Abstract
Ceramides are a class of sphingolipids which are implicated in skin disorders, obesity, and other metabolic diseases. As a class with pleiotropic effects, recent efforts have centred on discerning specific ceramide species and their effects on atopic dermatitis, obesity, type 2 diabetes, and cardiovascular diseases. This delineation has allowed the identification of disease biomarkers, with long acyl chain ceramides such as C16- and C18-ceramides linked to metabolic dysfunction and cardiac function decline, while ultra-long acyl chain ceramides (>25 carbon acyl chain) were reported to be essential for maintaining a functional skin barrier. Given the intricate link between free fatty acids with ceramides, especially the de novo synthetic pathway, intracellular lipid droplet formation is increasingly viewed as an important mechanism for preventing accumulation of toxic ceramide species. Here, we review recent reports of various ceramide species involved in skin abnormalities and metabolic diseases, and we propose that promotion of lipid droplet biogenesis can be seen as a potential protective mechanism against deleterious ceramides.
Keywords: ceramides, lipid droplets, intracellular fatty acids, metabolic disease, skin disorders
1. Introduction
Recently, there has been considerable interest in a subtype of sphingolipids known as ceramides, and in particular ceramide level derangements in skin and metabolic health. While the pleiotropic effects of ceramides have been reviewed extensively [1,2,3,4,5,6,7,8,9], knowledge of its abundance in relation to intracellular lipid partitioning and regulation remains patchy. This review starts with a summary on the significance of ceramides in well-studied areas such as skin abnormalities and metabolic disease, before a focus on current knowledge surrounding intracellular lipid regulation and its relation to intracellular ceramide levels.
2. Ceramides Synthesis
Ceramides are a class of bioactive lipids which comprise of a sphingoid base (commonly sphingosine) and a fatty acyl chain, and they were largely generalised as ER stress, apoptotic triggering molecules in various cell types [10,11,12]. The toxicity of a ceramide species is linked to the length, and the degree of saturation, of fatty acyl chains. For example, saturated C18:0 and C16:0 ceramides were more toxic to HeLa cells compared to C18:1 and C24:1 ceramides [13]. Of note, the sphingoid base of the sphingolipids is also a crucial determinant of its toxicity, as dihydroceramide, which lacks a double bond in its sphingoid base in comparison to ceramides, does not share similar cytotoxicity as observed in ceramides [14,15].
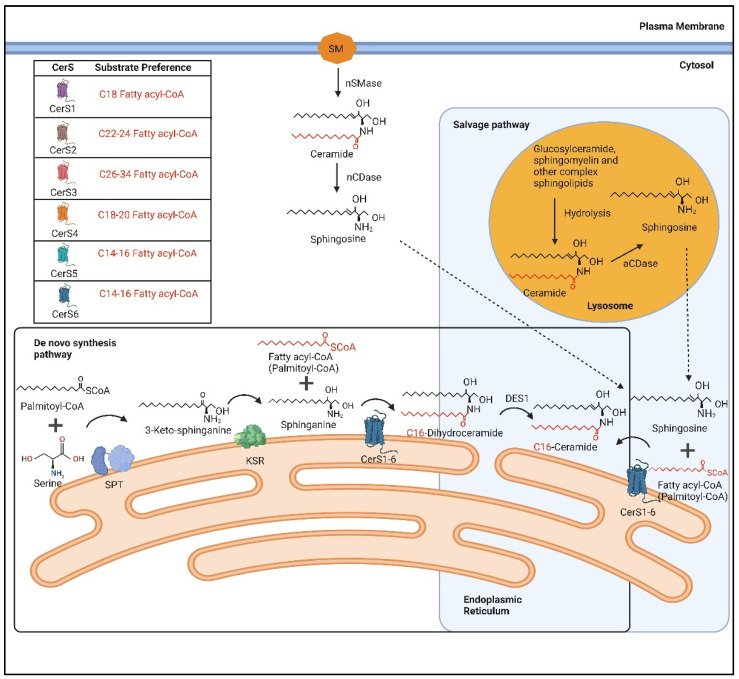
The generation of ceramides occurs through three pathways: the de novo synthesis pathway; the salvage pathway; and the sphingomyelinase pathway. The de novo synthesis pathway occurs at the ER and begins with the condensation of serine with palmitoyl-CoA to form 3-keto-sphinganine, a process catalysed by serine-palmitoyltransferase (SPT) (Figure 1) [16]. Subsequently, 3-keto-sphinganine is reduced to sphinganine by 3-keto-sphinganine reductase (KSR) (Figure 1) [16]. Following this process, an acyl-CoA chain will be added to sphinganine to form dihydroceramides, and this process is facilitated by a group of ER resident enzymes known as ceramide synthases (CerS) (Figure 1) [16]. Lastly, dihydroceramides are converted to ceramides by dihydroceramide desaturase 1 (DES1) (Figure 1) [16].
Figure 1.
An illustration of the different ceramides generated either through the de novo synthesis, or the salvage pathway. aCDase: acid ceramidase; nCDase: neutral ceramidase; nSMase: neutral sphingomyelinase; CerS: ceramide synthases; DES1: dihydroceramide desaturase-1; KSR: 3-ketosphinganine reductase; SPT: serine palmitoyltransferase. Created with BioRender.com.
The salvage pathway involves the degradation of glucosylceramide, sphingomyelin, and other complex sphingolipids, in acidic organelles such as the lysosome or late endosome, to form ceramides (Figure 1) [17]. These ceramides are then broken down by acid ceramidase to form a sphingosine base and a free fatty acid chain (Figure 1). The sphingosine base can exit the lysosome, and ceramide can be formed by the attachment of a fatty acid chain to the sphingosine base by CerS, in the ER (Figure 1) [17]. In the sphingomyelinase pathway, ceramides are derived from the hydrolysis of sphingomyelins by neutral sphingomyelinase (nSMase), an enzyme found in Golgi, microsomal, plasma membrane, and nuclear fractions (Figure 1) [17,18].
CerSs, as highlighted previously, are a group of ER-resident enzymes which are important gatekeepers of ceramide levels, as they largely account for ceramides from the de novo and salvage pathways [19,20]. There are a total of six mammalian ceramide synthases (CerS1-6), with each CerS member showing a substrate preference for different lengths of fatty acid chains and the subsequent acyl length of ceramides (Table 1) [19,20,21,22,23,24]. Importantly, the expression of the mammalian CerS differs in various types of tissue. CerS1 is abundant in mouse brain and skeletal tissues; CerS2 is ubiquitously expressed in most mouse tissue types, and is most abundant in mouse liver and kidney tissues; CerS3 has the highest expression in mouse testis and skin tissues; CerS4 is most highly expressed in mouse skin, leukocytes, heart, and liver tissues; CerS5 is expressed at low levels in most tissue types, with slightly higher expression found in skeletal muscle, testis, and kidney tissues; CerS6 is expressed at low levels in most tissues, with slightly higher expression in intestine and kidney tissues [22]. This difference in CerS expression in various tissues may perhaps explain the variation in ceramide species and their relative abundance in different tissue types. In addition, the dysregulation of these CerS expression in specific tissues would implicate the development of disease (Table 1) [25,26,27,28,29,30,31,32,33].
Table 1.
The different ceramide synthases (CerS) characterized to date, their substrate preferences, and their associations with different diseases.
| CerS | Acyl Chain Length Preference | Disease Implicated | Reference |
|---|---|---|---|
| CerS1 | C18 | ↑ Type 2 Diabetes: Skeletal muscle | Turpin-Nolan et al., 2019 [25] |
| ↑ Heart Failure: Myocardium | Carrillo et al., 2021 [26] | ||
| CerS2 | C22–C24 | ↓ Heart Failure: Myocardium | Ji et al., 2017 [27] |
| CerS3 | C26–C34 | ↓ Congenital Ichthyosis: Skin | Eckl et al., 2013 [28] |
| CerS4 | C18–C20 | ↑Atopic dermatitis: Skin | Ito et al., 2017 [29] |
| ↑ Type 2 Diabetes: β-cells | Véret et al., 2011 [30] | ||
| CerS5 | C14–C16 | ↑ Obesity and Type 2 Diabetes: White adipose tissue | Gosejacob et al., 2016 [31] |
| ↑ Type 2 Diabetes: β-cells | Manukyan et al., 2015 [32] | ||
| CerS6 | C14–C16 | ↑ Obesity and Type 2 Diabetes: White adipose tissue, Liver | Turpin et al., 2014 [33] |
| ↑ Type 2 Diabetes: β-cells | Manukyan et al., 2015 [32] |
3. Ceramides in Disease
Ceramides are linked to numerous diseases, but it is important to appreciate that its reported diverse impact on pathophysiology is related to the length of its fatty acid chain moiety. For example, ceramides are commonly associated with skin diseases as it is suggested to constitute approximately 50% of all lipids in the stratum corneum (SC) of the skin [34]. In atopic dermatitis (AD), the loss of a proper water barrier was found to be associated with altered lipid composition in the SC. Ceramides with ultra-long fatty acyl chains of >24 carbon atoms (C25 and above) were reduced, while the long acyl chain ceramides (C24 and below), were increased in the SC of AD patients [35,36]. This suggests that an increase in skin long chain ceramides at the expense of ultra-long chain ceramides may contribute to skin pathologies [35,36]. Indeed, the dysregulation of ceramide profiles in AD was supported by a randomized control trial (RCT) on 18 female participants with AD, where the topical application of a ceramide cream significantly ameliorated the severity of skin lesions after 4 weeks of treatment [37]. In another trial conducted by Spada and colleagues, transepidermal water loss (TEWL) was shown to be significantly lower in eczema patients on a ceramide-dominant moisturizing cream and cleanser when compared to the placebo group. Despite no significant difference in the assessment of the eczema area severity index (EASI) between the two groups [38], it does highlight the importance of (restoring) ultra-long acyl chain ceramides in regulating water balance in the skin. Such an observation was further corroborated by Ito et al., who showed that ceramide synthase 4 (CerS4), a key enzyme in the biosynthesis of long acyl chain ceramides, C18–C20 ceramides, was significantly higher in changed skin (affected sites) of AD patients compared to unchanged skin sites [29]. Conversely, mice lacking CerS3, an enzyme involved in the biosynthesis of ultra-long acyl chain ceramides (C26–C34 ceramides), exhibited more than a two-fold increase in TEWL rate than in control mice, with a concomitant reduction in C26-, C28-, and C30-ceramides, highlighting that loss of ultra-long acyl chain ceramides accompanied epidermal water permeability barrier disruption [39]. An autosomal recessive mutation leading to the inactivation of CerS3 was also reported to result in congenital ichthyosis, further highlighting the importance of CerS3 and the synthesis of ultra-long ceramides in maintaining skin physiology [28].
Within domains of obesity and type-2 diabetes mellitus (T2DM), an increase in C18- and C16-ceramides are associated with metabolic dysfunction, again linking long acyl chain ceramides to pathology. The knockout of CerS1 in the skeletal muscle of C57BL/6 mice was reported to prevent C18-ceramide accumulation with improvements in insulin resistance and glucose tolerance [25]. Pancreatic beta-cells are particularly sensitive to changes in extracellular lipids. Under glucolipotoxic conditions of T2D, CerS4 expression was upregulated, with parallel increases in C18-, C22-, and C24:1-ceramides in the rat insulinoma cell line INS-1 [30]. Similarly, high glucose and palmitate increased expression of CerS5 and CerS6 in the mouse pancreatic beta cell line MIN6, and knockdown of either CerS5 or CerS6 abrogated the glucolipotoxic response [32]. Separately, a global knockout of mouse CerS5 reduced C16:0 ceramide levels in muscle, liver, and epididymal white adipose tissues, leading to reduced high-fat diet-associated insulin insensitivity and glucose intolerance [31]. CerS6 (the enzyme that synthesizes C14 and C16 ceramides) expression was elevated in adipose tissues of obese humans, while there was global ablation of CerS6 in mice protected against glucose intolerance when challenged with a high-fat diet [33]. Similarly, higher levels of plasma and hepatic C16 ceramides, as well as higher levels of CerS6 expression, were found in liver and subcutaneous adipose tissues of male Ob/Ob mice [40]. Separately, Kim and colleagues showed that C57BL/6 mice on a high-fat diet for 18 weeks had elevated hepatic expression of CerS1, CerS5, and CerS6, and the latter coincided with increased hepatic C16 ceramide levels and liver steatosis [41].
This detrimental impact of long acyl chain ceramides on T2DM was corroborated by several human cohort studies. A 6-year longitudinal population study revealed that several circulating ceramides, including C18:1-ceramide, C20-ceramide, C20:1-ceramide, and C22:1-ceramide were found to be positively associated with T2DM incidence and inversely associated with HOMA-B, the latter an assessment of the pancreatic β-cell function [42]. Similarly, a prospective study showed that higher levels of plasma C16-, C18-, C20-, and C22- ceramides were each associated with increased risk of developing T2DM [43]. Independently, a higher C18-ceramide/C16-ceramide ratio in the plasma was found to be a predictor of T2DM, with C18-ceramide levels correlated with T2DM incidence [44]. Wittenbecher et al. also found that plasma C18-ceramide, C22-ceramide, C20-dihydroceramide, and C22-dihydroceramide were associated with T2DM incidence [45].
Indeed, long acyl chain ceramides are linked to cardiovascular diseases. The accumulation of endothelial cell long acyl chain ceramides in high-fat diet-fed mice was linked to vascular dysfunction. The inhibition of ceramide generation from the de novo synthesis pathway, through the heterozygous deletion of DES1, was sufficient to ameliorate the endothelial nitric oxide synthase (eNOS) activity and vascular dysfunction [46]. Inhibition of ceramide synthesis by myriocin, a pharmacological inhibitor of serine palmitoyl transferase (SPT), was also shown to be beneficial in reducing atherosclerotic lesions in high-fat diet-fed apolipoprotein E (apoE) knockout mice [47,48]. Similarly, the inhibition of ceramide synthesis protected against heat failure, as the myriosin treatment of LpLGPI mice (overexpressing glycosylphosphatidylinositol (GPI)-anchored human lipoprotein lipase specifically in the heart, leading to higher lipid uptake and cardiomyopathy) improved heart function and lowered gene expression of heart failure-associated genes such as Glut1, Glut4, Pdk4, Cd36, Acs1, and Fatbp1 [49].
Dysregulation of plasma ceramide levels were also reported to be correlated to the development of cardiovascular diseases [45,50]. A prospective study performed on a European cohort found that plasma C16-ceramide, C18-ceramide and C24:1-ceramide were associated with increased risk of coronary artery disease [50]. Similarly, a separate cohort study also reported that C16-ceramide and C22:2 dihydroceramide were associated with higher risk of cardiovascular diseases [45]. In another study, C16:1-, C16:0-, and C24:1- ceramides were found to be elevated in myocardium samples obtained from advanced heart failure patients when compared to control myocardial samples [27]. Elevated CerS1 expression (involved in synthesis of C18-ceramide) was also found in cardiac tissues of patients with heart failure when compared to tissues from non-diseased patients [26]. In a study looking at the effects of liraglutide treatment, a glucagon-like peptide-1 agonist known to reduce CVD risk in T2DM patients, plasma ceramides with acyl lengths of 19 and 20 carbon atoms were lowered by GLP-1 treatment, suggesting that the lowering of long acyl chain ceramides may contribute to reported GLP-1 cardio-protection [51]. Indeed, a study performed on European and American cohorts found that a higher ratio of ultra-long acyl chain ceramides, C24-ceramide, to long acyl chain ceramides, C16-ceramide, was inversely correlated with coronary heart disease and heart failure [52].
4. Linking Circulating Ceramides with Intracellular Lipid Regulation
It has become increasingly clear that the levels of circulating ceramides are intricately linked to intracellular lipid regulation, particularly at the ER, due to the organelle localization of the various ceramide synthases. Apart from this de novo synthesis pathway, ceramides can be taken up by cells with shorter chain ceramide species, such as C2- and C6-ceramides being far more efficient at passing through the plasma membrane compared to ceramides with an acyl chain length of 16 carbons and above [53,54]. Hence, when studying the contribution of ceramides to cell physiology and pathology, it is important to consider them against a backdrop of intracellular lipid regulation and the activity of ER fatty acid enzymes. Circulating lipids remain the reservoir of substrates for ceramide synthesis [55]. However, lipids, especially free fatty acids (FFAs), have a preferred evolved route of shuttling into the ER for either assembly/esterification into neutral lipids or for generation of metabolic intermediates and signalling molecules [56,57]. The availability and retention of lipids within the ER is therefore very much dependent on the activity of enzymes and scaffold proteins that are responsible for shuttling fatty acids and other lipid intermediates into other compartments such as the Golgi apparatus, lipid droplets, peroxisomes, and/or lysosomes.
5. Are Ceramides Responsible for Certain Fatty Acid-Induced ER Stress and Apoptosis?
Certain ceramides, produced after exposure to exogenous fatty acids such as palmitate, were found to induce pancreatic β-cell ER stress and apoptosis. Zheng et al. showed that C16 ceramides accompany heightened ER stress and apoptosis when lipid droplet biogenesis was disabled [58]. Fumonisin B1, a non-specific inhibitor of CerS, reduced ER stress in palmitate-exposed MIN6 cells [58]. Manukyan et al. showed that inhibition of C14- and C16-ceramide formation, using either fumonisin B1 or CerS5/CerS6-knockdown, rescued palmitate-induced apoptosis in mouse insulinoma cells and human pancreatic islets [32]. Similarly, the treatment of a rat myoblast cell line, L6 cells, with palmitate was also found to increase C18-ceramides, with a concomitant induction of caspase-3 activity [59]. The inhibition of ceramide biosynthesis in L6 cells, with fumonisin B1, protected against palmitate-induced apoptosis, and cells co-treated with fumonisin B1 and palmitate had significantly lower caspase-3 activity when compared to cells treated with palmitate alone [59]. Similarly, palmitate treatment of mouse C2C12 myoblast cells elevated C16-ceramides with a corresponding induction of apoptosis [60]. Importantly, this impact of ceramide accumulation was reported to be associated with insulin resistance, as the prevention of C18-ceramide accumulation in the muscle, through either global or skeletal-specific knockout of CerS1, significantly improved glucose homeostasis and insulin sensitivity in high-fat diet-fed mice [25].
C16-ceramide accumulation was also linked to hepatocyte ER stress following palmitate treatment [41]. Either overexpression of CerS6 or the exogenous addition of C16- and C18-ceramides exacerbated palmitate-induced ER stress in liver cell lines with an induction of UPR markers, including p-eIF2a, p-PERK, CHOP, and GRP78 proteins [41]. Interestingly, the overexpression of CerS2, which catalyses the formation of ultra-long acyl chain ceramides (C24- and C26-ceramides), protected the cells against palmitate-mediated ER stress [41]. In a separate study, the inhibition of the ceramide de novo biosynthesis pathway, through the intraperitoneal injection of myriosin, ameliorated hepatic apoptosis and liver inflammation in high-fat diet-fed rats, with reduced cleaved caspase-3, TNFα, IL-1β, and IL6 in liver tissues of myriocin-treated steatosis rats [61].
Similarly, C16-ceramides drive apoptosis in human coronary artery endothelial cells (HCAECs). Under hyperglycemic conditions, Zietzer and colleagues showed that C16-ceramides dominated HCAEC-derived large extracellular vesicles (lEVs), the latter of which triggered HCAEC apoptosis [62]. Inhibition of neutral sphingomyelinase 2 (nSMase2), significantly reduced C16-ceramides (as well as other less dominant ceramide species) in lEVs, which resulted in the abrogation of hyperglycemic-induced apoptosis in HCAECs [62].
6. Mechanisms Underpinning Ceramide-Mediated Apoptosis
Ceramides were shown to activate both extrinsic and intrinsic apoptotic pathways in various cancer models [63,64,65,66,67,68,69,70,71]. In the extrinsic apoptotic pathway, the generation of ceramides was linked to the activation of Fas-, as well as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-dependent apoptotic cascades in cancer cells [63,64,72]. Furthermore, ceramides were observed to reduce abundance of FLICE inhibitory proteins (FLIPs), an inhibitor of caspase 8, in glioblastoma and renal carcinoma cells [65,66].
In the intrinsic apoptotic pathway, ceramides were suggested to form large channels on the outer membrane of the isolated rat liver mitochondria, leading to increased permeability of small proteins, including cytochrome c, and triggering of the apoptotic cascade [73]. Furthermore, ceramides were shown to induce the phosphorylation of p38 mitogen-activated protein kinase (MAPK), leading to de-phosphorylation (inactivation) of Akt, and subsequent translocation of pro-apoptotic protein, Bax, to the mitochondria. Bax then facilitates cytochrome c release from the mitochondria into the cytosol, triggering apoptosis [68]. In addition, ceramides were observed to downregulate the Akt signaling cascade by binding to the inhibitor of protein phosphatase 2A (I2PP2A), leading to the activation of protein phosphatase 2A (PP2A), and subsequent inhibition of Akt [67,74]. Activated PP2A is linked to the de-phosphorylation (inactivation) of the anti-apoptotic Bcl2 protein [69]. Ceramides were separately linked to reduced levels of the anti-apoptotic protein, survivin, leading to increased expression of pro-apoptotic Bax [70,71].
As mentioned earlier, the acyl length of ceramides is linked to its toxicity, with C16- and C18-ceramides being notorious in the development of various diseases [29,32,41,62]. Saddoughi et al. reported that I2PP2A preferentially binds to C18-ceramides over C14-, C16-, C20-, C22-, and C24-ceramides [75], suggesting that C18-ceramides are a relatively more potent inhibitor of Akt compared to other ceramides. Hence, C18 ceramides may activate downstream apoptotic pathways more effectively. It was also reported that only C16-ceramides are able bind to p53, preventing its degradation by the E3 ubiquitin ligase, MDM2 [76]. Furthermore, the transient overexpression of CerS6 resulted in the stabilisation of p53 protein, while CerS1-5 overexpression did not [76]. This stabilisation of p53 could potentially increase binding of p53 to Bcl2, facilitate Bax translocation to mitochondria, and lead to the subsequent activation of apoptosis [69]. Separately, the formation of a mitochondrial outer membrane pore by C16-ceramides was found to be disrupted by ultra-long acyl chains (C22- and C24-ceramides) [77]. Conversely, such ultra-long acyl chain ceramides could also induce the formation of smaller membrane channels which were vice versa disrupted by increased C16-ceramides [77]. Therefore, apart from its interaction with proteins linked to apoptosis, the channel forming function of long acyl chain- and ultra-long acyl chain-ceramides perhaps require further investigation, especially with regards to the disruption of mitochondrial permeability and cytochrome c release.
7. Lipid Droplet Biogenesis: A Potential Protective Mechanism against C18- and C16-Ceramide Accumulation?
While inhibitors of ceramide biosynthesis, such as the mycotoxin fumonisin B1, was demonstrated to be effective in preventing ceramide-mediated apoptosis in vitro, there were concerns of adverse effects in vivo, especially inflammation and necrosis of the liver and kidney in mice [78]. Apart from the direct modulation of ceramide synthase, handling of excess intracellular fatty acids may offer another avenue for preventing long-chain ceramide accumulation, ER stress, and apoptosis in cells. Proper sequestration of fatty acids as neutral lipids, such as triglycerides, within lipid droplets (LDs) could prevent ceramide accumulation as it reduces the availability of free fatty acid substrates at the ER for ceramide synthesis. This was evident from a study performed by Zheng and colleagues, where the reduction of LD biogenesis, through the β-cell specific knockout of FITM2 protein in high-fat diet-fed mice, resulted in a significant increase in C16-ceramides in β-cells [58]. Similarly, the disruption of LD biogenesis, through the adipose-specific knockout of SEIPIN, also resulted in a significant increase in C18- and C20-ceramides [79].
Indeed, the sequestration of palmitate, the most abundant saturated fatty acid found in human serum, in the form of neutral lipids was found to protect the cells against palmitate-mediated apoptosis in multiple studies [80,81,82,83,84,85,86]. Exposure of the unsaturated fatty acid, oleate, prevented palmitate-induced apoptosis by promoting the incorporation of palmitate into triglycerides in CHO-, 1.1B4-, and INS1e cells, with increased intracellular neutral lipid accumulation [81,82,83]. In addition to oleate, the polyunsaturated fatty acid, C20:4 arachidonic acid, was also reported to have a similar beneficial impact on C2C12 cells, a mouse skeletal muscle cell line [84]. The exogenous addition of linoleate, oleate, α-linolenate, and docosahexaenoate to microglial cells also protected the cells from palmitate-induced cell death, with a concomitant increase in neutral lipid formation [85]. Apart from its effect on the sequestration of FFAs, LDs were also reported to store ceramides, in the form of acylceramide, in the livers of high-fat diet-fed mice [86].
LDs facilitate sequestration of ceramides, and therefore the stabilization of protein targets involved in LD formation may mitigate ceramide-induced apoptosis. The formation of LD occurs at the ER and begins with the accumulation of triglycerides between the ER membranes, and the subsequent curving and formation of an oil lens. With sufficient accumulation of triglycerides, the oil lens will then bud off from the ER as LD [87]. LD biogenesis involves many proteins, but three ER proteins—seipin, perilipin, and FITM2—are critical for LD formation. Seipin recruits and promotes the accumulation of triglycerides at the LD biogenesis site, while perilipins (PLIN2 and PLIN3) protect these triglyceride aggregates from lipolysis [88,89]. FITM2, other than its acyl coA diphosphatase activity, promotes membrane curvature at the LD biogenesis sites by interacting with ER tubule-forming proteins and septins [90,91].
8. Conclusions
Emerging data point towards the benefit of accelerating lipid droplet biogenesis to sequester fatty acid substrates away from CerS1 and CerS4–CerS6 in the ER. This could lower the generation of long acyl chain length ceramides, notably C18- and C16-ceramides. However, this is predicated upon rapid hydrolysis of the lipid droplets by the mitochondria to prevent lipid droplet accumulation, which may result in steatosis. Hence, much more research is needed to understand the evolutionary importance of long acyl chain ceramides and on how knowledge on intracellular lipid regulation can be harnessed to dampen its negative impact on various cells.
Acknowledgments
Y.A. is additionally supported by the LKCMedicine Healthcare Research Fund (Diabetes Research), established through the generous support of alumni of Nanyang Technological University, Singapore.
Author Contributions
Q.W.C.H. and Y.A. wrote the manuscript; X.Z. and Y.A. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Ministry of Education Singapore (MOE2018-T2-1-085, MOE-T2EP30221-0003) (Y.A.) and Tier 1 (2019-T1-001-059) (Y.A.). This work is also partly supported by the Program of National Natural Science Foundation of China (82070846) (X.Z.), the Program for Overseas High-Level Talents Introduction of Sichuan Province of China (21RCYJ0046) (X.Z.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cha H.J., He C., Zhao H., Dong Y., An I.S., An S. Intercellular and intracellular functions of ceramides and their metabolites in skin (Review) Int. J. Mol. Med. 2016;38:16–22. doi: 10.3892/ijmm.2016.2600. [DOI] [PubMed] [Google Scholar]
- 2.Blaess M., Deigner H.P. Derailed ceramide metabolism in atopic dermatitis (AD): A causal starting point for a personalized (basic) therapy. Int. J. Mol. Sci. 2019;20:3967. doi: 10.3390/ijms20163967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii M. The pathogenic and therapeutic implications of ceramide abnormalities in atopic dermatitis. Cells. 2021;10:2386. doi: 10.3390/cells10092386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Smeden J., Janssens M., Gooris G.S., Bouwstra J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta. 2014;1841:295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Galadari S., Rahman A., Pallichankandy S., Galadari A., Thayyullathil F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids Health Dis. 2013;12:98. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolowska E., Blachnio-Zabielska A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019;10:577. doi: 10.3389/fendo.2019.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandal N., Grambergs R., Mondal K., Basu S.K., Tahia F., Dagogo-Jack S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J. Diabetes Complicat. 2021;35:107734. doi: 10.1016/j.jdiacomp.2020.107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi R.H., Tatum S.M., Symons J.D., Summers S.A., Holland W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021;18:701–711. doi: 10.1038/s41569-021-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meeusen J.W., Donato L.J., Kopecky S.L., Vasile V.C., Jaffe A.S., Laaksonen R. Ceramides improve atherosclerotic cardiovascular disease risk assessment beyond standard risk factors. Clin. Chim. Acta. 2020;511:138–142. doi: 10.1016/j.cca.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Sassa T., Suto S., Okayasu Y., Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta. 2012;1821:1031–1037. doi: 10.1016/j.bbalip.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E.V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seumois G., Fillet M., Gillet L., Faccinetto C., Desmet C., Francois C., Dewals B., Oury C., Vanderplasschen A., Lekeux P., et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. Leukoc. Biol. 2007;81:1477–1486. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 13.Rudd A.K., Devaraj N.K. Traceless synthesis of ceramides in living cells reveals saturation-dependent apoptotic effects. Proc. Natl. Acad. Sci. USA. 2018;115:7485–7490. doi: 10.1073/pnas.1804266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breen P., Joseph N., Thompson K., Kraveka J.M., Gudz T.I., Li L., Rahmaniyan M., Bielawski J., Pierce J.S., Van Buren E., et al. Dihydroceramide desaturase knockdown impacts sphingolipids and apoptosis after photodamage in human head and neck squamous carcinoma cells. Anticancer Res. 2013;33:77–84. [PMC free article] [PubMed] [Google Scholar]
- 15.Siddique M.M., Bikman B.T., Wang L., Ying L., Reinhardt E., Shui G., Wenk M.R., Summers S.A. Ablation of dihydroceramide desaturase confers resistance to etoposide-induced apoptosis in vitro. PLoS ONE. 2012;7:e44042. doi: 10.1371/journal.pone.0044042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen P.J., Tennagels N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 2014;3:252–260. doi: 10.1016/j.molmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitatani K., Idkowiak-Baldys J., Hannun Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Airola M.V., Hannun Y.A. Sphingolipid metabolism and neutral sphingomyelinases. Handb. Exp. Pharmacol. 2013;215:57–76. doi: 10.1007/978-3-7091-1368-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizutani Y., Kihara A., Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Pt 1Biochem. J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J.C., Sullards M.C., Merrill A.H., Jr., Futerman A.H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- 21.Mizutani Y., Kihara A., Igarashi Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 2006;398:531–538. doi: 10.1042/BJ20060379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laviad E.L., Albee L., Pankova-Kholmyansky I., Epstein S., Park H., Merrill A.H., Jr., Futerman A.H. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 23.Riebeling C., Allegood J.C., Wang E., Merrill A.H., Jr., Futerman A.H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- 24.Lahiri S., Futerman A.H. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J. Biol. Chem. 2005;280:33735–33738. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- 25.Turpin-Nolan S.M., Hammerschmidt P., Chen W., Jais A., Timper K., Awazawa M., Brodesser S., Bruning J.C. CerS1-Derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 2019;26:1–10.e17. doi: 10.1016/j.celrep.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Carrillo L., Gimenez-Escamilla I., Martinez-Dolz L., Sanchez-Lazaro I.J., Portoles M., Rosello-Lleti E., Tarazon E. Implication of sphingolipid metabolism gene dysregulation and cardiac sphingosine-1-phosphate accumulation in heart failure. Biomedicines. 2022;10:135. doi: 10.3390/biomedicines10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji R., Akashi H., Drosatos K., Liao X., Jiang H., Kennel P.J., Brunjes D.L., Castillero E., Zhang X., Deng L.Y., et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight. 2017;2:e82922. doi: 10.1172/jci.insight.82922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckl K.M., Tidhar R., Thiele H., Oji V., Hausser I., Brodesser S., Preil M.L., Onal-Akan A., Stock F., Muller D., et al. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J. Investig. Dermatol. 2013;133:2202–2211. doi: 10.1038/jid.2013.153. [DOI] [PubMed] [Google Scholar]
- 29.Ito S., Ishikawa J., Naoe A., Yoshida H., Hachiya A., Fujimura T., Kitahara T., Takema Y. Ceramide synthase 4 is highly expressed in involved skin of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2017;31:135–141. doi: 10.1111/jdv.13777. [DOI] [PubMed] [Google Scholar]
- 30.Veret J., Coant N., Berdyshev E.V., Skobeleva A., Therville N., Bailbe D., Gorshkova I., Natarajan V., Portha B., Le Stunff H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 beta-cells. Biochem. J. 2011;438:177–189. doi: 10.1042/BJ20101386. [DOI] [PubMed] [Google Scholar]
- 31.Gosejacob D., Jager P.S., Vom Dorp K., Frejno M., Carstensen A.C., Kohnke M., Degen J., Dormann P., Hoch M. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 2016;291:6989–7003. doi: 10.1074/jbc.M115.691212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manukyan L., Ubhayasekera S.J., Bergquist J., Sargsyan E., Bergsten P. Palmitate-induced impairments of beta-cell function are linked with generation of specific ceramide species via acylation of sphingosine. Endocrinology. 2015;156:802–812. doi: 10.1210/en.2014-1467. [DOI] [PubMed] [Google Scholar]
- 33.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M., Mauer J., Xu E., Hammerschmidt P., Bronneke H.S., et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Coderch L., Lopez O., de la Maza A., Parra J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003;4:107–129. doi: 10.2165/00128071-200304020-00004. [DOI] [PubMed] [Google Scholar]
- 35.Janssens M., van Smeden J., Gooris G.S., Bras W., Portale G., Caspers P.J., Vreeken R.J., Hankemeier T., Kezic S., Wolterbeek R., et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012;53:2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Smeden J., Janssens M., Kaye E.C., Caspers P.J., Lavrijsen A.P., Vreeken R.J., Bouwstra J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014;23:45–52. doi: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 37.Draelos Z.D. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J. Cosmet. Dermatol. 2011;10:185–188. doi: 10.1111/j.1473-2165.2011.00568.x. [DOI] [PubMed] [Google Scholar]
- 38.Spada F., Harrison I.P., Barnes T.M., Greive K.A., Daniels D., Townley J.P., Mostafa N., Fong A.T., Tong P.L., Shumack S. A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: A randomized trial. Dermatol. Ther. 2021;34:e14970. doi: 10.1111/dth.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U., Bayerle A., van der Hoeven F., Imgrund S., Kirsch J., et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 40.Raichur S., Brunner B., Bielohuby M., Hansen G., Pfenninger A., Wang B., Bruning J.C., Larsen P.J., Tennagels N. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol. Metab. 2019;21:36–50. doi: 10.1016/j.molmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.R., Lee E.J., Shin K.O., Kim M.H., Pewzner-Jung Y., Lee Y.M., Park J.W., Futerman A.H., Park W.J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019;51:1–16. doi: 10.1038/s12276-019-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun H., Sun L., Wu Q., Zong G., Qi Q., Li H., Zheng H., Zeng R., Liang L., Lin X. Associations among circulating sphingolipids, beta-cell function, and risk of developing type 2 diabetes: A population-based cohort study in China. PLoS Med. 2020;17:e1003451. doi: 10.1371/journal.pmed.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fretts A.M., Jensen P.N., Hoofnagle A.N., McKnight B., Howard B.V., Umans J., Sitlani C.M., Siscovick D.S., King I.B., Djousse L., et al. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J. Lipid Res. 2021;62:100119. doi: 10.1016/j.jlr.2021.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilvo M., Salonurmi T., Havulinna A.S., Kauhanen D., Pedersen E.R., Tell G.S., Meyer K., Teeriniemi A.M., Laatikainen T., Jousilahti P., et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61:1424–1434. doi: 10.1007/s00125-018-4590-6. [DOI] [PubMed] [Google Scholar]
- 45.Wittenbecher C., Cuadrat R., Johnston L., Eichelmann F., Jager S., Kuxhaus O., Prada M., Del Greco M.F., Hicks A.A., Hoffman P., et al. Dihydroceramide- and ceramide-profiling provides insights into human cardiometabolic disease etiology. Nat. Commun. 2022;13:936. doi: 10.1038/s41467-022-28496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q.J., Holland W.L., Wilson L., Tanner J.M., Kearns D., Cahoon J.M., Pettey D., Losee J., Duncan B., Gale D., et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glaros E.N., Kim W.S., Wu B.J., Suarna C., Quinn C.M., Rye K.A., Stocker R., Jessup W., Garner B. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 2007;73:1340–1346. doi: 10.1016/j.bcp.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Park T.S., Panek R.L., Mueller S.B., Hanselman J.C., Rosebury W.S., Robertson A.W., Kindt E.K., Homan R., Karathanasis S.K., Rekhter M.D. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 49.Park T.S., Hu Y., Noh H.L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X.C., Abel E.D., et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigruener A., Kleber M.E., Heimerl S., Liebisch G., Schmitz G., Maerz W. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen risk and cardiovascular health (LURIC) study. PLoS ONE. 2014;9:e85724. doi: 10.1371/journal.pone.0085724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zobel E.H., Wretlind A., Ripa R.S., Rotbain Curovic V., von Scholten B.J., Suvitaival T., Hansen T.W., Kjaer A., Legido-Quigley C., Rossing P. Ceramides and phospholipids are downregulated with liraglutide treatment: Results from the LiraFlame randomized controlled trial. BMJ Open Diabetes Res. Care. 2021;9:e002395. doi: 10.1136/bmjdrc-2021-002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson L.R., Xanthakis V., Duncan M.S., Gross S., Friedrich N., Volzke H., Felix S.B., Jiang H., Sidhu R., Nauck M., et al. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 2018;7:e007931. doi: 10.1161/JAHA.117.007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon C.G., Jr., Holloway P.W., Gear A.R. Exchange of C(16)-ceramide between phospholipid vesicles. Biochemistry. 1999;38:14676–14682. doi: 10.1021/bi991537w. [DOI] [PubMed] [Google Scholar]
- 54.Kjellberg M.A., Lonnfors M., Slotte J.P., Mattjus P. Metabolic conversion of ceramides in HeLa cells—A cholesteryl phosphocholine delivery approach. PLoS ONE. 2015;10:e0143385. doi: 10.1371/journal.pone.0143385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabielski P., Blachnio-Zabielska A.U., Wojcik B., Chabowski A., Gorski J. Effect of plasma free fatty acid supply on the rate of ceramide synthesis in different muscle types in the rat. PLoS ONE. 2017;12:e0187136. doi: 10.1371/journal.pone.0187136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milger K., Herrmann T., Becker C., Gotthardt D., Zickwolf J., Ehehalt R., Watkins P.A., Stremmel W., Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. Pt 22J. Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 57.Shimura M., Shindou H., Szyrwiel L., Tokuoka S.M., Hamano F., Matsuyama S., Okamoto M., Matsunaga A., Kita Y., Ishizaka Y., et al. Imaging of intracellular fatty acids by scanning X-ray fluorescence microscopy. FASEB J. 2016;30:4149–4158. doi: 10.1096/fj.201600569R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng X., Ho Q.W.C., Chua M., Stelmashenko O., Yeo X.Y., Muralidharan S., Torta F., Chew E.G.Y., Lian M.M., Foo J.N., et al. Destabilization of beta Cell FIT2 by saturated fatty acids alter lipid droplet numbers and contribute to ER stress and diabetes. Proc. Natl. Acad. Sci. USA. 2022;119:e2113074119. doi: 10.1073/pnas.2113074119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turpin S.M., Lancaster G.I., Darby I., Febbraio M.A., Watt M.J. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2006;291:E1341–E1350. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- 60.Henique C., Mansouri A., Fumey G., Lenoir V., Girard J., Bouillaud F., Prip-Buus C., Cohen I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 2010;285:36818–36827. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang M., Li C., Liu Q., Wang A., Lei M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front. Endocrinol. 2019;10:665. doi: 10.3389/fendo.2019.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zietzer A., Jahnel A.L., Bulic M., Gutbrod K., Dusing P., Hosen M.R., Dormann P., Werner N., Nickenig G., Jansen F. Activation of neutral sphingomyelinase 2 through hyperglycemia contributes to endothelial apoptosis via vesicle-bound intercellular transfer of ceramides. Cell Mol. Life Sci. 2021;79:48. doi: 10.1007/s00018-021-04049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park M.A., Zhang G., Martin A.P., Hamed H., Mitchell C., Hylemon P.B., Graf M., Rahmani M., Ryan K., Liu X., et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol. Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang S.T., Yang R.C., Chen M.Y., Pang J.H. Phyllanthus urinaria induces the Fas receptor/ligand expression and ceramide-mediated apoptosis in HL-60 cells. Life Sci. 2004;75:339–351. doi: 10.1016/j.lfs.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 65.Yoon G., Kim K.O., Lee J., Kwon D., Shin J.S., Kim S.J., Choi I.H. Ceramide increases Fas-mediated apoptosis in glioblastoma cells through FLIP down-regulation. J. Neurooncol. 2002;60:135–141. doi: 10.1023/A:1020604705831. [DOI] [PubMed] [Google Scholar]
- 66.Asakuma J., Sumitomo M., Asano T., Asano T., Hayakawa M. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365–1370. [PubMed] [Google Scholar]
- 67.Mukhopadhyay A., Saddoughi S.A., Song P., Sultan I., Ponnusamy S., Senkal C.E., Snook C.F., Arnold H.K., Sears R.C., Hannun Y.A., et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H.J., Oh J.E., Kim S.W., Chun Y.J., Kim M.Y. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett. 2008;260:88–95. doi: 10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Deng X., Gao F., May W.S. Protein phosphatase 2A inactivates Bcl2’s antiapoptotic function by dephosphorylation and up-regulation of Bcl2-p53 binding. Blood. 2009;113:422–428. doi: 10.1182/blood-2008-06-165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X., Ryland L., Yang J., Liao A., Aliaga C., Watts R., Tan S.F., Kaiser J., Shanmugavelandy S.S., Rogers A., et al. Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116:4192–4201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temme A., Rodriguez J.A., Hendruschk S., Gunes S., Weigle B., Schakel K., Schmitz M., Bachmann M., Schackert G., Rieber E.P. Nuclear localization of Survivin renders HeLa tumor cells more sensitive to apoptosis by induction of p53 and Bax. Cancer Lett. 2007;250:177–193. doi: 10.1016/j.canlet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 72.Dumitru C.A., Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- 73.Siskind L.J., Kolesnick R.N., Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo Y.C., Huang K.Y., Yang C.H., Yang Y.S., Lee W.Y., Chiang C.W. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 75.Saddoughi S.A., Gencer S., Peterson Y.K., Ward K.E., Mukhopadhyay A., Oaks J., Bielawski J., Szulc Z.M., Thomas R.J., Selvam S.P., et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fekry B., Jeffries K.A., Esmaeilniakooshkghazi A., Szulc Z.M., Knagge K.J., Kirchner D.R., Horita D.A., Krupenko S.A., Krupenko N.I. C16-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat. Commun. 2018;9:4149. doi: 10.1038/s41467-018-06650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stiban J., Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: A plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim. Biophys. Acta. 2015;1848:561–567. doi: 10.1016/j.bbamem.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z., Zhang F., Jiang L., Chen Z., Sun H. Toxic effects of mycotoxin Fumonisin B1 at six different doses on female BALB/c mice. Toxins. 2021;14:21. doi: 10.3390/toxins14010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu L., Jiang Q., Wang X., Zhang Y., Lin R.C., Lam S.M., Shui G., Zhou L., Li P., Wang Y., et al. Adipose-specific knockout of SEIPIN/BSCL2 results in progressive lipodystrophy. Diabetes. 2014;63:2320–2331. doi: 10.2337/db13-0729. [DOI] [PubMed] [Google Scholar]
- 80.Abdelmagid S.A., Clarke S.E., Nielsen D.E., Badawi A., El-Sohemy A., Mutch D.M., Ma D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE. 2015;10:e0116195. doi: 10.1371/journal.pone.0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S., Schaffer J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oberhauser L., Jimenez-Sanchez C., Madsen J.G.S., Duhamel D., Mandrup S., Brun T., Maechler P. Glucolipotoxicity promotes the capacity of the glycerolipid/NEFA cycle supporting the secretory response of pancreatic beta cells. Diabetologia. 2022;65:705–720. doi: 10.1007/s00125-021-05633-x. [DOI] [PubMed] [Google Scholar]
- 83.Nemecz M., Constantin A., Dumitrescu M., Alexandru N., Filippi A., Tanko G., Georgescu A. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: The elucidation of associated mechanisms and effector molecules. Front. Pharmacol. 2018;9:1554. doi: 10.3389/fphar.2018.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheon H.G., Cho Y.S. Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channeling of palmitic acid into triglycerides in C2C12. J. Biomed. Sci. 2014;21:13. doi: 10.1186/1423-0127-21-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urso C.J., Zhou H. Palmitic acid lipotoxicity in microglia cells is ameliorated by unsaturated fatty acids. Int. J. Mol. Sci. 2021;22:9093. doi: 10.3390/ijms22169093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senkal C.E., Salama M.F., Snider A.J., Allopenna J.J., Rana N.A., Koller A., Hannun Y.A., Obeid L.M. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017;25:686–697. doi: 10.1016/j.cmet.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fader Kaiser C.M., Romano P.S., Vanrell M.C., Pocognoni C.A., Jacob J., Caruso B., Delgui L.R. Biogenesis and breakdown of lipid droplets in pathological conditions. Front. Cell Dev. Biol. 2021;9:826248. doi: 10.3389/fcell.2021.826248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bell M., Wang H., Chen H., McLenithan J.C., Gong D.W., Yang R.Z., Yu D., Fried S.K., Quon M.J., Londos C., et al. Consequences of lipid droplet coat protein downregulation in liver cells: Abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Listenberger L.L., Ostermeyer-Fay A.G., Goldberg E.B., Brown W.J., Brown D.A. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 90.Chen F., Yan B., Ren J., Lyu R., Wu Y., Guo Y., Li D., Zhang H., Hu J. FIT2 organizes lipid droplet biogenesis with ER tubule-forming proteins and septins. J. Cell Biol. 2021;220:e201907183. doi: 10.1083/jcb.201907183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Becuwe M., Bond L.M., Pinto A.F.M., Boland S., Mejhert N., Elliott S.D., Cicconet M., Graham M.M., Liu X.N., Ilkayeva O., et al. FIT2 is an acyl-coenzyme A diphosphatase crucial for endoplasmic reticulum homeostasis. J. Cell Biol. 2020;219:e202006111. doi: 10.1083/jcb.202006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.