Abstract
Triple-negative breast cancer is considered the most aggressive type of breast cancer among women and the lack of expressed receptors has made treatment options substantially limited. Recently, various types of nanoparticles have emerged as a therapeutic option against TNBC, to elevate the therapeutic efficacy of the existing chemotherapeutics. Among the various nanoparticles, lipid-based nanoparticles (LNPs) viz. liposomes, nanoemulsions, solid lipid nanoparticles, nanostructured lipid nanocarriers, and lipid–polymer hybrid nanoparticles are developed for cancer treatment which is well confirmed and documented. LNPs include various therapeutic advantages as compared to conventional therapy and other nanoparticles, including increased loading capacity, enhanced temporal and thermal stability, decreased therapeutic dose and associated toxicity, and limited drug resistance. In addition to these, LNPs overcome physiological barriers which provide increased accumulation of therapeutics at the target site. Extensive efforts by the scientific community could make some of the liposomal formulations the clinical reality; however, the relatively high cost, problems in scaling up the formulations, and delivery in a more targetable fashion are some of the major issues that need to be addressed. In the present review, we have compiled the state of the art about different types of LNPs with the latest advances reported for the treatment of TNBC in recent years, along with their clinical status and toxicity in detail.
Keywords: liposomes, nanoemulsion, solid lipid nanoparticles, nanostructured lipid carriers, lipid–polymer hybrid nanoparticles, triple-negative breast cancer, targeted therapy
1. Introduction
The American Cancer Society and National Cancer Institute reported approximately 276,480 new cases of breast cancer in the year 2020, out of which approximately 42,170 women died. Further, it was reported that in comparison to every 87 new cases per 100,000 women of HR+/HER2− type breast cancer, TNBC accounts for 13 new cases per 100,000 women. In addition, the American Cancer Society also reported that TNBC exhibited the worst 5-year relative survival rate (≈76.7%), compared to other types of breast cancer (≈90%). Moreover, as TNBC has a reputation for showing metastasis to the brain, lungs, and bones, its stage plays an important role in determining survival outcomes, which states that if the cancer is localized, then survival rate is ≈80.2%, but it eventually drops to ≈11.5% if the cancer is metastasized [1]. TNBC is characterized by alteration in signaling pathways, overexpression of oncogenes, and absence of hormonal (ER, PR, HER-2) expressions, which restrict the treatment to surgery, chemotherapy, radiation, and immunotherapy [2,3]. However, when TNBC gets distant, chemotherapy is considered the most widely employed treatment option with increased survival rates [4].
Although chemotherapy is a widely employed anticancer treatment strategy, it does display certain significant toxicities such as immunosuppression, bone marrow suppression, mucositis, alopecia, gastrointestinal discomfort, ventricular dysfunction, anemia, fatigue, etc., mainly due to its non-selectivity and non-specific cytotoxic effects over healthy cells of bone marrow, gastrointestinal epithelial, and hair follicles. Moreover, chemotherapy also shows multi-drug resistance (MDR) that decreases the anticancer activity of the chemotherapeutics which hinders their efficacy at the cancer site [5]. In addition to this, the drugs used in chemotherapy also display certain limited biopharmaceutical attributes such as large size, metabolic instability, poor aqueous solubility, and susceptibility to p-glycoproteins that hinder their delivery to the cancer site [6,7]. Such findings necessitate the development of a smart therapeutic strategy that will improve the intrinsic biopharmaceutical attributes of the chemotherapeutics as well as facilitate targeted delivery of chemotherapeutics to the cancer site preventing the proliferation and metastasis and enhancing their therapeutic efficacy [8].
This situation led to the emergence of cancer nanotechnology that overcomes the drawbacks of conventional drug delivery systems starting from small-scale barricades such as intracellular trafficking and site-specific targeting to large-scale barriers such as biodistribution [9,10]. Further, to initiate various studies and carry them to clinical translation, in the year 2000, the National Nanotechnology Initiative (NNI) was launched by the US National Science and Technology Council (NSTC) for supporting and improving nanotechnology research. For such initiation, nanoparticles (NPs) have gained much attention in the drug delivery system against various diseases such as cancer [11], diabetes [12,13,14,15], and bacterial infection [16,17,18]. Recently, nanoparticles were employed for the delivery of vaccines [19,20], proteins [21], nucleic acid [22,23], etc. Such application has enhanced the concept of nanotechnology in the field of medical science. Among various nanoparticles (NPs) for cancer therapy, lipid-based NPs (LNPs) are recognized as the most widely approved class of nanoparticles by FDA due to various advantages such as simplicity in their fabrication, ability to self-assemble in aqueous media, offering enhanced bioavailability, being biocompatible, ability to load both hydrophilic and hydrophobic drugs, increased loading capacity, ability to modulate their surface characteristics, etc. [24].
In this review, we discuss the various LNPs employed for the treatment of TNBC such as liposomes, nanoemulsions (NEs), solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), lipid–polymer hybrid nanoparticles (LPH-NPs), and exosomes (Exo) with emphasis on properties of various lipids employed in the fabrication of LNPs, that display their characteristic mechanism of loading of drugs and delivering them to the cancer site. We also mention the current challenges and future perspectives of LNPs in the effective treatment of TNBC.
2. Lipid-Based Nanoparticles: A Versatile Drug Delivery System
Rapid advances in lipid-based nanoparticles show an immense impact on cancer treatment and management. Looking into the structural makeup of LNPs, we found that LNPs are composed of lipids that are both biodegradable and biocompatible such as phospholipids, cholesterol, and triglycerides. The inclusion of such less toxic substances, as well as limited use of organic solvents, makes LNPs a safe drug delivery system against TNBC as compared to polymeric and inorganic NPs [4,8]. According to the general prototype, LNPs consist of API, lipids that are designed to sequester, deliver, and promote functionality. Further, to maintain the stability of the dispersions formed by the LNPs, in environmental stresses, surfactants or a combination of surfactants are used, depending on their HLB values [25]. Further, to safeguard the LNPs from the reticuloendothelial system (RES) and to render biological stability, LNPs are often coated with a stealth or biocompatible polymeric layer such as polyethylene glycol (PEG) [6,26,27]. Finally, for possessing an enhanced targeting property, the LNPs may bind with a targeting moiety such as a biological receptor-specific ligand. Such orientation of LNPs and their small size help the LNPs to accumulate frequently on the tumor sites [6].
In cancer research, it was observed that approximately 40% of new chemical entities show poor aqueous solubility, which further limits their therapeutic efficiency. Thus, the utilization of hydrophobic LNPs as a vehicle either encapsulates or solubilizes the drug moiety that will further improve the stability of poorly aqueous soluble drugs in the surrounding aqueous media and will prevent the precipitation of the drugs both in vitro and in vivo. Hence, it could be inferred that the development of LNPs shows improved chemotherapeutic efficacy by increasing their solubility [28,29]. It was further revealed that the absorption of the poorly water-soluble chemotherapeutics in the LNPs is due to the increased solubilizing capacity of the lipids/oils. The other well-established strategies employed by LNPs in increasing the solubility of the poorly water-soluble chemotherapeutics, thereby increasing their bioavailability, include preservation of the chemotherapeutics within the lipidic matrix against chemical and enzymatic degradation, alteration in the permeability of the gastrointestinal membrane, and facilitation of the lymphatic drug transport [25], as described in Table 1.
Table 1.
Strategies employed for increasing the solubility of incorporated poorly water-soluble drugs by using LNPs.
| Strategy | Mechanisms | Ref. |
|---|---|---|
| Enhanced solubilization | The lipids present in the gastrointestinal tract (GIT) increase the excretion of cholesterol and phospholipids (endogenous bile lipids), which further mediates the emulsification of the lipids present within the carrier system and solubilizes the drug. | [30] |
| Alteration in the biochemical barrier | Certain lipids and surfactants can decrease the intestinal secretions of the gastrointestinal wall, and prevent the metabolic activity of the enterocytes and lumen by alternating the P-glycoprotein, cytochromes, etc., thereby increasing the absorption of the drugs that are considered substrates of the stated efflux pump, and enzymes. | [25] |
| Alteration in the physical barrier | Some lipids and surfactants can promote intestinal absorption and membrane permeability by fluidizing the intestinal cell membrane and breaching the tight junctions. | [31] |
| Facilitation of lymphatic transport system | Lipids such as LCT (long-chain triglycerides) facilitate lipoprotein formation, which further facilitates their lymphatic transport. Hence, it could be stated that LNPs composed of LCT mediate lymphatic transport of poorly aqueous soluble drugs, thus bypassing the first-pass metabolism. | [25] |
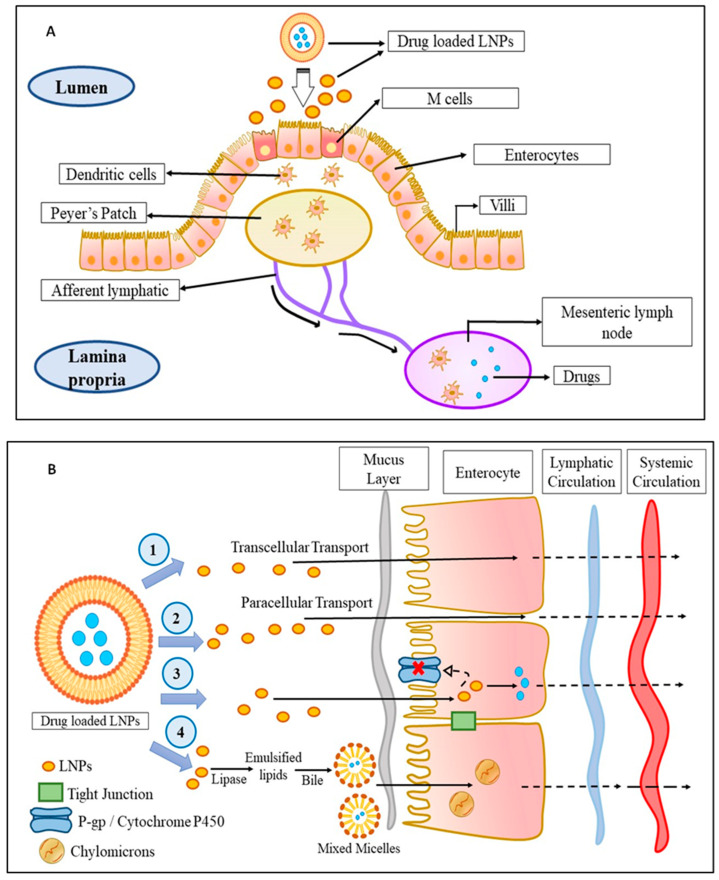
Talking in terms of oral delivery, most chemotherapeutics are suffered from first-pass metabolism which limits their concentration in blood and subsequently at the cancer site, which restricts their therapeutic efficacy. In this context, it was found that drug delivery through the lymphatic system overturns the first-pass metabolism and improves their targeting to the cancer site. From various studies, it was observed that the triglycerides, cholesterol esters, and lipid-soluble vitamins are easily taken up by the lymphatic system and as the LNPs are mostly composed of triglycerides and arranged similarly to that of chylomicrons, the LNPs are easily taken up by the lymphatic system, thereby circumventing the first-pass metabolism, improving their targeting, and reducing toxicity. Physiologically, it was observed that the lymphatic uptake of LNPs occurs via three routes as shown in Figure 1. First is through the gaps occurred within the lymphatic capillaries, the second is through the Peyer’s patches (Figure 1A), which are either isolated or aggregated lymphoid follicles, and the third is through the intestinal wall which follows four different mechanisms, namely, transcellular absorption, paracellular absorption, inhibition of the activity of P-glycoprotein, and cytochrome P450 and generation of chylomicrons (Figure 1B) [32].
Figure 1.
The mechanism of LNPs uptake into the lymphatic circulation: (A) Uptake of drug-loaded LNPs by Peyer’s patch into the lymphatic system: The drug-loaded LNPs are taken up by the M-cells of the enterocytes which are then taken up by the dendritic cells followed by Peyer’s patch from where the drug-loaded LNPs enter into the lymphatic system via afferent lymphatic. (B) Uptake of drug-loaded LNPs by an intestinal wall into the lymphatic system: The drug-loaded LNPs enter the lymphatic system through the intestinal wall in fours ways—(1) transcellular transport, (2) paracellular transport, (3) by inhibiting P-gp glycoprotein and cytochrome P450, or (4) by the production of chylomicrons. Abbreviations: M cell: membranous cell, LNPs: lipid-based nanoparticles; P-gp: P-glycoprotein.
Like cellular uptake, the in vivo fate of the LNPs also plays an important role in the drug delivery system. Basically, the entrapped drug gets released into the physiological surrounding only after breaking of the lipidic matrix. Such phenomenon occurs via lipolysis wherever lipases are found in abundance, especially in the GIT or surface erosion, in case the lipids are insensitive to lipolysis. It was observed that the LNPs composed of aliphatic esters are rapidly degraded by lipases, especially in the small intestine, while the LNPs comprised of triglycerides were first broken down by lysosomal acid lipases into diglycerides, which was then broken down into monoglycerides, and finally into fatty acids in the GIT, followed by endocytosis. Then, the lipolysates form and along with the encapsulated drugs, both are transported to the epithelial surfaces in the form on vesicles or micelles for absorption. It was further observed that apart from GIT, the lipolysis also takes place within tissues and cells. In the process of erosion, the lipid matrix undergoes either hydrolysis or dissolution which eventually plays a role in complete degradation of lipid matrices based on fatty acids and are insensitive to lipolysis. It was observed that as the chain length of the fatty acid increases, the rate of erosion of lipid matrix declines, and drug release becomes slow and steady, while the lipids with medium chain length significantly increases the erosion rate of the lipid matrix, which thereby increases the drug release from the lipid matrix [33].
It was further observed that the membrane-like structure of LNPs provides flexibility in their particle sizes which enables them to stay in the systemic circulation for a longer period by bypassing the immune responses, resulting in improved passive accumulation of LNPs in the cancer site [34]. Moreover, the particle sizes of LNPs are generally greater than 10 nm in diameter, which restricts their elimination by the kidney but allows the elimination via capillaries situated at the leaky microvasculature. Since leaky microvasculature is a common characteristic of solid tumors such as TNBC, many potent chemotherapeutics have been encapsulated in LNPs to extract the advantage of the EPR effect, which further results in increased drug accumulation at the tumor site with reduced dose regimen and systemic side effects. In this context, the encapsulation of doxorubicin (DOX) within LNPs (Doxil®/Caelyx®) increased their tumor accumulation and reduced their distribution to the myocardium, thereby decreasing the doxorubicin-induced cardiotoxicity, as compared to conventional doxorubicin solution (Adriamycin®, Pfizer, Manhattan, NY, USA) [28].
It was observed that the challenging task in the preparation of LNPs is obtaining proper size and polydispersity of the LNPs and uniform loading of chemotherapeutics within the LNPs. It was observed that the desired shape and size was obtained by controlling the process parameters employed in the various types of preparation procedure of LNPs. For instance, high pressure homogenization is one of the methods employed for the preparation of LNPs. It was observed that during hot high-pressure homogenization, very narrow particle size distribution was obtained, while in cold high-pressure homogenization, a broad size distribution was obtained. It was inferred that the size distribution of LNPs in high-pressure homogenization depends upon the temperature and pressure provided, type of homogenizer employed, and number of homogenization cycles applied. Likewise, in solvent emulsification evaporation method, the particle size of LNPs were controlled by the type and concentration of lipids, and surfactant mixture within the organic phase. It was observed that the particle size ranges between 30 and 100 nm when the lipid concentration is employed up to 5% w/v, above which the particle size increases beyond 100 nm. In case of solvent emulsification diffusion method, the particle size obtained was below 100 nm with narrow particle size distribution. It was observed that particle size increases on usage of non-ionic surfactant, while it decreases on using ionic surfactant. However, it was suggested to use a combination of two or more surfactants for better control of the particle size. Lastly, in the ultrasonication method, the particle size is obtained in the range of 30–200 nm with broad particle size distribution. It was observed that the particle size can be controlled by varying the frequency, intensity, and time of ultrasonication [35].
For the loading of the chemotherapeutics within the LNPs, the active incorporation method can be used, i.e., loading of drugs after LNPs formation, or passive i.e., loading of drugs during LNPs formation [36]. The active method involves adsorption or absorption methods that are achieved by incubating the LNPs with concentrated drug solution [37]. The passive method involves the mechanical method, solvent dispersion method, and detergent removal method [36]. It was observed that the drug loading depends upon the solubility of the drugs within the lipid matrix, which is further associated with the composition of the lipid matrix, molecular weight of the drug, the interaction between the drug and lipids and the presence of end functional groups (i.e., ester or carboxyl) in either the drug or lipid matrix [37]. LNPs were also used for the loading of nucleic acid (siRNA, mRNA, and pDNA) proteins. It was observed that fabrication of nucleic acid loaded LNPs include detergent dialysis and ethanol loading technique. However, the rapid-mixing method and T-mixing method have gained more popularity as it assures >90% entrapment efficiency. In recent times, microfluidic mixing approaches were designed based on rapid-mixing approach which further promises to fabricate nucleic acid, and protein loaded LNPs in a more reproducible and scalable fashion. It was further observed that all the mentioned methods allow rapid mixing of lipid containing organic phase into aqueous phase comprised of nucleic acid, and proteins, and resulting in an enhanced entrapment efficiency [38].
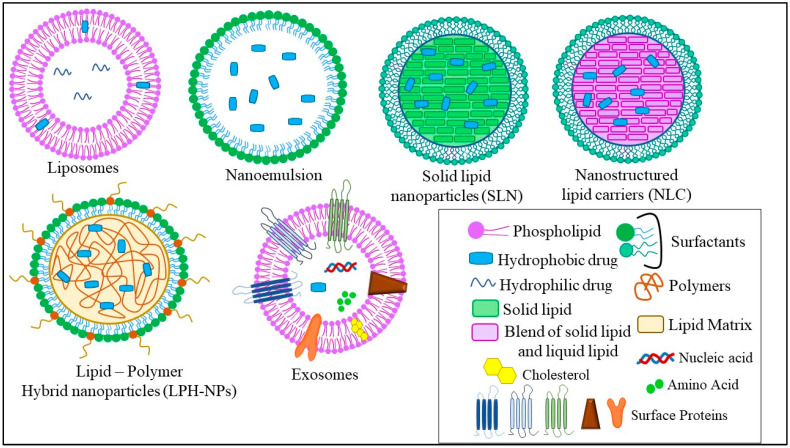
The different types of LNPs developed for the treatment of TNBC are liposomes, nanoemulsions (NEs), solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), lipid–polymer hybrid nanoparticles (LPH-NPs), and exosomes (Exo). Briefly, liposomes are microscopic phospholipid bilayer nanovesicles while NEs are colloidal nanosystems with lipophilic surfaces and a negative charge. SLNs are colloidal nanosuspensions, while NLCs are colloidal blends of solid and liquid lipids. LPH-NPs are colloidal blends of lipids and polymers (non-lipid substances) [4]. Exosomes (Exo) are biological nanosized vesicles composed of lipid bilayers with embedded surface proteins [39]. The distinct characteristics, advantages, and disadvantages of each type of LNPs are mentioned in Table 2 and described in Figure 2.
Table 2.
Various types of lipid-based nanoparticles (LNPs) employed for the treatment of TNBC.
| LNPs | Composition | Features | Advantages | Disadvantages | Status | Refs. |
|---|---|---|---|---|---|---|
| Liposomes | Phospholipids and cholesterol | Forms 1–20 phospholipid bilayers (vesicles) with globule size 30 nm to 3000 nm. Encapsulate hydrophilic and hydrophobic drugs. |
Induce a controlled release profile. Enhances solubility of hydrophobic drugs, thereby increasing bioavailability |
The structure is rigid. Controlled conditions are employed for reproducibility. Stability problems |
Some are commercialized, while some are under clinical trials. | [40] |
| Nanoemulsions (NEs) | Oils, surfactants, and co-surfactants | Kinetically stable o/w dispersions. Have high surface area with small size (50–500 nm). Encapsulate both lipophilic and lipophobic drugs. |
Form spontaneously. Increased reproducibility |
Require high concentration of surfactant, hence can lead to toxicity. Scare choices of surfactants, as the surfactant used must be GRAS recommended. |
Some are commercialized, while some are under clinical trials. | [41] |
|
Solid-lipid nanoparticles
(SLNs) |
Solid lipids (fats), surfactants | Solid lipids instead of oil improve the lipidic core and provide stability and mobility to the drug within the lipidic core. | Exhibits delayed degradation of lipidic matrices allowing controlled release of the drug. | Exhibit reduced drug loading due to crystalline structure of the lipidic matrix, facilitating drug expulsion. Chances of agglomeration and polymorphic transitions. |
Pre-clinical | [42] |
| Nanostructured lipid carriers (NLCs) | Solid lipids (fats), liquid lipids (fats or oils), and surfactants | NLC has a distorted structure which makes the matrix structure imperfect and creates spaces for the accommodation of active compounds. | Increased entrapment efficiency, with reduced drug leaking on storage. | Optimization required of the binary mixture, i.e., the ratio of solid and liquid lipids, otherwise, it would lead to cytotoxicity associated with the nature and concentration of lipid matrix. |
Pre-clinical | [43] |
| Lipid–polymer hybrid nanoparticles (LPH-NPs) | Polymers, lipids | Hybrid vesicular structures integrate advantageous characteristics of polymers and liposomes in a single moiety. | Load efficiently one or multiple drugs with different properties. | - | Pre-clinical | [44] |
|
Exosomes
(Exo) |
Cholesterol, diacylglycerol, surface proteins, heat shock proteins, lysosomal proteins, nucleic acids | Homogenous nanosized vesicles with size ranges from 30–150 nm. Formed by multivesicular bodies (MVB) after fusing with plasma membrane. |
Immunocompatible | Rapid clearance from circulation after in vivo administration. No current manufacturing method. |
Pre-clinical | [45,46] |
Figure 2.
Different types of LNPs used for the treatment of TNBC.
2.1. Liposomes
Liposomes are a vesicular-type drug delivery system obtained spontaneously by dispersing the lipids (phospholipids) in aqueous media. The liposomes were first discovered in 1963 by Alec Bangham [47]. In terms of lipid shell, phospholipids form the main element of liposomes [48]. It was found that phospholipids are also considered an important constituent of the biological membrane. The phospholipids are comprised of a polar head, which is composed of hydrophilic moieties, and a non-polar tail, which is comprised of hydrophobic moieties [49,50]. Depending on the presence or absence of charges or the type of charges, liposomes are classified as uncharged, positively charged, negatively charged, and amphiphilic (zwitterionic). The positively charged lipids used in the formation of liposomes are N-[1-(2,3-dioleyloxy)propyl]-N,N,N-triethylammonium (DOTMA), and 1,2-dioleoyl-3-trimethylammoniopropane (DOTAP), while the negatively charged lipids employed in liposomes are phosphatic acid, phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, and dicetylphosphate). The various zwitterionic lipids used are phosphatidylethanolamine, phosphatidylcholine, etc. [51]. It was found that the administration of the charged lipids increases the interlamellar distance between the phospholipid bilayers, which provides enhanced drug entrapment efficiency and physical stability. Further, in a study, it was observed that the presence of DMPC (dimyristoylphosphatidylcholine, a derivative of phosphatidylcholine) induced apoptosis in various cancer cell lines such as breast cancer, lung cancer, etc. [47]. Apart from phospholipids, cholesterol also exists as one of the elements of liposomes and plays a vital role in maintaining and preserving the fluidity, permeability, and stability of the phospholipids both in vitro and in vivo. Further, it was observed that using derivatives of cholesterol such as 6-aminomannose-cholesterol or glycosylated cholesteryl bypasses the RES uptake and increases the targetability of the liposomes towards cancer cells [47]. It was further found that the liposome also improves the aqueous solubility of poorly water-soluble drugs [28].
Therefore, from the above reports, it was inferred that the stability of the phospholipid bilayer, the entrapment efficiency, and the drug loading of the liposomes as well as their tissue distribution and renal clearance ultimately depend on the composition of the lipid membranes and the content of the cholesterol.
Various poorly water-soluble drugs have been administered using liposomes as a delivery system such as indomethacin, amphotericin B, and azidothymidine, which have already reached the commercial market [28].
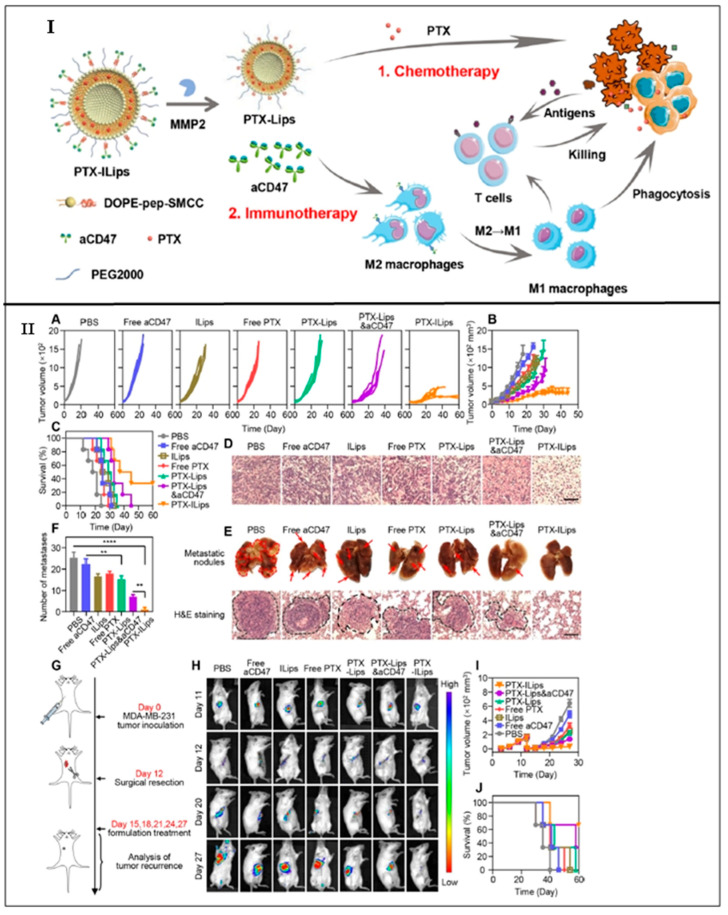
Guo et al., 2019 developed a dual complementary liposome (DCL) composed of lipids such as 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy (polyethylene glycol)-2000] (DSPE-PEG-COOH), encapsulating doxorubicin and further surface-functionalized with antibodies against intercellular adhesion molecule–1 (ICAM1) and epithelial growth factor receptor (EGFR) for effective treatment of TNBC. It was observed that DCLs showed an average particle size of 130 ± 30 nm with a zeta potential of −6 and −10 mV. Further, it was revealed that DCLs showed enhanced internalization to MDA-MB-231 cells and MDA-MB-436 cells (42.7% and 60.9% respectively), along with a significant reduction of proliferation in vitro (30–40%). Moreover, the cancer cells (MDA-MB-231 and MDA-MB-436) treated with DCLs showed a reduction in their cell count by 64% and 46%, respectively. DCLs displayed enhanced tumor targetability and antitumor efficacy with reduced lung metastasis, depicting that the DCLs could be served as an effective therapeutic nanoplatform against TNBC [52]. Yan et al., 2019 fabricated tLyp-1-peptide modified liposomes composed of DSPE-PEG2000. The modified liposomes were prepared to encapsulate the miRNA responsible for silencing the slug gene. It was found from the previous studies that the slug gene is responsible for activating the TGF-β1/Smad pathway, causing invasion and proliferation of TNBC cells. It was found that the modified miRNA liposomes showed a particle size of 120 nm and exhibited an enhanced cellular uptake by TNBC cells in vitro, targeting mitochondria. Moreover, the modified miRNA liposomes showed enhanced anticancer activity and silenced the expression of the slug gene. In addition to these, the modified miRNA liposomes showed increased internalization to TNBC cells (48.79 ± 0.42), as compared to free miRNA complexes (3.69 ± 0.08). In addition, modified miRNA liposomes showed increased inhibitory rates (64.33 ± 8.18%), as compared to free miRNA complexes [53]. Chen et al., 2021 prepared detachable immune liposomes (ILips) as an immunochemotherapeutic approach for delivering paclitaxel and anti-CD47 in the TNBC. The lipids used for the preparation of ILips include DOPE (dioleoylphosphatidylethanolamine). It was observed that the ILips facilitate the release of CD-47 in response to MMP2 eventually polarized the M2 phenotype to M1 macrophage enhancing phagocytosis of TNBC cells and activating the responses of the T cell immune system. Paclitaxel and CD-47 showed a synergistic anticancer effect along with reduced metastasis, compared to paclitaxel liposomes and free CD-47 (2.3- and 3.1-fold, respectively). Furthermore, a lower IC50 was observed in the case of ILips as compared to paclitaxel-liposomes and free paclitaxel (2.8- and 6.4-fold respectively), which indicated that the ILips showed significant inhibition of TNBC cell proliferation. In addition to these, ILips showed an increased expression of CD80 (1.5-fold) as compared to free CD-47, indicating that the binding of PTX and CD-47 led to the effective delivery of CD47 to cancer sites along with increased polarization of macrophages (Figure 3) [54]. Alawak et al., 2021 engineered thermoresponsive liposomes encapsulating doxorubicin for the effective treatment of TNBC. The engineered thermoresponsive liposomes were further surface-functionalized by linking with the MAB1031 antibody via covalent coupling (LipTS–GD–MAB). It was observed that the MAB1031 antibody was employed to target ADAM8, found to be overexpressed in TNBC patients. The lipids used for the fabrication of liposomes include DPPC, DSPC, cholesterol, and DSPE. The cellular toxicity study revealed that 80% of cells were found viable when the cells were treated with LipTS–GD–MAB. In addition to this, the LipTS–GD–MAB showed increased cellular internalization as compared to doxorubicin liposomes [55]. El-Senduny et al., 2021 prepared Azadiradione-loaded liposomes (AZD-lipo) for effective treatment of TNBC. It was observed that the AZD-lipo showed enhanced anti-cancer activity along with increased oral bioavailability as compared to free AZD. In addition, AZD-lipo showed less expression of proteins responsible for the proliferation of TNBC cells, and angiogenesis in TNBC cells such as cyclin D1, COX-2 (0.024 ± 0.005 at 50 μM), survivin, and VEGF-A (0.302 ± 0.01 at 25 μM) as compared to free AZD, where the concentration of COX-2 is 0.553 ± 0.015 at 50 μM, and that of VEGF-A is 0.801 ± 0.011 at 25 μM. In addition, the AZD-lipo showed decreased IC50 values (26.85 ± 3.48 μM), as compared to free AZD (44.88 ± 2.57 μM). Such observations indicated an increased bioavailability of AZD in the biological system from AZD-lipo. Hence, it could be inferred that liposomes provide an effective therapeutic strategy for increased delivery of low bioavailable drugs and effective treatment against TNBC [56].
Figure 3.
(I) An illustration displaying the construction of PTX-ILips and the release of PTX and aCD47 from the liposome for effective chemotherapy and immunotherapy respectively against TNBC. (II) Anticancer efficacy study and recurrence inhibition study in vivo: (A) Individual tumor growth curves in different groups. (B) Tumor growth kinetics of MDA-MB-231 tumors in mice treated with different formulations. (C) Survival rates of animals in various groups. (D) H&E staining of tumor slices collected from mice after the treatment of 21 days. Scale bar = 100 μm. (E) Photographs of lung metastatic nodules and histological assessment of lung metastatic nodules via H&E staining. Scale bar = 100 μm. (F) Numbers of lung metastatic nodules from each group. (G) Schematic illustration of the establishment of tumor recurrence model and therapy with different formulations. (H) Representative IVIS images of MDA-MB-231 tumor-bearing mice in each group. (I) Tumor volume growth curves after tumor implantation, subsequent surgery, and therapy. (J) Survival of mice in different treatment groups. Data are displayed as the mean ± SD. ** p < 0.01; **** p < 0.0001. Reprinted (adapted) with permission from [54]. Copyright (2021) American Chemical Society.
2.2. Nanoemulsions (NEs)
Nanoemulsions (NEs) are an isotropic thermodynamically stable system composed of two immiscible liquids that are equilibrated into a monophase using surfactants or a mixture of surfactants and co-surfactants. The nanoemulsions are composed of oils, surfactants, or their mixture and aqueous phase. The oil phase is used as a solubilizer for hydrophobic drugs [57]. For the delivery of the hydrophobic drugs via NE, the drugs are incorporated into the oil phase to form nanodroplets dispersed into the continuous aqueous phase and provide an oil in water (O/W) NE system [58]. It was further observed that the rate of drug release from the NE primarily depends on the oil/water partition coefficient of the drug molecule, oil/lipidic content as well as the water content of the NE system [28]. In NE, 5–20 wt% of the oil or lipid is considered as the dispersed phase. Hence, screening of oil or lipids is proven to be an important aspect of nanoemulsion formation, as the API must get freely solubilized in the oils/lipids before their dispersion to the aqueous phase. The various oils/lipids used for the lipid phase are glycerides, medium-chain triglycerides, long-chain unsaturated fatty acids, vegetable oils, and polyalcohol esters of medium-chain fatty acids [59]. It was observed from various studies that the nanoemulsions developed using long-chain triglycerides (LCT) showed an average particle size of 120 nm, while those prepared using short-chain triglycerides (SCT) showed smaller particle sizes (40 nm), compared to the former ones. In addition, the lipidic core of the nanoemulsion exhibits an impact on drug loading, stability, and physicochemical attributes. The surfactant or the mixture of surfactants and co-surfactants is employed to reduce the interfacial tension between the lipid phase and aqueous phase for the development of a thermodynamically stable monophasic system. Moreover, it was found apart from reducing the surface tension, a suitable surfactant stabilizes the interfacial surface via electrostatic interactions. The various emulsifying agents used in nanoemulsion preparation include surfactants such as Tween 80, sodium dodecyl sulfate, phospholipids such as soy lecithin, zwitterionic proteins such as caseinate, polysaccharides such as modified starch, and polymers such as PEGs [60]. Various studies showed that the small size, large surface area, and tunable surface characteristics help the nanoemulsion in increasing their circulation half-life, and specific targetability towards cancer sites. As cancer cells are fenced by leaky vasculature, the nanoemulsions can easily bypass the physiological barriers and accumulate within the cancer cells [61]. Various anticancer drugs such as tamoxifen and dacarbazine have been administered in the NE system [28].
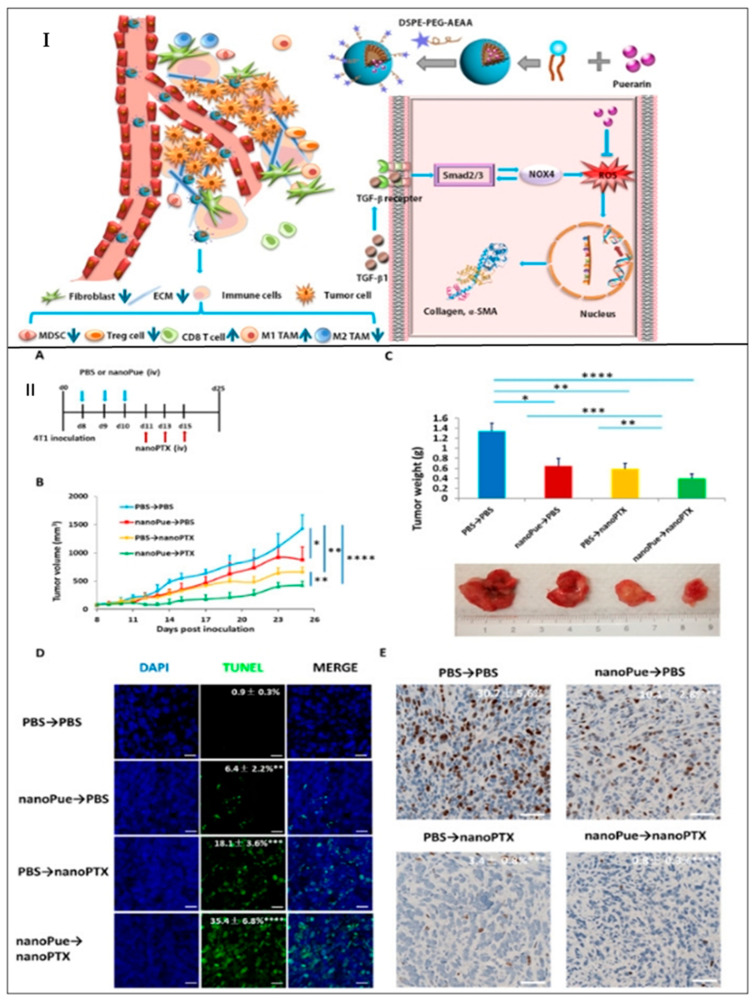
Kim et al., 2019 developed decitabine (DAC)- and panobinostat (PAN)-loaded nanoemulsion which was further coated with lysophophatidylcholine and lysophophatidic acid for targeting LPC receptor and LPAR1 receptor that is overexpressed in TNBC cells. It was observed that the DAC-PAN-LNEs restored CDH1/E-cadherin and suppressed the expression of FOXM1 which eventually inhibited the growth of TNBC cells. Further, it was observed that DAC/PAN LNEs decreased the cell viability of MDA-MB-231 by 55% which indicated the increased therapeutic activity of NEs against TNBC. In addition, DAC/PAN-LNEs synergistically decreased the expression of FOXM1 mRNA and FOXM1 protein expressions by 80% [62]. Xu et al., 2020 developed puerarin nanoemulsion (NanoPue) using soya lecithin and Kolliphor® HS15 for increased oral bioavailability and therapeutic efficacy against TNBC. It was observed that the NanoPue reduced the expression of tumor-associated fibroblast (TAFs) and enhanced the intra-tumoral infiltrations (ITLs) of cytotoxic T cells by 6-fold and 2-fold, respectively, as compared to control, and mediated chemotherapy effect of nano-paclitaxel in the desmoplastic triple-negative breast cancer (TNBC) model. Such an activated immune-microenvironment caused by NEs treatment facilitated a synergistic PD-L1 blockage approach for the treatment of TNBC (Figure 4) [63]. Han et al., 2021 fabricated elemene nanoemulsion (E-NE) for the treatment of TNBC as well as to inhibit their metastasis to the lung. The lipidic phase is comprised of soybean phospholipids and cholesterol. It was observed that E-NE reduced the stabilization of HIF-1α by effectively scavenging ROS. Additionally, the E-NE limited angiogenesis and NLRP3 inflammasomes and IL-1β [64]. Saraiva et al., 2021 developed edelfosine nanoemulsion (ET-NEs) containing lipids such as miglyol 812 and phosphatidylcholine. It was observed that the ET-NEs decreased tumor growth in vitro and in vivo. Furthermore, it was observed that the ET-NEs penetrated the physiological barriers of MDA-MB 231 xenografted zebrafish embryos, resulting in a significant reduction of cancer cell proliferation, which was further confirmed by confocal laser microscopy. Further, it was observed that the ET-NEs showed a dose-dependent IC50 which was found to be 6.9 μg/mL at 13.2 μM after 24 h of incubation, whereas the free ET showed a higher IC50 which is 13.9 μg/mL at 26.5 μM [65].
Figure 4.
(I) Schematic diagram of TME remodulation by targeted delivery of puerarin-loaded nanoemulsion. (II) Combination treatment of nanoPue and nanoPTX on 4T1 tumor model: (A) nanoPue and nanoPTX combination treatment scheme. (B) Tumor growth curves of 4T1 tumors in different treatment groups. (C) The tumor weight and the representative tumor image at the end of the experiment in different treatment groups. (D) TUNEL staining of differently treated 4T1 tumor tissues. (E) Comparison of Ki67 expression of 4T1 tumors in different treatment groups. Scale bar represents 20 μm * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001. Reprinted from Biomaterials, 235, Xu, et al., Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model, 1-12, Copyright (2020), with permission from Elsevier [63].
2.3. Solid Lipid Nanoparticles (SLNs)
Solid lipid nanoparticles (SLNs) are O/W type of colloidal nanoparticles consisting of an inner lipid-based phase and outer aqueous-based phase that are stabilized by surfactants or a mixture of surfactants [66,67]. It was observed from various studies that SLNs can either solubilize lipophilic chemotherapeutics homogeneously within the lipidic matrix or develop a drug-enriched shell surrounding the lipidic core [68,69]. Further, it was observed that based on the type of drug deposition pattern within the lipid matrices, i.e., either chemotherapeutics-enriched core or chemotherapeutics-enriched shell, the drug release profile can be regulated to our advantage for the fulfillment of desired release profile [70]. For example, SLNs with drug-enriched shells exhibit a biphasic drug-release profile through initial burst release from the outer shell followed by gradual release from the lipid core. However, in SLNs with drug-enriched core, a more prolonged sustained release profile was observed due to enhanced drug diffusional distance from the lipidic core [28]. The lipid-based phase used is the solid lipids including triglycerides, fatty acids, steroids, and waxes [71]. It was further reported that the lipids and surfactants used for the preparation of SLNs should fall under GRAS (Generally Recognized as Safe) regulations. The most widely used solid lipids are palmitic acid, stearic acid (SA), glyceryl monostearate (GMS), compritol 888 ATO, trimyristin, Capmul®MCM C10, soybean lecithin, etc. In terms of structure, SLNs show similarity with emulsion except for the fact that the SLNs replaced the oily core with a lipid-based core [8]. Moreover, the SLNs also show a certain amount of similarity as well as dissimilarity with the conventional liposomes. It was observed that like liposomes, SLNs are composed of lipids and unlike liposomes, the SLNs do not have a lipidic bilayer instead composed of a micelle-like structure. It was stated that lipids used in the SLNs should remain in solid form at room temperature and body temperature [3]. Such characteristics help in encapsulating lipophilic drugs in the melted lipid phase which further help in increasing the drug-loading capacity of SLNs and altering the physicochemical properties of drugs both in vitro and in vivo [72,73]. Such features also aid in reducing the degradation profile of the lipids making them suitable for the fabrication of the controlled release formulation. Rheologically, it was observed that the formation of SLNs depends on the interfacial tension (adhesive forces) between the two phases, where the addition of one or more surfactants the interfacial tension by reducing the surface energy and facilitates the formation of stable SLNs [72]. From various studies, it was observed that typically, SLNs are comprised of 0.1–30% solid lipid and 0.5–30% surfactant or surfactant blend [74]. SLNs exhibit increased loading capacity, entrapment efficiency, and less toxicity as compared to polymeric nanoparticles. Therapeutically, SLNs show enhanced targetability to cancer cells via passive targeting as well as active targeting with some external modifications. Additionally, SLNs could encapsulate various moieties such as drugs, proteins, nucleic acids, etc., and improve their pharmacokinetic attributes and physicochemical stability [71]. SLNs have been fabricated for the delivery of various lipophilic chemotherapeutics such as camptothecin, all-trans retinoic acid, etc. Despite the advantages provided by the SLNs, they do exhibit certain physical instability upon storage. It was observed that the solid lipids undergo crystallization during storage which limits the movement of active moieties within the lipidic core, mediating an expulsion of active moieties into the dispersion media and affecting the entrapment efficiency of the lipid-based nanosystem [66].
Eskiler et al., 2018 prepared BMN-673 loaded SLNs using GMS as solid lipids and Tween 80 as a surfactant to improve its therapeutic index and to overcome the BRCA1 mutated sensitive and resistant TNBC. It was observed that compared to native BMN 673, BMN 673-SLNs showed a significant decrease in HCC1937 and HCC1937-R cells with less damage to TNBC cells. In addition, BMN 673-SLNs induced significant toxicity in TNBC cells via breaking of double-stranded DNA, arresting of G2/M cell cycle, and cleaving of PARP moieties [75]. Siddhartha et al., 2018 developed di-allyl-disulfide (DADS)-loaded solid lipid nanoparticles using palmitic acid as solid lipid and pluronic F-68 and soy lecithin as surfactant mix, which was further conjugated with RAGE antibody to enhance the targetability and delivery of DADS to TNBC cells. It was observed that the DADS-RAGE-SLNs significantly increased the cytotoxicity and apoptosis (61.8%) as compared to DADS (15%). Additionally, DADS-RAGE-SLNs showed enhanced cellular internalization via receptor-mediated endocytosis as the SLNs bypassed P-gp efflux proteins as compared to DADS [76]. Kothari et al., 2019 fabricated docetaxel (DTX)–alpha-lipoic acid (ALA) co-loaded SLNs using GMS, SA, and Compritol ATO 888 as solid lipids and Tween 80 as a surfactant to treat TNBC. It was observed that the DTX-ALA-SLNs showed increased cytotoxicity to 4T1 cells as compared to DTX-SLNs, ALA-SLNs, and free drugs. Moreover, the DTX-ALA SLNs showed increased apoptosis of 32% as compared to free DTX which is only 11% [77]. Pindiprolu et al., 2019 prepared niclosamide-loaded SLNs (Niclo-SLNs) using stearyl amine as solid lipid and Tween 80, and pluronic F-68 as surfactant mix for the treatment of TNBC. It was observed that the Niclo-SLNs showed increased cytotoxicity and enhanced cellular internalization (77.06%) at the G0/G1 phase of the cell cycle as compared to free Niclo (69.50%). It was inferred that Niclo-SLNs showed increased cellular uptake due to their ability to bypass the efflux pump and increased absorption of drugs within the cancer cells [78]. In this context, Pindiprolu et al., 2020 fabricated phenylboronic acid-modified Niclo-SLNs (PBA-Niclo-SLNs) to enhance the targetability of Niclo to TNBC cells, thereby increasing its therapeutic efficacy toward TNBC cells. It was observed that PBA-Niclo-SLNs showed increased cytotoxicity (CTC50 7.311 ± 2.1 μM), inhibition of cell proliferation at G0/G1 cell cycle (74.01 ± 0.60%) and apoptosis (21.3 ± 1.0%) as compared to Niclo-SLNs (CTC50 18.49 ± 2.5 μM; 61.01 ± 1.10%; 12.3 ± 1.1%), and free Niclo (CTC50 31.17 ± 3.2 μM; 54.21 ± 0.90%; 10.8 ± 0.9%), respectively. Additionally, PBA-Niclo-SLNs significantly inhibited STAT3, TNBC stem cell populations (CD44+/CD24−), and EMT (epithelial–mesenchymal transition) markers along with increased tumor-site accumulation with significant tumor regression and enhanced survivability of TNBC-bearing mice [79].
2.4. Nanostructured Lipid Carriers (NLCs)
Nanostructured lipid carriers (NLCs) are considered second-generation lipid-based nanoparticles. It is composed of a mixture of solid lipid and liquid lipid which is further stabilized in the aqueous phase via one or more surfactants [80]. The incorporation of liquid lipid and solid lipid within the lipid matrix forms a massive crystal imperfection or amorphous structure that facilitates enhanced loading of drugs into the lipid matrix with less pronounced drug expulsion [8,74]. It was further observed that the NLCs obtained should remain solid at a temperature higher than 40 °C. Generally, NLCs encapsulate approximately 5% of drug w/v where approximately 3 to 4% drug loading is obtained (entrapment efficiency of ≈70%) [74]. The various solid lipids employed in NLCs preparation are glyceryl tripalmitate, softisan 154, glyceryl monostearate, compritol ATO 888, stearic acid, precirol, PEG-DSPE, soybean phosphatidylcholine, etc., and the liquid lipids used for the preparation of NLCs include glyceryl tridecanoate, olive oil, labrafil WL 2609 BS, oleic acid, labrafil M2125 Cs, labrafac PG, polyoxyl castor oil, etc. From various studies, it was observed that the NLCs show high tolerability due to the existence of lipids that are bio-compatible and tunable. Such characteristics enable NLCs to show increased drug loading capacity, decreased risk of gelation, and restricted leakage of the drug upon storage. In addition to these, NLCs also extend their exposure period over tumor cells via the EPR effect, thereby increasing the therapeutic efficacy of the antitumor drug on the tumor site [8]. The NLCs are further multi-functionalized to increase the drug payload, increase targetability to the cancer site, and release the drug in a more controlled way [74].
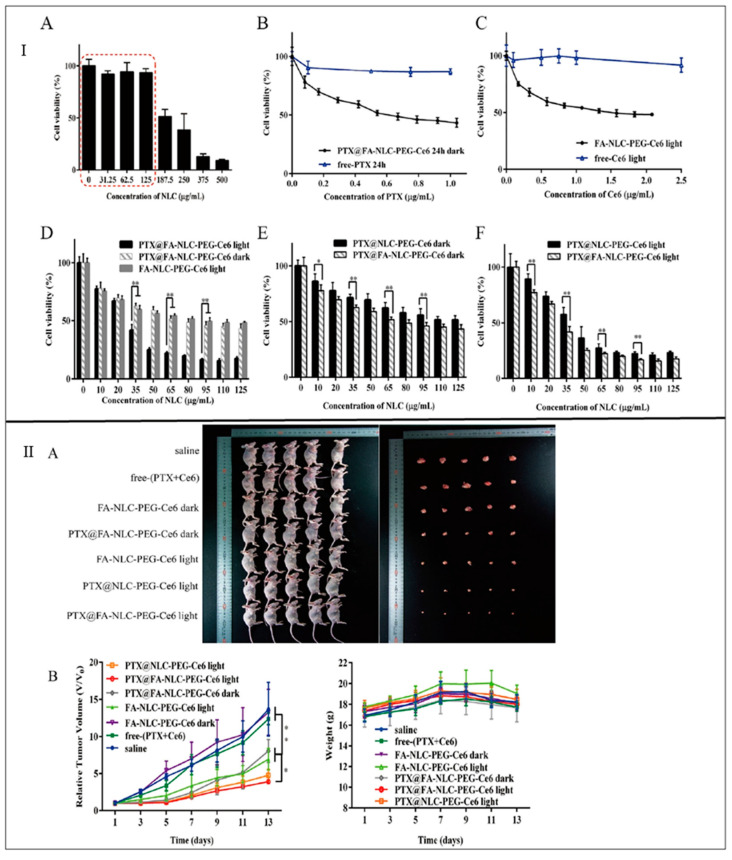
Pedro et al., 2019 prepared paclitaxel-loaded NLCs (PTX-NLCs) using compritol ATO 888 as solid lipid and MCT as liquid lipid to increase its therapeutic efficacy against TNBC. The NLCs were further stabilized by using Tween 80 and soya lecithin. It was observed that the PTX-NLCs showed increased in-vitro cell cytotoxicity and anti-clonogenic activity against MDA-MB-231 cells as compared to free PTX. Further, from the cell viability assay, it was observed that the free PTX showed more cell viability which is 56.0 ± 3.2% as compared to PTX-NLCs (38.0 ± 5.0%). Additionally, PTX-NLCs exhibited 1.5- and 1.7-fold increased tumor site accumulation after 30 and 120 min, respectively, in tumor-bearing mice, as compared to free PTX [81]. Zhang et al., 2019 fabricated folic acid (FA)-functionalized paclitaxel (PTX) and chlorin e6 (Ce6)-loaded NLC (PTX-Ce6-NLC) to increase their targetability and therapeutic efficacy against TNBC. The NLCs were prepared using Precirol ATO 5 as solid lipid and Maisine 35-1 as liquid lipid, stabilized by Cremophor RH40. It was observed that FA-PTX-Ce6-NLC showed enhanced MDA-MB-231 cellular uptake via FR-mediated endocytosis as compared to free PTX. Moreover, it was observed that Ce6 dissociated and evenly distributed in tumor cells. Additionally, from the pharmacodynamic study, it was observed that the NLCs showed enhanced drug-loading without side effects as compared to free PTX (Figure 5) [82].
Figure 5.
(I) In vitro cytotoxicity assays: (A) Cell viability of MDA-MB-231 cells after incubation with FA-NLC-PEG-Ce6 (dark) NPs of different concentrations. (B) Cytotoxicity evaluation of free-PTX and PTX@FA-NLC-PEG-Ce6 in dark in MDA-MB-231 cells by MTT. (C) Cytotoxicity evaluation of free-PTX and PTX@FA-NLC-PEG-Ce6 in light in MDA-MB-231 cells by MTT. (D) Cytotoxicity comprehensive evaluation of single drug NLCs and combination drug NLCs in MDA-MB-231 cells by MTT. (E) Cytotoxicity evaluation of PTX@NLC-PEG-Ce6 and PTX@FA-NLC-PEG-Ce6 in MDA-MB-231 cells with red laser by MTT. (F) Cytotoxicity evaluation of PTX@NLC-PEG-Ce6 and PTX@FA-NLC-PEG-Ce6 in MDA-MB-231 cells without red laser by MTT. * p < 0.05, ** p < 0.01. (II). In vivo anti-cancer activity of NLCs in tumor-bearing nude mice after intravenous administration of saline, free (PTX + Ce6) and different kinds of NLCs, with red laser after 24 h of injection (each mouse for 30 min): (A) Photographs of sacrificed nude mice and the tumor tissues collected from them. (B) Changes of relative tumor volumes in MDA-MB-231 tumor-bearing nude mice of each group. Note: * p < 0.05, ** p < 0.01 (n = 5). Reprinted from International Journal of Pharmaceutics, 569, Zhang, et al., Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6, 1-12, Copyright (2019), with permission from Elsevier [82].
Lages et al., 2020 developed doxorubicin- and α-tocopherol succinate-loaded NLCs using compritol 888 ATO as solid lipid and docosahexaenoic acid (DHA) as liquid lipid to increase their anti-cancer activity against TNBC. Tween 80 was employed as a surfactant to further stabilize the lipid phased in aqueous media. It was observed that the NLCs showed a controlled release profile with an increased release in acidic media. Further, the NLCs showed decreased mortality in mice, reduced metastasis to lungs, and prevented drug-induced toxicity to vital organs (heart and liver) as observed from biochemical and histological assays. In addition, the NLCs showed a higher tumor inhibition ratio (76.6%) as compared to free doxorubicin (64.6%) [83]. Gadag et al., 2021 prepared resveratrol-loaded NLCs (RVT-NLC) using GMS as solid lipid and caproyl 90 as liquid lipid for increasing the therapeutic efficacy of resveratrol against TNBC. The NLCs were stabilized using labrasol as a surfactant. From the cell viability study, it was observed that the RVT-NLCs showed decreased MDA-MB-231 cell-viability (IC50 = 27.50 ± 3.43 μg/mL), as compared to free RVT (IC50 = 33.93 ± 7.34 μg/mL), which indicated that the RVT-NLCs were found to be more potent as compared to free RVT. Further, to increase the therapeutic efficacy via the dermal route, the RVT-NLCs were loaded within microneedle. It was observed that the RVT-NLCs loaded microneedle showed increased skin permeation, improved cellular internalization, and prevented metastasis as compared to free RVT. In addition, the RVT-NLCs increased pharmacokinetic attributes (Cmax = 343.75 ± 31.89 ng/mL; AUC0-t = 4529.2 ± 299.67 h∗ng/mL), as compared to free RVT (Cmax = 269.30 ± 30.26 ng/mL; AUC0-t = 458.3 ± 21.21 h∗ng/mL) [84]. Gilani et al., 2021 prepared luteolin-loaded NLCs (LTN-NLC) using GMS as solid lipid and caproyl 90 as liquid lipid to treat TNBC, stabilized by poloxamer 188. Further, to obtain a sustained release profile, the NLCs were surface functionalized by chitosan (LTN-CS-NLCs). It was observed that LTN-CS-NLCs exhibited a slow-release profile of LTN during a 24 h study. Moreover, LTN-CS-NLCs showed increased mucoadhesion, improved gastrointestinal stability, and intestinal permeation as compared to free LTN. In addition, from the MTT assay, it was observed that LTN-CS-NLCs showed decreased MDA-MB-231 cell viability (IC50 = 11.48 ± 2.38 μM), as compared to free LTN (IC50 = 29.64 ± 3.84 μM) after 48 h treatment. Additionally, LTN-CS-NLCs exhibited 4.3-fold increased intestinal permeation as compared to LTN suspension, indicating the superiority of NLCs in overcoming P-gp efflux pump-mediating elimination, in comparison to suspension [85].
2.5. Lipid Polymer Hybrid Nanoparticles (LPH-NPs)
Lipid–polymer hybrid nanoparticles (LPH-NPs) are considered new-generation nanoparticles exploiting the advantages of both polymeric nanoparticles and lipid-based nanoparticles in a single nanosystem [86]. Such a system is comprised of a polymeric core surrounded by a lipidic monolayer, which could be further surface-functionalized via PEGylation to prolong its circulation or via ligand to enhance its targetability. LPH-NPs impart certain characteristics of lipid-based nanoparticles which include enhanced drug-loading capacity, biodegradable and biomimetic nature, and certain traits of polymeric nanoparticles such as controlled/sustained drug release profile and a variety of surface functionalization or modification. The various polymers used in the preparation of LPH-NPs are approved by the Food and Drug Administration (FDA) which include polycaprolactone (PCL), poly (lactic-co-glycolic acid) (PLGA), polylactic acid (PLA), poly β-amino ester (PbAE), etc. In terms of lipidic components, charged or zwitterionic lipids are selected as they could be exploited to mediate covalent or non-covalent bonds with the desired ligands, antibodies, and nucleic acids (DNA, RNA), proteins, or peptides. Moreover, the presence of charged moieties helps to facilitate electrostatic interaction between lipids and oppositely charged polymers (core), which would result in the development of self-assembling nanostructures. The various lipids used are DOTAP/DOPE, stearic acid, cholesterol, lecithin, lipoid GmbH, DSPE-PEG, and PEG2000-Mal [44]. It was further observed that the lipidic monolayer acts as a molecular barricade that alleviates the loss of loaded drugs throughout the LPH-NP preparation and safeguards the polymeric core from deterioration by inhibiting the diffusion of water into the polymeric core. Due to the hybrid nanostructure, LPH-NPs can incorporate anticancer drugs of different physicochemical profiles. They are also able to conjugate ligands that are overexpressed on cancer cells, resulting in enhanced targetability and therapeutic efficacy with restricted off-site toxicities. In addition, LPH-NPs exhibit improved mechanical stability upon storage. Further, it was observed that the LPH-NPs of dimensions ≤ 100 nm display increased intra-cellular release of drug(s), resulting in decreased cytotoxicity.
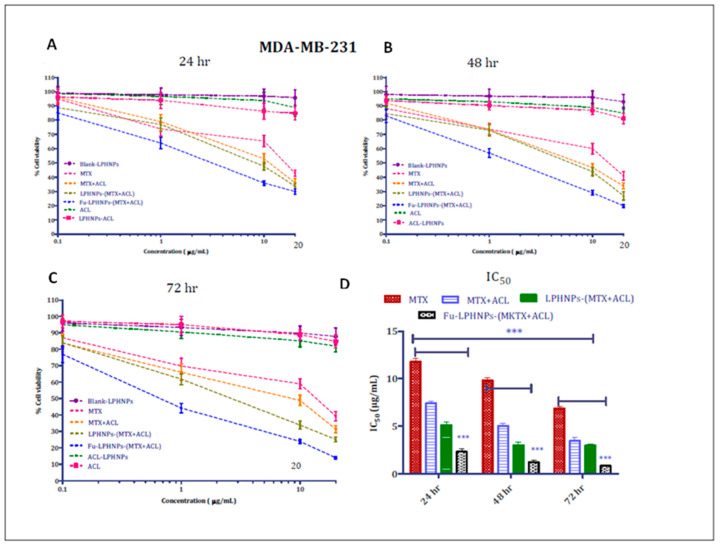
Zhang et al., 2017 prepared RGD-conjugated doxorubicin (DOX) and mitomycin C (MMC) co-loaded lipid–polymer hybrid nanoparticles (DMPLN) to treat TNBC. In the preparation of DMPLN, HPESO (hydrolyzed polymer of epoxidized soyabean oil) was used as a polymeric core and myristic acid was used as a lipid layer. It was observed that the RGD-DMPLN facilitated certain morphological changes and induced cytotoxicity. Moreover, compared to free drugs, RGD-DMPLN showed increased cellular accumulation, restricted lung metastasis (31-fold), remarkably decreased toxicity to the liver and heart, and improved median survival time (57%) [87]. Zhou et al., 2017 formulated calcium-phosphate-based lipid–polymer hybrid nanoparticles (LPH-NPs) co-loaded with paclitaxel and inhibitors of microRNA-221/222 for the treatment of TNBC. The LPH-NPs were prepared by using PLGA and PEG as polymers and dioleoylphosphatidic acid (DOPA) as anionic lipid. It was observed that the cell viability got decreased by approximately 80% in the case of co-loaded LPH-NPs as compared to free paclitaxel at the same dose of 0.67 μg/mL. Additionally, the co-loaded LPH-NPs showed enhanced intracellular activity as compared to free paclitaxel [88]. Garg et al., 2017 prepared fucose-anchored lipid–polymer hybrid nanoparticles (LPH-NPs) co-loaded with methotrexate (MTX) and aceclofenac (ACL) for the treatment of TNBC. LPH-NPs were prepared using gelucire 48/16 (lipid layer), phospholipid 90NG, and phospholipid S100 (polymer layer). Further, the LPH-NPs were conjugated with DSPE-PEG (2000)-NH-gructose. It was observed that LPH-NPs exhibited rapid MDA-MB-231 cellular internalization within 2 h and showed 10-fold increased bioavailability as compared to free drugs. Additionally, LPH-NPs showed ~21–25% less MDA-MB-231 cell growth, and 5–6 times increased mean residence time (MRT) as compared to free drugs (Figure 6) [89]. Bakar-Ates et al., 2020 developed cucurbitacin B-loaded lipid polymer hybrid nanoparticles (CuB-NPs) by using polymers such as PLGA, DSPE-PEG, and lipids such as lecithin for the treatment of TNBC by inducing apoptosis in MDA-MB-231 cells. It was observed that the CuB-NPs showed decreased cell viability at 0.1 and 5 μM concentrations as compared to the control. Additionally, the treatment with CuB-NPs showed increased cell population at G0/G1 phase (56.50 ± 1.23%), and apoptosis (20.66 ± 1.99%) as compared to free CuB (47.20 ± 1.02% and; 3.69 ± 0.57%, respectively) [90].
Figure 6.
The in vitro anti-cancer activity of MTX- and ACL-based free and co-encapsulated LPHNPs after 24 (A), 48 (B), and 72 h (C) in MDA-MB-231 cell lines. The image (D) depicts the comparison of IC50 of different samples obtained after 24, 48, and 72 h (*** p < 0.05). The statistics were run to determine significance in IC50 by 2-way ANOVA, and data are presented as the mean of three independent experiments (SD, n = 6). Reprinted (adapted) with permission from [89]. Copyright (2017) American Chemical Society.
2.6. Exosomes
Exosomes are nanosized extracellular vesicles that are enclosed by lipidic bilayers with diameter ranging from 30–150 nm, and are released by almost all kinds of cells [39]. In this context, our lab has isolated exosomes from bovine milk with particle size of 75 ± 0.6 nm [91,92], and colostrum with particle size 59 ± 1.1 nm [93]. The isolated exosomes further exhibited an increased therapeutic efficiency of anticancer agents to the targeted site [94,95]. They are basically generated by two invaginations of the plasma membrane. Exosomes are comprised of various surface proteins that are specific to the endosomal pathway, and can enclose nucleic acid, receptors, cytosolic proteins, and drugs [96,97]. The lipidic layer of exosomes varies from other types of extracellular vesicles such as apoptotic bodies and microvesicles as they are enriched in cholesterol and diacylglycerol [45,98]. Exosomes are considered one of the encouraging natural carriers of antineoplastics or biomolecules as they bypass their elimination through circulation and enhance cell-specificity towards cancer cells after modification via surface proteins [99]. It was observed that exosomes are biodistributed via body fluids to transport drugs or biomolecules to the cancer cells within the vicinity or dwelling remotely, which offers an advantage in recognizing potential pathological situations. The exosomes follow various uptake mechanisms namely direct membrane fusion, or endocytosis [100].
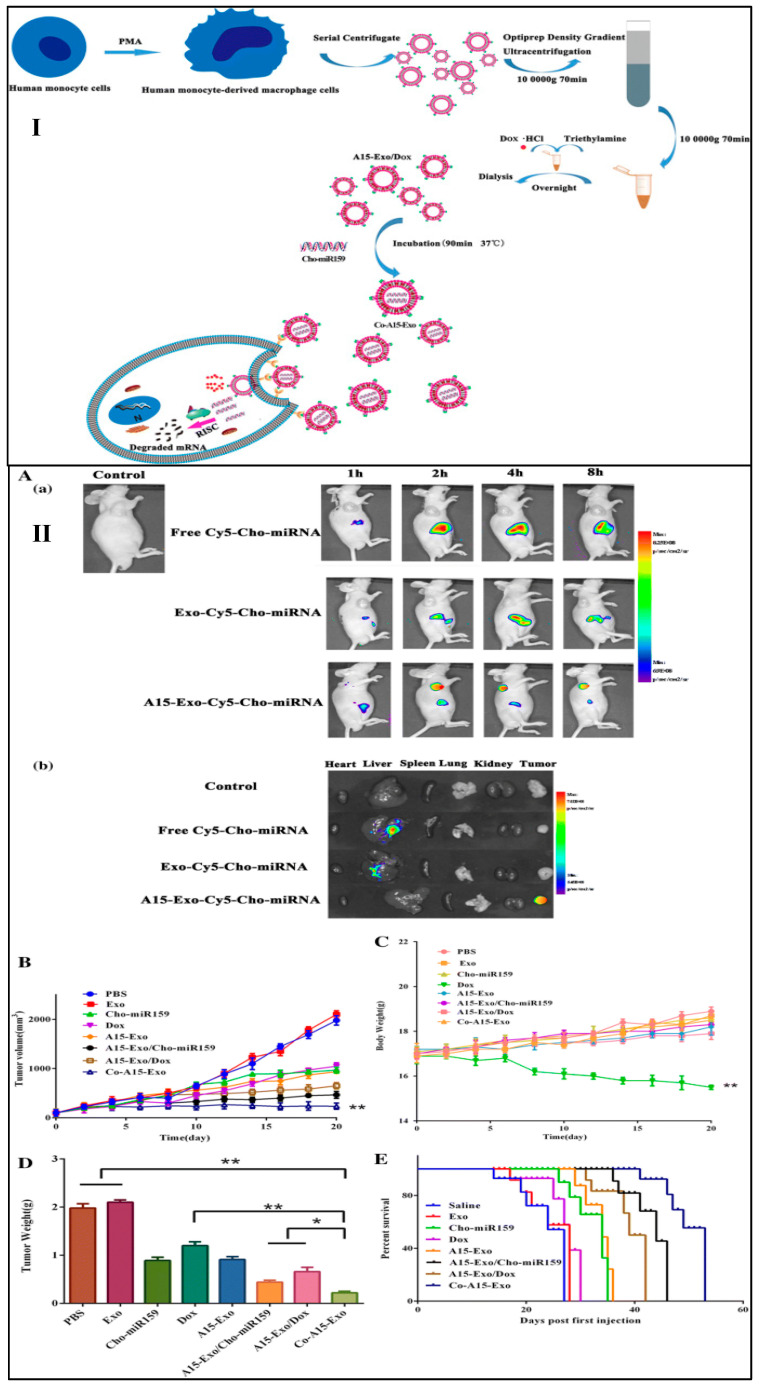
Naseri, et al., 2018 isolated exosomes from bone marrow-derived mesenchymal stem cells, loaded with locked nucleic acid (LNA)-modified anti-miR-142-3p oligonucleotides (MSCs-Exo) to diminish the expression of miR-142-3p in 4T1 breast cancer cell lines. It was observed from the in vitro and in vivo results that the MSCs-Exo showed efficient delivery and enhanced penetration of anti-miR-142-3p in breast cancer cells, respectively, along with increased transcription of regulatory target genes [101]. Gong et al., 2019 isolated exosomes from human leukemia monocytic cell line (THP-1), co-loaded with doxorubicin hydrochloride (Dox), and cholesterol-modified miRNA (Cho-miR159) to treat TNBC. Further to increase the targetability of co-loaded exosomes, the system was further conjugated with modified version of a disintegrin and metalloproteinase 15 (Co-A15-Exo) (Figure 7I). It was observed that flow cytometry data that the A15-Exo exhibited increased cellular uptake (78.60%), as compared to Exo (15.23%). Moreover, Co-A15-Exo showed enhanced apoptosis in MDA-MB-231 cells as compared to Dox-treated group. Further, Co-A15-Exo showed increased inhibitory rates of tumor volume (92.8%), as compared to Dox (49.5%), and Cho-miR159 (53.7%), revealing a potent synergism among Dox and Cho-miR159 (Figure 7II) [102]. Yu et al., 2019 fabricated erastin-loaded HFL-1 (human fetal lung fibroblast) derived exosomes conjugated with folic acid (FA) to target TNBC cells with overexpressed FA receptors (Erastin@FA-exo). It was observed that Erastin@FA-exo increased the cellular uptake of erastin into MDA-MB-231 cells, compared to free erastin. In addition, Erastin@FA-exo exhibited significant inhibition of TNBC cells proliferation and migration and promoted ferroptosis along with depletion of intracellular glutathione and ROS production [103]. Li et al., 2020 developed c-Met binding protein conjugated engineered exosomes for the treatment of TNBC. The author developed doxorubicin (Dox)-loaded polymeric nanoparticles and incorporated them into the macrophages-derived exosomes. It was observed that the engineered exosomes exhibited increased cellular uptake of Dox (2.28 times and 3.31 times), as compared to free-Dox and Dox-loaded polymeric nanoparticles, respectively. Further, the engineered exosomes showed increased apoptosis rate (39.73%), as compared to free Dox (10.58%) and Dox-loaded polymeric nanoparticles (11.33%) [104]. In this context, it is a noteworthy development that our lab also developed paclitaxel- and 5-fluorouracil-loaded exosomes, isolated from bovine milk and surface conjugated with folic acid for offering an effective treatment regimen against breast cancer. It was observed that developed exosomes showed an average particle size of 80–100 nm, with 82% entrapment efficiency. Moreover, the surface functionalized loaded exosomes showed increased cellular uptake and higher apoptotic index, compared to free drugs [97].
Figure 7.
(I) Schematic diagram of isolation of exosomes, loading of Dox.Hcl and Cho-miR159 within the exosomes and release of Dox and Cho-miR159-loaded A15-Exo (Co-A15-Exo). (II) Biodistribution and antitumor efficacy of Co-A15-Exo in vivo: (A(a)). Images were taken 1 h, 2 h, 4 h, or 8 h after the administration of free Cy5-Cho-miRNA, Exo-Cy5-Cho-miRNA, or A15-Exo-Cy5-Cho-miRNA. (A(b)) Ex vivo imaging of tumor and organs collected at the end of the experiment (8 h post-injection). (B) Tumor growth curves of mice receiving different therapeutic regimens (n = 5, mean ± SD). (C) Body weight changes during treatment. Data are expressed as the mean ± SD (n = 5). ** p < 0.01, vs. PBS. (D). The weights of the excised tumor tissues from all groups. Data are expressed as the mean ± SD (n = 5). * p < 0.05 and ** p < 0.01 when compared with the indicated groups. (E) Survival rate of MDA-MB-231 tumor-bearing BALB/c nude mice [102].
The various LNPs fabricated in the last decade to enhance therapeutic efficacy against TNBC have been summarized in Table 3.
Table 3.
Summary of different LNPs employed for the treatment of TNBC.
| Excipients | Results | Ref. |
|---|---|---|
| Liposomes | ||
| 1,2-dioleoyl-snglycero- 3-phosphocholine (DOPC) 1,2-distearoyl-sn-glycero- 3-phosphoethanolamine-N-[carboxy (polyethylene glycol)-2000] (DSPE-PEG-COOH) |
|
[52] |
| DSPE-PEG2000 |
|
[53] |
| Dioleoylphosphatidylethanolamine (DOPE) |
|
[54] |
| Dipalmitoylphosphatidylcholine (DPPC) Distearoylphosphatidylcholine (DSPC) Cholesterol Distearoylphosphatidylethanolamine (DSPE) |
|
[55] |
| LecithinCholesterol |
|
[56] |
| Nanoemulsion (NEs) | ||
| Cod liver oil Lysophophatidylcholine (LPC), lysophophatidic acid (LPA), DSPE-PEG (2000) |
|
[62] |
| Soya lecithin Kolliphor® HS15 |
|
[63] |
| Soybean phospholipids Cholesterol |
|
[64] |
| Miglyol 812 Phosphatidylcholine |
|
[65] |
| Solid lipid nanoparticles (SLNs) | ||
| GMS (Glyceryl monostearate) Tween 80 |
|
[75] |
| Palmitic acid Pluronic F-68 Soy lecithin |
|
[76] |
| GMS SA (Stearic acid) Compritol ATO 888 Tween 80 as a surfactant |
|
[77] |
| Stearyl amine Tween 80, Pluronic F-68 |
|
[78] |
|
[79] | |
| Nanostructured lipid carriers (NLCs) | ||
| Compritol ATO 888 Medium chain triglycerides (MCT) Tween 80 Soya lecithin |
|
[81] |
| Precirol ATO 5 Maisine 35-1 Cremophor RH40 |
|
[82] |
| Compritol 888 ATO Docosahexaenoic acid (DHA) Tween 80 |
|
[83] |
| GMS Caproyl 90 Labrasol |
|
[84] |
| GMS Caproyl 90 Poloxamer 188 |
|
[85] |
| Lipid– Polymer hybrid nanoparticles (LPH-NPs) | ||
| HPESO (hydrolyzed polymer of epoxidized soyabean oil) Myristic acid |
|
[87] |
| Poly lactide glycolic acid (PLGA) Polyethylene glycol (PEG) Dioleoylphosphatidic acid (DOPA) |
|
[88] |
| Gelucire 48/16, Phospholipid 90NG Phospholipid S100 |
|
[89] |
| PLGA, DSPE-PEG Lecithin |
|
[90] |
| Exosomes (Exo) | ||
| Mesenchymal stem cells (MSCs), Surface proteins: tetraspanins (CD63, CD9, CD81), heat shock proteins (Hsc70), lysosomal proteins (Lamp2b), and fusion proteins (flotillin, annexin). |
|
[101] |
| Human monocyte-derived macrophage cells Surface proteins (Exosomal marker proteins): CD81 and CD63. |
|
[102] |
| Human fetal lung fibroblast Surface proteins (Exosomal markers): TSG101 and CD81 |
|
[103] |
| Macrophage Surface proteins |
|
[104] |
3. Clinical Status
Liposomes have been employed in clinical applications for many years. From the survey, it was observed that there are approximately 21 approved liposomal formulations, out of which Doxil first reached clinics and opened the door for other nanoformulations by FDA viz. Myocet®, Lipodox®, and liposomal doxorubicin for the treatment of breast neoplasms, where Myocet® is the conventional liposomal formulation, whereas Doxil®, Lipodox®, and Doxorubicin are the PEG-conjugated liposomes, otherwise called stealth liposomes. For the treatment of solid tumors and acute lymphoblastic leukemia, the FDA has approved another liposomal formulation of vincristine named Marqibo®. As cancer research progresses, the concept of combination therapy has evolved and is subject to much attention, as compared to monotherapy. Taking this into account, Vyxeos® was formulated and approved in 2017 for the effective treatment of acute myeloid leukemia, as it provided the synergistic anticancer activity of daunorubicin and cytarabine [105]. The various LNPs approved by FDA are shown in Table 4.
Table 4.
FDA-approved LNPs for various diseases including cancer.
| S.No. | Brand Name |
Formulation | Company of Manufacture | Use | Approval Year |
Ref. |
|---|---|---|---|---|---|---|
| 1 | Doxil | Liposomal doxorubicin HCl (PEGylated) |
Janssen | Kaposi’s sarcoma, ovarian cancer, multiple myeloma |
1995 | [107] |
| 2 | DaunoXome | Liposomal daunorubicin | Galen | Kaposi’s sarcoma | 1996 | |
| 3 | DepoCyt© | Liposomal cytarabine | Pacira Pharms Inc. | Lymphoma | 1996 | |
| 4 | Myocet | Liposomal doxorubicin (non-PEGylated) | Teva UK | Metastatic breast cancer | 2000 | [108] |
| 5 | MEPACT | Liposomal Mifamurtide | Takeda | Osteo-sarcoma | 2009 | [109] |
| 6 | Marqibo | Liposomal vincristine | Acrotech Biopharma | Acute lympho-blastic leukaemia | 2012 | [110] |
| 7 | Onivyde | Liposomal irinotecan | Ipsen | Metastatic pancreatic cancer | 2015 | [111] |
| 8 | Vyxeos | Liposome encapsulating Cytarabine: daunorubicin in fixed-dose | Jazz Pharmaceuticals | Acute myeloid leukemia | 2017 | [112] |
Various other liposomes are under clinical trials, namely ThermoDox®, a thermosensitive liposome encapsulating doxorubicin. As it is well known that the tumor microenvironment experiences increased temperature than the usual body temperature, ThermoDox® utilizes this concept and releases the drug at the tumor site that experiences a higher temperature (>40 °C), hence increasing the targetability of the anticancer drug. Such formulation was fabricated for the treatment of liver cancer. ThermoDox® procured US FDA Fast Track Designation and was granted orphan drug designation in both the US and Europe for the treatment of primary liver cancer [106]. Recently, ThermoDox® completed a phase III trial (NCT02112656), where ThermoDox® is combined with standard radiofrequency ablation [105]. While the liposomes are occupied in clinical studies, the other LNPs are still under pre-clinical trials (in-vitro and in-vivo), as presented in Table 5.
Table 5.
List of LNPs subjected to pre-clinical trials and clinical trials.
| S.No. | Cancer Type |
LNPs | Route; Size |
Status | Ref. |
|---|---|---|---|---|---|
| 1. | Glioblastoma | Curcumin-loaded NE | Oral; 67 ± 6 nm |
In vitro and In vivo | [113] |
| Transferrin conjugated liposome encapsulating doxorubicin and erlotinib | 158.7–165.05 nm | In vitro | [114] | ||
| naI- IRI loaded liposome targeting topoisomerase I | Intravenous; 88–95 nm |
Phase I clinical trial | [115] | ||
| Docetaxel-loaded SLN targeting LRP1 | Intravenous; 79–111.4 nm |
In vitro and In vivo | [116] | ||
| Ferulic acid-loaded NLCs | <50 nm | In vitro | [117] | ||
| Lactoferrin and RGD peptide conjugated NLCs encapsulating temozolomide and vincristine | Intravenous; 96 nm |
In vitro and In vivo | [118] | ||
| 2. | Esophageal | Rhenium loaded liposomes | Intravenous; <100 nm |
In vitro and In vivo | [119] |
| LY294002 and 5-FU co-loaded Liposome (PEGylated) targeting thymidylate synthase | Intravenous; 110 nm |
In vitro and In vivo | [120] | ||
| 3. | Lung | 9-bromo-noscapine-loaded NE | Inhalation; 13.4 ± 3.2 nm |
In vitro and In vivo | [121] |
| Diferuloylmethane-loaded NE | Oral; ∼232.7 nm |
In vitro and In vivo | [122] | ||
| PEG-lecithin and nRGD peptide conjugated NE-loaded lycobetaine and oleic acid | Intravenous; 158.42 ± 2.87 nm |
In vitro and In vivo | [123] | ||
| Paclitaxel–Carboplatin–Gemcitabine-loaded liposome targeting tubulin | Percutaneous; 130 nm |
Phase III clinical trial | [124] | ||
| miR-34a conjugated Paclitaxel-loaded SLNs | Intravenous; 218.2 nm |
In vitro and In vivo | [125] | ||
| Transferrin-conjugated SLNs encapsulating Docetaxel and Baicalin | Intravenous; 135.5 nm |
In vitro and In vivo | [126] | ||
| Gemcitabine and Paclitaxel co-loaded NLC with surface functionalized via glucose receptor-targeting ligand | 120.3 ± 1.3 nm | In vitro | [127] | ||
| 4. | Breast | Doxorubicin and bromotetrandrine (W198)-loaded NE | Intravenous; 99.5–152.6 nm |
In vitro and In vivo | [128] |
| Doxorubicin and lapatinib-loaded liposome (PEGylated) | Intravenous; 100 nm |
Phase Ib clinical trial | [129] | ||
| Hyaluronic acid-coated Paclitaxel-pDNA-loaded SLNs | Intravenous; 156.3 ± 5.5 nm |
In vitro and In vivo | [130] | ||
| Fucose-conjugated Methotrexate-loaded SLNs | Intravenous; 174.51 ± 5.1 nm |
In vitro and In vivo | [131] | ||
| Lapachone and Doxorubicin loaded NLCs | Intravenous; 100.2 ± 6.8 nm |
In vitro and In vivo | [132] | ||
| 5. | Liver | Cantharidin-loaded liposomes (PEGylated) | Intravenous; 129.9 ± 2.5 nm |
In vitro and In vivo | [133] |
| Glycyrrhetinic acid-functionalized curcumin-loaded liposomes | Intravenous; 194 ± 0.25 nm |
In vitro and In vivo | [134] | ||
| miR-34a surface-functionalized liposomes | Intravenous; 120.21 ± 5 nm |
Phase I clinical trial | [135] | ||
| Sorafenib-loaded SLNs | 248 ± 113 nm | In vitro | [136] | ||
| Paclitaxel-loaded NLCs | Oral; 153.8 ± 5.58 nm |
In vitro and In vivo | [137] | ||
| 6. | Gastric | Indocyanine green-loaded liposome (PEGylated) | Intravenous; ~106 nm |
In vitro and In vivo | [138] |
| CD44 antibody-conjugated SATB1 siRNA-loaded liposome | 159.3 nm | In vitro | [139] | ||
| Etoposide-loaded SLNs | 30–50 nm | In vitro | [140] | ||
| Sorafenib and miR-542-3p-loaded SLNs (PEGylated) | Intravenous; ~156 nm |
In vitro and In vivo | [141] | ||
| Etoposide and curcumin co-loaded NLCs | Intravenous; 114 nm |
In vitro and In vivo | [142] | ||
| 7. | Pancreatic | Gemcitabine-loaded NE | ~150 nm, | In vitro | [143] |
| Gemcitabine and synthetic curcumin (EF24) combined loaded liposomes (PEGylated) | Intravenous; < 150 nm |
In vitro and In vivo | [144] | ||
| HSA-conjugated liposomes encapsulating Paclitaxel and Ellagic acid | Intravenous; 176.2 nm |
In vitro and In vivo | [145] | ||
| naI-IRI, 5-FU, and Leucovorin co-loaded liposomes | Intravenous; <200 nm |
Phase III clinical trial | [146] | ||
| 8. | Colorectal | Folic acid-conjugated 5-FU-loaded liposome | Intraperitoneal; 114 ± 4.58 nm |
In vitro and In vivo | [147] |
| Omega 3-fatty acid (DHA) and resveratrol-loaded SLNs | 100 ± 1.8 nm | In vitro | [148] | ||
| Folic acid and dextran-conjugated SLNs encapsulating Doxorubicin | Oral; 99–144 nm |
In vitro and In vivo | [149] | ||
| Hyaluronic acid-conjugated Irinotecan-loaded NLCs | 386 ± 2.2 nm | In vitro | [150] | ||
| 9. | Prostrate | Omega 3-fatty acid-conjugated Taxoid prodrug-loaded NE | Intravenous; 228 ± 7 nm |
In vitro and In vivo | [151] |
| Catechin extract-loaded NE | 11.45 nm | In vitro | [152] | ||
| Oleuropein-loaded liposome (PEGylated) | Intravenous; 184.2 ± 9.16 nm |
In vitro and In vivo | [153] | ||
| LRP1-targeted docetaxel-loaded liposome (PEGylated) | Intravenous; 163.2 ± 1.83 nm |
In vitro and In vivo | [154] |
From Table 5, it was observed that most of the LNPs employed in pre-clinical trials for the treatment of different types of cancers were mostly administered via intravenous route followed by oral and inhalation. Thus, if we just analyze the impact of LNPs over route of administration and targeting tumor site, based on the pre-clinical and clinical trials, we can conclusively state that for targeting lung cancer, pulmonary/inhalational route of administered was considered because the alveolar region of the lungs has larger surface area (~100 m2), extensive vasculature, thin alveolar epithelium (0.1–0.2 μm), and fewer drug-metabolizing enzymes, which allows enhanced absorption and bioavailability of nanosized LNPs loaded with chemotherapeutics. Moreover, the mucus membrane present within the alveolar region is composed of phospholipids (lipids) that are the major components of LNPs; as a result, LNPs are considered more biocompatible than other types of nanoparticles [155]. Similarly, brain targeting possesses a challenge for the delivery of hydrophilic chemotherapeutics, as they were unable to bypass blood–brain-barrier (BBB), hence LNPs were prepared to deliver hydrophilic drugs into the brain. It was observed that LNPs increased the lipophilicity of the drug which facilitates their transportation to brain by crossing the BBB. It was further observed that liposomes can cross BBB via receptor-mediated endocytosis (RMT), which facilitates enhanced accumulation of chemotherapeutics within tumor site. As a result, the off-target side effects were reduced. It was also observed from various studies that for the treatment of brain cancer, LNPs were administered by oral, intravenous as well as intranasal delivery [156]. The cellular uptake of LNPs by the oral route was already mentioned in Section 2. Briefly, on oral and intravenous administration, the LNPs enters the lymphatic and systemic circulation respectively, after which it targets the brain cancer cells via active (ligand-mediated cellular internalization) or passive (general cellular uptake via EPR effect) targeting [157]. Similar route and the associated approaches were employed for targeting breast, pancreas, and prostate cancer cells [24,158]. During intranasal administration for brain targeting, the LNPs binds with the mucus layer, which were then taken up by the neurons and translocated in the nerve axons to enter into brain cells, where the LNPs get degraded by the enzymes and drugs get released [156]. LNPs also ensure distinct drug delivery to the lesion site of the colon and rectum for the treatment of colorectal cancer. Most of the LNPs employed for the treatment of colorectal cancer are via oral route. It was observed that the LNPs are absorbed from the intestinal lumen into the circulation of colorectal region through endocytosis or via carrier-mediated transport [159]. Similarly, for treating hepatocellular carcinoma (liver cancer), LNPs are presently under pre-clinical and clinical trials. Like other cancer types, most of the chemotherapeutics are administered orally or intravenously for the treatment of hepatocellular carcinoma. It was observed that after administration of drug loaded LNPs, they non-specifically bind with the serum proteins leading to aggregation and opsonization, also causing a chance to get blocked within sinusoidal fenestrations. Therefore, to overcome the drawbacks, the LNPs were shielded with PEG, which provide shielding from plasma protein recognition as well as minimizing their sizes to <100 nm in order to cross the sinusoidal fenestrations. Moreover, liver targeting can be achieved by active or passive targeting [160].
The clinical achievement of LNPs with chemotherapeutics and nucleic acids revealed the potential of LNPs in the treatment of different types of cancer, However, the number of fruitful products reaching the market does not accurately represent the number of LNPs employed in pre-clinical trials, which indicated that the LNPs still suffers certain challenges while translating from animals to humans. Currently, various strategies have been developed to overcome such challenges. To further improve the stability and protect the drug from leaking, the lipidic structures have been modified that effectively form complex with the encapsulated chemotherapeutic via ionic attraction. Further stability of LNPs in systemic circulation was accomplished by PEGylating the LNPs, which safeguard them by reducing their recognition via RES. However, such approach leads to the production of anti-PEG antibodies which reduces the therapeutic efficacy of the LNPs. This incidence results in finding an alternative for PEGylation upon repeated administration. In addition to establish safety and therapeutic efficacy during prolonged circulation, the LNPs must also exhibit enhanced targetability and cellular internalization at the site of action [105]. To accomplish such objectives, the LNPs are fabricated with selective ligands which enables release of drug on targeted site when triggered by aberrations in pH, temperature, oxidation, or reduction within the tumor microenvironment [105,161].
4. Toxicity of Lipid-Based Nanoparticles (LNPs)
Despite the potential therapeutical efficacy, the LNPs do give a certain level of toxicity which includes cytotoxicity, and genotoxicity. It was observed from animal models that the presence of lipid-based nanoparticles activates the complement cascade leading to acute hypersensitivity reactions and anaphylaxis in almost 45% of patients. Further studies revealed that the toxicity of most of the FDA-approved nanoformulations is associated with their surface charge [162]. In this context, it was observed that cationic LNPs are therapeutically useless, as they activate immunological responses and inflammatory reactions. From previous studies, it was observed that LNPs are associated with 48–53% cytotoxicity in cell culture and animal models. Thus, to overcome such toxicities, the LNPs are encouraged to be fabricated in a solvent-free process employing only GRAS (Generally Recognized as Safe) approved ingredients. Lipid micelles, nanoemulsions, SLNs, and NLCs are considered well-tolerated LNPs as compared to liposomes. Orlando et al., 2013 experimented with SLNs and it was observed that the highest concentration of SLNs (1500 μg/mL) showed the highest concentration of NO in the macrophages, while in another study, it was observed that SLNs showed 10-fold and 100-fold less cytotoxicity as compared to poly-lactic acid nanoparticles (PLA NPs) and butyl cyanoacrylate nanoparticles (BC-NPs), respectively. Such findings revealed that the toxicity of LNPs is associated with the type and concentration of lipid used as a matrix. Herein, triglycerides exhibited no such cytotoxicity, whereas stearic acid exhibits a significant amount of toxicity.
In addition to cytotoxicity, LNPs also exhibit unpredictable genotoxicity as they are smaller in size with larger surface area and surface charges. Love et al., 2010 prepared SLNs using Witepsol and Carnauba waxes for loading siRNA against cancer. It was observed that moderate concentration of loaded SLNs showed no in vitro cyto- or genotoxicity in addition to in vivo safety profile. Löbrich et al., 2010 evaluated the genotoxicity of three SLNs formulations in hepatocarcinoma (HepG2) cells which revealed that minimal DNA damage was observed at 0.1 mg/mL of SLNs without any significantly increased DNA damage suggesting the fact that no genotoxicity at concentrations that do not reduce cell viability. Moreover, it was factualized that SLNs and NLCs are considered a safe delivery system for topical, ocular, and oral administration at lipid concentration of <1 mg/mL of total lipids. Hence, it was concluded that the cytotoxicity and genotoxicity of LNPs are dependent on the composition of the lipid matrix, the type and concentration of surfactant, and the surface electrical charge [163].
5. Conclusions
The underlying concept of LNPs as a well-tolerated carrier system is well established and documented. It was observed that the lipids employed in the preparation of LNPs such as liposomes, nanoemulsion, SLNs, NLCs, and LPH-NPs are non-toxic, biocompatible, and biodegradable with poor or no immunogenicity. The lipids have the capacity of forming nanostructure and have been investigated extensively as a nanocarrier for cancer-targeted drug delivery system. It was further concluded that the physicochemical properties of lipids provide the opportunity to optimize the drug delivery system by customizing their geometrical parameters which include particle size, morphology, entrapment efficiency, drug loading, and in vitro drug release profile. Further, the LNPs show passive targeting as well as active targeting if surface-functionalized, which enhances the therapeutic efficacy as well as targeting the cancer site. However, there exists certain reluctance regarding the efficacy and safety of LNPs which are overturned by the advent of reliable GRAS-regulated lipids, cGMP-grade manufacturing processes, and preclinical data related to their ADME status and toxicity, followed by fruitful first-in-man studies. It was further expected that the LNPs might be the first nanoparticle forming an impact on the management and treatment of cancer. Likewise, Doxil nanoparticles were observed as the first of a wave of novel LNP-mediated drug delivery systems that could deliver a transformative impact on anticancer therapeutics in the years to come.
6. Future Perspective
LNPs are a diverse and far-reaching type of nanoparticles that have been employed for the treatment of different types of diseases, especially cancer. However, liposomes were found to be the most successfully developed LNPs due to their flexibility and biocompatibility with biological systems. The various liposomes that shined out the lot for the treatment of cancers are Doxil®, Abraxane®, and Myocet®. Apart from liposomes, there are solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) that have gained a lot of interest for the treatment of malignancies from the success obtained from in vitro and in vivo studies and are considered 2nd generation LNPs. It was found that the success of such LNPs might be due to the employment of biomaterial, and environmentally safe ingredients. It was further observed that SLNs and NLCs can be used for the loading of biological drugs and imaging agents, apart from synthetic drugs. Future research is warranted in the area of targeted nanomedicine to make the concept of “Magic Bullet” a clinical reality. Interdisciplinary research is also required for the development of clinically prosperous theranostic nanoformulations.
Acknowledgments
The Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work under grant no. (IFPRP: 508-166-1442) and King Abdulaziz University, DSR, Jeddah, Saudi Arabia. The authors are also grateful to the Indian Institute of Technology (Banaras Hindu University), Varanasi for providing infrastructure facilities.
Author Contributions
A.C.: conceptualization, writing—original draft preparation; D.N.K.: writing—review and editing; R.A.S.: writing—review and editing; B.G.E.: writing—review and editing; A.B.A.-N.: writing—review and editing; S.M.: writing—review and editing; A.A.: writing—review and editing; A.K.A.: supervision, refinement, and improvement of the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data availability statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFPRP: 508-166-1442 and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gallo C. Triple Negative Breast Cancer Epidemiology. 2018. [(accessed on 1 April 2020)]. Available online: https://oncologynurse-ce.com/triple-negative-breast-cancer-epidemiology.
- 2.Aqil F., Munagala R., Agrawal A.K., Jeyabalan J., Tyagi N., Rai S.N., Gupta R.C. Anthocyanidins Inhibit Growth and Chemosensitize Triple-Negative Breast Cancer via the NF-kappaB Signaling Pathway. Cancers. 2021;13:6428. doi: 10.3390/cancers13246248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Z., Li M., Dey R., Chen Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021;14:85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Moukhtari S.H., Rodriguez-Nogales C., Blanco-Prieto M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021;173:238–251. doi: 10.1016/j.addr.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Yan L., Shen J., Wang J., Yang X., Dong S., Lu S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response. 2020;18:1559325820936161. doi: 10.1177/1559325820936161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller A.D. Lipid-based nanoparticles in cancer diagnosis and therapy. J. Drug Deliv. 2013;2013:165981. doi: 10.1155/2013/165981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aqil F., Munagala R., Agrawal A.K., Gupta R. New Look to Phytomedicine. Elsevier Inc.; Cambridge, MA, USA: 2019. Anticancer Phytocompounds; pp. 237–272. [Google Scholar]
- 8.Talluri S.V., Kuppusamy G., Karri V.V.S.R., Tummala S., Madhunapantula S. Lipid-based nanocarriers for breast cancer treatment-comprehensive review. Drug Deliv. 2016;23:1291–1305. doi: 10.3109/10717544.2015.1092183. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri A., Kumar D.N., Dehari D., Singh S., Kumar P., Bolla P.K., Kumar D., Agrawal A.K. Emergence of Nanotechnology as a Powerful Cavalry against Triple-Negative Breast Cancer (TNBC) Pharmaceuticals. 2022;15:542. doi: 10.3390/ph15050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negi S., Chaudhuri A., Kumar D.N., Dehari D., Singh S., Agrawal A.K. Nanotherapeutics in autophagy: A paradigm shift in cancer treatment. Drug Deliv. Transl. Res. 2022:1–24. doi: 10.1007/s13346-022-01125-6. [DOI] [PubMed] [Google Scholar]
- 11.Jain S., Spandana G., Agrawal A.K., Kushwah V., Thanki K. Enhanced Antitumor Efficacy and Reduced Toxicity of Docetaxel Loaded Estradiol Functionalized Stealth Polymeric Nanoparticles. Mol. Pharm. 2015;12:3871–3884. doi: 10.1021/acs.molpharmaceut.5b00281. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal A.K., Kumar K., Swarnakar N.K., Kushwah V., Jain S. “Liquid Crystalline Nanoparticles”: Rationally Designed Vehicle To Improve Stability and Therapeutic Efficacy of Insulin Following Oral Administration. Mol. Pharm. 2017;14:1874–1882. doi: 10.1021/acs.molpharmaceut.6b01099. [DOI] [PubMed] [Google Scholar]
- 13.Karunanidhi P., Verma N., Kumar D.N., Agrawal A.K., Singh S. Triphenylphosphonium functionalized Ficus religiosa L. extract loaded nanoparticles improve the mitochondrial function in oxidative stress induced diabetes. AAPS PharmSciTech. 2021;22:158. doi: 10.1208/s12249-021-02016-8. [DOI] [PubMed] [Google Scholar]
- 14.Singh S., Kushwah V., Agrawal A.K., Jain S. Insulin- and quercetin-loaded liquid crystalline nanoparticles: Implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine. 2018;13:521–537. doi: 10.2217/nnm-2017-0278. [DOI] [PubMed] [Google Scholar]
- 15.Urimi D., Agrawal A.K., Kushwah V., Jain S. Polyglutamic Acid Functionalization of Chitosan Nanoparticles Enhances the Therapeutic Efficacy of Insulin Following Oral Administration. AAPS PharmSciTech. 2019;20:131. doi: 10.1208/s12249-019-1330-2. [DOI] [PubMed] [Google Scholar]
- 16.Anjum M.M., Patel K.K., Dehari D., Pandey N., Tilak R., Agrawal A.K., Singh S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improves antimicrobial activity. Drug Deliv. Transl. Res. 2021;11:305–317. doi: 10.1007/s13346-020-00795-4. [DOI] [PubMed] [Google Scholar]
- 17.Patel K.K., Agrawal A.K., Anjum M., Tripathi M., Pandey N., Bhattacharya S., Tilak R., Singh S. DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Appl. Nanosci. 2019;10:563–575. [Google Scholar]
- 18.Patel K.K., Surekha D.B., Tripathi M., Anjum M.M., Muthu M.S., Tilak R., Agrawal A.K., Singh S. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol. Pharm. 2019;16:3916–3925. doi: 10.1021/acs.molpharmaceut.9b00527. [DOI] [PubMed] [Google Scholar]
- 19.Harde H., Agrawal A.K., Jain S. Development of stabilized glucomannosylated chitosan nanoparticles using tandem crosslinking method for oral vaccine delivery. Nanomedicine. 2014;9:2511–2599. doi: 10.2217/nnm.13.225. [DOI] [PubMed] [Google Scholar]
- 20.Harde H., Agrawal A.K., Jain S. Tetanus toxoids loaded glucomannosylated chitosan based nanohoming vaccine adjuvant with improved oral stability and immunostimulatory response. Pharm. Res. 2015;32:122–134. doi: 10.1007/s11095-014-1449-5. [DOI] [PubMed] [Google Scholar]
- 21.Rai V.K., Mishra N., Agrawal A., Jain S., Yadav N.P. Novel drug delivery system: An immense hope for diabetics. Drug Deliv. 2016;23:2371–2390. doi: 10.3109/10717544.2014.991001. [DOI] [PubMed] [Google Scholar]
- 22.Dehari D., Chaudhuri A., Singh S., Agrawal A.K. RNA-Based Vaccines for Infectious Disease. CRC Press; Boca Raton, FL, USA: 2022. [Google Scholar]
- 23.Jain S., Kumar S., Agrawal A.K., Thanki K., Banerjee U.C. Hyaluronic acid–PEI–cyclodextrin polyplexes: Implications for in vitro and in vivo transfection efficiency and toxicity. RSC Adv. 2015;5:41144–41154. [Google Scholar]
- 24.Jain S., Raza K., Agrawal A.K., Vaidya A. Nanotechnology Applications for Cancer Chemotherapy. Elsevier; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 25.Cerpnjak K., Zvonar A., Gašperlin M., Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63:427–445. doi: 10.2478/acph-2013-0040. [DOI] [PubMed] [Google Scholar]
- 26.Kushwah V., Katiyar S.S., Agrawal A.K., Gupta R.C., Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine. 2018;14:1629–1641. doi: 10.1016/j.nano.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Kushwah V., Katiyar S.S., Agrawal A.K., Saraf I., Singh I.P., Lamprou D.A., Gupta R.C., Jain S. Implication of linker length on cell cytotoxicity, pharmacokinetic and toxicity profile of gemcitabine-docetaxel combinatorial dual drug conjugate. Int. J. Pharm. 2018;548:357–374. doi: 10.1016/j.ijpharm.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Lim S.B., Banerjee A., Onyuksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release. 2012;163:34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Palei N.N., Mohanta B.C., Sabapathi M.L., Das M.K. Organic Materials as Smart Nanocarriers for Drug Delivery. Elsevier Inc.; Cambridge, MA, USA: 2018. Lipid-based nanoparticles for cancer diagnosis and therapy; pp. 415–470. [Google Scholar]
- 30.Porter C.J., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 31.Mohsin K., Shahba A.A., Alanazi F.K. Lipid Based Self Emulsifying Formulations for Poorly Water Soluble Drugs-An Excellent Opportunity. Indian J. Pharm. Educ. Res. 2012;46:88–96. [Google Scholar]
- 32.Ali Khan A., Khan A.A., Mudassir J., Mohtar N. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013;8:2733–2744. doi: 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi J., Zhuang J., Lu Y., Dong X., Zhao W., Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov. Today. 2017;22:166–172. doi: 10.1016/j.drudis.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Kim M.W., Kwon S.-H., Choi J.H., Lee A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018;19:3859. doi: 10.3390/ijms19123859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paliwal R., Babu R.J., Palakurthi S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech. 2014;15:1527–1534. doi: 10.1208/s12249-014-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R., Lillard J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendes B.B., Conniot J., Avital A., Yao D., Jiang X., Zhou X., Sharf-Pauker N., Xiao Y., Adir O., Liang H., et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022;2:25. doi: 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar D.N., Chaudhuri A., Aqil F., Dehari D., Munagala R., Singh S., Gupta R.C., Agrawal A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers. 2022;14:1435. doi: 10.3390/cancers14061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabizon A.A., Shmeeda H., Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006;16:175–183. doi: 10.1080/08982100600848769. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: Formation, properties and applications. Soft Matter. 2016;12:2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- 42.Kaur T., Slavcev R. Novel Gene Therapy Approaches. IntechOpen; London, UK: 2013. Solid Lipid Nanoparticles: Tuneable Anti-Cancer Gene/Drug Delivery Systems. [Google Scholar]
- 43.Iqbal M.A., Md S., Sahni J.K., Baboota S., Dang S., Ali J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 44.Persano F., Gigli G., Leporatti S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express. 2021;2:012006. doi: 10.1088/2632-959X/abeb4b. [DOI] [Google Scholar]
- 45.Antimisiaris S.G., Mourtas S., Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y.S., Chen Y.-S., Lin E.-Y., Chiou T.-W. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med. J. 2020;32:113–120. doi: 10.4103/tcmj.tcmj_182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias J.L., Clares B., Morales M.E., Gallardo V., Ruiz M.A. Lipid-Based Drug Delivery Systems for Cancer Treatment. Curr. Drug Targets. 2011;12:1151–1165. doi: 10.2174/138945011795906570. [DOI] [PubMed] [Google Scholar]
- 48.Kushwah V., Jain D.K., Agrawal A.K., Jain S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Coll. Surf. B Biointerfaces. 2018;172:213–223. doi: 10.1016/j.colsurfb.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 49.Jain S., Harde H., Indulkar A., Agrawal A. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine. 2014;10:431–440. doi: 10.1016/j.nano.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Jain S., Patil S.R., Swarnakar N.K., Agrawal A.K. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes) Mol. Pharm. 2012;9:2626–2635. doi: 10.1021/mp300202c. [DOI] [PubMed] [Google Scholar]
- 51.Olusanya T.O.B., Haj Ahmad R.R., Ibegbu D.M., Smith J.R., Elkordy A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules. 2018;23:907. doi: 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo P., Yang J., Liu D., Huang L., Fell G., Huang J., Moses M.A., Auguste D.T. Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci. Adv. 2019;5:eaav5010. doi: 10.1126/sciadv.aav5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan Y., Li X.-Q., Duan J.-L., Bao C.-J., Cui Y.-N., Su Z.-B., Xu J.-R., Luo Q., Chen M., Xie Y., et al. Nanosized functional miRNA liposomes and application in the treatment of TNBC by silencing Slug gene. Int. J. Nanomed. 2019;14:3645–3667. doi: 10.2147/IJN.S207837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M., Miao Y., Qian K., Zhou X., Guo L., Qiu Y., Wang R., Gan Y., Zhang X. Detachable Liposomes Combined Immunochemotherapy for Enhanced Triple-Negative Breast Cancer Treatment through Reprogramming of Tumor-Associated Macrophages. Nano Lett. 2021;21:6031–6041. doi: 10.1021/acs.nanolett.1c01210. [DOI] [PubMed] [Google Scholar]
- 55.Alawak M., Abu Dayyih A., Mahmoud G., Tariq I., Duse L., Goergen N., Engelhardt K., Pinnapireddy S.R., Jedelská J., Awak M., et al. ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. Eur. J. Pharm. Biopharm. 2021;158:390–400. doi: 10.1016/j.ejpb.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 56.El-Senduny F.F., Altouhamy M., Zayed G., Harsha C., Jalaja R., Somappa S.B., Nair M.S., Kunnumakkara A.B., Alsharif F.M., Badria F.A. Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2021;65:102665. doi: 10.1016/j.jddst.2021.102665. [DOI] [Google Scholar]
- 57.Souto E.B., Nayak A.B., Murthy R.S.R. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie. 2011;66:473–478. [PubMed] [Google Scholar]
- 58.Jain S., Garg T., Kushwah V., Thanki K., Agrawal A.K., Dora C.P. alpha-Tocopherol as functional excipient for resveratrol and coenzyme Q10-loaded SNEDDS for improved bioavailability and prophylaxis of breast cancer. J. Drug Target. 2017;25:554–565. doi: 10.1080/1061186X.2017.1298603. [DOI] [PubMed] [Google Scholar]
- 59.Gawin-Mikolajewicz A., Nartowski K.P., Dyba A.J., Gołkowska A.M., Malec K., Karolewicz B. Ophthalmic Nanoemulsions: From Composition to Technological Processes and Quality Control. Mol. Pharm. 2021;18:3719–3740. doi: 10.1021/acs.molpharmaceut.1c00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ganta S., Talekar M., Singh A., Coleman T.P., Amiji M.M. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15:694–708. doi: 10.1208/s12249-014-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez-Lopez E., Guerra M., Dias-Ferreira J., Lopez-Machado A., Ettcheto M., Cano A., Espina M., Camins A., Garcia M.L., Souto E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials. 2019;9:821. doi: 10.3390/nano9060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim B., Pena C.D., Auguste D.T. Targeted Lipid Nanoemulsions Encapsulating Epigenetic Drugs Exhibit Selective Cytotoxicity on CDH1−/FOXM1+ Triple Negative Breast Cancer Cells. Mol. Pharm. 2019;16:1813–1826. doi: 10.1021/acs.molpharmaceut.8b01065. [DOI] [PubMed] [Google Scholar]
- 63.Xu H., Hu M., Liu M., An S., Guan K., Wang M., Li L., Zhang J., Li J., Huang L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. 2020;235:119769. doi: 10.1016/j.biomaterials.2020.119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han B., Wang T., Xue Z., Wen T., Lu L., Meng J., Liu J., Wu S., Yu J., Xu H. Elemene Nanoemulsion Inhibits Metastasis of Breast Cancer by ROS Scavenging. Int. J. Nanomed. 2021;16:6035–6048. doi: 10.2147/IJN.S327094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saraiva S.M., Gutiérrez-Lovera C., Martínez-Val J., Lores S., Bouzo B.L., Díez-Villares S., Alijas S., Pensado-López A., Vázquez-Ríos A.J., Sánchez L., et al. Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci. Rep. 2021;11:9873. doi: 10.1038/s41598-021-87968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallan S.S., Sguizzato M., Esposito E., Cortesi R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics. 2021;13:549. doi: 10.3390/pharmaceutics13040549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel K.K., Gade S., Anjum M., Singh S.K., Maiti P., Agrawal A.K. Effect of penetration enhancers and amorphization on transdermal permeation flux of raloxifene-encapsulated solid lipid nanoparticles: An ex vivo study on human skin. Appl. Nanosci. 2019;9:1383–1394. doi: 10.1007/s13204-019-01004-6. [DOI] [Google Scholar]
- 68.Parvez S., Yadagiri G., Gedda M.R., Singh A., Singh O.P., Verma A., Sundar S., Mudavath S.L. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: An effective oral combination against experimental murine visceral leishmaniasis. Sci. Rep. 2020;10:12243. doi: 10.1038/s41598-020-69276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh A., Yadagiri G., Parvez S., Singh O.P., Verma A., Sundar S., Mudavath S.L. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater. Sci. Eng. C. 2020;117:111279. doi: 10.1016/j.msec.2020.111279. [DOI] [PubMed] [Google Scholar]
- 70.Harde H., Agrawal A.K., Katariya M., Kale D., Jain S. Development of a topical adapalene-solid lipid nanoparticle loaded gel with enhanced efficacy and improved skin tolerability. RSC Adv. 2015;5:43917–43929. doi: 10.1039/C5RA06047H. [DOI] [Google Scholar]
- 71.Edis Z., Wang J., Waqas M.K., Ijaz M., Ijaz M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021;16:1313–1330. doi: 10.2147/IJN.S289443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan Y., Dhar A., Patel C., Khimani M., Neogi S., Sharma P., Kumar N.S., Vekariya R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777–26791. doi: 10.1039/D0RA03491F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong H.L., Bendayan R., Rauth A.M., Li Y., Wu X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Puri A., Loomis K., Smith B., Lee J.H., Yavlovich A., Heldman E., Blumenthal R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/CritRevTherDrugCarrierSyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guney Eskiler G., Cecener G., Egeli U., Tunca B. Synthetically Lethal BMN 673 (Talazoparib) Loaded Solid Lipid Nanoparticles for BRCA1 Mutant Triple Negative Breast Cancer. Pharm. Res. 2018;35:218. doi: 10.1007/s11095-018-2502-6. [DOI] [PubMed] [Google Scholar]
- 76.Siddhartha V.T., Pindiprolu S.K.S.S., Chintamaneni P.K., Tummala S., Kumar S.N. RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: In vitro studies. Artif. Cells Nanomed. Biotechnol. 2018;46:387–397. doi: 10.1080/21691401.2017.1313267. [DOI] [PubMed] [Google Scholar]
- 77.Kothari I.R., Mazumdar S., Sharma S., Italiya K., Mittal A., Chitkara D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther. Deliv. 2019;10:227–240. doi: 10.4155/tde-2018-0074. [DOI] [PubMed] [Google Scholar]
- 78.Pindiprolu S., Chintamaneni P.K., Krishnamurthy P.T., Ratna Sree Ganapathineedi K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev. Ind. Pharm. 2019;45:304–313. doi: 10.1080/03639045.2018.1539496. [DOI] [PubMed] [Google Scholar]
- 79.Pindiprolu S.K.S.S., Krishnamurthy P.T., Ghanta V.R., Chintamaneni P.K. Phenyl boronic acid-modified lipid nanocarriers of niclosamide for targeting triple-negative breast cancer. Nanomedicine. 2020;15:1551–1565. doi: 10.2217/nnm-2020-0003. [DOI] [PubMed] [Google Scholar]
- 80.Gade S., Patel K.K., Gupta C., Anjum M., Deepika D., Agrawal A.K., Singh S. An Ex Vivo Evaluation of Moxifloxacin Nanostructured Lipid Carrier Enriched In Situ Gel for Transcorneal Permeation on Goat Cornea. J. Pharm. Sci. 2019;108:2905–2916. doi: 10.1016/j.xphs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Pedro I.D.R., Almeida O.P., Martins H.R., Lemos J.D.A., de Barros A.L., Leite E.A., Carneiro G. Optimization and in vitro/in vivo performance of paclitaxel-loaded nanostructured lipid carriers for breast cancer treatment. J. Drug Deliv. Sci. Technol. 2019;54:101370. doi: 10.1016/j.jddst.2019.101370. [DOI] [Google Scholar]
- 82.Zhang Q., Zhao J., Hu H., Yan Y., Hu X., Zhou K., Xiao S., Zhang Y.T., Feng N. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int. J. Pharm. 2019;569:118595. doi: 10.1016/j.ijpharm.2019.118595. [DOI] [PubMed] [Google Scholar]
- 83.Lages E.B., Fernandes R.S., Silva J.O., de Souza Â.M., Cassali G.D., de Barros A.L.B., Miranda Ferreira L.A. Co-delivery of doxorubicin, docosahexaenoic acid, and alpha-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020;132:110876. doi: 10.1016/j.biopha.2020.110876. [DOI] [PubMed] [Google Scholar]
- 84.Gadag S., Narayan R., Nayak A.S., Ardila D.C., Sant S., Nayak Y., Garg S., Nayak U.Y. Development and preclinical evaluation of microneedle-assisted resveratrol loaded nanostructured lipid carriers for localized delivery to breast cancer therapy. Int. J. Pharm. 2021;606:120877. doi: 10.1016/j.ijpharm.2021.120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilani S.J., Bin-Jumah M., Rizwanullah, Imam S., Imtiyaz K., Alshehri S., Rizvi M. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings. 2021;11:158. doi: 10.3390/coatings11020158. [DOI] [Google Scholar]
- 86.Jain S., Kumar S., Agrawal A.K., Thanki K., Banerjee U.C. Enhanced transfection efficiency and reduced cytotoxicity of novel lipid-polymer hybrid nanoplexes. Mol. Pharm. 2013;10:2416–2425. doi: 10.1021/mp400036w. [DOI] [PubMed] [Google Scholar]
- 87.Zhang T., Prasad P., Cai P., He C., Shan D., Rauth A.M., Wu X.Y. Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol. Sin. 2017;38:835–847. doi: 10.1038/aps.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Z., Kennell C., Lee J.Y., Leung Y.K., Tarapore P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine. 2017;13:403–410. doi: 10.1016/j.nano.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 89.Garg N.K., Tyagi R.K., Sharma G., Jain A., Singh B., Jain S., Katare O.P. Functionalized Lipid-Polymer Hybrid Nanoparticles Mediated Codelivery of Methotrexate and Aceclofenac: A Synergistic Effect in Breast Cancer with Improved Pharmacokinetics Attributes. Mol. Pharm. 2017;14:1883–1897. doi: 10.1021/acs.molpharmaceut.6b01148. [DOI] [PubMed] [Google Scholar]
- 90.Bakar-Ates F., Ozkan E., Sengel-Turk C.T. Encapsulation of cucurbitacin B into lipid polymer hybrid nanocarriers induced apoptosis of MDAMB231 cells through PARP cleavage. Int. J. Pharm. 2020;586:119565. doi: 10.1016/j.ijpharm.2020.119565. [DOI] [PubMed] [Google Scholar]
- 91.Agrawal A.K., Aqil F., Jeyabalan J., Spencer W.A., Beck J., Gachuki B.W., Alhakeem S.S., Oben K., Munagala R., Bondada S., et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Aqil F., Munagala R., Jeyabalan J., Agrawal A.K., Kyakulaga A.-H., Wilcher S.A., Gupta R.C. Milk exosomes-Natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–195. doi: 10.1016/j.canlet.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Kandimalla R., Aqil F., Alhakeem S., Jeyabalan J., Tyagi N., Agrawal A., Yan J., Spencer W., Bondada S., Gupta R. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers. 2021;13:3700. doi: 10.3390/cancers13153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aqil F., Jeyabalan J., Agrawal A.K., Kyakulaga A.-H., Munagala R., Parker L., Gupta R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017;8:4100–4107. doi: 10.1039/C7FO00882A. [DOI] [PubMed] [Google Scholar]
- 95.Aqil F., Kausar H., Agrawal A.K., Jeyabalan J., Kyakulaga A.-H., Munagala R., Gupta R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016;101:12–21. doi: 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 96.Anjum M.M., Kumar D.N., Chaudhuri A., Singh S., Agrawal A.K. Extracellular Vesicles for Nucleic Acid Delivery-Progress and Prospects for Safe RNA-Based Gene Therapy. 1st ed. CRC Press; Boca Raton, FL, USA: 2022. [Google Scholar]
- 97.Kumar D.N., Chaudhuri A., Dehari D., Shekher A., Gupta S.C., Majumdar S., Krishnamurthy S., Singh S., Kumar D., Agrawal A.K. Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer. Life. 2022;12:1143. doi: 10.3390/life12081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munagala R., Aqil F., Jeyabalan J., Agrawal A.K., Mudd A.M., Kyakulaga A.H., Singh I.P., Vadhanam M.V., Gupta R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. doi: 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aqil F., Munagala R., Jeyabalan J., Agrawal A., Gupta R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017;19:1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 100.Wang X., Sun C., Huang X., Li J., Fu Z., Li W., Yin Y. The Advancing Roles of Exosomes in Breast Cancer. Front. Cell Dev. Biol. 2021;9:731062. doi: 10.3389/fcell.2021.731062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naseri Z., Oskuee R.K., Jaafari M.R., Forouzandeh M. Exosome-mediated delivery of functionally active miRNA-142–3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018;13:7727–7747. doi: 10.2147/IJN.S182384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gong C., Tian J., Wang Z., Gao Y., Wu X., Ding X., Qiang L., Li G., Han Z., Yuan Y., et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019;17:93. doi: 10.1186/s12951-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu M., Gai C., Li Z., Ding D., Zheng J., Zhang W., Lv S., Li W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110:3173–3182. doi: 10.1111/cas.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li S., Wu Y., Ding F., Yang J., Li J., Gao X., Zhang C., Feng J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12:10854–10862. doi: 10.1039/D0NR00523A. [DOI] [PubMed] [Google Scholar]
- 105.Thi T.T.H., Suys E., Lee J., Nguyen D., Park K., Truong N. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines. 2021;9:359. doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Church J.W. Celsion Provides Update on ThermoDox® in the Phase III OPTIMA Study of Primary Liver Cancer. Celsion Corporation; Lawrenceville, NJ, USA: 2022. [Google Scholar]
- 107.Choi Y.H., Han H.K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018;48:43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020;154–155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Venkatakrishnan K., Liu Y., Noe D., Mertz J., Bargfrede M., Marbury T., Farbakhsh K., Oliva C., Milton A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment. Br. J. Clin. Pharmacol. 2014;77:986–997. doi: 10.1111/bcp.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silverman J.A., Deitcher S.R. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001–3007. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar A., Ahuja A., Ali J., Baboota S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016;23:214–229. doi: 10.3109/10717544.2014.909906. [DOI] [PubMed] [Google Scholar]
- 114.Lakkadwala S., Singh J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Coll. Surf. B Biointerfaces. 2019;173:27–35. doi: 10.1016/j.colsurfb.2018.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clarke J.L., Molinaro A.M., Cabrera J.R., DeSilva A.A., Rabbitt J.E., Prey J., Drummond D.C., Kim J., Noble C., Fitzgerald J.B., et al. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer Chemother. Pharmacol. 2017;79:603–610. doi: 10.1007/s00280-017-3247-3. [DOI] [PubMed] [Google Scholar]
- 116.Kadari A., Pooja D., Gora R.H., Gudem S., Kolapalli V.R.M., Kulhari H., Sistla R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur. J. Pharm. Biopharm. 2018;132:168–179. doi: 10.1016/j.ejpb.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 117.Carbone C., Campisi A., Musumeci T., Raciti G., Bonfanti R., Puglisi G. FA-loaded lipid drug delivery systems: Preparation, characterization and biological studies. Eur. J. Pharm. Sci. 2014;52:12–20. doi: 10.1016/j.ejps.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 118.Zhang J., Xiao X., Zhu J., Gao Z., Lai X., Zhu X., Mao G. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomed. 2018;13:3039–3051. doi: 10.2147/IJN.S161163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y.-J., Chang C.H., Chi C.-W., Yu H.-L., Chang T.-J., Tsai T.-H., Lee T.-W., Liu S.-Y. External beam radiotherapy synergizes 188Re-liposome against human esophageal cancer xenograft and modulates 188Re-liposome pharmacokinetics. Int. J. Nanomed. 2015;10:3641–3649. doi: 10.2147/IJN.S80302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng Y., Gao Y., Wang D., Xu Z., Sun W., Ren P. Autophagy Inhibitor (LY294002) and 5-fluorouracil (5-FU) Combination-Based Nanoliposome for Enhanced Efficacy Against Esophageal Squamous Cell Carcinoma. Nanoscale Res. Lett. 2018;13:325. doi: 10.1186/s11671-018-2716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jyoti K., Kaur K., Pandey R.S., Jain U.K., Chandra R., Madan J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015;445:219–230. doi: 10.1016/j.jcis.2014.12.092. [DOI] [PubMed] [Google Scholar]
- 122.Sun L., Wan K., Hu X., Zhang Y., Yan Z., Feng J., Zhang J. Functional nanoemulsion-hybrid lipid nanocarriers enhance the bioavailability and anti-cancer activity of lipophilic diferuloylmethane. Nanotechnology. 2016;27:085102. doi: 10.1088/0957-4484/27/8/085102. [DOI] [PubMed] [Google Scholar]
- 123.Chen T., Gong T., Zhao T., Fu Y., Zhang Z., Gong T. A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as therapeutic adjuvant for lung cancer therapy. Eur. J. Pharm. Sci. 2018;111:293–302. doi: 10.1016/j.ejps.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 124.Lu B., Sun L., Yan X., Ai Z., Xu J. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: A new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med. Oncol. 2015;32:345. doi: 10.1007/s12032-014-0345-5. [DOI] [PubMed] [Google Scholar]
- 125.Shi S., Han L., Deng L., Zhang Y., Shen H., Gong T., Zhang Z., Sun X. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J. Control. Release. 2014;194:228–237. doi: 10.1016/j.jconrel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Li S., Wang L., Li N., Liu Y., Su H. Combination lung cancer chemotherapy: Design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017;95:548–555. doi: 10.1016/j.biopha.2017.08.090. [DOI] [PubMed] [Google Scholar]
- 127.Liang Y., Tian B., Zhang J., Li K., Wang L., Han J., Wu Z. Tumor-targeted polymeric nanostructured lipid carriers with precise ratiometric control over dual-drug loading for combination therapy in non-small-cell lung cancer. Int. J. Nanomed. 2017;12:1699–1715. doi: 10.2147/IJN.S121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao X., Luo J., Gong T., Zhang Z.-R., Sun X., Fu Y. Coencapsulated doxorubicin and bromotetrandrine lipid nanoemulsions in reversing multidrug resistance in breast cancer in vitro and in vivo. Mol. Pharm. 2015;12:274–286. doi: 10.1021/mp500637b. [DOI] [PubMed] [Google Scholar]
- 129.Rocca A., Cecconetto L., Passardi A., Melegari E., Andreis D., Monti M., Maltoni R., Sarti S., Pietri E., Schirone A., et al. Phase Ib dose-finding trial of lapatinib plus pegylated liposomal doxorubicin in advanced HER2-positive breast cancer. Cancer Chemother. Pharmacol. 2017;79:863–871. doi: 10.1007/s00280-017-3279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu D., Li W., Zhang Y., Zhang B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed. Pharmacother. 2016;83:1428–1435. doi: 10.1016/j.biopha.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 131.Garg N.K., Singh B., Jain A., Nirbhavane P., Sharma R., Tyagi R.K., Kushwah V., Jain S., Katare O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Coll. Surf. B Biointerfaces. 2016;146:114–126. doi: 10.1016/j.colsurfb.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 132.Li X., Jia X., Niu H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018;13:4107–4119. doi: 10.2147/IJN.S163929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang X., Lin C.-C., Chan W.-K.-N., Liu K.-L., Yang Z.-J., Zhang H.-Q. Augmented Anticancer Effects of Cantharidin with Liposomal Encapsulation:In Vitro and In Vivo Evaluation. Molecules. 2017;22:1052. doi: 10.3390/molecules22071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang M., Wu M., Li H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018;25:1984–1995. doi: 10.1080/10717544.2018.1526227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.-K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grillone A., Riva E.R., Mondini A., Forte C., Calucci L., Innocenti C., de Julian Fernandez C., Cappello V., Gemmi M., Moscato S., et al. Active Targeting of Sorafenib: Preparation, Characterization, and In Vitro Testing of Drug-Loaded Magnetic Solid Lipid Nanoparticles. Adv. Healthc. Mater. 2015;4:1681–1690. doi: 10.1002/adhm.201500235. [DOI] [PubMed] [Google Scholar]
- 137.Harshita, Barkat M.A., Rizwanullah M., Beg S., Pottoo F.H., Siddiqui S., Ahmad F.J. Paclitaxel-loaded Nanolipidic Carriers with Improved Oral Bioavailability and Anticancer Activity against Human Liver Carcinoma. AAPS PharmSciTech. 2019;20:87. doi: 10.1208/s12249-019-1304-4. [DOI] [PubMed] [Google Scholar]
- 138.Ding J., Feng M., Wang F., Wang H., Guan W. Targeting effect of PEGylated liposomes modified with the Arg-Gly-Asp sequence on gastric cancer. Oncol. Rep. 2015;34:1825–1834. doi: 10.3892/or.2015.4142. [DOI] [PubMed] [Google Scholar]
- 139.Yang F., Zheng Z., Zheng L., Qin J., Li H., Xue X., Gao J., Fang G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. OncoTargets Ther. 2018;11:6811–6825. doi: 10.2147/OTT.S182437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J., Zhu R., Sun X., Wang T., Liu H., Wang S.-L. Intracellular uptake of etoposide-loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int. J. Nanomed. 2014;9:3987–3998. doi: 10.2147/IJN.S64103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li T., Zhang Y., Meng Y.-P., Bo L.-S., Ke W.-B. miR-542-3p Appended Sorafenib/All-trans Retinoic Acid (ATRA)-Loaded Lipid Nanoparticles to Enhance the Anticancer Efficacy in Gastric Cancers. Pharm. Res. 2017;34:2710–2719. doi: 10.1007/s11095-017-2202-7. [DOI] [PubMed] [Google Scholar]
- 142.Jiang H., Geng D., Liu H., Li Z., Cao J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug Deliv. 2016;23:3665–3673. doi: 10.1080/10717544.2016.1217954. [DOI] [PubMed] [Google Scholar]
- 143.Abu-Fayyad A., Nazzal S. Gemcitabine-vitamin E conjugates: Synthesis, characterization, entrapment into nanoemulsions, and in-vitro deamination and antitumor activity. Int. J. Pharm. 2017;528:463–470. doi: 10.1016/j.ijpharm.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bisht S., Schlesinger M., Rupp A., Schubert R., Nolting J., Wenzel J., Holdenrieder S., Brossart P., Bendas G., Feldmann G. A liposomal formulation of the synthetic curcumin analog EF24 (Lipo-EF24) inhibits pancreatic cancer progression: Towards future combination therapies. J. Nanobiotechnol. 2016;14:57. doi: 10.1186/s12951-016-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei Y., Wang Y., Xia D., Guo S., Wang F., Zhang X., Gan Y. Thermosensitive Liposomal Codelivery of HSA-Paclitaxel and HSA-Ellagic Acid Complexes for Enhanced Drug Perfusion and Efficacy Against Pancreatic Cancer. ACS Appl. Mater. Interfaces. 2017;9:25138–25151. doi: 10.1021/acsami.7b07132. [DOI] [PubMed] [Google Scholar]
- 146.Wang-Gillam A., Li C.-P., Bodoky G., Dean A., Shan Y.-S., Jameson G., Macarulla T., Lee K.-H., Cunningham D., Blanc J.F., et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 147.Moghimipour E., Rezaei M., Ramezani Z., Kouchak M., Amini M., Angali K.A., Dorkoosh F.A., Handali S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharm. Sci. 2018;114:166–174. doi: 10.1016/j.ejps.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 148.Serini S., Cassano R., Corsetto P.A., Rizzo A.M., Calviello G., Trombino S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018;19:586. doi: 10.3390/ijms19020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen M.Y., Liu T.-I., Yu T.-W., Kv R., Chiang W.-H., Tsai Y.-C., Chen H.-H., Lin S.-C., Chiu H.-C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials. 2019;197:86–100. doi: 10.1016/j.biomaterials.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 150.Negi L.M., Talegaonkar S., Jaggi M., Verma A.K., Verma R., Dobhal S., Kumar V. Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part I. Synthesis, characterization and in vitro investigation. Coll. Surf. B Biointerfaces. 2014;123:600–609. doi: 10.1016/j.colsurfb.2014.09.062. [DOI] [PubMed] [Google Scholar]
- 151.Ahmad G., El Sadda R.R., Botchkina G., Ojima I., Egan J., Amiji M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017;406:71–80. doi: 10.1016/j.canlet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsai Y.J., Chen B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016;11:1907–1926. doi: 10.2147/IJN.S103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nassir A.M., Ibrahim I.A.A., Md S., Waris M., Tanuja Ain M.R., Ahmad I., Shahzad N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: Invitro and invivo activity. Life Sci. 2019;220:136–146. doi: 10.1016/j.lfs.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 154.Hua H., Zhang N., Liu D., Song L., Liu T., Li S., Zhao Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int. J. Nanomed. 2017;12:7869–7884. doi: 10.2147/IJN.S143977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abdulbaqi I.M., Assi R., Yaghmur A., Darwis Y., Mohtar N., Parumasivam T., Saqallah F., Wahab H. Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update. Pharmaceuticals. 2021;14:725. doi: 10.3390/ph14080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hegde M.M., Prabhu S., Mutalik S., Chatterjee A., Goda J.S., Rao B.S.S. Multifunctional lipidic nanocarriers for effective therapy of glioblastoma: Recent advances in stimuli-responsive, receptor and subcellular targeted approaches. J. Pharm. Investig. 2021;52:49–74. doi: 10.1007/s40005-021-00548-6. [DOI] [Google Scholar]
- 157.Laquintana V., Trapani A., Denora N., Wang F., Gallo J.M., Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin. Drug Deliv. 2009;6:1017–1032. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kushwah V., Agrawal A.K., Dora C.P., Mallinson D., Lamprou D.A., Gupta R.C., Jain S. Novel Gemcitabine Conjugated Albumin Nanoparticles: A Potential Strategy to Enhance Drug Efficacy in Pancreatic Cancer Treatment. Pharm. Res. 2017;34:2295–2311. doi: 10.1007/s11095-017-2238-8. [DOI] [PubMed] [Google Scholar]
- 159.Ying K., Bai B., Gao X., Xu Y., Wang H., Xie B. Orally Administrable Therapeutic Nanoparticles for the Treatment of Colorectal Cancer. Front. Bioeng. Biotechnol. 2021;9:670124. doi: 10.3389/fbioe.2021.670124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bottger R., Pauli G., Chao P.-H., AL Fayez N., Hohenwarter L., Li S.-D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020;154–155:79–101. doi: 10.1016/j.addr.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 161.Das M., Jain R., Agrawal A., Thanki K., Jain S. Macromolecular bipill of gemcitabine and methotrexate facilitates tumor-specific dual drug therapy with higher benefit-to-risk ratio. Bioconjugate Chem. 2014;25:501–509. doi: 10.1021/bc400477q. [DOI] [PubMed] [Google Scholar]
- 162.Yildirimer L., Thanh N.T., Loizidou M., Seifalian A.M. Toxicology and clinical potential of nanoparticles. Nano Today. 2011;6:585–607. doi: 10.1016/j.nantod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Azarnezhad A., Samadian H., Jaymand M., Sobhani M., Ahmadi A. Toxicological profile of lipid-based nanostructures: Are they considered as completely safe nanocarriers? Crit. Rev. Toxicol. 2020;50:148–176. doi: 10.1080/10408444.2020.1719974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.