Abstract
Background:
During the gathering of demographic data for the biobank on Buerger’s Disease (BD), we found that, after the clinical manifestation of BD, the patients usually became infertile, and the age of their last child was compatible with the time of disease diagnosis. The aim of this study was to evaluate the underlying cause of secondary infertility in BD patients.
Methods:
Anti-sperm antibodies (ASA), testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) in the sera of 39 male BD patients were measured and compared with 39 age-matched Caucasian male controls.
Results:
Six patients declared that they suffered from impotency. The ASA level was positive in 25.6% of the patients and 2.4% of the controls (p= 0.003, CC= 6.96). The mean levels of testosterone in the patients and controls were 393.12±32.9 ng/dl and 354.37±30.9 ng/dl, respectively. The mean levels of LH in the patients and controls were 0.88±0.12 mIU/r and 0.85±0.1 mIU/r, respectively. The mean levels of FSH in the patients and controls were 4.1± 0.35 mIU/r and 3.56±0.33 mIU/r, respectively. No significant difference in the serum levels of testosterone, LH, or FSH was found between the patients and controls (p> 0.05). The spermograms of three ASA-negative patients demonstrated impaired sperm motility.
Discussion
Anti-sperm antibodies, disturbed genital circulation, autonomic dysfunction and sperm motility may be responsible for secondary infertility in Buerger’s Disease.
Key Words: Anti-sperm antibody, Buerger’s Disease, Infertility, Thromboangiitis Obliterans
Introduction
Thromboangiitis Obliterans, or Buerger’s Disease (BD), is an inflammatory-occlusive peripheral vascular disease which may lead to tissue gangrene and limb loss (1). BD has a worldwide distribution, although it is more common in the Middle-East, South-East Asia, Eastern Europe and South-America. BD usually is diagnosed in young men, and it appears that tobacco use is a risk factor for disease development and prognosis (2). However, smoking cessation during the disease flare-up might not prevent limb amputation and limb loss. Until recently, the trigger for developing BD was unknown, and it is not yet known if BD is an autoimmune vasculitis, or a peripheral vascular disease induced by an unknown etiological factor besides smoking (3). Neither it is not clear if BD is a systemic disease or a local vasculopathy
During the gathering of demographic data for the biobank on Buerger’s Disease, we found that, after the clinical manifestation of BD, the patients became infertile and the age of their last child was compatible with the time of disease diagnosis.
Notably, reproduction is value in Eastern culture and secondary infertility in BD patients who are almost always young males, could impose frustration, depression, and guilt in them (4) and consequently influence on their quality of life along with their physical disability.
The aim of this study was to evaluate the underlying cause of BD patients’ secondary infertility.
Materials and Methods
Subjects
During a cross-sectional study between February 2016 and February 2018, the patients who reported to the clinic with the diagnosis of Buerger’s Disease were included in this study. BD diagnosis was according to Shionoya’s criteria including 1-age of disease onset before 50y, 2-history of smoking, 3-occlusion of infra-popliteal arteries, 4-involevemnt of upper limbs or thrombophlebitis migrans, 5- absence of atherosclerotic risk factors other than smoking (5) with angiography or computed tomography angiography (CTA) confirmation. Of the reporting patients, those who had at least one child before disease onset and did not use any birth control method were enrolled in the study. Written informed consent was obtained from the patients (ethical code: IR.MUMS.fm.REC.1395.301).
The control group was selected randomly from the serum bank of healthy men who were age-matched with the BD group (39 out of 487 samples). The controls were diagnosed as healthy according to medical history, physical examination and normal laboratory tests including complete blood count, fast blood sugar, liver and renal function tests and lipid profile.
The serum levels of anti-sperm antibodies (ASA), testosterone, luteinizing hormone (LH), and follicle stimulating hormone (FSH) were measured in both the patients and controls.
A solid-phase sandwich enzyme immunoassay (ELISA) was used for the quantitative determination of anti-spermatozoa antibodies in the serum samples (Genway, San Diego, USA). The normal range for ASA is 0–60 U/ml, and above 60U/ml was considered positive based on the kit’s protocol.
Testosterone was measured by radioimmunoassay (RIA) kit (Parc Marcel Boiteux, Codolet, France). The normal range for healthy males is 280–1100 ng/dl.
The LH and FSH were also measured by RIA kits (PadyaTeb, Iran), and the normal ranges for LH and FSH are 0.8–11 mIU/r and 1–13 mIU/r, respectively.
Statistical Analysis
Statistical analysis was performed using SPSS version 11.5. The descriptive data are presented as the mean±standard deviation (mean±SE). Kolmogorov-Smirnov tests were used to determine the normality distribution of data. An independent sample T-test was used to compare the serum levels of testosterone, LH, and FSH between the BD group and controls. A chi-square test was considered to compare the positive ASA tests in the BD patients and the controls. A p value less than 0.05 was considered statistically significant
Results
In total, 42 patients with the diagnosis of BD reported to the clinic due to flare-up (71.4%) or follow-up (28.6%). Of these 42 patients, 39 patients enrolled in the study according to the inclusion criteria, of which two were single with no children and also did not intend to have children, and another patient had two children after the onset of the disease. Thus, the study was conducted with 39 patients and 39 aged-matched controls. The mean age of the BD patients and controls was 41.43±7.6 and 41.9±9.2 years, respectively. All of the patients were active smokers. The patients who reported to the clinic due to flare-ups suffered from progressive claudication, chronic ulcers, local gangrene, or burning pain. The patients who reported for follow-up suffered from unchanged claudication and cold sensitivity. The mean number of children of the patients was 2±1 child/children ranging from 1 to 4. Six patients who had suffered from Buerger’s Disease for more than 15 years complained of erectile dysfunction but not libido. Only one of them was satisfied with sildenafil before sexual intercourse. Four patients suffered from orgasmic dysfunction soon after their disease diagnosis. For two of the patients, secondary infertility had led to divorce.
The ASA level was positive in ten patients (25.60%) and in one (2.4%) control (p= 0.003, CC: 6.96). The mean age of the patients with and without the ASA-positive test result was 43.6±2.2 and 41.1±1.4 years, respectively (p= 0. 38). There was no correlation between adverse event outcome (major amputation) and the positive ASA test (p= 0.6).
Three patients with negative and one patient with positive ASA test results had spermograms. All of negative ASA patients had normal sperm volume, sperm count, and sperm shape, but there was a decrease in sperm motility in three patient and sperm agglutination in one patient with positive ASA (Figure 1). Unfortunately, the rest of the patients did not cooperate in undergoing the spermogram test.
Fig. 1.
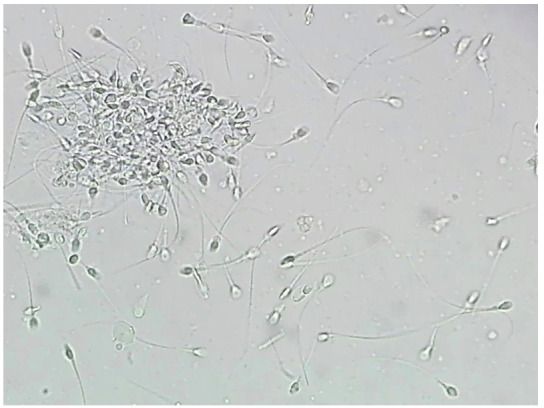
Sperm agglutination in a Buerger’s disease (BD) patient with negative serum anti-sperm antibody (X400 magnification).
The mean levels of testosterone in the BD group and the control group were 393.12 ±32.9 ng/dl and 354.3730.9 ng/dl, respectively. No significant difference was found between the testosterone levels in the two groups (p= 0.39). The mean levels of testosterone in patients with and without major amputation were 520±144.8ng/dl and 374.4±31.1 ng/dl, respectively (p= 0.1).
The mean levels of LH in the BD patients and the controls was 0.8± 0.12 mIU/r and 0.87±0.6 mIU/r, respectively. No significant difference was found between the LH serum levels of the two groups (p= 0.91).
The mean serum levels of FSH in the BD patients and the controls were 4.14±0.32 mIU/r and 3.53±0.31 mIU/r, respectively. There was no significant difference in the FSH levels of the patients and controls (p= 0.2).
Discussion
During the gathering of demographic data for the Buerger’s Disease biobank, we found that infertility after disease diagnosis is one of the problems that BD patients face. They usually tried to conceal it from their spouses, and those patients without children were concerned about it. Notably, we do not know how reliable the self-reporting on impotency and libido is, because infertility is a source of stigma for men in Iranian culture. This stigma is demonstrated by the fact that only three patients collaborated in the spermogram test. For two patients, the infertility had led to getting divorced and re-marrying, believing that their first wives were the infertile partners.
Because we did not have access to semen samples of more than three patients, to evaluate for secondary infertility in Buerger’s Disease, we first tested the serum levels of testosterone, LH, and FSH because, in disturbed genital circulation such as arteriosclerosis, the levels of these hormones are typically abnormal (4). However, there was no statistically significant difference in the serum levels of gonadal and sexual hormones between the patients and healthy controls. Yet, approximately 15% of the patients complained of erectile dysfunction without favorable response to Sildenafil, which may have been due to impaired genital circulation. Also, 9.5% of the patients complained of orgasmic dysfunction early after disease diagnosis which may have been due to autonomic dysfunction in BD patients (5).
There are a few case reports involving spermatic arteries in Buerger’s Disease (6-8). In these reports, the occlusion of a spermatic artery led to genital lesions. However, we do not yet know whether BD leads to impaired circulation of genitalia that does not involve thrombotic or ischemic lesions.
Immunological infertility was also evaluated in the patients. Because cultural concerns prevented most of the patients from cooperating in a spermogram test, we therefore conducted only the study on serum samples.
According to our findings, ASA was positive in approximately 25% of the patients. The prevalence of anti-sperm antibodies in the general population has been reported to be between 0 and 2.5% (9,10), which aligns with the results of our control group. Therefore, it appears that ASA may play a role in the secondary infertility of the patients. However, the origin of ASA in the serum of patients is still unknown, and we do not know if this type of autoantibody formation is due to an infectious pathogen and cross-reactions with auto-antigens, or because of an inflammation as a result of autoimmunity in Buerger’s Disease. The prevalence of ASA in various conditions is summarized in Table 1.
Table 1.
The prevalence of ASA in various conditions or diseases.
| Condition | Anti-Sperm Antibody Percentage | Secondary infertility? |
|---|---|---|
| Unexplained infertility (18) | 7% (male) | Primary and secondary |
| Infection (19) | 16.3% | Secondary |
| Systemic autoimmune diseases (20) | 7.1% | Secondary |
| Vasectomy (21) | 50-70% | Secondary |
| Cystic fibrosis (22) | 75% | Primary and secondary |
| Varicoceles (23) | 32% | Primary and secondary |
| Dysfunctional seminal vesicles (24) | 38.3% | Primary and secondary |
Several studies that evaluated ASA in both sperm and serum appear to indicate that the ASA serum level is about half of the ASA sperm (9). Therefore, if we consider that even half of the patients suffered from immunological infertility, and approximately 15% suffered from impotency due to impaired genitalia circulation, the question regarding underlying cause of infertility would remain in terms of the rest of the study subjects.
Impaired sperm motility was the only finding in the spermograms of the three ASA-negative patients. Various causes explain low sperm motility, such as varicocele, disturbed sex hormones, genetic disorders, and lifestyle and environmental factors, such as smoking.
Genetic disorders (11) and varicocele (12) are usually responsible for primary infertility, yet the gonadal and sexual hormones of the patients were in a normal range. Therefore, these two causes may not be responsible for the secondary infertility of the patients. In addition, many factors associated with lifestyle, such as nutrition, psychological stress, environmental and occupational exposures (13), and addiction to smoking (14) or illegal drugs (15) may influence fertility. Notably, most patients with Buerger’s Disease are heavy smokers (16). Some studies show the negative effects of smoking on sperm count, motility, and morphology (17), whilst others claim that there is no significant relationship between smoking and infertility (18). However, the BD patients began smoking many years before disease onset, and many had children who were born whilst the patients were smokers, before illness manifestation.
Notably, thrombogenicity and high oxidative stress, independent from smoking, has been demonstrated in BD patients (19,20). Interestingly, semen contains clotting and fibrinolysis factors which have a significant role in male reproductive physiology including the motility and function of sperms (21). Besides, high oxidative stress in semen could lead to male infertility by influencing on DNA, lipids, and proteins of sperms (22).
It is still unknown, if the serum level changes in coagulation and oxidative stress of BD patients also happened in the semen of these patients. But such changes might be responsible for secondary infertility of the ASA negative patients.
All in all, secondary infertility is a problem that many BD patients face. Owing to the fact that BD is a multifactorial disease and genetic background may play a role in developing the disease, secondary infertility may explain low BD prevalence, particularly in developing countries, as well as the lack of positive family history of Buerger’s disease. According to this study, impotency due to impaired genital circulation and ASA may play a role in a patient’s secondary infertility. In addition, the impaired sperm motility in three of the patients with negative ASA may be a clue to uncover the reason for the infertility of the rest of the patients.
However, the major limitation of this study was that we did not obtain sperm samples from most of the patients. But it is highly recommended to evaluate oxidative stress and coagulation and fibrinolytic activity in the semen of BD patients suffering from infertility. Nonetheless, the results of this study may be considered a step toward better understanding the pathophysiology of Buerger’s Disease. Because only one out of 40 patients had children after disease onset, we could not compare the BD patients with and without secondary infertility.
Acknowledgements
The authors would like to thank Prof. S. A. Rezaee and Mrs. Saeede Mehraban for their helpful comments and assistance in this project.
References
- 1.Fazeli B, Poredos P, Patel M, Klein-Weigel P, Catalano M, et al. Milestones in thromboangiitis obliterans: a position paper of the VAS-European independent foundation in angiology/vascular medicine. Int Angiol. 2021;40(5):395–408. doi: 10.23736/S0392-9590.21.04712-X. [DOI] [PubMed] [Google Scholar]
- 2.Fazeli B. Buerger's disease as an indicator of socioeconomic development in different societies, a cross-sectional descriptive study in the North-East of Iran. Arch Med Sci. 2010;6(3):343–7. doi: 10.5114/aoms.2010.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazeli B, Ligi D, Keramat S, Maniscalco R, Sharebiani H, Mannello F. Recent Updates and Advances in Winiwarter-Buerger Disease (Thromboangiitis Obliterans): Biomolecular Mechanisms, Diagnostics and Clinical Consequences. Diagnostics (Basel). 2021;11(10):1736. doi: 10.3390/diagnostics11101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeap BB. Androgens and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):269–276. doi: 10.1097/MED.0b013e3283383031. [DOI] [PubMed] [Google Scholar]
- 5.Singh K, Sood S. Autonomic functions in Buerger's disease. Indian J Physiol Pharmacol. 2001;45(4):470–4. [PubMed] [Google Scholar]
- 6.Kuwahara M, Matsushita K, Nakamura K, Yoshinaga H, Aki M, Fujisaki N, et al. (Thromboangiitis obliterans of the spermatic cord: a case report). Hinyokika kiyo. 1993;39(4):369–72. [PubMed] [Google Scholar]
- 7.Brehmer-Andersson E, Andersson L, Johansson JE. Hemorrhagic infarctions of testis due to intimal fibroplasia of spermatic artery. Urology. 1985;25(4):379–82. doi: 10.1016/0090-4295(85)90493-5. [DOI] [PubMed] [Google Scholar]
- 8.Fakour F, Fazeli B. Visceral bed involvement in thromboangiitis obliterans: a systematic review. Vasc Health Risk Manag. 2019;15:317–353. doi: 10.2147/VHRM.S182450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazumdar S, Levine AS. Antisperm antibodies: etiology, pathogenesis, diagnosis, and treatment. Fertility and sterility. 1998;70(5):799–810. doi: 10.1016/s0015-0282(98)00302-1. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich A, Bonfig R, Wilbert DM, Strohmaier WL, Engelmann UH. Risk factors for antisperm antibodies in infertile men. Am J Reprod Immunol. 1994;31(2-3):69–76. doi: 10.1111/j.1600-0897.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 11.Maduro MR, Lamb DJ. Understanding new genetics of male infertility. J Urol. 2002;168(5):2197–205. doi: 10.1016/S0022-5347(05)64355-8. [DOI] [PubMed] [Google Scholar]
- 12.Miyaoka R, Esteves SC. A critical appraisal on the role of varicocele in male infertility. Adv Uro. 2012;597495 doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calogero A, Polosa R, Perdichizzi A, Guarino F, La Vignera S, Scarfia A, et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod Biomed Online. 2009;19(4):564–71. doi: 10.1016/j.rbmo.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Rossato M, Pagano C, Vettor R. The cannabinoid system and male reproductive functions. J Neuroendocrinol. 2008;20(Suppl1):90–3. doi: 10.1111/j.1365-2826.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 16.Fleshman K. Buerger's disease in Nepal. Trop Doct. 1998 Oct;28(4):203–6. doi: 10.1177/004947559802800405. [DOI] [PubMed] [Google Scholar]
- 17.Colagar AH, Jorsaraee GA, Marzony ET. Cigarette smoking and the risk of male infertility. Pak J Biol Sci. 2007;10(21):3870–4. doi: 10.3923/pjbs.2007.3870.3874. [DOI] [PubMed] [Google Scholar]
- 18.Vine MF. Smoking and male reproduction: a review. Int J Androl. 1996;19(6):323–37. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 19.Adar R, Papa M Z, Schneiderman J. Thromboangiitis obliterans: an old disease in need of a new look. Int J Cardiol. 2000;75(Suppl 1):S167–70. doi: 10.1016/s0167-5273(00)00185-6. discussion S171-3. [DOI] [PubMed] [Google Scholar]
- 20.Sharebiani H, Fazeli B, Maniscalco R, Ligi D, Mannello F. The Imbalance among Oxidative Biomarkers and Antioxidant Defense Systems in Thromboangiitis Obliterans (Winiwarter-Buerger Disease). J Clin Med. 2020;9(4):1036. doi: 10.3390/jcm9041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lwaleed BA, Greenfield R, Stewart A, Birch B, Cooper AJ. Seminal clotting and fibrinolytic balance: a possible physiological role in the male reproductive system. Thromb Haemost. 2004;92(4):752–66. doi: 10.1160/TH04-03-0142. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


