Abstract
Optimal management of atopic dermatitis requires a comprehensive assessment of response to treatment in order to inform therapeutic decisions. In a realworld setting, successful response to atopic dermatitis treatment is measured by sustained improvements in signs, symptoms, and quality of life. Post-hoc analyses of a 1-year, randomized, double-blinded, placebo-controlled trial (NCT02260986) of dupilumab with concomitant topical corticosteroids in 421 adults with moderate-to-severe atopic dermatitis (of whom 315/106 received placebo/dupilumab (of whom 315 received placebo and 106 received dupilumab) was performed to assess the proportion of responders to dupilumab through a multidimensional composite endpoint. At 6-months, 80.2% of dupilumab-treated vs 40.0% placebo patients (p < 0.0001) achieved improvement in signs (Eczema Area and Severity Index ≤ 7), symptoms (worst itch score ≤ 4), or quality of life (Dermatology Life Quality Index ≤5), representative of minimal/clear atopic dermatitis. All 3 endpoints, indicative of no/minimal atopic dermatitis, were achieved by 44.3% of dupilumab-treated vs 10.2% placebo patients (p < 0.0001) and sustained through 1 year. Dupilumab treatment provided sustained clinically meaningful improvement in signs, symptoms, and quality of life in adults with moderate-to-severe atopic dermatitis.
Key words: atopic dermatitis, treat-to-target, responder, Eczema Area and Severity Index, pruritus, Dermatology Life Quality Index,
The ultimate goal in the management of moderate-to-severe atopic dermatitis (AD), for both physicians and patients, is to achieve disease control. The Harmonizing Outcome Measures for Eczema initiative recommends a multidimensional assessment that includes clinician-reported signs, patient-reported symptoms, quality of life (QoL), and long-term control (1); a recommendation recently supported by the European Task Force on Atopic Dermatitis (ETFAD) (2).
SIGNIFICANCE
In accordance with an international consensus, this study estimated the proportion of responders to dupilumab through a multidimensional composite endpoint assessing atopic dermatitis signs, symptoms (pruritus), and quality of life in a 1-year phase 3 trial of dupilumab with concomitant topical corticosteroids in adults with moderate-to-severe atopic dermatitis. Most dupilumab-treated patients (64%) achieved improvement equivalent to minimal disease in ≥1 atopic dermatitis domain after the first dose, and 84% were responders at 4 months. This response was sustained over 1 year. This comprehensive view of the efficacy of dupilumab from the perspective of both patients and physicians might provide better guidance to clinicians in daily practice.
Currently, there is no single tool available to assess all salient aspects of AD and treatment responses (Table I). Eczema Area and Severity Index (EASI) ≤ 7.0 (10) and objective SCORing Atopic Dermatitis (o-SCORAD) < 24 are interpreted as mild disease activity (11, 12). Itch can be measured using the Peak Pruritus Numerical Rating Scale (NRS). A score ≤ 4 indicates mild or no pruritus (13) and a change of ≥ 2 to 4 points from baseline is the threshold for defining a clinically relevant (within-person) response in Peak Pruritus NRS (6, 14). The Patient-Oriented Eczema Measure (POEM) (7) assesses patient-reported disease activity over the previous week, with a value ≤ 7 considered mild AD (4). QoL can be assessed by the dermatological disease-specific Dermatology Life Quality Index (DLQI), with values ≤ 5 consistent with a small or no effect on patient’s health-related QoL (13, 15) and a change of ≥ 4 points from baseline defined as clinically relevant (8, 9).
Table I.
Tools used to assess signs, symptoms, and quality of life in atopic dermatitis trials
| Clinical tool | MCID | Skin lesions | Pruritus | Sleep | Quality of life |
|---|---|---|---|---|---|
| IGA (3) | n/a | ✓ | |||
| EASI (4) | 6.6 | ✓ | |||
| SCORAD (4, 5) | 8.7 | ✓ | ✓ | ✓ | |
| Pruritus NRS (6) | 2–4 | ✓ | |||
| POEM (4, 7) | 4 | ✓ | ✓ | ✓ | |
| DLQI (8, 9)a | 4 | ✓ | ✓ |
DLQI question 1 includes itch; however, this is mixed with other symptoms (Over the last week, how itchy, sore, painful, or stinging has your skin been?)
DLQI: Dermatology Life Quality Index; EASI: Eczema and Severity Index; IGA: Investigator’s Global Assessment; MCID: minimum clinically important difference; n/a: not applicable; NRS: numerical rating scale; POEM: Patient-Oriented Eczema Measure; SCORAD: SCORing Atopic Dermatitis; ✓: measured by assessment tool.
Dupilumab is a fully human VelocImmune®-derived monoclonal antibody (16, 17), which blocks the shared receptor component for interleukin (IL)-4 and IL-13, thus inhibiting signalling of both IL-4 and IL-13, which are key and central drivers of type 2 inflammatory diseases, such as AD, asthma, allergic rhinitis, and food allergies (18). Dupilumab is a targeted, systemic, non-immunosuppressant treatment with a favorable benefit/risk profile, appropriate for long-term management of AD, as shown by prevention of AD flares in 85% of patients and providing well-controlled disease in > 85% of weeks in patients treated for 1 year (19, 20). The safety of dupilumab has been evaluated in patients treated for up to 3 years (21), during which time there was no need for routine laboratory monitoring (22), and in children aged 6–11 years with severe AD (23). Real-world evidence shows high persistence of treatment with dupilumab, with 91% and 88% of patients continuing treatment after 1 and 2 years, respectively (24).
The ETFAD states that multidimensional assessment is important for identification of relevant treatment targets (2), and an international consensus, through an eDelphi process, recently published a treat-to-target framework guidance for systemic treatment in adults with moderate-to-severe AD (25). To better characterize the multidimensional benefits of dupilumab treatment in AD over time, post hoc analyses were performed on data from a randomized, placebo-controlled, double-blind, 1-year phase 3 trial (LIBERTY AD CHRONOS (NCT02260986)) to assess the proportion of patients achieving short-term clinically meaningful improvement, as well as the proportion achieving no or mild/minimal disease, based on signs, symptoms, and QoL at 6 months and 1 year. This study also evaluated the percentage of patients with improvement in all 3 domains of AD (signs, symptoms, and QoL) in response to dupilumab treatment.
MATERIALS AND METHODS
Study design
The study design, patient populations, and efficacy and safety results for LIBERTY AD CHRONOS have been reported previously (26). Briefly, a total of 740 patients were randomized (1:3:3) to receive 300 mg every 2 weeks (q2w, n = 106), dupilumab 300 mg weekly (qw, n = 319), or placebo for 52 weeks (n = 315). All patients received concomitant medium-potency topical corticosteroids (TCS) or low-potency TCS for areas where continued treatment with medium-potency TCS is not recommended. The study design allowed for tapering of TCS after clearance of AD skin lesions and TCS reinstitution if lesions recurred, which would reflect real-world use of TCS (26). Eligible patients had moderate-to-severe AD with inadequate response to topical treatment, baseline EASI ≥ 16, Investigator’s Global Assessment (IGA) scores of 3 or 4 (moderate or severe), and body surface area affected by AD ≥ 10%.
The trial was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided signed written informed consent before performing any study procedures. Institutional review boards and independent ethics committees reviewed and approved the protocol, informed consent form, and patient information before study initiation.
Outcomes
Consistent with the ETFAD (2) and an international consensus discussion on treatment targets (25), 2 composite endpoints were defined for an initial acceptable target of “clinically meaningful response” at week 16 (4, 6, 14, 27) and an optimal treatment target of “no or mild/minimal disease” at 6 months and 1 year (6, 27, 28), as follows :
Initial acceptable target (week 16): Patients achieving a 50% improvement from baseline in EASI (EASI-50), or ≥ 3-point improvement from baseline in Peak Pruritus NRS scores, or ≥ 4-point improvement from baseline in DLQI. The proportion of patients achieving all the aforementioned responses in all 3 domains of AD from baseline through week 16 of treatment was also investigated. By definition, patients with high disease burden at baseline who achieved this endpoint may still have had moderate-to-severe AD at week 16.
Optimal target: Patients achieving EASI ≤ 7, or Peak Pruritus NRS score ≤ 4, or DLQI ≤ 5 at 6 months and 1 year. The proportion of patients achieving minimal or no disease in all 3 domains of AD from baseline through 1 year of treatment was also investigated.
In addition, according to the international consensus for defining treat-to-target outcomes in patients with moderate-to-severe AD, this study analyzed the proportion of patients achieving clinically relevant improvement in at least one specific domain measured with a validated outcome assessment instrument (POEM questionnaire, DLQI, EASI, Peak Pruritus NRS) and any improvement in Patient’s Global Assessment of Disease Status (PGADS) at 4 months, 6 months, and 1 year.
Statistical analysis
This analysis focused on patients treated with dupilumab 300 mg q2w + TCS and the corresponding placebo + TCS-treatment group, as 300 mg q2w is the approved treatment dose of dupilumab for adults with moderate-to-severe AD. All randomized patients were included in the full analysis set, and efficacy parameters were calculated among these patients. p-values for the categorical endpoints from this analysis were derived by a Cochran–Mantel–Haenszel (CMH) test stratified by region and baseline disease severity (IGA = 3 vs IGA = 4). Values after first rescue treatment used were set to missing (censored); patients with missing values were considered as non-responders. Analyses were conducted using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patients
A total of 421 patients who received placebo + TCS or dupilumab 300 mg q2w + TCS for 52 weeks were included in this analysis. Baseline demographics and characteristics were balanced between the treatment groups and showed considerable baseline disease burden (Table II).
Table II.
Baseline demographics and disease characteristics
| Placebo qw + TCS (n = 315) | Dupilumab 300 mg q2w + TCS (n = 106) | |
|---|---|---|
| Age, years, mean ± SD | 36.6 ± 13.0 | 39.6 ± 14.0 |
| Male sex, n (%) | 193 (61.3) | 62 (58.5) |
| EASI (range 0–72), mean ± SD | 32.6 ± 12.9 | 33.6 ± 13.3 |
| Peak Pruritus NRS score (range 0–10), mean ± SD | 7.3 ± 1.8 | 7.4 ± 1.7 |
| DLQI (range 0–30), mean ± SD | 14.7 ± 7.4 | 14.5 ± 7.3 |
DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; NRS: numerical rating scale; q2w: every 2 weeks; qw: weekly; SD: standard deviation; TCS: topical corticosteroids.
Efficacy
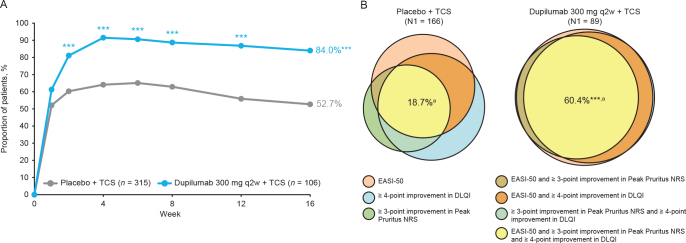
At week 16, 84.0% of dupilumab + TCS-treated patients achieved clinically meaningful improvement, while using less TCS (16% of dupilumab-treated patients received TCS as rescue medication during the 52-week period, vs 48% in the placebo group (26)), in signs, symptoms, or QoL, defined as achieving EASI-50, or ≥ 3-point improvement from baseline in Peak Pruritus NRS, or ≥ 4-point improvement from baseline in DLQI compared with placebo + TCS (52.7%; p < 0.0001) (Fig. 1A). A higher proportion of patients treated with dupilumab + TCS compared with placebo + TCS achieved clinically meaningful improvements in all 3 domains of AD at week 16 (60.4% vs 18.7%; p < 0.0001) (Fig. 1B).
Fig. 1.
(A) Percentage of patients achieving Eczema Area and Severity Index (EASI)-50 or ≥ 3-point improvement in Peak Pruritus NRS score or ≥ 4-point improvement in Dermatology Life Quality Index (DLQI) from baseline at week 16. (B) Percentage of patients achieving EASI-50 and ≥ 3-point improvement in Peak Pruritus NRS score and ≥ 4-point improvement in DLQI from baseline at week 16. Venn diagrams generated using areas proportional to the number of patients achieving 1, 2, or 3 of the individual endpoints relative to the number of patients achieving ≥ 1 endpoints (N1; placebo n = 166, dupilumab q2w n = 89). aPercentages in the Venn diagrams are calculated over the population in the treatment arm. ***p < 0.0001 vs placebo + TCS. DLQI: Dermatology Life Quality Index; EASI-50: ≥ 50% improvement from baseline in Eczema Area and Severity Index; NRS: numerical rating scale; q2w: every 2 weeks; qw: weekly; TCS: topical corticosteroids.
Altogether, the proportion of patients who achieved clinically meaningful improvement in at least one of the 5 endpoints (EASI, Peak Pruritus NRS, DLQI, POEM, or PGADS) was significantly higher in the dupilumab group compared with the TCS-only group at week 16 (85.8% vs 56.5%) (Fig. S1A1).
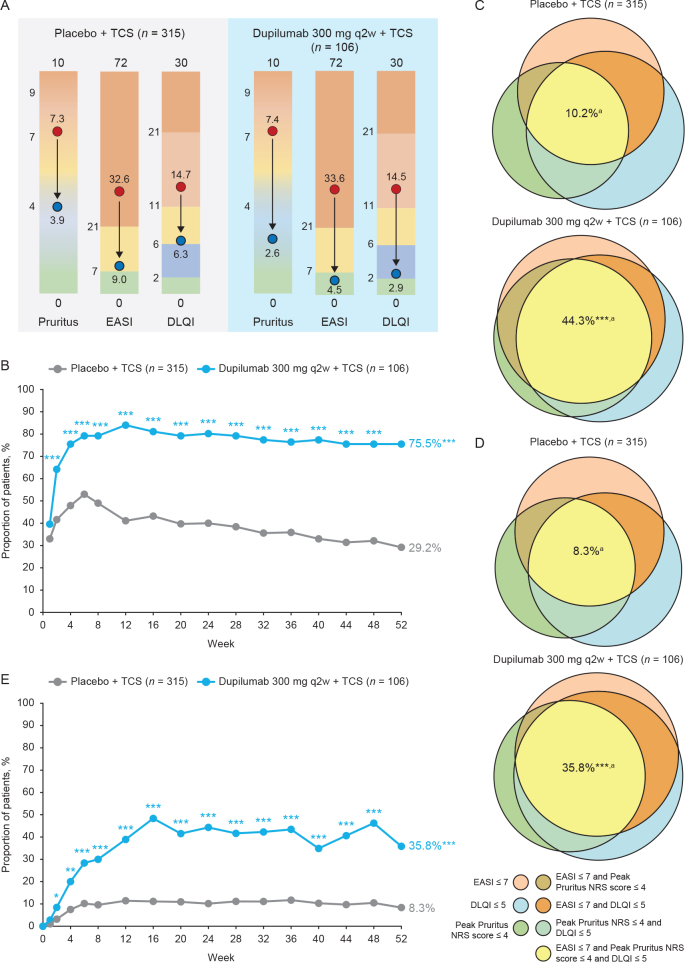
Overall, from baseline through 1 year, more dupilumab + TCS-treated patients achieved clinically relevant improvement in Peak Pruritus NRS, EASI, and DLQI vs control (Fig. 2A).
Fig. 2.
Clinically meaningful improvement in signs (Eczema Area and Severity Index; EASI), symptoms (Peak Pruritus numerical rating scale (NRS)), and quality of life (Dermatology Life Quality Index; DLQI) throughout the treatment period. (A) Changes in Peak Pruritus NRS score, EASI, and DLQI from baseline to week 52 among all patients included in the CHRONOS study. (B) Percentage of patients achieving EASI ≤ 7, or Peak Pruritus NRS score ≤ 4, or DLQI ≤ 5 from baseline through week 52. (C) Percentage of patients achieving EASI ≤ 7, and Peak Pruritus NRS score ≤ 4, and DLQI ≤ 5 at week 24. (D) Percentage of patients achieving EASI ≤ 7, and Peak Pruritus NRS score ≤ 4, and DLQI ≤ 5 at week 52. (E) Percentage of patients achieving EASI ≤ 7, and Peak Pruritus-NRS score ≤ 4, and DLQI ≤ 5, baseline through week 52. The color scale graphic displays the changes in absolute values from baseline (red) to week 52 (blue) for each outcome. Venn diagrams generated using areas proportional to the number of patients achieving 1, 2, or 3 of the individual endpoints relative to the number of patients achieving ≥ 1 of the endpoints (N1; week 24: placebo n = 126, dupilumab every 2 weeks (q2w) n = 85; week 52: placebo n = 92, dupilumab q2w n = 80). aPercentages in the Venn diagrams are calculated over the population in the treatment arm. *p < 0.05; **p < 0.001; ***p < 0.0001 vs placebo + topical corticosteroids (TCS).
Most patients (64.2%) treated with dupilumab + TCS achieved no or minimal disease in at least one of the AD domains as early as after the first dose (vs 41.6% in the placebo + TCS group; p < 0.0001). This proportion increased through 6 months (80.2% of dupilumab + TCS-treated and 40.0% of placebo + TCS-treated patients; p < 0.0001, Fig. 2B) and was sustained through 1 year (75.5% of dupilumab + TCS-treated and 29.2% of placebo + TCS-treated patients; p < 0.0001; Fig. 2B). Interestingly, among patients who achieved no or minimal disease in at least one of the AD domains at week 16, a higher proportion of patients in the dupilumab + TCS group, vs placebo + TCS, also achieved no or minimal disease in at least one other domain at 6 months (82.4% vs 59.6%). This proportion was sustained through 1 year (80.0% vs 56.0%). In addition, a significantly higher proportion of dupilumab + TCS-treated patients achieved no or minimal disease in all 3 AD domains (EASI ≤ 7 and Peak Pruritus NRS scores ≤ 4 and DLQI ≤ 5) compared with placebo + TCS as early as week 2. These improvements were sustained at 6 months (44.3% vs 10.2%) and 1 year (35.8% vs 8.3%), while using less TCS/calcineurin inhibitors (TCI) or systemic rescue therapy, as previously reported (26) (Fig. 2C–E).
Finally, significantly more dupilumab-treated patients reported a PGADS of “good, very good, or excellent” at 6 months compared with patients who received TCS only (67.9% vs 35.9%; p < 0.0001) (Fig. S1B1). Together, the proportion of patients who achieved no or mild/minimal disease in at least one of the 5 outcome measures was significantly higher in the dupilumab group compared with the placebo group at 6 months (80.2% vs 45.4%) (Fig. S1B1). This rate of responders was sustained at 1 year (79.2% vs 32.7%; p < 0.0001) while using less TCS/TCI or systemic rescue therapy, as previously reported (26) (Fig. S1C1).
Fig. 3 is an example photograph of a patient treated with dupilumab q2w + TCS in the CHRONOS study who achieved initial accepted target (≥ 4-point improvement in DLQI) at week 16 and achieved minimal disease (Peak Pruritus NRS score ≤ 4 and DLQI ≤ 5) at week 52.
Fig. 3.
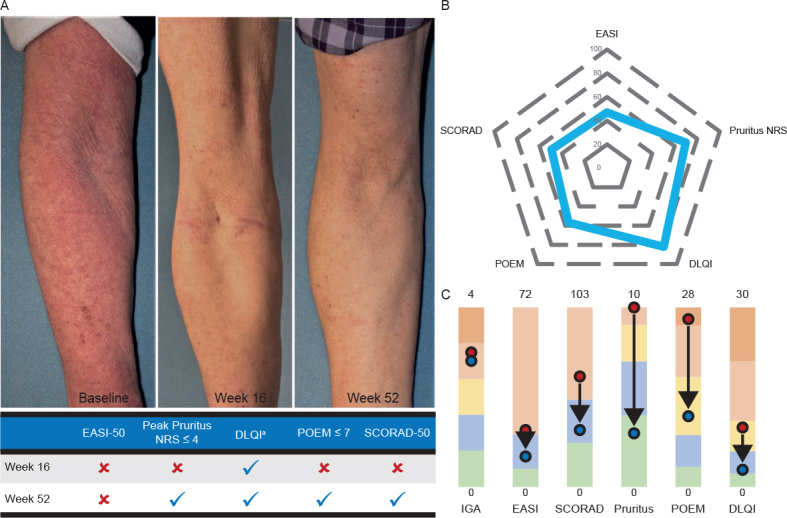
(A) Patient before–after photographs. (B) Achieving only Dermatology Life Quality Index (DLQI) ≥ 4-point improvement at week 16 with Peak Pruritus numerical rating scale (NRS) score ≤ 4, DLQI ≤ 5, Patient-Oriented Eczema Measure (POEM) total score ≤ 7, and SCORing Atopic Dermatitis (SCORAD)-50 at week 52. (C) Changes from baseline in Investigator’s Global Assessment (IGA) score, Eczema Area and Severity Index (EASI), SCORAD total score, Peak Pruritus NRS score, POEM total score, and DLQI at week 52. aThe patient achieved ≥ 4-point improvement from baseline in DLQI at week 16 and DLQI ≤ 5 at week 52. The spider graph shows percentage improvement in each outcome. The color scale graphic displays the changes in absolute values from baseline (red) to week 52 (blue) for each outcome.
Safety
In this trial, dupilumab was well tolerated overall and had an acceptable safety profile, as reported previously (26). Adverse events that were more commonly reported with dupilumab treatment than placebo included conjunctivitis (14% dupilumab q2w vs 8% placebo) and injection-site reactions (15% dupilumab q2w vs 8% placebo), whereas exacerbations of AD were reported more commonly with placebo treatment (20% dupilumab q2w vs 47% placebo) (26). Most adverse events related to dupilumab were of mild or moderate severity and did not lead to drug discontinuation. All cases of conjunctivitis in the dupilumab q2w group were mild or moderate, resolved with topical eye treatment, and did not lead to treatment discontinuation (26).
DISCUSSION
In clinical practice, physicians and patients work together toward achieving optimal disease control and share decisions about continuing, switching, combining, increasing dose, or stopping therapy depending on multiple factors (2, 25).
In these analyses, treatment of adults with moderate-to-severe AD with dupilumab + TCS provided a rapid and sustained multidimensional benefit, improving not only skin signs, but also intensity and frequency of symptoms as well as health-related QoL. Most patients treated with dupilumab achieved these improvements soon after the first dose of treatment, and the rate of response to treatment improved and/or was sustained through the entire 1-year treatment period, despite reduction in TCS use. In line with an international consensus to provide a framework for a treat-to-target approach for systemic treatment in AD (25), two approaches were investigated in these analyses. The first represented an initial acceptable treatment target at week 16, defined as minimal clinically meaningful improvements in AD signs (EASI-50), symptoms (≥ 3-point improvement in Peak Pruritus NRS), and QoL (≥ 4-point improvement in DLQI). However, by definition of this composite endpoint, patients with very high disease burden at baseline may still experience moderate-to-severe AD at week 16 despite clinically meaningful improvements. Consequently, the second approach was designed to represent an optimal treatment target, defined as achieving no or mild disease characteristics in AD signs, symptoms, or QoL at 6 months and 1 year.
Because the impact of AD is multidimensional, and no single instrument captures the full burden of disease and benefit of treatment, composite assessments are relevant for a holistic approach to disease assessment and management (25, 29); these assessments provide relevant information to physicians when selecting the best treatment options for their patients. This is particularly relevant for patients who do not achieve regulatory endpoints, which measure only the extent and severity of skin lesions.
Indeed, a pooled analysis of dupilumab phase 3 clinical trials in a subpopulation of adult patients with moderate-to-severe AD who did not achieve the regulatory endpoint of clear or almost clear skin (IGA 0/1) showed that most of these patients achieved clinically meaningful and statistically significant improvements in multiple measures, such as a reduction in Peak Pruritus NRS or an improvement in POEM score or DLQI (30). These findings were subsequently confirmed in another post hoc analysis of a trial in adolescent patients, in which patients who still had an IGA >1 (mild disease) after 4 months of treatment with dupilumab had achieved clinically significant improvement in Peak Pruritus NRS and Children’s Dermatology Life Quality Index compared with placebo (31).
These observations from phase 3 studies were also supported by the real-world evidence. In another realworld data study, most adults with moderate-to-severe AD resistant to immunosuppressive systemic treatment achieved response to dupilumab treatment in all 3 AD domains investigated: AD signs (75% improvement in EASI (EASI-75)), symptoms (≥ 4point improvement in Peak Pruritus NRS), and QoL (≥ 4-point improvement in DLQI) after 16 weeks of treatment (32). These improvements were sustained over 1 year of treatment with dupilumab. In addition, the perceived clinical benefit was confirmed by the very low rate of treatment discontinuations after 1 year due to ineffectiveness (4.3%) in a population in which most patients had failed multiple oral immunosuppressive treatments before initiating dupilumab). Another real-world evidence study in 543 adult patients with AD treated with dupilumab 300 mg q2w, reported that 98% of patients achieved improvement in at least one of the AD domains (EASI-75 or ≥ 4-point improvement in Peak Pruritus NRS or ≥ 4-point improvement in DLQI) at week 16 (33). Finally, in a systematic review and meta-analysis including 22 studies and 3,303 adult patients with AD in a real-world setting, up to 85% of patients treated with dupilumab achieved EASI-50 at week 16. In addition, patients achieved 48% and 68% reduction in weekly Peak Pruritus NRS and DLQI at week 16, respectively (34). Together, these data highlight the utility of a broader set of multiple measures, to evaluate response to treatments beyond the stricter and conventional IGA “clearance” endpoint, and to more comprehensively characterize improvements in AD signs, symptoms, and QoL in all patients.
Strengths and limitations
The data used in this analysis were derived from a randomized, double-blind, placebo-controlled trial and assessed both clinician- and patient-reported outcomes of AD severity. Robust randomized, controlled studies provide the most rigorous assessment of a drug’s benefit/risk profile. Measures of Peak Puritus NRS and DLQI, which are easy to perform in the clinical setting in addition to measure of clinical severity, can provide evidence of a more complete response to therapy compared with assessing the effect of therapy on disease signs alone. Because “shared decision-making” is strongly encouraged by patient advocate groups, multidimensional measures are more likely to resonate with patients and caregivers in any discussions of therapeutic options compared with endpoints required by regulatory agencies.
This analysis builds on previous findings, which showed that dupilumab provides important clinical benefits in adult patients over 16 weeks of treatment (30), provides sustained long-term control in most patients (19, 20), and highlights the need to evaluate patients with moderate-to-severe AD with long-standing disease over an extended period. A limitation of this study is that many of the outcomes presented here were not part of prespecified analyses.
Conclusion
A large majority of patients (84%) with moderate-to-severe AD treated with dupilumab every other week in combination with TCS achieved a satisfactory treatment response in at least one AD domain (signs, symptoms, or QoL) after 4 months of treatment, and 64% achieved improvement equivalent to minimal disease in at least one AD domain after the first dose. Most importantly for a chronic relapsing disease, in patients achieving minimal disease in one domain at 4 months, this response was sustained over 1 year of treatment, with the majority of patients achieving minimal disease in at least one other domain. Given the multidimensional nature of AD, evaluation based solely on AD signs does not capture the full treatment benefit of dupilumab relevant to patients with AD and their clinicians. Hence, a definition of response based on broader criteria, which incorporate signs, symptoms, and QoL, is more representative of the patient experience and will empower more impactful clinical decision-making.
ACKNOWLEDGEMENTS
Written informed consent for publication of the clinical images in Fig. 3 was obtained from the patient.
The authors thank the patients for their participation in these studies and their colleagues. Linda Williams, RPh (Regeneron Pharmaceuticals, Inc.), Adriana Mello, PharmD, Tracy Chew, PhD, and El-Bdaoui Haddad, PhD (Sanofi), contributed to the study.
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Spidergram and rainbow graphics developed by Ana B. Rossi and Marthe Vuillet of Sanofi Genzyme. Medical writing and editorial support were provided by Lola MacRae, PhD, and Alexandre Houzelle, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline.
Conflicts of interest. JIS has been an investigator for AbbVie, AnaptysBio, Arena Pharmaceuticals, Asana Biosciences, Boehringer Ingelheim, Dermira, Dermavant, DS Biopharma, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi; a consultant for AbbVie, Eli Lilly, Galderma, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Pfizer, Realm Therapeutics, Regeneron Pharmaceuticals, Inc., and Sanofi; and a speaker for Regeneron Pharmaceuticals, Inc., and Sanofi. ELS has been a consultant for AbbVie, Anacor Pharmaceuticals, Bristol Myers Squibb, Dermira, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, LEO Pharma, MedImmune, Menlo Therapeutics, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, and Valeant; and reports grants/research funding from Amgen, Anacor Pharmaceuticals, Bristol Myers Squibb, Chugai, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roivant Sciences, Sanofi, Tioga Pharmaceuticals, and Vanda Pharmaceutical. MB has been a consultant and reports grants/research funding for Regeneron Pharmaceuticals, Inc., and Sanofi. MdB-W has been a principal investigator for AbbVie, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme; an advisory board member for AbbVie, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, and UCB; a consultant for Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme; and reports research support and honoraria for lecturing for Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme. PF has been an investigator and/or advisory board member and reports honoraria and/or research grants for AbbVie, Amgen, Arcutis Biotherapeutics, Astra Zeneca, Bristol Myers Squibb, Boehringer Ingelheim, Botanix Pharmaceuticals, Bristol Myers Squibb, Celtaxsys, Cutanea, Dermira, Eli Lilly, Galderma, Genentech, GSK, Hexima, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Reistone Biopharma, Roche, Sanofi, Sun Pharma, UCB Pharma, and Valeant. YK reports research grants for AbbVie, Eli Lilly, LEO Pharma, Pfizer, Maruho, Otsuka, and Sanofi; and honoraria for lecturing for Sanofi. GB-LB is an employee of Sanofi and may hold stock and/or stock options in the company. ZC, BS, and JC are employees and shareholders of Regeneron Pharmaceuticals, Inc. ABR is an employee of Sanofi Genzyme and may hold stock and/or stock options in the company.
https://doi.org/10.2340/actadv.v101.307
REFERENCES
- 1.Leshem YA, Chalmers JR, Apfelbacher C, Furue M, Gerbens LAA, Prinsen CAC, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the Harmonising Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol 2020; 82: 1181–1186. [DOI] [PubMed] [Google Scholar]
- 2.Thyssen JP, Vestergaard C, Deleuran M, de Bruin-Weller MS, Bieber T, Taieb A, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: e839–e842. [DOI] [PubMed] [Google Scholar]
- 3.Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985–2010. PLoS One 2011; 6: e17520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schram ME, Spuls PI, Leeflang MMG, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012; 67: 99–106. [DOI] [PubMed] [Google Scholar]
- 5.Stalder JF, Taïeb A, Atherton DJ, Bieber P, Bonifazi E, Broberg A, et al. Severity scoring of atopic dermatitis: the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31. [DOI] [PubMed] [Google Scholar]
- 6.Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019; 181: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 8.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33. [DOI] [PubMed] [Google Scholar]
- 9.Badia X, Mascaró JM, Lozano R. Measuring health-related quality of life in patients with mild to moderate eczema and psoriasis: clinical validity, reliability and sensitivity to change of the DLQI. The Cavide Research Group. Br J Dermatol 1999; 141: 698–702. [DOI] [PubMed] [Google Scholar]
- 10.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015; 172: 1353–1357. [DOI] [PubMed] [Google Scholar]
- 11.Barbarot S, Wollenberg A, Silverberg JI, Deleuran M, Pellacani G, Armario-Hita JC, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results of four randomized phase 3 trials. J Dermatolog Treat 2020. Jun 8 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017; 177: 1316–1321. [DOI] [PubMed] [Google Scholar]
- 13.Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for five patient-reported outcomes in adults with atopic dermatitis. Br J Dermatol 2018; 178: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br J Dermatol 2021; 184: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald LE, Karow M, Stevens S, Auerbach W, Poueymirou WT, Yasenchak J, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A 2014; 111: 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A 2014; 111: 5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi N, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016; 15: 35–50. [DOI] [PubMed] [Google Scholar]
- 19.Merola JF, Sidbury R, Wollenberg A, Chen Z, Zhang A, Shumel B, et al. Dupilumab prevents flares in adults with moderateto-severe atopic dermatitis in a 52-week randomized controlled phase 3 trial. J Am Acad Dermatol 2020; 84: 495–497. [DOI] [PubMed] [Google Scholar]
- 20.Wu JJ, Spelman L, Tan JL, Etoh T, Zhang H, Shumel B, et al. Dupilumab maintains long-term disease control in adults with moderate-to-severe atopic dermatitis as measured by well-controlled weeks: results from the LIBERTY AD CHRONOS clinical trial. Dermatol Ther (Heidelb) 2021; 11: 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deleuran M, Thaçi D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol 2020; 82: 377–388. [DOI] [PubMed] [Google Scholar]
- 22.Wollenberg A, Beck LA, Blauvelt A, Simpson EL, Chen Z, Chen Q, et al. Laboratory safety of dupilumab in moderateto-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol 2020; 182: 1120–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paller AS, Siegfried EC, Thaçi D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020; 83: 1282–1293. [DOI] [PubMed] [Google Scholar]
- 24.Spekhorst LS, Ariëns LFM, van der Schaft J, Bakker DS, Kamsteeg M, Oosting AJ, et al. Two-year drug survival of dupilumab in a large cohort of difficult-to-treat adult atopic dermatitis patients compared to cyclosporine A and methotrexate: results from the BioDay registry. Allergy 2020; 75: 2376–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruin-Weller M, Biedermann T, Bissonnette R, Deleuran M, Foley P, Girolomoni G, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol 2021; 101: adv00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 27.Basra MKA, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 28.2020 Regeneron Pharmaceuticals. DUPIXENT® (dupilumab) [Prescribing Information]. [accessed February 17, 2021] Available from: https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf.
- 29.Simpson EL, de Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017; 77: 623–633. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg JI, Simpson EL, Ardeleanu M, Thaçi D, Barbarot S, Bagel J, et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: a pooled analysis of data from two phase III trials. Br J Dermatol 2019; 181: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paller AS, Bansal A, Simpson EL, Boguniewicz M, Blauvelt A, Siegfried EC, et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: post-hoc analyses from a randomized clinical trial. Am J Clin Dermatol 2020; 21: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ariens LFM, van der Schaft J, Spekhorst LS, Bakker DS, Romeijn GLE, Kouwenhoven TA, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-weeks results from the Dutch BioDay registry. J Am Acad Dermatol 2021; 84: 1000–1009. [DOI] [PubMed] [Google Scholar]
- 33.Nettis E, Ferrucci SM, Ortoncelly M, Pellacani G, Foti C, Di Leo E, et al. Use of dupilumab for 543 adult patients with moderate-to-severe atopic dermatitis: a multicenter, retrospective study. J Investig Allergol Clin Immunol 2020; Aug 26 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol 2021; 84: 139–147. [DOI] [PubMed] [Google Scholar]