Abstract
Polyploid cells demonstrate biological plasticity and stress adaptation in evolution; development; and pathologies, including cardiovascular diseases, neurodegeneration, and cancer. The nature of ploidy-related advantages is still not completely understood. Here, we summarize the literature on molecular mechanisms underlying ploidy-related adaptive features. Polyploidy can regulate gene expression via chromatin opening, reawakening ancient evolutionary programs of embryonality. Chromatin opening switches on genes with bivalent chromatin domains that promote adaptation via rapid induction in response to signals of stress or morphogenesis. Therefore, stress-associated polyploidy can activate Myc proto-oncogenes, which further promote chromatin opening. Moreover, Myc proto-oncogenes can trigger polyploidization de novo and accelerate genome accumulation in already polyploid cells. As a result of these cooperative effects, polyploidy can increase the ability of cells to search for adaptive states of cellular programs through gene regulatory network rewiring. This ability is manifested in epigenetic plasticity associated with traits of stemness, unicellularity, flexible energy metabolism, and a complex system of DNA damage protection, combining primitive error-prone unicellular repair pathways, advanced error-free multicellular repair pathways, and DNA damage-buffering ability. These three features can be considered important components of the increased adaptability of polyploid cells. The evidence presented here contribute to the understanding of the nature of stress resistance associated with ploidy and may be useful in the development of new methods for the prevention and treatment of cardiovascular and oncological diseases.
Keywords: polyploidy, epigenetic regulation, Myc, chromatin opening, adaptation to stress, gene regulatory network, cancer, cardiovascular diseases, neurodegeneration, hypertranscription
1. Introduction
Somatic polyploidy exists in tissues of nearly all multicellular organisms, including higher and lower plants, invertebrates, and vertebrates [1,2,3,4,5]. In humans and mammals, polyploidy may be a part of normal developmental programs and may result from stress caused by a variety of pathological conditions. In the normal mammalian development, cell polyploidization accompanies the early postnatal organogenesis of the neocortex, neuroglia, heart, retina, blood vessels, blood, liver, skin, placenta, kidneys, and other organs [4,6,7,8,9]. In humans, polyploidy develops mostly in the heart, where almost every normal cardiomyocyte contains 4–16 genomes [10]. Pathological conditions that enhance the accumulation of genomes in cells include neurodegenerative disorders, cardiovascular diseases, diabetes, wound healing, etc. [8,11,12,13,14,15,16,17,18,19,20]. Recent studied have indicated that polyploidy promotes cancer initiation, progression, metastasis, and drug resistance [21,22,23,24]. The main features of polyploidy include the association with stress and the ability to enhance stress adaptation under normal and pathological conditions [25,26]. The data confirming the relationships between polyploidy and stress under normal conditions only recently appeared, significantly contributing to the understanding of the biological role of genome duplications. It has become clear that in the development of mammals, the accumulation of genomes in somatic cells coincides with critical periods of postnatal growth when cells combine proliferation and differentiation and are subjected to stress associated with global physiological rearrangements [11,12,14,27,28]. For example, developmental cardiomyocyte polyploidization coincides with metabolic rearrangements accompanied by the transition to the microenvironment, with high oxygen content associated with DNA damage response, oxidative stress, and a decrease in expression of nuclear lamina filament lamin B2 (Lmnb2), which regulates nuclear breakdown prior to cell division [29,30]. Accordingly, in macrophages, polyploidization induced by inflammation is triggered by replication stress and DNA damage response [31]. The data obtained by single-cell sequencing of human tetraploid cell lines provide clear evidence that these cells undergo high rates of DNA damage during DNA replication in the first S phase following induction of tetraploidy [32]. In pathological conditions, polyploidy is associated with and aids in survival when faced with different types of stress. Thus, in cardiovascular diseases, including tetralogy of Fallot, cardiomyopathy, hypertension, and ischemic heart disease, polyploidy helps to cope with hypoxia and mechanical tension [10,33,34,35,36,37,38]. In neurodegeneration, polyploidy promotes adaptation to oxidative damage, ischemia, and inflammation [8]. In wound healing and myocardial infarction, genome accumulation stimulates adaptation to DNA damage, mechanical stress, and inflammation [9,34]. Moreover, in polyploid giant cancer cells, multiple genomes confer resistance to chemo- and radiotherapy [23,39,40,41,42,43,44,45,46,47]. These data are in agreement with previous observations of plant polyploid cells, indicating that multiple genomes can live under conditions under which diploid cells do not survive [25].
Notwithstanding the benefits under stress, polyploid cells cannot outcompete their diploid counterparts under normal conditions because of their slower proliferation, DNA instability, high energetic cost, and mitotic defects [17,25,48,49]. The ability of various types of polyploid cells to survive under extremely stressful conditions despite multiple detrimental effects suggests that genome accumulation creates new phenotypic features via specific pathways of gene regulation. Polyploidy does not alter gene dosage balance. Therefore, it cannot exert strong effects on the expression of separate genes. Furthermore, a tight association with stress suggests that polyploidy can operate via epigenetic changes.
Ploidy-associated epigenetic regulation has attracted increasing attention because it enables genome reorganization and cell fate change in adverse environments and in response to extreme stress [39,41,42,50]. Moreover, this mode of regulation operating via ploidy-related epigenetic rearrangements is characteristic of carcinogenesis and resistance to therapy [42,43]. In this review, we extensively analyze the literature, which indicates that polyploidy provokes global genome reorganization via chromatin relaxation induced by stress-related Myc overexpression and ploidy-related architectural rearrangements. The relaxation is observed at both high and low levels of chromatin organization. We also provide evidence that polyploidy activates global transcription amplifiers belonging to Myc-family oncogenes, which further promote chromatin opening.
2. Stress-Induced Myc Promotes Polyploidy and Vice Versa
Oncogenes of the Myc family (c-Myc and N-myc) are the most powerful and well-studied stress-response-related amplifiers of global transcription [51,52,53]. Recent studies indicate that, on the one hand, polyploidy is associated with Myc upregulation and, on the other hand, that Myc can promote polyploidy. Many features associated with polyploidy are also manifestations of activated Myc. Below, we outline the literature providing evidence that polyploidy can upregulate Myc and vice versa and that overexpressed Myc and polyploidy have many common manifestations.
2.1. Overexpressed Myc Induces Polyploidy
Stress-related Myc overexpression can trigger and enhance polyploidy. Supraphysiological Myc expression is sufficient to trigger polyploidy in cells with various mitotic potentials. Myc induces DNA replication in quiescent, terminally differentiated cells, including mammalian cardiomyocytes, neurons, kidney cells, and post-mitotic epithelial cells of Drosophila [8,54]. Myc enforces genome accumulation in normal cells with weak mitotic capacity (hepatocytes, neurons, megakaryocytes, keratinocytes, and trophoblast cells) [55,56] and in cycling polyploid cancer cells of various origins [43,45,57,58,59,60]. Moreover, N-Myc upregulation was recently documented in a wide variety of cancer cells with polyploid genomes [61]. Overexpressed Myc promotes polyploidy and endoreduplication via the induction of S-phase regulators, such as cyclins of CCNE and CCND families, cyclin-dependent kinases CDK2 and CDK4, and transcription factors of the E2F family [62,63,64,65]. Myc also interacts with DNA replication origins and activates them epigenetically through histone modifications and nucleosome remodeling, promoting re-replication in specific loci and chromosomal regions by acting as an illegitimate replication-licensing factor [65,66].
Surprisingly, Myc-associated S-phase enforcement is not accompanied by the acceleration of mitotic cellular division [52,58,67]. Recent observations indicate that blocking of mitotic progression can occur as a result of Myc-induced DNA instability, disturbance of mitotic spindle geometry, and metaphase and anaphase duration [64,68]. Another reason for the link between overexpressed Myc and polyploidy is that the latter can abrogate G1 and G2 checkpoints controlling DNA damage and DNA replication [52,57,58,65].
2.2. Polyploidy Upregulates C-Myc
In normal cells, polyploidy is associated with the induction of Myc and its interactants in human and mouse heart, liver, and placenta [16,43,55,69,70,71,72,73] (Figure 1). Endopolyploidy and Myc are coupled in polyploid cells of Drosophila arising in development and wound healing [74,75,76]. Polyploidy also upregulates Myc in cancer cells. For example, in high-grade diffuse large B-cell lymphoma, drug-induced and drug-resistant polyploid cells also show c-Myc overexpression [77]. A similar finding was reported with respect to polyploid melanoma cells generated by paclitaxel [58]. These cells were found to overexpress c-Myc and reduce the expression of MAD2, an essential component of the molecular core of the spindle assembly checkpoint (SAC), indicating impairment of this checkpoint [58]. An extensive bioinformatics study performed with 10,000 primary human cancer samples and essentiality data from 600 cancer cell lines provided evidence that polyploidy in cancer is associated with induction of N-myc [61] (Supplementary Table S1 from [61]). Accordingly, the authors revealed the steady upregulation of c-Myc targets (Figure 1E in [61]), which was not accompanied by c-Myc induction (Supplementary Table S1 in [61]), confirming that chromatin opening can enhance the efficiency of amplifiers, even if their expression is not changed or is even decreased. The authors observed the induction of gene modules related to DNA repair, proliferation, and cell cycle, as well as the downregulation of gene modules involved in immune response and allograft rejection [61]. These data confirm that polyploidy is associated with the upregulation of c-Myc in various conditions.
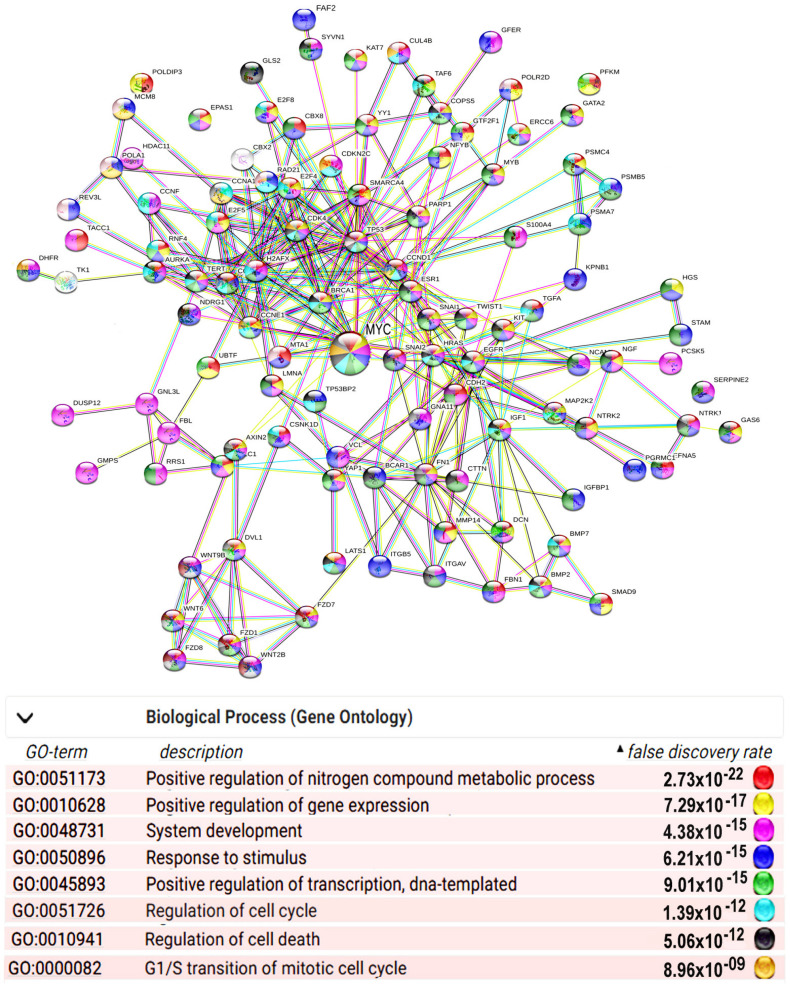
Figure 1.
The most connected component of protein interaction networks of significantly ploidy- induced genes in the c-Myc interactome of human and mouse heart, liver, and placenta. The network was constructed using the String server (https://string-db.org/, assessed on 5 May 2022). The data for network construction were taken from [72]. Color coding reflects the Biological Process of Gene Ontology (GO) database. The gene symbols containing portions of various colors indicate that a gene is involved in several GO biological processes. The fraction of a circle that is a particular color does not convey any meaning; the circle is simply divided into a number of partitions to reflect the number of GO processes involved.
Thus, Myc and polyploidy respond to similar stimuli, including a wide variety of stressful and pathological conditions. Overexpressed Myc promotes polyploidy via the stimulation of rapid S-phase entry, DNA replication, and mitotic spindle abnormalities, whereas polyploidy activates Myc through stress response, genetic instability, and replicative stress associated with chromatin opening [64,78,79].
3. Myc and Polyploidy Increase Stress Resistance
It is well-established that Myc enhances stress tolerance to various environmental clues. Myc promotes protection from hypoxia, oxidative stress, drugs, and DNA instability [80,81,82]. Moreover, Myc confers cells with resistance to apoptosis. An association between overexpressed Myc and protection from apoptosis was observed in tumors of various origins [77,83,84,85,86]. Another important protective feature of Myc is that when overexpressed, it enables tumor cells to deregulate their microenvironment and evade the host immune response [82].
Polyploidy is also associated with stress and protects cells from hypoxic, hyperoxic, and genotoxic environments and increases resistance to drugs [39,40,44,46,57,80,81,87]. Furthermore, polyploidy protects cells from aging related stress. This association has been well-established in cells of insects and mammals. For example, the increased polyploidization was discovered in spermathecal glands of honeybee queens during senescence, accompanied by genotoxic and oxidative stress [88]. The authors accounted for this phenomenon with a selective repression or induction of gene expression [88]. In mammals, the tight association between polyploidy and senescence was documented in neurons, hepatocytes, vascular smooth muscle cells, and even in cancer cells [16,43,47,89,90]. Importantly, polyploidy can also safeguard cell survival under energy depletion. For example, the giant polyploid nuclei originating from nuclear and cellular fusion arise in the insect vectors of Chagas disease, especially under starving-stress conditions [91]. The epithelial cells of Malpighian tubes of blood-sucking insects demonstrate polyploidization via nucleus fusion after 4.5 month of starvation [92,93].
Polyploidy mitigates consequences of DNA instability via the upregulation of pathways related to DNA damage response and DNA repair. This connection was well-established based on mRNA sequencing data from roughly 10,000 primary human cancer samples and essentiality data from approximately 600 cancer cell lines [61]. The link between polyploidy, DNA instability, and related pathways was also indicated for giant polyploid cancer cells [40,94,95], tumor-initiating cells in vivo, RPE1 cell in culture [32], and yeast [96]. Polyploidy also confers resistance to apoptosis. This feature was documented for polyploid cancer cells, giant cancer cells, and polyploid cells of normal tissues [40,61,72,97]. Another unexpected protective feature of polyploidy that is also common with Myc is immune evasion. The data of experimental studies and extensive transcriptome analyses indicate that in thousands of tumors and in normal tissues, polyploidy is associated with the downregulation of biological pathways and markers related to immunity [61,71,72,77,98]. Thus, ploidy-associated protection from stress confers polyploid cells with the ability to survive under conditions that are not suitable for diploid cells. An important cause of particular stress resistance of polyploid cells is the association between polyploidy and overexpressed Myc. On the one hand, stress-induced Myc can trigger polyploidy; on the other hand, polyploidy can upregulate Myc. Thus, there is a link between polyploidy, overexpressed Myc, and stress resistance.
4. Myc and Polyploidy Promote Chromatin Opening
4.1. Myc Promotes Chromatin Repositioning from the Nuclear Periphery to the Inner Part
Data obtained from B cells indicate that c-Myc can accelerate chromatin decondensation via its repositioning from the nuclear periphery to the inner part and by promoting a nuclear architectural shift from long-range to short-range contacts, leading to a near doubling of loops and topologically associated domains (TADs) [64,99]. Data recently derived from skeletal muscle stem cells provide evidence that Myc can regulate TAD composition and structure via TAD splitting, merging, rebuilding, rearranging, or disappearing [99,100]. Thus, overexpressed Myc can relax chromatin at the periphery of polyploid cells.
4.2. Polyploidy Stimulates Chromatin Transition from the Outer Part to the Inner Part of the Nucleus
Polyploidy can also potentially boost the effect of overexpressed Myc related to chromatin remodeling. It is well-established that genome duplication decreases the nucleus surface-to-volume ratio, which suggests the ability to affect high levels of chromatin organization [101,102]. Initial evidence was recently presented showing that this decrease affects the architecture of chromatin located at the periphery of the nucleus. Hi-C data obtained from KBM7 cells indicate that genome accumulation leads to preferential loss of nuclear lamina (NL) interactions of lamina-associated domains (LADs) [101,102]. The loss occurs as a result of increased competition for NL contacts originating from the decrease in nucleus surface-to-volume ratio [101,102]. The LADs exhibit heterochromatic features, including low gene density, low transcriptional activity, and late replication timing [103]. In the LADs detached from the lamina, some “repressed LAD promoters” became active as a result of their removal from the LAD context and transitioned to the nuclear interior [104]. This transition increases the number of compartments with open A-chromatin at the expense of compartments with closed B-chromatin [104]. B-to-A chromatin compartmental transition is a universal mechanism of topological activation of gene expression in development and differentiation [105]. For example, ploidy-related gene activation via large-scale chromatin topology rearrangement operates in cardiogenesis. Chromatin regions transitioning from B to A are strongly enriched for heart developmental genes upregulated during human and mouse cardiogenesis and in cardiac differentiation of hPSCs [105]. Accordingly, data obtained from synthetic autotetraploid plants (watermelon, soybean, and Arabidopsis) also indicate that polyploidy shifts the A-to-B chromatin balance toward the actively transcribed A-chromatin [106,107]. Moreover, recent studies provide evidence that genome duplications reorganize topologically associated domains (TAD) packaged within A- and B-chromatin compartments via the increase in intra-TAD interaction and reorganization of chromatin loops [106,108]. In addition, polyploidy in giant cancer cells can enlarge chromosome territories [109]. The ability of polyploidy to relax chromatin via its repositioning from the periphery to the center of the nucleus, as well as to alter LADs and TADs geometry, is also characteristic of cancer cells [109,110]. Thus, both c-Myc and polyploidy can reposition chromatin from the nuclear periphery to the inner part of the nucleus, promoting its relaxation (Figure 2).
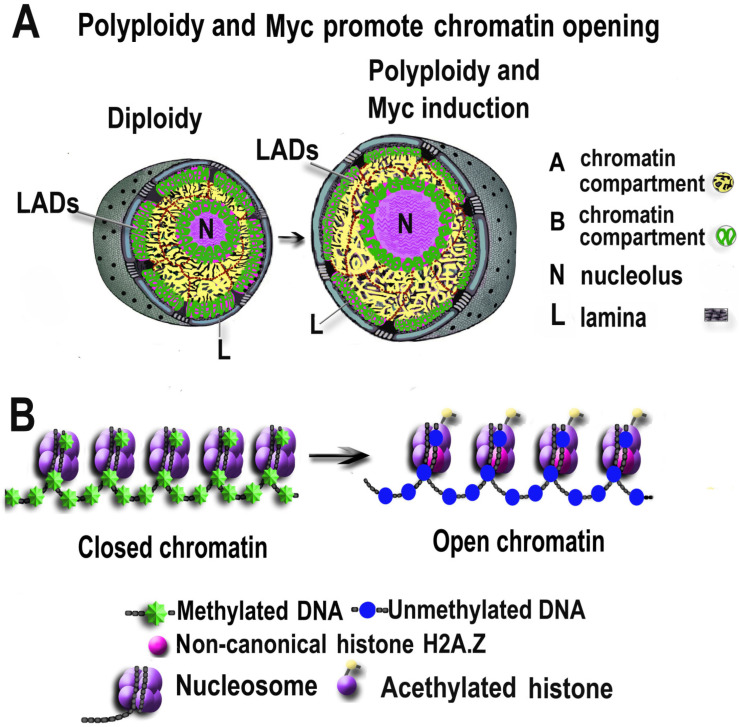
Figure 2.
Polyploidy and overexpressed Myc promote chromatin opening via common effects at high (A) and low (B) levels of organization. A-chromatin opening due to lamina-associated domain detachment from the lamina and chromatin transition from B (closed) to A (open) state. B-chromatin opening due to DNA hypomethylation, histone acetylation, and substitution of canonical histones with non-canonical histone H2A.Z.
5. Myc and Polyploidy Open Chromatin at the Low Level of Organization and Activate Transcription
5.1. Myc Opens Chromatin and Reinforces Expression of Already Actively Transcribed Genes via Binding of E Boxes
Myc proteins bind enhancer-box (E-box)-containing CACGTC sites [111]. E-box-containing genes can be divided into genes with “high-affinity E boxes” and “low-affinity E boxes” [65,112]. Researchers discovered that the two classes of E boxes can be differentiated by a marked enrichment of CpG islands and open chromatin marks, including DNA hypomethylation and histone modifications [65,113]. Consistently with this observation, Myc target genes were reported to demonstrate higher basal expression (even in the absence of Myc) relative to non-target genes [112]. Thus, Myc promotes transcription by further reinforcing the expression of already intensely working genes and that of genes with bivalent chromatin marks (i.e., genes containing both active and silent chromatin H3K4me3 and H3K27me3 marks) [15,51,73,114,115]. This phenomenon explains why overexpressed Myc shows different faces in various tissues [52,80,116]. Because the set of actively transcribed genes varies depending on the cell type, manifestations of Myc overexpression differ according to cell type. This feature indicates that Myc is not a specific transcription factor but a general amplifier that increases RNA content [117,118].
5.2. Myc Activates Chromatin via the Induction of Pol I, II, and III
Myc oncogenes can also activate chromatin via the induction of Pol I-, II-, and III-transcribing rRNA, tRNA, and mRNA [119,120]. Pol I, II, and III locally open the double-stranded DNA so that one strand of the exposed nucleotides can be used as a template for the RNA synthesis [119,121]. The effects of MYC on genes transcribed by all three polymerases are mostly inducing [111,122,123]. Myc activates genes participating in growth, proliferation, cell cycle (G1/S transition), energy metabolism, purine biosynthesis, ribogenesis, protein turnover, and other pathways, whereas Myc-repressed genes include cyclin-dependent kinase inhibitors and genes involved in apoptosis and adhesion [111,124]. Owing to these properties, Myc oncogenes can reverse DNA-damage-induced proliferation arrest and activate processes related to adaptation, wound healing, and tumorigenesis [75,125,126]. Almost all of the effects of Myc on the expression of specific target genes are weak and are often below the twofold threshold for expression difference, even when Myc levels are manipulated to increase several orders of magnitude [111,125,126].
5.3. Myc Interacts with Chromatin-Remodeling Partners
Myc-related facilitation of transcription can also be executed via the interaction with chromatin-remodeling partners. Myc isoforms interact with transformation/transcription-domain-associated protein (TRRAP), which is a scaffold protein of several large protein complexes involved in chromatin remodeling [127]. TRRAP also cooperates with p400 chromatin-remodeling helicase, which is the ATP-hydrolyzing subunit of the chromatin-remodeling Tip60/Ep400 complex that substitutes the canonical histone H2A for histone H2A.Z [128]. Histone H2A.Z increases enhancer activity, facilitating the binding of transcription factors and chromatin remodelers to trigger transcription and changes in the 3D chromatin structure [129]. H2A.Z histone also plays an important role in chromosome segregation and cell cycle progression, whereby H2A.Z upregulates the expression of key cell cycle genes, such as c-Myc, Myc-N, KIi67, and AURKA [130]. In addition, H2A.Z fine tunes processes of cell renewal and mediates the establishment of bivalent promoters of developmental genes during embryonic stem cell differentiation [131]. The reduction in H2A.Z in mESCs results in loss of pluripotency, premature differentiation, and senescence [130]. Another important Myc partner implicated in chromatin opening is WDR5, which facilitates histone H3 Lys4 (H3K4) methylation and increases the affinity of MYC for active promoters [65]. Myc can also be involved in chromatin activation via interaction with INI1 (SMARCB1/hSNF5/BAF47), a core member of the SWItch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complex [132]. It was also shown that the basic helix–loop–helix region associated with Myc interacts with INI1 repeat 1 (Rpt1) in ATP-dependent chromatin organization and transactivation [53].
Thus, the literature provides evidence that Myc overexpression can promote chromatin opening via the repositioning from the surface to the periphery of the nucleus (high level of chromatin organization) and via interaction with E boxes, RNA polymerases, and chromatin remodelers (low level). Because polyploidy can open chromatin, we speculate that when Myc overexpression coincides with polyploidy, their effects on chromatin can be cooperative. Therefore, it is reasonable to suggest that overexpressed c-Myc and polyploidy can cause prominent chromatin decompaction, leading to global activation of transcription and alteration of gene regulatory networks and cell fate decision. The abundance, diversity, and evolutionary conservatism of biological processes and functions that are coregulated by polyploidy and Myc indicate that they are responsible for transcriptional regulation via global epigenetic changes.
5.4. Polyploidy Promotes DNA Hypomethylation, Histone Modification, and Substitution of Canonical Histones with Non-Canonical Histones
Ploidy-associated chromatin activation and opening at high levels of organization induce a coherent response at lower levels. Genome duplications promote DNA hypomethylation and an increase in the amount of various open chromatin marks activating gene expression. For example, an study performed with colorectal cancer cells (line LS174T) showed that the onset of tetraploidy is associated with the demethylation of non-mobile pericentromeric repetitive elements SST1 and transposable elements LINE-1, comprising approximately 17% of the entire genome [133]. In line with these results, data obtained from ovarian cancer cells show a strong statistical association between polyploidy, increasing quantitative LINE1 DNA hypomethylation, and hypomethylation of centromeric DNAs Chr1 and Sat2 [134]. LINE-1 methylation is usually considered a marker of genome methylation [133]. Therefore, the association between polyploidy and hypomethylation of LINE-1 suggests that genome duplication decreases DNA methylation and opens chromatin in a substantial part of the genome. The concomitance of polyploidy and global hypomethylation was also found in trophoblast giant cells and mouse embryonic fibroblasts [135,136]. In concordance, data obtained from plants, including Medicago truncatula symbiotic nodule cells, neotetraploid rice, Arabidopsis, and soybean, reveal causal relationships between polyploidy and chromatin opening, mostly via DNA hypomethylation and/or demethylation of DNA packaging protein histone H3 (specifically the H3K27me3 mark) [137,138]. Polyploidy was also found to activate histone acetylation in human embryonic kidney cells and in bread wheat Triticum aestivum L [139,140]. In addition to DNA hypomethylation, histone acetylation, and histone demethylation, polyploidy can promote the substitution of canonical histones with non-canonical histone H2AZ, which is necessary for chromatin relaxation [135,141]. Thus, Myc and polyploidy can promote chromatin opening and activation of transcription at various levels of organization.
6. Common Biological Effects of Overexpressed Myc and Polyploidy Manifest in Embryonic Phenotype and Metabostemness
Chromatin opening affects basic biological processes and cell fate. In multicellular organisms, open chromatin is a feature of undifferentiated pluripotent stem cells that maintain plasticity in biological regulation, specific metabolic state, and dual capacity to self-renew and differentiate into all cell types [142,143,144,145]. In differentiated cells, open chromatin awakens silent genes, enabling sensing of multiple, simultaneous, and often opposing signals (e.g., adult and fetal) in the environment [142,145]. In addition, chromatin opening causes dedifferentiation and activates programs of embryonality and pluripotency [142]. These features are acquired via the remodeling of chromatin at various levels of organization, including nuclear periphery, TADs, LADs, posttranslational modifications of histones, and DNA methylation [143,144,146]. Polyploidy and overexpressed Myc are associated with many manifestations related to chromatin opening. Importantly, most of these features relate to pluripotency.
6.1. Myc and Polyploidy Activate Programs of Embryonality
Myc oncogenes activate signaling pathways involved in pluripotency and embryogenesis (including NOTCH, BMP, TGFb, PI3K, HIPPO, and WNT), as well as pathways of epithelial to mesenchymal transition and cell cycle progression, contributing to self-renewal through maintenance of undifferentiated states [83,147,148]. Moreover, Myc is one of the four Yamanaka factors that reprogram differentiated cells to an embryonic-like state [148]. A polyploid state, as well as the Myc oncogene, can maintain stemness. The activation of pluripotency programs was observed in polyploid giant cancer cells (PGCCs) of ovarian, breast, prostate, and colorectal cancers, as well as Burkitt’s lymphoma [39,44,45,46,60,95,149,150,151,152,153,154,155]. Moreover, in these cells, polyploidy was accompanied by the expression of the key embryonic stem cell markers Oct4/Nanog, Sox2, SCF, and c-kit, as well as markers of cancer stem cells (CD44 and CD133) [156,157]. In addition, endocycling cells of Drosophila epidermis and mouse cornea endothelial cells that participate in wound healing promote stemness through the activation of the Hippo pathway [75]. Researchers suggest that wound-induced polyploidization enables tissue repair when cell division is not a viable option [19,75,158]. Data recently obtained via mRNA sequencing of human and mouse heart, liver, and placenta and from isolated hepatocytes and cardiac interstitial cells provide evidence that polyploidy is associated with the induction of signaling pathways related to stress response, growth, G1/S transition, and multipotency, including NOTCH, BMP, TGFb, PI3K, HIPPO, and WNT, as well as epithelial-to-mesenchymal transition [2,48,71,72,73,159,160]. Thus, both, Myc and polyploidy can enhance programs of stemness.
6.2. Myc and Polyploidy Upregulate Genes with Bivalent Promoters
Another common feature of polyploidy and Myc is the upregulation of bivalent genes [73,114,161]. These genes harbor two opposite epigenetic modifications of histone H3, the repressing H3K27me3 mark and the activating H3K4me3 mark, in their promoters or enhancers, [162]. Bivalent genes are poised for transcription and are capable of rapid activation [162]. Prevalent in embryonic stem cells, bivalency is postulated to poise/prime lineage-controlling developmental genes for rapid activation during embryogenesis while maintaining a transcriptionally repressed state in the absence of activation cues [163]. This is particularly important for key developmental genes and enhancers, the activation of which in a short time window during differentiation may be crucial [164].
The activation of genes with bivalent chromatin was also observed in pathologies associated with cell polyploidization and manifestations of stemness, including cardiovascular diseases and cancer [73,162,165,166]. Moreover, the tight link between polyploidy, bivalent genes, and programs of embryonality seems to be a fundamental and evolutionarily conserved phenomenon. Thus, human ohnologs (genes retained in duplicates after whole-genome duplications) are most strongly enriched in the bivalent genes and genes implicated in development, showing an analogy with somatic polyploidy [16]. The biased retention of expression-regulating and developmental genes can be explained by their particular importance indicated by the strong purifying selection on them [167,168].
6.3. Myc and Polyploidy Are Associated with Metabostemness and Hypertranscription
It is well-known that Myc induces metabolic modification with features of metabostemness observed in enhanced glycolysis, glutaminolysis, ribosome biogenesis, and hypertranscription, which collectively enable cells to acquire continuous energy supply via reserved energy-producing pathways [9,111,169,170,171,172,173]. Additional sources of metabolites serve as essential cofactors for epigenetic enzymes regulating DNA methylation, posttranslational modifications of histones, and nucleosome position [174,175].
Metabolic pathways of polyploid cells also demonstrate manifestations of stemness [11,71,72]. Similarly to pluripotent stem cells, polyploid cells can simultaneously derive energy from pathways that are incompatible with differentiated cells, promoting adaptation to stress associated with energy deprivation [49,72,176,177,178,179,180,181,182]. Recent data obtained from polyploid cells from normal tissues of animals and plants, various tumors, and yeasts indicate that these cells possess energetically flexible metabolism combining glycolysis glutaminolysis and oxidative phosphorylation [43,57,72,183,184,185]. Thus, in addition to stem cells, polyploid cells can adjust their energy metabolism to the environment, which is impossible for differentiated cells.
Under hypoxia, lack of nutrients, starvation, or severe genotoxic stress, polyploid cells can be in a state of dormancy with predominantly glycolytic energy supply, which is a feature of metabostemness [40,43,46,88,92,93]. Under normoxia, polyploidy is usually accompanied by enhanced glycolysis, glutaminolysis, hypertranscription, active protein synthesis, and ribosome biogenesis, which are also manifestations of embryonality [2,6,71,79,186,187]. All these features of metabostemness have also been described in endopolyploid cells of Drosophila in development and wound healing, in cancer polyploid cells, and in cells of normal mammalian tissues [7,57,178,184,188].
6.4. Myc and Polyploidy Awaken Programs of Unicellularity
According to the ancient origin of Myc, it can be traced to unicellular organisms using deep phylostratigraphy [189], and several Myc-associated traits are also characteristic of unicellular organisms. For example, as in unicellular organisms, Myc activates glycolysis, glutaminolysis, ribogenesis, and features of epithelial-to-mesenchymal transition, confirming the association between Myc and the reactivation of evolutionary ancient gene modules [82,190,191]. Recent phylostratigraphic data indicate that both polyploidy and Myc shift the expression of genes toward unicellularity [43,73]. Polyploidy-associated features of stemness and metabostemness are also observed in unicellular primitive organisms, further confirming that polyploidy promotes dedifferentiation and primitive ancient traits, including the activation of ancestral gene modules related to glycolysis, epithelial-to-mesenchymal transition, housekeeping genes, cell cycle, ribosome biogenesis, and flexible adaptive reaction to stress [22,190,191,192,193,194,195,196]. The connection between polyploidy and unicellularity is not surprising because polyploidy is an ancient phenomenon that appeared together with reproductive cysts of unicellular organisms [197]. Moreover, Vladimir Niculescu considers polyploidization to be part of the already reactivated unicellular programs and acquired unicellular lifestyle [196,197]. Recent data obtained via phylostratigraphy indicate that in human and mouse heart and liver, polyploidy shifts the evolutionary age balance of the expressed genes from the late metazoan phylostrata toward the upregulation of unicellular and early metazoan phylostrata [73]. It has been shown that the human interactome consists of unicellular and multicellular giant clusters [167,192]. In cancer cells, the expression of the unicellular cluster is enhanced, whereas the multicellular cluster is suppressed [167,198]. Accordingly, in polyploid cancer cells, the expression of the unicellular cluster is upregulated, whereas the multicellular cluster is downregulated, even compared with diploid cells of the same cancer, indicating that polyploidization of cancer cells enhances their unicellular properties [16,199]. The tight connection between cancer polyploidy and unicellularity confirms the atavistic theory of oncogenesis, which suggests that cancer is a reversal from a multicellular to a unicellular state [200,201,202,203].
7. Myc and Polyploidy Are Possibly Evolutionarily Conserved Partners Increasing Adaptation to Stress via Epigenetic Plasticity, Metabolism, and DNA Damage Protection
7.1. Myc Increases the Ability of Polyploid Cells to Outcompete Diploid Cells under Stressful Conditions
Polyploidy and overexpressed Myc appear in response to physiological and pathological stress. Both exert similar effects on chromatin architecture and many biological processes, including chromatin relaxation, phenotype plasticity, stemness, metabolic rearrangements, regulation of gene expression, and adaptation. Myc is a sensor of intrinsic and extrinsic stress belonging to early response master regulators that reacts to a wide variety of stimuli [204]. In response to stress, Myc promotes adaptive reactions, increasing cell resistance and flexibility. Under long-term and severe activation, Myc can also cause unwanted effects, including DNA instability, hypertranscription, replicative stress, and cell cycle disturbance [65]. These flaws can stimulate polyploidy, which also exerts beneficial and detrimental effects [62]. On the one hand, polyploidy increases stress resistance and adaptability (similarly to Myc), and on the other hand, it promotes genetic instability and chromatin relaxation (also similarly to Myc), contributing to further Myc induction [43,57,71,72]. Thus, it is reasonable to suggest that Myc and polyploidy can reinforce each other, facilitating the manifestation of both adaptive and adverse effects. Likely as a result of this duality, polyploid cells acquire competitive advantages only under stressful conditions [25].
7.2. Epigenetic Phenotypic Plasticity, Energy Reserve, and Protection from DNA Instability Boosted by Myc Might Help Polyploid Cells to Adapt
Why might the Myc–polyploidy partnership enable particular adaptation to extreme stress? Data obtained from therapy-resistant giant polyploid cancer cells (GPCCs) suggest that the polyploid-related ability to “survive at the brink” [43] originates from three main sources. First, stress-related polyploidy can promote rapid adaptation to changing environments via global genome reorganization (also termed ‘genome chaos’), leading to high epigenetic and phenotypic plasticity and dedifferentiation or stemness [41,43,46,205,206]. Secondly, it protects cells from genomic instability [25,40,57,69]. Thirdly, it provides enhanced energy supply via additional pathways of ATP and HADH production [49,72,185] (Figure 3). Epigenetic phenotypic plasticity, stemness, and genome reorganization can be caused by chromatin opening, DNA damage, and bivalent gene induction promoting the ability for rapid transitions from one state to another via network self-organization and adaptive search for an energetically favorable attractor state [41,44,206,207,208,209,210,211,212,213]. Ploidy-associated cell protection from DNA instability is provided by additional genomes buffering DNA damage and by an efficient but error-prone DNA repair system, which is a characteristic of unicellular organisms [214,215]. Additional energy is provided by the activation of pathways of glycolysis, glutaminolysis, and NADH production, as well as by the induction of Warburg and Crabtree effects [72,183,184,185]. All three features enabling rapid identification of attractors are also related to ancient, unicellular organisms, confirming a tight link between polyploidy and recapitulation of evolutionarily ancient programs [200,201]. Thus, dedifferentiated state, error-prone DNA repair, and a particular metabolic patterns with enhanced Warburg and Crabtree effects were previously well-established in yeast, amoeba, and other organisms [183,197].
Figure 3.
The most important common features of polyploidy and overexpressed Myc that promote resistance to extreme stress and confer the ability to rapidly adapt to new environments.
In humans, polyploidy, in partnership with Myc, may help cells to survive under pathologic conditions associated with various diseases, including hypertension, congenital heart diseases, neurodegeneration, inflammation, and even cancer [8,14,17,43]. In the case of cancer, polyploidy confers particular resistance to therapy and drugs. Therefore, it is of particular importance to identify effective therapies that target polyploid cancer cells. These three ploidy-specific features are promising targets for therapy directed at the elimination of polyploid cells, particularly in cancer. Specifically, therapy can be directed toward the correction of metabolism (via impairment of glycolysis or glutaminolysis), the weakening of stemness through the changing of epigenetic state, and the removal of DNA instability protection by blocking of error-prone primitive DNA repair pathways. It is also tempting to suggest that Myc silencing could be effective. However, such an approach is controversial. On the one hand, Myc silencing can be beneficial, as in endopolyploid cells, it causes depolyploidization, leading to a reduction in cell size and ploidy [216]; on the other hand, Myc silencing can be detrimental because silent Myc can cause deep-cell dormancy accompanied by severe metabolic deprivation and permanent therapy resistance [217]. Therefore, more data and knowledge are needed to come to conclusion about Myc-silencing therapy for polyploidy targeting.
8. Conclusions
Polyploid cells demonstrate particular plasticity and adaptation to stress. On the one hand, this feature is beneficial because it helps to adapt to various pathological states, including cardiovascular diseases, neurodegeneration, inflammation, wound healing, and regeneration. On the other hand, this feature is detrimental because it promotes carcinogenesis, metastasis, and cancer relapse caused by cancer therapy resistance. Currently, the nature of ploidy-related adaptability is not completely understood. Here, we highlight literature data indicating that polyploidy can regulate gene expression via extensive epigenetic changes promoting chromatin relaxation. Owing to increased nuclear volume in polyploid cells, the decreased surface-to-volume ratio results in partial detachment of LADs from the nuclear lamina, thereby changing the structure of LADs and TADs and increasing the proportion of euchromatin. Polyploidy also promotes DNA hypomethylation and chromatin modifications, relaxing chromatin. Altogether, these changes awaken bivalent genes that are rapidly activated in response to stress or signals of morphogenesis. Contributing to the opening of chromatin, polyploidy also activates global transcription amplifier oncogenes of the Myc family, which, like polyploidy, contribute to the opening of chromatin. Myc oncogenes can also accelerate the accumulation of genomes, enhancing polyploidization. As a result of these cooperative effects of polyploidy and activated Myc, genetic instability occurs, which, together with chromatin opening and induction of bivalent genes, can result in genomic chaos, increasing epigenetic and phenotypic plasticity and the ability to search for adaptive states of cellular programs through gene regulatory network rewiring. Ploidy-related regulatory and phenotypic plasticity is manifested in (1) traits of stemness, dedifferentiation, and unicellularity; (2) flexible energy metabolism; and (3) effective protection from genome instability consisting via buffering of DNA damage and mutation effects and in complex DNA repair that combines primitive unicellular error-prone repair pathways and advanced multicellular error-free repair pathways. We suggest that these three features are important components of the increased adaptability of polyploid cells. The presented evidence can be useful in the development of new types of therapy with the aim of eliminating polyploid cancer cells. The evidence presented herein could also be useful for the development of new measures of preventive medicine with the aim of preventing excessive polyploidization of cardiomyocytes and neurons in order to reduce the risk of cardiovascular and neurodegenerative diseases.
Acknowledgments
The authors are grateful to Jekaterina Erenpreisa for helpful advice and valuable comments. The authors also highly appreciate important suggestions and recommendations of anonymous Reviewers.
Author Contributions
O.V.A.: conceptualization, writing original draft, review and editing, and figure preparation. A.E.V.: writing original draft, review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-1075, signed 28 September 2021).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anatskaya O.V., Vinogradov A.E., Kudryavtsev B.N. Hepatocyte Polyploidy and Metabolism/Life-History Traits: Hypotheses Testing. J. Theor. Biol. 1994;168:191–199. doi: 10.1006/jtbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- 2.Vinogradov A.E., Anatskaya O.V., Kudryavtsev B.N. Relationship of Hepatocyte Ploidy Levels with Body Size and Growth Rate in Mammals. Genome. 2001;44:350–360. doi: 10.1139/g01-015. [DOI] [PubMed] [Google Scholar]
- 3.Van de Peer Y., Mizrachi E., Marchal K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017;18:411–424. doi: 10.1038/nrg.2017.26. [DOI] [PubMed] [Google Scholar]
- 4.Fox D.T., Soltis D.E., Soltis P.S., Ashman T.-L., Van de Peer Y. Polyploidy: A Biological Force From Cells to Ecosystems. Trends Cell Biol. 2020;30:688–694. doi: 10.1016/j.tcb.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anatskaya O.V., Vinogradov A.E. Myocyte Ploidy in Heart Chambers of Birds with Different Locomotor Activity. J. Exp. Zool. 2002;293:427–441. doi: 10.1002/jez.10114. [DOI] [PubMed] [Google Scholar]
- 6.Anatskaya O.V., Vinogradov A.E. Paradoxical Relationship between Protein Content and Nucleolar Activity in Mammalian Cardiomyocytes. Genome. 2004;47:565–578. doi: 10.1139/g04-015. [DOI] [PubMed] [Google Scholar]
- 7.Anatskaya O.V., Vinogradov A.E. Heart and Liver as Developmental Bottlenecks of Mammal Design: Evidence from Cell Polyploidization: Heart and Liver as Bottlenecks of Mammal Design. Biol. J. Linn. Soc. 2004;83:175–186. doi: 10.1111/j.1095-8312.2004.00377.x. [DOI] [Google Scholar]
- 8.Nandakumar S., Rozich E., Buttitta L. Cell Cycle Re-Entry in the Nervous System: From Polyploidy to Neurodegeneration. Front. Cell Dev. Biol. 2021;9:698661. doi: 10.3389/fcell.2021.698661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey E.C., Kobielski S., Park J., Losick V.P. Polyploidy in Tissue Repair and Regeneration. Cold Spring Harb. Perspect. Biol. 2021;13:a040881. doi: 10.1101/cshperspect.a040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky V.Y., Sarkisov D.S., Arefyeva A.M., Panova N.W., Gvasava I.G. Polyploidy in Cardiac Myocytes of Normal and Hypertrophic Human Hearts; Range of Values. Virchows Arch. 1994;424:429–435. doi: 10.1007/BF00190566. [DOI] [PubMed] [Google Scholar]
- 11.Anatskaya O.V., Sidorenko N.V., Vinogradov A.E., Beyer T.V. Impact of Neonatal Cryptosporidial Gastroenteritis on Epigenetic Programming of Rat Hepatocytes. Cell Biol. Int. 2007;31:420–427. doi: 10.1016/j.cellbi.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Anatskaya O.V., Sidorenko N.V., Beyer T.V., Vinogradov A.E. Neonatal Cardiomyocyte Ploidy Reveals Critical Windows of Heart Development. Int. J. Cardiol. 2010;141:81–91. doi: 10.1016/j.ijcard.2008.11.158. [DOI] [PubMed] [Google Scholar]
- 13.Anatskaya O.V., Matveev I.V., Sidorenko N.V., Kharchenko M.V., Kropotov A.V., Vinogradov A.E. Changes in the Heart of Neonatal Rats after Cryptosporidial Gastroenteritis of Different Degrees of Severity. J. Evol. Biochem. Phys. 2013;49:509–518. doi: 10.1134/S0022093013050071. [DOI] [PubMed] [Google Scholar]
- 14.Lazzeri E., Angelotti M.L., Conte C., Anders H.-J., Romagnani P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol. Med. 2019;25:366–381. doi: 10.1016/j.molmed.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Anatskaya O.V., Vinogradov A.E. Whole-Genome Duplications in Evolution, Ontogeny, and Pathology: Complexity and Emergency Reserves. Mol. Biol. 2021;55:813–827. doi: 10.1134/S0026893321050022. [DOI] [PubMed] [Google Scholar]
- 16.Anatskaya O.V., Vinogradov A.E. Polyploidy as a Fundamental Phenomenon in Evolution, Development, Adaptation and Diseases. Int. J. Mol. Sci. 2022;23:3542. doi: 10.3390/ijms23073542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neiman M., Beaton M.J., Hessen D.O., Jeyasingh P.D., Weider L.J. Endopolyploidy as a Potential Driver of Animal Ecology and Evolution. Biol. Rev. Camb. Philos. Soc. 2017;92:234–247. doi: 10.1111/brv.12226. [DOI] [PubMed] [Google Scholar]
- 18.Silva I.S., Ghiraldini F.G., Veronezi G.M.B., Mello M.L.S. Polyploidy and Nuclear Phenotype Characteristics of Cardiomyocytes from Diabetic Adult and Normoglycemic Aged Mice. Acta Histochem. 2018;120:84–94. doi: 10.1016/j.acthis.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Gjelsvik K.J., Besen-McNally R., Losick V.P. Solving the Polyploid Mystery in Health and Disease. Trends Genet. 2019;35:6–14. doi: 10.1016/j.tig.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirillova A., Han L., Liu H., Kühn B. Polyploid Cardiomyocytes: Implications for Heart Regeneration. Development. 2021;148:dev199401. doi: 10.1242/dev.199401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walen K.H. Cell Cycle Stress in Normal Human Cells: A Route to “First Cells” (with/without Fitness Gain) and Cancer-like Cell-Shape Changes. Semin. Cancer Biol. 2022;81:73–82. doi: 10.1016/j.semcancer.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Erenpreisa J., Salmina K., Huna A., Jackson T.R., Vazquez-Martin A., Cragg M.S. The “Virgin Birth”, Polyploidy, and the Origin of Cancer. Oncoscience. 2014;2:3–14. doi: 10.18632/oncoscience.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J. Giant Cells: Linking McClintock’s Heredity to Early Embryogenesis and Tumor Origin throughout Millennia of Evolution on Earth. Semin. Cancer Biol. 2022;81:176–192. doi: 10.1016/j.semcancer.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Loftus L.V., Amend S.R., Pienta K.J. Interplay between Cell Death and Cell Proliferation Reveals New Strategies for Cancer Therapy. Int. J. Mol. Sci. 2022;23:4723. doi: 10.3390/ijms23094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van de Peer Y., Ashman T.-L., Soltis P.S., Soltis D.E. Polyploidy: An Evolutionary and Ecological Force in Stressful Times. Plant Cell. 2021;33:11–26. doi: 10.1093/plcell/koaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archetti M. Polyploidy as an Adaptation against Loss of Heterozygosity in Cancer. IJMS. 2022;23:8528. doi: 10.3390/ijms23158528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porrello E.R., Olson E.N. A Neonatal Blueprint for Cardiac Regeneration. Stem Cell Res. 2014;13:556–570. doi: 10.1016/j.scr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donne R., Saroul-Aïnama M., Cordier P., Celton-Morizur S., Desdouets C. Polyploidy in Liver Development, Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:391–405. doi: 10.1038/s41575-020-0284-x. [DOI] [PubMed] [Google Scholar]
- 29.Puente B.N., Kimura W., Muralidhar S.A., Moon J., Amatruda J.F., Phelps K.L., Grinsfelder D., Rothermel B.A., Chen R., Garcia J.A., et al. The Oxygen-Rich Postnatal Environment Induces Cardiomyocyte Cell-Cycle Arrest through DNA Damage Response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han P., Li W., Yang J., Shang C., Lin C.-H., Cheng W., Hang C.T., Cheng H.-L., Chen C.-H., Wong J., et al. Epigenetic Response to Environmental Stress: Assembly of BRG1-G9a/GLP-DNMT3 Repressive Chromatin Complex on Myh6 Promoter in Pathologically Stressed Hearts. Biochim. Biophys. Acta. 2016;1863:1772–1781. doi: 10.1016/j.bbamcr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrtwich L., Nanda I., Evangelou K., Nikolova T., Horn V., Sagar S., Erny D., Stefanowski J., Rogell L., Klein C., et al. DNA Damage Signaling Instructs Polyploid Macrophage Fate in Granulomas. Cell. 2016;167:1264–1280.e18. doi: 10.1016/j.cell.2016.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Gemble S., Basto R. CHRONOCRISIS: When Cell Cycle Asynchrony Generates DNA Damage in Polyploid Cells. Bioessays. 2020;42:e2000105. doi: 10.1002/bies.202000105. [DOI] [PubMed] [Google Scholar]
- 33.Bensley J.G., Stacy V.K., De Matteo R., Harding R., Black M.J. Cardiac Remodelling as a Result of Pre-Term Birth: Implications for Future Cardiovascular Disease. Eur. Heart J. 2010;31:2058–2066. doi: 10.1093/eurheartj/ehq104. [DOI] [PubMed] [Google Scholar]
- 34.Derks W., Bergmann O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020;126:552–565. doi: 10.1161/CIRCRESAHA.119.315408. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann O. Cardiomyocytes in Congenital Heart Disease: Overcoming Cytokinesis Failure in Tetralogy of Fallot. J. Thorac. Cardiovasc. Surg. 2021;161:1587–1590. doi: 10.1016/j.jtcvs.2020.05.091. [DOI] [PubMed] [Google Scholar]
- 36.Sukhacheva T.V., Serov R.A., Nizyaeva N.V., Burov A.A., Pavlovich S.V., Podurovskaya Y.L., Samsonova M.V., Chernyaev A.L., Shchegolev A.I., Kim A.I., et al. Accelerated Growth, Differentiation, and Ploidy with Reduced Proliferation of Right Ventricular Cardiomyocytes in Children with Congenital Heart Defect Tetralogy of Fallot. Cells. 2022;11:175. doi: 10.3390/cells11010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensley J.G., Moore L., De Matteo R., Harding R., Black M.J. Impact of Preterm Birth on the Developing Myocardium of the Neonate. Pediatr. Res. 2018;83:880–888. doi: 10.1038/pr.2017.324. [DOI] [PubMed] [Google Scholar]
- 38.Bensley J.G., De Matteo R., Harding R., Black M.J. Three-Dimensional Direct Measurement of Cardiomyocyte Volume, Nuclearity, and Ploidy in Thick Histological Sections. Sci. Rep. 2016;6:23756. doi: 10.1038/srep23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amend S.R., Torga G., Lin K.-C., Kostecka L.G., de Marzo A., Austin R.H., Pienta K.J. Polyploid Giant Cancer Cells: Unrecognized Actuators of Tumorigenesis, Metastasis, and Resistance. Prostate. 2019;79:1489–1497. doi: 10.1002/pros.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirzayans R., Murray D. Intratumor Heterogeneity and Therapy Resistance: Contributions of Dormancy, Apoptosis Reversal (Anastasis) and Cell Fusion to Disease Recurrence. Int. J. Mol. Sci. 2020;21:1308. doi: 10.3390/ijms21041308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erenpreisa J., Giuliani A. Resolution of Complex Issues in Genome Regulation and Cancer Requires Non-Linear and Network-Based Thermodynamics. Int. J. Mol. Sci. 2019;21:240. doi: 10.3390/ijms21010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erenpreisa J., Krigerts J., Salmina K., Gerashchenko B.I., Freivalds T., Kurg R., Winter R., Krufczik M., Zayakin P., Hausmann M., et al. Heterochromatin Networks: Topology, Dynamics, and Function (a Working Hypothesis) Cells. 2021;10:1582. doi: 10.3390/cells10071582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erenpreisa J., Salmina K., Anatskaya O., Cragg M.S. Paradoxes of Cancer: Survival at the Brink. Semin. Cancer Biol. 2022;81:119–131. doi: 10.1016/j.semcancer.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Erenpreisa J., Salmina K., Anatskaya O., Vinogradov A., Cragg M. The Enigma of Cancer Resistance to Treatment. Org. J. 2022;5:71–75. doi: 10.13133/2532-5876/17613. [DOI] [Google Scholar]
- 45.Liu J. The Dualistic Origin of Human Tumors. Semin. Cancer Biol. 2018;53:1–16. doi: 10.1016/j.semcancer.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J., Erenpreisa J., Sikora E. Polyploid Giant Cancer Cells: An Emerging New Field of Cancer Biology. Semin. Cancer Biol. 2022;81:1–4. doi: 10.1016/j.semcancer.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Sikora E., Czarnecka-Herok J., Bojko A., Sunderland P. Therapy-Induced Polyploidization and Senescence: Coincidence or Interconnection? Semin. Cancer Biol. 2022;81:83–95. doi: 10.1016/j.semcancer.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Anatskaya O.V., Vinogradov A.E. Somatic Polyploidy Promotes Cell Function under Stress and Energy Depletion: Evidence from Tissue-Specific Mammal Transcriptome. Funct. Integr. Genom. 2010;10:433–446. doi: 10.1007/s10142-010-0180-5. [DOI] [PubMed] [Google Scholar]
- 49.Kimmel G.J., Dane M., Heiser L.M., Altrock P.M., Andor N. Integrating Mathematical Modeling with High-Throughput Imaging Explains How Polyploid Populations Behave in Nutrient-Sparse Environments. Cancer Res. 2020;80:5109–5120. doi: 10.1158/0008-5472.CAN-20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krigerts J., Salmina K., Freivalds T., Zayakin P., Rumnieks F., Inashkina I., Giuliani A., Hausmann M., Erenpreisa J. Differentiating Cancer Cells Reveal Early Large-Scale Genome Regulation by Pericentric Domains. Biophys. J. 2021;120:711–724. doi: 10.1016/j.bpj.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabò A., Amati B. Genome Recognition by MYC. Cold Spring Harb. Perspect. Med. 2014;4:a014191. doi: 10.1101/cshperspect.a014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabay M., Li Y., Felsher D.W. MYC Activation Is a Hallmark of Cancer Initiation and Maintenance. Cold Spring Harb. Perspect. Med. 2014;4:a014241. doi: 10.1101/cshperspect.a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amjadi-Moheb F., Paniri A., Akhavan-Niaki H. Insights into the Links between MYC and 3D Chromatin Structure and Epigenetics Regulation: Implications for Cancer Therapy. Cancer Res. 2021;81:1925–1936. doi: 10.1158/0008-5472.CAN-20-3613. [DOI] [PubMed] [Google Scholar]
- 54.Bywater M.J., Burkhart D.L., Straube J., Sabò A., Pendino V., Hudson J.E., Quaife-Ryan G.A., Porrello E.R., Rae J., Parton R.G., et al. Reactivation of Myc Transcription in the Mouse Heart Unlocks Its Proliferative Capacity. Nat. Commun. 2020;11:1827. doi: 10.1038/s41467-020-15552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baena E., Gandarillas A., Vallespinós M., Zanet J., Bachs O., Redondo C., Fabregat I., Martinez-A C., de Alborán I.M. C-Myc Regulates Cell Size and Ploidy but Is Not Essential for Postnatal Proliferation in Liver. Proc. Natl. Acad. Sci. USA. 2005;102:7286–7291. doi: 10.1073/pnas.0409260102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bluteau O., Langlois T., Rivera-Munoz P., Favale F., Rameau P., Meurice G., Dessen P., Solary E., Raslova H., Mercher T., et al. Developmental Changes in Human Megakaryopoiesis. J. Thromb. Haemost. 2013;11:1730–1741. doi: 10.1111/jth.12326. [DOI] [PubMed] [Google Scholar]
- 57.Pienta K.J., Hammarlund E.U., Austin R.H., Axelrod R., Brown J.S., Amend S.R. Cancer Cells Employ an Evolutionarily Conserved Polyploidization Program to Resist Therapy. Semin. Cancer Biol. 2022;81:145–159. doi: 10.1016/j.semcancer.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Gatti G., Maresca G., Natoli M., Florenzano F., Nicolin A., Felsani A., D’Agnano I. MYC Prevents Apoptosis and Enhances Endoreduplication Induced by Paclitaxel. PLoS ONE. 2009;4:e5442. doi: 10.1371/journal.pone.0005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Li X., Pu J., Yang Q., Guan H., Ji M., Shi B., Chen M., Hou P. C-Myc Is a Major Determinant for Antitumor Activity of Aurora A Kinase Inhibitor MLN8237 in Thyroid Cancer. Thyroid. 2018;28:1642–1654. doi: 10.1089/thy.2018.0183. [DOI] [PubMed] [Google Scholar]
- 60.Nehme Z., Pasquereau S., Haidar Ahmad S., El Baba R., Herbein G. Polyploid Giant Cancer Cells, EZH2 and Myc Upregulation in Mammary Epithelial Cells Infected with High-Risk Human Cytomegalovirus. EBioMedicine. 2022;80:104056. doi: 10.1016/j.ebiom.2022.104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinton R.J., DiDomizio A., Vittoria M.A., Kotýnková K., Ticas C.J., Patel S., Koga Y., Vakhshoorzadeh J., Hermance N., Kuroda T.S., et al. Whole-Genome Doubling Confers Unique Genetic Vulnerabilities on Tumour Cells. Nature. 2021;590:492–497. doi: 10.1038/s41586-020-03133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q., Dang C.V. C-Myc Overexpression Uncouples DNA Replication from Mitosis. Mol. Cell. Biol. 1999;19:5339–5351. doi: 10.1128/MCB.19.8.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bretones G., Delgado M.D., León J. Myc and Cell Cycle Control. Biochim. Biophys. Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Littler S., Sloss O., Geary B., Pierce A., Whetton A.D., Taylor S.S. Oncogenic MYC Amplifies Mitotic Perturbations. Open Biol. 2019;9:190136. doi: 10.1098/rsob.190136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curti L., Campaner S. MYC-Induced Replicative Stress: A Double-Edged Sword for Cancer Development and Treatment. Int. J. Mol. Sci. 2021;22:6168. doi: 10.3390/ijms22126168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swarnalatha M., Singh A.K., Kumar V. The Epigenetic Control of E-Box and Myc-Dependent Chromatin Modifications Regulate the Licensing of Lamin B2 Origin during Cell Cycle. Nucleic Acids Res. 2012;40:9021–9035. doi: 10.1093/nar/gks617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deb-Basu D., Karlsson A., Li Q., Dang C.V., Felsher D.W. MYC Can Enforce Cell Cycle Transit from G1 to S and G2 to S, but Not Mitotic Cellular Division, Independent of P27-Mediated Inihibition of Cyclin E/CDK2. Cell Cycle. 2006;5:1348–1355. doi: 10.4161/cc.5.12.2860. [DOI] [PubMed] [Google Scholar]
- 68.Rohrberg J., Van de Mark D., Amouzgar M., Lee J.V., Taileb M., Corella A., Kilinc S., Williams J., Jokisch M.-L., Camarda R., et al. MYC Dysregulates Mitosis, Revealing Cancer Vulnerabilities. Cell Rep. 2020;30:3368–3382.e7. doi: 10.1016/j.celrep.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pienta K.J., Hammarlund E.U., Brown J.S., Amend S.R., Axelrod R.M. Cancer Recurrence and Lethality Are Enabled by Enhanced Survival and Reversible Cell Cycle Arrest of Polyaneuploid Cells. Proc. Natl. Acad. Sci. USA. 2021;118:e2020838118. doi: 10.1073/pnas.2020838118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conner E.A., Lemmer E.R., Sánchez A., Factor V.M., Thorgeirsson S.S. E2F1 Blocks and C-Myc Accelerates Hepatic Ploidy in Transgenic Mouse Models. Biochem. Biophys. Res. Commun. 2003;302:114–120. doi: 10.1016/S0006-291X(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 71.Anatskaya O.V., Vinogradov A.E. Genome Multiplication as Adaptation to Tissue Survival: Evidence from Gene Expression in Mammalian Heart and Liver. Genomics. 2007;89:70–80. doi: 10.1016/j.ygeno.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Vazquez-Martin A., Anatskaya O.V., Giuliani A., Erenpreisa J., Huang S., Salmina K., Inashkina I., Huna A., Nikolsky N.N., Vinogradov A.E. Somatic Polyploidy Is Associated with the Upregulation of C-MYC Interacting Genes and EMT-like Signature. Oncotarget. 2016;7:75235–75260. doi: 10.18632/oncotarget.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anatskaya O.V., Vinogradov A.E., Vainshelbaum N.M., Giuliani A., Erenpreisa J. Phylostratic Shift of Whole-Genome Duplications in Normal Mammalian Tissues towards Unicellularity Is Driven by Developmental Bivalent Genes and Reveals a Link to Cancer. Int. J. Mol. Sci. 2020;21:8759. doi: 10.3390/ijms21228759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgar B.A., Orr-Weaver T.L. Endoreplication Cell Cycles: More for Less. Cell. 2001;105:297–306. doi: 10.1016/S0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 75.Grendler J., Lowgren S., Mills M., Losick V.P. Wound-Induced Polyploidization Is Driven by Myc and Supports Tissue Repair in the Presence of DNA Damage. Development. 2019;146:dev173005. doi: 10.1242/dev.173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Almeida Machado Costa C., Wang X.-F., Ellsworth C., Deng W.-M. Polyploidy in Development and Tumor Models in Drosophila. Semin. Cancer Biol. 2022;81:106–118. doi: 10.1016/j.semcancer.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam S., Paek A.L., Hammer M., Rangarajan S., Ruijtenbeek R., Cooke L., Weterings E., Mahadevan D. Drug-Induced Aneuploidy and Polyploidy Is a Mechanism of Disease Relapse in MYC/BCL2-Addicted Diffuse Large B-Cell Lymphoma. Oncotarget. 2018;9:35875–35890. doi: 10.18632/oncotarget.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zajac-Kaye M. Myc Oncogene: A Key Component in Cell Cycle Regulation and Its Implication for Lung Cancer. Lung Cancer. 2001;34((Suppl. S2)):S43–S46. doi: 10.1016/S0169-5002(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 79.Losick V.P. Wound-Induced Polyploidy Is Required for Tissue Repair. Adv. Wound Care. 2016;5:271–278. doi: 10.1089/wound.2014.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fatma H., Maurya S.K., Siddique H.R. Epigenetic Modifications of C-MYC: Role in Cancer Cell Reprogramming, Progression and Chemoresistance. Semin. Cancer Biol. 2022;83:166–176. doi: 10.1016/j.semcancer.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Wolpaw A.J., Dang C.V. MYC-Induced Metabolic Stress and Tumorigenesis. Biochim. Biophys. Acta Rev. Cancer. 2018;1870:43–50. doi: 10.1016/j.bbcan.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Dhanasekaran R., Deutzmann A., Mahauad-Fernandez W.D., Hansen A.S., Gouw A.M., Felsher D.W. The MYC Oncogene—The Grand Orchestrator of Cancer Growth and Immune Evasion. Nat. Rev. Clin. Oncol. 2022;19:23–36. doi: 10.1038/s41571-021-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poole C.J., van Riggelen J. MYC-Master Regulator of the Cancer Epigenome and Transcriptome. Genes. 2017;8:142. doi: 10.3390/genes8050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scafuro M., Capasso L., Carafa V., Altucci L., Nebbioso A. Gene Transactivation and Transrepression in MYC-Driven Cancers. Int. J. Mol. Sci. 2021;22:3458. doi: 10.3390/ijms22073458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmadi S.E., Rahimi S., Zarandi B., Chegeni R., Safa M. MYC: A Multipurpose Oncogene with Prognostic and Therapeutic Implications in Blood Malignancies. J. Hematol. Oncol. 2021;14:121. doi: 10.1186/s13045-021-01111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Llombart V., Mansour M.R. Therapeutic Targeting of “Undruggable” MYC. EBioMedicine. 2022;75:103756. doi: 10.1016/j.ebiom.2021.103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moein S., Adibi R., da Silva Meirelles L., Nardi N.B., Gheisari Y. Cancer Regeneration: Polyploid Cells Are the Key Drivers of Tumor Progression. Biochim. Biophys. Acta Rev. Cancer. 2020;1874:188408. doi: 10.1016/j.bbcan.2020.188408. [DOI] [PubMed] [Google Scholar]
- 88.Peres L.M.C., Falco J.R.P., Aguirra T.J., Mello M.L.S. Feulgen-DNA Content and Chromatin Organization in the Spermathecal Glands of Apis Mellifera (Hymenoptera, Apoidea) Queens with Aging. Apidologie. 2014;45:601–609. doi: 10.1007/s13592-014-0277-2. [DOI] [Google Scholar]
- 89.Moraes A.S., Guaraldo A.M.A., Mello M.L.S. Chromatin Supraorganization and Extensibility in Mouse Hepatocytes with Development and Aging. Cytometry A. 2007;71:28–37. doi: 10.1002/cyto.a.20356. [DOI] [PubMed] [Google Scholar]
- 90.Ghiraldini F.G., Silva I.S., Mello M.L.S. Polyploidy and Chromatin Remodeling in Hepatocytes from Insulin-Dependent Diabetic and Normoglycemic Aged Mice. Cytometry A. 2012;81:755–764. doi: 10.1002/cyto.a.22102. [DOI] [PubMed] [Google Scholar]
- 91.Wigglesworth V.B. Polyploidy and Nuclear Fusion in the Fat Body of Rhodnius (Hemiptera) J. Cell Sci. 1967;2:603–616. doi: 10.1242/jcs.2.4.603. [DOI] [PubMed] [Google Scholar]
- 92.Mello M.L.S. Nuclear Fusion and Change in Chromatin Packing State in Response to Starvation in Triatoma Infestans. Rev. Bras. Genet. 1989;12:495–498. [Google Scholar]
- 93.Mello M.L., Raymundo H.H. Nuclear Fusion in the Malpighian Tubes of a Blood-Sucking Hemipteran. Cytologia. 1980;45:203–209. doi: 10.1508/cytologia.45.203. [DOI] [PubMed] [Google Scholar]
- 94.Vainshelbaum N.M., Salmina K., Gerashchenko B.I., Lazovska M., Zayakin P., Cragg M.S., Pjanova D., Erenpreisa J. Role of the Circadian Clock “Death-Loop” in the DNA Damage Response Underpinning Cancer Treatment Resistance. Cells. 2022;11:880. doi: 10.3390/cells11050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salmina K., Bojko A., Inashkina I., Staniak K., Dudkowska M., Podlesniy P., Rumnieks F., Vainshelbaum N.M., Pjanova D., Sikora E., et al. “Mitotic Slippage” and Extranuclear DNA in Cancer Chemoresistance: A Focus on Telomeres. Int. J. Mol. Sci. 2020;21:2779. doi: 10.3390/ijms21082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Storchová Z., Breneman A., Cande J., Dunn J., Burbank K., O’Toole E., Pellman D. Genome-Wide Genetic Analysis of Polyploidy in Yeast. Nature. 2006;443:541–547. doi: 10.1038/nature05178. [DOI] [PubMed] [Google Scholar]
- 97.Mittal K., Donthamsetty S., Kaur R., Yang C., Gupta M.V., Reid M.D., Choi D.H., Rida P.C.G., Aneja R. Multinucleated Polyploidy Drives Resistance to Docetaxel Chemotherapy in Prostate Cancer. Br. J. Cancer. 2017;116:1186–1194. doi: 10.1038/bjc.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Filatova N.A., Knyazev N.A., Skarlato S.O., Anatskaya O.V., Vinogradov A.E. Natural Killer Cell Activity Irreversibly Decreases after Cryptosporidium Gastroenteritis in Neonatal Mice. Parasite Immunol. 2018;40:e12524. doi: 10.1111/pim.12524. [DOI] [PubMed] [Google Scholar]
- 99.Kieffer-Kwon K.-R., Nimura K., Rao S.S.P., Xu J., Jung S., Pekowska A., Dose M., Stevens E., Mathe E., Dong P., et al. Myc Regulates Chromatin Decompaction and Nuclear Architecture during B Cell Activation. Mol. Cell. 2017;67:566–578.e10. doi: 10.1016/j.molcel.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He L., Ding Y., Zhao Y., So K.K., Peng X.L., Li Y., Yuan J., He Z., Chen X., Sun H., et al. CRISPR/Cas9/AAV9-Mediated in Vivo Editing Identifies MYC Regulation of 3D Genome in Skeletal Muscle Stem Cell. Stem. Cell Rep. 2021;16:2442–2458. doi: 10.1016/j.stemcr.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kind J., Pagie L., de Vries S.S., Nahidiazar L., Dey S.S., Bienko M., Zhan Y., Lajoie B., de Graaf C.A., Amendola M., et al. Genome-Wide Maps of Nuclear Lamina Interactions in Single Human Cells. Cell. 2015;163:134–147. doi: 10.1016/j.cell.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stevens T.J., Lando D., Basu S., Atkinson L.P., Cao Y., Lee S.F., Leeb M., Wohlfahrt K.J., Boucher W., O’Shaughnessy-Kirwan A., et al. 3D Structures of Individual Mammalian Genomes Studied by Single-Cell Hi-C. Nature. 2017;544:59–64. doi: 10.1038/nature21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malashicheva A., Perepelina K. Diversity of Nuclear Lamin A/C Action as a Key to Tissue-Specific Regulation of Cellular Identity in Health and Disease. Front. Cell Dev. Biol. 2021;9:761469. doi: 10.3389/fcell.2021.761469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briand N., Collas P. Lamina-Associated Domains: Peripheral Matters and Internal Affairs. Genome Biol. 2020;21:85. doi: 10.1186/s13059-020-02003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bertero A., Rosa-Garrido M. Three-Dimensional Chromatin Organization in Cardiac Development and Disease. J. Mol. Cell Cardiol. 2021;151:89–105. doi: 10.1016/j.yjmcc.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sas-Nowosielska H., Bernas T. Spatial Relationship between Chromosomal Domains in Diploid and Autotetraploid Arabidopsis Thaliana Nuclei. Nucleus. 2016;7:216–231. doi: 10.1080/19491034.2016.1182277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Lozano M., Natarajan P., Levi A., Katam R., Lopez-Ortiz C., Nimmakayala P., Reddy U.K. Altered Chromatin Conformation and Transcriptional Regulation in Watermelon Following Genome Doubling. Plant J. 2021;106:588–600. doi: 10.1111/tpj.15256. [DOI] [PubMed] [Google Scholar]
- 108.Wang X.-F., Yang S.-A., Gong S., Chang C.-H., Portilla J.M., Chatterjee D., Irianto J., Bao H., Huang Y.-C., Deng W.-M. Polyploid Mitosis and Depolyploidization Promote Chromosomal Instability and Tumor Progression in a Notch-Induced Tumor Model. Dev. Cell. 2021;56:1976–1988.e4. doi: 10.1016/j.devcel.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwarz-Finsterle J., Scherthan H., Huna A., González P., Mueller P., Schmitt E., Erenpreisa J., Hausmann M. Volume Increase and Spatial Shifts of Chromosome Territories in Nuclei of Radiation-Induced Polyploidizing Tumour Cells. Mutat. Res. 2013;756:56–65. doi: 10.1016/j.mrgentox.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 110.Bellanger A., Madsen-Østerbye J., Galigniana N.M., Collas P. Restructuring of Lamina-Associated Domains in Senescence and Cancer. Cells. 2022;11:1846. doi: 10.3390/cells11111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baluapuri A., Wolf E., Eilers M. Target Gene-Independent Functions of MYC Oncoproteins. Nat. Rev. Mol. Cell Biol. 2020;21:255–267. doi: 10.1038/s41580-020-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guccione E., Martinato F., Finocchiaro G., Luzi L., Tizzoni L., Dall’ Olio V., Zardo G., Nervi C., Bernard L., Amati B. Myc-Binding-Site Recognition in the Human Genome Is Determined by Chromatin Context. Nat. Cell Biol. 2006;8:764–770. doi: 10.1038/ncb1434. [DOI] [PubMed] [Google Scholar]
- 113.Samoylova E.M., Baklaushev V.P. Cell Reprogramming Preserving Epigenetic Age: Advantages and Limitations. Biochemistry. 2020;85:1035–1047. doi: 10.1134/S0006297920090047. [DOI] [PubMed] [Google Scholar]
- 114.Neri F., Zippo A., Krepelova A., Cherubini A., Rocchigiani M., Oliviero S. Myc Regulates the Transcription of the PRC2 Gene to Control the Expression of Developmental Genes in Embryonic Stem Cells. Mol. Cell Biol. 2012;32:840–851. doi: 10.1128/MCB.06148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ullius A., Lüscher-Firzlaff J., Costa I.G., Walsemann G., Forst A.H., Gusmao E.G., Kapelle K., Kleine H., Kremmer E., Vervoorts J., et al. The Interaction of MYC with the Trithorax Protein ASH2L Promotes Gene Transcription by Regulating H3K27 Modification. Nucleic Acids Res. 2014;42:6901–6920. doi: 10.1093/nar/gku312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf E., Lin C.Y., Eilers M., Levens D.L. Taming of the Beast: Shaping Myc-Dependent Amplification. Trends Cell Biol. 2015;25:241–248. doi: 10.1016/j.tcb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martinato F., Cesaroni M., Amati B., Guccione E. Analysis of Myc-Induced Histone Modifications on Target Chromatin. PLoS ONE. 2008;3:e3650. doi: 10.1371/journal.pone.0003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pellanda P., Dalsass M., Filipuzzi M., Loffreda A., Verrecchia A., Castillo Cano V., Thabussot H., Doni M., Morelli M.J., Soucek L., et al. Integrated Requirement of Non-Specific and Sequence-Specific DNA Binding in Myc-Driven Transcription. EMBO J. 2021;40:e105464. doi: 10.15252/embj.2020105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amati B., Frank S.R., Donjerkovic D., Taubert S. Function of the C-Myc Oncoprotein in Chromatin Remodeling and Transcription. Biochim. Biophys. Acta. 2001;1471:M135–M145. doi: 10.1016/S0304-419X(01)00020-8. [DOI] [PubMed] [Google Scholar]
- 120.de Pretis S., Kress T.R., Morelli M.J., Sabò A., Locarno C., Verrecchia A., Doni M., Campaner S., Amati B., Pelizzola M. Integrative Analysis of RNA Polymerase II and Transcriptional Dynamics upon MYC Activation. Genome Res. 2017;27:1658–1664. doi: 10.1101/gr.226035.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kress T.R., Sabò A., Amati B. MYC: Connecting Selective Transcriptional Control to Global RNA Production. Nat. Rev. Cancer. 2015;15:593–607. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 122.Gomez-Roman N., Grandori C., Eisenman R.N., White R.J. Direct Activation of RNA Polymerase III Transcription by C-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- 123.Grandori C., Gomez-Roman N., Felton-Edkins Z.A., Ngouenet C., Galloway D.A., Eisenman R.N., White R.J. C-Myc Binds to Human Ribosomal DNA and Stimulates Transcription of RRNA Genes by RNA Polymerase I. Nat. Cell Biol. 2005;7:311–318. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 124.Seoane J., Le H.-V., Massagué J. Myc Suppression of the P21(Cip1) Cdk Inhibitor Influences the Outcome of the P53 Response to DNA Damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 125.Barna M., Pusic A., Zollo O., Costa M., Kondrashov N., Rego E., Rao P.H., Ruggero D. Suppression of Myc Oncogenic Activity by Ribosomal Protein Haploinsufficiency. Nature. 2008;456:971–975. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D’Andrea A., Gritti I., Nicoli P., Giorgio M., Doni M., Conti A., Bianchi V., Casoli L., Sabò A., Mironov A., et al. The Mitochondrial Translation Machinery as a Therapeutic Target in Myc-Driven Lymphomas. Oncotarget. 2016;7:72415–72430. doi: 10.18632/oncotarget.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao L.-J., Loewenstein P.M., Green M. Enhanced MYC Association with the NuA4 Histone Acetyltransferase Complex Mediated by the Adenovirus E1A N-Terminal Domain Activates a Subset of MYC Target Genes Highly Expressed in Cancer Cells. Genes Cancer. 2017;8:752–761. doi: 10.18632/genesandcancer.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hota S.K., Bruneau B.G. ATP-Dependent Chromatin Remodeling during Mammalian Development. Development. 2016;143:2882–2897. doi: 10.1242/dev.128892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colino-Sanguino Y., Clark S.J., Valdes-Mora F. The H2A.Z-Nuclesome Code in Mammals: Emerging Functions. Trends Genet. 2022;38:273–289. doi: 10.1016/j.tig.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 130.Sales-Gil R., Kommer D.C., de Castro I.J., Amin H.A., Vinciotti V., Sisu C., Vagnarelli P. Non-Redundant Functions of H2A.Z.1 and H2A.Z.2 in Chromosome Segregation and Cell Cycle Progression. EMBO Rep. 2021;22:e52061. doi: 10.15252/embr.202052061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu Y., Lai Y., Chen X., Zhou D.-X., Zhao Y. Distribution Pattern of Histone Marks Potentially Determines Their Roles in Transcription and RNA Processing in Rice. J. Plant Physiol. 2020;249:153167. doi: 10.1016/j.jplph.2020.153167. [DOI] [PubMed] [Google Scholar]
- 132.Stojanova A., Tu W.B., Ponzielli R., Kotlyar M., Chan P.-K., Boutros P.C., Khosravi F., Jurisica I., Raught B., Penn L.Z. MYC Interaction with the Tumor Suppressive SWI/SNF Complex Member INI1 Regulates Transcription and Cellular Transformation. Cell Cycle. 2016;15:1693–1705. doi: 10.1080/15384101.2016.1146836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.González B., Navarro-Jiménez M., Alonso-De Gennaro M.J., Jansen S.M., Granada I., Perucho M., Alonso S. Somatic Hypomethylation of Pericentromeric SST1 Repeats and Tetraploidization in Human Colorectal Cancer Cells. Cancers. 2021;13:5353. doi: 10.3390/cancers13215353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeimet A.G., Fiegl H., Goebel G., Kopp F., Allasia C., Reimer D., Steppan I., Mueller-Holzner E., Ehrlich M., Marth C. DNA Ploidy, Nuclear Size, Proliferation Index and DNA-Hypomethylation in Ovarian Cancer. Gynecol. Oncol. 2011;121:24–31. doi: 10.1016/j.ygyno.2010.12.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zybina T.G. Genome Modifications Involved in Developmental Programs of the Placental Trophoblast. In: Larramendy M.L., Soloneski S., editors. Cytogenetics—Classical and Molecular Strategies for Analysing Heredity Material. IntechOpen; London, UK: 2021. [Google Scholar]
- 136.Zybina T.G., Zybina E.V. Role of Cell Cycling and Polyploidy in Placental Trophoblast of Different Mammalian Species. Reprod. Domest. Anim. 2020;55:895–904. doi: 10.1111/rda.13732. [DOI] [PubMed] [Google Scholar]
- 137.Nagymihály M., Veluchamy A., Györgypál Z., Ariel F., Jégu T., Benhamed M., Szűcs A., Kereszt A., Mergaert P., Kondorosi É. Ploidy-Dependent Changes in the Epigenome of Symbiotic Cells Correlate with Specific Patterns of Gene Expression. Proc. Natl. Acad. Sci. USA. 2017;114:4543–4548. doi: 10.1073/pnas.1704211114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang L., Jia G., Jiang X., Cao S., Chen Z.J., Song Q. Altered Chromatin Architecture and Gene Expression during Polyploidization and Domestication of Soybean. Plant Cell. 2021;33:1430–1446. doi: 10.1093/plcell/koab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng M., Lin J., Liu X., Chu W., Li J., Gao Y., An K., Song W., Xin M., Yao Y., et al. Histone Acetyltransferase TaHAG1 Acts as a Crucial Regulator to Strengthen Salt Tolerance of Hexaploid Wheat. Plant Physiol. 2021;186:1951–1969. doi: 10.1093/plphys/kiab187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bernhard S.V., Seget-Trzensiok K., Kuffer C., Krastev D.B., Stautmeister L.-M., Theis M., Keuper K., Boekenkamp J.-E., Kschischo M., Buchholz F., et al. Loss of USP28 and SPINT2 Expression Promotes Cancer Cell Survival after Whole Genome Doubling. Cell Oncol. 2022;45:103–119. doi: 10.1007/s13402-021-00654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hayakawa K., Terada K., Takahashi T., Oana H., Washizu M., Tanaka S. Nucleosomes of Polyploid Trophoblast Giant Cells Mostly Consist of Histone Variants and Form a Loose Chromatin Structure. Sci. Rep. 2018;8:5811. doi: 10.1038/s41598-018-23832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schlesinger S., Meshorer E. Open Chromatin, Epigenetic Plasticity, and Nuclear Organization in Pluripotency. Dev. Cell. 2019;48:135–150. doi: 10.1016/j.devcel.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 143.Lim P.S.L., Meshorer E. Organization of the Pluripotent Genome. Cold Spring Harb. Perspect. Biol. 2021;13:a040204. doi: 10.1101/cshperspect.a040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng Y., Liu X., Pauklin S. 3D Chromatin Architecture and Epigenetic Regulation in Cancer Stem Cells. Protein Cell. 2021;12:440–454. doi: 10.1007/s13238-020-00819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meshorer E., Plath K. Chromatin and Nuclear Architecture in Stem Cells. Stem Cell Rep. 2020;15:1155–1157. doi: 10.1016/j.stemcr.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dixon J.R., Jung I., Selvaraj S., Shen Y., Antosiewicz-Bourget J.E., Lee A.Y., Ye Z., Kim A., Rajagopal N., Xie W., et al. Chromatin Architecture Reorganization during Stem Cell Differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meškytė E.M., Keskas S., Ciribilli Y. MYC as a Multifaceted Regulator of Tumor Microenvironment Leading to Metastasis. Int. J. Mol. Sci. 2020;21:7710. doi: 10.3390/ijms21207710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 149.Erenpreisa J.A., Cragg M.S., Fringes B., Sharakhov I., Illidge T.M. Release of Mitotic Descendants by Giant Cells from Irradiated Burkitt’s Lymphoma Cell Line. Cell Biol. Int. 2000;24:635–648. doi: 10.1006/cbir.2000.0558. [DOI] [PubMed] [Google Scholar]
- 150.Salmina K., Jankevics E., Huna A., Perminov D., Radovica I., Klymenko T., Ivanov A., Jascenko E., Scherthan H., Cragg M., et al. Up-Regulation of the Embryonic Self-Renewal Network through Reversible Polyploidy in Irradiated P53-Mutant Tumour Cells. Exp. Cell Res. 2010;316:2099–2112. doi: 10.1016/j.yexcr.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 151.Pirsko V., Čakstiņa I., Nitiša D., Samoviča M., Daneberga Z., Miklaševičs E. Alterations of The Stem-Like Properties in The Breast Cancer Cell Line MDA-MB-231 Induced by Single Pulsed Doxorubicin Treatment. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2019;73:89–99. doi: 10.2478/prolas-2019-0015. [DOI] [Google Scholar]
- 152.Niu N., Mercado-Uribe I., Liu J. Dedifferentiation into Blastomere-like Cancer Stem Cells via Formation of Polyploid Giant Cancer Cells. Oncogene. 2017;36:4887–4900. doi: 10.1038/onc.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salmina K., Huna A., Kalejs M., Pjanova D., Scherthan H., Cragg M.S., Erenpreisa J. The Cancer Aneuploidy Paradox: In the Light of Evolution. Genes. 2019;10:83. doi: 10.3390/genes10020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erenpreisa J., Cragg M.S. Three Steps to the Immortality of Cancer Cells: Senescence, Polyploidy and Self-Renewal. Cancer Cell Int. 2013;13:92. doi: 10.1186/1475-2867-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ērenpreisa J. Oïìerts Çrenpreiss and His School of Cancer Research: Commemorating The 90th Anniversary. Proc. Latv. Acad. Sci. B Nat. Exact Appl. Sci. 2019;73:533–537. doi: 10.2478/prolas-2019-0081. [DOI] [Google Scholar]
- 156.Díaz-Carballo D., Saka S., Klein J., Rennkamp T., Acikelli A.H., Malak S., Jastrow H., Wennemuth G., Tempfer C., Schmitz I., et al. A Distinct Oncogenerative Multinucleated Cancer Cell Serves as a Source of Stemness and Tumor Heterogeneity. Cancer Res. 2018;78:2318–2331. doi: 10.1158/0008-5472.CAN-17-1861. [DOI] [PubMed] [Google Scholar]
- 157.Erenpreisa J., Cragg M.S. MOS, Aneuploidy and the Ploidy Cycle of Cancer Cells. Oncogene. 2010;29:5447–5451. doi: 10.1038/onc.2010.310. [DOI] [PubMed] [Google Scholar]
- 158.Besen-McNally R., Gjelsvik K.J., Losick V.P. Wound-Induced Polyploidization Is Dependent on Integrin-Yki Signaling. Biol. Open. 2021;10:bio055996. doi: 10.1242/bio.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Broughton K.M., Sussman M.A. Adult Cardiomyocyte Cell Cycle Detour: Off-Ramp to Quiescent Destinations. Trends Endocrinol. Metab. 2019;30:557–567. doi: 10.1016/j.tem.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katsuda T., Hosaka K., Matsuzaki J., Usuba W., Prieto-Vila M., Yamaguchi T., Tsuchiya A., Terai S., Ochiya T. Transcriptomic Dissection of Hepatocyte Heterogeneity: Linking Ploidy, Zonation, and Stem/Progenitor Cell Characteristics. Cell Mol. Gastroenterol. Hepatol. 2020;9:161–183. doi: 10.1016/j.jcmgh.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Evan G. Taking a Back Door to Target Myc. Science. 2012;335:293–294. doi: 10.1126/science.1217819. [DOI] [PubMed] [Google Scholar]
- 162.Court F., Arnaud P. An Annotated List of Bivalent Chromatin Regions in Human ES Cells: A New Tool for Cancer Epigenetic Research. Oncotarget. 2017;8:4110–4124. doi: 10.18632/oncotarget.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar D., Cinghu S., Oldfield A.J., Yang P., Jothi R. Decoding the Function of Bivalent Chromatin in Development and Cancer. Genome Res. 2021;31:2170–2184. doi: 10.1101/gr.275736.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blanco E., González-Ramírez M., Alcaine-Colet A., Aranda S., Di Croce L. The Bivalent Genome: Characterization, Structure, and Regulation. Trends Genet. 2020;36:118–131. doi: 10.1016/j.tig.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 165.Sonnenschein C., Soto A.M. Cancer Metastases: So Close and So Far. J. Natl. Cancer Inst. 2015;107:djv236. doi: 10.1093/jnci/djv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Valenzuela N., Fan Q., Fa’ak F., Soibam B., Nagandla H., Liu Y., Schwartz R.J., McConnell B.K., Stewart M.D. Cardiomyocyte-Specific Conditional Knockout of the Histone Chaperone HIRA in Mice Results in Hypertrophy, Sarcolemmal Damage and Focal Replacement Fibrosis. Dis. Models Mech. 2016;9:335–345. doi: 10.1242/dmm.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vinogradov A.E., Anatskaya O.V. Evolutionary Framework of the Human Interactome: Unicellular and Multicellular Giant Clusters. Biosystems. 2019;181:82–87. doi: 10.1016/j.biosystems.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 168.Vinogradov A.E., Anatskaya O.V. Gene Golden Age Paradox and Its Partial Solution. Genomics. 2019;111:115–126. doi: 10.1016/j.ygeno.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 169.Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 171.Kolodziejczyk A.A., Kim J.K., Tsang J.C.H., Ilicic T., Henriksson J., Natarajan K.N., Tuck A.C., Gao X., Bühler M., Liu P., et al. Single Cell RNA-Sequencing of Pluripotent States Unlocks Modular Transcriptional Variation. Cell Stem Cell. 2015;17:471–485. doi: 10.1016/j.stem.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Percharde M., Bulut-Karslioglu A., Ramalho-Santos M. Hypertranscription in Development, Stem Cells, and Regeneration. Dev. Cell. 2017;40:9–21. doi: 10.1016/j.devcel.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tang H.-Y., Goldman A.R., Zhang X., Speicher D.W., Dang C.V. Measuring MYC-Mediated Metabolism in Tumorigenesis. Methods Mol. Biol. 2021;2318:231–239. doi: 10.1007/978-1-0716-1476-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Menendez J.A. The Metaboloepigenetic Dimension of Cancer Stem Cells: Evaluating the Market Potential for New Metabostemness-Targeting Oncology Drugs. Curr. Pharm. Des. 2015;21:3644–3653. doi: 10.2174/1381612821666150710150327. [DOI] [PubMed] [Google Scholar]
- 175.Menendez J.A., Corominas-Faja B., Cuyàs E., Alarcón T. Metabostemness: Metaboloepigenetic Reprogramming of Cancer Stem-Cell Functions. Oncoscience. 2014;1:803–806. doi: 10.18632/oncoscience.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dudek J., Kutschka I., Maack C. Metabolic and Redox Regulation of Cardiovascular Stem Cell Biology and Pathology. Antioxid Redox Signal. 2021;35:163–181. doi: 10.1089/ars.2020.8201. [DOI] [PubMed] [Google Scholar]
- 177.Ivanova J.S., Lyublinskaya O.G. Redox Homeostasis and Regulation in Pluripotent Stem Cells: Uniqueness or Versatility? Int. J. Mol. Sci. 2021;22:10946. doi: 10.3390/ijms222010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koshkin S.A., Anatskaya O.V., Vinogradov A.E., Uversky V.N., Dayhoff G.W., Bystriakova M.A., Pospelov V.A., Tolkunova E.N. Isolation and Characterization of Human Colon Adenocarcinoma Stem-Like Cells Based on the Endogenous Expression of the Stem Markers. Int. J. Mol. Sci. 2021;22:4682. doi: 10.3390/ijms22094682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vinogradov A.E., Shilina M.A., Anatskaya O.V., Alekseenko L.L., Fridlyanskaya I.I., Krasnenko A., Kim A., Korostin D., Ilynsky V., Elmuratov A., et al. Molecular Genetic Analysis of Human Endometrial Mesenchymal Stem Cells That Survived Sublethal Heat Shock. Stem Cells Int. 2017;2017:2362630. doi: 10.1155/2017/2362630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sinenko S.A., Starkova T.Y., Kuzmin A.A., Tomilin A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021;9:714370. doi: 10.3389/fcell.2021.714370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Selenina A.V., Tsimokha A.S., Tomilin A.N. Proteasomes in Protein Homeostasis of Pluripotent Stem Cells. Acta Nat. 2017;9:39–47. doi: 10.32607/20758251-2017-9-3-39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anatskaya O. Polyploidy Activates Biological Pathways Related to Morphogenesis in Mammalian Tissues. MOJI. 2018;6:1. doi: 10.15406/moji.2018.06.00200. [DOI] [Google Scholar]
- 183.Mayfield-Jones D., Washburn J.D., Arias T., Edger P.P., Pires J.C., Conant G.C. Watching the Grin Fade: Tracing the Effects of Polyploidy on Different Evolutionary Time Scales. Semin. Cell Dev. Biol. 2013;24:320–331. doi: 10.1016/j.semcdb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 184.Kasperski A. Life Entrapped in a Network of Atavistic Attractors: How to Find a Rescue. Int. J. Mol. Sci. 2022;23:4017. doi: 10.3390/ijms23074017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kasperski A., Kasperska R. Bioenergetics of Life, Disease and Death Phenomena. Theory Biosci. 2018;137:155–168. doi: 10.1007/s12064-018-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pandit S.K., Westendorp B., de Bruin A. Physiological Significance of Polyploidization in Mammalian Cells. Trends Cell Biol. 2013;23:556–566. doi: 10.1016/j.tcb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 187.Vinogradov A.E., Anatskaya O.V. Phenological Resonance and Quantum Life History. J. Theor. Biol. 2004;228:417–420. doi: 10.1016/j.jtbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 188.Øvrebø J.I., Edgar B.A. Polyploidy in Tissue Homeostasis and Regeneration. Development. 2018;145:dev156034. doi: 10.1242/dev.156034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vinogradov A.E., Anatskaya O.V. Growth of Biological Complexity from Prokaryotes to Hominids Reflected in the Human Genome. Int. J. Mol. Sci. 2021;22:11640. doi: 10.3390/ijms222111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Young S.L., Diolaiti D., Conacci-Sorrell M., Ruiz-Trillo I., Eisenman R.N., King N. Premetazoan Ancestry of the Myc-Max Network. Mol. Biol. Evol. 2011;28:2961–2971. doi: 10.1093/molbev/msr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahani A., Henriksson J., Wright A.P.H. Origins of Myc Proteins--Using Intrinsic Protein Disorder to Trace Distant Relatives. PLoS ONE. 2013;8:e75057. doi: 10.1371/journal.pone.0075057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vinogradov A.E., Anatskaya O.V. Cell-Cycle Dependence of Transcriptome Gene Modules: Comparison of Regression Lines. FEBS J. 2020 doi: 10.1111/febs.15257. in press . [DOI] [PubMed] [Google Scholar]
- 193.Trigos A.S., Pearson R.B., Papenfuss A.T., Goode D.L. How the Evolution of Multicellularity Set the Stage for Cancer. Br. J. Cancer. 2018;118:145–152. doi: 10.1038/bjc.2017.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Trigos A.S., Pearson R.B., Papenfuss A.T., Goode D.L. Somatic Mutations in Early Metazoan Genes Disrupt Regulatory Links between Unicellular and Multicellular Genes in Cancer. Elife. 2019;8:e40947. doi: 10.7554/eLife.40947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kozlov A.P. Mammalian Tumor-like Organs. 2. Mammalian Adipose Has Many Tumor Features and Obesity Is a Tumor-like Process. Infect Agent Cancer. 2022;17:15. doi: 10.1186/s13027-022-00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Niculescu V.F. Cancer Genes and Cancer Stem Cells in Tumorigenesis: Evolutionary Deep Homology and Controversies. Genes Dis. 2022;9:1234–1247. doi: 10.1016/j.gendis.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Niculescu V.F. ACLS Cancers: Genomic and Epigenetic Changes Transform the Cell of Origin of Cancer into a Tumorigenic Pathogen of Unicellular Organization and Lifestyle. Gene. 2020;726:144174. doi: 10.1016/j.gene.2019.144174. [DOI] [PubMed] [Google Scholar]
- 198.Vinogradov A.E., Anatskaya O.V. Systemic Evolutionary Changes in Mammalian Gene Expression. Biosystems. 2020;198:104256. doi: 10.1016/j.biosystems.2020.104256. [DOI] [PubMed] [Google Scholar]
- 199.Anatskaia O.V., Sidorenko N.V., Beĭer T.V., Vinogradov A.E. Neonatal gastroenteritis triggers long-term cardiomyocyte atrophy, remodeling and irreversible hyperpolyploidization. Kardiologiia. 2010;50:35–44. [PubMed] [Google Scholar]
- 200.Lineweaver C.H., Davies P.C.W., Vincent M.D. Targeting Cancer’s Weaknesses (Not Its Strengths): Therapeutic Strategies Suggested by the Atavistic Model. Bioessays. 2014;36:827–835. doi: 10.1002/bies.201400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vincent M.D. Cancer: Beyond Speciation. Adv. Cancer Res. 2011;112:283–350. doi: 10.1016/B978-0-12-387688-1.00010-7. [DOI] [PubMed] [Google Scholar]
- 202.Vincent M.D. Optimizing the Management of Advanced Non-Small-Cell Lung Cancer: A Personal View. Curr. Oncol. 2009;16:9–21. doi: 10.3747/co.v16i4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Trigos A.S., Pearson R.B., Papenfuss A.T., Goode D.L. Altered Interactions between Unicellular and Multicellular Genes Drive Hallmarks of Transformation in a Diverse Range of Solid Tumors. Proc. Natl. Acad. Sci. USA. 2017;114:6406–6411. doi: 10.1073/pnas.1617743114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fowler T., Suh H., Buratowski S., Roy A.L. Regulation of Primary Response Genes in B Cells. J. Biol. Chem. 2013;288:14906–14916. doi: 10.1074/jbc.M113.454355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Heng J., Heng H.H. Two-Phased Evolution: Genome Chaos-Mediated Information Creation and Maintenance. Prog. Biophys. Mol. Biol. 2021;165:29–42. doi: 10.1016/j.pbiomolbio.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 206.Heng J., Heng H.H. Genome Chaos: Creating New Genomic Information Essential for Cancer Macroevolution. Semin. Cancer Biol. 2022;81:160–175. doi: 10.1016/j.semcancer.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 207.Huang S. Reconciling Non-Genetic Plasticity with Somatic Evolution in Cancer. Trends Cancer. 2021;7:309–322. doi: 10.1016/j.trecan.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 208.Huang S., Ernberg I., Kauffman S. Cancer Attractors: A Systems View of Tumors from a Gene Network Dynamics and Developmental Perspective. Semin. Cell Dev. Biol. 2009;20:869–876. doi: 10.1016/j.semcdb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.De Smet R., Van de Peer Y. Redundancy and Rewiring of Genetic Networks Following Genome-Wide Duplication Events. Curr. Opin. Plant Biol. 2012;15:168–176. doi: 10.1016/j.pbi.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 210.Tsuchiya M., Giuliani A., Hashimoto M., Erenpreisa J., Yoshikawa K. Emergent Self-Organized Criticality in Gene Expression Dynamics: Temporal Development of Global Phase Transition Revealed in a Cancer Cell Line. PLoS ONE. 2015;10:e0128565. doi: 10.1371/journal.pone.0128565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tsuchiya M., Giuliani A., Hashimoto M., Erenpreisa J., Yoshikawa K. Self-Organizing Global Gene Expression Regulated through Criticality: Mechanism of the Cell-Fate Change. PLoS ONE. 2016;11:e0167912. doi: 10.1371/journal.pone.0167912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Defoort J., Van de Peer Y., Vermeirssen V. Function, Dynamics and Evolution of Network Motif Modules in Integrated Gene Regulatory Networks of Worm and Plant. Nucleic Acids Res. 2018;46:6480–6503. doi: 10.1093/nar/gky468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mojtahedi M., Skupin A., Zhou J., Castaño I.G., Leong-Quong R.Y.Y., Chang H., Trachana K., Giuliani A., Huang S. Cell Fate Decision as High-Dimensional Critical State Transition. PLoS Biol. 2016;14:e2000640. doi: 10.1371/journal.pbio.2000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zheng L., Dai H., Zhou M., Li X., Liu C., Guo Z., Wu X., Wu J., Wang C., Zhong J., et al. Polyploid Cells Rewire DNA Damage Response Networks to Overcome Replication Stress-Induced Barriers for Tumour Progression. Nat. Commun. 2012;3:815. doi: 10.1038/ncomms1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shilina M.A., Grinchuk T.M., Anatskaya O.V., Vinogradov A.E., Alekseenko L.L., Elmuratov A.U., Nikolsky N.N. Cytogenetic and Transcriptomic Analysis of Human Endometrial MSC Retaining Proliferative Activity after Sublethal Heat Shock. Cells. 2018;7:184. doi: 10.3390/cells7110184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tamori Y., Deng W.-M. Tissue Repair through Cell Competition and Compensatory Cellular Hypertrophy in Postmitotic Epithelia. Dev. Cell. 2013;25:350–363. doi: 10.1016/j.devcel.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dhimolea E., de Matos Simoes R., Kansara D., Al’Khafaji A., Bouyssou J., Weng X., Sharma S., Raja J., Awate P., Shirasaki R., et al. An Embryonic Diapause-like Adaptation with Suppressed Myc Activity Enables Tumor Treatment Persistence. Cancer Cell. 2021;39:240–256.e11. doi: 10.1016/j.ccell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]