Abstract
Long non-coding RNAs (lncRNA) have recently been identified as key regulators of oxidative stress in several malignancies. The level of reactive oxygen species (ROS) must be constantly regulated to maintain cancer cell proliferation and chemoresistance and to prevent apoptosis. This review will discuss how lncRNAs alter the ROS level in cancer cells. We will first describe the role of lncRNAs in the nuclear factor like 2 (Nrf-2) coordinated antioxidant response of cancer cells. Secondly, we show how lncRNAs can promote the Warburg effect in cancer cells, thus shifting the cancer cell’s “building blocks” towards molecules important in oxidative stress regulation. Lastly, we explain the role that lncRNAs play in ROS-induced cancer cell apoptosis and proliferation.
Keywords: lncRNAs, ROS, antioxidant response, cancer metabolism, chemoresistance
1. Introduction
Cancer remains the second leading cause of mortality worldwide, lagging only to cardiovascular disease [1]. Globally, an estimated 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) occurred in 2020 [2]. Moreover, the median age of cancer diagnosis is on a decreasing trend [3] and estimates suggest that this century could witness cancer surpassing cardiovascular disease as the number one cause of premature death in most countries [4]. However, constant research in the not-so-long-ago cryptic mechanisms of cancer cells has led to significant progress over the last decade, which can be readily seen in a 50% decrease (from 186.9 to 86.3) in the age-standardized mortality rate [2,5]. With that in mind, this review aims to coalesce the current knowledge and highlight the latest advancements regarding two important molecules in cancer cells: long non-coding RNAs (lncRNAs) and reactive oxygen species (ROS).
ROS are oxygen containing molecules characterized by their high reactivity, playing the role of oxidant or reductor in a wide range of redox reactions [6]. Though a heterogenous group, a few representatives are of significant importance in cancer: superoxide anion (•O2−), hydrogen peroxide (H2O2) and hydroxyl radical (•OH) [7]. These molecules can easily derive from one another through commonly occurring reactions throughout the cell. For example, •O2− can be oxidized to H2O2 by superoxide dismutase (SOD) and H2O2 can be reduced by Fenton reaction to form •OH—a highly reactive and DNA damaging ROS [8].
In physiological conditions, ROS are not merely a byproduct of the cell’s redox homeostasis but do in fact carry out important functions [9]. For example, through the formation of disulfide bonds between thiol groups in adjacent cysteine residues, ROS can alter the function of proteins in key signaling pathways such as such as the PI3K/Akt, NF-κB, and p53 pathways [10,11,12]. However, due to their high reactivity, ROS need to be kept in check by well-orchestrated antioxidant machinery. An imbalance in oxidative stress regulation is a core pathophysiological process in multiple pathologies such as cardiovascular disease [13], diabetes [14], inflammatory disorders [15], and cancer.
Elevated levels of ROS are considered essential in promoting cancer cell growth and proliferation by augmenting oncogenes expression or inhibiting tumor suppressor genes, either by direct DNA damage or by post-translational modifications in key signaling proteins or enzymes [7,16,17,18]. Figure 1 reviews the most important ROS-coordinated pathways in the hallmarks of cancer [19,20,21]. On the other hand, high levels of ROS can overwhelm the antioxidant machinery of cancer cells and lead the cell to apoptosis [22]. As such, the ROS balancing mechanism is of paramount importance in cancer [23]. For example higher activity of antioxidant enzymes such as SOD and catalase (CAT) and higher levels of antioxidant molecules such as malondialdehyde (MDA) correlate with melanoma disease progression [24,25]. The same correlation was observed with breast cancer cell survival and epithelial mesenchymal transition (EMT) [26]. To coordinate the myriad of functions that ROS have in determining cancer cells’ fate, lncRNAs modulate a fine tuning of the cells’ redox balance.
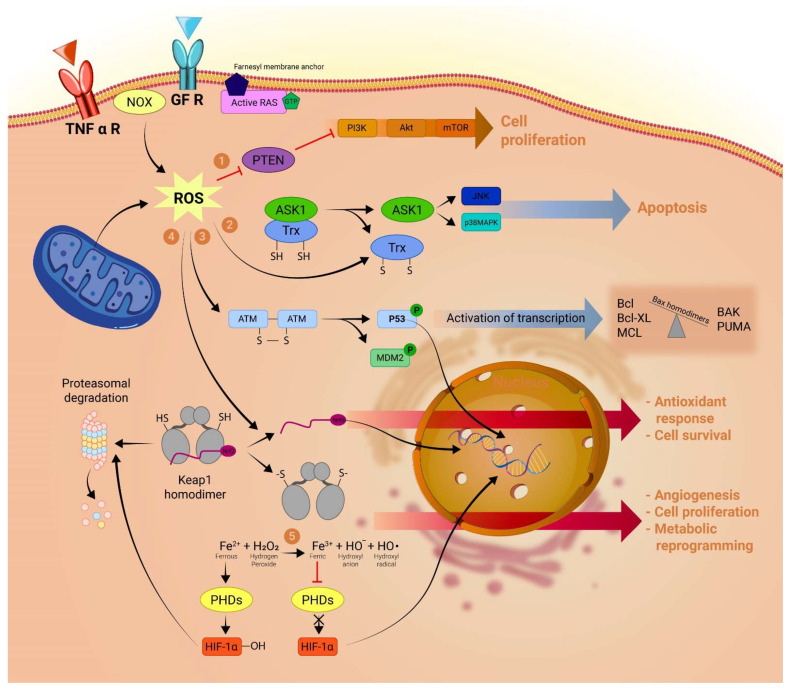
Figure 1.
ROS functions in cancer cells: The production of ROS is elevated in tumor cells as a consequence of increased metabolic rate, gene mutation, extracellular growth factor signaling and relative hypoxia. This increase in the baseline ROS level plays a crucial role in determining cancer cells’ fate: (1) ROS can drive cancer cell proliferation by inactivating PTEN, a tumor suppressor that acts as a brake on the pro-growth PI3K/Akt pathway. (2) Inversely, increased ROS can have a nefarious effect on the cancer cell by activating apoptotic pathways. As such, oxidative stress may lead to the formation of disulfide bonds in the thioredoxin molecule, which results in the activation of the kinase Ask1 and subsequent activation of key apoptotic transcription factors like JNK and p38. (3) A similar process occurs in the case of p53 activation, where an ROS-triggered formation of disulfide bonds at the Cys 2999 residues of ATM homodimer results in activation and subsequent phosphorylation of p53 and its inhibitory molecule MDM2. P53 alongside JNK, p38, and other transcription factors will shift the balance towards apoptosis by expressing proteins, such as Bax homodimers, BAK and PUMA, to the detriment of antiapoptotic proteins such as Bcl-2, Bcl-XL or MCL. (4) ROS can prevent the proteasomal degradation of transcription factor Nrf2 by dissociating the KEAP1-Nrf2 complex and thus allowing Nrf2 to translocate to the nucleus and activate antioxidant pathways that can mitigate the proapoptotic effect of ROS. (5) By oxidizing iron from its ferrous (Fe2+) to its ferric (Fe3+) state, ROS dampens the enzymatic activity of PHDs which would normally hydroxylase the HIF-1α protein causing its proteasomal degradation. The non-hydroxylated form of HIF-1α has a major role in promoting cancer cell survival, angiogenesis and metabolic reprogramming under hypoxic conditions. PTEN, phosphatase and tensin homolog; Ask1, apoptosis signal-regulating kinase 1; ATM, ataxia-telangiectasia mutated; MDM2, mouse double minute 2 homolog; Bax, Bcl-2-associated protein X; Bcl-2, B-cell lymphoma 2; KEAP1, Kelch-like ECH-associated protein 1; PHDs, prolyl hydroxylase domain enzymes; HIF-1α, hypoxia-inducible factor α.
LncRNAs are noncoding RNA transcripts of 200 or more nucleotides in length of which, until recently, little was known. However, recent studies estimate the number of such molecules to exceed coding mRNAs by as much as 20-fold [27], and the variety of functions that lncRNAs undertake is also significant [28]. Similar to their smaller microRNA (miRNA) counterparts, lncRNAs’ main function is to regulate gene expression, which can be achieved in multiple ways: (1) lncRNAs can induce gene activation and subsequent transcription into mRNA by acting as a liaison between transcription factors and specific gene promoters; (2) lncRNAs can inhibit gene expression by binding to chromatin regions and thus restricting RNA polymerase access to genes within the region; (3) lncRNAs may modulate gene expression by binding and directing enzymes such as histone acetyl transferases or histone methylation enzymes, conversely promoting or inhibiting epigenetic changes; (4) lncRNAs can act as scaffoldings to stabilize multi-subunit complexes that dictate gene expression [29]. Furthermore, in the cytoplasm, lncRNAs can also influence protein activity in a posttranscriptional manner, either by direct interaction with the protein (e.g., allosterically regulating enzymes activities) or by acting as competing endogenous RNAs (ceRNAs) for miRNAs and modifying the translation of mRNA transcripts [30,31].
Thus, not surprisingly, lncRNAs play a pivotal role in cancer cells, acting in different instances either as a part of their arsenal used toward proliferation or as key components in tumor-suppressing mechanisms. Meticulous work has been put into identifying hundreds of lncRNAs over/under expressed in a variety of cancers including but not limited to hepatocarcinoma (HCC) [32,33,34], lung cancer [35,36,37,38], prostate cancer [39,40,41], gastric cancer [42] and renal cell carcinoma (RCC) [43].
Recent studies show that various lncRNAs, such as HOTAIR, MALAT1, NRAL and TUG1, play a pivotal role in the regulation of cancer cells’ oxidative stress, highlighting a possible crossroad between lncRNAs and ROS. As such, it is now indicated that multiple key functions of lncRNAs, such as coordinating cancer cells towards proliferation or pro-apoptotic pathways [44], are either triggered or accomplished via variations in the cancer cell’s level of ROS [45]. This is in accordance to the already established property of ROS to drive cancer cells proliferation, while also triggering apoptosis when the level reaches a threshold [46,47,48].
Several lncRNAs could play a role in oxidative stress regulation or could act as responders to oxidative stress. Table 1 summarizes these lncRNAs, along with a brief description of their interaction with the redox system of the cell.
Table 1.
Important lncRNAs in the regulation of oxidative stress and their impact on cancer cells.
| LncRNA | Expression in Cancer Cells | Role in Oxidative Stress Regulation | ROS Level | Impact on Cancer Cells | Refs. |
|---|---|---|---|---|---|
| lncRNA HOTAIR | ↑ (increased) in lung cancer | confers structural stability to complex III | ↓(decreased) | ↑ chemoresistance and survival | [49,50,51] |
| ↑ in glioblastoma ↑ in cervical cancer |
↑ invasion and metastasis | ||||
| lncRNA SAMMSON | ↑ in breast cancer | inhibits complex I protein transcription and translation | ↓ | ↑ chemoresistance and survival | [52] |
| lncRNA TUG1 | ↑ in oesophageal SCC | ↑ antioxidant response by potentiating Nrf2 expression expression is also stimulated by Nrf2 |
↓ | ↑ chemoresistance and survival | [53,54,55] |
| ↑ in urothelial carcinoma of the bladder | ↑ cell proliferation ↓ apoptosis |
||||
| lncRNA SLC7A11-AS1 | ↑ in pancreatic adenocarcinoma | prevents proteosomal degradation of Nrf2 | ↓ | ↑ chemoresistance | [56] |
| lncRNA MIR4435-2HG | ↑ in colon cancer | increases Nrf2 expression | ↓ | ↑ chemoresistance ↑ cell proliferation |
[57] |
| lncRNA NRAL | ↑ in HCC | acts as an ceRNA by binding miRNA-miR340–5p and thereby freeing Nrf2 antioxidant effects | ↓ | ↑ chemoresistance | [58] |
| lncRNA KRAL | ↓ in HCC | acts as an ceRNA by binding miRNA miR-141 thereby freeing KEAP1 and preventing nuclear translocation of Nrf2 | ↑ | ↑ chemoresistance | [59] |
| lncRNA MALAT1 | ↑ in NSCLC | HIF 1 α dependent transcription | ↓ | ↑ cell survival and proliferation | [60,61,62,63,64,65] |
| ↓ KEAP1 activity | possible tumor suppressive role | ||||
| lncRNA SCAL1 | ↑ after exposure to cigarette smoke | effector of Nrf2 antioxidant response | ↓ | ↑cryoprotection against cigarette smoke–induced toxicity | [66,67] |
| ↑ in NSCLC | ↓ apoptosis in NSCLC | ||||
| lncRNA ODRUL | ↑ in osteosarcoma | mediates Nrf2 pro-apoptotic effects | ↓ | ↑ doxorubicin resistance | [68,69] |
| lncRNA SOX-2-OT | ↑ in HCC | inducing the PKM2 isoform of the enzyme PK | ↓ | metabolic reprogramming | [70] |
| ↑ invasion and metastasis | |||||
| lncRNA HULC | ↑ in HCC | inducing the PKM2 isoform of the enzyme PK | ↓ | metabolic reprogramming | [71,72] |
| lncRNA H19 | ↑ in HCC | decreases SOD activity via MAPK/ERK pathway | ↓ | ↑ cell viability | [33] |
| ↓ cell apoptosis | |||||
| ↑ chemoresistance | |||||
| lncRNA NLUCAT | ↑ in lung adenocarcinoma | effector of Nrf2 antioxidant response | ↓ | ↑ cell proliferation | [73] |
| ↓ cisplatin susceptibility | |||||
| lncRNA XIST | ↑ in NSCLC ↑ in osteosarcoma |
acts as a sponge for miR-335, inducing an increase in SOD2 expression acts as a sponge for miR-153, increasing Snail expression |
↓ | prevents pyroptosis ↑ cell proliferation ↑ EMT |
[74,75,76,77] |
| lincRNA-p21 | ↓ in NSCLC | induces apoptosis as a result of ROS-mediated p53 activation | ↑ | ↓chemoresistance ↓cell survival |
[78] |
| ↓ in HCC | |||||
| ↓ in breast cancer | |||||
| lncRNA NEAT1 | ↑ in HCC | induces apoptosis as a result of ROS-mediated p53 activation | ↑ apoptosis | [79] | |
| lncRNA ROR | ↑ in HCC | Inhibits p53 activity | ↓ cell apoptosis | [80] | |
| lncRNA NORAD | ↑ in gastric cancer | increases the expression of autophagy related genes ATG-5 and ATG-12 | ↓ | ↑cell survival | [81] |
| lincRNA 00963 | ↑ in breast cancer | sponges miR324-3p | ↓ | ↑cell survival ↑ chemoresistance |
[82] |
| lncRNA GAS-5 | ↓ in breast cancer | inhibits NOX4 protein expression Inhibits G6PD protein expression |
↑/↓ | ↓ tumor suppressor effect | [83,84,85,86,87,88,89] |
| ↓ in prostate cancer | |||||
| ↓ in gastric cancer | ↑cell survival and proliferation | ||||
| ↓ in melanoma |
2. LncRNAs and the Generation of Mitochondrial ROS in Cancer
The main intracellular source of ROS is the mitochondrial electron transport chain (ETC) [90,91]. A brief description of the ETC and its importance in ROS generation is depicted in Figure 2.
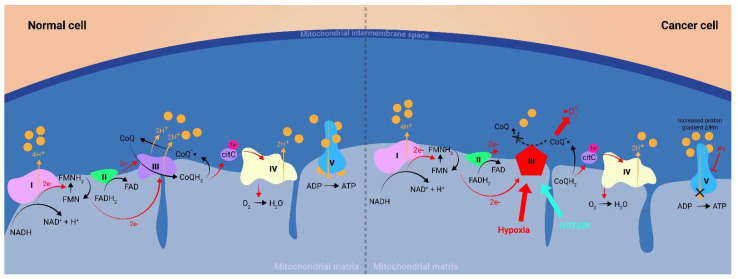
Figure 2.
Mitochondrial Electron transport chain (ETC): The ETC consists of five electron exchanging protein complexes bound to the inner mitochondrial membrane. The NADH and FADH2 formed in the tricarboxylic acid chain provide the electrons to complex I and II, respectively, and the electrons are passed on from one complex to another until they reach complex IV where four electrons together with four H+ from the mitochondrial matrix react with oxygen to form two water molecules. The purpose of the ETC is that at each complex–apart from complex, II–H+ are pumped in the intermembrane space (IMS) thus creating a proton gradient between the IMS and the mitochondrial matrix, which polarizes the inner mitochondrial membrane (ΔΨm). This gradient is then used to power the complex V ATP-synthetase mechanism, storing energy in the phosphodiester bound formed between ADP and a molecule of H3PO4. For a more complex depiction of the ETC, see reference [93]. The hypoxic cancer cell environment alongside the increased ΔΨm caused by oxidative phosphorylation inhibitors, such as inhibitory factor 1 (IF1), alter the ETC in such a way that the probability of ROS species formation increases as compared to a normal cell. Certain lncRNAs, such as HOTAIR, stabilize complex III and decrease the probability of ROS formation.
In cancer cells, alterations in the ROS homeostasis can derive either from internal mitochondrial alterations or from external signaling pathways [92,93]. In different cancer cells, the amount of mitochondria-generated ROS is higher than in normal cells [94,95]. One possible explanation is that in phases of hypoxic conditions (such as when the proliferation of tumor cells is high), the availability of O2 as an electron acceptor at complex IV is low, causing the accumulation of electrons and increased reduction of complexes I–III. Indeed, several studies have proven that ROS are increased in hypoxic conditions [96,97]. However, given that electrons react quite readily with O2 at complex IV (Km < 1 μM), such a low concentration of O2 at complex IV should also limit oxygen available for reduction to •O2− at other complexes [98].
A possible explanation is that hypoxia also affects the conformation of complex III in such a manner that ubisemiquinone (CoQ−•) is stabilized at the Qi site of complex III, transforming it into an excellent electron donor and leading to the generation of •O2− [93,99]. Furthermore, evidence suggests that lncRNA HOTAIR (HOX transcription RNA) plays a role in the structural stability of complex III. Although HOTAIR has been shown to induce tumorigenesis and metastasis in numerous tumors [100,101], Zheng P. et al. came to the interesting finding that HOTAIR inhibition leads to further depolarization of mitochondrial membrane potential, complex III ultrastructural abnormalities and enhanced intracellular ROS production in human cervical cancer cells [50]. This could indicate that HOTAIR acts as an antioxidant regulator lncRNA and prevents the cancer cell from surpassing the threshold ROS level that would activate apoptosis. This could also explain HOTAIR’s role in chemoresistance observed in lung cancer [51]. It was also observed that lncRNA HOTAIR correlates with the expression of transglutaminase 2 (TGM2) in glioblastoma cells [49]. TGM2 is e key enzyme in maintaining mitochondrial homeostasis under stressful conditions [102], suggesting that its activity plays a part in the stabilization of complex III by HOTAIR. In another recent study, Charlotte Orre et al. proved that lncRNA SAMMSON acts by inhibiting oxidative phosphorylation in MCF-7 doxorubicin-resistant breast cancer cells. This is achieved by inhibiting the transcription and translation of complex I structural proteins, which, in turn, blocks electrons’ passage down the ETC to form ROS [52].
Another theory that could explain the altered mitochondrial ROS metabolism in cancer cells is that the increased mitochondrial membrane potential (ΔΨm) observed in different cancer cell lines is responsible for the increase in ROS production [103,104] thus conferring higher tumorigenicity [105,106,107]. In the context where the ETC is incapacitated by the high ΔΨm, complex I and complex III again seem to be the most important sources of ROS [93,99,108].
3. LncRNAs and Nrf2. A Key Point in Cancer Cell’s Oxidative Stress Modulation and Chemoresistance
3.1. LncRNAs as Regulators of Nrf2
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)—a nuclear transcription factor for cellular anti-oxidation responses—positively correlates with various cancers’ progression and chemoresistance [109,110]. Increasing evidence suggests that lncRNAs play a key part in regulating Nrf2 expression [111]. This can be achieved either directly, by epigenetic alterations and posttranslational interactions with Nrf2, or indirectly, by influencing the expression of other molecules that are important regulators of Nrf2 (Figure 3).
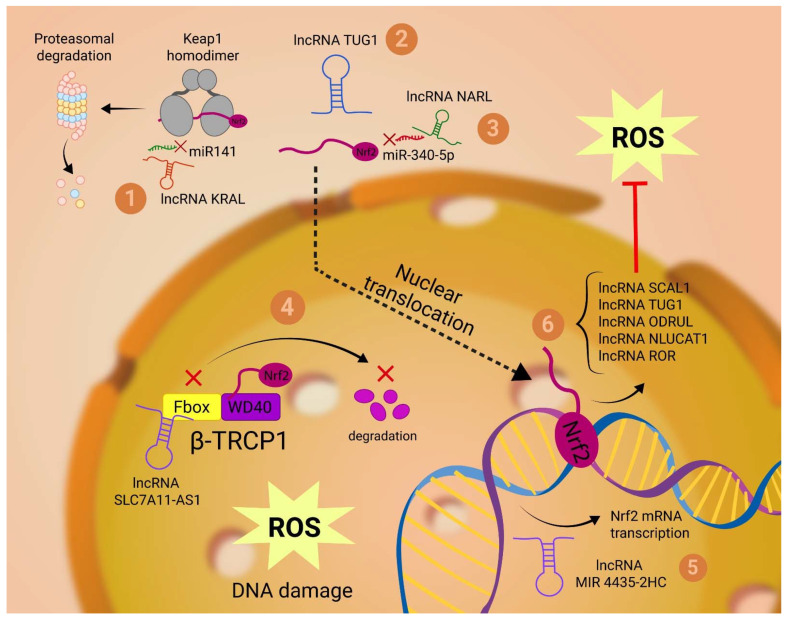
Figure 3.
Nrf2-associated lncRNAs: As regulators of Nrf2, lncRNAs can act both at the cytoplasmatic and the nuclear level. In the cytoplasm, lncRNA KRAL and lncRNA NARL act as ceRNAs for miR 141 and miR-340-5p, respectively. While KRAL promotes Nrf2 proteasomal degradation by freeing up the KEAP 1 homodimer (1), NARL prevents Nrf2 inactivation in the cytoplasm and facilitates its translocation to the nucleus (2). At the cytoplasmatic level, lncRNA TUG1 directly binds to the Nrf2 protein and potentiates its activity (3). In the nucleus, lncRNA SLC7A11-AS1 prevents the recruitment of β-TRCP1 to the SCFβ-TRCP E3 complex, which would otherwise initiate ubiquitination and proteasomal degradation of Nrf2 in the nucleus (4). LncRNA MIR4435-2HG induces Nrf2 gene expression and subsequent transcription into mRNA (5). Nrf2 induces the transcription of multiple lncRNAs that regulate the level of ROS (6).
LncRNA TUG1 post-translationally potentiates the Nrf2 effect in esophageal squamous cell carcinoma (SCC) cells, by directly binding to Nrf2 protein and elevating its expression [54].
LncRNA SLC7A11-AS1 confers chemoresistance in pancreatic adenocarcinoma cells by augmenting the expression of Nrf-2. By interacting with the F-box motif of β-TRCP1, the exon 3 of SLC7A11-AS1 prevents the recruitment of β-TRCP1 to the SCFβ-TRCP E3 complex, which would otherwise initiate ubiquitination and proteasomal degradation of Nrf2 in the nucleus [56,112].
Another study carried out by Luo P. et al. found that small interfering RNA (siRNA) targeting lncRNA MIR4435-2HG significantly reduced cisplatin resistance in colon cancer, which was attributed to the decrease in Nrf2 mRNA observed using qPCR [57].
Cisplatin-resistant HCC cells also presented specific lncRNA signatures in microarray and qPCR analyses. Following Luciferase reporter assays, it was confirmed that Nrf2 mRNA was targeted and inactivated by a miRNA-miR340–5p- and that Nrf2-associated lncRNA (NRAL) could function as a ceRNA for Nrf2 by binding to miR340–5p, thereby freeing Nrf2 antioxidant effects [58].
Another way by which lncRNAs influence the expression of Nrf2 is by modulating Kelch-like ECH-associated protein 1 (KEAP1) activity, a cytosolic protein that prevents Nrf2 translocation to the nucleus and accomplishing its role as a transcription factor. One example is lncRNA KRAL, which—similar to NARL lncRNA—acts as a ceRNA by competitively binding miR-141 and thereby freeing KEAP1. Given that KRAL is downregulated in 5-fluorouracil-resistant HCC cells, this leads to increased levels of Nrf2, thus also helping the cell mitigate its increased ROS level [59]. On the other hand, lncRNA MALAT-1 was observed to be overexpressed in different cancer cell lines [113] and to lower KEAP1 activity [114].
Thus, the decrease in KRAL and the increase in MALAT1 regulate KEAP1 and along with the overexpression of TUG1, NRAL and MIR4435-2HG observed in cancer cells, converge to increase the antioxidant potential of the cell, permitting it to tolerate higher oxidative stress, and once again substantiating the important influence of lncRNA on ROS in cancer.
3.2. LncRNAs Regulated by Nrf2
Inversely, the idea that Nrf2 antioxidant functions could be accomplished by lncRNAs has recently appeared.
One of the first lncRNAs that was identified to be regulated by Nrf2 was lncRNA SCAL1 (smoke and cancer-associated lncRNA 1). Thati P. et al. demonstrated that siRNA knockdown of Nrf2 caused a significant decrease in SCAL1, with subsequent increased susceptibility to oxidative stress caused by cigarette smoke [67].
LncRNA TUG1, which we previously noted increases the expression of Nrf2, has also been shown to be positively regulated by Nrf2 in urothelial carcinoma of the bladder cells [54], thus completing a possible positive feedback loop that sustains the antioxidant response of cancer cells.
Another study conducted by Gao M. et al. [69], validated the binding of Nrf2 transcription factor to lncRNA ODRUL (Osteosarcoma doxorubicin-resistance related upregulated lncRNA) gene promoter as a response to oxidative stress. In this case, ODRUL-mediated the pro-apoptotic effect of NFf2 by diminishing anti-apoptotic Bcl-2 protein levels.
Furthermore, Moreno Leon L. et al. [73] found that pharmacological inhibition of NF-κB-dependent transcription or siRNA-mediated depletion of Nrf2 downregulates the hypoxia dependent expression of lncRNA NLUCAT1. To explore the impact of this NLUCAT 1 downregulation without the bias of other lost functions due to inhibition of NF-κB or NRF2 transcription, they used CRISPR-Cas9 gene editing to invalidate NLUCAT-1 gene and found that NLUCAT-1 knockdown lung adenocarcinoma cells showed lower proliferation rates, higher level of ROS and thus an increased susceptibility to cisplatin-induced apoptosis. It follows that NLUCAT-1 represents another lncRNA induced by NRF2 with an important role in the antioxidant response of cancer cells.
The importance of such antioxidant responses conducted by lncRNAs is uncanny as it confers the property of chemoresistance to drugs or radiation therapy that often act by increasing the cell oxidative stress, triggering apoptosis.
4. A LncRNA Perspective on the Warburg Effect. Tipping the Redox Balance
Now generally accepted as a core hallmark of cancer, metabolic reprogramming (Warburg effect) shifts the use of glucose from an energy-producing molecule, to a biomass source, used in the synthesis of essential proteins and nucleic acids for the rapidly dividing cancer cells [115]. This is attained by redirecting a large part of the glucose intake of the cell away from oxidative phosphorylation and towards the glycolytic pathway. While in normal cells that are not actively growing, this process happens only under hypoxic conditions, in the cancer cell, through activation of oncogenes such as MYC and PI3K/AKT signaling, this happens even in normoxic conditions, hence the term aerobic glycolysis [115]. Recently, certain lncRNAs have emerged as important regulators of the metabolic reprogramming seen in cancer cells (Figure 4). LncRNA SOX-2-OT promotes HCC invasion and metastasis by orchestrating such a metabolic shift. Lyang Y. et al. [70] found that the overexpressed SOX-2-OT acts by inducing the PKM2 isoform of the enzyme PK (Pyruvate Kinase), which catalyzes the transformation of phosphoenolpyruvate into pyruvic acid, a rate limiting step of glycolysis. Despite its association with highly proliferative and metabolic active cells, the PKM2 isoform has lower intrinsic enzymatic activity than its PKM1 counterpart. Moreover, PKM2 is also sensible to particular inhibitory factors such as ROS formed in the ETC [116,117,118]. As such, the glucose metabolism will be partly shifted towards the pentose phosphate pathway form which NADPH will be formed as a byproduct. Consequently, NADPH can be used as an electron donor for most of the antioxidant mechanisms the cancer cell is reliant on to keep its high level of ROS in check [119,120].
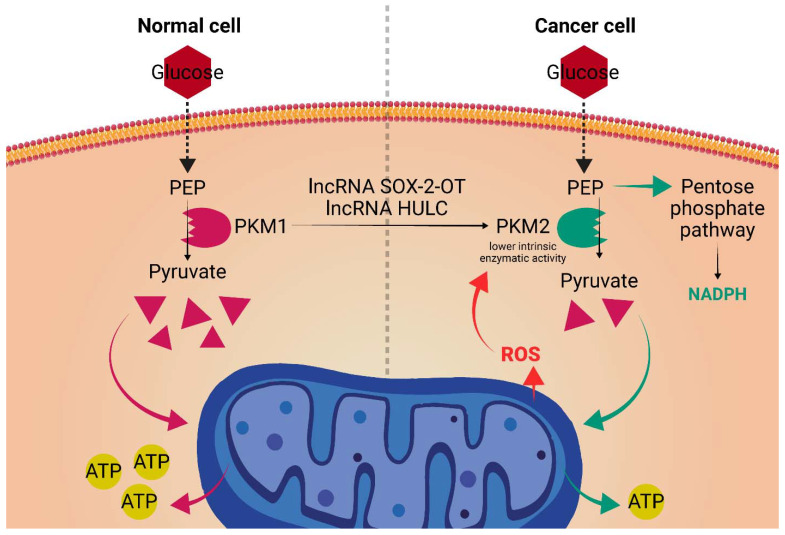
Figure 4.
LncRNAs’ role in the Warburg effect: SOX-2-OT and HULC lncRNAs carry out an antioxidant effect by inducing the PKM2 isoform of pyruvate kinase. Given its lower intrinsic enzymatic activity and its susceptibility to inhibition by ROS, the expression of the PKM2 isoform will allow for the synthesis of key antioxidant molecules such as NADPH. PEP, phosphoenolpyruvate; PKM1, pyruvate kinase M1 isoform; PKM2, pyruvate kinase M2 isoform.
LncRNAs’ role in the Warburg effect: SOX-2-OT and HULC lncRNAs carry out an antioxidant effect by inducing the PKM2 isoform of pyruvate kinase. Given its lower intrinsic enzymatic activity and its susceptibility to inhibition by ROS, the expression of the PKM2 isoform will allow for the synthesis of key antioxidant molecules such as NADPH. PEP, phosphoenolpyruvate; PKM1, pyruvate kinase M1 isoform; PKM2, pyruvate kinase M2 isoform.
Moreover, LncRNA that is highly upregulated in liver cancer (HULC) has been shown to sustain metabolic reprogramming and tumorigenesis in HCC by inducing the PKM2 isoform [71,121]. Multiple mechanisms have been proposed. One study [71] used a combination of tobramycin affinity purification and mass spectrometric analysis (TOBAP-MS) and found that HULC directly binds PKM2, augmenting its interaction with fibroblast growth factor receptor 1 (FGFR1). This causes increased enzyme phosphorylation with subsequent reduced formation of the tetrameric, more active form [122]. Furthermore, HULC can promote the expression of the PKM2 isoform via inhibition of the tumor suppressor phosphatase and tensin homolog (PTEN). Mechanistically, Xin X et al. [72] found that HULC inhibited the expression of PTEN on the translational level, but not on the transcriptional level, leading to the supposition that HULC acts by promoting cancer cell autophagy. This supposition was later confirmed by the observation that HULC could not alter the expression of PTEN after administration of MG132 (ubiquitin–proteasome inhibitor). Taken together, HULC promotes hepatocarcinogenesis by augmenting autophagy, depressing the PTEN tumor suppressor and inducing the PKM2 isoform, thus altering the redox potential of the cancer cell.
5. ROS and LncRNAs Determine Cancer Cell Fate. Possible Therapeutic Strategies
Besides being important regulators of the antioxidant response in cancer cells, lncRNAs take part in different ROS-dependent pathways essential for sustaining the hallmarks of cancer.
5.1. ROS, lncRNAs and Cell Proliferation
MALAT1 (metastasis-associated lung adenocarcinoma transcript 1, also known as NEAT2 or nuclear enriched abundant transcript 2) is directly correlated with the aggressiveness and mortality of lung adenocarcinoma [29,113,114]. The correlation between ROS and MALAT1 may be attributed to the effect of ROS on decreasing the degradation of hypoxia-inducible factor (HIF 1) α, a key transcriptional factor of MALAT-1 [123]. The preservation of HIF-1-α is achieved by the ROS-mediated inactivation of prolyl hydroxylase (PHDs) [124,125], which would otherwise determine the hydroxylation of specific prolyl residues in the HIF-1-α molecule, resulting in binding to the von Hippel–Lindau (VHL) protein of the E3 ubiquitin ligase complex and subsequent degradation via the polyubiquitination/proteasomal degradation pathway [126]. However, the role of MALAT1 in cell proliferation has been recently contradicted. While several studies have suggested that MALAT1 has a pro-growth effect on tumor cells [60,61,127] and that MALAT1 depleted cells undergo G1/S cell cycle arrest [62], recent studies postulate a tumor suppressive role of MALAT1 [63,64,65]. Nevertheless, MALAT1 has an important role in tumor progression and ROS can modulate its expression through HIF-1-α.
HIF-1-α also promotes the transcription of other lncRNAs correlated with tumorigenesis, such as HOTAIR [128], NUTF2P3-001 [129], UCA1 [130] and BX111 [131], but their direct correlation with the level of ROS has not yet been documented.
LncRNA XIST is another oncogenic lncRNA correlated with non-small cell lung cancer (NSCLC) proliferation, invasion, and metastasis [74,75]. The level of expression of XIST in NSCLC was also found to influence the cancer cells’ oxidative stress [76]. As such, Liu J et al. concluded that XIST knockdown NSCLC cells undergo pyroptosis due to a significant increase in the intracellular level of ROS. Treatment of such cells with N acetylcysteine (NAC), a potent antioxidant due to its thiol group, prevented pyroptosis. In researching why the loss of XIST distorts cancer cells’ oxidative stress control, they have found that it can act as a sponge for miR-335, inducing an increase in SOD2 expression, a crucial enzyme in the antioxidant process of cells, transforming •O2− to H2O2. Another study on osteosarcoma cancer cells found that XIST can also sponge miR-153, leading to oxidative stress-induced proliferation and invasion by potentiating Snail expression, one of the master regulators of EMT [77].
5.2. ROS, lncRNAs and Cell Death
Growth arrest-specific transcript 5 lncRNA (GAS-5) has been known to act as a tumor suppressor and to be notably downregulated in various cancers such as breast [83], prostate [84] and gastric cancer [85]. However, GAS-5 effect on the cellular level of ROS has been a subject of controversy. Chen et al. [86] used dihydroethidium (DHE) fluorescent staining and flow cytometry for the measurement of •O2− and qRT-PCR for the measurement of GAS5 in A375 and SK-Mel-28 human MM cell lines. Their results suggested that GAS5 expression was inversely proportional to the intracellular ROS level, resulting in disease progression and increased cell viability in GAS5 deficient cells, which is in accordance with previous studies on the effect of ROS on MM cell lines [132,133,134,135]. However, contrary results were obtained by Xu U. et al. [87], finding that A375 cells with low expression of GAS5 exhibited suppressed oxidative stress. In addition, studies assessing the effect of GAS5 in non-cancerous cells have also found that it can decrease the level of intracellular ROS by sponging different miRNAs (miR 452-5p, miR135a) [88,89]. It transpires that the GAS5 effect of inhibiting tumor growth can be partly attributed to its effect on the level of cellular ROS, but further research is needed to conclude whether this is achieved by augmenting ROS expression and promoting apoptosis or by mitigating the pro-growth effect of ROS.
Moreover, the apoptosis mediated by ROS p53 activation seems to be dependent on the transcription of lncRNAs. LincRNA-p21 (long intergenic noncoding RNa-p21) is a long interfering noncoding RNA that is under-expressed in different cancers [78]. Huarte M. et al. [136] indicated that RNAi-mediated knockdown of lincRNA-p21 severely decreases apoptosis. To further reinforce the importance of the ROS/p53/lincRNa-p21 pathway in cancer cell apoptosis, an impact of ROS inhibition on lincRNa-p21 should be assessed. LncRNA NEAT1 is another important cell death regulator that lays downstream of p53 in the ROS-induced ferroptosis of HCC. By sponging miR-362-3p, NEAT1 promoted Myo-inositol oxygenase (MIOX) expression, resulting in increased ROS production and ferroptosis [79]. Inversely, lncRNA ROR can inhibit apoptosis in liver cancer cells by suppressing the p-53 response to ROS exposure [80]. LncRNA NORAD increases the expression of autophagy-related genes ATG-5 and ATG-12, which reduces oxidative stress and DNA damage in gastric cancer cells [81,137]. In breast cancer cells, the lncRNA linc00963 sponges miR324-3p, an inhibitor of ACK1, a non-receptor-tyrosine-kinase that drives tumor progression [82]. In nasopharyngeal carcinoma, miR324-3p has also been shown to inhibit WNT2B, part of the WNT family of protein ligands, which are important in cancer cells’ stemness [138,139]. As such, linc00963 allows the cell to mitigate the DNA damage induced by ROS and avoid apoptosis.
5.3. The Road Forward. Possible Therapeutic Strategies
Given how quintessential lncRNAs are proving to be for oxidative stress regulation and how important ROS are to sustain cancer cells’ hallmarks, it follows that therapies targeting these lncRNAs are increasingly studied.
ROS has long been a target of the arsenal used by medical practitioners against cancer. Drugs such as cisplatin, anthracyclines and cyclophosphamide [140] all attain their chemotherapeutic effect, at least partly, by dysregulating the redox balance in cancer cells and leading the cell to oxidative stress-triggered apoptosis. For example, cisplatin is known to increase the intracellular level of ROS through disruption of mitochondrial membrane potential [141,142,143], while anthracyclines appear to cause direct •O2− formation trough the metabolism of the molecule due to its quinone and hydroquinone groups [144,145]. Moreover, it is indicated that both drugs could also cause depletion of cells’ glutathione reserves, thus preventing cancer cells’ antioxidant machinery from mitigating the increase in ROS [146,147]. Even external therapies such as radiotherapy also exert a cytotoxic effect through the ROS generated by the radiolysis of extracellular water [148,149]. However, one of the major disadvantages of these therapies is the toxicity exerted by the same mechanisms on healthy cells. For example, both the neurotoxicity [150,151,152,153] and nephrotoxicity [154,155] induced by cisplatin have been demonstrated to be correlated with the level of generated ROS.
In the search for novel therapeutic targets, lncRNAs have emerged as valid contenders due to their cancer cell line specificity and low level of expression in other cells. A perfect lncRNA target from a therapeutic standpoint would check 4 essential boxes: predominant expression of a single isoform (insignificant alternative splicing), cancer cell restricted expression, specific function, and having a highly conserved structure throughout species to allow for extrapolation from in vivo studies [156,157]. Although no single lncRNA satisfies all the above conditions, several intriguing targets have been identified. Multiple methods for such targeting have been proposed. Firstly, silencing lncRNA gene transcription using systems, such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 or zinc finger nucleases (ZFNs), has been successfully utilized in vitro, resulting in suppression of the targeted lncRNA effect [158,159]. Nonetheless, given that lncRNAs can share promoters with other coding or non-coding genes, using the CRISPR/Cas9 system for targeting lncRNAs comes with the risk of inadvertently deregulating neighboring genes [160]. Secondly, disrupting the interaction between a lncRNA and its specific target would, in theory, alter the transcription of the genes normally regulated by the interaction. For example, using a newly designed molecule named AC1N0D4Q, Ren et al. managed to block the interaction between lncRNA HOTAIR and the EZH2 catalytic subunit of the PCR2 complex, which resulted in in a reduction of breast cancer cell migration and invasion in both in vitro and animal models [161]. However, controversy still exists over the specificity of some of these lncRNA-gene regulating complex interactions, given that they function based on scaffolding rather than complementarity, as RNA-DNA interactions do [162,163,164,165]. Thirdly, reducing the level of expression of lncRNAs by degradation of the non-coding transcripts per se is another approach that has proved efficient in several studies. This can be achieved by siRNA or antisense oligonucleotides (ASO). Indeed, administration of MALAT1 targeting ASOs reduced metastasis in both human lung cancer xenograft models [60] and mouse mammary tumor models [166], while siRNA knockdown of MALAT1 reduced cell motility in vitro models [167]. Moreover, clinical trials using these methods have also rendered positive results. The nanocomplex delivery of MALAT1 targeting siRNA in glioblastoma patients increased the sensitivity of the tumor to temozolomide (TMZ) treatment [168], while a novel ASO drug against non-coding mitochondrial RNA demonstrated good tolerance in a phase one clinical study [169,170].
6. Conclusions and Further Perspectives
The dysregulation of oxidative stress is an essential aspect that allows cancer cells to take an advantage over neighboring cells. However, ROS is a double-edged sword, as excess oxidative stress induces growth inhibition and cell death. Conversely, cancer cells have developed elaborate mechanisms to calibrate the level of oxidative stress. LncRNAs are indispensable in this process, accommodating the increased level of ROS through multiple mechanisms. Several lncRNAs regulate the ROS homeostasis at the mitochondrial level, influencing the level of ROS generated in the ETC. Furthermore, by inducing Nrf2 gene expression, acting as ceRNA for Nrf2 mRNA targeting miRNA, preventing Nrf2 protein cytoplasmatic and nuclear inactivation and acting as effectors in the Nrf2-induced antioxidant response, lncRNAs sustain the activity of the main transcription factor of antioxidant response. LncRNAs also sustain the Warburg effect in cancer cells by promoting PKM2 isoform expression, another effective mechanism that perpetuates the dysregulated oxidative balance. Moreover, lncRNAs can act in synergy with ROS in orientating the cancer cell toward proliferation or apoptosis by modulating the expression of numerous important genes in the ROS pathways (e.g., TP53, HIF-1-α).
The specificity of an unstable redox balance and patterns of lncRNA expression to cancer cells offer a great therapeutic opportunity in the fight against cancer. However, although some clinical trials targeting lncRNAs have shown encouraging results, the majority of the research is still in the preclinical phase and further investigation and development through in vitro studies and animal models is required before lncRNA-targeted medicine can become a mainstream approach.
Acknowledgments
1. H2020-MSCA-RISE-2018 “Excellence in research and development of non-coding RNA DIAGnostics in Oncology” RNADIAGON. 2. Clinical and economical impact of personalized targeted anti-microRNA therapies in reconverting lung cancer chemoresistance—CANTEMIR grant no. 35/01.09.2016. 3. Advanced preclinical validation of microRNA-205-5p-based therapeutic model for inhibition of epithelial to mesenchymal transition in melanoma metastasis MELAMET, 539PED (PN-III-P2-2.1-PED-2019-3640).
Author Contributions
Conceptualization: T.P.K. and I.B.-N.; investigation: T.P.K., R.Z., A.T. and E.V.P.; writing—original draft preparation: T.P.K., R.Z., A.T. and E.V.P.; writing—review and editing: I.B.-N. and T.P.K.; visualization: T.P.K. and A.N.; supervision: I.B.-N.; project administration: T.P.K. and I.B.-N.; funding acquisition: I.B.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7/ATTACHMENT/0AAA0B77-F3E8-452C-A5C6-4DEBEAEAE82D/MMC2.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Cancer over Time. [(accessed on 11 April 2022)]. Available online: https://gco.iarc.fr/overtime/en/dataviz/cohorts?populations=84000&sexes=2&cancers=0&multiple_populations=0&key=total&age_end=16&years=2017&cohort=cohort&cohort_type=time.
- 4.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A., Siegel R., Xu J., Ward E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Hayyan M., Hashim M.A., Alnashef I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 7.Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imlay J.A., Chin S.M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 9.D’Autréaux B., Toledano M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira V., Park Y., Chen C.C., Xu P.Z., Chen M.L., Tonic I., Unterman T., Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458. doi: 10.1016/J.CCR.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103. doi: 10.1038/CR.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B., Chen Y., St. Clair D.K. ROS and p53: Versatile partnership. Free Radic. Biol. Med. 2008;44:1529. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Murugesan P., Huang K., Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020;17:170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P., Li T., Wu X., Nice E.C., Huang C., Zhang Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 15.Hamarsheh S., Osswald L., Saller B.S., Unger S., De Feo D., Vinnakota J.M., Konantz M., Uhl F.M., Becker H., Lübbert M., et al. Oncogenic Kras G12D causes myeloproliferation via NLRP3 inflammasome activation. Nat. Commun. 2020;11:1659. doi: 10.1038/s41467-020-15497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanicka J., Russell E.G., Woolley J.F., Cotter T.G. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J. Biol. Chem. 2015;290:9348–9361. doi: 10.1074/jbc.M113.510495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szatrowski T.P., Nathan C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 18.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X., Jia Y., Dong X., Shen J., Jin Y., Li Y., Wang F., Anenberg E., Zhou J., Zhu J., et al. Diplatin, a Novel and Low-Toxicity Anti-Lung Cancer Platinum Complex, Activation of Cell Death in Tumors via a ROS/JNK/p53-Dependent Pathway, and a Low Rate of Acquired Treatment Resistance. Front. Pharmacol. 2019;10:982. doi: 10.3389/fphar.2019.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 21.Huang C., Yang M., Deng J., Li P., Su W., Jiang R. Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol. Rep. 2018;40:2363–2370. doi: 10.3892/or.2018.6585. [DOI] [PubMed] [Google Scholar]
- 22.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29:323–333. doi: 10.1016/S0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 23.Denicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander C.S., Hamm F., Elsner P., Thiele J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003;148:913–922. doi: 10.1046/j.1365-2133.2003.05303.x. [DOI] [PubMed] [Google Scholar]
- 25.Bisevac J.P., Djukic M., Stanojevic I., Stevanovic I., Mijuskovic Z., Djuric A., Gobeljic B., Banovic T., Vojvodic D. Association Between Oxidative Stress and Melanoma Progression. J. Med. Biochem. 2018;37:12. doi: 10.1515/jomb-2017-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison C.A., Durbin S.M., Thau M.R., Zellmer V.R., Chapman S.E., Diener J., Wathen C., Leevy W.M., Schafer Z.T. Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res. 2013;73:3704–3715. doi: 10.1158/0008-5472.CAN-12-2482. [DOI] [PubMed] [Google Scholar]
- 27.Uszczynska-Ratajczak B., Lagarde J., Frankish A., Guigó R., Johnson R. Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 2018;19:535–548. doi: 10.1038/s41576-018-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X., Wang W., Zhu W., Dong J., Cheng Y., Yin Z., Shen F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019;20:5573. doi: 10.3390/ijms20225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K.C., Chang H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp F., Mendell J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 32.Yousef M.H., El-Fawal H.A.N., Abdelnaser A. Hepigenetics: A Review of Epigenetic Modulators and Potential Therapies in Hepatocellular Carcinoma. Biomed Res. Int. 2020;2020:9593254. doi: 10.1155/2020/9593254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding K., Liao Y., Gong D., Zhao X., Ji W. Effect of long non-coding RNA H19 on oxidative stress and chemotherapy resistance of CD133+ cancer stem cells via the MAPK/ERK signaling pathway in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018;502:194–201. doi: 10.1016/j.bbrc.2018.05.143. [DOI] [PubMed] [Google Scholar]
- 34.Yang T., He X., Chen A., Tan K., Du X. LncRNA HOTAIR contributes to the malignancy of hepatocellular carcinoma by enhancing epithelial-mesenchymal transition via sponging miR-23b-3p from ZEB1. Gene. 2018;670:114–122. doi: 10.1016/j.gene.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 35.Ginn L., Shi L., La Montagna M., Garofalo M. LncRNAs in non-small-cell lung cancer. Non-Coding RNA. 2020;6:25. doi: 10.3390/ncrna6030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Zitello E., Guo R., Deng Y. The function of LncRNAs and their role in the prediction, diagnosis, and prognosis of lung cancer. Clin. Transl. Med. 2021;11:e367. doi: 10.1002/ctm2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L., Wang Y., Song G., Zhang X., Gao S., Liu H. HOX cluster-embedded antisense long non-coding RNAs in lung cancer. Cancer Lett. 2019;450:14–21. doi: 10.1016/j.canlet.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 38.Esfandi F., Taheri M., Omrani M.D., Shadmehr M.B., Arsang-Jang S., Shams R., Ghafouri-Fard S. Expression of long non-coding RNAs (lncRNAs) has been dysregulated in non-small cell lung cancer tissues. BMC Cancer. 2019;19:222. doi: 10.1186/s12885-019-5435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takayama K.I., Fujimura T., Suzuki Y., Inoue S. Identification of long non-coding RNAs in advanced prostate cancer associated with androgen receptor splicing factors. Commun. Biol. 2020;3:393. doi: 10.1038/s42003-020-01120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen S., Wei Y., Zen C., Xiong W., Niu Y., Zhao Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol. Cancer. 2020;19:171. doi: 10.1186/s12943-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jianfeng W., Yutao W., Jianbin B. Long non-coding RNAs correlate with genomic stability in prostate cancer: A clinical outcome and survival analysis. Genomics. 2021;113:3141–3151. doi: 10.1016/j.ygeno.2021.06.029. [DOI] [PubMed] [Google Scholar]
- 42.Song Y., Wang R., Li L.W., Liu X., Wang Y.F., Wang Q.X., Zhang Q. Long non-coding RNA HOTAIR mediates the switching of histone H3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer. Int. J. Oncol. 2019;54:77–86. doi: 10.3892/ijo.2018.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi-Dong X., Yang X., Lu J.L., Liu C.Q., Sun J.X., Li C., Wang S.G. Development and Validation of a Nine-Redox-Related Long Noncoding RNA Signature in Renal Clear Cell Carcinoma. Oxid. Med. Cell. Longev. 2020;2020:6634247. doi: 10.1155/2020/6634247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi Y., Wang D., Wang J., Yu W., Yang J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells. 2019;8:1015. doi: 10.3390/cells8091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative Stress in Cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Chen D., Piao H.L., Liu H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmadov U., Picard D., Bartl J., Silginer M., Trajkovic-Arsic M., Qin N., Blümel L., Wolter M., Lim J.K.M., Pauck D., et al. The long non-coding RNA HOTAIRM1 promotes tumor aggressiveness and radiotherapy resistance in glioblastoma. Cell Death Dis. 2021;12:885. doi: 10.1038/s41419-021-04146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng P., Xiong Q., Wu Y., Chen Y., Chen Z., Fleming J., Gao D., Bi L., Ge F. Quantitative Proteomics Analysis Reveals Novel Insights into Mechanisms of Action of Long Noncoding RNA Hox Transcript Antisense Intergenic RNA (HOTAIR) in HeLa Cells. Mol. Cell. Proteomics. 2015;14:1447. doi: 10.1074/mcp.M114.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loewen G., Jayawickramarajah J., Zhuo Y., Shan B. Functions of lncRNA HOTAIR in lung cancer. J. Hematol. Oncol. 2014;7:1–10. doi: 10.1186/s13045-014-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orre C., Dieu X., Guillon J., Gueguen N., Ahmadpour S.T., Dumas J.F., Khiati S., Reynier P., Lenaers G., Coqueret O., et al. The long non-coding RNA SAMMSON is a regulator of chemosensitivity and metabolic orientation in MCF-7 doxorubicin-resistant breast cancer cells. Biology. 2021;10:1156. doi: 10.3390/biology10111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z., Xiong R., Li C., Xu M., Guo M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim. Biophys. Sin. 2019;51:826–833. doi: 10.1093/abbs/gmz069. [DOI] [PubMed] [Google Scholar]
- 54.Sun Z., Huang G., Cheng H. Transcription factor Nrf2 induces the up-regulation of lncRNA TUG1 to promote progression and adriamycin resistance in urothelial carcinoma of the bladder. Cancer Manag. Res. 2019;11:6079. doi: 10.2147/CMAR.S200998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashrafizaveh S., Ashrafizadeh M., Zarrabi A., Husmandi K., Zabolian A., Shahinozzaman M., Aref A.R., Hamblin M.R., Nabavi N., Crea F., et al. Long non-coding RNAs in the doxorubicin resistance of cancer cells. Cancer Lett. 2021;508:104–114. doi: 10.1016/j.canlet.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q., Li K., Huang X., Zhao C., Mei Y., Li X., Jiao L., Yang H. lncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCFβ-TRCP-Mediated Degradation of NRF2 in Pancreatic Cancer. Mol. Ther. Nucleic Acids. 2020;19:974–985. doi: 10.1016/j.omtn.2019.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo P., Wu S., Ji K., Yuan X., Li H., Chen J., Tian Y., Qiu Y., Zhong X. LncRNA MIR4435-2HG mediates cisplatin resistance in HCT116 cells by regulating Nrf2 and HO-1. PLoS ONE. 2020;15:e0223035. doi: 10.1371/JOURNAL.PONE.0223035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L., Cai W., Lei X., Shi K., Lin X., Shi L. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J. Cell Commun. Signal. 2019;13:99. doi: 10.1007/s12079-018-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu L., Pan C., Wei X., Shi Y., Zheng J., Lin X., Shi L. lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun. Signal. 2018;16:1–15. doi: 10.1186/s12964-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutschner T., Hämmerle M., Eißmann M., Hsu J., Kim Y., Hung G., Revenko A., Arun G., Stentrup M., Groß M., et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Katsaros D., Biglia N., Shen Y., Fu Y., Loo L.W.M., Jia W., Obata Y., Yu H. High expression of long non-coding RNA MALAT1 in breast cancer is associated with poor relapse-free survival. Breast Cancer Res. Treat. 2018;171:261. doi: 10.1007/s10549-018-4839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathi V., Shen Z., Chakraborty A., Giri S., Freier S.M., Wu X., Zhang Y., Gorospe M., Prasanth S.G., Lal A., et al. Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J., Piao H.L., Kim B.J., Yao F., Han Z., Wang Y., Xiao Z., Siverly A.N., Lawhon S.E., Ton B.N., et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018;50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwok Z.H., Roche V., Chew X.H., Fadieieva A., Tay Y. A non-canonical tumor suppressive role for the long non-coding RNA MALAT1 in colon and breast cancers. Int. J. Cancer. 2018;143:668–678. doi: 10.1002/ijc.31386. [DOI] [PubMed] [Google Scholar]
- 65.Eastlack S.C., Dong S., Mo Y.Y., Alahari S.K. Expression of long noncoding RNA MALAT1 correlates with increased levels of Nischarin and inhibits oncogenic cell functions in breast cancer. PLoS ONE. 2018;13:e0198945. doi: 10.1371/journal.pone.0198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz C.R.V., Ferrer J.L.M., Garcia R.L. Concomitant and decoupled effects of cigarette smoke and SCAL1 upregulation on oncogenic phenotypes and ROS detoxification in lung adenocarcinoma cells. Sci. Rep. 2021;11:18345. doi: 10.1038/s41598-021-97869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thai P., Statt S., Chen C.H., Liang E., Campbell C., Wu R. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am. J. Respir. Cell Mol. Biol. 2013;49:204–211. doi: 10.1165/rcmb.2013-0159RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C.-L., Zhu K.-P., Shen G.-Q., Zhu Z.-S. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumor Biol. 2016;37:2737–2748. doi: 10.1007/s13277-015-4130-7. [DOI] [PubMed] [Google Scholar]
- 69.Gao M., Zhao B., Chen M., Liu Y., Xu M., Wang Z., Liu S., Zhang C. Nrf-2-driven long noncoding RNA ODRUL contributes to modulating silver nanoparticle-induced effects on erythroid cells. Biomaterials. 2017;130:14–27. doi: 10.1016/j.biomaterials.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 70.Liang Y., Zhang D., Zheng T., Yang G., Wang J., Meng F., Liu Y., Zhang G., Zhang L., Han J., et al. lncRNA-SOX2OT promotes hepatocellular carcinoma invasion and metastasis through miR-122-5p-mediated activation of PKM2. Oncogenesis. 2020;9:54. doi: 10.1038/s41389-020-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C., Li Y., Yan S., Wang H., Shao X., Xiao M., Yang B., Qin G., Kong R., Chen R., et al. Interactome analysis reveals that lncRNA HULC promotes aerobic glycolysis through LDHA and PKM2. Nat. Commun. 2020;11:3162. doi: 10.1038/s41467-020-16966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xin X., Wu M., Meng Q., Wang C., Lu Y., Yang Y., Li X., Zheng Q., Pu H., Gui X., et al. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol. Cancer. 2018;17:94. doi: 10.1186/s12943-018-0843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leon L.M., Gautier M., Allan R., Ilié M., Nottet N., Pons N., Paquet A., Lebrigand K., Truchi M., Fassy J., et al. The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress. Oncogene. 2019;38:7146–7165. doi: 10.1038/s41388-019-0935-y. [DOI] [PubMed] [Google Scholar]
- 74.Wang X., Zhang G., Cheng Z., Dai L., Jia L., Jing X., Wang H., Zhang R., Liu M., Jiang T., et al. Knockdown of LncRNA-XIST Suppresses Proliferation and TGF-β1-Induced EMT in NSCLC Through the Notch-1 Pathway by Regulation of miR-137. Genet. Test. Mol. Biomark. 2018;22:333–342. doi: 10.1089/gtmb.2018.0026. [DOI] [PubMed] [Google Scholar]
- 75.Zhou K., Yang J., Li X., Chen W. Long non-coding RNA XIST promotes cell proliferation and migration through targeting miR-133a in bladder cancer. Exp. Ther. Med. 2019;18:3475. doi: 10.3892/etm.2019.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Yao L., Zhang M., Jiang J., Yang M., Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging. 2019;11:7830–7846. doi: 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen J.F., Jiang Y.Q., Li C., Dai X.K., Wu T., Yin W.Z. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol. Int. 2020;44:1991–2001. doi: 10.1002/cbin.11405. [DOI] [PubMed] [Google Scholar]
- 78.Chen S., Liang H., Yang H., Zhou K., Xu L., Liu J., Lai B., Song L., Luo H., Peng J., et al. LincRNa-p21: Function and mechanism in cancer. Med. Oncol. 2017;34:98. doi: 10.1007/s12032-017-0959-5. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y., Luo M., Cui X., O’Connell D., Yang Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022;29:1850–1863. doi: 10.1038/s41418-022-00970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Sun D., Zhao T., Zhang Z. Long non-coding RNA ROR confers arsenic trioxide resistance to HepG2 cells by inhibiting p53 expression. Eur. J. Pharmacol. 2020;872:172982. doi: 10.1016/j.ejphar.2020.172982. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Sun Y., Zhang X., Cai H., Zhang C., Qu H., Liu L., Zhang M., Fu J., Zhang J., et al. Oxidative stress activates NORAD expression by H3K27ac and promotes oxaliplatin resistance in gastric cancer by enhancing autophagy flux via targeting the miR-433-3p. Cell Death Dis. 2021;12:90. doi: 10.1038/s41419-020-03368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang N., Zeng X., Sun C., Guo H., Wang T., Wei L., Zhang Y., Zhao J., Ma X. LncRNA LINC00963 Promotes Tumorigenesis and Radioresistance in Breast Cancer by Sponging miR-324-3p and Inducing ACK1 Expression. Mol. Ther. Nucleic Acids. 2019;18:871–881. doi: 10.1016/j.omtn.2019.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Mourtada-Maarabouni M., Pickard M.R., Hedge V.L., Farzaneh F., Williams G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 84.Pickard M.R., Mourtada-Maarabouni M., Williams G.T. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Sun M., Jin F., Xia R., Kong R., Li J., Xu T., Liu Y., Zhang E., Liu X., De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L., Yang H., Yi Z., Jiang L., Li Y., Han Q., Yang Y., Zhang Q., Yang Z., Kuang Y., et al. LncRNA GAS5 regulates redox balance and dysregulates the cell cycle and apoptosis in malignant melanoma cells. J. Cancer Res. Clin. Oncol. 2019;145:637. doi: 10.1007/s00432-018-2820-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu W., Yan Z., Hu F., Wei W., Yang C., Sun Z. Long non-coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2-mediated CDKN1C downregulation. Cancer Cell Int. 2020;20:116. doi: 10.1186/s12935-020-01167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie C., Wu W., Tang A., Luo N., Tan Y. IncRNA GAS5/miR-452-5p reduces oxidative stress and pyroptosis of high-glucose-stimulated renal tubular cells. Diabetes Metab. Syndr. Obes. Targets Ther. 2019;12:2609–2617. doi: 10.2147/DMSO.S228654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y., Lu X., Yang M., Shangguan J., Yin Y. GAS5 knockdown suppresses inflammation and oxidative stress induced by oxidized low-density lipoprotein in macrophages by sponging miR-135a. Mol. Cell. Biochem. 2021;476:949–957. doi: 10.1007/s11010-020-03962-w. [DOI] [PubMed] [Google Scholar]
- 90.Solaini G., Baracca A., Lenaz G., Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta Bioenerg. 2010;1797:1171–1177. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Moloney J.N., Cotter T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 93.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moloney J.N., Stanicka J., Cotter T.G. Subcellular localization of the FLT3-ITD oncogene plays a significant role in the production of NOX- and p22phox-derived reactive oxygen species in acute myeloid leukemia. Leuk. Res. 2017;52:34–42. doi: 10.1016/j.leukres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Kumari S., Badana A.K., G M.M., G S., Malla R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights. 2018;13:1177271918755391. doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guzy R.D., Schumacker P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 97.Waypa G.B., Schumacker P.T. Oxygen sensing in hypoxic pulmonary vasoconstriction: Using new tools to answer an age-old question. Exp. Physiol. 2008;93:133–138. doi: 10.1113/expphysiol.2007.041236. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman D.L., Salter J.D., Brookes P.S. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: Implications for hypoxic cell signaling. Am. J. Physiol. Hear. Circ. Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 99.JF T., A A., AL L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J., Zhang P., Wang L., Piao H.L., Ma L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim. Biophys. Sin. 2014;46:1–5. doi: 10.1093/abbs/gmt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wan Y., Chang H.Y. HOTAIR: Flight of noncoding RNAs in cancer metastasis. Cell Cycle. 2010;9:3391–3392. doi: 10.4161/cc.9.17.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Eletto M., Rossin F., Occhigrossi L., Farrace M.G., Faccenda D., Desai R., Marchi S., Refolo G., Falasca L., Antonioli M., et al. Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 2018;25:3573–3581.e4. doi: 10.1016/j.celrep.2018.11.094. [DOI] [PubMed] [Google Scholar]
- 103.Suski J.M., Lebiedzinska M., Bonora M., Pinton P., Duszynski J., Wieckowski M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012;810:183–205. doi: 10.1007/978-1-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- 104.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 105.Sgarbi G., Barbato S., Costanzini A., Solaini G., Baracca A. The role of the ATPase inhibitor factor 1 (IF1) in cancer cells adaptation to hypoxia and anoxia. Biochim. Biophys. Acta Bioenerg. 2018;1859:99–109. doi: 10.1016/j.bbabio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 106.Faccenda D., Nakamura J., Gorini G., Dhoot G.K., Piacentini M., Yoshida M., Campanella M. Control of Mitochondrial Remodeling by the ATPase Inhibitory Factor 1 Unveils a Pro-survival Relay via OPA1. Cell Rep. 2017;18:1869–1883. doi: 10.1016/j.celrep.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 107.Santacatterina F., Sánchez-Cenizo L., Formentini L., Mobasher M.A., Casas E., Rueda C.B., Martínez-Reyes I., de Arenas C.N., García-Bermúdez J., Zapata J.M., et al. Down-regulation of oxidative phosphorylation in the liver by expression of the ATPase inhibitory factor 1 induces a tumor-promoter metabolic state. Oncotarget. 2015;7:490–508. doi: 10.18632/oncotarget.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boveris A., Cadenas E., Stoppani A.O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hammad A., Namani A., Elshaer M., Wang X.J., Tang X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019;467:40–49. doi: 10.1016/j.canlet.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 110.Zhu J., Wang H., Chen F., Fu J., Xu Y., Hou Y., Kou H.H., Zhai C., Nelson M.B., Zhang Q., et al. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free Radic. Biol. Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 111.Bhattacharjee S., Li J., Dashwood R.H. Emerging crosstalk between long non-coding RNAs and Nrf2 signaling. Cancer Lett. 2020;490:154–164. doi: 10.1016/j.canlet.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 112.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji P., Diederichs S., Wang W., Böing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E., et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 114.Zeng R., Zhang R., Song X., Ni L., Lai Z., Liu C., Ye W. The long non-coding RNA MALAT1 activates Nrf2 signaling to protect human umbilical vein endothelial cells from hydrogen peroxide. Biochem. Biophys. Res. Commun. 2018;495:2532–2538. doi: 10.1016/j.bbrc.2017.12.105. [DOI] [PubMed] [Google Scholar]
- 115.Ward P.S., Thompson C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 2012;21:297. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christofk H.R., Vander Heiden M.G., Wu N., Asara J.M., Cantley L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 117.Hitosugi T., Kang S., Vander Heiden M.G., Chung T.W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G.Z., et al. Tyrosine phosphorylation inhibits PKM2 to promote the warburg effect and tumor growth. Sci. Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirsch M., Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 120.Halliwell B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning) Free Radic. Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H., Liao Z., Liu F., Su C., Zhu H., Li Y., Tao R., Liang H., Zhang B., Zhang X. Long noncoding RNA HULC promotes hepatocellular carcinoma progression. Aging. 2019;11:9111. doi: 10.18632/aging.102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dombrauckas J.D., Santarsiero B.D., Mesecar A.D. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 123.Ikeda S., Kitadate A., Abe F., Takahashi N., Tagawa H. Hypoxia-inducible KDM3A addiction in multiple myeloma. Blood Adv. 2018;2:323–334. doi: 10.1182/bloodadvances.2017008847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Callapina M., Zhou J., Schmid T., Köhl R., Brüne B. NO restores HIF-1α hydroxylation during hypoxia: Role of reactive oxygen species. Free Radic. Biol. Med. 2005;39:925–936. doi: 10.1016/j.freeradbiomed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 125.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 2009;21:894. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pan Y., Mansfield K.D., Bertozzi C.C., Rudenko V., Chan D.A., Giaccia A.J., Simon M.C. Multiple Factors Affecting Cellular Redox Status and Energy Metabolism Modulate Hypoxia-Inducible Factor Prolyl Hydroxylase Activity In Vivo and In Vitro. Mol. Cell. Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jadaliha M., Zong X., Malakar P., Ray T., Singh D.K., Freier S.M., Jensen T., Prasanth S.G., Karni R., Ray P.S., et al. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget. 2016;7:40418–40436. doi: 10.18632/oncotarget.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou C., Ye L., Jiang C., Bai J., Chi Y., Zhang H. Long noncoding RNA HOTAIR, a hypoxia-inducible factor-1α activated driver of malignancy, enhances hypoxic cancer cell proliferation, migration, and invasion in non-small cell lung cancer. Tumor Biol. 2015;36:9179–9188. doi: 10.1007/s13277-015-3453-8. [DOI] [PubMed] [Google Scholar]
- 129.Li X., Deng S.J., Zhu S., Jin Y., Cui S.P., Chen J.Y., Xiang C., Li Q.Y., He C., Zhao S.F., et al. Hypoxia-induced lncRNA-NUTF2P3-001 contributes to tumorigenesis of pancreatic cancer by derepressing the miR-3923/KRAS pathway. Oncotarget. 2016;7:6000–6014. doi: 10.18632/oncotarget.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xue M., Li X., Li Z., Chen W. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumor Biol. 2014;35:6901–6912. doi: 10.1007/s13277-014-1925-x. [DOI] [PubMed] [Google Scholar]
- 131.Deng S.J., Chen H.Y., Ye Z., Deng S.C., Zhu S., Zeng Z., He C., Liu M.L., Huang K., Zhong J.X., et al. Hypoxia-induced LncRNA-bx111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene. 2018;37:5811–5828. doi: 10.1038/s41388-018-0382-1. [DOI] [PubMed] [Google Scholar]
- 132.Cesi G., Walbrecq G., Zimmer A., Kreis S., Haan C. ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol. Cancer. 2017;16:102. doi: 10.1186/s12943-017-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu-Smith F., Dellinger R., Meyskens F.L. Updates of reactive oxygen species in melanoma etiology and progression. Arch. Biochem. Biophys. 2014;563:51–55. doi: 10.1016/j.abb.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamaura M., Mitsushita J., Furuta S., Kiniwa Y., Ashida A., Goto Y., Shang W.H., Kubodera M., Kato M., Takata M., et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 135.Wittgen H.G.M., Van Kempen L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007;17:400–409. doi: 10.1097/CMR.0b013e3282f1d312. [DOI] [PubMed] [Google Scholar]
- 136.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., Khalil A.M., Zuk O., Amit I., Rabani M., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu Y.-Z., Su Y.-H., Kuo C.-Y. Stressing the Regulatory Role of Long Non-Coding RNA in the Cellular Stress Response during Cancer Progression and Therapy. Biomedicines. 2022;10:1212. doi: 10.3390/biomedicines10051212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu C., Li G., Yang N., Su Z., Zhang S., Deng T., Ren S., Lu S., Tian Y., Liu Y., et al. miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma. Cancer Cell Int. 2017;17:1–7. doi: 10.1186/s12935-016-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sahu K., Langeh U., Singh C., Singh A. Crosstalk between anticancer drugs and mitochondrial functions. Curr. Res. Pharmacol. Drug Discov. 2021;2:100047. doi: 10.1016/j.crphar.2021.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dasari S., Bernard Tchounwou P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mirzaei S., Hushmandi K., Zabolian A., Saleki H., Torabi S.M.R., Ranjbar A., Seyedsaleh S., Sharifzadeh S.O., Khan H., Ashrafizadeh M., et al. Elucidating Role of Reactive Oxygen Species (ROS) in Cisplatin Chemotherapy: A Focus on Molecular Pathways and Possible Therapeutic Strategies. Molecules. 2021;26:2382. doi: 10.3390/molecules26082382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi Y.M., Kim H.K., Shim W., Anwar M.A., Kwon J.W., Kwon H.K., Kim H.J., Jeong H., Kim H.M., Hwang D., et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE. 2015;10:e0135083. doi: 10.1371/JOURNAL.PONE.0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shandilya M., Sharma S., Das P.P., Charak S. Advances in Precision Medicine Oncology. IntechOpen; London, UK: 2020. Molecular-Level Understanding of the Anticancer Action Mechanism of Anthracyclines. [DOI] [Google Scholar]
- 145.Beretta G.L., Zunino F. Molecular mechanisms of anthracycline activity. Top. Curr. Chem. 2008;283:1–19. doi: 10.1007/128_2007_3. [DOI] [PubMed] [Google Scholar]
- 146.Siddik Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 147.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 148.Ozben T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 149.Kim W., Lee S., Seo D., Kim D., Kim K., Kim E., Kang J., Seong K.M., Youn H., Youn B. Cellular stress responses in radiotherapy. Cells. 2019;8:1105. doi: 10.3390/cells8091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Look M.P., Musch E. Lipid Peroxides in the Polychemotherapy of Cancer Patients. Chemotherapy. 1994;40:8–15. doi: 10.1159/000239163. [DOI] [PubMed] [Google Scholar]
- 151.McDonald E.S., Windebank A.J. Cisplatin-Induced Apoptosis of DRG Neurons Involves Bax Redistribution and Cytochrome cRelease But Not fas Receptor Signaling. Neurobiol. Dis. 2002;9:220–233. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 152.Cashman C.R., Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Areti A., Yerra V.G., Naidu V.G.M., Kumar A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aydinoz S., Uzun G., Cermik H., Atasoyu E.M., Yildiz S., Karagoz B., Evrenkaya R. Effects of different doses of hyperbaric oxygen on cisplatin-induced nephrotoxicity. Ren. Fail. 2007;29:257–263. doi: 10.1080/08860220601166487. [DOI] [PubMed] [Google Scholar]
- 155.Baliga R., Zhang Z., Baliga M., Ueda N., Shah S.V. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;54:1562–1569. doi: 10.1046/j.1523-1755.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 156.Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y., Barrette T.R., Prensner J.R., Evans J.R., Zhao S., et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gutschner T., Richtig G., Haemmerle M., Pichler M. From biomarkers to therapeutic targets-the promises and perils of long non-coding RNAs in cancer. Cancer Metastasis Rev. 2018;37:83–105. doi: 10.1007/s10555-017-9718-5. [DOI] [PubMed] [Google Scholar]
- 158.Thakore P.I., D’Ippolito A.M., Song L., Safi A., Shivakumar N.K., Kabadi A.M., Reddy T.E., Crawford G.E., Gersbach C.A. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods. 2015;12:1143–1149. doi: 10.1038/nmeth.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gutschner T., Baas M., Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goyal A., Myacheva K., Groß M., Klingenberg M., Duran Arqué B., Diederichs S. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12. doi: 10.1093/nar/gkw883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren Y., Wang Y.F., Zhang J., Wang Q.X., Han L., Mei M., Kang C.S. Targeted design and identification of AC1NOD4Q to block activity of HOTAIR by abrogating the scaffold interaction with EZH2. Clin. Epigenetics. 2019;11:29. doi: 10.1186/s13148-019-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blanco M.R., Guttman M. Re-evaluating the foundations of lncRNA-Polycomb function. EMBO J. 2017;36:964–966. doi: 10.15252/embj.201796796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Portoso M., Ragazzini R., Brenčič Ž., Moiani A., Michaud A., Vassilev I., Wassef M., Servant N., Sargueil B., Margueron R. PRC2 is dispensable for HOTAIR-mediated transcriptional repression. EMBO J. 2017;36:981. doi: 10.15252/embj.201695335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science. 2010;329:689. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Somarowthu S., Legiewicz M., Chillón I., Marcia M., Liu F., Pyle A.M. HOTAIR forms an intricate and modular secondary structure. Mol. Cell. 2015;58:353. doi: 10.1016/j.molcel.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S., Kim Y., MacLeod A.R., Krainer A.R., Norton L., et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tano K., Mizuno R., Okada T., Rakwal R., Shibato J., Masuo Y., Ijiri K., Akimitsu N. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 168.Kim S.S., Harford J.B., Moghe M., Rait A., Pirollo K.F., Chang E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018;46:1424–1440. doi: 10.1093/nar/gkx1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dhawan M.S., Aggarwal R.R., Boyd E., Comerford K., Zhang J., Méndez B., Valenzuela P., Grabowsky J., Thomas S., Munster P.N. Phase 1 study of ANDES-1537: A novel antisense oligonucleotide against non-coding mitochondrial DNA in advanced solid tumors. J. Clin. Oncol. 2018;36:2557. doi: 10.1200/JCO.2018.36.15_suppl.2557. [DOI] [Google Scholar]
- 170.Zuo J., Zhang Z., Li M., Yang Y., Zheng B., Wang P., Huang C., Zhou S. The crosstalk between reactive oxygen species and noncoding RNAs: From cancer code to drug role. Mol. Cancer. 2022;21:30. doi: 10.1186/s12943-021-01488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.