Abstract
Background:
Atopic dermatitis (AD) is an itchy, chronic or chronically relapsing, inflammatory skin condition.
Aims:
To study the effectiveness of probiotic supplementation (Bacillus clausii) in achieving clinical remission, preventing relapse and its effect on immunological profile in children with AD.
Methods:
In this randomized controlled study, 114 children with AD were randomized into two groups (57 each): Group A received conventional treatment, along with Bacillus clausii (Strains O/C, N/R, SIN and T) suspension available as Enterogermina® at the dose 2 billion spores/5 ml twice daily for 8 weeks and Group B receiving conventional treatment only. Baseline and follow-up SCORAD were assessed at 0, 4, 8, 12, 16, 20 and 24 weeks. Serum IL-17A levels were measured at baseline and 12 weeks.
Results:
There was no significant difference in mean SCORAD between the two groups at baseline, 12 weeks (p = 0.21) and 24 weeks (p = 0.26). The two groups did not differ significantly in terms of the number of patients who achieved SCORAD 90 (p = 0.19), SCORAD 75 (p = 0.59), and those who relapsed (p = 0.5). IL-17A levels were not significantly different between the two groups at baseline and 12 weeks (p = 0.7). There was no statistically significant correlation between IL-17A levels and AD severity.
Limitations:
Lack of double-blinding, lack of the use of placebo and a short follow-up period were the limitations of the present study.
Conclusion:
Administration of the probiotic Bacillus clausii in addition to conventional treatment does not offer any additional benefit in inducing remission or prevention relapse in AD.
KEY WORDS: Atopic eczema, bacillus clausii, probiotics
Introduction
Atopic dermatitis (AD) is an itchy and chronically relapsing, inflammatory skin condition which has a profound negative impact on the quality of life (QoL) of the affected children. Two morphological phenotypes are acute AD with polymorphic lesion and intense pruritus with a Th2 predominant response, and chronic AD with lichenification of the lesions and a Th1 predominant response. More recently, other proinflammatory cytokines like IL-17 and IL-23 have also been found to be correlated with AD severity as measured by SCORAD (scoring atopic dermatitis).[1] Topical corticosteroids and topical calcineurin inhibitors form the backbone of long-term management of AD. However, there is some evidence to suggest the role of probiotics in the prevention of AD although the studies on treatment are insufficient to reach any conclusion.[2,3] Probiotics can mediate their immuno-modulating action by restoring the physiological Th1 polarization and suppressing the Th2 response, thus inhibiting cytokines released by Th2 cells, i.e., IL-4, IL-5 and IL-13.[4] In the present study, we aimed to further explore the role of the probiotic Bacillus clausii in the treatment of AD and prevention of relapse and its effect on the immunologic milieu of the patients.
Methods
Consecutive children attending the Pediatric Dermatology Clinic were screened for recruitment into the study during the period between July 2016 and November 2017. The study was initiated after obtaining approval from the Institute's Ethics Committee and registration to the Clinical Trial Registry of India (Registration number CTRI/2017/08/009236).
The sample size was calculated by comparing the relative change of SCORAD scores according to observations in the study by Fölster-Holst et al.[5] Using the formula for comparing means, the required sample size was estimated at 114 patients, i.e., 57 in the arm receiving conventional treatment appropriate for disease severity with probiotic supplementation group and 57 in the other arm receiving conventional treatment appropriate for disease severity only.
Inclusion criteria
Children with AD diagnosed according to Hanifin and Rajka criteria between the ages of 6 months and 12 years.
All children were eligible irrespective of the disease severity.
Exclusion criteria
Age <6 months and >12 years.
Immunocompromised children.
Children with severe hepatic, renal or other systemic diseases.
Parents not consenting to their child's inclusion in the study.
Children having received any other probiotic in the preceding 6 months.
After obtaining informed assent/consent from the children/parents, detailed history and clinical examination including the disease severity using SCORAD scores of all patients were noted at the time of recruitment and the disease severity was graded according to the SCORAD index as mild (<25), moderate (26–50) and severe (>50) AD. Besides, the quality of life of the children was measured using the IDQOL and CDLQI questionnaire at baseline, 12 weeks and 24 weeks. We used IDQOL scoring also for children between the ages of 1 and 4 years.
The children who satisfied the inclusion and exclusion criteria were randomized into two parallel treatment groups (1:1):Group A (who received conventional treatment plus Bacillus clausii 2 billion spores per 5 ml suspension twice daily for 8 weeks; available as the brand name Enterogermina® manufactured by SANOFI; a French multinational pharmaceutical company), and Group B (who received conventional treatment only). Conventional treatments were similar in both the groups according to the severity of disease that included topical corticosteroids (fluticasone propionate 0.05% cream), topical calcineurin inhibitors (tacrolimus 0.03% ointment once night-time application in children <2 years of age and tacrolimus 0.1% ointment as once night-time application in children >2 years of age), oral steroids (oral prednisolone 0.3 mg/kg/day for 7–14 days) and other immunosuppressants like methotrexate (fixed weekly dose of 0.3 mg/kg/week), azathioprine (2 mg/kg/day) and emollients to be applied generously of 3–4 times/day or more. Random allocation was performed by block randomization using a computer-generated sequence. To prevent any recruitment bias, allocation concealment was conducted. Patients/parents were not blinded to treatment. All the patients followed up at 4 weeks of interval until 24 weeks from the date of recruitment. Patients who achieved complete clearance of skin lesions (defined as 90% reduction in SCORAD) were tapered off topical corticosteroids and other medications while maintaining only on emollients and were defined as being in remission. Besides, patients who achieved >75% reduction in SCORAD were said to be cleared though not completely cleared. Time of relapse was noted for any patients who suffered relapse with or without active treatment (defined as an increase in SCORAD to 50% of baseline value), and they were followed up. These patients were prescribed treatment as per the severity of relapse.
IL-17A was measured (by chemiluminescent ELISA) at baseline and week 12. The adverse effects of treatment were recorded and treated. For statistical analysis, data were entered in Microsoft Excel and analysis was performed by the statistical packages SPSS, release 20.0 (SPSS Inc. Chicago, IL, USA). For descriptive statistics, frequency, percentage and mean ± S.D. were used. For comparison of proportions, Fisher's exact test was performed. The paired t test was used to test for a significant difference between baseline and endpoint within the same group. Spearman's correlation test was used for comparison of mean scores where applicable. ANOVA test was used to compare three variables in IL-17A with duration, severity and age of onset of disease. Fisher's exact test was used for comparison of relapse between the two groups. All statistical tests were carried out at a significance level of 0.05. All the children were included in an intention-to-treat analysis regardless of compliance or withdrawal from the study. Children who were lost to follow-up were still included, and the data were included until they continued to follow up; a censoring method was applied for the data values when they lost to follow-up. Eleven children lost to follow-up with the attrition percentage of 9.64%.
Results
This was a prospective, parallel-group, randomized, single/observer-blinded study of probiotics supplementation in atopic dermatitis for 8 weeks with follow-up until 24 weeks from the day of recruitment. Of the 57 patients in Group A, 49 patients completed the study as 8 patients were lost to follow-up. In Group B, 54 patients completed the study as 3 patients were lost to follow-up. Five patients were lost to follow-up due to the far distance of the health facility and opted for treatment at nearby clinics, whereas two opted for homeopathic treatment and four had a complete improvement in the symptoms [Figure 1]. There were no significant differences between the two groups at baseline with respect to age, gender, disease severity, quality of life and other demographic variables [Table 1]. Fifty-four (47.4%) children had a family or personal history of atopy.
Figure 1.
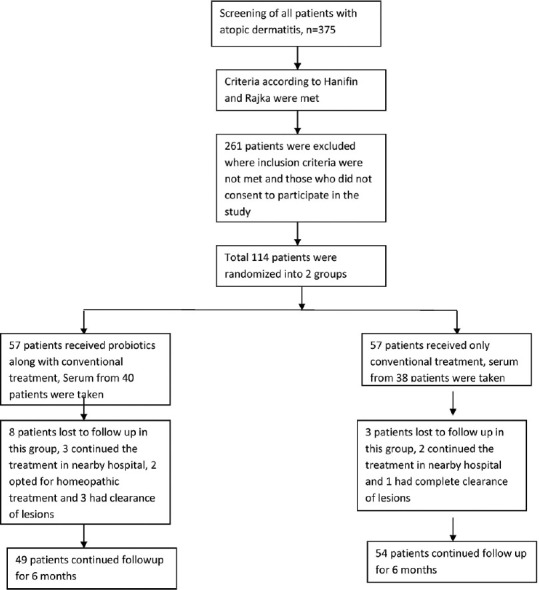
Flow chart of the study
Table 1.
Baseline characteristics of the study population
| Disease characteristic | Group A, n=57 mean±SD | Group B, n=57 mean±SD | P |
|---|---|---|---|
| Mean age of patients | 5.75±3.34 years (range 6 months–12 years) | 5.33±4.10 years (range 6 months–12 years) | 0.06 |
| M/F | 7.4:4 | 8.4:3 | |
| Duration of conventional treatments | 24 weeks | 24 weeks | |
| Types of conventional treatments | Topical steroids, topical calcineurin inhibitors, oral steroids, immunosuppressants | Topical steroids, topical calcineurin inhibitors, oral steroids, immunosuppressants | |
| Mean baseline SCORAD | 21.82±11.16 | 20.61±11.780 | 0.635 |
| Mean baseline CDLQI | 5.44±4.43 | 5.09±4.5 | 0.95 |
| Mean baseline DLQI | 8.87±6.24 | 6.25±4.98 | 0.26 |
| Mean baseline IL-17A | 384±1118.8 | 175±303.9 | 0.26 |
SCORAD—scoring atopic dermatitis; CDLQI—Children’s Dermatology Quality of Life Index; IDQOL—Infants’ Dermatitis Quality of Life Index; DLQI—Dermatology Quality of Life Index
Response to treatment
We could not find any significant difference in the primary clinical outcome (SCORAD) between Group A and Group B [Figures 2-3]. Intragroup comparison of mean SCORAD showed a significant difference from baseline to 12 and 24 weeks (p < 0.001) in both Group A and Group B. As there was a significant difference from the baseline to 24 weeks in both the groups, we could not find significant differences in intergroup analysis. When we compared the number of patients achieving >90% improvement in baseline mean SCORAD and >75% improvement in baseline mean SCORAD at 12 weeks and 24 weeks, there was no significant difference between the two groups. Similarly, there were no significant differences between the two groups concerning the disease severity [Table 2]. As a subgroup analysis, we assessed the effect of probiotics in the severity of disease in infants (ages <1 year). The mean baseline SCORAD among infants was found to be 20 ± 8.3 in Group A versus 18.2 ± 11.1 in Group B (p = 0.6). At weeks 12 and 24, SCORAD fell to 7.1 ± 8.5 in Group A and 8.4 ± 6.1 in Group B (P = 0.6). At week 24, mean SCORAD was 6.7 ± 7.5 in Group A and 8.5 ± 9.2 in Group B (P = 0.6). We compared the number of patients who relapsed after achieving remission. Five patients relapsed at 24 weeks—three in Group A (8.77%) and two in Group B, i.e., 3.5% (p = 0.5).
Figure 2.
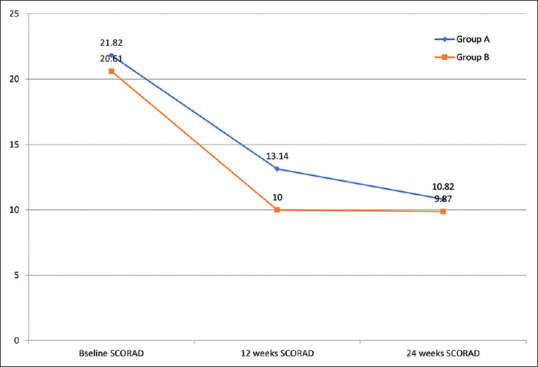
Comparison of mean SCORAD in Group A and Group B
Figure 3.
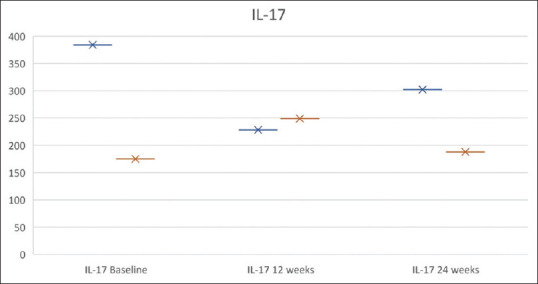
Response to treatment in terms of change in SCORAD and serum IL-17A levels in Group A and Group B at baseline, 12 weeks and 24 weeks
Table 2.
Effectiveness of probiotic supplementation in mild, moderate and severe disease
| Response to treatment in mild AD | |||
|---|---|---|---|
|
| |||
| Group A, n=37 | Group B, n=39 | P | |
| Mean baseline SCORAD | 15.16±4.317 | 14.13±5.44 | 0.363 |
| Mean SCORAD at 12 weeks | 11.03±9.647 | 7.84±6.109 | 0.098 |
| Mean SCORAD at 24 weeks | 9.25±7.886 | 7.18±8.269 | 0.291 |
| SCORAD 90 at 12 weeks | 3 | 7 | 0.231 |
| SCORAD 90 at 24 weeks | 6 | 15 | 0.067 |
| SCORAD 75 at 12 weeks | 8 | 10 | 0.56 |
| SCORAD 75 at 24 weeks | 10 | 18 | 0.175 |
| Mean baseline CDLQI | 4.48±3.429 | 3.75±1.962 | 0.368 |
| CDLQI at 12 weeks | 3.41±2.538 | 2.58±2.244 | 0.248 |
| CDLQI at 24 weeks | 3.77±3.161 | 2.71±2.579 | 0.216 |
| Mean baseline IDQOL | 7.33±6.228 | 4.13±1.959 | 0.071 |
| IDQOL at 12 weeks | 4.3±5.736 | 3.93±2.78 | 0.835 |
| IDQOL at 24 weeks | 4.70±6.76 | 3.71±2.92 | 0.630 |
|
| |||
| Response to treatment in moderate AD | |||
|
| |||
| Group A, n=18 | Group B, n=16 | P | |
|
| |||
| Mean baseline SCORAD | 31.56±4.87 | 32.19±6.14 | 0.74 |
| Mean SCORAD at 12 weeks | 15.6±8.28 | 13.29±10.75 | 0.52 |
| Mean SCORAD at 24 weeks | 12.73±10.42 | 16±16.9 | 0.53 |
| SCORAD 90 at 12 weeks | 1 | 2 | 0.5 |
| SCORAD 90 at 24 weeks | 3 | 3 | 0.59 |
| SCORAD 75 at 12 weeks | 3 | 5 | 0.33 |
| SCORAD 75 at 24 weeks | 7 | 6 | 0.65 |
| Mean baseline CDLQI | 7.25±5.37 | 7.7±6.51 | 0.86 |
| CDLQI at 12 weeks | 5.6±5.14 | 4.86±3.8 | 0.75 |
| CDLQI at 24 weeks | 6.8±5.94 | 6.57±4.86 | 0.93 |
| Mean baseline IDQOL | 9.5±5.75 | 11±6.7 | 0.68 |
| IDQOL at 12 weeks | 8.8±5.6 | 6.8±7.08 | 0.62 |
| IDQOL at 24 weeks | 7.2±3.89 | 7.5±7.47 | 0.93 |
|
| |||
| Response to treatment in severe AD | |||
|
| |||
| Group A, n=2 | Group B, n=2 | P | |
|
| |||
| Mean baseline SCORAD | 57.5±3.53 | 54.5±0.7 | 0.36 |
| Mean SCORAD at 12 weeks | 28.5±6.36 | 28±6.36 | 0.92 |
| Mean SCORAD at 24 weeks | 21.5±3.53 | 18±12.72 | 0.74 |
| SCORAD 90 at 12 weeks | 0 | 0 | |
| SCORAD 90 at 24 weeks | 0 | 0 | |
| SCORAD 75 at 12 weeks | 0 | 0 | |
| SCORAD 75 at 24 weeks | 0 | 1 | 0.5 |
| Mean baseline DLQI | 5.5±0.7 | 14.5±4.95 | 0.12 |
| DLQI at 12 weeks | 6±0.0 | 14.5±4.9 | 0.13 |
| DLQI at 24 weeks | 4±2.8 | 15±1.41 | 0.03 |
SCORAD—scoring atopic dermatitis; CDLQI—Children’s Dermatology Quality of Life Index; IDQOL—Infants’ Dermatitis Quality of Life Index; DLQI—Dermatology Quality of Life Index
There were no significant differences between the groups in secondary clinical outcomes, i.e., quality of life measured with CDLQI and IDQOL from baseline to 12 and 24 weeks though in intragroup comparison there was a significant improvement in IDQOL from baseline to 12 and 24 weeks in both groups and in CDLQI from baseline to 12 weeks in Group B only [Tables 1 and 2]. There was a positive correlation between CDLQI and SCORAD at baseline, 12 weeks and 24 weeks and a positive correlation between IDQOL and SCORAD at baseline, 12 weeks and 24 weeks [Figures 2 and 3].
The IL-17A levels were not significantly different between the two groups after the probiotic supplementation at 12 weeks though the mean IL-17A level decreased from baseline in Group A with probiotic supplementation, whereas the mean IL-17A concentration increased in Group B at 12 weeks and the mean difference between two groups was not significant. On comparing IL-17 and the SCORAD at baseline, there was no correlation of IL-17A concentration and the disease severity (SCORAD) (p = 0.09). Comparing IL-17A concentration based on age groups, patients under 1 year of age had mean IL-17A concentration of 141 ± 318.5 pg/ml, between 2 and 5 years had 225.4 ± 295.9 pg/ml, and >5 years had 192 ± 266 pg/ml at baseline. There was no significant difference in IL-17A according to the age with the P value 0.6 and according to the duration of disease (p = 0.9).
Discussion
There are numerous studies on the role of probiotics in AD where they are useful in the prevention of atopic dermatitis in younger children and infants, whereas the role of probiotics in the treatment of AD is debatable. Although the most widely used probiotics in previous studies are Lactobacillus rhamnosus GG, in the present study, we evaluated the effectiveness of Bacillus clausii in atopic eczema. Bacillus clausii is a 'spore-forming probiotic’ that has resistance to commonly used antibiotics and is involved in anti-microbial and immunomodulatory activity, regulation of cell growth and differentiation, cell signalling, cell adhesion, signal transcription and transduction.[6] The antimicrobial substances secreted by Bacillus clausii are active against Staphylococcus aureus. Bacillus clausii strains have also been shown to possess immunomodulatory activities in vitro studies by modulating nitric oxide (NOS II) synthetase activity and IFN-gamma production.[7] It has been previously used for the treatment of acute diarrhoeal diseases in children and in allergic rhinitis and also in the prevention of late onset sepsis, recurrent respiratory infections in children and bone loss in post-menopausal women.[8,9,10,11,12] Based on these properties, we expected Bacillus clausii to have a beneficial effect in AD. However, in this RCT we did not observe any benefit of supplementation with Bacillus clausii. Although no adverse event was reported in our study, one needs to be cautious and monitor stringently as septicemia secondary to Bacillus clausii has been reported previously in the paediatric age group subsequent to its use as a probiotic.[13]
These findings are in contrast to the study by Isolauri et al.[14] where the authors found a significant improvement in the SCORAD in infantile AD in Lactobacillus GG or Bifidobacterium lactis Bb-12 group compared to placebo. However, we did not find any significant differences in the SCORAD on follow-up in this subgroup too. Another study by Wang et al.,[15] where older children were included, studied the comparison of SCORAD in AD patients receiving LP (Lactobacillus paracasei), LF (Lactobacillus fermentum), LP+LF mixture, and placebo for 3 months and found that SCORAD was substantially reduced after treatment in each group, at 12 weeks both within the group (intragroup comparisons P < 0.05) and in comparison with placebo (intergroup comparisons, P < 0.001). Our observations are in concordance with the study by Gruber et al.[16] who also studied its effect in AD in infants (3–12 months) and found a significant reduction in the severity of SCORAD in both probiotics (Lactobacillus rhamnosus GG) and placebo group though there was no significant difference between the groups at 12 weeks with the P value 0.28. We could not find any previous studies on the use of Bacillus clausii strain as probiotics for the treatment of the prevention of AD for directly comparing the results of our study. One outcome which could not be adequately evaluated in the present study was the frequency of relapse between the two groups because of short follow-up as very few patients, i.e., only 5 patients, relapsed at 6 months.
One explanation regarding the lack of any benefit of the use of Bacillus clausii supplementation could be the choice of the probiotic as a majority of the studies with a beneficial effect have used Lactobacillus rhamnosus GG. Besides, the beneficial effect may be achieved by using an even higher concentration of probiotics. Probiotic amounts in currently used formulations show a wide variability which likely implies a high variability in the number of viable cells included in the products. Therefore, only formulations with a very high bacterial load may exert a positive effect, while those with a low bacterial content have no effect. Regarding IL-17A levels, we observed a difference in trend regarding a change in IL-17A levels between two groups as the mean IL-17A concentration decreased from baseline to 12 weeks in Group A, whereas there was an increase in the mean IL-17A concentration from baseline to 12 weeks in Group B. Hence, we could not conclusively observe any effect of treatment on the change in IL-17A levels in AD. Indrio et al.[17] studied the role of early probiotics supplementation in preterm babies and found that the concentration of IL-17A in the faecal sample was significantly low in the probiotics supplementation group when compared to placebo after 3 months.
The mean CDLQI at baseline was 5.3 ± 4.4 which was lower than that observed in few other studies such as the study by Wang et al. where CDLQI ranged from 10 to 12 out of 30 in AD. Both groups recorded an improvement in QoL with treatment. On comparison of CDLQI and IDQOL between groups, mean CDLQI at 12 weeks and 24 weeks was not significantly different between the two groups. In our study, we observed a significant correlation between CDLQI and IDQOL with the severity of the disease, consistent with the study by Ben-Gashir et al.[18] There are very few studies of the quality-of-life indices in AD in the Indian population. We can infer that these quality-of-life indices can be used as a marker of the severity of disease in an inflammatory condition like AD. One of the main limitations of the study was the lack of double-blinding and the lack of the use of placebo. As mentioned earlier, a longer follow-up would have given more information on the effect of probiotics on the frequency of disease relapse. Another limitation was the use of conventional treatment in the form of various topical and systemic agents as per disease severity that may bias the results in favour of conventional treatment rather than interventional product. However, this also reflects the real-life treatment scenario where a variety of therapeutic modalities are employed in treating AD.
Concluding, based on the observations made in the present study, we did not find any evidence regarding the usefulness of adding the probiotic Bacillus clausii in addition to the conventional treatment in the management of AD. Moreover, serum levels of IL-17A neither correlated with the severity of AD nor did change significantly with an improvement in disease severity, thereby implying a relative lack of pathogenetic correlation between IL-17A levels and AD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Leonardi S, Cuppari C, Manti S, Filippelli M, Parisi GF, Borgia F, et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): Association with clinical severity and phenotype. Allergy Asthma Proc. 2015;36:74–81. doi: 10.2500/aap.2015.36.3808. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–21.e1. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, Murrell DF, Tang MLK, Roberts A, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135. doi: 10.1002/14651858.CD006135.pub3. doi: 10.1002/14651858.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SO, Kim HJ, Kim YJ, Kang MJ, Kwon JW, Seo JH, et al. Asthma prevention by lactobacillus rhamnosus in a mouse model is associated with CD4(+) CD25(+) Foxp3(+) T cells. Allergy Asthma Immunol Res. 2012;4:150–6. doi: 10.4168/aair.2012.4.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fölster-Holst R, Müller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol. 2006;155:1256–61. doi: 10.1111/j.1365-2133.2006.07558.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopetuso LR, Scaldaferri F, Franceschi F, Gasbarrini A. Bacillus clausii and gut homeostasis: State of the art and future perspectives. Expert Review Gastroenterol Hepatol. 2016;10:943–8. doi: 10.1080/17474124.2016.1200465. [DOI] [PubMed] [Google Scholar]
- 7.Urdaci MC, Bressollier P, Pinchuk I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J Clin Gastroenterol. 2004;38:S86–90. doi: 10.1097/01.mcg.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 8.Ianiro G, Rizzatti G, Plomer M, Lopetuso L, Scaldaferri F, Franceschi F, et al. Bacillus clausii for the treatment of acute diarrhea in children: A systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10:1074. doi: 10.3390/nu10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar HY, Pal S, Shukla P, Mishra PK, Tomar GB, Chattopadhyay N, et al. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition. 2018;54:118–28. doi: 10.1016/j.nut.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Marseglia GL, Tosca M, Cirillo I, Licari A, Leone M, Marseglia A, et al. Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: A pilot study. Ther Clin Risk Manag. 2007;3:13–7. doi: 10.2147/tcrm.2007.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii effects in children with allergic rhinitis. Allergy. 2005;60:702–3. doi: 10.1111/j.1398-9995.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 12.Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: A randomized controlled trial. J Trop Pediatr. 2015;61:377–85. doi: 10.1093/tropej/fmv050. [DOI] [PubMed] [Google Scholar]
- 13.Joshi S, Udani S, Sen S, Kirolikar S, Shetty A. Bacillus clausii septicemia in a pediatric patient after treatment with probiotics. Pediatr Infect Dis J. 2019;38:e228–30. doi: 10.1097/INF.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 14.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604–10. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin Exp Allergy. 2015;45:779–87. doi: 10.1111/cea.12489. [DOI] [PubMed] [Google Scholar]
- 16.Gruber C, Wendt M, Sulser C, Lau S, Kulig M, Wahn U, et al. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy. 2007;62:1270–6. doi: 10.1111/j.1398-9995.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 17.Indrio F, Riezzo G, Tafuri S, Ficarella M, Carlucci B, Bisceglia M, et al. Probiotic supplementation in preterm: Feeding intolerance and hospital cost. Nutrients. 2017;9:965. doi: 10.3390/nu9090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284–90. doi: 10.1111/j.1365-2133.2004.05776.x. [DOI] [PubMed] [Google Scholar]


