and the tyrosinase-related proteins TYRP1 and TYRP2 are melanogenic enzymes required in the biosynthesis of melanin. The production of these enzymes is driven by the MITF transcription factor. Activation of MITF is regulated by various signaling pathways, which are stimulated by receptors, such as c-kit and MC1R. c-kit is a tyrosine kinase receptor for stem cell factors, and MC1R is a receptor for α-melanocyte-stimulating hormone (α-MSH), adrenocorticotropic hormone (ACTH) and acylation-stimulating protein (ASP). Pmel17 and MART1 have a role in forming melanocyte structure.[13]
In this study, the expression of tyrosinase, MITF, MC1R and c-kit in mice with UVB irradiation was decreased by the cryotherapy device under the following conditions: −5°C (for 5, 10 or 20 s), 0°C (for 5, 10 or 20 s), 5°C (for 5, 10 or 20 s), or 10°C (for 5, 10 or 20 s), with 0°C for 10 sec as the best condition. Cryotherapy is a useful therapeutic modality for the treatment of pigmentary disorders of the skin.[14] Pigmented cells are highly susceptible to freezing at temperatures of -4°C to -7°C. Although pigmentation changes are usually transient, prolonged freezing for longer than 30 s may result in permanent pigment loss. Available cryogens include liquid nitrogen (boiling point -195.8°C), carbon dioxide (boiling point -79.0°C), and nitrous oxide (boiling point -89.5°C). Cryotherapy has been used at a fixed temperature of each cryogen under various conditions of time and freeze-thaw cycle. In this study, we could control not only time but also the temperature in cryotherapy using the precise cryotherapy device.
Burge et al.[15] observed that cryotherapy can make absence of melanosomes in keratinocytes through decreased numbers of melanocytes, a reduction in synthesis of melanosomes or a block in transfer of melanosomes. Aktaş et al.[16] reported the effect of open spray cryotherapy using liquid nitrogen for 30 s for the treatment of long-standing drug-induced lip pigmentation. Liquid nitrogen cryotherapy was useful for the treatment of gingival physiologic pigmentation in adolescents.[17] Hosaka et al.[18] reported that liquid nitrogen cryotherapy is effective in the treatment of Ota nevus, senile lentigines, nevus spilus, and blue nevus. Elmelegy and Elghamry[19] found that carbon dioxide cryotherapy is an effective treatment modality for nasal and perinasal congenital melanocytic nevi. Carbon dioxide cryotherapy was also used for the treatment of Ota nevus and acquired bilateral nevus of Ota-like macules.[20] Cryotherapy has been involved in combination therapies of pigmentary skin disorders.[21] Cryotherapy can be used in patients with generalized vitiligo for depigmentation treatment of the pigmented patches.[22,23]
Traditional therapeutic modalities, including topical whitening agents and chemical peels, have also been used for the treatment of skin pigmentation. However, because of the refractory and recurrent nature of skin pigmentation, alternative treatment strategies, such as laser and light therapy, have been developed.[24] Although effective, both can cause post-inflammatory hyperpigmentation or hypopigmentation and have a risk of recurrence. Cryotherapy may also be associated with hyperpigmentation and hypopigmentation.[25] Burge et al,[15] reported pigment changes in the human skin after cryotherapy, which developed hypopigmentation with a peripheral rim of hyperpigmentation, and hypopigmentation persisted despite the presence of functional melanocytes.
The limitation of this study is as follows: there is no clinical photo and pigmentation level before and after treatment with precision cryotherapy device because we focused on changes in the expression of pigmented-related biomarkers. There is no data about preventative effects of precision cryotherapy device on UVB-induced pigmentation. Although we conducted this study under the various temperature and time conditions, mice was treated with precise cryotherapy device only one time at each condition.
In conclusion, the expression of melanin pigment-related biomarkers in mice with UVB irradiation was decreased by the cryotherapy device. Therefore, cryotherapy may be a useful option for the treatment and prevention of skin pigmentary disorders. In this study, the best condition for cryotherapy was 0°C for 10 s.
Statement of ethics
Our animal care and treatment protocols were in accordance with the guidelines for the use of Laboratory Animals. Animal experiments were approved by the Institutional Animal Care and Use Committee of the Kyungpook National University (IRB No. KNU 2018-167), and this study was carried out in compliance with the ARRIVE guidelines.
Author contributions
MHK, SL, EHL and GUH designed and performed the experiments. GHK and WJL contributed essential reagents and analyzed the data and wrote the manuscript. GHK and WJL supervised the research.
Financial support and sponsorship
This work was partly supported by the Technological innovation R&D program of MSS (S2689541) funded by the Ministry of SMEs and Startups (MSS, Korea) and National Research Foundation of Korea (NRF-2020R1A4A2002728).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Plensdorf S, Livieratos M, Dada N. Pigmentation disorders: Diagnosis and management. Am Fam Physician. 2017;96:797–804. [PubMed] [Google Scholar]
- 2.Bonaventure J, Domingues MJ, Larue L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigment Cell Melanoma Res. 2013;26:316–25. doi: 10.1111/pcmr.12080. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs S, Murli S, de Boer G, Mulder A, Mommaas AM, Ponec M. Melanosome capping of keratinocytes in pigmented reconstructed epidermis - effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000;13:458–66. doi: 10.1034/j.1600-0749.2000.130608.x. [DOI] [PubMed] [Google Scholar]
- 4.Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: Biology and development. Postepy Dermatol Alergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passeron T, Coelho SG, Miyamura Y, Takahashi K, Hearing VJ. Immunohistochemistry and in situ hybridization in the study of human skin melanocytes. Exp Dermatol. 2007;16:162–70. doi: 10.1111/j.1600-0625.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- 6.Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14:236–42. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 8.Delevoye C. Melanin transfer: The keratinocytes are more than gluttons. J Investig Dermatol. 2014;134:877–9. doi: 10.1038/jid.2013.487. [DOI] [PubMed] [Google Scholar]
- 9.Amico-Ruvio SA, Paganelli MA, Myers JM, Popescu GK. Ifenprodil effects on GluN2B-containing glutamate receptors. Mol Pharmacol. 2012;82:1074–81. doi: 10.1124/mol.112.078998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RR, Parrish JA. The optics of human skin. J Investig Dermatol. 1981;77:13–9. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 11.Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks MS, Seabra MC. The melanosome: Membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:738–48. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–61. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- 14.Chan HH, Kono T. Nevus of Ota: Clinical aspects and management. Skinmed. 2003;2:89. doi: 10.1111/j.1540-9740.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- 15.Burge SM, Bristol M, Millard PR, Dawber RP. Pigment changes in human skin after cryotherapy. Cryobiology. 1986;23:422–32. doi: 10.1016/0011-2240(86)90027-1. [DOI] [PubMed] [Google Scholar]
- 16.Aktaş H, Yılmaz OE, Ertuğrul G. Cryotherapy for long-standing drug-induced lip pigmentation: A fast, safe and inexpensive procedure. Clin Exp Dermatol. 2021;46:1130–1. doi: 10.1111/ced.14674. [DOI] [PubMed] [Google Scholar]
- 17.Shirazi AS, Moeintaghavi A, Khorakian F, Talebi M. Treatment of gingival physiologic pigmentation in adolescents by liquid nitrogen cryosurgery: 24-month follow-up. Int J Periodontics Restorative Dent. 2012;32:e142–6. [PubMed] [Google Scholar]
- 18.Hosaka Y, Onizuka T, Ichinose M, Yoshimoto S, Okubo F, Hori S, et al. Treatment of nevus Ota by liquid nitrogen cryotherapy. Plast Reconstr Surg. 1995;95:703–11. doi: 10.1097/00006534-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Elmelegy N, Elghamry S. Carbon dioxide cryotherapy for treatment of nasal and perinasal congenital melanocytic nevi. Ann Plast Surg. 2020;85:107–9. doi: 10.1097/SAP.0000000000002145. [DOI] [PubMed] [Google Scholar]
- 20.Hata Y, Matsuka K, Ito O, Matsuda H, Furuichi H, Ishizu N, et al. Treatment of nevus Ota: Combined skin abrasion and carbon dioxide snow method. Plast Reconstr Surg. 1996;97:544–54. doi: 10.1097/00006534-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Campanati A, Giannoni M, Scalise A, De Blasio S, Giuliano A, Giuliodori K, et al. Efficacy and safety of topical pidobenzone 4% as adjuvant treatment for solar lentigines: Result of a randomized, controlled, clinical trial. Dermatology. 2016;232:478–83. doi: 10.1159/000447356. [DOI] [PubMed] [Google Scholar]
- 22.van Geel N, Depaepe L, Speeckaert R. Laser (755 nm) and cryotherapy as depigmentation treatments for vitiligo: A comparative study. J Eur Acad Dermatol Venereol. 2015;29:1121–7. doi: 10.1111/jdv.12762. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Kumari R, Thappa DM. Depigmentation therapies in vitiligo. Indian J Dermatol Venereol Leprol. 2012;78:49–58. doi: 10.4103/0378-6323.90946. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11–20. doi: 10.1016/j.ijwd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datrice N, Ramirez-San-Juan J, Zhang R, Meshkinpour A, Aguilar G, Nelson JS, et al. Cutaneous effects of cryogen spray cooling on in vivo human skin. Dermatol Surg. 2006;32:1007–12. doi: 10.1111/j.1524-4725.2006.32223.x. [DOI] [PubMed] [Google Scholar]