Abstract
Parkinson’s disease is the second most common neurodegenerative disease. Insidious and progressive, this disorder is secondary to the gradual loss of dopaminergic signaling and worsening neuroinflammation, affecting patients’ motor capabilities. Gold standard treatment includes exogenous dopamine therapy in the form of levodopa–carbidopa, or surgical intervention with a deep brain stimulator to the subcortical basal ganglia. Unfortunately, these therapies may ironically exacerbate the already pro-inflammatory environment. An alternative approach may involve cell-based therapies. Cell-based therapies, whether endogenous or exogenous, often have anti-inflammatory properties. Alternative strategies, such as exercise and diet modifications, also appear to play a significant role in facilitating endogenous and exogenous stem cells to induce an anti-inflammatory response, and thus are of unique interest to neuroinflammatory conditions including Parkinson’s disease. Treating patients with current gold standard therapeutics and adding adjuvant stem cell therapy, alongside the aforementioned lifestyle modifications, may ideally sequester inflammation and thus halt neurodegeneration.
Keywords: Parkinson’s disease, stem cell, neuroinflammation
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, with an incidence rate of 61.21 and 37.55 per 100,000 people in males and females over 40, respectively [1]. The determinants of this disease continue to be investigated; however, its pathophysiology appears to be multifactorial, with various genetic components [2,3,4] and environmental factors, such as chemical exposure, increasing one’s risk of acquiring the disease [5]. The disease is classically characterized by the death of dopaminergic (DA) neurons in the basal ganglia, but the neurodegeneration extends beyond this structure, causing symptoms such as bradykinesia, resting tremors, and rigidity [6,7]. The aggregation of α-synuclein in neurons during the development of PD has been implicated as a critical cell death mechanism, although the exact role such aggregates contribute to its pathogenesis remains to be fully elucidated [8,9,10,11]. A crosstalk may exist between the activation of microglia and the overaccumulation or improper folding of α-synuclein. The neurodegenerative cascade may initiate with the migration of microglia to the site of aggregation, where they phagocytose the α-synuclein and induce an inflammatory immune response with downstream effects [12,13].
Inflammatory processes play a major role in the development of PD pathology and its symptoms. Indeed, a high concentration of glutathione peroxidase in glial cells surrounding DA neurons suggests a protective response to oxidative stress [4]. In contrast, higher levels of microglia in older mice are associated with significant neurodegeneration in the substantia nigra [14]. Microglia are key mediators in neuroinflammation, so this finding offered new insight into the role of inflammation in PD [15]. Activated microglia release a flood of cytokines and pro-inflammatory molecules such as IL-1, TNF-α, nitric oxide (NO), prostaglandin E2, and superoxide, all of which can have wide-ranging and damaging downstream effects [16]. Upon the deeper investigation of these cytokines, scientists note that inhibiting inducible nitric oxide synthase, the enzyme responsible for NO production, in rat models of PD induces a significant reduction in neurotoxicity, as shown by the increased integrity of the nigrostriatal pathway and decreased microglial count when compared to control rats [17]. Similarly, NADPH-oxidase participates in the development of neurotoxicity in rat PD models [18]. Aside from microglia, T cells circumvent the blood–brain barrier, infiltrating the brains of PD patients along with splenocytes [19,20]. Indeed, the increased propagation of microglia closely accompanies infiltrating T cells, suggesting that microglia act as the antigen-presenting cells to the T cells after they have crossed the blood–brain barrier [21].
A genetic basis may also mediate the aberrant inflammation in the development of PD. Mice with leucine-rich repeat kinase 2 (LRRK2) knock-down display an inhibited microglial inflammatory response when compared to control mice [22]. On the other hand, mice with DJ-1 knock-down astrocytes exhibit significantly more NO production and demonstrate apoptotic behavior, indicating that the gene plays an important role in mediating the inflammatory response [23]. Other genes associated with inflammation and neurotoxicity include PINK1, Parkin, and others not mentioned in this review [24,25,26]. Any attempt to regulate the inflammatory mechanisms in PD should recognize the complexity and multifactorial nature of neuroinflammation.
The aim of this review is to explore the most recent research on the topic of neuroinflammation, which seeks to understand the role of stem cell therapy as a potential therapeutic mediator of the inflammatory damage caused during the natural progression of PD. As later sections will discuss, current therapies can often exacerbate neuroinflammation, and thus finding a treatment that can sequester inflammation and halt the progression of PD would be a major development in the management of the disease.
2. Pathophysiology and Therapeutics for Parkinson’s Disease
In the basal ganglia, there are two motor pathways: the direct pathway and the indirect pathway. The direct pathway is excitatory for movement, and involves the striatum inhibiting the globus pallidus internus (GPi) and thus attenuating the inhibitory effect of the GPi on the thalamus. The thalamus is excitatory to the motor cortex, initiating movement. Contrarily, the indirect pathway is inhibitory for movement. The striatum inhibits the globus pallidus externus (GPe), thus attenuating the inhibitory effect of the GPe on the subthalamic nucleus (STN). The STN then excites the GPi, which inhibits the thalamus. The excitatory thalamic signaling to the motor cortex is lost, resulting in the inhibition of movement.
Both the indirect and direct pathways can be impacted by dopamine neurotransmission. Dopamine is an endogenous neurotransmitter, critical for the movement mediated by the basal ganglia. Dopamine is a catecholamine and is derived from L-DOPA via the enzyme DOPA decarboxylase, which itself is derived from L-tyrosine via the enzyme tyrosine hydroxylase. The substantia nigra pars compacta, located in the midbrain, releases dopamine onto the striatum via the nigrostriatal pathway. Dopamine binds to the D1 and D2 receptors in the striatum; subsequent D1 signaling stimulates the direct (excitatory) pathway, and D2 signaling inhibits the indirect (inhibitory) pathway. Ultimately, this signaling encourages movement initiation.
As noted above, PD presents with the degeneration of DA neurons in the pars compacta, which severely affects nigrostriatal signaling. To ameliorate the motor symptoms of PD, such as tremor, rigidity, and bradykinesia, exogenous therapy is utilized. Unfortunately, dopamine cannot cross the blood–brain barrier. Thus, a dopamine precursor, L-DOPA, is administered as the prodrug levodopa, which is converted in the central nervous system to dopamine via DOPA decarboxylase. Of note, levodopa is co-administered with carbidopa, which inhibits DOPA decarboxylase activity in the peripheral tissues, with the goal of increasing levodopa’s bioavailability in the brain. Another therapeutic option is deep brain stimulation (DBS). DBS was originally used to localize the target of a resection, but now subcortical neurostimulation is utilized as the first-line surgical treatment of movement disorders [27,28]. DBS stimulation, commonly in the STN or GPi, can modulate signaling within the basal ganglia, thalamus, and motor cortex to improve the motor dysregulation seen in PD.
Inflammation in the setting of PD contributes to the neurodegenerative process and has both acute and chronic phases. As PD worsens, reactive microglia increasingly localize in the substantia nigra, putamen, hippocampus, and cortex [29,30,31,32,33]. As chronic neuroinflammation progresses, microglia lose their reparatory M2 phenotype, and preferentially differentiate to their inflammatory M1 phenotype [34,35]. IL-6 is a proinflammatory cytokine local to the CNS, released by neurons, astrocytes, endothelial cells, and microglia [36]. IL-6 concentrations are positively correlated with motor scores in PD patients, as measured using the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [37]. PD patients with fatigue have significantly increased serum levels of IL-6 compared to PD patients without fatigue, as measured with the Parkinson’s Disease Fatigue Scale. Fatigued patients were reported to have worsened signs and symptoms of PD and increased functional dependence [38]. PD patients with higher IL-6 concentrations at baseline were seen to have worsened depression scores two years later [39]. There is a negative correlation between IL-6 levels and scores on the Activities of Daily Living scale, indicating that patients with worsened PD have greater IL-6 concentrations [40]. Interestingly, the literature indicates microglia and inflammatory cytokines such as IL-6 are involved in both neurodegeneration (typically in chronic settings) and neuronal survival (typically in acute settings) [33,35,41].
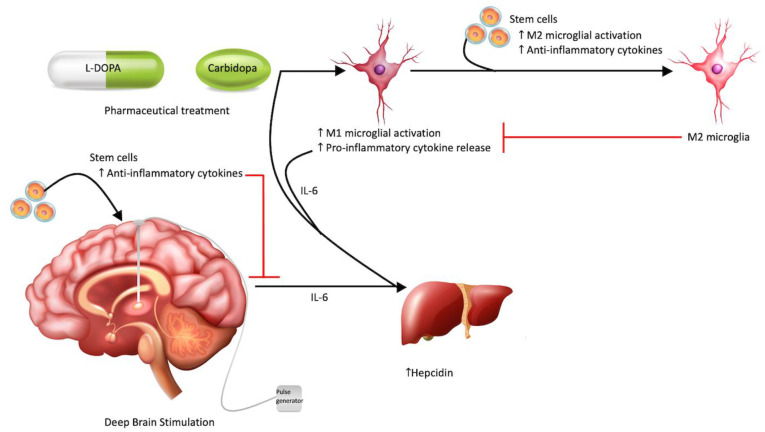
Unfortunately, L-DOPA and DBS therapy may worsen neuroinflammation. It is almost ironic that chronic L-DOPA therapy may precede L-DOPA induced dyskinesia (LID) [42]. After L-DOPA administration, there is excessive dopamine that saturates vesicles, resulting in a plethora of free cytosolic dopamine that is degraded by monoamine oxidase, leading to an accumulation of cytotoxic reactive oxygen species [43,44]. L-DOPA in the setting of PD leads to aberrant D1 signaling in the striatum, with the subsequent hyperactivation of cAMP and PKA [45]. In LID rat models, glucocorticoid treatment reduced abnormal involuntary movements [46], demonstrating L-DOPA’s role in the neuroinflammation seen to worsen Parkinsonism dyskinesia. In the same model, glucocorticoids also normalized the increased expression of proinflammatory cytokines, such as IL-1β, in the striatum. Additionally, the direct administration of IL-1 receptor antagonists to the striatum reduced abnormal involuntary movements [46]. Hepcidin, an acute phase reactant synthesized in the liver, is upregulated by IL-6 in settings of inflammation and is responsible for decreasing iron absorption through the destruction of ferroportin and the reduction in iron release from macrophages. Pro-hepcidin serum concentrations were significantly higher in PD patients treated with DBS and pharmacological therapy than in PD patients treated with pharmacological therapy only and in control patients [47,48].
Figure 1 demonstrates the aforementioned contribution of levodopa–carbidopa and deep brain stimulation to neuroinflammation.
Figure 1.
Therapeutic approaches to Parkinson’s disease. Typical treatment modalities for Parkinson’s disease, such as DBS and pharmaceuticals (L-DOPA and Carbidopa), may amplify the pro-inflammatory environment of PD by preferentially stimulating M1 microglial differentiation and inflammatory cytokine release. These cytokines can also increase hepcidin, an acute phase protein, from the liver. Stem cells may act via the bystander effect to minimize this neuroinflammation by shifting microglial differentiation to M2 and increasing anti-inflammatory cytokines.
3. Rewiring the Inflammatory Responses
3.1. Anti-Inflammatory Based Therapies
While most existing therapies for PD focus on restoring DA neuron loss or using DBS to restore basal ganglia pathways, evolving evidence for PD as an inflammatory disease prompts the use of anti-inflammatory therapies to ameliorate PD progression [49,50]. Several studies in clinical and pre-clinical populations have demonstrated a reduced PD risk and attenuated striatal DA neuron loss using anti-inflammatory drugs, such as ibuprofen, acetylsalicylic acid, and NSAIDs [51]. Focusing on immune cell proliferation, other groups have designed dendritic cell (DC) vaccines to modulate the typically inflammatory immune response to α-synuclein. In PD, DCs phagocytose toxic α-synuclein buildup to present to lymphocytes in the nearby cervical lymph nodes. T cells respond to the toxic α-synuclein and induce a pro-inflammatory environment [52,53] composed of M1 microglial and A1 astrocytic differentiation and inflammatory cytokines [54,55]. Using primed DCs, differentiation can be swayed towards anti-inflammatory M2 microglia, A2 astrocytic, and Th2 CD4+ T-cell phenotypes [54]. In mice, locomotor function is improved after sensitizing DCs to α-synuclein [56]. Decreased blood DCs are correlated with worse motor impairment, further linking the potential for DC vaccines in modulating PD prognosis [57]. Antibodies also demonstrate similarly successful immunomodulation in animal models of PD [58,59,60]. The administration of anti- α-synuclein antibodies prompts microglial clearance in mouse models of PD, reduces neuronal and glial protein accumulation, and translates to improved functionality [61]. In clinical studies, the administration of α-synuclein monoclonal antibodies, BIIB054 and PRX002, has also shown encouraging results [62,63,64]. Thus, anti-inflammatory pharmaceuticals may be important therapies to consider in treating PD, either alone or in combination with other PD therapeutics.
3.2. Cell-Based Therapies
Another promising route for inducing anti-inflammatory responses in PD may use stem cells. Many of the therapeutic effects of stem cells arise from their inherent secreted anti-inflammatory signals among other cell survival molecules, deemed the bystander effects [65]. Mesenchymal stem cells (MSCs), for example, demonstrate anti-inflammatory, anti-apoptotic, and regenerative capabilities [66,67]. ESC–MSCs and iPSC–MSCs can inhibit lymphocyte and natural killer cell proliferation, also showing promise for immunoregulatory cell-based therapies [68,69,70,71]. As noted above, exosomes show promise for PD therapies [66]. MSC-derived exosomes have anti-inflammatory, antioxidant, and neurotrophic properties, ultimately leading to functional recovery in animal models of PD [72,73]. An appealing feature of stem cell treatments includes their ability to be modified prior to implantation, for instance, to promote anti-inflammatory actions. A pathway of interest includes interferon-γ signaling via the aforementioned gene, LRRK2. LRRK2 G2019S is the most common genetic mutation associated with inherited PD. In PD patient-derived iPSCs possessing LRRK2 G2019S, IFN-γ signaling increases immunomodulated neuronal damage [74]. MSCs are initially activated by IFN-γ but then exert anti-inflammatory properties [75]. Thus, MSCs may effectively modulate this pathological signaling.
Furthermore, recognizing the underlying DA neuron loss in PD, it is logical to develop treatments that may reestablish these neuronal populations and connections. Cell-based therapies, however, show mixed results in PD, suggesting that additional anti-inflammatory enhancements of the cells may improve their viability. The use of grafts to restore DA neurons shows mixed results, with some reports demonstrating survival, innervation, and improved functionality, and others showing no clinical benefit [76,77,78,79,80,81,82,83,84]. The studies demonstrating the therapeutic value of stem cells for PD, however, prompt further investigation [85,86]. For instance, exosomes from human umbilical cord mesenchymal stem cells (hucMSCs) reduce apoptosis and encourage proliferation in an in vitro PD cell culture. In rats, hucMSC treatment shows a similar prevention of DA neuron loss and recovered DA levels [66]. To modulate the altered DA signaling, GABAergic cells from the medial ganglionic eminence (MGE) can also be targeted. MGE cells show potential to migrate and differentiate into GABAergic neurons, restoring function to mouse models treated with 6-hydroxydopamine (6-OHDA), a selective neurotoxin of DA neurons to mimic PD [87]. Human embryonic stem cells (hESCs) in rat models of PD can project long distances in the rodent brain to mitigate symptoms, offering hopeful clinical translation [88]. Even closer to the goal of clinical application, human parthenogenetic embryonic stem cell (hPESC)-derived DA neurons improve mobility in monkey models of PD [89]. Additionally, in monkey 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models recreating PD, human-induced pluripotent stem cells (iPSCs) improve movement and demonstrate ample neurite formation. Whether grafted from PD or control donors, iPSCs survive, make connections, and lead to functional recovery in monkeys [90]. There is still room for improvement in stem cell PD treatments, as full recovery is not typically achieved in animal models [91,92,93].
A comprehensive list of pre-clinical anti-inflammatory stem cell studies for PD, along with the cell types, dosages, and findings, is outlined in Table 1. The studies were completed in rodent models in vivo (6-OHDA or MPTP technique) or in vitro (A53T α-synuclein overexpressing cells).
Table 1.
Pre-clinical anti-inflammatory stem cell studies for Parkinson’s disease.
| Citation | Model | Cell type | Dosage | Findings |
|---|---|---|---|---|
| Haney et al. (2013) [94] | 6-OHDA mice | Modified macrophages with antioxidant plasmid | 5 × 106 cells/mouse in 100 µL PBS | Macrophages released exosomes containing antioxidant genetic material prompting neurons to increase protein synthesis. Mice had improved motor function. |
| Ugen et al. (2015) [56] | A53T α-synuclein overexpressing cells and mice | Bone marrow-derived dendritic cells | 106 cells | α-synuclein sensitized DCs induced α-synuclein antibodies, improved motor function in mice, and had lower pro-inflammatory cytokine levels. |
| Oh et al. (2017) [95] | A53T α-synuclein overexpressing cells and mice | Mesenchymal stem cells | 1 × 106 cells in 200 µL saline in tail | Eukaryotic elongation factor 1A-2 from MSCs increased neuronal survival by improving axonal transport and monitoring α-synuclein pathological phosphorylation |
| Kojima et al. (2018) [96] | 6-OHDA mice | Catalase mRNA in designer exosomes | Four hundred microliters of the cell/Matrigel mixture | Reduced neuroinflammation and neurotoxicity in Parkinson’s mice. |
| L’Episcopo et al. (2018) [97] | MPTP mice | Neural stem cells | 100 × 103 cells | NSCs that became astrocytic expressed Wnt1 and prompted Wnt/ β-catenin signaling in substantia nigra pars compacta midbrain dopaminergic neurons and microglia. This allowed for dopaminergic neuron rescue and decreased microglial inflammation. |
| Lee et al. (2019a) [98] | MPTP mice | Human umbilical cord blood stem cells | 500 µL cord blood plasma | Mice showed improved motor and GI function, ameliorated dopamine cell loss, and reduced neurological and GI inflammation. |
| Lee et al. (2019b) [99] | 6-OHDA mice | Human umbilical cord blood stem cells | Three separate doses of 4 × 106 cells | Mice showed improved motor and GI function, ameliorated dopamine cell loss, and reduced neurological and GI inflammation. |
| Serapide et al. (2020) [100] | MPTP Mice | Engrafted astrocytes | 150 × 103 ventral midbrain-Astrocytes | Grafted astrocytes can rescue dying dopaminergic neurons, likely via antioxidant and anti-inflammatory Nrf2/ARE/Wnt/β-catenin signaling. |
3.3. A Potential Synergistic Niche
While already anti-inflammatory by nature, stem cell treatments may be further enhanced in clinical effectivity and viability by modulating these cells to express additional anti-inflammation properties. The potential for MSCs to impact cell signaling can also be applied to the Wnt/β-catenin pathway. The Wnt/β-catenin signaling pathway is involved in promoting DA neurorepair [101]. In patients with a LRRK2 mutation, the neurodegenerative phenotype may arise from altered Wnt signaling. This alteration increases the inhibitory phosphorylation of β-catenin using GSK-3β [102]. Normally, Wnt signaling functions to upregulate β-catenin, and to promote the transcription of genes associated with cellular survival, proliferation, and differentiation [103]. Wnt signaling can be stimulated in PD treatment models using chemical Wnt activators to induce the differentiation of iPSCs to midbrain dopaminergic (mDA) neurons [86,104]. Further enhancing the stimulation of iPSCs into mDA neurons can be carried out through the co-activation of both canonical and non-canonical Wnt pathways. The overexpression of Wnt3a or Wnt5a has been shown to significantly promote ESC differentiation to DA neurons. Compared to the activation of Wnt signaling with only FGF2/FGF8/Shh, adding Wnt5a overexpression enhanced DA neuron production 4-fold [105]. Wnt3a is a component of the canonical route of mDA neurogenesis, and when overexpressed, prevents differentiation from reaching its full potential. However, when Wnt3a is administered first to an undifferentiated cell line to promote proliferation, followed by a “wash-out” phase and then exposure to Wnt5a to promote cell cycle exit and Nurr+ differentiation, a large rise in Tyrosine Hydroxylase + (TH+) DA neurons is seen [105,106]. The neuroregenerative signaling associated with Wnt/β-catenin is further established in MPTP-induced PD mice models. Transplanting neural stem cells (NSCs) to host substantia nigra pars compacta (SNpc) results in a differentiation of 30% of NSCs to the astrocyte phenotype, allowing the host microenvironment of SNpc to enhance DA neuronal plasticity [97,107,108]. Furthermore, it should be noted that through the antagonism of Wnt/β-catenin signaling in MPTP mice, the therapeutic effects of the transplanted NSCs were abolished [107,108]. In line with previously discussed benefits of anti-inflammatory modulators [65], NSC-mediated Wnt/β-catenin signaling downregulates microglia and reduces inflammatory mRNA species, including Tnf and Tnfrsf1a, Il1, Nos2, Nfkb, and phagocyte oxidase Cybb (gp91phox) [97]. The potential for unique therapeutic effects from NSC grafts may be realized considering the growing findings of the Wnt/β-catenin pathway in PD [109,110]. The use of cell therapies to amplify anti-inflammatory effects is a promising direction for future PD therapies given the immense neuroinflammation and dopaminergic cell death contributing to the disease’s pathophysiology.
3.4. Neurotrophic Factors and Stem Cell Therapy
Neurotrophic factors may demonstrate protective properties in the setting of stem cell therapy and neurodegenerative disease. Of particular interest, glial cell line-derived neurotrophic factor (GDNF) nurtures the survival of dopaminergic neurons commonly affected in PD [111]. GDNF signaling is complicated, but importantly, it binds to GDNF family receptor 1α, which subsequently binds to RET, promoting neuronal survival and plasticity [112]. Murine and primate dopaminergic neurons were spared from 6-OHDA- and MPTP-induced cell death upon treatment with GDNF [113,114,115]. Due to the success in animal models, seven human clinical trials have been completed; GDNF was administered, often via putamen infusion, and UPDRS III scores were measured [116,117,118,119,120,121,122]. The results were unfortunately ambivalent; some patients had dramatic improvements in motor function, but in the three studies with placebo control groups, there were no significant differences compared to the treatment group. Neurotrophic factors are a worthy consideration in the treatment of PD through interactions with stem cells.
4. Lifestyle Factors Facilitating the Stem Cell Response
4.1. Exercise and Physical Therapy
Exercise and physical therapy are critical strategies known to facilitate endogenous and exogenous stem cells in inducing an anti-inflammatory response, which thus offer potential benefits alongside concomitant stem cell therapy for PD [123,124,125,126,127,128].
Physical therapy and exercise training are significantly implicated in anti-inflammatory activity independent of age [129,130] or overall health status [131]. Physical activity shows benefits in many chronic conditions, such as cardiovascular disease [132,133], cancer [134,135], and neuroinflammatory diseases [136]. Even exercise surrogates, such as whole-body vibration training, are shown to attenuate inflammation and mobilize stem cell populations associated with vascular health [137]. Further, a recent systematic review and meta-analysis highlights the anti-inflammatory role of exercise when controlling for variables such as type, intensity, and volume of exercise, suggesting the mechanism is related to an induction of antioxidant indicators [138]. In mouse models, exercise is implicated in stem cell driven anti-inflammatory effects through changes to the epigenome and transcriptome of hematopoietic stem and progenitor cells, diminishing the proliferative yield of inflammatory immune cells [139]. Importantly, this finding is subsequently noted in adult populations independent of BMI [140]. The effects on neuroinflammation are mediated through the downregulation of microglia via the direct inhibition or suppression of pro-inflammatory cytokines released from a number of cell types [141]. Exercise in both animal models and PD patients has shown promise for lessening the neuroinflammatory processes associated with PD. A rat model receiving unilateral striatal injections of 6-OHDA to induce PD demonstrates that treadmill exercise downregulates the activation of microglia, astrocytes, and oxidative species, and indicates that regular exercise may preserve dopamine levels in PD [142]. This mechanism of reduced neuroinflammation is further elucidated in MPTP-induced mice, in which treadmill exercise downregulates the expression of Toll-like receptor 2 and its downstream molecules, resulting in reduced inflammatory markers, such as TNF-α, NF-κB, IL-1β, and NADPH oxidase, while enhancing plasma dopamine levels and dopamine transporter expression [143]. Moderate exercise also demonstrates reduced levels of TNF-α in patients with PD [144]. Treadmill activity in 6-OHDA rats is also seen to enhance the expression of BDNF, SERCA II, superoxide dismutase, and catalase, all of which decrease neuroinflammation and promote an antioxidant or regenerative response [145].
In addition to neuroinflammatory modulation, exercise has been shown to enhance the viability of endogenous NSCs. Physical exercise is shown in a number of rodent models to trigger the proliferation of endogenous NSCs [146,147,148]. Moderate exercise in mouse models with inflammation-induced hippocampal suppression specifically restores neurogenesis in the dentate area of the hippocampus [149]. Exercise also induces the release of a number of the neurotrophic factors described above, with GDNF concentrations particularly increasing in 6-OHDA rats, MPTP mice, and MPTP monkeys to promote endogenous cell survival for vulnerable dopaminergic neurons [150]. Further, MPTP mouse models placed on an exercise regimen show improved neurogenesis in the subventricular zone, subgranular zone, substantia nigra, and striatum [151]. These effects have been replicated, to a moderate extent, in studies on human PD patients. In these patients, intensive exercise elevates serum BDNF by 16%, enhancing the overall proliferative capacity of endogenous NSCs [152].
Exogenous stem cells also show significant benefits pertaining to exercise therapy. For instance, exercise in mouse models undergoing bone marrow transplantation results in enhanced stem cell survival and recipient blood cell reconstitution [153]. Additionally, physical therapy is proven to be a key aspect linked to inflammation and survival rates in cancer patients undergoing hematopoietic stem cell transplantation [154]. Transplanted exogenous GABAergic neural progenitor cells result in neuropathic pain reduction when coupled with intensive locomotor training [155]. When taken together, exercise therapy is, therefore, a promising field for future research into PD with potential to sequester inflammation-associated cell death and promotes both an endogenous and exogenous stem cell response.
4.2. Diet and the Gastrointestinal Microbiome
Dietary consumption has recently taken a prominent role in research into neuroinflammation and PD, in which food intake, supplementation, and the gut microbiome may have significant therapeutic potential for decreasing inflammation and enhancing stem cell survival and proliferation.
Amassing evidence indicates the role of diet in suppressing neuroinflammation. The ketogenic diet, consisting of high fats, low proteins, and low carbohydrates, has garnered interest for potential roles in neuroprotection and the inflammatory response. In MPTP-induced mice, the ketogenic diet is shown to be both neuroprotective and anti-inflammatory, alleviating motor dysfunction and inhibiting microglial activation, thereby reducing proinflammatory cytokines in the substantia nigra [156]. This underlying effect is likely attributed, in part, to the elevation of the ketone β-hydroxybutyrate, which has been shown to be neuroprotective for dopaminergic models and involved in the downregulation of microglial-induced neuroinflammation [157], having been documented as blocking the NLRP3-inflammasome [158]. Such ketone bodies have also been shown to reduce oxygen-free radicals of the mitochondrial respiration chain in DA neurons, further sequestering PD-associated inflammation [159,160]. Pilot studies in PD patients demonstrate an early confirmation of the role of ketosis in enhancing cognitive performance, though have failed to impact motor function [161]. A number of other dietary patterns and supplementations implicate a role for diet in the neuroinflammation and protection of neural stem cells. For instance, omega-3 fatty acids and vitamin E co-supplementation in PD patients show reduced inflammation (as measured by C-Reactive Protein) and enhanced total antioxidant response, thereby promoting neuron survival [162]. Polyphenols found in green tea have been studied within PD models, with potential mechanisms that include preventing α-synuclein aggregation (restoring the normal differentiation and apoptotic suppression of endogenous precursor cells associated with the protein) [163,164], increasing tyrosine hydroxylase and dopamine levels [165], and sequestering iron (known to trigger oxidative stress, α-synuclein aggregation, inflammation, and neurodegeneration) [166,167]. Resveratrol, a stilbene produced by various fruits and vegetables, is shown to mitigate neuroinflammation in the substantia nigra of PD-induced rats through the downregulation of TNF-α and COX-2 [168].
Additionally, dietary patterns have been implicated in the endogenous stem cell response. For example, intermittent fasting may possess utility in the management of PD, in which mouse models subjected to alternate day fasting prove to be more resistant to the MPTP induction of PD, potentially indicating a neuroprotective effect on existing DA neurons or the enhanced proliferation of precursor cells [169]. Generally, dietary restriction appears to stimulate endogenous stem cell neurogenesis and enhance synaptic plasticity [170], which could represent an important mechanism for resisting injury associated with PD. It is important to note that diets high in antioxidants may also benefit exogenous stem cell survival. For instance, the survival of grafted fetal ventral mesencephalic tissue is increased when a Sprague Dawley rat model of PD is given a diet of 2% blueberries, a fruit high in antioxidants. Therefore, these adjunctive diet regimens can ultimately be applied to human grafting as a PD combination treatment to promote overall stem cell viability [171].
However, diet can also have negative implications for neuroinflammation and PD. Wistar rat models on a high-fat diet for 25 weeks to model obesity demonstrate decreased tyrosine hydroxylase and increased microglial activity, TNF-α, oxidative stress and astrogliosis [172]. Similarly, a mouse model on a similar diet yields a loss of DA neurons in the substantia nigra [173]. Nevertheless, given the easily implicated and accessible benefits of certain dietary adjustments, this may be a promising initiative to lessen neuroinflammation and the symptoms of PD.
In recent years, the gastrointestinal microbiome has been found to be increasingly connected with inflammatory processes and the central nervous system. The neurodegeneration association with PD is associated with a shift in the gut microbiome towards a pro-inflammatory environment with bacteria that produce endotoxins (such as LPS) and participate in methanogenesis [174,175]. While the precise mechanism of this interaction is not fully understood, it is known that there exists bidirectional communication between the enteric and central nervous systems, known as the gut–brain axis, which allows for brain lesions to arise from the gut microbiome [176]. As further evidence of this peripheral inflammation infiltrating the brain, recent experimental mouse models show that glial activation is associated with the infiltration of peripheral adaptive immune cells [177], with Th17 lymphocytes invading the substantia nigra and enhancing DA neuron death [178]. Thus, treatment directed towards improving the microbiome, or intake that disrupts it, could have profound implications for PD. For instance, antibiotic and antifungal exposure is shown to increase the risk of PD through microbiome dysbiosis [179]. Meanwhile, a recent case report describes a fecal microbiota transplantation to relieve intractable constipation, an important symptom of PD, which resulted not only in unobstructed defecation but also a nearly two-month reprieve from tremors in the lower extremities [180]. In MPTP-induced mice, such a procedure exhibits neuroprotection through the inhibition of glial cells and neural inflammation [181]. Additionally, smoking and coffee consumption contribute to a reduced risk of PD development over the life course through their impact on the gut microbiome [182]. Probiotics have become important to PD research to mitigate peripheral inflammation, with studies in patients resulting in improved bowel symptoms and improved disease states according to the MDS-UPDRS [183,184,185,186,187]. Overall, diet represents an important approach in mediating inflammation to promote neural stem cell survival and proliferation, while simultaneously opening newfound research avenues related to modulating gut dysbiosis toward the sequestration of peripheral, as well as central, inflammation.
5. Conclusions and Future Directions
This review summarizes current and experimental treatments for PD, with a focus on stem cell therapies that address the neurotoxicity and inflammation that is prevalent throughout the disease’s progression. The neuroinflammation seen in the disease is the product of the crosstalk between local and peripheral inflammatory pathways, which play a critical and interwoven role in its pathophysiology. Current gold standard treatments, such as levodopa–carbidopa and DBS, can exacerbate inflammation, so patients may benefit from anti-inflammatory cell-based treatments, such as the transplantation of human stem cells or stem-cell-derived DA neurons. Aside from their anti-inflammatory properties, these treatments benefit from their inherent versatility, in some cases allowing them to both downregulate inflammation and simultaneously promote regeneration. Combining existing therapies with emerging stem cell treatments and lifestyle modifications that promote the stem cell-meditated sequestration of neuroinflammation appears to be a promising new direction that warrants further investigation.
Acknowledgments
We acknowledge the work of the artists Brgfx, Macrovector, and Frimufilms, whose vector art was downloaded from freepik.com and used within the figure design.
Author Contributions
Conceptualization, J.G., G.L., M.M., A.A., H.C. and C.V.B.; investigation, J.G., G.L., M.M., A.A. and H.C.; supervision, C.V.B.; visualization, J.G., G.L., M.M., A.A. and H.C.; writing—original draft preparation, J.G., G.L., M.M., A.A. and H.C.; writing—review and editing, J.G., G.L., M.M., A.A., H.C. and C.V.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirsch L., Jette N., Frolkis A., Steeves T., Pringsheim T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;46:292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 2.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 3.Bonifati V., Rizzu P., van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 4.Damier P., Hirsch E.C., Zhang P., Agid Y., Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-F. [DOI] [PubMed] [Google Scholar]
- 5.Tanner C.M., Goldman S.M., Ross G.W., Grate S.J. The disease intersection of susceptibility and exposure: Chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014;10:S213–S225. doi: 10.1016/j.jalz.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 7.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 8.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 9.Beach T.G., Adler C.H., Lue L., Sue L.I., Bachalakuri J., Henry-Watson J., Sasse J., Boyer S., Shirohi S., Brooks R., et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionnet A., Leclair-Visonneau L., Neunlist M., Murayama S., Takao M., Adler C.H., Derkinderen P., Beach T.G. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135:1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 11.Brundin P., Melki R. Prying into the Prion Hypothesis for Parkinson’s Disease. J. Neurosci. 2017;37:9808–9818. doi: 10.1523/JNEUROSCI.1788-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klegeris A., Pelech S., Giasson B.I., Maguire J., Zhang H., McGeer E.G., McGeer P.L. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim S., Cho S.H., Kim K.Y., Shin K.Y., Kim H.S., Park C.H., Chang K.A., Lee S.H., Cho D., Suh Y.H. Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J. Neurochem. 2009;109:1483–1496. doi: 10.1111/j.1471-4159.2009.06075.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugama S., Yang L., Cho B.P., DeGiorgio L.A., Lorenzl S., Albers D.S., Beal M.F., Volpe B.T., Joh T.H. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/S0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y.S., Joh T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 16.Block M.L., Hong J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Broom L., Marinova-Mutafchieva L., Sadeghian M., Davis J.B., Medhurst A.D., Dexter D.T. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic. Biol. Med. 2011;50:633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miklossy J., Doudet D.D., Schwab C., Yu S., McGeer E.G., McGeer P.L. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Benner E.J., Banerjee R., Reynolds A.D., Sherman S., Pisarev V.M., Tsiperson V., Nemachek C., Ciborowski P., Przedborski S., Mosley R.L., et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Depboylu C., Stricker S., Ghobril J.P., Oertel W.H., Priller J., Höglinger G.U. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 2012;238:183–191. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Kim B., Yang M.S., Choi D., Kim J.H., Kim H.S., Seol W., Choi S., Jou I., Kim E.Y., Joe E.H. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS ONE. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waak J., Weber S.S., Waldenmaier A., Görner K., Alunni-Fabbroni M., Schell H., Vogt-Weisenhorn D., Pham T.T., Reumers V., Baekelandt V., et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. Faseb. J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 24.Lücking C.B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B.S., Meco G., Denèfle P., Wood N.W., et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 25.Kim J., Byun J.W., Choi I., Kim B., Jeong H.K., Jou I., Joe E. PINK1 Deficiency Enhances Inflammatory Cytokine Release from Acutely Prepared Brain Slices. Exp. Neurobiol. 2013;22:38–44. doi: 10.5607/en.2013.22.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank-Cannon T.C., Tran T., Ruhn K.A., Martinez T.N., Hong J., Marvin M., Hartley M., Treviño I., O’Brien D.E., Casey B., et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albe-Fessard D. Electrophysiological methods for the identification of thalamic nuclei. Z. Neurol. 1973;205:15–28. doi: 10.1007/BF00315957. [DOI] [PubMed] [Google Scholar]
- 28.Groiss S.J., Wojtecki L., Südmeyer M., Schnitzler A. Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009;2:20–28. doi: 10.1177/1756285609339382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhard A. TSPO imaging in parkinsonian disorders. Clin. Transl. Imaging. 2016;4:183–190. doi: 10.1007/s40336-016-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koshimori Y., Ko J.H., Mizrahi R., Rusjan P., Mabrouk R., Jacobs M.F., Christopher L., Hamani C., Lang A.E., Wilson A.A., et al. Imaging Striatal Microglial Activation in Patients with Parkinson’s Disease. PLoS ONE. 2015;10:e0138721. doi: 10.1371/journal.pone.0138721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGeer P.L., McGeer E.G. Glial reactions in Parkinson’s disease. Mov. Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 32.Ouchi Y., Yagi S., Yokokura M., Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15((Suppl. S3)):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 33.Sawada M., Imamura K., Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006;70:373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- 34.Barcia C., Hunot S., Guillemin G.J., Pitossi F. Inflammation and Parkinson’s disease. Parkinsons Dis. 2011;2011:729054. doi: 10.4061/2011/729054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joers V., Tansey M.G., Mulas G., Carta A.R. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 2017;155:57–75. doi: 10.1016/j.pneurobio.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green H.F., Khosousi S., Svenningsson P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. J. Parkinsons Dis. 2019;9:705–709. doi: 10.3233/JPD-191699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira J.R., Santos L.V.D., Santos R.M.S., Campos A.L.F., Pimenta A.L., de Oliveira M.S., Bacheti G.G., Rocha N.P., Teixeira A.L., Christo P.P., et al. IL-6 serum levels are elevated in Parkinson’s disease patients with fatigue compared to patients without fatigue. J. Neurol. Sci. 2016;370:153–156. doi: 10.1016/j.jns.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Veselý B., Dufek M., Thon V., Brozman M., Királová S., Halászová T., Koriťáková E., Rektor I. Interleukin 6 and complement serum level study in Parkinson’s disease. J. Neural. Transm. (Vienna) 2018;125:875–881. doi: 10.1007/s00702-018-1857-5. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann K.W., Schuh A.F., Saute J., Townsend R., Fricke D., Leke R., Souza D.O., Portela L.V., Chaves M.L., Rieder C.R. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 2009;34:1401–1404. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- 41.Pisanu A., Lecca D., Mulas G., Wardas J., Simbula G., Spiga S., Carta A.R. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol. Dis. 2014;71:280–291. doi: 10.1016/j.nbd.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Pisanu A., Boi L., Mulas G., Spiga S., Fenu S., Carta A.R. Neuroinflammation in L-DOPA-induced dyskinesia: Beyond the immune function. J. Neural. Transm. (Vienna) 2018;125:1287–1297. doi: 10.1007/s00702-018-1874-4. [DOI] [PubMed] [Google Scholar]
- 43.Sulzer D., Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox Res. 2000;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 44.Zucca F.A., Segura-Aguilar J., Ferrari E., Muñoz P., Paris I., Sulzer D., Sarna T., Casella L., Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feyder M., Bonito-Oliva A., Fisone G. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front. Behav. Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnum C.J., Eskow K.L., Dupre K., Blandino P., Jr., Deak T., Bishop C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: Role for interleukin-1beta. Neuroscience. 2008;156:30–41. doi: 10.1016/j.neuroscience.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwiatek-Majkusiak J., Geremek M., Koziorowski D., Tomasiuk R., Szlufik S., Friedman A. Higher serum levels of pro-hepcidin in patients with Parkinson’s disease treated with deep brain stimulation. Neurosci. Lett. 2018;684:205–209. doi: 10.1016/j.neulet.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 48.Kwiatek-Majkusiak J., Geremek M., Koziorowski D., Tomasiuk R., Szlufik S., Friedman A. Serum levels of hepcidin and interleukin 6 in Parkinson’s disease. Acta Neurobiol. Exp. (Wars) 2020;80:297–304. doi: 10.21307/ane-2020-026. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong M.J., Okun M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 50.Zella M.A.S., Metzdorf J., Ostendorf F., Maass F., Muhlack S., Gold R., Haghikia A., Tonges L. Novel Immunotherapeutic Approaches to Target Alpha-Synuclein and Related Neuroinflammation in Parkinson’s Disease. Cells. 2019;8:105. doi: 10.3390/cells8020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramaniam S.R., Federoff H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017;9:176. doi: 10.3389/fnagi.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindestam Arlehamn C.S., Dhanwani R., Pham J., Kuan R., Frazier A., Rezende Dutra J., Phillips E., Mallal S., Roederer M., Marder K.S., et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 2020;11:1875. doi: 10.1038/s41467-020-15626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sulzer D., Alcalay R.N., Garretti F., Cote L., Kanter E., Agin-Liebes J., Liong C., McMurtrey C., Hildebrand W.H., Mao X., et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabahi M., Joshaghanian A., Dolatshahi M., Jabbari P., Rahmani F., Rezaei N. Modification of Glial Cell Activation through Dendritic Cell Vaccination: Promises for Treatment of Neurodegenerative Diseases. J. Mol. Neurosci. 2021;71:1410–1424. doi: 10.1007/s12031-021-01818-6. [DOI] [PubMed] [Google Scholar]
- 55.Li J.Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Bjorklund A., et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 56.Ugen K.E., Lin X., Bai G., Liang Z., Cai J., Li K., Song S., Cao C., Sanchez-Ramos J. Evaluation of an alpha synuclein sensitized dendritic cell based vaccine in a transgenic mouse model of Parkinson disease. Hum. Vaccin. Immunother. 2015;11:922–930. doi: 10.1080/21645515.2015.1012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciaramella A., Salani F., Bizzoni F., Pontieri F.E., Stefani A., Pierantozzi M., Assogna F., Caltagirone C., Spalletta G., Bossu P. Blood dendritic cell frequency declines in idiopathic Parkinson’s disease and is associated with motor symptom severity. PLoS ONE. 2013;8:e65352. doi: 10.1371/journal.pone.0065352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran H.T., Chung C.H., Iba M., Zhang B., Trojanowski J.Q., Luk K.C., Lee V.M. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahaduzzaman M., Nash K., Hudson C., Sharif M., Grimmig B., Lin X., Bai G., Liu H., Ugen K.E., Cao C., et al. Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson’s disease. PLoS ONE. 2015;10:e0116841. doi: 10.1371/journal.pone.0116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Games D., Valera E., Spencer B., Rockenstein E., Mante M., Adame A., Patrick C., Ubhi K., Nuber S., Sacayon P., et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci. 2014;34:9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae E.J., Lee H.J., Rockenstein E., Ho D.H., Park E.B., Yang N.Y., Desplats P., Masliah E., Lee S.J. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schenk D.B., Koller M., Ness D.K., Griffith S.G., Grundman M., Zago W., Soto J., Atiee G., Ostrowitzki S., Kinney G.G. First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Mov. Disord. 2017;32:211–218. doi: 10.1002/mds.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brys M., Fanning L., Hung S., Ellenbogen A., Penner N., Yang M., Welch M., Koenig E., David E., Fox T., et al. Randomized phase I clinical trial of anti-alpha-synuclein antibody BIIB054. Mov. Disord. 2019;34:1154–1163. doi: 10.1002/mds.27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jankovic J., Goodman I., Safirstein B., Marmon T.K., Schenk D.B., Koller M., Zago W., Ness D.K., Griffith S.G., Grundman M., et al. Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-alpha-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2018;75:1206–1214. doi: 10.1001/jamaneurol.2018.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stonesifer C., Corey S., Ghanekar S., Diamandis Z., Acosta S.A., Borlongan C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H.X., Liang F.C., Gu P., Xu B.L., Xu H.J., Wang W.T., Hou J.Y., Xie D.X., Chai X.Q., An S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11:288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanna T., Sachan V. Mesenchymal stem cells: Potential in treatment of neurodegenerative diseases. Curr. Stem Cell Res. Ther. 2014;9:513–521. doi: 10.2174/1574888X09666140923101110. [DOI] [PubMed] [Google Scholar]
- 68.Yen B.L., Chang C.J., Liu K.J., Chen Y.C., Hu H.I., Bai C.H., Yen M.L. Brief report--human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells. 2009;27:451–456. doi: 10.1634/stemcells.2008-0390. [DOI] [PubMed] [Google Scholar]
- 69.Trivedi P., Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan Z., Su Z.Y., Wu R.R., Gu B., Liu Y.K., Zhao X.L., Zhang M. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J. Zhejiang Univ. Sci. B. 2011;12:18–27. doi: 10.1631/jzus.B1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao F., Chiu S.M., Motan D.A., Zhang Z., Chen L., Ji H.L., Tse H.F., Fu Q.L., Lian Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.d’Angelo M., Cimini A., Castelli V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:5241. doi: 10.3390/ijms21155241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leggio L., Paterno G., Vivarelli S., L’Episcopo F., Tirolo C., Raciti G., Pappalardo F., Giachino C., Caniglia S., Serapide M.F., et al. Extracellular Vesicles as Nanotherapeutics for Parkinson’s Disease. Biomolecules. 2020;10:1327. doi: 10.3390/biom10091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panagiotakopoulou V., Ivanyuk D., De Cicco S., Haq W., Arsic A., Yu C., Messelodi D., Oldrati M., Schondorf D.C., Perez M.J., et al. Interferon-gamma signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 2020;11:5163. doi: 10.1038/s41467-020-18755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Kordower J.H., Goetz C.G., Chu Y., Halliday G.M., Nicholson D.A., Musial T.F., Marmion D.J., Stoessl A.J., Sossi V., Freeman T.B., et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann. Neurol. 2017;81:46–57. doi: 10.1002/ana.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., Shannon K.M., Nauert G.M., Perl D.P., Godbold J., et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 78.Liu Z., Cheung H.H. Stem Cell-Based Therapies for Parkinson Disease. Int. J. Mol. Sci. 2020;21:8060. doi: 10.3390/ijms21218060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitada M., Dezawa M. Parkinson’s disease and mesenchymal stem cells: Potential for cell-based therapy. Parkinsons Dis. 2012;2012:873706. doi: 10.1155/2012/873706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindvall O., Sawle G., Widner H., Rothwell J.C., Bjorklund A., Brooks D., Brundin P., Frackowiak R., Marsden C.D., Odin P., et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann. Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 81.Kordower J.H., Freeman T.B., Snow B.J., Vingerhoets F.J., Mufson E.J., Sanberg P.R., Hauser R.A., Smith D.A., Nauert G.M., Perl D.P., et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 82.Freed C.R., Breeze R.E., Rosenberg N.L., Schneck S.A., Kriek E., Qi J.X., Lone T., Zhang Y.B., Snyder J.A., Wells T.H., et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N. Engl. J. Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 83.Mendez I., Vinuela A., Astradsson A., Mukhida K., Hallett P., Robertson H., Tierney T., Holness R., Dagher A., Trojanowski J.Q., et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., Olanow C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y., Xiong M., Dong Y., Haberman A., Cao J., Liu H., Zhou W., Zhang S.C. Chemical Control of Grafted Human PSC-Derived Neurons in a Mouse Model of Parkinson’s Disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A., et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez-Cerdeno V., Noctor S.C., Espinosa A., Ariza J., Parker P., Orasji S., Daadi M.M., Bankiewicz K., Alvarez-Buylla A., Kriegstein A.R. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grealish S., Diguet E., Kirkeby A., Mattsson B., Heuer A., Bramoulle Y., Van Camp N., Perrier A.L., Hantraye P., Bjorklund A., et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y.K., Zhu W.W., Wu M.H., Wu Y.H., Liu Z.X., Liang L.M., Sheng C., Hao J., Wang L., Li W., et al. Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson’s Disease. Stem Cell Reports. 2018;11:171–182. doi: 10.1016/j.stemcr.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T., Mizuma H., Takara S., Takahashi R., Inoue H., et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 91.Ben-Hur T., Idelson M., Khaner H., Pera M., Reinhartz E., Itzik A., Reubinoff B.E. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 92.Hargus G., Cooper O., Deleidi M., Levy A., Lee K., Marlow E., Yow A., Soldner F., Hockemeyer D., Hallett P.J., et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang D., Zhang Z.J., Oldenburg M., Ayala M., Zhang S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haney M.J., Zhao Y., Harrison E.B., Mahajan V., Ahmed S., He Z., Suresh P., Hingtgen S.D., Klyachko N.L., Mosley R.L., et al. Specific transfection of inflamed brain by macrophages: A new therapeutic strategy for neurodegenerative diseases. PLoS ONE. 2013;8:e61852. doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Oh S.H., Lee S.C., Kim D.Y., Kim H.N., Shin J.Y., Ye B.S., Lee P.H. Mesenchymal Stem Cells Stabilize Axonal Transports for Autophagic Clearance of alpha-Synuclein in Parkinsonian Models. Stem Cells. 2017;35:1934–1947. doi: 10.1002/stem.2650. [DOI] [PubMed] [Google Scholar]
- 96.Kojima R., Bojar D., Rizzi G., Hamri G.C., El-Baba M.D., Saxena P., Auslander S., Tan K.R., Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.L’Episcopo F., Tirolo C., Peruzzotti-Jametti L., Serapide M.F., Testa N., Caniglia S., Balzarotti B., Pluchino S., Marchetti B. Neural Stem Cell Grafts Promote Astroglia-Driven Neurorestoration in the Aged Parkinsonian Brain via Wnt/β-Catenin Signaling. Stem Cells. 2018;36:1179–1197. doi: 10.1002/stem.2827. [DOI] [PubMed] [Google Scholar]
- 98.Lee J.Y., Tuazon J.P., Ehrhart J., Sanberg P.R., Borlongan C.V. Gutting the brain of inflammation: A key role of gut microbiome in human umbilical cord blood plasma therapy in Parkinson’s disease model. J. Cell Mol. Med. 2019;23:5466–5474. doi: 10.1111/jcmm.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J.Y., Tuazon J.P., Corey S., Bonsack B., Acosta S., Ehrhart J., Sanberg P.R., Borlongan C.V. A Gutsy Move for Cell-Based Regenerative Medicine in Parkinson’s Disease: Targeting the Gut Microbiome to Sequester Inflammation and Neurotoxicity. Stem Cell Rev. Rep. 2019;15:690–702. doi: 10.1007/s12015-019-09906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serapide M.F., L’Episcopo F., Tirolo C., Testa N., Caniglia S., Giachino C., Marchetti B. Boosting Antioxidant Self-defenses by Grafting Astrocytes Rejuvenates the Aged Microenvironment and Mitigates Nigrostriatal Toxicity in Parkinsonian Brain via an Nrf2-Driven Wnt/beta-Catenin Prosurvival Axis. Front. Aging Neurosci. 2020;12:24. doi: 10.3389/fnagi.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.L’Episcopo F., Tirolo C., Testa N., Caniglia S., Morale M.C., Serapide M.F., Pluchino S., Marchetti B. Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson’s disease. Stem Cells. 2014;32:2147–2163. doi: 10.1002/stem.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berwick D.C., Javaheri B., Wetzel A., Hopkinson M., Nixon-Abell J., Grannò S., Pitsillides A.A., Harvey K. Pathogenic LRRK2 variants are gain-of-function mutations that enhance LRRK2-mediated repression of β-catenin signaling. Mol. Neurodegener. 2017;12:9. doi: 10.1186/s13024-017-0153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marchetti B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018;19:3743. doi: 10.3390/ijms19123743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L., Deng J., Pan Q., Zhan Y., Fan J.B., Zhang K., Zhang Z. Targeted methylation sequencing reveals dysregulated Wnt signaling in Parkinson disease. J. Genet. Genomics. 2016;43:587–592. doi: 10.1016/j.jgg.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 105.Parish C.L., Thompson L.H. Modulating Wnt signaling to improve cell replacement therapy for Parkinson’s disease. J. Mol. Cell Biol. 2014;6:54–63. doi: 10.1093/jmcb/mjt045. [DOI] [PubMed] [Google Scholar]
- 106.Andersson E.R., Saltó C., Villaescusa J.C., Cajanek L., Yang S., Bryjova L., Nagy I.I., Vainio S.J., Ramirez C., Bryja V., et al. Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:E602–E610. doi: 10.1073/pnas.1208524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hermann A., Maisel M., Wegner F., Liebau S., Kim D.W., Gerlach M., Schwarz J., Kim K.S., Storch A. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949–964. doi: 10.1634/stemcells.2005-0192. [DOI] [PubMed] [Google Scholar]
- 108.Hermann A., Suess C., Fauser M., Kanzler S., Witt M., Fabel K., Schwarz J., Höglinger G.U., Storch A. Rostro-caudal gradual loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells. 2009;27:928–941. doi: 10.1002/stem.21. [DOI] [PubMed] [Google Scholar]
- 109.Arenas E. Wnt signaling in midbrain dopaminergic neuron development and regenerative medicine for Parkinson’s disease. J. Mol. Cell Biol. 2014;6:42–53. doi: 10.1093/jmcb/mju001. [DOI] [PubMed] [Google Scholar]
- 110.Joksimovic M., Awatramani R. Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J. Mol. Cell Biol. 2014;6:27–33. doi: 10.1093/jmcb/mjt043. [DOI] [PubMed] [Google Scholar]
- 111.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 112.Sariola H., Saarma M. Novel functions and signalling pathways for GDNF. J. Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 113.Gash D.M., Zhang Z., Ovadia A., Cass W.A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P.A., Collins F., et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 114.Kearns C.M., Gash D.M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-P. [DOI] [PubMed] [Google Scholar]
- 115.Tomac A., Lindqvist E., Lin L.F., Ogren S.O., Young D., Hoffer B.J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 116.Gill S.S., Patel N.K., Hotton G.R., O’Sullivan K., McCarter R., Bunnage M., Brooks D.J., Svendsen C.N., Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 117.Heiss J.D., Lungu C., Hammoud D.A., Herscovitch P., Ehrlich D.J., Argersinger D.P., Sinharay S., Scott G., Wu T., Federoff H.J., et al. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov. Disord. 2019;34:1073–1078. doi: 10.1002/mds.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lang A.E., Gill S., Patel N.K., Lozano A., Nutt J.G., Penn R., Brooks D.J., Hotton G., Moro E., Heywood P., et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 119.Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr., Lozano A.M., Penn R.D., Simpson R.K., Jr., Stacy M., et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/WNL.60.1.69. [DOI] [PubMed] [Google Scholar]
- 120.Slevin J.T., Gash D.M., Smith C.D., Gerhardt G.A., Kryscio R., Chebrolu H., Walton A., Wagner R., Young A.B. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: Response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 2007;106:614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- 121.Whone A., Luz M., Boca M., Woolley M., Mooney L., Dharia S., Broadfoot J., Cronin D., Schroers C., Barua N.U., et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain. 2019;142:512–525. doi: 10.1093/brain/awz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whone A.L., Boca M., Luz M., Woolley M., Mooney L., Dharia S., Broadfoot J., Cronin D., Schroers C., Barua N.U., et al. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Parkinsons Dis. 2019;9:301–313. doi: 10.3233/JPD-191576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berlet R., Galang Cabantan D.A., Gonzales-Portillo D., Borlongan C.V. Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson’s Disease, and Huntington’s Disease. Front. Cell Dev. Biol. 2022;10:798826. doi: 10.3389/fcell.2022.798826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jacotte-Simancas A., Costa-Miserachs D., Coll-Andreu M., Torras-Garcia M., Borlongan C.V., Portell-Cortes I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J. Neurotrauma. 2015;32:739–751. doi: 10.1089/neu.2014.3502. [DOI] [PubMed] [Google Scholar]
- 125.Kingsbury C., Shear A., Heyck M., Sadanandan N., Zhang H., Gonzales-Portillo B., Cozene B., Sheyner M., Navarro-Torres L., Garcia-Sanchez J., et al. Inflammation-relevant microbiome signature of the stroke brain, gut, spleen, and thymus and the impact of exercise. J. Cereb. Blood Flow Metab. 2021;41:3200–3212. doi: 10.1177/0271678X211039598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pianta S., Lee J.Y., Tuazon J.P., Castelli V., Mantohac L.M., Tajiri N., Borlongan C.V. A Short Bout of Exercise Prior to Stroke Improves Functional Outcomes by Enhancing Angiogenesis. Neuromolecular Med. 2019;21:517–528. doi: 10.1007/s12017-019-08533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watson N., Ji X., Yasuhara T., Date I., Kaneko Y., Tajiri N., Borlongan C.V. No pain, no gain: Lack of exercise obstructs neurogenesis. Cell Transplant. 2015;24:591–597. doi: 10.3727/096368915X687723. [DOI] [PubMed] [Google Scholar]
- 128.Yasuhara T., Hara K., Maki M., Matsukawa N., Fujino H., Date I., Borlongan C.V. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007;149:182–191. doi: 10.1016/j.neuroscience.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 129.McFarlin B.K., Flynn M.G., Campbell W.W., Craig B.A., Robinson J.P., Stewart L.K., Timmerman K.L., Coen P.M. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 130.Ye Y., Lin H., Wan M., Qiu P., Xia R., He J., Tao J., Chen L., Zheng G. The Effects of Aerobic Exercise on Oxidative Stress in Older Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2021;12:701151. doi: 10.3389/fphys.2021.701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flynn M.G., McFarlin B.K., Markofski M.M. The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007;1:220–235. doi: 10.1177/1559827607300283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adamopoulos S., Parissis J., Kroupis C., Georgiadis M., Karatzas D., Karavolias G., Koniavitou K., Coats A.J., Kremastinos D.T. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur. Heart J. 2001;22:791–797. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 133.Adamopoulos S., Parissis J., Karatzas D., Kroupis C., Georgiadis M., Karavolias G., Paraskevaidis J., Koniavitou K., Coats A.J., Kremastinos D.T. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J. Am. Coll Cardiol. 2002;39:653–663. doi: 10.1016/S0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 134.Murphy E.A., Enos R.T., Velázquez K.T. Influence of Exercise on Inflammation in Cancer: Direct Effect or Innocent Bystander? Exerc. Sport Sci. Rev. 2015;43:134–142. doi: 10.1249/JES.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 135.Daou H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2020;318:R296–R310. doi: 10.1152/ajpregu.00147.2019. [DOI] [PubMed] [Google Scholar]
- 136.Seo D.Y., Heo J.W., Ko J.R., Kwak H.B. Exercise and Neuroinflammation in Health and Disease. Int. Neurourol. J. 2019;23:S82–S92. doi: 10.5213/inj.1938214.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jawed Y., Beli E., March K., Kaleth A., Loghmani M.T. Whole-Body Vibration Training Increases Stem/Progenitor Cell Circulation Levels and May Attenuate Inflammation. Mil. Med. 2020;185:404–412. doi: 10.1093/milmed/usz247. [DOI] [PubMed] [Google Scholar]
- 138.de Sousa C.V., Sales M.M., Rosa T.S., Lewis J.E., de Andrade R.V., Simões H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017;47:277–293. doi: 10.1007/s40279-016-0566-1. [DOI] [PubMed] [Google Scholar]
- 139.Frodermann V., Rohde D., Courties G., Severe N., Schloss M.J., Amatullah H., McAlpine C.S., Cremer S., Hoyer F.F., Ji F., et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019;25:1761–1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niemiro G.M., Allen J.M., Mailing L.J., Khan N.A., Holscher H.D., Woods J.A., De Lisio M. Effects of endurance exercise training on inflammatory circulating progenitor cell content in lean and obese adults. J. Physiol. 2018;596:2811–2822. doi: 10.1113/JP276023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mee-Inta O., Zhao Z.W., Kuo Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells. 2019;8:691. doi: 10.3390/cells8070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Real C.C., Garcia P.C., Britto L.R.G. Treadmill Exercise Prevents Increase of Neuroinflammation Markers Involved in the Dopaminergic Damage of the 6-OHDA Parkinson’s Disease Model. J. Mol. Neurosci. 2017;63:36–49. doi: 10.1007/s12031-017-0955-4. [DOI] [PubMed] [Google Scholar]
- 143.Koo J.H., Jang Y.C., Hwang D.J., Um H.S., Lee N.H., Jung J.H., Cho J.Y. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-κB signaling pathway. Neuroscience. 2017;356:102–113. doi: 10.1016/j.neuroscience.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 144.Zoladz J.A., Majerczak J., Zeligowska E., Mencel J., Jaskolski A., Jaskolska A., Marusiak J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol Pharmacol. 2014;65:441–448. [PubMed] [Google Scholar]
- 145.Tuon T., Valvassori S.S., Lopes-Borges J., Luciano T., Trom C.B., Silva L.A., Quevedo J., Souza C.T., Lira F.S., Pinho R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience. 2012;227:305–312. doi: 10.1016/j.neuroscience.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 146.Kronenberg G., Reuter K., Steiner B., Brandt M.D., Jessberger S., Yamaguchi M., Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 147.Steiner B., Zurborg S., Hörster H., Fabel K., Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 148.Pérez-Domínguez M., Tovar Y.R.L.B., Zepeda A. Neuroinflammation and physical exercise as modulators of adult hippocampal neural precursor cell behavior. Rev. Neurosci. 2018;29:1–20. doi: 10.1515/revneuro-2017-0024. [DOI] [PubMed] [Google Scholar]
- 149.Wu C.W., Chen Y.C., Yu L., Chen H.I., Jen C.J., Huang A.M., Tsai H.J., Chang Y.T., Kuo Y.M. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J. Neurochem. 2007;103:2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x. [DOI] [PubMed] [Google Scholar]
- 150.Zigmond M.J., Cameron J.L., Leak R.K., Mirnics K., Russell V.A., Smeyne R.J., Smith A.D. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 2009;15((Suppl. S3)):S42–S45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- 151.Leem Y.H., Park J.S., Park J.E., Kim D.Y., Kim H.S. Neurogenic effects of rotarod walking exercise in subventricular zone, subgranular zone, and substantia nigra in MPTP-induced Parkinson’s disease mice. Sci. Rep. 2022;12:10544. doi: 10.1038/s41598-022-14823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frazzitta G., Maestri R., Bertotti G., Riboldazzi G., Boveri N., Perini M., Uccellini D., Turla M., Comi C., Pezzoli G., et al. Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil. Neural. Repair. 2015;29:123–131. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- 153.De Lisio M., Baker J.M., Parise G. Exercise promotes bone marrow cell survival and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp. Hematol. 2013;41:143–154. doi: 10.1016/j.exphem.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 154.Steinberg A., Asher A., Bailey C., Fu J.B. The role of physical rehabilitation in stem cell transplantation patients. Support. Care Cancer. 2015;23:2447–2460. doi: 10.1007/s00520-015-2744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dugan E.A., Jergova S., Sagen J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020;327:113208. doi: 10.1016/j.expneurol.2020.113208. [DOI] [PubMed] [Google Scholar]
- 156.Yang X., Cheng B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. J. Mol. Neurosci. 2010;42:145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 157.Fu S.P., Wang J.F., Xue W.J., Liu H.M., Liu B.R., Zeng Y.L., Li S.N., Huang B.X., Lv Q.K., Wang W., et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation. 2015;12:9. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Masino S.A., Rho J.M. Mechanisms of Ketogenic Diet Action. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2012. [PubMed] [Google Scholar]
- 160.Tieu K., Perier C., Caspersen C., Teismann P., Wu D.C., Yan S.D., Naini A., Vila M., Jackson-Lewis V., Ramasamy R., et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003;112:892–901. doi: 10.1172/JCI200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krikorian R., Shidler M.D., Summer S.S., Sullivan P.G., Duker A.P., Isaacson R.S., Espay A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Park Relat. Disord. 2019;1:41–47. doi: 10.1016/j.prdoa.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Taghizadeh M., Tamtaji O.R., Dadgostar E., Daneshvar Kakhaki R., Bahmani F., Abolhassani J., Aarabi M.H., Kouchaki E., Memarzadeh M.R., Asemi Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 163.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 164.Xu Y., Zhang Y., Quan Z., Wong W., Guo J., Zhang R., Yang Q., Dai R., McGeer P.L., Qing H. Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease. Neurochem. Res. 2016;41:2788–2796. doi: 10.1007/s11064-016-1995-9. [DOI] [PubMed] [Google Scholar]
- 165.Chen M., Wang T., Yue F., Li X., Wang P., Li Y., Chan P., Yu S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience. 2015;286:383–392. doi: 10.1016/j.neuroscience.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 166.Batista-Nascimento L., Pimentel C., Menezes R.A., Rodrigues-Pousada C. Iron and neurodegeneration: From cellular homeostasis to disease. Oxid. Med. Cell Longev. 2012;2012:128647. doi: 10.1155/2012/128647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao J., Xu L., Liang Q., Sun Q., Chen C., Zhang Y., Ding Y., Zhou P. Metal chelator EGCG attenuates Fe(III)-induced conformational transition of α-synuclein and protects AS-PC12 cells against Fe(III)-induced death. J. Neurochem. 2017;143:136–146. doi: 10.1111/jnc.14142. [DOI] [PubMed] [Google Scholar]
- 168.Rangarajan P., Karthikeyan A., Dheen S.T. Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromolecular Med. 2016;18:453–464. doi: 10.1007/s12017-016-8430-x. [DOI] [PubMed] [Google Scholar]
- 169.Duan W., Mattson M.P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 170.Mattson M.P., Duan W., Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: Cellular and molecular mechanisms. J. Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 171.Berglöf E., Small B.J., Bickford P.C., Strömberg I. Beneficial effects of antioxidant-enriched diet for tyrosine hydroxylase-positive neurons in ventral mesencephalic tissue in oculo grafts. J. Comp. Neurol. 2009;515:72–82. doi: 10.1002/cne.22002. [DOI] [PubMed] [Google Scholar]
- 172.Bittencourt A., Brum P.O., Ribeiro C.T., Gasparotto J., Bortolin R.C., de Vargas A.R., Heimfarth L., de Almeida R.F., Moreira J.C.F., de Oliveira J., et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr. Neurosci. 2022;25:1026–1040. doi: 10.1080/1028415X.2020.1831261. [DOI] [PubMed] [Google Scholar]
- 173.Kao Y.C., Wei W.Y., Tsai K.J., Wang L.C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2019;21:207. doi: 10.3390/ijms21010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tan L.Y., Yeo X.Y., Bae H.G., Lee D.P.S., Ho R.C., Kim J.E., Jo D.G., Jung S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life. 2021;11:698. doi: 10.3390/life11070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Milan Manani S., Virzì G.M., Giuliani A., Baretta M., Corradi V., De Cal M., Biasi C., Crepaldi C., Ronco C. Lipopolysaccharide Evaluation in Peritoneal Dialysis Patients with Peritonitis. Blood Purif. 2020;49:434–439. doi: 10.1159/000505388. [DOI] [PubMed] [Google Scholar]
- 176.Wang Q., Luo Y., Ray Chaudhuri K., Reynolds R., Tan E.K., Pettersson S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 177.Iba M., Kim C., Sallin M., Kwon S., Verma A., Overk C., Rissman R.A., Sen R., Sen J.M., Masliah E. Neuroinflammation is associated with infiltration of T cells in Lewy body disease and α-synuclein transgenic models. J. Neuroinflammation. 2020;17:214. doi: 10.1186/s12974-020-01888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu Z., Huang Y., Cao B.B., Qiu Y.H., Peng Y.P. Th17 Cells Induce Dopaminergic Neuronal Death via LFA-1/ICAM-1 Interaction in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2017;54:7762–7776. doi: 10.1007/s12035-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 179.Mertsalmi T.H., Pekkonen E., Scheperjans F. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov. Disord. 2020;35:431–442. doi: 10.1002/mds.27924. [DOI] [PubMed] [Google Scholar]
- 180.Huang H., Xu H., Luo Q., He J., Li M., Chen H., Tang W., Nie Y., Zhou Y. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Medicine (Baltimore) 2019;98:e16163. doi: 10.1097/MD.0000000000016163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun M.F., Zhu Y.L., Zhou Z.L., Jia X.B., Xu Y.D., Yang Q., Cui C., Shen Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 182.Scheperjans F., Pekkonen E., Kaakkola S., Auvinen P. Linking Smoking, Coffee, Urate, and Parkinson’s Disease—A Role for Gut Microbiota? J. Parkinsons Dis. 2015;5:255–262. doi: 10.3233/JPD-150557. [DOI] [PubMed] [Google Scholar]
- 183.Cassani E., Privitera G., Pezzoli G., Pusani C., Madio C., Iorio L., Barichella M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva. Gastroenterol. Dietol. 2011;57:117–121. [PubMed] [Google Scholar]
- 184.Barichella M., Pacchetti C., Bolliri C., Cassani E., Iorio L., Pusani C., Pinelli G., Privitera G., Cesari I., Faierman S.A., et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87:1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 185.Georgescu D., Ancusa O.E., Georgescu L.A., Ionita I., Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging. 2016;11:1601–1608. doi: 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tamtaji O.R., Taghizadeh M., Daneshvar Kakhaki R., Kouchaki E., Bahmani F., Borzabadi S., Oryan S., Mafi A., Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 187.Gazerani P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019;20:4121. doi: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.