Significance
Large animals (megafauna) have cascading effects on populations, communities, and ecosystems. The magnitude of these effects is often unknown because native megafauna are missing from most ecosystems. We found that reintroducing bison—a formerly dominant megafauna and the national mammal of the United States—doubles plant diversity in a tallgrass prairie. These plant communities have few nonnative species and were resilient to an extreme drought. Domesticated megafauna (cattle), which have replaced native herbivores in many grasslands, produced less than half of this increase in plant species richness. Our results suggest that many grasslands in the Central Great Plains have substantially lower plant biodiversity than before widespread bison extirpation. Returning or “rewilding” native megafauna could help to restore grassland biodiversity.
Keywords: biodiversity, keystone predation, top-down, rewilding, resistance
Abstract
The widespread extirpation of megafauna may have destabilized ecosystems and altered biodiversity globally. Most megafauna extinctions occurred before the modern record, leaving it unclear how their loss impacts current biodiversity. We report the long-term effects of reintroducing plains bison (Bison bison) in a tallgrass prairie versus two land uses that commonly occur in many North American grasslands: 1) no grazing and 2) intensive growing-season grazing by domesticated cattle (Bos taurus). Compared to ungrazed areas, reintroducing bison increased native plant species richness by 103% at local scales (10 m2) and 86% at the catchment scale. Gains in richness continued for 29 y and were resilient to the most extreme drought in four decades. These gains are now among the largest recorded increases in species richness due to grazing in grasslands globally. Grazing by domestic cattle also increased native plant species richness, but by less than half as much as bison. This study indicates that some ecosystems maintain a latent potential for increased native plant species richness following the reintroduction of native herbivores, which was unmatched by domesticated grazers. Native-grazer gains in richness were resilient to an extreme drought, a pressure likely to become more common under future global environmental change.
Well-documented top-down effects in terrestrial and aquatic ecosystems (1–6) suggest that past extirpation of megafauna could have had significant effects on modern-day biodiversity of ecological communities (7–10). Consumers can increase biodiversity by preferentially preying on species that are otherwise competitively dominant (11, 12), a process known as “keystone predation” (13) or, in the case of herbivores, “keystone herbivory.” Native megagrazers (i.e., herbivores over 25 kg that consume mostly graminoids) evolved digestive systems and selective foraging that can reduce the abundance of palatable grasses that dominate in the absence of grazers (14–17), resulting in increased species richness in many grasslands globally (18). Megagrazers can also create small-scale heterogeneity in nutrient availability [e.g., defecation and urination (19, 20)] and grazing frequency (21–23), which should theoretically increase plant diversity (24)—though these same processes can facilitate the expansion of invasive species (25, 26).
A potential alternative to reintroducing native megafauna is the strategic use of domesticated livestock as a food production and conservation tool (27). Cattle are ubiquitous in grasslands and might have similar effects as native megagrazers on plant communities because they share some key traits, notably a preference for consuming dominant grasses (28, 29). On the other hand, domesticated cattle are distinct from native megagrazers in several ways, including their grazing patch selection and their lack of adaptation to local plant defenses and climate extremes (28–30). Previous work from dry Great Plains grasslands suggests that cattle rely more on nongrass forage than bison (31–33), which could reduce the impact of keystone herbivory. To date, only a handful of studies have quantified whether cattle and native megagrazers differ in their effects on plant communities (e.g., refs. 34 and 35), and most have been short compared to ecological timescales (18), making it difficult to quantify if changes in plant diversity under different grazing regimes are lasting or ephemeral (36).
Before European colonization, bison were one of the most dominant and widespread megafaunal species in the grasslands of the North America (37). In the last two centuries, bison were nearly hunted to extinction, creating a gap in our basic and applied understanding of how native megagrazers shaped one of most extensive grassland biomes (38). A previous 10-y study compared the effects of plains bison (Bison bison, hereafter bison) and cattle grazing on tallgrass prairie while holding grazer biomass and grazing duration constant in replicated 5-ha enclosures (34). Plant species richness in ungrazed control areas changed little during the study, whereas both the bison- and cattle-grazed treatments saw linear decreases in grass cover and linear increases in plant species richness over time. In the bison treatment, grass cover decreased more and the increase in species richness was greater compared to the cattle treatment (34).
Here, we report results from a separate field experiment implemented at a much larger scale (>18 ha per replicate; Fig. 1A) and longer study period of 29 y. The study took place in the Flint Hills ecoregion, which is the largest remaining landscape of tallgrass prairie (39). We examined plant community composition and diversity in three treatments that were designed to capture characteristic management regimes: 1) no megagrazers present (ungrazed), 2) bison were reintroduced and graze year-round, or 3) domestic cattle were introduced and graze during the growing season. We used this study to address our first question: How do bison and cattle impact dominant grasses and plant species diversity over time?
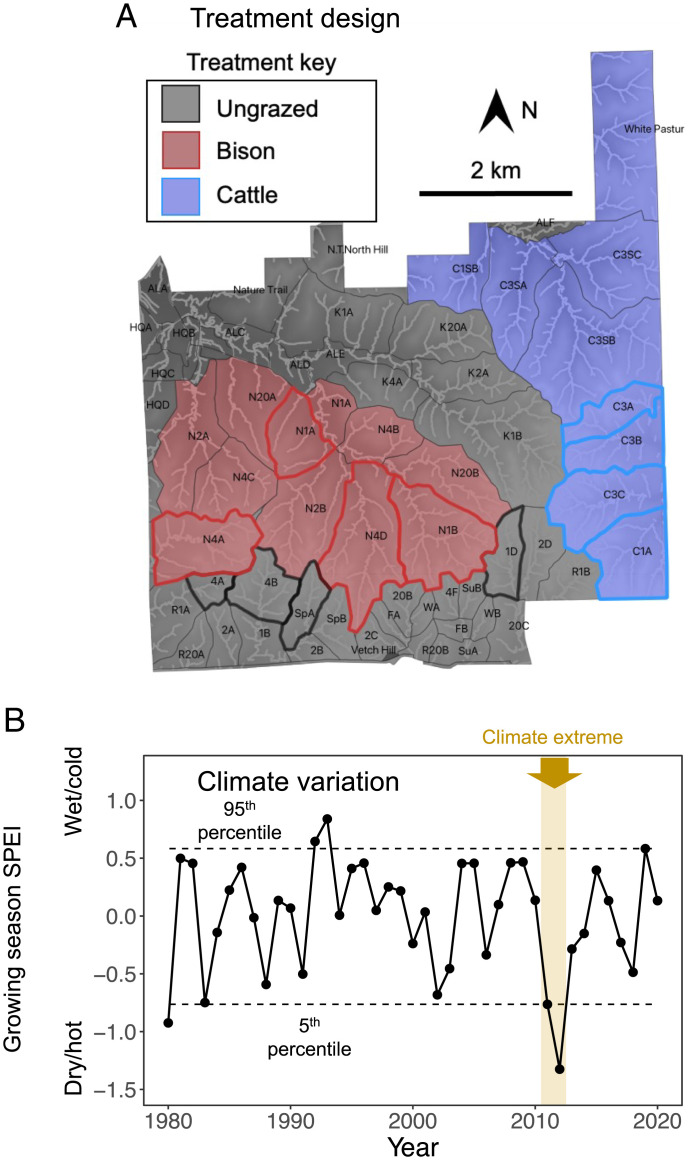
Fig. 1.
(A) A map of KPBS’s grazing treatments: ungrazed (gray), bison-grazed (red), and cattle-grazed (blue). Catchments used in this study have a boldface outline of their respective treatment color. (B) Changes in standardized precipitation evapotranspiration index (SPEI) from 1980 to 2020. Each point is the annual average over the growing season (June through August). Dashed horizontal lines denote the 5th to 95th percentiles. The vertical gold bar denotes the period identified as a climate extreme (as defined by ref. 87). Note that the earlier bison–cattle study (34) ran from 1995 to 2004.
Alongside shifting land use, climate change is one the greatest threats to grassland conservation. Our second question asks, how do bison affect plant community resilience to extreme drought? Whether communities persist in the face of changing climate will depend on their resilience, defined as the ability to avoid lasting ecological shifts in the face of environmental variation, including pulse disturbances such as heatwaves and droughts, and gradual environmental change, such as long-term warming (40–42). Climate change impacts resilience in many ways, but climate extremes, such as extreme droughts, are among the most acute threats to ecological resilience (43). Resilience to droughts can be achieved by 1) high resistance, where the community changes little during extreme events, or 2) low resistance to the extreme event followed by rapid recovery afterward (40–42). It is unknown how reintroducing megafauna affects plant community resilience to extreme drought (44), but ungrazed grasslands often have reduced species richness following extreme droughts (45), potentially lowering the functional resistance to future climate change (46).
During the earlier bison–cattle comparison (34) climate conditions deviated little from the long-term historical average (Fig. 1B), leaving open the question of whether bison or cattle alter the community’s resilience to climate extremes. With the longer duration of our study, we were fortunate to capture one the most extreme drought events that have occurred in the Great Plains since the 1930s Dust Bowl—the 2011 and 2012 droughts (47). In 2011 and 2012, years 20 and 21 of the experiment, the growing season standardized precipitation index—rainfall minus estimated evapotranspiration—was below the fifth percentile, and 2012 was the most extreme dry year on record (Fig. 1B). We assessed the initial resistance and long-term resilience of plant communities to this 2-y extreme event.
Study Design
In the past several decades, growing recognition of the keystone role that bison played in grasslands of North America (48) has led to their reintroduction in hundreds of grassland sites (49). It is important to note that bison are managed differently than domestic cattle. Nationally, regionally, and at our site, bison graze throughout the year (49), whereas cattle are only present during the growing season (between April and November), but at a higher density to compensate for different durations of grazing (50, 51). In the winter, cattle operations typically switch to supplemental feeding (50). Thus, the two grazer treatments in our experiment represent two different land-use types, which reflect how humans typically manage bison versus cattle across most of the region (50, 51). Seasonal grazing by bison or year-round grazing by cattle is neither realistic nor likely to occur. Our experimental treatments reflect that reality. For all analyses we considered two spatial scales: 1) the plot scale (10 m2, n = 80), which represents the scale at which grassland plant species typically interact (52), and 2) the catchment scale, composed of 20 plots each (n = 4; Fig. 1A).
Results
Changes over Time, Resistance, and Resilience.
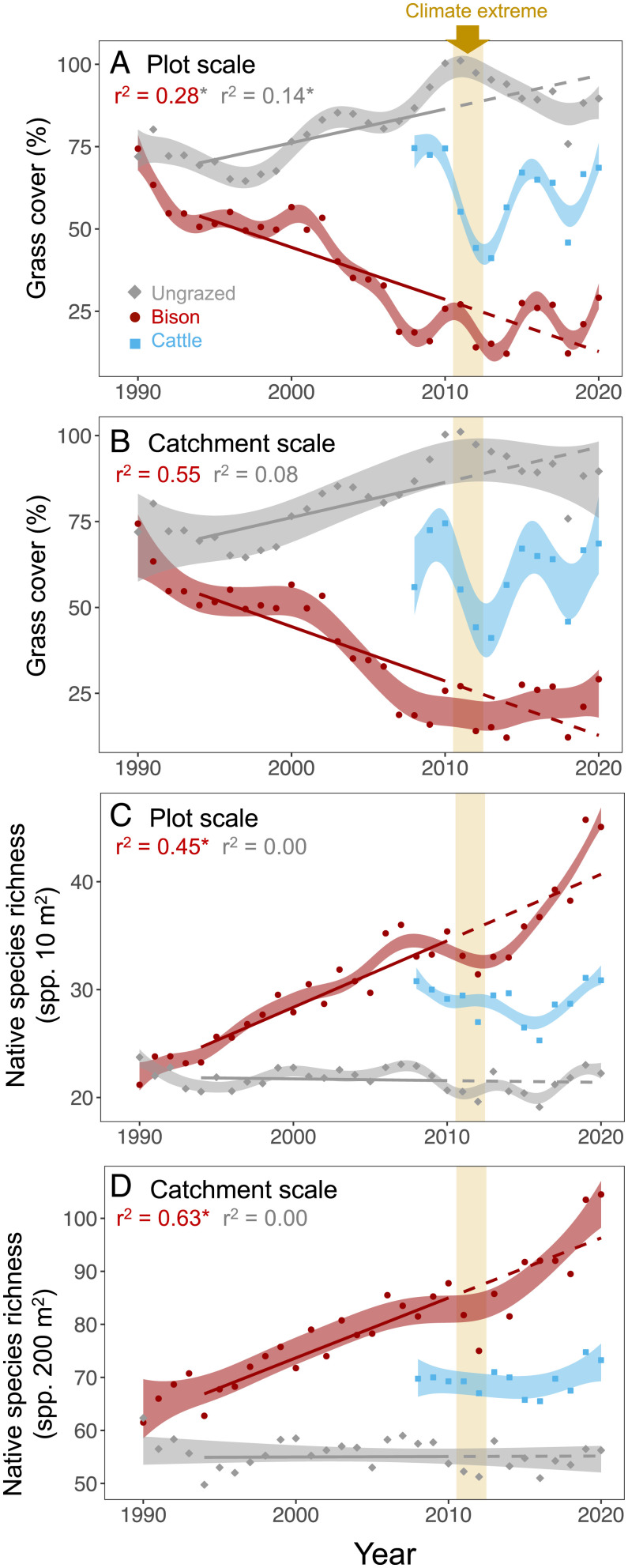
In the absence of grazers four grass species (Andropogon gerardii, Sorghastrum nutans, Panicum virgatum, and Schizachyrium scoparium) comprise ∼80% of annual net primary production (53). In the ungrazed treatment, using general additive models (GAMs), we found that the cover of these dominant grasses (“dominant grass cover” from here on) increased slightly over time (r2 = 0.14 for plots and r2 = 0.08 for catchments, P < 0.001 for both; Fig. 2). Native plant species richness did not change significantly at either scale (P > 0.12; Fig. 2 and SI Appendix, Tables S2–S4 and Figs. S1–S5 for other community and diversity metrics). Dominant grass cover remained high before, during, and after the climate extreme, whereas species richness remained low across these periods (Fig. 2).
Fig. 2.
Dominant grass cover at the plot (A) and catchment scales (B), as a function of time and grazing treatment (gray = ungrazed, red = bison, blue = cattle). (C and D) Changes in native species richness at the plot and catchment scale. Points are annual averages and shaded areas span the 95th confidence interval of GAMs. Solid lines are a linear fit based on data from 1994 to 2010. Dashed lines are an extrapolation of the linear model to the remaining years of data. r2 values are for the linear model for 1994 to 2010 where * indicates a correlation of P < 0.05. The vertical gold bar marks the extreme dry period. Data from the cattle treatment were not available until 2008 and therefore no linear model was fit to cattle data before the climate extreme.
In the bison treatment, dominant grass cover declined initially, stabilized for 9 y, and then declined for another 7 y (Fig. 2). During the final 11 y, dominant grass cover oscillated around a mean cover of 20 to 25%. Native species richness increased linearly from 1994 to 2010 at average rates of 0.58 species per year at the plot scale and 1.06 species per year at the catchment scale (Fig. 2 and SI Appendix, Table S3). To determine if the 2011–2012 climate extreme altered the long-term trajectory of species richness gains, we fit a linear model of time and native species richness before the climate extreme (r2 = 0.45 and P < 0.001 for plots; r2 = 0.63 and P < 0.001 for catchments in the bison treatment) and then extrapolated the model through the remaining years (shown as a dashed line in Fig. 2). In the bison treatment, the climate extreme in 2011–2012 coincided with declines in native species richness, which was the only period when the confidence interval of the GAMs fell below the linear model. This suggests limited resistance of species richness to extreme drought. After the climate extreme, native species richness in the bison treatment converged with the linear model of increasing species richness, with recovery taking 4 y at the plot scale and 2 y at the catchment scale (Fig. 2). This result provides quantitative evidence that native species richness gains were resilient to drought, indicated by the relatively rapid recovery of species richness to its predrought trajectory.
In the cattle treatment, dominant grass cover followed no directional trend leading up to the climate extreme, and before this event dominant grass cover was intermediate to the ungrazed and bison treatment (Fig. 2). During the climate extreme dominant grass cover decreased sharply and eventually recovered at both spatial scales. Before the climate extreme, species richness changed little from year to year and was at values in between the other two treatments. From 2011 to 2020, species richness oscillated from year to year, but we found no net changes in species richness at either scale, as the confidence intervals for the first and last year of data overlapped (Fig. 2).
Net Effects of Bison and Cattle on Species Richness and Community Composition.
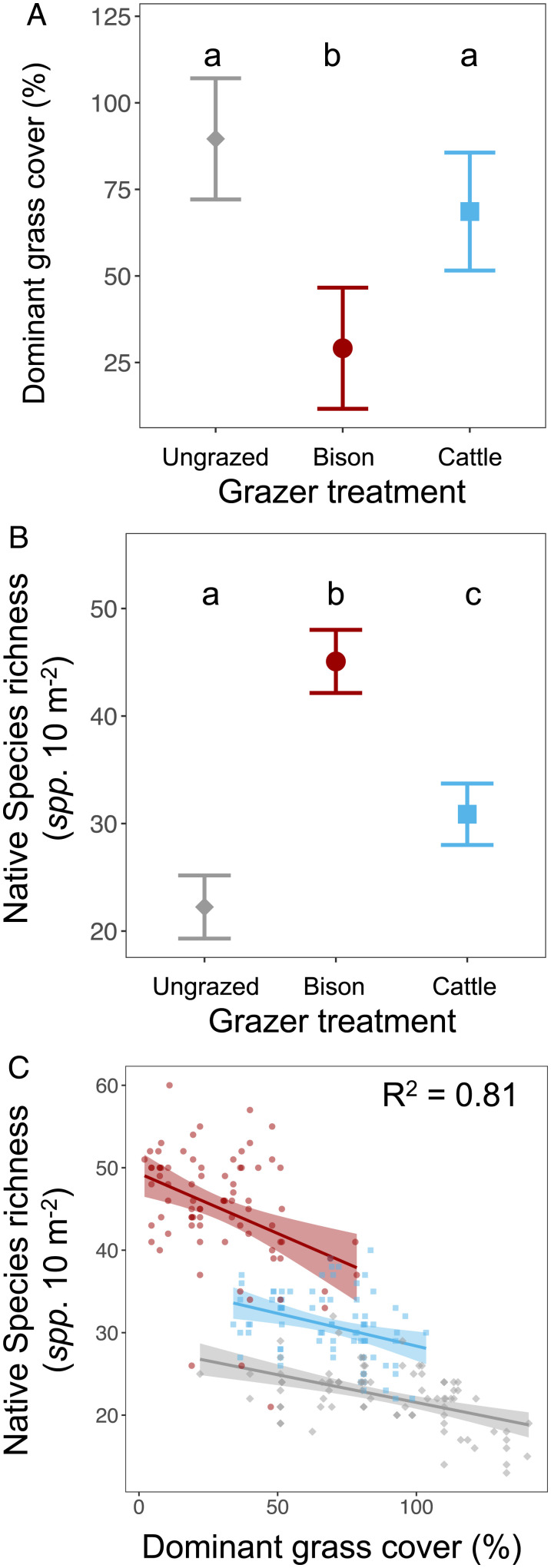
To quantify the net long-term effects of grazing, we compared treatments using the final year of data (SI Appendix, Table S3). Compared to the ungrazed treatment, dominant grass cover was lower in the bison treatment by the last year (2020), but bison did not eliminate dominant grasses (Fig. 3A and SI Appendix, Table S3). Dominant grass cover in the cattle treatment was not significantly lower than in the ungrazed treatment by the final year (Fig. 3B). Compared to the ungrazed treatment, native species richness in the bison treatment was 103% higher at the plot scale and 86% higher at the catchment scale (Fig. 3B). Cattle also caused a significant increase in native species richness but less than half as much as bison (P < 0.001 at both scales; Fig. 3B). Compared to the ungrazed treatment, native species richness in the cattle treatment was 41% higher at the plot scale and 30% higher at the catchment scale (Fig. 3B).
Fig. 3.
The effects of grazing treatments as of year 30 of the experiment (year 2020) on (A) dominant grass cover and (B) native species richness. Points mark averages and error bars span 95th percent confidence intervals. Treatments that share the same lettering at the top of the graph did not have statistically significant differences at an alpha of 0.05. (C) The relationship between dominant grass cover and native species richness in 2020 (gray = ungrazed, red = bison, blue = cattle). Lines in C show within-treatment relationships between dominant grass cover and species richness. All panels are at the plot scale.
We hypothesized that keystone herbivory of dominant grasses was a key mechanism driving increases in species richness under grazing. An analysis of covariance (ANCOVA) showed a significant effect of grazing treatment and dominant grass cover, with a negative correlation between dominant grass cover and species richness and an insignificant interaction between grazing treatment and dominant grass cover (R2 = 0.78 and P < 0.001 for the model; Fig. 3C and SI Appendix, Table S4).
Changes in species richness alone do not capture the full spectrum of bison effects on the plant community. For instance, all species richness values reported so far only included native species, which do not reveal if megagrazers also promoted nonnative species. Nevertheless, gains in species richness were not due to increases in exotic species; in the bison and cattle treatments, nonnative species remained scarce, with an average of fewer than four nonnative species per plot (SI Appendix, Fig. S4), compared to 48.9 native species per plot in the bison treatment (Fig. 3). Instead, the number of native species with a fidelity for native prairie habitat increased in frequency in bison and cattle treatments and remained flat in the ungrazed treatment (SI Appendix, Fig. S2). Forb cover, a key group of typically subdominant plants, also increased in the bison treatment (SI Appendix, Fig. S3).
Bison-grazed communities now include a set of plant species that are nearly absent in the ungrazed and cattle treatments (SI Appendix, Text S3 and Table S7 for all single species analyses). These include tall forbs from the Asteraceae family, such as rigid goldenrod (Solidago rigida), and 11 new annual species. Compared to both the ugnrazed and cattle treatment, bison increased the frequency of Bouteloua gracilis and Bouteloua dactyloides, which are grass species that are abundant in drier Great Plains grasslands (54). These species also have fine foliage, which should also make them less efficient to graze (55). Cattle also promoted perennial forbs but in general increased the abundance of half as many forb species as bison and did not increase the abundance of annuals (SI Appendix, Text S3 and Table S7).
Discussion
Plains bison, previously one of the most dominant and widespread species of megafauna in North America, were nearly driven to extinction in the late 19th century and currently occupy less than 1% of their pre-European range (56). Over a century after their near-extinction, we found that reintroducing bison into a North American tallgrass prairie led to three decades of continued increases in native plant species richness (Fig. 2).
Long-Term Effects of Bison.
Theory on top-down control of communities indicates that consumers can increase diversity through keystone consumption of dominant species (11–13). The negative relationship between dominant grass cover and species richness in mesic grasslands is consistent with this mechanism (Fig. 3 and ref. 18). Keystone herbivory could also increase the abundance of weedy and nonnative species, as seen in several studies showing an increase of nonnative species after the introduction of cattle (25, 26). This was not the case here—grazing has promoted plant species targeted for conservation (SI Appendix, Fig. S2) and nonnative plant species have remained uncommon (SI Appendix, Fig. S4).
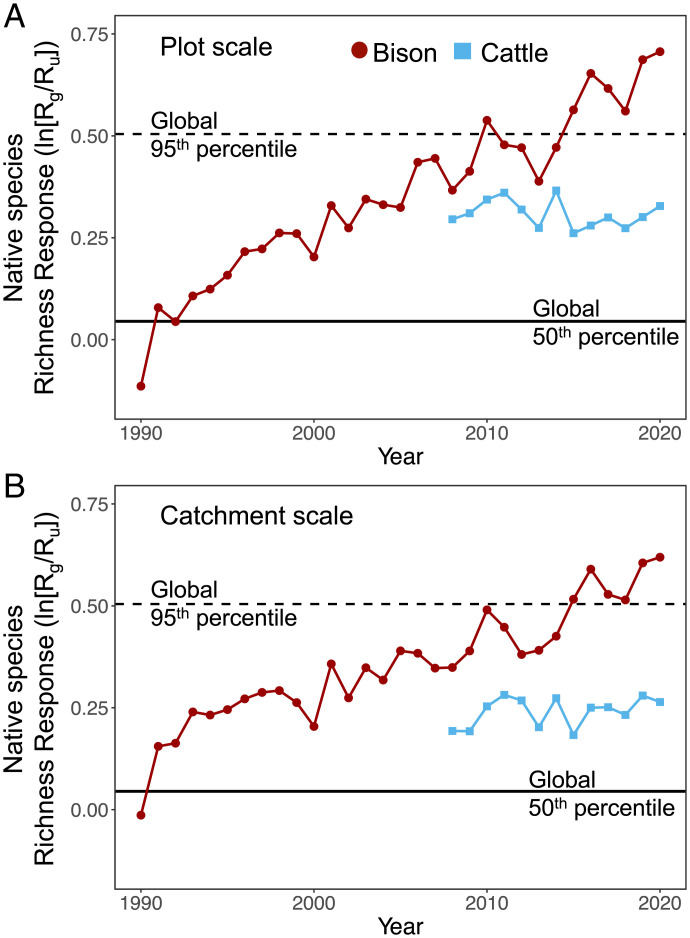
Across global grasslands, the magnitude of competitive release from keystone herbivory varies over an order of magnitude (18). Compared to a global database of grazing impacts on species richness (18), species richness gains in the bison treatment are now above the 95th percentile of the global dataset (Fig. 4), and therefore these gains in native species richness are among the largest recorded increases in plant species richness due to grazing (Fig. 4). We likely saw such a large response because the long duration of our study allowed the community response to manifest more fully. Also, tallgrass prairie is one of the most productive terrestrial grasslands in the Great Plains (53), and theoretically the competitive release from keystone herbivory is greater in more productive ecosystems (57).
Fig. 4.
Native species richness response ratios, ln(Rg/Ru), are the log response ratio of grazed versus ungrazed areas at the (A) plot and (B) catchment scales. Larger positive values indicate that grazing is associated with larger increases in native plant species richness. We compared ln(Rg/Ru) from bison (red) and cattle treatments (blue) to a global database of ln(Rg/Ru) values. The solid horizontal line is the median value of the global dataset set and the dashed line is the 95th percentile of the global dataset.
In contrast, species richness gains in the cattle treatment were well below the 95th percentile of this global dataset (Fig. 4), indicating that site characteristics cannot fully explain the magnitude of response seen in the bison treatment. Several differences between bison and cattle behavior potentially explain why bison increased species richness more than cattle. While the existence of DNA fragments from forbs and woody plants has been identified within bison dung (58), nongrass species comprise a minor portion of bison diet in comparison to grasses, as evidenced by direct identification of forage consumed (59), carbon isotope values of bison hair (59), and microhistology of bison dung (60). In contrast, the higher dominant grass cover in the cattle treatment suggests that these domesticated grazers likely consume more nongrass species than bison. This is consistent with studies of cattle diets in tallgrass prairie and elsewhere which found forbs comprise 10 to 34% of cattle diet (61, 62). In addition to differences in bison and cattle foraging behavior, bison also create physical disturbances (e.g., wallows) and grazing lawns (34) that increase habitat heterogeneity (20) and thus species richness across scales (63), whereas cattle do not form soil disturbances similar to bison wallows.
A caveat to this study is that we cannot isolate the effects of management (year-round grazing by bison vs. growing season only for cattle) and grazer identity (bison versus cattle). Also, to create large-enough areas that allow for realistic and heterogenous grazing patterns (e.g., ref. 64) we necessarily have some degree of pseudoreplication (65). However, we argue that the added realism of mimicking common land-use systems and an ecosystem-scale approach outweigh the disadvantages (e.g., ref. 66). Moreover, small-scale studies suggest that inherent differences between these grazer species are the primary explanation for why species richness increased so much more in the bison treatment. First, a smaller-scale study at our study site where bison and cattle had identical management and blocked grazing treatments also reported higher plant species richness in bison-grazed compared to cattle-grazed areas (34). Also, numerous studies in a variety of grassland ecosystems find that grazing, per se, increases plant species richness (18, 67), including one study using smaller-scale bison exclosures within bison-grazed watersheds at Konza (68).
Resistance and Resilience.
We found that gains in species richness from bison reintroduction were resilient to the most extreme dry period to affect this grassland for at least four decades (Fig. 3). The resilience in the bison treatment is consistent with theory that diversity promotes ecological resilience through mechanisms such as the portfolio effect and increased niche complementarity (46, 69–72). For instance, grasses primarily use shallow soil water at our site, whereas forbs (SI Appendix, Fig. S3) often use deeper soil water (73, 74). This niche partitioning reduces competition, especially during dry years when shallow soil water is scarce (73). In contrast, the ungrazed treatment was mostly dominated by several C4 grass species (Fig. 2, SI Appendix, Fig. S3, and ref. 75), which rely almost exclusively on shallow soil water (73, 74). This homogeneous functional composition should increase competition during drought through higher niche overlap (76) and thus decrease resilience. Some of the resilience in the bison treatment also reflects how bison grazing has promoted species that should be more resilient following drought—including C4 grasses that are typical of drier grasslands, annual species that flower early in the growing season, and native perennial forbs that respond rapidly when rainfall returns (SI Appendix, Text S3 and Table S7).
Overall, we found that long-term, year-round grazing by bison resulted in plant communities that were diverse and resilient to a 2-y extreme drought, with a postdrought recovery period of 2 to 4 y (Fig. 2). Responses from the Dust Bowl drought of the 1930s suggest that ungrazed Great Plains grasslands are potentially resilient to a 4-y-long drought (54, 77). Yet, due to anthropogenic climate change, the chance of extreme, decade-long droughts will double by the end of the century (78). Therefore, further work is needed to measure resilience to longer and more intense dry periods.
Conclusions
A pattern of megafauna extirpation or extinction has been repeated many times throughout human history, altering plant communities and potentially reducing biodiversity. We show that reintroducing native bison to tallgrass prairie can still lead to sustained biodiversity gains in native plant species that exceed those of domesticated herbivores without lowering grassland conservation value. Additionally, plant biodiversity gains were resilient to the type of climate extremes—heatwaves and a multiyear drought—that are projected to increase in frequency and intensity in this region in the future. Thus, restoring natural processes, such as grazing by native megaherbivores, can increase both the diversity and stability of mesic grassland ecosystems under projected global environmental change.
Methods
Site Description.
Konza Prairie Biological Station (KPBS) is an unplowed native tallgrass prairie located in eastern Kansas. KPBS is in the Flint Hills, the largest remaining landscape of tallgrass prairie in North America. The soils on the site were not glaciated during the Wisconsin glaciation, and most plots from this study are located on shallow upland soils underlain by limestone (usually less than 1 m deep), representative of the Florence soil series—a common soil type throughout the Flint Hills region. The climate is midcontinental, with hot summers and 51% of 812 mm precipitation falling during the growing season. Great Plains grasslands experience high interannual variability in precipitation and net primary production (79). Dominant native grass species comprise the majority of biomass, but forbs and subdominant grasses constitute the bulk of plant diversity (74).
KPBS includes a landscape-scale factorial experiment manipulating fire frequency and presence/absence of bison or cattle (Fig. 1). Bison were introduced in 1987, but the population was small (30 adults) and initially restricted to under half of its current spatial extent. In 1992, bison were introduced to the remaining area (SI Appendix, Table S1). At the time there were 121 bison (44.1 kg grazer⋅ha−1), which grew to a density of 99.9 kg grazer⋅ha−1. Cattle were also introduced to their treatment in 1992, with a long-term stocking rate of 127.9 kg of adult grazer⋅ha−1. SI Appendix, Text S1 gives more details on the site, its experimental design, the design of permanent plots, and climate variation.
Plant Biodiversity Data.
We used cover data from permanent monitoring plots [found at (81,82) ]. We focus on response variables that capture dominant plant species groups and plant biodiversity. Dominant grass cover was calculated as the summed cover of four dominant C4 perennial grass species in this system (A. gerardii, S. nutans, S. scoparium, and P. virgatum) (80). In the absence of grazing, these four species can constitute up to 80% of annual net primary productivity (53). Species richness was calculated as the total number of native species at the plot or catchment scale. SI Appendix, Text S3 and Figs. S1–S7 document changes in Shannon’s index (as another measure of species diversity), key species groups (species with high fidelity for tallgrass prairie, forb species cover, and invasive species richness), and changes in the frequency of individual species.
Statistics.
We tested four hypotheses: 1) grazing-induced decreases in dominant grass cover and increases in species richness have continued over time, despite an extreme climate event; 2) bison and cattle grazing result in a net decrease in dominant grass cover and net increase in species richness over time; 3) grazing-induced increases in species richness directly corresponded with decreases in dominant grass cover; and 4) the effect of bison and/or cattle on species richness was large relative to similar studies of megagrazers.
For the first hypothesis, we compared flexible GAMs to more rigid linear models (SI Appendix, Tables S2 and S3 for statistical summaries) to see if the GAMs’ predictions overlapped the linear models’ predictions following the climate extreme. GAMs were used because they can identify changes in complex time series with replication and are relatively robust to violations of model assumptions (ref. 83; see similar applications of splines in time series with replication in refs. 84 and 85). To quantify trajectories of change over time we built a separate GAM for every combination of response variable, treatment, and spatial scale (SI Appendix, Table S1 for further details). For GAMs we used all data available after 1989, which provides some pretreatment data while not overweighting the minority of catchments that had data available before 1992. For each GAM model we report the estimated 95% confidence interval of the mean, which we used to describe how the mean value of response variables changed over time. SI Appendix, Text S2 includes GAM model parameters.
To quantify the consistency of changes before, during, and after the climate extreme, we performed a linear fit between year and each response variable, for every combination of treatment, response variable, and spatial scale (SI Appendix, Table S3 includes model performances). The cattle treatment was excluded from these analyses because data were not available until 2008. The year 1994 was used as the first year of the linear models because one catchment had no data collection before this date and several catchments had limited data before 1992 (SI Appendix, Table S1). The year 2010 was used as the final year to end the analysis before the climate extreme. Within the bison treatment, changes in species richness over time conformed to a linear model (r2 = 0.45 at the plot scale and r2 = 0.63 at the catchment scale; SI Appendix, Table S3). For species richness, there was also a close match between the linear and GAM models, indicated by the GAM confidence overlapping with the linear model in all but 2 y in the bison treatment. We extrapolated linear model fits to 2011 through 2020, allowing us to compare this linear extrapolation to the more flexible GAM models. When the GAM confidence overlapped with the linear projection, we interpreted this as strong evidence of resilience. In the case of species richness this indicates resilience of increasing species richness over time, not a static state of elevated species richness.
To measure the net effects of grazing treatments, we compared plots as of the last year of available data (SI Appendix, Fig. S5 and Table S3). Results were similar when we used more years of data (SI Appendix, Table S5). We only considered the plot scale for these analyses because n = 4 at the catchment scale. For each response variable we performed a random-effects ANOVA for each of the following for dominant grass cover and species richness (other response variables are found in SI Appendix, Text S3). For each ANOVA, grazing treatment was a fixed effect and watershed was a random effect. When main effects were significant at an alpha of 0.05 we performed post hoc comparisons between groups using Tukey’s test of all pairwise comparisons at an alpha level of 0.05, with an honest squares difference adjustment to P values. These analyses were performed in R using the lmer package (86).
For the third hypothesis, we performed ANCOVA on the final year of data to determine the effect of both dominant grass cover and grazing treatment on species richness. ANCOVA was only performed at the plot scale because competition is a localized process. We used this model to generate confidence intervals of the linear relationship between dominant grass cover and species richness within each treatment (Fig. 3C and SI Appendix, Table S5). As before, results were similar if we included multiple years of data (SI Appendix, Table S6).
Finally, we placed our study within the context of a global database on grazing and grassland diversity, which compares plant species richness in grazed and ungrazed areas (18). We used this database to derive a statistical distribution of the log ratio of species richness (grazed/ungrazed). For each year of data, we compared the log response ratios of our grazing treatments to the statistical distribution of the global dataset and considered richness response ratios to be above average when they exceeded the 95th percentile of the global dataset.
Supplementary Material
Acknowledgments
This work was funded by NSF awards 2025849, 1440484, 0823341, 0218210, and 9632851; the Division of Biology at Kansas State University; and the Agricultural Research Station at Kansas State University. We thank the many people that have gathered long-term data for the Konza Prairie LTER program and the many staff, students, faculty, and volunteers that have maintained the fire and grazing treatments that are integral to this study. We thank Keith Gido for statistical advice. This is contribution no. 23-011-J from the Kansas Agricultural Experiment Station.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2210433119/-/DCSupplemental.
Data, Materials, and Software Availability
References
- 1.Pace M. L., Cole J. J., Carpenter S. R., Kitchell J. F., Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Borer E. T., et al. , What determines the strength of a trophic cascade? Ecology 86, 528–537 (2005). [Google Scholar]
- 3.Pringle R. M., Young T. P., Rubenstein D. I., McCauley D. J., Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl. Acad. Sci. U.S.A. 104, 193–197 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staver A. C., Bond W. J., Cramer M. D., Wakeling J. L., Top-down determinants of niche structure and adaptation among African Acacias. Ecol. Lett. 15, 673–679 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Sankaran M., Augustine D. J., Ratnam J., Native ungulates of diverse body sizes collectively regulate long‐term woody plant demography and structure of a semi‐arid savanna. J. Ecol. 101, 389–1399 (2013). [Google Scholar]
- 6.Louthan A., et al. , Large mammals generate both top-down effects and extended trophic cascades on floral-visitor assemblages. J. Trop. Ecol. 35, 185–198 (2019). [Google Scholar]
- 7.Estes J. A., et al. , Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ripple W. J., et al. , Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bråthen K. A., Pugnaire F. I., Bardgett R. D., The paradox of forbs in grasslands and the legacy of the mammoth steppe. Front. Ecol. Environ. 19, 584–592 (2021). [Google Scholar]
- 10.Guyton J. A., et al. , Trophic rewilding revives biotic resistance to shrub invasion. Nat. Ecol. Evol. 4, 712–724 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Leibold M. A., A graphical model of keystone predators in food webs: Trophic regulation of abundance, incidence, and diversity patterns in communities. Am. Nat. 147, 784–812 (1996). [Google Scholar]
- 12.Terborgh J. W., Toward a trophic theory of species diversity. Proc. Natl. Acad. Sci. U.S.A. 112, 11415–11422 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paine R. T., Food web complexity and species diversity. Am. Nat. 100, 65–75 (1966). [Google Scholar]
- 14.Milchunas D. G., Lauenroth W. K., Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 63, 327–366 (1993). [Google Scholar]
- 15.Collins S. L., Knapp A. K., riggs J. M. B., Blair J. M., Steinauer E. M., Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 280, 745–747 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Strömberg C. A., Evolution of grasses and grassland ecosystems. Annu. Rev. Earth Planet. Sci. 39, 517–544 (2011). [Google Scholar]
- 17.Kartzinel T. R., et al. , DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Natl. Acad. Sci. U.S.A. 112, 8019–8024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koerner S. E., et al. , Change in dominance determines herbivore effects on plant biodiversity. Nat. Ecol. Evol. 2, 1925–1932 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Augustine D. J., Frank D. A., Effects of migratory grazers on spatial heterogeneity of soil nitrogen properties in a grassland ecosystem. Ecology 82, 3149–3162 (2001). [Google Scholar]
- 20.Collins S. L., Barber S. C., Effects of disturbance on diversity in mixed-grass prairie. Vegetatio 64, 87–94 (1986). [Google Scholar]
- 21.Adler P., Raff D., Lauenroth W., The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 128, 465–479 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Archibald S., Bond W. J., Stock W. D., Fairbanks D. H. K., Shaping the landscape: Fire–grazer interactions in an African savanna. Ecol. Appl. 15, 96–109 (2005). [Google Scholar]
- 23.Thakur M. P., Bakker E. S., Veen G. C., Harvey J. A., Climate extremes, rewilding, and the role of microhabitats. One Earth 2, 506–509 (2020). [Google Scholar]
- 24.Chesson P., General theory of competitive coexistence in spatially-varying environments. Theor. Popul. Biol. 58, 211–237 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Hobbs R. J., Synergisms among habitat fragmentation, livestock grazing, and biotic invasions in southwestern Australia. Conserv. Biol. 15, 1522–1528 (2001). [Google Scholar]
- 26.Williamson M. A., et al. , Fire, livestock grazing, topography, and precipitation affect occurrence and prevalence of cheatgrass (Bromus tectorum) in the central Great Basin, USA. Biol. Invasions 22, 663–680 (2020). [Google Scholar]
- 27.Gordon I. J., Manning A. D., Navarro L. M., Rouet-Leduc J., Domestic livestock and rewilding: Are they mutually exclusive? Front. Sustain. Food Syst. 5, 68 (2021). [Google Scholar]
- 28.Allred B. W., Fuhlendorf S. D., Hamilton R. G., The role of herbivores in Great Plains conservation: Comparative ecology of bison and cattle. Ecosphere 2, 1–17 (2011). [Google Scholar]
- 29.Kohl M. T., Krausman P. R., Kunkel K., Williams D. M., Bison versus cattle: Are they ecologically synonymous? Rangeland Ecol. Manag. 66, 721–731 (2013). [Google Scholar]
- 30.Ranglack D. H., Du Toit J. T., Wild bison as ecological indicators of the effectiveness of management practices to increase forage quality on open rangeland. Ecol. Indic. 56, 145–151 (2015). [Google Scholar]
- 31.Schwartz C. C., Ellis J. E., Feeding ecology and niche separation in some native and domestic ungulates on the shortgrass prairie. J. Appl. Ecol. 18, 343–353 (1981). [Google Scholar]
- 32.Van Vuren D., Summer diets of bison and cattle in southern Utah. Rangeland Ecol. Manag. 37, 260–261 (1984). [Google Scholar]
- 33.Plumb G. E., Dodd J. L., Foraging ecology of bison and cattle on a mixed prairie: Implications for natural area management. Ecol. Appl. 3, 631–643 (1993). [DOI] [PubMed] [Google Scholar]
- 34.Towne E. G., Hartnett D. C., Cochran R. C., Vegetation trends in tallgrass prairie from bison and cattle grazing. Ecol. Appl. 15, 1550–1559 (2005). [Google Scholar]
- 35.Riginos C., Porensky L. M., Veblen K. E., Young T. P., Herbivory and drought generate short-term stochasticity and long-term stability in a savanna understory community. Ecol. Appl. 28, 323–335 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Bestelmeyer B. T., et al. , Analysis of abrupt transitions in ecological systems. Ecosphere 2, 1–26 (2011). [Google Scholar]
- 37.Flores D. L.,American Serengeti: The Last Big Animals of the Great Plains (Tantor Media, 2017). [Google Scholar]
- 38.Dixon A. P., Faber‐Langendoen D., Josse C., Morrison J., Loucks C. J., Distribution mapping of world grassland types. J. Biogeogr. 41, 2003–2019 (2014). [Google Scholar]
- 39.Samson F., Knopf F., Prairie conservation in North America. Bioscience 44, 418–421 (1994). [Google Scholar]
- 40.Holling C. S., Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23 (1973). [Google Scholar]
- 41.Walker B., Holling C. S., Carpenter S. R., Kinzig A., Resilience, adaptability and transformability in social–ecological systems. Ecol. Soc. 9, 5 (2004). [Google Scholar]
- 42.Ratajczak Z., et al. , Abrupt change in ecological systems: Inference and diagnosis. Trends Ecol. Evol. 33, 513–526 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Turner M. G., et al. , Climate change, ecosystems and abrupt change: Science priorities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratajczak Z., Ladwig L. M., “Will climate change push grasslands past critical thresholds” in Grasslands and Climate Change, Gibson D. J., Newman J. A., Eds. (Cambridge University Press, 2019), pp. 98–114. [Google Scholar]
- 45.Tilman D., El Haddi A., Drought and biodiversity in Grasslands. Oecologia 89, 257–264 (1992). [DOI] [PubMed] [Google Scholar]
- 46.Isbell F., et al. , Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Hoerling M., et al. , Causes and predictability of the 2012 Great Plains drought. Bull. Am. Meteorol. Soc. 95, 269–282 (2014). [Google Scholar]
- 48.Knapp A. K., et al. , The keystone role of bison in North American tallgrass prairie: Bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience 49, 39–50 (1998). [Google Scholar]
- 49.US Department of Agriculture, “Health and management practices on U.S. ranched-Bison operations” (#702.1216, APHIS, USDA, 2016).
- 50.Hoy J., Cattle in the Flint Hills. Symphony in the Flint Hills Field Journal (2010). https://newprairiepress.org/sfh/2009/flinthills/6. Accessed 18 December 2021.
- 51.Beam M., Beef production in the Flint Hills. Symphony in the Flint Hills Field Journal (2010). https://newprairiepress.org/sfh/2010/nature/5. Accessed 16 December 2021.
- 52.Koerner S. E., Collins S. L., Small-scale patch structure in North American and South African grasslands responds differently to fire and grazing. Landsc. Ecol. 28, 1293–1306 (2013). [Google Scholar]
- 53.Smith M. D., Knapp A. K., Dominant species maintain ecosystem function with non‐random species loss. Ecol. Lett. 6, 509–517 (2003). [Google Scholar]
- 54.Weaver J. E., Albertson F. W., Grasslands of the Great Plains: Their Nature and Use (University of Nebraska Press, 1956). [Google Scholar]
- 55.Brown V. K., Lawton J. H., Herbivory and the evolution of leaf size and shape. Phil. Trans. Royal Soc. B 333, 265–272. [Google Scholar]
- 56.Sanderson E. W., et al. , The ecological future of the North American bison: Conceiving long-term, large-scale conservation of wildlife. Conserv. Biol. 22, 252–266 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Leibold M. A., Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am. Nat. 134, 922–949 (1989). [Google Scholar]
- 58.Craine J. M., Towne E. G., Miller M., Fierer N., Climatic warming and the future of bison as grazers. Sci. Rep. 5, 16738 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raynor E. J., Joern A., Nippert J. B., Briggs J. M., Foraging decisions underlying restricted space use: Effects of fire and forage maturation on large herbivore nutrient uptake. Ecol. Evol. 6, 5843–5853 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coppedge B. R., Leslie D. M., Shaw J. H., Botanical composition of bison diets on tallgrass prairie in Oklahoma. Rangeland Ecol. Manag. 51, 379–382 (1998). [Google Scholar]
- 61.Sproul N. A., et al. , Forage selection preferences by multiparous and primiparous beef cows grazing native tallgrass range during winter. Proc. West. Sec. Am. Soc. Anim. Sci 61, 15–20 (2010). [Google Scholar]
- 62.Sowers C. A., Gatson G. A., Wolf J. D., Fick W. H., Olson K. C., Botanical composition of yearling-steer and mature-ewe diets in the Kansas Flint Hills. Rangeland Ecol. Manag. 72, 126–135 (2019). [Google Scholar]
- 63.Collins S. L., Smith M. D., Scale-dependent interaction of fire and grazing on community heterogeneity in tallgrass prairie. Ecology 87, 2058–2067 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Fuhlendorf S. D., et al. , Should heterogeneity be the basis for conservation? Grassland bird response to fire and grazing. Ecol. Appl. 16, 1706–1716 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Hurlbert S. H., Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54, 187–211 (1984). [Google Scholar]
- 66.Schindler D. W., Whole-ecosystem experiments: Replication versus realism: The need for ecosystem-scale experiments. Ecosystems (N. Y.) 1, 323–334 (1998). [Google Scholar]
- 67.Borer E. T., et al. , Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508, 517–520 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Koerner S. E., et al. , Plant community response to loss of large herbivores differs between North American and South African savanna grasslands. Ecology 95, 808–816 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Wang L., et al. , Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl. Acad. Sci. U.S.A. 116, 6187–6192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilcox K. R., et al. , Rapid recovery of ecosystem function following extreme drought in a South African savanna grassland. Ecology 101, e02983 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Hautier Y., et al. , General destabilizing effects of eutrophication on grassland productivity at multiple spatial scales. Nat. Comm. 11, 5375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muraina T. O., et al. , Species asynchrony stabilises productivity under extreme drought across Northern China grasslands. J. Ecol. 109, 1665–1675 (2021). [Google Scholar]
- 73.Nippert J. B., Knapp A. K., Soil water partitioning contributes to species coexistence in tallgrass prairie. Oikos 116, 1017–1029 (2007). [Google Scholar]
- 74.O’Keefe K., Nippert J. B., Grazing by bison is a stronger driver of plant ecohydrology in tallgrass prairie than fire history. Plant Soil 411, 423–436 (2017). [Google Scholar]
- 75.Collins S. L., Calabrese L. B., Effects of fire, grazing and topographic variation on vegetation structure in tallgrass prairie. J. Veg. Sci. 23, 563–575 (2012). [Google Scholar]
- 76.O’Keefe K., Nippert J. B., McCulloh K. M., Plant water uptake along a diversity gradient provides evidence for complementarity in hydrological niches. Oikos 128, 1748–1760 (2019). [Google Scholar]
- 77.Knapp A. K., et al. , Resolving the Dust Bowl paradox of grassland responses to extreme drought. Proc. Natl. Acad. Sci. U.S.A. 117, 22249–22255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook B. I., Ault T. R., Smerdon J. E., Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 1, e1400082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knapp A. K., Smith M. D., Variation among biomes in temporal dynamics of aboveground primary production. Science 291, 481–484 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Knapp A. K., Briggs J. M., Hartnett D. C., Collins S. L., Grassland Dynamics: Long-Term Ecological Research in Tallgrass Prairie (Oxford University Press, 1998). [Google Scholar]
- 81.D. Hartnett, S. Collins, and Z. Ratajczak. 2022. PVC02 Plant species composition on selected watersheds at Konza Prairie ver 19. Environmental Data Initiative. 10.6073/pasta/b768b10f9b17bafc68194a4aaa8e53c2 (Accessed 25 August 2021). [DOI] [Google Scholar]
- 82.J. Blair, PBG01 Plant species composition in the Patch Burning-grazing Experiment at Konza Prairie. Environmental Data Initiative. 10.6073/pasta/b1e152cc621a32c7aa623bafc016ce6c. Accessed 11 August 2022. [DOI] [Google Scholar]
- 83.Simpson G. L., Modelling palaeoecological time series using generalized additive models. Front. Ecol. Evol. 6, 149 (2018). [Google Scholar]
- 84.Isbell F., Tilman D., Polasky S., Binder S., Hawthorne P., Low biodiversity state persists two decades after cessation of nutrient enrichment. Ecol. Lett. 16, 454–460 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Collins S. L., et al. , Fire frequency, state change and hysteresis in tallgrass prairie. Ecol. Lett. 24, 636–647 (2021). [DOI] [PubMed] [Google Scholar]
- 86.Bates D., Maechler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015) [Google Scholar]
- 87.Smith M. D., The ecological role of climate extremes: Current understanding and future prospects. J. Ecol. 99, 651–655 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.