Abstract
The enantioselective preparation of the two isomers of 4-hydroxy-2-cyclohexanone derivatives 1a,b was achieved, starting from a common cyclohexenone, through asymmetric transfer hydrogenation (ATH) reactions using bifunctional ruthenium catalysts. From these versatile intermediates, a stereoselective route to a cytosine analogue built on a bicyclo [4.1.0]heptane scaffold is described. Nucleoside kinase activity assays with this cyclopropyl-fused cyclohexane nucleoside, together with other related nucleosides (2a–e), were performed, showing that thymine- and guanine- containing compounds have affinity for herpes simplex virus Type 1 (HSV-1) thymidine kinase (TK) but not for human cytosolic TK-1, thus pointing to their selectivity for herpetic TKs but not cellular TKs.
Keywords: asymmetric synthesis, carbocyclic nucleosides, HSV-1 thymidine kinase, enzymatic assays
1. Introduction
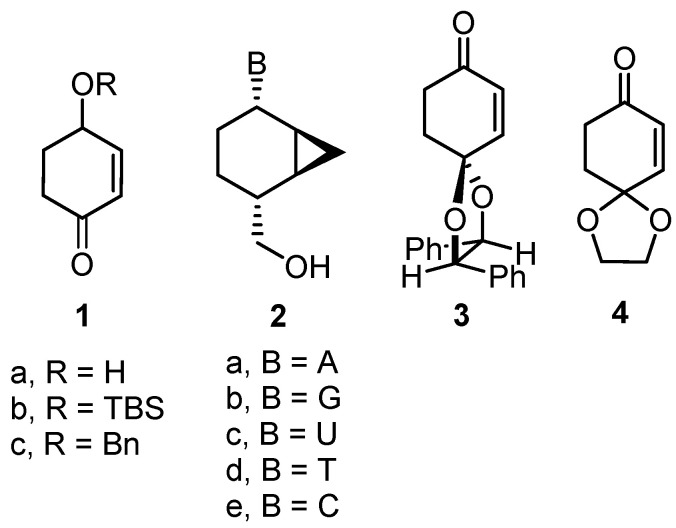
Optically active ϒ-substituted cycloalkenones are compounds of synthetic importance that are used as precursors in the synthesis of natural products and pharmaceutically active molecules [1,2,3,4]. In particular, both enantiomers of 4-hydroxy-2-cyclohexenone 1a and their O-protected derivatives, such as 1b,c, have been extensively used in organic synthesis (Figure 1) [5,6,7]. These chiral cycloalkenones have been prepared by different methodologies that involve enzymatic transformations [8], kinetic resolutions [9], chiral auxiliaries [10], asymmetric catalysis [11], or chiral pool compounds [12,13]. However, most of these approaches do not provide access to both enantiomers. Therefore, the development of efficient routes to such relevant building blocks is of considerable interest.
Figure 1.
Chiral cyclohexenone building blocks 1a–c, nucleoside analogues 2a–e built on a bicyclo [4.1.0]heptane scaffold and synthetic precursors 3 and 4.
In a previous work, we reported the synthesis of the nucleoside analogues (NAs) 2a–d built on a bicyclo [4.1.0]heptane scaffold, starting from cyclohexenone 3 bearing a dihydrobenzoin moiety as the chiral auxiliary [14]. These NAs were designed as potential antiherpetic agents by a molecular modeling study on the HSV-1 TK active site that is involved in the first phosphorylation step of the activation process. However, none of the compounds showed significant antiviral activity at subtoxic concentrations (~250 μM). The lack of activity indicated that these functionalized NAs might, eventually, fail to reach the HSV DNA polymerase interaction step. Therefore, further studies were needed to identify the molecular basis of the antiherpetic inactivity of the prepared compounds; in particular, single enzymatic assays were required to test the suitability of these compounds to pass the usual rate-limiting first phosphorylation step [15].
In order to perform these enzymatic assays, to complete the 5′-hydroxymethylbicyclo [4.1.0]heptanyl family 2 by preparing the cytosine analogue 2e, and considering our work on the synthesis of bioactive products bearing a cyclohexane unit [16,17,18], general and easy access to both enantiomers of 1a and 1b was required. We envisaged that the asymmetric transfer hydrogenation (ATH) on 1,4-cyclohexenedione monoethylene ketal 4 could be applied to prepare such chiral cyclohexenones [19]. ATH with bifunctional ruthenium catalysts has become one of the most practical and versatile tools for accessing enantiomerically enriched alcohols in organic synthesis, due to its excellent selectivity, practical simplicity, and wide substrate scope [20,21]. In this article, we disclose a concise entry to both enantiomers of ϒ-hydroxycyclohexenone by ATH via catalyst control, their use as precursors in the preparation of the conformationally restricted cytosine analogue 2e, and the results of the enzymatic affinity of compounds 2a–e on HSV-1 thymidine kinase.
2. Results and Discussion
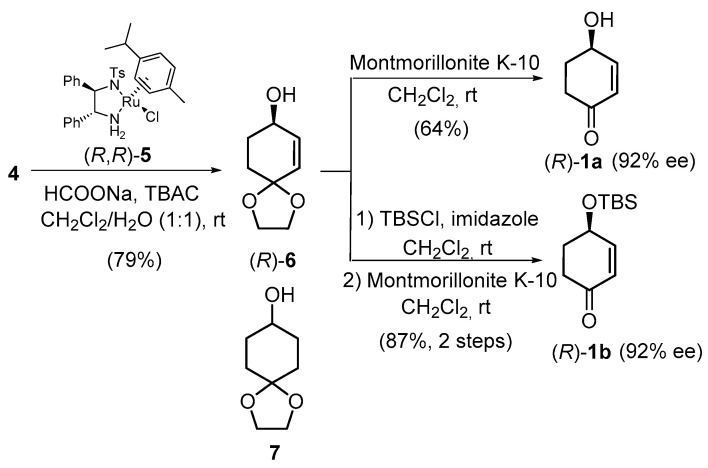
The synthesis of both enantiomers of 1a and 1b (Scheme 1) started with enone 4, which was easily prepared on a multigram scale from the commercially available 1,4-cyclohexanedione monoethylene acetal in 88% yield, through a two-step optimized dehydrogenation protocol that involved the formation of the corresponding silyl enol ether followed by oxidation with IBX·MPO complex [17]. The asymmetric reduction of enone 4 was first carried out in a biphasic medium (CH2Cl2/H2O 1:1), using (R,R)-Noyori-I, (R,R)-5 as catalyst, TBAC as a phase-transfer agent, and HCOONH4 as the hydrogen source [22,23,24]. Under these experimental conditions, allylic alcohol (R)-6 was obtained, along with its totally hydrogenated derivative 7 in a 6:1 ratio and 67% yield. The reduction also resulted in the removal of the ketal protecting group, providing variable amounts of 1a. However, when the reaction was performed using HCOONa [25], the desired allylic alcohol (R)-6 was isolated in 79% yield. Next, deprotection of the carbonyl group was achieved by treatment with montmorillonite K-10 in dichloromethane at room temperature, affording the volatile 4-hydroxy-2-cyclohexenone (R)-1a in 64% yield and 92% ee (determined by CHPLC). Protection of the alcohol 4 as a silyl ether with imidazole and TBSCl in dichloromethane and subsequent hydrolysis of the ketal using montmorillonite K-10 led to (R)-1b in 87% yield for the two steps.
Scheme 1.
Enantioselective preparation of cyclohexanones (R)-1a and (R)-1b.
In a similar way, the asymmetric reduction was performed with the enantiomeric (S,S)-5 catalyst, providing allylic alcohol (S)-6 in 78% yield, along with traces of the totally hydrogenated derivative. Hydrolysis of the ketal delivered (S)-1a in 62% yield and 92% ee (determined by CHPLC), while silyl ether protection and ketal cleavage afforded (S)-1b in 88% yield. Thus, we established a practical enantioselective procedure to prepare both enantiomers of 4-hydroxy-2-cyclohexenone (R)- and (S)-1a in four steps, ca. 44% overall yield and 92% ee, and their O-silyl derivatives, (R)- and (S)-1b, in five steps and 60% overall yield, which compared with other published methodologies [5,6,7,8,9,10,11,12,13]. Both enantiomers of the product could be obtained from the same precursor only by selecting either the (S,S)- or (R,R)-5 catalyst.
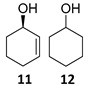
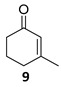
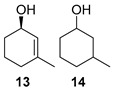
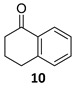
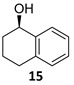
The satisfactory results achieved in the ATH step prompted us to examine the scope of the reaction with other cyclohexenones 8–10 using the optimized conditions ((R,R)-5, TBAC and HCOONa in CH2Cl2/H2O 1:1, Table 1). The ATH reaction on 2-cyclohexenone 8 delivered a 2:1 mixture of the corresponding alcohol 11 and the fully hydrogenated cyclohexanol 12 in good yield with an enantiomeric excess of 92% (entry 1). The ATH on cyclohexenone 9 bearing a methyl group at the β position, provided a 10:1 mixture of the corresponding allylic alcohol 13 and its fully hydrogenated derivative 14, from which the major compound 13 could be isolated by column chromatography (entry 2). The enantioselectivity of the reaction was slightly lower (88% ee). Finally, the ATH reaction with 1-tetralone 10 (entry 3) delivered allylic alcohol 15 in good yield with the highest enantioselectivity (94% ee). Overall, the ATH reaction on 8–10 in the presence of (R,R)-5 proved to be highly effective, providing access to the corresponding alcohols as the major products with excellent levels of enantioselectivity. The enantio- and chemoselectivities of the ATH reaction were sensitive to the structure of the cyclohexenones. In cyclohexanone 8, the formation of the fully hydrogenated compound was detrimental for the yield. The presence of a vinylic methyl group enhances the chemoselectivity of the ATH reaction. These chiral cyclohexanols are useful platforms for the synthesis of more elaborate products [26,27,28]. The ATH reaction has also been studied on cycloalkenones, with five- and seven-membered rings obtaining lower values of enantio- and chemoselectivity (see the Supplementary Materials).
Table 1.
Asymmetric Hydrogenation of Cyclic Ketones.
| Entry | Substrate | Products | Yield (%) | Ratio a | ee (%) b |
|---|---|---|---|---|---|
| 1 |
|
|
82 c | 2:1 | 92 |
| 2 |
|
|
65 | 10:1 | 88 |
| 3 |
|
|
87 | - | 94 |
a Determined by 1H-NMR. b Determined by chiral high-pressure liquid chromatography (CHPLC). c From the mixture of compounds 11 and 12.
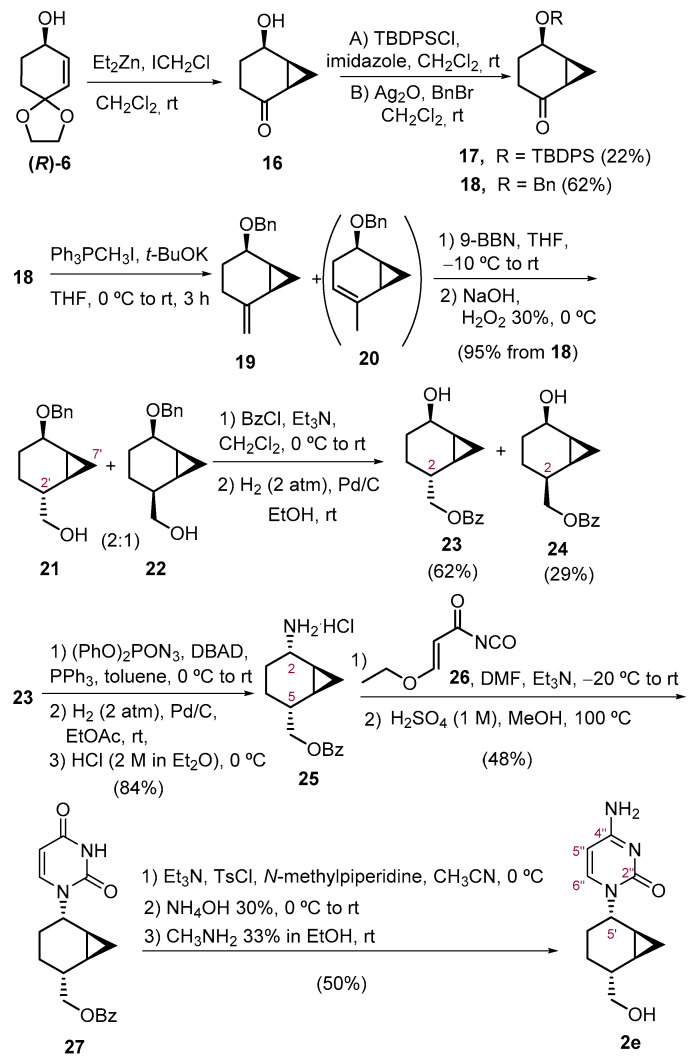
Next, we turned our attention to the use of chiral cyclohexanol R-(6) as the starting material in the preparation of the cytosine analogue 2e, following an adaptation of our earlier work [14]. Accordingly, the synthesis of 2e (Scheme 2) started with the cyclopropanation reaction on (R)-6, which was first attempted using Shi’s carbenoid (CF3COOZn CH2I) [29,30]. Under these conditions, the cyclopropanation took place with concomitant removal of the ketal to deliver the known bicyclic keto alcohol 16 albeit in very low yield (16%). Better results were obtained using Furukawa’s procedure [31,32] (Et2Zn and ICH2Cl), which, after purification by column chromatography, afforded 16 in 78% yield. This compound was highly volatile; therefore, the next protection step was performed immediately after the cyclopropanation reaction without further purification. To continue with the synthesis, two protecting groups were evaluated. First, protection of the alcohol as a tert-butyldiphenylsilyl ether furnished 17 in 22% yield for the two steps. All attempts to increase the yield failed. On the other hand, benzyl protection under standard conditions (BnBr, NaH, THF) did not provide better results, delivering only unidentified decomposition products. After some experimentation, it was found that the O-benzyl-protected 18 could be obtained in 62% overall yield over the two steps, using BnBr and Ag2O. Next, olefination of the ketone via a Wittig reaction led to the expected alkene 19, which was rapidly submitted to the next step, as it easily isomerizes to the endocyclic isomer 20. Accordingly, the subsequent hydroboration (9-BBN) -oxidation (H2O2) process furnished a chromatographically inseparable 2:1 mixture of diastereomers 21 and 22 in 95% yield. After several purifications by column chromatography, an enriched fraction of the main product was obtained and analyzed by NMR. The anti relative configuration of C-2′ of the main product was determined by a NOESY experiment that showed cross peaks between H-7′endo and H-2′, indicating that the approach of the borane through the syn face was favored, due to the steric hindrance between the alkylborane and the cyclopropane in the four-center transition state that resulted from the approach of the borane to the more accessible anti face. After purification by column chromatography, benzoyl protection, followed by the removal of the benzyl-protecting group, delivered alcohol 23 in 62% yield and its diastereomer 24 in 29% yield. The primary amine 25 required for the nucleobase construction was accomplished in 84% yield, starting from 23 through a Mitsunobu reaction using diphenylphosphoryl azide (DPPA) [33], followed by catalytic hydrogenation and hydrochloride salt formation.
Scheme 2.
Synthesis of compound 2e.
The cytosine nucleoside was prepared through the amination of the corresponding uridine derivative, which was prepared by a two-step protocol that involved the addition of the amine 25 to the isocyanate 26, followed by acid-mediated cyclization, to furnish the protected uracil nucleoside 27 [34]. Then, a one-pot amination in the presence of TsCl, Et3N and N-methylpiperidine at 0 °C [35], followed by ammonolysis with 30% NH4OH solution in water and, finally, removal of the benzoyl protection (CH3NH2, 33% in EtOH), afforded, after purification by column chromatography, the desired cytosine nucleoside analogue 2e.
Compound 2e was examined for antiherpetic activity (herpes simplex virus-1 (HSV-1; strain KOS) and herpes simplex virus-2 (strain G) in human embryonic lung (HEL) cell cultures. Unfortunately, however, it did not show significant antiviral activity at subtoxic concentrations (~100 μM) (see Supplementary Materials). These results were similar to those obtained for the previously synthesized compounds 2a–d. As previously mentioned, the lack of significant activity against herpes simplex virus by the 5′-hydroxymethylbicyclo [4.1.0]heptanyl NAs may arise from a low affinity (if any) or from substrate activity for the viral-encoded or cellular nucleoside kinases. Therefore, to evaluate this last point, we performed further studies to test the suitability of these compounds to pass the usual rate-limiting first phosphorylation step. Thus, the affinity of the synthesized compounds 2a–e for the cellular and HSV-1 thymidine kinases (TKs) were investigated (Table 2). Based on these results, it can be concluded that compounds 2b and, to a lesser extent, 2d, were nicely recognized by herpes simplex virus-TK (IC50 = 1.6 ± 0.1 µg/mL and 52 ± 28 µg/mL, respectively), in agreement with our modelling studies [14]. Only compound 2b showed some marginal affinity for mitochondrial TK-2, in addition to its excellent affinity for HSV-1 TK. The 2b analogue was also evaluated for substrate activity by HPLC technology, and it was found to be a good substrate for HSV-1 TK with an efficient conversion to the monophosphate metabolite. The cytosine derivative 2e did not display affinity for HSV-1 TK. It was expected that this cytosine analogue might show affinity for HSV-1 TK, as the natural nucleoside (2-deoxycytidine, 2-dC) is known to be recognized by several kinases, including HSV-1 TK; however, that was apparently not the case. Interestingly, none of the analogues showed significant affinity to human cellular kinases. Therefore, because some of the synthesized analogues were recognized by the virus-encoded nucleoside kinase, the lack of antiherpetic activity could be attributed to the lack of further conversion of the 5′-monophosphate derivative to the 5′-triphosphate or to a low affinity, if any, of the 5′-triphosphate metabolite to the virus-encoded DNA polymerase.
Table 2.
Evaluation of the synthesized compounds for affinity against nucleoside kinases.
| Compound | Conc. Unit | IC50 a | ||
|---|---|---|---|---|
| Human TK-1 | Human TK-2 | HSV-1 TK | ||
| 2a | µg/mL | >200 | >200 | 150 ± 15 |
| 2b | µg/mL | >200 | 93 ± 7 | 1.6 ± 0.1 |
| 2c | µg/mL | >200 | >200 | >200 |
| 2d | µg/mL | >200 | >200 | 52 ± 28 |
| 2e | µg/mL | >100 | >100 | >100 |
a 50% inhibitory concentration, or compound concentration required to inhibit TK-catalyzed conversion of [3H]dThd to its 5′-monophosphate derivative.
3. Materials and Methods
General Methods. Commercially available reagents were used as received. Solvents were dried by distillation over the appropriate drying agents. All of the reactions were monitored by analytical thin-layer chromatography (TLC), using silica gel 60 F254 pre-coated aluminum plates (0.25 mm thickness). TLC spots were detected under UV light and/or by charring with a KMnO4/KOH aqueous solution or vanillin solution. Flash column chromatography was performed using silica gel (230–400 mesh). 1H NMR spectra were recorded using 250 or 400 MHz and were referenced to the residual proton signals of CDCl3, 7.26 ppm, and MeOH-d4, 3.31 ppm. 13C [1H] NMR spectra were recorded at 90 MHz or 100 MHz and were referenced to the residual 13C signal of CDCl3, 77.16 ppm, and MeOH-d4, 49.00 ppm. NMR signals were assigned with the help of COSY, HSQC, HMBC, and NOESY experiments. Melting points were determined on a hot stage and were uncorrected. Optical rotations were measured at 20 ± 2 °C at the sodium D line (589 nm) in a microcell (0.1 dm). Infrared spectra were recorded on a spectrophotometer equipped with a Golden Gate Single Refraction Diamond ATR (attenuated total reflectance) accessory. High-resolution mass spectra were recorded using electrospray ionization (ESI).
Thymidine Kinase Assay Using [CH3-3H]dThd as the Natural Substrate [36]. The activity of recombinant thymidine kinase 1 (TK-1), TK-2, and herpes simplex virus-1 (HSV-1) TK, and the 50% inhibitory concentration of the test compounds, were assayed in a 50-μL reaction mixture containing 50 mM Tris/HCl, pH 8.0, 2.5 mM MgCl2, 10 mM dithiothreitol, 0.5 mM CHAPS, 3 mg/mL bovine serum albumin, 2.5 mM ATP, 1 µM [methyl-3H]dThd, and enzyme. The samples were incubated at 37 °C for 30 min in the presence or absence of different concentrations (five-fold dilutions) of the test compounds. At this time, the enzyme reaction still proceeded linearly. Aliquots of 45 µL of the reaction mixtures were spotted on Whatman DE-81 filter paper disks (Whatman, Clifton, NJ, USA). The filters were washed three times for 5 min each in 1 mM ammonium formate, once for 1 min in water, and once for 5 min in ethanol. The radioactivity was determined by scintillation counting.
Synthetic Procedures
(8R)-1,4-Dioxaspiro [4.5]dec-6-en-8-ol [(R)-6]. To a stirred solution of enone 4 (104 mg, 0.68 mmol) in a biphasic medium of CH2Cl2 (2.3 mL) and water (2.3 mL), tetrabutylammonium chloride (57 mg, 0.20 mmol), sodium formate (139 mg, 2.04 mmol), and (R,R)-Noyori-I [(R,R)-5] catalyst (13 mg, 0.020 mmol) were sequentially added at room temperature. The reaction mixture was allowed to stir for 3 h. Then, the phases were separated, and the aqueous layer was extracted with additional CH2Cl2 (2 × 2 mL) and the organic layers were concentrated under reduced pressure, affording a yellow oil that was purified by column chromatography (diethyl ether) to furnish allylic alcohol (R)-6 (83 mg, 0.53 mmol, 79%): [α]D20 = +39.0 (c 1.2, CHCl3) [lit: [37] [α]D20 = +39.8 (c 1.21, CHCl3), ee = 93.1%] as a yellowish oil: 1H NMR (250 MHz, CDCl3) δ 5.94 (ddd, J7,6 = 10.1 Hz, J7,8 = 2.8 Hz, J7,9 = 1.1 Hz, 1H, H-7), 5.61 (dt, J6,7 = 10.1 Hz, J6,8 = J6,10 =1.5 Hz, 1H, H-6), 4.29–4.12 (m, 1H, H-8), 4.05–3.85 (m, 4H, H-2, H-3), 2.17–2.02 (m, 1H, H-9), 2.00–1.89 (m, 1H, H-9), 1.85–1.65 (m, 2H, H-10); 13C NMR (90 MHz, CDCl3) δ 135.3 (C-7), 129.0 (C-6), 105.2 (C-5), 66.0 (C-8), 64.8/64.6 (C-2, C-3), 31.0/30.6 (C-9, C-10).
(8S)-1,4-Dioxaspiro [4.5]dec-6-en-8-ol [(S)-6]. To a stirred solution of enone 4 (76 mg, 0.49 mmol) in a biphasic medium of CH2Cl2 (1.3 mL) and water (1.3 mL), tetrabutylammonium chloride (45 mg, 0.16 mmol), sodium formate (140 mg, 2.06 mmol), and (S,S)-Noyori-I catalyst, [(S,S)-5], (9.1 mg, 0.015 mmol) were sequentially added at room temperature. The reaction mixture was allowed to stir for 24 h. Then, the phases were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The organic layers were concentrated under reduced pressure, affording a yellow oil that was purified by column chromatography (diethyl ether) to obtain allylic alcohol (S)-6 (60 mg, 0.38 mmol, 78% yield) as a yellow oil: [α]D20 = −38.7 (c 1.3, CHCl3) [lit: [38] [α]D20 = −40.5 (c 1.24, CHCl3) ee > 98.4%]; 1H NMR (250 MHz, CDCl3) δ: 5.93 (ddd, J7,6 = 10.1 Hz, J7,8 = 2.8 Hz, J7,9 = 1.0 Hz, 1H, H-7), 5.60 (dt, J6,7 = 10.1 Hz, J6,8 = J6,10 = 1.4 Hz, 1H, H-6), 4.26–4.16 (m, 1H, H-8), 4.05–3.85 (m, 4H, H-2, H-3), 2.19–2.05 (m, 1H, H-9), 2.04–1.90 (m, 1H, H-9), 1.90–1.68 (m, 2H, H-10).
(4R)-4-Hydroxycyclohex-2-en-1-one [(R)-1a]. Montmorillonite K-10 (832 mg) was added to a solution of (R)-6 (77 mg, 0.49 mmol) in CH2Cl2 (7.6 mL) and the mixture was stirred at room temperature for 4 h. Then, it was filtered, and the solvent was removed under vacuum to furnish the enone (R)-1a (34 mg, 0.31 mmol, 64% yield) as a yellowish oil: CHPLC (Daicel Chiralpak IC): 92% ee; [α]D20 = +90.0 (c 0.2, CHCl3) [lit: [39] [α]D20 = +92.3 (c 0.7, CHCl3) ee > 99%]; 1H NMR (250 MHz, CDCl3) δ 6.95 (ddd, J3,2 = 10.2 Hz, J3,4 = 2.1 Hz, J3,5eq = 1.6 Hz, 1H, H-3), 5.98 (ddd, J2,3 = 10.2 Hz, J2,4 = 2.1 Hz, J2,6 = 1.0 Hz, 1H, H-2), 4.58 (ddt, J4,5ax = 9.2 Hz, J4,5eq = 4.4 Hz, J4,2 =J4,3 =2.1 Hz, 1H, H-4), 2.60 (dt, Jgem = 9.8 Hz, J6eq,5 = J6eq,4 = 4.5 Hz, 1H, H-6eq), 2.45–2.28 (m, 2H, H-6ax, H-5eq), 2.10–1.90 (m, 1H, H-5ax); 13C NMR (90 MHz, CDCl3) δ 199.4 (C-1), 153.6 (C-3), 129.0 (C-2), 66.2 (C-4), 35.4 (C-6), 32.4 (C-5).
(4S)-4-Hydroxycyclohex-2-en-1-one [(S)-1a]. Montmorillonite K-10 (852 mg) was added to a solution of (S)-6 (79 mg, 0.50 mmol) in CH2Cl2 (7.8 mL) and the mixture was stirred at rt for 4 h. Then, it was filtered and the solvent was removed under vacuum to furnish the known enone (S)-1a (35 mg, 0.31 mmol, 62% yield) as a yellowish oil: CHPLC (Daicel Chiralpak IC): 92% ee; [α]D 20 =−92.3 (c 0.50, CHCl3) [lit: [9] [α]D20= −92.0 (c 0.50, CHCl3) ee > 99%]; 1H NMR (250 MHz, CDCl3) δ 6.93 (ddd, J3,2 = 10.2 Hz, J3,4 = 2.4 Hz, J3,5eq = 1.6 Hz, 1H, H-3), 5.95 (ddd, J2,3 = 10.2 Hz, J2,4 = 2.0 Hz, J2,6 = 1.0 Hz, 1H, H-2), 4.68–4.46 (m, 1H, H-4), 2.80–2.68 (m, 1H, OH), 2.57 (ddd, Jgem = 9.8 Hz, J6eq,4 = 5.0Hz, J6eq,5 = 4.0 Hz, 1H, H-6eq), 2.47–2.24 (m, 2H, H-6ax, H-5eq), 2.00 (ddd, Jgem = 12.8 Hz, J5ax,6ax = 9.5 Hz, J5ax,4 = 5.3 Hz, 1H, H-5ax).
(4R)-4-((tert-Butyldimethylsilyl)oxy)cyclohex-2-en-1-one [(R)-1b]. To a stirred solution of (R)-6 (102 mg, 0.65 mmol) in CH2Cl2 (0.5 mL), imidazole (56 mg, 0.82 mmol) and a solution of TBSCl (124 mg, 0.82 mmol) in CH2Cl2 (0.4 mL) were added at room temperature. The mixture was allowed to stir overnight. Then, a saturated aqueous NaHCO3 solution (1 mL) was added, the aqueous layer was extracted with CH2Cl2 (3 × 1 mL), and the organic layers were dried and concentrated under vacuum. The crude was dissolved in CH2Cl2 (4 mL) and montmorillonite K-10 (513 mg) was added. The reaction mixture was allowed to stir at room temperature for 1 h. Then, it was filtered, and the solvent was removed under vacuum to furnish enone (R)-1b (128 mg, 0.56 mmol, 87% yield) as a yellowish oil: [α]D20 = +92.3 (c 1.02, CHCl3) [lit: [39] [α]D20 = +97.9 (c 1.2, CHCl3) ee > 99%]; 1H NMR (400 MHz, CDCl3) δ 6.82 (ddd, J3,2 = 10.2 Hz, J3,4 = 2.1 Hz, J3,5eq = 1.6 Hz, 1H, H-3), 5.91 (ddd, J2,3 = 10.2 Hz, J2,4 = 2.1 Hz, J2,6eq = 1.0 Hz, 1H, H-2), 4.51 (ddt, J4,5ax = 9.1 Hz, J4,5eq = 4.6 Hz, J4,2 = J4,3 = 2.1 Hz, 1H, H-4), 2.56 (dt, Jgem = 16.8 Hz, J6eq,5ax = J6eq,5eq = 4.5 Hz, 1H, H-6eq), 2.33 (ddd, Jgem = 16.8 Hz, J6ax,5ax = 12.8 Hz, J6ax,5eq = 4.7 Hz, 1H, H-6ax), 2.20 (dqd, Jgem = 12.8 Hz, J5eq,4 = J5eq,6eq = J5eq,6ax = 4.6 Hz, J5eq,3 = 1.6 Hz, 1H, H-5eq), 1.99 (tdd, Jgem = J5ax,6ax = 12.8 Hz, J5ax,4 = 9.1 Hz, J5ax,6eq = 4.5 Hz, 1H, H-5ax), 0.90 (s, 9H, H-tBu), 0.12 (s, 3H, SiCH3), (s, 3H, SiCH3).
(4S)-4-((tert-Butyldimethylsilyl)oxy)cyclohex-2-en-1-one [(S)-1b]. To a stirred solution of (S)-6 (49 mg, 0.32 mmol) in CH2Cl2 (0.4 mL), imidazole (34 mg, 0.49 mmol) and a solution of TBSCl (74 mg, 0.49 mmol) in CH2Cl2 (0.2 mL) were added at room temperature. The mixture was allowed to stir overnight. Then, a saturated aqueous NaHCO3 solution (1 mL) was added, the aqueous layer was extracted with more CH2Cl2 (3 × 1 mL), and the organic layers were dried and concentrated under vacuum. The crude was dissolved in CH2Cl2 (4 mL) and montmorillonite K-10 (294 mg) was added. The reaction mixture was allowed to stir at room temperature for 1 h. Then, it was filtered, and the solvent was removed under reduced pressure to afford enone (S)-1b (64 mg, 0.28 mmol, 88% yield) as a yellowish oil: [α]D20= −93.7 (c 0.70, CHCl3) [lit: [6] [α]D20 = −97.0 (c 1.26, CHCl3), ee >99%]; 1H NMR (250 MHz, CDCl3) δ 6.81 (ddd, J3,2 = 10.2 Hz, J3,4 = 2.1 Hz, J3,5eq = 1.6 Hz, 1H, H-3), 5.90 (ddd, J2,3 = 10.2 Hz, J2,4 = 2.1 Hz, J2,6eq = 1.0 Hz, 1H, H-2), 4.50 (ddt, J4,5ax = 9.0 Hz, J4,5eq = 4.8 Hz, J4,3 = J4,2 = 2.1 Hz, 1H, H-4), 2.55 (dt, Jgem = 16.7 Hz, J6eq.5ax = J6eq,5eq = 4.8 Hz, 1H, H-6eq), 2.32 (ddd, Jgem = 16.7 Hz, J6ax,5ax = 12.8 Hz, J6ax,5eq = 4.8 Hz, 1H, H-6ax), 2.19 (dqd, Jgem = 12.8 Hz, J5eq,6eq = J5eq,4 = J5eq,6ax = 4.8 Hz, J5eq,3 = 1.6 Hz, 1H, H-5eq), 1.97 (tdd, Jgem = J5ax,6ax = 12.8 Hz, J5ax,4 = 9.0 Hz, J5ax,6eq = 4.8 Hz, 1H, H-5ax), 0.89 (s, 9H, H-tBu), 0.11 (s, 3H, SiCH3), 0.10 (s, 3H, SiCH3).
(1R)-2-Cyclohexen-1-ol [(R)-11]. To a stirred solution of enone 8 (250 µL, 2.59 mmol) in a biphasic medium of CH2Cl2 (8.6 mL) and water (8.6 mL) and under a nitrogen flux, tetrabutylammonium chloride (222 mg, 0.8 mmol), sodium formate (532 mg, 7.82 mmol), and (R,R)-Noyori-I catalyst, [(R,R)-5] (49.6 mg, 0.08 mmol) were sequentially added at room temperature. The reaction mixture was allowed to stir for 24 h. Then, CH2Cl2 (10 mL) and water (10 mL) were added, the two phases were separated, and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The volatiles of the organic phase were removed under reduced pressure and the resulting residue was purified by column chromatography (CHCl3), affording an inseparable mixture of allylic alcohol (R)-11 and cyclohexanol 12 (210 mg, 2.14 mmol, 82% yield) in a 2:1 ratio as a yellowish oil that was analyzed by NMR spectroscopy and CHPLC (Daicel Chiralpak IC): 92% ee; 1H NMR (250 MHz, CDCl3) ((R)-11) δ 5.82 (dtd, J3,2 = 10.0 Hz, J3,4eq = J3,4ax = 3.3 Hz, J3,1 = 1.1 Hz, 1H, H-3), 5.73 (ddt, J2,3 = 10.0, J2,1 = 3.4, J2,4 = J2,6 = 1.8 Hz, 1H, H-2), 4.42–4.13 (m, 1H, H-1), 2.07–1.89 (m, 1H, H-6), 1.92–1.81 (m, 1H, H-6), 1.79–1.64 (m, 2H, H-4, H-5), 1.64–1.48 (m, 2H, H-4, H-5); (12) δ 3.66–3.52 (m, 1H, H-1′), 1.64–1.48 (m, 4H, H-2′, H-6′), 1.38–0.96 (m, 6H, H-3′, H-4′, H-5′).
(1R)-3-Methyl-2-cyclohexen-1-ol [(R)-13]. To a stirred solution of enone 9 (510 µL, 495 mg, 4.49 mmol) in a biphasic medium of CH2Cl2 (14.7 mL) and water (14.7mL) and under a nitrogen flux, tetrabutylammonium chloride (367 mg, 1.32 mmol), sodium formate (899 mg, 13.2 mmol), and (R,R)-Noyori-I catalyst [(R,R)-5] (84 mg, 0.13 mmol) were sequentially added at room temperature. The reaction mixture was allowed to stir for 72 h. Then, CH2Cl2 (10 mL) and water (10 mL) were added, the two phases were separated, and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The volatiles of the organic phase were removed under reduced pressure and the resulting residue was purified by column chromatography (CHCl3), yielding allylic alcohol (R)-13 (327 mg, 2.92 mmol, 65% yield) as a yellowish oil: CHPLC (Daicel Chiralpak IC): 88% ee; [α]D20 = +89.8 (c 0.1, CHCl3) [lit: [40] [α]D20 = +62.4 (c 1.0, CHCl3), ee = 96%]; 1H NMR (250 MHz, CDCl3) δ 5.47 (dq, J2,1 = 3.3 Hz, J2,1′ = 1.6 Hz, 1H, H-2), 4.22–4.02 (m, 1H, H-1), 2.06–1.81 (m, 2H, H-6), 1.86–1.57 (m, 2H, H-5), 1.67 (s, 3H, H-1′), 1.64–1.34 (m, 2H, H-4).
(1R)-1,2,3,4-Tetrahydro-1-naphthalenol [(R)-15]. To a stirred solution of α-tetralone 10 (100 µL, 110 mg, 0.73 mmol) in a biphasic medium of CH2Cl2 (2.4 mL) and water (2.4 mL) and under a nitrogen flux, tetrabutylammonium chloride (61 mg, 0.22 mmol), sodium formate (149 mg, 2.19 mmol), and (R,R)-Noyori-I catalyst [(R,R)-5] (13.9 mg, 0.022 mmol) were sequentially added at room temperature. The reaction mixture was allowed to stir for 24 h. Then, CH2Cl2 (2 mL) and water (2 mL) were added, the two phases were separated, and the aqueous layer was extracted with CH2Cl2 (3 × 1 mL). The volatiles of the organic phase were removed under reduced pressure and the resulting residue was purified by column chromatography (CHCl3), yielding alcohol (R)-15 (97 mg, 0.65 mmol, 87% yield) as a yellowish oil: CHPLC (Daicel Chiralpack OD-H): 94% ee; [α]D20 = −36.5 (c 0.98, CHCl3) [lit: [40] [α]D20 = −33.2 (c 1.0, CHCl3), ee = 99%]; 1H NMR (250 MHz, CDCl3) δ 7.43 (dd, J8,7 = 5.5 Hz, J8,6 = 3.5 Hz, 1H, H-8), 7.25–7.17 (m, 2H, H-6, H-7), 7.11 (dd, J5,6 = 5.5 Hz, J5,7 = 3.5 Hz 1H, H-5), 4.76 (t, J1,2ax = J1,2eq = 4.5 Hz, 1H, H-1), 2.94–2.62 (m, 2H, H-4), 2.05–1.84 (m, 4H, H-2, H-3); 13C NMR (100 MHz, CDCl3) δ 138.9 (C-8a), 137.3 (C-4a), 129.2/128.8 (C-5, C-8), 127.7/126.3 (C-6, C-7), 68.3 (C-1), 32.4 (C-2), 29.4 (C-4), 18.9 (C-3).
(1R,5R,6S)-5-(Benzyloxy)bicyclo [4.1.0]heptan-2-one(18). To a stirred solution of allylic alcohol (R)-6 (314 mg, 2.01 mmol) in anhydrous CH2Cl2 (10 mL), Et2Zn (4 mL, 4.02 mmol, 1 M in hexane) at 0 ᵒC was added and the mixture was stirred for 5 min at this temperature. Then, a solution of ICH2Cl (600 µL, 8.30 mmol) in CH2Cl2 (3 mL) was added dropwise via syringe at 0 ᵒC, and the mixture was stirred overnight, allowing it to warm to room temperature. Then, a saturated aqueous NaHCO3 solution (10 mL) was added, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The organic layer was dried (Na2SO4), concentrated under reduced pressure (with vacuum controller) and purified by column chromatography (hexane-EtO2, 4:1) furnishing ketone 16 (198 mg, 1.57 mmol, 78% yield) as a colorless oil. Due to the volatility of compound 16, the crude was used without further purification prior to the next step.
To a solution of crude of the hydroxyacetone 16 in CH2Cl2 (4 mL), Ag2O (560 mg, 2.41 mmol) and benzyl bromide (340 µL, 2.81 mmol) were added. The mixture was stirred at room temperature for 24 h, then it was filtered through a Celite® pad and concentrated in vacuo. The crude was purified by column chromatography (hexanes-EtOAc, 6:1) to furnish 18 (270 mg, 1,24 mmol, 62% yield from (R)-6) as a pale oil.
16: [α]D20 = +78.5 (c 0.65, CHCl3) [lit: [41] [α]D20 = −80.7 (c 1.37, CHCl3), for its enantiomer, ee = 100%]; 1H NMR (400 MHz, CDCl3) δ 4.42 (dddd, J5,4ax = 10.1 Hz, J5,6 = 5.2 Hz, J5,4eq = 4.1 Hz, J5,3ax = 0.8 Hz, 1H, H-5), 2.37 (ddd, Jgem = 18.4 Hz, J3eq,4ax = 5.6 Hz, J3eq,4eq = 3.6 Hz, 1H, H-3eq), 2.15 (dddd, Jgem = 18.4 Hz, J3ax,4ax = 12.1 Hz, J3ax,4eq = 6.6 Hz, J3ax,5 = 0.8 Hz, 1H, H-3ax), 1.98–1.83 (m, 3H, H-1, H-6, H-4eq), 1.63 (dddd, Jgem = 13.8 Hz, J4ax,3ax = 12.1 Hz, J4ax,5 = 10.3 Hz, J4ax,3eq = 5.6 Hz, 1H, H-4ax), 1.44 (q, Jgem = J7endo,1 = J7endo,6 = 5.4 Hz, 1H, H-7endo), 1.14 (ddd, J7exo,1/6 = 9.8 Hz, J7exo,1/6 = 7.7 Hz, Jgem = 5.4 Hz, 1H, H-7exo); 13C NMR (100 MHz, CDCl3) δ 207.5 (C-2), 65.3 (C-5), 34.8 (C-3), 26.8 (C-1), 26.7 (C-4), 23.3 (C-6), 8.0 (C-7); IR (ATR) ν 3359, 2919, 2850, 1659, 1345, 1066, 1041 (cm−1). HRMS (ESI+) Calcd. for [C7H10O2+Na]+ 149.0573, found: 149.0576.
18: [α]D20 = +62.6 (c 1.45, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 4H, H-Ar), 7.32–7.28 (m, 1H, H-Ar), 4.73 (d, Jgem = 11.9 Hz, 1H, CH2-Ph), 4.64 (d, Jgem = 11.9 Hz, 1H, CH2-Ph), 4.15 (dt, J5,4ax = 9.3 Hz, J5,4eq = J5,6 = 4.9 Hz, 1H, H-5), 2.42 (dt, Jgem = 17.7 Hz, J3eq,4 = 5.5 Hz, 1H, H-3eq), 2.12 (ddd, Jgem = 17.7 Hz, J3ax,4x = 10.7 Hz, J3ax,4eq = 6.5 Hz, 1H, H-3ax), 2.05–1.93 (m, 2H, H-4), 1.88–1.71 (m, 2H, H-1, H-6), 1.50 (q, Jgem = J7endo,6 = J7endo,1 = 5.4 Hz, 1H, H-7endo), 1.24 (td, J7exo,6 = J7exo,1 = 9.1 Hz, Jgem = 5.4 Hz, 1H, H-7exo); 13C NMR (100 MHz, CDCl3) δ 208.2 (C-2), 138.5 (C-Ar), 128.6/128.2/127.9/127.8 (C-Ar), 71.3 (C-5), 70.6 (CH2-Ph), 34.4 (C-3), 26.5 (C-4), 25.5 (C-1), 21.3 (C-6), 9.5 (C-7); IR (ATR) ν 3028, 2857, 1692 (C=O), 1342, 1075, 1028, 881, 631 cm−1. HRMS (ESI+) Calcd. for [C14H16O2+H]+ 217.1229, Found: 217.1231.
[(1S’,2R’,5R’,6S’)-5′-(Benzyloxy)bicyclo [4.1.0]hept-2′-yl]methanol (21) and its (2′S)-diastereoisomer (22). To a stirring solution of Ph3PCH3I (1.452 g, 3.59 mmol) in anhydrous THF (4 mL) at 0 °C, t-BuOK (402 mg, 3.58 mmol) was added, under nitrogen atmosphere, and the resulting yellow mixture was allowed to react for 1 h. Then, a solution of ketone 18 (155 mg, 0.71 mmol) in dry THF (1 mL) was added and the mixture was allowed to warm to room temperature and stirred for 3 h. Then, diethyl ether (10 mL) was added and the crude was filtered through a short pad of silica and Celite®, using additional diethyl ether as eluent. The volatiles were removed under vacuum to obtain an orange oil of alkene 19 that was used for the next step without further purification, as it isomerizes to the more stable endocyclic regioisomer 20 at room temperature.
The crude of alkene 19 was rapidly dissolved in anhydrous THF (7 mL) and 9-BBN (4.30 mL, 2.15 mmol, 0.5 M in THF) was added at −10 °C. The mixture was allowed to warm to room temperature and stirred overnight. Then, water (1.2 mL), NaOH (1.5 mL, 3 M in water), and H2O2 (1.4 mL, 30% in water) were added at 0 °C. After stirring for 15 min, the mixture was diluted with brine (15 mL) and CH2Cl2 (15 mL) and the aqueous phase was extracted with additional CH2Cl2 (2 × 10 mL). The organic layers were dried (Na2SO4), concentrated under reduced pressure, and purified by column chromatography (gradient hexane-EtOAc, 5:1 → 2:1) to provide a chromatographically inseparable mixture of alcohols 21 and 22 (158 mg, 0.68 mmol, 95% overall yield from 18) in a ca. 2:1 diastereomeric ratio as a colorless oil. After repeated purification by column chromatography, enriched fractions were obtained and were analyzed by NMR.
19: 1H NMR (400 MHz, CDCl3) δ 7.46–7.25 (m, 5H, H-Ar), 4.90 (br s, 1H, H-1’), 4.79 (br s, 1H, H-1′), 4.74 (d, Jgem = 11.9 Hz, 1H, CH2-Ph), 4.56 (d, Jgem = 11.9 Hz, 1H, CH2-Ph), 4.05 (q, J2,3ax = J2,3eq = J2,1 = 5.9 Hz, 1H, H-2), 2.21 (dddt, Jgem = 14.9 Hz, J4ax,3ax = 8.5 Hz, J4ax,3eq = 4.4 Hz, J4ax,1′ = 1.4 Hz, 1H, H-4ax), 2.03 (dddt, Jgem = 14.9 Hz, J4eq,3ax = 7.2 Hz, J4eq,3eq = 4.3 Hz, J4eq,1′ = 1.4 Hz, 1H, H-4eq), 1.88–1.75 (m, 1H, H-6), 1.71–1.50 (m, 3H, H-1, H-3), 0.94–0.84 (m, 2H, H-7); 13C NMR (100 MHz, CDCl3) δ 146.3 (C-5), 139.1 (C-ipso), 128.4/127.7/127.5 (C-Ar), 108.1 (C-1′), 72.0 (C-2), 69.7 (CH2-Ph), 28.8 (C-4), 27.7 (C-3), 19.9 (C-1), 17.0 (C-6), 8.7 (C-7); IR (ATR) ν 3070, 2930, 1471, 1427, 1106, 806, 741 cm−1. HRMS (ESI+) Calcd. for [C15H18O+H]+ 215.1436, Found: 215.1465.
20: 1H NMR (400 MHz, CDCl3) δ 7.42–7.36 (m, 3H, H-Ar), 7.35–7.25 (m, 2H, H-Ar), 5.01 (dq, J3,4eq = 5.3 Hz, J3,4ax = J3,1′ = 1.6 Hz, 1H, H-3), 4.69 (d, Jgem = 12.0 Hz, 1H, CH2-Ph), 4.65 (d, Jgem = 12.0 Hz, 1H, CH2-Ph), 3.98 (ddd, J5,4ax = 9.1 Hz, J5,4eq = 6.8 Hz, J5,6 = 4.1 Hz, 1H, H-5), 2.32 (ddd, Jgem = 15.3 Hz, J4eq,5 = 6.8 Hz, J4eq,3 = 5.2 Hz, 1H, H-4eq), 1.89–1.75 (m, 4H, H-1′, H-4ax) 1.64–1.51 (m, 1H, H-6), 1.30–1.23 (m, 1H, H-1), 0.90–0.85 (m, 2H, H-7); 13C NMR (100 MHz, CDCl3): δ 139.6 (C-ipso), 136.7 (C-2), 128.8/128.2/127.9 (C-Ar), 114.3 (C-3), 73.4 (C-5), 70.6 (CH2-Ph), 28.0 (C-4), 23.5 (C-1′), 18.0/17.8 (C-1, C-6), 10.1 (C-7); IR (ATR) ν 3064, 2910, 2852, 1667, 1452, 1070, 734, 697 cm−1. HRMS (ESI+) Calcd. for [C15H18O+H]+ 215.1436, Found: 215.1434.
21 and 22 mixture: 1H NMR (400 MHz, CDCl3) (ca. 84% trans-isomer 21) δ 7.40–7.31 (m, 4H, H-Ar), 7.30–7.24 (m, 1H, H-Ar), 4.71 (d, Jgem = 12.0 Hz, 1H, CH2-Ph), 4.59 (d, Jgem = 12.0 Hz, 1H, CH2-Ph), 3.95 (dt, J5′,4′ax = 10.0 Hz, J5′,4′eq = J5′,6′ = 5.7 Hz, 1H, H-5′), 3.57 (d, J1,2′ = 6.6 Hz, 2H, H-1), 1.79 (dtd, Jgem = 13.3 Hz, J4′eq,5′ = J4′eq,3′ax = 5.7 Hz, J4′eq,3′eq = 2.4 Hz, 1H, H-4′eq), 1.70 (dddd, J2′,3′ax = 8.5 Hz, J2′,1 = 6.6 Hz, J2′,3′eq = 5.4 Hz, J2′,1′ = 1.9 Hz, 1H, H-2′), 1.55 (dtd, Jgem = 13.2 Hz, J3′eq,4′ax = J3′eq,2′ = 5.4 Hz, J3′eq,4′eq = 2.4 Hz, 1H, H-3′eq), 1.29–1.26 (m, 1H, H-6′), 1.04 (tdd, Jgem = J4′ax,3′ax = 13.3 Hz, J4′ax,5′ = 10.0 Hz, J4′ax,3′eq = 5.4 Hz, 1H, H-4′ax), 0.99–0.83 (m, 2H, H-1′, H-3′ax), 0.74 (td, J7′exo,6′ = J7′exo,1′ = 8.8 Hz, Jgem = 5.4 Hz, 1H, H-7′exo), 0.46 (q, Jgem = J7′endo,6′ = J7′endo,1′ = 5.4 Hz, 1H, H-7′endo); 1H NMR (400 MHz, CDCl3) (ca. 16% cis-isomer 22, observable signals) δ 7.40–7.31 (m, 4H, H-Ar), 7.30–7.24 (m, 1H, H-Ar), 4.72 (d, Jgem = 11.8 Hz, 1H, CH2-Ph), 4.47 (d, Jgem = 11.8 Hz, 1H, CH2-Ph), 4.01 (ddd, J5′,4′ax = 7.0 Hz, J5′,4′eq = 5.1 Hz, J5′,6′ = 3.4 Hz, 1H, H-5′), 3.57 (m, 1H, H-1a), 3.49 (dd, Jgem = 10.5 Hz, J1b,2′ = 6.6 Hz, 1H, H-1b), 2.05 (dddd, J2′,3′ax = 12.3 Hz, J2′,1a = 10.2 Hz, J2′,1b = 6.6 Hz, J2′,1′ = 5.4 Hz, 1H, H-2′), 1.66–1.57(m, 1H, H-4′a), 1.44–1.33 (m, 2H, H-3′a, H-4′b), 1.18 (tt, J1′,6′ = J1′,7′exo = 8.5 Hz, J1′,7′endo = J1′,2′ = 5.4 Hz, 1H, H-1′), 1.02–0.98 (m, 1H, H-3′b), 0.75 (m, 1H, H-6′), 0.60–0.51 (m, 2H, H-7′); 13C NMR (101 MHz, CDCl3) (ca. 84% trans-isomer 20): δ 139.25 (C-ipso), 128.43 (C-meta), 127.82 (C-para), 127.49 (C-orto), 74.44 (C-5′), 69.62 (CH2-Ph), 67.98 (C-1), 38.10 (C-2′), 25.31 (C-4′), 25.23 (C-3′), 15.90 (C-1′), 14.88 (C-6′), 8.21 (C-7′); 13C NMR (100 MHz, CDCl3) (ca. 16% cis-isomer 21, observable signals) δ 139.22 (C-ipso), 128.43 (C-meta), 127.80 (C-para), 127.44 (C-orto), 70.87 (C-5′), 69.57 (CH2-Ph) 67.78 (C-1), 35.64 (C-2′), 28.43 (C-4′), 18.93 (C-3′), 14.19 (C-1′), 13.37 (C-6′), 1.98 (C-7′). HRMS (ESI+) Calcd. for [C15H20O2+H]+ 233.1542, Found: 233.1537.
[(1′S,2′R,5′R,6′S)-5′-Hydroxybicyclo [4.1.0]hept-2′-yl]methyl benzoate (23) and its (2′S)-diastereoisomer (24). To a stirred solution of a 2:1 mixture of 21 and 22 (158 mg, 0.68 mmol) in dry CH2Cl2 (7 mL) at 0 °C, anhydrous Et3N (100 µL, 0.71 mmol) and benzoyl chloride (91 µL, 0.78 mmol) were sequentially added under argon atmosphere. The mixture was subsequently allowed to attain room temperature overnight. Then, the solution was treated with HCl 10% solution (7 mL) and CH2Cl2 (7 mL), the two phases were separated, and the aqueous phase was extracted with CH2Cl2 (3 × 7 mL). The organic layers were washed with brine (30 mL), dried (Na2SO4), and concentrated under reduced pressure to obtain a colorless oil that was directly used for the next step without further purification. Accordingly, the crude was dissolved in EtOH (7 mL) and hydrogenated in the presence of Pd/C (23 mg, 10% wt.) at 2 atm overnight. The mixture was filtered through a short pad of Celite® and washed with additional EtOH. The solvent was evaporated under reduced pressure and purified by column chromatography (hexanes-EtOAc, 10:1 → 5:1) to afford alcohol 23 (104 mg, 0.42 mmol, 62% yield) as a colorless syrup and its isomer 24 (49 mg, 0.20 mmol, 29% yield) as a colorless syrup.
23: [α]D20 = +48.7 (c 1,02, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (d, Jorto,meta = 7.6 Hz, 2H, H-orto), 7.61–7.51 (m, 1H, H-para), 7.44 (t, Jmeta,orto = Jmeta,para = 7.6 Hz, 2H, H-meta), 4.28 (dd, J1,2′ = 6.8 Hz, J1,1′ = 2.0 Hz, 2H, H-1), 4.20 (dt, J5′,4′ax = 9.5 Hz, J5′,4′eq = J5′,6′ = 5.7 Hz, 1H, H-5′), 2.0 (dddd, J2′,3′ax = 13.8 Hz, J2′,1 = 6.8 Hz, J2′,3′eq = 4.4 Hz, J2′,1′ = 1.9 Hz, 1H, H-2′), 1.86–1.72 (m, 1H, H-4′), 1.70–1.61 (m, 2H, H-3′), 1.33 (tt, J6′,7′endo = J6′,7′exo = 8.8 Hz, J6′,5′ = J6′,1′ = 5.7 Hz, 1H, H-6′), 1.13–0.87 (m, 2H, H-1′, H-4′), 0.71 (td, J7′exo,1′ = J7′exo,6′ = 8.8 Hz, Jgem = 4.9 Hz, 1H, H-7′exo), 0.40 (q, J7′endo,1′ = J7′endo,6′ = Jgem = 5.3 Hz, 1H, H-7′endo); 13C NMR (100 MHz, CDCl3) δ 166.8 (C=O), 133.1 (C-para), 130.4 (C-ipso), 129.7 (C-orto), 128.5 (C-meta), 69.4 (C-1), 68.0 (C-5′), 34.5 (C-2′), 28.0 (C-4′), 26.0 (C-3′), 18.1 (C-6′), 16.2 (C-1′), 6 (C-7′).
24: [α]D20 = −12.3 (c 1.16, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (d, Jorto,meta = 7.2 Hz, 2H, H-orto), 7.56 (t, Jpara,meta = 7.4 Hz, 1H, H-para), 7.44 (t, Jmeta,orto = Jmeta,para = 7.6 Hz, 2H, H-meta), 4.34 (dt, J5′,4′ax = 7.3 Hz, J5′,4′eq = J5′,6′ = 4.7 Hz, 1H, H-5′), 4.29 (dd, Jgem = 10.7 Hz, J1a,2′ = 6.9 Hz, 1H, H-1a), 4.18 (dd, Jgem = 10.7 Hz, J1b,2′ = 7.3 Hz, 1H, H-1b), 2.36 (dq, J2′,3′ax = 12.6 Hz, J2′,1a = J2′,1b = J2′,3′eq = 6.7 Hz, 1H, H-2′), 1.51–1.37 (m, 4H, H-6′, 2H-4′, H-3′), 1.27 (tt, J1′,2′ = J1′,7′exo = 8.5 Hz, J1′,7′endo = J1′,6′ = 5.7 Hz, 1H, H-1′), 1.23–1.13 (m, 1H, H-3′), 0.57 (q, Jgem = J7′endo,6′ = J7′endo,1′ = 5.3 Hz, 1H, H-7′endo), 0.46 (td, J7′exo,1′ = J7′exo,6′ = 8.9 Hz, Jgem = 5.1 Hz, 1H, H-7′exo); 13C NMR (100 MHz, CDCl3) δ 166.8 (C=O), 133.0 (C-para), 130.6 (C-ipso), 129.7 (C-orto), 128.5 (C-meta), 68.9 (C-1), 64.7 (C-5′), 31.7 (C-2′), 29.9 (C-4′), 19.4 (C-3′), 17.1 (C-1′), 14.7 (C-6′), 1.7 (C-7′).
(1S,2S,5R,6R)-5-[(Benzoyloxy)methyl)]bicyclo [4.1.0]heptan-2-amonium chloride (25). To a stirred solution of Ph3P (170 mg, 0.65 mmol) in dry toluene (4.5 mL), DBAD (150 mg, 0.65 mmol) was slowly added under argon atmosphere and the mixture was stirred for 45 min at 0 °C. After 15 min, a white suspension appeared. Then, diphenylphosphoryl azide (DPPA, 100 µL, 0.45 mmol) and a solution of 23 (105 mg, 0.43 mmol) in dry toluene (1 mL) were sequentially added. The mixture was allowed to warm to room temperature and stirred overnight. Then, the solvent was removed, and the crude was purified by column chromatography (hexanes-EtOAc, 20:1 to 15:1) to obtain a crude that was dissolved in EtOAc (2 mL) and hydrogenated in the presence of Pd/C (15 mg, 10% wt.) at 2 atm for 24 h. Then, the mixture was filtered through a short pad of Celite® and washed with additional EtOAc. The solvent was evaporated under reduced pressure and the crude was treated with 2 M HCl in Et2O (2 mL, 4 mmol) at 0 °C, stirred for 2 h, and filtered to furnish the ammonium salt 25 (101 mg, 0.36 mmol, 84% yield) as a brown solid: Mp 130–132 °C (from EtO2); [α]D20 = +14.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 3H, NH3+), 8.08–7.97 (m, 2H, H-Ar), 7.56–7.50 (m, 1H, H-Ar), 7.44–7.36 (m, 2H, H-Ar), 4.45 (d, J1′,5 = 7.7 Hz, 2H, H-1′), 3.84 (br s, 1H, H-2), 2.15–1.89 (m, 2H, H-5, H-4), 1.60–1.46 (m, 3H, H-3, H-4), 1.31–1.22 (m, 1H, H-1), 1.12–1.02 (m, 1H, H-6), 0.91 (td, J7exo,1 = J7exo,6 = 9.1 Hz, Jgem = 5.1 Hz, 1H, H-7exo), 0.19 (q, Jgem = J7endo,1 = J7endo,6 = 5.5 Hz, 1H, H-7endo); 13C NMR (100 MHz, CDCl3) δ 166.6 (C=O), 133.1 (C-para), 130.4 (C-ipso), 129.7 (C-orto), 128.5 (C-meta), 69.0 (C-1′), 47.1 (C-2), 33.9 (C-5), 23.2 (C-4), 19.4 (C-3), 14.0 (C-6), 12.5 (C-1), 10.4 (C-7); IR (ATR) ν 3404, 2929, 1712, 1273, 1113, 713 cm−1. HRMS (ESI+) Calcd. for [C15H18NO2]+ 244.1338, Found: 244.1334.
(E)-3-Ethoxyacryloyl isocyanate (26). Vinyl ether (5.75 mL, 60 mmol) was added dropwise to oxalyl chloride (7.60 mL, 90 mmol) at 0 °C. The reaction mixture was maintained for 2 h at 0 °C and then warmed to room temperature overnight. Excess oxalyl chloride was distilled off and the black residue was heated at 120 °C for 30 min. Then, the residue was purified by vacuum distillation, using a short Vigreux column, to obtain (2E)-3-ethoxyacryloyl chloride (4.30 g, 31.97 mmol, 53% yield) as a colorless liquid.
Silver cyanate (90 mg, 0.60 mmol), previously dried over phosphorus pentoxide at 80 °C for 3 h, in dry benzene (2 mL), was heated to reflux for 30 min and a solution of (2E)-3-ethoxyacryloyl chloride (45 mg, 0.31 mmol) in dry benzene (0.8 mL) was then added dropwise. The mixture was stirred for 30 min before allowing the solid to settle. The supernatant, which is a solution of the isocyanate 25, was then decanted and used directly in the next reaction.
[(1′R,2′R,5′S,6′S)-5′-(2″,4″-dioxo-3″,4″-dihydropyrimidin-1″(2H)-yl] bicyclo [4.1.0]heptan-2′-yl)methyl benzoate (27). The ammonium chloride 25 (43 mg, 0.153 mmol) was dissolved in dry DMF (1.8 mL), and Et3N (22 µL, 0.157 mmol) was added. The mixture was cooled to −20 °C and a freshly prepared solution of the isocyanate 26 was added slowly to avoid an increase in the temperature. The reaction mixture was stirred overnight at room temperature. The solvent was evaporated in vacuo, and then water (2 mL) was added. The residue was extracted with EtOAc (2 × 2 mL), washed with brine (2 mL), dried (Na2SO4), filtered, and evaporated under reduced pressure. The residue was dissolved in MeOH (0.40 mL), H2SO4 (1 M, 0.62 mL) was added, and the mixture was heated to reflux for 3 h. Then, the mixture was concentrated under reduced pressure and purified by column chromatography (CH2Cl2 100% to CH2Cl2-MeOH, 20:1) to provide 27 (25 mg, 0.073 mmol, 48% yield) as a yellowish oil: 1H NMR (400 MHz, MeOH-d4) δ 8.05 (d, Jorto,meta = 7.6 Hz, 2H, H-orto), 7.96 (d, J6″,5″ = 8.0 Hz, 1H, H-6″), 7.64 (d, Jpara,meta = 7.6 Hz, 1H, H-para), 7.52 (t, Jmeta,orto = Jmeta,para = 7.6 Hz, 2H, H-meta), 5.46 (d, J5″,6″ = 8.0 Hz, 1H, H-5″), 4.83 (dt, J5′,4′ax = 4.3 Hz, J5′,4eq = J5′,6′ = 2.3 Hz, 1H, H-5′), 4.45 (d, J1,2′ = 6.0 Hz, 2H, H-1), 2.21 (dq, J2′,3′ax = 11.9 Hz, J2′,3′eq = J2′1 = 6.0 Hz, 1H, H-2′), 1.78–1.68 (m, 1H, H-4′eq), 1.54 (tt, Jgem = J4′ax,3′ax = 14.7 Hz, J4′ax,5′ = J4′ax,3′eq = 4.3 Hz, 1H, H-4′ax), 1.49–1.40 (m, 1H, H-3′), 1.32–1.21 (m, 2H, H-3′, H-6′), 1.12–1.04 (m, 1H, H-1′), 0.97 (td, J7exo,1 = J7exo,6 = 9.4 Hz, Jgem = 5.0 Hz, 1H, H-7′exo), 0.39 (q, Jgem = J7endo,1 = J7endo,6 = 5.3 Hz, 1H, H-7′endo); 13C NMR (101 MHz, MeOH-d4): δ 168.1 (C=O), 166.3 (C-4″), 152.7 (C-2″), 145.1 (C-6″), 134.4 (C-para), 131.5 (C-ipso), 130.5 (C-orto), 129.7 (C-meta), 101.6 (C-5″), 69.9 (C-1), 52.5 (C-5′), 34.7 (C-2′), 24.5 (C-4′), 20.1 (C-3′), 15.3 (C-6′), 13.7 (C-1′), 10.4 (C-7′). HRMS (ESI+) Calcd. for [C19H20N2O4+Na]+ 363.1321, Found: 363.1312.
4-Amino-1-[(1′S,2′S,5′R,6′R)-5′-(hydroxymethyl)bicyclo [4.1.0]hept-2′-yl]pyrimidin-2(1H)-one (2e). A solution of TsCl (31 mg, 0.16 mmol) in dry CH3CN (104 µL) was added to a mixture of 27 (27 mg, 0.08 mmol), Et3N (22 µL, 0.16 mmol), and N-methylpiperidine (12 µL, 0.1 mmol) in dry CH3CN (135 µL) at 0 °C, and the reaction mixture was stirred for 3 h. Then, 30% NH4OH was added at 0 °C, and the reaction solution was stirred at room temperature overnight. The mixture was diluted with water (1 mL) and EtOAc (1 mL) and the aqueous phase was extracted with additional CH2Cl2 (2 × 1 mL). The organic layers were dried (Na2SO4), concentrated under reduced pressure, and purified by column chromatography (CH2Cl2-EtOAc, 10:2 → CH2Cl2-MeOH 15:1) to provide the protected cytosine analogue as a yellowish oil that was dissolved in a 33% solution of methylamine in EtOH (17 mL) and stirred for 24 h. Then, the mixture was concentrated under reduced pressure and purified by column chromatography (CH2Cl2-MeOH, 20:1) to provide cytosine nucleoside analogue 2e (9 mg, 38 μmol, 50% yield) as a yellowish solid: 2e: [α]D20 = +36.2 (c 0.4, MeOH-d4); 1H NMR (400 MHz, MeOH-d4) δ 8.04 (d, J6,5 = 7.4 Hz, 1H, H-6), 5.90 (d, J5,6 = 7.4 Hz, 1H, H-5), 4.91–4.87 (m, 1H, H-2′) 3.64 (dd, Jgem = 10.7 Hz, J1″a,5′ = 5.9 Hz, 1H, H-1″a), 3.59 (dd, Jgem = 10.7 Hz, J1″a,5′ = 5.9 Hz, 1H, H-1″b), 1.85 (dq, J5′,4′ax = 11.4 Hz, J5′4′eq = J5′,1″a = J5′,1″b = 5.9 Hz, 1H, H-5′), 1.68 (dt, Jgem = 13.7 Hz, J3′eq,4′ax = J3′eq,5′ = 4.4 Hz, 1H, H-3′eq), 1.48 (tt, Jgem = J3′ax,4′ax = 13.7 Hz, J3′ax, 4′eq = J3′ax,5′ = 3.6 Hz, 1H, H-3′ax), 1.34–1.26 (m, 2H, H-1′, H-4′a), 1.19–1.07 (m, 1H, H-4′b), 1.00 (td, J6′,7′exo = J6′,1′ = 9.4 Hz, J6′,7′endo = 5.2 Hz, 1H, H-6′), 0.89 (td, J7exo,1 = J7exo,6 = 9.4, Jgem = 5.2 Hz, 1H, H-7′exo), 0.30 (q, Jgem = J7endo,1 = J7endo,6 = 5.2 Hz, 1H, H-7′endo); 13C NMR (100 MHz, MeOH-d4): δ 167.3 (C-4), 159.0 (C-2), 145.6 (C-6), 95.0 (C-5), 67.6 (C-1″), 52.8 (C-2′), 37.5 (C-5′), 24.6 (C-3′), 19.6 (C-4′), 15.7 (C-1′), 13.81 (C-6′), 10.26 (C-7′). HRMS (ESI+) Calcd. for [C12H17N3O2+H]+ 236.1399, Found: 236.1394.
4. Conclusions
In this article, an easy route to enantiomerically pure 4-hydroxy-2-cyclohexanone derivatives 1a,b, which are commonly used as chiral building blocks, was identified on the basis of ATH with bifunctional ruthenium catalysts. This approach provided convenient access to both enantiomers from the same precursor by selecting either the (S,S)- or (R,R)-5 catalyst. In addition, a stereoselective route to the cytosine analogue, built on a bicyclo [4.1.0]heptane scaffold, was finely tuned, starting from cyclohexanol (R)-6. Finally, the kinase activity assays of compounds 2a–e showed that compounds 2b and 2d display affinity for HSV-1 TK but not for human TK-1, thus validating our previous molecular modeling study and pointing to their selectivity for herpetic TKs but not cellular TKs.
Acknowledgments
We acknowledge the support of Generalitat de Catalunya (2017-SGR-191). We thank Lizette van Berckelaer and Ria van Berwaer for their excellent technical assistance. S.J. and B.D.-P. acknowledge the financial support provided by the Universitat Autònoma de Barcelona and Generalitat de Catalunya, respectively.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179704/s1.
Author Contributions
S.J. and B.D.-P. contributed equally to this article; F.B. and R.A. devised the project’s concept and designed the experiments; S.J. and B.D.-P. synthesized compounds, supervised by O.I., F.B.; and R.A.; J.B. performed the biological assays; R.A. wrote the manuscript with contributions of the other authors. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Spanish Ministry of Economy, Industry, and Competitiveness (MINECO) (projects: CTQ2016-75363-R and PID2019-106403RB-I00), co-financed with the European Fund for Regional Development (FEDER).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huber T., Preuhs T.A., Gerlinger C.K.G., Magauer T. Development of a β-C−H Bromination Approach toward the Synthesis of Jerantinine E. J. Org. Chem. 2017;82:7410–7419. doi: 10.1021/acs.joc.7b01095. [DOI] [PubMed] [Google Scholar]
- 2.White J.D., Li Y., Kim J., Terienk M. A Novel Synthesis of (-)-Huperzine A via Tandem Intramolecular Aza-Prins Cyclization-Cyclobutane Fragmentation. Org. Lett. 2013;15:882–885. doi: 10.1021/ol400012s. [DOI] [PubMed] [Google Scholar]
- 3.Kawasumi M., Kanoh N., Iwabuchi Y. Concise Entry to Both Enantiomers of 8-Oxabicyclo[3.2.1]oct-3-en-2-one Based on Novel Oxidative Etherification: Formal Synthesis of (-)-Sundiversifolide. Org. Lett. 2011;13:3620–3623. doi: 10.1021/ol201273b. [DOI] [PubMed] [Google Scholar]
- 4.Hughes C.C., Miller A.K., Trauner D. An Electrochemical Approach to the Guanacastepenes. Org. Lett. 2005;7:3425–3428. doi: 10.1021/ol047387l. [DOI] [PubMed] [Google Scholar]
- 5.Wehlauch R., Grendelmeier S.M., Miyatake-Ondozabal H., Sandtorv A.H., Scherer M., Gademann K. Investigating Biogenetic Hypotheses of the Securinega Alkaloids: Enantioselective Total Syntheses of Secu’amamine E/ent-Virosine A and Bubbialine. Org. Lett. 2017;19:548–551. doi: 10.1021/acs.orglett.6b03716. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima H., Sakai M., Kaneko Y., Kobayashi Y. Further Study on Synthesis of the Cyclobakuchiols. Tetrahedron. 2015;71:2387–2392. [Google Scholar]
- 7.Burns A.R., Taylor R.J.K. Synthetic Approaches to Enantiomerically Enriched 4-Hydroxycyclohex-2-en-1-one—A Key Chiral Building Block in Complex Natural Product Synthesis. Synthesis. 2011;2011:681–707. [Google Scholar]
- 8.Hua Z., Yu W., Su M., Jin Z. Synthetic Studies toward the Construction of the cis-Decalin Portion of Superstolides A and B. Application of a Sequential Double Michael Reaction and an Anionic Oxy-Cope Rearrangement. Org. Lett. 2005;7:1939–1942. doi: 10.1021/ol050339w. [DOI] [PubMed] [Google Scholar]
- 9.Bayón P., Marjanet G., Toribio G., Alibés R., De March P., Figueredo M., Font J. An Efficient Protocol for the Enantioselective Preparation of a Key Polyfunctionalized Cyclohexane. New Access to (R)- and (S)-4-Hydroxy-2-cyclohexenone and (R)- and (S)-trans-Cyclohex-2-ene-1,4-diol. J. Org. Chem. 2008;73:3486–3491. doi: 10.1021/jo800107h. [DOI] [PubMed] [Google Scholar]
- 10.Fudickar W., Vorndran K., Linker T. Auxiliary Controlled Singlet-Oxygen Ene Reactions of Cyclohexenes. Tetrahedron. 2006;62:10639–10646. [Google Scholar]
- 11.Trost B.M., Masters J.T., Lumb J.-P., Fateen D. Asymmetric Synthesis of Chiral Cycloalkenone Derivatives via Palladium Catalysis. Chem. Sci. 2014;5:1354–1360. doi: 10.1039/C3SC53250J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthurs C.L., Morris G.A., Piacenti M., Pritchard R.G., Stratford I.J., Tatic T., Whitehead R.C., Williams K.F., Wind N.S. The Synthesis of 2-Oxyalkyl-cyclohex-2-enones, Related to the Bioactive Natural Products COTC and Antheminone A, which Possess Anti-Tumour Properties. Tetrahedron. 2010;66:9049–9060. doi: 10.1016/j.tet.2010.08.072. [DOI] [Google Scholar]
- 13.Audia J.E., Boisvert L., Patten A.D., Villalobos A., Danishefsky S.J. Synthesis of Two Useful, Enantiomerically Pure Derivatives of (S)-4-Hydroxy-2-cyclohexenone. J. Org. Chem. 1989;8:3738–3740. doi: 10.1021/jo00276a043. [DOI] [Google Scholar]
- 14.Domínguez-Pérez B., Ferrer E., Figueredo M., Maréchal J.D., Balzarini J., Alibés R., Busqué F. Synthesis of Novel Nucleoside Analogues Built on a Bicyclo[4.1.0]heptane Scaffold. J. Org. Chem. 2015;80:9495–9505. doi: 10.1021/acs.joc.5b01413. [DOI] [PubMed] [Google Scholar]
- 15.Eyer L., Nencka R., De Clercq E., Seley-Radtke K., Růžek D. Nucleoside Analogs as a Rich Source of Antiviral Agents Active Against Arthropod-Borne Flaviviruses. Antivir. Chem. Chemother. 2018;26:1–28. doi: 10.1177/2040206618761299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toribio G., Marjanet G., Alibés R., de March P., Font J., Bayón P., Figueredo M. Divergent Approach to Gabosines and Anhydrogabosines: Enantioselective Syntheses of (+)-Epiepoformin, (+)-EpoKformin, (+)-Gabosine A, and Gabosines B and F. Eur. J. Org. Chem. 2011;2011:1534–1543. doi: 10.1002/ejoc.201001527. [DOI] [Google Scholar]
- 17.Ferrer E., Alibés R., Busqué F., Figueredo M., Font J., de March P. Enantiodivergent Synthesis of Cyclohexenyl Nucleosides. J. Org. Chem. 2009;74:2425–2432. doi: 10.1021/jo802492g. [DOI] [PubMed] [Google Scholar]
- 18.Bardaji G.G., Cantó M., Alibés R., Bayón P., Busqué F., de March P., Figueredo M., Font J. Diastereoselective Synthesis of Allosecurinine and Viroallosecurinine from Menisdaurilide. J. Org. Chem. 2008;73:7657–7662. doi: 10.1021/jo801470u. [DOI] [PubMed] [Google Scholar]
- 19.Noyori R., Hashiguchi S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997;30:97–102. doi: 10.1021/ar9502341. [DOI] [Google Scholar]
- 20.Wang D., Astruc D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015;115:6621–6686. doi: 10.1021/acs.chemrev.5b00203. [DOI] [PubMed] [Google Scholar]
- 21.Foubelo F., Nájera C., Yus M. Catalytic Asymmetric Transfer Hydrogenation of Ketones: Recent Advances. Tetrahedron: Asymmetry. 2015;26:769–790. doi: 10.1016/j.tetasy.2015.06.016. [DOI] [Google Scholar]
- 22.Ferrie L., Boulard L., Pradaux F., Bouzbouz S., Capdevielle P., Cossy J. Synthetic Efforts toward the Macrolactone Core of Leucascandrolide A. J. Org. Chem. 2008;73:1864–1880. doi: 10.1021/jo701315h. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura K., Hashiguchi S., Ikariya T., Noyori R. Asymmetric Transfer Hydrogenation of α, β-Acetylenic Ketones. J. Am. Chem. Soc. 1997;119:8738–8739. doi: 10.1021/ja971570a. [DOI] [Google Scholar]
- 24.Fujii A., Hashiguchi S., Uematsu N., Ikariya T., Noyori R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid-Triethylamine Mixture. J. Am. Chem. Soc. 1996;118:2521–2522. doi: 10.1021/ja954126l. [DOI] [Google Scholar]
- 25.Wu J., Wang F., Ma Y., Cui X., Cun L., Zhu J., Deng J., Yu B. Asymmetric Transfer Hydrogenation of Imines and Iminiums Catalyzed by a Water-Soluble Catalyst in Water. Chem. Commun. 2006:1766–1768. doi: 10.1039/b600496b. [DOI] [PubMed] [Google Scholar]
- 26.Holub N., Neidhöfer J., Blechert S. Total Synthesis of (+)-trans-195A. Org. Lett. 2005;7:1227–1229. doi: 10.1021/ol0474610. [DOI] [PubMed] [Google Scholar]
- 27.Xu B., Xun W., Wang T., Qiu F.G. Total Synthesis of (+)-Aplykurodinone-1. Org. Lett. 2017;19:4861–4863. doi: 10.1021/acs.orglett.7b02350. [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum A.M., Meissner A., Harding R.A., Wong C.A., Remmel R.P. Synthesis, Ph-Dependent, and Plasma Stability of Meropenem Prodrugs for Potential Use Against Drug-Resistant Tuberculosis. Bioorg. Med. Chem. 2013;21:5605–5617. doi: 10.1016/j.bmc.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebel H., Marcoux J.F., Molinaro C., Charette A.B. Stereoselective cyclopropanation reactions. Chem. Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z., Lorenz J.C., Shi Y. Exploring New Reactive Species for Cyclopropanation. Tetrahedron Lett. 1998;39:8621–8624. doi: 10.1016/S0040-4039(98)01954-6. [DOI] [Google Scholar]
- 31.Kim H.Y., Walsh P. Efficient Approaches to the Stereoselective Synthesis of Cyclopropyl Alcohols. Acc. Chem. Res. 2012;45:1533–1547. doi: 10.1021/ar300052s. [DOI] [PubMed] [Google Scholar]
- 32.Furukawa J., Kawabata N., Nishimura J. Synthesis of Cyclopropanes by The Reaction of Olefins with Dialkylzinc and Methylene Iodide. Tetrahedron Lett. 1968;24:53–58. doi: 10.1016/0040-4020(68)89007-6. [DOI] [Google Scholar]
- 33.Scott J.P., Alam M., Bremeyer N., Goodyear A., Lam T., Wilson R.D., Zhou G. Inversion of a Secondary Alcohol with Diphenylphosphoryl azide. Application to the Enantioselective Multikilogram Synthesis of a HCV Polymerase Inhibitor. Org. Process Res. Dev. 2011;15:1116–1123. doi: 10.1021/op200002u. [DOI] [Google Scholar]
- 34.Fernández F., García-Mera X., Morales M., Rodríguez-Borges J.E. A Short, Efficient Synthesis of Substituted Uracil: An Indane Carbocyclic Nucleoside. Synthesis. 2001;2:239–242. doi: 10.1055/s-2001-10811. [DOI] [Google Scholar]
- 35.Komatsu H., Morizane K., Kohno T., Tanikawa H. An Efficient Amination Method for Manufacturing Cytidines. Org. Process Res. Dev. 2004;8:564–567. doi: 10.1021/op0499371. [DOI] [Google Scholar]
- 36.Balzarini J., Van Daele I., Negri N., Solaroli N., Karlsson A., Liekens S., Gago F., Van Calenbergh S. Human Mitochondrial Thymidine Kinase Is Selectively Inhibited by 3′-Thiourea Derivatives of β-Thymidine: Identification of Residues Crucial for Both Inhibition and Catalytic Activity. Mol. Pharmacol. 2009;75:1127–1136. doi: 10.1124/mol.108.053785. [DOI] [PubMed] [Google Scholar]
- 37.Ren K., Zhao M., Hu B., Xie X., Ratovelomamana-Vidal V., Zhang Z. An Enantioselective Approach to 4-O-Protected-2-Cyclopentene-1,4-Diol Derivatives via Rhodium-Catalyzed Redox-Isomerization Reaction. J. Org. Chem. 2015;80:12572–12579. doi: 10.1021/acs.joc.5b02519. [DOI] [PubMed] [Google Scholar]
- 38.Tachihara T., Kitahara T. Total Synthesis of (1)-Epiepoformin, (1)-Epiepoxydon and (1)-Bromoxone Employing a Useful Chiral Building Block, Ethyl (1R,2S)-5,5-Ethylenedioxy-2-hydroxycyclohexanecarboxylate. Tetrahedron. 2003;59:1773–1780. doi: 10.1016/S0040-4020(03)00119-4. [DOI] [Google Scholar]
- 39.Matsuzawa M., Kayeka H., Yamaguchi J., Shoji M., Onose R., Osada H., Hayashi Y. Enantio- and Diastereoselective Total Synthesis of (+)-Panepophenanthrin, a Ubiquitin-Activating Enzyme Inhibitor, and Biological Properties of Its New Derivatives. Chem. Asian J. 2006;1:845–851. doi: 10.1002/asia.200600199. [DOI] [PubMed] [Google Scholar]
- 40.Touge T., Hakamata T., Nara H., Kobayashi T., Sayo N., Saito T., Kayaki Y., Ikariya T. Oxo-tethered Ruthenium(II) Complex as a Bifunctional Catalyst for Asymmetric Transfer Hydrogenation and H2-Hydrogenation. J. Am. Chem. Soc. 2011;133:14960–14963. doi: 10.1021/ja207283t. [DOI] [PubMed] [Google Scholar]
- 41.Kitahara T., Kurata H., Mori K. Efficient Synthesis of The Natural Enantiomer of Sporogen-AO 1 (13-Desoxyphomenone) a Sporogenic Sesquiterpene from Aspergillus Oryzae. Tetrahedron. 1988;44:4339–4349. doi: 10.1016/S0040-4020(01)86136-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.