Abstract
Cardiovascular disease (CVD) has become the leading cause of death worldwide. Many recent studies have pointed out that Lactiplantibacillus plantarum (Lb. plantarum) has great potential in reducing the risk of CVD. Lb. plantarum is a kind of lactic acid bacteria (LAB) widely distributed in fermented food and the human intestinal tract, some strains of which have important effects on human health and the potential to be developed into probiotics. In this review, we summarize the mechanism of potential probiotic strains of Lb. plantarum against CVD. It could regulate the body’s metabolism at the molecular, cellular, and population levels, thereby lowering blood glucose and blood lipids, regulating blood pressure, and ultimately reducing the incidence of CVD. Furthermore, since Lb. plantarum is widely utilized in food industry, we highlight some of the most important new developments in fermented food for combating CVD; providing an insight into these fermented foods can assist scientists in improving the quality of these foods as well as alleviating patients’ CVD symptoms. We hope that in the future functional foods fermented by Lb. plantarum can be developed and incorporated into the daily diet to assist medication in alleviating CVD to some extent, and maintaining good health.
Keywords: cardiovascular disease, Lactiplantibacillus plantarum, fermentation, probiotic functional foods, probiotics, nutriceuticals
1. Introduction
Cardiovascular disease (CVD) has become much more prevalent and complicated as our modern society develops. CVD is one of the largest contributors to the burden of chronic disease, with an estimated 17.9 million people dying from it in 2019 globally [1]. It is also reported that CVD was the leading cause of death in 2018 in China, accounting for 46.66% and 43.81% of all deaths in rural and urban areas, respectively [2]. Also, despite the lowered incidence of CVD in adults aged >50 years, the incidence of CVD has either been steady or increasing among younger adults (aged 18–50 years) in Western countries over the past two decades [3]. Thus, not only the elderly but also young people should attach importance to cardiovascular health.
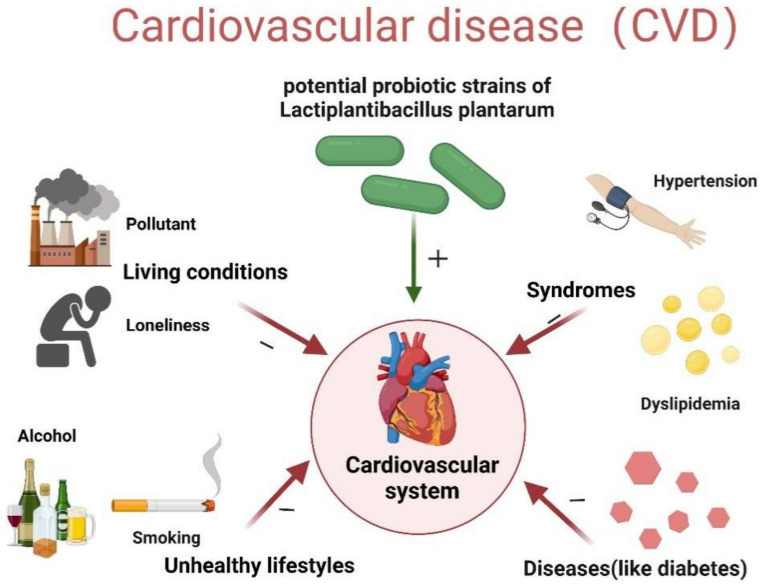
The factors that influence our cardiovascular health are complicated and multiple, as illustrated in Figure 1. Diseases, syndromes, personal lifestyles, and living conditions will all have an impact on our cardiovascular health. CVD is the most common complication in certain diseases, such as diabetes mellitus, and has now become the leading cause of death in people with diabetes [4]. In the meantime, according to a review study in 2022 [5], syndromes like hypertension and dyslipidemia are important CVD risk factors for people. Moreover, adhering to a healthy lifestyle, including regular fruit intake, less alcohol consumption, and non-smoking, could statistically significantly reduce the overall incidence of CVD by 66% [6]. Different kinds of environmental stressors, such as discrimination, social isolation, low socioeconomic status, can alter an individual’s biology through many kinds of pathways, such as the neuro-hematopoietic axis and epigenetic modification. This will lead to chronic inflammation and, finally, result in the development and progression of CVD [7]. Scientists also find that people who are continuously exposed to hazardous waste will have a tendency to get high blood pressure [8]. As a result, finding a way to reduce the risk of CVD is an important health-related topic in modern people’s lives nowadays.
Figure 1.
Impacts of different factors on cardiovascular health. There are mainly many causes that will lead to cardiovascular disease (CVD) and potential probiotic strains of Lactiplantibacillus plantarum (Lb. plantarum), one kind of lactic acid bacteria (LAB), may have the ability to protect the cardiovascular system. (“−“means that this factor is harmful to the health of the cardiovascular system, and “+” means that this factor promotes the health of the cardiovascular system) (Created with Biorender.com).
In these circumstances, besides the urgent need for new effective medicines, the development of probiotics and probiotic foods has also become a potential way to protect people against CVD. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [9]. The Lactobacillus spp. are widely considered to be probiotics because of their generally probiotic properties [10]. Lactiplantibacillus plantarum (Lb. plantarum), a species of the Lactobacillus spp., is also considered to be a potentially probiotic strain, with some Lb. plantarum strains able to help maintain the balance of blood glucose, lipids, and blood pressure, and to regulate metabolic disorders [11], thus having anti-cardiovascular potential. The entry of probiotics into the human gastrointestinal tract requires the use of carriers, usually including capsules and food products, with the latter offering better acceptance and marketing than the encapsulation technology. Fermentation is an effective method to produce probiotic foods. Lb. plantarum with probiotic potential can be used as a fermentation strain for a variety of foods, such as dairy products, meat products, vegetables and fruits, and cereals. Several studies have shown that foods fermented with Lb. plantarum have higher health benefits, such as better antioxidant capacity and higher active substance content [12,13]. In addition, scientists have also found that Lb. plantarum-fermented foods may also be endowed with certain probiotic effects, and may be developed into probiotic functional foods that can be useful for human health through daily dietary supplementation.
This review shows the probiotic potential of Lb. plantarum and analyzes the possible mechanisms by which Lb. plantarum can prevent or help improve the condition of CVD. In addition, this review focuses specifically on the great potential of Lb. plantarum-fermented foods to be developed as probiotic functional foods, providing an insight into how these fermented foods could assist scientists in improving their quality and ultimately alleviating patients’ CVD symptoms.
2. Probiotic Potential of Lb. plantarum and Foods Fermented with It
Lb. plantarum belongs to the genus Lactobacillus, which is widely considered to be a probiotic, and may have probiotic potential from species analysis. However, a prerequisite for probiotics to function in the host’s gastrointestinal tract is a good tolerance to unfavorable factors such as gastric acid, bile salts, and degrading enzymes, as well as the ability to complete colonization in the gastrointestinal tract [14]. Therefore, Lb. plantarum must have a high gastrointestinal survival rate to meet the basic requirements for development as a probiotic. Kriti Ghatani and Buddhiman Tamang [15] isolated a strain of Lb. plantarum from fermented yak milk products, and then added cholesterol and bile salts to MRS broth to determine the tolerance of the selected strain. Combined with acid resistance, bile salt hydrolase activity and cell surface hydrophobicity, they confirmed that this strain of Lb. plantarum was tolerant to the corrosive and toxic effects of gastric acid and bile in humans, and can adhere to host cells to complete colonization. Wang, G.Q. and Chen, Y. et al. [16] recorded the images of mouse intestines at different times after oral administration of Lb. plantarum, confirming that Lb. plantarum AR17-1 can colonize and play a role in the intestinal tract, implying that Lb. plantarum has probiotic potential.
As a carrier for Lb. plantarum to enter the human body, Lb. plantarum-fermented foods with high live counts have the potential to be developed into probiotic foods that can play a healthy role in the human body. However, the ability of these fermented foods to function as probiotic foods also depends on the gastrointestinal survival of the probiotic bacteria in the food. Different food matrices also have significant impacts on the gastrointestinal survival rate of Lb. plantarum. The most widely used matrices are dairy products including yogurt, cheese, etc. The higher fat and whey protein content in dairy products result in their better buffering capacity against gastric acid, which can improve the gastrointestinal survival rate of probiotics [17,18]. In addition, other fermented foods, such as fruit and vegetable juices, oats and cereals, and meat products are also commonly used as food matrices to create fermented foods that bring probiotics into the human gastrointestinal tract. The fat in meat products protects probiotics from low pH and bile salts [18], the higher sugar content in cereals allows probiotics to better tolerate intestinal conditions [19], and the relatively short digestion time of fruit and vegetable juices can greatly reduce the adverse effects of the gastric environment on probiotics [20,21]. Current Lb. plantarum-fermented foods that may have probiotic potential include dairy products, meat products, soy products, fruits and vegetables, and cereals, where the gastrointestinal survival of Lb. plantarum. is high. However, studies on the effect of food matrices on the gastrointestinal survival rate of probiotics are still relatively few, and it is hoped that more research will focus on this in the future.
3. Possible Mechanism of Lb. plantarum against CVD
3.1. Mechanisms of Antioxidant Abilities by Lb. plantarum
Free radical generation has been linked in studies to the development of CVD. It has been demonstrated that several strains of Lb. plantarum achieve antioxidant capacity by scavenging free radicals. Therefore, we speculate that the Lb. plantarum strains with antioxidant capacity may have the potential to mitigate CVD [22].
The antioxidant mechanisms of Lb. plantarum are complex and diverse. For example, Lb. plantarum JM113 can increase the mRNA levels of Nrf2 (a protein that regulates endogenous antioxidant systems) and its corresponding downstream HO-1 gene (HO-1, an important antioxidant enzyme that mainly catalyzes the decomposition and metabolism of heme to ferrous, carbon monoxide, and bile green) to achieve antioxidation [23]. Lb. plantarum KCCP11226 can produce C-30 carotenoid 4,4’-diaponeurosporene, which has antioxidant capacity [24,25,26]. Lb. plantarum DM5 has been reported to directly scavenge superoxide anion radical, hydroxyl radical, and DPPH radical [27,28]. Further mechanisms for the antioxidant activity of Lb. plantarum are shown in Table 1.
Table 1.
Possible mechanisms by which Lb. plantarum protects cells from the attack of free radicals.
| Name of Lb. plantarum | Resource | In Vivo Studies(a) or In Vitro Studies(b) | Antioxidant Mechanism | Reference |
|---|---|---|---|---|
| Lb. plantarum CCFM10 | Different storage centers (edible fungi strains) | a | Increase levels of GSH, CAT, SOD, and TOC in serum of mice with oxidative damage | [35] |
| Lb. plantarum CCFM242 | Different storage centers (edible fungi strains) | a | Improve total antioxidant capacity of liver | [35] |
| Lb. plantarum RS15-3 | Different storage centers (edible fungi strains) | a | Increase levels of GSH, CAT, SOD, and TOC in serum of mice with oxidative damage | [35] |
| Lb. plantarum FEED8 | Intestinal tract of longevity elderly in Bama, Guangxi | a | Increase levels of glutathione (GSH) and other indicators | [34] |
| Lb. plantarum NCFM, ATCC 14917 and NDC 75017 | a | Increase SOD activity, GSH-Px activity, and T-AOC content in serum, brain, and liver, decreasing MDA content. | [26] | |
| Lb. plantarum C88 | Traditional fermented milk tofu in Inner Mongolia | a | Regulation of intracellular antioxidant enzyme activity in oxidative damaged Caco-2 cells | [36] |
| Lb. plantarum Y-20 | Chopped pepper by natural fermentation | a | Protect the Caco-2 cells against H2O2 and induce oxidative stress by renewing the enzymatic and non-enzymatic antioxidant defense system. | [37] |
| Lb. plantarum JMCC0017 | Xinjiang traditional fermented dairy products | b | Metabolites have significant antioxidant activity | [38] |
| Lb. plantarum DM5 | Fermented beverage Marcha of Sikkim | a | Scavenge free radicals and superoxide anion | [27,28] |
| Lb. plantarum KM1 | Natural fermentation products | a | Scavenge hydroxyl radical, superoxide anion radical, and DPPH radical | [31] |
| Lb. plantarum AR501 | a | Scavenge hydroxyl radical, superoxide anion radical, and DPPH | [39] | |
| Lb. plantarum JM113 | healthy intestinal contents of Tibetan chicken | a | Change the expression levels of Phosphoglycerin kinase, α-glycerophosphate oxidase, pyruvate oxidase, and NADH peroxidase to alleviate oxidative stress. | [23] |
| Lb. plantarum NC8 | a | Up-regulation of antioxidant gene expression | [40] | |
| Lb. plantarum SM4. | kimchi and fermented with white Taraxacum coreanum | a | Increase the mRNA levels of Nrf2 and its corresponding downstream HO-1 gene | [41] |
| Lb. plantarum KCCP11226 | a | Regulation of Bcl-2 family members and activation of Bcl-2/Bax signaling pathway | [24,25] | |
| Lb. plantarum ZLP001 | b | Produce white T. coreanum fermented product which shows higher bioactive properties of oxidation resistance | [42] | |
| Lb. plantarum KLDS1.0202 | Cheddar cheese | b | Produce C-30 carotenoid 4,4′-diaponeurosporene. | [43,44] |
| Lb. plantarum isolated from traditional sourdough | Traditional Sourdough | a | Supplementation of Lb. plantarum ZLP001 increases the concentration of superoxide dismutase (p < 0.05), glutathione peroxidase (p < 0.01), and catalase in serum (p < 0.10), while decreasing the concentration of malondialdehyde (p < 0.05). | [45] |
| Lb. plantarum NJAU-01 | jinhua ham | The strain not only itself has a certain antioxidant activity, but also promotes the decomposition of protein of Cheddar cheese | [29,30] |
On the other hand, a single Lb. plantarum may have several different antioxidant mechanisms. For example, Lb. plantarum NJAU-01 can not only alleviate and protect the body from oxidative stress by regulating the protein expression in the metabolic pathways, but also regulate the composition of the intestinal flora. Additionally, its extracts also show powerful antioxidant capacity [29,30].
In addition, the antioxidant capacity and antioxidant mechanisms of Lb. plantarum may be affected by several environmental conditions and interactions with other strains. For example, hydrogen peroxide (H2O2) is one of the antagonism factors to regulate the gut microbiota composition, and after being stimulated by H2O2, the redox balance in the Lb. plantarum KM1 is destroyed, resulting in the down-regulation of the expression of α-glycerophosphate oxidase and pyruvate oxidase, and the reduction of H2O2 production in the bacteria, thus achieving an antioxidant effect [31].
The antioxidant function of Lb. plantarum has been proved in animal experiments, with several strains of Lb. plantarum exerting stable antioxidant effects in mice [32,33]. For example, in the experiment of Yan, L. et al. [34], a certain dose of Lb. plantarum FEED8 solution and the corresponding dose of skimmed milk were intragastrically administered to mice in the two groups. The antioxidant effect was evaluated by detecting the superoxide dismutase (SOD) activity, glutathione peroxidase (GSH-Px) activity, total antioxidant capacity (T-AOC) content, and malonaldehyde (MDA) content in the serum, brain, and liver of mice. The results showed that compared with the control group, the SOD activity, GSH-Px activity, and T-AOC content in serum, brain, and liver of mice in the medium and high dose Lb. plantarum FEED8 groups were significantly increased, and the MDA content was significantly decreased. This difference grew as the concentration of Lb. plantarum FEED8 increased. It can be concluded that Lb. plantarum FEED8 has good antioxidant effect on mice. This gives some indication of whether Lb. plantarum can exert antioxidant effects on humans.
3.2. Mechanisms of Blood Pressure Lowering by Lb. plantarum
Hypertension, defined as systolic blood pressure (BP) ≥130 mmHg and/or diastolic BP ≥80 mm Hg [46], has become much more prevalent in recent years. Due to increases in unhealthy lifestyles, including high sodium diets, low potassium intake, alcohol consumption, overweight condition, and lack of exercise, hypertension is now becoming a leading cause of CVD [47]. According to the statistics generated by M. H. Forouzanfar, et al. [48], hypertension led to the deaths of 7.8 million people (14.0% of all deaths) in 2015.
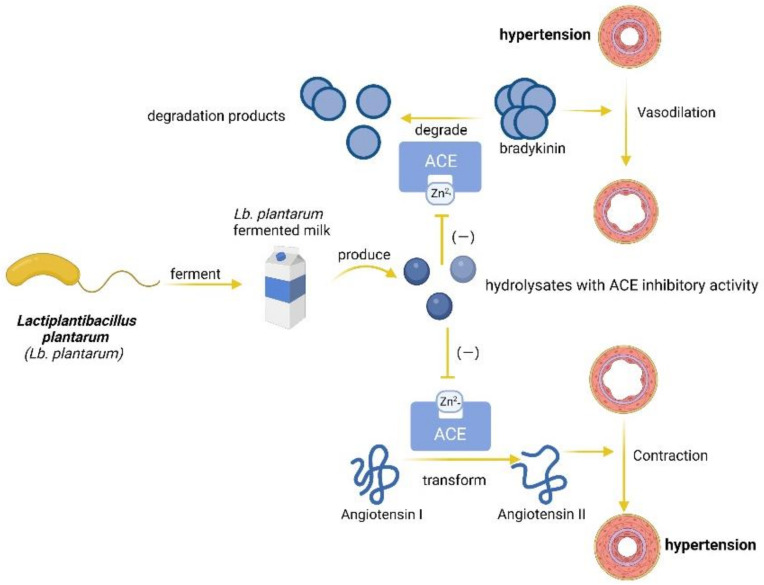
Angiotensin-converting enzyme (ACE) contributes significantly to regulating blood pressure. According to the research conducted by Eriksson in 2002 [49], with the elevation of ACE activity, BP will rise because ACEs can promote the production of angiotensin, which can cause vascular contraction. Recently, some studies have shown that foods fermented by Lb. plantarum could assist in lowering patients’ BP. In a systematic review and meta-analysis, the authors found that when hypertensive patients ingested Lb. plantarum supplementation, their diastolic BP reduced significantly compared with normal people [50]. According to Chen et al. [51], goat milk fermented by L. plantarum 69 could inhibit ACE activity, with the calculated inhibition rate reaching 88.91%. Some other findings regarding the inhibitory capacity of these functional foods on ACE activity are presented in Table 2.
Table 2.
The ACEI activity of different kinds of foods fermented by Lb. plantarum.
| Lactiplantibacillus plantarum | Fermented Food | Fermentation Condition | ACE Inhibition Effect | References |
|---|---|---|---|---|
| Lb. plantarum L69 together with Directed Vat Set starter containing Lactobacillus bulgaricus and Streptococcus thermophilus (1:1) | skim milk powder | 4.5 h, 42 °C, pH = 4.65 |
Lb. plantarum L69 contributed to ACEI substances production compared with single Directed Vat Set starter, with ACEI activity reaching 87.14%. | [66] |
| Lb. plantarum SPS109 | whey beverage | 72 h, 35 °C, pH = 5.5 |
ACEI activity = 25.70 ± 1.20% | [67] |
| Lb. plantarum previously isolated and identified from Chiapas double cream cheese | reconstituted whole milk. | 48 h, 37 °C, pH = 9 |
59.3 ± 1.6% ACEI activity | [68] |
| Lb. plantarum K25 together with yogurt starter L. delbrueckiissp. bulgaricus and Streptococcus thermophilus) | yogurt | 37 °C, pH = 4.5 ± 0.5, then storing in 4 °C for 21 days |
Lb. plantarum K25 significantly raising the ACEI ratio in contrast to fermentation with the starter only, with the estimation of ACEI activity = 49.3% | [69] |
| Lb. plantarum BG 112 | soymilk containing okara flour | 32 h | 50% ACEI activity | [70] |
| Lb. Plantarum which NCBI Accession is KF806535 | soy milk | 24 h, 37 °C, 100 rpm | ACEI activity (in-vitro) of peptides all above 70% | [71] |
| Lb. plantarum (TISTR 858) | eggshell membranes | 30 °C, 120 rpm |
ACE-inhibition corresponding to 49.3% with the concentration of the protein hydrolysates obtained after fermentation up to 2 mg/mL | [72] |
| Lb. plantarum 70810 | navy bean milk | 2 h, 31 °C | 50% inhibiting concentration (IC50) = 109 ± 5.1μg protein/ml | [73] |
| Lb. plantarum B1-6 | navy bean milk | 3 h, 37 °C | IC50 = 101 ± 2.2 μg protein/mL, in vitro gastrointestinal simulation IC50 = 21 ± 2.1 μg protein/ml | [73] |
| Lb. plantarum (NCDO 1193) | freeze-dried camu-camu powder and soymilk combination |
37 °C, 72 h | 94.0 ± 1.0% ACEI activity | [74] |
| Lb. plantarum 69 | goat milk | 35 °C, CaCl2 concentration of 0.07%, and Tween-80 concentration of 0.04% | 88.91%ACEI activity | [51] |
| Lb. plantarum KU15003 | yogurt | fermentation was terminated when the pH reached 4.4 ± 0.1. | IC50 = 0.68 mg/mL | [75] |
| Lb. plantarum KU15031 (T3) | yogurt | fermentation was terminated when the pH reached 4.4 ± 0.1. | IC50 = 0.79 mg/mL | [75] |
| Lb. plantarum NK181 (T4) | yogurt | fermentation was terminated when the pH reached 4.4 ± 0.1. | IC50 = 0.48 mg/mL | [75] |
| Lb. plantarum QS670 | milk | 37 °C, 48 h | IC50 = 1.26 mg/mL | [57] |
The specific proteolytic system of potentially probiotic strain Lb. plantarum might be the main reason for its inhibition of ACE activity [52]. According to Fabiola Sanchez-Lopez et al. [53], the product obtained after 8 h of hydrolysis of amaranth proteins by Lb. plantarum exhibited favorable ACE-inhibitory (ACEI) activity. These hydrolysates, whose chemical essence is mainly peptides, could chelate Zn2+ to eventually reduce the activity of ACE. Moreover, Yi-Yen Liu et al. [54] reported that one kind of bioactive ACEI peptide extracted from soy milk could promote nitric oxide production after being fermented by Lb. plantarum TWK10. With the increase of nitric oxide level in cells, blood vessels can be relaxed, contributing to further improvement in hypertension. Nahariah, N. et al. [55] reported that egg albumen fermented by Lb. plantarum FNCC 0027 for 18 h was rich in ACEI peptides. However, the longer fermentation periods (24, 30, and 36 h) reduced the ACEI peptides’ activities in fermented egg albumen. In addition, Shu, Guowei [56] reported that the optimal temperature for one kind of milk to ferment and induce ACEI peptide activity was 35 °C. In the meantime, digestive enzymes also have a great impact on these functional foods. If digestive enzymes, such as pepsin and chymotrypsin, can destroy these ACEI peptides, these functional foods will scarcely have any benefits. According to Yanan Xia et al. [57], ACEI peptides isolated from whey proteins of milk fermented with Lb. plantarum QS670 exhibited stability toward chymotrypsin, pepsin, and trypsin, which meant that the fermented milk can be used as a potential functional food to help relieve the symptoms of hypertension. The mechanism by which Lb. plantarum lowers ACE activity and reduces blood pressure is illustrated in Figure 2. However, the impact of digestive enzymes is sometimes overlooked by researchers in in vitro tests, so some results of these kinds of experiments will be further illustrated through in vivo tests.
Figure 2.
The blood pressure-lowering mechanism of Lactiplantibacillus plantarum fermented food.Lactiplantibacillus plantarum (Lb. plantarum) fermented foods can produce angiotensin-converting enzyme (ACE) inhibitors, which can prevent bradykinin from degrading and angiotensin I from transforming, thus relieving hypertension symptoms. (“−“ means inhibition) (Created with Biorender.com).
In the meantime, γ-aminobutyric acid (GABA), a non-protein amino acid, can act as a hypotensive agent. Some studies have indicated that GABA can reduce high blood pressure in both animals and humans [58]. Since the GAD enzyme (a key enzyme to synthesize GABA) is highly active in the cells of LAB, they have high GABA production. Mohsen Zareian et al. [59] reported that using Lb. plantarum MNZ to ferment a bowl of wheat-based rice (dosa) for 120 h could enhance the GABA content (about 143 mg/kg) of the dosa. GABA could also be found when different types of Lb. plantarum are used to ferment milk [60] and lentils [61].
Apart from ACEI peptides and GABA, certain kinds of phenolic acids can also contribute to lowering blood pressure. According to Irini Lazou Ahrén et al. [62], blueberries fermented by Lb. plantarum DSM 15313 can produce three kinds of phenolic acids (hydroxyphenyllactic acid, 3,4-dixydroxyphenyl-propionic acid, and phenyllactic acid). They separated rats into six groups. Three groups had normal blood pressure and were then fed with standard chow, fermented blueberry products with a low concentration of the phenolic acids (product A), and products with a high level of the same (product B). Another three groups were induced hypertension and then fed with the same three kinds of foods. The authors found that after feeding normal rats with product A for four weeks, their blood pressure dropped more obviously than the other two groups. Nevertheless, product B can significantly reduce hypertensive rats’ blood pressure compared with product A. The conclusion was that phenolic acids have the ability to reduce blood pressure, but the reason why higher concentrations of phenolic acids have more impact on normal rats than hypertensive ones remain to be investigated.
Besides the bioactive substances Lb. plantarum can produce in fermented foods, researchers have found that Lb. plantarum can also help enhance drug absorption. Febrina A. Saputri et al. [63] reported that providing male New Zealand rabbits with Lb. plantarum for 14 days can help enhance the absorption of Amlodipine, which can be used to treat hypertension. The blood concentrations of amlodipine in the Lb. plantarum IS-10506 group had a higher level compared with those in the control group. This survey suggests that when the animals are supplemented with Lb. plantarum IS-10506, their blood flow can be enhanced, which may increase the absorption of amlodipine.
Gut microbiota dysbiosis is another factor that is related to hypertension. According to Yang T. et al., microbial richness and diversity were significantly reduced in the spontaneously hypertensive rats [64]. Additionally, fecal samples of some human hypertension patients were also tested in this research, and a similar dysbiosis pattern was found. The mechanism of this relationship has not been fully clarified. According to Robles-Vera et al., some lactobacillus strains can synthesize products such as short-chain fatty acids and lipopolysaccharides [65]. These products can potentially influence host cell physiology. Still, more scientific research is required in this area.
3.3. Mechanisms of Lipid Lowering by Lb. plantarum
High cholesterol is also one of the risk factors for CVD, which can cause a variety of high mortality diseases such as atherosclerosis and hyperlipidemia. Atherosclerosis is a chronic inflammatory disease, often accompanied by endothelial dysfunction, endometrial lipid deposition, smooth muscle cell proliferation, apoptosis, and necrosis, as well as local and systemic inflammation with the participation of innate and acquired immunity [76]. Hypercholesterolemia is a pathological condition clinically diagnosed with concentrations of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) exceeding the standards, along with concentrations of high-density lipoprotein (HDL) being lower than the standards [77]. One of the main causes of these diseases is the accumulation of cholesterol in the arteries. Studies have shown that lowering serum cholesterol concentration by 1% can reduce the incidence of CVD by 2% to 3%, particularly when LDL cholesterol level can be lowered and HDL cholesterol level raised; for patients with atherosclerosis, the low-density lipoprotein cholesterol (LDL-C) goal is <1.8 mmol/L (<70 mg/dL), or a reduction of at least 50% if the baseline level is between 1.8 and 3.5 mmol/L (70 and 135 mg/dL) [78].
Therefore, modern medicine believes that finding drugs that can reduce the lipid levels in the blood and liver is an effective way to prevent CVD. So far, pharmacological treatments such as statins, 3-hydroxy-3-methylglutaryl CoA (HMG CoA) reductase inhibitors, are the most widely used treatments to effectively lower cholesterol, especially LDL-C. However, these drugs are expensive and have harmful side effects, such as liver enzyme abnormalities and rhabdomyolysis [79]. Therefore, scientists hope that a safe substance of natural origin can be found as an alternative to drugs. Probiotics isolated from foods or intestines have great beneficial characteristics, and can be used as functional strains in the treatment and prevention of many diseases. In addition, most lactobacilli have a long history of safe use and are considered Generally Recognized as Safe (GRAS) strains with a high safety profile and low side effects compared to drugs. [80]. As one of them, Lb. plantarum has a high cholesterol-lowering capacity in vivo and in vitro, and can significantly improve serum cholesterol levels and reduce the risk of disease [81,82,83,84,85,86,87,88].
The cholesterol-lowering ability of Lb. plantarum in vitro has been widely reported, and its possible mechanism has been verified by experiments, including enzymatic deconjugation of bile acids by bile salt hydrolase (BSH), assimilation of cholesterol by probiotics, co-precipitation of cholesterol with deconjugated bile, incorporation of cholesterol into the cellular membranes of probiotics during growth, conversion of cholesterol into coprostanol through cholesterol reductase, and production of short-chain fatty acids upon fermentation [89,90]. However, the mechanism of lipid-lowering in vivo has not been fully clarified. We summarize the possible mechanisms as: (1) regulation of lipid metabolism through signaling pathways; (2) cholesterol reduction through bile acids; (3) regulation of lipid metabolism through intestinal flora; (4) cholesterol reduction through conjugated linoleic acid (CLA) isomerase.
3.3.1. Lowering Cholesterol through Signaling Pathways
Potential probiotic strains of Lb. plantarum can regulate lipid metabolism through signal pathways, and the AMPK signaling pathway is one of the classical pathways [91]. Lb. plantarum can activate adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK), and alter the expression of proteins downstream, thereby regulating the synthesis of cholesterol and fatty acids. In earlier studies, Lee, Choi, et al. [92] found that the way Lb. plantarum activates the AMPK pathways was through covalent modification, rather than affecting the expression of AMPK mRNA. By covalent modification, Lb. plantarum could increase the phosphorylation levels of AMPK to activate it. Acetyl-CoA carboxylase (ACC) is a receptor protein of AMPK, as well as a key enzyme in fatty acid synthesis, which can be inhibited by the activated AMPK, thus inhibiting lipid synthesis. Besides, activated AMPK signaling promotes fatty acid oxidation by increasing peroxisome proliferation-activated receptor alpha (PPARα) expression, which can avoid the accumulation of fatty acids in the form of TG [93]. Scientists also found that Lb. plantarum can significantly down-regulate the expression of lipogenic proteins in liver tissue, such as sterol regulatory element-binding protein 1c (SREBP-1c) and stearoyl-CoA desaturase 1 (SCD-1) [94]. All of this suggests that Lb. plantarum can regulate liver lipid metabolism to some extent through the AMPK pathway, increasing fatty acid oxidation while decreasing lipid biosynthesis.
This mechanism has been confirmed in many species of Lb. plantarum. Yue Teng et al. [95] conducted experiments using Lb. plantarum LP104 isolated from kimchi. They randomly divided the mice into the normal-fat diet (NFD) group, a high-fat diet (HFD) group, and HFD + LP104 group. They found that in the HFD + Lb. plantarum 104 group, the phosphorylation level of AMPK was increased, and the level of ACC was reduced compared to the HFD group. Furthermore, the expression of lipogenic proteins SREBP-1c and SCD-1 was down-regulated, while the expression of PPARα was significantly increased. Meanwhile, experimental data showed that supplementation with Lb. plantarum LP104 significantly reduced the elevated TC, TG, and LDL levels caused by the high-fat diet. The same results have been found in Lb. plantarum NCU116 [96], Lb. plantarum NA136 [97], and Lb. plantarum S58 [94].
3.3.2. Lowering Cholesterol through Bile Acids
Lb. plantarum can also lower serum cholesterol levels through bile acids. Bile acid is one of the components of bile, and in alkaline bile, it is often present as a potassium salt or sodium salt, hence the name bile salt. Bile salt is closely related to the balance of cholesterol in the body. Clinical studies have shown that bile acid levels are significantly higher in patients with hyperlipidemia, non-alcoholic steatohepatitis, and fatty liver than in normal people [98], and since cholesterol is a precursor to the de novo synthesis of bile salts [99], promoting the catabolism of bile salts may be an effective way to lower cholesterol.
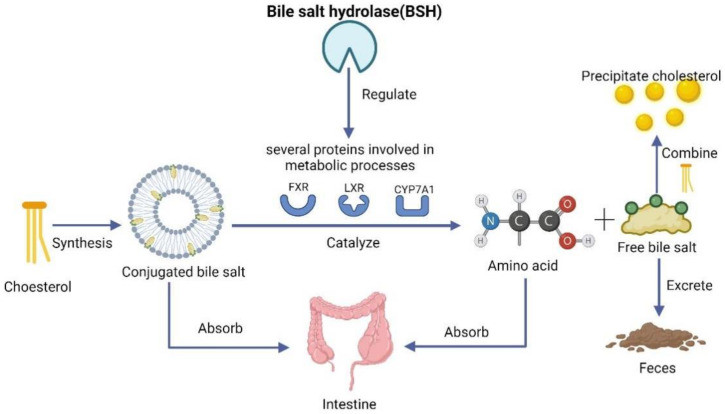
A 2009 study by Sridevi and Prabhune [100] demonstrated that many Lb. plantarum have high bile salt hydrolase (BSH) activity, which can regulate bile acid metabolism and lower cholesterol levels. BSH is an enzyme of high biological significance belonging to the family chologlycine hydrolase (EC 3.5.1.1 1) that catalyzes the conversion of conjugated bile salts into free bile salts and amino acids. Conjugated bile salts are very soluble, and most of them can be reabsorbed through enterohepatic circulation. In contrast, unconjugated bile salts or free bile salts are insoluble and are reabsorbed less efficiently, so they will be excreted with feces [101]. After BSH catalysis, the bile acid content in the body decreases, and more cholesterol is needed if bile salts are to be formed. With the high activity of BSH, Lb. plantarum can increase bile acid consumption and promote the de novo synthesis of cholesterol into bile acids, ultimately reducing the body’s cholesterol levels.
This process is adjusted by the expression of a variety of proteins, including farnesoid X receptor (FXR), liver X receptor (LXR), and cholesterol 7α-hydroxylase (CYP7A1). Wang Huang et al. [102] fed mice with Lb. plantarum AR113, which has high BSH activity. They found that the serum TC and LDL-C levels decreased significantly, and the serum HDL-C level was increased in the Lb. plantarum AR113 group compared with mice in the control groups. Meanwhile, the mRNA levels of FXR were significantly down-regulated, and mRNA expression of CYP7A1 and LXR were significantly upregulated in the AR113 group, which means that Lb. plantarum AR113 can regulate the metabolism of bile acids to affect cholesterol content. Other studies have obtained similar findings in other Lb. plantarum with high BSH activity [103,104].
In addition to regulating bile acid metabolism through high BSH activity, Lb. plantarum can decrease serum cholesterol levels by promoting the combination of free bile salt and cholesterol to form precipitation, which is hard to absorb. Aarti [105] isolated several strains from breast-fed infants’ feces and cultured them in MRS broth to which sodium glycinamide and sodium taurocholate were added. The content of bile acids released from the conjugated bile salts sodium glycinamide and sodium taurocholate were then utilized as criteria to determine the ability of different strains to remove cholesterol. The method described by Walker and Gilliland was used to detect the cholic acid level and colorimetric analyses to detect cholesterol level. The experimental results proved that among all these strains, Lb. plantarum GD2 had the best capacity to precipitate cholesterol, while it could survive at low PH and high bile concentration, which indicates that Lb. plantarum GD2 has a good ability for cholesterol scavenging. The exact mechanism still needs to be determined by further studies.
The two ways in which Lb. plantarum regulates bile acid levels and lowers cholesterol are shown in Figure 3. These findings indicate that Lb. plantarum can regulate bile acid metabolism through BSH, and promote the combination of free bile salts and cholesterol to reduce lipids. Therefore, cholesterol-lowering via bile acids may be a promising approach for the use of Lb. plantarum in the treatment of CVD.
Figure 3.
Mechanism of cholesterol lowering by Lactiplantibacillus plantarum via bile acids.Lactiplantibacillus plantarum reduces cholesterol by regulating the metabolism of bile acids that consume cholesterol, as well as promoting the combination of bile acids and cholesterol into precipitates. (FXR—farnesoid X receptor, LXR—liver X receptor, CYP7A1—cholesterol 7α-hydroxylase) (Created with Biorender.com).
3.3.3. Lowering Cholesterol through Intestinal Flora
Accumulating evidence suggests that gut dysbiosis induced by a high-fat diet promotes the development of hypercholesterolemia, obesity, and other metabolic syndromes. Recent studies have highlighted the importance of the gastrointestinal microbiome in regulating host health and disease [106]. Scientists have suggested that Lb. plantarum could increase the species richness and biodiversity of intestinal flora, as evidenced by an increase in beneficial microorganisms and a decrease in pathogenic bacteria. Shao, Y., Huo, D., Peng, Q., et al. [107] applied a metagenomic approach to indicate that the consumption of probiotics can regulate the intestinal microbial structure in hyperlipidaemic patients. Their research showed that the genera Bifidobacterium, Lactobacillus, Akkermansia, and Faecalibacterium were significantly increased in the groups fed with Lb. plantarum, while the genera Clostridium, Natranaerovirga, Fervidicella, Roseburia, Gemella, Escherichia/Shigella, and Odoribacter were significantly increased in the hyperlipidemic group. The intrinsic mechanism of lipid-lowering by intestinal flora has not been fully clarified, although it is a current research hotspot. We look forward to the latest research results in the future.
3.3.4. Lowering Cholesterol through Conjugated Linoleic Acid (CLA) Isomerase
In addition to the mechanism above, Lb. plantarum can produce conjugated linoleic acid (CLA) isomerase to synthetic CLA, thus reducing cholesterol and treating hyperlipidaemia.
CLA is not a single kind of linoleic acid but a series of linoleic acid isomers, whose functions are caused by the coordinated regulation of metabolic pathways by multiple CLA isomers, such as cis-9, trans-11, trans-10, and cis-12, playing an important role in fat uptake and lipid metabolism. However, the requirements for the chemical synthesis of linoleic acid are complex and there are many by-products. Thus, the biosynthesis of linoleic acid isomerase through microorganisms is believed to be a great alternative approach. Some bacteria, especially Lb. plantarum ZS2058, screened from Chinese traditional food pickles, had high linoleate isomerase activity, and has proved to be the most efficient strain of lactobacillus. Through localization and purification of linoleic acid isomerase, it was determined that CLA isomerase catalyzed hydrated reaction in microbial cells to form 10- HOE, which is the intermediate of CLA synthesis, and after multi-step reaction generated the CLA with biological activity [108].
The same transformation mechanism exists in Lb. plantarum AKU1009A [109]. In addition, a study of L. plantarum CGMCC8198 showed that it had a homologous sequence with the isomerase gene of L. plantarum ZS2058, and linoleic acid isomer could be produced by fermentation with Acer Truncatum Bunge seeds oil. This has laid a certain foundation for microbial production of Lb. plantarum [110]. In the experiment of Chen L. et al. [111], dietary supplementation of conjugated CLA can increase the abundance of lipid metabolism-related bacteria and significantly reduce fat deposition in mice, further evidence of its cholesterol-lowering effect.
3.4. Mechanisms of Glucose Lowering by Lb. plantarum
The concentration of glucose in the blood is called the serum glucose level. A normal serum glucose level plays an important role in maintaining the human body’s metabolism and the basic functioning of all organs. Thus, if the blood glucose level is too high or too low, it can cause damage to people’s health. Hyperglycemia can increase the risk of diabetes, lead to poor immunity, and cause CVD. Meanwhile, if glucose is not utilized by the body in a timely manner, it may lead to low energy supply, weariness, and depression.
Lb. plantarum can regulate the serum glucose level and inhibit diabetes [112]. Ahtesham Hussain [113] used Lb. plantarum LB818 to feed mice and found that high-fat diet (HFD) mice had a lower serum glucose level when treated with Lb. plantarum LB818. Taking the changes in TC, TG, HDL, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) into account, it can be concluded that Lb. plantarum LB818 can display anti-diabetic effects by preventing weight gain, lowering fat, and balancing glucose levels. Similar results were obtained in a study conducted by U. Andersson et al. [114]. They found that feeding with Lb. plantarum DSM 15313 can enhance the glucose elimination and insulin response of mice. Moreover, in another report, rats showed a decrease in serum glucose levels after 28 days of taking Lb. plantarum 49 and Lb. plantarum 201 [115].
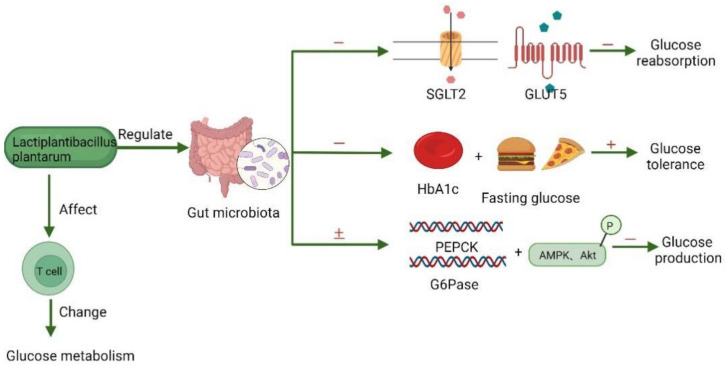
Numerous related experiments were conducted to validate the hypoglycemic effect of Lb. plantarum and explore the underlying mechanism of Lb. plantarum. Although the glucose-lowering mechanism remained to be elucidated, the most likely explanation was related to gut microbiota. Sen Lin [116] detected the changes in glucose concentrations of piglets that were fed with Lb. plantarum and analyzed the microbiota profile in fecal samples. Based on the results, they speculated that the way Lb. plantarum affected glucose homeostasis may be associated with modulating the relative abundances of gut microbial genera. Lb. plantarum can alter the host’s gut microbiota, leading to the upregulation of specific glucose, which means a decrease in glucose reabsorption and a decrease in fasting glucose and glycated hemoglobin (HbA1c) levels. Moreover, with the higher glucose tolerance, the protein kinase B (Akt) and AMPK phosphorylation, as well as the mRNA expression levels of Phosphoenolpyruvate carboxykinase (PEPCK) and Glu-cose-6-phosphatase (G6Pase) are altered, resulting in the decrease of glucose production. The possible mechanism by which gut microbiota affect glucose metabolism is shown in Figure 4.
Figure 4.
The blood glucose lowering mechanism of Lactiplantibacillus plantarum.Lactiplantibacillus plantarum lowering glucose level through gut microbiota, which can regulate the expression of Phosphoenolpyruvate carboxykinase (PEPCK) and Glucose-6-phosphatase (G6Pase), as well the Adenosine 5‘-monophosphate activated protein kinase (AMPK)/protein kinase B (Akt) signaling pathway, meanwhile reducing the level of transporter SGLT2, GLUT5, glycated hemoglobin (HbA1c), and fasting glucose. Immune cell function is regarded as a possible mechanism but still needs further study. (“+” means upregulation, “−“ means downregulation and “±“ means upregulation or downregulation) (Created with Biorender.com).
In addition, studies have shown that Lb. plantarum can regulate signal pathways through intestinal flora [117,118,119]. It is reported that mice fed with Lb. plantarum HAC01 had a lower level of fasting glucose and glycated hemoglobin (HbA1c) level and performed better in the oral glucose tolerance test (OGTT), which suggests the anti-hyperglycemic effect of the strain, and implies that it can improve glucose intolerance. Through the study of the expression of genes and proteins related to glucose metabolism in the liver, scientists found that it was able to increase Akt and AMPK phosphorylation, and decrease PEPCK andG6Pase mRNA expression levels in the liver, therefore affecting endogenous glucose production in the liver [120]. Some researchers have also proved that Lb. plantarum could improve the utilization of glucose. Rats fed with a high-fructose diet exhibited upregulation of specific glucose transporter SGLT2 and specific fructose transporter GLUT5, which may lead to the higher fructose level in the blood and kidney of rats. A supplement of Lb. plantarum may restrict the reabsorption of glucose [121]. Apart from utilizing glucose, Lb. plantarum can also adapt to glucose-limited conditions. Studies of the genotypic and proteomic changes in Lb. plantarum P-8 in response to long-term (3 years) glucose limitation, explored various mechanisms for survival, which consist of altering the cell envelope, activating the phosphotransferase system, accumulating and consuming amino acids, reducing glucose intake, and increasing the generation of glucose or ATP in response to glucose starvation [122].
Aside from that, Lb. plantarum OLL2712 could regulate glucose metabolism without the reduction of body weight, and this effect may be connected with the functioning of immune cells, such as T cells. However, whether immune cells can change glucose metabolism requires further study [123].
4. Recent Developments in Lb. plantarum-Fermented Food
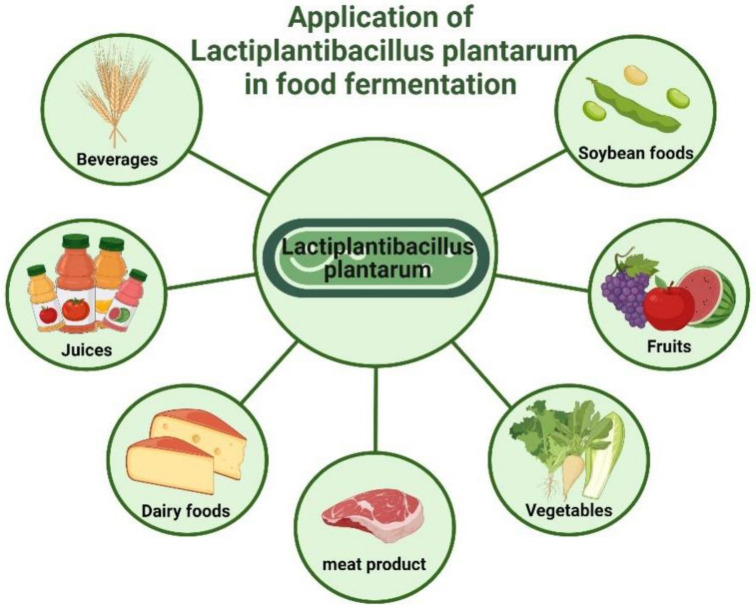
As mentioned above, Lb. plantarum has the potential to be developed as a probiotic because of its excellent anti-cardiovascular properties, as well as its good tolerance in the gastrointestinal tract, which is important for human health. In addition, some studies have demonstrated that foods fermented with it have better health benefits than before fermentation, and may have the ability to be developed into probiotic functional foods. The applications of Lb. plantarum in different types of foods, and the corresponding effects of its fermented foods, are shown in Figure 5 and Table 3.
Figure 5.
The application of Lactiplantibacillus plantarum in several different types of food.Lactiplantibacillus plantarum has been widely used as a fermenting strain in the production of a wide range of fermented foods. (Created with Biorender.com).
Table 3.
The effects of Lb. plantarum fermented foods in the prevention of different diseases.
| Types of Food | Fermented Food | Lb. plantarum | Function | Mechanism | References |
|---|---|---|---|---|---|
| Fruit and vegetable juice |
charantia juice | Lb. plantarum NCU116 | antioxidant | increase the content of phenolic compounds and promote the biotransformation to provide stronger antioxidant properties | [124] |
| papaya juice | Lb. plantarum GIM1.140 | antioxidant | increase the content of total flavonoids and improve inhibition of DPPH free radicals | [125] | |
| blueberry juice | A variety of mixed strains including Lb. plantarum | anti-diabetes | maintain glucose homeostasis and promote glucose consumption | [126] | |
| green loofah | Lb. plantarum SU4 | cholesterol lowering |
high bile acid lowering capacity in vitro and in vivo to promote cholesterol consumption | [127] | |
| Aquatic product |
laminaria japonica | Lb. plantarum FZU3013 | cholesterol lowering |
reduce expression levels of genes involved in lipid metabolism and bile acid homeostasis to promote cholesterol consumption | [128] |
| Soybean products |
black Soymilk | Lb. plantarum BCRC 10357 | antioxidant | increase the ferric reducing antioxidant capacity | [129] |
| soy milk added with cuminum cyminum essential oil |
Lb. plantarum
A7 (KC 355240) |
anti-diabetes and cholesterol lowering |
significantly reduces postprandial serum glucose concentrations and TG levels. | [130] | |
| soy extract | Lb. plantarum WW | cholesterol lowering |
regulate the expression levels of genes involved in lipid metabolism and oxidation-reduction processes to promote cholesterol catabolism | [131] | |
| Dairy products |
orange juice-milk based beverage |
Lb. plantarum (TR-7, TR-71, TR-14) | antioxidant | increase the content of carotenoids and the total antioxidant activity | [132] |
| kalari cheese | Lb. plantarum NCDC 012 | anti-diabetes | produce a variety of bioactive peptides to enhance the inhibitory activity of α-amylase and α-glucosidase, and inhibit carbohydrate decomposition to lower glucose | [133] | |
| goat milk | Lb. plantarum C4 | blood pressure lowering | enhance the ACE inhibitory activity by fermentation | [134] | |
| skim milk | Lb. plantarum WW | cholesterol lowering |
regulate the intestinal flora and lower cholesterol levels | [135] | |
| cheese | Lb. plantarum VC213 | cholesterol lowering |
significantly lower cholesterol content than before fermentation | [136] | |
| Cereal grains | rice bran and wheat bran |
Lb. plantarum NCU116 | antioxidant | enhance the hydroxyl radical-scavenging activity and the oxygen radical-quenching activity | [137] |
| whole-grain oats | Lb. plantarum B1-6 | blood pressure lowering | present higher ACE inhibitory activities | [138] | |
| Meat products |
Chinese fermented sausages | Lb. plantarum CD101 | antioxidant | reduce pH, and promote the formation of antioxidant peptides | [139] |
| fermented meat patty | Lb. plantarum PTCC 1745 | antioxidant | radical scavenging activity significantly higher than before fermentation | [140] | |
| fermented camel sausages | Lb. plantarum KX881772 | anti-diabetes | higher α-amylase and higher α-glucosidase inhibitions to control diabetes by reducing carbohydrate hydrolysis | [141] | |
| fermented sausage | Lb. plantarum CD101 | blood pressure lowering | significantly increase the ACE inhibitory activity | [142] |
4.1. Fermented Food with Antioxidant Function
Fruits and vegetables are rich in phenolic substances, carotenoids, flavonoids, and vitamin C, which have good antioxidant effects. Therefore, scientists hope to develop functional beverages based on fruit and vegetable juices and improve their antioxidant capacity through fermentation. In earlier research, some fermented juices with antioxidant functions, such as apple, orange, jujube, and coconut juices have been produced and proven to have a better ability to scavenge free radicals. In recent years, there have also been many reports on the antioxidant functional juice fermented by Lb. plantarum. Wu, Li, et al. [143] compared the antioxidant activity of blueberry and blackberry juices before and after fermentation based on ABTS methods, and they found that the ABTS radical scavenging activity of blackberry and blueberry juices fermented with Lactobacillus plantarum increased by 53.3% and 64.0%, respectively, compared to the juice before fermentation. The same results were also reported in fermented vegetable juice. Zhang, Duan, et al. [144] also found that the DPPH radical scavenging rate and ABTS free radical scavenging rate of carrot juice after 72 h of fermentation by Lb. plantarum WZ-01 were increased to some extent compared to their pre-fermentation state. These results suggest that fermentation by Lb. plantarum can effectively enhance the free radical scavenging activity of juices, and fermented fruit and vegetable juices can be developed as a good antioxidant functional beverage.
In addition to fruit and vegetable juice, there are also reports on the application of Lb. plantarum in the fermentation of other types of foods, including dairy products [132], soybean products [129], fermented sausages [139]. All these types of fermented foods show better antioxidant capacity in vitro compared with prior to fermentation.
4.2. Fermented Food with Cholesterol-Lowering Function
The development of Lb. plantarum-fermented foods with cholesterol-lowering effects have achieved some results, including dairy products, soybean products, fruits and vegetables, aquatic products. As early as 2015, Jeon, Lee, et al. [145] isolated a strain of lactic acid bacteria from kimchi that could lower cholesterol in vitro, named Lb. plantarum EM. Four years later, they utilized Lb. plantarum EM as a fermenting strain in cabbage-apple juice to investigate the health-promoting effects of fermented juice on high-cholesterol diet rats. It was found that the serum levels of TG, TC, and LDL-C were greatly reduced, and HDL-C levels were increased in rats fed with fermented juice compared to unfermented juice. Many scientists have obtained similar results with different types of fermented foods. Cao, Wu, et al. [131] pointed out that soy extract fermented by Lb. plantarum could regulate lipid metabolism through signaling pathways and thus lower cholesterol. Li, Wu, et al. [135] analyzed the microbial diversity of rat feces and found that skim milk fermented with Lb. plantarum WW could regulate intestinal flora and lower cholesterol levels. Hu, Zheng, et al. [128] found that Lb. plantarum FZU3013-fermented Laminaria japonica could affect the expression of genes involved in lipid metabolism and bile acid homeostasis in rats.
4.3. Fermented Food with Blood Pressure Lowering Function
There are few reports on the hypotensive effects of Lb. plantarum-fermented foods, and most of those reports focused on fermented foods that can inhibit ACE activity and are mainly fermented dairy products. Some research results are shown in Table 2 above. in addition to dairy products, the same findings have been reported for other fermented foods, such as soy milk [71], fermented sausages [146], and fruits like guava [147]. However, due to the lack of relevant studies, it cannot be ascertained whether fermented foods are effective in in vivo experiments.
4.4. Fermented Food with Hypoglycemic Function
Several studies have confirmed the hypoglycemic effect of Lb. plantarum-fermented foods. Zhong, Abdullah, et al. [126] used blueberry as a fermentation substrate. Blueberries are widely considered to have a good antidiabetic ability and the potential to treat early-stage diabetes. In their research, they found that, compared with the non-fermented blueberry juice, the fumarate contents in blueberry juice fermented by Lb. plantarum were significantly increased, which could maintain glucose homeostasis. In addition, fermentation altered the concentrations of phenolic compounds in blueberry juice, which promoted glucose consumption. They employed HepG2 cell lines as a model to confirm that fermented juice significantly enhanced cellular glucose consumption [148]. In addition to this, Lb. plantarum fermentation also increases α-Amylase and α-glucosidase inhibitory activity, thus reducing carbohydrate hydrolysis for the effective control of diabetes [149].
4.5. Other Applications of Lb. plantarum
In conclusion, it can be seen that Lb. plantarum-fermented foods show good effects in in vitro experiments, and have the potential to be developed into probiotic products. Moreover, in addition to conferring additional functional characteristics to the foods, Lb. plantarum fermentation may improve the properties of traditional fermented foods. Compared with unfermented food, fermented food can produce bacteriocins with good antimicrobial properties and extended storage time [150]; it may also have better stability [151], and its flavor will be improved to meet consumers’ demands [124]. At present, there are various kinds of functional fermented foods reported covering the basic diet, such as dairy products, fruits, vegetables, meat products, soybeans, and cereals. These factors comprehensively show that developing Lb. plantarum-fermented foods and supplementing these foods in daily life may be of great significance to human health.
5. Conclusions
In the context of high CVD incidence worldwide, in addition to common pharmacological treatments, probiotics and probiotic functional foods have become important means of preventing and alleviating such diseases. Lb. plantarum has great potential in the prevention and treatment of CVD and could be developed as a probiotic to play a healthy role in the human body. Its mechanisms include increasing antioxidant levels and maintaining the balance of blood glucose, blood lipids, and blood pressure. At the same time, studies have found that Lb. plantarum has a wide range of applications in fermented foods such as beverages and cheese. A variety of fermented foods have the effect of preventing and alleviating CVD. Research and optimization of the composition, technology, properties, and other related characteristics of Lb. plantarum-fermented foods can assist scientists in improving the quality of these foods and their probiotic activity, ultimately alleviating patients’ CVD symptoms. Therefore, Lb. plantarum functional fermented foods have broad application prospects in reducing the risk of CVD and its treatment. In the future, we can reduce the risk of CVD and alleviate CVD by supplementing Lb. plantarum fermented foods to our daily diet. However, the therapeutic effects of Lb. plantarum-fermented food on CVD have not been clinically verified, and the function of Lb. plantarum in actual production is affected by temperature, pH value, food composition, and other food microorganisms. Therefore, in order to develop functional foods better, it is necessary to explore the optimal production conditions further.
Acknowledgments
We are grateful to Fujian GTR Biotechnology Co. for the information and theoretical guidance.
Author Contributions
Conceptualization, Z.W. and Z.T.; methodology, J.W. and Y.S.; software, Z.W., Z.T. and Y.S.; writing—original draft preparation, Z.W., J.W., Z.T. and Y.S.; writing—review and editing, Z.W., Z.T., J.W. and H.C.; visualization, Z.W., Z.T. and Y.S.; supervision, H.C. and J.G.; project administration, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the Natural Science Foundation of Shandong Province (No. ZR2019BC053), the National Natural Science Foundation of China (No. 32102129), and the Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, Jiangnan University (No. 1022050205219730/008), Young Scholars Program of Shandong University, Weihai.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Cardiovascular Diseases (CVDs) 2021. [(accessed on 11 June 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Shengshou H. Report on Cardiovascular Health and Diseases Burden in China: An Updated Summary of 2020. Chin. Circ. J. 2021;36:521–545. [Google Scholar]
- 3.Andersson C., Vasan R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2017;15:230–240. doi: 10.1038/nrcardio.2017.154. [DOI] [PubMed] [Google Scholar]
- 4.Khanal M.K., Bhandari P., Dhungana R.R., Gurung Y., Rawal L.B., Pandey G., Bhandari M., Devkota S., de Courten M., de Courten B. Poor glycemic control, cardiovascular disease risk factors and their clustering among patients with type 2 diabetes mellitus: A cross-sectional study from Nepal. PLoS ONE. 2022;17:e0271888. doi: 10.1371/journal.pone.0271888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciumărnean L., Milaciu M.V., Negrean V., Orășan O.H., Vesa S.C., Sălăgean O., Iluţ S., Vlaicu S.I. Cardiovascular Risk Factors and Physical Activity for the Prevention of Cardiovascular Diseases in the Elderly. Int. J. Environ. Res. Public Health. 2021;19:207. doi: 10.3390/ijerph19010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaresko J., Rienks J., Nöthlings U. Lifestyle Indices and Cardiovascular Disease Risk: A Meta-analysis. Am. J. Prev. Med. 2018;55:555–564. doi: 10.1016/j.amepre.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Powell-Wiley T.M., Baumer Y., Baah F.O., Baez A.S., Farmer N., Mahlobo C.T., Pita M.A., Potharaju K.A., Tamura K., Wallen G.R. Social Determinants of Cardiovascular Disease. Circ. Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habeeb E., Aldosari S., Saghir S.A., Cheema M., Momenah T., Husain K., Omidi Y., Rizvi A.S.A., Akram M., Ansari R.A. Role of environmental toxicants in the development of hypertensive and cardiovascular diseases. Toxicol. Rep. 2022;9:521–533. doi: 10.1016/j.toxrep.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenster K., Freeburg B., Hollard C., Wong C., Laursen R.R., Ouwehand A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms. 2019;7:83. doi: 10.3390/microorganisms7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasika D., Vidanarachchi J., Luiz S., Azeredo D., Cruz A., Ranadheera C. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture. 2021;11:599. doi: 10.3390/agriculture11070599. [DOI] [Google Scholar]
- 11.Wu W., Wang L., Zhao J., Zhang H., Chen W. Research progress on physiological characteristics and health. Food Ferment. Ind. 2019;45:1–13. [Google Scholar]
- 12.Oh Y., Kim T., Moon H., Lee S., Lee S., Ji G., Hwang K. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules. 2020;25:5056. doi: 10.3390/molecules25215056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimoto-Nira H., Moriya N., Nogata Y., Sekiyama Y., Toguchi Y. Fermentation of Shiikuwasha (Citrus depressa Hayata) pomace by lactic acid bacteria to generate new functional materials. Int. J. Food Sci. Technol. 2018;54:688–695. doi: 10.1111/ijfs.13980. [DOI] [Google Scholar]
- 14.Han S., Lu Y., Xie J., Fei Y., Zheng G., Wang Z., Liu J., Lv L., Ling Z., Berglund B., et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021;11:609722. doi: 10.3389/fcimb.2021.609722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghatani K., Tamang B. Assessment of probiotic characteristics of lactic acid bacteria isolated from fermented yak milk products of Sikkim, India: Chhurpi, Shyow, and Khachu. Food Biotechnol. 2017;31:210–232. doi: 10.1080/08905436.2017.1335212. [DOI] [Google Scholar]
- 16.Wang G., Chen Y., Fei S., Xie C., Xia Y., Ai L. Colonisation with endogenous Lactobacillus reuteri R28 and exogenous Lactobacillus plantarum AR17-1 and the effects on intestinal inflammation in mice. Food Funct. 2021;12:2481–2488. doi: 10.1039/D0FO02624G. [DOI] [PubMed] [Google Scholar]
- 17.Galdino I.K.C.P.D.O., Oliveira M.M., Oliveira A.T., da Silva G.M., de Oliveira T.A., dos Santos K.M.O., Egito A.S.D., Buriti F.C.A. Fermentative behavior of native lactobacilli in goat milk and their survival under in vitro simulated gastrointestinal conditions. LWT. 2020;135:109905. doi: 10.1016/j.lwt.2020.109905. [DOI] [Google Scholar]
- 18.Flach J., van der Waal M., Nieuwboer M.V.D., Claassen H., Larsen O.F.A. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Crit. Rev. Food Sci. Nutr. 2017;58:2570–2584. doi: 10.1080/10408398.2017.1334624. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R., Mokhtari S., Jafari S.M., Sharma S. Barley-based probiotic food mixture: Health effects and future prospects. Crit. Rev. Food Sci. Nutr. 2021:1–15. doi: 10.1080/10408398.2021.1921692. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W., Lyu F., Naumovski N., Ajlouni S., Ranadheera C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages. 2020;6:13. doi: 10.3390/beverages6010013. [DOI] [Google Scholar]
- 21.De Oliveira P.M., de Castro Leite Junior B.R., Martins E.M.F., Martins M.L., Vieira N.R., de Barros F.A.R., Cristianini M., de Almeida Costa N., Ramos A.M. Mango and carrot mixed juice: A new matrix for the vehicle of probiotic lactobacilli. J. Food Sci. Technol. 2020;58:98–109. doi: 10.1007/s13197-020-04518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldwin C.M., Bootzin R.R., Schwenke D.C., Quan S.F. Antioxidant nutrient intake and supplements as potential moderators of cognitive decline and cardiovascular disease in obstructive sleep apnea. Sleep Med. Rev. 2005;9:459–476. doi: 10.1016/j.smrv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang X., Li L., Duan Y., Yang X. Antioxidant activity of Lactobacillus plantarum JM113 in vitro and its protective effect on broiler chickens challenged with deoxynivalenol1. J. Anim. Sci. 2017;95:837–846. doi: 10.2527/jas.2016.0789. [DOI] [PubMed] [Google Scholar]
- 24.Kim M., Seo D.-H., Park Y.-S., Cha I.-T., Seo M.-J. Isolation of Lactobacillus plantarum subsp. plantarum Producing C30 Carotenoid 4,4′-Diaponeurosporene and the Assessment of Its Antioxidant Activity. J. Microbiol. Biotechnol. 2019;29:1925–1930. doi: 10.4014/jmb.1909.09007. [DOI] [PubMed] [Google Scholar]
- 25.Kim M., Jung D.-H., Seo D.-H., Park Y.-S., Seo A.M.-J. 4,4′-Diaponeurosporene from Lactobacillus plantarum subsp. plantarum KCCP11226: Low Temperature Stress-Induced Production Enhancement and In Vitro Antioxidant Activity. J. Microbiol. Biotechnol. 2021;31:63–69. doi: 10.4014/jmb.2010.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S., Jiang C.Y. Antioxidant activity of three strains of lactic acid bacteria and investigate the antioxidant mechanism primarily; Proceedings of the 9th International Symposium on Lactic Acid Bacteria and Health; Tianjing, China. 21–23 May 2014; pp. 68–69. [Google Scholar]
- 27.Das D., Goyal A. Potential probiotic attributes and antagonistic activity of an indigenous isolate Lactobacillus plantarum DM5 from an ethnic fermented beverage “Marcha” of North Eastern Himalayas. Int. J. Food Sci. Nutr. 2014;65:335–344. doi: 10.3109/09637486.2013.869792. [DOI] [PubMed] [Google Scholar]
- 28.Das D., Goyal A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT Food Sci. Technol. 2015;61:263–268. doi: 10.1016/j.lwt.2014.11.013. [DOI] [Google Scholar]
- 29.Yang B. Study on the Mechanism of Lactobacillus Plantarum NJAU-01 to Relieve Oxidative Stress in Mice. BMC Microbiol. 2021;21:182. doi: 10.1186/s12866-021-02248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L. Research on the Antioxidant Ability and Mechanism of Lactobacillus Plantarum NJAU-01. Yangzhou University; Yangzhou, China: 2020. [Google Scholar]
- 31.Tian Y., Wang Y., Zhang N., Xing X., Xiao M., Wang Y. Screening of Antioxidant Lactic Acid Bacteria and Its Antioxidant Mechanism; Proceedings of the 16th International Symposium on Probiotics and Health; Jiangsu, China. 25 May 2021; pp. 42–43. [Google Scholar]
- 32.Liu B., Xiao X., Zhou X., Zhou J., Lan L., Long X., Pan Y., Du M., Zhao X. Effects of Lactobacillus plantarum CQPC01-fermented soybean milk on activated carbon-induced constipation through its antioxidant activity in mice. Food Sci. Nutr. 2019;7:2068–2082. doi: 10.1002/fsn3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L., Long L., Sun J., Bu L., Cao J., Luo Y., Liu H., Wu Y., Meng X. Exploring the antioxidant effect of Lactobacillus plantarum SCS2 on mice with type 2 diabetes. J. Food Biochem. 2021;45:e13781. doi: 10.1111/jfbc.13781. [DOI] [PubMed] [Google Scholar]
- 34.Yan L., Wang X., Jing T., Liu Z., Liu L. Antioxidant effect of Lactobacillus plantarum FEED8 on mice. China Dairy Cattle. 2020:59–63. doi: 10.19305/j.cnki.11-3009/s.2020.10.014. [DOI] [Google Scholar]
- 35.Zhao J. Evaluation of Antioxidative Abilities of Lactobacillus Plantarum and the Antioxidative Mechanisms. J. Microbiol. Biotechnol. 2018;28:1052–1060. doi: 10.4014/jmb.1712.12022. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Li D., Zhao Y., Zhang X., Huang L. Protective effect of Lactobacillus plantarum C88 on H2O2-induced oxidative damage in Caco-2 cells. Sci. Agric. Sin. 2013;46:606–613. [Google Scholar]
- 37.Huang C. Studies on the Isolation, Identification of Lactobacillus Plantarum Y-20 and the Antioxidant Activity of Its Exopolysaccharides. Hunan Agricultural University; Hunan, China: 2019. [Google Scholar]
- 38.Xun Y., Zhang D., Feng L., Xue Y., Wang S., Zhu H. Study on the Antioxidant Effect of Lactobacillus plantarum JMCC0017. China Association for Science and Technology; Beijing, China: 2019. pp. 137–138. [Google Scholar]
- 39.Lv F., Wang G., Xia Y., Zhang H., Ai L., Xiong Z. Antioxidant Effect and Mechanism of Lactobacillus plantarum AR501. China Association for Science and Technology; Beijing, China: 2019. pp. 40–41. [Google Scholar]
- 40.Wang M. Master’s Thesis. Jilin Agricultural University; Jilin, China: 2019. Preliminary Study on the Anti-oxidative Stress Effect of Lactobacillus Plantarum and Its Mechanism. [Google Scholar]
- 41.Hong J.W., Park H.Y., Kim J.H., Yeom S.H., Kim J.W. Antioxidation and Anti-photoaging Effects of White Taraxacum Coreanum Extract by Lactobacillus plantarum. J. Korea Acad. Ind. Coop. Soc. 2021;22:554–562. doi: 10.5762/kais.2021.22.4.554. [DOI] [Google Scholar]
- 42.Wang J., Ji H.F., Wang S.X., Zhang D.Y., Liu H., Shan D.C., Wang Y.M. Lactobacillus plantarum ZLP001: In vitro Assessment of Antioxidant Capacity and Effect on Growth Performance and Antioxidant Status in Weaning Piglets. Asian Australas. J. Anim. Sci. 2012;25:1153–1158. doi: 10.5713/ajas.2012.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu S., Liu B., Sun X., Li X., Li D. Study on antioxidant activities of Lactobacillus plantarum and Lactobacillus casei. China Dairy Ind. 2018;46:4–8. [Google Scholar]
- 44.Li D., Li X., Han X., Li L., Wang L., Zhang X., Wang Y. Antioxidant Activity of Lactobacillus Plantarum and Lactobacillus Casei on Cheddar Cheese and the Number of Simulated Gastrointestinal Bacteria. J. Chin. Inst. Food Sci. Technol. 2018;18:130–138. doi: 10.16429/j.1009-7848.2018.06.017. [DOI] [Google Scholar]
- 45.Cheng X., Sun Y., Liu N., Huang W., Li N., Filip A. Antioxidant Properties and Flavor Characteristics of Steamed Bread Fermented by Lactobacillus plantarum Isolated from Traditional Sourdough. Food Sci. 2015;36:87–92. [Google Scholar]
- 46.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Himmelfarb C.D., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/hyp.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 47.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forouzanfar M.H., Liu P., Roth G.A. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:648. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson U., Danilczyk U., Penninger J.M. Just the Beginning: Novel Functions for Angiotensin-Converting Enzymes. Curr. Biol. 2002;12:R745–R752. doi: 10.1016/S0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 50.Lewis-Mikhael A.-M., Davoodvandi A., Jafarnejad S. Effect of Lactobacillus plantarum containing probiotics on blood pressure: A systematic review and meta-analysis. Pharmacol. Res. 2020;153:104663. doi: 10.1016/j.phrs.2020.104663. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Zhang Q., Ji Z., Shu G., Chen H. Production and fermentation characteristics of angiotensin-I-converting enzyme inhibitory peptides of goat milk fermented by a novel wild Lactobacillus plantarum 69. LWT. 2018;91:532–540. doi: 10.1016/j.lwt.2018.02.002. [DOI] [Google Scholar]
- 52.Omedi J.O., Huang W., Su X., Liu R., Tang X., Xu Y., Rayas-Duarte P. Effect of five lactic acid bacteria starter type on angiotensin-I converting enzyme inhibitory activity and emulsifying properties of soy flour sourdoughs with and without wheat bran supplementation. J. Cereal Sci. 2016;69:57–63. doi: 10.1016/j.jcs.2016.02.008. [DOI] [Google Scholar]
- 53.Sánchez-López F., Robles-Olvera V.J., Hidalgo-Morales M., Tsopmo A. Angiotensin-I converting enzyme inhibitory activity of Amaranthus hypochondriacus seed protein hydrolysates produced with lactic bacteria and their peptidomic profiles. Food Chem. 2021;363:130320. doi: 10.1016/j.foodchem.2021.130320. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y.-Y., Zeng S.-Y., Leu Y.-L., Tsai T.-Y. Antihypertensive Effect of a Combination of Uracil and Glycerol Derived from Lactobacillus plantarum Strain TWK10-Fermented Soy Milk. J. Agric. Food Chem. 2015;63:7333–7342. doi: 10.1021/acs.jafc.5b01649. [DOI] [PubMed] [Google Scholar]
- 55.Nahariah N., Legowo A.M., Abustam E., Hintono A. Angiotensin I-Converting Enzyme Inhibitor Activity on Egg Albumen Fermentation. Asian Australasian J. Anim. Sci. 2015;28:855–861. doi: 10.5713/ajas.14.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu G., Yang H., Chen H., Zhang Q., Tian Y. Effect of incubation time, inoculum size, temperature, pasteurization time, goat milk powder and whey powder on ACE inhibitory activity in fermented milk by L. plantarum LP69. Acta Sci. Polonorum. Technol. Aliment. 2015;14:107–116. doi: 10.17306/J.AFS.12. [DOI] [PubMed] [Google Scholar]
- 57.Xia Y., Yu J., Xu W., Shuang Q. Purification and characterization of angiotensin-I-converting enzyme inhibitory peptides isolated from whey proteins of milk fermented with Lactobacillus plantarum QS670. J. Dairy Sci. 2020;103:4919–4928. doi: 10.3168/jds.2019-17594. [DOI] [PubMed] [Google Scholar]
- 58.Diana M., Quílez J., Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods. 2014;10:407–420. doi: 10.1016/j.jff.2014.07.004. [DOI] [Google Scholar]
- 59.Zareian M., Oskoueian E., Forghani B., Ebrahimi M. Production of a wheat-based fermented rice enriched with γ-amino butyric acid using Lactobacillus plantarum MNZ and its antihypertensive effects in spontaneously hypertensive rats. J. Funct. Foods. 2015;16:194–203. doi: 10.1016/j.jff.2015.04.015. [DOI] [Google Scholar]
- 60.Liu C.F., Tung Y.T., Wu C.L., Lee B.-H., Hsu W.-H., Pan T.M. Antihypertensive Effects of Lactobacillus-Fermented Milk Orally Administered to Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2011;59:4537–4543. doi: 10.1021/jf104985v. [DOI] [PubMed] [Google Scholar]
- 61.Torino M.I., Limón R.I., Martínez-Villaluenga C., Mäkinen S., Pihlanto A., Vidal-Valverde C., Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2012;136:1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Ahrén I.L., Xu J., Önning G., Olsson C., Ahrné S., Molin G. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin. Nutr. 2015;34:719–726. doi: 10.1016/j.clnu.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Saputri F.A., Kang D., Kusuma A.S.W., Rusdiana T., Hasanah A.N., Mutakin, Surono I.S., Koyama H., Abdulah R. Lactobacillus plantarum IS-10506 probiotic administration increases amlodipine absorption in a rabbit model. J. Int. Med. Res. 2018;46:5004–5010. doi: 10.1177/0300060518788994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robles-Vera I., Toral M., Romero M., Jiménez R., Sánchez M., Pérez-Vizcaíno F., Duarte J. Antihypertensive Effects of Probiotics. Curr. Hypertens. Rep. 2017;19:26. doi: 10.1007/s11906-017-0723-4. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Shu G., Chen L., Dai C., Li Y., Niu J., Wan H. Directed-Vat-Set starter producing ACE-inhibitory peptides: Optimization and evaluation of stability. J. Food Process. Preserv. 2022;46:e16475. doi: 10.1111/jfpp.16475. [DOI] [Google Scholar]
- 67.Jitpakdee J., Kantachote D., Kanzaki H., Nitoda T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT. 2022;160:113269. doi: 10.1016/j.lwt.2022.113269. [DOI] [Google Scholar]
- 68.Hernández-Sánchez H., Nacional I.P., Fajardo-Espinoza F., Gutiérrez-López G., Ávila-Reyes S., Cano-Sarmiento C., Figueroa-Hernández C. ACE-Inhibitory and metal-binding activity produced during milk fermentation by three probiotic potential LAB strains isolated from Chiapas double cream cheese. Rev. Mex. De Ing. Quim. 2020;20:97–112. doi: 10.24275/rmiq/Alim1395. [DOI] [Google Scholar]
- 69.Min Z., Yunyun J., Miao C., Zhennai1 Y. Characterization and ACE Inhibitory Activity of Fermented Milk with Probiotic Lactobacillus plantarum K25 as Analyzed by GC-MS-Based Metabolomics Approach. J. Microbiol. Biotechnol. 2020;30:903–911. doi: 10.4014/jmb.1911.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filho M.L.M., Busanello M., Garcia S. Probiotic creamy soy sauce with Lactobacillus plantarum BG 112. Br. Food J. 2019;121:2746–2758. doi: 10.1108/BFJ-02-2019-0116. [DOI] [Google Scholar]
- 71.Singh B.P., Vij S. Growth and bioactive peptides production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT. 2017;86:293–301. doi: 10.1016/j.lwt.2017.08.013. [DOI] [Google Scholar]
- 72.Jain S., Anal A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017;54:1062–1072. doi: 10.1007/s13197-017-2530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rui X., Wen D., Li W., Chen X., Jiang M., Dong M. Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct. 2014;6:622–629. doi: 10.1039/C4FO00730A. [DOI] [PubMed] [Google Scholar]
- 74.Fujita A., Sarkar D., Genovese M.I., Shetty K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem. 2017;59:133–140. doi: 10.1016/j.procbio.2017.05.017. [DOI] [Google Scholar]
- 75.Kim E.-D., Lee H.-S., Kim K.-T., Paik H.-D. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation. Foods. 2021;10:2324. doi: 10.3390/foods10102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herrero-Fernandez B., Gomez-Bris R., Somovilla-Crespo B., Gonzalez-Granado J.M. Immunobiology of Atherosclerosis: A Complex Net of Interactions. Int. J. Mol. Sci. 2019;20:5293. doi: 10.3390/ijms20215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Wang Z., Jin G., Yang X., Zhou H. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017;101:107–116. doi: 10.1016/j.ijbiomac.2017.03.084. [DOI] [PubMed] [Google Scholar]
- 78.Hasenfuß G. Secondary prevention of cardiovascular diseases: Current state of the art. Kardiol. Pol. 2018;76:1671–1679. doi: 10.5603/KP.a2018.0198. [DOI] [PubMed] [Google Scholar]
- 79.Qu T., Yang L., Wang Y., Jiang B., Shen M., Ren D. Reduction of serum cholesterol and its mechanism by Lactobacillus plantarum H6 screened from local fermented food products. Food Funct. 2020;11:1397–1409. doi: 10.1039/C9FO02478F. [DOI] [PubMed] [Google Scholar]
- 80.Colautti A., Arnoldi M., Comi G., Iacumin L. Antibiotic resistance and virulence factors in lactobacilli: Something to carefully consider. Food Microbiol. 2022;103:103934. doi: 10.1016/j.fm.2021.103934. [DOI] [PubMed] [Google Scholar]
- 81.Ding P., Ding K., Yu Z., Li W., Li Y., Liu Y., He W., Zhao Z., Wang Y., Cheng X., et al. Screening of Lactobacillus with Cholesterol-Degrading Property and Its Effect on Serum Cholesterol in Mice. Food Sci. 2016;37:192–197. [Google Scholar]
- 82.Ding W., Shi C., Chen M., Zhou J., Long R., Guo X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J. Funct. Foods. 2017;32:324–332. doi: 10.1016/j.jff.2017.03.021. [DOI] [Google Scholar]
- 83.Liu C., Liu Q., Sun J., Jiang B., Yu J., Qi X. Screening and cholesterol removal ability of lactic acid bacteria DCel3 from Phasianus colchicus. Food Ferment. Ind. 2015;41:47–51. [Google Scholar]
- 84.Tang Y., Yu S., Guo L., Wang N., Huo G. Screening and study on probiotic characteristics of a cholesterol-lowering lactobacillus. Sci. Technol. Food Ind. 2016;37:142–144, 152. [Google Scholar]
- 85.Wang J., Gu Y., Ma W., Yang Z. Screening of Potential Probiotic Lactic Acid Bacteria from Inner Mongolian Dairy Tofu. Food Sci. 2014;35:171–177. [Google Scholar]
- 86.Xin J., Ni X., Zeng D., Wang H., Li T., Shen X. Screening of the hypocholesterolemic effect and body fat control ability of Lactobacillus plantarum F. J. China Agric. Univ. 2014;19:137–142. [Google Scholar]
- 87.Zhang K., Tian J. Screening of Cholesterol-Reducing Lactobacillus and Adverse Effects in Mice. J. Food Sci. Biotechnol. 2014;33:1222–1227. [Google Scholar]
- 88.Zhao F., Li Y., Li B. Screening of probiotic Lactobacillus in simulated gastrointestinal environment. Microbiol. China. 2016;43:1396–1403. [Google Scholar]
- 89.Ooi L.-G., Liong M.-T. Cholesterol-Lowering Effects of Probiotics and Prebiotics: A Review of in Vivo and in Vitro Findings. Int. J. Mol. Sci. 2010;11:2499–2522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lye H.-S., Rusul G., Liong M.-T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010;93:1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- 91.Qu L.-L., Yu B., Li Z., Jiang W.-X., Jiang J.-D., Kong W.-J. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phytotherapy Res. 2015;30:402–411. doi: 10.1002/ptr.5541. [DOI] [PubMed] [Google Scholar]
- 92.Lew L.-C., Choi S.-B., Khoo B.-Y., Sreenivasan S., Ong K.-L., Liong M.-T. Lactobacillus plantarum DR7 Reduces Cholesterol via Phosphorylation of AMPK That Down-regulated the mRNA Expression of HMG-CoA Reductase. Korean J. Food Sci. Anim. Resour. 2018;38:350–361. doi: 10.5851/kosfa.2018.38.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leung C., Rivera L., Furness J.B., Angus C.L.P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 94.Tang T., Song J.J., Li J., Wang H.W., Zhang Y., Suo H.Y. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley β-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr. Polym. 2021;243:116398. doi: 10.1016/j.carbpol.2020.116398. [DOI] [PubMed] [Google Scholar]
- 95.Teng Y., Wang Y., Tian Y., Chen Y.-Y., Guan W.-Y., Piao C.-H. Lactobacillus plantarum LP104 ameliorates hyperlipidemia induced by AMPK pathways in C57BL/6N mice fed high-fat diet. J. Funct. Foods. 2019;64:103665. doi: 10.1016/j.jff.2019.103665. [DOI] [Google Scholar]
- 96.Xu Y., Wu Y., Wang S., Lin X. Liver Protection Mechanism of Lactobacillus plantarum in Nonalcoholic Fatty Liver Mice. Genom. Appl. Biol. 2020;39:1845–1851. [Google Scholar]
- 97.Zhao Z., Wang C., Zhang L., Zhao Y., Duan C., Zhang X., Gao L., Li S. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 2019;103:5843–5850. doi: 10.1007/s00253-019-09703-4. [DOI] [PubMed] [Google Scholar]
- 98.Rosso C., Younes R., Eslam M., Chen F., Cucco M., Gaggini M., Coulter S., Carli F., Barbieri C., Della Latta V., et al. Bile acid composition modulates insulin resistance in non-diabetic patients with NAFLD. Dig. Liver Dis. 2018;50:17. doi: 10.1016/j.dld.2018.01.034. [DOI] [Google Scholar]
- 99.Kumar R., Rajkumar H., Kumar M., Varikuti S.R., Athimamula R., Shujauddin M., Ramagoni R., Kondapalli N. Molecular cloning, characterization and heterologous expression of bile salt hydrolase (Bsh) from Lactobacillus fermentum NCDO394. Mol. Biol. Rep. 2013;40:5057–5066. doi: 10.1007/s11033-013-2607-2. [DOI] [PubMed] [Google Scholar]
- 100.Sridevi N., Prabhune A.A. Brevibacillus sp: A Novel Thermophilic Source for the Production of Bile Salt Hydrolase. Appl. Biochem. Biotechnol. 2008;157:254–262. doi: 10.1007/s12010-008-8326-9. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y., Wang X., Wang J., Wu F., Sui Y., Yang L., Wang Z. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J. Dairy Sci. 2013;96:2746–2753. doi: 10.3168/jds.2012-6123. [DOI] [PubMed] [Google Scholar]
- 102.Wang G., Huang W., Xia Y., Xiong Z., Ai L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 2019;10:1684–1695. doi: 10.1039/C8FO02181C. [DOI] [PubMed] [Google Scholar]
- 103.Guo L., Wang L., Liu F., Li B., Tang Y., Yu S., Zhang D., Huo G. Effect of bile salt hydrolase-active Lactobacillus plantarum KLDS 1.0344 on cholesterol metabolism in rats fed a high-cholesterol diet. J. Funct. Foods. 2019;61:103497. doi: 10.1016/j.jff.2019.103497. [DOI] [Google Scholar]
- 104.Li Q., Zhang K., Zhao Y., Du R., Tian J., Jin Y. Research Progress of Regulatory Key Factors Involved in Cholesterol Metabolism by Lactic Acid Bacteria. J. Chin. Inst. Food Sci. Technol. 2021;21:341–350. [Google Scholar]
- 105.Aarti C., Khusro A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT. 2019;114:108387. doi: 10.1016/j.lwt.2019.108387. [DOI] [Google Scholar]
- 106.Marchesi J.R., Adams D.H., Fava F., Hermes G.D.A., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao Y., Huo D., Peng Q., Pan Y., Jiang S., Liu B., Zhang J. Lactobacillus plantarum HNU082-derived improvements in the intestinal microbiome prevent the development of hyperlipidaemia. Food Funct. 2017;8:4508–4516. doi: 10.1039/C7FO00902J. [DOI] [PubMed] [Google Scholar]
- 108.Yang B., Chen H., Gu Z., Tian F., Ross R.P., Stanton C., Chen Y.Q., Chen W., Zhang H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 2014;117:430–439. doi: 10.1111/jam.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kishino S., Takeuchi M., Park S.-B., Hirata A., Kitamura N., Kunisawa J., Kiyono H., Iwamoto R., Isobe Y., Arita M., et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen D.-J., Yan L.-H., Li Q., Zhang C.-J., Si C.-L., Li Z.-Y., Song Y.-J., Zhou H., Zhang T.-C., Luo X.-G. Bioconversion of conjugated linoleic acid by Lactobacillus plantarum CGMCC8198 supplemented with Acer truncatum bunge seeds oil. Food Sci. Biotechnol. 2017;26:1595–1611. doi: 10.1007/s10068-017-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen L., Zhao W., Zhang F., Yang X., Zhu X., Wang L., Gao P., Shu G., Jiang Q. Effects of different conjugated linoleic acid isomers on fat deposition, energy metabolism and intestinal microorganisms in mice. J. S. China Agric. Univ. 2022;43:1–8. [Google Scholar]
- 112.Hütt P., Songisepp E., Rätsep M., Mahlapuu R., Kilk K., Mikelsaar M. Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef. Microbes. 2015;6:233–243. doi: 10.3920/BM2014.0035. [DOI] [PubMed] [Google Scholar]
- 113.Hussain A., Kwon M.H., Kim H.K., Lee H.S., Cho J.S., Lee Y.I. Anti-Obesity Effect of Lactobacillus plantarum LB818 Is Associated with Regulation of Gut Microbiota in High-Fat Diet-Fed Obese Mice. J. Med. Food. 2020;23:750–759. doi: 10.1089/jmf.2019.4627. [DOI] [PubMed] [Google Scholar]
- 114.Andersson U., Bränning C., Ahrné S., Molin G., Alenfall J., Önning G., Nyman M., Holm C. Probiotics lower plasma glucose in the high-fat fed C57BL/6J mouse. Benef. Microbes. 2010;1:189–196. doi: 10.3920/BM2009.0036. [DOI] [PubMed] [Google Scholar]
- 115.da Costa W.K.A., Brandão L.R., Martino M.E., Garcia E.F., Alves A.F., de Souza E.L., Aquino J.D.S., Saarela M., Leulier F., Vidal H., et al. Qualification of tropical fruit-derived Lactobacillus plantarum strains as potential probiotics acting on blood glucose and total cholesterol levels in Wistar rats. Food Res. Int. 2018;124:109–117. doi: 10.1016/j.foodres.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 116.Lin S., Yang X., Long Y., Zhong H., Wang P., Yuan P., Zhang X., Che L., Feng B., Li J., et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 2020;124:797–808. doi: 10.1017/S0007114520001774. [DOI] [PubMed] [Google Scholar]
- 117.Lee E., Jung S.-R., Lee S.-Y., Lee N.-K., Paik H.-D., Lim S.-I. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients. 2018;10:643. doi: 10.3390/nu10050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saltiel A.R., Kahn C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 119.Pessin J.E., Thurmond D.C., Elmendorf J.S., Coker K.J., Okada S. Molecular Basis of Insulin-stimulated GLUT4 Vesicle Trafficking. Location! Location! Location! J. Biol. Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 120.Lee Y.-S., Lee D., Park G.-S., Ko S.-H., Park J., Lee Y.-K., Kang J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021;12:6363–6373. doi: 10.1039/D1FO00698C. [DOI] [PubMed] [Google Scholar]
- 121.Korkmaz O.A., Sumlu E., Koca H.B., Pektas M.B., Kocabas A., Sadi G., Akar F. Effects of Lactobacillus Plantarum and Lactobacillus Helveticus on Renal Insulin Signaling, Inflammatory Markers, and Glucose Transporters in High-Fructose-Fed Rats. Medicina. 2019;55:207. doi: 10.3390/medicina55050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He Q., Cao C., Hui W., Yu J., Zhang H., Zhang W. Genomic resequencing combined with quantitative proteomic analyses elucidate the survival mechanisms of Lactobacillus plantarum P-8 in a long-term glucose-limited experiment. J. Proteom. 2018;176:37–45. doi: 10.1016/j.jprot.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 123.Sakai T., Taki T., Nakamoto A., Shuto E., Tsutsumi R., Toshimitsu T., Makino S., Ikegami S. Lactobacillus plantarum OLL2712 Regulates Glucose Metabolism in C57BL/6 Mice Fed a High-Fat Diet. J. Nutr. Sci. Vitaminol. 2013;59:144–147. doi: 10.3177/jnsv.59.144. [DOI] [PubMed] [Google Scholar]
- 124.Gao H., Wen J.-J., Hu J.-L., Nie Q.-X., Chen H.-H., Nie S.-P., Xiong T., Xie M.-Y. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019;29:62–72. doi: 10.1016/j.fbio.2019.03.007. [DOI] [Google Scholar]
- 125.Chen R., Chen W., Chen H., Zhang G., Chen W. Comparative Evaluation of the Antioxidant Capacities, Organic Acids, and Volatiles of Papaya Juices Fermented by Lactobacillus acidophilus and Lactobacillus plantarum. J. Food Qual. 2018;2018:1–12. doi: 10.1155/2018/9490435. [DOI] [Google Scholar]
- 126.Zhong H., Abdullah, Zhao M., Tang J., Deng L., Feng F. Probiotics-fermented blueberry juices as potential antidiabetic product: Antioxidant, antimicrobial and antidiabetic potentials. J. Sci. Food Agric. 2021;101:4420–4427. doi: 10.1002/jsfa.11083. [DOI] [PubMed] [Google Scholar]
- 127.Shikano A., Kuda T., Shibayama J., Toyama A., Ishida Y., Takahashi H., Kimura B. Effects of Lactobacillus plantarum Uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res. Int. 2019;121:817–824. doi: 10.1016/j.foodres.2018.12.065. [DOI] [PubMed] [Google Scholar]
- 128.Hu J.-P., Zheng T.-T., Zeng B.-F., Wu M.-L., Shi R., Zhang Y., Chen L.-J., Cheng W.-J., Liang P. Effects of Lactobacillus plantarum FZU3013-Fermented Laminaria japonica on Lipid Metabolism and Gut Microbiota in Hyperlipidaemic Rats. Front. Nutr. 2021;8:786571. doi: 10.3389/fnut.2021.786571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsui C.-Y., Yang C.-Y. Evaluation of Semi-Solid-State Fermentation of Elaeocarpus serratus L. Leaves and Black Soymilk by Lactobacillus plantarum on Bioactive Compounds and Antioxidant Capacity. Foods. 2021;10:704. doi: 10.3390/foods10040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mirlohi M., Babashahi M., Ghiasvand R., Azadbakht L., Mosharaf L., Torki-Baghbadorani S. Effects of probiotic soy milk fermented by lactobacillus plantarum A7 (KC 355240) added with Cuminum Cyminum essential oil on fasting blood glucose levels, serum lipid profile and body weight in diabetic Wistar rats. Int. J. Prev. Med. 2020;11:8. doi: 10.4103/ijpvm.IJPVM_541_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao C., Wu R., Zhu X., Li Y., Li M., An F., Wu J. Ameliorative effect of Lactobacillus plantarum WW-fermented soy extract on rat fatty liver via the PPAR signaling pathway. J. Funct. Foods. 2019;60:103439. doi: 10.1016/j.jff.2019.103439. [DOI] [Google Scholar]
- 132.de la Fuente B., Luz C., Puchol C., Meca G., Barba F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2020;343:128414. doi: 10.1016/j.foodchem.2020.128414. [DOI] [PubMed] [Google Scholar]
- 133.Mushtaq M., Gani A., Masoodi F. Himalayan cheese (Kalari/Kradi) fermented with different probiotic strains: In vitro investigation of nutraceutical properties. LWT. 2019;104:53–60. doi: 10.1016/j.lwt.2019.01.024. [DOI] [Google Scholar]
- 134.Moreno-Montoro M., Olalla-Herrera M., Rufián-Henares J., Martínez R.G., Miralles B., Bergillos T., Navarro-Alarcón M., Jauregi P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017;8:2783–2791. doi: 10.1039/C7FO00666G. [DOI] [PubMed] [Google Scholar]
- 135.Li Y., Wu J., Cao C., Zhu X., Sun X., Wu R. Effects of skim milk fermented with Lactobacillus plantarum WW on the constitutions of rats fed a high-fat diet. J. Dairy Sci. 2020;103:5019–5029. doi: 10.3168/jds.2019-17560. [DOI] [PubMed] [Google Scholar]
- 136.Albano C., Morandi S., Silvetti T., Casiraghi M., Manini F., Brasca M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018;101:10807–10818. doi: 10.3168/jds.2018-15096. [DOI] [PubMed] [Google Scholar]
- 137.Wang M., Lei M., Samina N., Chen L., Liu C., Yin T., Yan X., Wu C., He H., Yi C. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020;330:127156. doi: 10.1016/j.foodchem.2020.127156. [DOI] [PubMed] [Google Scholar]
- 138.Wu H., Rui X., Li W., Xiao Y., Zhou J., Dong M. Whole-grain oats (Avena sativa L.) as a carrier of lactic acid bacteria and a supplement rich in angiotensin I-converting enzyme inhibitory peptides through solid-state fermentation. Food Funct. 2018;9:2270–2281. doi: 10.1039/C7FO01578J. [DOI] [PubMed] [Google Scholar]
- 139.Luan X., Feng M., Sun J. Effect of Lactobacillus plantarum on antioxidant activity in fermented sausage. Food Res. Int. 2021;144:110351. doi: 10.1016/j.foodres.2021.110351. [DOI] [PubMed] [Google Scholar]
- 140.Hashemi S., Raeisi S., Zanardi E., Paciulli M., Chiavaro E. Antimicrobial and Antioxidant Activities of Fermented Meat Patty with Lactobacillus Strains. Ital. J. Food Sci. 2018;30:268–279. doi: 10.14674/IJFS-993. [DOI] [Google Scholar]
- 141.Ayyash M., Liu S.-Q., Al Mheiri A., Aldhaheri M., Raeisi B., Al-Nabulsi A., Osaili T., Olaimat A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT. 2018;99:346–354. doi: 10.1016/j.lwt.2018.09.084. [DOI] [Google Scholar]
- 142.Kong Y.-W., Feng M.-Q., Sun J. Effects of Lactobacillus plantarum CD101 and Staphylococcus simulans NJ201 on proteolytic changes and bioactivities (antioxidant and antihypertensive activities) in fermented pork sausage. LWT. 2020;133:109985. doi: 10.1016/j.lwt.2020.109985. [DOI] [Google Scholar]
- 143.Wu Y., Li S., Tao Y., Li D., Han Y., Show P.L., Wen G., Zhou J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021;348:129083. doi: 10.1016/j.foodchem.2021.129083. [DOI] [PubMed] [Google Scholar]
- 144.Zhang X., Duan W., Zou J., Zhou H., Liu C., Yang H. Flavor and antioxidant activity improvement of carrot juice by fermentation with Lactobacillus plantarum WZ-01. J. Food Meas. Charact. 2019;13:3366–3375. doi: 10.1007/s11694-019-00260-y. [DOI] [Google Scholar]
- 145.Bin Jeon Y., Lee J., Chang H.C. Characterization of juice fermented with Lactobacillus plantarum EM and its cholesterol-lowering effects on rats fed a high-fat and high-cholesterol diet. Food Sci. Nutr. 2019;7:3622–3634. doi: 10.1002/fsn3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang L., Feng M., Sun J. Angiotensin-converting enzyme (ACE) inhibitory peptides from fermented sausages inoculated with Lactobacillus plantarum CD101 and Staphylococcus simulans NJ201. Int. J. Food Sci. Technol. 2022;57:4985–4997. doi: 10.1111/ijfs.15765. [DOI] [Google Scholar]
- 147.de Oliveira S.D., Araújo C.M., Borges G.D.S.C., Lima M.D.S., Viera V.B., Garcia E.F., de Souza E.L., de Oliveira M.E.G. Improvement in physicochemical characteristics, bioactive compounds and antioxidant activity of acerola (Malpighia emarginata D.C.) and guava (Psidium guajava L.) fruit by-products fermented with potentially probiotic lactobacilli. LWT. 2020;134:110200. doi: 10.1016/j.lwt.2020.110200. [DOI] [Google Scholar]
- 148.Zhang Z.-P., Ma J., He Y.-Y., Lu J., Ren D.-F. Antioxidant and hypoglycemic effects of Diospyros lotus fruit fermented with Microbacterium flavum and Lactobacillus plantarum. J. Biosci. Bioeng. 2018;125:682–687. doi: 10.1016/j.jbiosc.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 149.Ayyash M., Olaimat A., Al-Nabulsi A., Liu S.-Q. Bioactive Properties of Novel Probiotic Lactococcus lactis Fermented Camel Sausages: Cytotoxicity, Angiotensin Converting Enzyme Inhibition, Antioxidant Capacity, and Antidiabetic Activity. Korean J. Food Sci. Anim. Resour. 2020;40:155–171. doi: 10.5851/kosfa.2020.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hashemi S.M.B., Gholamhosseinpour A. Fermentation of table cream by Lactobacillus plantarum strains: Effect on fungal growth, aflatoxin M1 and ochratoxin A. Int. J. Food Sci. Technol. 2018;54:347–353. doi: 10.1111/ijfs.13943. [DOI] [Google Scholar]
- 151.Dan T., Chen H., Li T., Tian J., Ren W., Zhang H., Sun T. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019;9:3133. doi: 10.3389/fmicb.2018.03133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.