Abstract
The Rob protein of Escherichia coli is a member of the AraC-XylS family of prokaryotic transcriptional regulators and is expressed constitutively. Deletion of the rob gene increases susceptibility to organic solvents, while overexpression of Rob increases tolerance to organic solvents and resistance to a variety of antibiotics and to the superoxide-generating compound phenazine methosulfate. To determine whether constitutive levels of Rob regulate basal gene expression, we performed a MudJ transposon screen in a rob deletion mutant containing a plasmid that allows for controlled rob gene expression. We identified eight genes and confirmed that seven are transcriptionally activated by normal expression of Rob from the chromosomal rob gene (inaA, marR, aslB, ybaO, mdlA, yfhD, and ybiS). One gene, galT, was repressed by Rob. We also demonstrated by Northern analysis that basal expression of micF is significantly higher in wild-type E. coli than in a rob deletion mutant. Rob binding to the promoter regions of most of these genes was substantiated in electrophoretic mobility shift assays. However, Mu insertions in individual Rob-regulated genes did not affect solvent sensitivity. This phenotype may depend on changes in the expression of several of these Rob-regulated genes or on other genes that were not identified. Rob clearly affects the basal expression of genes with a broad range of functions, including antibiotic resistance, acid adaptation, carbon metabolism, cell wall synthesis, central intermediary metabolism, and transport. The magnitudes of Rob's effects are modest, however, and the protein may thus play a role as a general transcription cofactor.
The Rob protein of Escherichia coli is a member of the AraC-XylS family of prokaryotic transcriptional regulators (12). Rob was first identified by its ability to bind the right arm of the origin of chromosomal replication (oriC) (30). The N-terminal DNA binding region of Rob (100 residues) is closely related to the E. coli SoxS protein, a regulator of the superoxide stress regulon (14, 18), and MarA protein, a regulator of the multiple antibiotic resistance regulon (for a review, see reference 2). The 175-residue C-terminal region of Rob does not have extensive sequence homology to known proteins, and its function has not been established.
Rob is a constitutively expressed protein (estimated up to 5,000 molecules per cell) (30), but its function is unclear. At present, the only phenotype described for an E. coli rob null mutant is increased susceptibility to organic solvents (35). Overexpression of Rob, on the other hand, increases the tolerance of E. coli to organic solvents (24), a phenotype that may be related to Rob-regulated expression of acrAB, which encodes an efflux pump (32, 35). Overexpression of Rob also increases resistance to antibiotics and to the superoxide-generating compound phenazine methosulfate (5, 24, 32). The latter two phenotypes overlap with those associated with increased expression of the well-characterized homologs of Rob, the MarA and SoxS proteins (2, 4).
Normal levels of Rob are proposed to contribute to ∼65% of in vivo transcription levels from the marRAB promoter (21), which regulates resistance to diverse antibiotics and bacteriocidal agents in E. coli (2, 13). Furthermore, in vivo studies (5) have shown that overexpression of Rob (or its N-terminal domain alone) activates transcription of sodA (encoding manganese-containing superoxide dismutase), fumC (encoding fumarase C), inaA (encoding a weak acid-inducible protein), and micF (gene for an antisense RNA repressing the outer membrane porin OmpF). In vitro studies (15) demonstrated Rob-activated transcription of sodA, fumC, micF, zwf (encoding glucose-6-phosphate dehydrogenase), nfo (encoding DNA repair endonuclease IV), and fpr (encoding NADPH-ferredoxin oxidoreductase). However, it is not known whether normal expression of Rob contributes to the in vivo expression of these genes. The Rob homologs MarA and SoxS can activate transcription of a broad range of genes in vivo (reviewed in references 2 and 14), including sodA, fumC, micF, zwf, and inaA, suggesting broadly overlapping activities of these three regulators. However, the observation that in vivo zwf transcription can be activated by MarA and SoxS, but not by Rob (5), shows that control by these regulators may not overlap completely. To gain more insight into these questions, we screened a transposon library in E. coli for Rob-regulated insertions, which revealed eight genes under the control of Rob.
MATERIALS AND METHODS
Bacterial strains and media.
The E. coli strains and plasmids used in this study are listed in Table 1. E. coli was cultured in Luria-Bertani (LB) medium or in M9 minimal medium containing 0.01% thiamine and 0.4% glucose (M9 medium) (29) as indicated. Cells were grown at 37°C in a shaking incubator (250 rpm) unless stated otherwise.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| GC4468 | F− Δ(lac)U169 rpsL | 33 |
| RA4468 | As GC4468 but Δrob::kan | 5 |
| MB4468 | As GC4468 but Δrob | This work |
| N7926 | As GC4468 but inaA1::lacZ (Kanr) zei-723::Tn10 (Tetr) | 28 |
| MB9701 | As RA4468 but inaA1::lacZ | This work |
| DH5α | F− K80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK−) phoA supE44 λ− thi-1 gyrA96 relA1 | Gibco BRL |
| Plasmids | ||
| pKO3 | Temperature-sensitive pSC101 origin of replication; sacB Cmr | 20 |
| pJP105 | ColE1 lacI lacZp::soxS Ampr | 27 |
| pMB101 | ColE1 lacI lacZp::rob Ampr | This study |
| pSRob | 926-bp SalI-SacI fragment containing the rob gene inserted into pSE380 | 5, 30 |
Materials and recombinant DNA techniques.
Antibiotics, isopropyl-β-d-thiogalactopyranoside (IPTG), and organic solvents were obtained from Sigma Chemical Co. (St. Louis, Mo.). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was obtained from Amersham (Piscataway, N.J.). Oligonucleotides were obtained from Operon Technologies Inc. (Alameda, Calif.). Restriction endonucleases, T4 DNA ligase, and T4 polynucleotide kinase were obtained from New England Biolabs (Cambridge, Mass.). [γ-32P]ATP and [α-32P]dATP were obtained from NEN Life Sciences Products (Boston, Mass.).
E. coli genomic DNA was isolated using a QIAGEN-tip kit (Qiagen, Valencia, Calif.). Plasmid DNA preparation, gel extraction of DNA fragments, and purification of DNA amplified by PCR were performed using QIAprep, QIAEX II, and QIAquick kits, respectively (Qiagen). Taq polymerase (Promega, Madison, Wis.) or Pfu DNA polymerase (Stratagene, La Jolla, Calif.) was used as described by the enzyme supplier. After subcloning of the PCR-generated fragments, correct amplification was verified by DNA sequencing (Molecular Biology Core Facility of the Dana Farber Cancer Institute, Boston, Mass.).
Construction of E. coli MB4468 and MB9701.
The Δrob mutant E. coli MB4468 was constructed in a GC4468 (wild-type [wt]) background by using vector pKO3, which contains a thermosensitive replication origin and a counterselection marker (kindly provided by G. M. Church, Harvard Medical School, Boston, Mass.), as described by Link et al. (20). In short, 500-bp fragments encompassing the 5′ and 3′ flanking regions of the rob gene were PCR amplified from E. coli GC4468 genomic DNA by using primer set No (outside, forward primer) and Ni (inside, reverse primer) and primer set Co (outside, reverse primer) and Ci (inside, forward primer), respectively (see Table 2). A second crossover PCR was performed with primers No and Co, using the PCR-amplified 3′ and 5′ flanking regions of rob as templates. This yielded a DNA fragment of about 1,000 bp, encompassing 500 bp of the flanking regions of the rob gene and a complete in-frame deletion of rob. This fragment was digested with EcoRV and BamHI, gel purified, and ligated to pKO3 that had been digested with SmaI and BamHI. Transformations were performed using E. coli strain DH5α, and cells were plated onto LB plates containing 20 μg of chloramphenicol/ml and incubated at 30°C. A plasmid with the correct insert (screened by PCR) was subsequently used for transformation of E. coli GC4468 (incubation at 30°C). Single Cmr colonies were picked and cultured at 30°C in 1 ml of LB broth containing chloramphenicol, and samples were plated on LB agar containing chloramphenicol and 5% sucrose and were incubated at 42°C. Screening by PCR and Southern hybridization revealed a positive recombinant lacking the rob gene. This Δrob mutant was designated MB4468.
TABLE 2.
Oligonucleotides used in this study
| Primer name | Sequence |
|---|---|
| AslBdir | 5′-AGCGCAGCTTGGTAGCGCAACTGG-3′ |
| AslBrev | 5′-CGTTGGAACCTGTTGCAGCATGG-3′ |
| Ci primer | 5′-GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGCGTTAACGCTGCAGCTCATCTAATGCA-3′ |
| Co primer | 5′-CGCGGATCCGCATATCATCAGTAGCGATTTAGGACG-3′ |
| Galpromdir | 5′-TTAGCACCCTCTCCGGCCAACG-3′ |
| Galpromrev | 5′-ACCACCGGTAACCAGAACTCTC-3′ |
| Marpromdir | 5′-GTAAACAAGGATAAAGTGTCACTC-3′ |
| Marpromrev | 5′-ATCTTTCTTCTGATTAACCATATGG-3′ |
| Mbarb1 | 5′-GGCCACTAGTCGACTAGTACNNNNNNNNNNGATAT-3′ |
| Mbarb2 | 5′-GGCCACTAGTCGACTAGTACNNNNNNNNNNACGCC-3′ |
| Mbarb3 | 5′-GGCCACTAGTCGACTAGTAC-3′ |
| MdlAdir | 5′-TGTCACGGTGGTTACCGAAATGC-3′ |
| MdlArev | 5′-TTAATTGAGCAAATAATCGCACG-3′ |
| MicFdir | 5′-GCGGGAGTTATTCTAGTTGCG-3′ |
| MicFrev | 5′-GTCGGCAAGTCCATTCTCCCC-3′ |
| Muleft | 5′-AATAATCCAATGTCCTCCCGG-3′ |
| Muleftnest | 5′-ACTACAGGCTTGCAAGCCCCA-3′ |
| No primer | 5′-AGCTTTGATATCGTTTTTAATACGATCCGACAGTTCAATCG-3′ |
| Ni primer | 5′-CACGCAATAACCTTCACACTCCAAATTTATAACCATAAAATATCCTCATCCTTTCAACAA-3′ |
| Randomdir | 5′-TCGCTGACGGTGACGGTTTGAGGCTTTCCGGCGGAAGGTATCCACAGACGTTGATACTGG-3′ |
| Randomrev | 5′-CCAGTATCAACGTCTGTGGATACCTTCCGCCGGAAAGCCTCAAACCGTCACCGTCAGCGA-3′ |
| RobHd3Fwd2 | 5′-TGTCGAAAGCTTGTTGTTGAAAGGATGAGG-3′ |
| RobBamrev | 5′-ATTAGAGGAGCCGCAGCGTTAACGACGG-3′ |
| YbaOdir | 5′-GTTAGTCAGCGTCGGTAGCGG-3′ |
| YbaOrev | 5′-CACGACAATTTATTCTGTGCGG-3′ |
E. coli MB9701 (as RA4468 but inaA1::lacZ) was obtained by introducing inaA1::lacZ (Kanr) from N7926 (28) into RA4468 by P1 cotransduction with a linked zei723::Tn10 (Tetr) marker and screening for both tetracycline resistance and blue coloration on plates containing X-Gal. P1 transductions were performed by standard methods (22).
Construction of the Rob expression vector pMB101.
To construct the Rob expression vector pMB101, allowing for IPTG-regulated Rob expression, the E. coli rob gene was PCR amplified from pSRob (5) using primers RobHd3Fwd2 and RobBamrev (see Table 2). The PCR product was digested with HindIII and BamHI and was ligated to pJP105 (27) that had been digested with the same enzymes. This Rob expression vector was introduced by transformation into E. coli MB4468, and mRNA levels of rob were determined by Northern analysis. Plasmid pMB101 was also introduced by transformation into E. coli MB9701 (Δrob inaA1::lacZ), and β-galactosidase activity was measured after IPTG-induced expression of Rob.
MudJ transposon mutagenesis.
The phage MudJ (promoterless lacZ; Kanr) was delivered to the Δrob strain MB4468/pMB101 by lambda phage transduction as described by Bremer et al. (7). Transductants were isolated on LB plates containing kanamycin (50 μg/ml) and ampicillin (100 μg/ml). Single colonies were picked and streaked individually onto LB agar plates containing either (i) ampicillin, kanamycin, and X-Gal (70 μg/ml) or (ii) ampicillin, kanamycin, X-Gal (70 μg/ml), and IPTG (0.1 mM) to promote expression of rob located on plasmid pMB101. Colonies displaying differences in blue coloration between the two plates were selected as candidates to harbor lacZ insertions in Rob-regulated loci. Insertional mutants were repurified by single-colony isolation and assayed for β-galactosidase activity in LB broth in the presence or absence of IPTG. Reproducible differences in the β-galactosidase activities of the mutants confirmed the MudJ insertions at Rob-regulated loci. For each mutant, the MudJ insertion was backcrossed into the wild-type strain GC4468 by P1 transduction (22). The MudJ insertions were then transferred from the GC4468 background into MB4468 (Δrob) by P1 transduction, and each pair of strains was assayed for β-galactosidase activity. Finally, pMB101 was introduced by transformation into the strains containing the MudJ insertions in a strain MB4468 (Δrob) background, and the resulting transformants were assayed for β-galactosidase in the presence and absence of IPTG.
Arbitrary primed PCR to locate MudJ insertions.
A first round of PCR was performed on chromosomal DNA of the mutants, using primer Muleft (complementary to the 5′ end of phage MudJ) and two different arbitrary primers, Mbarb1 and Mbarb2 (see Table 2). PCRs were carried out in standard PCR buffer, with 0.2 mM deoxynucleoside triphosphates, 1 μM primers, 5% dimethyl sulfoxide, 1 μg of template DNA, and 5 U of Taq polymerase (Promega) in a total volume of 50 μl (3 min at 95°C; 11 cycles of 1 min at 95°C, 50 s at 40°C, and 1 min at 72°C; 35 cycles of 1 min at 95°C, 50 s at 55°C, and 1 min at 72°C; followed by 5 min at 72°C). A second round of PCR was performed with primers Mbarb3 and the nested primer Muleftnest (Table 2), using 5 μl of the product of the first reaction as a template. These second-round PCRs were performed as described above, in a total volume of 100 μl (3 min at 95°C; 35 rounds of 40 s at 95°C, 50 s at 55°C, and 1 min at 72°C; followed by 5 min at 72°C). The extension products were gel purified and subsequently sequenced, using primer Muleftnest.
β-Galactosidase activities.
The β-galactosidase activities of bacterial cultures were determined as described by Miller (22). Cultures were grown overnight in LB broth or M9 glucose medium with shaking at 37°C as indicated, diluted 100-fold, and then grown to an optical density at 600 nm (OD600) of approximately 0.5. At that point, cultures were exposed to IPTG (1mM) for 30 or 60 min as indicated or were left untreated. All assays were performed in triplicate.
Organic solvent tolerance assays.
Cultures were grown overnight at 30°C in LB broth, subcultured (1/100) in LB broth, and grown for 2.5 h at 30°C. Serial 10-fold dilutions were made in 0.85% NaCl–0.1% (wt/vol) peptone. Aliquots (50 μl) were plated in duplicate onto LB agar in glass petri dishes and allowed to dry. The plates were overlaid with organic solvents (n-hexane, cyclohexane, or a 1:1 mixture of n-hexane–cyclohexane) to a depth of 2 to 3 mm and subsequently sealed with silicone rings. After incubation for 24 h at 30°C, colonies were counted, and the CFU per milliliter were calculated.
Northern blot analysis.
Cultures were grown overnight in LB medium, diluted 100-fold in 3 ml of LB broth, and grown at 37°C to an OD600 of 0.6. At this point, cultures were induced with different concentrations of IPTG for 30 min at 37°C or were left untreated. Total RNA was extracted using an RNeasy kit (Qiagen). The RNA was resuspended in diethyl pyrocarbonate-treated water and was quantified by measuring the A260. A total of 2 μg of RNA was run in a 1% agarose gel containing formaldehyde and was subsequently transferred to Nytran membranes using a Turboblotter setup. The RNA was cross-linked to the membrane by UV irradiation, followed by hybridization at 68°C with 32P-labeled DNA fragments using Quickhyb solution (Stratagene) and visualization by autoradiography. Probes were generated by standard techniques (29): a 52-mer derived from the micF sequence (custom synthesized by Operon Technologies Inc.) was end labeled and used as a micF probe; the rob probe was obtained by labeling PCR-amplified full-length rob (see “Construction of the Rob expression vector pMB101” above) using Klenow fragment.
Purification of Rob-His6 in E. coli.
The Rob protein with a C-terminal hexahistidine tag (Rob-His6) was purified as described by Kwon et al. (17). The purified Rob-His6 protein was assayed for its DNA binding activity by performing band shift assays with a 263-bp DNA fragment that was PCR amplified from the promoter region of micF (see below). Native Rob protein (kindly provided by K. Skarstad, University of Oslo, Oslo, Norway) was used as a control.
EMSA.
Promoter fragments of the mar operon, the gal operon, aslB, ybaO, and mdlA were amplified by PCR with E. coli GC4468 genomic DNA as the template by using the following respective primer sets (see Table 2): Marpromdir and Marpromrev; Galpromdir and Galpromrev; AslBdir and AslBrev; YbaOdir and YbaOrev; MdlAdir and MdlArev. MicFdir and MicFrev were used to PCR amplify the micF promoter region. These PCR-amplified fragments encompassed the (predicted) start of transcription and the 240- to 260-bp regions upstream of the respective genes. The gel-purified PCR fragments were 5′ end labeled with [γ-32P]ATP. DNA binding reaction mixtures (20 μl) contained 10 mM Tris-HCl (pH 8.0), 75 mM KCl, 10% (vol/vol) glycerol, 1 fmol of a 32P-labeled DNA fragment, and different amounts of Rob-His6 protein as indicated. All electrophoretic mobility shift assays (EMSA) were performed using Rob-His6 protein. In control assays, the binding affinity of Rob-His6 protein to the micF promoter region was compared to that of native Rob protein, and the apparent binding affinity for both proteins was confirmed to be the same. Where indicated, competitor DNA was added in 1:10 or 1:100 molar ratios over the probe DNA. Double-stranded, nonspecific competitor DNA was obtained by annealing 60-mer oligonucleotides containing no similarities to previously reported Rob binding sites (Randomdir and Randomrev [Table 2]). As competitor DNA, we used the double-stranded 35-mer micF promoter site 1, containing a known Rob binding site (5′-GTATTTGACAGCACTGAATGTCAAAACAAAACCTT-3′, kindly provided by H. J. Kwon, Harvard Medical School, Boston, Mass.). The binding reaction mixtures were incubated at room temperature for 15 min and then subjected to electrophoresis in 6% nondenaturing polyacrylamide gels (0.5 × Tris-borate-EDTA [TBE]) at 200 V for 2 to 3 h. The gels were dried and visualized by autoradiography.
RESULTS
To evaluate whether Rob controls the expression of proteins in E. coli, we compared the total cellular protein of the E. coli wt strain GC4468 and Δrob strain RA4468 in a pulse-labeling experiment. We observed at least five protein bands on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel with higher intensities in the wt strain than in the Δrob strain in late-exponential-phase cultures (data not shown), indicating that several genes in E. coli are, directly or indirectly, controlled by Rob.
Regulated Rob expression from plasmid pMB101.
To identify Rob-regulated genes, a MudJ-lacZ transposon library was created in strain MB4468 (Δrob/pMB101). Plasmid pMB101 allows for IPTG-inducible activation of the expression of Rob protein under the control of the lac promoter (see Materials and Methods). To test the transcriptional activation of the rob gene from pMB101 in the Δrob strain MB4468, we used Northern analysis to compare rob mRNA expressed from pMB101 with the native rob mRNA in wt E. coli GC4468. The expression of rob from pMB101 was observed at IPTG concentrations as low as 0.05 mM, with mRNA levels exceeding those of wt E. coli (Fig. 1A). The rob message from pMB101 was slightly longer than the wt message, due to the displaced transcriptional start site provided by the lacZ promoter. The untransformed Δrob strain showed no detectable rob-specific mRNA (Fig. 1A).
FIG. 1.
Regulated rob expression. (A) Northern blot of rob mRNA. RNA was extracted from cultures of E. coli GC4468 (wt), MB4468 (Δrob), or MB4468/pMB101 after exposure to different concentrations of IPTG for 30 min at 37°C. Autoradiograms were exposed for ∼8 h. (B) To confirm that equivalent amounts of RNA were loaded, rRNA bands were visualized by ethidium bromide staining. (C) Expression of the rob-regulated gene inaA. The β-galactosidase activity of E. coli MB9701 (Δrob inaA1::lacZ) containing plasmid pMB101 was quantified as a function of IPTG concentration in the growth medium.
To test whether Rob expressed from pMB101 could activate transcription in vivo, we measured the transcriptional induction of the inaA1::lacZ fusion in E. coli MB9701 containing pMB101 at different IPTG concentrations in LB broth (Fig. 1C) and evaluated the blue coloration on LB plates containing different IPTG concentrations after overnight incubation. The visual discrimination between uninduced and induced replicas of E. coli MB9701/pMB101 grown on plates was optimal at 0.1 mM IPTG. This concentration of IPTG resulted consistently in a 1.5-fold increase in β-galactosidase activity from the inaA1::lacZ fusion (Fig. 1C) and was used for screening for MudJ insertions responsive to Rob.
Isolation of Rob-regulated insertion mutants.
To identify genes regulated by Rob, we screened a library of random lacZ transcriptional fusions (generated by MudJ) for differential expression in the presence and absence of Rob. After 11,000 MudJ insertion mutants were screened on plates, 109 colonies were further analyzed in liquid medium, and 20 of these showed ≥2-fold differences in β-galactosidase expression in the presence of 1 mM IPTG. To confirm regulation by Rob in a physiologically relevant setting, the MudJ insertions from these mutants were transferred into the wt strain GC4468 and subsequently into the Δrob strain MB4468 by P1 transduction. Plasmid pMB101 was introduced in the Δrob strains to allow controlled Rob expression.
After assays of strains grown in LB broth, MudJ insertions were chosen that showed (i) ≥1.5-fold differences in β-galactosidase activity between the wt and Δrob backgrounds and (ii) ≥2-fold differences in β-galactosidase activity in the Δrob/pMB101 background dependent on IPTG. These criteria were met by 12 MudJ insertions (listed in Table 3). Of these, 11 showed 1.5- to 2.2-fold higher levels of β-galactosidase activity in the wt background than in the Δrob background, indicative of Rob-induced expression. Significantly higher expression of all these insertions was also observed after IPTG induction of Rob from pMB101, ranging from 2.8- to 19.5-fold (Table 3). A single insertion (mb63) showed lower β-galactosidase activity in the wt than in the Δrob background (1.5-fold), indicating negative regulation. Rob repression of this fusion in the Δrob/pMB101 background was 2.3-fold (Table 3).
TABLE 3.
β-Galactosidase activities of Rob-dependent promoter-lacZ fusions in MudJ mutants grown in rich mediuma
| Insertion | Insertion site | β-Galactosidase activity (Miller units) in LB broth in the indicated background
|
|||||
|---|---|---|---|---|---|---|---|
| rob+ (wt) | Δrob | Expression ratio | Δrob/pMB101
|
||||
| Uninduced | Induced | Expression ratio | |||||
| mb46 | inaA | 14.4 ± 1.3 | 7.6 ± 0.3 | 1.9 | 10.3 ± 0.4 | 87.7 ± 1.2 | 8.5 |
| mb107 | inaA | 19.9 ± 1.8 | 10.1 ± 0.2 | 2.0 | 19.5 ± 1.0 | 134 ± 5 | 6.9 |
| mb12 | marR | 111 ± 6 | 45.3 ± 0.6 | 2.5 | 112 ± 3 | 314 ± 21 | 2.8 |
| mb63 | galT | 516 ± 16 | 763 ± 34 | 1.5 (−)b | 628 ± 28 | 275 ± 25 | 2.3 (−) |
| mb38 | aslB | 2.6 ± 0.4 | 1.2 ± 0.4 | 2.1 | 2.2 ± 0.1 | 33.0 ± 0.5 | 14.8 |
| mb17 | ybaO | 3.4 ± 0.3 | 1.5 ± 0.02 | 2.2 | 2.6 ± 0.4 | 49.9 ± 3.1 | 19.5 |
| mb55 | ybaO | 1.9 ± 0.2 | 1.0 ± 0.3 | 1.9 | 1.2 ± 0.3 | 17.9 ± 1.3 | 15.2 |
| mb83 | mdlA | 11.0 ± 0.9 | 7.5 ± 0.3 | 1.5 | 12.3 ± 0.3 | 38.1 ± 0.9 | 3.1 |
| mb33 | yfhD | 17.5 ± 1.5 | 9.5 ± 0.6 | 1.8 | 17.1 ± 0.3 | 79.0 ± 4.9 | 4.6 |
| mb41 | yfhD | 11.5 ± 0.8 | 6.4 ± 0.3 | 1.8 | 11.4 ± 0.5 | 50.8 ± 2.2 | 4.5 |
| mb48 | yfhD | 15.3 ± 0.1 | 8.1 ± 0.2 | 1.9 | 15.5 ± 0.6 | 53.4 ± 4.2 | 3.4 |
| mb108 | ybiS | 49.2 ± 2.4 | 30.3 ± 1.8 | 1.6 | 47.9 ± 1.6 | 142 ± 3 | 3.0 |
Strains were grown in LB medium (overnight at 37°C), subcultured (1/100), grown to an OD600 of 0.5, and incubated for an additional 1 h prior to sampling. Strains carrying pMB101 were grown to an OD600 of 0.5, when IPTG (1 mM) was added to the cultures. Incubation was continued for 1 h, and samples were taken. Values represent averages of three different measurements, and standard deviations are indicated.
For the mb63 insertion (galT), (−) indicates negative control by Rob.
Identities of the Rob-regulated genes.
To identify the genetic loci of the 12 Rob-regulated MudJ insertions, the DNA sequences of the junctions between the 5′ end of MudJ and the DNA proximal to the fusion junction were determined and compared with the E. coli genomic DNA sequence (6). The positions of the MudJ insertions are shown schematically in Fig. 2.
FIG. 2.
Locations of insertions of transcriptional fusions of MudJ containing promoterless lacZ in Rob-regulated genes identified in this study. Arrows indicate the direction of transcription of genes and of lacZ.
Insertions mb46 and mb107 both mapped to inaA, a gene that encodes a weak-acid-inducible protein of unknown function (36). The mb12 insertion mapped to the 3′ end of the first gene of the marRAB operon, marR. The multiple antibiotic resistance (mar) locus controls an adaptive response to antibiotics and other environmental stresses (reviewed in reference 2). Both inaA and marRAB are known Rob-regulated genes (5, 21). The other nine insertions mapped to genes that were not known to be regulated by Rob. The mb63 insertion was located in the second member of the galETKM operon, galT, encoding galactose-1-phosphate uridylyltransferase (1). Interestingly, the galT insertion was the only Rob-repressed gene identified in this study (Table 3). The mb38 insertion was located in aslB, encoding a potential positive regulator of arylsulfatase (6, 23). Insertions mb17 and mb55 were both located in ybaO. This gene codes for a predicted protein that has a strong similarity to leucine-responsive regulatory protein (Lrp) from E. coli and to Lrp homologs in several other bacteria (25, 34). Insertion mb83 mapped in mdlA, directly downstream of ybaO. The mdlA gene encodes a protein related to the ATP-binding component of a multiple drug resistance transport system (3, 6). Insertions mb33, mb41, and mb48 all mapped to the yfhD gene. This gene codes for a predicted protein that has been annotated as a putative periplasmic binding transport protein (6). The mb108 insertion was located directly upstream of the ybiS open reading frame, which has no known function or homolog.
The positions of the insertions downstream from the start codons were as follows: inaA (651 bp), insertion mb46 at bp 572, mb107 at bp 350; marR (378 bp), insertion mb12 at bp 304; galT (1,047 bp), insertion mb63 at bp 812; aslB (1,236 bp), insertion mb38 at bp 1004; ybaO (546 bp), insertion mb17 at bp 176, mb55 at bp 174; mdlA (1,773 bp), insertion mb83 at bp 921; yfhD (1,419 bp), insertion mb33 at bp 119, mb41 at bp 57, mb48 at bp 891; ybiS (921 bp), insertion mb108 at 31 bp upstream of start codon ybiS and 178 bp upstream of start codon ybiT.
As indicated above, metabolic labeling of late-exponential cultures of E. coli showed at least five protein bands with higher intensities in the wt strain than in the Δrob strain, with Mrs of approximately 30,000, 35,000, 55,000, 60,000, and 115,000 (data not shown). These values were compared with the predicted masses of the proteins encoded by the Rob-regulated genes and operons identified in this study and with those of proteins encoded by genes known to be induced by Rob in vivo (5) or in vitro (15, 32), namely, InaA (25.3 kDa), the mar operon products (13.9 kDa [MarR], 15.4 kDa [MarA], and 7.5 kDa [MarB]), the gal operon products (37.3 kDa [GalE], 39.6 kDa [GalT], 41.4 kDa [GalK], and 38.2 kDa [GalM]), AslB (46.6 kDa), YbaO (20.9 kDa), the mdl operon products (66.0 kDa [MdlA] and 65.2 kDa [MdlB]), YfhD (53.2 kDa), YbiS (33.3 kDa), AcrA (42.2 kDa), AcrB (114 kDa), Fpr (27.8 kDa), FumC (50.5 kDa), Nfo (31.5 kDa), SodA (23.1 kDa), and Zwf (55.7 kDa).
Either the Mr 30,000 or the Mr 35,000 protein band may correspond with the Rob protein, which has a predicted mass of 33.1 kDa, or with proteins encoded by ybiS (33.3 kDa), fpr (27.8 kDa), or nfo (31.5). The band with an Mr of ∼55,000 could correspond with proteins encoded by yfhD (53.2 kDa) or zwf (55.7 kDa); however, previous studies showed no in vivo induction of zwf by Rob (5). The band with an Mr of ∼60,000 could correspond with MdlAB (66.0 and 65.2 kDa). In this study, we did not identify a Rob-regulated gene encoding a protein with an Mr of ∼115,000, but this band might correspond with AcrB (114 kDa). No candidate proteins corresponding to proteins encoded by the mar operon, inaA, ybaO, fumC, sodA, or acrA were observed.
Expression of Rob-dependent genes in minimal medium.
We determined the effect of Rob on transcription of the MudJ insertions in minimal medium. The Rob-dependent expression ratios in wt compared with Δrob backgrounds were significantly lower in M9 medium than in LB broth for all fusions except for ybiS, dropping below 1.5-fold for the following insertions: mb107 (inaA), mb63 (galT), mb83 (mdlA), mb33 and mb48 (yfhD), and mb108 (ybiS) (Table 4). Furthermore, the inducibility of the transcriptional lacZ fusions in response to Rob induction in the Δrob/pMB101 background was reduced: induction levels of the inaA, aslB, ybaO, mdlA, and yfhD fusions were, respectively, 2-, 7-, 2.5-, 1.5-, and 2-fold lower in M9 medium than in LB broth (Table 3 versus Table 4). The expression of the fusion directly upstream of ybiS remained unchanged in the two different media, while no Rob-mediated repression of the galT fusion was observed in M9 medium (Table 4). These results indicated that Rob-dependent effects on gene expression are strongly influenced by the growth medium.
TABLE 4.
β-Galactosidase activities of Rob-dependent promoter-lacZ fusions in MudJ mutants grown in minimal mediuma
| Insertion | Insertion site | β-Galactosidase activity (Miller units) in M9 minimal medium supplemented with 0.4% glucose in the indicated background
|
|||||
|---|---|---|---|---|---|---|---|
| rob+ (wt) | Δrob | Expression ratio | Δrob/pMB101
|
||||
| Uninduced | Induced | Expression ratio | |||||
| mb46 | inaA | 10.0 ± 0.3 | 6.5 ± 0.2 | 1.6 | 7.0 ± 0.4 | 27.4 ± 0.4 | 3.9 |
| mb107 | inaA | 12.6 ± 0.5 | 9.6 ± 0.6 | 1.3 | 9.6 ± 1.3 | 31.6 ± 1.4 | 3.3 |
| mb12 | marR | NDb | ND | ND | ND | ND | ND |
| mb63 | galT | 12.3 ± 1.4 | 14.9 ± 0.8 | 1.2 (−)c | 14.6 ± 1.0 | 12.8 ± 1.1 | 0.9 |
| mb38 | aslB | 2.0 ± 0.3 | 1.2 ± 0.1 | 1.7 | 1.7 ± 0.2 | 3.2 ± 0.4 | 1.9 |
| mb17 | ybaO | 1.1 ± 0.1 | 0.6 ± 0.05 | 1.9 | 0.9 ± 0.2 | 7.5 ± 0.5 | 7.9 |
| mb55 | ybaO | 1.0 ± 0.1 | 0.4 ± 0.1 | 2.2 | 0.6 ± 0.1 | 3.4 ± 0.8 | 5.5 |
| mb83 | mdlA | 5.4 ± 0.03 | 4.9 ± 0.2 | 1.1 | 13.7 ± 0.5 | 30.8 ± 1.1 | 2.2 |
| mb33 | yfhD | 11.0 ± 1.5 | 8.7 ± 0.4 | 1.3 | 9.5 ± 0.7 | 24.4 ± 0.7 | 2.6 |
| mb41 | yfhD | 8.5 ± 2.0 | 5.3 ± 0.4 | 1.6 | 6.2 ± 0.7 | 17.0 ± 2.7 | 2.7 |
| mb48 | yfhD | 9.3 ± 1.6 | 6.9 ± 0.5 | 1.4 | 8.5 ± 1.3 | 20.8 ± 0.9 | 2.5 |
| mb108 | ybiS | 12.6 ± 0.8 | 12.2 ± 0.3 | 1.0 | 43 ± 2 | 109 ± 8 | 2.5 |
Strains were grown in M9 minimal medium containing 0.4% glucose (overnight at 37°C), subcultured (1/100), grown to an OD600 of 0.5, and incubated for an additional 1 h prior to sampling. Strains carrying pMB101 were grown to an OD600 of 0.5, and IPTG (1 mM) was added to the cultures. Incubation was continued for 1 h, and samples were taken. Values represent averages of three different measurements, and standard deviations are indicated.
ND, not determined.
For the mb63 insertion (galT), (−) indicates negative control by Rob.
Solvent sensitivity of insertion mutants.
Since Rob controls basal resistance to organic solvents (35), the MudJ insertion mutants might have disrupted genes that contribute to this solvent resistance. To test this hypothesis, we determined the sensitivities of E. coli GC4468 (wt), MB4468 (Δrob), and the 12 Rob-dependent insertion mutants to n-hexane and to a 1:1 mixture of n-hexane–cyclohexane. Plating of the strains under n-hexane resulted in a 104-fold-decreased survival rate of the Δrob strain compared with that of the unexposed control. n-Hexane did not affect the survival of the wt or the insertion mutants. When the strains were exposed to the mixture of n-hexane and cyclohexane, an ∼106-fold reduction in colony-forming ability was observed for the Δrob strain, whereas the wt and all the insertion mutants showed only 104-fold reductions in plating efficiency. Thus, disruptions of the individual genes identified in this study may not be sufficient to affect sensitivity to solvents.
Direct binding of Rob to target genes.
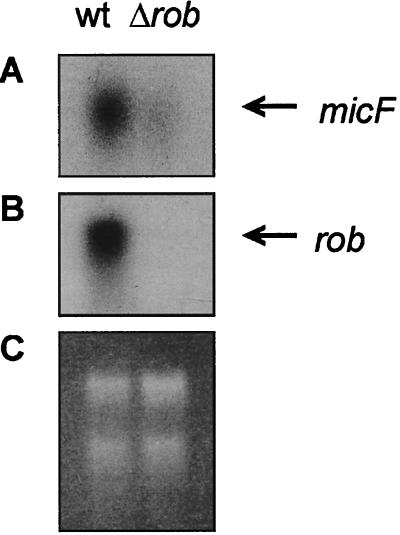
The regulatory effect of Rob on the various MudJ insertions could be either direct or indirect, through another regulator, such as MarA. We tested this point by using EMSA to assay in vitro binding of Rob to the promoter regions of genes identified in this study. To establish specific binding of Rob protein to the DNA fragments, we used a double-stranded DNA fragment lacking known Rob binding sites (Randomdir plus Randomrev; see Table 2) as a nonspecific competitor DNA and the micF promoter (double-stranded 35-mer micF promoter site 1; see Materials and Methods) as a specific competitor DNA. In vitro binding of Rob to the micF promoter has been demonstrated previously (5, 19), and Rob activates the transcription of micF in vitro (15). Here, the relevance of Rob-dependent expression of micF was further established by Northern blotting of micF mRNA (Fig. 3), which showed a higher micF mRNA level in the wt than in a Δrob mutant.
FIG. 3.
Expression of micF depends on rob. Total RNA was extracted from cultures of E. coli GC4468 (wt) and MB4468 (Δrob) that were grown in LB medium to an OD600 of 0.6 at 37°C. RNA was hybridized with probes specific for micF (A) and rob (B). Autoradiograms were exposed for ∼20 h. To confirm that equivalent amounts of RNA were loaded, rRNA bands were visualized by ethidium bromide staining (C).
The concentrations of Rob required to form complexes with the different DNA fragments were determined by assaying Rob-His6 in a series of EMSA experiments (data not shown). These experiments showed that the fragments with the highest apparent affinity were the mar fragment, which produced DNA-protein complexes at Rob-His6 concentrations of 1.5 to 3 nM, and the micF (control) and ybaO fragments, both of which showed binding at 3 to 5 nM Rob-His6. For the remaining fragments, binding was observed at Rob-His6 concentrations of ≥9 nM: 9 nM for the gal fragment, 13.5 nM for the aslB fragment, and 11 nM for the mdlA fragment. The ∼250-bp sequence directly upstream of the mdlA gene has low affinity for Rob. Even though this DNA region contains a putative Rob-binding site, this region is probably not involved in Rob-activated transcription: The ybaO and mdlA genes are annotated by Blattner et al. (6) as part of a predicted operon and are probably transcribed from a common promoter upstream of ybaO.
Results of EMSA of the selected DNA fragments with Rob-His6 are shown in Fig. 4. Multiple protein-DNA complexes were observed with the gal, aslB, and mdlA probes in our experiments but not with the mar and ybaO fragments (Fig. 4). It has been shown previously that Rob forms multiple complexes with micF and other promoters, probably as a result of the presence of multiple, independent Rob-binding sites (5, 19). The addition of nonspecific competitor DNA in a 100-fold molar excess (Fig. 4, lanes 4) did not eliminate binding of Rob to the different probes (Fig. 4, lanes 2). The strongest effect of the nonspecific competitor was in the gal promoter fragment, which nonetheless showed some binding to Rob-His6 even with a 100-fold excess of nonspecific competitor (Fig. 4B). These results indicate that the observed complexes are not due to nonspecific DNA-protein interactions. When micF competitor DNA (35-mer) was present at a 100-fold molar excess, binding to all the fragments was strongly diminished or eliminated (Fig. 4, lanes 5 and 6 versus lanes 2). For the gal promoter fragment, the micF competitor was much more effective than the nonspecific competitor (Fig. 4B). The mar fragment showed the most effective resistance to competition by the micF fragment: even at a 100-fold excess of this competitor, some residual mar promoter-Rob-His6 complex was still observed (Fig. 4A, lane 6).
FIG. 4.
Binding of Rob-His6 to the promoter regions of Rob-regulated genes. F, free probe; C1 through C5, DNA-Rob-His6 complexes; X, contaminating labeled DNA strand. Lanes 1, probe alone (no protein); lanes 2 through 6, Rob-His6 (see below for amounts) with no competitor (lanes 2), a 10-fold excess of nonspecific competitor (lanes 3), a 100-fold excess of nonspecific competitor (lanes 4), a 10-fold excess of specific competitor (lanes 5), or a 100-fold excess of specific competitor (lanes 6). (A) Rob-His6 (3 nM) binding to mar promoter DNA; (B) Rob-His6 (9 nM) binding to gal promoter DNA; (C) Rob-His6 (13.5 nM) binding to aslB promoter DNA; (D) Rob-His6 (3 nM) binding to ybaO upstream DNA region (free probe of lane 1 ran off gel); (E) Rob-His6 (11 nM) binding to mdlA upstream DNA region.
DISCUSSION
Rob protein contains a domain strongly related to the SoxS and MarA proteins, which is conserved in the entire AraC-XylS family of transcriptional regulators (12). Although Rob is expressed constitutively in E. coli (30), its biological function has remained obscure. We have now shown that the normal expression of several proteins depends on the rob gene. Furthermore, using transposon mutagenesis, we have identified eight genes under Rob control, including six not previously connected to Rob, MarA, or SoxS.
Our analysis revealed that inaA, marRAB, aslB, ybaO, mdlA, yfhD, and ybiS were transcriptionally activated by constitutive levels of Rob, while galT expression was repressed. Rob contributes significantly to the expression of the marRAB operon, which regulates the intrinsic resistance of E. coli to various antibiotics, bactericidal agents, and organic solvents (5, 10, 13). Our finding that 60% of mar expression depends on the presence of Rob agrees with previous observations that ∼65% of mar transcription depends on Rob, and that mar transcription can be further induced by Rob overexpression (21). Similarly, the demonstrated dependence of inaA on Rob is consistent with previously reported transcriptional activation of inaA by Rob overexpression (5). Other genes reported to be activated by Rob in vitro (fpr, fumC, micF, nfo, sodA, and zwf) (15) or by Rob overexpression in vivo (fumC, inaA, micF, and sodA) (5) were not found in our study; thus, it appears that the mutagenesis and screening procedure was not saturating.
Among functions that could contribute to cellular resistance to environmental agents, Rob enhances expression of mdlA, which encodes a multiple-drug-resistance-like ATP-binding component of a transport system (3), and strongly enhances expression of micF, the antisense RNA that downregulates the outer membrane porin OmpF. The protein encoded by yfhD, predicted to be involved in periplasmic transport (6), could also be involved in cellular resistance. The role of Rob in basal expression of marRAB, mdlA, and micF was further substantiated by in vitro binding assays of Rob to the respective promoter regions of these genes, where Rob showed the highest apparent binding affinity to the marRAB and micF promoter fragments. It is likely that the increased antibiotic resistance observed after overexpression of Rob is, at least in part, mediated through activation of these genes. The fact that insertions in the individual genes do not affect solvent sensitivity suggests either that multiple genes are involved in this phenotype or that other Rob-regulated genes are important. A good candidate that was not identified in this study is acrAB, which encodes an efflux pump that can contribute to Rob-, MarA-, and SoxS-induced organic solvent resistance (32, 35).
Several Rob-regulated genes, such as marA, noted above, seem to encode other regulatory proteins. Rob overproduction elicited the strongest transcriptional activation of ybaO and aslB (15- to 20-fold). The ybaO locus encodes a predicted protein of 181 amino acids with strong similarity to E. coli leucine-responsive regulatory protein (Lrp; 33% identity over 150 residues). Lrp is a global regulator of various operons (25, 34). The ybaO-encoded protein is most closely related to another Lrp homolog: the glutamate uptake regulatory protein (Grp) of Zymomonas mobilis (44% identity over 150 residues) (26). The aslB gene encodes a putative regulator of the E. coli arylsulfatase gene (aslA). Homologous systems include Klebsiella pneumoniae AtsB (which is involved in posttranslational activation of the arylsulfatase AtsA) (31), Klebsiella aerogenes AtsB (controlling arylsulfatase) (23), and ChuR from Bacteroides thetaiotaomicron (controlling chondro-6-sulfatase) (8). Rob could thus affect the expression of many more genes indirectly, through its effects on other regulatory proteins.
In this study, galT was the only gene repressed by Rob. This gene is part of the galETKM operon, encoding galactokinase (galK), galactose-1-phosphate uridylyl transferase (galT), UDP-galactose 4-epimerase (galE), and galactose mutarotase (galM). These enzymes are required in galactose metabolism and mediate the conversion of d-galactose to glucose-1-phosphate. Furthermore, these enzymes play an essential role in the production of galactosyl units needed for the biosynthesis of complex carbohydrates in glycoproteins, glycolipids, and the cell wall (1, 11). Transcription of the gal operon involves two promoters and operators and can be repressed by GalR (1). For complete repression of both gal promoters, DNA looping is reported to be essential (9). We demonstrated that Rob forms multiple complexes with the gal promoter in vitro, suggesting the existence of multiple Rob binding sites. The observed Rob-dependent galT repression might be related to facilitating the looping of the DNA in conjunction with GalR. Structural aspects of Rob (see below) suggest that this connection merits further investigation.
While normal expression of Rob contributes to the basal expression of the genes identified in this study, it is possible that this group of genes plays a more prominent role in an undiscovered cellular response that activates Rob. We have recently elucidated the crystal structure of Rob complexed with a fragment of the micF promoter, which reveals that the C-terminal domain of Rob contains a region that is highly homologous to the ligand binding region of the galT-encoded galactose-1-phosphate uridylyl transferase (17). Thus, it is tempting to speculate that the C-terminal region of Rob is involved in binding an effector molecule that regulates Rob activity. We have not yet identified possible ligands for this domain of Rob.
Rob clearly contributes to the expression of genes with a broad range of functions. The effects on basal expression levels by Rob are modest, but there is a possibility that Rob-induced gene expression might be enhanced upon binding of an effector molecule to the C-terminal region of Rob. Additional effects on the expression of many genes may occur through Rob-mediated activation of other regulatory proteins (MarA, AslB, and YbaO). In this capacity, Rob would have a truly global influence on gene expression in E. coli.
ACKNOWLEDGMENTS
We thank the members of our research group, T. Ellenberger and H. J. Kwon (Harvard Medical School), and L. E. N. Quadri (Weill Medical College of Cornell University, New York, N.Y.) for helpful discussions and advice.
This work was supported by grant CA37831 from the National Institutes of Health (B.D.) and research grants from the Agrotechnological Research Institute, Wageningen, The Netherlands (M.H.J.B.).
REFERENCES
- 1.Adhya S. The galactose operon. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1503–1512. [Google Scholar]
- 2.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allikmets R, Gerrard B, Court D, Dean M. Cloning and organization of the abc and mdl genes of Escherichia coli: relationship to eukaryotic multidrug resistance. Gene. 1993;136:231–236. doi: 10.1016/0378-1119(93)90470-n. [DOI] [PubMed] [Google Scholar]
- 4.Amábile Cuevas C F, Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991;19:4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariza R R, Li Z, Ringstad N, Demple B. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J Bacteriol. 1995;177:1655–1661. doi: 10.1128/jb.177.7.1655-1661.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shoa Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 7.Bremer E, Silhavy T J, Weinstock G M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985;162:1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Q, Hwa V, Salyers A A. A locus that contributes to localization of the intestinal tract by Bacteroides thetaiotaomicron contains a single regulatory gene (chuR) that links two polysaccharide utilization pathways. J Bacteriol. 1992;174:7185–7193. doi: 10.1128/jb.174.22.7185-7193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choy H E, Adhya S. Control of gal transcription through DNA looping: inhibition of the initial transcribing complex. Proc Natl Acad Sci USA. 1992;89:11264–11268. doi: 10.1073/pnas.89.23.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S P, Haechler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey P A. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon atom in galactose. FASEB J. 1996;10:461–470. [PubMed] [Google Scholar]
- 12.Gallegos M T, Schleif R, Bairoch A, Hofmann K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman J D, White D G, Levy S B. The multiple antibiotic resistance (mar) locus protects Escherichia coli from rapid cell killing by fluoroquinolones. Antimicrob Agents Chemother. 1996;40:1266–1269. doi: 10.1128/aac.40.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo E, Demple B. Adaptive responses to oxidative stress: the soxRS and oxyR regulons. In: Lin E C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 435–452. [Google Scholar]
- 15.Jair K-W, Yu X, Skarstad K, Thöny B, Fujita N, Ishihama A, Wolf R E., Jr Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of the chromosomal replication. J Bacteriol. 1996;178:2507–2513. doi: 10.1128/jb.178.9.2507-2513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakeda M, Ueguchi C, Yamada H, Mizuno T. An Escherichia coli curved DNA-binding protein whose expression is affected by the stationary phase-specific sigma factor ςs. Mol Gen Genet. 1995;248:629–634. doi: 10.1007/BF02423459. [DOI] [PubMed] [Google Scholar]
- 17.Kwon H J, Bennik M H J, Demple B, Ellenberger T. Crystal structure of the Escherichia coli Rob transcription factor complexed to DNA. Nat Struct Biol. 2000;7:424–430. doi: 10.1038/75213. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Demple B. SoxS, an activator of superoxide stress genes in Escherichia coli. J Biol Chem. 1994;269:18371–18377. [PubMed] [Google Scholar]
- 19.Li Z, Demple B. Sequence specificity for DNA binding by Escherichia coli SoxS and Rob proteins. Mol Microbiol. 1996;20:937–945. doi: 10.1111/j.1365-2958.1996.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 20.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin R G, Rosner J L. Fis, an accessorial factor for transcriptional activation of the mar (multiple antibiotic resistance) promoter of Escherichia coli in the presence of the activator MarA, SoxS, or Rob. J Bacteriol. 1997;179:7410–7419. doi: 10.1128/jb.179.23.7410-7419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 23.Murooka Y, Ishibashi K, Yasumoto M, Sasaki M, Sugino H, Azakami H, Yamashita M. A sulfur- and tyramine-related Klebsiella aerogenes operon containing the arylsulfatase (atsA) gene and the atsB gene. J Bacteriol. 1990;172:2131–2140. doi: 10.1128/jb.172.4.2131-2140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima H, Kobayashi K, Kobayashi M, Asako H, Aono R. Overexpression of the robA gene increases organic solvent tolerance and multiple antibiotic and heavy metal ion resistance in Escherichia coli. Appl Environ Microbiol. 1995;61:2302–2307. doi: 10.1128/aem.61.6.2302-2307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman E B, Lin R. The Leucine/Lrp regulon. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 419–429. [Google Scholar]
- 26.Peekhuis N, Tolner B, Poolman B, Kraemer R. The glutamate uptake regulatory protein (Grp) of Zymomonas mobilis and its relation to the global regulator Lrp of Escherichia coli. J Bacteriol. 1995;177:5140–5147. doi: 10.1128/jb.177.17.5140-5147.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomposiello P J, Demple B. Identification of SoxS-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182:23–29. doi: 10.1128/jb.182.1.23-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosner J L, Slonczewski J L. Dual regulation of inaA by the multiple antibiotic resistance (Mar) and superoxide (SoxRS) stress response systems of Escherichia coli. J Bacteriol. 1994;176:6262–6269. doi: 10.1128/jb.176.20.6262-6269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Skarstad K, Thöny B, Hwang D S, Kornberg A. A novel binding protein of the origin of the Escherichia coli chromosome. J Biol Chem. 1993;268:5365–5370. [PubMed] [Google Scholar]
- 31.Szameit C, Miech C, Balleininger M, Schmidt B, von Figura K, Dierks T. The iron sulfur protein AtsB is required for posttranslational formation of formylglycine in the Klebsiella sulfatase. J Biol Chem. 1999;274:15375–15381. doi: 10.1074/jbc.274.22.15375. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Horii T, Shibayama K, Sato K, Ohsuka S, Arakawa Y, Yamaki K, Takagi K, Ohta M. RobA-induced multiple antibiotic resistance largely depends on the activation of the AcrAB efflux. Microbiol Immunol. 1997;41:697–702. doi: 10.1111/j.1348-0421.1997.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 33.Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983;155:1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Wu J, Friedberg D, Plakto J, Calvo J M. Regulation of the Escherichia coli lrp gene. J Bacteriol. 1994;176:1831–1839. doi: 10.1128/jb.176.7.1831-1839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White S, Tuttle F E, Blankenhorn D, Dosch D C, Slonczewski J L. pH dependence and gene structure of inaA in Escherichia coli. J Bacteriol. 1992;174:1537–1543. doi: 10.1128/jb.174.5.1537-1543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]