Abstract
BACKGROUND:
Multiple chronic conditions (MCC) are common among older patients with cancer; however, the exclusion of these patients from clinical trials has resulted in scarce knowledge concerning outcomes, resulting in variations in treatment. Superficial bladder cancer (SBC) disproportionately affects older adults, yet to the authors’ knowledge few studies to date have examined whether treatment improves long-term survival. In the current study, the authors evaluated the association between treatment of SBC and 10-year mortality in medically complex older adults.
METHODS:
The authors identified 1800 older (aged ≥60 years) patients with SBC (American Joint Committee on Cancer stage ≤I) from 2 community-based health systems who received treatment (bladder instillation and/or transurethral resection) or observation. Cox proportional hazards regression was performed adjusting for age, sex, race, health system, stage of disease/grade, and MCC (≥2 baseline chronic conditions). Propensity score analysis using stabilized inverse probability of treatment weights was used to compare 10-year mortality in the 2 treatment groups with adjustment for covariates.
RESULTS:
Overall, 1485 patients (82.5%) and 315 patients (17.5%) received treatment and observation, respectively. In unweighted multivariable analysis, treatment was associated with a 30% reduction in death (adjusted hazard ratio [HR], 0.70; 95% confidence interval [95% CI], 0.58-0.85 [P<.01]) and MCC with a 72% increase in death (adjusted HR, 1.72; 95% CI, 1.44-2.05 [P<.01]). Weighted analysis with adjustment (doubly robust) also demonstrated a survival benefit for treatment (adjusted HR, 0.66; 95% CI, 0.52-0.84 [P<.01]).
CONCLUSIONS:
The results of the current study demonstrated a clinically meaningful association between cancer treatment and survival benefit in older, medically complex patients with SBC, even after adjustment for medical complexity. These data provide a foundation for future work aimed at personalizing the treatment guidance of older patients with cancer with MCC.
Keywords: bladder cancer, geriatric oncology, geriatrics, multiple chronic conditions
INTRODUCTION
Greater than two-thirds of cancers are diagnosed in adults aged ≥60 years; this group also accounts for approximately 70% of all cancer deaths.1,2 A large majority of older adults with cancer have multiple chronic conditions (MCC), which we define as the presence of ≥2 chronic conditions lasting at least 1 year that require ongoing medical attention and/or limit activities of daily living.3-7 Among other things, MCC in patients with cancer strongly affect treatment decision making, health care use, and quality of life.8-12 Despite the rising prevalence of cancer among older adults, to the best of our knowledge, the majority of treatment and mortality data and clinical practice guidelines are derived from clinical trials that exclude medically complex older adults.13 This has resulted in a lack of information with which to guide decision making for treatment when older patients with cancer and physicians seek to balance quality of life against survival within the context of MCC. Several professional societies have expressed an urgent need to understand the outcomes of cancer treatment in older adults with MCC to facilitate patient-centered treatment decision making.14,15
In older adults, superficial bladder cancer (SBC) is an ideal condition with which to examine the interplay between MCC and cancer treatment, and may potentially inform this dynamic for other cancers. To our knowledge, bladder cancer has the highest median age at diagnosis (73 years) of all cancer sites, and its incidence is projected to increase by 54% by 2030.2,16,17 The increase will disproportionately affect older adults, as evidenced by Surveillance, Epidemiology, and End Results data trends that suggest that the incidence of the lowest stage disease (American Joint Committee on Cancer [AJCC] stage 0a/low grade) will rise fastest among adults aged ≥85 years.2,17 Among the oldest old patients with low-grade SBC, the risk of death from cancer is exceedingly low; however, treatment itself can negatively impact quality of life.18 Although rates of disease progression and cancer-specific death are low, rates of disease recurrence are high (30%-70%).19 With a median of 8 chronic conditions, patients with bladder cancer have high rates of medical complexity.20
Based on current guidelines, the standard treatment of SBC depends on stage of disease and grade.19 Transurethral resection of bladder tumor (TURBT), the initial treatment, is an ambulatory surgical procedure performed under general anesthesia, which carries risks for older, medically complex adults. Other complications may result from TURBT, such as hematuria and bladder perforation. A recent study from the National Surgical Quality Improvement Project demonstrated that frail older adults undergoing TURBT for large tumors had higher rates of postoperative complications.21 TURBT and intravesical treatments for bladder cancer lead to changes in urinary function, and for individuals with serious illness, urinary incontinence is considered a “state worse than death.”22,23
Although the treatment of SBC is associated with reduced rates of disease recurrence and possibly progression, a lack of data regarding the effect on mortality has led to significant treatment variation in older adults.24-26 The objective of the current study was to estimate the association between standard SBC treatment and 10-year mortality among older adults with adjustment for MCC. This association was estimated by combining propensity score analysis with proportional hazards regression in a cohort of older patients with SBC derived from 2 large, community-based health systems.
MATERIALS AND METHODS
Data/Study Population
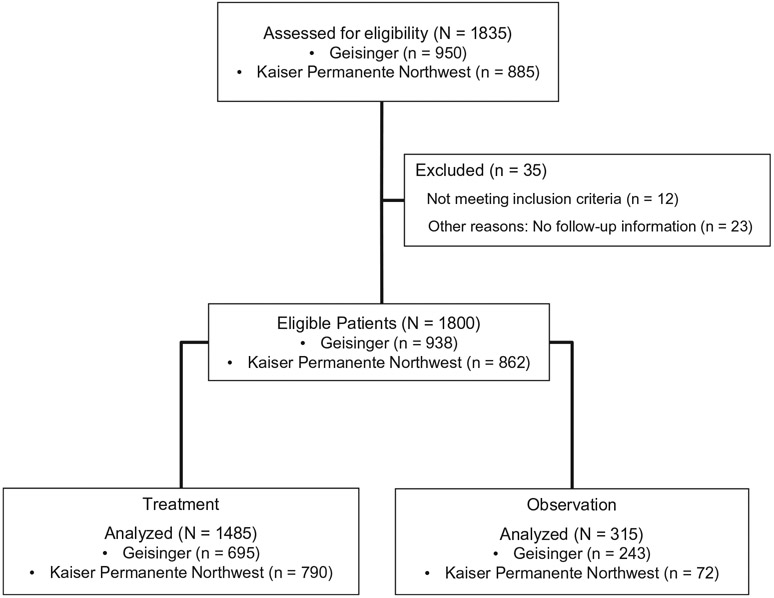
After establishing institutional review board approval and data use agreements at 2 community-based health systems, Geisinger and Kaiser Permanente Northwest (KPNW), we used tumor registry data to identify 1835 older adults (aged ≥60 years at the time of diagnosis) who were diagnosed with SBC (AJCC stage ≤I) between 2003 and 2014. Only individuals with urothelial carcinoma were considered, and 12 patients were excluded due to nonurothelial histology. Individuals with missing follow-up data also were excluded (23 individuals). The final study population included a total of 1800 patients: 938 from Geisinger and 862 from KPNW (Fig. 1).
Figure 1.
Consolidated Standards Of Reporting Trials (CONSORT) diagram describing cohort selection.
Definition of Treatment Groups
We dichotomized the study population into treatment and observation groups. Based on American Urological Association guidelines for the treatment of SBC, we defined SBC treatment as the presence of Current Procedural Terminology procedure codes for TURBT (codes 52204, 52224, 52235, and 52240) and/or intravesical instillation (codes 90586 and 51720) within 6 months of the date of diagnosis.19 Individuals without treatment-related Current Procedural Terminology codes were assigned to the observation group.
Other Covariates
In multivariable analyses, we controlled for age at diagnosis, sex, race/ethnicity, body mass index, smoking status, and health system (Geisinger or KPNW). Body mass index was determined using height and weight measurements from electronic health record data in the 24 months prior to the SBC diagnosis. In those patients with missing data, diagnosis codes for obesity were used. Individuals lacking height and weight measurements who had diagnosis codes for obesity were classified into the “nonobese” category. Because the prognosis and treatment of SBC are determined by both clinical stage and histologic grade, we developed a categorical measure combining AJCC stage of disease and histologic grade (0A/low grade, 0A/high grade, 0Is/high grade, and I/high grade) to describe disease severity.
MCC Measurement
From the Agency for Healthcare Research and Quality’s Chartbook on MCC and other literature, a total of 48 chronic conditions were selected for analysis.27,28 Based on our prior work, all baseline chronic conditions were identified using the Agency for Healthcare Research and Quality’s Chronic Condition Indicator and Clinical Classifications Software tools applied to all International Classification of Diseases, Ninth Edition codes from clinical encounters preceding the SBC diagnosis or within 30 days after diagnosis.20 For individuals diagnosed with SBC in the calendar year of 2003, we limited International Classification of Diseases, Ninth Edition codes to the year preceding diagnosis because of the limited availability of electronic health record data at each site.
When analyzing clinical encounters within the first 30 days after the diagnosis of SBC, we noted that patients were diagnosed with new chronic conditions at a high rate, most likely due to increased health system contact resulting from medical evaluation in preparation for cancer treatment. We considered these chronic conditions as preexisting conditions that were “unmasked” by the SBC diagnosis. For this reason, chronic conditions first identified within the 30-day period after the date of the SBC diagnosis were treated as baseline conditions.
MCC was defined as the presence of ≥2 chronic conditions at baseline and codified as a binary variable (yes/no).
Outcomes
The primary outcome variable was time to death within 10 years of diagnosis (ie, overall 10-year survival [OS]). For participants who survived but did not have a full 10 years of follow-up, this was calculated as time from the date of SBC diagnosis to the date of last follow-up listed in the tumor registry. Individuals surviving >10 years were censored at the end of the observation period. For decedents, the outcome was calculated as the time from diagnosis to the date of death.
Statistical Analysis
Demographic and baseline clinical characteristics were compared by treatment status, continuous variables were compared using the Student t test, and categorical variables were compared with a chi-square statistic. Frequencies and percentages of the type of chronic condition and the number of chronic conditions at baseline for all patients, by health system, by status, and by treatment were calculated (see Supporting Tables 1 and 2).
We used Kaplan-Meier curves to estimate survival time from diagnosis through 10 years among treatment groups. Cox proportional hazards was used to model time to death and was regressed on treatment status and adjusted for age, sex, health system, race, stage of disease/grade, and baseline MCC. Checks of functional form, linearity, and the assumption of proportional hazards confirmed acceptable compliance.
To balance the measurable confounders that could affect the estimate of treatment on time to death, we performed a propensity score analysis. Logistic regression of treatment calculated each person’s propensity for treatment based on age and the following chronic conditions: cataracts, dyspepsia, hearing loss, osteoarthritis, heart failure, hyperlipidemia, thyroid disorder, hypertension, depression, back problems, cancer, anxiety disorders, arrhythmias, chronic sinusitis, diverticular disease of the intestine, irritable bowel syndrome, obesity, and dementia/schizophrenia. These conditions were selected because they exhibited high correlation with treatment using chi-square analyses and a preselected cutoff P value of .05. Diabetes, peripheral vascular disease, and stroke/transient ischemic attack were included later after checking for balance using the initial inverse probability of treatment weights, a technique used to balance covariates in survival analysis. They then were included in the final logistic regression model and the initial inverse probability of treatment weights was recalculated (see Supporting Tables 1 and 2). Subsequently, we calculated stabilized inverse probability of treatment weights (SIPTW) to stabilize outlier propensity scores. The balance of covariates was verified across quintiles of the SIPTW.29 In doubly robust analysis, the SIPTW weighting of the multivariable model of time to death included adjustment for age, sex, health system, race, stage of disease/grade, and baseline MCC.30,31 A sensitivity analysis using E-values assessed how strong any unmeasured confounder would have to be to eliminate the significance of the association. We calculated E-values for the hazard ratio (HR) and confidence limit closest to one of the treatment effects.32
RESULTS
Of the total cohort, 1485 patients (82.5%) received treatment for SBC. Table 1 shows that the mean age of the cohort was 73.6 years, with slightly greater than three-quarters of patients being male (78.8%), and the majority were white (97.6%); these trends are very consistent with national demographics for patients with bladder cancer.33 The patients in the treatment group had higher rates of obesity, had a majority of current/former smokers (74.3%), and more frequently met criteria for MCC (70.4%). Fewer patients from Geisinger received treatment (77.1% of the observation group) compared with those at KPNW. When comparing treatment groups, there were no significant differences noted with regard to mean age, sex, or race, although mean age was found to be slightly higher in the observation group (74.1 years vs 73.5 years). Those patients receiving treatment had a higher severity of SBC, with 26.3% diagnosed with stage I/high-grade disease versus 19% in the observation group (P<.01). The median follow-up for the entire cohort was 6.6 years (95% confidence interval [95% CI], 6.26-7.07 years). At 10 years of follow-up, 932 patients in the treatment group (62.8%) and 143 patients in the observation group (45.4%) were still alive (P<.01).
TABLE 1.
Baseline Characteristics of Study Cohort Stratified by Treatment and Observation Groups
| Group |
||||
|---|---|---|---|---|
| All N = 1800 |
Observation N = 315 |
Treatment N = 1485 |
P a | |
| Mean age at diagnosis (SD), y | 73.6 (8.2) | 74.1 (8.6) | 73.5 (8.2) | .29 |
| Male sex, no. (%) | 1418 (78.8) | 248 (78.7) | 1170 (78.8) | .98 |
| White race/ethnicity, no. (%) | 1757 (97.6) | 312 (99.1) | 1445 (97.3) | .07 |
| Health system, no. (%) | ||||
| Geisinger | 938 (52.1) | 243 (77.1) | 695 (46.8) | <.01 |
| Kaiser Permanente Northwest | 862 (47.9) | 72 (22.9) | 790 (53.2) | |
| Obese BMI, no. (%) | 594 (33.0) | 80 (25.4) | 514 (34.6) | <.01 |
| Smoking status, no. (%) | ||||
| Current/former smoker | 1322 (73.4) | 219 (69.5) | 1103 (74.3) | <.01 |
| Never smoker | 435 (24.2) | 71 (22.5) | 364 (24.5) | |
| Never assessed/unknown if ever smoked | 43 (2.4) | 25 (7.9) | 18 (1.2) | |
| Multiple chronic conditions at baseline: yes, no. (%) | 1204 (66.9) | 159 (50.5) | 1045 (70.4) | <.01 |
| AJCC stage of disease/grade, no. (%) | ||||
| 0A/low grade | 921 (51.2) | 216 (68.6) | 705 (47.5) | <.01 |
| 0A/high grade | 318 (17.7) | 22 (7.0) | 296 (19.9) | |
| 0IS/high grade | 92 (5.1) | 16 (5.1) | 76 (5.1) | |
| I/high grade | 451 (25.1) | 60 (19.0) | 391 (26.3) | |
| Unknown | 18 (1.0) | 1 (0.32) | 17 (1.1) | |
| Alive status within 10 y of index date, no. (%) | 1075 (59.7) | 143 (45.4) | 932 (62.8) | <.01 |
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index; SD, standard deviation.
Comparing the treatment and observation groups.
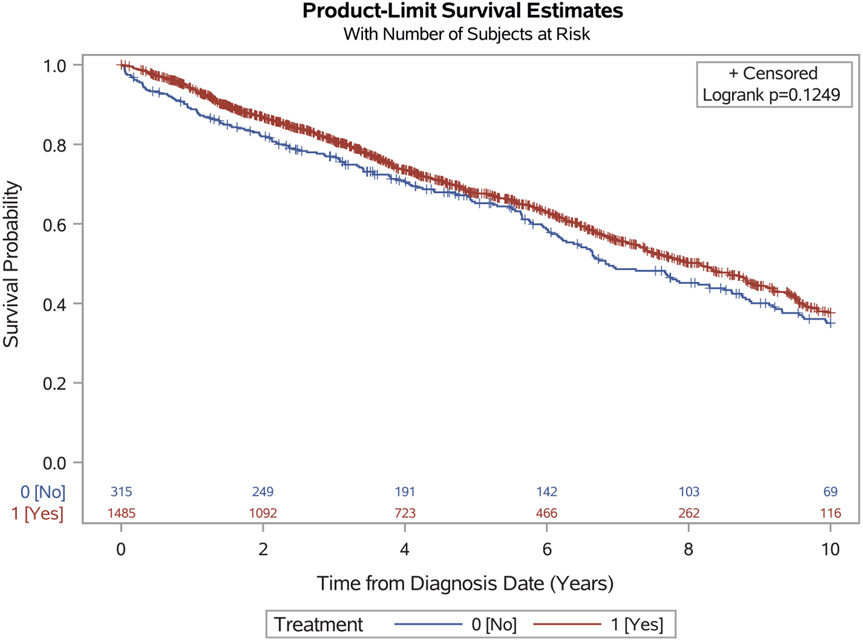
The median survival time for the entire cohort was 7.8 years (95% CI, 7.31-8.42 years). In an unadjusted comparison, although survival time was found to be higher for the treatment group (8.2 years) compared with the observation group (6.8 years), the difference was not statistically significant (log-rank P=.12) (Fig. 2). In the unweighted, multivariable Cox proportional hazards models, treatment was found to be associated with a 30% reduction in the hazard of death within 10 years (adjusted hazard ratio [HR], 0.70; 95% CI, 0.58-0.85 [P<.01]) (Table 2).
Figure 2.
Kaplan-Meier curve of 10-year survival stratified by treatment and observation groups.
TABLE 2.
Unweighted Multivariable Cox Proportional Hazards Model of the Association Between Treatment and 10-Year Mortalitya
| Variable | Reference | HR (95% CI) | P |
|---|---|---|---|
| Treatment 6 mo after diagnosis | Yes vs no (reference) | 0.70 (0.58-0.85) | <.01 |
| Multiple chronic conditions at baseline | Yes vs no (reference) | 1.72 (1.44-2.05) | <.01 |
| Age | 1.07 (1.06-1.08) | <.01 | |
| Sex | Female vs male (reference) | 0.73 (0.61-0.89) | <.01 |
| Health system | KPNW vs Geisinger (reference) | 1.11 (0.94-1.30) | .23 |
| Race/ethnicity | Nonwhite vs white (reference) | 1.35 (0.85-2.14) | .21 |
| AJCC stage of disease/grade | 0A/high vs 0A/low (reference) | 1.24 (0.99-1.53) | .05 |
| 0IS/high vs 0A/low (reference) | 1.01 (0.71-1.43) | .98 | |
| I/high vs 0A/low (reference) | 1.58 (1.33-1.89) | <.01 |
Abbreviations: 95% CI, 95% confidence interval; AJCC, American Joint Committee on Cancer; HR, hazard ratio; KPNW, Kaiser Permanente Northwest.
The analysis excluded 18 patients for whom information regarding stage of disease and grade was missing.
In quintiles of the pseudopopulation created by weighting of the cohort with SIPTW, propensity scores between the treatment and observation groups were comparable. In an unadjusted Cox proportional hazards model with SIPTW weighting to balance the sample, the causal association between treatment and 10-year mortality was a 23% reduction in the hazard of death (HR, 0.77; 95% CI, 0.61-0.98 [P=.03]). Demonstrating consistent results, doubly robust analysis (Table 3) showed a survival benefit for the treatment group (adjusted HR, 0.66; 95% CI, 0.52-0.84 [P<.01]). The hazard of death from MCC also was strong and highly statistically significant (adjusted HR, 1.76; 95% CI, 1.46-2.13 [P<.01]). Higher age and severity of disease (stage I/high grade) also were found to be significantly associated with an increased hazard of death. As expected, female sex was protective against death. Neither health system nor race/ethnicity were found to be significantly associated with death.
TABLE 3.
SIPTW Multivariable Cox Proportional Hazards Model of the Association Between Treatment and 10-Year Mortality in Doubly Robust Analysisa
| Variable | Reference | HR (95% CI) | P |
|---|---|---|---|
| Treatment 6 mo after diagnosis | Yes vs no (reference) | 0.66 (0.52-0.84) | <.01 |
| Multiple chronic conditions at baseline | Yes vs no (reference) | 1.76 (1.46-2.13) | <.01 |
| Age | 1.08 (1.07-1.09) | <.01 | |
| Sex | Female vs male (reference) | 0.77 (0.62-0.96) | .02 |
| Health system | KPNW vs Geisinger (reference) | 1.14 (0.95-1.37) | .16 |
| Race/ethnicity | Nonwhite vs white (reference) | 1.20 (0.84-1.73) | .31 |
| AJCC stage of disease/grade | 0A/high vs 0A/low (reference) | 1.13 (0.89-1.44) | .30 |
| 0IS/high vs 0A/low (reference) | 1.10 (0.78-1.54) | .60 | |
| I/high vs 0A/low (reference) | 1.68 (1.38-2.04) | <.01 |
Abbreviations: 95% CI, 95% confidence interval; AJCC, American Joint Committee on Cancer; HR, hazard ratio; KPNW, Kaiser Permanente Northwest; SIPTW, stabilized inverse probability of treatment weights.
The analysis excluded 18 patients for whom information regarding stage of disease and grade was missing.
Using the method of VanderWeele and Ding,32 the E-value for the relative risk equivalent to the inverted HR of the doubly robust treatment effect was 1.99, with a lower confidence limit of 1.54. This indicates that the protective effect of treatment on survival potentially could be removed by an unmeasured confounder with a relative risk of ≥1.54, indicating moderate robustness.
DISCUSSION
In this large cohort of older, medically complex adults with SBC from 2 community-based health systems, the objective was to determine whether there was a survival benefit associated with cancer treatment within the larger context of MCC. We found that cancer treatment was associated with a 30% reduction in the hazard of death. However, this survival benefit was countered by the strong positive association between baseline MCC and death. Although existing clinical trial data have demonstrated improvement in short-term outcomes such as disease recurrence after treatment of SBC, to the best of our knowledge the current study is one of the few to examine the association between SBC treatment and OS in an older cohort while adjusting for medical complexity by comprehensive accounting of baseline chronic conditions.
The majority of new cancers are diagnosed in older adults, most of whom have ≥2 chronic conditions, or MCC. As patients and physicians balance the risks and benefits of treatment within the larger context of MCC, helping medically complex older adults make comprehensive decisions regarding their cancer treatment is especially challenging. Because older adults with MCC are excluded from cancer clinical trials, there are a paucity of outcome data on which to base decision making. To facilitate cancer treatment decision making, an expert panel from the American Society of Clinical Oncology has expressed an urgent need for new tools with which to understand the cumulative effect of MCC on life expectancy to facilitate cancer treatment decision making.15
The presence of MCC impacts cancer treatment decision making in several important ways: 1) MCC and corresponding treatments may change cancer behavior; 2) treatments may interact with MCC; 3) the presence of MCC may suggest that treatment is too risky; 4) MCC may change treatment outcomes; and 5) MCC may change life expectancy.8 A prior hospital registry study of 19,268 patients with cancer found that chronic conditions were associated with reduced OS in older patients in a dose-dependent relationship independent of cancer stage.34 Similar to the current study, patients with urinary system cancers and severe comorbidity had nearly double the hazard of death compared with those without comorbidities (adjusted HR, 1.97; 95% CI, 1.49-2.62).
In addition, older patients with cancer prioritize health-related quality of life, and cancer treatment may affect a patient’s ability to engage with activities they enjoy by limiting physical and/or cognitive function.35 A telephone survey of 1457 older patients with cancer found that higher Charlson Comorbidity Index scores were associated with lower physical health-related quality of life.36 Because older, medically complex patients with cancer already may be experiencing a compromised quality of life, they tend to make treatment decisions that emphasize health-related quality of life over prolonged survival.37
The National Comprehensive Cancer Network guidelines for oncology in older adults provide a total of 5 recommendations for patients with bladder cancer.8 These sparse guidelines originated from only 6 publications, reflecting an overall lack of trial data and of practice-based evidence in the field. In addition, although there is evidence that SBC treatment prevents disease recurrence and possibly progression to advanced disease, to the best of our knowledge randomized clinical trials of treatment of SBC published to date have lacked long-term follow-up and have failed to examine OS as an endpoint.19,38 Given this dearth of evidence-based information, physicians and medically complex older adults lack information with which to guide treatment decisions, which may result in either overtreatment or undertreatment due to the treatment-risk paradox.39
For example, in a population-based study of 23,932 patients with SBC from the Surveillance, Epidemiology, and End Results–Medicare database, an age at diagnosis >80 years and a Charlson Comorbidity Index of ≥2 each were found to be associated with the decreased use of intravesical treatment, which is the standard of care to prevent recurrence of high-grade disease among patients with AJCC stage 0a, 0Is, or I disease. That same study also demonstrated an OS and bladder cancer–specific survival benefit for older adults who received treatment. Corroborating the results of the current study, the survival benefit was offset by an increasing number of chronic conditions.25
SBC may provide an opportunity to redesign care and decision making into a patient-centered approach that takes into account an individual’s MCC profile, goals, and preferences. For older adults with MCC and low-risk SBC, active surveillance is emerging as a new option.40 In a prospective observational study of 625 patients with SBC, approximately 62% of patients were able to remain on active surveillance for a median of 11.9 months, with none stopping active surveillance due to anxiety.41
The current study has several notable strengths. To the best of our knowledge, it is one of the few studies to date to examine the association between cancer treatment and mortality in medically complex older adults. Despite the high prevalence of MCC among older patients with SBC, to our knowledge few studies have examined the causal association between cancer treatment and OS in this population. A second strength is that the current study sample was drawn from 2 geographically disparate community-based health systems. The large sample size with long-term follow-up allowed for robust analyses to accomplish the study objective. The E-value indicated that our treatment effect has moderate robustness to unmeasured confounders.
The current study also must be interpreted within the context of certain limitations. We were unable to ascertain why the observation group opted against treatment. We speculate that the choice may have been influenced by variations in practice patterns among urologists, other patient factors (eg, transportation and socioeconomic factors), or insurance coverage. The catchment areas of both health systems cover large geographic areas, encompassing urban and rural populations. KPNW is a closed system, in contrast to Geisinger, which accepts a variety of insurance plans. The current study data did not capture patients with lapses in coverage or who obtained treatment outside of the system. Because we were unable to ascertain the cause of death, we were unable to assess cancer-specific survival. The current study cohort was predominantly white, which, although tracking closely with national demographics for bladder cancer, may limit its generalizability. Last, due to the retrospective nature of the current study, we were unable to assess functional status, which may impact OS. However, because MCC is closely related to functional status, we believe that adjustment for MCC is a reasonable surrogate. We accounted for baseline chronic conditions and, in future work, we will consider examining MCC trajectories and severity.
Conclusions
In the current study of older adults with SBC, many of whom had MCC, we found a clinically meaningful association between cancer treatment and survival benefit. This survival benefit was retained after adjustment for MCC and clinically important patient characteristics. These data provide a foundation for future work aimed at providing guidance to medically complex older adults with SBC as they weigh the risks and benefits of cancer treatment. Future studies will focus on developing patient-centered interventions to incorporate individualized MCC profiles and preferences into cancer treatment decisions.
Supplementary Material
FUNDING SUPPORT
Supported by the Health Care Systems Research Network (HCSRN)–Older Americans Independence Center (OAICs) AGING Initiative (grant R24AG045050) and the Yale Claude D. Pepper Older Americans Independence Center (grant P30AG021342; Principal Investigator: Gill).
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST DISCLOSURES
Tullika Garg, Amanda J. Young, and Terrence E. Murphy report grant support from the National Institute on Aging for work performed as part of the current study. Matthew E. Nielsen has acted as a paid consultant for the High Value Care Task Force for the American College of Physicians; received grants from the Agency for Healthcare Research and Quality, the National Institutes of Health, and the Patient-Centered Outcomes Research Institute; and acted as a paid member of the medical advisory board for Grand Rounds for work performed outside of the current study.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. HHS initiative on multiple chronic conditions. https://www.hhs.gov/ash/about-ash/multiple-chronic-conditions/about-mcc/index.html. Accessed August 4, 2018. [Google Scholar]
- 4.Hudson SV, Miller SM, Hemler J, et al. Cancer survivors and the patient-centered medical home. Transl Behav Med. 2012;2:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88:653–663. [DOI] [PubMed] [Google Scholar]
- 6.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Senior adult oncology (Version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed August 4, 2018. [Google Scholar]
- 9.Zulman DM, Asch SM, Martins SB, Kerr EA, Hoffman BB, Goldstein MK. Quality of care for patients with multiple chronic conditions: the role of comorbidity interrelatedness. J Gen Intern Med. 2014;29:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy GP Jr, Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic burden of chronic conditions among survivors of cancer in the United States. J Clin Oncol. 2017;35:2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ploeg J, Matthew-Maich N, Fraser K, et al. Managing multiple chronic conditions in the community: a Canadian qualitative study of the experiences of older adults, family caregivers and healthcare providers. BMC Geriatr. 2017;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rim SH, Guy GP Jr, Yabroff KR, McGraw KA, Ekwueme DU. The impact of chronic conditions on the economic burden of cancer survivorship: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer AJ, Freeman M, Giambarresi L. Introduction to the national urology research agenda: a roadmap for priorities in urological disease research. J Urol. 2010;184:823–824. [DOI] [PubMed] [Google Scholar]
- 15.Somerfield MR, Bohlke K, Browman GP, et al. Innovations in American Society of Clinical Oncology practice guideline development. J Clin Oncol. 2016;34:3213–3220. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Cancer Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Accessed August 4, 2018. [Google Scholar]
- 17.Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. 2014;120:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylvester RJ, van derMeijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–475; discussion 475-477. [DOI] [PubMed] [Google Scholar]
- 19.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. [DOI] [PubMed] [Google Scholar]
- 20.Garg T, Young AJ, Kost KA, et al. Burden of multiple chronic conditions among patients with urological cancer. J Urol. 2018;199:543–550. [DOI] [PubMed] [Google Scholar]
- 21.Suskind AM, Walter LC, Jin C, et al. Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int. 2016;117:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green TC, Gilbert M. States worse than death among hospitalized patients with serious illnesses OR Counterfeit Medications and Fentanyl. JAMA Intern Med. 2016;176:1555–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg T, Connors JN, Ladd IG, Bogaczyk TL, Larson SL. Defining priorities to improve patient experience in non–muscle invasive bladder cancer. Bladder Cancer. 2018;4:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelley MD, Court JB, Kynaston H, Wilt TJ, Fish RG, Mason M. Intravesical bacillus Calmette-Guerin in Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2000;(4):CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer BA, McBride RB, Hershman DL, et al. Adjuvant intravesical bacillus Calmette-Guerin therapy and survival among elderly patients with non–muscle-invasive bladder cancer. J Oncol Pract. 2013;9:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. [DOI] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality. Multiple Chronic Conditions Chartbook 1–52. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 28.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Schaubel DE. Contrasting treatment-specific survival using double-robust estimators. Stat Med. 2012;31:4255–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 34.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 35.Naik AD, Martin LA, Moye J, Karel MJ. Health values and treatment goals of older, multimorbid adults facing life-threatening illness. J Am Geriatr Soc. 2016;64:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisu M, Azuero A, Halilova KI, et al. Most impactful factors on the health-related quality of life of a geriatric population with cancer. Cancer. 2017;124:596–605. [DOI] [PubMed] [Google Scholar]
- 37.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. [DOI] [PubMed] [Google Scholar]
- 38.Network National Comprehensive Cancer. NCCN Bladder Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2018:1–96. [Google Scholar]
- 39.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291:1864–1870. [DOI] [PubMed] [Google Scholar]
- 40.Klaassen Z, Soloway MS. European Association of Urology and American Urological Association/Society of Urologic Oncology guidelines on risk categories for non–muscle-invasive bladder cancer may lead to overtreatment for low-grade Ta bladder tumors. Urology. 2017;105:14–17. [DOI] [PubMed] [Google Scholar]
- 41.Hurle R, Lazzeri M, Vanni E, et al. Active surveillance for low risk nonmuscle invasive bladder cancer: a confirmatory and resource consumption study from the BIAS Project. J Urol. 2018;199:401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.