Abstract
Purpose:
Our understanding of thyroid-associated ophthalmopathy (TAO, A.K.A Graves’ orbitopathy, thyroid eye disease) has advanced substantially since one of us (TJS) wrote the 2010 update on TAO, appearing in this journal.
Methods:
Pubmed was searched for relevant articles.
Results:
Recent insights have resulted from important studies conducted by many different laboratory groups around the World. A clearer understanding of autoimmune diseases in general and TAO specifically emerged from the use of improved research methodologies. Several key concepts have matured over the past decade. Among them, those arising from the refinement of mouse models of TAO, early-stage investigation into restoring immune tolerance in Graves’ disease, and a hard-won acknowledgement that the insulin-like growth factor I receptor (IGF-IR) might play a critical role in the development of TAO, stand out as important. The therapeutic inhibition of IGF-IR has blossomed into an effective and safe medical treatment. Teprotumumab, a β-arrestin biased agonist monoclonal antibody inhibitor of IGF-IR has been studied in two multicenter, double masked, placebo-controlled clinical trials demonstrated both effectiveness and a promising safety profile in moderate to severe, active TAO. Those studies led to the approval by the US FDA of teprotumumab, currently marketed as Tepezza for TAO. We have also learned far more about the putative role that CD34+ fibrocytes and their derivatives, CD34+ orbital fibroblasts, play in TAO.
Conclusion:
The past decade has been filled with substantial scientific advances that should provide the necessary springboard for continually accelerating discovery over the next 10 years and beyond.
Keywords: Graves’ ophthalmopathy, autoimmune, orbit, fibrocyte, insulin-like growth factor receptor
Introduction
The recently accelerating rate of discovering how thyroid–associated ophthalmopathy (TAO) or Graves’ orbitopathy (GO) as it is referred to in Europe, develops and how it might be effectively treated has been propelled by a persistent group of clinical investigators over the past decade.[1] As a consequence of improved research techniques, development of refined preclinical models and more successfully accessed and used patient-derived biological samples, we now better understand the pathogenesis of GO. These insights have resulted directly in the development of fact-based and scientifically plausible medical therapies. In this brief review of advances over the past decade, we attempt to describe which discoveries might directly impact the management of GO. We attempt to cover contributions made by multiple laboratory and clinical investigations including those emanated from our own laboratory at the University of Michigan. We first cover the basic and relevant translational science conducted in the recent past. We then discuss some iof the currently utilized therapeutic approaches. Finally, we briefly describe our view of the future treatments for GO. We acknowledge our selection biases but have attempted to remain balanced in our assessments. We too appreciate the limitations imposed by limited word count. This has resulted unfortunately in many important topics and findings remaining unmentioned. Ultimately, we seek to provide a fact-based review of how several key concepts in this field have matured recently.
Methods:
Pubmed was searched for relevant articles published in the most recent two decades using search terms such as: thyroid-associated ophthalmopathy, Graves’ disease, orbit, autoimmune, Graves’ orbitopathy, fibroblast, TSH receptor, IGF-I receptor, thyroid cytokines, biological therapies, thyroid
Results
Studies on the Genetic and Epigenetic Basis for TAO
Potential importance of genetic contributions to the development of Graves’ disease (GD) and GO has been emphasized for many years, a realization arising in part from recognizing ethnic and family clustering of the disease. Those patients with GD experience a higher prevalence of other autoimmune conditions in themselves and in their family members.[2–7] Genome-wide association studies (GWAS) have detected polymorphisms associated with GD susceptibility, including thyrotropin receptor (TSHR), CD25, HLA-DRβ-Arg74, PTPN22, ARID5B, CTLA4, CD40, and thyroglobulin (Tg). Some candidates are shared with other autoimmune thyroid diseases, especially Hashimoto’s thyroiditis. [8] Single-nucleotide polymorphisms (SNPs) of IL-1β and IL-12 family members, including the IL-23 receptor, have also been identified as candidates for TAO susceptibility. [9–11] Characterization of SNPs suggest that they may not lead to TAO susceptibility specifically, but rather may be linked to GD. [12]
Dysregulated gene expression in GO has been identified with microarray analysis. When compared with healthy orbital tissue, explants and cultured orbital preadipocytes from TAO orbits exhibit overexpression of adipogenesis related genes, the stimulatory Wnt/β-catenin signaling gene, secreted frizzled-related protein-1 (sFRP-1), and intermediate early genes such as CYR61 and EGR1.[13,14] Ezra et al. reported that Wnt signaling, IGF-1R, IGF-I, and the downstream transcription factors SGK-1 and c-JUN emerged as prominently expressed.[15] mRNA levels in formalin-fixed adipose tissues from patients with TAO, sarcoidosis, non-specific orbital inflammation, granulomatosis with polyangiitis, and healthy tissues [16] were compared. A greater similarity between inflammatory gene expression profiles was identified in TAO and healthy tissues as compared with those in the other diseases studied. Potentially important limitations of that study, and correctly acknowledged by the authors, included differing disease durations, lack of uniform thyroid function status, and varying steroid and radiotherapy treatment among the tissue donors.
It would appear that genetic variations thus far found in GD and GO account for only ~30% of disease risk. Exploration of epigenetic alterations in GD has been even more limited. Multiple examples of gene hyper-methylation have been reported, including sites within the first TSHR intron and CpG regions of signaling protein-encoding genes expressed by CD4+ and CD8+ T cells.[17] Increased global methylation of orbital fibroblasts from patients with GO (termed GD-OF) have also been observed when compared with control analogues.[18] A potential utility for post-transcriptional repressor microRNAs (miRNAs) and epigenetic modifications as biomarkers in autoimmune disease has been suggested.[19,20] Initial studies of GO orbital fibroblasts have revealed increased expression of pro-lipogenic miR-155 [21] and miR-130a, [22] as well as reduced regulatory microRNA-146a [21,23] and microRNA-27a/b [24] levels. Larger and more comprehensive studies will be necessary before a mechanistic link between variations in epigenetic loci and disease-defining specific protein expression in GO can be made. Thus, although studies have identified potential genetic and epigenetic susceptibility signatures in GD and GO, our understanding of how these might underpin disease pathogenesis remains uncertain.
Orbital fibroblasts as key players in TAO
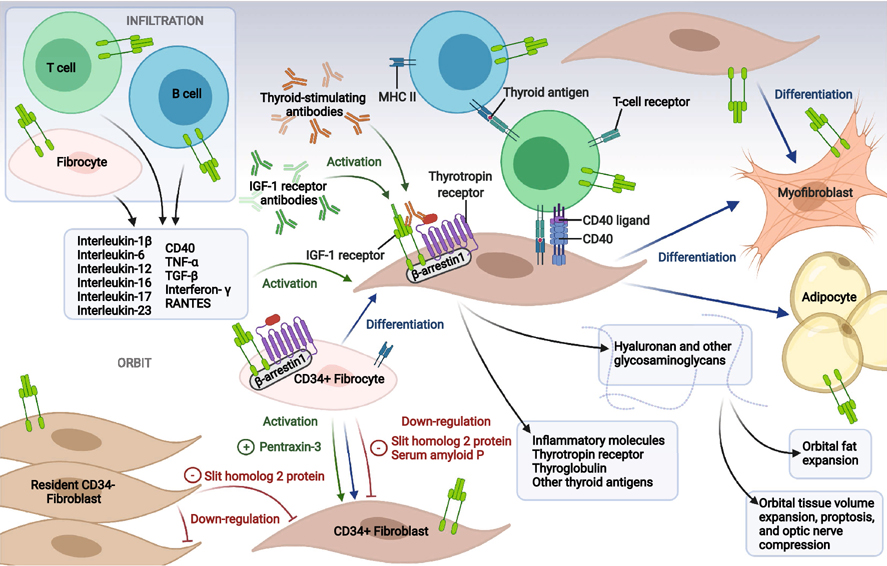
The characteristic pattern of inflammation, volume expansion, and remodeling in GO [25,26] has been widely postulated as resulting from the unique properties of orbital fibroblasts (Fig. 1). Among these unusual attributes are their remarkably robust responses to multiple inflammatory mediators. GD-OF express extremely low levels of secreted IL-1 receptor antagonist (sIL-1RA) when induced by inflammatory cytokines and TSH [27,28] as compared to their counterparts from other anatomic regions. The reduced capacity to generate this antagonist may underlie the exaggerated induction of genes including those encoding prostaglandin endoperoxide H synthase 2 (PGHS-2), and proinflammatory cytokines such as IL-6 and IL-1β.[29,30] Initially recognized determinants of GD-OF heterogeneity included divergent display of the glycoprotein, Thy-1 (CD90).[31] Thy1+ OFs can differentiate into myofibroblasts when treated with TGF-β. In contrast, Thy 1− GD-OF undergo adipogenesis in response to thiazolidinediones.[31,32] Douglas et al. later discovered presence of CD34+CXCR4+Col I+ GD-OF uniquely in the GO orbit. [33] These CD34+ OFs appear to derive from circulating CD34+ fibrocytes and can be detected in situ in GO orbital fat but are uniformly absent in healthy tissues. Fibrocytes express relatively high constitutive levels of MHC class II [34] and can induce antigen-specific T cell proliferation through their display of the co-stimulatory molecules CD80 and CD86, [35,36] and production of adhesion molecules including CD11a, CD54, and CD58.[35] Fibrocytes and CD34+ OF express several proteins historically considered unique to thyroid epithelial cells. These include TSHR, Tg, sodium iodide symporter (NIS), and thyroid peroxidase (TPO).[37–39] The expression of these proteins appears to depend on the actions of thymic autoimmune regulator (known as autoimmune polyendocrinopathy candidiasis ectodermal dystrophy protein, encoded by the AIRE gene). [38] Fibrocytes activated through the TSHR pathway produce high levels of IL-1β, IL-1RA, TNF-α, IL-6, IL-8, and MCP, in response to TSH and M22, an agonist human monoclonal anti-TSHR antibody (TSI). [26,33,40,41] Once in the GO orbit, fibrocytes masquerade as CD34+ OF where they coexist with CD34− OF. In distinction to circulating fibrocytes, GD-OF express substantially lower MHC class II and the thyroid proteins mentioned above.[37,38,42]
Figure 1.
Theoretical concept of TAO pathogenesis. CD34+ fibrocytes of the monocyte lineage infiltrate the orbit where they can differentiate into CD34+ fibroblasts, adipocytes, and myofibroblasts. CD34+ fibroblasts co-populate the orbit with CD34− fibroblasts in an admixture (GD-OF). CD34+ fibrocytes express thyroid autoantigens, including thyrotropin receptor (TSHR), thyroglobulin (Tg), thyroperoxidase (TPO), and sodium-iodide symporter (NIS), the expression of which depends on the autoimmune regulator (AIRE) protein. Fibrocytes can present antigens to T cells which in turn support IgG1 production in B cells. When activated, fibrocytes and orbital fibroblasts produce many cytokines, including interleukins 1β, 6, 8, 10, 12, 16, 23, TGF-β, tumor necrosis factor α, regulated on activation, normal T expressed and secreted (RANTES,) CXCL-12 and CD40 ligand (CD154). Many genes expressed by fibrocytes are also detected in CD34+ fibroblasts but at considerably lower levels. These lower levels result from actions of Slit2 which is expressed and released by CD34− orbital fibroblasts. Orbital fibroblasts display the tyrosine kinase, insulin-like growth factor-I receptor (IGF-IR), a therapeutic target for TAO. Cytokine-activated orbital fibroblasts express all three mammalian hyaluronan synthase isozymes and UDP glucose dehydrogenase (UGDH), with the majority of hyaluronan synthesis attributable to activities of HAS2 expressed by CD34− fibroblasts. Created with BioRender.com
Cytometric sorting into pure CD34+ OF and CD34− OF has revealed divergent expression of hyaluronan biosynthetic enzymes and cytokines in the two subsets.[43,44] For instance, IL-12p35 is preferentially expressed in CD34− OF while IL-23p19, TNF-α, and IL-6 levels are substantially higher in CD34+ OF.[43,44] Besides inflammatory changes, orbital congestion and tissue volume expansion are the consequence in part of accumulating hyaluronan. The three mammalian hyaluronan synthase isoenzymes and UDP-glucose dehydrogenase are expressed by GD-OF. Basal and bTSH-induced HAS1 expression localizes to CD34+ OF and fibrocytes, while HAS2 and UDP-glucose dehydrogenase are predominately expressed by CD34− OF.[44] HAS2 appears primarily responsible for HA synthesis in both cell subsets and the relatively low levels of HAS2 in CD34+ OF appears to represent the basis for substantially higher levels of hyaluronan synthesized in CD34− OF [44].
Several factors modulate fibrocyte differentiation into fibroblasts, myofibroblasts and adipocytes. Among these, the axonal guidance glycoprotein Slit2, acts through its binding to the second leucine-rich repeat region of roundabout 1 (ROBO1). [45,46] Slit2 inhibits fibrocyte differentiation and attenuates pulmonary fibrosis following bleomycin aspiration in mice.[47] In addition to Slit2, the decameric serum amyloid P (SAP) [48] glycoprotein inhibits fibrocyte differentiation.[49] Long pentraxin-3 (PTX-3) [50] shares a common carboxy-terminal “pentraxin signature” with SAP.[51–53] In contrast to SAP, PTX-3 potentiates fibrocyte differentiation in a FcgR-mediated manner.[54]
Because pure CD34+ OF express higher levels of several genes than do mixed population GD-OF comprising CD34+ OF and CD34− OF,[37] we postulated that CD34− OF express and release a soluble modulatory factor(s) that attenuates gene expression. We identified Slit2 as the factor that down-regulates CD34+ OF gene expression.[42] Specifically, rhSlit2 dramatically represses the expression of AIRE, TSHR, Tg, NIS and TPO as well as TNF-α, IL-6, and IL-23 induction by TSH in fibrocytes and CD34+ OF. [42–44] Slit2 knockdown enhances HAS1 expression in CD34− OF, while reducing both basal and TSH-dependent HAS2 expression.[44] Basal and TSH-dependent HAS2 expression is augmented in fibrocytes by rhSlit2 and this may represent the central mechanism underlying the enhancement in those cells of hyaluronan synthesis.[44] Similar to Slit2, PTX3 was found to be inducible by TSH,[55] actions that can be blunted by IGF-IR inhibition. Teprotumumab, a monoclonal antibody that inhibits IGF-IR activity, reduces IGF-IR and TSHR plasma membrane display on fibrocytes.[56] The complexities of cell-cell interactions present in the heterogeneous GD-OF population highlight the importance of better understanding the characteristics and behavior of each GD-OF subset. Slit2 may represent a heretofore unrecognized endogenous molecular determinant in modulating hyaluronan levels and immune responses within connective tissues. It might be therapeutically targeted in severe TAO. Slit2 is highly TSH-inducible in CD34− OF [43].
Characterization of orbit-infiltrating mononuclear cells in GO
The histopathology of GO is characterized by orbital infiltration with professional immune cells, including T and B lymphocytes, macrophages, monocytes, and mast cells.[57] A positive correlation between total orbital tissue lymphocytes and CAS severity has been suggested.[58] An examination of extraocular muscle biopsies suggests that pathogenic CD4+ and CD8+ T cells are among these infiltrating cells and appear to be recruited early in the disease process.[59] Among the cytokines relevant to these T cells are IL-4, IL-10 and IFN-γ. [60] With antigenic stimulation, naive CD4+ T cells develop into Th1 or Th2 cell phenotypes that can be identified within extraocular muscles in a disease-phase-dependent manner.[61,62] CD3+CD82RORγt+CCR6+ Th17 cells appear to play important roles in active GO.[63] Increased expression of receptors for both IL-23 and IL-1 while lower IL-21 receptor levels are found on Th17 cells. CD34+ OF appear to enhance the Th17 phenotype through the prostaglandin E2-EP2/EP4-cAMP signaling pathway.[64] Whether GD is biased towards the Th1, Th2, or Th17 lineage continues to be uncertain.[65,66] It remains possible that a shift in Th bias occurs, at least peripherally, as the duration of GD increases. Peripheral regulatory T cell (Treg) phenotypes have recently been examined in vitro in GO.[67] Anti-T lymphocyte globulin stimulated Tregs in TAO display higher CD25, FoxP3, and IL-7 receptor (CD127) levels than do cells from patients with GD but without clinically detectable GO. IGF-I stimulation of PMBCs from these patients enhances Treg abundance.[68]
Preclinical Models of GO
Effort toward developing rodent models of GO has spanned 20 years and has included a number of near-misses. While several strategies at producing antibody-driven hyperthyroid mice have yielded variably successful models,[69–72] far less convincing has been the development of mice exhibiting periocular manifestations resembling GO. Female BALB/c mice [69,70] immunized with TSHR A and IGF- 1Rα subunit-encoding plasmids, delivered with muscle electroporation, induced orbital pathology resembling some characteristics of human GO. Anti-TSHR and IGF-1R autoantibodies were detected in animals challenged with hTSHR A, suggesting an association between the immune reactivity toward the two receptors. [70] Zhang et al.[71] subsequently modified the injection/electroporation protocol to include intramuscular Ad-TSHR A over 34 weeks compared with injections delivered over 12 weeks.[69,70] These modifications yielded orbital fibrosis and adipogenesis detected by the seventh Ad-TSHR A dosing. Exophthalmia, conjunctival redness, eyelid hyperplasia, and orbital T cell infiltration were also observed in a subset of these mice. Hyperplastic changes could be identified in thyroid glands after four injections. A three-dimensional organoid culture model [73] utilizing a hanging drop plate [74] has shown advantages over monolayer cultures in recapitulating the fibrotic extracellular matrix remodeling observed in GO.[75,76] Studies exploring the pathogenesis of GO in spheroid culture systems may more closely resemble the conditions found in vivo.
The road to specific, fact-based therapies for GO
Rationale for Glucocorticoid use in GO
Glucocorticoids remain the most widely used pharmacologic agents in the management of active GO. The European Thyroid Association (ETA) recommends intravenous glucocorticoid pulse dosing as first line treatment for active, moderate-to-severe GO. They recommend a cumulative dosage of 4.5g IV methylprednisolone administered over 12 weeks. [77–80] These steroids exert their anti-inflammatory actions largely through gene transcriptional activation and repression. [80] Activation of glucocorticoid-responsive genes occurs through interactions between DNA-bound receptors (GR) and transcriptional co-activator molecules.[81–83]
The effectiveness of glucocorticoids in GO has been assessed in several studies. A single-masked, controlled study of 70 consecutive patients with untreated, active and severe GO involved administration of either oral prednisone (4 g.) or intravenous methylprednisolone (4.5 g.).[84] Intravenous steroid was associated with improved quality of life, milder adverse events, and less-frequent requirement for additional treatment than was oral drug. Bartalena et al.[85] examined three different cumulative dosages of intravenous methylprednisolone (2.25, 4.98, 7.47 g). Divided doses were administered weekly for 12 weeks in a multicenter, double-masked randomized control trial. Overall improvement of GO was greatest in those receiving the highest dosage (7.47 g) (52%) when compared to lower dosages (4.98 g group: 35%; P = 0.03; 2.25 g group: 28%; P = 0.01). Although palpebral aperture changed minimally in all three treatment groups, the clinically inconsequential reduction of proptosis and improvement of subjective diplopia did not differ among the treatment groups. High-dosage glucocorticoids may improve symptoms and benefit dysthyroid optic neuropathy,[86] but are inconsistent in reducing the severity of active GO, including proptosis reduction. Much of the clinical response may be mediated through their attenuation of PGHS-2 expression and PGE2 production. [29,87–89] Among the widely recognized side effects associated with high-dose glucocorticoids is the potential for severe hepatic toxicity. [90,91] Those effects appear to be dose-dependent, with highest risk in patients receiving >8 g iv methylprednisolone,[92] but severe toxicity has been reported with lower dosages. [93] Given their limited effectiveness, including their inability to meaningfully modify the long-term outcomes of GO, their use should be carefully monitored and weighed against their potential for causing side effects.
Mycophenolate/Azathioprine
Antimetabolites have been repurposed from their niche in the immunosuppressive regimen of renal transplantation [94] to limited use in GO. Mycophenolate mofetil (MMF) is the pro-drug for mycophenolic acid (MPA).[95] MPA preferentially inhibits type II inosine monophosphate dehydrogenase, the rate limiting-enzyme in de novo GTP nucleotide synthesis that is expressed in activated B and T lymphocytes.[96–98] As an anti-epileptic intervention, MMF inhibits the PI3K/AKT/mTOR pathway through pre-translational actions in rodent models as well as attenuating IL-2 and IL-1β.[99] A recent observer-masked randomized trial compared methylprednisolone (cumulative 4.5 g) as a single agent versus steroid combined with mycophenolate sodium (cumulative 120 g) for 24 weeks with a 12 week follow up.[100] The primary outcomes were response rates at 12 and 24 weeks of reduction in ≥ 2 CAS parameters (eyelid swelling, proptosis, lid width, diplopia grade) without simultaneous deterioration in any parameter, and sustained response at week 36. The benefit of adding mycophenolate to steroid was not significant compared to steroid alone in the intention-to-treat population at 12 weeks or in the relapse rate at 24 and 46 weeks. However, post-hoc analysis suggested that mycophenolate plus methylprednisolone increased the rate of response at 24 weeks compared to steroid alone. Actions of azathioprine resemble those of MMF with anti-proliferative effects on B and T lymphocytes mediated through de novo purine synthesis. [101] As a monotherapy, azathioprine failed to improve proptosis, visual acuity, or palpebral aperture over a 2-year period in moderate to severe GO. [102] Subsequent trials have been inadequately powered statistically to draw firm conclusions. The limited therapeutic value of many agents thus far studied for GO and their potentially serious side effects have driven the search for more targeted therapies.
Repurposing of biological agents to GO
Among the earliest biologics used to treat GO was rituximab (RTX), a chimeric monoclonal antibody consisting of variable light- and heavy-chain murine anti-CD20 regions linked to a human IgG-k region that prolongs the t1/2.[103,104] CD20 represents a phosphorylated cell surface protein expressed by both immature and mature B cells yet is undetectable on plasma cells.[105,106] RTX depletes B cells through induction of caspase-dependent apoptosis, antibody-dependent cellular toxicity, and complement-dependent cytotoxicity.[107,108] Developed more than 2 decades ago as therapy for relapse/refractory CD20+ B cell malignancies including non-Hodgkin’s lymphoma,[109] it has since found an important place in the therapeutic armamentarium of several autoimmune diseases, achieving U.S. FDA registration for rheumatoid arthritis (RA), Wegener’s granulomatosis, microscopic polyangiitis, and granulomatosis with polyangiitis.[110] The available data from three controlled studies of RTX therapy in GD or GO suggested that TRAb levels decline following B-cell depletion, but not to a greater extent than that following treatment with methimazole or glucocorticoids. [111–113] Coetaneous randomized trials published in 2015 were the first to meaningfully test the efficacy of RTX in active GO. [93,114] Both involved a 24-week treatment period. One U.S. study included 13 patients treated with two bimonthly infusions of RTX (1000 mg each) and 12 with two placebo saline infusions. The study was conducted in euthyroid patients with CAS scores ≥ 4 (using a 7-point scale). [114] No differences were observed in the two treatment arms of patients in achieving the primary outcome of CAS reduction ≥2 at 24 weeks. 5/6 cases of moderate-to-severe adverse events were experienced in the RTX arm, including dysthyroid optic neuropathy, infection, vasculitis, and gastrointestinal problems (tongue pain, abdominal pain, and diarrhea), during and following trial completion. The other trial, conducted in Italy, randomized (1:1) euthyroid patients with CAS scores ≥ 3/7 to receive either 7.5 g (cumulative dosage) iv methylprednisolone (n=16) or 1000 mg rituximab twice in a two-week interval (n=15), though this protocol was later amended to a single 500 mg RTX and 11 placebo infusions. [93] A significant reduction in CAS was noted in the RTX arm at week 16 (p<0.04); week 20 (p<0.02); and week 24 (p<0.006). Neither trial yielded evidence that RTX treatment was associated with reductions in proptosis or diplopia. A subsequent post-hoc analysis revealed that the disparities in results from these two trials may have resulted from differences in baseline CAS. Further, the Italian study comprised younger patients with shorter disease duration and lower TRAb levels (P=0.036). [115] Differences in smoking habits and use of radioactive iodine therapy could have also impacted the differing results. Anti-CD19 antibodies may represent a more promising therapy since RTX fails to affect CD20 negative plasmablasts. [116] Inebilizumab (MEDI-551), an affinity-optimized and afucosylated mAb targeting the extracellular loop of human CD19, [117] has shown greater efficacy than RTX in depleting blood and splenic B cells in huCD19/CD20 double transgenic mice at lower mAb dosage, independent of complement-dependent cytotoxicity. [118]
Elevated serum BAFF levels have been described in autoimmune thyroid disease, such as GD, and Hashimoto’s thyroiditis. [119,120] The anti-BAFF monoclonal antibody, belimumab is being compared currently with methylprednisolone for active GO (EudraCT number 2015-002127-26). Interim analysis has failed to identify significant effects of either treatment on proptosis or diplopia. [121]
The MHC class I-related neonatal Fc receptor (FcRn) does not act as a signaling receptor but rather extends the half-life of IgGs in the peripheral circulation. [122] IgG-FcRn interactions are pH-dependent, promoting immunoglobulin recycling. [123] The central role of IgG1 autoantibodies targeting TSHR in GD provides plausible rationale for FcRn inhibitors in GO. Efgartigimod, a monoclonal IgG1 Fc fragment, and rozanolixizumab, a humanized IgG4 anti-FcRn monoclonal antibody, have demonstrated tolerability and accelerated serum IgG and IgG subtype clearance in phase 2 trials in primary immune thrombocytopenia [124] and myasthenia gravis. [125,126] Fully human IMVT-1401 (formerly RVT-1401) underwent a phase 2b trial for safety, tolerability, and pharmacodynamics in GO patients (NCT03938545). Unmasked 12-week data from approximately 40 patients showed dose-dependent increases in total cholesterol and low-density lipoprotein levels in the IMVT-1401 cohort, suspending the study as of this writing. This adverse effect may reflect a previously unrecognized link between GO and the liver. Should they come into clinical use, FcRn-inhibitors will require monitoring for prolonged hypogammaglobulinemia and increased susceptibility to infection.
Statins as potential therapeutics in GO
The potential of elevated serum cholesterol levels as an independent risk factor for GO has been assessed recently. [127,128] In a cross-sectional study of 250 patients with GD, 133 manifesting GO, a significant correlation was detected between total/LDL-cholesterol and development of GO. [127] A retrospective study found increased serum cholesterol in those with GO, although disease severity and CAS did not correlate with serum cholesterol levels. [128] TSH-β expression in adipose tissue has been linked to total and LDL-cholesterol levels in euthyroid patients with GD, an association that may represent a risk factor for GO. [129] Classically used to manage hypercholesterolemia, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known commonly as statins, decrease cholesterol production through inhibition of the mevalonate pathway. [130] Statins exhibit anti-inflammatory actions thought to be mediated through cholesterol-independent mechanisms. In this regard, they suppress TLR4/MyD88/NF-ĸB signaling and TLR2 and TLR4 expression. [131] The protective effect of statins in recently diagnosed GD was examined retrospectively in patients enrolled in a U.S. managed-care network. [132] Multivariable Cox regression revealed that statin use exceeding 60 days over a one-year observation was associated with decreased risk of GO by 40% (HR=0.60 [CI 0.37–0.93]). These effects may be mediated through pleiotropic mechanisms. These findings were supported by Nilsson et al. [133] in a registry-based study of more than 30,000 Swedish patients with hyperthyroid GD. In that study, atorvastatin decreased GO risk, with an adjusted HR of 0.78 and 0.91 for men and women, respectively. These effects were marginally superior to other lipid-lowering agents (HR=0.87 [CI 0.75–1.01]) [133] This therapeutic approach with statins carries potentially severe side effects, including liver dysfunction. [134] With the development of agents targeting pathways specific to GO, it seems unlikely that lipid-lowering agents will find widely-embraced traction in treating this disease.
Repurposed anti-cytokine therapy in GO
A number of studies examining the potential roles of cytokines in the pathogenesis of GO have been conducted over the last decade. In general, these have concentrated on molecules implicated previously in other autoimmune diseases. The CD40/CD40 ligand (aka CD154)/receptor cognate is among these targets. Increased CD40 levels were found in GD-OF. [30] When treated with interferon γ (IFN-γ) or TSH, CD40 expression in GD-OFs is further enhanced. [30,135,136] CD40 belongs to the tumor necrosis α (TNFα) receptor superfamily. It undergoes trimerization after binding to CD154, recruits TNFα receptor associated factors (TRAFS) to the cytoplasmic domain and interfaces with signal transduction pathways. [137] Constitutively expressed NF-κB1 p65 and the up-regulation of NF-κB2 p52 in GD thyrocyte cultures following CD40 activation implicates both canonical and non-canonical NF-κB activities in GD. [138] CD40-CD154 ligation results in transcriptional activation of the PGHS-2 gene, hyaluronan synthesis and production of PGE2and proinflammatory cytokines.. [30,87,139,140] These include ICAM-1, IL-6, IL-8, and macrophage chemoattractant protein (MCP-1). Confocal microscopy recently revealed TSHR-CD40 co-localization on the surface of fibrocytes. [136] In autoimmune diseases such as systemic lupus erythematosus and RA, aberrant CD40-CD154 interactions may result in abnormal humoral- and cell-mediated immunity. [141] The CD40 pathway has consequently become an attractive therapeutic target. Unfortunately, early-stage clinical trials of a monoclonal antibody targeting CD40L have revealed platelet-targeted thromboembolic adverse events. [142,143] The human anti-CD40 monoclonal IgG1 blocking antibody, iscalimab (CFZ533), can suppress T cell-dependent Ab responses and abrogated germinal center formation in cynomolgus monkeys. [144] CFZ533 contains a N297A silencing mutation that disables the mAb from binding Fcγ receptors, impeding antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). [145] An open-label phase II trial of CFZ533 in which a 12-week treatment period was conducted in 15 hyperthyroid patients receiving the drug. [146] This was followed by a 24 week follow up period. The antibody bound CD40 displayed on peripheral blood B cells for up to 20 weeks. Its administration was associated with euthyroid status in 7/15 patients (47%). TSHR-Ab levels normalized in 4 (27%) patients at week 20. 4/7 responders relapsed over the follow-up period, the majority of whom required rescue antithyroid drugs. Placebo-controlled studies will be necessary to more fully evaluate whether targeting the CD40–CD40L pathway in GD and GO might be effective and safe. Anti TNF-α biologics have shown effectiveness in RA. [147,148] Pilot studies in GO have been inadequately powered. [149–151]
IL-6 is pleiotropic cytokine that promotes Th17 development in naïve T cells in conjunction with TGF-β and IL-23 [152]. It can inhibit TGF-β-induced Treg differentiation. [153] IL-6 classically activates early acute response genes through the STAT-3 pathway. [152] Serum IL-6 levels are elevated in GD [154,155] and particularly high in GO. [156] Both TSH and the TSIs can induce IL-6 expression in GD-OFs and fibrocytes, effects mediated through gene promoter activation and enhanced mRNA stability.[40] IL-6 production in fibrocytes is up-regulated in a cAMP independent mechanism in fibrocytes. Instead, it is mediated through TSH activation of AKT, PKC, and PDK1 pathways.[40] PKCβII, a PKC isozyme associated with lymphoproliferative disorders, [157,158] becomes phosphorylated in fibrocytes following TSH stimulation. Dominant PKC isoenzyme expression appears to switch from PKCβII to PKCμ isoenzyme as fibrocytes differentiate into GD-OFs. CD34− fibroblasts may impose this shift in expression which reverts to that resembling fibrocytes following CD34+ OF isolation. [40,157,158] Tocilizumab (TCZ) is an inhibitory recombinant, humanized IL-6 receptor monoclonal antibody that binds both soluble and membrane-bound receptors. The IL-6 receptor has been implicated in multiple autoimmune diseases including RA. [159,160] In an uncontrolled study of 18 steroid-resistant patients with active GO, CAS improved in all subjects, proptosis decreased in 72%, and ocular motility improved in 83.3%. [161] No relapses were observed at the end of a 9-month follow-up period. A subsequent, randomized, placebo-controlled trial conducted by these same investigators in Spain included 32 patients with glucocorticoid-resistant GO (defined as CAS ≥ 4 on a 10 point scale) for a mean duration of one year. [162] TCZ was administered intravenously over 12 weeks, followed by a 28 week follow up period. 93.3% patients receiving TCZ achieved the primary outcome of improved CAS ≥ 2 points at week 16 compared to 58.8% of those receiving placebo (P = 0.04). The median baseline CAS in both treatment arms was 5, with patients receiving TCZ achieving CAS < 3 at week 16 (P=0.005). Those receiving TCZ experienced a greater reduction in proptosis (median of −1.5 mm) compared with placebo (0.0 mm, P = 0.01) but proptosis reduction was minimal and transient. The authors correctly considered their study limited by the relatively small cohort studied, prolonged duration of GO prior to trial enrollment, and a potentially insufficient treatment period. [162] A retrospective study of 54 steroid-resistant patients with GO duration <24 months [163] revealed significant differences from baseline at weeks 4, 8, 12, 16 and follow-up (mean= 22 month) in CAS and TRAb levels (p<0.001 at all- time points). 37/50 (74%) patients achieved an absolute CAS of 0 or 1, 42/54 (78%) experienced proptosis reduction ≥ 2 mm, 33/44 (75%) had eyelid retraction reduction ≥ 2 mm, and diplopia improved in 19/28 (68%, p<0.001 for all variables). 7.4% of patients experienced disease relapses. Twenty-six patients experienced mild to moderate adverse events. Future prospective, placebo-controlled studies might help clarify the potential benefit of IL-6 receptor inhibition in glucocorticoid resistant TAO.
TSHR as a therapeutic target
Loss of immune tolerance to TSHR and generation of TSI are central events underlying development of hyperthyroidism in GD. [76] Among the pieces of circumstantial evidence that these anti-TSHR antibodies also play roles in GO are the closely associated onset of both thyroid dysfunction and ocular manifestations. [1,164] TSHR is a member of the rhodopsin-like G protein coupled receptor family, all members of which possess seven plasma membrane spanning regions. [165] TSHR undergoes intramolecular proteolytic cleavage at the hinge region, where ~50 amino acids are removed, leaving two components; an A-subunit containing a leucine-rich domain and a B-subunit consisting of the transmembrane domain. These subunits are linked by two disulfide bonds. [166,167] Cleavage occurs through actions of a metalloprotease and multimerization of the shed A-subunit, resulting in enhancing induction and affinity maturation of pathogenic anti-TSHR antibodies. [166,168] Unique from other members of this glycoprotein hormone receptor family (including follicle-stimulating receptor and luteinizing receptor), TSHR is characterized by its relatively high constitutive (basal) activity. [169] TSH and GD-IgG actions in GD-OF and fibrocytes are mediated through surface TSHR and result in the induction of several cytokines, including IL-1 receptor antagonist, IL-6, IL-8, IL-12 and IL-23. [27,40,56,170] In recent years, interest in treatments for GD and GO have focused on interrupting TSHR signaling, either with monoclonal antibodies or allosteric small-molecule inhibitory ligands. Anti-TSHR monoclonal antibodies with antagonist (K1–70), agonist (M22 and K1–18), and dual antagonist and inverse agonist (5C9) activities have been developed and are at various stages of testing. [171–175] K1–70 was derived from the serum of a hypothyroid patient demonstrating both TSHR-cAMP stimulating and blocking activity in TSHR-transfected CHO cells. [171,172,176] It binds the concave surface of the leucine-rich repeat domain of TSHR. [177] K1–70 reduces total and free serum thyroxine (T4) in rats [176] and is currently being evaluated in a phase I clinical trial for GD hyperthyroidism (NCT02904330). 5C9 inhibits both constitutive and agonist-driven cAMP production in TSHR-transfected CHO cells. [175] Further, it blocks activities of gain-of-function TSHR mutants, including Ser281Ile, Ile568Thr, and Ala623Ile. [175] In contrast to activating anti-TSHR antibodies that bind the extracellular region of the receptor, small molecule antagonists bind the cleft between helices and extracellular loops of the transmembrane domain. In so doing, they can modulate ectodomain-mutated receptor activity at micromolar concentrations, both in vivo and in cultured cells. [178–181] ANTAG3 decreases endogenous TSH-driven serum FT4, as well as the induction by thyroid releasing hormone of NIS and TPO expression in an in vivo murine model. [181] This inhibition lacks specificity for TSHR as cross-reactivity with the luteinizing and follicular stimulating hormone receptors was detected. Another TSHR antagonist, s37a, was identified using high-throughput screening. [178] This molecule exhibits TSHR-selectivity and awaits further study.
Targeting IGF-IR pathway may shift the treatment paradigm for GO
The absence of detectable TSIs in a small subset of patients with GO [182,183] has suggested the possible involvement of another autoantigen target in the development of GO. Thus, we began our examination of other cell-surface receptors that might play roles in the disease and therefore might represent therapeutic targets for GD and GO. [184,185] Besides its behavior as a prototypical tyrosine kinase receptor, IGF-IR can signal through non-canonical mechanisms as a partner of the G-protein-coupled receptor (GCPR) family. [186–188] Although β-arrestins are recognized as adaptors of GCPR endocytosis and transducers of signaling from seven-transmembrane receptors, [189] they can also bind to agonist-occupied IGF-IR and facilitate clathrin-mediated receptor internalization. [190] Girnita et al. [191,192] advanced the important concept that β-arrestin 1 represents a cofactor through its association with the oncoprotein E3 ubiquitin ligase Mdm2161–400. It can facilitate IGF-IR ubiquitination and degradation. In addition to direct receptor desensitization, β-arrestin 1 initiates MAPK/ERK activation, which can occur in the absence of detectable IGF-IR tyrosine kinase activity. [192] These effects mirrored those of an IGF-IR inhibiting therapeutic antibody that incompletely inhibited the IGF-IR complex, instead forming a partially active receptor which induced β-arrestin-dependent phosphorylated ERK signaling. [193] Deletion of the β-arrestin 1 binding site on the IGF-IR C-terminal tail (Δ1245) in fibroblasts rendered those cells resistant to either anti-IGF-IR antibody- or IGF-I induced degradation, thus demonstrating biased signaling.[193]
Anti-IGFIR autoantibodies were insinuated in the pathogenesis of GO in 1993 by Weightman et al, [194] when these authors demonstrated that GD-IgG could displace radiolabeled IGF-I from its high affinity surface binding sites on fibroblasts. The identity of those binding sites was subsequently established by Pritchard et al. [184,185] as IGF-IR and not IGF binding proteins. Further, GD-IgG could mimic rhIGF-I in inducing expression of IL-16, a CD4-specific chemoattractant, and RANTES, a C-C chemokine, in GD-OF. Those effects are mediated through activation of the Akt/mTOR/FRAP/p70s6K pathway. The effects of GD-IgG on IL-16 expression were attenuated with rapamycin, a macrolide specifically targeting mTOR protein kinase. [184] Further, the murine monoclonal anti-IGF-IR inhibitory antibody (1H7) [185] and transfection of GD-OF with a dominant-negative mutant IGF-IR (486/STOP) [195] could both attenuate induction of IL-16 and RANTES expression. Subsequent studies revealed that induction by GD-IgG of hyaluronan accumulation in GD-OF could be attenuated by 1H7. [196] IGF-I could mimic the effects of GD-IgG, but rhTSH failed to do so. Using multi-parameter flow cytometry, IGF-1R was found to be overexpressed in GD-OF, [185] and by peripheral lymphocytes [197,198] when compared to cells from healthy donors. Later studies from Tsui et al. [199] demonstrated that GD-IgG can activate Erk p42/p44 in GD-OF, as can IGF-I, effects that are attenuated by 1H7. In contrast, rhTSH failed to activate Erk in vitro. Orbital fibroblasts from healthy donors failed to exhibit an Erk response to either GD-IgG or IGF-I. Thus, it appears that the phenotype of GD-OF differs from that of orbital fibroblasts from healthy donors.
Subsequent studies performed in other laboratories have examined the ability of GD-IgG to provoke tyrosine phosphorylation in IGF-IR, an issue left unexplored by Pritchard et al. [184,185,199] Those later studies by in large have been unsuccessful in demonstrating that GD-IgG can activate IGF-IR tyrosine kinase. These findings have sparked debate as to whether increased levels of anti-IGF-IR antibodies are relevant to GO. The reports of Varewijck et al. [200] and Minich et al. [201] described studies using transfected HEK 293 cells to detect anti-IGF-IR antibodies but arrived at disparate results. Using an immunoprecipitation-based assay, Minich et al. [201] found similar anti-IGF-IR autoantibody levels in sera from healthy and GO patients. The authors treated HepG2 and MCF-7 cells with GD-IgG alone or with IGF-I. The Igs failed to stimulate IGF-IR autophosphorylation in Hep G2 and attenuated IGF-I-dependent IGF-IR activation. GD-IgGs also inhibited MCF7 breast cancer cell proliferation, suggesting that GD-IgG contains an IGF-IR antagonist/inhibitor. [201] That study used purified GD-IgGs, potentially removing endogenous serum factors that could affect cellular responses to Igs. It should be noted that both HepG2 and MCF-7 cell lines synthesize IGF-II. [202] Thus, endogenous IGF-II could alter IGF-1R receptor activation, potentially masking any effects of GD-IgG. In other studies, [203] GD-IgG stimulated HA secretion in GD-OFs in the absence of detectable IGF-IR autophosphorylation. No evidence of IGF-IR-stimulating activity was found in GD-IgG. [203] 1H7 and another IGF-IR inhibiting monoclonal antibody inhibited IGF-I-induced HA secretion in primary GD-OF cultures, leading the authors to conclude that GD-IgG does not bind to or activate IGF-IR. Further, they speculate that TSHR mediated signaling comprises both IGF-IR dependent and independent components. The use of non-standardized experimental conditions and study designs, uncertain culture medium content, and different cellular targets and assays for detecting IGF-IR activation remain possible explanations for widely dissimilar results. The potential impact of endogenous IGF and IGFBP production in these cell models may also confound interpretation of the experimental results thus far reported.
Growing understanding of how GCPRs and TKRs interact [204,205] has coincided with the evolving concept of IGF-IR playing a meaningful role in GO. Using confocal microscopy, Tsui et al. [199] reported that IGF-IRβ forms a signaling complex with TSHR on the plasma membranes and within the cytoplasm and perinuclear compartments of GD-OF, thyroid epithelial cells, and orbital fat in situ. Immunoprecipitation with either anti-IGF-IRβ or anti-TSHR monoclonal antibodies brought both proteins out of solution. [199] Of note, IH7 blocked the actions of IGF-I, rhTSH and GD-IgG in provoking ERK1/2 phosphorylation in primary human thyrocytes, indicating that signaling initiated through either receptor is dependent on IGF-IR activity. [199] In aggregate, these observations provided a rationale for interrupting the IGF-IR pathway as therapy in GD and GO. More recently, Krieger et al. [206] suggested that β-arrestin 1 could provide scaffolding for the TSHR-IGF-IR protein complex as the basis for receptor crosstalk.
Therapeutic Targeting of IGF-IR in GO
Teprotumumab (R1507) is a 150 kDa fully human monoclonal anti-IGF-IR antibody without detectable agonistic activity toward canonical IGF-IR signaling or binding affinity for the insulin receptor. [207] It binds with selectivity and high affinity to the extracellular α-chain of IGF-IR, [208] blocking its binding pocket and thus precluding IGF-I and IGF-II ligation. Once bound, teprotumumab promotes internalization of the antibody-receptor complex, promoting its degradation. [207] Its pharmacokinetic disposal exhibits a dose-dependent dual elimination pathway; non-linear (saturable) kinetics at lower concentrations and linear (nonsaturable) clearance at higher concentrations, with a uniform steady-state volume of distribution across all doses. [209] The aberrant signaling of IGF-IR and its binding proteins in tumors provided rationale for targeting the IGF-pathway in cancer, [210,211] yet clinical trials involving several anti-IGF-IR monoclonal antibodies have failed to improve disease outcomes. [212]
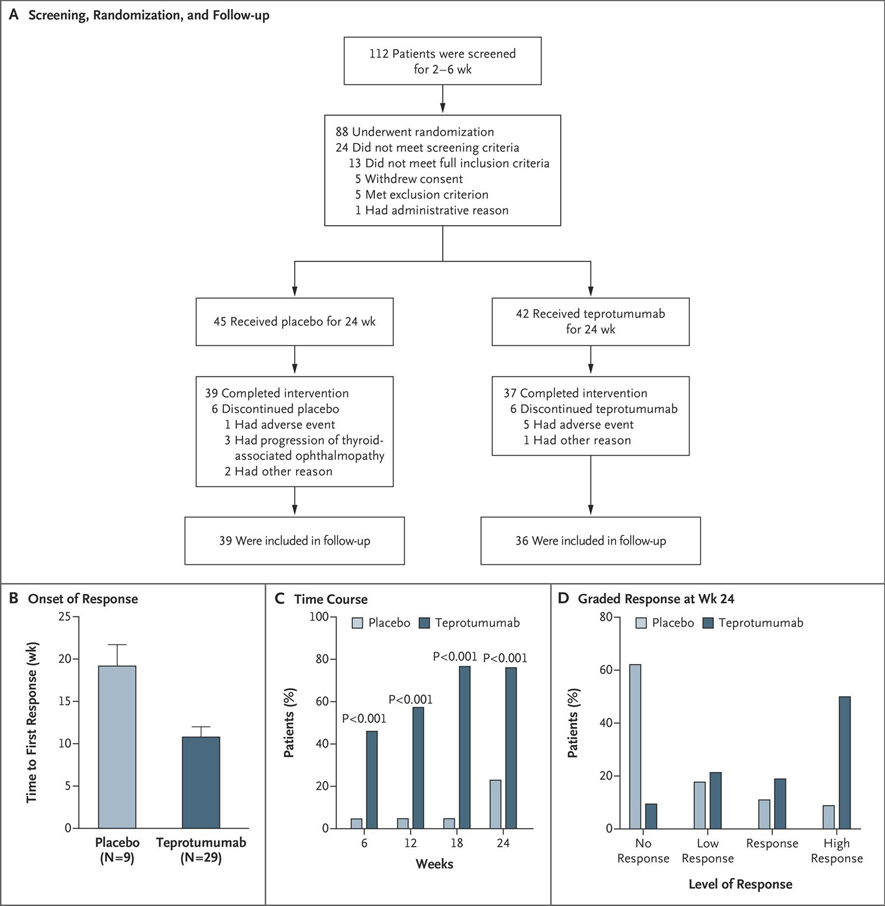
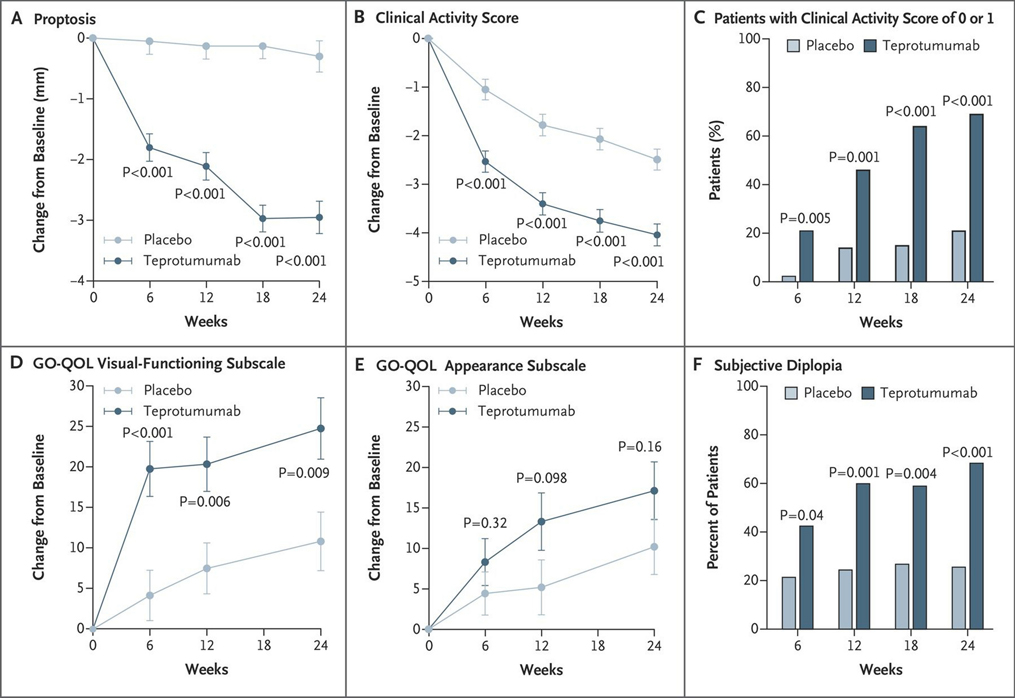
In collaboration with the study sponsor, River Vision Development, a randomized, multicenter, double-masked, placebo-controlled therapeutic trial (NCT01868997) for teprotumumab was organized for active, progressive and moderate to severe GO, based almost entirely on in vitro studies (Fig. 2). [213] Eighty-eight patients with active (defined by clinical activity score (CAS) ≥4 on a seven-point scale), moderate-severe GO were randomly assigned to receive either placebo or active drug. Inclusion criteria included ages 18–75 years with onset of periocular manifestations within 9 months of enrollment, chemical euthyroidy (serum FT4 and FT3 within 50% above or below the normative upper and lower assay limits) and prior cumulative glucocorticoid dosage ≤ 1 g with a 6-week washout period. Exclusion criteria included pregnancy, prior surgical intervention for GO and previous immunotherapy with RTX. Also excluded from the trial were patients with optic neuropathy. Patients were stratified at each institution with regard to smoking status. The intention-to-treat cohort consisted of 87 patients, 42 received teprotumumab and 45 were assigned to the placebo arm. Patients were assessed clinically at baseline and at 6-week intervals over a 24-week span during which they received 8 infusions of either placebo or teprotumumab. The initial drug dosage was 10 mg/kg of body weight, which was increased to 20 mg/kg for the remaining infusions if the initial infusion was uncomplicated. The primary response was the aggregate at 24 weeks of a reduction in CAS ≥ 2 points and proptosis ≥ 2 mm in the study eye (the more severely affected eye) without contralateral (fellow) eye worsening. Secondary responses included reduction from baseline in CAS ≥ 2 points and reduction in proptosis ≥ 2 mm measured as continuous variables, as well as improvement in quality of life score (GO-Qol questionnaire). [214] Thirty-seven (88%) patients receiving teprotumumab and 39 (87%) receiving placebo completed the intervention phase of the study. Those patients who did not complete the week 24 clinical assessment for any reason were judged as treatment failures in the intention-to-treat analysis. Twenty-nine out of 42 (69%) patients in the teprotumumab cohort met the primary response compared to 9/45 (20%) of those receiving placebo at week 24 (p < 0.001) (Fig. 3). The interval from baseline to achieving primary response was significantly shorter in the teprotumumab group; patients in this treatment arm had greater responses at weeks 6, 12, and 18 (p < 0.001). A “high response” of ≥3 mm proptosis reduction and ≥3 CAS reduction occurred in 21/42 (50%) patients in the teprotumumab group as compared to those receiving placebo (4/45, 9%) (p<0.001). The visual-functioning GO-QOL score also improved significantly at 24 weeks (p < 0.001) with teprotumumab. In contrast, the appearance subscale failed to achieve statistical significance despite marked improvement with treatment duration. Seventeen of 42 patients (40%) receiving teprotumumab experienced proptosis reduction ≥ 4 mm at week 24. This magnitude of proptosis reduction is unprecedented among previously developed medical therapies and is equivalent to the best reported surgical outcomes.
Figure 2.
Phase II trial design and therapeutic response. (a) Strategy for screening patients, their randomization into the two treatment arms for a 24-week intervention phase, and follow-up. One patient after screening failed to meet inclusion criteria, and withdrew prior to treatment initiation. (b) Analysis of the primary response (a reduction of ≥ 2 mm in proptosis and an improvement of ≥ 2 points on a 7-point scale in clinical activity score) in the study eye. (c) Time course in patients meeting response criteria at 6,12,18 and 24 weeks during treatment. (d) Grading the response at week 24. A high response denoted decrease of proptosis ≤ 3 mm and clinical activity score reduction ≤ 3 points. A response indicates a reduction ≥ 2 mm but < 3 mm in proptosis, and ≥ 2 points but < 3 points in Clinical Activity Score. A low response indicates reductions ≥ 1 mm but < 2 mm in proptosis and ≥ 1 point but < 2 points in Clinical Activity Score. No response indicates that the patient did not meet any response criteria or missed evaluation at week 24. Reprinted with permission from Smith TJ et al N Engl J Med. 2017; copyright 2017 Massachusetts Medical Society
Figure 3.
Secondary efficacy points of Phase II trial. Vertical bars in panels a,b,d, and e represent standard error (SE). (a) Reduction in proptosis from baseline over 24 weeks. (b) Changes in clinical activity score from baseline. (c) Percentage of patients with clinical activity score of 0 or 1 at week 24. (d) Change from baseline in visual-functioning subscale of the Graves’ ophthalmopathy–specific quality-of-life scale (GO-QOL) from baseline. Scores on the visual-functioning subscale range from 0 to 100, with a change of 8 points considered clinically relevant. e) Change from baseline in GO-QOL appearance subscale. (f) Response in subjective diplopia. Reprinted with permission from Smith TJ et al N Engl J Med. 2017; copyright 2017 Massachusetts Medical Society.
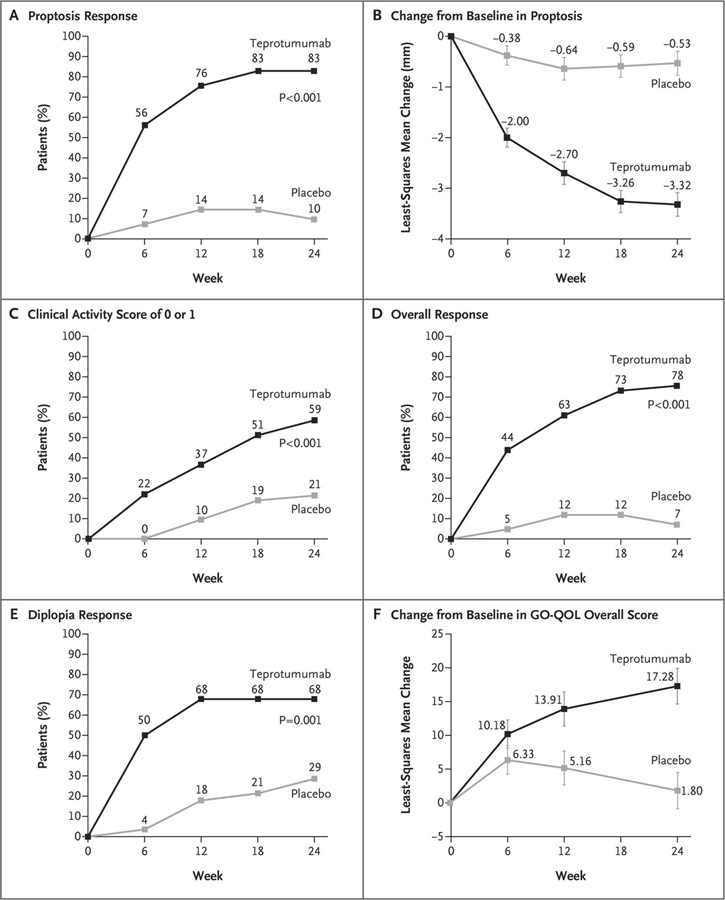
Subsequently, a pivotal multicenter phase 3 OPTIC trial (NCT03298867) was conducted with a similar design as that of phase 2 with minor modifications. Age eligibility was extended to 18–80 years and excluded individuals with a history of inflammatory bowel syndrome. [215] 83 patients underwent randomization in the intention-to-treat population, with 41 assigned to teprotumumab and 42 to placebo. The primary endpoint was proptosis responder rate of ≥ 2 mm reduction in the study eye at week 24. Following the 24 week treatment period, an open-label extension trial, OPTIC-X (NCT03461211,) was offered to proptosis non-responders and to relapsing responders, irrespective of the treatment the received during OPTIC. This 24 week extension included 8 additional infusions of teprotumumab every 3 weeks. The fraction of patients with proptosis response of ≥ 2 mm in those receiving teprotumumab at week 24 (83%) in OPTIC was significantly greater than in the placebo arm (10%; p < 0.001). The number needed to treat was 1.36 (Fig. 4). Thus the vast majority of patients in the teprotumumab group achieved the primary outcome. The magnitude of proptosis reduction with teprotumumab was −2.82 mm vs. −0.54 mm in placebo. Six patients treated with the active drug who underwent orbital MRI at baseline and again at week 24 experienced a mean reduction in extraocular muscle and orbital fat volumes of 1.56 cm3 (35%) and 2.67 cm3 (17%), respectively (Fig. 5). Follow-up of integrated responses at 7 weeks and 51 weeks after final dose for proptosis in 62/71 (87%) and 38/57 (67%) of patients respectively; 38/58 (66%) and 33//48 (69%) for diplopia, respectively [215]. An ophthalmic composite outcome included improvement in ≥1 eye from baseline without deterioration in either eye in ≥2 of the following: absence of eyelid swelling; CAS ≥2; proptosis ≥2 mm; lid aperture ≥2 mm; diplopia disappearance or grade change; or 8 degrees of globe motility improvement. The composite response was achieved in 66/72 (92%) at 7 weeks and 48/58 (83%) at 51 weeks.
Figure 4.
Efficacy outcomes for phase III trial. The p values for the between-group differences at week 24 (panels a, c, d, and e) were adjusted for multiplicity. I bars in panels c and f represent SE. (a) The primary response of proptosis response (b) the change from baseline in proptosis (c) The percentage of patients with a Clinical Activity Score of 0 or 1 at week 24 (d) The percentage of patients with an overall response (e) The percentage of patients with a diplopia response (f) The change from baseline in the overall score on the GO-QOL questionnaire. Reprinted with permission from Douglas RS et al N Engl J Med. 2020; copyright 2020 Massachusetts Medical Society
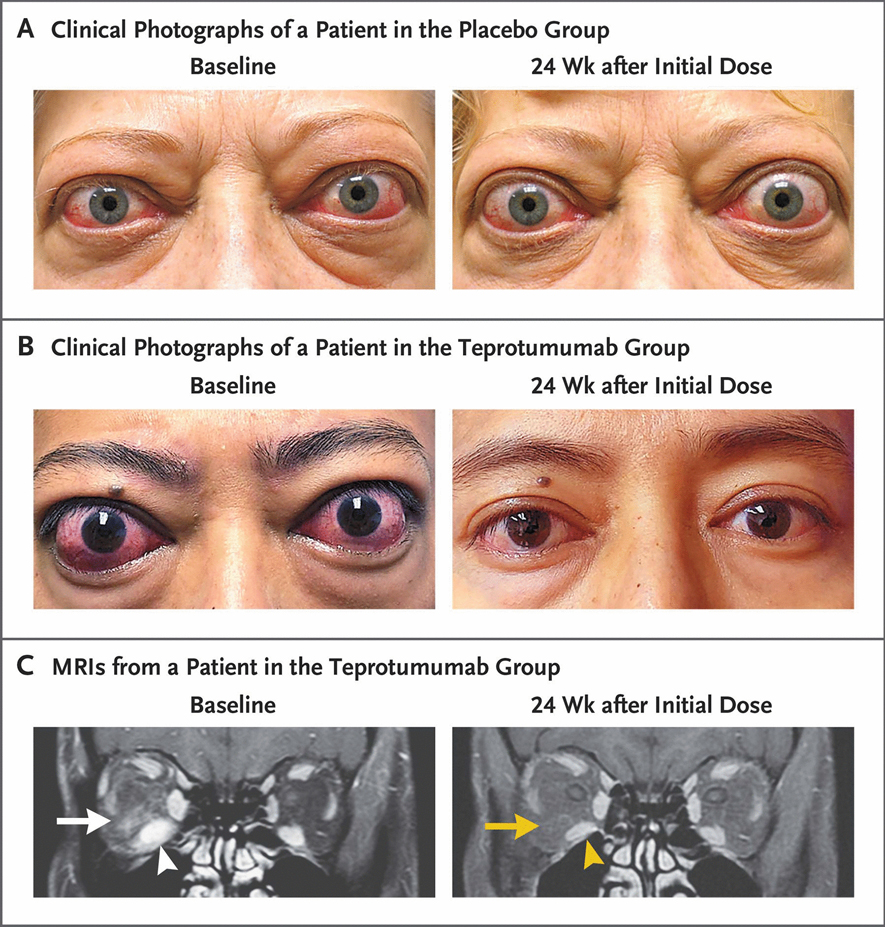
Figure 5.
Phase III trial facial appearance and orbital MRIs at baseline and 24 weeks after initial saline or teprotumumab infusion. (a) Frontal view of a patient receiving placebo. At baseline, considerable bilateral proptosis (OD, 29 mm and OS, 27 mm) and inflammatory signs (Clinical Activity Score 7 OD and 5 OS). Proptosis and inflammation persist at week 24. (b) Frontal view of a patient receiving teprotumumab. At baseline, proptosis (24 mm OU), edema, upper and lower eyelid retraction, and multiple inflammatory signs (Clinical activity score 5 OU) are present. Reductions of proptosis of 5 mm OU and reduction of CAS 4 points OU observed at 24 weeks (c) T1-weighted coronal and contrast-enhanced, magnetic resonance images at baseline and week 24 in a patient treated with teprotumumab. Enhancement of inferior rectus muscle (OD, white arrowhead) and adipose tissue (white arrow) reflect findings of inflammation and edema. The respective inferior rectus muscle (yellow arrowhead) and orbital fat are shown at week 24 (yellow arrow). The inferior rectus volume was reduced by 49% by Materialise software (yellow arrowhead). Proptosis reduction decreased 5 mm from baseline at week 24. Reprinted with permission from Douglas RS et al N Engl J Med. 2020; copyright 2020 Massachusetts Medical Society
Adverse events (AEs) during treatment in the intention-to-treat populations from both phase 2 and 3 studies were pooled for analysis. [216] Sixty-three (94%) of 67 patients receiving teprotumumab and 59 (98%) of 60 patients in the placebo group experienced mild to moderate (grade 1 or 2) AEs, with 3 patients (4%) experiencing serious AEs related or possibly related to teprotumumab. These included diarrhea, infusion reactions, and Hashimoto’s encephalopathy (co-incident) which prompted study discontinuation. The prominent AEs with risk difference within the teprotumumab cohort were muscle spasm (18%), hearing loss (10%), and hyperglycemia (8%). As IGF-I and IGF-IR signaling are intimately involved in glucose homeostasis, [217] these were associated with pre-existing diabetes mellitus. Glycemic control was restored with diabetes medication adjustment. Increased drug requirements returned to those at baseline following the end of exposure to teprotumumab.
In January 2020, teprotumumab became the first medical therapy approved by the US FDA for the treatment of GO.[218] Following its approval, case reports have been published suggesting that teprotumumab may effectively treat compressive optic neuropathy secondary to GO. [219–221] The effectiveness of the drug must now be studied in formal trials and compared with the orthodox regimen of high-dosage steroids and urgent surgical decompression. Teprotumumab might also be effective in chronic, non-progressive GO. [222,223]
Concluding Remarks
The past decade has witnessed substantial progress in understanding and treating GO. For the first time, a specific therapy, based on recognition of the immunologic pathogenesis of GO, has been developed and was recently approved by the U.S. FDA. Teprotumumab, market as Tepezza, is now available in North America but has not yet been introduced elsewhere. Importantly, that drug, an IGF-IR inhibitory monoclonal antibody, was developed as a therapeutic based entirely on insights into the pathogenesis of GO. It therefore serves as example of the power of basic and translational science that can result in effective treatment. We envision future insights providing a springboard for development of even better therapeutic options over the coming decade. Further, we predict that the ultimate treatment for GD and GO will involve the restoration of immune tolerance to the relevant autoantigen(s), sparing patients chronic exposure to immunomodulatory agents. Among the new approaches in early-stage development are those using synthetic peptides resembling T cell epitopes, some of which have shown promise in protecting against autoimmune induction. [224,225] The potential for capturing CAAR-T cell technology [226,227] to generate memory cells against pathogenic B cells and thus promoting long term remission in GO, is an exciting possibility based on our growing understanding of how the disease develops.
Funding
National Institutes of Health grants EY008976,
Glossary
- Ad-TSHR A
Adenovirus expressing the extracellular A-subunit of the human TSH receptor (TSHR)
- AE
Adverse event
- AIRE
thymic autoimmune regulator
- AKT
Protein kinase B
- AP-1
Activator protein-1
- ARID5B
AT-Rich Interaction Domain 5B
- BAFF
B-cell activating factor
- CAAR-T cells
Chimeric autoantibody receptor expressing T cells
- cAMP
Cyclic adenosine monophosphate
- CAS
Clinical activity score
- CFZ533
Iscalimab
- CHO
Chinese hamster ovary cell
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- COX-2
Cyclooxygenase-2
- CYR61
Cysteine-rich angiogenic inducer 61
- Dsg3
Desmoglein-3
- EGR-1
Early growth response protein 1
- ERK
Extracellular signal-regulated kinases
- ETA
European Thyroid Association
- GcgR
Fc gamma receptor
- FcgRI
High affinity immunoglobulin gamma Fc receptor I
- FcgRIIa
Low affinity immunoglobulin gamma Fc region receptor II-a
- FcRn
Neonatal Fc receptor
- FRAP
FK506-binding protein 12-rapamycin-associated protein 1
- FT3
Free triiodothyronine
- FT4
Free thyroxine
- GD-IgG
Graves’ disease immunoglobulins
- GD-OF
Graves’ disease orbital fibroblasts
- GO
Graves’ orbitopathy
- GO-QOL
Graves’ ophthalmopathy quality-of-life scale
- GR
Glucocorticoid receptor
- HAS1
Hyaluronan synthase 1
- HAS2
Hyaluronan synthase 2
- HLA-DRβ-Arg74
Human Leukocyte Antigen – DR isotype -- variant containing Arginine at position 74
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme
- IFN-γ
Interferon gamma
- IGF-I
Insulin-like growth factor 1
- IGF-II
Insulin-like growth factor 2
- IGF-1R
Insulin-Like Growth Factor 1 Receptor
- IgG-k
Immunoglobulin G kappa light chain
- LRR
Leucine-rich repeat
- MHC class I
Major histocompatibility complex I
- MHC class II
Major histocompatibility complex II
- MMF
Mycophenolate motefil
- MPA
Mycophenolic acid
- NF-κB
Nuclear factor-κB
- NIS
Sodium iodide symporter
- OD
Oculus dexter
- OF
Orbital fibroblast
- OS
Oculus sinister
- p70s6K
Ribosomal protein S6 kinase beta-1
- PDK1
Pyruvate dehydrogenase kinase 1
- PGE2
Prostaglandin E2
- PGHS-2
Prostaglandin endoperoxide H synthase 2
- PI3K/AKT/mTOR
Phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR)
- PKC
Protein kinase C
- PKCβII
Protein kinase C beta type 2
- PMBC
Peripheral blood mononuclear cell
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTPN22
Protein tyrosine phosphatase, non-receptor type 22
- PTX-3
Pentraxin-3
- qRT-PCR
Real-Time Quantitative Reverse Transcription PCR
- RA
Rheumatoid Arthritis
- RANTES
Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted
- RTX
Rituximab
- ROBO1
Roundabout 1
- RhSlit2
Recombinant human Slit homolog 2 protein
- SAP
Serum amyloid P
- sFRP-1
Secreted frizzled-related protein-1
- SGK-1
Serine/threonine-protein kinase Sgk1
- sIL-1RA
Secreted IL-1 receptor antagonist
- SLE
systemic lupus erythematosus
- Slit2
Slit homolog 2 protein
- SNP
Single-nucleotide polymorphisms
- STAT-3
Signal transducer and activator of transcription 3
- TAO
Thyroid associated Ophthalmopathy
- TCZ
Tocilizumab
- Tg
Thyroglobulin
- TNFα
Tumor necrosis factor
- TPO
thyroid peroxidase
- Trab
Thyrotropin receptor antibody
- TRAFS
TNFα receptor associated factors
- Treg
T regulatory cells
- TSH-β
Thyroid stimulating hormone beta protein
- TSHR
Thyrotropin Receptor
- TSI
Thyroid stimulating immunoglobulin
- UGDH
UDP-glucose dehydrogenase
- Wnt
Wingless/Integrated
- 1H7
Murine monoclonal anti-IGF-IR inhibitory antibody
- 486/STOP
Dominant-negative mutant IGF-IR
Footnotes
Conflicts of interest/Competing interests TJS was issued US patents for the use of IGF-I receptor inhibition in the treatment of autoimmune diseases. These are held by UCLA and the Lundquist Institute. He is a paid consultant for Horizon Therapeutics.
Declarations
Availability of data and material N/A
Code availability N/A
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals N/A
Ethics approval N/A
Consent to participate N/A
Consent for publication N/A
Permissions: Appear in the figure legends.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Smith TJ, Hegedüs L. Graves’ Disease. N Engl J Med. 2016. Oct 20;375(16):1552–1565. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 2.Tellez M, Cooper J, Edmonds C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf). 1992. Mar;36(3):291–4. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 3.Khalilzadeh O, Noshad S, Rashidi A, Amirzargar A. Graves’ ophthalmopathy: a review of immunogenetics. Curr Genomics. 2011. Dec;12(8):564–75. doi: 10.2174/138920211798120844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003. Aug;13(8):761–4. doi: 10.1089/105072503768499653. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen H, Li X, Sundquist K, Sundquist J, Försti A, Hemminki K. Familial risks between Graves disease and Hashimoto thyroiditis and other autoimmune diseases in the population of Sweden. J Transl Autoimmun. 2020. Jun 1;3:100058. doi: 10.1016/j.jtauto.2020.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari SM, Fallahi P, Ruffilli I, Elia G, Ragusa F, Benvenga S, Antonelli A. The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): Review of the literature and report of a large series. Autoimmun Rev. 2019. Mar;18(3):287–292. doi: 10.1016/j.autrev.2018.10.001. Epub 2019 Jan 11. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of Graves’ disease: evidence of a genetic and an environmental contribution. J Autoimmun. 2010. May;34(3):J307–13. doi: 10.1016/j.jaut.2009.11.019. Epub 2009 Dec 28. [DOI] [PubMed] [Google Scholar]
- 8.Tomer Y Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–56. doi: 10.1146/annurev-pathol-012513-104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, Chen RH, Wu HH, Liao WL, Chen WC, Tsai Y, Tsai CH, Wan L, Tsai FJ. Association of interleukin-1beta (IL1B) polymorphisms with Graves’ ophthalmopathy in Taiwan Chinese patients. Invest Ophthalmol Vis Sci. 2010. Dec;51(12):6238–46. doi: 10.1167/iovs.09-4965. Epub 2010 Jul 29. [DOI] [PubMed] [Google Scholar]
- 10.Liu YH, Chen CC, Liao LL, Wan L, Tsai CH, Tsai FJ. Association of IL12B polymorphisms with susceptibility to Graves ophthalmopathy in a Taiwan Chinese population. J Biomed Sci. 2012. Nov 19;19(1):97. doi: 10.1186/1423-0127-19-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber AK, Jacobson EM, Jazdzewski K, Concepcion ES, Tomer Y. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. 2008. Mar;93(3):1077–81. doi: 10.1210/jc.2007-2190. Epub 2007 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin X, Latif R, Bahn R, Davies TF. Genetic profiling in Graves’ disease: further evidence for lack of a distinct genetic contribution to Graves’ ophthalmopathy. Thyroid. 2012. Jul;22(7):730–6. doi: 10.1089/thy.2012.0007. Epub 2012 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Leontovich A, Coenen MJ, Bahn RS. Gene expression profiling of orbital adipose tissue from patients with Graves’ ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J Clin Endocrinol Metab. 2005. Aug;90(8):4730–5. doi: 10.1210/jc.2004-2239. Epub 2005 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantz M, Vondrichova T, Parikh H, Frenander C, Ridderstråle M, Asman P, Aberg M, Groop L, Hallengren B. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005. Aug;90(8):4784–91. doi: 10.1210/jc.2004-2275. Epub 2005 May 31. [DOI] [PubMed] [Google Scholar]
- 15.Ezra DG, Krell J, Rose GE, Bailly M, Stebbing J, Castellano L. Transcriptome-level microarray expression profiling implicates IGF-1 and Wnt signalling dysregulation in the pathogenesis of thyroid-associated orbitopathy. J Clin Pathol. 2012. Jul;65(7):608–13. doi: 10.1136/jclinpath-2012-200719. Epub 2012 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum JT, Choi D, Wong A, Wilson DJ, Grossniklaus HE, Harrington CA, Dailey RA, Ng JD, Steele EA, Czyz CN, Foster JA, Tse D, Alabiad C, Dubovy S, Parekh PK, Harris GJ, Kazim M, Patel PJ, White VA, Dolman PJ, Edward DP, Alkatan HM, Al Hussain H, Selva D, Yeatts RP, Korn BS, Kikkawa DO, Stauffer P, Planck SR. The Role of the Immune Response in the Pathogenesis of Thyroid Eye Disease: A Reassessment. PLoS One. 2015. Sep 15;10(9):e0137654. doi: 10.1371/journal.pone.0137654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limbach M, Saare M, Tserel L, Kisand K, Eglit T, Sauer S, Axelsson T, Syvänen AC, Metspalu A, Milani L, Peterson P. Epigenetic profiling in CD4+ and CD8+ T cells from Graves’ disease patients reveals changes in genes associated with T cell receptor signaling. J Autoimmun. 2016. Feb;67:46–56. doi: 10.1016/j.jaut.2015.09.006. Epub 2015 Oct 12. [DOI] [PubMed] [Google Scholar]
- 18.Rotondo Dottore G, Bucci I, Lanzolla G, Dallan I, Sframeli A, Torregrossa L, Casini G, Basolo F, Figus M, Nardi M, Marcocci C, Marinò M. Genetic Profiling of Orbital Fibroblasts from Patients with Graves’ Orbitopathy. J Clin Endocrinol Metab. 2021. Apr 23;106(5):e2176–e2190. doi: 10.1210/clinem/dgab035. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Saito H, Liang G, Friedman JM. Epigenetic alterations and microRNA misexpression in cancer and autoimmune diseases: a critical review. Clin Rev Allergy Immunol. 2014. Oct;47(2):128–35. doi: 10.1007/s12016-013-8401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedrich CM, Tsokos GC. Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med. 2011. Dec;17(12):714–24. doi: 10.1016/j.molmed.2011.07.005. Epub 2011 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, Du Y, Jiang BL, He JF. Increased microRNA-155 and decreased microRNA-146a may promote ocular inflammation and proliferation in Graves’ ophthalmopathy. Med Sci Monit. 2014. Apr 18;20:639–43. doi: 10.12659/MSM.890686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond CL, Roztocil E, Gonzalez MO, Feldon SE, Woeller CF. MicroRNA-130a Is Elevated in Thyroid Eye Disease and Increases Lipid Accumulation in Fibroblasts Through the Suppression of AMPK. Invest Ophthalmol Vis Sci. 2021. Jan 4;62(1):29. doi: 10.1167/iovs.62.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Ma C, Li HY, Chen L, Yuan SS, Li KJ. MicroRNA-146a downregulates the production of hyaluronic acid and collagen I in Graves’ ophthalmopathy orbital fibroblasts. Exp Ther Med. 2020. Nov;20(5):38. doi: 10.3892/etm.2020.9165. Epub 2020 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang SY, Chae MK, Lee JH, Lee EJ, Yoon JS. MicroRNA-27 inhibits adipogenic differentiation in orbital fibroblasts from patients with Graves’ orbitopathy. PLoS One. 2019. Aug 15;14(8):e0221077. doi: 10.1371/journal.pone.0221077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989. Aug;10(3):366–91. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014. Mar 20;55(3):1735–48. doi: 10.1167/iovs.14-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Smith TJ. Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J Clin Endocrinol Metab. 2014. Apr;99(4):E625–33. doi: 10.1210/jc.2013-3977. Epub 2014 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34⁺ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013. Jul;98(7):2783–90. doi: 10.1210/jc.2013-1245. Epub 2013 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HS, Cao HJ, Winn VD, Rezanka LJ, Frobert Y, Evans CH, Sciaky D, Young DA, Smith TJ. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996. Sep 13;271(37):22718–28. [PubMed] [Google Scholar]
- 30.Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, Chen B, Phipps RP, Goldberg RA, Smith TJ, Douglas RS. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50:2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003. Oct;163(4):1291–300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002. Mar;12(3):197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 33.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010. Jan;95(1):430–8. doi: 10.1210/jc.2009-1614. Epub 2009 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Göbel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009. Oct 20;106(42):17892–7. doi: 10.1073/pnas.0906070106. Epub 2009 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997. Jun 10;94(12):6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005. Jun;77(6):923–33. doi: 10.1189/jlb.1204701. Epub 2005 Mar 14. [DOI] [PubMed] [Google Scholar]
- 37.Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, Smith TJ. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012. May 8;109(19):7427–32. doi: 10.1073/pnas.1202064109. Epub 2012 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014. Jul;99(7):E1236–44. doi: 10.1210/jc.2013-4271. Epub 2014 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Atkins SJ, Fernando R, Trierweiler A, Mester T, Grisolia ABD, Mou P, Novaes P, Smith TJ. CD34- Orbital Fibroblasts From Patients With Thyroid-Associated Ophthalmopathy Modulate TNF-α Expression in CD34+ Fibroblasts and Fibrocytes. Invest Ophthalmol Vis Sci. 2018. May 1;59(6):2615–2622. doi: 10.1167/iovs.18-23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013. Sep 25;8(9):e75100. doi: 10.1371/journal.pone.0075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK, Goncalves AC, Zhao SX, Ginter A, Lu Y, Smith TJ, Douglas RS. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012. May;97(5):E740–6. doi: 10.1210/jc.2011-2514. Epub 2012 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernando R, Grisolia ABD, Lu Y, Atkins S, Smith TJ. Slit2 Modulates the Inflammatory Phenotype of Orbit-Infiltrating Fibrocytes in Graves’ Disease. J Immunol. 2018. Jun 15;200(12):3942–3949. doi: 10.4049/jimmunol.1800259. Epub 2018 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernando R, Atkins SJ, Smith TJ. Slit2 May Underlie Divergent Induction by Thyrotropin of IL-23 and IL-12 in Human Fibrocytes. J Immunol. 2020. Apr 1;204(7):1724–1735. doi: 10.4049/jimmunol.1900434. Epub 2020 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernando R, Smith TJ. Slit2 Regulates Hyaluronan & Cytokine Synthesis in Fibrocytes: Potential Relevance to Thyroid-Associated Ophthalmopathy. J Clin Endocrinol Metab. 2021. Jan 1;106(1):e20–e33. doi: 10.1210/clinem/dgaa684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999. Mar 19;96(6):785–94. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 46.Ypsilanti AR, Chedotal A. Roundabout receptors. Adv Neurobiol. 2014;8:133–64. doi: 10.1007/978-1-4614-8090-7_7. [DOI] [PubMed] [Google Scholar]
- 47.Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci U S A. 2014. Dec 23;111(51):18291–6. doi: 10.1073/pnas.1417426112. Epub 2014 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohenester E, Hutchinson WL, Pepys MB, Wood SP. Crystal structure of a decameric complex of human serum amyloid P component with bound dAMP. J Mol Biol. 1997. Jun 20;269(4):570–8. doi: 10.1006/jmbi.1997.1075. [DOI] [PubMed] [Google Scholar]
- 49.Cox N, Pilling D, Gomer RH. Distinct Fcγ receptors mediate the effect of serum amyloid p on neutrophil adhesion and fibrocyte differentiation. J Immunol. 2014. Aug 15;193(4):1701–8. doi: 10.4049/jimmunol.1400281. Epub 2014 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994. Nov 15;84(10):3483–93. [PubMed] [Google Scholar]
- 51.Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, Bastone A, Salvatori G, Mantovani A, Siracusa G, Salustri A. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007. Oct 12;282(41):30161–70. doi: 10.1074/jbc.M703738200. Epub 2007 Aug 2. [DOI] [PubMed] [Google Scholar]
- 52.Bonacina F, Baragetti A, Catapano AL, Norata GD. Long pentraxin 3: experimental and clinical relevance in cardiovascular diseases. Mediators Inflamm. 2013;2013:725102. doi: 10.1155/2013/725102. Epub 2013 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D’Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997. Dec 26;272(52):32817–23. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 54.Pilling D, Cox N, Vakil V, Verbeek JS, Gomer RH. The long pentraxin PTX3 promotes fibrocyte differentiation. PLoS One. 2015. Mar 16;10(3):e0119709. doi: 10.1371/journal.pone.0119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Atkins SJ, Fernando R, Wei RL, Smith TJ. Pentraxin-3 Is a TSH-Inducible Protein in Human Fibrocytes and Orbital Fibroblasts. Endocrinology. 2015. Nov;156(11):4336–44. doi: 10.1210/en.2015-1399. Epub 2015 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014. Sep;99(9):E1635–40. doi: 10.1210/jc.2014-1580. Epub 2014 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves’ ophthalmopathy. Clin Exp Immunol. 1989. Feb;75(2):222–7. [PMC free article] [PubMed] [Google Scholar]
- 58.Rotondo Dottore G, Torregrossa L, Caturegli P, Ionni I, Sframeli A, Sabini E, Menconi F, Piaggi P, Sellari-Franceschini S, Nardi M, Latrofa F, Vitti P, Marcocci C, Basolo F, Marinò M. Association of T and B Cells Infiltrating Orbital Tissues With Clinical Features of Graves Orbitopathy. JAMA Ophthalmol. 2018. Jun 1;136(6):613–619. doi: 10.1001/jamaophthalmol.2018.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pappa A, Lawson JM, Calder V, Fells P, Lightman S. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000. May;84(5):517–22. doi: 10.1136/bjo.84.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994. Jun;93(6):2738–43. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang S, Huang Y, Zhong S, Li Y, Zhang Y, Li Y, Sun J, Liu X, Wang Y, Zhang S, Xu T, Sun X, Gu P, Li D, Zhou H, Li B, Fan X. Regulation of Orbital Fibrosis and Adipogenesis by Pathogenic Th17 Cells in Graves Orbitopathy. J Clin Endocrinol Metab. 2017. Nov 1;102(11):4273–4283. doi: 10.1210/jc.2017-01349. [DOI] [PubMed] [Google Scholar]
- 62.Fang S, Huang Y, Wang N, Zhang S, Zhong S, Li Y, Sun J, Liu X, Wang Y, Gu P, Li B, Zhou H, Fan X. Insights Into Local Orbital Immunity: Evidence for the Involvement of the Th17 Cell Pathway in Thyroid-Associated Ophthalmopathy. J Clin Endocrinol Metab. 2019. May 1;104(5):1697–1711. doi: 10.1210/jc.2018-01626. Erratum in: J Clin Endocrinol Metab. 2020 Jan 1;105(1): [DOI] [PubMed] [Google Scholar]
- 63.Rapoport B, McLachlan SM. Graves’ hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. J Clin Endocrinol Metab. 2014. Nov;99(11):4060–1. doi: 10.1210/jc.2014-3011. Epub 2014 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng D, Xu B, Wang Y, Guo H, Jiang Y. A high frequency of circulating th22 and th17 cells in patients with new onset graves’ disease. PLoS One. 2013. Jul 11;8(7):e68446. doi: 10.1371/journal.pone.0068446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000. Feb;85(2):776–80. doi: 10.1210/jcem.85.2.6333. [DOI] [PubMed] [Google Scholar]
- 66.Shen J, Li Z, Li W, Ge Y, Xie M, Lv M, Fan Y, Chen Z, Zhao D, Han Y. Th1, Th2, and Th17 Cytokine Involvement in Thyroid Associated Ophthalmopathy. Dis Markers. 2015;2015:609593. doi: 10.1155/2015/609593. Epub 2015 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahaly GJ, Shimony O, Gellman YN, Lytton SD, Eshkar-Sebban L, Rosenblum N, Refaeli E, Kassem S, Ilany J, Naor D. Regulatory T-cells in Graves’ orbitopathy: baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab. 2011. Feb;96(2):422–9. doi: 10.1210/jc.2010-1424. Epub 2010 Dec 8. [DOI] [PubMed] [Google Scholar]
- 68.Pawlowski P, Grubczak K, Kostecki J, Ilendo-Poskrobko E, Moniuszko M, Pawlowska M, Rejdak R, Reszec J, Mysliwiec J. Decreased Frequencies of Peripheral Blood CD4+CD25+CD127-Foxp3+ in Patients with Graves’ Disease and Graves’ Orbitopathy: Enhancing Effect of Insulin Growth Factor-1 on Treg Cells. Horm Metab Res. 2017. Mar;49(3):185–191. doi: 10.1055/s-0042-122780. Epub 2017 Feb 21. [DOI] [PubMed] [Google Scholar]
- 69.Zhao SX, Tsui S, Cheung A, Douglas RS, Smith TJ, Banga JP. Orbital fibrosis in a mouse model of Graves’ disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011. Sep;210(3):369–77. doi: 10.1530/JOE-11-0162. Epub 2011 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013. Sep;154(9):3008–15. doi: 10.1210/en.2013-1576. Epub 2013 Jul 30. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Ding X, Wu LP, He MQ, Chen ZY, Shi BY, Wang Y. A Promising Mouse Model of Graves’ Orbitopathy Induced by Adenovirus Expressing Thyrotropin Receptor A Subunit. Thyroid. 2021. Apr;31(4):638–648. doi: 10.1089/thy.2020.0088. Epub 2021 Jan 5. [DOI] [PubMed] [Google Scholar]
- 72.Nakahara M, Johnson K, Eckstein A, Taguchi R, Yamada M, Abiru N, Nagayama Y. Adoptive transfer of antithyrotropin receptor (TSHR) autoimmunity from TSHR knockout mice to athymic nude mice. Endocrinology. 2012. Apr;153(4):2034–42. doi: 10.1210/en.2011-1846. Epub 2012 Feb 14. [DOI] [PubMed] [Google Scholar]
- 73.Hikage F, Atkins S, Kahana A, Smith TJ, Chun TH. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology. 2019. Jan 1;160(1):20–35. doi: 10.1210/en.2018-00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol (Camb). 2013. Sep;5(9):1149–61. doi: 10.1039/c3ib40040a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo L, Reed J, Shin A, Kung J, Gimzewski JK, Poukens V, Goldberg RA, Mancini R, Taban M, Moy R, Demer JL. Characterization of ocular tissues using microindentation and hertzian viscoelastic models. Invest Ophthalmol Vis Sci. 2011. Jun 1;52(6):3475–82. doi: 10.1167/iovs.10-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weetman AP. Graves’ disease. N Engl J Med. 2000. Oct 26;343(17):1236–48. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 77.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM; European Group on Graves’ Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008. Mar;158(3):273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 78.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM; European Group on Graves’ Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016. Mar;5(1):9–26. doi: 10.1159/000443828. Epub 2016 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ponto KA, Pitz S, Mann WJ, Weber MM, Pfeiffer N, Kahaly GJ. Vorgehen bei endokriner Orbitopathie. Evidenzbasierte Empfehlungen [Management of Graves’ orbitopathy: evidence-based recommendations]. Dtsch Med Wochenschr. 2009. Dec;134(49):2521–4. German. doi: 10.1055/s-0029-1243057. Epub 2009 Nov 25. [DOI] [PubMed] [Google Scholar]
- 80.Längericht J, Krämer I, Kahaly GJ. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther Adv Endocrinol Metab. 2020. Dec 14;11:2042018820958335. doi: 10.1177/2042018820958335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000. Sep;20(18):6891–903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell. 2008. Mar 14;29(5):611–24. doi: 10.1016/j.molcel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005. Mar;25(3):552–63. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 84.Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005. Sep;90(9):5234–40. doi: 10.1210/jc.2005-0148. Epub 2005 Jul 5. [DOI] [PubMed] [Google Scholar]
- 85.Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Currò N, Boschi A, Bernard M, von Arx G; European Group on Graves’ Orbitopathy. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012. Dec;97(12):4454–63. doi: 10.1210/jc.2012-2389. Epub 2012 Oct 4. [DOI] [PubMed] [Google Scholar]
- 86.Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S, Dazzi D, Guastella C, Pignataro L, Beck-Peccoz P, Ratiglia R, Salvi M. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014. May;24(5):897–905. doi: 10.1089/thy.2013.0445. Epub 2014 Mar 6. [DOI] [PubMed] [Google Scholar]
- 87.Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998. Nov 6;273(45):29615–25. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 88.Thorén S, Jakobsson PJ. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Inhibition by NS-398 and leukotriene C4. Eur J Biochem. 2000. Nov;267(21):6428–34. doi: 10.1046/j.1432-1327.2000.01735.x. [DOI] [PubMed] [Google Scholar]
- 89.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997. Jul;49:15–9. [PubMed] [Google Scholar]
- 90.Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campennì A, Morelli S, Puxeddu E, Sisti E, Trimarchi F, Vermiglio F, Marinò M. Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy. Endocrine. 2016. Oct;54(1):259–268. doi: 10.1007/s12020-016-0928-3. Epub 2016 Mar 22. [DOI] [PubMed] [Google Scholar]
- 91.Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid. 2007. Apr;17(4):357–62. doi: 10.1089/thy.2006.0267. [DOI] [PubMed] [Google Scholar]
- 92.Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, Bartalena L; European Group of Graves’ Orbitopathy. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012. Feb;166(2):247–53. doi: 10.1530/EJE-11-0779. Epub 2011 Nov 4. [DOI] [PubMed] [Google Scholar]
- 93.Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422–431. doi: 10.1210/jc.2014-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meier-Kriesche HU, Morris JA, Chu AH, Steffen BJ, Gotz VP, Gordon RD, Kaplan B. Mycophenolate mofetil vs azathioprine in a large population of elderly renal transplant patients. Nephrol Dial Transplant. 2004. Nov;19(11):2864–9. doi: 10.1093/ndt/gfh445. [DOI] [PubMed] [Google Scholar]
- 95.Gabardi S, Tran JL, Clarkson MR. Enteric-coated mycophenolate sodium. Ann Pharmacother. 2003. Nov;37(11):1685–93. doi: 10.1345/aph.1D063. [DOI] [PubMed] [Google Scholar]
- 96.Allison AC. Mechanisms of action of mycophenolate mofetil in preventing chronic rejection. Transplant Proc. 2002. Nov;34(7):2863–6. doi: 10.1016/s0041-1345(02)03538-8. [DOI] [PubMed] [Google Scholar]
- 97.Fujiyama N, Miura M, Kato S, Sone T, Isobe M, Satoh S. Involvement of carboxylesterase 1 and 2 in the hydrolysis of mycophenolate mofetil. Drug Metab Dispos. 2010. Dec;38(12):2210–7. doi: 10.1124/dmd.110.034249. Epub 2010 Sep 7. [DOI] [PubMed] [Google Scholar]
- 98.Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993. Dec 25;268(36):27286–90. [PubMed] [Google Scholar]
- 99.Mazumder AG, Patial V, Singh D. Mycophenolate mofetil contributes to downregulation of the hippocampal interleukin type 2 and 1β mediated PI3K/AKT/mTOR pathway hyperactivation and attenuates neurobehavioral comorbidities in a rat model of temporal lobe epilepsy. Brain Behav Immun. 2019. Jan;75:84–93. doi: 10.1016/j.bbi.2018.09.020. Epub 2018 Sep 20. [DOI] [PubMed] [Google Scholar]
- 100.Kahaly GJ, Riedl M, König J, Pitz S, Ponto K, Diana T, Kampmann E, Kolbe E, Eckstein A, Moeller LC, Führer D, Salvi M, Curro N, Campi I, Covelli D, Leo M, Marinò M, Menconi F, Marcocci C, Bartalena L, Perros P, Wiersinga WM; European Group on Graves’ Orbitopathy (EUGOGO). Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018. Apr;6(4):287–298. doi: 10.1016/S2213-8587(18)30020-2. Epub 2018 Jan 31. [DOI] [PubMed] [Google Scholar]
- 101.Kelley WN, Rosenbloom FM, Seegmiller JE. The effects of azathioprine (imuran) on purine synthesis in clinical disorders of purine metabolism. J Clin Invest. 1967. Sep;46(9):1518–29. doi: 10.1172/JCI105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perros P, Weightman DR, Crombie AL, Kendall-Taylor P. Azathioprine in the treatment of thyroid-associated ophthalmopathy. Acta Endocrinol (Copenh). 1990. Jan;122(1):8–12. doi: 10.1530/acta.0.1220008. [DOI] [PubMed] [Google Scholar]
- 103.Traullé C, Coiffier BB. Evolving role of rituximab in the treatment of patients with non-Hodgkin’s lymphoma. Future Oncol. 2005. Jun;1(3):297–306. doi: 10.1517/14796694.1.3.297. [DOI] [PubMed] [Google Scholar]
- 104.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003. Oct 20;22(47):7359–68. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 105.Piro LD, White CA, Grillo-López AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1999. Jun;10(6):655–61. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 106.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994. Sep;15(9):450–4. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen CH, El Fassi D, Hasselbalch HC, Bendtzen K, Hegedüs L. B-cell depletion with rituximab in the treatment of autoimmune diseases. Graves’ ophthalmopathy the latest addition to an expanding family. Expert Opin Biol Ther. 2007. Jul;7(7):1061–78. doi: 10.1517/14712598.7.7.1061. [DOI] [PubMed] [Google Scholar]
- 108.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002. Feb 1;99(3):1038–43. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 109.Anderson DR, Grillo-López A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma. Biochem Soc Trans. 1997. May;25(2):705–8. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 110.Storz U Rituximab: how approval history is reflected by a corresponding patent filing strategy. MAbs. 2014. Jul-Aug;6(4):820–37. doi: 10.4161/mabs.29105. Epub 2014 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L. B lymphocyte depletion with the monoclonal antibody rituximab in Graves’ disease: a controlled pilot study. J Clin Endocrinol Metab. 2007. May;92(5):1769–72. doi: 10.1210/jc.2006-2388. Epub 2007 Feb 6. [DOI] [PubMed] [Google Scholar]
- 112.Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, Bonara P, Rossi S, Sina C, Guastella C, Ratiglia R, Beck-Peccoz P. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007. Jan;156(1):33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 113.Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, Romijn JA, Smit JW. Rituximab in relapsing Graves’ disease, a phase II study. Eur J Endocrinol. 2008. Nov;159(5):609–15. doi: 10.1530/EJE-08-0084. Epub 2008 Jul 15. [DOI] [PubMed] [Google Scholar]
- 114.Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015. Feb;100(2):432–41. doi: 10.1210/jc.2014-2572. Epub 2014 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stan MN, Salvi M. MANAGEMENT OF ENDOCRINE DISEASE: Rituximab therapy for Graves’ orbitopathy - lessons from randomized control trials. Eur J Endocrinol. 2017. Feb;176(2):R101–R109. doi: 10.1530/EJE-16-0552. Epub 2016 Oct 19. [DOI] [PubMed] [Google Scholar]
- 116.Chen D, Gallagher S, Monson NL, Herbst R, Wang Y. Inebilizumab, a B Cell-Depleting Anti-CD19 Antibody for the Treatment of Autoimmune Neurological Diseases: Insights from Preclinical Studies. J Clin Med. 2016. Nov 24;5(12):107. doi: 10.3390/jcm5120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gallagher S, Turman S, Yusuf I, Akhgar A, Wu Y, Roskos LK, Herbst R, Wang Y. Pharmacological profile of MEDI-551, a novel anti-CD19 antibody, in human CD19 transgenic mice. Int Immunopharmacol. 2016. Jul;36:205–212. doi: 10.1016/j.intimp.2016.04.035. Epub 2016 May 7. [DOI] [PubMed] [Google Scholar]
- 118.Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, Woods R, Rowe DC, Cheng L, Cook K, Evans K, Sims GP, Pfarr DS, Bowen MA, Dall’Acqua W, Shlomchik M, Tedder TF, Kiener P, Jallal B, Wu H, Coyle AJ. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther. 2010. Oct;335(1):213–22. doi: 10.1124/jpet.110.168062. Epub 2010 Jul 6. Erratum in: J Pharmacol Exp Ther. 2011 Jan;336(1):294. Dall’Aqua, William [corrected to Dall’Acqua, William]. [DOI] [PubMed] [Google Scholar]
- 119.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004. Apr;20(4):441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 120.Lin JD, Wang YH, Fang WF, Hsiao CJ, Chagnaadorj A, Lin YF, Tang KT, Cheng CW. Serum BAFF and thyroid autoantibodies in autoimmune thyroid disease. Clin Chim Acta. 2016. Nov 1;462:96–102. doi: 10.1016/j.cca.2016.09.004. Epub 2016 Sep 8. [DOI] [PubMed] [Google Scholar]
- 121.Endocrine Abstracts (2020) 70 YI9 | DOI: 10.1530/endoabs.70.YI9 [DOI] [Google Scholar]
- 122.Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J Allergy Clin Immunol. 2020. Sep;146(3):467–478. doi: 10.1016/j.jaci.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 123.Henne KR, Ason B, Howard M, Wang W, Sun J, Higbee J, Tang J, Matsuda KC, Xu R, Zhou L, Chan JC, King C, Piper DE, Ketchem RR, Michaels ML, Jackson SM, Retter MW. Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J Pharmacol Exp Ther. 2015. Apr;353(1):119–31. doi: 10.1124/jpet.114.221242. Epub 2015 Feb 4. [DOI] [PubMed] [Google Scholar]
- 124.Newland AC, Sánchez-González B, Rejtő L, Egyed M, Romanyuk N, Godar M, Verschueren K, Gandini D, Ulrichts P, Beauchamp J, Dreier T, Ward ES, Michel M, Liebman HA, de Haard H, Leupin N, Kuter DJ. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020. Feb;95(2):178–187. doi: 10.1002/ajh.25680. Epub 2019 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Howard JF Jr, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, Beydoun S, Garrido FJRR, Piehl F, Rottoli M, Van Damme P, Vu T, Evoli A, Freimer M, Mozaffar T, Ward ES, Dreier T, Ulrichts P, Verschueren K, Guglietta A, de Haard H, Leupin N, Verschuuren JJGM; Efgartigimod MG Study Group. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019. Jun 4;92(23):e2661–e2673. doi: 10.1212/WNL.0000000000007600. Epub 2019 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B, Kiessling P, Woltering F, Griffin L, Van den Bergh P; MG0002 Investigators. Efficacy and Safety of Rozanolixizumab in Moderate to Severe Generalized Myasthenia Gravis: A Phase 2 Randomized Control Trial. Neurology. 2021. Feb 9;96(6):e853–e865. doi: 10.1212/WNL.0000000000011108. Epub 2020 Nov 20. Schnitzer T, Kuenkele KP, Rebers F, et al. Characterization of a recombinant, fully human monoclonal antibody directed against the human insulin-like growth factor-1 receptor. Eur J Cancer Suppl. 2006;4:66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sabini E, Mazzi B, Profilo MA, Mautone T, Casini G, Rocchi R, Ionni I, Menconi F, Leo M, Nardi M, Vitti P, Marcocci C, Marinò M. High Serum Cholesterol Is a Novel Risk Factor for Graves’ Orbitopathy: Results of a Cross-Sectional Study. Thyroid. 2018. Mar;28(3):386–394. doi: 10.1089/thy.2017.0430. Epub 2018 Feb 9. [DOI] [PubMed] [Google Scholar]
- 128.Lanzolla G, Sabini E, Profilo MA, Mazzi B, Sframeli A, Rocchi R, Menconi F, Leo M, Nardi M, Vitti P, Marcocci C, Marinò M. Relationship between serum cholesterol and Graves’ orbitopathy (GO): a confirmatory study. J Endocrinol Invest. 2018. Dec;41(12):1417–1423. doi: 10.1007/s40618-018-0915-z. Epub 2018 Jun 19. [DOI] [PubMed] [Google Scholar]
- 129.Moreno-Navarrete JM, Moreno M, Ortega F, Xifra G, Hong S, Asara JM, Serrano JCE, Jové M, Pissios P, Blüher M, Ricart W, Portero-Otin M, Fernández-Real JM. TSHB mRNA is linked to cholesterol metabolism in adipose tissue. FASEB J. 2017. Oct;31(10):4482–4491. doi: 10.1096/fj.201700161R. Epub 2017 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15(5):467–78. doi: 10.2174/138161209787315684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP, Sahebkar A. Anti-inflammatory Action of Statins in Cardiovascular Disease: the Role of Inflammasome and Toll-Like Receptor Pathways. Clin Rev Allergy Immunol. 2021. Apr;60(2):175–199. doi: 10.1007/s12016-020-08791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, Smith TJ, Douglas R. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmol. 2015. Mar;133(3):290–6. doi: 10.1001/jamaophthalmol.2014.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nilsson A, Tsoumani K, Planck T. Statins Decrease the Risk of Orbitopathy in Newly Diagnosed Patients with Graves Disease. J Clin Endocrinol Metab. 2021. Apr 23;106(5):1325–1332. doi: 10.1210/clinem/dgab070. [DOI] [PubMed] [Google Scholar]
- 134.Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Statins: pros and cons. Med Clin (Barc). 2018. May 23;150(10):398–402. doi: 10.1016/j.medcli.2017.11.030. Epub 2017 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998. Mar;274(3):C707–14. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 136.Mester T, Raychaudhuri N, Gillespie EF, Chen H, Smith TJ, Douglas RS. CD40 Expression in Fibrocytes Is Induced by TSH: Potential Synergistic Immune Activation. PLoS One. 2016. Sep 15;11(9):e0162994. doi: 10.1371/journal.pone.0162994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baccam M, Woo SY, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-kappa B, AP-1, and C/EBP. J Immunol. 2003. Mar 15;170(6):3099–108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 138.Lee HJ, Lombardi A, Stefan M, Li CW, Inabnet WB 3rd, Owen RP, Concepcion E, Tomer Y. CD40 Signaling in Graves Disease Is Mediated Through Canonical and Noncanonical Thyroidal Nuclear Factor κB Activation. Endocrinology. 2017. Feb 1;158(2):410–418. doi: 10.1210/en.2016-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao LQ, Wei RL, Cheng JW, Cai JP, Li Y. The expression of intercellular adhesion molecule-1 induced by CD40-CD40L ligand signaling in orbital fibroblasts in patients with Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2010. Sep;51(9):4652–60. doi: 10.1167/iovs.09-3789. Epub 2010 Jan 27. [DOI] [PubMed] [Google Scholar]
- 140.Douglas RS, Mester T, Ginter A, Kim DS. Thyrotropin receptor and CD40 mediate interleukin-8 expression in fibrocytes: implications for thyroid-associated ophthalmopathy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:26–37. [PMC free article] [PubMed] [Google Scholar]
- 141.Alaaeddine N, Hassan GS, Yacoub D, Mourad W. CD154: an immunoinflammatory mediator in systemic lupus erythematosus and rheumatoid arthritis. Clin Dev Immunol. 2012;2012:490148. doi: 10.1155/2012/490148. Epub 2011 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shock A, Burkly L, Wakefield I, Peters C, Garber E, Ferrant J, Taylor FR, Su L, Hsu YM, Hutto D, Amirkhosravi A, Meyer T, Francis J, Malcolm S, Robinson M, Brown D, Shaw S, Foulkes R, Lawson A, Harari O, Bourne T, Maloney A, Weir N. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther. 2015. Sep 3;17(1):234. doi: 10.1186/s13075-015-0757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000. Feb;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 144.Cordoba F, Wieczorek G, Audet M, Roth L, Schneider MA, Kunkler A, Stuber N, Erard M, Ceci M, Baumgartner R, Apolloni R, Cattini A, Robert G, Ristig D, Munz J, Haeberli L, Grau R, Sickert D, Heusser C, Espie P, Bruns C, Patel D, Rush JS. A novel, blocking, Fc-silent anti-CD40 monoclonal antibody prolongs nonhuman primate renal allograft survival in the absence of B cell depletion. Am J Transplant. 2015. Nov;15(11):2825–36. doi: 10.1111/ajt.13377. Epub 2015 Jul 2. [DOI] [PubMed] [Google Scholar]
- 145.Ristov J, Espie P, Ulrich P, Sickert D, Flandre T, Dimitrova M, Müller-Ristig D, Weider D, Robert G, Schmutz P, Greutmann B, Cordoba-Castro F, Schneider MA, Warncke M, Kolbinger F, Cote S, Heusser C, Bruns C, Rush JS. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am J Transplant. 2018. Dec;18(12):2895–2904. doi: 10.1111/ajt.14872. Epub 2018 May 24. [DOI] [PubMed] [Google Scholar]
- 146.Kahaly GJ, Stan MN, Frommer L, Gergely P, Colin L, Amer A, Schuhmann I, Espie P, Rush JS, Basson C, He Y. A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial. J Clin Endocrinol Metab. 2020. Mar 1;105(3):dgz013. doi: 10.1210/clinem/dgz013. [DOI] [PubMed] [Google Scholar]
- 147.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999. Jan 28;340(4):253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 148.den Broeder AA, Joosten LA, Saxne T, Heinegård D, Fenner H, Miltenburg AM, Frasa WL, van Tits LJ, Buurman WA, van Riel PL, van de Putte LB, Barrera P. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002. Apr;61(4):311–8. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005. Jun;24(2):117–9. doi: 10.1080/01676830590912562. [DOI] [PubMed] [Google Scholar]
- 150.Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves’ ophthalmopathy: a pilot study. Eye (Lond). 2005. Dec;19(12):1286–9. doi: 10.1038/sj.eye.6701768. [DOI] [PubMed] [Google Scholar]
- 151.Ayabe R, Rootman DB, Hwang CJ, Ben-Artzi A, Goldberg R. Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2014. Sep-Oct;30(5):415–9. doi: 10.1097/IOP.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 152.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol. 2003. Sep 15;171(6):3194–201. doi: 10.4049/jimmunol.171.6.3194. [DOI] [PubMed] [Google Scholar]
- 153.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 154.Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, Braverman LE, Roti E. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. J Clin Endocrinol Metab. 1996. Aug;81(8):2976–9. doi: 10.1210/jcem.81.8.8768861. [DOI] [PubMed] [Google Scholar]
- 155.Salvi M, Pedrazzoni M, Girasole G, Giuliani N, Minelli R, Wall JR, Roti E. Serum concentrations of proinflammatory cytokines in Graves’ disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol. 2000. Aug;143(2):197–202. doi: 10.1530/eje.0.1430197. [DOI] [PubMed] [Google Scholar]
- 156.Molnár I, Balázs C. High circulating IL-6 level in Graves’ ophthalmopathy. Autoimmunity. 1997;25(2):91–6. doi: 10.3109/08916939708996275. [DOI] [PubMed] [Google Scholar]
- 157.Azar AA, Michie AM, Tarafdar A, Malik N, Menon GK, Till KJ, Vlatković N, Slupsky JR. A novel transgenic mouse strain expressing PKCβII demonstrates expansion of B1 and marginal zone B cell populations. Sci Rep. 2020. Aug 4;10(1):13156. doi: 10.1038/s41598-020-70191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Decouvelaere AV, Morschhauser F, Buob D, Copin MC, Dumontet C. Heterogeneity of protein kinase C beta(2) expression in lymphoid malignancies. Histopathology. 2007. Apr;50(5):561–6. doi: 10.1111/j.1365-2559.2007.02666.x. [DOI] [PubMed] [Google Scholar]
- 159.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, Sawaqed R, Perkins D, Shahrara S, Sweiss NJ. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014. Mar 28;8:349–64. doi: 10.2147/DDDT.S41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010. Jul;40(7):1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 161.Pérez-Moreiras JV, Alvarez-López A, Gómez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic Plast Reconstr Surg. 2014. Mar-Apr;30(2):162–7. doi: 10.1097/IOP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 162.Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, Castillo Laguarta JM, Del Estad Cabello A, Gessa Sorroche M, España Gregori E, Sales-Sanz M; Tocilizumab in Graves Orbitopathy Study Group. Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol. 2018. Nov;195:181–190. doi: 10.1016/j.ajo.2018.07.038. Epub 2018 Aug 4. [DOI] [PubMed] [Google Scholar]
- 163.Pérez-Moreiras JV, Varela-Agra M, Prada-Sánchez MC, Prada-Ramallal G. Steroid-Resistant Graves’ Orbitopathy Treated with Tocilizumab in Real-World Clinical Practice: A 9-Year Single-Center Experience. J Clin Med. 2021. Feb 11;10(4):706. doi: 10.3390/jcm10040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khoo DH, Eng PH, Ho SC, Tai ES, Morgenthaler NG, Seah LL, Fong KS, Chee SP, Choo CT, Aw SE. Graves’ ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000. Dec;10(12):1093–100. doi: 10.1089/thy.2000.10.1093. [DOI] [PubMed] [Google Scholar]
- 165.Núñez Miguel R, Sanders J, Furmaniak J, Smith BR. Structure and activation of the TSH receptor transmembrane domain. Auto Immun Highlights. 2017. Dec;8(1):2. doi: 10.1007/s13317-016-0090-1. Epub 2016 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rapoport B, McLachlan SM. TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective. Endocr Rev. 2016. Apr;37(2):114–34. doi: 10.1210/er.2015-1098. Epub 2016 Jan 22. Erratum in: Endocr Rev. 2020 Dec 1;41(6): [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Chazenbalk GD, Tanaka K, Nagayama Y, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology. 1997. Jul;138(7):2893–9. doi: 10.1210/endo.138.7.5259. [DOI] [PubMed] [Google Scholar]
- 168.Rapoport B, Aliesky HA, Chen CR, McLachlan SM. Evidence that TSH Receptor A-Subunit Multimers, Not Monomers, Drive Antibody Affinity Maturation in Graves’ Disease. J Clin Endocrinol Metab. 2015. Jun;100(6):E871–5. doi: 10.1210/jc.2015-1528. Epub 2015 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang M, Phuong K, Tong T, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW (2000) The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517. doi: 10.1210/en.141.9.351433. [DOI] [PubMed] [Google Scholar]
- 170.Wu T, Mester T, Gupta S, Sun F, Smith TJ, Douglas RS. Thyrotropin and CD40L Stimulate Interleukin-12 Expression in Fibrocytes: Implications for Pathogenesis of Thyroid-Associated Ophthalmopathy. Thyroid. 2016. Dec;26(12):1768–1777. doi: 10.1089/thy.2016.0243. Epub 2016 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Evans M, Sanders J, Tagami T, Sanders P, Young S, Roberts E, Wilmot J, Hu X, Kabelis K, Clark J, Holl S, Richards T, Collyer A, Furmaniak J, Smith BR. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf). 2010. Sep;73(3):404–12. doi: 10.1111/j.1365-2265.2010.03831.x. Epub 2010 Jun 9. [DOI] [PubMed] [Google Scholar]
- 172.Rees Smith B, Sanders J, Evans M, Tagami T, Furmaniak J. TSH receptor - autoantibody interactions. Horm Metab Res. 2009. Jun;41(6):448–55. doi: 10.1055/s-0029-1220913. [DOI] [PubMed] [Google Scholar]
- 173.Sanders J, Evans M, Premawardhana LD, Depraetere H, Jeffreys J, Richards T, Furmaniak J, Rees Smith B. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003. Jul 12;362(9378):126–8. doi: 10.1016/s0140-6736(03)13866-4. [DOI] [PubMed] [Google Scholar]
- 174.Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Núñez Miguel R, Blundell TL, Furmaniak J, Rees Smith B. Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid. 2004. Aug;14(8):560–70. doi: 10.1089/1050725041692918. [DOI] [PubMed] [Google Scholar]
- 175.Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, Summerhayes S, Harris R, Reeve M, Coco G, Zanchetta R, Chen S, Furmaniak J, Smith BR. A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid. 2008. Jul;18(7):735–46. doi: 10.1089/thy.2007.0327. [DOI] [PubMed] [Google Scholar]
- 176.Furmaniak J, Sanders J, Young S, Kabelis K, Sanders P, Evans M, Clark J, Wilmot J, Rees Smith B. In vivo effects of a human thyroid-stimulating monoclonal autoantibody (M22) and a human thyroid-blocking autoantibody (K1–70). Auto Immun Highlights. 2011. Sep 14;3(1):19–25. doi: 10.1007/s13317-011-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sanders P, Young S, Sanders J, Kabelis K, Baker S, Sullivan A, Evans M, Clark J, Wilmot J, Hu X, Roberts E, Powell M, Núñez Miguel R, Furmaniak J, Rees Smith B. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol. 2011. Feb 15;46(2):81–99. doi: 10.1530/JME-10-0127. [DOI] [PubMed] [Google Scholar]
- 178.Marcinkowski P, Hoyer I, Specker E, Furkert J, Rutz C, Neuenschwander M, Sobottka S, Sun H, Nazare M, Berchner-Pfannschmidt U, von Kries JP, Eckstein A, Schülein R, Krause G. A New Highly Thyrotropin Receptor-Selective Small-Molecule Antagonist with Potential for the Treatment of Graves’ Orbitopathy. Thyroid. 2019. Jan;29(1):111–123. doi: 10.1089/thy.2018.0349. Epub 2018 Dec 15. [DOI] [PubMed] [Google Scholar]
- 179.Latif R, Ali MR, Ma R, David M, Morshed SA, Ohlmeyer M, Felsenfeld DP, Lau Z, Mezei M, Davies TF. New small molecule agonists to the thyrotropin receptor. Thyroid. 2015. Jan;25(1):51–62. doi: 10.1089/thy.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Neumann S, Eliseeva E, McCoy JG, Napolitano G, Giuliani C, Monaco F, Huang W, Gershengorn MC. A new small-molecule antagonist inhibits Graves’ disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011. Feb;96(2):548–54. doi: 10.1210/jc.2010-1935. Epub 2010 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Neumann S, Nir EA, Eliseeva E, Huang W, Marugan J, Xiao J, Dulcey AE, Gershengorn MC. A selective TSH receptor antagonist inhibits stimulation of thyroid function in female mice. Endocrinology. 2014. Jan;155(1):310–4. doi: 10.1210/en.2013-1835. Epub 2013 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tabasum A, Khan I, Taylor P, Das G, Okosieme OE. Thyroid antibody-negative euthyroid Graves’ ophthalmopathy. Endocrinol Diabetes Metab Case Rep. 2016;2016:160008. doi: 10.1530/EDM-16-0008. Epub 2016 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wall JR, Lahooti H, El Kochairi I, Lytton SD, Champion B. Thyroid-stimulating immunoglobulins as measured in a reporter bioassay are not detected in patients with Hashimoto’s thyroiditis and ophthalmopathy or isolated upper eyelid retraction. Clin Ophthalmol. 2014. Oct 9;8:2071–6. doi: 10.2147/OPTH.S67098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002. Jan 15;168(2):942–50. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 185.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003. Jun 15;170(12):6348–54. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 186.Crudden C, Girnita A, Girnita L. Targeting the IGF-1R: The Tale of the Tortoise and the Hare. Front Endocrinol (Lausanne). 2015. Apr 27;6:64. doi: 10.3389/fendo.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reiter E, Ayoub MA, Pellissier LP, Landomiel F, Musnier A, Tréfier A, Gandia J, De Pascali F, Tahir S, Yvinec R, Bruneau G, Poupon A, Crépieux P. β-arrestin signalling and bias in hormone-responsive GPCRs. Mol Cell Endocrinol. 2017. Jul 5;449:28–41. doi: 10.1016/j.mce.2017.01.052. Epub 2017 Feb 4. [DOI] [PubMed] [Google Scholar]
- 188.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013. Mar;12(3):205–16. doi: 10.1038/nrd3954. Epub 2012 Feb 15. [DOI] [PubMed] [Google Scholar]
- 189.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009. May 1;284(18):11953–62. doi: 10.1074/jbc.M808176200. Epub 2009 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lin FT, Daaka Y, Lefkowitz RJ. beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem. 1998. Nov 27;273(48):31640–3. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 191.Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ, Larsson O. {beta}-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005. Jul 1;280(26):24412–9. doi: 10.1074/jbc.M501129200. Epub 2005 May 3. [DOI] [PubMed] [Google Scholar]
- 192.Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A, Lefkowitz RJ, Larsson O. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007. Apr 13;282(15):11329–38. doi: 10.1074/jbc.M611526200. Epub 2007 Feb 15. [DOI] [PubMed] [Google Scholar]
- 193.Zheng H, Shen H, Oprea I, Worrall C, Stefanescu R, Girnita A, Girnita L. β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing’s sarcoma. Proc Natl Acad Sci U S A. 2012. Dec 11;109(50):20620–5. doi: 10.1073/pnas.1216348110. Epub 2012 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16(4):251–7. doi: 10.3109/08916939309014643. [DOI] [PubMed] [Google Scholar]
- 195.Reiss K, Tu X, Romano G, Peruzzi F, Wang JY, Baserga R. Intracellular association of a mutant insulin-like growth factor receptor with endogenous receptors. Clin Cancer Res. 2001. Jul;7(7):2134–44. [PubMed] [Google Scholar]
- 196.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004. Oct;89(10):5076–80. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 197.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007. Mar 1;178(5):3281–7. doi: 10.4049/jimmunol.178.5.3281. [DOI] [PubMed] [Google Scholar]
- 198.Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008. Oct 15;181(8):5768–74. doi: 10.4049/jimmunol.181.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008. Sep 15;181(6):4397–405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Varewijck AJ, Boelen A, Lamberts SW, Fliers E, Hofland LJ, Wiersinga WM, Janssen JA. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013. Feb;98(2):769–76. doi: 10.1210/jc.2012-2270. Epub 2013 Jan 7. [DOI] [PubMed] [Google Scholar]
- 201.Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Köhrle J, Eckstein A, Schomburg L. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013. Feb;98(2):752–60. doi: 10.1210/jc.2012-1771. Epub 2012 Dec 21. [DOI] [PubMed] [Google Scholar]
- 202.Yee D, Cullen KJ, Paik S, Lippman ME, Rosen N (1989) Growth Regulation of Human Breast Cancer by Insulin-Like Growth Factors. In: LeRoith D, Raizada MK (eds) Molecular and Cellular Biology of Insulin-like Growth Factors and Their Receptors. Springer, Boston, MA. 10.1007/978-1-4684-5685-1_21 [DOI] [Google Scholar]
- 203.Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. TSH/IGF-1 Receptor Cross Talk in Graves’ Ophthalmopathy Pathogenesis. J Clin Endocrinol Metab. 2016. Jun;101(6):2340–7. doi: 10.1210/jc.2016-1315. Epub 2016 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chaplin R, Thach L, Hollenberg MD, Cao Y, Little PJ, Kamato D. Insights into cellular signalling by G protein coupled receptor transactivation of cell surface protein kinase receptors. J Cell Commun Signal. 2017. Jun;11(2):117–125. doi: 10.1007/s12079-017-0375-9. Epub 2017 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gavi S, Shumay E, Wang HY, Malbon CC. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab. 2006. Mar;17(2):48–54. doi: 10.1016/j.tem.2006.01.006. Epub 2006 Feb 7. [DOI] [PubMed] [Google Scholar]
- 206.Krieger CC, Boutin A, Jang D, Morgan SJ, Banga JP, Kahaly GJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC. Arrestin-β−1 Physically Scaffolds TSH and IGF1 Receptors to Enable Crosstalk. Endocrinology. 2019. Jun 1;160(6):1468–1479. doi: 10.1210/en.2019-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Smith TJ. Challenges in Orphan Drug Development: Identification of Effective Therapy for Thyroid-Associated Ophthalmopathy. Annu Rev Pharmacol Toxicol. 2019. Jan 6;59:129–148. doi: 10.1146/annurev-pharmtox-010617-052509. Epub 2018 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, Zakowski MF, Frankel S, Peifer M, Thomas RK, Ladanyi M, Pao W. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One. 2009. Oct 6;4(10):e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, Eckhardt SG, Eid JE, Greig G, Habben K, McCarthy CD, Gore L. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010. Apr 15;16(8):2458–65. doi: 10.1158/1078-0432.CCR-09-3220. Epub 2010 Apr 6. [DOI] [PubMed] [Google Scholar]
- 210.Hua H, Kong Q, Yin J, Zhang J, Jiang Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol. 2020. Jun 3;13(1):64. doi: 10.1186/s13045-020-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sarfstein R, Belfiore A, Werner H. Identification of Insulin-Like Growth Factor-I Receptor (IGF-IR) Gene Promoter-Binding Proteins in Estrogen Receptor (ER)-Positive and ER-Depleted Breast Cancer Cells. Cancers (Basel). 2010. Mar 25;2(2):233–61. doi: 10.3390/cancers2020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Qu X, Wu Z, Dong W, Zhang T, Wang L, Pang Z, Ma W, Du J. Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy. Oncotarget. 2017. Apr 25;8(17):29501–29518. doi: 10.18632/oncotarget.15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for Thyroid-Associated Ophthalmopathy. N Engl J Med. 2017. May 4;376(18):1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves’ ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998. Jul;82(7):773–9. doi: 10.1136/bjo.82.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, Fleming JC, Fowler BT, Marcocci C, Marinò M, Antonelli A, Dailey R, Harris GJ, Eckstein A, Schiffman J, Tang R, Nelson C, Salvi M, Wester S, Sherman JW, Vescio T, Holt RJ, Smith TJ. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020. Jan 23;382(4):341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 216.Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021. Apr 15:S2213-8587(21)00056-5. doi: 10.1016/S2213-8587(21)00056-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 217.Okuyama T, Kyohara M, Terauchi Y, Shirakawa J. The Roles of the IGF Axis in the Regulation of the Metabolism: Interaction and Difference between Insulin Receptor Signaling and IGF-I Receptor Signaling. Int J Mol Sci. 2021. Jun 24;22(13):6817. doi: 10.3390/ijms22136817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Markham A Teprotumumab: First Approval. Drugs. 2020. Apr;80(5):509–512. doi: 10.1007/s40265-020-01287-y. [DOI] [PubMed] [Google Scholar]
- 219.Sears CM, Azad AD, Dosiou C, Kossler AL. Teprotumumab for Dysthyroid Optic Neuropathy: Early Response to Therapy. Ophthalmic Plast Reconstr Surg. 2020. Sep 22. doi: 10.1097/IOP.0000000000001831. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 220.Slentz DH, Smith TJ, Kim DS, Joseph SS. Teprotumumab for Optic Neuropathy in Thyroid Eye Disease. JAMA Ophthalmol. 2021. Feb 1;139(2):244–247. doi: 10.1001/jamaophthalmol.2020.5296. [DOI] [PubMed] [Google Scholar]
- 221.Hwang CJ, Nichols EE, Chon BH, Perry JD. Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. Eur J Ophthalmol. 2021. Feb 1:1120672121991042. doi: 10.1177/1120672121991042. [DOI] [PubMed] [Google Scholar]
- 222.Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Rep. 2020. May 15;19:100744. doi: 10.1016/j.ajoc.2020.100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic thyroid eye disease. Orbit. 2021. Jun 1:1–8. doi: 10.1080/01676830.2021.1933081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 224.Larché M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005. Apr;11(4 Suppl):S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 225.Anderton SM, Viner NJ, Matharu P, Lowrey PA, Wraith DC. Influence of a dominant cryptic epitope on autoimmune T cell tolerance. Nat Immunol. 2002. Feb;3(2):175–81. doi: 10.1038/ni756. Epub 2002 Jan 14. [DOI] [PubMed] [Google Scholar]
- 226.Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005. Apr;115(4):888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, Payne AS. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016. Jul 8;353(6295):179–84. doi: 10.1126/science.aaf6756. Epub 2016 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]