Abstract
Nucleic acid-based electrochemical sensors are a versatile technology enabling affinity-based detection of a great variety of molecular targets, regardless of inherent electrochemical activity or enzymatic reactivity. Additionally, their modular interface and ease of fabrication enable rapid prototyping and sensor development. However, the technology has inhibiting limitations in terms of long-term stability that have precluded translation into clinically valuable platforms like continuous molecular monitors. In this opinion, we discuss published methods to address various aspects of sensor stability, including thiol-based monolayers and anti-biofouling capabilities. We hope the highlighted works will motivate the field to develop innovative strategies for extending the long-term operational life of nucleic acid-based electrochemical sensors.
Keywords: Electrochemical sensors, Nucleic acids, Thiol monolayers, Biofouling, Long-term stability
Introduction
Since the development of the continuous glucose monitor, and its subsequent commercial success [1], electrochemical biosensors have gained widespread attention for their potential value in biomedical applications. While the glucose monitor achieves selectivity from a surface-immobilized enzyme, glucose oxidase [2], another class of electrochemical biosensors uses nucleic acids in place of enzymes. These nucleic acid-based electrochemical sensors (NBEs) rely on affinity of surface-bound oligonucleotides for a specific target molecule [3,4]. Upon target binding, signal is generated through a change in electron transfer kinetics of either a covalently-attached or solvated redox reporter [5]. Because NBEs rely on affinity instead of target reactivity, they can be developed for many molecules of interest: from a complementary nucleic acid [4] to small molecule drugs [6], protein biomarkers [7], and even whole viruses [8] or cells [9]. Additionally, NBEs have achieved popularity due to their ease of fabrication and modular interface, which allow changing the sensing element (i.e., the nucleic acid sequence) without individually optimizing other interface components [10]. However, NBEs have limitations preventing their translation into clinically valuable platforms, such as wearable molecular monitors [11].
One such critical limitation of NBEs is their lack of monolayer stability over time [12]. Most often, NBEs leverage the chemistry of thiol on gold self-assembly to form mixed monolayers containing: a) thiol- and redox reporter-modified nucleic acids for target binding and signal generation; and b) short-chain alkylthiols for electrode surface passivation (Figure 1a) [6]. Unfortunately, these chemistries desorb over time when exposed to environmental or experimental factors like, for example, dry air [13], high temperatures [14], voltage pulsing [15], and biological fluids [16]. This desorption process simultaneously removes sensing moieties and passivating thiols from the electrode surface, prohibiting their deployment for more than a few hours [17]. Despite this restriction, many NBE studies, especially those in the sub-field of electrochemical aptamer-based (E-AB) sensors, claim long-term, continuous sensing as a key application [18,19]. However, if affinity NBEs are to be translated for such a purpose, the issue of long-term monolayer stability must be addressed, including in buffered solutions and dry air for the extended shelf-life storage needed for successful commercialization.
Figure 1. Degradation of the NBE sensor interface.
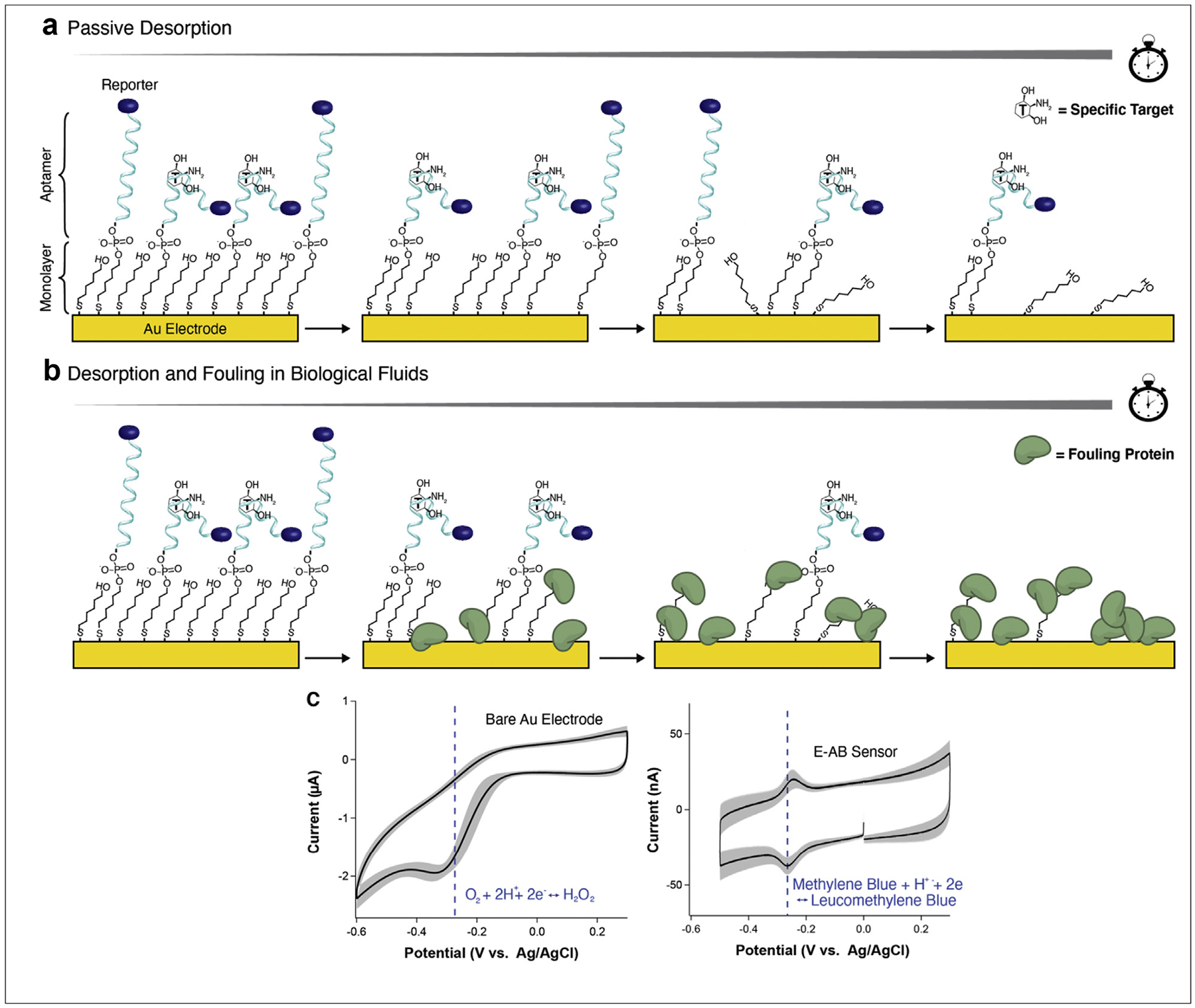
Using E-AB sensors as an example, we highlight (a) the passive desorption of short chain thiols and thiol-modified aptamers from the gold surface; (b) the simultaneous desorption of thiols and fouling by proteins when sensors are exposed to biological fluids; and (c) the overlap between the reduction of methylene blue and the reduction of molecular oxygen on a gold electrode.
A second critical limitation of the NBE sensor interface is that of fouling in biological fluids [12]. When NBEs are exposed to blood or serum, proteins from those fluids will deposit on the monolayer surface (Figure 1b) [20,21]. These proteins, or fouling layer, simultaneously restrict the conformational dynamics of electrode-attached nucleic acids and the electron transfer efficiency between the electrode and redox reporter. Fouling-induced hindering of conformational dynamics reduces the total signal gain possible from target binding, while worse electron transfer reduces overall signal output, regardless of target concentration [22,23]. Taken together, these effects limit the signaling lifetime of NBEs. For example, continuous in-vivo measurements using E-AB sensors have only been demonstrated for ~12 h to date [18,19]. Thus, in addition to reducing monolayer desorption, NBE antifouling capabilities must also be enhanced [24].
Other remaining limitations for NBE stability relate to the attached redox reporter and electrochemical method employed for the interrogation of sensors. Specifically, only one reporter (methylene blue) is primarily deployed in NBEs [6,12,25]. In fact, methylene blue does reveal superior stability over dozens of other reporters when attached to an NBE interface [26]. However, its negative reduction potential overlaps with the reduction of molecular oxygen (which includes the formation of the highly reactive radical superoxide, Figure 1c), a reaction that likely accelerates the degradation of the underlying monolayer [12]. Additionally, because methylene blue undergoes a proton transfer during reduction, signaling with methylene blue is pH dependent [27]. Therefore, finding alternative redox reporters with a more positive reduction potential and insensitivity to sample pH remains of interest in the field. As for electrochemical interrogation, the most used method is square-wave voltammetry (SWV). While SWV achieves high signal-to-noise electron transfer measurements [28], its square wave-based voltage pulsing can accelerate monolayer desorption [29,30], thereby limiting long-term stability. As such, a balance between maximizing signal versus maximizing lifetime should be considered.
The focus of this opinion is to critically evaluate published approaches that address the above issues of monolayer stability, fouling resistance, and stable continuous sensing. We specifically highlight works that focus on: a) thiol-based strategies to prolong monolayer stability; b) approaches to reduce NBE sensor biofouling; and c) insights into sensor signal loss driving factors. We hope this work will spotlight the need for innovative strategies to prolong NBE sensor lifetime, in addition to serving as a reference for researchers looking to tailor their sensor interface to a specific application.
Thiol-based strategies to prolong monolayer stability
Thiol-gold monolayers have been extensively studied and applied to a variety of technologies [31]. For NBEs specifically, the most deployed thiols are 6-mercapto-hexanol for the passivation of the electrode [12], and an analogous six carbon thiol for immobilization of nucleic acids. However, these hydrophilic, monodentate thiols rapidly desorb from the electrode surface when removed from their highly concentrated deposition solutions [29,32–35]. We highlight here three approaches demonstrated to enhance the stability of thiol self-assembled monolayers: multi-dentate anchoring groups [36,37], crosslinking [38,39], and hydrophobic thiols [12,40]. While not fully overcoming the issue of thiol desorption, these methodologies address a fundamental limitation of NBEs. As such, their deployment extends both their shelf-life and operational stability for long-term sensing.
The first methodology for enhancing monolayer stability is to increase the number of attachment points to the electrode surface. Multidentate interactions are well-known to increase binding energy due to the chelate effect. Because of this knowledge, groups have formed monolayers from di- and trithiol anchoring groups with a variety of carbon chain lengths [41], number of carbon chains [36], and additional functionalities [37,42]. Such multidentate monolayers can enable prolonged shelf-life [43] and increased thermal stability [37]. In a specific example we highlight here, both flexible and rigid trithiols were used to immobilize DNA onto an electrode surface, then compared to the traditional monothiol in terms of sensor signaling, shelf-life, and stability under repeated electrochemical interrogation (Figure 2a) [37]. Sensors using DNA immobilized via flexible trithiols retained 94% of their original signal after 50 scans of alternating current voltammetry. In contrast, sensors using monothiols only retained ~75%. Of note, these sensors still utilized 6-mercapto-hexanol as the passivating thiol. It is possible that use of multidentate anchoring groups for both nucleic acid immobilization AND electrode passivation could further extend monolayer stability and sensor lifetime.
Figure 2. Thiol-based strategies to prolong monolayer stability.
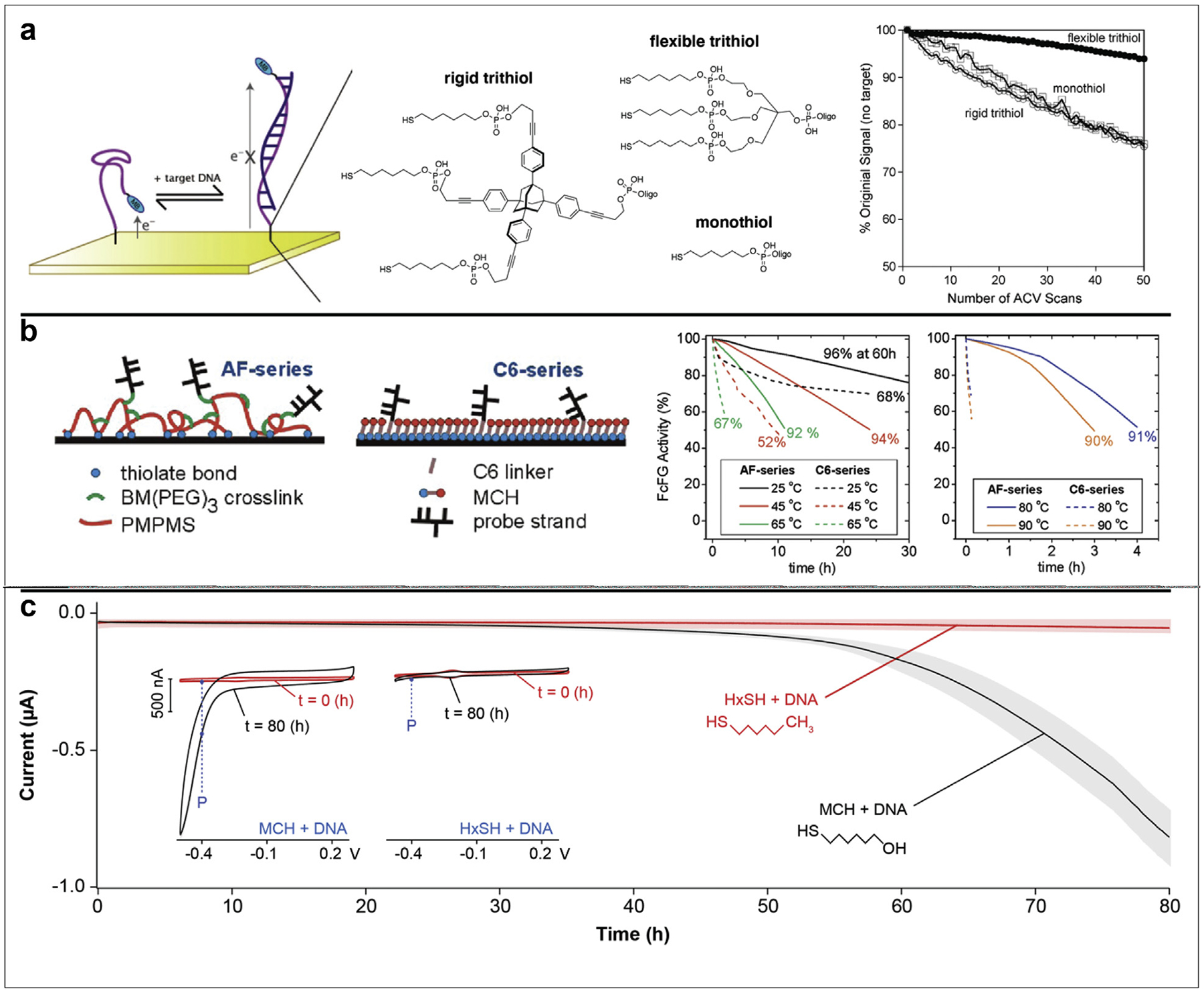
(a) Plaxco et al. compared rigid and flexible trithiol anchoring groups to the most used monothiol nucleic acid modification for immobilization on the electrode surface. In doing so, they demonstrated greater signal retention upon repetitive interrogation of sensors with the flexible trithiol. (b) Levickey et al. crosslinked a film of PMPMS with BM(PEG)3 to create a multidentate monolayer. After further conjugating ferrocenyl formylglycine-modified (FcFG) DNA strands, they demonstrated superior thermal stability during exposure of sensors to a range of temperatures. Percentages at the end of each trace correspond to the hybridization efficiency after thermal treatment, a measure to control for thermal degradation of FcFG. (c) Shaver et al. replaced the commonly used 6-mercapto-hexanol (MCH) with hexanethiol (HxSH) to demonstrate that hydrophobic monolayers remain on an electrode surface for days. They reveal this by tracking the contribution of oxygen reduction to the voltametric current at −0.4 V vs Ag/AgCl, an indicator of monolayer coverage on the electrode surface. Panels were adapted with permission from references 36 (copyright 2009), 39 (copyright 2012), and 12 (copyright 2020), American Chemical Society.
As an additional method for creating multidentate sensor interfaces, some thiol moieties can be crosslinked after surface deposition. This idea was first demonstrated by Crooks et al., in which they used ultraviolet light to crosslink diacetylenic thiols and form monolayers with superior resistance to electrochemically induced desorption [38]. A more recent study from Levicky et al. used 1,11-bis-maleimidotriethyleneglycol (BM(PEG)3) to crosslink a surface-deposited layer of poly(mercaptopropyl)methylsiloxane (PMPMS) on the electrode surface (Figure 2b) [39]. Unreacted maleimide groups from the BM(PEG)3 were then conjugated to thiol-functionalized oligonucleotides for sensor fabrication. By determining the extent of target strand hybridization before and after thermal treatments, the authors found that crosslinked sensors retained >90% hybridization capacity after 3 h at 90 °C, compared to undetectable hybridization for monothiol sensors. The retention of capture strands on the electrode surface likely arises due to the increased strength of multi-dentate interactions compared to monodentate. Similar trends were seen for other temperatures tested.
A final methodology for enhancing monolayer stability is to decrease its solubility. Hydrophobic thiols have long been known to impart higher monolayer packing density compared to hydrophilic thiols [40]. A recent study in our group leveraged this knowledge to create E-AB sensors that retained signal for more than 80 h (Figure 2c) [12]. Specifically, we exchanged the traditional 6-mercapto-hexanol for its methyl-terminated analog, hexanethiol. By tracking the voltametric current at −0.4 V versus Ag/AgCl (corresponding to the reduction of molecular oxygen on gold), we demonstrated that the hydrophobic monolayer remains on the electrode surface, enabling signal resolution the entire period. Of note, however, this strategy can only be applied in buffered solutions, as deployment of the hydrophobic surface in biological fluids leads to immediate fouling and loss of sensor signal.
Approaches to reduce nucleic acid-based sensor biofouling
Sensor fouling presents a major limitation for technologies deployed in protein-containing fluids, such as serum or whole blood [20]. This issue is especially pertinent when the application involves long-term sensing [22]. We highlight here three different methods for either mitigating sensor fouling or preventing fouling from affecting signaling: nanoporous gold [44], zwitterionic monolayers [22,23], and hydrogel coatings [18,45]. The specific use of any such strategy depends greatly on the sensor application, and we discuss their limitations.
One strategy to enable sensor signaling even in the presence of fouling is to use porous materials that exclude proteins and cells based on size. For example, Seker et al. employed nanoporous gold electrodes for the detection of nucleic acids in 10% fetal bovine serum (Figure 3a) [44]. While short oligonucleotides can diffuse into pores and hybridize with immobilized capture strands, proteins from the serum are restricted to the surface. Thus, they demonstrated only 10% signal suppression on planar Au electrodes in FBS, whereas full 40% signal suppression is maintained on unannealed nanoporous electrodes, and an intermediate 20% for annealed electrodes. The larger pores of annealed electrodes likely allow some proteins to block access of target oligos, diminishing the maximum signal suppression. One obvious limitation of this strategy is the target molecule size. While applicable to small molecule and short oligonucleotide detection, sensing of larger nucleic acids or protein targets requires alternative methods.
Figure 3. Approaches to reduce nucleic acid-based sensor biofouling.
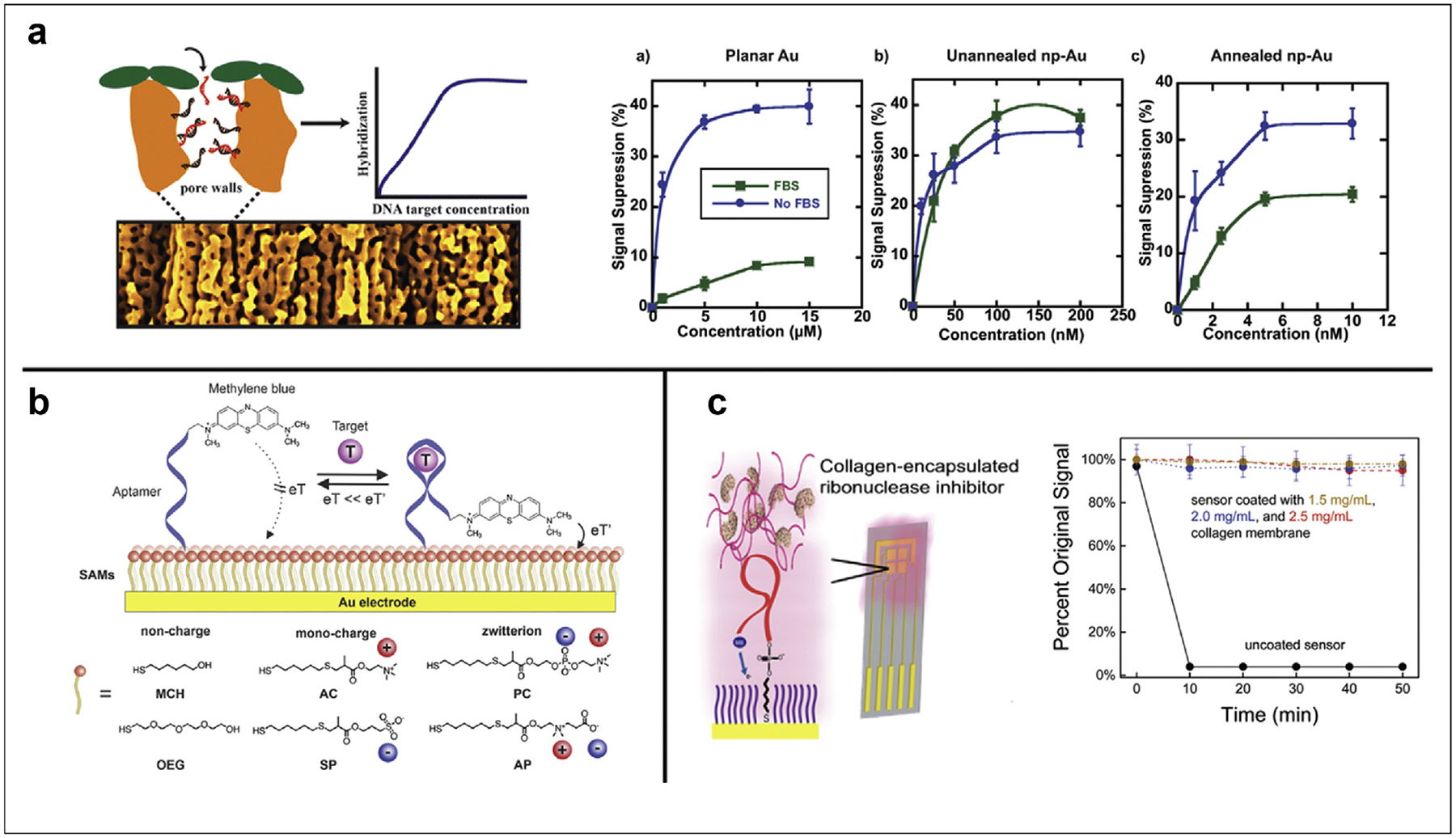
(a) Seker et al. created nanoporous Au electrodes for the detection of short oligonucleotides. Since proteins are restricted from entering the small, unannealed pores, this strategy enabled full signal suppression (compared to buffer) in 10% fetal bovine serum. When they annealed the Au to form larger pores, some proteins were able to block access of target oligos, diminishing the maximum signal suppression. (b) Hui Li et al. evaluated the effect of uncharged (mercaptohexanol/MCH, oligoethyleneglycol/OEG), singly charged (trimethyl ammonium chloride/AC, sulfopropylmethacrylate/SP), and zwitterionic (phosphorylcholine/PC, dimethylammonio propane sulfonate/AP) monolayers on the signal sensitivity, anti-fouling capability, and signal stability over time. (c) Santos–Cancel and White entrapped an RNAse inhibitor in collagen membranes of three different densities to enable signaling via RNA aptamer in undiluted serum. Panels were adapted with permission from references 44 (copyright 2015), 23 (copyright 2021), and 45 (copyright 2017), American Chemical Society.
A more general and versatile strategy to mitigate sensor fouling is to deploy zwitterionic thiols in the blocking monolayer. This idea was first explored by Whitesides et al., who demonstrated that zwitterionic monolayers resist the adsorption of proteins [46,47]. One recent example we highlight here involves the characterization of several charged thiols on the signaling and stability of E-AB sensors [23]. Specifically, Hui Li et al. compared monolayers of uncharged, singly charged, and zwitterionic thiols (Figure 3b). They evaluated the performance of E-ABs with each monolayer in terms of signaling, fouling resistance, and stability over 12 h in three different biological fluids, finding the phosphorylcholine (PC) monolayer most effective at mitigating protein adsorption. Although undoubtedly effective at reducing sensor fouling by protein adsorption, zwitterionic monolayers are still limited by the long-term stability issues described above [22]. As such, this strategy is only useful for short-term applications in biofluids.
A more ubiquitous method to reduce fouling is through the deposition of hydrogels or membranes on top of a sensor surface. This strategy can be applied to nearly any NBE sensor because of the ability to customize hydrogel composition, extent of crosslinking, and membrane thickness [48]. Hydrogels are stable over long periods, having even shown utility when deployed in vivo on E-AB sensors [18]. An additional benefit of hydrogel membranes is the ability to physically trap beneficial molecules during deposition. For example, Santose–Cancel and White entrapped an RNAse inhibitor in a collagen membrane to enable sensing with an RNA aptamer in undiluted serum (Figure 3c) [45]. Although sensor stability was only evaluated for 50 min, this approach could be used in conjunction with other strategies to extend the lifetime of NBEs.
Insights into sensor signal loss driving factors
In this final section, we highlight three manuscripts that provide insight into the driving factors of progressive sensor signal loss, both in buffered solutions and biological fluids. The first of these, authored by Shaver et al. [17], reports the functionalization of E-AB sensors with aptamers of either naturally occurring (and traditionally synthesized) D-DNA, or its nuclease-resistant enantiomer L-DNA (Figure 4a, left). In this work, they demonstrated that the nuclease resistance of L-DNA seen in other applications [49] translates to the NBE platform by tracking the peak current from cyclic voltammograms (CV) both before and after injection of an excess of nuclease into buffer (Figure 4a, center). The authors repeated the experiment in undiluted serum, where exposure to excess nuclease does not cause additional sensor signal loss (Figure 4a, right). These results indicate that NBE sensor signal loss is not driven by nuclease hydrolysis of surface immobilized nucleic acids, something previously speculated in the field. Additionally, the differences in first hour signal loss between nuclease-resistant sensors deployed in buffer versus serum (center vs right panels, red traces) suggests that multiple factors contribute to signal loss in biological fluids.
Figure 4. Insights into sensor signal loss driving factors.
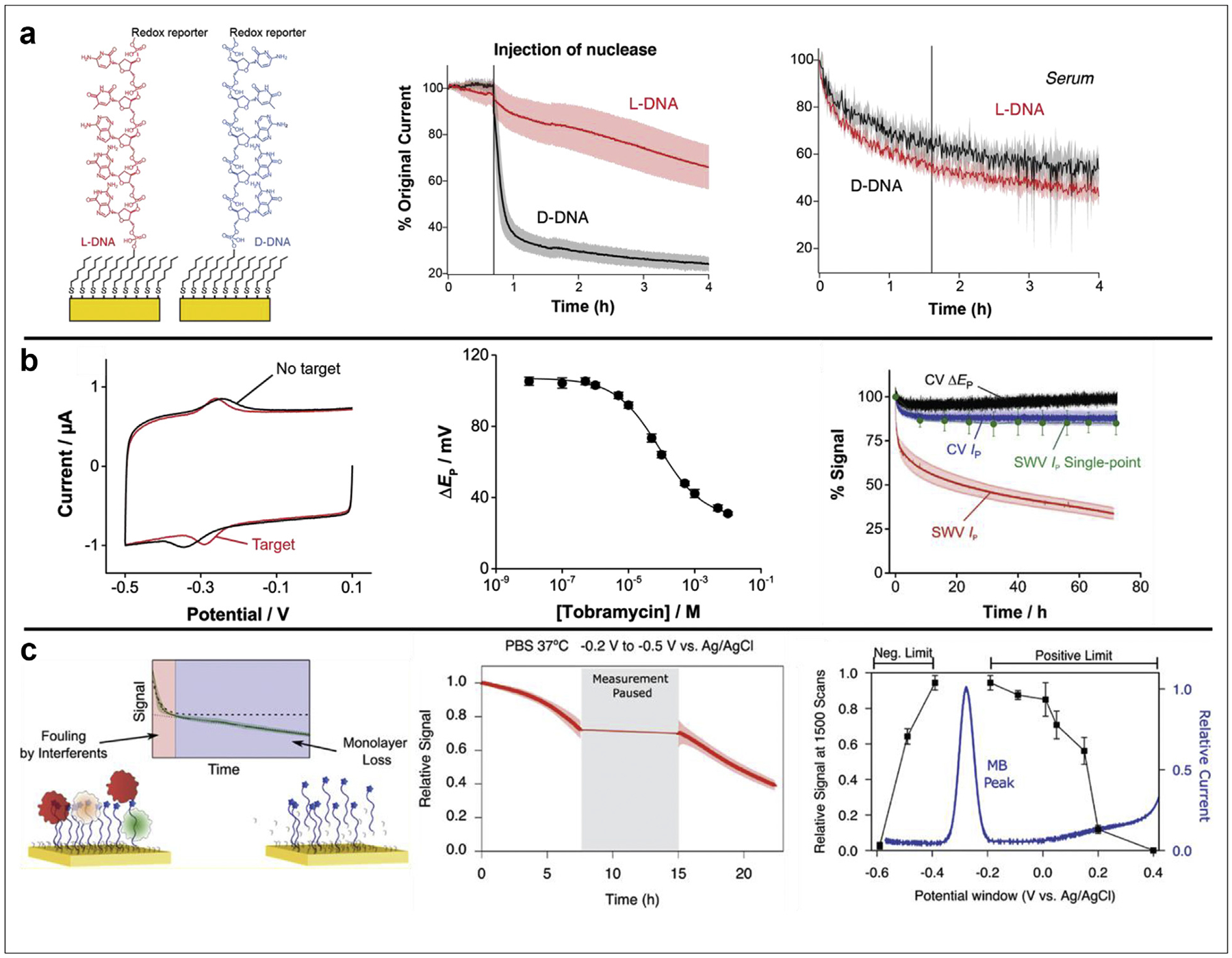
(a) Shaver et al. functionalized E-AB sensors with either D-DNA or its nuclease-resistant enantiomer L-DNA (left). After demonstrating that the L-DNA sensors maintained signaling upon exposure to an excess of nuclease in buffer (center), they deployed sensors in undiluted serum to reveal a lack of signal stability difference between D- and L-DNA sensors in biological fluids (right). (b) Pellitero et al. demonstrated that E-AB sensor target binding can be accurately tracked via peak-to-peak separation (ΔEp) in CV (left), with signaling similar to sensors interrogated via SWV (center). This method presents a benefit over the traditionally used SWV because interrogation every 5 s by CV enables retention of >80% signal after 72 h, as opposed to ~30% signal retention with SWV every 5 s, or limiting SWV to just 10 single points to match the >80% signal retention (right). (c) Plaxco et al. revealed competing mechanisms of sensor interface fouling and monolayer desorption on the signal loss of NBE sensors in biological fluids (left). They also demonstrated that monolayer desorption is: (center) caused by electrochemical interrogation; and (right) dependent on the potential window used. Panels were adapted with permission from references 17 (copyright 2021), 30 (copyright 2021), and 29 (copyright 2021), American Chemical Society.
The second insight was reported by Pellitero et al. [30], who investigated the effects of CV versus SWV on the long-term stability of NBE sensor signal. Specifically, they first demonstrated that E-AB sensor binding to target can be accurately monitored via peak-to-peak separation in CV (Figure 4b, left, center), as opposed to the more commonly used peak height in SWV. By then continuously interrogating sensors every 5 s for 72 h (Figure 4b, right), they revealed that peak current (blue trace) and peak-to-peak separation (black trace) in CV retain >80% signal after 3 days, whereas peak current from SWV (red trace) falls to ~30% signal in the same period. However, if SWV is run only ten times in the same timeframe (green trace), sensors again retain >80% signal. These results indicate that both electrochemical method and measurement frequency play vital roles in the stability of NBE sensor signal, with the most common method of SWV driving rapid signal loss.
The third article providing insight on progressive NBE sensor signal loss comes from the Plaxco group [29]. They determined that when sensors are deployed in biological fluids, the initial exponential decrease in sensor signal is caused by fouling of the sensor interface, while the subsequent continuous linear decrease in signal is caused by monolayer desorption from the electrode surface (Figure 4c, left). This conclusion is in line with previous results highlighted in Figure 4a, where Shaver et al. [17] demonstrated linear signal loss in buffer and biphasic signal loss in biological fluids. Plaxco et al. also determined the parameters that drive monolayer desorption. Specifically, they isolated two driving factors: a) by continuously interrogating sensors for 7 h, pausing for 7 h, then resuming for 7 more hours, they revealed that signal output is the same just before and just after the pause (Figure 4c, center). This result indicates that electrochemical interrogation is the major driving force for monolayer desorption, supporting work highlighted in Figure 4b [30]; and b) they also went a step further to determine that the potential window used during interrogation has a significant effect on signal stability (Figure 4c, right). Their results suggest that both oxidative and reductive desorption of thiols occurs on the NBE surface, and that the potential window should be minimized to reduce these effects. Of note, all experiments in this study ran SWV, so it’s possible that simultaneously minimizing potential windows while running CV could further enhance NBE signal stability.
The challenge of long-term stability for NBEs is a complex issue, especially when deployed in biological fluids. In this opinion, we highlighted some strategies that can be used to prolong monolayer stability, as well as minimize sensor fouling. We hope these examples serve as a guide for others looking to tailor their sensor interface for a specific application. Moreover, with the discussion of NBE sensor signal loss driving factors, we hope to motivate the field toward designing innovative methods of overcoming progressive signal loss. Hopefully, NBE sensor operational life can be extended to last for days, thus enabling the development of continuous molecular monitors.
Funding
A.S. and N.A.C. thank the National Institute of General Medical Sciences of the National Institutes of Health for supporting this work under award number R01GM140143.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Newman JD, Turner APF: Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron 2005, 20: 2435–2453, 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Mcgarraugh G: The chemistry of commercial continuous glucose monitors. Diabetes Technol Therapeut 2009, 11. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Dauphin-ducharme P, Ortega G, Plaxco KW: Calibration-Free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J Am Chem Soc 2017, 139:11207–11213, 10.1021/jacs.7b05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soleymani L, Fang Z, Lam B, Bin X, Vasilyeva E, Ross AJ, Sargent EH, Kelley SO: Hierarchical nanotextured microelectrodes overcome the molecular transport barrier to achieve rapid, direct bacterial detection. ACS Nano 2011, 5: 3360–3366, 10.1021/nn200586s. [DOI] [PubMed] [Google Scholar]
- 5.Pellitero MA, Shaver A, Arroyo-Currás N: Critical review—approaches for the electrochemical interrogation of DNA-based sensors: a critical review. J Electrochem Soc 2020, 167, 037529, 10.1149/2.0292003jes. [DOI] [Google Scholar]
- 6.Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ, Plaxco KW: An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc 2006, 128:3138–3139, 10.1021/ja056957p. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Lubin AA, Heeger AJ, Plaxco KW: Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew Chem Int Ed 2005, 44:5456–5459, 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
- 8.Peinetti AS, Lake RJ, Cong W, Cooper L, Wu Y, Ma Y, Pawel GT, Toimil-Molares ME, Trautmann C, Rong L, Mariñas B, Azzaroni O, Lu Y: Direct detection of human adenovirus or SARS-CoV-2 with ability to inform infectivity using DNA aptamer-nanopore sensors. Sci Adv 2021, 7:1–13, 10.1126/sciadv.abh2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu L, Xu J, Tan X, Liu Z, Xu L, Peng R: Dual-aptamer modification generates a unique interface for highly sensitive and specific electrochemical detection of tumor cells. ACS Appl Mater Interfaces 2014, 6:7309–7315, 10.1021/am5006783. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Xie S, Liang H, Wu C, Cui L, Huan SY, Zhang X: Generation of biostable L-aptamers against achiral targets by chiral inversion of existing D-aptamers. Talanta 2017, 164: 662–667, 10.1016/j.talanta.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo-Currás N, Dauphin-Ducharme P, Scida K, Chávez JL: From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Anal Methods 2020, 12:1288–1310, 10.1039/d0ay00026d. [DOI] [Google Scholar]
- 12.*.Shaver A, Curtis SD, Arroyo-Currás N: Alkanethiol monolayer end groups affect the long-term operational stability and signaling of electrochemical, aptamer-based sensors in biological fluids. ACS Appl Mater Interfaces 2020, 12: 11214–11223, 10.1021/acsami.9b22385. [DOI] [PubMed] [Google Scholar]; The authors compared long-term stability of sensors with either 6-mercapto-hexanol or 1-hexanethiol as the passivating monolayer. Hexanethiol functionalized sensors enabled peak resolution for >80 h, compared to ~24 h stability for 6-mercapto-hexanol functionalized sensors.
- 13.Willey TM, Vance AL, Van Buuren T, Bostedt C, Terminello LJ, Fadley CS: Rapid degradation of alkanethiol-based self-assembled monolayers on gold in ambient laboratory conditions. Surf Sci 2005, 576:188–196, 10.1016/j.susc.2004.12.022. [DOI] [Google Scholar]
- 14.Civit L, Fragoso A, O’Sullivan CK: Thermal stability of diazonium derived and thiol-derived layers on gold for application in genosensors. Electrochem Commun 2010, 12:1045–1048, 10.1016/j.elecom.2010.05.020. [DOI] [Google Scholar]
- 15.Mulder WH, Calvente JJ, Andreu R: A kinetic model for the reductive desorption of self-assembled thiol monolayers. Langmuir 2001, 17:3273–3280, 10.1021/la001283b. [DOI] [Google Scholar]
- 16.Maciel J, Martins MCL, Barbosa MA: The stability of self-assembled monolayers with time and under biological conditions. J Biomed Mater Res 2010, 94:833–843, 10.1002/jbm.a.32746. [DOI] [PubMed] [Google Scholar]
- 17.* *.Shaver A, Kundu N, Young BE, Vieira PA, Sczepanski JT, Arroyo-Currás N: Nuclease hydrolysis does not drive the rapid signaling decay of DNA aptamer-based electrochemical sensors in biological fluids. Langmuir 2021, 37:5213–5221, 10.1021/acs.langmuir.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors fabricated sensors with either D- or L-DNA to determine if nuclease hydrolysis of immobilized nucleic acids contributes to sensor signal loss. They found that signal loss does not differ between the two types of sensor when deployed in biological fluids, even upon injection of excess nuclease.
- 18.Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW: Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci USA 2017, 114:645–650, 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dauphin-Ducharme P, Zhang Y, Gerson J, Kurnik M, Kippin TE, Stojanovic MN: Electrochemical aptamer-based sensors for improved therapeutic drug monitoring and high-precision, feedback-controlled drug delivery. ACS Sens 2019, 4: 2832–2837, 10.1021/acssensors.9b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostuni E, Grzybowski BA, Mrksich M, Roberts CS, Whitesides GM: Adsorption of proteins to hydrophobic sites on mixed self-assembled monolayers. Langmuir 2003, 19: 1861–1872, 10.1021/la020649c. [DOI] [Google Scholar]
- 21.Whitesides GM, Prime KL: Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science 1991, 252:1164–1167. http://adsabs.harvard.edu/abs/1991Sci…252.1164P. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Dauphin-Ducharme P, Arroyo-Currás N, Tran CH, Vieira PA, Li S, Shin C, Somerson J, Kippin TE, Plaxco KW: A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors. Angew Chem Int Ed 2017, 56:7492–7495, 10.1002/anie.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*.Li S, Wang Y, Zhang Z, Wang Y, Li H, Xia F: Exploring end-group effect of alkanethiol self-assembled monolayers on electrochemical aptamer-based sensors in biological fluids. Anal Chem 2021, 93:5849–5855, 10.1021/acs.analchem.1c00085. [DOI] [PubMed] [Google Scholar]; The authors employed uncharged, singly charged, and zwitterionic thiols for the fabrication of sensor monolayers. They evaluated each type of sensor in terms of sensitivity, antifouling capability, and stability over time in biological fluids, finding the phosphorylcholine monolayer to be the best at resisting protein adsorption.
- 24.Russo MJ, Han M, Desroches PE, Manasa CS, Dennaoui J, Quigley AF, Kapsa RMI, Moulton SE, Guijt RM, Greene GW, Silva SM: Antifouling strategies for electrochemical biosensing: mechanisms and performance toward point of care based diagnostic applications. ACS Sens 2021, 6:1482–1507, 10.1021/acssensors.1c00390. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Y, Lai RY, Plaxco KW: Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat Protoc 2007, 2:2875–2880, 10.1038/nprot.2007.413. [DOI] [PubMed] [Google Scholar]
- 26.Kang D, Ricci F, White RJ, Plaxco KW: Survey of redox-active moieties for application in multiplexed electrochemical biosensors. Anal Chem 2016, 88:10452–10458, 10.1021/acs.analchem.6b02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahlum JD, Pellitero MA, Arroyo-Currás N: Chemical equilibrium-based mechanism for the electrochemical reduction of DNA-bound methylene blue explains double redox waves in voltammetry. J Phys Chem C 2021, 125: 9038–9049, 10.1021/acs.jpcc.1c00336. [DOI] [Google Scholar]
- 28.Dauphin-Ducharme P, Plaxco KW: Maximizing the signal gain of electrochemical-DNA sensors. Anal Chem 2016, 88: 11654–11662, 10.1021/acs.analchem.6b03227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.* *.Leung KK, Downs AM, Ortega G, Kurnik M, Plaxco KW: Elucidating the mechanisms underlying the signal drift of electrochemical aptamer-based sensors in whole blood. ACS Sens 2021, 17, 10.1021/acssensors.1c01183. [DOI] [PubMed] [Google Scholar]; The authors distinguished sensor fouling and monolayer desorption as independent machanisms for signal loss. Moreover, they demonstrated that monolayer desorption is accelerated by electrochemical interrogation, a process dependent on the potential window used.
- 30.* *.Pellitero MA, Curtis SD, Arroyo-Currás N: Interrogation of electrochemical aptamer-based sensors via peak-to-peak separation in cyclic voltammetry improves the temporal stability and batch-to-batch variability in biological fluids. ACS Sens 2021, 6:1199–1207, 10.1021/acssensors.0c02455. [DOI] [PubMed] [Google Scholar]; The authors demonstrated that sensors interrogated via CV retain signal for longer than sensors interrogated via SWV. Additionally, they show that interrogation by CV enhances batch-to-batch reproducibility.
- 31.Gooding JJ, Darwish N: The rise of self-assembled monolayers for fabricating electrochemical biosensors-an interfacial perspective. Chem Rec 2012, 12:92–105, 10.1002/tcr.201100013. [DOI] [PubMed] [Google Scholar]
- 32.Xue Y, Li X, Li H, Zhang W: Quantifying thiol-gold interactions towards the efficient strength control. Nat Commun 2014, 5, 10.1038/ncomms5348. [DOI] [PubMed] [Google Scholar]
- 33.Satjapipat M, Sanedrin R, Zhou F: Selective desorption of alkanethiols in mixed self-assembled monolayers for subsequent oligonucleotide attachment and DNA hybridization. Langmuir 2001, 17:7637–7644, 10.1021/la010989i. [DOI] [Google Scholar]
- 34.Xu X, Makaraviciute A, Kumar S, Wen C, Sjödin M, Abdurakhmanov E, Danielson UH, Nyholm L, Zhang Z: Structural changes of mercaptohexanol self-assembled monolayers on gold and their influence on impedimetric aptamer sensors. Anal Chem 2019, 91:14697–14704, 10.1021/acs.analchem.9b03946. [DOI] [PubMed] [Google Scholar]
- 35.Collard DM, Fox MA: Use of electroactive thiols to study the formation and exchange of alkanethiol monolayers on gold. Langmuir 1991, 7:1192–1197, 10.1021/la00054a029. [DOI] [Google Scholar]
- 36.Rittikulsittichai S, Park CS, Jamison AC, Rodriguez D, Zenasni O, Lee TR: Bidentate aromatic thiols on gold: new insight regarding the influence of branching on the structure, packing, wetting, and stability of self-assembled monolayers on gold surfaces. Langmuir 2017, 33:4396–4406, 10.1021/acs.langmuir.7b00088. [DOI] [PubMed] [Google Scholar]
- 37.*.Phares N, White RJ, Plaxco KW: Improving the stability and sensing of electrochemical biosensors by employing trithiol-anchoring groups in a six-carbon self-assembled monolayer. Anal Chem 2009, 81:1095–1100, 10.1021/ac8021983. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors immobilized aptamers via flexible or rigid trithiol anchoring groups. Compared to traditional monolthiol anchors, sensors with felxible trithiols revealed superior stability under buffered storage, repeated PCR cycles, and repeated voltammetric scanning.
- 38.Kim T, Chan KC, Crooks RM: Self-assembled diacetylenic and polydiacetylenic monolayers. Langmuir 1997, 7863:189–193. [Google Scholar]
- 39.*.Ge D, Wang X, Williams K, Levicky R: Thermostable DNA immobilization and temperature effects on surface hybridization. Langmuir 2012, 28:8446–8455, 10.1021/la301165a. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used 1,11-bis-maleimidotriethyleneglycol to crosslink a surface-deposited layer of poly(mercaptopropyl)methylsiloxane on the electrode surface. Subsequent conjugation of aptamers to the multi-dentate monolayer created sensors with improved thermal stability.
- 40.Chidsey CED, Loiacono DN: Chemical functionality in self-assembled monolayers: structural and electrochemical properties. Langmuir 1990, 6:682–691, 10.1021/la00093a026. [DOI] [Google Scholar]
- 41.Singhana B, Jamison AC, Hoang J, Lee TR: Self-assembled monolayer films derived from tridentate cyclohexyl adsorbates with alkyl tailgroups of increasing chain length. Langmuir 2013, 29:14108–14116, 10.1021/la401899q. [DOI] [PubMed] [Google Scholar]
- 42.Staderini M, González-Fernández E, Murray AF, Mount AR, Bradley M: A tripod anchor offers improved robustness of peptide-based electrochemical biosensors. Sensor Actuator B Chem 2018, 274:662–667, 10.1016/j.snb.2018.07.100. [DOI] [Google Scholar]
- 43.Kuralay F, Campuzano S, Wang J: Greatly extended storage stability of electrochemical DNA biosensors using ternary thiolated self-assembled monolayers. Talanta 2012, 99: 155–160, 10.1016/j.talanta.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 44.*.Daggumati P, Matharu Z, Wang L, Seker E: Biofouling-resilient * nanoporous gold electrodes for DNA sensing. Anal Chem 2015, 87:8618–8622, 10.1021/acs.analchem.5b02969. [DOI] [PubMed] [Google Scholar]; The authors employed nanoporous gold electrodes for the immobilization of capture probes. Because the pores exclude large proteins from entering while still allowing diffusion of smaller targets, they were able to sense small oligoncleotides in 10% FBS.
- 45.*.Santos-Cancel M, White RJ: Collagen membranes with ribonuclease inhibitors for long-term stability of electrochemical aptamer-based sensors employing RNA. Anal Chem 2017, 89: 5598–5604, 10.1021/acs.analchem.7b00766. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors physically entrap RNase inhibitors within a collagen membrane on top of RNA-based sensors. Doing so enables prolonged RNA-based sensing in undiluted serum.
- 46.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM: Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17: 2841–2850, 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 47.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM: Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir 2001, 17:6336–6343, 10.1021/la010552a. [DOI] [Google Scholar]
- 48.Ahmed EM: Hydrogel: preparation, characterization, and applications: a review. J Adv Res 2015, 6:105–121, 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young BE, Kundu N, Sczepanski JT: Mirror-image oligonucleotides: history and emerging applications. Chem - A Eur J 2019, 25:7981–7990, 10.1002/chem.201900149. [DOI] [PMC free article] [PubMed] [Google Scholar]


