Abstract
Conjugative transfer of Enterococcus faecalis-specific sex pheromone plasmids relies on an adhesin, called aggregation substance, to confer a tight cell-to-cell contact between the mating partners. To analyze the dependence of pAD1-encoded aggregation substance, Asa1, on pheromone induction, a variety of upstream fragments were fused to an α-amylase reporter gene, amyL, by use of a novel promoter probe vector, pAMY-em1. For pheromone-regulated α-amylase activity, a total of at least six genes, traB, traC, traA, traE1, orfY, and orf1, are required: TraB efficiently represses asa1 (by a mechanism unrelated to its presumptive function in pheromone shutdown, since a complete shutdown is observed exclusively in the presence of traC); only traC can relieve traB-mediated repression in a pheromone-dependent manner. In addition to traB, traA is required but not sufficient for negative control. Mutational inactivation of traE1, orfY, or orf1, respectively, results in a total loss of α-amylase activity for constructs normally mediating constitutive expression. Inversion of a fragment covering traA, P0, and traE1 without disrupting any gene or control element switches off amyL or asa1 expression, indicating the involvement of a cis-acting, orientation-dependent factor (as had been shown for plasmid pCF10). Unexpectedly, pAD1 represses all pAMY-em1 derivatives in trans, while its own pheromone-dependent functions are unaffected. The discrepancy between the new data and those of former studies defining TraE1 as a trans-acting positive regulator is discussed.
Facultatively pathogenic microorganisms differ largely with respect to the mechanisms by which they affect their host. However, they all face the common problem that they have to strictly distinguish between two totally different “lifestyles,” i.e., between living as commensals or even free in nature and being involved in the process of colonizing tissues or blood, which demands an alternate equipment of cell surface components, exoproteins, and metabolic enzymes.
The gram-positive bacterium Enterococcus faecalis is a commensal of the intestine, but under different circumstances may infect the urinary tract, blood, or endocardium. The conditions indispensable for the infectious pathway are still unknown, and there is no common factor identified for all clinical isolates. However, sex pheromone plasmid-encoded aggregation substance is a widespread adhesin shown to be involved in the colonization of various tissues (21, 28, 30). In addition, it plays an essential role in the conjugative transfer of the sex pheromone plasmid on which it is encoded in that it confers a tight contact between donor and recipient cells, visible as large clumps. (For reviews of the sex pheromone system, see references 7, 10, and 37.)
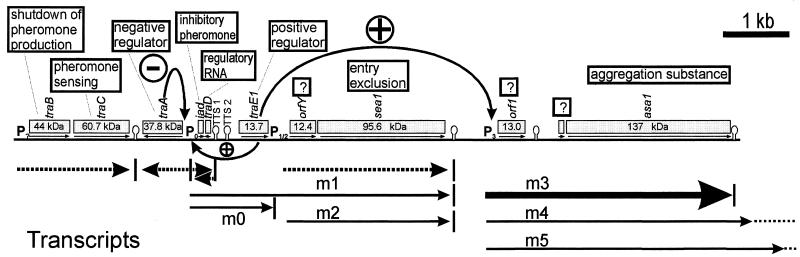
Therefore, regulation of aggregation substance is on three different levels. Under normal growth conditions, its expression is totally shut down. During the operation of the infectious pathway, there may be various environmental factors inducing aggregation substance, among them a component of blood serum (23) and several antibiotics (16, 39). For conjugative plasmid transfer, the corresponding gene is transcribed in response to a plasmid-specific oligopeptide, called sex pheromone, secreted by recipient cells not containing the corresponding plasmid. The latter phenomenon has been known for a long time and has been investigated by several groups (7, 10, 37). Especially for two different plasmids, pAD1and pCF10, rather detailed data on the regulatory circuits are available. Surprisingly, despite a similar overall organization of the regulatory genes and highly homologous DNA regions (15), induction of aggregation substance seems to involve two mechanistically distinct strategies. While for the pAD1-encoded aggregation substance, Asa1, a trans-acting protein, TraE1, obviously serves as the general inducer of transcription (25, 32) (a survey of the genetic data available for pAD1 is given in Fig. 1), the pCF10-encoded aggregation substance, Asc10, is expressed via transcriptional readthrough which involves a cis-acting, orientation-dependent factor (6), a regulatory RNA molecule interacting with ribosomal proteins, and a small proteinaceous regulator (4, 5).
FIG. 1.
Genetic organization of a pheromone-regulated pAD1 region and functions of several gene products according to previous publications. Interrupted arrows indicate constitutive transcripts. m0 to m5, pheromone-inducible transcripts; Px, presumptive promoters as localized by primer extension experiments. rho-independent transcriptional terminators are indicated as stem-loop structures.
The question is whether two related plasmids may have developed totally different strategies to regulate the same adhesin, or whether there are common basic pathways only slightly modified to ensure a specific response to the corresponding sex pheromone. This study presents data on the regulation of sex pheromone plasmid pAD1, supporting in part the second idea and addressing the function of several pAD1-specific genes. These data were obtained by use of a newly constructed α-amylase-based promoter-probe vector.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
E. faecalis strains OG1X (19) and OG1X(pAD1) were grown in Todd-Hewitt-broth (THB; Oxoid). Escherichia coli cloning strain TOP10F′ (Invitrogen) was grown in Luria-Bertani broth (24). Selective antibiotics were added as follows: erythromycin, 800 μg/ml for E. coli and 20 μg/ml for E. faecalis; chloramphenicol, 20 μg/ml for both organisms.
Construction of pAMY-em1 vector and derivatives containing pAD1 fragments.
pAMY-em1 is composed of the same genetic elements used for the construction of expression vector pERM-ex1 (27). In addition, a promoterless chloramphenicol acetyltransferase gene (cat) protected by a downstream terminator (1) was integrated adjacent to the polylinker, but in the opposite orientation relative to amyL.
A variety of pAD1 fragments were cloned into MCSI of pAMY-em1; Table 1 summarizes the constructs obtained and their genotypes. For stable maintenance in E. faecalis, constructs were linearized with AvrII and ligated to E. coli-E. faecalis shuttle vector pWM401 (38) cut with NheI (which at the same time removes the P15A ori from pWM401). The recombinant product obtained after double selection with erythromycin and chloramphenicol in E. coli was electrotransformed into E. faecalis and selected for by use of the same antibiotics. The integrity of constructs was tested as described previously (25).
TABLE 1.
pAMY-em1 constructs and their pAD1-related genotypes
| Construct | Description | Genotype |
|---|---|---|
| pBCAEYP31 | Complete sequence of regulatory regionsa from traB (BspHI site) to the asa1 start codon (BspHI site) | traBCAE1orfY1 |
| pCAEYP31 | ΔtraB (PvuI/PacI fragment deleted from pBCAEYP31) | traCAE1orfY1 |
| pBAEYP31 | ΔtraC (PvuII fragment deleted from pBCAEYP31) | traBAE1orfY1 |
| pBEYP31 | ΔtraC/A (ca. 300-bp deletion within traA introduced into pBAEYP31) | traBCAE1orfY1 |
| pAEYP31 | Complete sequence of regulatory region from traA (HincII site) to the asa1 start codon (BspHI site) | traAE1orfY1 |
| pP0EYP31 | Complete sequence of regulatory region from P0 (NheI site) to the asa1 start codon (BspHI site) | traE1orfY1 |
| pP1/2YP31 | Complete sequence of regulatory region from P1/2 (NsiI site) to the asa1 start codon (BspHI site) | orfY1 |
| pAEP31 | orfY (NsiI-Sau3a fragment) deleted from pAEYP31 | traAE1orf1 |
| pAEY-RBSamy | Complete sequence of regulatory region from traA (HincII site) to orfY (Sau3a site): traAE1orfY; amyL controlled by its original RBS (RBSamy) | traAE1orfY |
| pP31 | Fragment from P3 (HpaII site) to the asa1 start codon (BspHI site): orf1 | orf1 |
| pEA/YP31 | pAEYP31 with inversion of a HincII-NsiI fragment covering traA through traE1 | traAE1(Inv)-orfY1 |
| pEA/P31 | orfY (NsiI-Sau3a fragment) deleted from pEA/YP31 | traAE1(Inv)-orf1 |
| pEA/RBSamy | HincII-NsiI fragment covering traA through traE1 inverted relative to amyL; amyL controlled by its original RBS (RBSamy) | traAE1(Inv) |
| pEP0/P31 | NdeI-NsiI fragment covering P3 through traE1 inverted relative to amyL; sequence from P3 (HpaII site) to the asa1 start codon (BspHI site) in original orientation | traE1(Inv)-orfY1 |
| pAEYP3(1) | pAEYP31 with a stop codon introduced into orf1 (1-nucleotide exchange creating a BamHI site near the orf1 start codon) | traAE1orfY-Δorf1 |
| pA(E)YP31 | pAEYP31 containing a −2 frameshift within traE1 | traA-ΔE1-orfY1 |
| pA(E)Y′-P31 | pA(E)YP31 with orfY 3′-end deleted (AciI-Sau3a fragment) | traA-ΔE1-orfYΔ3′-orf1 |
| pA(E)P31 | pA(E)YP31 lacking P1/2orfY (NsiI-Sau3a fragment) | traA-ΔE1-orf1 |
| pP0(E)P31 | pA(E)P31 with traA 3′-end deleted (NheI-HincII fragment) | ΔtraE1-orf1 |
| pAT1-′EP31 | pAT1-′EYP31 lacking P1/2orfY (NsiI-Sau3a fragment) | traA-E1Δ5′-orf1 |
| pP0T1-′EP31 | pAT1-′EP31 with traA 3′-end deleted (NheI-HincII fragment) | traE1Δ5′-orfY1 |
| pAE′-P2YP31 | 3′-end of traE1 and P1 promoter deleted from pAEYP31 (FspI-BspEI fragment) | traA-E1Δ3′-orfY1 |
| pAP0P1/2YP31 | pAEYP31 with traE1 deleted (XbaI-NsiI fragment) | traAorfY1 |
| pAP0P31 | pAP0P1/2YP31 lacking P1/2orfY (NsiI-Sau3a fragment) | traAorf1 |
| pP0P1/2YP31 | pP0EYP31 with traE1 deleted (XbaI-NsiI fragment) | orfY1 |
| pRBSamy | Control plasmid corresponding to the pAMY-em1/pWM401 cointegrate | |
| pPermRBSamy | Control plasmid corresponding to the pERM-ex1/pWM401 cointegrate (26) |
“Complete sequence of regulatory regions” indicates that the original sequence within the given fragment remained unchanged, with the following exceptions: (i) the NsiI site downstream of traE1 contains 8 additional bp derived from cloning vector pOK12 (TGCAGGCA), and (ii) sea1 encoding surface exclusion protein (36) is completely lacking and has been replaced by 8 bp derived from cloning vector pUC19 (TCTAGAGT).
Mutational inactivation of pAD1 genes.
pAD1-encoded orf1 was mutated according to Kunkel et al. (22); with a single nucleotide exchange, a BamHI site was introduced that converted the fourth orf1 codon into a stop codon.
traE1 was inactivated as follows. The pAEYP31 construct was linearized by FspI digestion, normally causing a blunt-ended cut within traE1. To select for incorrect restoration of the restriction site, ligation products were submitted to a second round of FspI digestion linearizing all plasmids containing the correct recognition sequence and transformed into E. coli TOP10F′. Sequencing of a selected plasmid [pA(E)YP31] nonsusceptible to FspI revealed a −2 frameshift (the TGCGCA recognition sequence had been changed to TGCA).
orfY was subcloned as a HindIII-AciI fragment (removing 84 bp from the 3′ end of orfY) into pUC19 linearized with HindIII-AccI, excised as an NsiI-XbaI fragment, and reintroduced into the original constructs partially digested with the same enzymes.
To introduce a mutation into traA, pBAEYP31 was cut with NheI, and the single-stranded overhangs were filled in with Klenow fragment of DNA polymerase I and ligated. After transformation into E. coli Top10F′, only a derivative containing a deletion of about 300 bp within traA (not affecting neighboring sequences) was obtained (pBEYP31).
α-Amylase activity assay.
Overnight cultures of E. faecalis strains containing pAMY-em1 constructs were inoculated 1:100 into 1 ml of fresh THB medium supplemented with 20-μg/ml concentrations of chloramphenicol and erythromycin (with or without synthetic sex pheromone cAD1). After ca. 5 h of incubation at 37°C, cultures were placed on ice, and 200 μl of each cell suspension was removed for the determination of turbidity at a wavelength of 600 nm. (Note that to dissolve cell aggregates grossly influencing the turbidity, suspensions are mixed with 1 ml of 8 M urea; this treatment does not detectably lyse E. faecalis cells or break cell chains.) Turbidity should not exceed a value of about 0.7 to ensure that cells are still exponentially growing. The rest of the cultures were centrifuged at 4°C, and 700 μl of supernatant was mixed with 200 μl of a precooled slurry of Phadebas reagent (Pharmacia) in H2O. The mixtures were heavily stirred at 75°C on an Eppendorf shaker (Thermomixer 5436) until not more than half of the slurry had lost its blue color (in order to guarantee linearity of activity values). The reaction was stopped by placing the suspensions on ice for several minutes, spinning them briefly in a cooled centrifuge, and transferring 700 μl of supernatant to a plastic cuvette containing 500 μl of 0.5 M NaOH (which stops the reaction). Optical densities at 620 nm (OD620) were determined, and the resulting values were equalized to standard conditions (i.e., cell densities of OD600 = 1.0 and 15 min of amylase reaction time).
Activities in units per liter were deduced from the conversion table supplied by the manufacturer (which, taking into account the change in assay conditions, suggested the values have to be multiplied by a factor of 0.085 [calculated from the altered dilution rates]). One unit is defined as the amount of enzyme catalyzing the hydrolysis of 1 μmol of glucosidic linkage per min.
Clumping assay.
Supernatants from overnight cultures of OG1X strains containing pAMY-em1 constructs were boiled for 5 min to kill residual E. faecalis cells and diluted with fresh THB in steps of 1:2 with 24-well microtiter plates used as a reservoir. After 1:100 inoculation with an overnight culture of OG1X(pAD1/pRBSamy) (the second plasmid required because supernatants contain chloramphenicol and erythromycin), the microtiter plates were gently shaken at 37°C for several hours, and the formation of macroscopically visible aggregates was determined.
Nucleotide sequence accession number.
The nucleotide sequence of pAMY-em1 has been submitted to GenBank (accession no. AJ243541).
RESULTS
Construction of a new α-amylase-based promoter probe vector.
Previous analyses of asa1 regulation mainly relied on three different methods: (i) Northern blotting (2, 13, 25) (Fig. 1), (ii) clumping assays using aggregation substance itself as a marker (11, 25), and (iii) transcriptional lacZ fusions based on a Tn917-derivative bearing a ′lacZ gene at one end (29, 34). Transcriptional analysis is too time-consuming for routine investigations and difficult to quantify. Clumping assays are easy to execute, but quantification is even less sensitive than Northern blotting. The use of Tn917lac suffers from the random location of transposition sites and from the polar effect of the 8.5-kb insert inactivating the possibly essential downstream sequence. Furthermore, only the transposition site itself was analyzed for transcription, while the effects of mutations on distant DNA regions are difficult to address. Our own previous attempts to present the lacZ gene on a definite promoter probe vector failed because of the instability of constructs. In addition, for assaying very weak promoter activities, β-galactosidase may be inadequate, since this cytoplasmic enzyme possibly is not quantitatively extracted from the highly rigid E. faecalis cells.
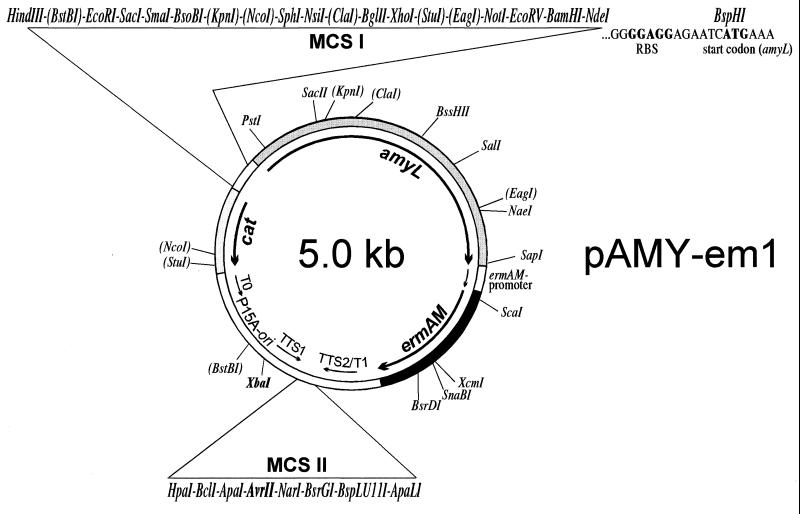
Here, a new vector, pAMY-em1 (Fig. 2), was constructed by using α-amylase (amyL) from Bacillus licheniformis (14) as a quantifiable marker. This enzyme has been successfully used previously in E. faecalis (17) and combines several advantages. As an exoprotein, its activity can be determined from the supernatant without cell extraction by using a very simple assay (Phadebas reagent from Pharmacia). Since AmyL is a thermophilic enzyme with a temperature optimum of 75°C, a quantitative removal of residual cells (which might change results by enzyme production during long-run assays) is not necessary. The promoterless amyL gene was cloned adjacent to a large multiple cloning site (MCSI) containing many restriction sites for the integration of DNA fragments. To allow analysis of countertranscription from the same culture, a promoterless cat gene (1) was introduced in the opposite orientation to MCSI. (This marker gene was not used in the present work.) An erythromycin resistance gene (ermAM) downstream of amyL allows selection in E. coli and many gram-positive bacteria (9), and a second MCS protected on both sides by strong transcriptional terminators the integration of species-specific plasmid replicons. A similar vector, pERM-ex1, has been constructed for the purpose of gene expression (27).
FIG. 2.
Promoter probe vector pAMY-em1 (GenBank accession no. AJ243541). amyL, promoterless α-amylase gene from B. licheniformis; cat: promoterless chloramphenicol acetyltransferase gene from Staphylococcus aureus plasmid pC194; ermAM: erythromycin resistance gene from E. faecalis plasmid pAMβ1; T0, T1, TTS1, and TTS2, transcriptional terminators. Selected restriction sites present only once or twice are indicated, the latter in parentheses.
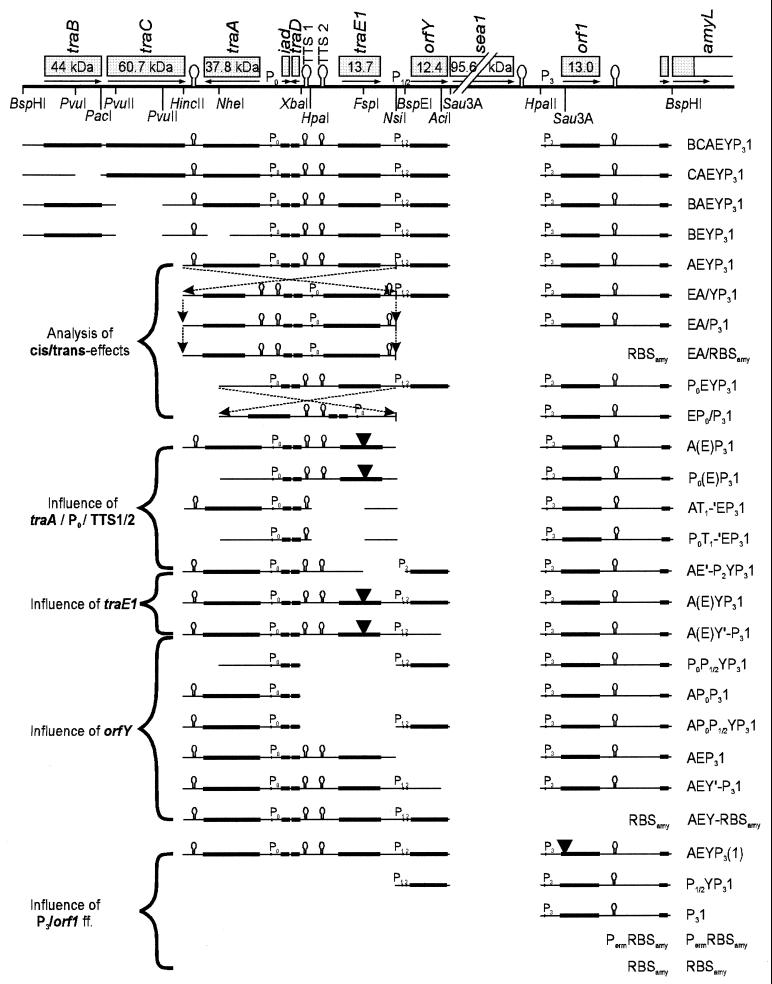
After testing the vector for the absence of nonspecific promoter activity—neither E. faecalis nor E. coli expressed detectable amounts of α-amylase—a variety of DNA fragments derived from the pAD1 sequence upstream of asa1 were cloned into pAMY-em1. In most cases, the start codon of asa1 was fused to the start codon of amyL by use of the BspHI sites covering both ATG initiation sites. Thus, true translational fusions were created retaining all original nucleotides upstream of asa1, including the ribosome binding site (RBS). Only for those constructs designed to test the role of the region between orf1 and asa1 was the RBS of amyL used (RBSamy). From all constructs, the 3-kb gene for surface exclusion protein, sea1 (36), was excluded, since it encodes a cell surface protein very probably not involved in asa1 regulation. A survey of all constructs is given in Fig. 3 (also see Table 1). To propagate the plasmids in E. faecalis, they were integrated into the low-copy shuttle vector pWM401 (see Materials and Methods). Culture supernatants of E. faecalis OG1X or OG1X(pAD1) containing one of the constructs were submitted to amylase assays as described in Materials and Methods. Each construct was tested at least three times.
FIG. 3.
Survey of pAMY-em1 constructs used in this study. (Fusions involving the ermAM promoter [Perm] were constructed by use of the related vector pERM-ex1 [27].) The nomenclature of constructs is given as follows: genes are indicated by a one-letter code (B, traB; C, traC; A, traA; E, traE1; Y, orfY; and 1, orf1); promoters (Px) and terminator T1 (TTS1) are given only when they border disruptions of the original sequence; genes given in parentheses have been destroyed by point mutations (graphically marked by black triangles), while primes indicate the removal of a few nucleotides from the 5′ or 3′ ends, respectively. The diagrams of the constructs (in this and the following figures) are aligned to the graphical representation of the pAD1 sequence given on the top. Only complete genes (and those disrupted by point mutations) are accented by thick lines. Restriction sites involved in the various constructions are indicated.
traB and traC are both required for sex pheromone-dependent asa1 expression.
In the first approach, the complete transcriptional units defined by the promoters determined previously for the regulatory region of pAD1 (13) were successively added to the asa1-amyL fusion (Fig. 4). P3 and P1/2 turned out to be largely inactive. With the addition of P0-traE1, α-amylase was constitutively expressed at a high level (pEYP31). These data are consistent with previous results showing that activation of the P3 promoter (responsible for asa1 transcription) is dependent on the activity of traE1 which was transcribed from P0 in the absence of the negative regulator TraA (25, 32). Addition of the traA gene (pAEYP31) reduced amyL expression only slightly; this is not surprising, since the strains all produce sex pheromone, cAD1, which inactivates TraA (12).
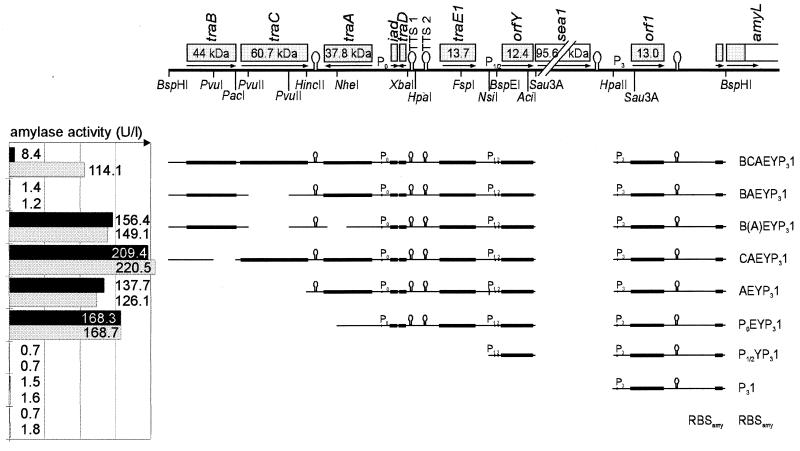
FIG. 4.
Effects of various pAD1 fragments confined by presumptive pAD1-specific promoters in the presence or absence of sex pheromone cAD1. Black bars, α-amylase activities of noninduced E. faecalis OG1X cultures containing one of the pAMY-em1 constructs; grey bars, α-amylase activities of E. faecalis cultures induced by synthetic pheromone cAD1 (with pheromone concentration about 100-fold of the minimal inductory concentration). Activities (units per liter) always refer to the constructs given immediately on the right.
Complementation of the constructs with original pAD1 should change results fundamentally: since transcription from P3 was shown to be activated in trans by TraE1 gene product (25), even the smallest fragment (pP31) originating at P3 should allow inducible α-amylase expression via pAD1-born TraE1. Surprisingly, not only cAD1-induced OG1X(pAD1/pP31) failed to produce detectable amounts of α-amylase (not shown); pAD1 shut down amyL activity of all pAMY-em1 derivatives—even those which per se resulted in strong constitutive expression (e.g., pAEYP31). This effect is not due to plasmid incompatibility or instability, since in all cases, the intact pAMY-em1 constructs could be isolated and retransformed into E. coli TOP10F′, where α-amylase activity was restored. (In E. coli, amyL expression probably originates nonspecifically from one of the many promoter-like structures found in the low-G+C DNA of E. faecalis.) In all of these strains, pAD1-encoded aggregation substance remained normally inducible by cAD1, since the characteristic cell clumping was observed after exposure to sex pheromone.
In the next step, the pAD1 sequence fused to amyL was further extended for two key elements involved in sex pheromone control: traC, encoding a cell surface lipoprotein responsible for sex pheromone sensing (31), and traB, with a presumptive function of the gene product in the shutdown of chromosomally encoded cAD1 (35). With these additional genes, amyL expression was considerably reduced (about 14-fold) when compared to that expressed by pAEYP31 (Fig. 4). Addition of cAD1 to the nutrient broth increased expression about 10-fold, but to a level below that of the constitutively expressing construct pAEYP31. Deletion of traC from the traB-traC operon (without affecting the common promoter) totally shut down amyL expression, irrespective of the presence of sex pheromone, but with dependence on traA, the additional deletion of which (pBEYP31) completely relieved repression. A deletion of traB (pCAEYP31) further increased constitutive expression compared to that of pAEYP31.
These data still may be explained with the predicted functions of traB and traC (compare with the results in Fig. 1): traB gene product switches off cAD1 production and therefore self-inducibility. If externally added cAD1 is internalized exclusively by TraC, the deletion of traC would make the cells totally insensitive to sex pheromone induction. However, some doubts about this interpretation emerge when the supernatants of the corresponding strains are tested for cAD1 content: OG1X(pBAEYP31) containing the complete traB gene still secretes active sex pheromone [although in a markedly reduced amount, since the supernatant was active down to a 1:64 dilution, compared to a still active 1:256 dilution for OG1X(pAEYP31) supernatant]. Very surprisingly, the only strains completely defective in cAD1 production are OG1X(pCAEYP31) and OG1X(pBCAEYP31) expressing TraC. Here, no clumping could be induced even when the pAD1-containing cells were grown in undiluted or slightly THB-enriched supernatant. This total lack of active cAD1 cannot be explained by a possible overproduction of inhibitory pheromone iAD1: when supernatants from OG1X(pAEYP31) are mixed 1:1 with supernatants from OG1X(pBCAEYP31), the minimal inductory concentration is reduced by a factor of about (or slightly greater than) 2, as expected for a diluent lacking both cAD1 and iAD1.
asa1 regulation involves a cis-acting factor in an orientation-dependent manner.
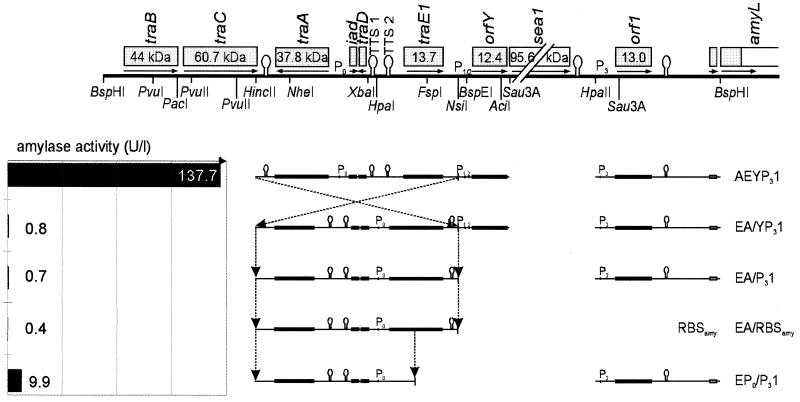
The most obvious discrepancy between the regulation models of pAD1 and pCF10 lies in the fact that pCF10 is submitted to the action of a cis-acting factor probably tracking along the DNA in an orientation-dependent manner (6), while for pAD1, the TraE1 protein was shown to act in trans as a diffusible activator of the P3 promoter (25, 32). To verify the model for pAD1 by using the amyL-based assay system, a fragment of the constitutive pAEYP31 construct covering the complete sequence from traA to traE1 was inverted relative to the amyL gene (Fig. 5). A strain containing the resulting construct (pEA/YP31) failed to produce α-amylase (irrespective of the presence of cAD1). Further removal of the P1/2-orfY and P3-orf1 regions (pEA/YP1, pEA/P31, and pEA/RBSamy) did not restore α-amylase activity. The possibility that the disruption of the original sequence necessary for the rearrangement may have affected an essential structure can be largely excluded, since the used NsiI site (i) lies within a sequence lacking any open reading frame (ORF) or striking nucleotide sequence motif and (ii) even in the highly active constructs contains some additional nucleotides from a polylinker used for intermediate cloning. Only when part of traA and its downstream terminator were deleted in addition (pEP0/P31) could some basic α-amylase activity be measured, probably representing the constitutive countertranscription from the bidirectional P0 promoter responsible for permanent traA expression.
FIG. 5.
Nondisruptive inversion of the region from traA through traE1. The mode of inversion involving a HincII-NsiI fragment (or NheI-NsiI for EP0/P31) is indicated by broken arrows. (Since there is no difference between induced and noninduced cultures, only results for noninduced cultures are indicated in this and the subsequent figures.)
Therefore, a cis-acting orientation-dependent factor similar to that of pCF10 has to be claimed for pAD1 too. Whatever its nature may be, it obviously is encoded within the 2.3-kb region from traA to traE1 or at least initiates its cis-acting function within this region.
traE1, orfY, and orf1 regions are essential.
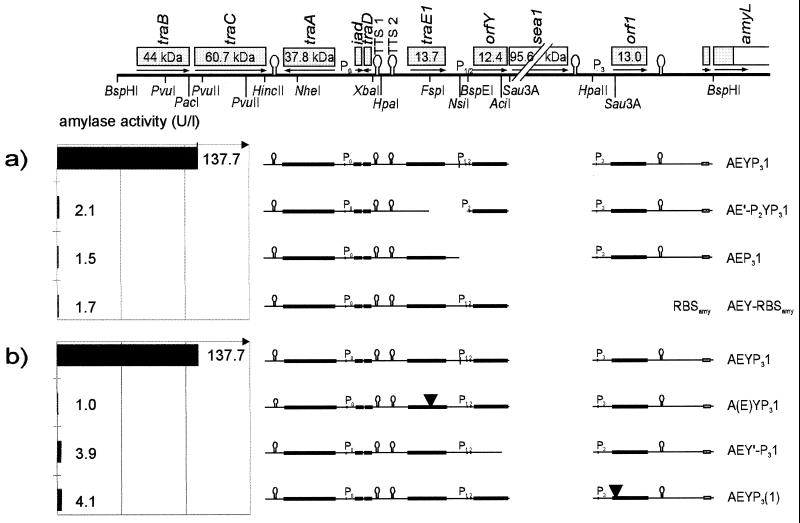
There are at least three small ORFs carried on pAD1 which are not present on pCF10. While traE1 and orf1 are unique to pAD1 (and related cAD1-inducible plasmids) (15), orfY is ubiquitous for most sex pheromone plasmids, but at least in the special case of pCF10, it is disrupted by a stop codon, leaving only a truncated 198-bp ORF (prgT) (20). These ORFs might be responsible for pheromone specificity of induction. To test their involvement in asa1 regulation, each of them (in common with some bordering sequences) was independently deleted from the constitutive construct pAEYP31. As shown in Fig. 6a, none of them could be removed without a nearly complete loss of α-amylase activity.
FIG. 6.
Deletions of traE1, orfY, and orf1 genes from the constitutive pAEYP31 construct. (a) Gross deletions covering a great part of the gene (traE1) or the complete DNA region (orfY or orf1). (b) Point mutations of traE1 and orf1 and 3′ truncation of orfY (see Materials and Methods for details).
traE1, orfY, and orf1 gene products are essential.
The essential roles of traE1, orfY, and orf1 regions need not necessarily be connected with the respective gene products, but might be dependent on other genetic information encoded on the corresponding sequence. It therefore was necessary to specifically mutate the peptides without grossly changing the nucleotide sequence. traE1 was mutated by the introduction of a −2-bp frameshift, and orf1 was mutated by creating a stop codon near its 5′ end (see Materials and Methods). In the case of orfY, the complete 3′ end downstream of the unique AciI site (84 bp) was removed. Again, OG1X strains containing any of these constructs [and a double mutant, pA(E)Y′P31, given in Fig. 3] largely failed to produce α-amylase (Fig. 6b), which implies an indispensable positive involvement of all three translation products in asa1 expression.
orfY and traA contribute to negative regulation.
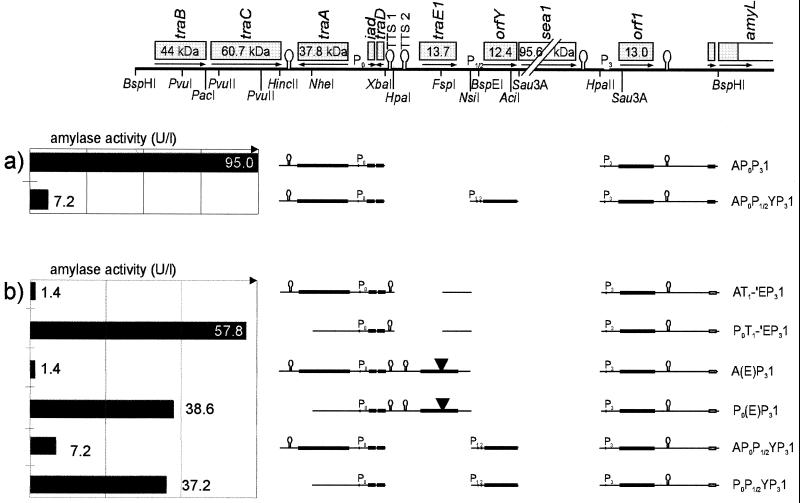
Interestingly, orfY seems to exhibit additional negative effects: Fig. 7a shows a pair of constructs differing with respect to the presence of orfY. Here, expression was achieved by removal of transcriptional terminators TTS1 and TTS2 along with traE1. In these cases, the presence of orfY reduced rather than increased α-amylase activity. The same may hold true for orf1, but measurable effects are so weak that activity values may be considered as nonsignificant (not shown). traE1 could not be tested for possible negative effects, since removal of one or both of the upstream terminators without deleting traE1 has toxic effects on E. faecalis.
FIG. 7.
Negative effects of orfY (a) and traA (b). Several pairs of constructs are compared, differing only with respect to the corresponding gene.
TraA has been known for a long time as a key negative regulator of aggregation substance; its disruption within pAD1 results in a constitutively clumping strain (18, 29). In this study, these data could largely be confirmed. It was shown that (in the absence of the traB-traC operon) TraA is not sufficient for a shutdown of asa1 expression, but helps to keep the TTS1 and -2 terminators locked. These effects become visible when traE1, known to exhibit autoinduction (25), is deleted from several constructs (three pairs of constructs differing with respect to the presence of traA are shown in Fig. 7b). The site of action is not located within the terminator region, since the constitutive amyL expression observed when both terminators are deleted still is modified by traA. This is consistent with data defining a binding site for TraA in the P0 promoter region (32).
DISCUSSION
In the present study, it could be shown that regulation of pAD1-encoded aggregation substance at least involves a total of 10 kb upstream of the corresponding asa1 gene. All ORFs of >100 bp within this region, except for sea1 encoding the surface exclusion protein, are required for sex pheromone-controlled asa1 expression. On the other hand, several important structures were excluded from a detailed analysis: iad (8) coding for inhibitory sex pheromone; traD, which encodes immediately downstream from iad but is transcribed in the opposite direction (2, 33); and a small ORF immediately upstream of asa1, which encodes an RAPC amino acid motif conserved for most, if not all, sex pheromone plasmids (27). The roles of these ORFs were not addressed independently of the genes investigated in detail; therefore, their functions might further complicate the picture of pAD1 regulation presented here.
Mutation of any of the genes tested either results in constitutive expression (traB or traA) or totally shuts down expression (traC, traE1, orfY, or orf1). As for pCF10, regulation involves a cis-acting orientation-dependent factor encoded—or at least initiating its function—within the 2.3-kb region covering traA through traE1, since its pure inversion results in a total loss of expression. In probably all cases, the proteins encoded by the ORFs rather than their nucleotide sequences are necessary for regulation. For orfY, a specific role of the deleted 3′-terminal 84 bp on the nucleotide level cannot totally be excluded, although there are no hints at all from the DNA sequence (e.g., repeats, conserved motifs, or possible secondary structures).
orfY (and possibly orf1) plays an ambiguous role in that it exhibits an additional negative effect. The function of TraA as a negative regulator of asa1 expression could be established; however, it was not sufficient for a complete repression, since it probably is inactivated by cAD1 still produced by the tested strains—provided that the pheromone is internalized despite the lack of a specific uptake system (see below). If this is the case, nonspecific internalization must be rather efficient, since addition of cAD1 to the culture broth of OG1X(pAEYP31) does not significantly raise α-amylase activity (Fig. 4).
The role of the remaining genes which have been investigated previously will have to be modified or newly defined according to the data presented. TraB efficiently represses aggregation substance, but not simply by avoiding self-induction via its assumed function as a repressor of cAD1 production. This can be excluded, since constructs containing traB still promote expression and secretion of active cAD1 in E. faecalis. Only the addition of traC, irrespective of the presence of traB, switches off cAD1 production, an effect that cannot simply be explained by the primary function of TraC lipoprotein as a pheromone-specific oligopeptide transporter. It may be argued that TraC surface protein traps all self-produced cAD1 from the supernatant—but why does this not induce asa1 expression (in the case of pBCAEYP31 [Fig. 4]) while externally added pheromone does?
Last but not least, and contrary to our own previous results (25), TraE1 cannot be simply a trans-acting protein directly effecting transcription from the P3 promoter. If this were the case, the inversion of a fragment containing the complete traE1 gene, including its original upstream sequence, which covers the corresponding P0 promoter, should not alter activity. Instead, this manipulation switches off asa1 expression, proving the involvement of an orientation-dependent mechanism, as has been shown for pCF10 (6).
The most puzzling result was that even complete pAD1 cannot activate the P3 promoter in trans, a strategy which has proven successful previously (25, 26). The fact that pAD1 inactivates the otherwise constitutive pAMY-em1 constructs would imply the existence of a pAD1-encoded, trans-acting super-repressor. However, even the control plasmid (pPermRBSamy) constitutively expressing amyL and not containing any pAD1 sequence is affected by pAD1, in that α-amylase activity is reduced to about 50% of its normal value (data not shown). Maybe the cellular secretion system mediating α-amylase export out of the cell is influenced by pAD1 (possibly via simple competition by pAD1-encoded surface proteins). This effect should be independent of pAD1-specific sequences and therefore cannot explain either why amyL preceded by pAD1 sequences is nearly completely switched off, contrary to the incomplete inactivation of pPermRBSamy control plasmid.
Updating of the model for asa1 regulation.
How can the data presented here and in previous publications be integrated into a conclusive regulation model? The following view combines all of the negative and positive effects shown for the various regulatory genes. Under noninducing conditions, transcription within the regulatory region is locked by a cooperative action of TraB and TraA. While TraA binds directly to the P0 promoter region (32), TraB acts in a still unclear way (but not simply by the shutdown of sex pheromone production). OrfY and Orf1 proteins could occupy specific positions upstream from their own coding regions, thus contributing to repression by preventing accidental transcription events. Additional factors help to keep the TTS1 and TTS2 terminators locked. Inhibitory pheromone iAD1 (8) competes with trace amounts of the sex pheromone cAD1, possibly present in the environment or produced by the cell itself, and traD countertranscription (2) interferes with transcription from the P0 promoter in the direction of traE1.
TraA does not prevent transcription from P0, since both iad and traA itself are constitutively expressed (however, transcription stops at the transcriptional terminators located downstream of these genes); it is more likely that TraA prevents residual TraE1 molecules from binding to its recognition site in the P0 region. This at the same time is the clue to the rapid induction by very low concentrations of sex pheromone: the affinity of TraA for its DNA binding site is directly relieved by cAD1 binding to TraA protein, and the site becomes accessible for TraE1. DNA-bound TraE1 modifies RNA polymerase in that it now can pass over the downstream terminators. Transcription of traE1 reinforces the initiation of transcription by positive feedback (25). When the active complex meets with OrfY and Orf1 gene products bound to their specific sites on the DNA (or to a DNA-binding cofactor), the proteins form an active complex initiating transcription at P2 and P3, respectively. (P1 is a weakly constitutive promoter causing a basic orfY-sea1 transcription terminating downstream of sea1 [13].) If only one of the proteins is lacking, transcription of asa1 is prevented.
Alternatively, the active complex may be required for the extension of a super-transcript initiating at P0. Such a mechanism is discussed for sex pheromone plasmid pCF10 (in references 3 and 4). If this were the case, however, the transcript would be processed immediately after RNA polymerase has transcribed the processing sites, since a precursor transcript cannot be detected by Northern blotting of mRNA isolated shortly after induction (13).
In addition, the involvement of a pAD1-encoded RNA molecule or molecules as for the pCF10 system (4) must be considered. The pCF10-encoded prgQ transcripts have been shown to interact directly with ribosomal proteins, which probably results in a posttranscriptional control of Asc10 expression (the pCF10-encoded aggregation substance). prgQ is carried within the region most conserved among all sex pheromone plasmids (15). It seems unlikely that a function attributed to this region for one plasmid should be totally irrelevant to the others. This mechanism may be rather a common principle of the regulation of all sex pheromone plasmids, while specificity is guaranteed by minor sequence variations or specific proteins (such as TraE1 and Orf1 gene products in the pAD1 system).
The attractivity of the suggested model lies in the fact that it supports both possible functions of TraE1 as a trans-acting and cis-acting factor: As a typical protein, it diffuses readily through the cell, but only in its DNA-bound conformation is able to interact with its cofactors to form an active transcription initiation complex. This conformation is permanently maintained when TraE1 tracks along the DNA. Nevertheless, the idea of TraE1 having a dual function as a cis- and trans-acting factor is still speculative and must be checked on a molecular level.
ACKNOWLEDGMENTS
I am grateful to E. Silberhorn for excellent technical help, P. Hols for providing pGIP61 as a source of α-amylase, and R. Wirth for helpful discussions and continuous support.
REFERENCES
- 1.Band L, Yansura D G, Henner D J. Construction of a vector for cloning promoters in Bacillus subtilis. Gene. 1983;26:313–315. doi: 10.1016/0378-1119(83)90204-4. [DOI] [PubMed] [Google Scholar]
- 2.Bastos M C F, Tomita H, Tanimoto K, Clewell D B. Regulation of the Enterococcus faecalis pAD1-related sex pheromone response: analyses of traD expression and its role in controlling conjugation functions. Mol Microbiol. 1998;30:381–392. doi: 10.1046/j.1365-2958.1998.01074.x. [DOI] [PubMed] [Google Scholar]
- 3.Bensing B A, Meyer B J, Dunny G M. Sensitive detection of bacterial transcription initiation sites and differentiation from RNA processing sites in the pheromone-induced plasmid transfer system of Enterococcus faecalis. Proc Natl Acad Sci USA. 1996;93:7794–7799. doi: 10.1073/pnas.93.15.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensing B A, Dunny G M. Pheromone-inducible expression of an aggregation protein in Enterococcus faecalis requires interaction of a plasmid-encoded RNA with components of the ribosome. Mol Microbiol. 1997;24:295–308. doi: 10.1046/j.1365-2958.1997.3311709.x. [DOI] [PubMed] [Google Scholar]
- 5.Bensing B A, Manias D A, Dunny G M. Pheromone cCF10 and plasmid pCF10-encoded regulatory molecules act post-transcriptionally to activate expression of downstream conjugation functions. Mol Microbiol. 1997;24:285–294. doi: 10.1046/j.1365-2958.1997.3301710.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung J W, Dunny G M. cis-acting, orientation-dependent, positive control system activates pheromone inducible conjugation functions at distances greater than 10 kilobases upstream from its target in Enterococcus faecalis. Proc Natl Acad Sci USA. 1992;89:9020–9024. doi: 10.1073/pnas.89.19.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clewell D B. Sex pheromone systems in enterococci. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 47–65. [Google Scholar]
- 8.Clewell D B, Pontius L T, An F Y, Ike Y, Suzuki A, Nakayama J. Nucleotide sequence of the sex pheromone inhibitor (iAD1) determinant of Enterococcus faecalis conjugative plasmid pAD1. Plasmid. 1990;24:156–161. doi: 10.1016/0147-619x(90)90019-9. [DOI] [PubMed] [Google Scholar]
- 9.Courvalin P, Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet. 1987;206:259–264. doi: 10.1007/BF00333582. [DOI] [PubMed] [Google Scholar]
- 10.Dunny G M, Leonard B A. Cell-cell communication in Gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenfeld E E, Clewell D B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987;169:3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto S, Clewell D B. Regulation of the pAD1 sex pheromone response of Enterococcus faecalis by direct interaction between the cAD1 peptide mating signal and the negatively regulating, DNA-binding TraA protein. Proc Natl Acad Sci USA. 1998;95:6430–6435. doi: 10.1073/pnas.95.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli D, Friesenegger A, Wirth R. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol Microbiol. 1992;6:1297–1308. doi: 10.1111/j.1365-2958.1992.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 14.Gray G L, Mainzer S E, Rey M W, Lamsa M H, Kindle K L, Carmona C, Requadt C. Structural genes encoding the thermophilic α-amylases of Bacillus stearothermophilus and Bacillus licheniformis. J Bacteriol. 1986;166:635–643. doi: 10.1128/jb.166.2.635-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirt H, Wirth R, Muscholl A. Comparative analysis of eighteen sex pheromone plasmids: detection of a new insertion element on pPD1 and implications for the evolution of this plasmid family. Mol Gen Genet. 1996;252:640–647. doi: 10.1007/BF02173969. [DOI] [PubMed] [Google Scholar]
- 16.Höfner H, Wirth R, Marre R, Wanner G, Straube E. Subinhibitory concentrations of daptomycin enhance adherence of Enterococcus faecalis to in vitro cultivated renal tubuloepithelial cells and induce a sex pheromone plasmid-encoded adhesin. Med Microbiol Lett. 1995;4:140–149. [Google Scholar]
- 17.Hols P, Baulard A, Garmyn D, Delplace B, Hogan S, Delcour J. Isolation and characterisation of genetic expression and secretion signals from Enterococcus faecalis through the use of broad-host-range α-amylase probe vectors. Gene. 1992;118:21–30. doi: 10.1016/0378-1119(92)90244-j. [DOI] [PubMed] [Google Scholar]
- 18.Ike Y, Clewell D B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using Tn917 as an insertional mutagen. J Bacteriol. 1884;158:777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ike Y, Craig R A, White B A, Yagi Y, Clewell D B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci USA. 1983;80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao S-M, Olmsted S B, Viksnins A S, Gallo J C, Dunny G M. Molecular and genetic analysis of a region of plasmid pCF10 containing positive control genes and structural genes encoding surface proteins involved in pheromone-inducible conjugation in Enterococcus faecalis. J Bacteriol. 1991;173:7650–7664. doi: 10.1128/jb.173.23.7650-7664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkel T A, Roberts J D, Zabour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 23.Leonard B A, Hirt H, Dunny G M. Regulation of aggregation substance expression by bacterial and host factors. Adv Exp Med Biol. 1997;418:785–787. doi: 10.1007/978-1-4899-1825-3_185. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 25.Muscholl A, Galli D, Wanner G, Wirth R. Sex pheromone plasmid pAD1-encoded aggregation substance of Enterococcus faecalis is positively regulated in trans by traE1. Eur J Biochem. 1993;214:333–338. doi: 10.1111/j.1432-1033.1993.tb17928.x. [DOI] [PubMed] [Google Scholar]
- 26.Muscholl-Silberhorn A B. Analysis of the clumping-mediating domain(s) of sex pheromone plasmid pAD1-encoded aggregation substance. Eur J Biochem. 1998;258:515–520. doi: 10.1046/j.1432-1327.1998.2580515.x. [DOI] [PubMed] [Google Scholar]
- 27.Muscholl-Silberhorn A B. Cloning and functional analysis of Asa373, a novel adhesin unrelated to the other sex pheromone plasmid-encoded aggregation substances of Enterococcus faecalis. Mol Microbiol. 1999;34:620–630. doi: 10.1046/j.1365-2958.1999.01631.x. [DOI] [PubMed] [Google Scholar]
- 28.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 29.Pontius L T, Clewell D B. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J Bacteriol. 1992;174:3152–3160. doi: 10.1128/jb.174.10.3152-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlievert P M, Gahr P J, Assimacopoulos A P, Dinges M M, Stoehr J A, Harmala J W, Hirt H, Dunny G M. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanimoto K, An F Y, Clewell D B. Characterization of the traC determinant of the Enterococcus faecalis hemolysin-bacteriocin plasmid pAD1: binding of sex pheromone. J Bacteriol. 1993;175:5260–5264. doi: 10.1128/jb.175.16.5260-5264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimoto K, Clewell D B. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J Bacteriol. 1993;175:1008–1018. doi: 10.1128/jb.175.4.1008-1018.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita H, Clewell D B. A pAD1-encoded small RNA molecule, mD, negatively regulates Enterococcus faecalis pheromone response by enhancing transcription termination. J Bacteriol. 2000;182:1062–1073. doi: 10.1128/jb.182.4.1062-1073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver K E, Clewell D B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988;170:4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver K E, Clewell D B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: effects of host strain and traA, traB, and C region mutants on expression of an E region pheromone-inducible lacZ fusion. J Bacteriol. 1990;172:2633–2641. doi: 10.1128/jb.172.5.2633-2641.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidlich G, Wirth R, Galli D. Sex pheromone plasmid pAD1-encoded surface exclusion protein of Enterococcus faecalis. Mol Gen Genet. 1992;233:161–168. doi: 10.1007/BF00587575. [DOI] [PubMed] [Google Scholar]
- 37.Wirth R. The sex pheromone system of Enterococcus faecalis: more than “just” a plasmid collection mechanism? Eur J Biochem. 1994;222:235–246. doi: 10.1111/j.1432-1033.1994.tb18862.x. [DOI] [PubMed] [Google Scholar]
- 38.Wirth R, An F Y, Clewell D B. Highly efficient cloning system for Streptococcus faecalis: protoplast transformation, shuttle vectors, and applications. In: Ferretti J J, Curtiss III R, editors. Streptococcal genetics. Washington, D.C.: American Society for Microbiology; 1987. pp. 25–27. [Google Scholar]
- 39.Wu K, An F Y, Clewell D B. Enterococcus faecalis pheromone-responding plasmid pAD1 gives rise to an aggregation (clumping) response when cells are exposed to subinhibitory concentrations of chloramphenicol, erythromycin, or tetracycline. Plasmid. 1999;41:82–88. doi: 10.1006/plas.1998.1373. [DOI] [PubMed] [Google Scholar]