Lingering virus particles, microclots, and faulty immune signaling are likely culprits for many long-hauler symptoms. Anticoagulants, immune regulators, and antivirals show early promise
As the current crisis phase of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic winds down—and the world nervously awaits potentially dangerous new variants—research into the nature and treatment of so-called long coronavirus disease (COVID) is beginning to ramp up. The White House has promised funding and a federal research roadmap, and dedicated clinics have started cropping up at academic medical centers across the country.
Attempts to understand and treat long COVID have been underway almost since the pandemic began. Amid a maze of clues, some ideas are starting to coalesce. Image credit: Shutterstock/Lightspring.
But attempts to understand and treat long COVID have been underway almost since the pandemic began. For more than 2 years, clinicians have been coping—mostly on their own—with streams of patients complaining of persistent symptoms or mysterious new ones after a bout with COVID-19 had seemingly resolved (1). And collectively, doctors and researchers have already made headway toward identifying some of the mechanisms underlying the condition—formally known as post-acute sequelae of COVID (PASC).
That’s not to say that it’s been easy; estimates of the proportion of COVID-19 patients who will report symptoms 12–24 weeks after their infection range from about 30% to 70% (2–4). Some 200 different symptoms have been ascribed to long COVID, and the syndrome is yet to be fully defined. The most common complaints include fatigue, shortness of breath, and a dysfunctional sense of smell (5). For many, symptoms clear up on their own within 6 months. But one large study in China found that 55% of patients infected early in the pandemic were still struggling with at least one symptom 2 years after infection (6).
“Long COVID is very unlikely to be one thing,” says Michael VanElzakker, a neuroscience researcher at Harvard in Cambridge, MA, who co-authored a recent review of possible mechanisms behind the syndrome (7). Amid a maze of clues, some ideas are starting to coalesce. One leading theory, for example, suggests that tissue damage incurred during the acute infection can contribute to symptoms, but lingering virus and viral antigens could also be provoking prolonged, harmful immune responses including inflammation, autoimmunity, and disruption of the microbiome (8).
Worryingly, many long COVID symptoms align with other bewildering post-viral syndromes such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). “Immune dysregulation is at the heart of it,” says Bela Chheda, an infectious disease doctor at the Center for Complex Diseases in Mountain View, CA. Treating long COVID, she notes, entails finding the right combination of drugs to return the body to its homeostasis—a drug regimen that could include antivirals, antihistamines, drugs that defuse inflammatory immune cells, and treatments for the blood vessel inflammation and tiny blood clots that are hallmarks both of COVID-19 and long COVID.
Informed by experience with other post-infection syndromes and intensive study of how the novel coronavirus behaves in the body, researchers have many leads for long COVID therapy. Proven treatments are few. But a handful of clinical trials are starting to recruit participants. And as the pressing need keeps growing, so does confidence that the mysteries of long COVID can be cracked.
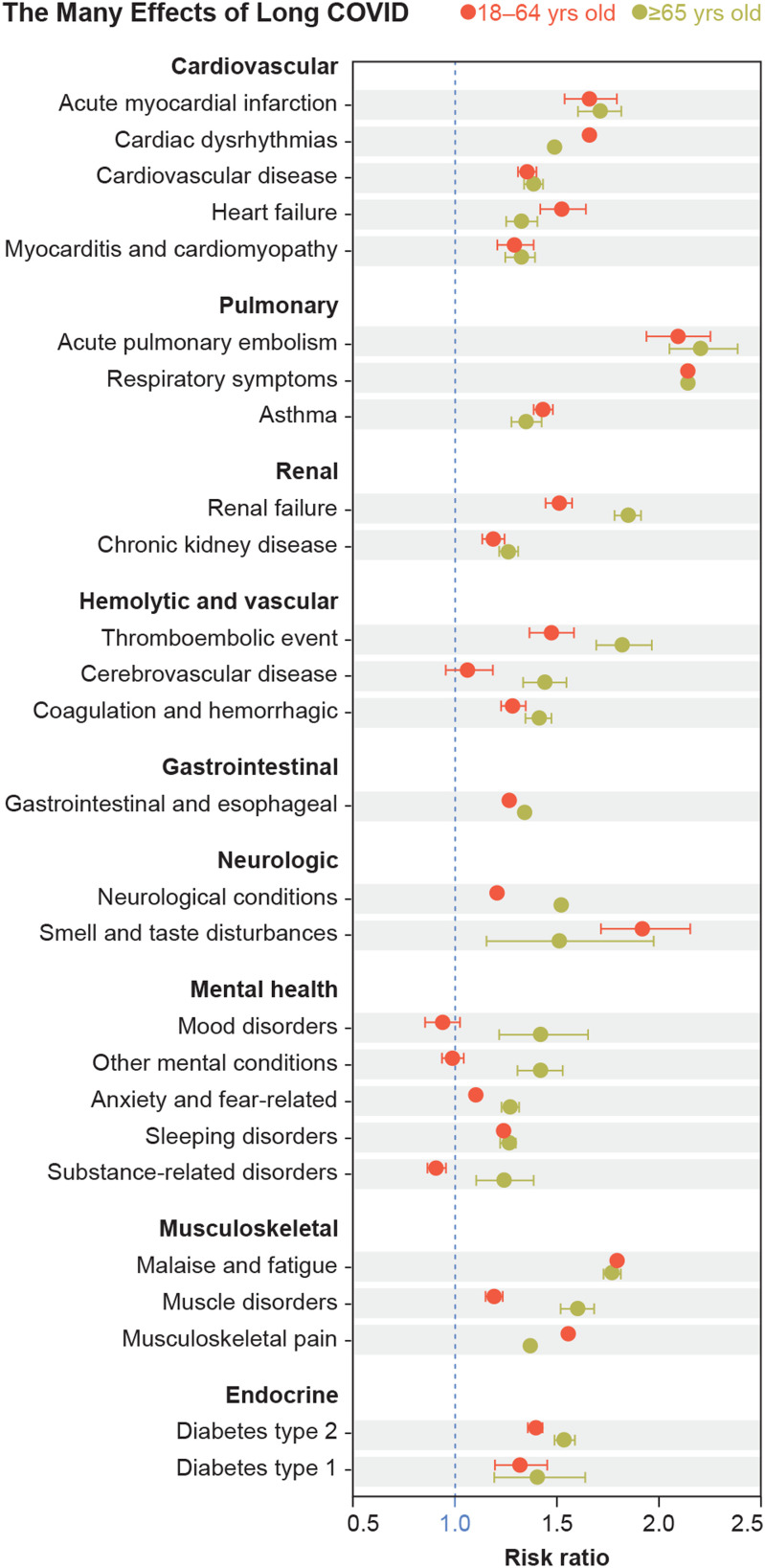
As shown here based on data from March 2020 through November 2021, adult patients have developed a wide variety of post-COVID conditions, with some clearly more pronounced in older age groups. Image credit: Reprinted with permission from Ref 2. Modified for style by Lucy Reading-Ikkanda (artist).
Hidden Virus
There is now ample evidence that SARS-CoV-2 infects diverse tissues throughout the body—far beyond the reach of a nasal swab—and that those infections can last long after obvious respiratory symptoms have cleared (9–11). A study by Stanford University researchers, for example, found that 4% of recovered COVID-19 patients were shedding viral RNA in their feces and reporting gastrointestinal (GI) symptoms 7 months after infection (12).
Lurking infection may seem like an obvious explanation for persistent long COVID symptoms, but it’s been one of the most difficult to prove. The presence of viral RNA doesn’t necessarily indicate live virus, which has to be cultured from tissue samples (13). And sampling tissues is more invasive and costly than just a nasal swab or blood test. “Immune markers (in blood) are easy to test for but virus in tissue is a harder test,” says VanElzakker. It’s also hard to determine whether long COVID is attributable to ongoing inflammation and damage from the original infection or is a response to lingering virus or viral remnants, he adds.
Anecdotal evidence for the viral reservoir hypothesis comes from case reports of long COVID patients seeing symptoms clear up after a course of antivirals. One 47-year-old woman had been experiencing debilitating symptoms for 6 months after an initial SARS-CoV-2 infection; then she was exposed and potentially infected again. She tested negative by nasal swab, but doctors considered her at risk for severe disease and prescribed a 5-day course of Pfizer’s protease-inhibitor pill combination Paxlovid (nirmatrelvir/ritonavir). The patient said all her old symptoms cleared up rapidly and completely, and she was able to return to work and even vigorous exercise for the first time in months (14).
Such anecdotes spread fast in the patient community and long-haulers are anxious to get their hands on antivirals, including Paxlovid and Merck’s Lagevrio (molnupiravir), a ribonucleoside prodrug that stymies viral replication by introducing errors into the viral genome. Both medications have FDA emergency use authorizations only for treating acute COVID in patients at risk of severe illness, and until this year, both were in short supply even for that indication.
Companies and clinicians haven’t yet tested antivirals in a clinical trial for long COVID. They ought to, says David Strain, a senior clinical researcher at the University of Exeter in the UK. Case reports and insights from other post-viral syndromes where hidden viral reservoirs are a factor justify trials of COVID-19 therapeutics for long COVID, he says. “I think it’s a relatively robust hypothesis and fits with what we’re currently observing in the moment as well.”
Strain is among the lead investigators on a multicenter UK trial, Symptoms, Trajectory, Inequalities and Management: Understanding Long-COVID to Address and Transform Existing Integrated Care Pathways (STIMULATE-ICP), launched in 2021 to study the progression of long COVID and to test therapies. One goal is to recruit 4,500 long COVID patients and randomly assign them either to standard care or a new clinical pathway that includes an app designed to simplify the process of connecting with doctors and finding individualized treatments. Within that project, another trial arm will test different treatments for 3 months at a time to see whether symptoms abate. Strain eventually hopes to include Gilead Sciences’ antiviral Veklury (remdesivir) among the therapies but is still in talks with the company.
Remdesivir was chosen partly because it has few interactions with other drugs, Strain explains. “I think Paxlovid is probably the better antiviral, but there’s lots of other drugs you can’t take it with,” he says, noting statins in particular.
A Vascular Disease
The virus need not be actively copying itself for it to trigger damaging inflammation and clotting in patients experiencing long COVID. Lingering bits and pieces of virus could still inflame blood vessels. The result: microclots that could account for a range of symptoms.
Fatigue, for instance, is one of the most common complaints for long-haulers. Strain says that fatigue and pain can stem from muscles being deprived of oxygen by microclots in the smallest blood vessels. Activated endothelial cells lining blood vessel walls can draw coagulation proteins to them, constricting blood flow and fostering the formation of tiny clots.
Persistent clotting may be widespread in recovered COVID patients. Soracha Ward and colleagues at the Royal College of Surgeons in Dublin, Ireland, measured markers for clot formation in 50 people who had COVID-19 in the preceding 10 weeks (15). She was surprised to see the clotting problem even in patients who had not been hospitalized for COVID-19. Levels of coagulation markers were highest in patients who had severe illness. But even those with mild illness still had elevated levels of D dimers, fragments of proteins left over from clots, along with high levels of factor VIII, a clotting protein. “When [these markers] are elevated, it’s a hint that these endothelial cells are inflamed and they’re active,” Ward says.
Although the microclots may explain the fatigue, the muscle aches and pains, and the brain fog, researchers still don’t know where these symptoms are in the “causative cycle,” Strain says. “If we get rid of those clots, will it do any good, if inflammation is still in place?” To find out, STIMULATE-ICP will be testing the anti-clotting drug rivaroxaban, along with the anti-inflammatory gout drug colchicine—which has a good track record with ME patients, although NIH treatment guidelines don’t endorse it for acute COVID-19—as well as antihistamines famotidine and loratadine (16,17).
Virologist Bruce Patterson, CEO of San Carlos, CA-based IncellDx, is going after inflammation directly in the hope of also reducing clotting. Patterson, a former Stanford University professor, believes fragments of the virus that remain attached to immune cells trigger immune signaling that keeps endothelial cells activated and blood vessels inflamed. He’s found that nonclassical and intermediate monocytes, immune cells that patrol blood vessels and play a role in vascular homeostasis, can carry the SARS-CoV-2 viral S1 protein, a portion of the viral “spike,” more than a year after infection (18). “This vascular inflammation seems to be at the heart of long COVID,” he says.
But the approaches of Patterson and incellDX—which produces cancer diagnostics and tests for immune biomarkers used in reference labs—have faced criticism. Some see an overhyped treatment regimen that has not gone through clinical trials. The company, which conducts telemedicine in consultation with primary care doctors who are treating long COVID patients, does not function as a clinic; instead, it connects patients with doctors in their provider network who will prescribe medications.
Patterson has “treated” long COVID patients using two types of drugs to target inflammation. Pravastatin, one of the statins typically associated with lowering cholesterol, can also suppress the expression of fractalkine, an endothelial-cell transmembrane protein that attracts immune cells to attach themselves to blood vessel walls. And the HIV drug Maraviroc blocks the monocytes’ chemokine receptor 5 (CCR5), which plays a role in inflammatory signaling.
“For long COVID, this microclotting is potentially a really big thing that is not allowing them to heal.”
—Bela Chheda
In a preprint, Patterson and colleagues describe their results with Maraviroc and Pravastatin in 18 patients, who showed a statistically significant decrease in levels of inflammatory immune markers and subjective symptoms in weeks 6 to 12. For instance, in the case of fatigue, most patients went from a symptom score greater than 60 to less than 40 (19). IncellDx has patented the use of Maraviroc for treating coronavirus infections including COVID-19 (20).
Work by professor of physiology Etheresia Pretorius at Stellenbosch University in South Africa supports the notion that these fragments of leftover viral antigen can indeed wreak havoc. Before the pandemic, Pretorius was investigating how bacteria that lurk in the bloodstream after an infection can set off blood-clotting cascades; she thinks the SARS-CoV-2 spike protein has a similar effect (21,22). Pretorius and colleagues tested the idea of targeting the resulting microclots in long COVID patients directly, using an existing blood thinner regimen for atherosclerosis. In a small study still in preprint, they used the standard “triple therapy” anti-platelet drugs clopidogrel and aspirin along with anticoagulation drug Apixiban on 23 patients. The team found that all the participants returned to their pre-COVID levels of platelet activity and reported that their symptoms of exhaustion and brain fog had resolved. The result aligns with the hypothesis that microclots are depriving tissue, including the brain, of oxygen, according to the study authors (23).
Chheda isn’t ready to recommend anti-clotting medication just yet, although she thinks that fixing the microclot problem could go a long way toward relieving exhaustion and brain fog. “For long COVID, this microclotting is potentially a really big thing that is not allowing them to heal,” she says.
Trial and Error
Despite high hopes for antivirals and promising results tackling vascular inflammation and microclots, researchers and clinicians like Chheda know that what helps one patient experiencing long COVID might do little for the next. Indeed, Patterson and other researchers predict that long COVID and other post-viral illnesses will eventually be broken down into subcategories with different underlying mechanisms, and treatment will be tailored accordingly.
That’s why Strain and colleagues are designing their clinical trials to be nimble in testing a wide variety of repurposed drugs, with 3-month courses of different therapies to quickly sort wheat from chaff.
Chheda, too, gives patients short trials of various drugs for 4 to 6 weeks, and then on to the next one if it’s not effective. Treating long COVID often entails this trial and error because of the variability among patients. Chheda has had some success with antihistamines such as famotidine (Pepcid) and diphenhydramine (Benadryl) targeting patients’ mast cells, immune-system first responders that release inflammatory mediator molecules such as leukotriene E4. “If you have a chronic inflammatory state, the question is how big a role are mast cells playing?” Chheda says. Treatments that diffuse the cells include antihistamines such as montelukast, along with H1, H2 receptor blockers like levocetirizine and famotidine.
In a small randomized, double-blind, phase 2 clinical trial involving 55 nonhospitalized patients with COVID-19, famotidine use led to an earlier reduction of inflammatory type-I interferon signaling without blunting anti-viral immune responses, according to researchers at Cold Spring Harbor Laboratory in New York. “Famotidine improved resolution of 14 of 16 assessed symptoms and led to a statistically significant increased rate of symptom recovery,” Tobias Janowitz and colleagues reported inGut(24). How the drug works against COVID-19 remains unclear, but some researchers think it might have less to do with mast cells than with famotidine’s action on the vagus nerve, which plays an important role in the body’s “inflammatory reflex”—the brain’s way of turning off and on inflammatory signals throughout the body.
Kevin Tracey, president and CEO of the Feinstein Institutes for Medical Research in Manhasset, NY, pioneered research into this reflex more than 20 years ago. His group has tested famotidine and other drugs in mice experiencing an induced immune “cytokine storm” of the kind seen in severe COVID-19. They reported that famotidine improved the animals’ survival and did not work via the mast cell mechanism (25). “It was really, really surprising, but when we treated mice with famotidine, it activated their vagus nerve,” says Tracey. Through this mechanism, the vagus nerve “turns on the brakes” on the cytokine storm.
A clinical trial in Spain of direct vagus nerve stimulation via the skin on the neck supported the idea that it can calm cytokine signaling in COVID-19 patients (26). And the FDA has issued an Emergency Use Authorization for the gammaCore Saffire CV vagus nerve stimulation device to be used in cases of acute COVID-19 where the patient is experiencing asthma symptoms.
VanElzakker agrees that vagus nerve stimulation also could potentially alleviate some of the misery of long COVID, noting that the nerves detect inflammation and send the signal to the brain that the body is sick. “This is the thing that makes us feel tired, and we lose our appetite,” he says about vagus nerve signaling. “You can use vagus nerve stimulation to try to support the ‘anti-inflammatory reflex,’” much like a pacemaker works to bring regulation to heart beats, he explains. But neither he nor Tracey currently has funding to scale up the research themselves.
Apart from better access to drugs and more funding to test promising long COVID therapies, many researchers are eager to see a central hub of long COVID data to help accelerate discovery (27). In April, President Biden pledged an additional $1 billion for long COVID research and treatment and gave the Secretary of Health and Human Services 90 days to produce a roadmap for developing “the first-ever interagency national research action plan on long COVID” (28).
It’s encouraging that help is on the way, researchers say, but they’re anxious to get started with clinical trials of the many potential treatments already showing promise.
For some patients, these existing therapies are enough to restore homeostasis, says Chheda. “The body actually does want to go back to health.” Still, it’s not necessarily going to be a straight path back to normal. “If 10 people are to be infected with the same virus or the same bacteria,” says Chheda, “everybody’s immune system will do something a little different.” Understanding those differences is an ongoing challenge—especially for an unpredictable virus with a maddening array of symptoms.
References
- 1.Mahase E., Covid-19: What do we know about “long covid”? BMJ 370, m2815 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Bull-Otterson L., et al. , Post-COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years — United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 71, 713–717 (2022). [Google Scholar]
- 3.Groff D., et al. , Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw. Open 4, e2128568 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M., et al. , Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 18, e1003773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo S. M., et al. , Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J. Gen. Intern. Med. 37, 1988–1995 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L., et al. , Health outcomes in people 2 years after surviving hospitalization with COVID-19: A longitudinal cohort study. Lancet Respir. Med., 10.1016/S2213-2600(22)00126-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proal A. D., VanElzakker M. B., Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 12, 698169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M., Blish C. A., Sallusto F., Iwasaki A., The immunology and immunopathology of COVID-19. Science 375, 1122–1127 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Stein SR, et al. , SARS-CoV-2 infection and persistence throughout the human body and brain. 10.21203/rs.3.rs-1139035/v1 (20 December 2020). [DOI]
- 10.Puelles V. G., et al. , Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 383, 590–592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartard C., et al. , Multiorgan and vascular tropism of SARS-CoV-2. Viruses 14, 515 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan A., et al. , Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med (N Y) 3, 371–387.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trypsteen W., Van Cleemput J., Snippenberg W. V., Gerlo S., Vandekerckhove L., On the whereabouts of SARS-CoV-2 in the human body: A systematic review. PLoS Pathog. 16, e1009037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng LN, Bonilla HF, Shafer RW, Miglis MG, Yang PC, Case report of breakthrough long COVID and the use of nirmatrelvir-ritonavir. 10.21203/rs.3.rs-1443341/v1 (15 March 2022). [DOI]
- 15.Fogarty H., et al. ; Irish COVID-19 Vasculopathy Study (iCVS) investigators, Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 19, 2546–2553 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.do Campo J., Taylor V., Clinical improvement in patients with ME/CFS with synergistic effect of colchicine and spironolactone targeting inhibition of inflammasome activity. Intern. Med. J. 52 (suppl. 1), 4–19 (2022).35514213 [Google Scholar]
- 17.COVID-19 Treatment Guidelines Panel, COVID-19 treatment guidelines: Colchicine. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/colchicine/. Accessed 1 July 2022.
- 18.Patterson B. K., et al. , Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front. Immunol. 12, 746021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson BK, et al. , Targeting the monocytic-endothelial-platelet axis with maraviroc and pravastatin as a therapeutic option to treat long COVID/post-acute sequelae of COVID (PASC). 10.21203/rs.3.rs-1344323/v1 (10 February 2022). [DOI]
- 20. IncellDx awarded patent for use of CCR5 antagonist maraviroc in treatment of COVID-19. https://www.businesswire.com/news/home/20211123005960/en/IncellDx-Awarded-Patent-for-Use-of-CCR5-Antagonist-Maraviroc-in-Treatment-of-COVID-19. Businesswire. Accessed 1 July 2022.
- 21.Page M. J., Kell D. B., Pretorius E., The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress (Thousand Oaks) 6, 24705470221076390 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobbelaar L. M., et al. , SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci. Rep. 41, BSR20210611 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pretorius E, Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. 10.21203/rs.3.rs-1205453/v1 (28 December 2021). [DOI]
- 24.Brennan C. M., et al. , Oral famotidine versus placebo in non-hospitalised patients with COVID-19: A randomised, double-blind, data-intense, phase 2 clinical trial. Gut 71, 879–888 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H., et al. , Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm. Mol. Med. 28, 57 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tornero C., et al. , Non-invasive vagus nerve stimulation for COVID-19: Results from a randomized controlled trial (SAVIOR I). Front. Neurol. 13, 820864 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albarracín D., et al. , ( 2022) Getting to and sustaining the next normal: A roadmap for living with COVID. https://www.covidroadmap.org Accessed 1 July 2022.
- 28. FACT SHEET: The Biden Administration accelerates whole-of-government effort to prevent, detect, and treat long COVID. https://www.whitehouse.gov/briefing-room/statements-releases/2022/04/05/fact-sheet-the-biden-administration-accelerates-whole-of-government-effort-to-prevent-detect-and-treat-long-covid/. The White House. Accessed 1 July 2022.