Abstract
Using a new-generation drug-eluting stent, we compared the 2-year clinical outcomes of patients with diabetes mellitus (DM) and non-DM concomitant with a non-ST-segment elevation myocardial infarction (NSTEMI) and an ST-segment elevation myocardial infarction (STEMI) who underwent percutaneous coronary intervention. A total of 11,798 patients with acute myocardial infarction were classified into two groups: DM (NSTEMI, n = 2399; STEMI, n = 2693) and non-DM (NSTEMI, n = 2694; STEMI, n = 4012). The primary clinical outcome was the occurrence of major adverse cardiac events (MACE), defined as all-cause death, recurrent myocardial infarction, or any coronary repeat revascularization. The secondary outcome was the occurrence of definite or probable stent thrombosis. In all the patients, both multivariable and propensity score-adjusted analyses revealed that the incidence rates of MACE (adjusted hazard ratio (aHR), 1.214; p = 0.006 and aHR, 1.298; p = 0.002, respectively), all-cause death, cardiac death (CD), and non-CD rate were significantly higher in the NSTEMI group than in the STEMI group. Additionally, among patients with NSTEMI, there was a higher non-CD rate (aHR, 2.200; p = 0.007 and aHR, 2.484; p = 0.004, respectively) in the DM group and a higher CD rate (aHR, 2.688; p < 0.001 and 2.882; p < 0.001, respectively) in the non-DM group. In this retrospective study, patients with NSTEMI had a significantly higher 2-year mortality rate than those with STEMI did. Furthermore, strategies to reduce the non-CD rate in patients with DM and the CD rate in patients without DM could be beneficial for those with NSTEMI.
Keywords: diabetes, non-ST-elevation myocardial infarction, outcomes, ST-elevation myocardial infarction
1. Introduction
Diabetes mellitus (DM), particularly type 2 DM (T2DM), is one of the most important threats to public health in the twenty-first century [1]. In the Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (AMI) trial [2], patients with DM and those with newly diagnosed DM had higher 3-year death rates than non-DM patients (11%, 12%, and 6%, respectively). Thrombus formation after rupture or erosion of vulnerable atherosclerotic plaques is a common pathophysiology in both non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI) [3]. However, patients with NSTEMI have partial or intermittent occlusion of the coronary artery, whereas patients with STEMI often have complete occlusion [4]. Additionally, after a certain duration of complete ischemia, there are no interventions that can salvage the ischemic myocardium [5]. Because cardiogenic shock complicates an increasing number of STEMI cases [6], Polonski et al. suggested that in-hospital outcomes were worse in patients with STEMI than in those with NSTEMI. Patients with NSTEMI have a greater prevalence of comorbidities [7] and have received relatively fewer guideline-based treatments than patients with STEMI [8]. Hence, long-term mortality is higher in patients with NSTEMI than in those with STEMI [9,10]. Until now, comparative results between NSTEMI and STEMI have been conflicting, and further discussion is needed [10,11]. Because recent reports concerning long-term clinical outcomes in patients with and without DM are confined to patients with NSTEMI [12] or STEMI [13], data on head-to-head comparisons between long-term clinical outcomes in NSTEMI and STEMI in patients with and without DM are scarce. Currently, new-generation drug-eluting stents (DESs) have nearly replaced bare-metal stents (BMSs) and first-generation DESs (1G-DESs) for routine percutaneous coronary intervention (PCI) [14]. New-generation DESs are more effective than 1G-DESs in reducing major clinical outcomes in patients with DM [14]. Furthermore, to the best of our knowledge, no specific large-scale study has compared the long-term clinical outcomes between the NSTEMI and STEMI groups in patients with and without DM after PCI using new-generation DESs to reflect current real-world practice. Hence, this study, using a new-generation DES, evaluated the 2-year comparative clinical outcomes between two different types of AMI (NSTEMI versus STEMI) in patients with DM and non-DM who underwent successful PCI.
2. Methods
2.1. Study Population
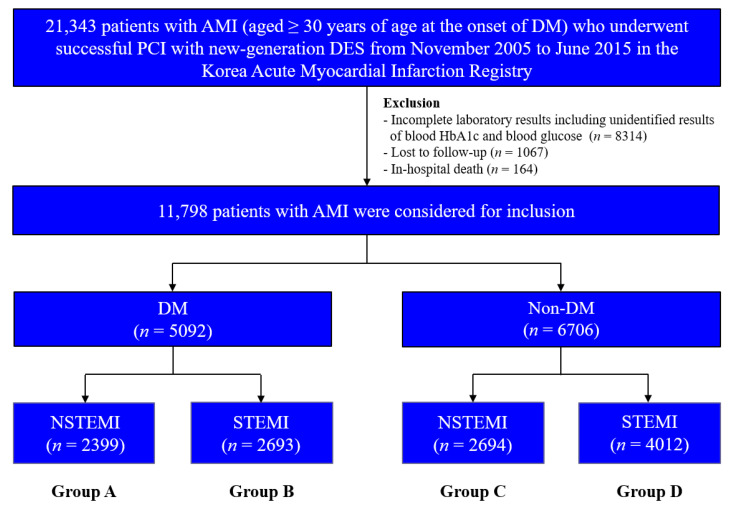
This non-randomized multicenter observational retrospective cohort study enrolled 21,343 patients with AMI aged ≥30 years at the onset of DM who underwent successful PCI with newer-generation DESs between November 2005 and June 2015; data was obtained from the Korea AMI Registry (KAMIR). To ensure that only individuals with T2DM were included, patients aged <30 years at the onset of DM were excluded based on a previous study [15]. KAMIR was established in November 2005 and involves more than 50 communities and teaching hospitals in South Korea [16]. Patients with the following conditions were excluded: incomplete laboratory results including unidentified results of blood hemoglobin (Hb) A1c and blood glucose (n = 8314; 39.0%), lost to follow-up (n = 1067; 5.0%), and in-hospital death (n = 164; 0.8%). After exclusion, 11,798 patients with AMI who underwent successful PCI using new-generation DESs were included. The patients were classified into DM (n = 5092; 43.2%) and non-DM (n = 6706; 56.8%) groups (Figure 1). Thereafter, these two groups were further sub-classified into NSTEMI (group A, n = 2399 (47.1%) and group C, n = 2694 (40.2%), respectively) and STEMI (group B, n = 2693 (52.9%) and group D, n = 4012 (59.8%), respectively). This study was conducted in accordance with the ethical guidelines of the 2004 Declaration of Helsinki and was approved by the ethics committee of each participating center and the Chonnam National University Hospital Institutional Review Board ethics committee (CNUH-2011-172). All 11,798 patients included in the study provided written informed consent prior to enrollment and completed a 2-year clinical follow-up through face-to-face interviews, phone calls, or chart reviews. An independent event adjudication committee evaluated all the clinical events. The event adjudication process has been previously described by KAMIR investigators [16].
Figure 1.
Flowchart. AMI, acute myocardial infarction; DM, diabetes mellitus; DES, drug-eluting stent; Hb, hemoglobin; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
2.2. Percutaneous Coronary Intervention and Medical Treatment
Diagnostic coronary angiography and PCI were performed according to the general guidelines [17]. Loading doses of aspirin (200–300 mg), clopidogrel (300–600 mg), ticagrelor (180 mg), and prasugrel (60 mg) were administered to all the enrolled patients prior to PCI. Subsequently, dual antiplatelet therapy (a combination of aspirin (100 mg/day) with clopidogrel (75 mg/day), or ticagrelor (90 mg twice a day), or prasugrel (5–10 mg/day)) was recommended for >12 months. Based on previous reports [18,19], triple antiplatelet therapy was administered (100 mg of cilostazol was administered twice a day in addition to the dual antiplatelet therapy) at the discretion of the individual operator. Moreover, the access site, revascularization strategy, and DES selection were assigned to the individual operators and were to be performed at their own discretion.
2.3. Study Definitions and Clinical Outcomes
The DM group included patients with HbA1c, fasting plasma glucose, and/or random plasma glucose levels of ≥6.5%, ≥126 mg/dL (7.0 mmol/L), and ≥200 mg/dL (11.1 mmol/L) at index hospitalization, respectively, according to the American Association’s clinical practice recommendations [20]. In addition to their medical history, patients with known diabetes, for which they received medical treatment (insulin or antidiabetic), or newly diagnosed diabetes were also included in the DM group. NSTEMI was defined as the absence of persistent ST-segment elevation with increased levels of cardiac biomarkers and the appropriate clinical context [21,22]. STEMI was defined as follows: ongoing chest pain and electrocardiogram findings on admission showing an ST-segment elevation in at least two contiguous leads of ≥2 mm (0.2 mV) in men or ≥1.5 mm (0.15 mV) in women in leads V2–V3 and/or ≥1 mm (0.1 mV) in other contiguous chest leads, limb leads, or new-onset left bundle branch block [23,24]. The primary clinical outcome of this study was the occurrence of major adverse cardiac events (MACE), defined as all-cause death, recurrent myocardial infarction (re-MI), or repeat coronary revascularization, including target lesion revascularization, target vessel revascularization (TVR), and non-TVR. The secondary outcome was definite or probable stent thrombosis during the 2-year follow-up period. All-cause death was considered as cardiac death (CD) unless an undisputed non-cardiac cause was present [25]. The definitions of re-MI, target lesion revascularization, TVR, and non-TVR have previously been published [26]. The types of new-generation DESs used are listed in Table 1. Glomerular function was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation for the estimated glomerular filtration rate (eGFR) [27]. In our study, patients who required multivessel PCI included those who underwent PCI of the non-infarct-related artery (IRA) during index PCI of the IRA or who underwent staged PCI for the non-IRA within the index hospitalization. Therefore, patients who underwent staged PCI after discharge were excluded from this study because reperfusion timing could have acted as a bias.
Table 1.
Baseline clinical, laboratory, angiographic, and procedural characteristics.
| Variables | Overall (n = 11,798) |
DM (n = 5092) |
Non-DM (n = 6706) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| NSTEMI (n = 5093) |
STEMI (n = 6705) |
p Value | NSTEMI (n = 2399) |
STEMI (n = 2693) |
p Value | NSTEMI (n = 2694) |
STEMI (n = 4012) |
p Value | |
| Male, n (%) | 3626 (71.2) | 5184 (77.3) | <0.001 | 1585 (66.1) | 1996 (74.1) | <0.001 | 2041 (75.8) | 3188 (79.5) | 0.001 |
| Age, years | 64.4 ± 12.0 | 62.1 ± 12.5 | <0.001 | 65.5 ± 11.3 | 63.0 ± 11.9 | <0.001 | 63.5 ± 12.6 | 61.4 ± 13.0 | <0.001 |
| LVEF, % | 54.1 ± 11.1 | 50.7 ± 11.0 | <0.001 | 52.6 ± 11.9 | 50.0 ± 11.1 | <0.001 | 55.4 ± 10.2 | 51.2 ± 10.8 | <0.001 |
| BMI, kg/m2 | 24.1 ± 3.2 | 24.2 ± 3.1 | 0.076 | 24.3 ± 3.1 | 24.5 ± 3.1 | 0.017 | 24.0 ± 3.2 | 24.0 ± 3.1 | 0.404 |
| SBP, mmHg | 134.8 ± 26.6 | 127.7 ± 27.9 | <0.001 | 134.5 ± 26.8 | 128.1 ± 28.6 | <0.001 | 135.0 ± 26.5 | 127.5 ± 27.5 | <0.001 |
| DBP, mmHg | 80.7 ± 15.2 | 78.4 ± 16.8 | <0.001 | 79.8 ± 15.1 | 77.8 ± 17.0 | <0.001 | 81.5 ± 15.7 | 78.7 ± 16.7 | <0.001 |
| Cardiogenic shock, n (%) | 113 (2.2) | 395 (5.9) | <0.010 | 61 (2.5) | 176 (6.5) | <0.001 | 52 (1.9) | 219 (5.5) | <0.001 |
| Killip class I/II, n (%) | 4440 (87.2) | 5613 (83.7) | <0.001 | 2013 (83.9) | 2211 (82.1) | 0.087 | 2427 (90.1) | 3402 (84.8) | <0.001 |
| CPR on admission, n (%) | 122 (2.4) | 374 (5.6) | <0.001 | 60 (2.5) | 128 (4.8) | <0.001 | 62 (2.3) | 246 (6.1) | <0.001 |
| Hypertension, n (%) | 2779 (54.6) | 3120 (46.5) | <0.001 | 1540 (64.2) | 1532 (56.9) | <0.001 | 1239 (46.0) | 1588 (39.6) | <0.001 |
| Dyslipidemia, n (%) | 661 (13.0) | 736 (11.0) | 0.001 | 375 (15.6) | 356 (13.2) | 0.014 | 286 (10.6) | 380 (9.5) | 0.134 |
| Previous MI, n (%) | 234 (4.6) | 196 (2.9) | <0.001 | 149 (6.2) | 98 (3.6) | <0.001 | 85 (3.2) | 98 (2.4) | 0.079 |
| Previous PCI, n (%) | 386 (7.6) | 297 (4.4) | <0.001 | 239 (10.0) | 154 (5.7) | <0.001 | 147 (5.5) | 143 (3.6) | <0.001 |
| Previous CABG, n (%) | 31 (0.6) | 20 (0.3) | 0.015 | 25 (1.0) | 14 (0.5) | 0.036 | 6 (0.2) | 6 (0.1) | 0.561 |
| Previous HF, n (%) | 72 (1.4) | 55 (0.8) | 0.002 | 50 (2.1) | 28 (1.0) | 0.003 | 22 (0.8) | 27 (0.7) | 0.498 |
| Previous CVA, n (%) | 387 (7.6) | 326 (4.9) | <0.001 | 224 (9.3) | 168 (6.2) | <0.001 | 163 (6.1) | 158 (3.9) | <0.001 |
| Current smokers, n (%) | 1922 (37.7) | 3194 (47.6) | <0.001 | 803 (33.5) | 1182 (43.9) | <0.001 | 1119 (41.5) | 2012 (50.1) | <0.001 |
| Peak CK-MB, mg/dL | 23 (7.0–82.9) | 124.0 (34.6–268.5) | <0.001 | 18.3 (6.0–63.4) | 102.4 (26.9–236.2) | <0.001 | 29.5 (8.7–103.1) | 140.5 (39.6–290.8) | <0.001 |
| Peak Troponin-I, ng/mL | 11.0 (2.2–47.8) | 46.8 (16.0–61.2) | <0.001 | 8.7 (1.7–42.0) | 48.1 (18.5–67.8) | <0.001 | 12.7 (2.6–47.8) | 47.8 (12.7–52.1) | <0.001 |
| Blood glucose, mg/dL | 171.2 ± 88.6 | 184.6 ± 83.3 | <0.001 | 216.5 ± 103.1 | 234.3 ± 95.9 | <0.001 | 130.6 ± 43.4 | 150.8 ± 50.8 | <0.001 |
| Hemoglobin A1c, % | 6.64 ± 2.08 | 6.57 ± 2.22 | 0.074 | 7.72 ± 2.60 | 7.90 ± 3.00 | 0.022 | 5.67 ± 0.45 | 5.67 ± 0.45 | 0.800 |
| NT-ProBNP, pg/mL | 658.0 (160.0–2740.5) | 265.0 (59.0–1443.0) | <0.001 | 1325.5 (301.0–5417.0) | 453.5 (89.0–2621.8) | <0.001 | 484.0 (130.5–1857.5) | 225.0 (53.0–1195.0) | <0.001 |
| Hs-CRP, mg/dL | 10.1 ± 46.8 | 9.4 ± 37.9 | 0.427 | 10.7 ± 43.9 | 11.3 ± 43.1 | 0.596 | 9.5 ± 49.2 | 8.2 ± 33.9 | 0.210 |
| Serum creatinine, mg/dL | 1.17 ± 1.70 | 1.06 ± 1.20 | <0.001 | 1.34 ± 2.21 | 1.10 ± 0.86 | <0.001 | 1.03 ± 1.06 | 1.03 ± 1.39 | 0.822 |
| eGFR, mL/min/1.73 m2 | 88.3 ± 45.3 | 87.3 ± 37.2 | 0.215 | 83.1 ± 46.0 | 85.3 ± 41.3 | 0.074 | 92.8 ± 44.1 | 88.6 ± 34.1 | <0.001 |
| Total cholesterol, mg/dL | 181.8 ± 46.3 | 184.0 ± 43.7 | 0.013 | 177.3 ± 49.8 | 180.5 ± 45.8 | 0.015 | 186.0 ± 42.4 | 186.3 ± 42.1 | 0.795 |
| Triglyceride, mg/L | 136.3 ± 117.5 | 137.9 ± 109.8 | 0.445 | 150.2 ± 136.2 | 151.0 ± 124.0 | 0.814 | 123.9 ± 96.3 | 129.0 ± 98.2 | 0.032 |
| HDL cholesterol, mg/L | 43.0 ± 14.1 | 43.3 ± 15.4 | 0.291 | 41.6 ± 13.5 | 42.0 ± 14.4 | 0.359 | 44.2 ± 14.4 | 44.1 ± 16.0 | 0.869 |
| LDL cholesterol, mg/L | 114.7 ± 41.7 | 115.9 ± 39.5 | 0.096 | 109.3 ± 38.8 | 111.8 ± 37.2 | 0.018 | 119.5 ± 43.6 | 118.7 ± 40.8 | 0.467 |
| Diabetes management | |||||||||
| Diet, n (%) | 166 (3.3) | 244 (3.6) | 0.287 | 166 (6.9) | 244 (9.0) | 0.005 | |||
| Oral agent, n (%) | 1492 (29.3) | 1681 (25.1) | <0.001 | 1492 (62.2) | 1681 (62.4) | 0.885 | |||
| Insulin, n (%) | 160 (3.1) | 128 (1.9) | <0.001 | 160 (6.7) | 128 (4.8) | 0.003 | |||
| Untreated, n (%) | 581 (11.4) | 640 (9.5) | 0.001 | 581 (24.2) | 640 (23.8) | 0.718 | |||
| Discharge medications | |||||||||
| Aspirin, n (%) | 4940 (97.2) | 6484 (96.7) | 0.370 | 2326 (97.0) | 2596 (96.4) | 0.268 | 2614 (97.0) | 3888 (96.9) | 0.777 |
| Clopidogrel, n (%) | 4325 (84.9) | 5780 (86.2) | 0.049 | 2115 (88.2) | 2335 (86.7) | 0.118 | 2210 (82.0) | 3445 (85.9) | <0.001 |
| Ticagrelor, n (%) | 484 (9.5) | 607 (9.1) | 0.403 | 183 (7.6) | 219 (8.1) | 0.532 | 301 (11.2) | 388 (9.7) | 0.047 |
| Prasugrel, n (%) | 236 (4.6) | 366 (5.5) | 0.047 | 101 (4.2) | 139 (5.2) | 0.112 | 135 (4.4) | 227 (5.7) | 0.270 |
| Cilostazol, n (%) | 887 (17.4) | 1242 (18.5) | 0.122 | 462 (19.3) | 538 (20.0) | 0.525 | 425 (15.8) | 704 (17.5) | 0.058 |
| ACEIs, n (%) | 2581 (50.7) | 3849 (57.4) | <0.001 | 1133 (47.2) | 1455 (54.0) | <0.001 | 1448 (53.7) | 2394 (59.7) | <0.001 |
| ARBs, n (%) | 1558 (30.6) | 1576 (23.5) | <0.001 | 828 (34.5) | 712 (26.4) | <0.001 | 730 (27.1) | 864 (21.5) | <0.001 |
| BBs, n (%) | 4219 (82.8) | 5641 (84.1) | 0.061 | 2007 (83.7) | 2266 (84.1) | 0.639 | 2212 (82.1) | 3375 (84.1) | 0.030 |
| CCBs, n (%) | 501 (9.8) | 243 (3.6) | <0.001 | 265 (11.0) | 115 (4.3) | <0.001 | 236 (8.8) | 128 (3.2) | <0.001 |
| Lipid lowering agents, n (%) | 4427 (86.9) | 5746 (85.7) | 0.056 | 2038 (85.0) | 2262 (84.0) | 0.347 | 2389 (88.7) | 3484 (86.8) | 0.025 |
| Year of index MI | <0.001 | <0.001 | <0.001 | ||||||
| Before 2013 | 2959 (58.1) | 4372 (65.2) | 1480 (61.7) | 1834 (68.1) | 1479 (54.9) | 2538 (63.3) | |||
| After 2013 | 2134 (41.9) | 2333 (34.8) | 919 (38.3) | 859 (31.9) | 1215 (45.1) | 1474 (36.7) | |||
| IRA | |||||||||
| Left main, n (%) | 127 (2.5) | 75 (1.1) | <0.001 | 66 (2.8) | 31(1.2) | <0.001 | 61 (2.3) | 44 (1.1) | <0.001 |
| LAD, n (%) | 2178 (42.8) | 3548 (52.9) | <0.001 | 1006 (41.9) | 1343 (49.9) | <0.001 | 1172 (43.5) | 2205 (55.0) | <0.001 |
| LCx, n (%) | 1364 (26.8) | 592 (8.8) | <0.001 | 625 (26.1) | 233 (8.7) | <0.001 | 739 (27.4) | 359 (8.9) | <0.001 |
| RCA, n (%) | 1424 (28.0) | 2490 (37.1) | <0.001 | 702 (29.3) | 1086 (40.3) | <0.001 | 722 (26.8) | 1404 (35.0) | <0.001 |
| Treated vessel | |||||||||
| Left main, n (%) | 214 (4.2) | 115 (1.7) | <0.001 | 105 (4.4) | 44 (1.6) | <0.001 | 109 (4.0) | 71 (1.8) | <0.001 |
| LAD, n (%) | 2888 (56.7) | 4035 (60.2) | <0.001 | 1378 (57.4) | 1582 (58.7) | 0.346 | 1510 (56.1) | 2453 (61.1) | <0.001 |
| LCx, n (%) | 2016 (39.6) | 1076 (16.0) | <0.001 | 957 (39.9) | 454 (16.9) | <0.001 | 1059 (39.3) | 622 (15.5) | <0.001 |
| RCA, n (%) | 1868 (36.7) | 2824 (42.1) | <0.001 | 937 (39.1) | 1236 (45.9) | <0.001 | 931 (34.6) | 1588 (39.6) | <0.001 |
| ACC/AHA lesion type | |||||||||
| Type B1, n (%) | 703 (13.8) | 857 (12.8) | 0.105 | 327 (13.6) | 327 (12.1) | 0.113 | 376 (14.0) | 530 (13.2) | 0.381 |
| Type B2, n (%) | 1797 (35.3) | 2031 (30.3) | <0.001 | 835 (34.8) | 825 (30.6) | 0.002 | 962 (35.7) | 1206 (30.1) | <0.001 |
| Type C, n (%) | 2124 (41.7) | 3139 (46.8) | <0.001 | 1025 (42.7) | 1280 (47.5) | 0.001 | 1099 (40.8) | 1859 (46.3) | <0.001 |
| Extent of CAD | |||||||||
| 1-vessel, n (%) | 2208 (43.4) | 3551 (53.0) | <0.001 | 910 (37.9) | 1267 (47.0) | <0.001 | 1298 (48.2) | 2284 (56.9) | <0.001 |
| 2-vessel, n (%) | 1724 (33.9) | 2010 (30.0) | <0.001 | 848 (35.3) | 845 (31.4) | 0.003 | 876 (32.6) | 1165 (29.0) | 0.002 |
| ≥3-vessel, n (%) | 1161 (22.8) | 1144 (17.1) | <0.001 | 641 (26.7) | 581 (21.6) | <0.001 | 520 (19.3) | 563 (14.0) | <0.001 |
| Pre-PCI TIMI 0/1, n (%) | 2047 (40.2) | 4781 (71.3) | <0.001 | 903 (37.6) | 1884 (70.0) | <0.001 | 1144 (42.5) | 2897 (72.2) | <0.001 |
| PCI within 24 h, n (%) | 4396 (86.3) | 6477 (96.6) | <0.001 | 2040 (85.0) | 2599 (96.5) | <0.001 | 2356 (87.5) | 3878 (96.7) | <0.001 |
| GP IIb/IIIa inhibitor, n (%) | 480 (9.4) | 1458 (21.7) | <0.001 | 202 (8.4) | 527 (19.6) | <0.001 | 278 (10.3) | 931 (23.2) | <0.001 |
| Transradial approach, n (%) | 1833 (36.0) | 1218 (18.2) | <0.001 | 815 (34.0) | 493 (18.3) | <0.001 | 1018 (37.8) | 725 (18.1) | <0.001 |
| IVUS, n (%) | 1241 (24.4) | 1352 (20.2) | <0.001 | 539 (22.5) | 542 (20.1) | 0.041 | 702 (26.1) | 810 (20.2) | <0.001 |
| OCT, n (%) | 67 (1.3) | 22 (0.3) | <0.001 | 26 (1.1) | 10 (0.4) | 0.004 | 41 (1.5) | 12 (0.3) | <0.001 |
| FFR, n (%) | 75 (1.5) | 61 (0.9) | 0.448 | 38 (1.6) | 24 (0.9) | 0.029 | 37 (1.4) | 37 (0.9) | 0.095 |
| Types of DES a | |||||||||
| ZES, n (%) | 1647 (32.3) | 2372 (35.4) | 0.206 | 789 (32.9) | 976 (36.2) | 0.012 | 858 (31.8) | 1396 (34.8) | 0.012 |
| EES, n (%) | 2710 (53.2) | 3374 (50.3) | 0.193 | 1289 (53.7) | 1336 (49.6) | 0.003 | 1421 (52.7) | 2038 (50.8) | 0.117 |
| BES, n (%) | 783 (15.4) | 873 (13.0) | 0.078 | 335 (14.0) | 330 (12.3) | 0.071 | 448 (16.6) | 543 (13.5) | <0.001 |
| Others, n (%) | 124 (2.4) | 190 (2.8) | 0.710 | 72 (3.0) | 89 (3.3) | 0.575 | 52 (1.9) | 101 (2.5) | 0.133 |
| Stent diameter, mm | 3.18 ± 0.42 | 3.07 ± 0.42 | <0.001 | 3.15 ± 0.42 | 3.04 ± 0.41 | <0.001 | 3.19 ± 0.41 | 3.09 ± 0.42 | <0.001 |
| Stent length, mm | 27.8 ± 12.8 | 26.7 ± 10.2 | 0.019 | 28.0 ± 12.9 | 27.0 ± 10.5 | 0.002 | 27.6 ± 12.6 | 26.5 ± 10.0 | <0.001 |
| Number of stents | 1.61 ± 0.89 | 1.40 ± 0.70 | <0.001 | 1.67 ± 0.90 | 1.44 ± 0.74 | <0.001 | 1.56 ± 0.88 | 1.40 ± 0.66 | <0.001 |
Values are means ± standard deviation or median (interquartile range) or numbers and percentages. The p values for continuous data were obtained from the unpaired t-test. The p values for categorical data were obtained from the chi-squared or Fisher’s exact test. DM, diabetes mellitus; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; LVEF, left ventricular ejection fraction; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; HF, heart failure; CVA, cerebrovascular accidents; eGFR, estimated glomerular filtration rate; CK-MB, creatine kinase myocardial band; NT-ProBNP, N-terminal pro-brain natriuretic peptide; Hs-CRP, high sensitivity-C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BBs, beta-blockers; CCBs, calcium channel blockers; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; RCA, right coronary artery; ACC/AHA, American College of Cardiology/American Heart Association; CAD, coronary artery disease; TIMI, thrombolysis in myocardial infarction; ZES, zotarolimus-eluting stent; EES, everolimus-eluting stent; BES, biolimus-eluting stent; GP, glycoprotein; IVUS, intravascular ultrasound; OCT, optical coherence tomography; FFR, fractional flow reserve; a Drug-eluting stents were composed of ZES (Resolute Integrity stent; Medtronic, Inc., Minneapolis, MN, USA), EES (Xience Prime stent, Abbott Vascular, Santa Clara, CA, USA; or Promus Element stent, Boston Scientific, Natick, MA, USA), and BES (BioMatrix Flex stent, Biosensors International, Morges, Switzerland; or Nobori stent, Terumo Corporation, Tokyo, Japan).
2.4. Statistical Analyses
For continuous variables, between-group differences were evaluated using unpaired t-tests. Data are expressed as mean ± standard deviation. For discrete variables, between-group differences were expressed as counts and percentages and analyzed using the chi-squared or Fisher’s exact test. We tested all variables with a p value of <0.001 in the univariate analysis between the NSTEMI and STEMI groups. After the univariate analysis, we performed a multicollinearity test [28] between the included variables to confirm that there was no definite collinearity between them (Supplementary Table S1). The variance inflation factor values were calculated to measure the degree of multicollinearity among the variables. A variance inflation factor of >5 indicates a high correlation [29]. Multicollinearity was considered when the tolerance value was <0.1 [30] or the condition index was >10 [29]. The variables included in the multivariable Cox regression analysis were as follows: male sex, age, left ventricular ejection fraction (LVEF), body mass index, systolic blood pressure, diastolic blood pressure, cardiogenic shock, Killip class I/II, cardiopulmonary resuscitation (CPR) on admission, hypertension, dyslipidemia, previous myocardial infarction, previous PCI, previous coronary artery bypass graft, previous heart failure, previous cerebrovascular accidents (CVA), current smoker, peak creatine kinase myocardial band (CK-MB), peak troponin-I, N-terminal pro-brain natriuretic peptide (NT-ProBNP), serum creatinine, eGFR, total cholesterol, triglyceride, low-density lipoprotein cholesterol, clopidogrel, ticagrelor, prasugrel, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), beta-blocker, calcium channel blocker, and lipid-lowering agent. Moreover, to adjust for potential confounders, a propensity score (PS)-adjusted analysis was performed using a logistic regression model. We tested all the available variables that could be of potential relevance, including baseline clinical, angiographic, and procedural factors (Table 1). The c-statistic for the PS-matched analysis in this study was 0.712. Patients in the NSTEMI group were matched to those in the STEMI group (1:1) according to PSs using the nearest available pair-matching method. The patients were matched using a caliper width of 0.01. This procedure yielded 5,768 well-matched pairs (Supplementary Table S2). Various clinical outcomes were estimated using the Kaplan–Meier curve analysis, and group differences were compared using the log-rank test. Statistical significance was defined as a 2-tailed p value of <0.05. All the statistical analyses were performed using the SPSS software version 20 (IBM, Armonk, NY, USA).
3. Results
3.1. Baseline Characteristics
Table 1 and Supplementary Table S3 show the baseline, laboratory, angiographic, and procedural characteristics of the study population. In both the DM and non-DM groups, patients in the NSTEMI group had a higher mean age, mean values of LVEF, systolic blood pressure, diastolic blood pressure, NT-ProBNP levels, diameter of the deployed stent, length of deployed stent, and mean number of deployed stents than patients in the STEMI group. The numbers of the following patients were higher in the NSTEMI group: with hypertension; dyslipidemia; previous histories of PCI and CVA; prescribed with ARB, left main coronary artery and left circumflex artery as the IRA and treated vessels; American College of Cardiology/American Heart Association type B2 lesions and multivessel diseases; and those who received transradial approach and underwent intravascular ultrasound examination. The STEMI group included the following patients: a higher number of men; cardiogenic shock; underwent CPR on admission; current smokers; prescribed with ACEI and glycoprotein II/IIIa; right coronary artery as an IRA and treated vessel; American College of Cardiology/American Heart Association type C lesions; single-vessel disease and pre-PCI thrombolysis in myocardial infarction (TIMI) flow grade 0/1; and those who underwent PCI within 24 h compared to those in the NSTEMI group. In both the NSTEMI and STEMI groups, patients in the DM group had a higher mean age, mean body mass index values, NT-ProBNP levels, triglyceride levels, and mean number of deployed stents than those in the non-DM group (Supplementary Table S3). The number of patients with hypertension; dyslipidemia; previous history of MI, PCI, coronary artery bypass graft, and CVA; those prescribed ARB; and those with RCA as a treated vessel and multivessel disease were also higher in the DM group. The non-DM group included the following patients: a higher number of men; underwent CPR on admission; current smokers; those prescribed with ticagrelor, ACEI, lipid-lowering agent, and glycoprotein II/IIIa; and those with single-vessel disease compared to those in the DM group. The mean values of LVEF, peak CK-MB, total cholesterol, low-density lipoprotein cholesterol, and number of patients with pre-PCI TIMI flow grade 0/1 were also higher in the non-DM group.
3.2. Clinical Outcomes
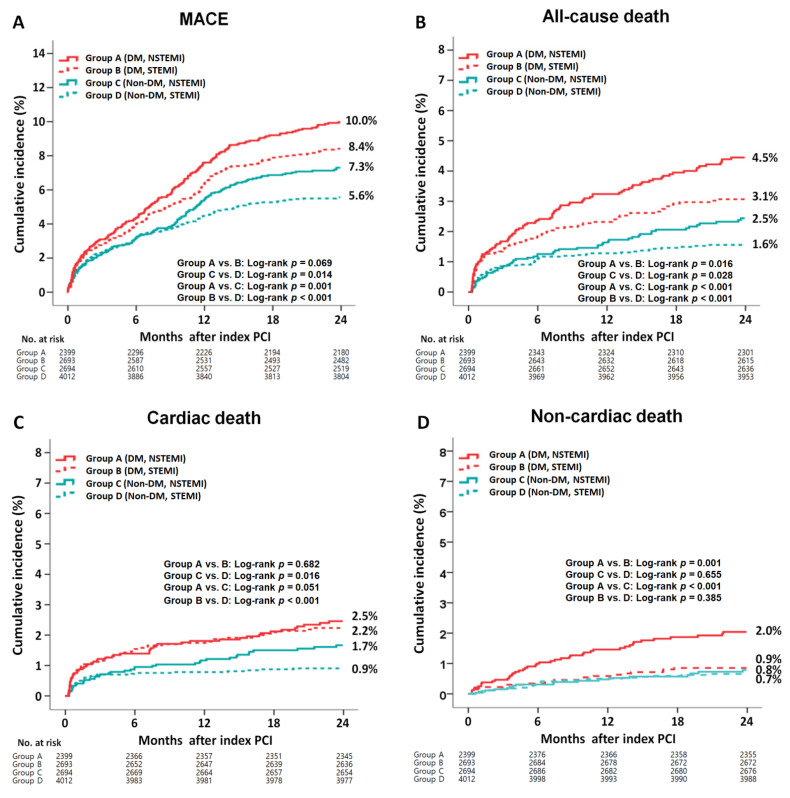
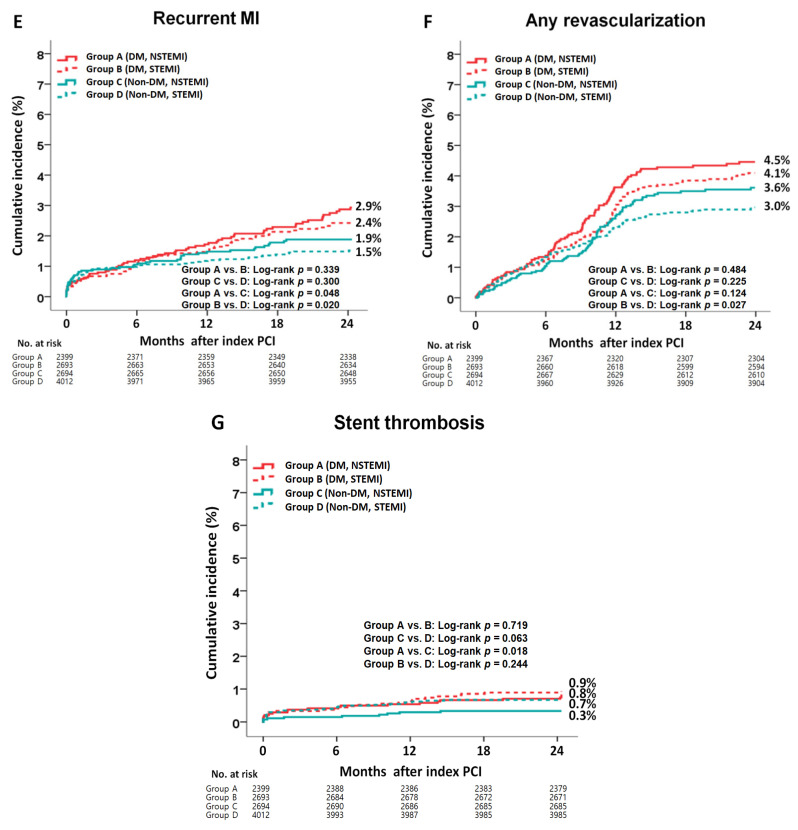
The cumulative incidences of major clinical outcomes during the 2-year follow-up period are summarized in Table 2 and Table 3 and Figure 2. In the DM group, after a multivariable-adjusted analysis, the incidence rates of MACE (adjusted hazard ratio (aHR), 1.098; 95% confidence interval [CI], 0.875–1.314; p = 0.401), all-cause death, CD, re-MI, any repeat revascularization, and ST were not significantly different between the NSTEMI and STEMI groups (Table 2). However, the non-CD rate was significantly higher in the NSTEMI group than that in the STEMI group (aHR, 2.200; 95% CI, 1.231–3.813; p = 0.007). After a PS-adjusted analysis, the non-CD rate was significantly higher in the NSTEMI group than in the STEMI group (aHR, 2.484; 95% CI, 1.326–4.651; p = 0.004). In the non-DM group, after a multivariable-adjusted analysis, the rates of MACE (aHR, 1.384; p = 0.002), all-cause death (aHR, 2.054; p < 0.01), and CD (aHR, 2.688; 95% CI, p < 0.001) were significantly higher in the NSTEMI group than in the STEMI group (Table 2). After a PS-adjusted analysis, the rates of MACE (aHR, 1.543; p < 0.001), all-cause death (aHR, 2.172; p < 0.001), and CD (aHR, 2.882; p < 0.001) were also higher in the NSTEMI group than in the STEMI group. In all the patients, after both multivariable- and PS-adjusted analyses, the rates of MACE (aHR, 1.214; p = 0.006 and aHR, 1.298; p = 0.002, respectively), all-cause death (aHR, 1.521; p < 0.001 and aHR, 1.653; p < 0.001, respectively), CD (aHR, 1.367; p = 0.041 and aHR, 1.499; p = 0.022, respectively), and non-CD rate (aHR, 1.745; p = 0.005 and aHR, 1.977; p = 0.004, respectively) were significantly higher in the NSTEMI group than in the STEMI group. Table 3 compares the clinical outcomes of the DM and non-DM groups. In the NSTEMI group, after a multivariable-adjusted analysis, the rates of MACE (aHR, 1.326; p = 0.007), all-cause death (aHR, 1.701; p = 0.001), non-CD rate (aHR, 2.549; p < 0.001), and ST (aHR, 2.272; p = 0.048) were significantly higher in the DM group than in the non-DM group. However, the CD rates were similar between the two groups. In the STEMI group, the rates of MACE (aHR, 1.481; p < 0.001), all-cause death (aHR, 1.869; p < 0.001), CD (aHR, 2.248; p < 0.001), re-MI (aHR, 1.537; p = 0.023), and any repeat revascularization (aHR, 1.374; p = 0.024) were significantly higher in the DM group than in the non-DM group. However, the non-CD rates were similar between the two groups. Overall, all of the clinical outcomes were worse in the DM group than those in the non-DM group. Supplementary Table S4 shows the causes of non-CD in this study. In the DM group, the multiple organ failure (0.7% vs. 0.2%, p = 0.008) and CVA (0.9% vs. 0.4%, p = 0.034) rates were significantly higher in the NSTEMI group than in the STEMI group. However, in the non-DM group, none of the causes of non-CD, including multiple organ failure and CVA, were significantly different between the NSTEMI and STEMI groups. Supplementary Table S5 shows the independent predictors of MACE. Reduced LVEF (<40%), cardiogenic shock, CPR on admission, peak troponin-I, NT-ProBNP, and lipid-lowering agents were common independent predictors of MACE in both the DM and non-DM groups. Supplementary Figure S1 shows the subgroup analysis for MACE in patients with and without DM. In the DM group, patients without cardiogenic shock, hypertension, or dyslipidemia and patients who received lipid-lowering agents or a deployed stent with a mean diameter of ≥3 mm had a lower MACE rate in the STEMI group than in the NSTEMI group. In the non-DM group, patients without dyslipidemia and those who received lipid-lowering agents had a lower MACE rate in the STEMI group than in the NSTEMI group.
Table 2.
Clinical outcomes between NSTEMI and STEMI groups at 2 years.
| Outcomes | DM (n = 5092) | Log-Rank | Unadjusted | Multivariable-Adjusted a | Propensity Score-Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|
| NSTEMI (n = 2399) |
STEMI (n = 2693) |
HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 219 (10.0) | 211 (8.4) | 0.069 | 1.192 (0.986–1.440) | 0.069 | 1.098 (0.875–1.314) | 0.401 | 1.074 (0.850–1.358) | 0.548 |
| All-cause death | 98 (4.5) | 78 (3.1) | 0.016 | 1.436 (1.067–1.934) | 0.017 | 1.275 (0.914–1.684) | 0.121 | 1.334 (0.926–1.922) | 0.102 |
| Cardiac death | 54 (2.5) | 57 (2.2) | 0.682 | 1.081 (0.745–1.568) | 0.682 | 1.068 (0.695–1.580) | 0.756 | 1.078 (0.720–1.690) | 0.732 |
| Non-cardiac death | 44 (2.0) | 21 (0.9) | 0.001 | 2.403 (1.429–4.041) | 0.001 | 2.200 (1.231–3.813) | 0.007 | 2.484 (1.326–4.651) | 0.004 |
| Recurrent MI | 61 (2.9) | 59 (2.4) | 0.339 | 1.191 (0.832–1.703) | 0.339 | 1.102 (0.764–1.612) | 0.580 | 1.104 (0.751–1.720) | 0.660 |
| Any repeat revascularization | 95 (4.5) | 99 (4.1) | 0.484 | 1.106 (0.835–1.465) | 0.484 | 1.021 (0.612–1.312) | 0.901 | 1.186 (0.839–1.675) | 0.334 |
| ST (definite or probable) | 20 (0.8) | 25 (0.9) | 0.719 | 0.898 (0.499–1.616) | 0.719 | 0.901 (0.512–1.702) | 0.745 | 0.946 (0.485–1.968) | 0.882 |
| Outcomes | Non-DM (n = 6706) | Log-Rank | Unadjusted | Multivariable-Adjusted a | Propensity score-Adjusted | ||||
|
NSTEMI
(n = 2694) |
STEMI
(n = 4012) |
HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 175 (7.3) | 208 (5.6) | 0.014 | 1.287 (1.052–1.574) | 0.014 | 1.384 (1.120–1.694) | 0.002 | 1.543 (1.211–1.965) | <0.001 |
| All-cause death | 58 (2.5) | 59 (1.6) | 0.028 | 1.497 (1.042–2.151) | 0.029 | 2.054 (1.399–3.031) | <0.001 | 2.172 (1.423–3.276) | <0.001 |
| Cardiac death | 40 (1.7) | 35 (0.9) | 0.016 | 1.734 (1.102–2.730) | 0.017 | 2.688 (1.698–4.331) | <0.001 | 2.882 (1.679–4.967) | <0.001 |
| Non-cardiac death | 18 (0.8) | 24 (0.7) | 0.655 | 1.150 (0.624–2.118) | 0.655 | 1.215 (0.575–2.182) | 0.598 | 1.250 (0.641–2.557) | 0.539 |
| Recurrent MI | 46 (1.9) | 57 (1.5) | 0.300 | 1.227 (0.832–1.810) | 0.301 | 1.194 (0.772–1.751) | 0.511 | 1.380 (0.865–2.201) | 0.177 |
| Any repeat revascularization | 84 (3.6) | 108 (3.0) | 0.225 | 1.193 (0.897–1.586) | 0.226 | 1.184 (0.788–1.532) | 0.278 | 1.299 (0.920–1.835) | 0.137 |
| ST (definite or probable) | 9 (0.3) | 27 (0.7) | 0.063 | 0.495 (0.233–1.053) | 0.068 | 0.623 (0.302–1.178) | 0.184 | 0.513 (0.252–1.245) | 0.140 |
| Outcomes | Overall (n = 11,798) | Log-Rank | Unadjusted | Multivariable-Adjusted a | Propensity score-Adjusted | ||||
|
NSTEMI
(n = 5093) |
STEMI
(n = 6705) |
HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 394 (8.6) | 419 (6.7) | 0.001 | 1.269 (1.106–1.456) | 0.001 | 1.214 (1.034–1.315) | 0.006 | 1.298 (1.097–1.535) | 0.002 |
| All-cause death | 156 (3.4) | 137 (2.2) | <0.001 | 1.529 (1.216–1.924) | <0.001 | 1.521 (1.208–1.994) | <0.001 | 1.653 (1.252–2.183) | <0.001 |
| Cardiac death | 94 (2.0) | 92 (1.4) | 0.032 | 1.369 (1.027–1.824) | 0.032 | 1.367 (1.009–1.684) | 0.041 | 1.499 (1.060–2.120) | 0.022 |
| Non-cardiac death | 62 (1.4) | 45 (0.7) | 0.001 | 1.859 (1.266–2.729) | 0.002 | 1.745 (1.207–2.596) | 0.005 | 1.977 (1.239–3.155) | 0.004 |
| Recurrent MI | 107 (2.4) | 116 (1.9) | 0.102 | 1.245 (0.957–1.619) | 0.103 | 1.214 (0.902–1.563) | 0.372 | 1.247 (0.905–1.719) | 0.177 |
| Any repeat revascularization | 179 (4.0) | 207 (3.4) | 0.123 | 1.170 (0.958–1.429) | 0.123 | 1.060 (0.682–1.297) | 0.521 | 1.068 (0.837–1.324) | 0.597 |
| ST (definite or probable) | 29 (0.6) | 52 (0.8) | 0.179 | 0.733 (0.466–1.155) | 0.181 | 0.746 (0.502–1.273) | 0.382 | 0.754 (0.433–1.313) | 0.319 |
MACE, major adverse cardiac events; DM, diabetes mellitus; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; HF, heart failure; CVA, cerebrovascular accidents; CK-MB, creatine kinase myocardial band; NT-ProBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; CCB, calcium channel blocker. a Adjusted by male sex, age, LVEF, BMI, SBP, DBP, cardiogenic shock, Killip class I/II, CPR on admission, hypertension, dyslipidemia, previous histories (MI, PCI, CABG, HF, and CVA), current smoker, peak CK-MB, peak troponin-I, NT-ProBNP, serum creatinine, eGFR, total cholesterol, triglyceride, LDL cholesterol, clopidogrel, ticagrelor, prasugrel, ACEI, ARB, BB, CCB, lipid-lowering agents, and year of index MI (Supplementary Table S1).
Table 3.
Clinical outcomes between the DM and non-DM groups at 2 years.
| Outcomes | NSTEMI (n = 5093) | Log-Rank | Unadjusted | Multivariable-Adjusted a | |||
|---|---|---|---|---|---|---|---|
| DM (n = 2399) |
Non-DM (n = 2694) |
HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 219 (10.0) | 175 (7.3) | 0.001 | 1.391 (1.140–1.696) | 0.001 | 1.326 (1.080–1.629) | 0.007 |
| All-cause death | 98 (4.5) | 58 (2.5) | <0.001 | 1.873 (1.354–2.592) | <0.001 | 1.701 (1.215–2.382) | 0.002 |
| Cardiac death | 54 (2.5) | 40 (1.7) | 0.051 | 1.498 (0.995–2.255) | 0.053 | 1.325 (0.866–2.027) | 0.195 |
| Non-cardiac death | 44 (2.0) | 18 (0.8) | <0.001 | 2.706 (1.564–4.683) | <0.001 | 2.549 (1.450–4.480) | 0.001 |
| Recurrent MI | 61 (2.9) | 46 (1.9) | 0.048 | 1.469 (1.002–2.154) | 0.049 | 1.469 (0.990–2.180) | 0.056 |
| Any repeat revascularization | 95 (4.5) | 84 (3.6) | 0.124 | 1.259 (0.938–1.688) | 0.125 | 1.223 (0.904–1.654) | 0.191 |
| ST (definite or probable) | 20 (0.8) | 9 (0.3) | 0.018 | 2.501 (1.139–5.492) | 0.022 | 2.272 (1.010–5.023) | 0.048 |
| Outcomes | STEMI (n = 6705) | Log-rank | Unadjusted | Multivariable-Adjusted a | |||
|
DM
(n = 2693) |
Non-DM
(n = 4012) |
HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 211 (8.4) | 208 (5.6) | <0.001 | 1.507 (1.244–1.825) | <0.001 | 1.481 (1.218–1.801) | <0.001 |
| All-cause death | 78 (3.1) | 59 (1.6) | <0.001 | 1.961 (1.399–2.751) | <0.001 | 1.869 (1.322–2.643) | <0.001 |
| Cardiac death | 57 (2.2) | 35 (0.9) | <0.001 | 2.419 (1.588–3.684) | <0.001 | 2.248 (1.462–3.458) | <0.001 |
| Non-cardiac death | 21 (0.9) | 24 (0.7) | 0.385 | 1.296 (0.721–2.327) | 0.386 | 1.307 (0.716–2.384) | 0.383 |
| Recurrent MI | 59 (2.4) | 57 (1.5) | 0.020 | 1.535 (1.067–2.209) | 0.021 | 1.537 (1.060–2.228) | 0.023 |
| Any repeat revascularization | 99 (4.1) | 108 (3.0) | 0.027 | 1.360 (1.035–1.786) | 0.027 | 1.374 (1.041–1.816) | 0.024 |
| ST (definite or probable) | 25 (0.9) | 27 (0.7) | 0.244 | 1.380 (0.801–2.377) | 0.246 | 1.381 (0.792–2.224) | 0.315 |
| Outcomes | Overall (n = 11,798) | Log-rank | Unadjusted | Multivariable-Adjusted a | |||
|
DM
(n = 5092) |
Non-DM
(n = 6706) |
HR (95% CI) | p | HR (95% CI) | p | ||
| MACE | 430 (9.1) | 383 (6.2) | <0.001 | 1.473 (1.283–1.690) | <0.001 | 1.434 (1.245–1.652) | <0.001 |
| All-cause death | 176 (3.7) | 117 (1.9) | <0.001 | 1.969 (1.558–2.488) | <0.001 | 1.866 (1.467–2.374) | <0.001 |
| Cardiac death | 111 (2.3) | 75 (1.2) | <0.001 | 1.939 (1.447–2.599) | <0.001 | 1.818 (1.345–2.457) | <0.001 |
| Non-cardiac death | 65 (1.4) | 42 (0.7) | <0.001 | 2.022 (1.372–2.981) | <0.001 | 1.957 (1.313–2.916) | 0.001 |
| Recurrent MI | 120 (2.7) | 103 (1.7) | 0.002 | 1.524 (1.171–1.983) | 0.002 | 1.527 (1.166–2.000) | 0.002 |
| Any repeat revascularization | 194 (4.0) | 192 (3.4) | 0.006 | 1.324 (1.085–1.617) | 0.006 | 1.314 (1.071–1.611) | 0.009 |
| ST (definite or probable) | 45 (0.9) | 36 (0.5) | 0.024 | 1.648 (1.063–2.554) | 0.026 | 1.521 (1.027–2.351) | 0.037 |
NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; DM, diabetes mellitus; HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiac events; ST, stent thrombosis; LVEF, left ventricular ejection fraction; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; HF, heart failure; CVA, cerebrovascular accidents; CK-MB, creatine kinase myocardial band; NT-ProBNP, N-terminal pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; ACEI, angiotensin converting enzyme inhibitor. a Adjusted by male sex, age, LVEF, BMI, SBP, DBP, cardiogenic shock, Killip class I/II, CPR on admission, hypertension, previous histories (MI, PCI, CABG, HF, and CVA), current smoker, peak CK-MB, peak troponin-I, NT-ProBNP, serum creatinine, eGFR, total cholesterol, LDL cholesterol, and year of index MI (Supplementary Tables S3 and S6).
Figure 2.
Kaplan–Meier curved analysis for MACE (A), all-cause death (B), cardiac death (C), non-cardiac death (D), recurrent MI (E), any repeat revascularization (F), and stent thrombosis (G). MACE, major adverse cardiac events; DM, diabetes mellitus; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention.
4. Discussion
The main findings of this study were as follows: (1) In the DM group, although MACE, all-cause death, CD, recurrent MI, any repeat revascularization, and ST rates were not significantly different between the NSTEMI and STEMI groups, the non-CD rate was significantly higher in the NSTEMI group than in the STEMI group; (2) in the non-DM group, the MACE, all-cause death, and CD rates were significantly higher in the NSTEMI group than in the STEMI group; (3) in the NSTEMI group, the MACE, all-cause death, non-CD, and ST rates were significantly higher in the DM group than in the non-DM group; (4) in the STEMI group, the MACE, all-cause death, CD, recurrent MI, and any repeat revascularization rates were significantly higher in the DM group than in the non-DM group; and (5) reduced LVEF (<40%), cardiogenic shock, CPR on admission, peak troponin-I level, NT-ProBNP level, and lipid-lowering agents were common independent predictors for MACE in both the DM and non-DM groups.
STEMI is the result of acute occlusion of the IRA and is associated with transmural ischemia, whereas NSTEMI is caused by transient or incomplete coronary artery occlusion, resulting in non-transmural subendocardial ischemia [4]. In previous studies [9,31], the 6-month post-discharge mortality (6.2% vs. 4.8%, respectively) and 1-year mortality (11.6% vs. 9.0%, respectively) rates were higher in the NSTEMI group than in the STEMI group. Although these two randomized studies [9,31] are valuable for estimating comparative clinical outcomes between NSTEMI and STEMI groups, they [9,31] were conducted before the new-generation DES era and were not limited to patients with DM. Hyperglycemia contributes to increased mortality and morbidity rates through an oxidative-linked mechanism [32] and may exert significant hemodynamic effects even in normal study participants [12]. Furthermore, hyperglycemia caused by oxidative stress, inflammation, apoptosis, endothelial dysfunction, hypercoagulation, and platelet aggregation [33] could damage the ischemic myocardium in patients with NSTEMI [12]. In a study by Hao et al. [12], among 890 patients with NSTEMI who underwent PCI, hyperglycemia upon admission was an independent predictor of a 30-day (aHR, 1.014; p < 0.001 and aHR, 1.018, p < 0.001, respectively) and 3-year MACE (aHR, 1.009; p < 0.001 and aHR, 1.017, p < 0.001, respectively) in patients with and without DM. Recently, Li et al. [13] demonstrated that the incidences of all-cause death (1.1%) and MACE (3.4%) were significantly lower in patients without a history of DM and an HbA1c level of <6.5% at admission. However, DM patients with poor glycemic control at admission experienced high rates of all-cause death (18.8%) and MACE (25%) in 350 consecutive patients with STEMI during a 2-year follow-up period. Similarly, in our study, both in the NSTEMI and STEMI groups, the rates of all-cause death (aHR, 1.701; p = 0.002 and aHR; 1.869, p < 0.001, respectively) and MACE (aHR, 1.326; p = 0.007 and aHR, 1.481; p < 0.001, respectively) were significantly higher in the DM group than in the non-DM group (Table 3). However, the study population in these studies [12,13] was limited to patients with NSTEMI or STEMI. DESs have been developed to overcome the limitations of BMS deployment, such as neointimal hyperplasia and repeat revascularization [22,24]. In the era of DES, second-generation DES is the most commonly used DES because it can solve the problems of 1G-DES, such as inflammation and restenosis, and decrease the mortality rate (aHR, 1.534; p = 0.009) [14]. Hence, to provide more meaningful results and compensate for the shortcomings of the previous studies [9,12,13,31], we compared the 2-year clinical outcomes between the NSTEMI and STEMI groups according to the presence or absence of DM. The study population was strictly confined to patients with AMI who underwent successful implantation of a new-generation DES to reflect the current PCI trend. Additionally, to evaluate the long-term outcomes of the NSTEMI and STEMI groups, we excluded patients who died in the hospital (Figure 1).
Patients with NSTEMI tend to be older and have a lower rate of acute revascularization than those with STEMI [34]. In our study, in both the DM and non-DM groups, the patients in the NSTEMI group had a higher mean age than those in the STEMI group (65.5 ± 11.3 years vs. 63.0 ± 11.9, p < 0.001 and 63.5 ± 12.6 years vs. 61.4 ± 13.0, p < 0.001, respectively) and the total number of patients who underwent PCI within 24 h was lower in the NSTEMI group than that in the STEMI group (85.0% vs. 96.5%, p < 0.001 and 87.5% vs. 96.7%, p < 0.001, respectively, Table 1). Although this study did not include in-hospital outcomes, it could be stated that STEMI is a higher-risk disease in the acute phase [6,7], for which we have gathered treatment knowledge, while NSTEMI is a higher-risk disease in the chronic phase, owing to the higher complexity of these patients [9,10]. Moreover, in our study, although HbA1c levels were higher in patients with STEMI, the number of patients undergoing insulin treatment was higher in the NSTEMI group. Patients with NSTEMI may have had greater decompensated diabetes at the time of MI. Insulin-treated patients with DM were associated with significantly higher short- and long-term adverse cardiovascular outcomes after PCI than those not treated with insulin therapy [35]. However, long-term comparative results between patients with insulin-treated DM and non-insulin-treated DM after NSTEMI and STEMI are very limited. Further studies are required to evaluate long-term clinical outcomes in the NSTEMI and STEMI groups. Okura et al. reported that the non-CD rate (15.1% vs. 8.4 %, p < 0.001) was significantly higher in the NSTEMI group than in the STEMI group during a median 4.3-year follow-up period [34]. In a recent study involving patients with chronic kidney disease [36], the non-CD rate (aHR, 1.960; p = 0.004) was significantly higher in the NSTEMI group than in the STEMI group. In our study, after both multivariable- (aHR, 2.223; p = 0.006) and PS-adjusted (aHR, 2.484; p = 0.004) analyses, the non-CD rate was significantly higher in the DM group than that in the STEMI group (Table 2). Furthermore, after multivariable-adjusted analysis, the non-CD rate in the NSTEMI group was significantly higher in the DM group than that in the non-DM group (aHR, 2.810; p < 0.001, Table 3). The main causes of non-CD in the DM group were multiple organ failure (0.7% vs. 0.2%, p = 0.008) and CVA (0.9% vs. 0.4%, p = 0.034) (Supplementary Table S4). In a previous report [37], CVA was independently predicted by DM (HR, 1.43; 95% CI, 1.04-1.97; p = 0.03) and was associated with a significantly increased 3-year mortality rate (aHR, 2.39; p = 0.004). DM accelerates atherosclerosis in multiple vascular beds and causes severe coronary atherosclerosis. Coronary and cerebrovascular diseases frequently coexist because of their similar pathogeneses [38]. In our study, in the non-DM group, the mortality rate (all-cause and CD) was significantly higher in the NSTEMI group than in the STEMI group; however, in the DM group, the mortality rate did not significantly differ between the two MI groups (Table 2). This result may be related to the relatively higher CD rate in the DM and STEMI groups than that in the non-DM and STEMI groups (aHR, 2.248; p < 0.001, Table 3). Additionally, this result may be related to the insignificantly different non-CD rates in the DM and STEMI groups compared to those in the non-DM and STEMI groups (aHR, 1.307; p = 0.383, Table 3).
The incidence of recurrent ischemic events after AMI is higher in the first year, and, in subsequent years, it is based on several cardiovascular risk factors [39]. Recently, Kim et al. [40] reported that in patients with AMI, the cumulative incidence of re-MI was significantly higher in the DM group than in the normoglycemia group (aHR, 1.752; 95% CI, 1.087–2.823; p = 0.021). Among all of the patients in our study, the re-MI rate was significantly higher in the DM group than in the non-DM group (aHR, 1.527; 95% CI, 1.166–2.000; p = 0.002, Table 3). The re-MI rate increased continuously in the DM group, regardless of AMI type (Figure 2). In a Danish study [41], the risk of ST (definite, probable, or possible) did not differ significantly between DM and non-DM groups in patients with STEMI (aHR, 1.50; 95% CI, 0.92–2.45). Recently, a subgroup analysis of the ultrathin strut biodegradable polymer sirolimus-eluting stent versus the durable polymer everolimus-eluting stent for percutaneous coronary revascularization trial [42] showed that the 5-year ST (definite or probable) was significantly higher in the DM group than in the non-DM group (rate ratio (RR), 2.05; 95% CI, 1.45–2.90; p < 0.001) in all the patients after new-generation DES implantation. Furthermore, in their study, the target lesion failure (RR, 1.87; p < 0.001) and target vessel failure (rate ratio, 1.76; p < 0.001) rates were significantly higher in the DM group than in the non-DM group [42]. In our study, although patients in the DM with STEMI group showed comparable ST (definite or probable) rates to those in the non-DM STEMI group (aHR, 1.381; 95% CI, 0.792–2.224; p = 0.315; Table 3), the overall ST rate was significantly higher in the DM group than in the non-DM group (aHR, 1.521; 95% CI, 1.027–2.351; p = 0.037, Table 3). Additionally, the repeat revascularization rate was significantly higher in the DM group than in the non-DM group (aHR, 1.310; 95% CI, 1.069–1.605; p = 0.009). In our study, reduced LVEF, cardiogenic shock, CPR on admission, peak troponin-I, NT-ProBNP, and lipid-lowering agents were common independent predictors of MACE in both the DM and non-DM groups (Supplementary Table S5). These are well-known independent predictors of MACE in patients with AMI [33,43,44].
Several previous studies have demonstrated that higher long-term mortality was observed in the NSTEMI group than in the STEMI group, regardless of the type of death [7,9,10,31,45]. Consistent with the results of previous studies [7,9,10,31,45], in all of the patients in our study, the rates of MACE, all-cause death, CD, and non-CD were significantly higher in the NSTEMI group than in the STEMI group. However, the higher non-CD rate in the NSTEMI group was more evident in the DM group, while the higher CD rate in the NSTEMI group was more evident in the non-DM group. Hence, we believe that strategies for reducing the non-CD rate in patients with DM and those for reducing the CD rate in patients without DM could be beneficial for the NSTEMI group after successful PCI with new-generation DES. Although the sample size of the study population was too small to demonstrate meaningful results, more than 50 high-volume universities and community hospitals with facilities for primary PCI and on-site cardiac surgery participated in this study. Regarding the relatively higher incidence of multiple organ failure and CVA in the DM and NSTEMI groups than in the non-DM and STEMI groups (Supplementary Table S4), more well-established and regular follow-ups [9] as well as more focused and diverse secondary prevention therapies [31], including those involving lipid-lowering agents (Supplementary Table S5 and Figure S1), are required to reduce the occurrence of non-CD in patients with NSTEMI. Since this study enrolled patients (from the registry) from 2005, many patients with diabetes did not experience the benefits of newer therapies (such as sodium–glucose cotransporter 2 inhibitor, glucagon-like peptide-1 receptor agonist). These medications have been shown to reduce the risk of cardiovascular events when compared with that of the controls [46,47]. Therefore, recent guidelines [48] support the incorporation of these newer agents with cardiovascular benefits into routine clinical practice and screening of patients who are at a high risk of cardiovascular disease. Unfortunately, because we could not obtain information about the various recently developed antidiabetic agents that had been prescribed from the KAMIR registry, we could not provide information concerning the effects of these drugs on the long-term clinical outcomes in this study. Additionally, due to the large temporal interval of this retrospective analysis, many patients did not experience the benefits brought from the newer therapies and sodium–glucose cotransporter 2 inhibitor, which was approved as a new alternative for the treatment of T2DM by the Food and Drug Administration in 2013 for use in Europe [49]. We stratified patients into two groups before and after 2013 according to the year of index myocardial infarction as shown in Table 1 and Supplementary Table S3. Finally, we believe that the results of this comparative study could provide interventional cardiologists with meaningful information regarding treatment strategies for patients with two different types of AMI according to the presence or absence of DM.
This study has several limitations. First, because we used registry data, there may have been some under-reported or missing data. Second, this study was based on discharge medications because we could not precisely determine the participants’ adherence or non-adherence to their antidiabetic drugs during the 2-year follow-up period. Third, although the interval from symptom onset to PCI was an important determinant of major clinical outcomes, this variable included many missing values in the registry data; therefore, we could not include this variable in our study. Fourth, although we performed multivariable- and PS-adjusted analyses to strengthen our results, variables not included in the KAMIR may have affected the study outcomes. Fifth, it is not certain that our population had 100% T2DM based only on the age at which diabetes was discovered. Occasionally, type 1 DM occurs in individuals aged over 30 years [50]. Moreover, there were some missing values concerning patient-reported history, such as the presence or absence of a history of ketoacidosis and other medical records indicating T2DM in the KAMIR data, owing to the registry-based nature of this study. These factors may be considered as critical limitations of this study. Sixth, this retrospective study was a long-term (November 2005 to June 2015) study of patients with AMI, which could have affected clinical outcomes. Finally, the 2-year follow-up period in this study was relatively short and may have been inadequate for determining long-term major clinical outcomes.
5. Conclusions
In this retrospective study, patients with NSTEMI had a significantly higher 2-year mortality rate than those with STEMI. Furthermore, strategies to reduce the non-CD rate in patients with DM and the CD rate in non-DM patients could be beneficial for those with NSTEMI. Hence, more regular follow-up and focused and diverse secondary prevention therapies to reduce the incidence of multiple organ failure and CVA are required in patients with DM and NSTEMI.
Acknowledgments
Korea Acute Myocardial Infarction Registry (KAMIR) investigators. Myung Ho Jeong, Youngkeun Ahn, Sung Chul Chae, Jong Hyun Kim, Seung-Ho Hur, Young Jo Kim, In Whan Seong, Donghoon Choi, Jei Keon Chae, Taek Jong Hong, Jae Young Rhew, Doo-Il Kim, In-Ho Chae, Junghan Yoon, Bon-Kwon Koo, Byung-Ok Kim, Myoung Yong Lee, Kee-Sik Kim, Jin-Yong Hwang, Myeong Chan Cho, Seok Kyu Oh, Nae-Hee Lee, Kyoung Tae Jeong, Seung-Jea Tahk, Jang-Ho Bae, Seung-Woon Rha, Keum-Soo Park, Chong Jin Kim, Kyoo-Rok Han, Tae Hoon Ahn, Moo-Hyun Kim, Ki Bae Seung, Wook Sung Chung, Ju-Young Yang, Chong Yun Rhim, Hyeon-Cheol Gwon, Seong-Wook Park, Young-Youp Koh, Seung Jae Joo, Soo-Joong Kim, Dong Kyu Jin, Jin Man Cho, Sang-Wook Kim, Jeong Kyung Kim, Tae Ik Kim, Deug Young Nah, Si Hoon Park, Sang Hyun Lee, Seung Uk Lee, Hang-Jae Chung, Jang-Hyun Cho, Seung Won Jin, Myeong-Ki Hong, Yangsoo Jang, Jeong Gwan Cho, Hyo-Soo Kim, and Seung-Jung Park.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11175079/s1, Supplementary Table S1: Results of collinearity test for MACE in patients with DM or non-DM; Supplementary Table S2: Baseline characteristics of the NSTEMI and STEMI groups before and after PSM analysis; Supplementary Table S3: Baseline clinical, laboratory, angiographic, and procedural characteristics of the NSTEMI and STEMI groups; Supplementary Table S4: Causes of non-cardiac death; Supplementary Table S5: Independent predictors for MACE; Supplementary Table S6: Results of the collinearity test for MACE between the NSTEMI and STEMI groups; Supplementary Figure S1: Subgroup analysis for MACE in patients with diabetes (A) and non-diabetes (B).
Author Contributions
Conceptualization, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J.; data curation, Y.H.K., A.-Y.H., S.-W.R., B.G.C., J.B.K., S.P. and D.O.K.; formal analysis, Y.H.K., A.-Y.H., S.-W.R., B.G.C., S.P. and D.O.K.; funding acquisition, M.H.J.; investigation, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J.; methodology, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J.; project administration, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., J.Y.P., S.-H.P. and M.H.J.; resources, S.-W.R., C.U.C., J.B.K., S.P., D.O.K. and M.H.J.; software, Y.H.K., A.-Y.H., B.G.C., S.P. and D.O.K.; supervision, Y.H.K., S.-W.R. and M.H.J.; validation, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J.; visualization, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J.; writing—original draft, Y.H.K. and A.-Y.H.; writing—review and editing, Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., J.Y.P., S.-H.P. and M.H.J. All authors contributed to the manuscript revision, read, and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Chonnam National University Hospital Institutional Review Board (IRB) ethics committee (protocol code CNUH-2011-172 and 1 March 2011).
Informed Consent Statement
Informed written consent was obtained from all subjects involved in this study.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Milazzo V., Cosentino N., Genovese S., Campodonico J., Mazza M., de Metrio M., Marenzi G. Diabetes Mellitus and Acute Myocardial Infarction: Impact on Short and Long-Term Mortality. Adv. Exp. Med. Biol. 2021;1307:153–169. doi: 10.1007/5584_2020_481. [DOI] [PubMed] [Google Scholar]
- 2.Ertelt K., Brener S.J., Mehran R., Ben-Yehuda O., McAndrew T., Stone G.W. Comparison of Outcomes and Prognosis of Patients With Versus Without Newly Diagnosed Diabetes Mellitus After Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction (the HORIZONS-AMI Study) Am. J. Cardiol. 2017;119:1917–1923. doi: 10.1016/j.amjcard.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 4.Bassand J.P., Hamm C.W., Ardissino D., Boersma E., Budaj A., Fernández-Avilés F., Fox K.A., Hasdai D., Ohman E.M., Wallentin L., et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 5.Basalay M.V., Yellon D.M., Davidson S.M. Targeting myocardial ischaemic injury in the absence of reperfusion. Basic Res. Cardiol. 2020;115:63. doi: 10.1007/s00395-020-00825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basir M.B., Schreiber T., Dixon S., Alaswad K., Patel K., Almany S., Khandelwal A., Hanson I., George A., Ashbrook M., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2018;91:454–461. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 7.Puymirat E., Simon T., Cayla G., Cottin Y., Elbaz M., Coste P., Lemesle G., Motreff P., Popovic B., Khalife K., et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136:1908–1919. doi: 10.1161/CIRCULATIONAHA.117.030798. [DOI] [PubMed] [Google Scholar]
- 8.Fox C.S., Muntner P., Chen A.Y., Alexander K.P., Roe M.T., Cannon C.P., Saucedo J.F., Kontos M.C., Wiviott S.D. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg R.J., Currie K., White K., Brieger D., Steg P.G., Goodman S.G., Dabbous O., Fox K.A., Gore J.M. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]) Am. J. Cardiol. 2004;93:288–293. doi: 10.1016/j.amjcard.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Chan M.Y., Sun J.L., Newby L.K., Shaw L.K., Lin M., Peterson E.D., Califf R.M., Kong D.F., Roe M.T. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation. 2009;119:3110–3117. doi: 10.1161/CIRCULATIONAHA.108.799981. [DOI] [PubMed] [Google Scholar]
- 11.Polonski L., Gasior M., Gierlotka M., Osadnik T., Kalarus Z., Trusz-Gluza M., Zembala M., Wilczek K., Lekston A., Zdrojewski T., et al. A comparison of ST elevation versus non-ST elevation myocardial infarction outcomes in a large registry database: Are non-ST myocardial infarctions associated with worse long-term prognoses? Int. J. Cardiol. 2011;152:70–77. doi: 10.1016/j.ijcard.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Hao Y., Lu Q., Li T., Yang G., Hu P., Ma A. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc. Disord. 2017;17:6. doi: 10.1186/s12872-016-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Li X., Zhang Y., Zhang L., Wu Q., Bai Z., Si J., Zuo X., Shi N., Li J., et al. Impact of glycemic control status on patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc. Disord. 2020;20:36. doi: 10.1186/s12872-020-01339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.H., Her A.Y., Jeong M.H., Kim B.K., Hong S.J., Kim S., Ahn C.M., Kim J.S., Ko Y.G., Choi D., et al. Effects of stent generation on clinical outcomes after acute myocardial infarction compared between prediabetes and diabetes patients. Sci. Rep. 2021;11:9364. doi: 10.1038/s41598-021-88593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.A., Cho S.J., Jeong M.H., Kim Y.J., Kim C.J., Cho M.C., Kim H.S., Ahn Y., Koh G., Lee J.M., et al. Hypoglycemia at admission in patients with acute myocardial infarction predicts a higher 30-day mortality in patients with poorly controlled type 2 diabetes than in well-controlled patients. Diabetes Care. 2014;37:2366–2373. doi: 10.2337/dc13-2856. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.H., Chae S.C., Oh D.J., Kim H.S., Kim Y.J., Ahn Y., Cho M.C., Kim C.J., Yoon J.H., Jeong M.H., et al. Multicenter Cohort Study of Acute Myocardial Infarction in Korea—Interim Analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ. J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 17.Grech E.D. ABC of interventional cardiology: Percutaneous coronary intervention. II: The procedure. BMJ. 2003;326:1137–1140. doi: 10.1136/bmj.326.7399.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K.Y., Rha S.W., Li Y.J., Poddar K.L., Jin Z., Minami Y., Wang L., Kim E.J., Park C.G., Seo H.S., et al. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2009;119:3207–3214. doi: 10.1161/CIRCULATIONAHA.108.822791. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.W., Park S.W., Hong M.K., Kim Y.H., Lee B.K., Song J.M., Han K.H., Lee C.W., Kang D.H., Song J.K., et al. Triple versus dual antiplatelet therapy after coronary stenting: Impact on stent thrombosis. J. Am. Coll. Cariol. 2005;46:1833–1837. doi: 10.1016/j.jacc.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes–2010. Diabetes Care. 2010;33((Suppl. 1)):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Jr., Ganiats T.G., Holmes D.R., Jr., Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cariol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F., Bax J.J., Borger M.A., Brotons C., Chew D.P., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 23.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 24.O’Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A., Ettinger S.M., Fang J.C., Fesmire F.M., Franklin B.A., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.M., Rhee T.M., Hahn J.Y., Kim H.K., Park J., Hwang D., Choi K.H., Kim J., Park T.K., Yang J.H., et al. Multivessel Percutaneous Coronary Intervention in Patients With ST-Segment Elevation Myocardial Infarction With Cardiogenic Shock. J. Am. Coll. Cariol. 2018;71:844–856. doi: 10.1016/j.jacc.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y.H., Her A.Y., Jeong M.H., Kim B.K., Lee S.Y., Hong S.J., Shin D.H., Kim J.S., Ko Y.G., Choi D., et al. Impact of renin-angiotensin system inhibitors on long-term clinical outcomes in patients with acute myocardial infarction treated with successful percutaneous coronary intervention with drug-eluting stents: Comparison between STEMI and NSTEMI. Atherosclerosis. 2019;280:166–173. doi: 10.1016/j.atherosclerosis.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., van Lente F., Greene T., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vatcheva K.P., Lee M., McCormick J.B., Rahbar M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology. 2016;6:e120. doi: 10.4172/2161-1165.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019;72:558–569. doi: 10.4097/kja.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalantari S., Khalili D., Asgari S., Fahimfar N., Hadaegh F., Tohidi M., Azizi F. Predictors of early adulthood hypertension during adolescence: A population-based cohort study. BMC Public Health. 2017;17:915. doi: 10.1186/s12889-017-4922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montalescot G., Dallongeville J., van Belle E., Rouanet S., Baulac C., Degrandsart A., Vicaut E. STEMI and NSTEMI: Are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry) Eur. Heart J. 2007;28:1409–1417. doi: 10.1093/eurheartj/ehm031. [DOI] [PubMed] [Google Scholar]
- 32.Marfella R., Nappo F., de Angelis L., Paolisso G., Tagliamonte M.R., Giugliano D. Hemodynamic effects of acute hyperglycemia in type 2 diabetic patients. Diabetes Care. 2000;23:658–663. doi: 10.2337/diacare.23.5.658. [DOI] [PubMed] [Google Scholar]
- 33.Odegaard A.O., Jacobs D.R., Jr., Sanchez O.A., Goff D.C., Jr., Reiner A.P., Gross M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016;15:51. doi: 10.1186/s12933-016-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okura N., Ogawa H., Katoh J., Yamauchi T., Hagiwara N. Long-term prognosis of patients with acute myocardial infarction in the era of acute revascularization (from the Heart Institute of Japan Acute Myocardial Infarction [HIJAMI] registry) Int. J. Cardiol. 2012;159:205–210. doi: 10.1016/j.ijcard.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 35.Bundhun P.K., Li N., Chen M.H. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2015;14:135. doi: 10.1186/s12933-015-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.H., Her A.Y., Jeong M.H., Kim B.K., Hong S.J., Lee S.J., Ahn C.M., Kim J.S., Ko Y.G., Choi D., et al. Two-year outcomes between ST-elevation and non-ST-elevation myocardial infarction in patients with chronic kidney disease undergoing newer-generation drug-eluting stent implantation. Catheter. Cardiovasc. Interv. 2022;99:1022–1037. doi: 10.1002/ccd.30049. [DOI] [PubMed] [Google Scholar]
- 37.Nikolsky E., Mehran R., Dangas G.D., Xu K., Parvataneni R., Witzenbichler B., Guagliumi G., Kornowski R., Généreux P., Brener S.J., et al. Cerebrovascular events after a primary percutaneous coronary intervention strategy for acute ST-segment-elevation myocardial infarction: Analysis from the HORIZONS-AMI Trial. Circ. Cardiovasc. Interv. 2015;8:e002283. doi: 10.1161/CIRCINTERVENTIONS.114.002283. [DOI] [PubMed] [Google Scholar]
- 38.Spencer F.A., Gore J.M., Yarzebski J., Lessard D., Jackson E.A., Goldberg R.J. Trends (1986 to 1999) in the incidence and outcomes of in-hospital stroke complicating acute myocardial infarction (The Worcester Heart Attack Study) Am. J. Cardiol. 2003;92:383–388. doi: 10.1016/S0002-9149(03)00654-4. [DOI] [PubMed] [Google Scholar]
- 39.Jernberg T., Hasvold P., Henriksson M., Hjelm H., Thuresson M., Janzon M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015;36:1163–1170. doi: 10.1093/eurheartj/ehu505. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y.H., Her A.Y., Jeong M.H., Kim B.K., Hong S.J., Kim S., Ahn C.M., Kim J.S., Ko Y.G., Choi D., et al. Effects of prediabetes on long-term clinical outcomes of patients with acute myocardial infarction who underwent PCI using new-generation drug-eluting stents. Diabetes Res. Clin. Pract. 2020;160:107994. doi: 10.1016/j.diabres.2019.107994. [DOI] [PubMed] [Google Scholar]
- 41.Jensen L.O., Maeng M., Thayssen P., Tilsted H.H., Terkelsen C.J., Kaltoft A., Lassen J.F., Hansen K.N., Ravkilde J., Christiansen E.H., et al. Influence of diabetes mellitus on clinical outcomes following primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am. J. Cardiol. 2012;109:629–635. doi: 10.1016/j.amjcard.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias J.F., Heg D., Roffi M., Tüller D., Lanz J., Rigamonti F., Muller O., Moarof I., Cook S., Weilenmann D., et al. Five-Year Outcomes in Patients With Diabetes Mellitus Treated With Biodegradable Polymer Sirolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents. J. Am. Heart Assoc. 2019;8:e013607. doi: 10.1161/JAHA.119.013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y.H., Her A.Y., Jeong M.H., Kim B.K., Hong S.J., Kim S., Ahn C.M., Kim J.S., Ko Y.G., Choi D., et al. Comparison of two-year clinical outcomes according to glycemic status and renal function in patients with acute myocardial infarction following implantation of new-generation drug-eluting stents. J. Diabetes Complicat. 2021;35:108019. doi: 10.1016/j.jdiacomp.2021.108019. [DOI] [PubMed] [Google Scholar]
- 44.Hassan S., Ali M.N., Ghafoor B. Evolutionary perspective of drug eluting stents: From thick polymer to polymer free approach. J. Cardiothorac. Surg. 2022;17:65. doi: 10.1186/s13019-022-01812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roe M.T., Parsons L.S., Pollack C.V., Jr., Canto J.G., Barron H.V., Every N.R., Rogers W.J., Peterson E.D. Quality of care by classification of myocardial infarction: Treatment patterns for ST-segment elevation vs non-ST-segment elevation myocardial infarction. Arch. Intern. Med. 2005;165:1630–1636. doi: 10.1001/archinte.165.14.1630. [DOI] [PubMed] [Google Scholar]
- 46.McKee A., Al-Khazaali A., Albert S.G. Glucagon-like Peptide-1 Receptor Agonists versus Sodium-Glucose Cotransporter Inhibitors for Treatment of T2DM. J. Endocr. Soc. 2020;4:bvaa037. doi: 10.1210/jendso/bvaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Htoo P.T., Buse J., Cavender M., Wang T., Pate V., Edwards J., Stürmer T. Cardiovascular Effectiveness of Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists in Older Patients in Routine Clinical Care With or Without History of Atherosclerotic Cardiovascular Diseases or Heart Failure. J. Am. Heart Assoc. 2022;11:e022376. doi: 10.1161/JAHA.121.022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das S.R., Everett B.M., Birtcher K.K., Brown J.M., Januzzi J.L., Jr., Kalyani R.R., Kosiborod M., Magwire M., Morris P.B., Neumiller J.J., et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asrih M., Gariani K. Impact of SGLT Inhibitors on Multiple Organ Defects in Diabetes. Curr. Diabetes Rev. 2020;16:411–418. doi: 10.2174/1573399815666191105151828. [DOI] [PubMed] [Google Scholar]
- 50.Harding J.L., Wander P.L., Zhang X., Li X., Karuranga S., Chen H., Sun H., Xie Y., Oram R.A., Magliano D.J., et al. The Incidence of Adult-Onset Type 1 Diabetes: A Systematic Review From 32 Countries and Regions. Diabetes Care. 2022;45:994–1006. doi: 10.2337/dc21-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.