Abstract
Aging causes a progressive decline in the structure and function of organs. With advancing age, an accumulation of senescent endothelial cells (ECs) contributes to the risk of developing vascular dysfunction and cardiovascular diseases, including hypertension, diabetes, atherosclerosis, and neurodegeneration. Senescent ECs undergo phenotypic changes that alter the pattern of expressed proteins, as well as their morphologies and functions, and have been linked to vascular impairments, such as aortic stiffness, enhanced inflammation, and dysregulated vascular tone. Numerous molecules and pathways, including sirtuins, Klotho, RAAS, IGFBP, NRF2, and mTOR, have been implicated in promoting EC senescence. This review summarizes the molecular players and signaling pathways driving EC senescence and identifies targets with possible therapeutic value in age-related vascular diseases.
Keywords: cellular senescence, endothelial cell, molecular player, signaling pathway, putative target, age-related vascular disease
1. Introduction
Cellular senescence is a state of indefinite cell cycle arrest implemented in response to sublethal stresses, such as DNA damage, telomere shortening, oxidative stress, and oncogene activation. The implementation of senescence involves dynamic changes in protein expression programs, as well as morphology and metabolism. These changes are coordinated by different signaling pathways, some of them culminating with the activation of p53 and a rise in the production of cyclin-dependent kinase (CDK) inhibitors, such as p16 (CDKN2A), p15 (CDKN2B), p21 (CDKN1A), and p27(CDKN1B) [1]. Inhibition of CDK-cyclin complexes is important to suppress growth through the hypophosphorylation of pRb (RB) [2]. Cellular senescence is beneficial for the organism during embryonic development, tissue morphogenesis, wound healing, and tumor suppression in young persons [1,2,3,4,5,6]. However, in older persons, the increased burden of senescent cells has many detrimental actions, exacerbating age-related diseases, including cancer, neurodegeneration, and cardiovascular disease.
A blood vessel consists of three layers, the tunica intima (inner layer), the tunica media (middle layer), and the tunica adventitia (outer layer). The tunica intima is a barrier between the vessel and blood flow and is composed of a layer of endothelial cells and an internal elastic lamina. Endothelial cells (ECs) play a significant physiological role in vascular homeostasis, maintaining blood flow, and regulating vascular tone, pro-inflammatory responses, and neovascularization [7]. Since ECs come into direct contact with the bloodstream, EC senescence can also be triggered by mechanical stimuli, such as disturbed flow [8,9]; although the molecular mechanisms are not completely characterized, disturbed flow induces DNA damage, telomere disfunction, and production of excessive reactive oxygen species (ROS) in ECs [9]. Senescent ECs generally exhibit typical traits of senescent cells, such as a flattened and enlarged morphology, increased polyploidy, decreased nitric oxide (NO) bioavailability, and secretion of a myriad of pro-inflammatory cytokines [10,11,12]. Consequently, endothelial senescence promotes the pathogenesis of vascular aging by decreasing vascular density, increasing intima-media layer thickness and collagen deposition, reducing elastin deposition, and expanding the vascular lumen [13,14,15,16]. As a result of these physiological changes, the vessel experiences increased vascular stiffness caused by decreased arterial elasticity, enhanced vascular inflammatory responses, in turn leading to impaired angiogenesis and vascular tone [8,17]. These alterations in aged vessels are significantly associated with age-related vascular diseases, such as atherosclerosis and hypertension.
Given that senescent ECs play crucial roles in age-related pathophysiological changes in the vessel wall, understanding the mechanisms that promote EC senescence is essential for developing therapeutic strategies to combat age-related vascular disease. This review focuses on the molecular mechanisms and emerging therapeutic targets of EC senescence and age-related vascular pathologies.
2. Characteristics of Endothelial Cell Senescence
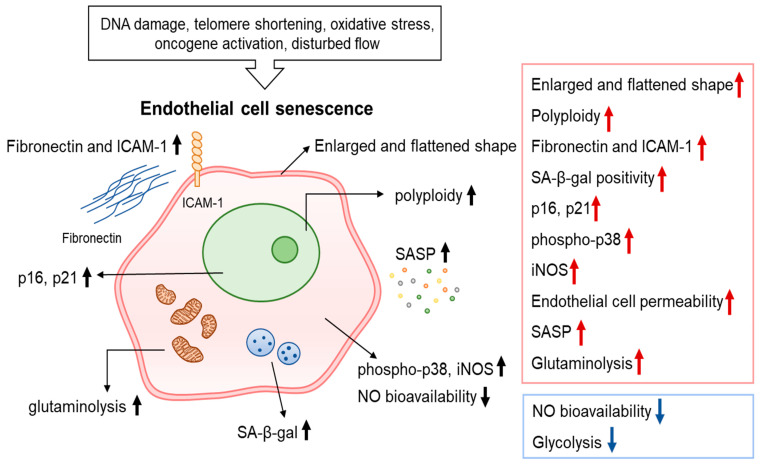
Senescent ECs display an enlarged and flattened morphology, a classic phenotype of senescent cells. In addition, senescent ECs exhibit increased polyploidy, senescence-associated-β-galactosidase (SA-β-Gal) activity, and telomere shortening [18,19,20,21,22]. In addition to classical senescence markers (e.g., p16, p21, and phosphorylated (phospho)-p38), other changes have been identified in senescent ECs, including higher production of fibronectin, intercellular adhesion molecule 1 (ICAM-1), and inducible nitric oxide synthase (iNOS) (Figure 1) [23,24,25,26,27].
Figure 1.
Characteristics of senescent endothelial cells. Various stimuli including DNA damage, telomere shortening, disturbed flow induce endothelial cell (EC) senescence. EC senescence is a state of indefinite cell cycle arrest accompanied by multiple biochemical and metabolic changes. Senescent cells generally become enlarged and flattened and display increased polyploidy, SA-β-gal activity, permeability, phosphorylation of p38, and expression of p16/p21. In addition, senescent ECs display elevated fibronectin, ICAM-1, and iNOS and reduced of NO bioavailability. Senescent ECs also produce a complex and unique SASP that includes secretion of IL-8, IL-6, PAI-1, MCP-1, IGFBP-5, and CXCL11. The metabolic alterations of senescent ECs include reduced glycolysis and increased glutaminolysis.
Senescent ECs produce a complex senescence-associated secretory phenotype (SASP) that varies depending on the type, duration, and intensity of damage, as well as on EC growth conditions [28]. For instance, replicatively senescent HUVECs show increased production of interleukin 8 (IL8), IL6, plasminogen activator inhibitor 1 (PAI-1 or SERPINE1), and monocyte chemotactic protein-1 (MCP-1/CCL2), and IL-1α (IL1A) [29,30,31]. By contrast, HUVECs rendered senescence by disturbed flow show moderate ROS production, which is a distinct SASP feature observed in replicatively senescent HUVECs [32]. In another example, exposure to chronic TNF-α (TNF) induces HUVEC senescence with production of many cytokines, such as IL6, IL8, PAI-1, and IGFBP5 (insulin-like growth factor binding protein-5) [33]. Other triggers of HUVEC senescence, including exposure to radiation or doxorubicin (Doxo), led to increases in the level of the C–X–C motif chemokine 11 (CXCL11), which promotes the aggressive activity of cancer cells by binding to the C–X–C motif chemokine receptor 3 (CXCR3) [34].
Compared with proliferating HUVECs, senescent HUVECs have extended and interconnected mitochondria associated with reduced expression of FIS1 and DRP1, two important proteins in mitochondrial fission [35]. Silencing DRP1 induces senescence in young HUVECs with increased SA-β-Gal staining, elongated mitochondria, impaired autophagy, and increased expression of p21 and p16. DRP1 abundance is reduced in the endothelium of aorta in old rats and is associated with impaired autophagy. Silencing DRP1 also impairs autophagic flux in the vascular endothelium of carotid arteries in rats [30] and excessive mitochondrial fission induces EC senescence [36]. Loss of protein disulfide isomerase A1 causes EC senescence by inducing the production of mitochondrial ROS and mitochondrial fragmentation through the DRP1 sulfenylation at cysteine 644 [36].
Oxidative stress is a well-known contributor to EC senescence [37]. ROS is not only generated through mitochondrial respiration, but also from peroxisomal β-oxidation of free fatty acids, xanthine oxidase, lipoxygenase, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), microsomal P-450 enzymes, cyclooxygenases, and prooxidant heme molecules [38]. Unlike mitochondria in other cells, EC mitochondria are not considered as the primary source of ROS generation, which could be associated with the small ratio of mitochondria to EC by mass [39]. Instead, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are considered to be the main source of ROS formation in ECs; additional sources include the lysosome, peroxisome, and endoplasmic reticulum (ER) [40,41,42,43]. NOX is one of the key enzymes responsible for much of the generation of ROS in cardiovascular disease (CVD). NOX1, NOX2, NOX4 and NOX5 isoforms have been found to induce EC dysfunction, inflammation and apoptosis in atherosclerosis, hypertension, and diabetes [44]. Although physiological ROS levels are required to maintain normal cellular function, overproduction of ROS induces deleterious effects, such as changes in DNA transcription, interruption of many redox-sensitive signaling pathways, impairment of cellular structure and function, inflammation, and organ dysfunction [45]. In late-passage ECs, a rise in the level of intracellular ROS further contributes to EC senescence by accelerating telomere shortening [46,47].
Senescent cells are metabolically active and generally require more energy from glycolysis. However, senescent ECs undergo senescence-associated metabolic shifts, with decreased glycolysis and oxidative phosphorylation [37,48,49,50]. A recent report [51] found that senescent cells increase the production of glutaminase and consume high levels of glutamine, in turn producing glutamate and ammonia. This process is believed to enhance senescent cell survival by helping to maintain internal cellular pH; accordingly, inhibiting glutaminolysis was found to selectively remove senescent ECs and alleviated age-associated organ dysfunction [51]. Investigation of glutaminolysis on the EC metabolism under different disease conditions may provide new targets to manage EC senescence. Dysfunctional ECs show a higher dependence on oxidative phosphorylation (OXPHOS) than on glycolysis [52]. Although OXPHOS yields more ATP than glycolysis, high levels of OXPHOS lead to an increase in ROS and EC damage [53]. Hyperglycemic stress markedly reduces glycolytic enzymes and shifts glycolysis to OXPHOS in the diabetic endothelium [52]. Haspula et al. found that angiogenic signaling was compromised in the presence of a high superoxide burden, possibly due to the rise in OXPHOS activity [52].
Senescent cells have been observed in a variety of mammalian vascular tissues associated with cellular stress, aging, and age-related pathologies. For example, single and double balloon denudations of rabbit carotid arteries caused EC senescence, as identified by SA-β-gal staining; the authors suggested that vascular cell senescence could affect atherogenesis and post-angioplasty restenosis [54]. In addition, senescent ECs were found in Zucker diabetic rats at six-fold higher levels by 22 weeks of age when compared with control rats, with dramatic increases in p53, p21, and p16 levels [55,56]; the number of SA-β-Gal-positive ECs declined after treatment with ebselen, a peroxyni-trite scavenger [56]. The aging process alone can increase senescence, as a single-cell RNA sequencing analysis found that ~10% of ECs in the brain microvasculature of 28-month-old mice were senescent [57]. Similarly, SA-β-gal-positive ECs exist in human atherosclerotic plaques of the aorta, coronary arteries, and adipose tissues of obese human subjects [26,58,59]. Taken together, these studies illustrate the prevalence and importance of senescent ECs in age-related vascular diseases.
3. Molecular Players and Pathways Associated with Endothelial Cell Senescence
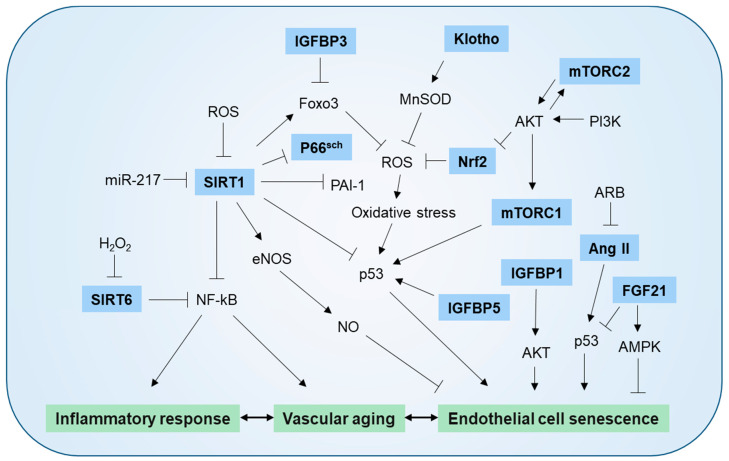
Many molecular mechanisms have been implicated in endothelial and vascular cell senescence. Those best characterized are described below and summarized in Figure 2.
Figure 2.
Molecular mechanism mediating endothelial cell senescence and vascular aging. SIRT1 protects against endothelial dysfunction by deacetylating eNOS and increasing endothelial NO bioavailability. SIRT1 also protects vascular inflammation by suppressing NF-κB signaling and exerts anti-oxidant action by increasing Foxo and down-regulating p66shc. Under conditions of age-related overexpression, miR-217 suppresses SIRT1 production. Klotho increases MnSOD activity and NO availability by negatively downregulating IGF-1 signaling. H2O2 induces endothelial cell (EC) senescence via downregulation of SIRT6 levels. SIRT6 deficiency raises the expression of endothelial proinflammatory cytokines and increases NF-κB transcriptional activity. Increased oxidative stress due to NRF2 deficiency promotes EC senescence. mTORC1 increases EC senescence through the PI3K/AKT pathway and mTORC2 induces EC senescence by suppressing NRF2 production via the AKT/GSK-3β/CEBPα signaling pathway. IGFBP1 and IGFBP5 induce senescence in HUVECs through AKT or p53-dependent mechanism. IGFBP3 promotes EC senescence by inhibiting the SIRT1-Foxo axis, ARB inhibits Ang II-induced EC senescence, and FGF21 represses EC senescence via inhibiting p53 signaling pathway in an AMPK-dependent manner.
3.1. Sirtuins
Sirtuins comprise a family of signaling proteins with metabolic functions, particularly nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase and ADP-ribosyltransferase activities [60]. Among the seven sirtuins, SIRT1 is particularly highly expressed in ECs of arteries, veins, and capillaries, and plays crucial roles in EC senescence, DNA repair, cell cycle regulation, gene silencing, stress resistance, apoptosis, organism longevity, and inflammation in the vascular system [60]. Dysfunction of the SIRT1–eNOS axis results in impaired NO production during the aging-associated downregulation of SIRT1 [61,62,63]. Furthermore, the protection against ROS-induced premature senescence by SIRT1 was linked to an increase in eNOS activity, as supported by observations that an endogenous suppressor of SIRT1 production, miR-217, suppressed eNOS activity and advanced endothelial senescence [62,63].
During inflammatory processes underlying the initiation and progression of vascular aging, ECs and activated immune cells exhibit chronically active NF-κB signaling. In this paradigm, reductions in the levels of SIRT1 lead to increased acetylation of Lys-310 on the p65 subunit of NF-κB, resulting in an increased inflammatory response in ECs and monocytes/macrophages [64,65,66]. In ECs, increased CDK5-mediated hyperphosphorylation of SIRT1 at Ser-47 prevents the nuclear export of SIRT1 and its interaction with telomeric repeat-binding factor 2-interacting protein 1 (TERF2IP), a regulator of telomere function and NF-κB signaling [67]. Other factors blocked by SIRT1, including p53, PAI-1, and p66Shc, participate in the protection against endothelial senescence [68,69,70]. Moreover, in aged mice, a decline in SIRT1 activity, due to impaired phosphorylation at Ser-154, accounts for the reduced expression of endothelium estrogen receptor β (ERβ) via binding of SIRT1-PPAR-c/RXR-p300 to a PPAR response element site on the ERβ promoter [71]. Consistent with the role for SIRT1 in endothelial senescence, a mouse model of vascular senescence produced by genetically excluding exon 4 of SIRT1 in ECs (SIRT1endo−/−) exhibited damaged endothelium-dependent angiogenesis and vasorelaxation [72].
More recently, another molecular mechanism underlying the protective effect of SIRT1 against endothelial senescence and vascular aging was reported. SIRT1 was found to prevent endothelial senescence by reducing the acetylation of stress-responsive serine/threonine liver kinase B1 (LKB1) via HERC2, a giant scaffold protein and E3 ubiquitin ligase [73]. In senescent ECs and aged arteries, loss of SIRT1 caused acetylated LKB1 to accumulate in the nucleus, with irreversible changes in the blood vessel wall, vascular stiffness, and adverse arterial remodeling. These changes suggest that the SIRT1/HERC2/LKB1 regulatory paradigm fine-tunes the communication between endothelial and vascular smooth muscle cells (VSMCs) to maintain vascular homeostasis [73]. Conversely, SIRT1 overexpression and increased LKB1 deacetylation prevent EC senescence in culture and stress-induced senescence in mice [70].
SIRT6, a chromatin-associated protein that stabilizes genomes and telomeres, protects cells from premature senescence by maintaining their replicative capacity and angiogenic ability in culture [74]. In ECs, SIRT6 was found to decrease during H2O2-induced senescence, while overexpression of SIRT6 partially reversed this process [75]. Knockdown of SIRT6 accelerated cell senescence and overactive NF-κB signaling, suggesting that SIRT6 plays a fundamental role in aging and inflammation [76]. SIRT6 prevents the formation of volatile atherosclerotic plaques in diabetic patients, and silencing SIRT6 suppresses EC replication and promotes EC senescence [77,78]. In addition, SIRT6 was proposed to inhibit heart failure and cardiac hypertrophy by regulating signaling through the insulin/insulin-like growth factor (IGF)-Akt [79]. SIRT6 was also postulated to protect hepatic low-density lipoprotein (LDL) receptors from degradation and reduced plasma LDL cholesterol levels in mice by inhibiting the transcriptional increase in proprotein convertase subtilisin/kexin type 9 (PCSK9), both of which may prevent atherogenesis [80]. Overall, SIRT1 and 6 engage in critical functions to maintain vascular function and integrity with age.
3.2. Klotho
Klotho is expressed primarily in the kidney and, to a lesser extent, in the vascular tissue [81]. Animal studies offer evidence of the protective capacity of Klotho in endothelial dysfunction via the upregulation of NO [82]. In support of this role, mice with ablated Klotho gene exhibit damaged endothelium and NO-dependent vasodilation in aorta and arterioles from when exposed to acetylcholine [83]. Klotho was also found to promote endothelial health by inducing anti-oxidative pathways; for example, it decreased H2O2-induced apoptosis in HUVECs by promoting manganese superoxide dismutase (MnSOD) activity through insulin/IGF signaling. Besides promoting resistance to oxidative stress, Klotho has anti-aging effects by suppressing caspase activity, reducing signaling through the p53/p21 pathway, and inducing resistance to oxidative stress [83,84,85,86].
Clinical trials showed that adults with high level of plasma Klotho have lower risk of coronary artery disease (CAD), heart failure, stroke, and peripheral arterial disease (PAD) [87]. Conversely, lower Klotho levels in serum correlated with the presence and severity of CAD separately from other well-known cardiovascular disease (CVD) risk factors, such as dyslipidemia, diabetes, and hypertension [88]. Furthermore, the level of Klotho in serum was an independent predictor of arterial stiffness [89]. Until now, overexpression of Klotho prevented renal and cardiovascular dysfunction related to aging and extended lifespan in experimental mice [86]. Klotho knockout mice showed premature aging characteristics, including changed calcium/phosphate metabolism, vascular calcification, and decreased lifespan [90]. These studies generally support the hypothesis that high levels of soluble Klotho may protect ECs and cardiovascular functions from age-related dysfunction, and extend human lifespan.
3.3. Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is a well-known regulator of salt and water homeostasis. Plasma renin converts angiotensinogen to angiotensin I, which is then converted by angiotensin-converting enzyme (ACE) on the surface of vascular endothelial cells to angiotensin II (Ang II) [91]. Angiotensin (Ang) II is a significant effector of the renin-angiotensin-aldosterone system (RAAS) and functions in controlling cardiovascular hemodynamics and structures, serving as a mitogenic factor and a vasoconstrictor [92]. Ang II acts through two main cell surface receptor subtypes, Ang II type 1 and Ang II type 2 receptors (AT1R and AT2R, respectively). The mitogenic function of Ang II is linked to initiating and maintaining the G1-S phase transition in the cell cycle, which is mediated through the AT1R [92,93]. Ang II increases ROS production, inflammation, extracellular matrix remodeling, and vessel tone through AT1R [10]. Ang II also promotes senescence of ECs, associated with age-related vascular impairments in humans and rodents [94,95,96], possibly linked to the presence of ECs with senescence-associated features in human atherosclerotic lesions [26]. Ang II induces EC senescence and inflammation, at least partly via the activation of MAPK [97,98]. Interestingly, AT1R blockers suppress Ang II-induced EC senescence and have a protective effect against the age-related vascular diseases, such as hypertension and atherosclerosis in rodents [99,100]. These results suggest that Ang II promotes EC senescence through AT1R-coupled signaling mechanisms. A study found that expressions of Ang II, AT1R, NOX2, collagen IV, and fibronectin increased, while the expression levels of the G protein-coupled receptor Mas and AT2R decreased in 24-month-old mice [101]. The heptapeptide Ang-(1-7), a key player of the so-called protective branch of the RAAS [102], counteracts the Ang II-induced senescence of cultured human ECs by acting upon Mas and attenuating the increase in SA-β-Gal [103]. Ang-(1-7) further reduces total and telomeric DNA damage, an early event that releases signals downstream to trigger growth arrest and senescence [103]. The anti-senescence action of Ang-(1-7) is further supported by evidence that p53 was markedly attenuated by the co-infusion of Ang-(1-7) in the aortas of C57BL6/J mice infused with IL-1β [104]. Overall, these findings underscore the pleiotropic benefits of the Ang-(1-7)/Mas receptor axis beyond Ang II and the RAAS, and reinforce the potential interest of Ang-(1-7) in suppressing EC senescence [105].
3.4. Insulin-like Growth Factor-Binding Proteins
Insulin-like growth factor (IGF)-binding protein 1 (IGFBP1) is a member of the IGFBP family, which consists of six structurally similar proteins (IGFBP1 through ~6) with strong affinity for IGF. IGFBP1 participates in metabolic homeostasis and cell growth [105,106]. Activation of the IGF1 receptor by IGF1 and IGF2 mediates radiation-induced senescence of human pulmonary artery endothelial cells (HPAECs) [107]; paradoxically, however, IGF1 prevents the onset of senescence by oxidative stress in human aortic endothelial cells (HAECs) [108]. IGFBP3 is upregulated in replicatively senescent ECs in culture [109]. The senescent phenotype of HUVECs was enhanced by IGFBP3 overexpression and was suppressed by IGFBP3 silencing [110]. IGFBP5 induced senescence in HUVECs through a p53-dependent mechanism and is a potential candidate for radiation-induced senescence in HUVECs [111,112,113].
3.5. Nuclear Factor-E2-Related Factor
Nuclear factor-E2-related factor 2 (NRF2) is a transcription factor sensitive to oxidative stress. NRF2 binds to antioxidant response elements (AREs) in the nucleus and enhances the transcription of several antioxidant genes [114]. Activation of NRF2 was found to prevent the induction of cellular senescence [106], and genetic ablation of NRF2 was found to aggravate cell senescence in older cerebral arteries, associated with increased expression of inflammatory cytokines in vessels and hippocampus [115]. Moreover, the effects of cardiovascular risk factors (e.g., obesity) were aggravated, at least partly due to accelerated cellular senescence, in genetically Nrf2-deficient mice [116,117,118]. Previous studies, including studies in non-human primates, reported that decreased NRF2-mediated antioxidant response and suppression of mitochondrial SOD2 induced chronic oxidative stress in EC senescence [119]. Ungvari et al. demonstrated that the carotid arteries of aged Rhesus macaques showed substantial oxidative stress compared to the vessels of younger animals [120]. Old monkeys showed impaired nuclear translocation of NRF2 and reduced expression of target genes NQO1, GCLC, and HMOX1. Moreover, in response to H2O2 and high-glucose conditions, VSMCs from young monkeys showed considerably higher expression of NRF2-regulated genes, while activation of the NRF2 signaling pathway was suppressed in VSMCs of aged monkeys [120]. Similar results were obtained in aged rats, which showed dampened NRF2 activity and expression of NRF2 target genes, and elevated expression of the NF-κB target genes in the vasculature [121]. In sum, senescence impairs NRF2 function, contributing to a vicious cycle that aggravates cellular damage induced by senescence-associated oxidative stress.
3.6. Mammalian Target of Rapamycin
Mammalian target of rapamycin (mTOR), a serine/threonine kinase of the phosphatidylinositol-3-OH kinase (PI3K)-associated family, is a core component of two distinct multi-protein complexes, mTORC1 and mTORC2 [122,123]. In HUVECs, alleviation of mTORC1 signaling with low doses of rapamycin delayed replicative senescence and increased proliferation and tube formation [124]. Similarly, treatment of primary human fibroblasts and fibroblasts that overexpressed the RAS oncogene with rapamycin reversed replicative and oncogene-induced senescence, due to the inhibition of mTORC1 activation. Mechanistically, rapamycin inhibits S6 kinase (S6K) and reduces the phosphorylation of the ribosomal subunit S6 at Ser 235 in senescent cells [125]. In aged mice under caloric restriction (CR), blockade of mTOR signaling enhanced endothelium function; these effects were linked to the CR-mediated reduction in aortic mTOR activation and the protection of age-associated arterial endothelium-dependent dilation (EDD) by increasing NO and preventing the superoxide-dependent suppression of EDD [126,127]. Similarly, treatment of old Wistar Kyoto (WKY) rats with resveratrol or rapamycin also suppressed ribosomal protein S6 kinase 1 (S6K1) activation and enhanced NO generation by endothelial cells [128], while inhibiting the PI3K/AKT/mTORC1 pathway in differentiating mouse embryonic stem cells increased vascular EC elongation triggered by VEGF [129]. In agreement with the fact that mTOR modulates SASP factor production [130,131,132], inhibiting the PI3K/AKT/mTOR pathway in Doxo-treated HUVECs was found to induce a senescence secretory response different from the canonical SASP response [133]. A self-limiting SASP response, mediated through the inhibition of PI3K/AKT/mTOR pathway, suppressed strong senescence-associated inflammation and served to maintain tissue homeostasis [133]. While many studies have explored the function of mTORC1 in aging and longevity, much less is known about the function of mTORC2. Yang et al. recently reported that mTORC2 activity increases during replicative senescence and H2O2-induced premature senescence in HUVECs and found that silencing mTORC2 and inhibiting AKT regulated NRF2 and alleviated HUVEC senescence [134].
3.7. Others Molecules
Many other endothelial senescence-related molecules have been identified. Fibro-blast growth factor 21 (FGF21) prevents high glucose-induced cellular damage and in-hibition of eNOS activity in cultured HUVECs [135]. FGF21 protects Ang II-induced cerebrovascular aging through inhibiting p53 activation and inducing mitochondrial biogenesis in an AMP-activated protein kinase (AMPK)-dependent manner [136]. The adaptor protein P66shc is a signaling molecule involved in the pathways that regulate redox metabolism [137]. In aortic rings from young and old mice, P66shc was implicated in protecting against an age-dependent impairment of EC relaxation in response to acetylcholine [138]. Guidance receptor UNC5B promotes EC senescence, potentially by activating the ROS-p53 pathway [139]. IL-17A is an inflammatory cytokine produced by Th17 cells (a subgroup of helper T cells), which contributes to EC senescence via activation of the NF-κB/p53/RB signaling pathway [140]. Peroxisome proliferator-activated receptor alpha (PPARα) inhibits EC senescence by promoting the expression of the aging-related protein growth differentiation factor 11 (GDF11), the levels of which decline with age in several organs in mice [141]. Deficiency in developmentally regulated GTP-binding protein 2 (DRG2) increases NOX2 expression, ROS generation, and senescence in ECs [142]. C1q/tumor necrosis factor-related protein 9 (CTRP9), an emerging potential cardiokine, contributes to the inhibition of hyperglycemia-induced endothelial senescence through an AMPKα/ Krüppel-like factor 4 (KLF4)-dependent signaling pathway [143]. Soluble dipeptidyl peptidase 4 (DPP4), secreted from visceral adipose tissue, induces EC senescence through the protease-activated receptor 2 (PAR2)–cyclooxygenase2 (COX-2)–thromboxane receptor (TP) axis and activates the NLRP3 inflammasome [144]. In cultured HAECs, senescence induced by apoC3-rich low-density lipoprotein (AC3RL) is mediated by intracellular ROS formation, H2A.X variant histone (H2AX) deposition, and F-box only protein 31 (FBXO31) activation, resulting in the inhibition of mouse double minute 2 homolog (MDM2), p53, and p21 activation [145]. Resolvin E1 (RvE1) reduces EC senescence induced by both Doxo and IL-1β through preventing the increase in levels of pP65, NLRP3, and pro-IL-1β and the formation of active NLRP3 inflammasome complexes [146]. While we have highlighted the numerous molecular players and pathways involved in EC senescence, we expect that many more will be discovered as we continue to dissect the mechanisms that foment senescence in vascular diseases.
4. Conclusions and Perspectives
EC senescence is induced in many different ways, including telomere shortening and damage to DNA and other cell components [147]. Increasing evidence shows an important role for EC senescence in vascular aging and age-related vascular disease. To develop therapeutic targets to treat or prevent vascular aging and age-related vascular disease, we must continue to elucidate the molecular mechanisms that promote EC senescence in cell and animal models. SIRT1 and SIRT6 prevent EC senescence by blocking oxidative stress-induced signaling. Interestingly, while Klotho and NRF2 prevent EC senescence, other factors, such as angiotensin, IGFBP3, IGFBP5, and mTOR, promote EC senescence. A comprehensive identification of druggable targets in this paradigm will broaden the therapeutic opportunities to attenuate EC senescence and improve vascular disease outcomes. In Ang II-induced senescence, olmesartan, a pharmacological inhibitor of Ang II, suppressed Ang II-induced vascular inflammation and premature senescence [95]. The identification of novel markers and improved methods to detect senescent ECs in vivo systems are necessary to accelerate progress towards finding more efficient ways to discover senescent ECs in various tissues and organs. Further studies focused on the molecular mechanisms and signaling pathways of EC senescence will accelerate the development of strategies to prevent age-related vascular disease.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants to JSL from RCIC (2021R1A5A2031612) and the Basic Research Science Program (2020R1A2B5B02002375) through the National Research Foundation (NRF), funded by the Korean government (MSIT).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muñoz-Espín D., Serrano M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 2.Chicas A., Wang X., Zhang C., McCurrach M., Zhao Z., Mert O., Dickins R.A., Narita M., Zhang M., Lowe S.W. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz-Espín D., Cañamero M., Maraver A., Gómez-López G., Contreras J., Murillo-Cuesta S., Rodríguez-Baeza A., Varela-Nieto I., Ruberte J., Collado M., et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.M., Vijg J., Van Steeg H., Dollé M.E., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng T., Meng J., Kou S., Jiang Z., Huang X., Lu Z., Zhao H., Lau L.F., Zhou B., Zhang H. CCN1-Induced Cellular Senescence Promotes Heart Regeneration. Circulation. 2019;139:2495–2498. doi: 10.1161/CIRCULATIONAHA.119.039530. [DOI] [PubMed] [Google Scholar]
- 6.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Jia G., Aroor A.R., DeMarco V.G., Martinez-Lemus L.A., Meininger G.A., Sowers J.R. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 2015;6:231. doi: 10.3389/fphys.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian X.L., Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41:485–495. doi: 10.1016/j.jgg.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Xinghui S., Mark W.F. Vascular Endothelial Senescence: Pathobiological Insights, Emerging Long Noncoding RNA Targets, Challenges and Therapeutic Opportunities. Front Physiol. 2021;12:693067. doi: 10.3389/fphys.2021.693067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G., Aroor A.R., Jia C., Sowers J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019;1865:1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Higashi Y., Kihara Y., Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35:1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Wang X., Liu T., Zhu X., Pan X. The multifaceted role of the SASP in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 2022;12:74. doi: 10.1186/s13578-022-00815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown W.R., Thore C.R. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonithon-Kopp C., Touboul P.J., Berr C., Leroux C., Mainard F., Courbon D., Ducimetière P. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler. Thromb. Vasc. Biol. 1996;16:310–316. doi: 10.1161/01.ATV.16.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Behnke B.J., Prisby R.D., Lesniewski L.A., Donato A.J., Olin H.M., Delp M.D. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J. Physiol. 2006;575:617–626. doi: 10.1113/jphysiol.2006.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.A., Lee K.H., Won H.Y., Park S., Chung J.H., Jang Y., Ha J.W. Quantitative assessment of aortic elasticity with aging using velocity-vector imaging and its histologic correlation. Arterioscler. Thromb. Vasc. Biol. 2013;33:1306–1312. doi: 10.1161/ATVBAHA.113.301312. [DOI] [PubMed] [Google Scholar]
- 17.Wang J.C., Bennett M. Aging and atherosclerosis: Mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 18.Erusalimsky J.D., Kurz D.J. Endothelial cell senescence. Handb. Exp. Pharmacol. 2006;176:213–248. doi: 10.1007/3-540-36028-x_7. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Goligorsky M.S. Premature senescence of endothelial cells: Methusaleh’s dilemma. Am. J. Physiol. Heart Circ. Physiol. 2006;290:1729–1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kurz D.J., Hong Y., Trivier E., Huang H.L., Decary S., Zang G.H., Lüscher T.F., Erusalimsky J.D. Fibroblast growth factor-2, but not vascular endothelial growth factor, upregulates telomerase activity in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:748–754. doi: 10.1161/01.ATV.0000069624.55424.61. [DOI] [PubMed] [Google Scholar]
- 21.Cho K.A., Ryu S.J., Oh Y.S., Park J.H., Lee J.W., Kim H.P., Kim K.T., Jang I.S., Park S.C. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004;279:42270–42278. doi: 10.1074/jbc.M402352200. [DOI] [PubMed] [Google Scholar]
- 22.Coleman P.R., Hahn C.N., Grimshaw M., Lu Y., Li X., Brautigan P.J., Beck K., Stocker R., Vadas M.A., Gamble J.R. Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood. 2010;116:4016–4024. doi: 10.1182/blood-2009-11-252700. [DOI] [PubMed] [Google Scholar]
- 23.Yin H., Pickering J.G. Cellular Senescence and Vascular Disease: Novel Routes to Better Understanding and Therapy. Can. J. Cardiol. 2016;32:612–623. doi: 10.1016/j.cjca.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Shelton D.N., Chang E., Whittier P.S., Choi D., Funk W.D. Microarray analysis of replicative senescence. Curr. Biol. 1999;9:939–945. doi: 10.1016/S0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 25.Kumazaki T., Mitsui Y. Alterations in transcription factor-binding activities to fibronectin promoter during aging of vascular endothelial cells. Mech. Ageing Dev. 1996;88:111–124. doi: 10.1016/0047-6374(96)01712-5. [DOI] [PubMed] [Google Scholar]
- 26.Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 27.Brandes R.P., Fleming I., Busse R. Endothelial aging. Cardiovasc. Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampel B., Fortschegger K., Ressler S., Chang M.W., Unterluggauer H., Breitwieser A., Sommergruber W., Fitzky B., Lepperdinger G., Jansen-Dürr P., et al. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp. Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Lin J.R., Shen W.L., Yan C., Gao P.J. Downregulation of dynamin-related protein 1 contributes to impaired autophagic flux and angiogenic function in senescent endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2015;35:1413–1422. doi: 10.1161/ATVBAHA.115.305706. [DOI] [PubMed] [Google Scholar]
- 31.Barinda A.J., Ikeda K., Nugroho D.B., Wardhana D.A., Sasaki N., Honda S., Urata R., Matoba S., Hirata K.I., Emoto N. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 2020;11:481. doi: 10.1038/s41467-020-14387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominic A., Banerjee P., Hamilton D.J., Le N.T., Abe J.I. Time-dependent replicative senescence vs. disturbed flow-induced pre-mature aging in atherosclerosis. Redox Biol. 2020;37:101614. doi: 10.1016/j.redox.2020.101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan S.Y., Awad E.M., Oszwald A., Mayr M., Yin X., Waltenberger B., Stuppner H., Lipovac M., Uhrin P., Breuss J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017;7:39501. doi: 10.1038/srep39501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang H.J., Lee Y.R., Kang D., Lee H.C., Seo H.R., Ryu J.K., Kim Y.N., Ko Y.G., Park H.J., Lee J.S. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 2020;490:100–110. doi: 10.1016/j.canlet.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Mai S., Klinkenberg M., Auburger G., Bereiter-Hahn J., Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y.M., Youn S.W., Sudhahar V., Das A., Chandhri R., Cuervo Grajal H., Kweon J., Leanhart S., He L., Toth P.T., et al. Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep. 2018;23:3565–3578. doi: 10.1016/j.celrep.2018.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeedi Saravi S.S., Eroglu E., Waldeck-Weiermair M., Sorrentino A., Steinhorn B., Belousov V., Michel T. Differential endothelial signaling responses elicited by chemogenetic H2O2 synthesis. Redox Biol. 2020;36:101605. doi: 10.1016/j.redox.2020.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia G., Habibi J., Bostick B.P., Ma L., DeMarco V.G., Aroor A.R., Hayden M.R., Whaley-Connell A.T., Sowers J.R. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65:531–539. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldosari S., Awad M., Harrington E.O., Sellke F.W., Abid M.R. Subcellular reactive oxygen species (ROS) in cardiovascular pathophysiology. Antioxidants. 2018;7:14. doi: 10.3390/antiox7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C. NADPH Oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schröder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 42.Shafique E., Choy W.C., Liu Y., Feng J., Arthur Lyra B.C., Arafah M. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging. 2013;5:515–530. doi: 10.18632/aging.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y.M., Kim S.J., Tatsunami R., Yamamura H., Fukai T., Fukai M.U. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017;312:C749–C764. doi: 10.1152/ajpcell.00346.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia G., Habibi J., Aroor A.R., Hill M.A., DeMarco V.G., Lee L.E., Ma L., Barron B.J., Whaley-Connell A., Sowers J.R. Enhanced endothelium epithelial sodium channel signaling prompts left ventricular diastolic dysfunction in obese female mice. Metabolism. 2018;78:69–79. doi: 10.1016/j.metabol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Chatterjee S., Ganini D., Tokar E.J., Kumar A., Das S., Corbett J., Kadiiska M.B., Waalkes M.P., Diehl A.M., Mason R.P. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haendeler J., Hoffmann J., Diehl J.F., Vasa M., Spyridopoulos I., Zeiher A.M., Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 47.Kurz D.J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell. Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 48.Unterluggauer H., Mazurek S., Lener B., Hütter E., Eigenbrodt E., Zwerschke W., Jansen-Dürr P. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology. 2008;9:247–259. doi: 10.1007/s10522-008-9134-x. [DOI] [PubMed] [Google Scholar]
- 49.Kuosmanen S.M., Sihvola V., Kansanen E., Kaikkonen M.U., Levonen A.L. MicroRNAs mediate the senescence-associated decline of NRF2 in endothelial cells. Redox Biol. 2018;18:77–83. doi: 10.1016/j.redox.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng X., Shihabudeen Haider Ali M.S., Moran M., Viana M.P., Schlichte S.L., Zimmerman M.C., Khalimonchuk O., Feinberg M.W., Sun X. Long non-coding RNA Meg3 deficiency impairs glucose homeostasis and insulin signaling by inducing cellular senescence of hepatic endothelium in obesity. Redox Biol. 2021;40:101863. doi: 10.1016/j.redox.2021.101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johmura Y., Yamanaka T., Omori S., Wang T.W., Sugiura Y., Matsumoto M., Suzuki N., Kumamoto S., Yamaguchi K., Hatakeyama S., et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371:265–270. doi: 10.1126/science.abb5916. [DOI] [PubMed] [Google Scholar]
- 52.Haspula D., Vallejos A.K., Moore T.M., Tomar N., Dash R.K., Hoffmann B.R. Influence of a Hyperglycemic Microenvironment on a Diabetic Versus Healthy Rat Vascular Endothelium Reveals Distinguishable Mechanistic and Phenotypic Responses. Front. Physiol. 2019;10:558. doi: 10.3389/fphys.2019.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liemburg-Apers D.C., Willems P.H., Koopman W.J., Grefte S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015;89:1209–1226. doi: 10.1007/s00204-015-1520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenton M., Barker S., Kurz D.J., Erusalimsky J.D. Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler. Thromb. Vasc. Biol. 2001;22:220–226. doi: 10.1161/01.ATV.21.2.220. [DOI] [PubMed] [Google Scholar]
- 55.Chen J., Brodsky S.V., Goligorsky D.M., Hampel D.J., Li H., Gross S.S., Goligorsky M.S. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ. Res. 2002;90:1290–1298. doi: 10.1161/01.RES.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- 56.Brodsky S.V., Gealekman O., Chen J., Zhang F., Togashi N., Crabtree M., Gross S.S., Nasjletti A., Goligorsky M.S. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ. Res. 2004;94:377–384. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- 57.Kiss T., Nyúl-Tóth Á., Balasubramanian P., Tarantini S., Ahire C., DelFavero J., Yabluchanskiy A., Csipo T., Farkas E., Wiley G., et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–444. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasile E., Tomita Y., Brown L.F., Kocher O., Dvorak H.F. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: Evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 59.Villaret A., Galitzky J., Decaunes P., Estève D., Marques M.A., Sengenès C., Chiotasso P., Tchkonia T., Lafontan M., Kirkland J.L., et al. Adipose tissue endothelial cells from obese human subjects: Differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arunachalam G., Yao H., Sundar I.K., Caito S., Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlomosti F., D’Agostino M., Beji S., Torcinaro A., Rizzi R., Zaccagnini G., Maimone B., Di Stefano V., De Santa F., Cordisco S., et al. Oxidative Stress-Induced miR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017;27:328–344. doi: 10.1089/ars.2016.6643. [DOI] [PubMed] [Google Scholar]
- 62.Menghini R., Casagrande V., Cardellini M., Martelli E., Terrinoni A., Amati F., Vasa-Nicotera M., Ippoliti A., Novelli G., Melino G., et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 63.Ota H., Eto M., Kano M.R., Ogawa S., Iijima K., Akishita M., Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- 64.Pan W., Yu H., Huang S., Zhu P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS ONE. 2016;11:e0147034. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshizaki T., Schenk S., Imamura T., Babendure J.L., Sonoda N., Bae E.J., Oh D.Y., Lu M., Milne J.C., Westphal C., et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:419–428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Onofrio N., Vitiello M., Casale R., Servillo L., Giovane A., Balestrieri M.L. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim. Biophys. Acta. 2015;1852:1311–1322. doi: 10.1016/j.bbadis.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Paschalaki K.E., Starke R.D., Hu Y., Mercado N., Margariti A., Gorgoulis V.G., Randi A.M., Barnes P.J. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan Y.Z., Gao P., Zhou S., Zhang Z.Q., Hao D.L., Lian L.S., Li Y.J., Chen H.Z., Liu D.P. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell. 2014;13:890–899. doi: 10.1111/acel.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou S., Chen H.Z., Wan Y.Z., Zhang Q.J., Wei Y.S., Huang S., Liu J.J., Lu Y.B., Zhang Z.Q., Yang R.F., et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 71.Kong D., Zhan Y., Liu Z., Ding T., Li M., Yu H., Zhang L., Li H., Luo A., Zhang D., et al. SIRT1-mediated ERβ suppression in the endothelium contributes to vascular aging. Aging Cell. 2016;15:1092–1102. doi: 10.1111/acel.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasko R., Xavier S., Chen J., Lin C.H., Ratliff B., Rabadi M., Maizel J., Tanokuchi R., Zhang F., Cao J., et al. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: Relevance to fibrosis of vascular senescence. J. Am. Soc. Nephrol. 2014;25:276–291. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai B., Man A.W., Yang K., Guo Y., Xu C., Tse H.F., Han W., Bloksgaard M., De Mey J.G., Vanhoutte P.M., et al. Endothelial SIRT1 prevents adverse arterial remodeling by facilitating HERC2-mediated degradation of acetylated LKB1. Oncotarget. 2016;7:39065–39081. doi: 10.18632/oncotarget.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maksin-Matveev A., Kanfi Y., Hochhauser E., Isak A., Cohen H.Y., Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp. Cell Res. 2015;330:81–90. doi: 10.1016/j.yexcr.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Liu R., Liu H., Ha Y., Tilton R.G., Zhang W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed. Res. Int. 2014;2014:902842. doi: 10.1155/2014/902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang N., Li Z., Mu W., Li L., Liang Y., Lu M., Wang Z., Qiu Y., Wang Z. Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-κB signaling. Cell Cycle. 2016;15:1009–1018. doi: 10.1080/15384101.2016.1152427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cardus A., Uryga A.K., Walters G., Erusalimsky J.D. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc. Res. 2013;97:571–579. doi: 10.1093/cvr/cvs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balestrieri M.L., Rizzo M.R., Barbieri M., Paolisso P., D’Onofrio N., Giovane A., Siniscalchi M., Minicucci F., Sardu C., D’Andrea D., et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: Effects of incretin treatment. Diabetes. 2015;64:1395–1406. doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 79.Sundaresan N.R., Vasudevan P., Zhong L., Kim G., Samant S., Parekh V., Pillai V.B., Ravindra P.V., Gupta M., Jeevanandam V., et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao R., Xiong X., DePinho R.A., Deng C.X., Dong X.C. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J. Biol. Chem. 2013;288:29252–29259. doi: 10.1074/jbc.M113.481473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 82.Saito Y., Yamagishi T., Nakamura T., Ohyama Y., Aizawa H., Suga T., Matsumura Y., Masuda H., Kurabayashi M., Kuro-o M., et al. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 83.Saito Y., Nakamura T., Ohyama Y., Suzuki T., Iida A., Shiraki-Iida T., Kuro-o M., Nabeshima Y., Kurabayashi M., Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem. Biophys. Res. Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 84.Ikushima M., Rakugi H., Ishikawa K., Maekawa Y., Yamamoto K., Ohta J., Chihara Y., Kida I., Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto M., Clark J.D., Pastor J.V., Gurnani P., Nandi A., Kurosu H., Miyoshi M., Ogawa Y., Castrillon D.H., Rosenblatt K.P., et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., McGuinness O.P., Chikuda H., Yamaguchi M., Kawaguchi H., et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Semba R.D., Cappola A.R., Sun K., Bandinelli S., Dalal M., Crasto C., Guralnik J.M., Ferrucci L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navarro-González J.F., Donate-Correa J., Muros de Fuentes M., Pérez-Hernández H., Martínez-Sanz R., Mora-Fernández C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40. doi: 10.1136/heartjnl-2013-304746. [DOI] [PubMed] [Google Scholar]
- 89.Kitagawa M., Sugiyama H., Morinaga H., Inoue T., Takiue K., Ogawa A., Yamanari T., Kikumoto Y., Uchida H.A., Kitamura S., et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindberg K., Olauson H., Amin R., Ponnusamy A., Goetz R., Taylor R.F., Mohammadi M., Canfield A., Kublickiene K., Larsson T.E. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS ONE. 2013;8:e60658. doi: 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saravi B., Li Z., Lang C.N., Schmid B., Lang F.K., Grad S., Alini M., Richards R.G., Schmal H., Südkamp N., et al. The Tissue Renin-Angiotensin System and Its Role in the Pathogenesis of Major Human Diseases: Quo Vadis? Cells. 2021;10:650. doi: 10.3390/cells10030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carey R.M., Siragy H.M. Newly recognized components of the renin-angiotensin system: Potential roles in cardiovascular and renal regulation. Endocr. Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 93.de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 94.Herbert K.E., Mistry Y., Hastings R., Poolman T., Niklason L., Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ. Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kunieda T., Minamino T., Nishi J., Tateno K., Oyama T., Katsuno T., Miyauchi H., Orimo M., Okada S., Takamura M., et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 96.Min L.J., Mogi M., Iwanami J., Li J.M., Sakata A., Fujita T., Tsukuda K., Iwai M., Horiuchi M. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc. Res. 2007;76:506–516. doi: 10.1016/j.cardiores.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Shan H., Bai X., Chen X. Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem. Funct. 2008;26:459–466. doi: 10.1002/cbf.1467. [DOI] [PubMed] [Google Scholar]
- 98.Li R., Mi X., Yang S., Yang Y., Zhang S., Hui R., Chen Y., Zhang W. Long-term stimulation of angiotensin II induced endothelial senescence and dysfunction. Exp. Gerontol. 2019;119:212–220. doi: 10.1016/j.exger.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 99.Basso N., Paglia N., Stella I., de Cavanagh E.M., Ferder L., del Rosario Lores Arnaiz M., Inserra F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul. Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 100.de Cavanagh E.M., Piotrkowski B., Fraga C.G. Concerted action of the renin-angiotensin system, mitochondria, and antioxidant defenses in aging. Mol. Aspects. Med. 2004;25:27–36. doi: 10.1016/j.mam.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Yoon H.E., Kim E.N., Kim M.Y., Lim J.H., Jang I.A., Ban T.H., Shin S.J., Park C.W., Chang Y.S., Choi B.S. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid. Med. Cell Longev. 2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valencia, Shamoon L., Romero A., De la Cuesta F., Sánchez-Ferrer C.F., Peiró C. Angiotensin-(1-7), a protective peptide against vascular aging. Peptides. 2022;152:170775. doi: 10.1016/j.peptides.2022.170775. [DOI] [PubMed] [Google Scholar]
- 103.Romero A., San Hipólito-Luengo Á., Villalobos L.A., Vallejo S., Valencia I., Michalska P., Pajuelo-Lozano N., Sánchez-Pérez I., León R., Bartha J.L., et al. The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell. 2019;18:e12913. doi: 10.1111/acel.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romero A., Dongil P., Valencia I., Vallejo S., Hipólito-Luengo Á.S., Díaz-Araya G., Bartha J.L., González-Arlanzón M.M., Rivilla F., de la Cuesta F., et al. Pharmacological Blockade of NLRP3 Inflammasome/IL-1β-Positive Loop Mitigates Endothelial Cell Senescence and Dysfunction. Aging. Dis. 2022;13:284–297. doi: 10.14336/AD.2021.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Kaay D., Deal C., de Kort S., Willemsen R., Leunissen R., Ester W., Paquette J., van Doorn J., Hokken-Koelega A. Insulin-like growth factor-binding protein-1: Serum levels, promoter polymorphism, and associations with components of the metabolic syndrome in short subjects born small for gestational age. J. Clin. Endocrinol. Metab. 2009;94:1386–1392. doi: 10.1210/jc.2008-1430. [DOI] [PubMed] [Google Scholar]
- 106.Hu D., Pawlikowska L., Kanaya A., Hsueh W.C., Colbert L., Newman A.B., Satterfield S., Rosen C., Cummings S.R., Harris T.B., et al. Health, Aging, and Body Composition Study. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: The Health, Aging, and Body Composition Study. J. Am. Geriatr. Soc. 2009;57:1213–1218. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panganiban R.A., Day R.M. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PLoS ONE. 2013;8:e78589. doi: 10.1371/journal.pone.0078589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Higashi Y., Pandey A., Goodwin B., Delafontaine P. Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1. Biochim. Biophys. Acta. 2013;1832:391–399. doi: 10.1016/j.bbadis.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grillari J., Hohenwarter O., Grabherr R.M., Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 2000;35:187–197. doi: 10.1016/S0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 110.Kim K.S., Kim M.S., Seu Y.B., Chung H.Y., Kim J.H., Kim J.R. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 111.Kim K.S., Seu Y.B., Baek S.H., Kim M.J., Kim K.J., Kim J.H., Kim J.R. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol. Biol. Cell. 2007;18:4543–4552. doi: 10.1091/mbc.e07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim K.S., Kim J.E., Choi K.J., Bae S., Kim D.H. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int. J. Radiat. Biol. 2014;90:71–80. doi: 10.3109/09553002.2014.859763. [DOI] [PubMed] [Google Scholar]
- 113.Rombouts C., Aerts A., Quintens R., Baselet B., El-Saghire H., Harms-Ringdahl M., Haghdoost S., Janssen A., Michaux A., Yentrapalli R., et al. Transcriptomic profiling suggests a role for IGFBP5 in premature senescence of endothelial cells after chronic low dose rate irradiation. Int. J. Radiat. Biol. 2014;90:560–574. doi: 10.3109/09553002.2014.905724. [DOI] [PubMed] [Google Scholar]
- 114.Niture S.K., Khatri R., Jaiswal A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fulop G.A., Kiss T., Tarantini S., Balasubramanian P., Yabluchanskiy A., Farkas E., Bari F., Ungvari Z., Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tarantini S., Valcarcel-Ares M.N., Yabluchanskiy A., Tucsek Z., Hertelendy P., Kiss T., Gautam T., Zhang X.A., Sonntag W.E., de Cabo R., et al. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ungvari Z., Bagi Z., Feher A., Recchia F.A., Sonntag W.E., Pearson K., de Cabo R., Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010;299:18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ungvari Z., Bailey-Downs L., Gautam T., Jimenez R., Losonczy G., Zhang C., Ballabh P., Recchia F.A., Wilkerson D.C., Sonntag W.E., et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am. J. Physiol. Heart Circ. Physiol. 2011;300:1133–1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El Assar M., Angulo J., Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 120.Ungvari Z., Bailey-Downs L., Gautam T., Sosnowska D., Wang M., Monticone R.E., Telljohann R., Pinto J.T., de Cabo R., Sonntag W.E., et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ungvari Z., Bailey-Downs L., Sosnowska D., Gautam T., Koncz P., Losonczy G., Ballabh P., de Cabo R., Sonntag W.E., Csiszar A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 2011;301:363–372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heitman J., Movva N.R., Hall M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 123.Lipton J.O., Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khor E.S., Wong P.F. Endothelial replicative senescence delayed by the inhibition of MTORC1 signaling involves MicroRNA-107. Int. J. Biochem. Cell Biol. 2018;101:64–73. doi: 10.1016/j.biocel.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 125.Kolesnichenko M., Hong L., Liao R., Vogt P.K., Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donato A.J., Walker A.E., Magerko K.A., Bramwell R.C., Black A.D., Henson G.D., Lawson B.R., Lesniewski L.A., Seals D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Donato A.J., Morgan R.G., Walker A.E., Lesniewski L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rajapakse A.G., Yepuri G., Carvas J.M., Stein S., Matter C.M., Scerri I., Ruffieux J., Montani J.P., Ming X.F., Yang Z. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: Inhibition by resveratrol. PLoS ONE. 2011;6:e19237. doi: 10.1371/journal.pone.0019237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuji-Tamura K., Ogawa M. Inhibition of the PI3K-Akt and mTORC1 signaling pathways promotes the elongation of vascular endothelial cells. J. Cell Sci. 2016;129:1165–1178. doi: 10.1242/jcs.178434. [DOI] [PubMed] [Google Scholar]
- 130.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta J.C., Innes A.J., Banito A., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laberge R.M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., Curran S.C., Davalos A.R., Wilson-Edell K.A., Liu S., et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Narita M., Young A.R., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., Hong S., Berry L.S., Reichelt S., Ferreira M., et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bent E.H., Gilbert L.A., Hemann M.T. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016;30:1811–3021. doi: 10.1101/gad.284851.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang H.W., Hong H.L., Luo W.W., Dai C.M., Chen X.Y., Wang L.P., Li Q., Li Z.Q., Liu P.Q., Li Z.M. mTORC2 facilitates endothelial cell senescence by suppressing Nrf2 expression via the Akt/GSK-3β/C/EBPα signaling pathway. Acta Pharmacol. Sin. 2018;39:1837–1846. doi: 10.1038/s41401-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X.M., Song S.S., Xiao H., Gao P., Li X.J., Si L.Y. Fibroblast growth factor 21 protects against high glucose induced cellular damage and dysfunction of endothelial nitric-oxide synthase in endothelial cells. Cell Physiol. Biochem. 2014;34:658–671. doi: 10.1159/000363031. [DOI] [PubMed] [Google Scholar]
- 136.Wang X.M., Xiao H., Liu L.L., Cheng D., Li X.J., Si L.Y. FGF21 represses cerebrovascular aging via improving mitochondrial biogenesis and inhibiting p53 signaling pathway in an AMPK-dependent manner. Exp. Cell Res. 2016;346:147–156. doi: 10.1016/j.yexcr.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 138.Francia P., delli Gatti C., Bachschmid M., Martin-Padura I., Savoia C., Migliaccio E., Pelicci P.G., Schiavoni M., Lüscher T.F., Volpe M., et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 139.Yang Z., Li H., Luo P., Yan D., Yang N., Zhang Y., Huang Y., Liu Y., Zhang L., Yan J., et al. UNC5B Promotes Vascular Endothelial Cell Senescence via the ROS-Mediated P53 Pathway. Oxid. Med. Cell. Longev. 2021;2021:5546711. doi: 10.1155/2021/5546711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang L., Liu M., Liu W., Hu C., Li H., Deng J., Cao Q., Wang Y., Hu W., Li Q. Th17/IL-17 induces endothelial cell senescence via activation of NF-κB/p53/Rb signaling pathway. Lab. Invest. 2021;101:1418–1426. doi: 10.1038/s41374-021-00629-y. [DOI] [PubMed] [Google Scholar]
- 141.Dou F., Wu B., Chen J., Liu T., Yu Z., Chen C. PPARα Targeting GDF11 Inhibits Vascular Endothelial Cell Senescence in an Atherosclerosis Model. Oxid. Med. Cell. Longev. 2021;2021:2045259. doi: 10.1155/2021/2045259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Le A.N., Park S.S., Le M.X., Lee U.H., Ko B.K., Lim H.R., Yu R., Choi S.H., Lee B.J., Ham S.Y., et al. DRG2 Depletion Promotes Endothelial Cell Senescence and Vascular Endothelial Dysfunction. Int. J. Mol. Sci. 2022;23:2877. doi: 10.3390/ijms23052877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang G., Han B., Zhang R., Liu Q., Wang X., Huang X., Liu D., Qiao W., Yang M., Luo X., et al. C1q/TNF-Related Protein 9 Attenuates Atherosclerosis by Inhibiting Hyperglycemia-Induced Endothelial Cell Senescence Through the AMPKα/KLF4 Signaling Pathway. Front. Pharmacol. 2021;12:758792. doi: 10.3389/fphar.2021.758792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Valencia I., Vallejo S., Dongil P., Romero A., San Hipólito-Luengo Á., Shamoon L., Posada M., García-Olmo D., Carraro R., Erusalimsky J.D., et al. DPP4 Promotes Human Endothelial Cell Senescence and Dysfunction via the PAR2-COX-2-TP Axis and NLRP3 Inflammasome Activation. Hypertension. 2022;79:1361–1373. doi: 10.1161/HYPERTENSIONAHA.121.18477. [DOI] [PubMed] [Google Scholar]
- 145.Tsai P.H., Chen L.Z., Tseng K.F., Chen F.Y., Shen M.Y. Apolipoprotein C3-Rich Low-Density Lipoprotein Induces Endothelial Cell Senescence via FBXO31 and Its Inhibition by Sesamol In Vitro and In Vivo. Biomedicines. 2022;10:854. doi: 10.3390/biomedicines10040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shamoon L., Espitia-Corredor J.A., Dongil P., Menéndez-Ribes M., Romero A., Valencia I., Díaz-Araya G., Sánchez-Ferrer C.F., Peiró C. Resolvin E1 attenuates doxorubicin-induced endothelial senescence by modulating NLRP3 inflammasome activation. Biochem. Pharmacol. 2022;201:115078. doi: 10.1016/j.bcp.2022.115078. [DOI] [PubMed] [Google Scholar]
- 147.Foreman K.E., Tang J. Molecular mechanisms of replicative senescence in endothelial cells. Exp. Gerontol. 2003;38:1251–1257. doi: 10.1016/j.exger.2003.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.