Abstract
Nucleoside diphosphate kinase (Ndk) is a ubiquitous enzyme which functions in balancing the nucleotide pool of the cell. We have recently reported that in addition to being intracellular in both mucoid and nonmucoid Pseudomonas aeruginosa, Ndk is also secreted into the extracellular environment by mucoid P. aeruginosa cells. This secreted Ndk has biochemical activity similar to the intracellular Ndk and is 16 kDa in size. To demonstrate that Ndk is indeed secreted and to localize the secretion motif, we constructed an ndk knockout mutant, which lacks both intracellular and extracellular forms of Ndk. In this study, we report the construction of deletion derivatives made from the carboxy-terminal region of Ndk. These deletion derivatives were introduced into the ndk::Cm knockout mutant and were examined for the intracellular and extracellular presence of Ndk. It was observed that the carboxy-terminal 8-amino-acid region is required for the secretion of Ndk into the extracellular region. This region has the sequence DXXX, where X is a predominantly hydrophobic residue. Such sequences represent a conserved motif in proteins secreted by the type I secretory pathway in gram-negative microorganisms. To investigate the significance of this motif in the secretion of Ndk, we constructed a fusion protein of Ndk and the blue fluorescent protein (BFP) as well as a fusion protein of mutated Ndk (whose DTEV motif has been changed to AAAA) and the BFP. The presence of extracellular Ndk was detected only in the ndk::Cm knockout mutant harboring the wild-type BFP-Ndk protein fusion. We could not detect the presence of extracellular Ndk in the ndk::Cm knockout mutant containing the mutated BFP-Ndk protein fusion. In addition, we have also used immunofluorescence microscopy to localize the wild-type and mutated BFP-Ndk proteins in the cell. The significance of these observations is discussed.
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen which causes infection primarily in patients with cystic fibrosis (CF) and in immunocompromised patients. Chronic lung infections with P. aeruginosa are the major cause of morbidity and mortality in CF patients (29). One of the striking properties of the P. aeruginosa strain encountered in the CF lung is its mucoid alginate-overproducing phenotype. The emergence of mucoid variants occurs at variable times upon the initial colonization with nonmucoid strains (9, 16) and is linked to the establishment of chronic infections in CF (22). Encapsulation of mucoid cells by alginate allows the cells to somehow evade the host immune system, but this process is not clearly understood (29, 31). Alginate biosynthesis requires large amounts of GTP, and one of the enzymes implicated in supplying GTP to the cell is nucleoside diphosphate kinase (Ndk). The role of Ndk in alginate synthesis has recently been reviewed (5).
Mucoid P. aeruginosa harbors two forms of Ndk, a 16-kDa form and a truncated 12-kDa form (32). This truncated 12-kDa form is generated by the proteolytic action of periplasmic elastase (20) and has been shown to allow predominant GTP synthesis through complex formation with other proteins (6). It has also been reported recently that mucoid strains of P. aeruginosa secrete a number of ATP-utilizing enzymes, including Ndk, into the extracellular environment (39). Similarly, in mammalian cells, Ndk has been shown to be present both as a membrane-bound enzyme (21) and as an ectoenzyme in the cell surface exposed to the outside medium (24). What is the implication of the presence of Ndk as an ectoenzyme? It has become apparent that mammalian cells extrude ATP into the extracellular fluid in order to carry out various functions that require ATP (2, 17). Many cellular functions that are mediated by external adenine nucleotides require the presence of specific receptors, called the P2 purinergic receptors (10). Among the P2 receptors, the P2Y and P2Z receptors are present on the surface of macrophages, which are the first line of defense against infection by bacterial pathogens. Macrophage-surface-associated P2Z receptors are known to be involved in macrophage cell death when they are activated in the presence of millimolar concentrations of external ATP (8, 23). Various ATP-utilizing ectoenzymes on the outer surface of mammalian cells convert the ATP to various adenine nucleotides, thus allowing activation of various purino receptors and maintaining a balance of adenine nucleotides in the external medium (40). It is interesting to note that many pathogens secrete ATP-utilizing enzymes similar to the mammalian ectoenzymes so as to alter the level of external ATP extruded by macrophages (38, 39). Such ATP-utilizing enzymes secreted by mucoid P. aeruginosa 8821 have been shown to cause cytotoxicity in macrophages through both P2Z-dependent and -independent pathways (39). Thus, the secretion of these enzymes by mucoid strain 8821, but not by nonmucoid strain PAO1, may be a factor conferring on mucoid P. aeruginosa cells protection from the host immune system (39).
The exact mechanism by which ATP-utilizing enzymes are secreted is not known. This report investigates the secretion of one of the ATP-utilizing enzymes, Ndk, and discusses the possibility of Ndk being secreted into the extracellular medium by virtue of the DXXX motif present in its carboxy-terminal region.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and P. aeruginosa strains were maintained in Luria-Bertani (LB) and Pseudomonas isolation agar (Difco) media, respectively. All strains were grown at 37°C in LB broth. For plasmid maintenance in E. coli, ampicillin was used at a concentration of 100 μg/ml, and 500 μg of carbenicillin per ml or 500 μg of chloramphenicol per ml was used for P. aeruginosa.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| P. aeruginosa strain 8821 ndk::Cm mutant | ndk gene deleted due to insertion of a chloramphenicol cassette | 39 |
| Plasmids | ||
| pSAK13 | ndk gene lacking the 3′ 63 bp cloned into pMMB67HE | This study |
| pSAK14 | ndk gene lacking the 3′ 42 bp cloned into pMMB67HE | This study |
| pSAK15 | ndk gene lacking the 3′ 24 bp cloned into pMMB67HE | This study |
| pSAK22 | Wild-type bfp-ndk fusion cloned into pMMB67HE | This study |
| pSAK30 | Mutated bfp-ndk fusion cloned into pMMB67HE | This study |
| pGWS95 | Whole ndk gene cloned into pMMB67HE | 33 |
Isolation of the cell-free supernatant.
A P. aeruginosa 8821 ndk knockout (ndk::Cm) mutant and complemented mutants (ndk::Cm/pGWS95, ndk::Cm/pSAK13, ndk::Cm/pSAK14, and ndk::Cm/pSAK15) were grown for 14 h in LB medium containing carbenicillin. The cells were removed by centrifugation at 5,000 × g for 10 min, and the supernatants obtained were filtered through 0.2-μm-pore-size filters and were used as the cell-free supernatants in subsequent experiments.
Isolation of the whole-cell extract and 45 to 70% ammonium sulfate fraction.
P. aeruginosa 8821 ndk::Cm mutant, complemented mutant (ndk::Cm/pGWS95), and ndk::Cm/pSAK15 cells were harvested from 14-h-old cultures by centrifugation at 5,000 × g for 10 min and were washed with cold sterile saline. The cells were suspended in buffer A (50 mM Tris-HCl, pH 7.5; 10 mM MgCl2) and were sonicated for four cycles of 30-s duration with a 15-s gap between pulses. The sonicated suspension was centrifuged at 10,000 × g for 10 min. The supernatant was used as the whole-cell extract. To isolate the 45 to 70% ammonium sulfate fraction, the whole-cell extract was first subjected to a 45% ammonium sulfate precipitation for 1 h. The pellet was removed by centrifugation, and ammonium sulfate was added to the supernatant to a final concentration of 70%. This suspension was stirred on ice for 1 h and then centrifuged. The pellet obtained was suspended in buffer A and dialyzed against the same buffer. This was used as the 45 to 70% fraction for subsequent analysis. Cytoplasmic and membrane fractions were isolated as reported earlier (20).
Construction of the 21-, 14-, and 8-amino-acid carboxy-terminal deletions of Ndk.
The 63-, 42-, and 24-bp deletion constructs from the 3′ end of the ndk gene (33) were designed by PCR by using the chromosomal DNA of wild-type P. aeruginosa 8821 as the template. A specific N-terminal primer with a PstI site was designed, and the sequence was TGCAGGACTAGCATAGGCCGCCC. The C-terminal primer sequences used were TCAATCCGCGAAGAAGTGACGAT, TCAGCGAGCGGCGGAAGCTTCGGA, and TCAACCGTGGACGGCGTCTC. The PCR conditions used were one cycle of 95°C for 5 min, 55°C for 5 min, and 72°C for 2 min followed by 30 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. These products were cloned into the pGEMTeasy vector (Promega), were excised by digestion with PstI and EcoRI, and were gel purified. The PstI/EcoRI-digested PCR fragments were then cloned into pMMB67HE to generate plasmids pSAK13, pSAK14, and pSAK15 (Table 1). These plasmids were then introduced into the P. aeruginosa 8821 ndk::Cm mutant by triparental matings (13).
Polyacrylamide gel electrophoresis and immunoblotting.
Approximately 5 μg of purified protein was separated by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis as described before (20) and was transferred onto a nitrocellulose membrane. The transfer was performed in a buffer containing Tris-glycine-methanol (25 mM Tris-HCl, pH 8.3; 192 mM glycine; 20% methanol) at 0.3 A for 1.5 h. The nitrocellulose membrane was first treated with TBST (10 mM Tris-HCl, pH 8.0; 50 mM NaCl; 0.05% Tween 20) containing 5% skim milk at room temperature for 1 h. The membrane was then incubated with anti-blue fluorescent protein (BFP) monoclonal antibody (Clontech) at a dilution of 1:4,000 in TBST at room temperature for 1 h. The blot was washed three times with TBST and was incubated with anti-mouse immunoglobulin G coupled to alkaline phosphatase at a dilution of 1:7,500 for 1 h. The blot was washed three times with TBST and was developed in a solution containing nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sigma).
Construction of the BFP-Ndk and mutated BFP-Ndk fusion.
The ndk gene was amplified by PCR by using plasmid pGWS95 as the template and NDKN (CTAGTCTAGAGCACTGCAACGC) and NDKC (CGGGAATTCTCAGCGAATGCGCTC) as primers. The first primer hybridizes to the 5′ end of ndk gene and contains an XbaI restriction site (underlined). The second primer contains the EcoRI restriction site (underlined) and hybridizes to the 3′ end of the ndk gene. The amplified DNA was cloned into pGEMTeasy vector (Promega) and was excised by digestion with XbaI and EcoRI and ligated into pUC19, which was digested with XbaI and EcoRI. This clone was used to create an in-frame gene fusion with bfp.
To construct plasmids that allow fusion of bfp to the 5′ end of the ndk gene, a fragment of bfp gene was amplified by PCR by using plasmid pBFP2 (Clontech) as the template and BFPN (ACATGCATGCGTACCGGTAGAAAAG) and BFPC (CTAGTCTAGATTTGTATAGTTCATC) as primers. The first primer hybridizes to the 5′ end of bfp and contains the SphI restriction site (underlined). The second primer hybridizes to the 3′ end of the bfp gene and contains an XbaI restriction site (underlined). The amplified DNA was cloned into the pGEMTeasy vector (Promega) and was excised out by digestion with SphI and XbaI. This fragment was then ligated into the above clone (containing the ndk gene) at the SphI and XbaI sites to generate the plasmid pSAK20. The bfp-ndk gene fusion was then excised from plasmid pSAK20 by digestion with SphI and EcoRI and was cloned into the Pseudomonas-compatible vector pMMB67HE to generate the plasmid pSAK22. This plasmid was then introduced into P. aeruginosa 8821 ndk::Cm mutant by triparental matings.
Growth and processing of cells for protein localization experiments.
To prepare cells for protein localization by immunofluorescence, overnight cultures of the strains were grown in LB medium containing carbenicillin at a concentration of 400 μg/ml. From the starting inoculum, the culture was inoculated into a flask containing fresh LB medium with 400 μg of carbenicillin per ml and 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG). The cells were harvested at various growth phases and were processed for immunofluorescence.
For immunofluorescence studies, the cells were fixed by a slight modification of the procedure described by Weiss et al. (35). A 1-ml sample of the cells was added directly to a microfuge tube containing 40 μl of 1 M Na3PO4 (pH 7.4) and 200 μl of 16% paraformaldehyde. Fixation was for 15 min at room temperature followed by 30 min on ice. Fixed cells were washed four times in 1 ml of phosphate-buffered saline (PBS) and were then resuspended in 500 μl of PBS. Cells (10 μl) were applied to 15-well multitest slides (ICN Biochemicals, Aurora, Ohio) that had been pretreated with poly-l-lysine (Sigma, St. Louis, Mo.). After a 10-min interval to allow cells to adsorb to the slide, the wells were washed three times with 10 μl of PBS for 5 min each wash. To each well, 10 μl of blocking reagent (2% bovine serum albumin in PBS) was added and the cells were incubated at room temperature for 20 min. To each well, 10 μl of primary antibody at a dilution of 1:50 (monoclonal antibody to BFP) was added and the cells were incubated at 4°C overnight in a humid chamber. The cells were then washed three times with PBS at room temperature for 5 min each wash. The cells were further incubated with the secondary antibody conjugated to Texas Red at a dilution of 1:100 at room temperature for 30 min. The wells were then washed three times with PBS at room temperature for 5 min each wash, after which they were mounted in Vectashield (Vector Labs).
Confocal microscopy.
Images were acquired by using a Carl Zeiss laser scanning confocal microscope LSM510 equipped with a 100× oil immersion objective. A single 568-nm beam from an argon-krypton laser was used for excitation. The emission from rhodamine isothiocyanate (RITC) was detected through an LP590 filter. At the same time, the differential interference contrast images were collected. The collected images were processed by using Adobe Photoshop version 4.0 and were printed on a codonic printer (NP-1600).
RESULTS
The carboxy-terminal 8 amino acids of Ndk are essential for its secretion into the extracellular medium.
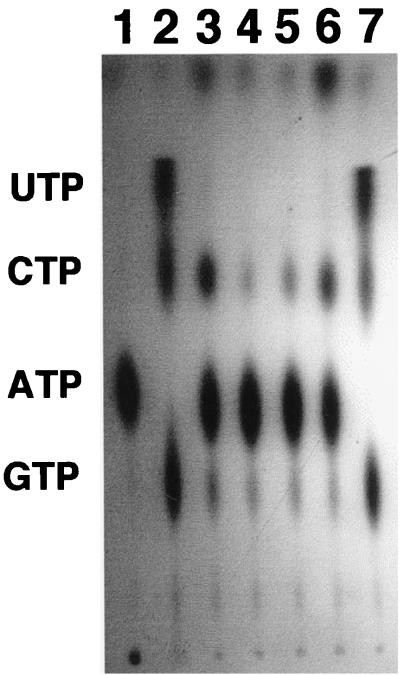
We have reported recently that mucoid cells of P. aeruginosa secrete the 16-kDa form of Ndk into the extracellular environment (39). The amino acid sequence of Ndk (33) lacks any N-terminal secretion signal known to be present in proteins secreted by the general secretory pathway (28). Consequently, it was of interest to determine the nature of any signal that might be required for Ndk secretion. In order to see if the carboxy-terminal region may be needed for secretion, we constructed three carboxy-terminal deletion derivatives of Ndk by PCR. These derivatives lack, respectively, 21, 14, and 8 amino acid residues from the carboxy terminus. These deletion constructs were cloned into the plasmid pMMB67HE and were termed pSAK13, pSAK14, and pSAK15, respectively (Table 1). All these constructs, as well as pGWS95, which harbors the complete ndk gene as part of plasmid pMMB67HE, were then introduced into the ndk::Cm knockout mutant. The strains harboring these constructs were then checked for their ability to secrete Ndk into the extracellular environment (Fig. 1). It should be noted that deletion of several carboxy-terminal amino acids does not affect Ndk activity, since the truncated 12-kDa Ndk, which has been postulated to have lost about 24 amino acids from the carboxy terminus, is fully functional in generating nucleoside triphosphates (NTPs) from nucleoside diphosphates (NDPs) (32). The results in Fig. 1 demonstrate that the cell-free supernatant (growth medium) of either the ndk::Cm mutant (lane 3) or the ndk::Cm mutant harboring plasmid pSAK13, pSAK14, or pSAK15 (lanes 4, 5, and 6) exhibits no Ndk activity with regard to production of NTPs from NDPs. The ndk::Cm mutant harboring pMMB67HE (vector control) showed similar results. The band comigrating with CTP is radioactive ADP produced by the combined activity of secreted adenylate kinase and 5′ nucleotidase (phosphatase) enzymes (39). Introduction of pGWS95 harboring the intact ndk gene, however, restores Ndk secretion (Fig. 1, lane 7). Thus, truncation by a minimum of eight carboxy-terminal amino acid residues completely inhibits Ndk secretion.
FIG. 1.
Lack of secretability of various truncated forms of Ndk missing their C-terminal 8, 14, or 21 amino acids. All reactions contained [γ-32P]ATP and a mixture of 100 μM each CDP, GDP, and UDP. Lane 1, [γ-32P]ATP control; lane 2, purified cytoplasmic Ndk; lane 3, cell-free supernatant (growth medium) of ndk::Cm mutant; lane 4, cell-free supernatant of ndk::Cm mutant harboring plasmid pSAK13; lane 5, cell-free supernatant of ndk::Cm mutant harboring plasmid pSAK14; lane 6, cell-free supernatant of ndk::Cm mutant harboring plasmid pSAK15; lane 7, cell-free supernatant of ndk::Cm mutant harboring plasmid pGWS95 (wild-type ndk gene). Note that the band migrating at the position of CTP in lanes 3, 4, 5, and 6 represents [32P]ADP formed by the combined action of secreted 5′ nucleotidase (phosphatase) and adenylate kinase from the ndk::Cm mutant on [γ-32P]ATP as reported earlier (39).
In order to confirm that our inability to detect extracellular Ndk was not due to instability of the protein, we expressed the mutant protein and examined its stability in vitro in the presence of the growth medium of the ndk::Cm mutant by Western blotting. No degradation of the enzyme was observed, suggesting that the absence of the mutant protein in the cell-free supernatant was not due to a lack of stability. We also checked intracellular Ndk activity in the ndk::Cm mutant harboring plasmid pSAK15 (lacking 8 amino acid residues) or pGWS95 (complete ndk gene). While the ndk::Cm mutant has no intracellular Ndk activity as measured in the partially purified cell extract (Fig. 2, lane 2), considerable Ndk activity is present in partially purified cell extracts of both ndk::Cm/pSAK15 and ndk::Cm/pGWS95 cells (Fig. 2, lanes 3 and 4).
FIG. 2.
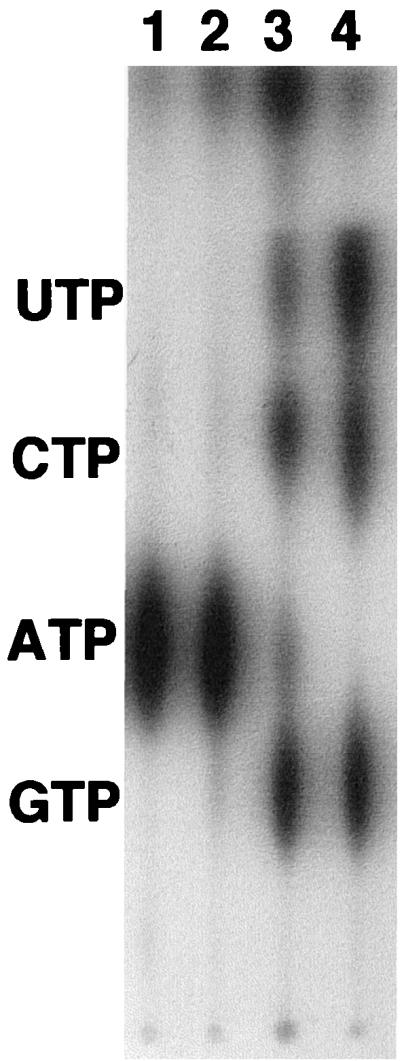
Detection of intracellular Ndk activity in the ndk::Cm mutant harboring the 8-amino-acid-truncated form of Ndk and the complete Ndk. All reactions contained [γ-32P]ATP and a mixture of 100 μM each CDP, GDP, and UDP. Lane 1, [γ-32P]ATP control; lane 2, 45 to 70% ammonium sulfate fraction of the cell extract of ndk::Cm mutant; lane 3, 45 to 70% ammonium sulfate fraction of the cell extract of ndk::Cm mutant expressing the truncated form of Ndk lacking its C-terminal 8 amino acids; lane 4, 45 to 70% ammonium sulfate fraction of the cell extract of ndk::Cm mutant expressing the complete Ndk protein. The 45 to 70% ammonium sulfate fraction was previously shown to harbor Ndk activity during Ndk purification (33). Equal amounts of proteins from the ammonium sulfate fractions were used in lanes 2, 3, and 4.
A 4-amino-acid motif located in the carboxy terminus of Ndk is required for its secretion.
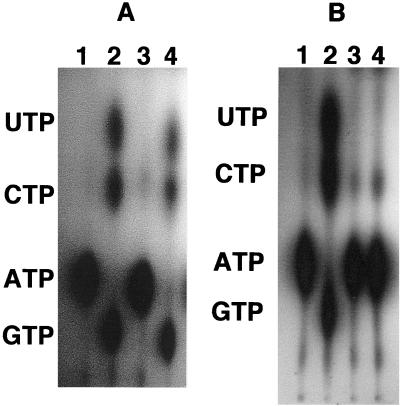
Since the terminal 8 amino acid residues were essential for Ndk secretion, we looked at this region to see if it had any characteristic motif present. Recently, motifs comprised of 4 amino acids (DXXX, where X is a predominantly hydrophobic amino acid) located in the carboxy terminus of proteins have been implicated in the secretability of proteins which are secreted by the type I machinery. Examples of these proteins are the Erwinia chrysanthemi metalloproteases PrtA, -B, and -C (15) and the P. aeruginosa alkaline protease (18). Interestingly, Ndk appears to harbor such a motif, which is DTEV, in its carboxy-terminal region (Fig. 3). In order to see if this motif was essential for secretion of Ndk, we constructed two fusions at the 5′ end of the ndk gene with the BFP gene. The first construct was the fusion of the wild-type ndk gene in frame with the bfp gene. The second construct was the fusion of the mutated ndk gene with the bfp gene. The mutated BFP-Ndk fusion protein had the DTEV motif replaced with AAAA. These fusions were then cloned into the plasmid pMMB67HE to generate plasmids pSAK22 (wild-type bfp-ndk) and pSAK30 (mutated bfp-ndk). These constructs were then introduced into the ndk::Cm mutant. These strains were assayed for the presence of extracellular Ndk by measuring NTP-synthesizing activity. The extracellular Ndk activity was detected in the ndk::Cm mutant expressing the wild-type BFP-Ndk fusion protein (Fig. 4A, lane 4), but this activity was not detected in the ndk::Cm mutant expressing the mutated BFP-Ndk fusion protein (Fig. 4B, lane 4). This indicates that the DTEV motif is important for secretion of Ndk. The possibility that the DTEV mutant protein was unstable because of defective folding was checked by incubating the mutant protein for 40 min with the cell-free supernatant. The enzyme was stable under such conditions.
FIG. 3.
Presence of the DXXX motif in the C-terminal region of Ndk. The complete amino acid sequence of Ndk (33) with two such putative motifs, shown in bold letters, is depicted.
FIG. 4.
(A) Ability of the wild-type BFP-Ndk protein to be secreted into the cell-free supernatant medium. All reactions contained [γ-32P]ATP and a mixture of 100 μM each CDP, GDP, and UDP. Lane 1, [γ-32P]ATP control; lane 2, purified cytoplasmic Ndk; lane 3, cell-free supernatant of ndk::Cm mutant; lane 4, cell-free supernatant of ndk::Cm mutant expressing the wild-type BFP-Ndk protein. (B) Inability of the C-terminally mutated BFP-Ndk protein to be secreted into the cell-free supernatant medium. All reactions contained [γ-32P]ATP and a mixture of 100 μM each CDP, GDP, and UDP. Lane 1, [γ-32P]ATP control; lane 2, purified cytoplasmic Ndk; lane 3, cell-free supernatant of ndk::Cm mutant; lane 4, cell-free supernatant of ndk::Cm mutant expressing the mutated BFP-Ndk protein.
Detection of intracellular wild-type and mutated BFP-Ndk fusion proteins by immunoblotting.
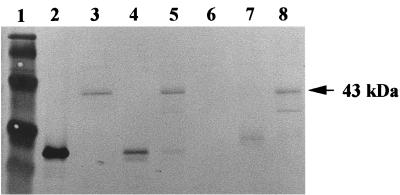
To ensure that the fusion proteins are indeed expressed and are stable, a Western blot analysis of the intracellular extracts and extracellular supernatants of ndk::Cm mutant/pSAK22 and ndk::Cm mutant/pSAK30 was performed. As seen in Fig. 5, lanes 3 and 5, a 43-kDa BFP-Ndk fusion protein was detected in the supernatant and membrane fractions of the ndk::Cm mutant expressing the wild-type BFP-Ndk fusion protein. In contrast, the mutated 43-kDa BFP-Ndk fusion protein was detected in the membrane fraction but not in the cell-free supernatant of the ndk::Cm mutant expressing the mutated BFP-Ndk fusion protein (Fig. 5, lanes 8 and 6), as was demonstrated earlier by the NTP-synthesizing assay of the mutated BFP-Ndk protein (Fig. 4B).
FIG. 5.
Immunoblotting to detect the intracellular wild-type BFP-Ndk and mutated BfP-Ndk proteins in the ndk::Cm mutant harboring the plasmids pSAK22 and pSAK30. To prepare cell extracts for protein detection by immunoblotting, overnight cultures of the respective strains were grown in LB medium containing carbenicillin at a concentration of 400 μg/ml. From this starting inoculum, 2% of the culture was inoculated into a flask containing fresh LB medium with carbenicillin (400 μg/ml). The cells were induced with 1 mM IPTG at an optical density at 600 nm of 0.6, and the cells were harvested 3 h after induction. Lane 1, low-molecular-weight markers; lane 2, purified GFP protein (the antibodies used for BFP and GFP are the same); lane 3, cell-free supernatant of ndk::Cm mutant/pSAK22; lane 4, cytoplasmic fraction of ndk::Cm mutant/pSAK22; lane 5, membrane fraction of ndk::Cm mutant/pSAK22; lane 6, cell-free supernatant of ndk::Cm mutant/pSAK30; lane 7, cytoplasmic fraction of ndk::Cm mutant/pSAK30; lane 8, membrane fraction of ndk::Cm mutant/pSAK30. About 5 μg of protein was loaded in each case.
Membrane localization of Ndk and secretion of Ndk are not interdependent.
The detection of the BFP-Ndk fusion protein in the membrane fraction (Fig. 5, lane 5) confirms our earlier observation that Ndk exists in P. aeruginosa strain 8830 in the membrane fractions, particularly at high cell density (32). Since our previous observations suggested that the secreted Ndk is 16 kDa in size (39), while the 12-kDa truncated form is obtained when the membrane-associated Ndk is cleaved by the periplasmic elastase (20), it was of interest to see if membrane association and secretion of Ndk are coupled. To address this question, we performed immunofluorescence microscopy with the ndk::Cm mutant strains expressing the wild-type and mutated BFP-Ndk fusion proteins taken from logarithmic and stationary phases of growth.
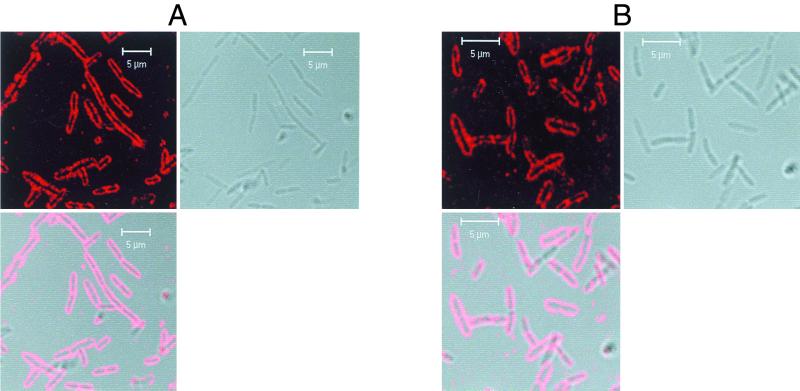
In both cases, the wild-type BFP-Ndk protein and the mutated BFP-Ndk protein were seen to be localized to the membrane (Fig. 6). As a control, we also carried out immunofluorescence microscopy with the ndk::Cm mutant expressing only the bfp gene. In this case, BFP was detected everywhere in the cell and was not localized to any specific region (data not shown). This suggests that secretion and membrane localization are independent events and that mutation of the carboxy-terminal DTEV motif inhibits secretion while having no effect on membrane localization of Ndk.
FIG. 6.
(A) Immunofluorescence microscopy at different growth stages of the ndk::Cm mutant expressing the wild-type BFP-Ndk protein. The cells were processed for immunofluorescence microscopy as described in Materials and Methods. Only mid-log- to stationary-phase cultures are shown. On the top left are the ndk::Cm mutant cells with wild-type BFP-Ndk protein that fluoresces red because of Texas Red-conjugated secondary antibody being bound to anti-BFP antibody cross-reacting with the membrane-associated BFP-Ndk fusion protein. On the top right are the pictures of the cells using differential interference contrast, and in the bottom left, the red fluorescence is superimposed on the cells. (B) Immunofluorescence microscopy at different growth stages of the ndk::Cm mutant expressing the mutated BFP-Ndk protein. The cells were processed for immunofluorescence microscopy as described in Materials and Methods and explained in the legend to Fig. 6A.
DISCUSSION
The secretion of Ndk by mucoid P. aeruginosa 8821 but not by the nonmucoid strain PAO1 (39) raises interesting questions. We previously reported the membrane localization as well as cytoplasmic location of Ndk in the mucoid strain 8821 (32). An important question, therefore, is whether membrane localization is a prerequisite for secretion. Since Ndk lacks a type II secretion signal at the N-terminal end, we looked for other secretion motifs. We noted that the carboxy terminus of Ndk has the DXXX motif present in proteins which are secreted by the type I secretory pathway present in gram-negative organisms. These proteins lack the N-terminal signal peptide and are usually transported by the signal-peptide-independent pathway and bypass the periplasm. The proteins secreted through this signal-peptide-independent pathway lack extensive regions of homology but share several common features. (i) Most of them possess a glycine-rich repeated motif close to their COOH terminus (36), but the role of these repeats in secretion is quite questionable. These repeats are not involved in secretion of small proteins but might be required for secretion of high-molecular-weight fusion proteins (27). (ii) They are secreted via similar membrane transporters composed of two inner membrane proteins and an outer membrane protein. (iii) There is a significant level of sequence homology between the protein components of these secretion systems, which are partially interchangeable (25). (iv) One of the inner membrane components has a conserved ATP-binding cassette (ABC) and is a member of a superfamily of transporters involved in the translocation of diverse substrates across membranes in both prokaryotes and eukaryotes (19). (v) In nearly all the cases studied so far, deletion analysis demonstrated that the secretion signal is located in the COOH-terminal part of these proteins (7, 14).
Similar to proteins secreted by a type I mechanism, deletion of the carboxy-terminal 8 amino acids of Ndk results in complete inhibition of secretion. Mutational alterations of these residues also result in inhibition of secretion. It appears that the carboxy-terminal 8 amino acids are essential for Ndk secretion. The upstream and downstream regions of the ndk gene do not show the presence of any genes coding for the ABC-type secretory components. It is possible that Ndk uses a heterologous secretion machinery. Secretion of extracellular proteins by heterologous secretion systems is a well-known phenomenon. The Prt system, composed of PrtD-PrtE-PrtF, which promotes E. chrysanthemi metalloprotease secretion (25), has been reported to similarly promote the secretion of Serratia marcescens PrtA and the P. aeruginosa alkaline protease (11, 18, 26). The secretion of Pseudomonas fluorescens lipase (34) is known to be facilitated by the P. aeruginosa alkaline protease secretion pathway AprDEF (12). Some hybrid exporters, which are composed of parts of the Lip, Prt, and Has systems, have also been reported to allow the secretion of secretory proteins, demonstrating that the secretion specificity depends largely on the ABC protein (1, 4).
The carboxy-terminal site-directed mutations of the ndk gene, while inhibiting secretion, do not affect membrane localization of the Ndk protein. Thus, the motif might be involved in allowing secretion of Ndk after its membrane localization. The 16-kDa size of the secreted Ndk suggests that the Ndk escapes cleavage by periplasmic elastase (20), presumably by bypassing the periplasmic space during secretion. Recently, there has been a report about dehalogenases like LinA and LinB of Sphingomonas paucimobilis UT26 being exported into the periplasm in a sec-independent mechanism (30). These proteins lack the N-terminal signal peptides present in proteins which are secreted into the periplasmic space. Thus, reports about proteins being secreted by novel secretion systems are being published, though the exact mechanism of secretion remains unknown. In addition, it has been shown that the flagellum export apparatus in Yersinia enterocolitica, consisting of only the basal body and hook, is capable of functioning as a secretion system for export of virulence-associated enzymes (37). Based on the results presented in this report, it is fair to speculate that Ndk has a carboxy-terminal motif that makes it secretion competent. It appears to resemble the motif present in proteins secreted by the type I secretory system, though it is not present at the extreme C-terminal end of the protein. Further investigation is needed to understand the mechanism of secretion of Ndk or the involvement of chaperones such as DnaK in the process (3).
ACKNOWLEDGMENTS
We thank Vinayak Kapatral for helpful discussions pertaining to this study. We also thank Dianah Jones-James for her help in typing the manuscript.
This work was supported by Public Health Service grant AI 16790-20 from the National Institutes of Health.
REFERENCES
- 1.Akatsuka H, Binet R, Kawai E, Wandersman C, Omori K. Lipase secretion by bacterial hybrid ATP-binding cassette exporters: molecular recognition of the LipBCD, PrtDEF, and HasDEF exporters. J Bacteriol. 1997;179:4754–4760. doi: 10.1128/jb.179.15.4754-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-AwQati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- 3.Barthel T K, Walker G C. Inferences concerning the ATPase properties of DnaK and other HSP70s are affected by the ADP kinase of copurifying nucleoside-diphosphate kinase. J Biol Chem. 1999;274:36670–36678. doi: 10.1074/jbc.274.51.36670. [DOI] [PubMed] [Google Scholar]
- 4.Binet R, Wandersman C. Protein secretion by hybrid bacterial ABC-transporters: specific functions of the membrane ATPase and the membrane fusion protein. EMBO J. 1995;14:2298–2306. doi: 10.1002/j.1460-2075.1995.tb07224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarty A M. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol. 1998;28:875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Chopade B A, Shankar S, Sundin G W, Mukhopadhyay S, Chakrabarty A M. Characterization of membrane-associated Pseudomonas aeruginosa Ras-like protein, Pra, a GTP-binding protein that forms complexes with truncated nucleoside diphosphate kinase and pyruvate kinase to modulate GTP synthesis. J Bacteriol. 1997;179:2181–2188. doi: 10.1128/jb.179.7.2181-2188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delepelaire P, Wandersman C. Protein secretion in gram-negative bacteria. The extracellular metalloprotease B from Erwinia chrysanthemi contains a C-terminal secretion signal analogous to that of Escherichia coli α-hemolysin. J Biol Chem. 1990;265:17118–17125. [PubMed] [Google Scholar]
- 8.DiVirgilio F. The P2Z purino receptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 9.Doggett R G, Harrison G M, Stillwell R N, Wallis E S. An atypical Pseudomonas aeruginosa associated with cystic fibrosis of the pancreas. J Pediatr. 1966;68:215–221. [Google Scholar]
- 10.Dubyak G R, El-Motassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 11.Duong F, Lazdunski A, Cami B, Murgier M. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene. 1992;121:47–54. doi: 10.1016/0378-1119(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 12.Duong F, Soscia C, Lazdunski A, Murgier M. The Pseudomonas fluorescens lipase has C-terminal secretion signal and is secreted by a three-component bacterial ABC-exporter system. Mol Microbiol. 1994;11:1117–1126. doi: 10.1111/j.1365-2958.1994.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 13.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghigo J-M, Wandersman C. Cloning, nucleotide sequence and characterization of the gene encoding the Erwinia chrysanthemi B374 PrtA metalloprotease: a third metalloprotease secreted via a C-terminal secretion signal. Mol Gen Genet. 1992;236:135–144. doi: 10.1007/BF00279652. [DOI] [PubMed] [Google Scholar]
- 15.Ghigo J-M, Wandersman C. A carboxy-terminal four-amino acid motif is required for the secretion of the metalloprotease PrtG through the Erwinia chrysanthemi protease secretion pathway. J Biol Chem. 1994;269:8979–8985. [PubMed] [Google Scholar]
- 16.Govan J R W, Nelson J. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 17.Guidotti G. ATP transport and ABC proteins. Chem Biol. 1996;3:703–706. doi: 10.1016/s1074-5521(96)90244-6. [DOI] [PubMed] [Google Scholar]
- 18.Guzzo J, Duong F, Wandersman C, Murgier M, Lazdunski A. The secretion genes of Pseudomonas aeruginosa alkaline protease are functionally related to those of Erwinia chrysanthemi proteases and Escherichia coli α-haemolysin. Mol Microbiol. 1991;5:447–453. doi: 10.1111/j.1365-2958.1991.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 19.Higgins C F. ABC transporters—from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 20.Kamath S, Kapatral V, Chakrabarty A M. Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol Microbiol. 1998;30:933–942. doi: 10.1046/j.1365-2958.1998.01121.x. [DOI] [PubMed] [Google Scholar]
- 21.Kimura N, Shimada N. Membrane-associated nucleoside diphosphate kinase from rat liver. J Biol Chem. 1988;263:4647–4653. [PubMed] [Google Scholar]
- 22.Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 23.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingan S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 24.Lazarowski E R, Homolya L, Boucher R C, Harden T K. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- 25.Letoffe S, Delepelaire P, Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli α-haemolysin. EMBO J. 1990;9:1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letoffe S, Delepelaire P, Wandersman C. Cloning and expression in Escherichia coli of the Serratia marcescens metalloprotease gene: secretion of the protease from E. coli in the presence of Erwinia chrysanthemi protease secretion functions. J Bacteriol. 1991;173:2160–2166. doi: 10.1128/jb.173.7.2160-2166.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letoffe S, Wandersman C. Secretion of CyaA-Prtb and HlyA-PrtB fusion proteins in Escherichia coli: involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J Bacteriol. 1992;174:4920–4927. doi: 10.1128/jb.174.15.4920-4927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lory S. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr Opin Microbiol. 1998;1:27–35. doi: 10.1016/s1369-5274(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 29.May T B, Shinaberger D, Maharaj R, Kato J, Chu L, DeVault J D, Roychoudhury S, Zielinski N A, Berry A, Rothmel R K, Misra T K, Chakrabarty A M. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections in cystic fibrosis patients. Clin Microbiol Rev. 1991;4:191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata Y, Futamura A, Miyauchi K, Takagi M. Two different types of dehalogenases, LinA and LinB, involved in gamma-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26 are localized in the periplasmic space without periplasmic processing. J Bacteriol. 1999;181:5409–5413. doi: 10.1128/jb.181.17.5409-5413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 32.Shankar S, Kamath S, Chakrabarty A M. Two forms of nucleoside diphosphate kinase of Pseudomonas aeruginosa 8830: altered specificity of nucleoside triphosphate synthesis by the cell membrane-associated form of the truncated enzyme. J Bacteriol. 1996;178:1777–1781. doi: 10.1128/jb.178.7.1777-1781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundin G W, Shankar S, Chugani S A, Chopade B, Kavanaugh-Black A, Chakrabarty A M. Nucleoside diphosphate kinase from Pseudomonas aeruginosa: characterization of the gene and its role in cellular growth and exopolysaccharide alginate synthesis. Mol Microbiol. 1996;20:965–979. doi: 10.1111/j.1365-2958.1996.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 34.Tan Y, Miller K J. Cloning, expression, and nucleotide sequence of a lipase gene from Pseudomonas fluorescens B52. Appl Environ Microbiol. 1992;58:1402–1407. doi: 10.1128/aem.58.4.1402-1407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss D S, Chen J C, Ghigo J M, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch R A. Pore forming cytolysins of gram negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 37.Young G M, Schmiel D H, Miller V L. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci USA. 1999;96:6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty A M. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 39.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, El-Azami El-Idrissi M, Prabhakar B S, Chakrabarty A M. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]