Abstract
The ftsH gene encodes an ATP- and Zn2+-dependent metalloprotease which is anchored to the cytoplasmic membrane via two transmembrane segments in such a way that the very short amino- and the long carboxy termini are exposed to the cytoplasm. Deletion of the ftsH gene in Bacillus subtilis results in a pleiotropic phenotype such as filamentous growth. This observation prompted us to ask whether ftsH is involved in cell division. A translational fusion was constructed between the complete coding region of ftsH and gfp+ the latter carrying five point mutations to obtain enhanced fluorescence. We detected that the FtsH protein accumulates in the midcell septum of dividing cells, and during sporulation first in the asymmetrically located septa of sporulating cells and later in the membrane which engulfs the forespore. These observations revealed a new function of FtsH.
Early in the Escherichia coli and Bacillus subtilis cell cycles, a complex of several cell division proteins assembles into a ring-like structure at the future site of septation (reviewed in references 7 and 17). This septation complex is formed at a medial position and includes the conserved proteins FtsZ and FtsA (2, 6, 15, 16) as well as enzymes required to synthesize septal peptidoglycan (17, 29, 30). These proteins remain at the cell midpoint until late in the cell cycle, when a so far unknown signal triggers the onset of septal biogenesis, and a ring composed of FtsZ molecules constructs as the septum grows inward (1, 6, 19).
In B. subtilis, the onset of sporulation is marked by the relocalization of the division site from the midcell position to sites near both cell poles, producing two daughter cells differing in size, the smaller forespore and the larger mother cell (recently reviewed in reference 25). This alteration in the site of division is mediated by the assembly of two apparently identical complexes of cell division proteins, one near each pole of the early sporangium (15). After the bipolar assembly of cell division complexes, one is activated, synthesizing the asymmetrically positioned sporulation septum which separates the mother cell from the forespore. The second potential division complex remains inactive and is later disassembled. Then, the mother cell membrane migrates around the forespore starting near the edge of the septum, and the membranes fuse when they meet on the distal side of the forespore. This process has been designated engulfment.
The ftsH gene was detected in E. coli as a temperature-sensitive mutant forming filaments during growth at the restrictive temperature (21). This gene encodes an ATP- and Zn2+-dependent metalloprotease with a molecular mass of about 70 kDa (reviewed in reference 23). The FtsH protein is anchored in the cytoplasmic membrane via two transmembrane segments where both the short N and the long C terminus with the ATP- and Zn2+-binding sites are exposed to the cytoplasm. Several substrate proteins have been identified in E. coli, among them the regulator proteins ς32 and λ CII and the uncomplexed integral membrane proteins SecY and subunit α of the F1F0 ATPase (4, 12, 13, 26). In B. subtilis, the ftsH gene has been identified as a salt-sensitive insertion mutant (11). In contrast to E. coli, the ftsH gene of B. subtilis is not essential but the mutant cells exhibit a pleiotropic phenotype including filamentous growth (10). This observation prompted us to ask whether ftsH might be involved in cell division. To approach this question, a translational ftsH-gfp fusion was constructed and microscopically analyzed.
Isolation of a modified version of GFP.
To improve the sensitivity of green fluorescent protein (GFP) detection, the folding mutations of GFPuv (F99S, M153T, and V163A) (9) were combined with the chromophore mutations of GFPmut1 (F64L and S65T) (8) by site-directed mutagenesis of the gfpuv gene of pMS5, resulting in the gfp+ gene of pMN402 (details will be published elsewhere). The absorption and emission maxima of GFP+ were determined as 491 and 512 nm, respectively, and are very similar to those of all other class 2 GFPs (28). Using wild-type GFP as a reference, the fluorescence of E. coli expressing GFPuv, GFPmut1, and GFP+ was increased about 16-, 35-, and 130-fold, respectively (data not shown). This demonstrates that gfp+ is superior to both parent genes for in vivo detection.
Construction of an ftsH-gfp+ translational fusion.
To obtain a gene fusion between ftsH and gfp, the coding region of gfp was fused in frame to the penultimate amino acid residue of FtsH. This hybrid gene was constructed as follows. First, the polylinker of pUC18 was replaced by a 28-bp linker carrying the three restriction sites BamHI-EcoRV-BglII (pEH01). Then, ftsH was amplified using chromosomal DNA as template, flanked by BamHI and BglII sites, and inserted into pEH01, resulting in pEH02. Third, the 3′-terminal 378-bp EcoRI-BglII fragment of ftsH was generated by PCR, and its stop codon was replaced by recognition sequences for NheI and PstI (pWW01). Fourth, the coding region of gfp was PCR amplified using pMN402 as the template, flanked by NheI and PstI sites, and fused in-frame to ftsH (pFtsH-GFP). In a last step, the hybrid gene was recovered as a 2.7-kb BglII fragment and ligated into BamHI-linearized expression vector pX (14) and subsequently integrated at the amyE locus of strain 1012 (14). Expression of the chimeric protein is controlled by PxylA and xylR as described previously (14); a schematic representation of the hybrid gene integrated at the amyE locus is presented in Fig. 1. The resulting B. subtilis strain WW02 carries both wild-type and ftsH-gfp+ alleles. When the ftsH-gfp+ gene was expressed in an ftsH knockout, cells did not exhibit fluorescence. This finding indicates that the fusion protein might be unstable by itself or, more likely, cannot form the oligomeric structure described (24). In all subsequent experiments, the B. subtilis strain WW02 was grown in the presence of 0.1% xylose to induce expression of the ftsH-gfp+ gene.
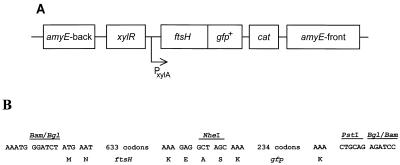
FIG. 1.
The ftsH-gfp+ gene fusion inserted at the chromosomal amyE locus of the B. subtilis chromosome. (A) Schematic drawing of the ftsH-gfp+ translational fusion fused to the xylose-regulatable promoter PxylA and sandwiched between amyE-front and amyE-back (not drawn to scale). (B) Partial DNA sequence of the ftsH-gfp+ fusion. Indicated is the DNA sequence at the immediate beginning of ftsH with the first two codons, the hybrid BamHI-BglII site, the last two codons of ftsH (K and E), two foreign codons introduced by the NheI restriction site (A and S), the first and the last codon of gfp, and the downstream PstI and the hybrid BglII-BamHI sites.
The FtsH-GFP protein accumulates in the midcell septum of dividing B. subtilis cells.
So far, there are no data concerning the localization of FtsH within the bacterial cell. Therefore, we asked whether FtsH is equally distributed within the B. subtilis cell or whether it might accumulate at specific sites, e.g., during cell division or during sporulation. To determine the location within the cell, we first analyzed bacterial cells carrying the ftsH-gfp+ fusion growing in the exponential phase in Luria broth at 37°C. In nondividing cells, FtsH-GFP is more or less equally distributed around the cell, though there seems to be some concentration of fluorescence in one region (Fig. 2A). Microscopic examination of cells in cross section revealed a strong fluorescence in the cell envelope (Fig. 2B). Both pictures are fully in agreement with published data that FtsH is anchored within the cytoplasmic membrane (27).
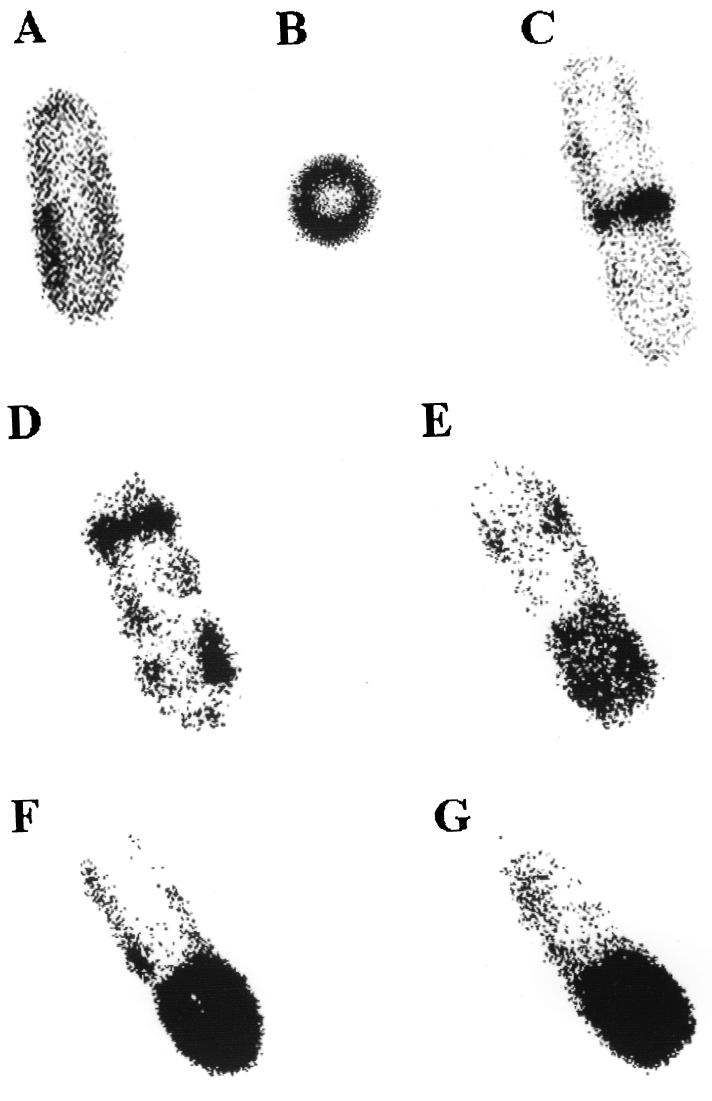
FIG. 2.
FtsH-GFP+ localization in live cells. Cells were grown either in Luria broth (A to C) or in DS medium (22) (D to G) in the presence of 0.1% xylose to induce ftsH-gfp+. FtsH-GFP was visualized in live cells as follows. Cells from 1.5 ml of growth medium were sedimented, resuspended in 200 μl of Tris-HCl buffer (pH 7.4), and mixed with 500 μl of 4% agarose; 10 to 20 μl was applied to a glass slide, covered with a coverglass, and allowed to cool for ∼1 min. A Leica (Heidelberg, Germany) TCS/SP confocal scanning laser microscope equipped with a argon-ion laser for excitation at 488 nm was used. Detection occurred at 510 nm, and the data from the channel were collected with fourfold averaging at a resolution of 512 by 512 pixels and processed using Corel Photo Point 7. Original magnification, ×20,000.
In dividing cells, we detected a strong accumulation of FtsH-GFP within the midcell septum (Fig. 2C). Inspection of many hundred cells revealed that about 15% of them exhibited accumulation of FtsH-GFP within the midcell septum. These observations indicate that FtsH concentrates in the midcell most probably within the membranes growing from the outside toward the interior of the dividing cells. What might be the function of FtsH during cell division? Three possibilities, not mutually exclusive, can be envisaged. Since FtsH belongs to the group of metalloproteases, it might be involved in the degradation of one or more proteins acting as inhibitors of septal biogenesis. In addition, there are indications that FtsH might also act as a molecular chaperone (3). Therefore, FtsH might aid insertion of one or more proteins into the cytoplasmic membrane, being part of the cell division complex. A third possibility might be that FtsH acts as a quality control system involved in the integration of integral membrane proteins and degrading those which fail to insert properly. Experiments are in progress to distinguish between these possibilities and to identify the target proteins of FtsH.
The FtsH-GFP protein accumulates in asymmetrically located septa in sporulating B. subtilis cells.
Sporulation involves a complex series of intracellular morphological events occurring in a temporal sequence and resulting in the formation of a heat-resistant endospore in B. subtilis. At the onset of sporulation, two polar ring-like structures can be seen, but only one is used for septation, which separates the forespore from the mother cell (15). As can be seen in Fig. 2D, there is strong fluorescence at one of these two polar ring-like structures and some labeling at the opposite pole. This picture is reminiscent of what Pogliano and coworkers have reported (18). Using time-lapse deconvolution microscopy, they showed that partial septa first appear and then disappear near the cell poles during sporulation. Later during sporulation, the fluorescence concentrates in one-third of the cell, most probably representing the forespore (Fig. 2E). In later stages, an intense fluorescence can be observed along the forespore, while that marking the second septum has disappeared (Fig. 2F and G). These sequences of pictures, not taken from one and the same cell, demonstrate, too, that FtsH accumulates in newly synthesized membranes. The mechanism by which this occurs is completely unknown. Newly synthesized FtsH might directly be channeled to the forespore. Alternatively, FtsH present in the forespore might be protected from degradation by deposition of the cortex, and those present in the mother cell might be degraded. Again, it can be assumed that the FtsH protein is involved in the degradation of one or more target proteins, in chaperoning integral membrane protein(s), or in both, thereby acting as a protein quality control system. It was recently published that the integral membrane protein SpoIVFA is substantially stabilized in the absence of FtsH, indicating that the metalloprotease might be involved in the degradation of this protein (20).
Conclusions.
The principal contribution of this study is the finding that FtsH accumulates in the midcell septum during vegetative cell division and at the onset of sporulation at positions near the cell poles that appear to coincide with future division sites. Then, FtsH becomes concentrated at the sporulation septum and disappears from the distal pole. This behavior is reminiscent of SpoIIE, which also first localizes near the two poles and later becomes concentrated at the sporulation septum (5). Later during the sporulation process, most of FtsH is found within the membrane engulfing the forespore. The function of FtsH during these two processes is not known but might involve its proven protease activity and/or its postulated chaperone function. What signal triggers accumulation of FtsH at these sites? Is only newly synthesized FtsH preferentially inserted at these sites, or does preformed FtsH migrate into these sites? In both cases, what causes either preferential insertion or migration of FtsH to its new location? These are some of the questions that remain to be answered.
Acknowledgments
We are indebted to C. Lehner for providing his confocal laser microscope and A. Herzig for his excellent introduction into the laser scanning microscopic technique. We thank Eva Harfst for constructing pEH01 and pEH02.
This work was supported by the DFG and the Fonds der Chemischen Industrie to W.S.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;78:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addinall S G, Lutkenhaus J. FtsZ is localized to theseptum in an FtsZ-dependent manner. J Bacteriol. 1996;178:7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama Y, Ehrmann M, Kihara A, Ito K. Polypeptide binding of Escherichia coli FtsH (HflB) Mol Microbiol. 1998;28:803–812. doi: 10.1046/j.1365-2958.1998.00843.x. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama Y, Kihara A, Ito K. Subunit a of proton ATPase F0 sector is a substrate of the FtsH protease in Escherichia coli. FEBS Lett. 1996;399:26–28. doi: 10.1016/s0014-5793(96)01283-5. [DOI] [PubMed] [Google Scholar]
- 5.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 6.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 7.Bouché J-P, Pick E. On the birth and fate of bacterial division sites. Mol Microbiol. 1998;29:19–26. doi: 10.1046/j.1365-2958.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 8.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 9.Crameri A, Whitehorn E A, Tate E, Stemmer W P C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 10.Deuerling E, Mogk A, Richter C, Purucker M, Schumann W. The ftsH gene of Bacillus subtilis is involved in major cellular processes such as sporulation, stress adaptation and secretion. Mol Microbiol. 1997;23:921–933. doi: 10.1046/j.1365-2958.1997.2721636.x. [DOI] [PubMed] [Google Scholar]
- 11.Geisler U, Schumann W. Isolation of stress mutants of Bacillus subtilis by a novel genetic method. FEMS Microbiol Lett. 1993;108:251–254. doi: 10.1111/j.1574-6968.1993.tb06110.x. [DOI] [PubMed] [Google Scholar]
- 12.Kihara A, Akiyama Y, Ito K. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc Natl Acad Sci USA. 1995;92:4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kihara A, Akiyama Y, Ito K. Host regulation of lysogenic decision in bacteriophage lambda: transmembrane modulation of FtsH (HflB), the cII degrading protease, by HflKC (HflA) Proc Natl Acad Sci USA. 1997;94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim L, Mogk A, Schumann W. A xylose-inducible Bacillus subtilis integration vector and its application. Gene. 1996;181:71–76. doi: 10.1016/s0378-1119(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 15.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 16.Ma X L, Ehrhardt D W, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanninga N. Morphogenesis of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogliano J, Orborne N, Sharp M, Abanes-De Mello A, Perez A, Sun Y-L, Pogliano K. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–1159. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogliano J, Pogliano K, Weiss D S, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnekov O. Role of the sporulation protein BofA, in regulating activation of the Bacillus subtilis developmental transcription factor ςK. J Bacteriol. 1999;181:5384–5388. doi: 10.1128/jb.181.17.5384-5388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos D, Almeida D F. Isolation and characterization of a new temperature-sensitive cell division mutant of Escherichia coli K-12. J Bacteriol. 1975;124:1502–1507. doi: 10.1128/jb.124.3.1502-1507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaeffer P, Millet J, Aubert J P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumann W. FtsH—a single-chain charonin? FEMS Microbiol Rev. 1999;23:1–11. doi: 10.1111/j.1574-6976.1999.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 24.Shotland Y, Koby S, Teff D, Mansur N, Oren D A, Tatematsu K, Tomoyasu T, Kessel M, Bukau B, Ogura T, Oppenheim A B. Proteolysis of the phage lambda CII regulatory protein by FtsH (HflB) of Escherichia coli. Mol Microbiol. 1997;24:1303–1310. doi: 10.1046/j.1365-2958.1997.4231796.x. [DOI] [PubMed] [Google Scholar]
- 25.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 26.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraga S, Ogura T. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomoyasu T, Yamanaka K, Murata K, Suzaki T, Bouloc P, Kato A, Niki H, Hiraga S, Ogura T. Topology and subcellular localization of FtsH protein in Escherichia coli. J Bacteriol. 1993;175:1352–1357. doi: 10.1128/jb.175.5.1352-1357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Lutkenhaus J. FtsZ-ring: the eubacterial division apparatus conserved in archaebacteria. Mol Microbiol. 1996;21:313–319. doi: 10.1046/j.1365-2958.1996.6421360.x. [DOI] [PubMed] [Google Scholar]
- 30.Weiss D S, Pogliano K, Carson M, Guzman L-M, Fraipont C, Nguyen-Distèche M, Losick R, Beckwith J. Localization of the Escherichia coli cell division protein FtsI (PBP3) to the division site and cell pole. Mol Microbiol. 1997;25:671–681. doi: 10.1046/j.1365-2958.1997.5041869.x. [DOI] [PubMed] [Google Scholar]