Abstract
Cellulose is a non-toxic, bio-degradable, and renewable biopolymer which is abundantly available in nature. The most common source of commercial microcrystalline cellulose is fibrous wood pulp. Cellulose and its derivatives have found wide commercial applications in the pharmaceutical, cosmetic, food, paper, textile, and engineering industries. This study aims to isolate and characterize cellulose forms from cocoa pod husk (CPH) and to assess its mechanical and disintegration properties as a direct compression excipient in metronidazole tablets. Two isolated cellulose types (i.e., cocoa alpha-cellulose (CAC) and cocoa microcrystalline cellulose (C-MCC)) were compared with avicel (AV). CAC and C-MCC were characterized for their physicochemical properties using Scanning Electron Microscopy (SEM), FTIR spectroscopy, Differential Scanning Calorimetry (DSC), and X-Ray Powder Diffraction (XRD). Metronidazole tablets were produced by direct compression with cellulose. The mechanical and disintegration properties of the tablets were evaluated. CAC and C-MCC yield was 42.3% w/w and 38.25% w/w, respectively. Particle diameters were significantly different with CAC (282.22 μm) > C-MCC (161.32 μm) > AV (72.51 μm). CAC and C-MCC had a better flow than AV. SEM revealed the fibrous nature of the cellulose. FTIR and XRD analysis confirmed the presence of cellulose with crystallinity index of 69.26%, 43.83%, and 26.32% for AV, C-MCC, and CAC, respectively. C-MCC and AV are more crystalline and thermally stable at high temperatures compared to CAC. The mechanical and disintegration properties of C-MCC and AV tablets complied with pharmacopeia specifications. Taken together, C-MCC isolated from CPH displayed some fundamental characteristics suitable for use as a pharmaceutical excipient and displayed better properties compared to that of AV.
Keywords: alpha cellulose, microcrystalline cellulose, cocoa pod husk, direct compression, metronidazole tablet
1. Introduction
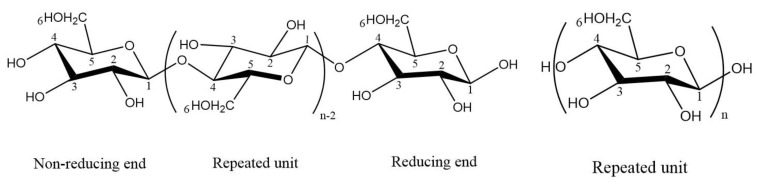
Cellulose is a non-toxic, bio-degradable, and renewable biopolymer derived from biomass, and so, is abundantly available in nature [1,2,3]. It is a long linear chain polysaccharide composed of β-(1-4)-linked D-anhydro-glucopyranose repeating units (AGU) with the formula (C6H10O5)n (Figure 1) where n is the number of glucose groups and thus represents the degree of polymerization [4,5]. It is present in small quantities in the cell walls of some bacteria and more abundant in plants [6].
Figure 1.
Chemical structure of cellulose (C6H10O5)n. ChemDraw Pro 8.0 was used to represent this linear homopolymer composed of repeated units of AUG linked together by β-(1-4)-glycosidic bonds.
Cellulose and its derivatives, such as microcrystalline cellulose, methylcellulose, ethylcellulose, carboxymethyl cellulose, hydroxylpropyl methyl cellulose, cellulose acetate, etc., have found wide commercial applications in the pharmaceutical, cosmetic, food, paper, textile, and engineering industries [7,8,9,10,11].
Four different polymorphs of cellulose exist, cellulose I, II, III, and IV. Cellulose l is the natural form of cellulose known as native cellulose (NC) and the most abundantly found in nature. Cellulose I can be converted to Cellulose II, also known as alpha cellulose, by either mercerization (alkali treatment) or regeneration (solubilization and subsequent recrystallization) [12]. Cellulose II is more thermodynamically stable than cellulose I. Cellulose III which is amorphous can be obtained by treatment of either cellulose I or II with amines, while Cellulose IV can be obtained by treatment of cellulose III with glycerol [13]. NC hydrolyzes in the presence of water and acid under heat and pressure and depolymerizes into small chain polymers or crystals [14]. Some components and other impurities like wax, hemicellulose, and lignin are dissolved during the hydrolytic reaction, and filtration and washing processes to obtain a pure cellulose known as alpha cellulose [15]. Alpha cellulose is a white, odorless, and tasteless powder that is insoluble in water. It is a widely used raw material in the manufacturing of propellants, paper, paperboards, fabrics, electrical cable insulators, and cellulose derivatives, among others [7,16].
Microcrystalline cellulose (MCC) is a purified, partially depolymerized cellulose derivative obtained from alpha cellulose. The most common source of commercial MCC is fibrous wood pulp [7]. MCC is the most widely used cellulose. It is used in the food industry as a stabilizer, anti-caking agent, and emulsifier [17], while in cosmetics and pharmaceuticals it is used as an abrasive, adsorbent, adhesive, anti-caking, binder, disintegrant, bulking agent, emulsion stabilizer, etc. [7,17,18,19,20].
CPH (Theobroma cacao L.) is a natural waste generated from the post-harvest processing of cocoa fruits into cocoa beans which constitutes about 67–76% weight of the fresh cocoa fruit [21]. CPH is rich in minerals and fibers like hemicellulose, cellulose, lignin, and pectin [22]. CPH has been utilized as a bioplastic film in food packaging [23], biofertilizers in cocoa farming [24], an energy source in electricity generation [25], animal feeds, as well as in biopharmaceutics [22]. Few studies on cellulose isolated from CPH, available in the literature, are the extraction of MCC from CPH using alkaline pretreatment combined with ultrasonication [26], and isolation of nanocrystalline cellulose (NCC) from CPH [27].
This study aims (i) at isolating and characterizing cellulose types from CPH, which can be considered as an alternative source of cellulose to the mostly used fibrous wood pulp currently used as a pharmaceutical excipient, and (ii) to assess their mechanical and disintegration properties as direct compression excipients in a metronidazole tablet.
2. Materials and Methods
2.1. Reagents
Dried cocoa pod husks (CPH), Avicel® 101 (AV) (Merck, Germany)—a brand of microcrystalline cellulose, Sodium hydroxide (Guangdong Guanghua Sci-Tech Company, Ltd., Shantou, China), Hydrochloric acid (BDH Lab Supplies, Poole, UK), and Sodium hypochlorite (Tolaram Africa Enterprises, Ltd., Lagos, Nigeria). All other reagents are of analytical grade.
2.2. Collection and Preparation of Cocoa Pod Husk (CPH)
Dried CPH were obtained from a local farm in Ago-Iwoye, Ogun State, Nigeria in the month of September. The CPH were further sun-dried for two weeks. The dried CPH were crushed, milled into fine powder, and screened through 850 μm sieve. It was air dried and stored in a closed container.
2.3. Phytochemical Screening of Cocoa Pod Husk (CPH)
Qualitative phytochemical screening of CPH was carried out by standard procedures to identify major phytoconstituents [28,29]. Frothing test was used to screen saponin while Molisch Test was used for carbohydrate. Mayers reagent, Draggendorff’s reagent, and Wagner’s reagent were used to screen alkaloids. Ferric chloride solution (FeCl3), lead acetate solution, gelatin solution tests were used to identify the presence of tannins. The presence of flavonoids was screened by adding a few drops of dilute sodium hydroxide (NaOH) solution to the aqueous extract of CPH followed by a few drops of dilute hydrochloric acid solution (HCl). The presence of triterpenoids was screened by dissolving CPH in chloroform, followed by the addition of acetic anhydride and concentrated sulphuric acid (H2SO4).
2.4. Extraction of Cocoa Alpha-Cellulose (CAC) from Cocoa Pod Husk (CPH)
CAC was extracted from the prepared CPH powder by a method previously described [15]. 500 g of the CPH powder was boiled in 3 L of distilled water (dH2O) for 10 min. The mixture was filtered, and the residue was then treated with 3 L of 1 M HCl for 30 min at 85 °C on a magnetic stirrer. The mixture was again filtered, and the residue was magnetically stirred with 3 L of 0.5 M HCl for 30 min at 85 °C. The mixture was further filtered, but the residue was treated this time with 3 L of 1 M NaOH solution for 30 min at 85 °C under constant stirring. Subsequently, the mixture was filtered, and this step was repeated three times. The residue was bleached with 2% sodium hypochlorite (NaOCl) solution for 60 min at 95–96 °C. This step was repeated twice to obtain alpha cellulose which was washed several times with hot dH2O until a neutral pH of the filtrate was obtained. The CAC was then dried in an oven at 60 °C for 16 h and the weight was noted. It was milled and screened through 650 μm sieve and stored at room temperature (RT). The yield of CAC was determined as follows:
2.5. Isolation of Cocoa Microcrystalline Cellulose (C-MCC) from Cocoa Pod Husk (CPH)
Fifty grams CAC were placed in a Pyrex glass beaker and hydrolyzed with 1.2 L of 2.5 N HCl for 15 min at 100 °C in hot water bath. The hot acid mixture was poured into cold tap water followed by vigorous stirring on a magnetic stirrer and was allowed to stand at RT overnight. The precipitate obtained was the C-MCC which was subsequently washed with dH2O until neutral. The precipitate was dried for 60 min in an oven at 60 °C. Thereafter, the dried C-MCC was milled with an Osterizer blender (Model 857 Williamette Industries, Bowing Green Kentucky, KY, USA) and screened through 650 μm sieve, and stored at RT in a desiccator [30]. The yield of C-MCC was determined as follows:
2.6. General Cellulose Identification Tests
2.6.1. Solubility Test
Solubility test was performed by mixing 250 mg of the cellulose in 10 mL of different solvents (dH2O, ethanol, chloroform, concentrated HCl, and concentrated H2SO4) [31,32].
2.6.2. Chemical Tests
Two chemical tests were performed as follow:
(i): 50 mg of cellulose was weighed into a test tube and 2 mL of iodine solution was added. The mixture was allowed to stand at RT for 5 min before filtration. Two drops of 66% v/v H2SO4 were added to the residue. Color change was noted and recorded [33].
(ii): 10 mg of cellulose was weighed into a watch glass and 2 mL of iodinated zinc chloride (ClIZn) solution was added and allowed to stand for some minutes. Color change was noted and recorded [34].
2.6.3. Degree of Polymerization Determination
The degree of polymerization (DP) was determined by measuring the viscosity of the cellulose using a capillary viscometer at 25 °C [35]. The DP was calculated using Equation (1).
| (1) |
where, ηspec (η/η0−1) = specific viscosity (η/η0 is relative viscosity)—η0 is the time used for the solvent to travel from the upper mark to the lower mark, η is the time used for the cellulose to travel from the upper mark to the lower mark and c = concentration in g/L.
2.6.4. Phytochemical Screening of Cocoa Alpha-Cellulose (CAC) and Cocoa Microcrystalline Cellulose (C-MCC)
The methods previously used for the phytochemical screening of the CPH were repeated for the screening of CAC and C-MCC (Section 2.3).
2.7. Physicochemical Properties of the Cellulose Types
2.7.1. pH Determination
One gram of each cellulose powder was dispersed in 50 mL of dH2O for 5 min and the pH of the supernatant liquid was taken using a pocket size pH meter (3510 model, Jenway, UK). The determination was done in triplicate to ensure data reliability.
2.7.2. Bulk and Tapped Density
Ten grams of each cellulose powder were placed into a 50 mL clean and dry measuring cylinder. The volume occupied without tapping was determined and noted as Vb. The measuring cylinder was then tapped manually on a soft surface bench about 300 times and the volume occupied after tapping was determined and noted as Vt. This was done in triplicate to ensure data reliability, and the average volume was calculated and recorded. The bulk and tap densities were determined using Equations (2) and (3), respectively:
| (2) |
| (3) |
2.7.3. True Density
The true density of each cellulose powder was determined by the pycnometer method with xylene as the displacement liquid, as previously described by Adeleye et al. [36]. A 50-mL empty pycnometer bottle was weighed with the stopper and noted as W0. This was filled with xylene to the brim and the excess was wiped off. The weight with the stopper was then noted as W1. The difference between these weights (W0–W1) was recorded as W2. 2 g of cellulose was weighed and noted as W3, before transfer into the pycnometer bottle, and excess solvent was wiped off. The bottle was weighed again with the stopper and noted as W4. The true density, Dt, was calculated using Equation (4).
| (4) |
2.7.4. Angle of Repose
The angle of repose, θ, was measured by the fixed height method, as described by Adeleye et al. [36]. A funnel was clamped to a retort stand with its tip 2 cm (h) above a graph paper placed on a flat horizontal surface. The cellulose was carefully poured until the apex of the conical pile touched the tip of the funnel. The diameter (D) of the base of the conical pile was measured and recorded. The angle of repose, θ, was calculated using Equation (5).
| (5) |
where r is half of (D)
The procedure was repeated thrice to ensure data reliability and the average was calculated.
2.7.5. Hausner Ratio Assesses the Inter-Particle Friction in a Powder Bed Giving an Insight into Powder Flow Properties
The Hausner ratio was calculated using Equation (6)
| (6) |
2.7.6. Carr’s Index
Carr’s index is a measure of the compressibility and flow properties of a powder.
Carr’s index was calculated using Equation (7)
| (7) |
2.7.7. Powder Porosity
The powder porosity was derived from Equation (8)
| (8) |
where Db is the bulk density, Dt is the true density, and E is the porosity.
2.7.8. Particle Size Distribution (PSD) Measurement
Twenty grams of each type of cellulose powder was placed on the top sieve of sieves arranged in a stack in descending degree of coarseness ranging from 1.0 mm to 90 µm. The nest of sieves was subjected to agitation on a mechanical shaker (Endecotts) at RT for 15 min. The percentage weight of the powder retained on each sieve was then determined and the mean particle size (PS) was calculated [37].
2.7.9. Swelling Capacity
Three grams of each type of cellulose were placed in a 50 mL measuring cylinder. Then, 20 mL of dH2O was added, and the cylinder was shaken vigorously at RT every 10 min for 1 h and then allowed to stand at RT for 5 h. The swelling capacity was calculated using Equation (9).
| (9) |
where S is the % swelling capacity, V2 is the volume of the hydrated cellulose and V1 is the tapped volume of the material prior to hydration.
2.7.10. Moisture Content
Moisture content was determined by the loss on drying method, as previously reported [30]. Five grams of cellulose were transferred into a tarred glass petri dish and then dried in an oven (Ketan Lab. Oven, model; 400097, Mumbai, India) at 105 °C until a constant weight was attained. The percentage moisture loss was calculated using Equation (10).
| (10) |
where M is the % moisture loss, Wb is the weight of the cellulose before drying and Wa is the weight of the cellulose after drying.
2.8. Physical Characterizations of the Cellulose Types
2.8.1. Scanning Electron Microscopy (SEM)
SEM was used to examine the morphology of each cellulose powder. Images were taken by Phenom Pharos Desktop SEM (Thermos Fisher Scientific, Eindhoven, The Netherlands). The sample was prepared by placing it on a sample stub and coated by quorum technologies model Q150R with 5 nm of gold. Thereafter the prepared sample was viewed in the SEM machine via NaVCam. The morphologies of the sample at different magnifications were saved on a USB stick.
2.8.2. Fourier-Transform Infrared (FTIR) Spectroscopy
FTIR was used to obtain the infrared spectrum of each cellulose powder using KBr method with FTIR spectrometer (Model: 530M, Maker: Buck Scientific, Norwalk, CT, USA). The absorbance of the powders was recorded between 1000 and 4000 cm−1.
2.8.3. X-ray Diffraction (XRD)
An XPERT-PRO diffractometer (PAN analytical, Almelo, The Netherlands) was applied to obtain XRD spectrum of the samples at 2θ between 10° and 70° using Kα Cu radiation (λ: 1.542 Ǻ) at 25 °C and 53 ± 2% relative humidity. For this analysis, all the tested samples were pulverized and spread on sample holder thoroughly to cover the whole area of the sample holder. The crystallinity of the samples was calculated from the ratio of the integrated area of XRD spectrum and crystalline peak areas, using Origin 18 software, considering Gaussian profiles for crystalline peaks, as described by Collazo-Bigliardi et al. [38].
2.8.4. Differential Scanning Calorimetry (DSC) Analysis
DSC analysis was performed on DSC3 apparatus (Mettler-Toledo GmbH, Gießen, Germany). About 10 mg of the sample was weighed into a 40 μL aluminum crucible and heated between 30 °C and 250 °C at a rate of 20 °C min−1 under nitrogen atmosphere flow of 20 mL·min−1.
2.9. Tablet Formulation and Compression
Two hundred milligrams (200 mg) of metronidazole tablet were formulated by blending 200 mg (50% w/w) metronidazole (active ingredient), 100 mg (25% w/w) cellulose (CAC, C-MCC or AV–dry binder), 50 mg (12.5% w/w) corn starch (disintegrant) and 50 mg (12.5% w/w) lactose (diluent) in a planetary mixer for 5 min. The blend was directly compressed on a Carver hydraulic hand press (model 38510E, Carver Inc., Wabash, IN, USA) using three different compression pressures (56.64 Mnm−2, 84.96 Mnm−2, and 113.28 Mnm−2) in a 10 mm die with a flat-faced punch lubricated with 1% dispersion of magnesium stearate in 96% ethanol before each compression.
2.10. Tablet Evaluation
2.10.1. Determination of Tablet Hardness
Tablets were selected randomly from each formulation and the force required to break the tablet at RT was determined with a Monsanto hardness tester (DKB instrument, Mumbai. Model EH 01). The reading of the pointer on the tester was noted. Ten tablets from each formulation were evaluated and the mean was recorded.
2.10.2. Determination of Tablet Friability
The weight of ten tablets selected randomly from each formulation was noted (W1) and transferred into the Friability test apparatus (Shivani scientific Ind., Mumbai, India). The apparatus was operated for 4 min at 25 revolutions per minute, and then the tablets were collected, dusted, and weighed (W2). The test was performed in triplicate and the mean was recorded. Percentage weight variation (friability) was calculated using Equation (11).
| (11) |
2.10.3. Determination of Disintegration Time of Tablets
The disintegration test was carried out in vitro using a tablet disintegration test apparatus (DBK Instruments, Mumbai, India) within 1 L of distilled water in a beaker as the disintegration medium at 37 ± 0.5 °C [19]. Three tablets from each batch were individually placed in the basket of each tube of the disintegration apparatus. The time taken for the tablets to completely break up and pass through the wire mesh was noted and recorded. Determinations were done in triplicate.
2.10.4. Statistical Analysis
Data were subjected to one-way ANOVA using GraphPad Prism Software version 5.01. p-values < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Yield of the Cellulose Types
The yield of CAC and C-MCC pod husk powders was 42.3% w/w and 38.25% w/w, respectively. The low yield of C-MCC in this study was expected since it is a product derived from alpha cellulose hydrolysis (amorphous regions in the alpha cellulose would have been hydrolyzed and eliminated). Interestingly, the yield of CAC and C-MCC reported in this study was higher when compared with some agro-industrial wastes such as groundnut husks [39], sorghum stalks [40], cornstalk [41], papaya stem [42], rice straw [16], and wheat straw [43].
3.2. Identification Parameters of the Cellulose Types
A violet-blue color was observed with all three cellulose types (CAC, C-MCC, and AV) when treated with iodinated ClIZn solution, and iodine (I) and H2SO4 indicating that they are all cellulose. They are all insoluble in dH2O, ethanol, and chloroform but soluble in concentrated HCl and concentrated H2SO4. This also indicated that the samples were more likely cellulose. The DP of the cellulose was determined for further identification. The result indicates that CAC had a DP of 1220 while C-MCC was 336. According to the Pharmacopeia standard, MCC is defined by a typical characteristic value of DP of less than 350 glucose units [18]. This signifies that the acid hydrolysis of CAC indeed led to the synthesis of MCC.
3.3. Phytochemical Screening of Cocoa Pod Husk (CPH), Cocoa Alpha-Cellulose (CAC), Cocoa Microcrystalline Cellulose (C-MCC)
The phytochemical screening of CPH revealed the presence of saponins, carbohydrates, alkaloids, tannins, flavonoids, and triterpenoids which were all absent from CAC and C-MCC (Table 1). The absence of phytochemical constituents from CAC and C-MCC is an indication that the isolated cellulose is pure and free of biological activity thus making them pharmacologically inert [7], which is a desirable property of an ideal excipient.
Table 1.
Phytochemical Screening of Cocoa Pod Husk (CPH), Cocoa Alpha-Cellulose (CAC), Cocoa Microcrystalline Cellulose (C-MCC).
| Constituent | CPH | CAC | C-MCC |
|---|---|---|---|
| Saponin | + | - | - |
| Tannins | + | - | - |
| Alkaloids | + | - | - |
| Carbohydrate | + | - | - |
| Flavonoids | + | - | - |
| Triterpenoids | + | - | - |
+ = present, - = absent.
3.4. Physicochemical Properties of the Cellulose Types
The physicochemical properties of CAC, C-MCC and AV are presented in Table 2.
Table 2.
Physicochemical properties of the cellulose types. Cocoa alpha-cellulose (CAC) and cocoa microcrystalline cellulose (C-MCC) were compared with avicel (AV).
| Parameters | CAC | C-MCC | AV |
|---|---|---|---|
| Color | Off white | White | White |
| Odor | Odorless | Odorless | Odorless |
| Mean particle diameter (μm) | 282.22 ± 0.12 | 161.32 ± 0.04 | 72.51 ± 0.53 |
| True density (g/cm3) | 1.535 ± 0.21 | 1.494 ± 0.13 | 1.507 ± 0.35 |
| Bulk density (g/cm3) | 0.273 ± 0.02 | 0.382 ± 0.06 | 0.316 ± 0.02 |
| Tapped density (g/cm3) | 0.337 ± 0.01 | 0.473 ± 0.04 | 0.469 ± 0.10 |
| Carr’s Index (%) | 18.93 | 19.11 | 32.62 |
| Hausner’s ratio | 1.23 | 1.24 | 1.48 |
| Angle of repose (°) | 35.05 ± 0.54 | 31.09 ± 0.33 | 44.29 ± 0.61 |
| Swelling Capacity (%) | 36.36 ± 0.05 | 27.5 ± 0.01 | 11.23 ± 0.08 |
| Moisture Content (%) | 7.20 ± 0.62 | 5.8 ± 0.37 | 6.6 ± 0.60 |
| pH | 6.7 ± 0.37 | 6.5 ± 0.14 | 6.5 ± 0.21 |
| Crystallinity Index (%) | 26.32 | 43.83 | 69.26 |
3.4.1. Organoleptic Properties of the Cellulose Types
The isolated cellulose types (CAC and C-MCC) were odorless, tasteless, and white in color. These observations are like the reference standard (AV).
3.4.2. Particle Size of the Cellulose Types
Their mean particle diameter was significantly different, with CAC (282.22 μm) > C-MCC (161.32 μm) > AV (72.51 μm). The mean particle diameter of AV was higher than the average mean size of 50 μm as specified in the official compendia for avicel 101. Studies led by Rojas et al. [44] and Doelker et al. [45] also reported smaller mean PS of 50 μm and 41.9 μm for avicel 101, respectively. Because of the small mean particle size of C-MCC and AV, they will easily deform plastically because of a process known as micro-squashing thereby enhancing cohesiveness and compactibility which may be desirable in direct compression [37].
3.4.3. Density Measurements of the Cellulose Types
The bulk densities of the cellulose types were significantly different, with C-MCC (0.382 g/cm3) > AV (0.316 g/cm3) > CAC (0.273 g/cm3). The tapped densities were also significantly different and followed a similar order like bulk densities with C-MCC (0.473 g/cm3) > AV (0.469 g/cm3) > CAC (0.337 g/cm3).
The true densities of cellulose types were significantly different, and in the following order: CAC (1.535 g/cm3) > AV (1.507 g/cm3) > C-MCC > (1.494 g/cm3).
The pH of the cellulose types ranged from 6.5–6.7 which conformed to the United States Pharmacopeia (USP)-National Formulary reference standard of 5.0–7.5 [34].
3.4.4. Moisture Content of the Cellulose Types
The moisture contents of the cellulose types were significantly different with CAC (7.2%) > AV (6.6%) > C-MCC (5.8%). However, they all complied with the USP-NF specification of a maximum of 8%.
3.4.5. Swelling Properties of the Cellulose Types
Swelling capacities of the cellulose types were significantly different with CAC (36.36%) > C-MCC (27.5%) > AV (11.23%). Swelling is one formulation attribute which is essential for tablet disintegration [46]. For faster tablet disintegration, CAC with the highest swelling capacity would be desirable. The swelling tendency of these cellulose types may also be utilized as fillers in wet granulation because of their ability to promote wetting of powder blend [46].
3.4.6. Flow Properties of the Cellulose Types
Powder flow is an important and significant factor in pharmaceutical dosage form designs such as blending, capsule filling, movement of material in the plant, tablet compression, and scale-up operations [47]. It enhances manufacturing efficiency and effectiveness in terms of uniformity in weight, content, and mechanical properties [48]; it also reduces the risk of capping and lamination in tablet production because it prevents entrapment of air [49,50]. Carr’s index, Hausner’s ratio, and angle of repose are powder material properties that give insight into powder flow. The results of these parameters, shown in Table 2, indicate that CAC and C-MCC had Carr’s index between 16–20%, Hausner’s ratio of 1.19–1.25, and an angle of repose of 30–40° which is an indication of a fair flow whereas AV had Carr’s index between 23–35%, Hausner’s ratio of 1.25–1.5 and angle of repose > 40° which is an indication of a poor flow. So, from these results, CAC and C-MCC have better flow than AV. The PS, particle shape, and moisture content of materials are related to the flow of powder materials. As expected, AV with the smallest PS and high moisture content had the least flow due to increased cohesiveness. The poor flow of AV confirms previous published reports [44,45,51].
3.5. Physical Characterization of the Cellulose Types
3.5.1. Scanning Electron Microscopy (SEM)
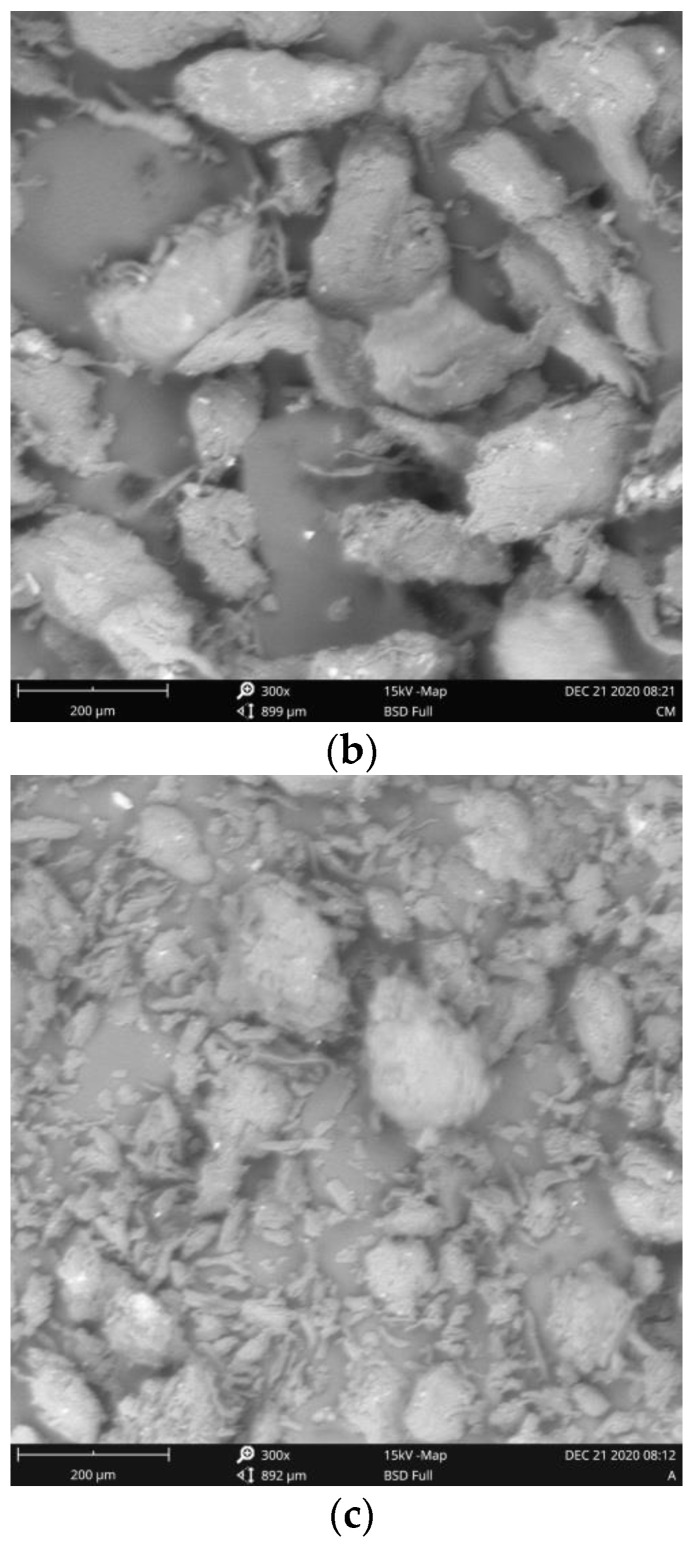
The morphology of the cellulose types was studied by SEM and the resulting micrographs revealed that the three cellulose samples are fibrous in nature, which is typical of cellulose (Figure 2a–c) [52]. The fibers are rough and irregularly shaped. CAC has elongated and aggregated fibers while C-MCC has short and aggregated fibers, and AV has short and non-aggregated fibers. The short fibers found in C-MCC and AV are due to acid hydrolysis of alpha cellulose which led to partial depolymerization of the cellulose.
Figure 2.
(a) SEM micrograph of cocoa alpha-cellulose (CAC), at 300× magnification. (b) SEM micrograph of cocoa microcrystalline cellulose (C-MCC) at 300× magnification. (c) SEM micrograph of avicel (AV) at 300× magnification.
3.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
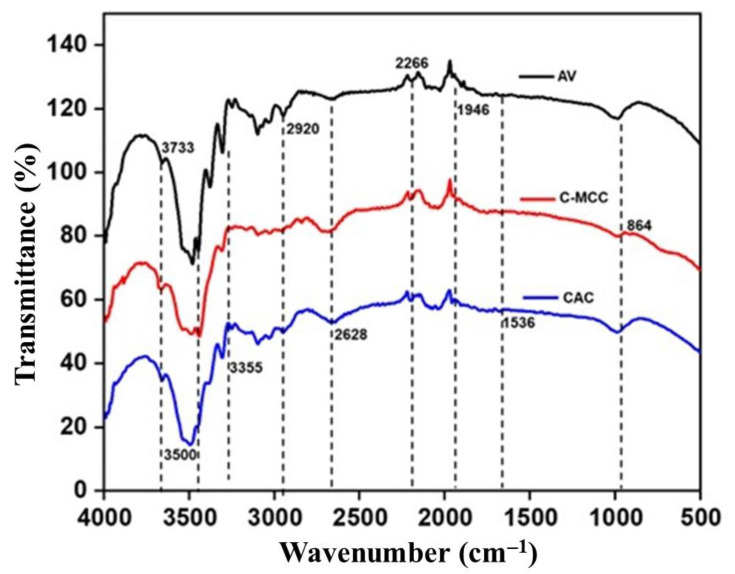
FTIR spectroscopy was used to characterize the cellulose types. FTIR spectra of CAC and C-MCC, isolated from CPH, showed similarities in peak positions with differences in peak height with the reference standard—Avicel 101 (Figure 3). The similarities in peak positions indicate that CAC and C-MCC are indeed cellulose. The presence of absorption peaks at about 3733, 3500, 3492, 2920, 2826, 1946, 1536, 1372, and 864 cm−1 were associated with the representative functional groups of cellulose [53,54]. Noticeably, the broad absorption peak at 3492 cm−1 for AV had slightly shifted to 3500 cm−1 for C-MCC and, CAC suggesting the increasingly exposed cellulose region through the treatments. Furthermore, the absorption peak at 864 cm−1 corresponds to the blending vibrations in C-OH groups [55]. Peaks in the 1500–2000 range are C=C in plane aromatic stretching vibrations. Peaks at 1536 cm−1 correspond to C-H bending (aromatic compounds) or C=C=C stretching (alkene). Peaks at 2628 cm−1 correspond to symmetric C-H stretching. Peaks at 3733.60 cm−1 correspond to O-H stretching vibrations for free OH. The broad band of the unassigned characteristics peaks at 3300–3400 cm−1 for the cellulose is due to inter and intra molecular O–H stretching vibrations which is characteristic of cellulose. A peak at 1936 cm−1 corresponds to O–H bending vibration of water adsorbed in cellulose and some hemicellulose. CH2 bending known as the crystallinity band is present in the cellulose at 1400–1315 cm−1 peak [56]. The presence of C–O–C ether stretching at a peak of 1257 cm−1 for CAC and C-MCC is an indication that the bleaching treatment used during the isolation of the cellulose did not effectively remove lignin from the cellulose. The presence of C–O–C pyranose stretching skeletal vibration in all the spectra confirms the presence of cellulose [55].
Figure 3.
FTIR spectra of avicel (AV), cocoa microcrystalline cellulose (C-MCC) and cocoa alpha-cellulose (CAC).
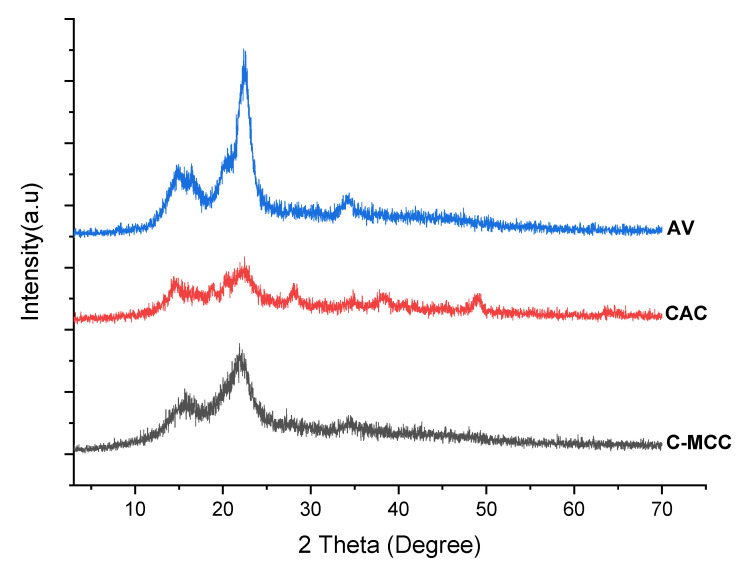
3.5.3. X-ray Diffraction (XRD)
Cellulose is a semi-crystalline polysaccharide which includes crystalline and amorphous regions [57]. There are different crystallite forms of cellulose which are CI, CII, CIII, and CIV [12]. Cellulose I is native cellulose and the most abundantly found in nature. Cellulose II can be prepared by either mercerization (alkali treatment) or regeneration (solubilization and subsequent recrystallization) [12]. Figure 4 displays the XRD spectrum of the C-MCC and CAC isolated from cocoa pod husk and AV cellulose used as standard. In the C-MCC sample, the typical crystalline peaks of cellulose 004, 200, and 002 were observed at 2θ: 15–16°, 22°, and 34° as described in previous literature [58,59,60]. However, the characteristic peak of C-MCC is broader compared to AV. This peak broadening may be due to an increase in the amorphous nature of C-MCC during the synthesis process. CAC shows only one broader peak at 2θ: 19.0–25.0° presumably due to the high amorphous nature of the synthesized cellulose. From the calculation, AV showed the highest crystallinity index (69.26%), followed by C-MCC (43.83%), and lastly by CAC (26.32%). The lowermost crystallinity for CAC was possibly related to the alkaline treatment that softened the structure of cellulose via the swelling process. The result of the swelling capacities of the cellulose is also confirmed by the crystallinity index result. CAC with the highest swelling capacity had more amorphous regions which are responsible for water uptake and thus swelling [61]. The results of the XRD and FTIR of different forms of cellulose agreed with the reported literature since both analyses confirmed the presence of cellulose.
Figure 4.
XRD patterns of avicel (AV), cocoa alpha-cellulose (CAC) and cocoa microcrystalline cellulose (C-MCC).
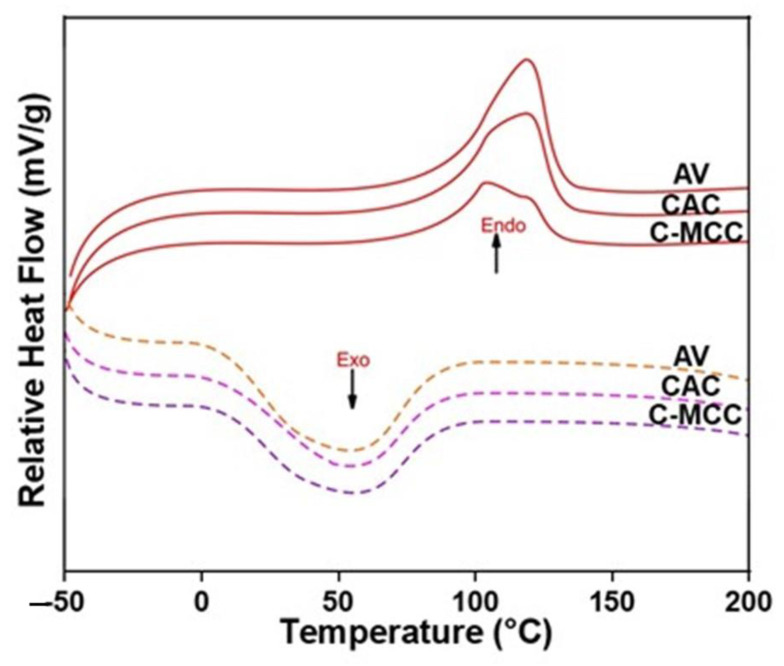
3.5.4. Differential Scanning Calorimetry (DSC)
DSC analysis was used to evaluate the energy consumption properties and thermal stability of the cellulose types (Figure 5). The temperature of 30–200 °C was selected since temperatures above 300 °C could cause decarboxylation reactions and depolymerization of cellulose. The thermal stability of cellulose depends upon the degree of crystallinity and the type and/or source of the cellulose. The broad endothermic peak characteristic of alpha cellulose was observed in the range of 90–130 °C due to the evaporation of moisture and other volatile components. The shift in the maximum temperature of dehydration/evaporation to higher values in the case of AV and CAC may be due to the decrease in their structural crystallinity index. This result is consistent with previous studies on cellulose extracted from different sources [62,63]. The glass transition temperature (Tg) peak of CAC was 68 °C which is within the glass transition temperature of amorphous cellulose (60–70 °C). The possible reason for the reduction of Tg may be due to an increase in the molecular mobility due to the disruption of hydrogen bonds in adjacent macro molecules leading to a decrease in Tg of the amorphous regions inducing a transformation from a hard and rigid to soft and flexible state. The glass transition peak of the amorphous region observed with CAC was absent in C-MCC and AV [64]. The disappearance of this peak in these samples suggests that C-MCC and AV are more crystalline and higher in purity compared to CAC. The observed exothermic peaks around 50 °C in C-MCC, CAC, and AV may be attributed to charring. As a result of various treatment methods, cellulose crystallites can undergo reorientation and rearrangement, leading to a more compact crystal structure and hence, improved thermal stability as observed with C-MCC and AV [65,66].
Figure 5.
DSC curves of avicel (AV), cocoa alpha-cellulose (CAC) and cocoa microcrystalline cellulose (C-MCC).
3.5.5. Evaluation of Tablets
The isolated cellulose types were employed at 25% concentration as a direct compression excipient in the formulation of a metronidazole tablet. The mechanical and disintegration properties of the tablets were assessed to check their suitability in tablet formulation as an excipient. The hardness, friability, and disintegration time of the formulated tablets are presented in Table 3. Tablet hardness is a property that ensures the appropriateness of tablet strength. In this study, there was generally a significant increase in the hardness of the tablets (<0.0001) with an increase in compression pressure due to an increase in inter-particulate bonding. The British Pharmacopeia specifies that the hardness of a conventional uncoated tablet is 4–10 kg/F [67]. None of the tablets formulated with CAC passed the test while all the tablets formulated with C-MCC and AV passed. The reason for the failure could be attributed to the poor thermoplastic properties of the cellulose influenced by the presence of some quantity of hemicellulose and lignin [68]. Suzuki and Nakagami [69] reported reduced crushing strength as crystallinity decreased while Ling et al. [70] reported amorphous materials to have less tensile strength. A similar trend was observed in this study with CAC having the least crystallinity and the smallest hardness. The friability—a measure which describes the tendency of a tablet to break or crumble during manufacturing, packaging, and transportation—is often related to hardness. The friability of the tablets increased with an increase in compression pressure although the increase was not significantly different. However, the tablets formulated with CAC and formulation F4 failed the friability test since their values were greater than 1% while formulations F5–F9 passed. Friability is often inversely related to hardness. As expected, as the hardness of the tablets increased, friability decreased. Disintegration time increased with an increase in compression pressure. AV formulation had a higher disintegration time compared to C-MCC formulations. However, both formulations complied with BP specifications. CAC did not produce tablets with satisfactory properties in terms of hardness and friability but shows a promising activity when formulating a fast-disintegrating conventional formulation or orally disintegrating formulations because of its high swelling capacity.
Table 3.
Tablet hardness, friability, and disintegration time.
| Formulation Code | Hardness (kg/F) | Friability % | Disintegration (min) |
|---|---|---|---|
| F1 | 2.8 ± 3.2 | 2.84 ± 0.03 | 0.82 ± 0.20 |
| F2 | 2.5 ± 3.4 | 2.12 ± 0.02 | 1.15 ± 0.14 |
| F3 | 3.1 ± 2.1 | 1.60 ± 0.04 | 1.20 ± 0.17 |
| F4 | 3.8 ± 2.7 | 1.25 ± 0.05 | 4.12 ± 0.38 |
| F5 | 4.7 ± 3.2 | 0.85 ± 0.01 | 4.92 ± 0.25 |
| F6 | 5.4 ± 2.0 | 0.68 ± 0.06 | 5.21 ± 0.44 |
| F7 | 5.8 ± 2.6 | 0.60 ± 0.01 | 5.70 ± 0.32 |
| F8 | 7.1 ± 3.0 | 0.48 ± 0.02 | 6.55 ± 0.30 |
| F9 | 8.7 ± 2.1 | 0.32 ± 010 | 8.24 ± 0.56 |
F1 = CAC at 56.64 Mnm−2 compression pressure, F2 = CAC at 84.96 Mnm−2 compression pressure, F3 = CAC at 113.28 Mnm−2 compression pressure, F4 = C-MCC at 56.64 Mnm−2 compression pressure, F5 = C-MCC at 84.96 Mnm−2 compression pressure, F6 = C-MCC at 113.28 Mnm−2 compression pressure, F7 = AV at 56.64 Mnm−2 compression pressure, F8 = AV at 84.96 Mnm−2 compression pressure, F9—AV at 113.28 Mnm−2 compression pressure.
4. Conclusions
The mechanical and disintegration properties of metronidazole tablets formulated by direct compression using C-MCC or AV complied with pharmacopeia specifications. C-MCC possessed some fundamental characteristics suitable to make it a pharmaceutical excipient comparable to AV in varieties of pharmaceutical processes and applications.
Author Contributions
Conceptualization, O.A.A., O.A.B., D.A.A. and S.S.; methodology, O.A.A., O.A.B., H.I., G.T., F.M., D.A.A., A.S.A., K.O.S. and Z.S.Y.; software, H.I., D.A.A., A.S.A., K.O.S. and F.M.; validation, O.A.A., O.A.B., S.S., F.M., D.A.A., A.S.A. and H.I.; formal analysis, O.A.A., D.A.A., G.T., F.M., A.S.A., H.I. and K.O.S.; investigation, O.A.A., O.A.B., S.S., K.O.S., Z.S.Y. and M.N.F.-O.; resources, O.A.A., F.M., O.A.B., S.S., K.O.S. and Z.S.Y.; data curation, O.A.A., O.A.B., H.I., G.T., F.M., S.S., K.O.S. and Z.S.Y.; writing—original draft preparation, O.A.A., S.S. and K.O.S.; writing—review and editing, M.N.F.-O., D.A.A., A.S.A., H.I. and F.M.; visualization, O.A.A., O.A.B., S.S., F.M., D.A.A., A.S.A. and H.I.; supervision, O.A.A., O.A.B., M.N.F.-O., F.M., D.A.A. and A.S.A.; project administration, O.A.A., O.A.B., S.S., M.N.F.-O. and F.M.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be requested from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adel A.M., El-shinnawy N.A. Hypolipidemic applications of microcrystalline cellulose composite synthesized from different agricultural residues. Int. J. Biol. Macromol. 2012;51:1091–1102. doi: 10.1016/j.ijbiomac.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhu H., Luo W., Ciesielski P.N., Fang Z., Zhu J.Y., Henriksson G., Himmel M.E., Hu L. Wood derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016;116:9305–9374. doi: 10.1021/acs.chemrev.6b00225. [DOI] [PubMed] [Google Scholar]
- 3.Du H., Liu W., Zhang M., Si C., Zhang X., Li B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019;209:130–144. doi: 10.1016/j.carbpol.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 4.French A.D. Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose. 2017;24:4605–4609. doi: 10.1007/s10570-017-1450-3. [DOI] [Google Scholar]
- 5.Zhao T., Chen Z., Lin X., Ren Z., Li B., Zhang Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018;184:164–170. doi: 10.1016/j.carbpol.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Klemm D., Heublein B., Fink H.P., Bohn A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005;44:3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
- 7.Shokri J., Adibkia K. Application of cellulose and cellulose derivatives in pharmaceutical industries. In: Ven T.V., Godbout L., editors. Cellulose—Medical, Pharmaceutical and Electronic Applications. IntechOpen; London, UK: 2013. [DOI] [Google Scholar]
- 8.Ullah H., Santos H.A., Khan T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose. 2016;23:2291–2314. doi: 10.1007/s10570-016-0986-y. [DOI] [Google Scholar]
- 9.Osorno D.M., Castro C. Cellulose application in food industry: A review. In: Somashekar R., Thejas G., editors. Emergent Research on Polymeric and Composite Materials. IgI Global Publisher; Hershey, PA, USA: 2018. pp. 38–77. [DOI] [Google Scholar]
- 10.Bianchet R.T., Cubas A.V., Machado M.M., Moecke E.S. Applicability of bacterial cellulose in cosmetics—Bibliometric review. Biotechnol. Rep. 2020;27:e00502. doi: 10.1016/j.btre.2020.e00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trache D., Thakur V.K., Boukherroub R. Cellulose nanocrystals/graphene hybrids—A promising new class of materials for advanced applications. Nanomaterials. 2020;10:1523. doi: 10.3390/nano10081523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal A., Katahira R., Himmel M.E., Johnson D.K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels. 2011;4:41. doi: 10.1186/1754-6834-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan A.O. Cellulose: The structure slowly unravels. Cellulose. 1997;4:173–207. doi: 10.1023/A:1018431705579. [DOI] [Google Scholar]
- 14.Kambli N.D., Mageshwaran V., Patil P.G., Saxena S., Deshmukh R.R. Synthesis and characterization of microcrystalline cellulose powder from corn husk fibres using bio-chemical route. Cellulose. 2017;24:5355–5369. doi: 10.1007/s10570-017-1522-4. [DOI] [Google Scholar]
- 15.Szymańska-Chargot M., Chylińska M., Gdula K., Kozioł A., Zdunek A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers. 2017;9:495. doi: 10.3390/polym9100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivai H., Hamdani A.S., Ramdani R., Lalfari R.S., Andayani R., Armin F., Djamaan A. Production and characterization of alpha cellulose derived from rice straw (Oryza sativa L.) Int. J. Pharm. Sci. Rev. Res. 2018;52:45–48. [Google Scholar]
- 17.Nsor-Atindana J., Chen M., Goff H.D., Zhong F., Sharif H.R., Li Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017;15:159–174. doi: 10.1016/j.carbpol.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Thoorens G., Krier F.B., Evrard C.B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014;473:64–72. doi: 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 19.Adedokun M., Essien G.E., Uwah T., Romanus A.U., Josiah I., Jackson C. Evaluation of the release properties of microcrystalline cellulose derived from Saccharum officinarum L. in paracetamol tablet formulation. J. Pharm. Sci. Res. 2014;6:342–346. [Google Scholar]
- 20.Belali N.G., Chaerunisaa A.Y., Rusdiana T. Isolation and characterization of microcrystalline cellulose derived from plants as excipient in tablet: A review. Indonesian J. Pharm. 2019;1:23–29. doi: 10.24198/idjp.v1i2.21515. [DOI] [Google Scholar]
- 21.Campos-Vega R., Nieto-Figueroa K.H., Oomah B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018;81:172–184. doi: 10.1016/j.tifs.2018.09.022. [DOI] [Google Scholar]
- 22.Lu F., Rodriguez-Garcia J., Damme I.V., Westwood N.J., Shaw L., Robinson J., Warren G., Chatzifragkou A., Mason S.M., Gomez L., et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018;14:80–88. doi: 10.1016/j.cogsc.2018.07.007. [DOI] [Google Scholar]
- 23.Lubis M., Gana A., Maysarah S., Ginting M.H., Harahap M.P. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2018;309:012100. doi: 10.1088/1757-899X/309/1/012100. [DOI] [Google Scholar]
- 24.Dahunsi S.O., Adesulu-Dahunsi A.T., Izebere J. Cleaner energy through liquefaction of cocoa (Theobroma cacao) pod husk: Pretreatment and process optimization. J. Clean Prod. 2019;226:578–588. doi: 10.1016/j.jclepro.2019.04.112. [DOI] [Google Scholar]
- 25.Kilam G., Lating P.O., Byaruhanga J., Biira S. Quantification and characterization of cocoa pod husks for electricity generation in Uganda. Energy Sustain. Soc. 2019;9:22. doi: 10.1186/s13705-019-0205-4. [DOI] [Google Scholar]
- 26.Jimat D.N., Jami M.S. Extraction of microcrystalline cellulose (MCC) from cocoa pod husk via alkaline pretreatment combined with ultrasonication. Int. J. Appl. Eng. Res. 2016;11:9876–9879. [Google Scholar]
- 27.Akinjokun A.I., Petrik L.F., Ogunfowokan A.O., Ajao J., Ojumu T.V. Isolation and characterization of nanocrystalline cellulose from cocoa pod husk (CPH) biomass wastes. Heliyon. 2017;7:e06680. doi: 10.1016/j.heliyon.2021.e06680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossain M.A., AL-Raqmi K.A., AL-Mijizy Z.H., Weli A.M., Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013;3:705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seow L.J., Behm H.K., Sadikun A., Asmawi M.Z. Preliminary phytochemical and physicochemical characterization of Gynura segetum (Lour) Merr (Compositae) leaf. Trop. J. Pharm. Res. 2013;12:777–782. doi: 10.4314/tjpr.v12i5.18. [DOI] [Google Scholar]
- 30.Ohwoavworhua F.O., Adelakun T.A. Some physical characteristics of microcrystalline cellulose obtained from raw cotton of Ochlospermum planchonii. Trop. J. Pharm. Res. 2005;4:501–507. doi: 10.4314/tjpr.v4i2.14626. [DOI] [Google Scholar]
- 31.Ejikeme P.M. Investigation of the physicochemical properties of microcrystalline cellulose from agriculture Wastes I: Orange Mesocarp. Cellulose. 2008;15:141–147. doi: 10.1007/s10570-007-9147-7. [DOI] [Google Scholar]
- 32.Guy A., Podczeck F. Handbook of Pharmaceutical Excipients. Pharmaceutical Press; London, UK: 2017. [Google Scholar]
- 33.Young F.S., Hindson W.R. 40—the identification of damage to lignified fibres a new microscopical test using iodine and sulphuric acid. J. Text. Inst. 1958;49:T554–T556. doi: 10.1080/19447025808662464. [DOI] [Google Scholar]
- 34.U.S. Pharmacopoeia . Microcrystalline cellulose. In: Rockville M.D., editor. National Formulary [USP 39 NF 24] Volume 4. United States Pharmacopeial Convention, Inc.; Rockville, MD, USA: 2015. p. 7231. [Google Scholar]
- 35.Karande V.S., Bharimalla A., Hadge G.B., Mhaske S.T., Vigneshwaran N. Nanofibrillation of cotton fibers by disc refiner and its characterization. Fibers Polym. 2011;12:399. doi: 10.1007/s12221-011-0399-3. [DOI] [Google Scholar]
- 36.Adeleye O.A., Femi-Oyewo M.N., Odeniyi M.A., Ajala T.O. Evaluation of Cissus populnea gum as a directly compressible matrix system for tramadol hydrochloride extended-release tablet. J. Appl. Pharm. Sci. 2019;9:105–111. doi: 10.7324/JAPS.2019.90214. [DOI] [Google Scholar]
- 37.Adeleye O.A., Odeniyi M.A., Femi-Oyewo M.N. Physicochemical and rheological characterization of Cissus populnea gum extracted by different solvents. West Afr. J. Pharm. 2015;26:113–126. [Google Scholar]
- 38.Collazo-Bigliardi S., Ortega-Toro R., Chiralt B.A. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018;191:205–215. doi: 10.1016/j.carbpol.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Ohwoavworhua F.O., Adelakun T., Okhamafe A.O. Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: Extraction methods and characterization. Int. J. Green Pharm. 2009;3:97–104. doi: 10.4103/0973-8258.54895. [DOI] [Google Scholar]
- 40.Ohwoavworhua F., Adelakun T. Non-wood fibre production of microcrystalline cellulose from sorghum caudatum: Characterisation and tableting properties. Indian J. Pharm. Sci. 2010;72:295–301. doi: 10.4103/0250-474X.70473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azubuike C., Silva B., Okhamafe A.O. Pharmacopoeial and physicochemical properties of α-cellulose and microcrystalline cellulose powders derived from cornstalks. Int. J. Green Pharm. 2012;6:193–198. doi: 10.4103/0973-8258.104930. [DOI] [Google Scholar]
- 42.Nwajiobi C.C., Otaigbe J.O.E., Oriji O. Isolation and characterization of microcrystalline cellulose from papaya stem. Der Pharma Chem. 2019;11:19–26. [Google Scholar]
- 43.Krstić M., Zoran M., Svetlana I., Jovana P., Slavica R., Tamara B. Lignocellulosic biomass as a source of microcrystalline cellulose-chemical and technological characterization and future perspectives. Cellul. Chem. Technol. 2018;52:577–588. [Google Scholar]
- 44.Rojas J., Lopez A., Guisao S., Ortiz C. Evaluation of several microcrystalline cellulose obtained from agricultural by-products. J. Adv. Pharm. Technol. Res. 2011;2:144–150. doi: 10.4103/2231-4040.85527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doelker E., Maswellel D., Veuillezl F., Humbert-Droz P. Morphological, packing, flow and tableting properties of new avicel types. Drug Dev. Ind. Pharm. 1995;21:643–661. doi: 10.3109/03639049509048132. [DOI] [Google Scholar]
- 46.Chaerunisaa A.Y., Sriwidodo S., Abdassah M. Microcrystalline cellulose as pharmaceutical excipient. In: Ahmad U., Akhtar J., editors. Pharmaceutical Formulation Design—Recent Practices. IntechOpen; London, UK: 2019. [DOI] [Google Scholar]
- 47.Sarraguça M.C., Cruz A.V., Soares S.O., Amaral H.R., Costa P.C., Lopes J.A. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J. Pharm. Biomed. Anal. 2010;52:484–492. doi: 10.1016/j.jpba.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 48.Lumay G., Pillitteri S., Marck M., Monsuur F., Pauly T., Ribeyre O., Francqui F., Vandewalle N. Influence of mesoporous silica on powder flow and electrostatic properties on short and long term. J. Drug Deliv. Sci. Technol. 2019;53:101192. doi: 10.1016/j.jddst.2019.101192. [DOI] [Google Scholar]
- 49.Staniforth J. Powder flow. In: Aulton M., editor. Pharmaceutics, the Science of Dosage Form Design. 2nd ed. Churchill Livingstone; London, UK: 2002. pp. 197–210. [Google Scholar]
- 50.McDonagh A.F., Duff B., Brennan L., Tajber L. The impact of the degree of intimate mixing on the compaction properties of materials produced by crystallo-co-spray drying. Eur. J. Pharm. Sci. 2020;154:105505. doi: 10.1016/j.ejps.2020.105505. [DOI] [PubMed] [Google Scholar]
- 51.Soppela I., Airaksinen S., Murtomaa M., Tenho M., Hatara J., Räikkönen H., Yliruusi J., Sandler N. Investigation of the powder flow behavior of binary mixtures of microcrystalline cellulose and paracetamol. J. Excip. Food Chem. 2010;1:55–67. [Google Scholar]
- 52.Tristantini D., Dewanti D.P., Sandra C. Isolation and characterization of α-cellulose from blank bunches of palm oil and dry jackfruit leaves with alkaline process NaOH continued with bleaching process H2O2. In: Tursiloadi S., Rinaldi N., editors. Proceedings of the 3rd International Symposium on Applied Chemistry [20001] (AIP Conference Proceedings; Vol. 1904); Jakarta, Indonesia. 23–24 October 2017; Jakarta, Indonesia: American Institute of Physics Inc.; 2017. [DOI] [Google Scholar]
- 53.Merci A., Urbano A., Grossmann M.E., Tischer C.A., Mali S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015;73:38–43. doi: 10.1016/j.foodres.2015.03.020. [DOI] [Google Scholar]
- 54.Hou W., Ling C., Shi S., Yan Z. Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019;123:363–368. doi: 10.1016/j.ijbiomac.2018.11.112. [DOI] [PubMed] [Google Scholar]
- 55.Kian L.K., Jawaid M., Ariffin H., Alothman O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017;103:931–940. doi: 10.1016/j.ijbiomac.2017.05.135. [DOI] [PubMed] [Google Scholar]
- 56.Pachuau L., Dutta R.S., Hauzel L., Devi T.B., Deka D. Evaluation of novel microcrystalline cellulose from Ensete glaucum (Roxb.) Cheesman biomass as sustainable drug delivery biomaterial. Carbohydr. Polym. 2019;206:336–343. doi: 10.1016/j.carbpol.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Niinivaara E., Faustini M., Tammelin T., Kontturi E. Mimicking the humidity response of the plant cell wall by using 2d systems: The critical role of amorphous and crystalline polysaccharides. Langmuir. 2016;32:2032–2040. doi: 10.1021/acs.langmuir.5b04264. [DOI] [PubMed] [Google Scholar]
- 58.Kallel F., Bettaieb F., Khiari R., García A., Bras J., Ellouz C.S. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw. Ind. Crops Prod. 2016;87:287–296. doi: 10.1016/j.indcrop.2016.04.060. [DOI] [Google Scholar]
- 59.Johar N., Ahmad I., Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012;37:93–99. doi: 10.1016/j.indcrop.2011.12.016. [DOI] [Google Scholar]
- 60.Savadekar N.M., Mhaske S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012;89:146–151. doi: 10.1016/j.carbpol.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 61.Anis S.F., Lalia B.S., Hashaikeh R. Controlling swelling behavior of poly (vinyl) alcohol via networked cellulose and its application as a reverse osmosis membrane. Desalination. 2014;336:138–145. doi: 10.1016/j.desal.2014.01.005. [DOI] [Google Scholar]
- 62.Ciolacu D., Ciolacu F., Popa V.I. Amorphous cellulose—Structure and characterization. Cellul. Chem. Technol. 2011;45:13–21. [Google Scholar]
- 63.Morán J.I., Alvarez V.A., Cyras V.P., Vazquez A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose. 2008;15:149–159. doi: 10.1007/s10570-007-9145-9. [DOI] [Google Scholar]
- 64.Keshk S.M., Haija M.A. A new method for producing microcrystalline cellulose from Gluconacetobacter xylinus and kenaf. Carbohydr. Polym. 2011;84:1301–1305. doi: 10.1016/j.carbpol.2011.01.024. [DOI] [Google Scholar]
- 65.Awadel-Karim S., Nazhad M.M., Paszner L. Factors affecting crystalline structure of cellulose during solvent purification treatment. Holzforschung. 1999;53:1–8. doi: 10.1515/HF.1999.001. [DOI] [Google Scholar]
- 66.Bhuiyan M.R., Hirai N., Sobue N. Changes of crystallinity in wood cellulose by heat treatment under dried and moist conditions. J. Wood Sci. 2000;46:431–436. doi: 10.1007/BF00765800. [DOI] [Google Scholar]
- 67.Altamimi M.J., Greenwood J.C., Wolff K., Hogan M.E., Lakhani A., Martin G.P., Royall P.G. Anti-counterfeiting DNA molecular tagging of pharmaceutical excipients: An evaluation of lactose containing tablets. Int. J. Pharm. 2019;571:118656. doi: 10.1016/j.ijpharm.2019.118656. [DOI] [PubMed] [Google Scholar]
- 68.Ornaghi H.L., Poletto M.P., Zattera A.J., Amico S.C. Correlation of the thermal stability and the decomposition kinetics of six different vegetal fibers. Cellulose. 2014;21:177–188. doi: 10.1007/s10570-013-0094-1. [DOI] [Google Scholar]
- 69.Suzuki T., Nakagami H. Effect of crystallinity of microcrystalline cellulose on the compactability and dissolution of tablets. Eur. J. Pharm. Biopharm. 1999;47:225–230. doi: 10.1016/S0939-6411(98)00102-7. [DOI] [PubMed] [Google Scholar]
- 70.Ling Z., Wang T., Makarem M., Santiago C.M., Cheng H.N., Kang X., Bacher M., Potthast A., Rosenau T., King H., et al. Effects of ball milling on the structure of cotton cellulose. Cellulose. 2019;26:305–328. doi: 10.1007/s10570-018-02230-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be requested from the corresponding authors.