Abstract
Background and Aims
Endoscopy and the use of faecal calprotectin [faecal CP] are among the least-favoured methods for assessing disease activity by inflammatory bowel disease [IBD] patients; the handling/processing of faecal samples is also impractical. Therefore, we sought to develop a novel neo-epitope serum calprotectin enzyme-linked immunosorbent assay [ELISA], CPa9-HNE, with the aim of quantifying neutrophil activity and neutrophil extracellular trap [NET]-osis and proposing a non-invasive method for monitoring disease activity in IBD patients.
Methods
In vitro cleavage was performed by mixing calprotectin [S100A9/S100A8] with human neutrophil elastase [HNE], and a novel HNE-derived calprotectin neo-epitope [CPa9-HNE] was identified by mass spectrometry for ELISA development. The CPa9-HNE ELISA was quantified in supernatants from ex vivo activated neutrophils and serum samples from patients with ulcerative colitis [UC, n = 43], Crohn’s disease [CD, n = 93], and healthy subjects [HS, n = 23]. For comparison, faecal CP and MRP8/14 biomarkers were also measured.
Results
CPa9-HNE was specific for activated neutrophils ex vivo. Serum CPa9-HNE levels were 4-fold higher in CD [p <0.0001] and UC [p <0.0001] patients than in HS. CPa9-HNE correlated well with the Simple Endoscopic Score [SES]-CD score [r = 0.61, p <0.0001], MES [r = 0.46, p = 0.0141], and the full Mayo score [r = 0.52, p = 0.0013]. CPa9-HNE was able to differentiate between CD and UC patients in endoscopic remission and moderate/severe disease activity (CD: area under the curve [AUC] = 0.82 [p = 0.0003], UC: AUC = 0.87 [p = 0.0004]). The performance of CPa9-HNE was equipotent or slightly better than that of faecal CP.
Conclusions
Serum CPa9-HNE levels were highly associated with CD and UC patients. CPa9-HNE correlated with the SES-CD score and the full Mayo score, indicating a strong association with disease activity.
Keywords: Calprotectin, neutrophil granulocyte, neutrophil elastase, neutrophil extracellular traps [NETs], biomarkers, inflammation, IBD
1. Introduction
Endoscopic remission, also referred to as mucosal healing, for inflammatory bowel disease [IBD] has become the desired endpoint for therapeutic interventions; ulcerative colitis [UC] and Crohn’s disease [CD] patients achieving endoscopic remission have a significantly reduced probability of disease relapse and hospitalisation, including surgery.1 Although sequential endoscopy to monitor disease activity and relapse is effective, it is not the preferred option because it is costly and currently ranked as the least acceptable tool for evaluating disease activity by patients with CD.1,2 There is a medical need for non-invasive biomarkers that can be applied to monitor disease activity and be used as objective measures to determine when an endoscopy is essential to perform,1–5 potentially reducing the number of sequential endoscopies. Calprotectin can be measured in the faeces [faecal CP] of patients with IBD6–8 and has proven to be a robust biomarker for distinguishing IBD patients from patients with irritable bowel syndrome [IBS].3,6,9–12 However, faecal calprotectin ranks among the least favourable markers among IBD patients, who strongly prefer blood-based biomarkers over faecal markers.1,13–15 The difficulties linked to stool sample collection and sample processing for the faecal calprotectin assays and their diminished usefulness for CD patients are some of their disadvantages; faecal samples and faecal consistency also affect faecal calprotectin accuracy, with their high day-to-day and sample-to-sample variations, which is why serum samples are preferred over faecal samples for biomarker analysis.1 Therefore, a medical need exists for blood-based biomarkers to monitor disease activity for IBD.1
S100 protein monomers, S100A8 and S100A9, can form heterodimers and heterotetramers, which constitute the structure of calprotectin.16 Calprotectin is expressed intracellularly, mainly by neutrophil granulocytes, and it comprises around 60% of neutrophil granulocytes’ total cytosolic matter.6 Calprotectin is part of the innate inflammasome and has been shown to play an important role as a chemokine in the activation and mobilisation of the innate immune response.17 Calprotectin’s ability to act as chemokine and an indirect mediator of CXCL-2 release through TLR-2 and 4 receptor activation emphasises its importance as an immune regulator.18,19 Neutrophil granulocytes are highly present in inflamed tissues and are one of the strongest mediators of inflammation and chronic tissue remodelling in IBD.20–22 Calprotectin and human neutrophil elastase [HNE] are secreted by the neutrophils in inflamed tissues as a result of neutrophil extracellular trap formation [NETosis],23–25 but calprotectin may also be secreted through a novel pathway requiring the activation of protein kinase C via an intact microtubule network.26 Consequently, the extracellular matrix [ECM] and other secreted proteins will be degraded by HNE and other proteases, resulting in the generation of neo-epitope containing protein fragments27,28 [Figure 1]. HNE has also been linked to tissue inflammation in IBD.29 An association between neo-epitope protein fragments of collagen degradation/formation with disease activity and severity has been demonstrated in IBD patients and pre-clinical models27,30–34 and in relation to treatment response.35,36 The aims of the present study were to: 1] generate a robust and reliable serum/plasma calprotectin biomarker that measures neutrophil granulocytes activity by developing a neo-epitope biomarker enzyme-linked immunosorbent assasy [ELISA] against the HNE-mediated degradation of calprotectin [CPa9-HNE]; and 2] evaluate the CPa9-HNE assay biological relevance in serum samples from patients with CD and UC and compare the performance of the calprotectin neo-epitope vs. conventional calprotectin protein assay and neutrophil count as a non-invasive tool for monitoring IBD disease activity.
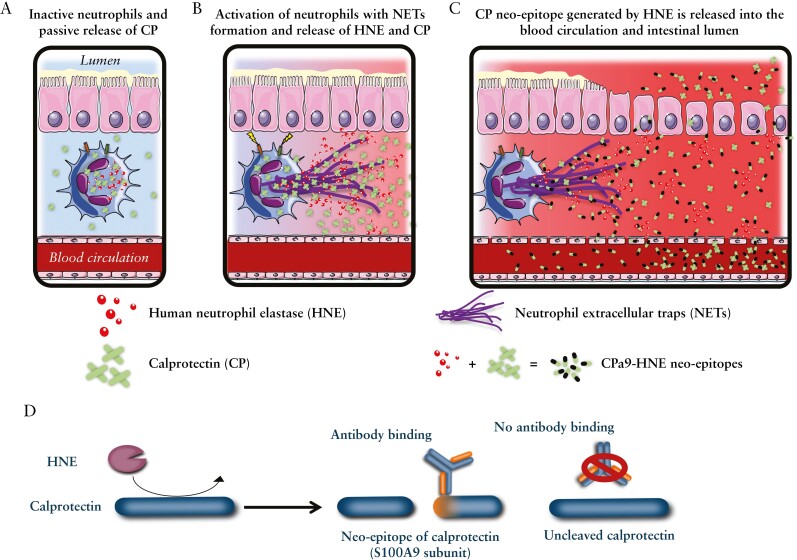
Figure 1.
Neutrophil granulocytes release both calprotectin [CP] and human neutrophil elastase [HNE]. A] Inactive neutrophils express CP and HNE, where CP is passively released. B] Upon activation, the neutrophils will initiate the process of neutrophil extracellular trap [NET] formation, which releases CP and HNE in huge amounts into the extracellular space, where HNE will begin to degrade the surrounding tissue and proteins, including CP. C] CP is cleaved by HNE, and small HNE-derived CP neo-epitope protein fragments [CPa9-HNE] are released into the blood and intestinal lumen and can be quantified by ELISA using protein fingerprint technology. D] Simplified depiction of how HNE cleaves CP and how specific monoclonal antibodies recognise only the specific HNE-derived CP neo-epitope fragment. ELISA, enzyme-linked immunosorbent assay.
2. Methods
2.1. In vitro cleavage of calprotectin S100A9/S100A8 by human neutrophil elastase
Calprotectin neo-epitopes were generated in vitro by adding active native HNE purified from human cells by SDS-page [>95% purity] [Abcam, cat. no, ab80475] to samples containing recombinant calprotectin purified from E. coli [Kerafast, cat. no. EVU204] to a final volume of 150 µL, a heterodimer composed of the proteins S100A9 and S100A8, in a protein/protease ratio of 100:1 [10 µg of calprotectin to 0.1 µg of HNE] and incubated for 24 h at 37°C [in darkness with shaking]. The proteolytic reaction was inhibited after 24 h by adding 5 mM ethylenediaminetetraacetic acid [EDTA] stop buffer. Vials containing only protease buffer, calprotectin without HNE, or HNE without calprotectin served as experimental controls.
2.2. Mass spectrometry analysis
Following the manufacturer’s instructions, 100 μl of the cleaved samples or controls were desalted with reverse-phase Vydac UltraMicro Spin C18 columns [Harvard Apparatus, cat. no. 74-7206]. Non-targeted mass spectrometry analysis was performed on a quadrupole Orbitrap benchtop mass spectrometer [QExactive, Thermo Scientific] equipped with an EASY nano-LC 1000 system [ThermoFisher Scientific]. For liquid chromatography, the mobile phase A consisted of Pierce™ water containing 0.1% formic acid [ThermoFisher Scientific, cat. no. PI85170] and mobile phase B consisting of acetonitrile containing 0.1% formic acid [ThermoFisher Scientific, cat. no. PI85174]. Separation was performed on 75 μm × 25 cm Acclaim Pepmap™ RSLC C18 capillary columns packed with 2-μm particles [ThermoFisher Scientific]. A spray voltage of + 2000 V was used with a heated ion transfer setting of 275°C for desolvation. The online reverse-phase separation was performed using a flow rate of 300 nl/min and a linear binary 85-min gradient [achieved by mixing mobile phase A with mobile phase B], starting with 3% mobile phase B for 4 min, then going to 35% mobile phase B in 64 min, and 45% mobile phase B in 5 min, followed by 90% mobile phase B for 5 min, and finally isocratic 90% solvent B for 7 min. Finally, the mobile phase B concentration was increased to 90% in 5 min and kept at 90% for 7 min. Mass spectrometry [MS] scans [400–1200 m/z] were recorded in the mass analyser set at a resolution of 70 000 at 200 m/z, 1 × 106 automatic gain control target, and 100 ms maximum ion injection time [44]. The MS was followed by data-dependent collision-induced dissociation MS/MS scans at a resolution of 17 500 on the 15 most intense multiply charged ions at a 2 × 104 intensity threshold, 2 m/z isolation width, and dynamic exclusion enabled for 30 s.
2.3. Calprotectin S100A9/S100A8 neo-epitope identification
Recorded raw files were analysed using Proteome Discoverer 2.1 [ThermoFisher Scientific]. The processing workflow nodes were as follows: Spectrum Selector for spectra pre-processing [precursor mass range: 100–10 000 Da; S/N threshold: 1.5], Sequest-HT search engine [Protein Database: UniProt proteome ID UP000005640, n20200 downloaded 12/06/2015; enzyme: no enzyme; maximum missed cleavage sites: 2, peptide length range 6–144 amino acids; precursor mass tolerance: 10 ppm; fragment mass tolerance: 0.02 Da; dynamic modification: oxidation; and Percolator for peptide validation [false-discovery rate [FDR] <0.01 based the on peptide q-value]. Peptide intensities were quantified using Proteome Discoverer’s proprietary algorithm.
2.4. Monoclonal antibody production and clone characterisation for HNE-generated calprotectin S100A9/S100A8 neo-epitope
The generation of monoclonal antibodies was carried out as follows. 6-7 week-old Balb/C mice were immunised subcutaneously with 200 μL emulsified antigen and 100 μg immunogenic peptide [KLGHPDTLNQ-GGC-Keyhole Limpet Hemocyanin] using Stimune Immunogenic Adjuvant [Invitrogen]. The mouse with the highest and best reactivity serum titre was selected for monoclonal antibody production. Splenocytes were fused with SP2/0 myeloma cells to produce hybridoma cells, and these were cultured into 96-well microtitre plates. The supernatants were screened for reactivity against the selection peptide [KLGHPDTLNQ] and elongated [VKLGHPDTLNQ], truncated [LGHPDTLNQ], and non-sense peptides [YRDDLKKLLET] as well as against native material [cleavage material] in an indirect competitive ELISA. The clone with the best reactivity was chosen for ELISA development, and antibodies were purified [99% purity] using protein-G-columns according to the manufacturer’s instructions [GE Healthcare Life Sciences, Little Chalfont, UK].
2.5. Principle of calprotectin S100A9 neo-epitope [CPa9-HNE] chemiluminescence ELISA in serum
CPa9-HNE levels in the samples were assessed with a solid-phase competitive chemiluminescence-based enzyme-linked immunosorbent assay [ELISA]. Plates with 96 wells, pre-coated with streptavidin [Roche Diagnostics cat. No. 11940279, Hvidovre, Denmark] were coated with a biotinylated antigen [KLGHPDTLNQ-K-Biotin] by incubation with this antigen for 30 min at room temperature. Unbound biotinylated coater antigen was discarded, and the wells were washed with washing buffer using a standardised ELISA plate washing machine [BioTek® Instruments, Microplate washer, ELx405 Select CW, Winooski, USA]. Pre-diluted samples in assay buffer [10mM PBS-BTB, pH 7.4], standards, and controls were added to the wells and incubated with the primary monoclonal antibody against the CPa9-HNE neo-epitope at 20°C for 1 h and agitated at 300 rpm. The unbound primary antibody and sample were discarded, and the wells were washed with buffer [20mM Tris, pH 7.2]. Subsequently, HRP-conjugated AffiniPure Rabbit anti-mouse IgG secondary antibodies [Jackson cat. No 315-035-045] were added to the wells and incubated for 1 h at 20°C. Unbound secondary antibody was discarded by washing the wells in washing buffer. Chemiluminescence substrate [Roche Diagnostic’s cat. No. 11582950001] was added to the wells [100 µl/well], and the plates were incubated for 3 min at room temperature before reading. Finally, an ELISA reader [SpectraMax M5; Molecular Devices, Wokingham Berkshire, UK] was used to quantify the relative light units emitted from the plates. A standard curve was plotted using a four-parametric mathematical fit model.
The procedures for the faecal CP [Inova, USA] and MRP8/14 ELISA [Bühlmann serum-CP] are available in the Supplementary data.
2.6. IBD patient demographics
In total, two patient cohorts were included in this together with 23 healthy subjects in a retrospective study. Serum was available for a total of 40 UC [Cohort 1] and 85 CD [Cohort 2] patients [patients recruited from Clinical Hospital Centre Zagreb, Department of Gastroenterology and Hepatology, Croatia] and 23 healthy subjects [HS; obtained from BioIVT, USA]. Serum samples and faecal samples were collected at the same visit after informed signed consent and approval by the local ethics committee. All samples were stored at minimum -70℃ and were shipped in a box containing dry ice. Upon arrival. the samples were stored at -70℃ until assayed. Demographical data for the UC and CD patients, disease history, and therapy were obtained from electronic medical records and questionnaires. Patients with comorbidities and extra-intestinal manifestations were excluded from the study. Anthropometric parameters were measured upon inclusion. For available patients, endoscopic disease activity was based on the simple endoscopic score for CD [SES-CD], and for UC, the Mayo Endoscopic Score [MES] and the full Mayo score were used. Endoscopy was performed within 3 months of blood sampling and endoscopic scores were prospectively validated based on routine endoscopy and scoring by an experienced IBD endoscopist. To minimise the effect of time lag, we have included patients without significant therapeutic intervention in this period of serum sampling and endoscopy [steroid induction, introduction of new biological drug, drug dosage or interval changes]. If endoscopic scoring was not available for UC patients, the partial Mayo [pMayo] score was applied. Patient stratification based on endoscopic scores was done as follows: SES-CD [remission = 0–2, mild = 3–6, moderate = 7–15, severe >15], MES [remission = 0, mild = 1, moderate = 2, severe = 3], full Mayo [remission = 0–1, mild = 2–5, moderate = 6–10, severe > 10]. Only information on gender and age was available for the HS.
2.7. Ex vivo model of neutrophil activity
An ex vivo model of neutrophil activation using purified neutrophils, cultured bacteria, and human cartilage was used as a tissue control. In brief, the cartilage was extracted from patients suffering hip or head of femur fracture, which underwent a hip replacement procedure. Only healthy cartilage with no macroscopic sign of damage was used. The same donor was used for this experiment by using five random discs per experimental condition to account for variation in size. Neutrophil granulocytes from three healthy volunteers were isolated from fresh peripheral blood in EDTA tubes using a negative selection isolation kit [EasySep Human Neutrophil Isolation Kit, StemCell Technologies cat. No. 17957]. Following isolation, 3 x 106 neutrophils were added to human tissue culture plates and then Staphylococcus aureus [1 x 106 cfu] in 1 mL RPMI. Cultures were incubated for 4–24 h in the presence or absence of neutrophil agonists, such as 1 μM/mL of phorbol myristate acetate [PMA] and 1 μM/mL of calcium ionophore [Cal ion]. Supernatants were collected 4 h and 24 h after neutrophil stimulation, for biomarker measurement.
2.8. Statistical analysis
To achieve normal distribution, log transformation of the data was applied prior to statistical analysis. Student’s t test and one-way analysis of variance [ANOVA] for normally distributed data were applied to analyse statistical differences. If a normal distribution was not achieved with log transformation, the Mann–Whitney U test and the Kruskal–Wallis test were applied. The FDR method [FDR = 5%] was used for multiple comparisons’ correction. The chi square test was applied for categorial variables. Spearman’s rho for correlations was carried out on non-parametric data and the Pearson correlation was applied for parametric data. Biomarker levels are presented as non-log transformed data. To assess the diagnostic power of the biomarker, receiver operating characteristic curves were calculated. A p-value of ≤0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 8.0 and MedCalc 19.3. Figures were made using GraphPad Prism version 8.0.
2.9. Ethical statement
All patients included in this study provided written informed consent, and the study protocol for including serum from Crohn’s disease and ulcerative colitis was approved by the Ethical Committee of University Hospital Centre Zagreb [ID number: 02/21 AG], and for including the human tissues and human neutrophil granulocytes for biomarker analysis was approved by West of Scotland Research Ethics Service. Ethical approval for the study was granted by the West of Scotland Research Ethics Service [project reference: REC14/WS/1035; ID number: 265704].
3. Results
3.1. Development of human neutrophil elastase-mediated degradation of calprotectin S100A9 [CPa9-HNE] ELISA
3.1.1. Calprotectin neo-epitope generation and identification
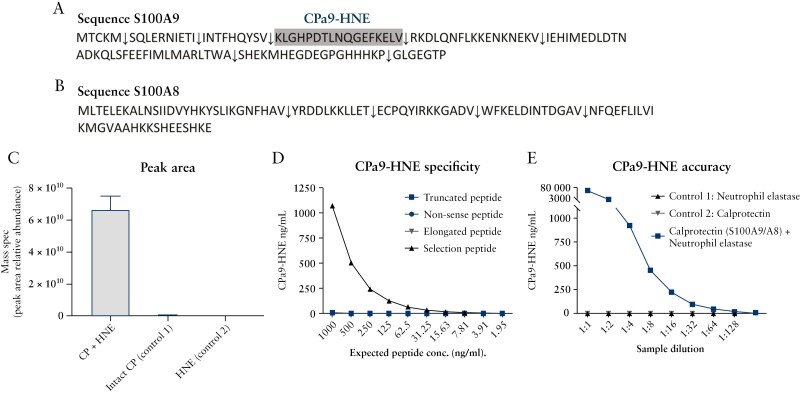
Initially calprotectin cleavage experiments with HNE were conducted to identify potential unique calprotectin neo-epitopes through MS analysis. The non-targeted MS analysis and subsequent neo-epitope identification applying UniProt proteome ID UP000005640 revealed that several neo-epitope calprotectin fragments were generated by HNE [Supplementary Table 1] from both S100A9 [Figure 2A] and S100A8 [Figure 2B] proteins. The MS analysis demonstrated that the HNE-derived calprotectin neo-epitope [KLGHPDTLNQGEFKELV] was the most abundant peptide and was present in samples containing full-length human calprotectin and HNE. Only minuscule amounts of the neo-epitope were present in the control samples exclusively containing full-length human calprotectin, and the CPa9-HNE fragment was not detectable in the sample containing HNE only [Figure 2C]. The identified unique calprotectin neo-epitope was named CPa9-HNE and selected for ELISA development for the quantification of HNE-mediated degradation of calprotectin [S100A9].
Figure 2.
Overview of the calprotectin sequence of both A] S100A9 and B] S100A8 dimers. The calprotectin fragments generated by human neutrophil elastase [HNE]-mediated cleavage and selected for ELISA development are depicted by an arrow down [↓], where the neo-epitope selected for ELISA development is depicted by the color grey. Relative peptide abundance of selected HNE-derived calprotectin neo-epitope cleavage fragments; antibody specificity test and biological evaluation of the CPa9-HNE ELISA. C] The relative peptide abundance for the CPa9-HNE sequence was tested using mass spectrometry in samples containing HNE-cleaved calprotectin, full-length calprotectin, or HNE. Furthermore, D] antibody specificity was tested against the selection peptide, elongated peptide, truncated peptide, non-sense peptide, and finally E] biological evaluation of the CPa9-HNE assay was tested in samples containing HNE + calprotectin, calprotectin [Control 1], and HNE [Control 2]. Error bars represent the standard error of the mean [SEM]. ELISA, enzyme-linked immunosorbent assay.
3.1.2. Specificity, accuracy, and precision of the CPa9-HNE assay
To evaluate the CPa9-HNE assay, the specificity of the monoclonal antibody was tested against elongated peptide, truncated peptide, and non-sense peptide [peptide sequence for NBH-225 [CPa8-HNE]]. The CPa9-HNE antibody demonstrated reactivity only toward the CPa9-HNE neo-epitope sequence in a dosage-dependent manner; however, no reactivity towards elongated, truncated, and non-sense peptides was observed [Figure 2D]. In samples containing HNE cleaved calprotectin only, intact full-length human calprotectin only, or HNE only, the CPa9-HNE antibody was able to identify the specific neo-epitope sequence only when calprotectin cleaved by HNE was present [Figure 2E]. The final specifications of the CPa9-HNE ELISA can be reviewed in Table 1, which includes the assay variability and analyte stability. Overall, the CPa9-HNE assay demonstrated low inter-/intra-variations below 10% and analyte recoveries for 24 h at 4°C/20°C of 91% and 95%, respectively, with interference only observed in samples containing high levels of haemoglobin [5 mg] [Table 1].
Table 1.
CPa9-HNE ELISA parameters.
| Species possibilities | Human |
|---|---|
| ELISA range [ng/ml] LLMR-ULMR | 9–493 ng/mL [adjusted: 36.3–1970 ng/mL] |
| IC50 [mean, ng/ml] | 78.34 |
| Slope [mean] | 0.94 |
| Intra-ELISA mean CV%, QC | 9% [5.2-15%] |
| Inter-ELISA mean CV%, QC | 4.3% [1.5-9.3%] |
| Analyte recovery [%], 24 h, 4°C/20°C | 91%/95% |
| Analyte recovery [%], 48 h, 4°C/20°C | 114%/138% |
| Sample recovery [%], freeze/thaw cycles, 3F/T cycles/4F/T cycles | 93%/91% |
| Dilution recovery [%], serum [MRD = 1:4] | 96% |
| Dilution recovery [%], EDTA [MRD = 1:0] | 92% |
| Spiking recovery [%], serum | 101% |
| Spiking recovery [%], EDTA | 99% |
| Interference, biotin recovery [%] [low/high] | 98%/95% |
| Interference, lipaemia recovery [%] [low/high] | 100%/97% |
| Interference, haemoglobin [%] recovery [low/high] | 91%/72% |
Percentages are reported as mean.
ELISA, enzyme-linked immunosorbent assay; EDTA, ethylene diamine tetraacetic acid; FT, freeze/thaw; LLMR, lower limit of measurement range; ULMR, upper limit of measurement range; IC50, 50% inhibitory concentration; CV%, coefficient of variance %; QC, quality control samples; MRD, maximum [or minimum] recovery diluent.
3.2. Patient demographics
All cohorts in this study were age- and body mass index-matched. However, gender, smoking, and endoscopic activity differed between the CD and UC cohorts [Table 2]. The SES-CD did not correlate with either the Harvey-Bradshaw index [HBI] score or the Crohn’s disease activity index [CDAI] [SES-CD vs. HBI: r = 0.25, p = 0.071, SES-CD vs. CDAI: r = 0.21, p = 0.13], so there was no consensus between clinical scores and endoscopic scores for disease activity. Thus, the SES-CD score was applied as the main tool for evaluating disease activity for CD for this study. In the overall IBD population, there was a significant difference in sample availability between serum and faecal samples (p = 0.0278, odds ratio [OR] = 2.31 confidence interval [CI]: [1.07–5.14]) between the obtained serum samples [n = 125, 92%] and the faecal samples [n = 113, 83%], indicating better compliance for serum samples.
Table 2.
Patient demographics and clinical information.
| Variables | CD [n = 93] | UC [n = 43] | HD [n = 23] |
|---|---|---|---|
| Age, years, IQR [median, 25% to 75%] | 35 [27 to 44.3] | 36 [21 to 49] | 37 [26 to 50] |
| Male gender, n [%] | 33 [61] | 23 [53] | 22 [55] |
| BMI, kg/m2, IQR [median, 25% to 75%] | 23.43 [19 to 33] | 22.9 [19.9 to 27.5] | |
| Smoking, n [%] | 24 [44] | 7 [16] | |
| Disease duration, IQR [median, 25% to 75%] | 9.01 [3.76 to 15.89] | 7.34 [0.04 to 28.6] | |
| Localisation CD, n [%] L1/L2/L3/L4 | 6 [11]/10 [19]/34 [63]/4 [7] | - | |
| Behaviour CD, n [%] B1/B2/B3 | 19 [37]/17 [33]/15 [30] | - | |
| Extension UC, n [%] E1/E2/E3 | - | 4 [10]/11 [28]/25 [63] | |
| Perianal disease, n [%] | 14 [26] | - | |
| Truelove and Witts score | 40 [93] | ||
| Partial Mayo Score | 42 [98] | ||
| Mayo Endoscopic Score | 30 [70] | ||
| Endoscopic severity, n [%] Remission Mild Moderate Severe |
SES-CD, 54 [55] 28 [52] 10 [19] 11 [20] 5 [9] |
Full Mayo Score, 30 [70] 7 [23] 12 [40] 9 [30] 2 [7] |
|
| Prior surgery, n [%] | 21 [39] | 5 [12] | |
| Immunosuppressive therapy, n [%] | 33 [61] | 16 [37] | |
| Biologic therapy, n [%] | 31 [57] | 28 [65] | |
| Serum sample availability, n [%] | 85 [91] | 40 [93] | 40 [100] |
| Faecal sample availability, n [%] | 76 [82] | 37 [86] | |
| Neutrophil, count, IQR [median, 25% to 75%] | 4.48 [3.41 to 5.48] | 4.43 [3.20 to 5.47] | |
| Leukocytes IQR [median, 25% to 75%] | 7 [5.6 to 8.4] | 6.70 [5.35 to 9.30] | |
| Lymphocytes IQR [median, 25% to 75%] | 1.53 [1.18 to 2.18] | 1.73 [1.29 to 2.44] | |
| Faecal CP, IQR [median, 25% to 75%] | 150 [32.8 to 399] | 308 [138 to 1325] | |
| C-reactive protein, IQR [median, 25% to 75%] | 2.5 [0.85 to 5.85] | 3.1 [0.60 to 8.4] | |
| Albumin, IQR [median, 25% to 75%] | 44.9 [42.2 to 46.8] | 43.9 [40.8 to 52.3] | |
| AST, IQR [median, 25% to 75%] | 23 [19 to 29.5 | 20 [16 to 25] | |
| ALT, IQR [median, 25% to 75%] | 21 [15 to 29] | 19 [14 to 27.5] |
CD, Crohn’s disease; UC, ulcerative colitis; IQR, interquartile range; BMI, body mass index; CP, calprotectin; SES-CD, Simple Endoscopic Score of Severity for CD; AST, aspartate transaminase; ALT, alanine transaminase; HD, healthy donors.
3.3. CPa9-HNE serum levels in IBD
3.3.1. CPa9-HNE is elevated in the serum of IBD patients compared with healthy controls
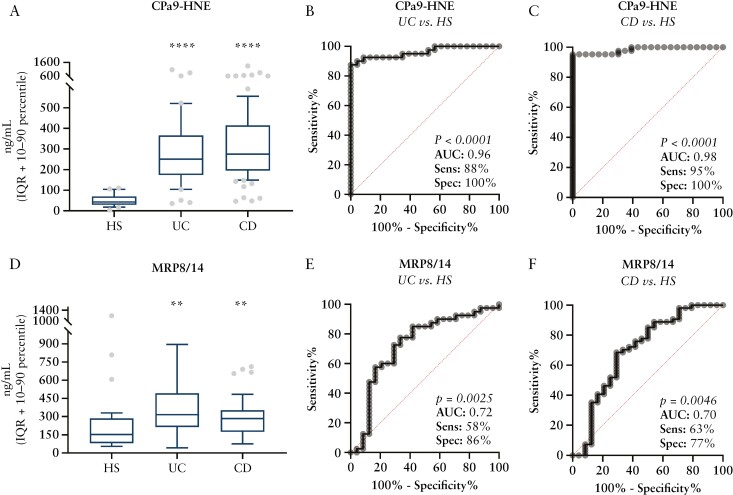
Next we evaluated the CPa9-HNE assay’s potential for monitoring disease activity in UC and CD together, and compared the results with other neutrophil and calprotectin proteins. Serum CPa9-HNE levels were equally elevated in UC (interquartile range [IQR] [251, 174-366]) and CD (IQR [275, 195-414]) patients and were approximately 4-fold higher in CD (area under the curve [AUC]: 0.98 [CI:0.97–1.00], p <0.0001) and UC (AUC: 0.96 [CI: 0.92–1.00], p <0.0001) patients compared with the HS (IQR: [42.7, 29.2-69.9]) [Figure 3A–C]. MRP8/14 serum-CP was also significantly elevated [approximately 1.4-fold higher] in patients with UC (IQR: [314, 212-492], AUC: 0.72 [CI:0.59–0.86], p = 0.0025) and CD (IQR: [283, 174-352], AUC: 0.70 [CI: 0.56–0.84], p = 0.0046) compared with the HS (IQR: [153, 80.9-285]) [Figure 3D–F].
Figure 3.
Biomarker levels of CPa9-HNE in Crohn’s disease [CD: n = 54] and ulcerative colitis [UC: n = 43] vs. healthy subjects [HS: n = 23]. A-C] CPa9-HNE biomarker and D-F] MRP8/14 biomarker were quantified in serum from ulcerative colitis and Crohn’s disease and compared with those of the healthy subjects. Data are depicted as interquartile range [IQR] with 10–90 percentile. Asterisks [*] depict significant differences between HS, CD, and UC patients, calculated using one-way ANOVA, **p <0.01. ***p <0.001, ****p <0.0001. ELISA, enzyme-linked immunosorbent assay; ANOVA, analysis of variance.
3.4. CPa9-HNE is associated with disease activity in UC and CD
Next we evaluated the CPa9-HNE assay as a potential biomarker for monitoring disease activity for UC and CD together with other calprotectin biomarkers, neutrophil count, and CRP. CPa9-HNE correlated with endoscopic disease activity for CD [SES-CD] and UC [full Mayo score and MES] and demonstrated high correlation to the endoscopic scores for UC and CD compared to the other markers [faecal CP, MRP8/14, neutrophil count, and CRP]. A direct comparison of the CPa9-HNE biomarker with the other biomarkers was performed in paired serum and faecal samples, demonstrating improved correlation with endoscopic score for UC and CD [Table 3].
Table 3.
Correlation matrix between calprotectin biomarkers and disease activity.
| Disease activity scoresc | CPa9-HNE | Faecal CP | MRP8/14 | Neutrophil count | CRP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDb | UCa | CDb | UCa | CDb | UCa | CDb | UCa | CDb | UCa | |||||||||||
| r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | |
| SES-CD | 0.61 | <0.0001 | 0.46 | 0.0023 | 0.018 | 0.89 | 0.22 | 0.11 | 0.29 | 0.0289 | ||||||||||
| Full Mayo score | 0.60 | <0.0001 | 0.53 | 0.001 | 0.14 | 0.41 | 0.33 | 0.0343 | 0.54 | 0.004 | ||||||||||
| MES | 0.53 | 0.0048 | 0.33 | 0.092 | 0.07 | 0.68 | 0.30 | 0.11 | 0.42 | 0.0189 | ||||||||||
| pMayo score | 0.51 | 0.001 | 0.56 | 0.0005 | 0.21 | 0.21 | 0.34 | 0.0342 | 0.53 | 0.005 | ||||||||||
| Truelove & Witts | 0.63 | <0.0001 | 0.63 | <0.0001 | 0.33 | 0.0422 | 0.36 | 0.0253 | 0.68 | <0.0001 | ||||||||||
| SES-CD | 0.39 | 0.0201 | 0.35 | 0.0300 | -0.013 | 0.93 | 0.27 | 0.0675 | 0.29 | 0.0743 | ||||||||||
| Full Mayo score | 0.63 | <0.0001 | 0.49 | 0.0042 | 0.18 | 0.32 | 0.28 | 0.12 | 0.55 | 0.005 | ||||||||||
| MES | 0.57 | 0.0040 | 0.35 | 0.085 | 0.009 | 0.96 | 0.26 | 0.23 | 0.41 | 0.0433 | ||||||||||
| pMayo score | 0.51 | 0.0028 | 0.49 | 0.0041 | 0.22 | 0.23 | 0.23 | 0.20 | 0.54 | 0.0009 | ||||||||||
| Truelove & Witts | 0.52 | 0.0035 | 0.78 | <0.0001 | 0.37 | 0.047 | 0.35 | 0.058 | 0.72 | <0.0001 | ||||||||||
CD, Crohn’s disease; UC, ulcerative colitis; CP, calprotectin; SES-CD, Simple Endoscopic Score of Severity for CD; MES, Mayo Endoscopic Score; CRP, C-reactive protein.
The biomarker was not normally distributed and the Spearman rho correlation was applied.
The biomarker was normally distributed and the Pearson correlation was applied.
Paired serum and faecal samples.
3.5. Discriminative power of CPa9-HNE to identify UC and CD patients in endoscopic remission
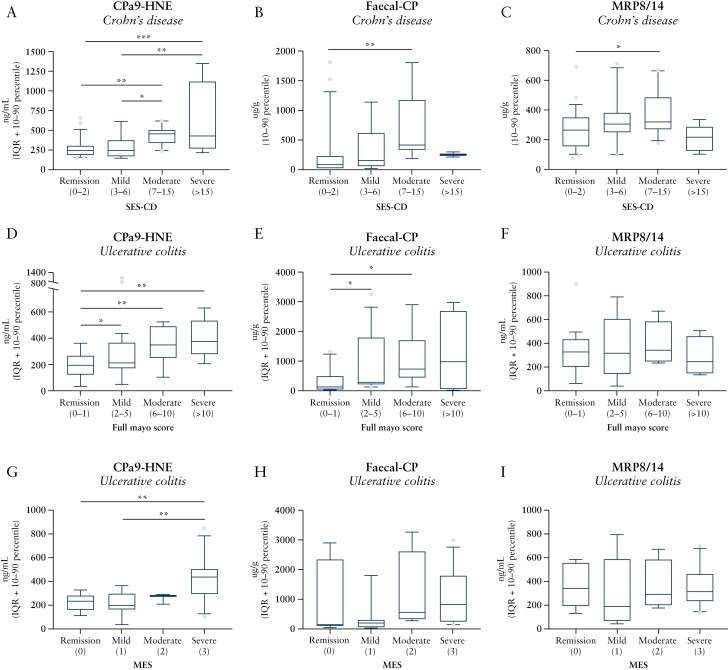
To assess the discriminative power of CPa9-HNE to discriminate IBD patients with different disease activity, UC and CD patients were stratified according to severity of endoscopic disease activity. UC and CD patients demonstrated a gradual increase in CPa9-HNE serum levels with increasing severity compared with patients in remission {SES-CD: in remission (IQR: [244, 188-306]), mild (IQR: [245, 167-375], not significant: [ns), moderate (IQR: [457, 342-504], p <0.01), severe (IQR: [429, 267-1124], p <0.001]); full Mayo: remission (IQR: [194, 123-268]), mild (IQR: [212, 172-366], p <0.05), moderate (IQR: [347, 277-532], p <0.01), severe (IQR: [315, 234-544], p <0.001)} and patients with SES-CD compared with moderate [p <0.05] and severe [p <0.01]; and mild full Mayo compared with severe [p <0.001] [Figure 4A and D]. The CPa9-HNE serum levels was also gradually increased in relation to MES severity compared with remission (IQR: [231, 160-282], ns), mild (IQR: [197, 164-298], ns), moderate (IQR: [278, 209-289], ns), severe (IQR: [434, 289-505], p <0.01), and severe vs. mild [p <0.01] [Figure 4G]. Faecal CP levels were significantly elevated in CD and UC patients with active endoscopic disease activity compared with patients in endoscopic remission {SES-CD: remission (IQR: [82, 20-231]), mild (IQR: [157, 61-621], ns), moderate (IQR: [410, 334-1172], p <0.01), severe (IQR: [254, 212-296], ns); full Mayo: remission (IQR [139, 41.5-497]), mild (IQR: [291, 217-1800], p <0.05), moderate (IQR: [733, 445-1709], p <0.05), severe (IQR [981, 55-2691], p <0.05)} [Figure 4B and E].
Figure 4.
Biomarker levels of CPa9-HNE, faecal CP, and MRP8/14 serum calprotectin stratified according to endoscopic disease severity based on the A-C] SES-CD for Crohn’s disease [n = 54], D-F], full Mayo score for ulcerative colitis [n = 43], and G-I] MES score for ulcerative colitis [n = 30]. Data are depicted as interquartile range [IQR] with 10–90 percentile. Statistical differences were calculated using one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. CP, calptotectin; ANOVA, analysis of variance; SES-CD, Simple Endoscopic Score for Crohn’s Disease; MES, Mayo Endoscopic Score.
Calprotectin quantified with the MRP8/14 ELISA was numerically elevated in the CD and UC patients with moderate disease activity compared with patients in remission based on the SES-CD score (remission IQR: [264, 156-349], mild IQR: [304, 250-380], moderate IQR: [318, 271-483], severe IQR: [218, 122-289]) and Full Mayo score (remission IQR: [326, 202-436], mild IQR: [315, 143-586], moderate IQR: [342, 248-586], severe IQR: [247, 234-544]), although the difference did not reach statistical significance [Figure 4C and F]. No differences were observed between disease activity based on the Mayo Endoscopic Score [MES] score for faecal CP, MRP8/14 [Figure 4H–I]. Similar observations with no statistically significant differences between disease severity were seen for neutrophil count in relation to the SES-CD score (remission IQR: [3.92, 2.90-5.19], mild IQR: [4.22, 2.39-6.90], moderate IQR: [5.03, 3.59-7.58], severe IQR: [5.85, 3.43-8.64]) and Full Mayo score (remission IQR: [3.65, 2.97-4.45], mild IQR: [4.67, 2.9-6.82], moderate IQR: [4.71, 3.84-5.62], severe IQR: [4.24, 3.6-4.77]).
CPa9-HNE showed discriminative power to differentiate between CD and UC patients in remission and those with active disease based on the SES-CD and Full Mayo score or moderate/severe disease activity [Table 4]. The discriminative performance of CPa9-HNE was similar to that of faecal CP for the CD cohort, whereas it was slightly better than that of faecal CP in the UC cohort [Table 4]. The comparison of serum and faecal biomarker performance based on the analysis of available paired serum and serum data demonstrated that the discriminative power of serum CPa9-HNE was superior to that of faecal calprotectin [see AUC results in Table 4]. Even though an improved performance of CPa9-HNE could be observed, the difference in the discriminative power between CPa9-HNE and faecal-CP was not statistically significant [data not shown].
Table 4.
Diagnostic accuracy of CPa9-HNE and faecal CP to differentiate between remission and active disease.
| CPa9-HNE | Faecal CP | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC [CI] | SENS % [CI] | SPEC % [CI] | t test [p-value] | AUC [CI] | SENS % [CI] | SPEC % [CI] | t test [p-value] | |
| Remission vs. active | ||||||||
| SES-CD | 0.68 [0.52 to 0.84] | 62 [41 to 80] | 86 [69 to 94] | 0.0152 | 0.73 [0.58 to 89] | 56 [34 to 75] | 88 [69 to 96] | 0.0137 |
| Full Mayo | 0.78 [0.63 to 0.93] | 62 [41 to 80] | 94 [73 to 100] | 0.0041 | 0.78 [0.62 to 0.94] | 88 [69 to 96] | 62 [36 to 82] | 0.0031 |
| MES | 0.69 [0.51 to 0.88] | 48 [26 to 67] | 92 [64 to 100] | 0.0752 | 0.64 [0.43 to 0.85] | 73 [52 to 87] | 64 [34 to 85] | 0.302 |
| SES-CDa | 0.68 [0.50 to 0.86] | 47 [26 to 69] | 96 [78 to 100 | 0.0149 | 0.72 [] | 56 [33 to 76] | 86 [67 to 95] | 0.0264 |
| Full Mayoa | 0.82 [0.67 to 0.96 | 66 [45 to 82] | 91 [62 to 100] | 0.0036 | 0.79 [0.64 to 0.96 | 48 [28 to 90] | 91 [62 to 100] | 0.0035 |
| MESa | 0.74 [0.54 to 0.94] | 74 [51 to 88] | 80 [38 to 99] | 0.22 | 0.55 [0.21 to 0.89] | 79 [56 to 92] | 60 [23 to 92] | 0.738 |
| Remission vs. moderate/severe | ||||||||
| SES-CD | 0.82 [0.69 to 0.95] | 79 [41 to 80] | 86 [73 to 100] | 0.0003 | 0.85 [0.71 to 0.98] | 78 [45 to 96] | 88 [69 to 96] | 0.0043 |
| Full Mayo | 0.87 [0.70 to 1.00] | 80 [49 to 96] | 94 [73 to 100] | 0.0004 | 0.85 [0.71 to 0.98] | 42 [19 to 68] | 100 [77 to 100] | 0.014 |
| MES | 0.76 [0.56 to 0.97] | 77 [50 to 92] | 75 [46 to 91] | 0.0328 | 0.65 [0.30 to 1.00] | 93 [70 to 100] | 60 [23 to 93] | 0.249 |
| SES-CDa | 0.85 [0.70 to 1.00] | 67 [35 to 88] | 95 [78 to 100] | 0.0002 | 0.83 [0.69 to 0.98] | 75 [41 to 96] | 86 [67 to 95] | 0.0090 |
| Full Mayoa | 0.94 [0.82 to 1.00] | 91 [62 to 100] | 91 [62 to 100] | 0.0021 | 0.79 [0.60 to 0.99] | 55 [28 to 79] | 90 [62 to 99] | 0.014 |
| MESa | 0.85 [0.67 to 1.00] | 67 [39 to 86] | 100 [56 to 100] | 0.0251 | 0.63 [0.26 to 0.99] | 92 [65 to 100] | 60 [23 to 93] | 0.235 |
CP, calprotectin; SES-CD, Simple Endoscopic Score of Severity for CD; MES, Mayo Endoscopic Score; CRP, C-reactive protein; AUC, area under the curve; SENS, sensitivity; SPEC, specificity; CI, confidence interval.
Paired serum and faecal samples.
3.6. Biomarker correlations with relevant clinical parameters
Supplementary Table 2 summarises the correlations of CPa9-HNE, faecal CP, and MRP8/14 biomarkers with patient demographics, several parameters, and biological markers. CPa9-HNE correlated mildly with neutrophil count, and CRP [UC cohort]. CRP correlated with faecal CP [Supplementary Table 2]. We observed a negative correlation trend with age for CPa9-HNE in the CD cohort but not in the UC group [Supplementary Table 2].
3.7. Proof of concept that CPa9-HNE quantifies neutrophil activity
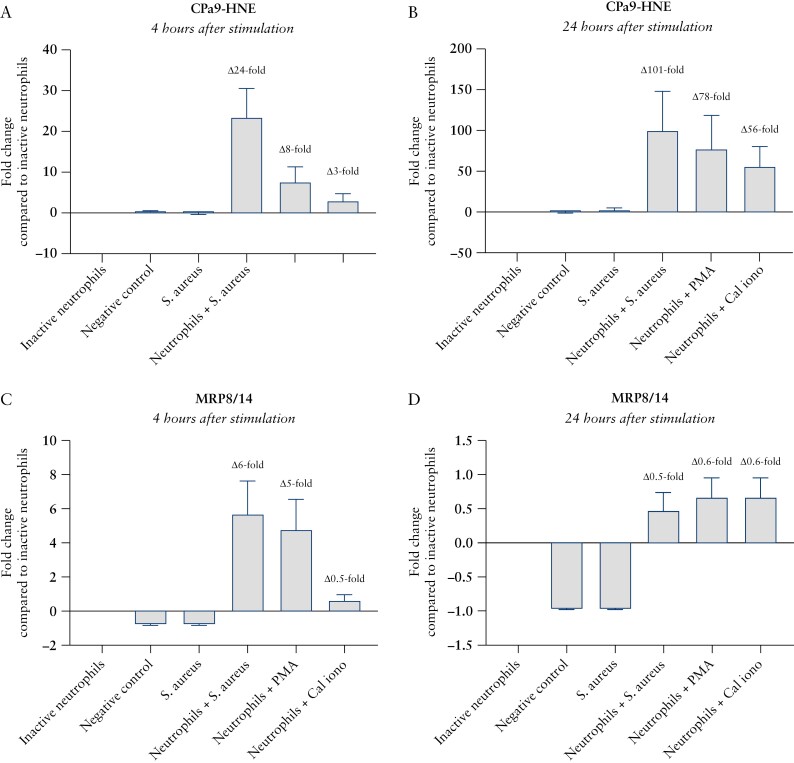
Finally, we wanted to investigate how the neo-epitope [CPa9-HNE assay] is different from the protein [MRP8/14 assay] in association with neutrophil activity, by quantifying the CPa9-HNE fragment and calprotectin protein in supernatant from inactive and activated neutrophil ex vivo cultures. CPa9-HNE fragments were only released into the supernatant by neutrophils in the presence of bacteria or neutrophil agonists. The fold-increases in CPa9-HNE supernatant levels between inactive neutrophils and neutrophils activated by S. aureus, PMA, and calcium ionophore at 4 h were 8-25-fold, and at 24 h were 57-101-fold [Figure 5A and B]. The fold-increases in calprotectin supernatant levels measured by the MRP8/14 ELISA between inactive neutrophils and neutrophils activated by S. aureus, PMA, and calcium ionophore at 4 h were 2-7-fold, and at 24 h were 1.5-1.7-fold [Figure 5C and D]. CPa9-HNE levels in the supernatant were significantly elevated in cultures of neutrophils after 4 h and 24 h of culture in the presence of: S. aureus (4 h: median 73.9 ng/mL, 68.5-89.2 ng/mL minimum-maximum [min-max]; 24 h: median 56.3 ng/mL, 55.9-63.0 ng/mL min-max); PMA: [4 h: median 21.7 ng/mL, 18.9-31.0 ng/mL min-max; 24 h: median 63.1 ng/mL, 23.3-177 ng/mL min-max]; and calcium ionophore [4 h: mean 10.7ng/mL, 8.71-14.5ng/mL min-max; 24 h: median 36.4 ng/mL, 27.8-104 ng/mL min-max]; compared with inactive neutrophils [4 h: median 3.67 ng/mL, 3.17-7.81 ng/mL min-max; 24 h: median 0.40 ng/mL, 0.40-8.21 ng/mL min-max] [Supplementary Figure 1]. The MRP8/14 ELISA detected calprotectin in supernatants from both inactive and activated neutrophils [Supplementary Figure 1].
Figure 5.
Ex vivo cultures of neutrophil granulocytes demonstrating CPa9-HNE biomarker level fold-change compared with inactive neutrophils, reflecting true neutrophil activity. CPa9-HNE biomarker levels after A] 4 h of cultivation with neutrophil agonists B] and 24 h of cultivation; MRP8/14 biomarker measurements after C] 4 h of cultivation with neutrophil agonists D] and 24 h of cultivation. Definition: negative control [only tissue], S. aureus [tissue + Staphylococcus aureus], inactive neutrophils [tissue + neutrophils], neutrophils + S. aureus [tissue + neutrophils + Staphylococcus aureus], neutrophils], neutrophils + PMA [tissue + neutrophils + phorbol myristate acetate], neutrophils + Cal iono [tissue + neutrophils + calcium ionophore]. Error bars represent the standard error of the mean [SEM].
4. Discussion
The CPa9-HNE neo-epitope biomarker was demonstrated to be highly abundant in the serum of CD and UC patients, indicating its potential as a novel marker for aiding IBD diagnosis. This is in concordance with the use of faecal CP ELISAs, which are highly associated with IBD and can reliably distinguish between IBD and IBS patients.3,6,7,9–12 CPa9-HNE also correlated with endoscopic measures [SES-CD and MES] for CD and UC and with clinical disease activity [pMayo and Truelove and Witts] for UC, indicating that the biomarker can be used to monitor disease activity and may be a surrogate marker for endoscopic assessment for CD and UC. This is an important finding, as endoscopic assessment is a necessity for evaluating mucosal healing. Importantly, CPa9-HNE was demonstrated to be specific for neutrophil activity ex vivo, with high discriminative power to differentiate IBD patients in endoscopic remission from IBD patients with endoscopically active disease. The performance of CPa9-HNE ELISA was equipotent with or better than that of faecal CP, and CPa9-HNE ELISA was superior in all aspects related to IBD compared with MRP8/14 ELISA [Figure 4, Table 3], neutrophil count [Table 3], and CRP. These data further strengthen the notion that CPa9-HNE ELISA quantifies neutrophil activity and is therefore a biologically and clinically relevant serum calprotectin biomarker with the potential to be applied for monitoring disease activity in UC and CD.
Calprotectin’s ability to bind metal ions, such as calcium and zinc ions, makes it highly resistant to matrix metalloproteinase [MMP] degradation, as MMPs require zinc ions for activation.22 HNE is a serine protease and does not require activation by metal ions,37–39 so calprotectin will not be able to inhibit the HNE-mediated degradation of calprotectin and the generation of HNE-derived calprotectin neo-epitope protein fragments. This was initially proven in in vitro cleavage experiments from the study at hand, where the CPa9-HNE fragments [and other S100A8 and S100A9 fragments] were only present in samples containing calprotectin and HNE. These in vitro data, combined with the ex vivo data showing a more than 100-fold increase in CPa9-HNE release from activated neutrophils compared with inactive neutrophils, demonstrate that the CPa9-HNE fragment is released only from activated neutrophils. This was further confirmed by showing that the MRP8/14 ELISA, which detected full-length calprotectin, demonstrated a 1.7-fold increase compared with inactive neutrophils [Figure 5]. These findings show that the CPa9-HNE fragment will only be generated by activated neutrophil granulocytes and in the presence of HNE, suggesting that the CPa9-HNE ELISA may represent the quantification of neutrophil activity and reflect local tissue inflammation. The ex vivo results from Figure 5 and Supplementary Figure 1 demonstrated that the calprotectin protein is passively released from inactive neutrophils, which could suggest a similar process in vivo, including circulating neutrophils and other leukocytes. This could to some extent explain the poor sensitivity and specificity of conventional serum calprotectin assays for IBD patients measuring the protein, and the poor correlation of CPa9-HNE with MRP8/14 and neutrophil count. These data, combined with the clinical data obtained from the CPa9-HNE assay, indicate that the neo-epitope may increase the levels of detail in relation to disease activity, allowing quantification of different biological processes, rather than the whole protein calprotectin ELISAs, for serological monitoring of neutrophil activity and tissue infiltration.
The ex vivo findings of this study may also suggest that CPa9-HNE is a marker of NETosis, as bacterial activation of neutrophils and PMA/calcium ionophore induces NETosis in neutrophil granulocytes, and calprotectin and HNE are components of NETs.40–42 The rapid increase in CPa9-HNE neo-epitope release after only 4 h may be linked to what is known as vital NETosis, in which the neutrophil granulocytes retain some of their functions, and the release of NET components becomes a controlled and rapid process and can be induced by S. aureus.43–45 This contrasts with terminal/suicidal NETosis, which is characterised by the total destruction of the neutrophil granulocytes and the release of all nuclear and cytosolic matter.43 PMA is a strong inducer of this process, which can take up to several hours. This is in line with the findings in this study, in which the CPa9-HNE neo-epitope was released after 24 h of PMA stimulation of neutrophil granulocytes.43–45 Therefore, these results suggest that the CPa9-HNE neo-epitope is associated with both vital and terminal/suicidal NETosis [Figure 5]. However, future experiments should investigate this more in depth by looking at co-localisation of CPa9-HNE and NETs in vitro and in tissues. It would also be relevant to investigate the biological activity of this calprotectin neo-epitope, as calprotectin is a known TLR2/4 ligand.
The data currently available on the utility of conventional serum calprotectin ELISAs as applicable biomarkers for IBD are controversial.46–49 The majority of available data show that serum/plasma calprotectin performs similarly to CRP and measures primarily systemic inflammation rather than local inflammation.46–49 Calprotectin also has a very short half-life in plasma [5 h], which further underlines its similarities with CRP.50 The CPa9-HNE ELISA is technically robust and has biological and clinical relevance in IBD, which positions it as a potential novel serum calprotectin biomarker for monitoring disease activity and flares.
This study also confirmed significantly lower patient compliance in delivering faecal samples compared with serum sampling, emphasising that serum sampling is preferred as the matrix for biomarker analysis.1 As specified in Table 1, the CPa9-HNE neo-epitope is stable at 4°C for a minimum of 48 h in serum/plasma, which also means that the neo-epitope fragment has a much longer half-life than intact calprotectin in plasma, which is only 5 h,50 despite being proven to be stable in faeces for 7 days6; this makes CPa9-HNE a suitable marker for monitoring disease activity.6 This might also explain the poor sensitivity and specificity of conventional calprotectin ELISAs when applied to serum, as well as the similarities with acute phase reactants, such as CRP46–48; only a few studies have demonstrated the superiority of serum calprotectin ELISAs over CRP.51,52 The high stability of the CPa9-HNE neo-epitope in serum may be explained by the fact that it is a degradation product/metabolite of calprotectin, making the fragment more resistant to further degradation.
This study has some limitations that should be addressed in future research. The findings of this study are promising, showing that the CPa9-HNE neo-epitope is a potential biomarker for monitoring IBD activity. However, as this study was exploratory in nature and had a fairly small sample size, these findings should be validated in larger and independent cohorts. The diagnostic potential for CPa9-HNE could not be determined, since no serum samples from non-IBD patients, such as irritable bowel patients, were included for this study. Therefore, future studies should also focus on the diagnostic potential by investigating IBD vs. IBS and IBD with extraintestinal manifestations. CPa9-HNE ELISA was superior to the MRP8/14 ELISA in all aspects, but the MRP8/14 biomarker is being marketed for use in rheumatoid arthritis, so it is possible that the ELISA has been optimised for arthropathies rather than gastrointestinal disorders. Nevertheless, we found the comparison valid, as the MRP8/14 ELISA is optimised specifically for serum measurement. Furthermore, as the predictive ability or dynamics of the CPa9-HNE ELISA in response therapy is not known at this stage, future studies should aim to address the effect of treatment over time and the capacity of the marker to predict and monitor treatment response in, for example, anti-TNF-alpha, anti-integrin, anti-IL-23, JAK-inhibitors, and lymphocyte trafficking inhibitors. In addition, future studies should also focus on the CPa9-HNE association with components of NETosis.
In conclusion, the CPa9-HNE ELISA was proven to have high specificity toward the HNE cleaved neo-epitope fragment of calprotectin and was able to quantify neutrophil activity ex vivo. It was able to distinguish CD and UC patients from healthy donors, demonstrating high discriminative power. The CPa9-HNE biomarker also correlated with endoscopic disease activity in CD by SES-CD and UC by full Mayo/MES, showing high accuracy in identifying IBD patients with moderate/severe disease activity. CPa9-HNE is a novel, clinically relevant biomarker with the potential to be applied for monitoring neutrophil activity and disease activity in IBD, and is a potential biomarker for identifying UC and CD patients who are more likely to achieve mucosal healing.
The data underlying this article will be shared on reasonable request to the corresponding author with permission from all authors and institutes.
Supplementary Material
Acknowledgements
We would like to thank and acknowledge Katharina Bering Bryld for excellent technical assistance in the development of the CPa9-HNE ELISA and for performing CPa9-HNE ELISA sample measurements for the IBD cohorts. We would also like to thank Mie Egelund Mortensen and Helene Sofie Hector for excellent technical assistance in performing the MRP8/14 ELISA sample measurements.
Contributor Information
Joachim Høg Mortensen, Nordic Bioscience A/S, Herlev, Denmark.
Dovile Sinkeviciute, Nordic Bioscience A/S, Herlev, Denmark; Lund University, Rheumatology and Molecular Skeletal Biology, Department of Clinical Sciences, Lund, Sweden.
Tina Manon-Jensen, Nordic Bioscience A/S, Herlev, Denmark.
Viktor Domislović, Clinical Hospital Centre Zagreb, Department of Gastroenterology and Hepatology, Zagreb, Croatia.
Kathryn McCall, University of Glasgow, Institute of Infection, Immunity and Inflammation, Glasgow, UK.
Christian S Thudium, Nordic Bioscience A/S, Herlev, Denmark.
Marko Brinar, Clinical Hospital Centre Zagreb, Department of Gastroenterology and Hepatology, Zagreb, Croatia.
Patrik Önnerfjord, Lund University, Rheumatology and Molecular Skeletal Biology, Department of Clinical Sciences, Lund, Sweden.
Carl S Goodyear, University of Glasgow, Institute of Infection, Immunity and Inflammation, Glasgow, UK.
Željko Krznarić, Clinical Hospital Centre Zagreb, Department of Gastroenterology and Hepatology, Zagreb, Croatia.
Morten Asser Karsdal, Nordic Bioscience A/S, Herlev, Denmark.
Anne-Christine Bay-Jensen, Nordic Bioscience A/S, Herlev, Denmark.
Funding
The biomarker portion of the study was supported by the Danish Research Foundation [den Danske Forskningsfond].
Conflict of Interest
JHM, TM-J, CT, MAK, and A-CB -J are employed at Nordic Bioscience A/S which is a company involved in the discovery and development of biochemical biomarkers. TM-J, MAK, and A-CB-J own stocks at Nordic Bioscience. DS, VD, KMcC, MB, PO, CSG, and ŽK have no competing interests.
Author Contributions
JHM: concept and design of the study, acquisition of data [in vitro cleavage, mass spectrometry, CPa9-HNE assay development, CPa9-HNE biomarker measurements], analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. DS, TM-J, VD, KMcC, CST, MB, PO, CSG, ŽK, MAK, and A-CB-J: concept and design of the study, interpretation of data, revising critically for important intellectual content. VD, MB, and ŽK: collecting IBD patient samples. DS and PO: acquisition of mass spectrometry data. KMcC, CST, and CSG: acquisition of ex vivo data.
References
- 1. D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology 2020;158:515–26.e10. [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb K, Daperno M, Usiskin K, et al. Endoscopy and central reading in inflammatory bowel disease clinical trials: achievements, challenges and future developments. Gut 2020:1–9. Doi: 10.1136/gutjnl-2020-320690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porter AC, Aubrecht J, Birch C, et al. Biomarkers of Crohn’ s disease to support the development of new therapeutic interventions. Inflamm Bowel Dis 2020;26:1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 5. Coskun M, Vermeire S, Nielsen OH.. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42. [DOI] [PubMed] [Google Scholar]
- 6. Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992;27:793–8. [DOI] [PubMed] [Google Scholar]
- 7. Tibble JJ, Teahon KK, Thjodleifsson BB, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut 2000;47:506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United Eur Gastroenterol J 2014;2:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gionchetti P, Dignass A, Danese S, et al. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 2: surgical management and special situations. J Crohns Colitis 2017;11:135–49. [DOI] [PubMed] [Google Scholar]
- 10. Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 2008;14:32–9. [DOI] [PubMed] [Google Scholar]
- 11. Chang MH, Chou JW, Chen SM, et al. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol Med Rep 2014;10:522–6. [DOI] [PubMed] [Google Scholar]
- 12. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD. Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 13. Kalla R, Boyapati R, Vatn S, et al. Patients’ perceptions of fecal calprotectin testing in inflammatory bowel disease: results from a prospective multicentre patient-based survey. Scand J Gastroenterol 2018;53:1437–42. [DOI] [PubMed] [Google Scholar]
- 14. Khakoo NS, Lewis A, Roldan GA, et al. Patient adherence to fecal calprotectin testing is low compared to other commonly ordered tests in patients with inflammatory bowel disease. Crohns Colitis 360 2021;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maréchal C, Aimone-Gastin I, Baumann C, et al. Compliance with the fecal calprotectin test in patients with inflammatory bowel disease. United Eur Gastroenterol J 2017;5:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephan JR, Nolan EM.. Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem Sci 2016;7:1962–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trivedi PJ, Adams DH.. Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; pitfalls and promise. J Crohns Colitis 2018;12:S641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moles A, Murphy L, Wilson CL, et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J Hepatol 2014;60:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrchen JM, Sunderkötter C, Foell D, et al. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 [calprotectin] as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 2009;86:557–66. [DOI] [PubMed] [Google Scholar]
- 20. Levine AP, Segal AW.. What is wrong with granulocytes in inflammatory bowel diseases. Dig Dis 2013;31:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geboes K. Histopathology of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2003;18:255–76. [Google Scholar]
- 22. Wéra O, Lancellotti P, Oury C.. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 2016;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–6. [DOI] [PubMed] [Google Scholar]
- 24. Kirov S, Sasson A, Zhang C, et al. Degradation of the extracellular matrix is part of the pathology of ulcerative colitis. Mol Omi 2019;15:67–76. [DOI] [PubMed] [Google Scholar]
- 25. Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009;5. Doi: 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rammes A, Roth J, Goebeler M, et al. Myeloid-related protein [MRP] 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem 1997;272:9496–502. [DOI] [PubMed] [Google Scholar]
- 27. Mortensen JH, Manon-Jensen T, Jensen MD, et al. Ulcerative colitis, Crohn’s disease, and irritable bowel syndrome have different profiles of extracellular matrix turnover, which also reflects disease activity in Crohn’s disease. PLoS One 2017;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kristensen JH, Karsdal MA, Sand JM, et al. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm Med 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer, surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017:1–115. Doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 30. Mortensen JH, Godskesen LE, Jensen MD, et al. Fragments of citrullinated and MMP-degraded vimentin and MMP-degraded Type III collagen are novel serological biomarkers to differentiate Crohn’s disease from ulcerative colitis. J Crohns Colitis 2015;9:863–72. [DOI] [PubMed] [Google Scholar]
- 31. van Haaften WT, Mortensen JH, Karsdal MA, et al. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating [Montreal B3] Crohn’s disease. Aliment Pharmacol Ther 2017;46:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goffin L, Fagagnini S, Vicari A, et al. Anti-MMP-9 antibody: a promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm Bowel Dis 2016;22:2041–57. [DOI] [PubMed] [Google Scholar]
- 33. Lindholm M, Manon-Jensen T, Madsen GI, et al. Extracellular matrix fragments of the basement membrane and the interstitial matrix are serological markers of intestinal tissue remodeling and disease activity in dextran sulfate sodium colitis. Dig Dis Sci 2019;64:3134–42. [DOI] [PubMed] [Google Scholar]
- 34. Mortensen JH, Lindholm M, Langholm LL, et al. The intestinal tissue homeostasis – the role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2019:977–93. Doi: 10.1080/17474124.2019.1673729. [DOI] [PubMed] [Google Scholar]
- 35. Mortensen JH, van Haaften WT, Karsdal MA, et al. The citrullinated and MMP-degraded vimentin biomarker [VICM] predicts early response to anti-TNFα treatment in Crohn’s disease. J Clin Gastroenterol 2021;55:59–66. [DOI] [PubMed] [Google Scholar]
- 36. van Haaften WT, Mortensen JH, Dige AK, et al. Serological biomarkers of tissue turnover identify responders to anti-TNF therapy in Crohn’ s disease: a pilot study. Clin Transl Gastroenterol 2020;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Döring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med 1994;150:S114–7. [DOI] [PubMed] [Google Scholar]
- 38. Alfakry H, Malle E, Koyani CN, et al. Neutrophil proteolytic activation cascades: a possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun 2016;22:85–99. [DOI] [PubMed] [Google Scholar]
- 39. Gouni-Berthold I. Neutrophil-elastase in chronic inflammatory bowel disease: a marker of disease activity? Hepatogastroenterology 1999;46:2315–20. [PubMed] [Google Scholar]
- 40. Li RHL, Ng G, Tablin F.. Lipopolysaccharide-induced neutrophil extracellular trap formation in canine neutrophils is dependent on histone H3 citrullination by peptidylarginine deiminase. Vet Immunol Immunopathol 2017;193–4:29–37. [DOI] [PubMed] [Google Scholar]
- 41. Hoppenbrouwers T, Autar ASA, Sultan AR, et al. In vitro induction of NETosis: comprehensive live imaging comparison and systematic review. PLoS One 2017;12:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Bont CM, Koopman WJH, Boelens WC, et al. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim Biophys Acta Mol Cell Res 2018;1865:1621–9. [DOI] [PubMed] [Google Scholar]
- 43. Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 2017;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Twaddell SH, Baines KJ, Grainge C, et al. The emerging role of neutrophil extracellular traps in respiratory disease. Chest 2019;156:774–82. [DOI] [PubMed] [Google Scholar]
- 45. Vorobjeva NV, Chernyak BV.. NETosis: molecular mechanisms. Biochemistry 2020;85:1178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kopi TA, Shahrokh S, Mirzaei A, et al. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: a review study. Gastroenterol Hepatol Bed Bench 2019;12:183–9. [PMC free article] [PubMed] [Google Scholar]
- 47. Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. Serum calprotectin as a biomarker for Crohn’s disease. J Crohns Colitis 2013;7:e678–83. [DOI] [PubMed] [Google Scholar]
- 48. McCann RK, Smith K, Gaya DR.. A prospective single centre pilot evaluation of a serum calprotectin assay in unselected GI patients. Clin Biochem 2017;50:533–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlsen K, Malham M, Hansen LF, et al. Serum calprotectin in adolescents with inflammatory bowel disease—a pilot investigation. J Pediatr Gastroenterol Nutr 2019;68:669–75. [DOI] [PubMed] [Google Scholar]
- 50. Hammer HB, Ødeg̊ard S, Fagerhol MK, et al. Calprotectin [a major leucocyte protein] is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis 2007;66:1093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malham M, Carlsen K, Riis L, et al. Plasma calprotectin is superior to serum calprotectin as a biomarker of intestinal inflammation in ulcerative colitis. Scand J Gastroenterol 2019;54:1214–9. [DOI] [PubMed] [Google Scholar]
- 52. Kalla R, Kennedy NA, Ventham NT, et al. Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am J Gastroenterol 2016;111:1796–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.