Abstract
Hemodialysis patients (HDPs) have higher blood pressure, higher levels of inflammation, a higher risk of cardiovascular disease, and unusually low plasma n-3 polyunsaturated fatty acid (PUFA) levels compared to healthy subjects. The objective of our investigation was to examine the levels of endocannabinoids (eCBs) and oxylipins (OxLs) in female HDPs compared to healthy matched female controls, with the underlying hypothesis that differences in specific PUFA levels in hemodialysis patients would result in changes in eCBs and OxLs. Plasma phospholipid fatty acids were analyzed by gas chromatography. Plasma was extracted and analyzed using ultra-performance liquid chromatography followed by electrospray ionization and tandem MS for eCBs and OxLs. The global untargeted metabolite profiling of plasma was performed by GCTOF MS. Compared to the controls, HDPs showed lower levels of plasma EPA and the associated OxL metabolites 5- and 12-HEPE, 14,15-DiHETE, as well as DHA derived 19(20)-EpDPE. Meanwhile, no changes in arachidonylethanolamide or 2-arachidonylglycerol in the open circulation were detected. Higher levels of multiple N-acylethanolamides, monoacylglycerols, biomarkers of progressive kidney disease, the nitric oxide metabolism-linked citrulline, and the uremic toxins kynurenine and creatine were observed in HDP. These metabolic differences in cCBs and OxLs help explain the severe inflammatory and cardiovascular disease manifested by HDPs, and they should be explored in future studies.
Keywords: hemodialysis patient, women, endocannabinoids, oxylipins, global metabolites, polyunsaturated fatty acids
1. Introduction
Hemodialysis patients (HDPs) suffer from malnutrition [1,2,3,4,5] and generalized systemic inflammation [4]. US HDPs have a blood distribution of long-chain n–3 PUFAs that is similar to that of the general population, but their n-3 PUFA blood content is among the lowest recorded in the medical literature [1]. Moreover, long-chain n-3 PUFAs are strongly and independently associated with a lower risk of sudden cardiac death in hemodialysis patients throughout the first year of hemodialysis [6]. What is less understood is if changes in endocannabinoids (eCBs) and oxylipins (OxLs) are contributing factors in, or a consequence of, kidney disease. The global analysis of blood metabolites can be useful to ascertain how malnutrition, the loss of kidney function, and inflammation influence macronutrient metabolism in hemodialysis patients [7].
The endocannabinoid system (ECS) functions with its bioactive lipid mediators and receptors to perform a host of behavioral and biochemical functions—for instance, modulating food intake [8], pain perception [9], and memory processes [10]. The ECS ligand arachidonoylethanolamine (anandamide, AEA), which is derived from arachidonic acid (AA), was the first endogenous ligand identified [11], followed by the identification of 2-arachidonoylglycerol (2-AG) [12,13]. Since then, numerous ECS ligands have been isolated and identified [14,15]. While the ECS is known to influence renal physiology [16,17,18], its role in HDPs has not been well described.
Similar to eCBs, OxLs are another group of endogenously produced lipid-derived biologically active signaling compounds that act locally with broad physiological and pathological effects on cellular function, acting through receptor- and ion channel-mediated processes, and that play both pro- and anti-inflammatory roles in a number of pathological processes [19,20,21]. The OxLs encompass a wide range of compounds with diverse chemical structures, such as prostaglandins, leukotrienes, di-and tri-hydroxy pro-resolving lipid mediators (e.g., lipoxins and resolvins), and various epoxides and their diol metabolites formed by the actions of cyclooxygenases, lipoxygenases, and cytochrome P450s and epoxide hydrolases, or through the interaction of PUFAs with reactive oxygen species [19]. Among the diverse functions of OxLs, many of these lipid mediators regulate smooth muscle tone, which affects small blood vessel dilation, influences inflammation, regulates blood pressure, and alters cardiovascular disease risk and kidney function [21,22,23].
The long-chain n-3 PUFA status and the effects of supplementing with fish oil have been extensively studied to show the link between dietary n-3 PUFA intake and their levels in circulation for subjects undergoing hemodialysis. Several investigators [6,24,25] have characterized the circulating levels of n-3 PUFA (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) in populations of hemodialysis patients and found that EPA and DHA are possible modifiable cardiovascular risk factors of sudden cardiac death. As precursor PUFAs for both eCBs and OxLs, changes in EPA, DHA, and the n-6 PUFA (arachidonic acid (AA)) status in a human would inevitably lead to changes in the types and levels of both eCBs and OxLs. Since the link between EPA/DHA and eCB/OxL has rarely been studied in hemodialysis patients, the present research endeavor was to explore the relationship between eCB and OxL levels, including those derived from n-3 PUFAs, in hemodialysis patients.
Previous studies have reported elevated levels of uremic toxins and markers of nitric oxide metabolism in subjects suffering from renal dysfunction that were being treated with hemodialysis [26,27,28]. Global metabolite analysis (metabolomics) provides a broad biochemical snapshot that can identify factors associated with the pathophysiology of the progression of disease and disease state [29]. Indeed, by applying the metabolomics in a cross-sectional study, Shah et al. [29] observed differences in the metabolites between the different stages of chronic kidney disease (CKD). Marked changes in arginine metabolism and elevated coagulation and inflammation were found as potential benchmarks of the CKD state. Although metabolomic analysis has been adopted in research on kidney disease patients [30,31,32], to the best of our knowledge, the metabolomics of HDPs at all stages have not been reported. Therefore, the current research is our attempt to fill the knowledge gap for this particular patient population and efficiently document co-occurring metabolic defects in HDPs. Moreover, this secondary effort allowed for the potential identification of novel biomarkers in HDPs and provided an opportunity to integrate any observed alterations in ECS and OxL metabolism to these changes.
The primary hypothesis explored in this preliminary study is that plasma PUFAs are associated with changes in the amounts of AA and long-chain n-3 PUFAs (EPA and DHA), which will reflect increases or decreases in the associated eCB and OxL levels in the blood. Moreover, we hypothesized that differences in the plasma global metabolite profiles between healthy controls and HDPs would highlight the metabolic and physiological consequences of kidney disease and allow a direct evaluation of links between these and the measured eCBs and OxLs. To test these hypotheses, chronic hemodialysis patients (nine female) and age-matched healthy controls (ten female) were recruited. Fatty acid species in the plasma and red blood cells (RBCs), along with an array of plasma eCB and OxL species derived from eighteen- to twenty-two-carbon PUFAs, were measured. In addition, plasma metabolomic analyses were performed. To our knowledge, this is the first study to examine the full array of eCBs and OxLs in hemodialysis patients.
2. Results
2.1. Fatty Acid Analysis of Plasma and RBCs
The plasma fatty acid and RBC fatty acid composition data are presented in Table 1. Between the healthy controls and the HDPs, the relative abundances of 15:0, 16:0, 20:3n-6, 20:5n-3, and 22:5n-3 were higher in the control group, while the relative levels of 18:0, 20:4n-6, and 22:1n-9 were higher in the female HDP group. The data for the FAME analysis of RBCs did show a lower 20:5n-3 in the HDP group compared to the control group (0.11 ± 0.17 HDPs and 0.49 ± 0.13 controls). The results of higher EPA levels in the plasma and RBCs for controls versus HDPs might have influenced the capacity for the biosynthesis of the related eCBs and OxLs derived from EPA [33], and possibly the downstream actions of these lipid mediators of pain and inflammation [34].
Table 1.
Plasma fatty acid percent composition of polar lipids and RBCs in female healthy control subjects and female hemodialysis patients.
| Fatty Acids | Plasma Polar Lipid Fatty Acids | RBC Fatty Acids | ||||
|---|---|---|---|---|---|---|
| Controls (n = 10) |
HDPs (n = 9) |
p-Values | Controls (n = 10) |
HDPs (n = 9) |
p-Values | |
| 14:0 | 1.23 ± 0.76 | 1.28 ± 0.63 | 0.9 | 0.17 ± 0.18 | 0.06 ± 0.13 | 0.14 |
| 15:0 | 0.09 ± 0.07 | ND | - | ND | ND | - |
| 16:0 | 24.15 ± 1.34 | 21.86 ± 1.44 | 0.0022 | 18.71 ± 1.17 | 18.37 ± 1.14 | 0.83 |
| t16:1n-7 | 0.15 ± 0.06 | 0.10 ± 0.09 | 0.2 | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.53 |
| 16:1n-7 | 0.67 ± 0.36 | 0.40 ± 0.26 | 0.08 | 0.03 ± 0.05 | ND | - |
| 17:0 | 0.34 ± 0.05 | 0.33 ± 0.04 | 0.6 | 0.32 ± 0.26 | 0.06 ± 0.12 | 0.014 |
| 18:0 | 18.59 ± 1.45 | 20.47 ± 1.61 | 0.016 | 12.95 ± 1.27 | 12.91 ± 0.88 | 0.50 |
| 18:1n-9 | 10.82 ± 1.56 | 10.95 ± 2.23 | 0.9 | 16.78 ± 1.79 | 15.69 ± 1.85 | 0.46 |
| 18:1n-7 | 1.42 ± 0.23 | 1.66 ± 0.28 | 0.06 | 1.32 ± 0.17 | 1.47 ± 0.19 | 0.05 |
| 18:2n-6 | 18.16 ± 3.29 | 18.40 ± 2.28 | 0.9 | 11.25 ± 1.48 | 11.31 ± 1.73 | 0.93 |
| 18:3n-6 | 0.15 ± 0.11 | 0.05 ± 0.08 | 0.042 | 0.03 ± 0.06 | ND | - |
| 18:3n-3 | 0.34 ± 0.12 | 0.35 ± 0.10 | 0.8 | 0.04 ± 0.07 | ND | - |
| 20:0 | 0.16 ± 0.02 | 0.18 ± 0.04 | 0.2 | 0.04 ± 0.06 | 0.06 ± 0.13 | 0.71 |
| 20:1n-9 | 0.15 ± 0.06 | 0.19 ± 0.03 | 0.05 | 0.09 ± 0.11 | 0.18 ± 0.28 | 0.36 |
| 20:2n-6 | 0.34 ± 0.07 | 0.32 ± 0.06 | 0.5 | 0.26 ± 0.10 | 0.19 ± 0.19 | 0.34 |
| 20:3n-6 | 3.14 ± 0.82 | 2.15 ± 0.81 | 0.017 | 1.74 ± 0.28 | 1.27 ± 0.45 | 0.013 |
| 20:4n-6 | 11.47 ± 1.71 | 13.61 ± 1.87 | 0.019 | 15.98 ± 1.40 | 15.71 ± 3.49 | 0.83 |
| 20:5n-3 | 0.71 ± 0.28 | 0.38 ± 0.09 | 0.005 | 0.49 ± 0.13 | 0.11 ± 0.17 | 0.001 |
| 22:0 | 0.08 ± 0.06 | 0.04 ± 0.08 | 0.2 | 0.33 ± 0.12 | 0.41 ± 0.33 | 0.47 |
| 22:1n-9 | 0.18 ± 0.10 | 0.31 ± 0.14 | 0.032 | ND | 0.03 ± 0.08 | - |
| 22:4n-6 | 0.55 ± 0.15 | 0.52 ± 0.06 | 0.5 | 3.73 ± 0.71 | 4.04 ± 0.95 | 0.44 |
| 22:5n-6 | 0.43 ± 0.21 | 0.35 ± 0.10 | 0.3 | 0.84 ± 0.23 | 0.68 ± 0.30 | 0.22 |
| 22:5n-3 | 0.88 ± 0.11 | 0.71 ± 0.08 | 0.0013 | 1.98 ± 0.28 | 1.77 ± 0.47 | 0.25 |
| 22:6n-3 | 3.11 ± 1.57 | 2.68 ± 0.78 | 0.5 | 4.06 ± 1.40 | 3.63 ± 1.45 | 0.52 |
| 24:0 | 0.06 ± 0.05 | 0.03 ± 0.07 | 0.4 | 0.95 ± 0.15 | 0.93 ± 0.73 | 0.94 |
| 24:1n-9 | 0.09 ± 0.08 | 0.13 ± 0.08 | 0.3 | 0.91 ± 0.14 | 1.10 ± 0.90 | 0.54 |
| Total SFA | 44.71 ± 1.33 | 44.19 ± 2.13 | 0.5 | 36.11 ± 0.86 | 36.83 ± 3.87 | 0.54 |
| Total MUFA | 13.33 ± 1.77 | 13.65 ± 2.52 | 0.8 | 15.76 ± 1.30 | 16.32 ± 2.05 | 0.38 |
| Total PUFA | 39.29 ± 1.83 | 39.51 ± 1.87 | 0.8 | 40.39 ± 1.45 | 38.90 ± 7.83 | 0.50 |
| Total n-6 PUFA | 34.25 ± 1.88 | 35.40 ± 1.74 | 0.2 | 33.82 ± 1.93 | 33.19 ± 5.43 | 0.75 |
| Total n-3 PUFA | 5.04 ± 1.66 | 4.11 ± 0.91 | 0.15 | 6.56 ± 1.29 | 5.51 ± 1.93 | 0.18 |
| LC n-6 PUFA | 15.94 ± 2.13 | 16.94 ± 1.58 | 0.26 | 22.5 ± 0.93 | 21.88 ± 4.34 | 0.68 |
| LC n-3 PUFA | 4.71 ± 1.67 | 3.77 ± 0.85 | 0.15 | 6.52 ± 1.30 | 5.51 ± 1.93 | 0.19 |
| LC n-6/n-3 Ratio | 3.72 ± 1.29 | 4.65 ± 0.86 | 0.087 | 3.61 ± 0.88 | 4.79 ± 2.73 | 0.24 |
The fatty acid data were measured as fatty acid methyl esters as area % means ± SD of plasma polar lipid fatty acids and RBCs. Authentic external standards were used for peak identification of chromatogram data during integration. The sensitivity of the GC detector is at 10 ng/peak. HDPs = hemodialysis patients; LC n-6 = 20:2n-6 + 20:3n-6 + 20:4n-6 + 22:4n-6 + 22:5n-6; LC n-3 = 20:5n-3 + 22:5n-3 + 22:6n-3. ND = not detected.
2.2. Impact of PMSF on Endocannabinoid (eCB) and Oxylipin (OxL) Data
The addition of PMSF to plasma had subtle impacts on the OxL and eCB results. However, in the presence of PMSF, eight compounds showed subtle decreases (p < 0.1) and a reduced variance when the analyses were adjusted for subject as a random effect. These included 2-AG, multiple prostaglandins, and the platelet degranulation-related TXB2, 12-HETE, and 15-HETE. Therefore, the subsequent analyses were conducted using only the PMSF-treated samples.
2.3. Endocannabinoids and Oxylipins
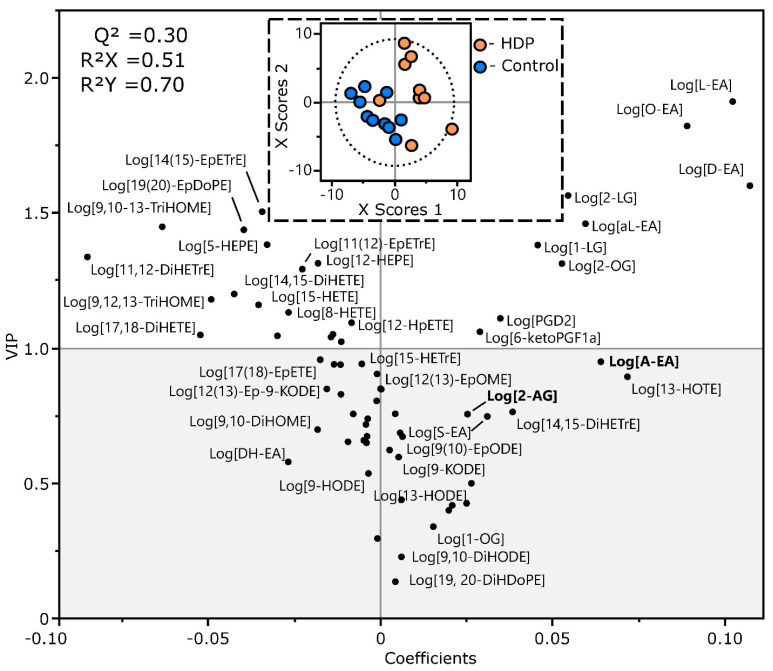
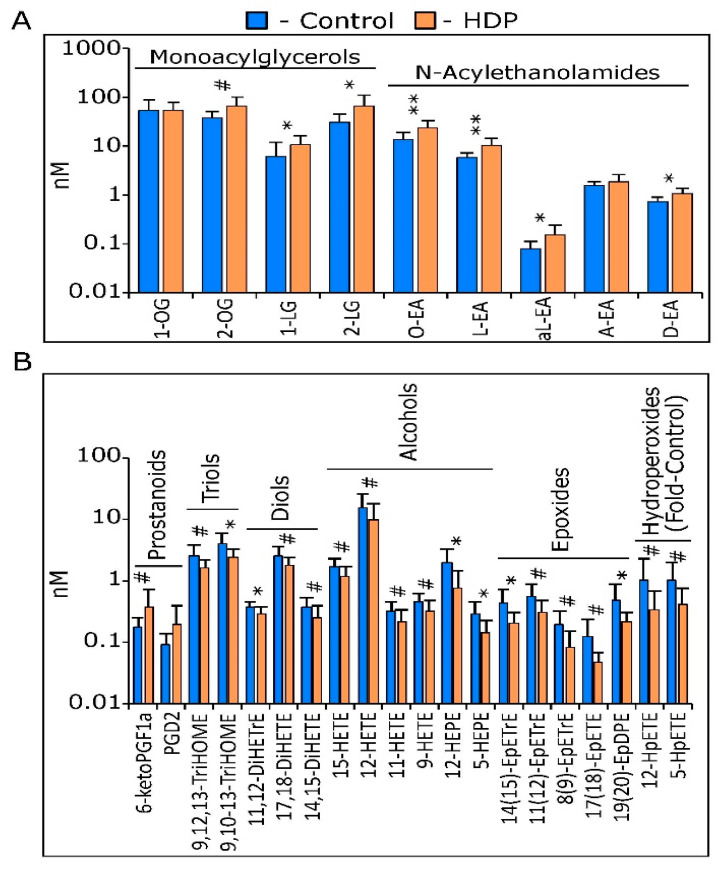
A total of 65 eCBs and OxLs were routinely detected in the plasma of study participants, including 15 of 17 (88%) eCBs and 50 of 75 (66%) OxLs. As seen in Figure 1, a PLS-DA did not fully segregate the study population into the control and HDP groups (Q2 = 0.30). However, only two members of the HDP group aggregated with the control group, and 29 of the 65 metabolites (45%) had VIP scores > 1. With regards to the eCBs, the HDP group showed higher levels of multiple monoacylglycerols and N-acylethanolamides, but not the canonical endocannabinoids 2-AG or A-EA (Figure 1 and Figure 2A). Of the seven compounds with VIPs < 1, six showed differences by 2-tailed t-tests at p ≤ 0.05. With regards to the OxLs, the HDP group showed slightly higher levels of two prostanoids, with 6-keto-PGF1alpha different between groups at p = 0.1. Of the remaining 18 discriminating metabolites, all showed lower levels, with six showing differences at p ≤ 0.05 and eleven reaching p ≤ 0.1, including triols, diols, alcohols, epoxides, and hydroperoxides (Figure 1 and Figure 2B). Given the small sample size and the pilot nature of these findings, these results are quite suggestive of differences between the HDP and control groups. The OxL analysis showed that the levels of six compounds were higher in the control females relative to the female HDPs: 11,12-DiHETrE, 14(15)-EpETrE, 14,15-DiHETE, 12-HEPE, 5-HEPE, and 19(20)-EpDPE (Figure 1). The complete results from these analyses, as shown in Table 2, demonstrate lower levels of linoleic acid, α-linolenic acid, oleic acid, and arachidonic acid-derived eCBs, but higher levels of OxLs derived from eicosapentaenoic acid and docosahexaenoic acid in the female controls compared to the female HDPs (Table 2). A complete list of the metabolites, their concentration group means, their VIP scores, and the p-values from 2-tailed t-tests are included in Supplemental Table S1.
Figure 1.
Partial least squares-discriminate analysis of control and hemodialysis patient (HDP) endocannabinoids and oxylipins.
Figure 2.
Selected plasma lipids in controls (n = 10) and hemodialysis patients (HDP; n = 9), measured by UPLC-tandem mass spectrometry. With the exception of 1-OG and AEA, all metabolites showed group discrimination power in a partial least squares-discriminate analysis with variable importance in projection scores > 1 (Figure 1). (A) Endocannabinoid and endocannabinoid-like compounds, including monoacylglycerols and N-acylethanolamides. (B) Oxylipins, including prostanoids, triols, diols, alcohols, epoxides, and hydroperoxides. Hydroperoxide levels were estimated from internal standard corrected-area responses and are expressed as fold-control. Results are expressed as the mean ± SD. The p-values < 0.05 are rounded to 1 significant figure. Group mean differences were assessed on log-transformed data by 2-tailed t-tests, with annotations indicating p-values (** ≤ 0.01; * ≤ 0.050, # ≤ 0. 10).
Table 2.
Plasma endocannabinoid and oxylipin subset levels in healthy controls and hemodialysis patients a,b.
| Compound | Parent Fatty Acid |
Units | Control | HDP | VIP | p-Value |
|---|---|---|---|---|---|---|
| Monoacylglycerols | ||||||
| 2-OG | OA | nM | 38.3 ± 13 | 65.8 ± 38 | 1.3 | 0.1 |
| 1-LG | LA | nM | 6.25 ± 6 | 10.6 ± 5.9 | 1.4 | 0.033 |
| 2-LG | ““ | nM | 30.5 ± 15 | 67 ± 42 | 1.6 | 0.026 |
| N-Acylethanolamides | ||||||
| O-EA | OA | nM | 14.1 ± 5.1 | 24.5 ± 9.7 | 1.8 | 0.0086 |
| L-EA | LA | nM | 5.8 ± 1.5 | 10.4 ± 4.1 | 1.9 | 0.0017 b |
| αL-EA | αLEA | nM | 0.082 ± 0.03 | 0.154 ± 0.091 | 1.5 | 0.024 |
| A-EA | AA | nM | 1.59 ± 0.3 | 1.88 ± 0.71 | 1.0 | 0.5 |
| D-EA | AdA | nM | 0.734 ± 0.19 | 1.06 ± 0.32 | 1.6 | 0.023 |
| Prostanoids | ||||||
| 6-ketoPGF1a | AA | nM | 0.177 ± 0.078 | 0.377 ± 0.34 | 1.1 | 0.1 |
| PGD2 | ““ | 0.0938 ± 0.046 | 0.196 ± 0.2 | 1.1 | 0.2 | |
| Triols | ||||||
| 9,12,13-TriHOME | LA | nM | 2.51 ± 1.3 | 1.59 ± 0.6 | 1.2 | 0.063 |
| 9,10-13-TriHOME | ““ | nM | 3.9 ± 1.9 | 2.35 ± 0.89 | 1.4 | 0.019 |
| Alcohols | ||||||
| 15-HETE | AA | nM | 1.66 ± 0.68 | 1.19 ± 0.51 | 1.1 | 0.1 |
| 12-HETE | ““ | nM | 15.6 ± 10 | 9.68 ± 8.4 | 1.0 | 0.1 |
| 11-HETE | ““ | nM | 0.318 ± 0.13 | 0.221 ± 0.12 | 1.0 | 0.1 |
| 9-HETE | ““ | nM | 0.46 ± 0.15 | 0.316 ± 0.17 | 1.2 | 0.070 |
| 12-HEPE | EPA | nM | 1.92 ± 1.4 | 0.76 ± 0.67 | 1.3 | 0.037 |
| 5-HEPE | ““ | nM | 0.295 ± 0.16 | 0.147 ± 0.077 | 1.4 | 0.026 |
| Hydroperoxides | ||||||
| 12-HpETE | AA | Fold-C | 1.0 ± 1.3 | 0.35 ± 0.35 | 1.1 | 0.087 |
| 5-HpETE | AA | Fold C | 1.0 ± 1.0 | 0.42 ± 0.33 | 1.1 | 0.1 |
| Epoxides | ||||||
| 14(15)-EpETrE | AA | nM | 0.437 ± 0.28 | 0.203 ± 0.11 | 1.5 | 0.014 |
| 11(12)-EpETrE | ““ | nM | 0.551 ± 0.32 | 0.309 ± 0.17 | 1.3 | 0.06 |
| 8(9)-EpETrE | ““ | nM | 0.195 ± 0.13 | 0.0822 ± 0.068 | 1.0 | 0.1 |
| 17(18)-EpETE | EPA | nM | 0.124 ± 0.11 | 0.0478 ± 0.019 | 1.0 | 0.1 |
| 19(20)-EpDPE | DHA | nM | 0.484 ± 0.38 | 0.212 ± 0.09 | 1.4 | 0.019 |
| Diols | ||||||
| 11,12-DiHETrE | AA | nM | 0.372 ± 0.084 | 0.287 ± 0.089 | 1.3 | 0.031 |
| 17,18-DiHETE | EPA | nM | 2.5 ± 1.1 | 1.79 ± 0.66 | 1.0 | 0.1 |
| 14,15-DiHETE | ““ | nM | 0.378 ± 0.16 | 0.247 ± 0.15 | 1.2 | 0.057 |
a—all values are mean ± SD. Variable importance in projection (VIP) scores are reported from partial least squares-discriminant models of all measured metabolites. The p-values are 2-tailed t-tests comparing group means. b—difference in L-EA between groups that survived false discover rate correction at q = 0.2. Abbreviations: AA = arachidonic acid; AdA = adrenic acid; αLEA = alpha-linoleic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; Fold-C = fold-control, i.e., control ÷ HDP; HDP = hemodialysis patient; LA = linoleic acid; OA = oleic acid.
The analysis score plot (inset) showing group discrimination and the variable coefficient plot showing metabolite strength in discrimination are displayed. Metabolites with a variable importance in projections (VIP) > 1 were considered to have discriminating power, and are investigated in detail in Figure 2A,B and Table 2. Leave-one-out cross-validation was used to build the model. While showing a tendency towards group discrimination (i.e., Q2 positive), it did not reach full group segregation (Q2 > 0.4). A complete list of metabolites, their concentration group means, their VIP scores, and the p-values from 2-tailed t-tests are included in Supplemental Table S1.
2.4. Global Metabolite Profiles
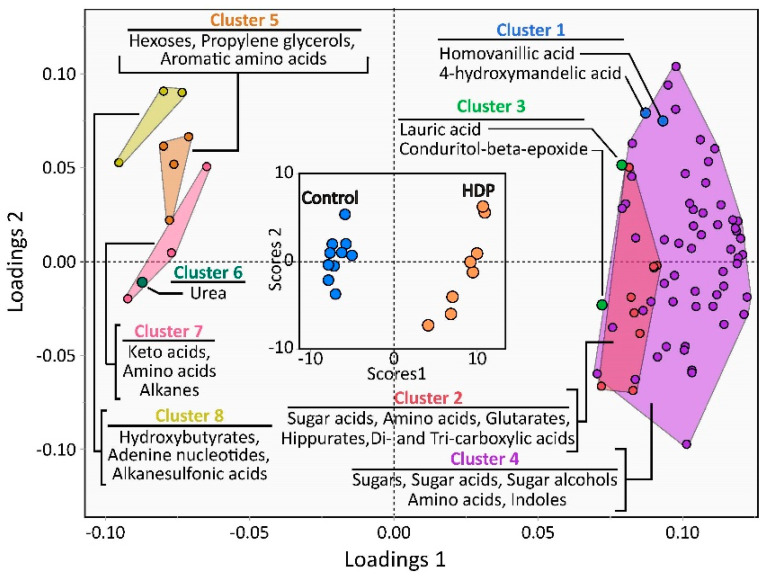
Plasma mass spectrometry-based untargeted metabolomic analysis of HDPs and controls detected 566 unique features, including 192 identified chemical compounds. Of the named metabolites, 82 were found to be different by a t-test when compared between the female HDPs and female controls, with 70 higher in female HDPs as presented in Figure 3 and Supplemental Table S1. The data were subjected to a partial least squares-discriminant analysis (PLS-DA) to visualize untargeted metabolite differences between the female hemodialysis and healthy female control groups (Figure 2). Upon further analysis, the hierarchical cluster dendrogram was pruned into eight clusters based on the screen plot, and the obtained clusters were used for a multivariate analysis of variance (MANOVA). As shown in Figure 3, the differences between the controls and HDPs revealed specific clusters of the metabolites measured. The data from the PLS were subjected to MANOVA and are presented in Supplemental Table S1 for ease of interpretation of the metabolites, and for the presentation of means ± SD and the t-test p-values.
Figure 3.
Partial least squares-discriminant analysis of untargeted metabolomic data.
All detected metabolomic features were used for the discriminant analysis, but only metabolites with variable importance in projection scores > 1 were displayed for clarity. The inset score plot demonstrates control and hemodialysis patient (HDP) discrimination, while the loadings plot shows metabolites driving group discrimination. Metabolites with negative and positive loadings in latent variable 1 were lower and higher in the HDP group than in the control group, respectively. A hierarchical cluster analysis of auto-scaled metabolomic data yielded eight unique clusters that were important in group discrimination. The loadings of individual metabolites are color-coded according to hierarchical cluster membership (see the MANOVA analysis in Table S2), as are their cluster names. Clusters having more than two members with VIPs > 1 are displayed within containing geometric shapes of the same color. The cumulative values for the two factors were: Q2 = 0.99; R2X = 0.39; and R2Y = 0.98.
2.5. Thematic Changes for the Metabolites
To effectively interpret the vast amount of data from the metabolomics analysis, a series of t-tests were performed to tease out the interrelationships among hundreds of compounds that are involved in many metabolic pathways. Because of the different representation of the study subjects, the data analyses were grouped based on HDP. Thus, statistical analyses were performed within each grouping, namely between controls and HDPs. The results of these analyses revealed several thematic changes, as illustrated in Figure 3. The principal changes in the metabolomics profiles pointed to an altered amino acid metabolism for essential and nonessential amino acids. This was also reflected by differences in the intermediates of the urea cycle and creatine, which makes them useful as biomarkers of kidney failure.
Amino Acids
In the female HDPs, the levels of several amino acids were found to be different compared to those of the female controls. Among these amino acids, leucine, isoleucine, phenylalanine, tryptophan, and valine—essential amino acids—were either higher in the female HDP group, or higher in the female control group (Supplemental Table S2). Glutamine, a conditional essential amino acid, was higher, while alanine was lower in the HDPs compared to the controls. A prominent difference was observed in the level of metabolites in the biochemical pathways of amino acids between the HDPs and controls. Only two metabolites in this group were lower in the female HDP group (taurine and 2-hydroxybutanoic acid) compared to the female controls. The data point to a heightened metabolic change in the HDPs compared to the healthy subjects (Supplemental Table S2). The levels of creatinine and its catabolic product 1-methylhydantoin were higher in HDPs, echoing the disease state with respect to the diminished kidney function associated with the progression of kidney disease. Other differences were also found between healthy female controls and female HDPs, with higher levels of pyrophosphate and adenosine-5-phosphate in the controls, but higher levels of citrulline and ornithine, two intermediates in the urea cycle, in the HDPs.
3. Discussion
3.1. Levels of Plasma PUFAs, eCBs, and OxLs in Female Controls and HDPs
Arachidonic acid is the most well-studied PUFA for the biosynthesis of eCBs and OxLs. The two most well-studied ECS ligands, AEA and 2-AG, are arachidonate derivatives. Further, arachidonic acid is the precursor for many immunomodulatory OxLs. In this study, we reported a higher amount of 20:5n-3 (EPA) in the plasma phospholipids of healthy female controls compared to the female HDPs. In contrast, female HDPs had higher arachidonate levels compared to the female controls in their plasma phospholipids. Phospholipid fatty acids are a primary target for evaluation in hemodialysis patients [35]. The differences in the levels of EPA and arachidonate consistently showed changes in both the eCB and OxL amounts between female controls and female HDPs. The observation of low n-3 PUFAs in the plasma of female HDPs is consistent with several previous reports on hemodialysis patients [6,24,25]. Moreover, differences in eCBs are associated with appetite in both male and female hemodialysis patients compared to healthy controls [36].
L-EA, a linoleic acid-derived eCB, has been shown to possess anti-inflammatory effects by inhibiting NF-κB signaling and the expression of pro-inflammatory cytokines (TNF-α, IL-1, and IL-6), as well as inhibiting COX-2 activity and the production of PGE2 in mouse RAW264.7 macrophages [37]. In the accompanying in vivo study with a mouse dermatitis model, L-EA successfully lessened 2,4-dinitrofluorobenzene-induced contact dermatitis on ear skin and pro-inflammatory cytokine expression at inflamed sites [37]. It is interesting to note the coexistence of potentially pro-inflammatory arachidonate and the anti-inflammatory L-EA, which were both higher in the female HDPs in this study. These findings might suggest a role for the participation of PUFAs and eCB-like compounds in the complex disease state of hemodialysis and the malfunction of the renal system. L-EA is one of the major N-acylethanolamine species found in peripheral tissues, especially in the intestine, where it is actually the N-acylethanolamine of the highest concentration [38,39]. With respect to the OxLs, arachidonate derivatives represent the well-known and most studied OxLs (eicosanoids), such as prostaglandins, leukotrienes, thromboxanes, and lipoxins. These arachidonate derivatives, especially the prostaglandins and leukotrienes, are generally pro-inflammatory, and their counterparts derived from n-3 PUFAs appear to be less pro-inflammatory [40]. In end-stage renal disease patients on hemodialysis, it has been found that 5-lipoxygenase activity and expression are greatly increased in peripheral blood mononuclear cells, which activates the arachidonate cascade that leads to the formation and release of the reactive oxygen species of pro-inflammatory and pro-atherogenic OxLs and cytokines [41].
Modifying the circulating levels of OxLs by dietary n-3 PUFA supplementation with fish oil improved the condition (measured by proteinuria) of immunoglobulin A nephropathy (IgAN) of the patients and shifted the profile of OxLs towards more EPA and DHA derivatives [23]. An analysis of blood OxLs underscored that the baseline n-3 PUFA status was a significant determinant of OxL status [42]. Grapov et al. examined the linkages between changes in circulating OxLs and eCBs by measuring > 150 plasma lipids in overweight to obese patients with type 2 diabetes and found that these plasma lipidomic profiles reflected the biochemical and physiological changes of this pathological state, independently of obesity [43]. Thus, it is likely that these relationships exist and must be investigated in hemodialysis patients supplemented with n-3 PUFAs. We report for HDPs that, in general, monoacylglycerols were higher, which is consistent with end-stage renal disease patients on maintenance hemodialysis (MHD) [17]. The eCB 2AG did not reach significance (p = 0.3; Supplemental Table S1). While AEA also appeared higher in the HDP group compared to the control group, with a PLS-DA VIP score of 0.95, this was not significant in a univariate analysis (p = 0.40). This is in contrast with the findings of Moradi et al. [18], and the difference between the univariate and multivariate analyses suggests the potential for interactions among the measured metabolites.
3.2. HDPs Had Lower Plasma EPA Compared to Controls and Lower 14,15-DiHETE, 12-HEPE, and 5-HEPE
EPA is another major PUFA substrate for a range of eicosanoids that are largely regarded as antagonistic and anti-inflammatory against the actions of those derived from the n-6 PUFA arachidonate with mostly pro-inflammatory activities. The finding that lower levels of EPA and its OxL derivatives were detected in the female HDP group could be an indication that this group of patients may be deficient in their n-3 PUFA status compared to the healthy controls. Indeed, it has been reported that dietary EPA supplementation is associated with an enhanced in vivo production of EPA-derived OxLs, e.g., 5-HEPE and 15-HEPE, as shown in 116 human subjects (68% female, 20–59 years old) given a fish oil supplement (2 g EPA + 1 g DHA, daily) in a randomized placebo-controlled study [44]. Supplementing with n-3 PUFAs increased the n-3 OxL levels from 2- to 5-fold and reduced those of n-6 origin by approximately 20% in healthy volunteers during 4 weeks of treatment with prescription n-3 PUFA ethyl esters (4 g/day) [45]. This supports our premise that n-3 PUFAs should be incorporated into the treatment regimen of hemodialysis patients [24,25]. Furthermore, the higher amounts of arachidonic acid-derived epoxy fatty acids in the healthy female controls compared to the female HDPs must be evaluated in future studies.
It is well-known that unsaturated fatty acids are often converted to epoxides by P450 monooxygenases [46,47,48,49]. Numerous studies have shown that these cytochrome P450-mediated arachidonate metabolites could play multiple physiological and pathological roles on the cerebral microvasculature to mediate or alleviate the pathogenesis of cerebrovascular diseases [50,51]. HDPs are known to be in an inflammatory state [3]. In support of the heightened state of inflammation, the pro-inflammatory cytokine IL-6 concentration is a robust predictor of death in hemodialysis patients [52]. Noori et al. corroborated this finding, reporting that higher serum albumin, prealbumin, and creatinine were associated with greater survival, whereas C-reactive protein and IL-6 were associated with increased mortality in hemodialysis patients [53]. Further supporting the link between the inflammatory state and kidney disease, Na et al. showed that a significant association exists between the WBC count and the risk for developing CKD in women, but not in men [54]. EETs are regulated at multiple levels, from production to epoxide hydrolase-dependent degradation [49,55,56]. They are also integrally linked to the renin–angiotensin system and blood pressure control.
3.3. Amino Acid and Metabolite Differences between Healthy Controls and HDPs
Proteins and amino acids are important nutrients for maintaining muscle mass and biosynthetic capacity in the body. Plasma amino acid levels are believed to directly reflect disturbances in protein and amino acid metabolism and their inter-organ exchanges in renal dysfunction, since compromised renal function disrupts metabolism and as a consequence, it alters circulating metabolite profiles [57,58]. Qi et al. have reported significant changes in endogenous metabolites, including glycolysis products (glucose and lactate), amino acids (valine, alanine, glutamate, and glycine), and organic osmolytes (betaine, myo-inositol, taurine, and glycerophosphocholine) in different stages of CKD in a pilot metabolic profiling study that compared 80 patients in four stages of CKD and 28 healthy controls [59]. In patients suffering from acute kidney injury, Sun et al. also showed increased serum acylcarnitines and amino acids (methionine, homocysteine, pyroglutamate, asymmetric dimethylarginine, and phenylalanine), deceased arginine, and several decreased lysophosphatidylcholines compared to healthy controls [60]. Among these, increases in homocysteine, asymmetric dimethylarginine, and pyroglutamate have been shown as biomarkers of cardiovascular and renal diseases, while augmented acylcarnitines could serve as biomarkers of defective oxidative fatty acid metabolism [60,61]. In the current study, the levels of several amino acids were found to be different between the female HDPs and the female controls, with two (phenylalanine and glutamine) higher in the HDPs, while another two (tryptophan and alanine) were lower in the HDPs.
It has been reported that free amino acids, either from dietary intake or from protein catabolism, are substantially retained in the plasma of patients with end-stage renal disease, where dialysis is adopted to correct the imbalance [57]. In our study, we found a similar phenomenon, where the major differences in amino acid metabolites were higher in HDPs compared to controls. It was clearly shown that there was a distinct difference in amino acid metabolism and the levels of their metabolites between the HDPs and the healthy female controls. These changes might be related to large differences in the systemic use of amino acids and intermediary metabolism. Recently, the upregulation of the CB2 receptor was found to play a primary role in the mitochondrial dysfunction of renal tubular cells [62]. Thus, understanding the full extent of differences in eCBs and OxLs in the present study helps identify the relationship between dietary PUFAs and these bioactive lipids in the HDPs.
The creatinine concentration rises when the kidney function is compromised. Therefore, blood creatinine alone or in conjunction with the 24 h urine creatinine level is traditionally used as a clinical biomarker for assessing kidney function [63]. In our investigation, the female HDPs had higher plasma levels of creatinine (6-fold increase) compared to the female controls, which clearly shows a distinct difference between patients with kidney disease who rely on hemodialysis and those without compromised renal function. However, creatinine concentrations in the blood could be affected by age, gender, muscle mass, medication, or hydration status [64]. In the end-product metabolism of amino acids, we found that the HDP group’s urea levels were twice that of controls, and two intermediates of the urea cycle, namely citrulline and ornithine, were elevated to the same extent. The findings of Shah et al. [29] are consistent with these results.
In a recent article [65], plasma metabolites were measured in 19 MHD patients that were receiving end-stage renal dialysis and 12 healthy controls to identify metabolites unique to the dialysis patients. The subjects and patients consisted of both males and females. The analysis revealed that 30 metabolites were higher and 33 lower in the MHD patients compared to the controls in the study; a few of these were observed in our current investigation. Here, in the plasma, we observed higher levels of hippuric acid and several indole-derivative compounds, and lower levels of lactic acid, in the MHD patients compared to the controls, which is consistent with the findings of Chen et al. [65]. The study by Chen and colleagues [65] did not include any eCBs or OxLs. Nonetheless, with the number of subjects in our study, some metabolite levels were similar, and our findings for OxLs emphasize the differences in the inflammatory state for HDPs.
3.4. Implications, Strengths, and Limitations
eCBs and OxLs are changed in HDPs compared to control women. These findings would implicate a possible link for OxLs and the ECS as candidate targets for chronic kidney disease due to their role in inflammation. The study limitation is the small sample size; however, many of our results for eCBs and metabolites are consistent with other investigators. The dialysis patients had a high BMI, as did the controls, and they were matched by age, height, and weight with the controls. Although the subjects had a high BMI and appeared to have adapted to the hemodialysis, our data might not be the same for patients with a lower-range BMI. Yet, both the controls and the HDP group had similar BMI values. The HDP group had a higher incidence of tobacco use (four of nine participants), which may have influenced some results. However, recent reports indicate few impacts on OxL levels [66]. While tobacco smoke exposure may influence the endocannabinoid system via nicotine exposure [67], to the best of our knowledge, its influences on circulating eCBs have not been reported.
4. Materials and Methods
4.1. Subjects and Design
Study subjects were recruited at Indiana University School of Medicine, Indianapolis, IN and included 9 female HDPs (hemodialysis patients) and 10 age-, BMI-, and diabetes status-matched non-HDP female controls (Table 3). All subjects were either overweight or obese, with a BMI of 31.0 ± 9.3 in the patients and 31.1 ± 5.4 in the control group. Furthermore, the differences in ethanol use and smoking between the groups could be a limitation. The average time on dialysis was 10.0 ± 11 years for the female patients. The study cohort was derived from our previous study [36]. The 9 HDPs were part of a larger cohort of 20 dialysis patients of mixed sex. After identifying this female cohort, we then recruited healthy female volunteers that were matched to our cohort using frequency matching by age. SNAQ appetite scores were obtained for all subjects, with values of 12.00 ± 3.39 for the female patients and 14.90 ± 2.60 for the control females [36]. Blood samples were obtained immediately pre-dialysis from arteriovenous blood sampling from the dialysis tubing for all HDPs, and from the cannulation of blood veins in the controls. Blood samples for eCBs and OxLs were treated with the protease inhibitor phenylmethanesulfonylfluoride (PMSF), a fatty acid amide hydrolase (FAAH) inhibitor added to a set of samples to prevent the breakdown of anandamide (i.e., arachidonoyl ethanolamide (AEA)) [36]. The samples for the FAME analysis of plasma and RBCs were not treated as such. This was a secondary analysis of an exploratory study to assess the differences between cohorts, and as such, no power calculations were needed. The severity of the patients’ kidney function was uniform among the hemodialysis population because they were all on dialysis, which indicates minimal to no residual kidney function remaining. No markers of inflammation were measured in these patients. The study was initiated after written informed consent from the study participants and approved by the Institutional Review Board at the University of Indiana, School of Medicine (ClinicalTrials.gov Identifier: NCT01477515).
Table 3.
Characteristics of female control subjects and female hemodialysis patients.
| Characteristics | Units | Controls (n = 10) |
Hemodialysis Patients (n = 9) |
|---|---|---|---|
| Diabetes mellitus | # positive | 2 | 2 |
| NSAID | # positive | 4 | 5 |
| Age | yr | 54.82 ± 4.99 | 59.31 ± 12.81 |
| Medical review | |||
| Time on dialysis | yr | 0 | 10.00 ± 10.52 |
| Age | yr | 54.43 ± 4.90 | 58.02 ± 15.06 |
| Weight | kg | 84.01 ± 11.5 | 82.71 ± 23.68 |
| Height | m | 1.65 ± 0.08 | 1.63 ± 0.06 |
| BMI | kg/m2 | 31.08 ± 5.43 | 31.03 ± 9.26 |
| 3 mo. weight change | kg | No value | 0.84 ± 1.82 |
| 6 mo. weight change | kg | No value | 0.67 ± 1.26 |
| Tobacco | # positive | 0 | 4 |
| ETOH | # positive | 5 | 1 |
| Marijuana | # positive | 0 | 0 |
Values are means ± SD. The # values for diabetes mellitus and NSAID are the number of cases.
4.2. Fatty Acid Analysis of Plasma and RBCs
Human plasma and RBC samples were processed to determine the fatty acid composition. The samples processed were from all 19 subjects. Briefly, 200 µL of RBCs or 100 µL of plasma were extracted with chloroform/methanol (2:1, vol/vol). Lipids from the plasma were further separated into polar and neutral fractions by solid-phase extraction using silica cartridges (300 mg filling, Alltech) after eluting the neutral fraction with chloroform and the polar fraction with methanol [68]. Only the polar lipids were used for further analysis, based on several fatty acid analyses of HDPs. The resulting extracted lipids were treated with 0.5 N NaOH in methanol, and fatty acid methyl esters (FAME) were prepared by esterification using boron trifluoride (BF3) in methanol (10% w/w, Supelco Inc. Bellefonte, PA, USA). The FAME were concentrated in isooctane (HPLC grade, Fisher Scientific, Pittsburg, PA, USA) and analyzed by gas chromatography (GC) (HP 7890A series, autosampler 7693, GC ChemStation Rev.B.04.03, Agilent Technologies, Palo Alto, CA, USA) with a DB-225 column (30 m, 0.25 mm i.d., 0.15 mm film thickness, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector [68]. Sample peaks were identified by comparison to authentic FAME standards (Nu-Chek-Prep Inc., Elysian, MN, USA). The results of the FAME analysis were obtained by area percentage reports.
4.3. Measurement of Endocannabinoids (eCBs) and Oxylipins (OxLs)
Measurements of eCBs and OxLs were performed by LC-MS/MS of 250 µL of plasma, thawed on ice, placed into solid-phase extraction column cartridges on a vacuum manifold, spiked with deuterated eCB/OxL internal standards, diluted to 20% MeOH/0.1% acetic acid, and gravity-loaded onto a 60 mg Oasis-HLB solid-phase extraction column, followed by vacuum-drying with ambient air. The columns were then wetted with 0.2 mL MeOH and eluted with 0.5 mL ethyl acetate by gravity. The solvent was removed by vacuum, the samples were reconstituted in 50 µL MeOH containing internal standards, samples were filtered at 0.1 µm, and they were then analyzed by UPLC-(ESI)MS/MS by back-to-back (+)-mode/(−)-mode injections for 17 eCBs and eCB-like compounds and 58 OxL profiles, respectively, in the Newman Laboratory, as previously reported [42,43].
4.4. Measurement of Metabolites
Human plasma samples were analyzed for global metabolites by following the method of Fiehn et al. [69]. An Agilent 6890 gas chromatograph, controlled using Leco ChromaTOF software, and a 30 m × 0.25 mm i.d. × 0.25 µm 95% dimethyl/5% diphenyl polysiloxane film Rtx-5Sil MS column with an additional 10 m integrated guard column were used. Pure helium (99.9999%) with a built-in purifier was used at a constant flow of 1 mL per min. The oven temperature was held constant at 50 °C for 1 min, and then ramped up by 20 °C per min to 330 °C and held constant for 5 min. The mass spectrometry instrument was a Leco Pegasus IV time-off light mass spectrometer, controlled using Leco ChromaTOF software, version 2.32. For the sample introduction, the transfer line temperature between the gas chromatograph and the mass spectrometer was set to 280 °C; electron impact ionization at 70 V was employed, with an ion-source temperature of 250 °C. Data acquisition was performed after a 290 s solvent delay, filament 1 was turned on, and the mass spectra were acquired at the mass resolving power R = 600 from m/z 85–500, at 20 spectra per second and a 1550 V detector voltage, without turning on the mass defect option. Recording ended after 1200 s. The instrument performed auto-tuning for mass calibration using FC43 (perfluorotributylamine) before starting the analysis sequences. The results of the eCB and OxL analysis of plasma samples (with and without PMSF) from 2 of the same subjects were similar [36].
4.5. Statistical Analyses
Data for plasma fatty acids, eCBs, OxLs, and metabolites were expressed as the means and standard deviations. Group differences in fatty acid analyses were evaluated by the t-test procedure using SAS (v 9.3; SAS Institute, Inc., Cary, NC, USA). Analyses of eCBs, OxLs, and metabolomics were conducted in JMP Pro (v 16.1; SAS Institute, Inc., Cary, NC, USA). Values were log-transformed and normality was evaluated prior to the analyses. The impact of PMSF on eCB and OxL measurements was assessed by least-squares mean regressions, with the subject as a random effect. Partial least squares-discriminate analysis (PLS-DA) was used to determine if a multivariate metabolite analysis could segregate experimental groups. The calculated model quality metrics included Q2, R2X, and R2Y, with Q2 > 0.4 indicating full group segregation. Metabolites with variable importance in projection (VIP) scores > 1 were considered significant discriminating variables. Group mean differences were assessed by 2-tailed t-tests, with and without an adjustment for multiple comparisons, using the Benjamini–Hochberg false discovery rate correction procedure at q = 0.2 [70]. Based on previous studies for the OxLs and eCBs, a power analysis revealed that an n value of 8–10 allowed for discrimination of these patients [36].
Metabolomic analyses used only the PMSF-containing sample set. Metabolomic data dimensionality was reduced by grouping highly correlated variables using hierarchical cluster analysis, as described by Ward [71]. Clusters were assigned using auto-scaled data to ensure that all metabolites were equally considered in the statistical models [72]. The hierarchical cluster dendrogram was pruned into 8 clusters based on the screen plot, and the obtained clusters were used for multivariate analysis of variance (MANOVA). MANOVA analysis was performed using hierarchical clusters, with the metabolite as a fixed effect and the subject as a random effect. Prior to analysis, data were normalized using the Johnson transformation. Clustering was performed separately for the targeted and untargeted analyses. MANOVAs and t-tests were used to evaluate group differences in metabolite abundances. Multiple comparison corrections of MANOVA and t-test p-values used the Benjamini–Hochberg false discovery rate correction at q = 0.2 [70]. A PLS-DA was used to visualize untargeted metabolite differences between the HDP and control groups. Analyses were performed using auto-scaled data after the imputation of metabolites missing was detected in > 70% of study participants. All metabolites were used to perform the analysis; however, only metabolites with VIP > 1 are displayed in figures for clarity of presentation. VIP scores were negatively correlated with the t-test p-values.
5. Conclusions
In summary, female dialysis patients have alterations in eCB and OxL species that are associated with plasma PUFAs, following our hypothesis. This important diet relationship should be further investigated to ascertain if these lipid biomarkers are associated with or help mediate disease conditions. Furthermore, in our study we found that plasma phospholipid EPA levels (and RBCs) were higher in the healthy control group compared to the HDP group. While the majority of OxLs also followed this pattern, the eCB-like compounds measured were generally higher in the HDP group. Thus, our results suggest that OxLs and the ECS are potential targets for altering the course of chronic kidney disease [73]. In addition, amino acid metabolism is significantly altered by the kidney disease state. Our investigation is the first to report a comprehensive examination of the status of eCBs, OxLs, and other metabolites in female control and female hemodialysis patients. Combined with our previous work on the benefits on long-chain n-3 PUFAs in this population, and the emphasis on the DHA and EPA status in chronic kidney disease [74], the current research, although preliminary, identifies new opportunities for evaluating the role of dietary PUFAs and eCBs/OxLs in clinical disease for kidney dialysis patients. Given the small number of subjects used in this study as a limitation (albeit the fact that we reported some similar findings to other investigators for eCBs and systemic metabolites), validation is needed in a larger cohort.
Abbreviations
| 1-AG | 1-arachidonoylglycerol |
| 1-LG | 1-linoleoyl glycerol |
| 1-OG | 1-oleoyl glycerol |
| 2-AG | 2-arachidonoylglycerol |
| 2-LG | 2-linoleoyl glycerol |
| 2-OG | 2-oleoyl glycerol |
| 5-HEPE | 5-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid; |
| 8,9-DiHETrE | 8,9-dihydroxy-5Z,11Z,14Z-eicosatrienoic acid; |
| 8(9)-EpETrE | 8(9)-epoxy-5Z,11Z,14Z-eicosatrienoic acid; |
| 9(10)-EpODE | 9(10)-epoxy-12Z,15Z-octadecadienoic acid; |
| 11,12-DiHETrE | 11,12-dihydroxy-5Z,8Z,14Z-eicosatrienoic acid; |
| 11(12)-EpETrE | 11(12)-epoxy-5Z,8Z,14Z-eicosatrienoic acid; |
| 12(13)-EpODE | 12(13)-epoxy-10E,15Z-octadecadienoic acid; |
| 12-HEPE | 12-hydroxy-5Z,8Z,10E,14Z,17Z-eicosapentaenoic acid; |
| 13-HODE | 13-hydroxy-9Z,11E-octadecadienoic acid; |
| 13-HpODE | 13-hydroperoxy-9Z,11E-octadecadienoic acid. |
| 14,15-DiHETE | 14,15-dihydroxy-5Z,8Z,11Z,17Z-eicosatetraenoic acid; |
| 14(15)-EpETrE | 14(15)-epoxy-5Z,8Z,11Z-eicosatrienoic acid; |
| 15(16)-EpODE | 15(16)-epoxy-9Z,12Z-octadecadienoic acid; |
| 17,18-DiHETE | 17,18-dihydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid; |
| 19(20)-EpDPE | 19(20)-epoxy-4Z,7Z,10Z,13Z,16Z-docosapentaenoic acid |
| αL-EA | alpha-linolenoyl ethanolamide |
| A-EA | arachidonoyl ethanolamide |
| AEA | anandamide |
| CKD | chronic kidney disease |
| D-EA | docosatetraenoyl ethanolamide |
| DAGL | diacylglycerol lipase |
| DGLA | dihomo-gamma-linolenic aid |
| DGLA-EA | dihomo-gamma-linolenoyl ethanolamide |
| DH-EA | docosahexaenoyl ethanolamide |
| DHA | docosahexaenoic acid |
| ECS | endocannabinoid system |
| EPA | eicosapentaenoic acid |
| FAME | fatty acid methyl esters |
| HDP | Hemodialysis patients |
| L-EA | linoleoyl ethanolamide |
| MAGL | monoacylglycerol lipase |
| NAPE-PLD | N-arachidonoyl phosphatidylethanolamine phospholipase D |
| O-EA | oleoyl ethanolamide |
| P-EA | palmitoyl ethanolamide |
| PMSF | phenylmethanesulfonylfluoride |
| PUFA | polyunsaturated fatty acids |
| S-EA | stearoyl ethanolamide |
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179781/s1.
Author Contributions
Conceptualization, B.A.W.; data curation, J.K., K.B. and S.K.; metabolomic data generation, O.F.; investigation, B.A.W. and J.W.N.; oxylipin and endocannabinoid data generation, J.W.N.; writing—original draft, B.A.W. and A.N.F.; writing—review and editing, B.A.W. and J.W.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All information and codes are with the website cited with the reference https://clinicaltrials.gov/ct2/history/NCT01477515 (accessed on 18 August 2022).
Informed Consent Statement
The study was initiated after written informed consent from the study participants and approved by the Institutional Review Board at the University of Indiana, School of Medicine (ClinicalTrials.gov Identifier: NCT01477515).
Data Availability Statement
The only available data can be found in the Supplemental Materials Tables.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by funding provided to B.A.W. and the metabolomics effort at the University of Connecticut Health Center, Center on Aging. Additional support was provided by the USDA, Intramural Projects 5306-51530-019-00D, 5306-51530-022-00D, and 5306-51530-025-00D, and the West Coast Metabolomics Center, Grant NIH U24 DK097154. The USDA is an equal-opportunity provider and employer.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bossola M., Leo A., Viola A., Carlomagno G., Monteburini T., Cenerelli S., Santarelli S., Boggi R., Miggiano G., Vulpio C., et al. Dietary intake of macronutrients and fiber in Mediterranean patients on chronic hemodialysis. J. Nephrol. 2012;26:912–918. doi: 10.5301/jn.5000222. [DOI] [PubMed] [Google Scholar]
- 2.Fernstrom A., Hylander B., Rossner S. Energy intake in patients on continuous ambulatory peritoneal dialysis and haemodialysis. J. Intern. Med. 1996;240:211–218. doi: 10.1046/j.1365-2796.1996.36865000.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang A.Y., Sea M.M., Ip R., Law M.C., Chow K.M., Lui S.F., Li P.K., Woo J. Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie, and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 2001;12:2450–2457. doi: 10.1681/ASN.V12112450. [DOI] [PubMed] [Google Scholar]
- 4.Wang A.Y.-M., Sea M.M.-M., Ng K., Kwan M., Lui S.-F., Woo J. Nutrient Intake during Peritoneal Dialysis at the Prince of Wales Hospital in Hong Kong. Am. J. Kidney Dis. 2007;49:682–692. doi: 10.1053/j.ajkd.2007.02.257. [DOI] [PubMed] [Google Scholar]
- 5.Wright M., Woodrow G., O’Brien S., King N., Dye L., Blundell J., Brownjohn A., Turney J. Disturbed appetite patterns and nutrient intake in peritoneal dialysis patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2004;23:550–556. doi: 10.1177/089686080302300606. [DOI] [PubMed] [Google Scholar]
- 6.Friedman A.N., Yu Z., Tabbey R., Denski C., Tamez H., Wenger J., Thadhani R., Li Y., Watkins B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013;83:1130–1135. doi: 10.1038/ki.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee E.P., Thadhani R. New insights into uremia-induced alterations in metabolic pathways. Curr. Opin. Nephrol. Hypertens. 2011;20:593–598. doi: 10.1097/MNH.0b013e32834b8a1d. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez C.A., Pera G., Quiros J.R., Lasheras C., Tormo M.J., Rodriguez M., Navarro C., Martinez C., Dorronsoro M., Chirlaque M.D., et al. Types of fat intake and body mass index in a Mediterranean country. Public Health Nutr. 2000;3:329–336. doi: 10.1017/S1368980000000379. [DOI] [PubMed] [Google Scholar]
- 9.Williams D.E.M., Prevost A.T., Whichelow M.J., Cox B.D., Day N.E., Wareham N.J. A cross-sectional study of dietary patterns with glucose intolerance and other features of the metabolic syndrome. Br. J. Nutr. 2000;83:257–266. doi: 10.1017/S0007114500000337. [DOI] [PubMed] [Google Scholar]
- 10.Brunner E., Wunsch H., Marmot M. What is an optimal diet? Relationship of macronutrient intake to obesity, glucose tolerance, lipoprotein cholesterol levels and the metabolic syndrome in the Whitehall II study. Int. J. Obes. 2001;25:45–53. doi: 10.1038/sj.ijo.0801543. [DOI] [PubMed] [Google Scholar]
- 11.Madsen L., Petersen R.K., Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta Mol. Basis Dis. 2005;1740:266–286. doi: 10.1016/j.bbadis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.He K., Rimm E.B., Merchant A., Rosner B.A., Stampfer M.J., Willett W.C., Ascherio A. Fish Consumption and Risk of Stroke in Men. JAMA. 2002;288:3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 13.Huang X.-F., Xin X., McLennan P., Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: Insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes. Metab. 2003;6:35–44. doi: 10.1111/j.1463-1326.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 14.Christiansen E., Schnider S., Palmvig B., Tauber-Lassen E., Pedersen O. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids. Effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care. 1997;20:881–887. doi: 10.2337/diacare.20.5.881. [DOI] [PubMed] [Google Scholar]
- 15.Coelho S.B., de Sales R.L., Iyer S.S., Bressan J., Costa N.M.B., Lokko P., Mattes R. Effects of peanut oil load on energy expenditure, body composition, lipid profile, and appetite in lean and overweight adults. Nutrition. 2006;22:585–592. doi: 10.1016/j.nut.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Chua J.T., Argueta D.A., DiPatrizio N.V., Kovesdy C.P., Vaziri N.D., Kalantar-Zadeh K., Moradi H. Endocannabinoid System and the Kidneys: From Renal Physiology to Injury and Disease. Cannabis Cannabinoid Res. 2019;4:10–20. doi: 10.1089/can.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moradi H., Park C., Igarashi M., Streja E., Argueta D.A., Soohoo M., Daglian J., You A.S., Rhee C.M., Kashyap M.L., et al. Serum Endocannabinoid Levels in Patients With End-Stage Renal Disease. J. Endocr. Soc. 2019;3:1869–1880. doi: 10.1210/js.2019-00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moradi H., Park C., Streja E., Argueta D.A., DiPatrizio N.V., You A.S., Rhee C.M., Vaziri N.D., Kalantar-Zadeh K., Piomelli D. Circulating Endocannabinoids and Mortality in He-modialysis Patients. Am. J. Nephrol. 2020;51:86–95. doi: 10.1159/000505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balgoma D., Checa A., Sar D.G., Snowden S., Wheelock C.E. Quantitative metabolic profiling of lipid mediators. Mol. Nutr. Food Res. 2013;57:1359–1377. doi: 10.1002/mnfr.201200840. [DOI] [PubMed] [Google Scholar]
- 20.Luo P., Wang M.H. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011;95:1–10. doi: 10.1016/j.prostaglandins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shearer G.C., Newman J.W. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr. Atheroscler. Rep. 2009;11:403–410. doi: 10.1007/s11883-009-0061-3. [DOI] [PubMed] [Google Scholar]
- 22.Luria A., Weldon S.M., Kabcenell A.K., Ingraham R.H., Matera D., Jiang H., Gill R., Morisseau C., Newman J., Hammock B.D. Compensatory Mechanism for Homeostatic Blood Pressure Regulation in Ephx2 Gene-disrupted Mice. J. Biol. Chem. 2007;282:2891–2898. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zivkovic A.M., Yang J., Georgi K., Hegedus C., Nording M.L., O’Sullivan A., German J.B., Hogg R.J., Weiss R.H., Bay C., et al. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman A.N. Omega-3 Fatty Acid Supplementation in Advanced Kidney Disease. Semin. Dial. 2010;23:396–400. doi: 10.1111/j.1525-139X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedman A.N., Yu Z., Tabbey R., Denski C., Tamez H., Wenger J., Thadhani R., Li Y., Watkins B.A. Low Blood Levels of Long-Chain n–3 Polyunsaturated Fatty Acids in US Hemodialysis Patients: Clinical Implications. Am. J. Nephrol. 2012;36:451–458. doi: 10.1159/000343741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim Y.J., Sidor N.A., Tonial N.C., Che A., Urquhart B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardi-ovascular Disease: Mechanisms and Therapeutic Targets. Toxins. 2021;13:142. doi: 10.3390/toxins13020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawlak D., Pawlak K., Malyszko J., Mysliwiec M., Buczko W. Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int. Urol. Nephrol. 2001;33:399–404. doi: 10.1023/A:1015238418500. [DOI] [PubMed] [Google Scholar]
- 28.Varshney A., Rehan M., Subbarao N., Rabbani G., Khan R.H. Elimination of Endogenous Toxin, Creatinine from Blood Plasma Depends on Albumin Conformation: Site Specific Uremic Toxicity & Impaired Drug Binding. PLoS ONE. 2011;6:e17230. doi: 10.1371/journal.pone.0017230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah V.O., Townsend R.R., Feldman H.I., Pappan K.L., Kensicki E., Vander Jagt D.L. Plasma Metabolomic Profiles in Different Stages of CKD. Clin. J. Am. Soc. Nephrol. 2012;8:363–370. doi: 10.2215/CJN.05540512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portilla D., Schnackenberg L., Beger R.D. Metabolomics as an Extension of Proteomic Analysis: Study of Acute Kidney Injury. Semin. Nephrol. 2007;27:609–620. doi: 10.1016/j.semnephrol.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee E.P., Clish C.B., Ghorbani A., Larson M.G., Elmariah S., McCabe E., Yang Q., Cheng S., Pierce K., Deik A., et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J. Am. Soc. Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyohara T., Akiyama Y., Suzuki T., Takeuchi Y., Mishima E., Tanemoto M., Momose A., Toki N., Sato H., Nakayama M., et al. Metabolomic profiling of uremic solutes in CKD patients. Hypertens. Res. 2010;33:944–952. doi: 10.1038/hr.2010.113. [DOI] [PubMed] [Google Scholar]
- 33.Watkins B.A., Kim J., Kenny A., Pedersen T.L., Pappan K.L., Newman J.W. Circulating levels of endocannabinoids and oxylipins altered by dietary lipids in older women are likely associated with previously identified gene targets. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2016;1861:1693–1704. doi: 10.1016/j.bbalip.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Watkins B.A. Endocannabinoids, exercise, pain, and a path to health with aging. Mol. Asp. Med. 2018;64:68–78. doi: 10.1016/j.mam.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Watkins B.A., Kim J., Tamez H., Wenger J., Thadhani R., Friedman A.N. Serum phospholipid fraction of polyunsaturated fatty acids is the preferred indicator for nutrition and health status in hemodialysis patients. J. Nutr. Biochem. 2016;38:18–24. doi: 10.1016/j.jnutbio.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Friedman A.N., Kim J., Kaiser S., Pedersen T.L., Newman J.W., Watkins B.A. Association between plasma endocannabinoids and appetite in hemodialysis patients: A pilot study. Nutr. Res. 2016;36:658–662. doi: 10.1016/j.nutres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida T., Nishiumi S., Tanahashi T., Yamasaki A., Yamazaki A., Akashi T., Miki I., Kondo Y., Inoue J., Kawauchi S., et al. Linoleoyl ethanolamide reduces lipopolysac-charide-induced inflammation in macrophages and ameliorates 2,4-dinitrofluorobenzene-induced contact dermatitis in mice. Eur. J. Pharmacol. 2013;699:6–13. doi: 10.1016/j.ejphar.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Artmann A., Petersen G., Hellgren L.I., Boberg J., Skonberg C., Nellemann C., Hansen S.H., Hansen H.S. Influence of dietary fatty acids on endocan-nabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Fu J., Astarita G., Gaetani S., Kim J., Cravatt B.F., Mackie K., Piomelli D. Food intake regulates oleoylethanolamide formation and deg-radation in the proximal small intestine. J. Biol. Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siriwardhana N., Kalupahana N.S., Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 41.Taccone-Gallucci M., Manca-di-Villahermosa S., Battistini L., Stuffler R.G., Tedesco M., Maccarrone M. N-3 PUFAs reduce oxi-dative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006;69:1450–1454. doi: 10.1038/sj.ki.5000291. [DOI] [PubMed] [Google Scholar]
- 42.Keenan A.H., Pedersen T.L., Fillaus K., Larson M.K., Shearer G.C., Newman J.W. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J. Lipid Res. 2012;53:1662–1669. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grapov D., Adams S.H., Pedersen T.L., Garvey W.T., Newman J.W. Type 2 Diabetes Associated Changes in the Plasma Non-Esterified Fatty Acids, Oxylipins and Endocannabinoids. PLoS ONE. 2012;7:e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephensen C.B., Armstrong P., Newman J.W., Pedersen T.L., Legault J., Schuster G.U., Kelley D., Vikman S., Hartiala J., Nassir R., et al. ALOX5 gene variants affect eicosanoid production and response to fish oil supplementation. J. Lipid Res. 2011;52:991–1003. doi: 10.1194/jlr.P012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shearer G.C., Harris W.S., Pedersen T.L., Newman J.W. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adas F., Berthou F., Picart D., Lozac’H P., Beaugé F., Amet Y. Involvement of cytochrome P450 2E1 in the (omega-1)-hydroxylation of oleic acid in human and rat liver microsomes. J. Lipid Res. 1998;39:1210–1219. doi: 10.1016/S0022-2275(20)32545-1. [DOI] [PubMed] [Google Scholar]
- 47.Laethem R.M., Koop D.R. Identification of rabbit cytochromes P450 2C1 and 2C2 as arachidonic acid epoxygenases. Mol. Pharmacol. 1992;42:958–963. [PubMed] [Google Scholar]
- 48.Oliw E.H. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Prog. Lipid Res. 1994;33:329–354. doi: 10.1016/0163-7827(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 49.Spector A.A., Fang X., Snyder G.D., Weintraub N. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2003;43:55–90. doi: 10.1016/S0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 50.Hasunuma K., Terano T., Tamura Y., Yoshida S. Formation of epoxyeicosatrienoic acids from arachidonic acid by cultured rat aortic smooth muscle cell microsomes. Prostagland. Leukot. Essent. Fat. Acids. 1991;42:171–175. doi: 10.1016/0952-3278(91)90153-V. [DOI] [PubMed] [Google Scholar]
- 51.Miller T.M., Donnelly M.K., Crago E.A., Roman D.M., Sherwood P.R., Horowitz M.B., Poloyac S.M. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J. Chromatogr. B. 2009;877:3991–4000. doi: 10.1016/j.jchromb.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimmel P.L., Chawla L.S., Amarasinghe A., Peterson R.A., Weihs K.L., Simmens S.J., Alleyne S., Burke H.B., Cruz I., Veis J.H. Anthropometric measures, cytokines and survival in haemodialysis patients. Nephrol. Dial. Transplant. 2003;18:326–332. doi: 10.1093/ndt/18.2.326. [DOI] [PubMed] [Google Scholar]
- 53.Noori N., Kovesdy C.P., Dukkipati R., Feroze U., Molnar M.Z., Bross R., Nissenson A.R., Kopple J.D., Norris K.C., Kalantar-Zadeh K. Racial and ethnic differences in mortality of hemo-dialysis patients: Role of dietary and nutritional status and inflammation. Am. J. Nephrol. 2011;33:157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Na H.-Y., Shim J.-Y., Lee H.-R., Jung D.-H., Kim H.-B., Park B.-J., Jung R.-J., Lee Y.-J. Sex Differences in the Relationship between Leukocyte Count and Chronic Kidney Disease: The 2007 Korean National Health and Nutrition Examination Survey. J. Women’s Health. 2011;20:99–105. doi: 10.1089/jwh.2010.2115. [DOI] [PubMed] [Google Scholar]
- 55.Newman J.W., Morisseau C., Hammock B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Roman R.J. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol. Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 57.Chuang C.-K., Lin S.-P., Chen H.-H., Chen Y.-C., Wang T.-J., Shieh W.-H., Wu C.-J. Plasma free amino acids and their metabolites in Taiwanese patients on hemodialysis and continuous ambulatory peritoneal dialysis. Clin. Chim. Acta. 2006;364:209–216. doi: 10.1016/j.cccn.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Metcoff J., Furst P., Scharer K., Distler G., Weber R., Mangold J., Graser T.A., Pfaff G., Schonberg D. Energy production, intracellular amino acid pools, and protein synthesis in chronic renal disease. J. Am. Coll. Nutr. 1989;8:271–284. doi: 10.1080/07315724.1989.10720302. [DOI] [PubMed] [Google Scholar]
- 59.Qi S., Ouyang X., Wang L., Peng W., Wen J., Dai Y. A pilot metabolic profiling study in serum of patients with chronic kidney disease based on (1) H-NMR-spectroscopy. Clin. Transl. Sci. 2012;5:379–385. doi: 10.1111/j.1752-8062.2012.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J., Shannon M., Ando Y., Schnackenberg L.K., Khan N.A., Portilla D., Beger R.D. Serum metabolomic profiles from patients with acute kidney injury: A pilot study. J. Chromatogr. B. 2012;893–894:107–113. doi: 10.1016/j.jchromb.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams S.H., Hoppel C.L., Lok K.H., Zhao L., Wong S.W., Minkler P.E., Hwang D.H., Newman J.W., Garvey W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou S., Ling X., Meng P., Liang Y., Shen K., Wu Q., Zhang Y., Chen Q., Chen S., Liu Y., et al. Cannabinoid receptor 2 plays a central role in renal tubular mitochondrial dysfunction and kidney ageing. J. Cell. Mol. Med. 2021;25:8957–8972. doi: 10.1111/jcmm.16857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dharnidharka V.R., Kwon C., Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 64.Cruz D.N., De Geus H.R., Bagshaw S.M. Biomarker Strategies to Predict Need for Renal Replacement Therapy in Acute Kidney Injury. Semin. Dial. 2011;24:124–131. doi: 10.1111/j.1525-139X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y., Wen P., Yang J., Niu J. Plasma Metabolomics Profiling in Maintenance Hemodialysis Patients Based on Liquid Chro-matography Quadrupole Time-of-Flight Mass Spectrometry. Kidney Dis. 2020;6:125–134. doi: 10.1159/000505156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckner T., Vanderlinden L.A., Johnson R.K., DeFelice B.C., Carry P.M., Seifert J., Waugh K., Dong F., Fiehn O., Clare-Salzler M., et al. Predictors of oxylipins in a healthy pediatric population. Pediatr. Res. 2020;89:1530–1540. doi: 10.1038/s41390-020-1084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gamaleddin I.H., Trigo J.M., Gueye A.B., Zvonok A., Makriyannis A., Goldberg S.R., Le Foll B. Role of the endogenous cannabinoid system in nicotine addiction: Novel insights. Front. Psychiatry. 2015;6:41. doi: 10.3389/fpsyt.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Seifert M.F., Lim S.-Y., Salem N., Watkins B.A. Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats. Br. J. Nutr. 2010;104:674–685. doi: 10.1017/S0007114510001133. [DOI] [PubMed] [Google Scholar]
- 69.Fiehn O., Wohlgemuth G., Scholz M., Kind T., Lee D.Y., Lu Y., Moon S., Nikolau B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008;53:691–704. doi: 10.1111/j.1365-313X.2007.03387.x. [DOI] [PubMed] [Google Scholar]
- 70.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 71.Ward J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963;58:236. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- 72.Van den Berg R.A., Hoefsloot H.C., Westerhuis J.A., Smilde A.K., van der Werf M.J. Centering, scaling, and transformations: Im-proving the biological information content of metabolomics data. BMC Genom. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Worth H., O’Hara D.V., Agarwal N., Collister D., Brennan F., Smyth B. Cannabinoids for Symptom Management in Patients with Kidney Failure: A Narrative Review. Clin. J. Am. Soc. Nephrol. 2022;17:911–921. doi: 10.2215/CJN.11560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sikorska-Wiśniewska M., Mika A., Sledzinski T., Małgorzewicz S., Stepnowski P., Rutkowski B., Chmielewski M. Disorders of serum omega-3 fatty acid composition in dialyzed patients, and their associations with fat mass. Ren. Fail. 2017;39:406–412. doi: 10.1080/0886022X.2017.1295870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The only available data can be found in the Supplemental Materials Tables.