Abstract
Background:
To explore the role and clinical significance of serum adiponectin and leptin levels in patients with psoriasis accompanied by atherosclerosis.
Methods:
Eighty patients diagnosed with psoriasis in our dermatology department and 40 healthy people in our physical examination centre were included as the study group and control group, respectively. All the included patients underwent fasting blood and serum tests. Levels of adiponectin, leptin, and the blood lipid content; colour Doppler ultrasonography of both common carotid arteries, internal carotid and external carotid arteries; and intimal-medial thickness (IMT) and carotid plaque were evaluated.
Results:
In the study group, the leptin level increased, and the serum adiponectin level decreased; these levels were statistically significantly different compared with those in the control group (t = 6.774, P < 0.001 and t = –3.511, P < 0.05, respectively). IMT was negatively correlated with adiponectin levels (r = –0.378, P < 0.001) and positively correlated with leptin levels (r = 0.581, P < 0.001).
Conclusions:
The imbalanced expression of serum and adiponectin levels will aggravate psoriasis and promote the occurrence of atherosclerosis. Serum levels can be used to assess the disease severity, detect vascular lesions early, and prevent the development of psoriasis to cardiovascular disease.
KEY WORDS: Adiponectin, atherosclerosis, dermatology, leptin, psoriasis
Introduction
Psoriasis is an inflammatory-related skin disease with a prevalence of about 1–3%.[1] In addition to being a skin disease, it is now considered as a kind of systemic disease related to co-morbidities, such as those associated with cardiovascular and cerebrovascular diseases, metabolic syndrome, and depression.[2,3,4] Several experiments have confirmed that the patients with psoriasis have an increased risk of cardiovascular diseases. However, the potential causal relationship between psoriasis and cardiovascular diseases has not been determined. Current research has suggested that the systemic inflammatory nature of psoriasis may be the causal link between psoriasis and cardiovascular disease. Systemic inflammation because of psoriasis may lead to vascular endothelial cell dysfunction and atherosclerosis, thereby promoting the development of cardiovascular disease.[5,6] Adiponectin and leptin are important regulators of the metabolism and inflammation. Studies have shown that in patients with psoriasis, the level of leptin increases, whereas that of adiponectin decreases.[7,8] Adiponectin and leptin may also be involved in the occurrence and development of cardiovascular complications.[9,10] At present, there is no related research on the prevalence of psoriasis and atherosclerosis in China. There are a few studies abroad about the correlation between serum adipokines and psoriatic atherosclerosis, and only the relationship between adiponectin or leptin and atherosclerosis in patients with psoriasis has been studied separately. Therefore, this study aimed to explore the role and clinical significance of serum adiponectin and leptin levels in patients with psoriasis accompanied by atherosclerosis. The occurrence and treatment of psoriasis provide a theoretical basis, and more effective methods are needed to detect cardiovascular disease secondary to psoriasis.
Materials and Methods
The study was approved by the Ethics Committee of Affiliated Hospital of Southwest Medical University.
1.1 Subjects
The study group consisted of 80 patients with moderate to severe psoriasis who were treated in our dermatology department from January 2018 to June 2018. The control group consisted of 40 healthy people who were routinely examined in our physical examination centre from May 2018 to June 2018.
The study group inclusion criteria were as follows: 1) age >18 years, 2) patients who meet the diagnostic criteria for psoriasis vulgaris[11] and have moderate to severe psoriasis, and 3) patients who understand the informed consent and agree to be selected for the experiment. The study group exclusion criteria were as follows: 1) patients with psoriasis and joint involvement; 2) those with a history of hypertension, diabetes, and cardiovascular and cerebrovascular diseases; 3) smokers; 4) patients with severe liver and kidney dysfunction and severe systemic inflammatory diseases; and 5) those who have used glucocorticoids or immunosuppressants systemically within the first 6 months.
The inclusion criteria of the control group were as follows: 1) healthy individuals aged >18 years of age and 2) those who can fully understand the informed consent and are willing to participate in.
The exclusion criteria for the control group were as follows: 1) individuals with a history of hypertension, diabetes, and cardiovascular and cerebrovascular diseases; 2) smokers; 3) those with severe liver and kidney dysfunction; 4) individuals suffering from severe systemic inflammatory diseases; and 5) those taking systemic glucocorticoids within the first 6 months of receiving hormones or immunosuppressants.
1.2 Data collection
The following general information was collected: sex, age, height, weight, and body mass index (BMI) (BMI = weight ÷ height2) (kg/m2). The research team also collected the time to disease.
1.2.1 PASI
The Psoriasis Area and Severity Index (PASI) was used to evaluate the diseased skin lesions (infiltration, desquamation, and erythema) and their locations (head and neck, upper and lower limbs, or trunk). There are four parts of PASI, and each part is scored separately first. 1) The area of each part is 100% and divided into seven portions according to the range of skin lesions: <10%, 10–29%, 30–49%, 50–69%, 70%–89%, and 90–100%. From low to high, 0 points, 1 point, 2 points, 3 points, 4 points, 5 points, and 6 points should be recorded. 2) The skin lesions of each part are divided into three manifestations of erythema, infiltration, and desquamation. Each manifestation is categorized into none, light, medium, severe, and very severe, which are recorded as 0, 1, 2, 3, and 4 minutes, respectively. 3) Each position has a position weighting number: 0.1 for the head and neck, 0.2 for the upper limbs, 0.3 for the trunk, and 0.4 for the lower limbs. 4) The scores of the degree of skin lesions are added to indicate a single location and multiplied by the area score and the weight of each location. 5) Then, the sum is the final PASI score. PASI scores range from 0 to 72, with higher scores indicating more severe psoriasis lesions: 0, no psoriasis; 1–18, mild psoriasis; 19–30, moderate psoriasis; 31–38, severe psoriasis; and ≥39, very severe psoriasis.
1.2.2 Lipid profile
After 8 hours of fasting, about 8 ml of blood from the antecubital vein was collected from all participants with an empty stomach in the morning, and the blood was divided into two biochemical tubes. One tube was sent to the laboratory for blood lipid testing. The other one used enzyme-linked immunosorbent assay to measure the levels of serum leptin and adiponectin. After the other tube was left at room temperature for 30 minutes, the blood sample was centrifuged at 3000 rpm for 5 minutes. The upper serum was collected and aliquoted into an Eppendorf tube and immediately placed in the –80°C refrigerator of the central laboratory for storage. Blood samples can be stored for up to 6 months.
1.2.3 Atherosclerosis
The carotid arteries were selected using a Philips iU22 colour Doppler ultrasound system (Amsterdam, the Netherlands) with the probe frequency set at 3–9 MHz. The following measurements and observations were recorded: 1) the presence of plaque formation in the lumen, 2) the maximum value of intimal-medial thickness (IMT) (the distance between the intima media and the media was measured at a distance of 1 cm from the common carotid artery bifurcation and 1 cm from the carotid bulb and internal carotid artery), and 3) the presence of atherosclerosis if IMT ≥1 mm. Atherosclerotic plaque was defined as a prominent protrusion into the lumen of a blood vessel.
1.3 Statistical analysis
The normality test was first performed to evaluate the measurement data. The natural logarithmic values of the skewed distribution variables were consistent with normal distribution. Continuous variables are described as mean ± standard deviation (x ± s) based on the independent sample t-test. The classification data are presented as frequency (f) or rate (%), and they were tested by x2-test.[2] The correlation between two continuous variables was determined in the Spearman correlation analysis. All statistical analyses were performed using SPSS 17 (IBM Corp., Armonk, NY, USA). The study was approved by the Ethics Committee of Affiliated Hospital of Southwest Medical University.
Results
2.1 Comparison of basic conditions between the study and control groups
2.1.1 Clinical data
In this study, 80 patients with psoriasis and 40 healthy controls were included. There was no significant difference in sex, age, and BMI between the two groups (both P > 0.05), and they were comparable [Table 1]. The shortest disease course in the study group was 6 months and the longest was 16 years, with an average course of 62.20 ± 44.84 months. The average PASI score was 27.24 ± 10.29; the lowest score was 19, and the highest score was 63.8. According to the PASI score, there were 62 cases of moderate psoriasis and 18 cases of severe psoriasis.
Table 1.
Comparison of clinical data, laboratory results, and other data between the study and control groups
| Study Group (n=80) | Control group (n=40) | t/χ2 | P | |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 43 (53.75) | 21 (52.5) | 0.17 | 0.897 |
| Female, n (%) | 37 (46.25) | 19 (47.5) | ||
| Age (years) | 43.54±11.82 | 43.88±11.79 | –0.148 | 0.883 |
| BMI (kg/m2) | 24.25±3.90 | 24.29±3.47 | –0.056 | 0.956 |
| Total cholesterol level (mmol/L) | 5.08±0.78 | 4.77±0.86 | 2.02 | 0.046 |
| Triglyceride level (mmol/L) | 1.59±0.95 | 1.57±0.88 | 0.079 | 0.937 |
| High-density lipoprotein level (mmol/L) | 1.19±0.25 | 1.49±0.37 | –5.377 | <0.001 |
| Low-density lipoprotein level (mmol/L) | 2.59±0.74 | 2.85±0.75 | –1.765 | 0.08 |
| Adiponectin level (ng/ml) | 28.72±16.69 | 39.50±14.01 | –3.511 | 0.001 |
| Leptin level (ng/ml) | 4.64±2.51 | 1.76±1.34 | 6.774 | <0.001 |
| Carotid IMT value (mm) | 1.02±0.16 | 0.81±0.15 | 6.901 | <0.001 |
| Positive carotid plaque, n (%) | 9 (11.3) | 2 (5) | 1.25 | 0.263 |
| Disease course (months) | 62.20±44.84 | - | ||
| PASI score | 27.24±10.29 | - |
BMI, body mass index; IMT, intimal-medial thickness; PASI, Psoriasis Area and Severity Index
2.1.2 Laboratory and imaging results
In the study group, the total cholesterol level increased, and the high-density lipoprotein level decreased. These levels were statistically significantly different between the study and control groups (t = 2.02, P < 0.05 and t = –5.377, P < 0.001, respectively). There was no significant difference in the triglyceride and low-density lipoprotein levels between the groups (P > 0.05). The average serum adiponectin level was 28.72 ± 16.69 ng/ml in the study group, which was lower than that in the control group (39.50 ± 14.01 ng/ml). The average serum leptin level was 4.64 ± 2.51 ng/ml in the study group, which was higher than that in the control group (1.76 ± 1.34 ng/ml); this difference was statistically significant (t = –3.511, P < 0.05; t = 6.774, P < 0.001). The IMT value of the carotid artery was 1.02 ± 0.16 mm in the study group, which was higher than that in the control group by 0.81 ± 0.15 mm, and the difference was statistically significant (t = 6.901, P < 0.001). According to the definition of atherosclerosis based on carotid ITM ≥1 mm, 37 patients in the study group had an atherosclerosis rate of 46.25%, and five patients in the control group had an atherosclerosis rate of 12.5%, which was significantly different (x2 = 13.35, P < 0.001). The number of positive carotid plaques in the study group was 9 (11.3%), and that in the control group was 2 (positive rate 5%); however, there was no significant difference between the groups (x2 = 1.25, P > 0.05).
2.2 Comparison of leptin and adiponectin levels between the study and control groups
There were significant sex differences in serum leptin levels between the groups. The overall leptin level in women was 5.10 ± 2.58 ng/ml, which was significantly higher than that in men by 2.44 ± 1.83 ng/ml; the difference was statistically significant (t = –6.574, P < 0.001) [Figure 1]. When further stratified by sex, the leptin level of men in the study group was 3.24 ± 1.97 ng/ml, which was higher than that of men in the control group by 1.09 ± 0.85 ng/ml, and the leptin level of women in the study group was 6.27 ± 2.05 ng/ml, which was higher than that of women in the control group by 2.50 ± 1.41 ng/ml; the differences were statistically significant (t = 4.75, P < 0.001 and t = 7.172, P < 0.001, respectively).
Figure 1.
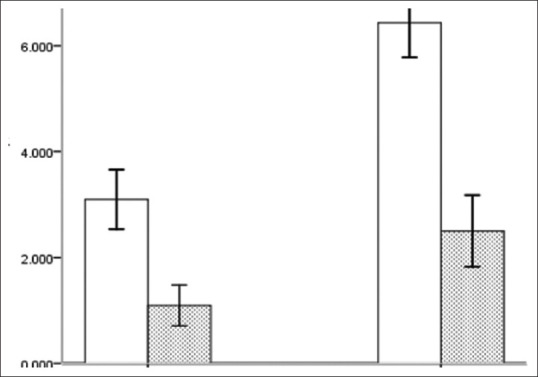
Comparison of leptin levels in men and women between the groups
The overall adiponectin level in women was 23.65 ± 14.59 ng/ml, which was lower than the overall adiponectin level in men of 39.89 ± 14.45 ng/ml, and the difference was statistically significant (t = 6.115, P < 0.001) [Figure 2]. The adiponectin level in men of the study group was 37.29 ± 15.87 ng/ml, which was lower than that in men of the control group (43.68 ± 12.17 ng/ml), and the difference was not statistically significant (t = –1.624, P > 0.05). The adiponectin level in women of the study group was 18.76 ± 11.29 ng/ml, which was lower than that in women of the control group (34.87 ± 14.73 ng/ml), and the difference was statistically significant (t = –4.552, P < 0.001).
Figure 2.
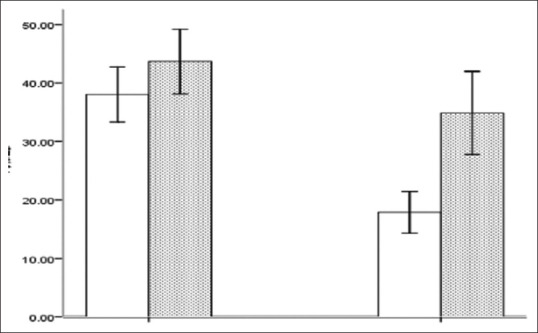
Comparison of adiponectin levels in men and women between the groups
2.3 Comparison of clinical variables between the psoriatic atherosclerosis and non-atherosclerosis groups
The patients with psoriasis were divided into two groups according to the ITM value: the group with atherosclerosis (group A, ITM ≥1 mm) and the group without atherosclerosis (group B, ITM <1 mm) [Table 2]. There were 37 patients in group A and 43 in group B. There was no significant difference in the disease course and PASI score between the groups (t = –0.358, P > 0.05 and t = 0.438, P > 0.05, respectively). The average age of group A was older than that of group B, and the difference was statistically significant (t = 3.488, P < 0.05). (t = 1.693, P > 0.05). Triglyceride and low-density lipoprotein levels were increased in group A, and they were statistically significantly higher than those in group B (t = 4.983, P < 0.001 and t = 2.555, P < 0.05, respectively). However, the total cholesterol level and high-density lipoprotein were not statistically significantly different between the groups (t = 0.391, P > 0.05 and t = –0.351, P > 0.05, respectively). In group A, the adiponectin level decreased, and the leptin level increased; compared with group B, these differences were statistically significant (t = –2.703, P < 0.05 and t = 3.557, P < 0.05, respectively). The average carotid IMT was higher in group A than in group B, and the difference was statistically significant (t = 14.717, P < 0.001). The positive rate of carotid plaque was higher in group A than in group B, but the difference was not statistically significant (2 = 4.055, P > 0.05).
Table 2.
Comparison of clinical variables between the psoriatic atherosclerosis group and non-atherosclerosis group
| Group A (n=37) | Group B (n=43) | t/χ2 | P | |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 19 (51.35) | 24 (55.81) | 0.159 | 0.69 |
| Female, n (%) | 18 (48.65) | 19 (44.19) | ||
| Age (years) | 48.19±10.95 | 39.53±11.17 | 3.488 | 0.001 |
| BMI (kg/m2) | 25.04±4.17 | 23.57±3.57 | 1.693 | 0.094 |
| Disease course (months) | 60.11±45.25 | 64.00±44.94 | –0.358 | 0.701 |
| PASI score | 27.78±12.27 | 26.77±8.33 | 0.438 | 0.663 |
| Total cholesterol level (mmol/L) | 5.12±0.89 | 5.05±0.68 | 0.391 | 0.697 |
| Triglyceride level (mmol/L) | 2.09±1.13 | 1.16±0.44 | 4.983 | <0.001 |
| High-density lipoprotein level (mmol/L) | 1.18±0.22 | 1.20±0.27 | –0.351 | 0.727 |
| Low-density lipoprotein level (mmol/L) | 2.81±0.80 | 2.41±0.62 | 2.555 | 0.013 |
| Adiponectin level (ng/ml) | 23.49±15.94 | 33.22±16.16 | –2.703 | 0.008 |
| Leptin level (ng/ml) | 5.64±2.65 | 3.78±2.05 | 3.557 | 0.001 |
| Carotid ITM value (mm) | 1.17±0.12 | 0.89±0.02 | 14.717 | <0.001 |
| Positive carotid plaque, n (%) | 7 (18.92) | 2 (4.65) | 4.055 | 0.073 |
BMI, body mass index; IMT, intimal-medial thickness; PASI, Psoriasis Area and Severity Index
2.4 Correlation analysis of carotid IMT with adiponectin and leptin levels
There was a positive correlation between IMT and the serum leptin level [Figure 3]. The correlation coefficient between them was r = 0.581, which was statistically significant (P < 0.001). ITM was negatively correlated with the serum adiponectin level [Figure 4]; the correlation coefficient was r = –0.378, which was statistically significant (P < 0.001).
Figure 3.
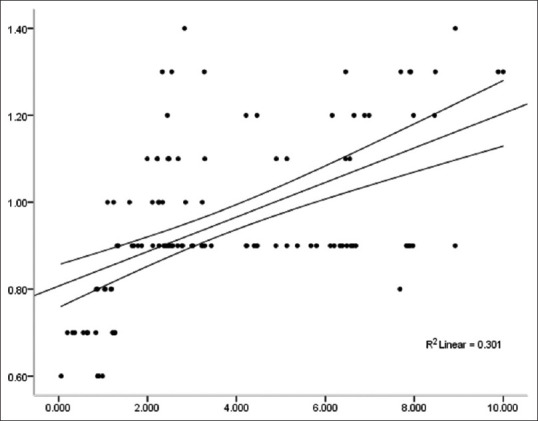
IMT is positively correlated with the serum leptin level
Figure 4.
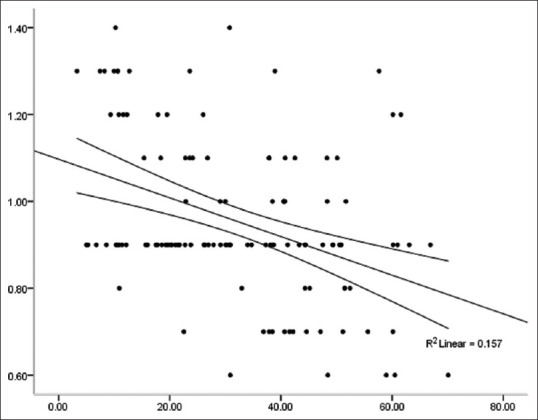
IMT is negatively correlated with the serum adiponectin level
Discussion
In recent years, several experiments have confirmed the increased risk of cardiovascular disease in patients with psoriasis. McDonald and Calabresi demonstrated for the first time that among 300 hospitalised patients, the risk associated with psoriatic vascular disease was 2.2 times higher than that in patients with other skin diseases.[12] Moreover, a recent study showed that the 10-year risk of cardiovascular disease in the psoriasis group was significantly higher than that of healthy people.[13] However, to date, the underlying causal link between psoriasis and cardiovascular disease has not been determined, and chronic inflammation may be a shared mechanism between psoriasis and atherosclerosis. In a study about the relationship between psoriasis and atherosclerosis, it was found that chronic systemic inflammation can induce endothelial cell dysfunction, affect the glucose metabolism, and produce insulin resistance. Inflammation plays a vital role in the occurrence and progression of atherosclerosis.[14] Psoriasis and atherosclerosis share a common immunological basis. T cells, macrophages, monocytes, dendritic cells, and mast cells infiltrate psoriatic skin lesions, and cells in atherosclerotic plaques have a similar composition.[15] Adiponectin and leptin can regulate the metabolism, angiogenesis, and inflammation, and both play important roles in the occurrence of psoriasis and atherosclerosis. Leptin activates inflammatory cells, increases the release of pro-inflammatory factors, causes vascular dysfunction and the activation of immune cells, increases the expression of adhesion molecules and angiogenesis, stimulates the proliferation of keratinocytes, and promotes the skin inflammatory response.[11,12,13,14,15,16] In contrast to leptin, adiponectin has anti-inflammatory effects, insulin sensitisation, and anti-inflammatory and anti-atherosclerotic functions.[17] Disorders in the balance of leptin and adiponectin levels are related to cardiovascular disease, atherosclerosis, and psoriasis.[18,19,20,21]
The results of this experiment showed that serum leptin levels in psoriasis are elevated and positively correlated with the severity score of psoriatic skin lesions, suggesting that leptin can promote the occurrence and development of psoriasis. These findings are consistent with previous results reported by Elhilaly et al.,[21] Singal et al.,[22] and Hoffman et al.,[23] who suggested that leptin might be a biomarker of psoriasis severity. Contrary to the results of leptin, we found that the adiponectin level decreased in the study group and was negatively correlated with the PASI score. This difference was statistically significant, suggesting that a decreased adiponectin level may be a risk factor for psoriasis, which is consistent with the results of Takahashi et al.'s study.[24] If the adiponectin level is reduced, its anti-inflammatory effect is weakened, and the inflammation process of psoriasis is accelerated. There was no difference in BMI between the study and control groups, suggesting that the correlation between adiponectin levels and psoriasis may not be related to BMI.
In summary, the imbalanced expressions of serum leptin and adiponectin will aggravate the condition of psoriasis and promote the occurrence of atherosclerosis. The mechanism may be related to the disorder of the lipid metabolism and increased release of inflammatory factors. Detecting the serum adiponectin and leptin levels in patients with psoriasis makes it possible to assess the severity of the disease and to detect vascular lesions early. Regulating the balance between leptin and adiponectin levels is of great significance to control the progression of psoriasis and reduce the progression of psoriasis to cardiovascular disease.
Abbreviations
intimal-medial thickness (IMT)
body mass index (BMI)
Psoriasis Area and Severity Index (PASI).
Consent for publication
Not applicable
Financial support and sponsorship
Luzhou-Southwest Medical University Joint Project.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wolk K, Sabat R. Adipokines in psoriasis: An important link between skin inflammation and metabolic alterations. Rev Endocr Metab Disord. 2016;17:305–17. doi: 10.1007/s11154-016-9381-0. [DOI] [PubMed] [Google Scholar]
- 2.Nickoloff BJ, Xin H, Nestle FO, Qin JZ. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568–73. doi: 10.1016/j.clindermatol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 4.Batacsorgo Z, Hammerberg C, Voorhees J, Cooper KD. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture. Cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest. 1995;95:317–27. doi: 10.1172/JCI117659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitoufella H, Taleb S, Mallat Z, Tedgui A. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi: 10.1007/s00281-009-0143-x. [DOI] [PubMed] [Google Scholar]
- 6.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery–infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robati RM, Partovikia M, Haghighatkhah HR, Younespour S, Abdollahimajd F. Increased serum leptin and resistin levels and increased carotid intima-media wall thickness in patients with psoriasis: Is psoriasis associated with atherosclerosis? J Am Acad Dermatol. 2014;71:642–8. doi: 10.1016/j.jaad.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kaur S, Zilmer K, Leping V, Zilmer M. The levels of adiponectin and leptin and their relation to other markers of cardiovascular risk in patients with psoriasis. J Eur Acad Dermatol Venereol. 2011:251328–33. doi: 10.1111/j.1468-3083.2011.03982.x. [DOI] [PubMed] [Google Scholar]
- 9.Wassink AMJ, Olijhoek JK, Visseren FLJ. The metabolic syndrome: Metabolic changes with vascular consequences. Eur J Clin Invest. 2007;37:8–17. doi: 10.1111/j.1365-2362.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- 10.Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: From pathophysiology to risk stratification. Int J Cardiol. 2010;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 11.Mcdonald CJ, Calabresi P. Occlusive vascular disease in psoriatic patients. N Engl J Med. 1973;288:912. [PubMed] [Google Scholar]
- 12.Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: A systematic review and meta-analysis. J Invest Dermatol. 2013;133:2340–6. doi: 10.1038/jid.2013.149. [DOI] [PubMed] [Google Scholar]
- 13.Hao LY. Preliminary Study on the Correlation between Psoriasis and the Risk of Cardiovascular Disease. Southern Medical University. 2017 [Google Scholar]
- 14.Sattar N, Mcinnes IB. Vascular comorbidity in rheumatoid arthritis: Potential mechanisms and solutions. Curr Opin Rheumatol. 2005;17:286–92. doi: 10.1097/01.bor.0000158150.57154.f9. [DOI] [PubMed] [Google Scholar]
- 15.Golden JB, Mccormick TS, Ward NL. IL-17 in psoriasis: Implications for therapy and cardiovascular co-morbidities. Cytokine. 2013;62:195–201. doi: 10.1016/j.cyto.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeki H, Shibata S, Tada Y, Karakawa M, Minatani Y, Tamaki K. Psoriasis arthropathica associated with severe obesity showing high serum leptin level. J Dermatol. 2009;36:364–6. doi: 10.1111/j.1346-8138.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chen J, Zhao Y, Geng L, Song F, Chen HD. Psoriasis is associated with increased levels of serum leptin. Br J Dermatol. 2008;158:1134–5. doi: 10.1111/j.1365-2133.2008.08456.x. [DOI] [PubMed] [Google Scholar]
- 18.Shailendra K. Comorbidities associated with leptin and psoriasis. J Drugs Dermatol. 2013;12:515. [PubMed] [Google Scholar]
- 19.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Al-Othman A, Draz HM, et al. Hypovitaminosis D associations with adverse metabolic parameters are accentuated in patients with type 2 diabetes mellitus: A body mass index-independent role of adiponectin? J Endocrinol Invest. 2013;36:1–6. doi: 10.3275/8183. doi: 10.3275/8183. [DOI] [PubMed] [Google Scholar]
- 20.Kim GW, Park HJ, Kim HS, Kim SH, Ko HC, Kim BS, et al. Analysis of cardiovascular risk factors and metabolic syndrome in Korean patients with psoriasis. Ann Dermatol. 2012;24:11–5. doi: 10.5021/ad.2012.24.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enany B, Zohiery AKE, Elhilaly R, Badr T. Carotid intima-media thickness and serum leptin in psoriasis. Herz. 2012;37:527–33. doi: 10.1007/s00059-011-3547-z. [DOI] [PubMed] [Google Scholar]
- 22.Asha K, Sharma SB, Singal A, Aggarwal A. Association of carotid intima-media thickness with leptin and apoliprotein b/apoliprotein a-I ratio reveals imminent predictors of subclinical atherosclerosis in psoriasis patients. Acta Medica. 2014;57:21–7. doi: 10.14712/18059694.2014.4. [DOI] [PubMed] [Google Scholar]
- 23.Herron MD, Hinckley M, Hoffman MS, Papenfuss J, Hansen CB, Callis KP, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527–34. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Tsuji H, Takahashi I, et al. Plasma adiponectin and leptin levels in Japanese patients with psoriasis. Br J Dermatol. 2008;159:1207–8. doi: 10.1111/j.1365-2133.2008.08823.x. [DOI] [PubMed] [Google Scholar]


