Abstract
The Arc two-component system, comprising a tripartite sensor kinase (ArcB) and a response regulator (ArcA), modulates the expression of numerous genes involved in respiratory functions. In this study, the steps of phosphoryl group transfer from phosphorylated ArcB to ArcA were examined in vivo by using single copies of wild-type and mutant arcB alleles. The results indicate that the signal transmission occurs solely by His-Asp-His-Asp phosphorelay.
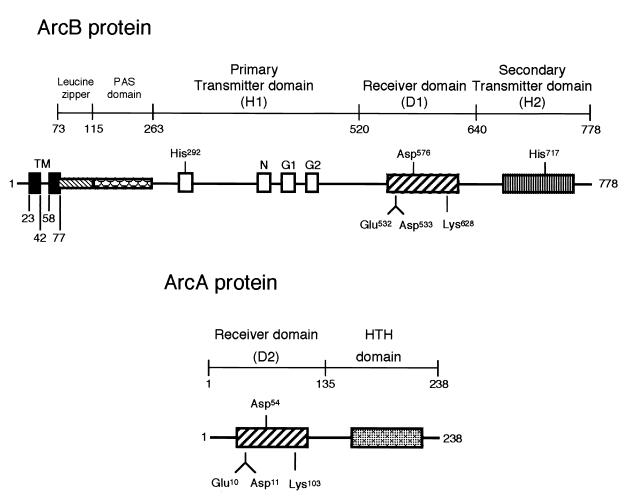
The ArcB-ArcA two-component signal transduction system of Escherichia coli regulates the expression of more than 30 operons depending on the redox conditions of growth (12, 17, 18). This system comprises ArcB as the membrane-bound sensor kinase and ArcA as the cognate response regulator (Fig. 1). The ArcB protein has three cytoplasmic domains: a primary transmitter domain (H1) containing a conserved His292, a receiver domain (D1) containing a conserved Asp576, and a secondary transmitter domain (H2) containing a conserved His717 (10, 13, 15, 27). ArcB thus belongs to the tripartite hybrid sensor kinase subfamily (23), which also includes BarA (21), EvgS (29), and TorS (14) of E. coli, BvgS of Bordetella pertussis (1), LemA of Pseudomonas syringae pv. syringae (9), and RteA of Bacteroides thetaiotaomicron (25).
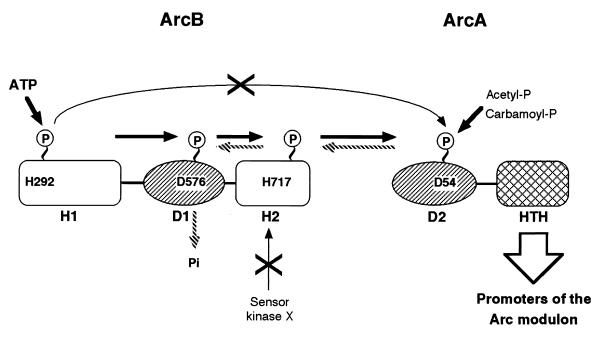
FIG. 1.
Schematic representation of ArcB and ArcA. (Top) The N-terminal transmembrane domain of ArcB was determined by alkaline phosphatase fusions (16). A putative leucine zipper (6) and a PAS domain (26) were predicted on the basis of amino acid sequence homology. The primary transmitter domain (H1) contains the conserved His292 and the catalytic determinants N, G1, and G2. The G1 and G2 sequences typify nucleotide-binding motifs. The receiver domain (D1) contains the conserved Asp576, and the secondary transmitter domain (H2) contains the conserved His717 (13, 27). (Bottom) ArcA consists of an N-terminal receiver domain (D2) containing the conserved Asp54 and a C-terminal helix-turn-helix (HTH) domain (12).
In vitro studies showed that the primary transmitter domain of ArcB is autophosphorylated at His292 at the expense of ATP (8, 11). The phosphoryl group is then sequentially transferred to Asp576 and His717 and from there to Asp54 of ArcA. However, the phosphoryl group on His292 could also be directly transferred in vitro to ArcA at a very low rate (8). An in vivo study utilizing ArcB domains borne by a low-copy-number plasmid led to the conclusion that the phosphoryl group from His292 could be transferred to ArcA and that this transfer was regulated by the nature of the carbon source. On the other hand, the phosphoryl group from His717 could also be transferred to ArcA, but this transfer was regulated by redox conditions (19). Possible misleading results caused by multiple gene dosage effect and different degrees of catabolite repression during utilization of various carbon sources, however, were not discussed. In yet another study, it was suggested that His717 received the phosphoryl group from an unknown sensor kinase (10). Here, we address the questions of whether ArcA can be phosphorylated by both H1 and H2 and whether H2 can be phosphorylated by a noncognate sensor kinase, under in vivo conditions in cells bearing a single copy of an arcB allele on the chromosome.
Strategy for the in vivo study with modified arcB.
The strains, phage, and plasmids used in this study are listed in Table 1. To determine the sequence of phosphotransfer in the Arc system, the arcB+ allele on the chromosome was replaced by various mutant sequences (Fig. 2). The strategy of single-copy replacement circumvents possible complementation, epistatic, and dosage effects. The phenotypic consequences of ArcB modification were analyzed by changes in the in vivo levels of phosphorylated ArcA (ArcA-P), as indicated by expressions of target operons. We employed a λΦ(cydA′-lacZ) operon fusion as an ArcA-P-activable reporter and a λΦ(lldP′-lacZ) operon fusion as an ArcA-P-repressible reporter. A Δfnr::Tn9(Cmr) allele was incorporated into the λΦ(cydA′-lacZ)-harboring strains to avoid its repression by Fnr (3).
TABLE 1.
E. coli K-12 strains, phage, and plasmids used in this study
| Strain, phage, or plasmid | Relevant genotype or characteristics | Reference or source |
|---|---|---|
| Strains | ||
| MC4100 | F− araD139 Δ(argF-lac) U169 rpsL150 relA1 flbB5301 deoC ptsF25 rbsR | 24 |
| JC7623 | recB21 recC22 sbcB15 sbcC201 | 22 |
| ECL5000 | ΔarcB::TetrrecB21 recC22 sbcB15 sbcC201 | 16 |
| ECL5001 | MC4100 but λΦ(cydA′-lacZ) | 16 |
| ECL5002 | MC4100 but λΦ(lldP′-lacZ) | 16 |
| ECL5003 | MC4100 but Δfnr::Tn9 (Cmr) λΦ(cydA′-lacZ) | 16 |
| ECL5004 | ΔarcB::Tetr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | 16 |
| ECL5005 | ΔarcB::Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | 16 |
| ECL5006 | arcB+ Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | 16 |
| ECL5012 | ΔarcB::Tetr λΦ(lldP′-lacZ) | 16 |
| ECL5013 | ΔarcB::Kanr λΦ(lldP′-lacZ) | 16 |
| ECL5014 | arcB+ Kanr λΦ(lldP′-lacZ) | 16 |
| ECL5022 | arcBH292Q Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5023 | arcBD576A Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5024 | arcBH717Q Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5025 | arcB1–661 Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5026 | arcB1–661, D576A Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5027 | arcB1–520 Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5028 | arcBD576A, H717Q Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5029 | arcBH292Q, D576A Kanr λΦ(cydA′-lacZ) Δfnr::Tn9(Cmr) | This study |
| ECL5030 | arcBH292Q Kanr λΦ(lldP′-lacZ) | This study |
| ECL5031 | arcBD576A Kanr λΦ(lldP′-lacZ) | This study |
| ECL5032 | arcBH717Q Kanr λΦ(lldP′-lacZ) | This study |
| ECL5033 | arcB1–661 Kanr λΦ(lldP′-lacZ) | This study |
| ECL5034 | arcB1–661, D576A Kanr λΦ(lldP′-lacZ) | This study |
| ECL5035 | arcB1–520 Kanr λΦ(lldP′-lacZ) | This study |
| ECL5036 | arcBD576A, H717Q Kanr λΦ(lldP′-lacZ) | This study |
| ECL5037 | arcBH292Q, D576A Kanr λΦ(lldP′-lacZ) | This study |
| Phage | ||
| P1vir | Laboratory stock | |
| Plasmids | ||
| pDB3 | ΔarcB::Tetrbla+ | 16 |
| pIB3 | ΔarcB::Kanrbla+ | 16 |
FIG. 2.
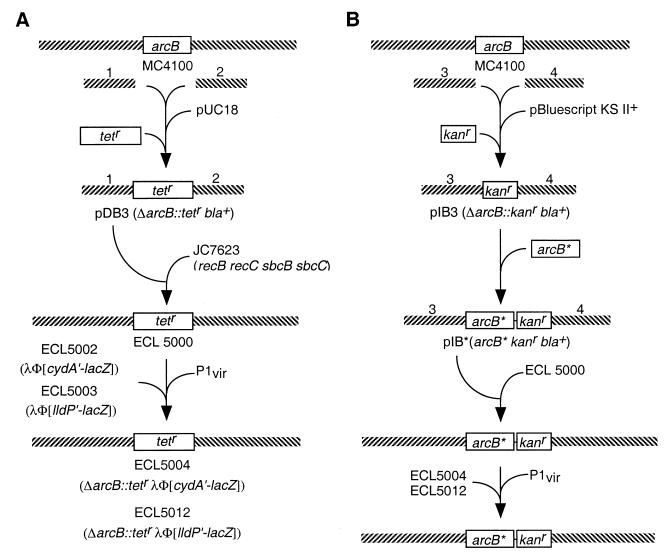
Allele replacement strategy. (A) Construction of the ΔarcB::Tetr strains. The 5′- and 3′-flanking DNA fragments of arcB (fragments 1 and 2) were prepared by PCR, using chromosomal DNA from strain MC4100 as the template and, respectively, the primer pairs DAB-5N–DAB-5C and DAB-3N–DAB-3C (16). The PCR products were cloned into pUC18. A Tetr cassette, isolated from pNK81 (30), was then inserted between the two arcB-flanking fragments to generate pDB3. This plasmid was transformed into strain JC7623 (22) to create a ΔarcB::Tetr strain (ECL5000) by homologous recombination. The ΔarcB::Tetr allele was then P1 transduced into strains ECL5002 and ECL5003, resulting in ECL5004 and ECL5012, respectively. (B) Introduction of modified arcB sequences into the ΔarcB::Tetr strain. The 5′- and 3′-flanking DNA fragments of arcB (fragments 3 and 4) were prepared by PCR, using chromosomal DNA from strain MC4100 as the template and, respectively, the primer pairs IAB-5N–IAB-5C and IAB-3N–IAB-3C (16). The PCR products were cloned into pBluescript II KS (+). A Kanr cassette, isolated from pUC4-KIXX (2), was then inserted between the two arcB-flanking fragments to generate pIB3. Fragment 3 includes the arcB promoter, the ribosome-binding site, and an introduced NdeI site that includes the initiation codon of arcB followed by a HindIII site. A modified arcB sequence (arcB*) was cloned into the pIB3 between the NdeI site and HindIII site, generating pIB*. This plasmid was transformed into strain ECL5000 to replace the ΔarcB::Tetr allele with arcB* Kanr by homologous recombination. Recombinants were selected by their Tets Kanr Amps phenotypes and confirmed by PCR. The arcB* Kanr construct was then P1 transduced into strains ECL5004 and ECL5012.
Requirement of all three conserved residues of ArcB for its ArcA-phosphorylating activity.
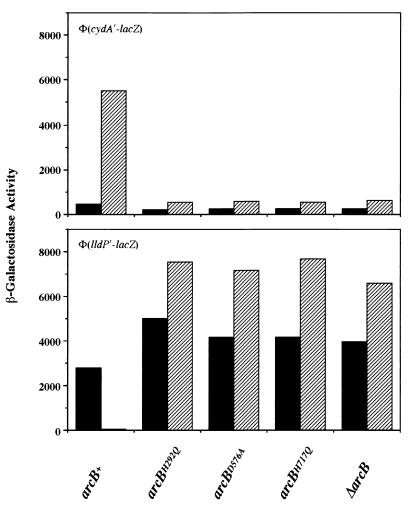
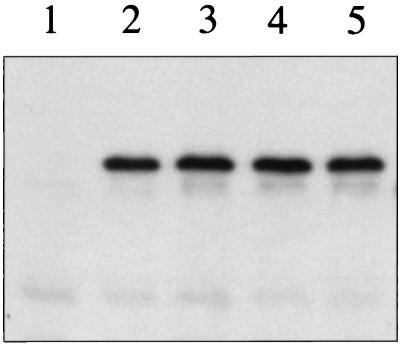
To verify the importance of His292, Asp576, and His717 in the phosphotransfer pathway leading to the formation of ArcA-P, we replaced the chromosomal arcB+ allele by arcBH292Q, arcBD576A, or arcBH717Q in a reporter strain bearing λΦ(cydA′-lacZ) or λΦ(lldP′-lacZ). The cells were grown aerobically or anaerobically and their β-galactosidase activity levels were assayed. It was found that all three mutants exhibited phenotypes indistinguishable from that of the ΔarcB mutant, suggesting that ArcB phosphorylates ArcA exclusively by the relay pathway (Fig. 3). Western analysis showed that all point mutants did produce wild-type levels of ArcB (Fig. 4). To confirm that H1 cannot mediate the phosphorylation of ArcA without H2, we replaced arcB+ by arcB1–520, arcB1–661, arcB1–661, D576A, or arcBD576A, H717Q. All of the tested alleles gave a null phenotype (Table 2).
FIG. 3.
Effects of mutations in the conserved amino acid residues of ArcB on the expressions of λΦ(cydA′-lacZ) or a λΦ(lldP′-lacZ). The cydAB operon encodes cytochrome d oxidase (5), and the lldPRD operon encodes proteins involved in l-lactate utilization (4). The Φ(cydA′-lacZ)-bearing strains were grown in Luria-Bertani broth containing 0.1 M MOPS (morpholinepropanesulfonic acid; pH 7.4) and 20 mM d-xylose. The Φ(lldP′-lacZ)-bearing strains were grown in the above medium supplemented with 20 mM l-lactate as an inducer (4). β-Galactosidase activity was assayed and expressed in Miller units (20). The data are averages from four experiments (variations were <10% from the mean). Solid bars, aerobically grown cells; hatched bars, anaerobically grown cells.
FIG. 4.
Western blot analysis. A 1-ml sample of cultures grown aerobically in Luria-Bertani broth was harvested at an optical density at 600 nm of 0.5. The pelleted cells were washed with 1 ml of 10 mM Tris-HCl (pH 8.0) and solubilized by incubation at 95°C for 5 min in 100 μl of 2× sodium dodecyl sulfate sample buffer. Samples of 10 μl were subjected to electrophoresis in a sodium dodecyl sulfate–12% polyacrylamide gel, and the resolved proteins were electrotransferred to a Hybond-ECL filter (Amersham). Immunoblot analyses were subsequently performed, using ArcB polyclonal antibodies as previously described (16). Lane 1, ΔarcB; lane 2, arcB+; lane 3, arcBH292Q; lane 4, arcBD576A; lane 5, arcBH717Q.
TABLE 2.
Effects of various mutant arcB alleles on the expressions of λΦ(cydA′-lacZ) or λΦ (lldP′-lacZ)a
| Relevant genotype | β-Galactosidase activity (U)b
|
|||
|---|---|---|---|---|
| Φ(cydA′-lacZ)
|
Φ(lldP′-lacZ)
|
|||
| +O2 | −O2 | +O2 | −O2 | |
| arcB+ | 440 | 5,500 | 2,800 | 50 |
| arcB1–661 | 230 | 690 | 4,200 | 6,600 |
| arcB1–661, D576A | 220 | 610 | 4,300 | 7,600 |
| arcB1–520 | 270 | 610 | 4,300 | 7,800 |
| arcBD576A, H717Q | 240 | 570 | 3,900 | 7,100 |
| arcBH292Q, D576A | 230 | 550 | 3,300 | 7,900 |
| ΔarcB | 260 | 630 | 3,900 | 6,600 |
The Φ(cydA′-lacZ)-bearing strains were grown in Luria-Bertani broth containing 0.1 M MOPS (pH 7.4) and 20 mM d-xylose. The Φ(lldP′-lacZ)-bearing strains were grown in the same medium supplemented with 20 mM l-lactate as an inducer (4).
β-Galactosidase activity was assayed and expressed in Miller units (20). The data are averages from four experiments (variations were <10% from the mean).
His717 of H2 derives its phosphoryl group exclusively from the relay.
To test whether His717 can be phosphorylated by a noncognate sensor kinase(s), we replaced arcB+ by arcBH292Q, D576A. The mutant showed an arcB-null phenotype, despite the presence of His717 in the ArcB protein (Table 2). Furthermore, when arcB638–778 (H2) was expressed from a low-copy-number plasmid in an ΔarcB background, an arcB-null phenotype was also found. On the other hand, when the same plasmid was tested in an arcB1–661 background, an arcB+ phenotype was obtained (data not shown). This latter result indicated that the phosphorylation of ArcA via His717 depended on the presence of His292 and Asp576 and that the phosphorelay involved an intermolecular reaction between different ArcB domains, in agreement with the results of our previous in vitro study (8).
Discussion and conclusion.
We were prompted to undertake this study not only because in vitro enzymatic data (8) and in vivo properties of cells with multiple gene dosage (19) may be misleading, but also because of certain conflicting results. For instance, in a study of purified proteins, the rate of ArcA phosphorylation catalyzed by ArcB78–661 (H1-D1) was less than an order of magnitude smaller than that catalyzed by a mixture of ArcB78–661 (H1-D1) and ArcB638–778 (H2), indicating a predominant role of the phosphorelay (8). By contrast, in a study of everted vesicles, the rate of ArcA phosphorylation catalyzed by ArcBD576Q was almost as high as that catalyzed by wild-type ArcB, indicating a predominant role of His292 as a direct phosphoryl group donor to ArcA (27). However, in a third study, the same ArcBD576Q mutant protein (encoded by a low-copy-number plasmid) was inactive in vivo as a phosphoryl group donor to ArcA (19). Paradoxically, in that same study, ArcBH717L apparently was able to serve as a phosphoryl group donor to ArcA (19).
The results from the present study, based on single-copy arcB alleles, indicate that the sole route of phosphotransfer from ArcB to ArcA is by a relay involving His292, Asp576, and His717 of the sensor kinase (Fig. 5). In particular, there is no evidence for direct phosphoryl group transfer from His292 to ArcA or for the phosphorylation of ArcA by an unknown kinase via the H2 domain of ArcB. Thus, the mode of signal transmission in the Arc system seems to be no more elaborate than that proposed for the Bvg (28) and Tor (14) systems.
FIG. 5.
Model for signal transduction by the Arc system. Heavy solid arrows indicate the forward phosphotransfer reactions leading to formation of ArcA-P. Hatched arrows indicate the reverse phosphotransfer reactions leading to signal decay (7). Arrows with crosses indicate phosphotransfer reactions not substantiated by this study.
Acknowledgments
This work was supported by U. S. Public Health Service grant GM40993 from NIGMS of the National Institutes of Health.
REFERENCES
- 1.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37:111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- 3.Cotter P A, Melville S B, Albrecht J A, Gunsalus R P. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol Microbiol. 1997;25:605–615. doi: 10.1046/j.1365-2958.1997.5031860.x. [DOI] [PubMed] [Google Scholar]
- 4.Dong J-M, Taylor J S, Latour D J, Iuchi S, Lin E C C. Three overlapping lct genes involved in l-lactate utilization by Escherichia coli. J Bacteriol. 1993;175:6671–6678. doi: 10.1128/jb.175.20.6671-6678.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu H-A, Iuchi S, Lin E C C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991;226:209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- 6.Georgellis D, Kwon O, Lin E C C. Amplification of signaling activity of the Arc two-component system of Escherichia coli by anaerobic metabolites: an in vitro study with different protein modules. J Biol Chem. 1999;274:35950–35954. doi: 10.1074/jbc.274.50.35950. [DOI] [PubMed] [Google Scholar]
- 7.Georgellis D, Kwon O, De Wulf P, Lin E C C. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- 8.Georgellis D, Lynch A S, Lin E C C. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J Bacteriol. 1997;179:5429–5435. doi: 10.1128/jb.179.17.5429-5435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrabak E M, Willis D K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishige K, Nagasawa S, Tokishita S-I, Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iuchi S. Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB of Escherichia coli. J Biol Chem. 1993;263:23972–23980. [PubMed] [Google Scholar]
- 12.Iuchi S, Lin E C C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iuchi S, Lin E C C. Mutational analysis of signal transduction by ArcB: a membrane sensor protein for anaerobic expression of operons involved in the central aerobic pathways in Escherichia coli. J Bacteriol. 1992;174:3972–3980. doi: 10.1128/jb.174.12.3972-3980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourlin C, Ansaldi M, Méjean V. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS sensor in Escherichia coli. J Mol Biol. 1997;267:770–777. doi: 10.1006/jmbi.1997.0919. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Mizuno T, Shimizu T, Hakoshima T. Insights into multistep phosphorelay from the crystal structure of the C-terminal HPt domain of ArcB. Cell. 1997;88:717–723. doi: 10.1016/s0092-8674(00)81914-5. [DOI] [PubMed] [Google Scholar]
- 16.Kwon O, Georgellis D, Lynch A S, Boyd D, Lin E C C. The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J Bacteriol. 2000;182:2960–2966. doi: 10.1128/jb.182.10.2960-2966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch A S, Lin E C C. Responses to molecular oxygen. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1526–1538. [Google Scholar]
- 18.Lynch A S, Lin E C C. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J Bacteriol. 1996;178:6238–6249. doi: 10.1128/jb.178.21.6238-6249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushika A, Mizuno T. A dual-signalling mechanism mediated by the ArcB hybrid sensor kinase containing the histidine-containing phosphotransfer domain in Escherichia coli. J Bacteriol. 1998;180:3973–3977. doi: 10.1128/jb.180.15.3973-3977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 21.Nagasawa S, Tokishita S, Aiba H, Mizuno T. A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol Microbiol. 1992;6:799–807. doi: 10.1111/j.1365-2958.1992.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 22.Oden K L, Deveaux L C, Vibat C R, Cronan J E, Jr, Gennis R B. Genomic replacement in Escherichia coli K-12 using covalently closed circular plasmid DNA. Gene. 1990;30:29–36. doi: 10.1016/0378-1119(90)90337-q. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 24.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 25.Stevens A M, Sanders J M, Shoemaker N B, Salyers A A. Genes involved in production of plasmid-like forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992;174:2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuzuki M, Ishege K, Mizuno T. Phosphotransfer circuitry of the putative multi-signal transducer, ArcB, of Escherichia coli: in vitro studies with mutants. Mol Microbiol. 1995;18:953–962. doi: 10.1111/j.1365-2958.1995.18050953.x. [DOI] [PubMed] [Google Scholar]
- 28.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 29.Utsumi R, Katayama S, Taniguchi M, Horie T, Ikeda M, Igaki S, Nakagawa H, Miwa A, Tanabe H, Noda M. Newly identified genes involved in the signal transduction of Escherichia coli K-12. Gene. 1994;140:73–77. doi: 10.1016/0378-1119(94)90733-1. [DOI] [PubMed] [Google Scholar]
- 30.Way J C, Davis M A, Morisato D, Roberts D E, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusion by transposition. Gene. 1984;32:369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]