Abbreviations
- AOM
azoxymethane
- CAC
colitis‐associated colorectal cancer
- CD
Crohn's disease
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- IBD
inflammatory bowel disease
- MAPK
mitogen‐activated protein kinase
- miR
microRNAs
- p38γ/p38δ
p38γ and p38δ
- UC
ulcerative colitis
- WT
Wild type
1.
Dear Editor,
Colorectal cancer (CRC) is the second leading cause of cancer death according to the World Health Organization. Patients with inflammatory bowel disease (IBD), ulcerative colitis (UC) or Crohn's disease (CD) are at increased risk of developing colitis‐associated CRC (CAC) [1]; however, our understanding of the inflammation‐cancer interplay at the molecular level is still limited.
p38 mitogen‐activated protein kinases (MAPKs) (p38α, p38β, p38γ and p38δ) modulate the inflammatory response which contribute to CAC development [2]. p38α (called p38) has been a therapeutic target in CD treatment, but these treatments have not progressed beyond phase I/II clinical trials [2]. Interestingly, the less studied p38γ and p38δ (p38γ/p38δ) are essential in the immune response and regulate inflammatory molecule production [2, 3, 4, 5, 6]. Moreover, the importance of p38γ/p38δ in CRC development has been established in vitro and in an azoxymethane‐dextran sodium sulfate (AOM‐DSS)‐induced CAC model using p38γ/p38δ‐knockout mice [2, 5]. However, their implication in human CRC and IBD is not yet well defined.
Here, we report that p38δ mRNA and protein levels were markedly decreased, whereas p38γ phosphorylation was increased, in AOM‐DSS‐treated wild‐type (WT) mice (Figure 1A, Supplementary Figure S1). Additionally, we performed a comparative analysis of p38γ/p38δ mRNA expression in human CRC samples, using The Cancer Genome Atlas (TCGA) data and Gene Expression Omnibus (GEO) dataset (GSE39582). p38γ expression was significantly increased in primary colorectal tumors compared to normal colon tissues, whereas p38δ was markedly decreased (Figure 1B, Supplementary Figure S2A‐B). High p38γ mRNA expression, but not p38δ, was associated with shorter overall survival of CRC patients (Figure 1C). p38γ/p38δ gene expression in CRC samples was not affected by patient gender or tumor stage (Supplementary Figure S2C‐D). Additionally, p38γ/p38δ genes showed no systematic loss‐of‐function mutations (Supplementary Figure S2E), in agreement with findings in other cancers [7].
FIGURE 1.
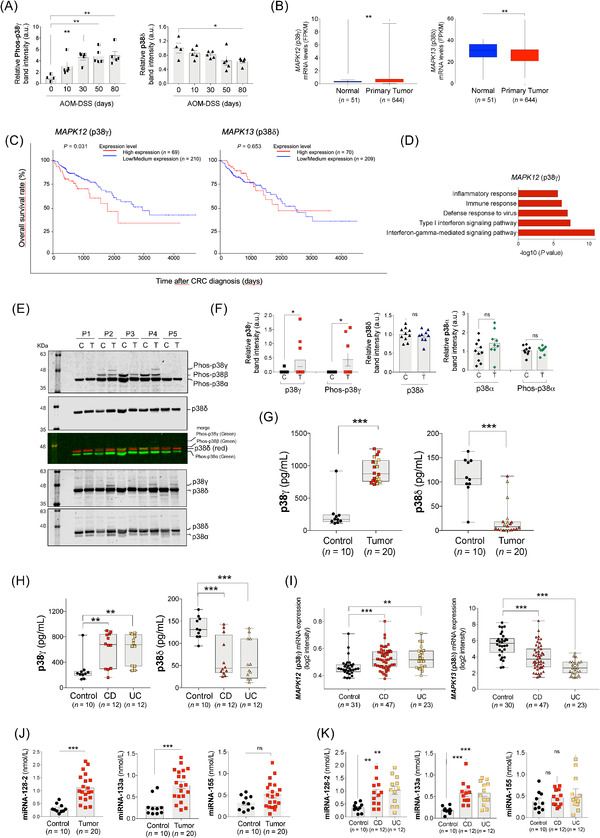
p38γ and p38δ regulation in CRC and IBD. (A) WT mice were treated with AOM and DSS as described in Supplementary Figure S1A. Colon extracts (50 μg) from AOM‐DSS‐treated WT mice were immunoblotted with antibodies to phosphorylated p38MAPKs and total p38α, p38γ and p38δ, as shown in Supplementary Figure S1D. Phospho‐p38γ and p38δ bands were analyzed and quantified. (B) MAPK12 (p38γ) and MAPK13 (p38δ) mRNA expression levels in normal and primary CRC tumor tissues from the TCGA database. (C) Survival analysis (Kaplan‐Meier plot with log‐rank test) of CRC patients from the TCGA dataset (in UALCAN portal) stratified by MAPK12 (p38γ) and MAPK13 (p38δ) mRNA expression levels. (D) Enrichment analysis of GO biological processes of the genes whose expression positively correlates with MAPK12 (p38γ). (E) Organoid lysates (20 μg) from tumors (T) or normal tissues (C) from CRC patients (P1‐5) were immunoblotted sequentially with the following antibodies, from top to bottom: 1) anti‐phos‐p38MAPK: secondary anti‐rabbit green (this antibody recognizes all phospho‐p38MAPK isoforms); 2) anti‐p38δ: secondary anti‐sheep red (merge image of these two blots is shown in the figure); 3) anti‐p38γ: secondary anti‐sheep red (total p38δ can be also visualized), and 4) anti‐p38α: secondary anti‐rabbit red (total p38δ can be also visualized). Blots were analyzed using the Odyssey infrared imaging system. The identity of the isoforms was determined, as in Supplementary Figure S1E‐F, by their different mobility in the SDS‐PAGE gel (to distinguish p38γ from p38α, p38δ and p38β) and by using simultaneously specific antibodies for a particular p38MAPK isoform (in red) and anti‐phospho‐p38MAPK antibody (in green). After analysis of the blots in the Odyssey infrared imaging system, phospho‐p38MAPK bands should be yellow when colors were merged. (F) Total and phospho‐protein bands of all organoid samples (9 organoids derived from tumor tissues and 10 from normal tissues) shown in panel (E) and Supplementary Figure S4B were quantified using ImageJ. As a control, p38α expression and phosphorylation were examined, and they were similar in organoids from normal and tumor tissues. (G) p38γ and p38δ expression in plasma samples from 20 colon cancer patients and 10 healthy controls were measured by ELISA. Black dots represent the data of healthy donors; red symbols represent the data of CRC patients without IBD; yellow symbols represent the data of CRC patients with IBD. (H) p38γ and p38δ protein expression in the plasma samples from IBD (CD and UC) patients were measured by ELISA. (I) MAPK12 (p38γ) and MAPK13 (p38δ) mRNA expression in IBD (UC and CD) patients and healthy controls using data from GEO (GSE179285). Colon tissues were collected from patients with UC; colon and ileum tissues were collected from patients with CD and healthy donors. (J, K) Expression of miRNAs in the plasma samples from colon cancer (J) and colitis patients (K). miRNA‐128‐2, miRNA‐133a and miRNA‐155 were measured by FLEET CTD analysis in plasma samples from 20 healthy donors (10 for CRC and 10 for IBD), 20 colon cancer patients, 12 UC and 12 CD patients. Data are shown as mean ± SEM. Student's t‐test was used for comparison. ns, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Abbreviations: AOM: azoxymethane; CD: Crohn's disease; CRC: colorectal cancer; DSS: dextran sodium sulfate; FPKM: fragments per kilobase of transcript per million mapped reads; ELISA: enzyme‐linked immunosorbent assay; GEO: gene expression omnibus; GO: gene ontology; IBD: inflammatory bowel disease; MAPK: mitogen‐activated protein kinase; miRNA: microRNAs; p38γ/p38δ: p38γ and p38δ; SDS‐PAGE: sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; SEM: standard error of the mean; TCGA: The Cancer Genome Atlas; UC: ulcerative colitis; WT: Wild type; au, arbitrary units
To understand the role of p38γ/p38δ in CRC, we analyzed the correlation of their expression with other genes. Using the UALCAN portal and Gene Ontology (GO) enrichment analysis, we found that p38γ was positively correlated with genes whose biological functions are related to the immune and inflammatory response (Figure 1D, Supplementary Figure S3A‐C, Supplementary Tables S1‐S4). p38γ expression was positively correlated with matrix metalloproteinase 9 (MMP9), tissue inhibitor of metalloproteinase 1 (TIMP1) and interleukin‐6 (IL‐6) (Supplementary Figure S3D), which were overexpressed in CRC (Supplementary Figure S3E). IL‐6 is a marker of colitis that may precede CRC development. These results suggest that p38γ plays a role in the inflammatory tumor microenvironment which is associated to tumor formation and progression. This agrees with the observation that p38γ is activated in response to inflammatory stimuli, leading to the production of mediators, such as cytokines and other genes, which regulate cell proliferation, death or transformation [2, 3, 4]. On the other hand, the expression of p38δ mRNA in CRC was positively correlated with genes involved in cell division and RNA/DNA processing, and negatively with MMP9 and TIMP1 (Supplementary Figure S3F‐J).
There are few databases containing comparative studies of protein expression in tumor versus normal tissue, and those available did not analyze p38γ/p38δ. However, in the Human Protein Atlas portal, we found immunohistochemical staining of p38γ/p38δ in CRC patients’ tumor sections (Supplementary Figure S4A). To further determine p38γ/p38δ protein expression and phosphorylation (which indicates protein activation) in CRC, we compared human colon organoids derived from CRC and normal colon tissues (Supplementary Table S5) by Western blotting (Figure 1E, Supplementary Figure S4B). Quantitative analysis revealed that p38γ was expressed and phosphorylated in 30% of the tumors examined, while p38δ expression was similar in tumor and normal tissues and was not phosphorylated (Figure 1F). These data indicate that protein expression and activation of p38γ, but not p38δ, is upregulated in CRC patients. An alternative method to evaluate protein expression changes in CRC is a blood‐based test. We then measured p38γ/p38δ protein levels, as well as p38MAPK phosphorylation, in plasma samples from healthy donors and CRC patients (Supplementary Table S6) by enzyme‐linked immunosorbent assay (ELISA). We found that p38γ levels in plasma samples from CRC patients were markedly increased compared with healthy donors (Figure 1G), which was associated with increased levels of the inflammatory molecules IL‐6, C‐C motif chemokine ligand 5 (CCL5), tumor necrosis factor α (TNFα), Interferon stimulated gene 15 (ISG15) and anti‐neutrophil cytoplasmic antibodies (ANCA) (Supplementary Figure S4C‐D). In contrast, p38δ levels were significantly decreased in plasma samples from CRC patients (Figure 1G). Phosphorylated p38MAPKs were decreased in CRC samples (Supplementary Figure S4E). These data agree with those observed for the p38γ/p38δ mRNA expression in CRC patients and those found for p38γ in human tissue‐derived organoids. The minimal changes in p38δ protein observed in organoids could be due to that, although organoids replicate characteristics of tumor source, they do not contain non‐tumor cells that could affect p38δ expression, accounting for the difference observed.
As all our data indicated that p38γ/p38δ, particularly p38γ, played an essential role in colon inflammation and since CRC is associated to or can be caused by chronic colon inflammation, we examined the levels of p38γ/p38δ in plasma samples from IBD (UC and CD) patients (Supplementary Table S7). p38γ protein levels in the plasma samples from IBD patients were markedly increased compared to healthy donors, while the amounts of p38δ and phosphorylated p38MAPKs were decreased (Figure 1H, Supplementary Figure S4F). We also assessed the mRNA expression of p38γ/p38δ in IBD patients versus healthy controls using the GEO datasets (GSE179285; GSE166925) [8]. p38γ mRNA expression was significantly increased in UC and CD compared to healthy controls, whereas p38δ was markedly decreased in UC and CD (Figure 1I, Supplementary Figure S4G). These results indicate that increased levels of p38γ protein in plasma and p38γ mRNA in colon samples as well as decreased levels of p38δ mRNA may be good indicators of both CRC and IBD. These data and previous findings strongly suggest the p38γ’s role in linking inflammation and tumor development.
p38γ/p38δ have a pro‐oncogenic function in CRC by regulating the production of inflammatory molecules and miRNAs [2, 5, 9, 10]. We then examined their effect on the levels of miR‐128‐2, miR133a and miR‐155, which are implicated in inflammation and cancer development [9, 10] and are detectable in plasma samples. miR‐128‐2 and miR133a were regulated by p38γ and p38δ in a mouse model of colitis and CAC (Supplementary Figure S5A‐D). In the plasma samples from IBD and CRC patients, miR128‐2 and miR133a, but not miR‐155, were increased compared to healthy donors (Figure 1J‐K); however, only miR128‐2 was correlated with p38γ/p38δ expression levels (Supplementary Figure S5E‐G). These results indicate a link between the expression of p38γ/p38δ and molecules implicated in inflammation and cancer development, reinforcing the described interplay of CRC and colitis (Supplementary Figure S6).
This study showed that p38γ/p38δ protein could be detected in human plasma. Given the high mortality of colon cancer, the easy access to early risk assessment would contribute to improved survival rates. Our work indicates that p38γ/p38δ could be useful biomarkers for CRC/IBD diagnostic blood‐based tests for colitis and early‐stage CRC. Additionally, our results support the role of p38γ/p38δ in promoting tumor growth and give further evidence that one of the mechanisms by which p38γ promotes tumorigenesis is linked to its elevated protein expression and activation, thus increasing inflammation and providing an inflamed tumor environment that would promote tumor growth.
DECLARATIONS
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
FUNDING
This research was funded by the MCIN/AEI/10.13039/501100011033 (PID2019‐108349RB‐100 and SAF2016‐79792R) to AC and JJSE; Villum Foundation, grant no. 13152 to KA; by Agencia Estatal de Investigación (PID2019‐104867RB‐I00/AEI/10.13039/501100011033) and the Instituto de Salud Carlos III‐ Fondo Europeo de Desarrollo Regional (CIBERONC/CB16/12/00273 and ICI20/00057) to AM and AB. PF received MCIN FPI fellowship (BES‐2017‐080139).
AUTHOR CONTRIBUTIONS
AC, KA and MT conceived and planned the experiments. PF, MT, MAMS, BS and AB carried out the experiments. PF, MT, MAMS, JH, SS, AM, AB, AS, JJSE, AH, JMG and AC contributed to sample collection and preparation. PF, MT, AKJ, MLW, JJSE, KA and AC contributed to the analysis and interpretation of the results. MT, AKJ, MLW, KA and AC contributed to writing the original draft; AC writing‐Review and editing. All authors provided critical feedback, helped shape the research, analysis and manuscript, and agreed to the published version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
De‐identified human colitis plasma samples were obtained from Stanford University (USA) under Stanford Institutional Review Board (IRB protocol 28427). Human colon cancer samples were provided by Danish Biobank RBGB Herlev, under EVK number 21015520. All the samples were fully anonymized prior to analyses. Samples from patients used for organoid cultures were provided by the Biobank IdiPAZ (PT20/00004) with the approval of the Ethics and Scientific Committees of La Paz University Hospital (HULP‐PI‐1425 and HULP‐PI‐3169). Normal tissue samples were obtained from the CRC‐adjacent non‐tumoral tissue. Informed consent was obtained from all participants. Mouse studies were conducted in specific pathogen‐free conditions in accordance with European Union regulations and the regulation of local Centro Nacional de Biotecnología and Consejo Superior de Investigaciones Científicas bioethics committees and were approved by the Community of Madrid PROEX316/15 and PROEX289.2/20.
CONSENT FOR PUBLICATION
Not applicable.
DATA AVAILABILITY STATEMENT
Not applicable.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank our colleagues Tirso Pons and Lorena Madraner for informatic support. We thank Prof. MD Elizabeth D Mellins, Stanford University School of Medicine and Prof. N. Heegaard, SSI, Denmark, for providing us with healthy human controls for this study. We acknowledge S. Khatri, Technical University of Denmark, for technical assistance with performing ELISA and bead‐based assay. We want to particularly acknowledge the patients and the Biobank IdiPAZ (PT20/00004) from the Spanish National Biobanks Network.
Pilar Fajardo and Maria Taskova contributed equally to this work
Contributor Information
Kira Astakhova, Email: kiraas@kemi.dtu.dk.
Ana Cuenda, Email: acuenda@cnb.csic.es.
REFERENCES
- 1. Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cuenda A, Sanz‐Ezquerro JJ. p38γ and p38δ: From Spectators to Key Physiological Players. Trends Biochem Sci. 2017;42, 431‐42. [DOI] [PubMed] [Google Scholar]
- 3. Alsina‐Beauchamp D, Escós A, Fajardo P, González‐Romero D, Díaz‐Mora E, Risco A, et al. Myeloid cell deficiency of p38γ/p38δ protects against candidiasis and regulates antifungal immunity. EMBO Mol Med. 2018;10.(5):e8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risco A, del Fresno C, Mambol A, Alsina‐Beauchamp D, MacKenzie K, Yang HT, et al. p38γ and p38δ kinases regulate the Toll‐like receptor 4 (TLR4)‐induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc Natl Acad Sci U S A 2012;109:11200‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Del Reino P, Alsina‐Beauchamp D, Escós A, Cerezo‐Guisado MI, Risco A, Aparicio N, et al. Pro‐oncogenic role of alternative p38 mitogen‐activated protein kinases p38γ and p38δ, linking inflammation and cancer in colitis‐associated colon cancer. Cancer Res. 2014;74:6150‐60. [DOI] [PubMed] [Google Scholar]
- 6. Barrio L, Román‐García S, Díaz‐Mora E, Risco A, Jiménez‐Saiz R, Carrasco YR, et al. B Cell Development and T‐Dependent Antibody Response Are Regulated by p38γ and p38δ. Front Cell Dev Biol. 2020;8:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pritchard AL, Hayward NK. Molecular pathways: mitogen‐activated protein kinase pathway mutations and drug resistance. Clin Cancer Res. 2013;19:2301‐9. [DOI] [PubMed] [Google Scholar]
- 8. Keir ME, Fuh F, Ichikawa R, Acres M, Hackney JA, Hulme G, et al. Regulation and Role of αE Integrin and Gut Homing Integrins in Migration and Retention of Intestinal Lymphocytes during Inflammatory Bowel Disease. J Immunol. 2021;207:2245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M, Fu W, Wo L, Shu X, Liu F, Li C. miR‐128 and its target genes in tumorigenesis and metastasis. Exp Cell Res. 2013;319:3059‐64. [DOI] [PubMed] [Google Scholar]
- 10. Necela BM, Carr JM, Asmann YW, Thompson EA. Differential expression of microRNAs in tumors from chronically inflamed or genetic (APCMin/+) models of colon cancer. PLoS One. 2011;6:e18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Not applicable.


